Abstract
Middle East respiratory syndrome coronavirus (MERS-CoV), an emerging infectious disease of growing global importance, has caused severe acute respiratory disease in more than 1600 people, resulting in more than 600 deaths. The high case fatality rate, growing geographic distribution and vaguely defined epidemiology of MERS-CoV have created an urgent need for effective public health countermeasures, paramount of which is an effective means of prevention through a vaccine or antibody prophylaxis. Despite the relatively few number of cases to-date, research and development of MERS-CoV vaccine candidates is advancing quickly. This review surveys the landscape of these efforts across multiple groups in academia, government and industry.
Keywords: Middle East Respiratory Syndrome, Coronavirus, Vaccine, Research, Development
1. Introduction
Middle East Respiratory Syndrome (MERS-CoV) was first isolated in September 2012 from a patient in Saudi Arabia who presented two months earlier with severe acute respiratory infection and acute renal failure [1]. Retrospective testing of samples in Jordan identified earlier cases from a nosocomial outbreak in April 2012 [2]. Although the majority of MERS-CoV cases (∼75%) have occurred in Saudi Arabia, 25 other countries have confirmed imported or autochthonously transmitted cases (Fig. 1 ) [3], [4]. The most recent and largest outbreak outside of Saudi Arabia occurred in South Korea in May 2015 [5], raising concern for an eruption of regional outbreaks or accelerated global spread, similar to the phylogenetically related severe acute respiratory syndrome coronavirus (SARS-CoV) that killed nearly a thousand people a decade earlier [6]. Although the definitive host for MERS-CoV has not yet been established, closely related coronaviruses have been isolated from bats across wide geographic areas [7], [8], [9]. Mounting evidence has strongly implicated dromedary camels as the intermediate animal reservoir, as serological surveys throughout the Middle East and North Africa have demonstrated them to have a high prevalence of MERS-CoV binding or neutralizing Abs [10], [11], [12], [13]. Additionally, outbreak investigations have suggested epidemiologic linkage between farm camels and human cases [14].
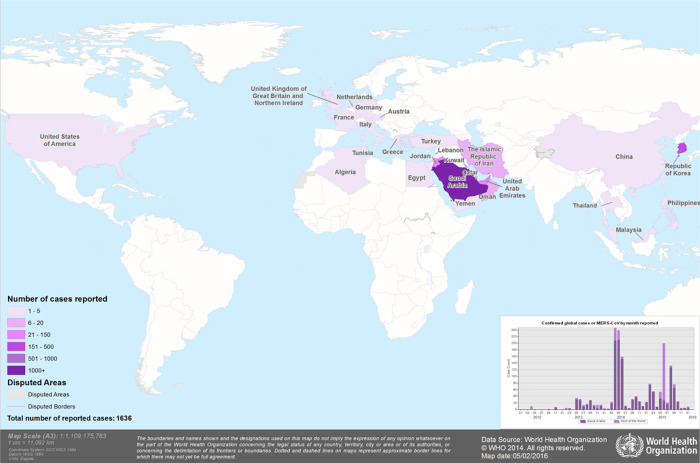
Fig. 1.
Geographic distribution (MERS-CoV) confirmed cases, 2012–2016. To date, there have been more than 1600 cases and 600 deaths from MERS-CoV. Shading corresponds to the last year a case was confirmed in that country. Red circles only indicate the number of cases confirmed in 2016. The number of cases before 2016 are not reflected in this map.
MERS-CoV is a spherical, enveloped, single-stranded, positive sense RNA beta-coronavirus [1], [15]. Its genome contains a replicase locus at the 5′ end and codes for structural proteins toward the 3′ end. The most immunogenic of the viral proteins is Spike (S), a trimeric, envelope-anchored, type I fusion glycoprotein that interfaces with its human host cognate receptor, dipeptidyl peptidase 4 (DPP4), to mediate viral entry [16], [17]. S comprises two subunits: S1, which contains the receptor-binding domain and determines cell tropism; and S2, the location of the cell fusion machinery. Although DPP4 has a broad tissue distribution, most of the clinical manifestations of MERS-CoV can be attributed to its localization to the lower respiratory tract [18], [19]. Much like other coronaviruses, MERS-CoV can also cause significant dysfunction of the gastrointestinal, cardiovascular, renal, and neurologic systems. MERS-CoV is distinct, though, in its tendency to cause greatest harm to older individuals with concurrent comorbidities of one or more of these organ-systems [20], [21].
Despite past efforts to develop coronavirus countermeasures in response to the SARS-CoV pandemic, there are currently no prophylactic or therapeutic interventions of proven efficacy for MERS-CoV or any other coronavirus infection. Although combination treatment with ribavirin and interferons were shown to improve clinical outcomes in MERS-CoV-infected non-human primates (NHPs), treatment was initiated very soon after viral challenge (∼8 h) and results have not been replicated in humans [22]. In fact, no experimental interventions have demonstrated appreciable benefit in acutely ill patients in a consistent or controlled manner. Rapidly scaled treatments based on naturally occurring neutralizing antibodies such as convalescent plasma or hyperimmune globulin, on the other hand, have demonstrated mortality reductions for other respiratory infections and may hold promise for MERS-CoV as well [23]. Their development, however, is limited by logistical challenges, local technical capacity, and donor supply. Supportive management, adapted from guidelines developed for SARS-CoV, has thus far been the mainstay of MERS-CoV treatment.
2. Prospective for an effective human vaccine
The global will to develop a coronavirus vaccine faded in the aftermath of SARS-CoV pandemic but has since gained renewed momentum in the face of the current MERS-CoV outbreak. Previous approaches to coronavirus vaccine development were broad and included whole-inactivated and live-attenuated viruses, recombinant vectors and protein subunits, as well as DNA and RNA based platforms [6]. Most developers based their immunogen designs on the S surface glycoprotein, the primary target for neutralizing antibodies during any natural coronavirus infection. A number of preclinical and clinical studies showed that the SARS-CoV S1 protein subunit, and specifically the RBD at its core, could serve as a dominant target for neutralizing antibodies in mice, non-human primates, and humans [24]. S1, therefore, became the basis for a number of promising SARS-CoV vaccine candidates.
The S1 protein subunit and RBD have also been the basis for several MERS-CoV vaccine candidates (Fig. 2 ) [25], [26], [27], [28], [29]. Resolution of RBD crystal structures alone or in complex with the DPP4 receptor [25], [30], [31] have informed the design of immunogens that have been expressed either as recombinant protein fragments or conjugates to the fragment crystallizable (Fc) region of human antibodies. Both types of constructs, in formulation with aluminum salt or oil-in-water adjuvants, have elicited neutralizing antibodies of high potency across multiple viral strains. Despite their demonstrated immunogenicity in animal models and anticipated safety in humans, RBD or S1-subunit based vaccine candidates are limited in their epitope breadth. Although the coronavirus genomes are not as variable as other RNA viruses, the RBD is the most mutable region, containing mutation sites that define antibody escape variants [25], [32]. Thus, vaccine candidates that elicit a more diverse antibody repertoire as well as a robust cellular immune response may offer the advantage of broader and more durable protection.
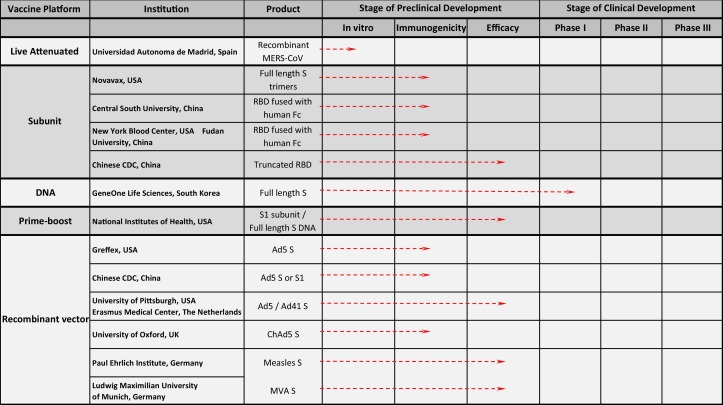
Fig. 2.
Summary of active MERS-CoV vaccine candidates in development. Currently, there are more than a dozen published active vaccine candidates across five general platforms in development for MERS-CoV. To date, all but one of the candidates are in various stages of preclinical development. The DNA-alone candidate has entered Phase I human testing. S: Spike glycoprotein; Fc: crystallizeable fraction of a human antibody; RBD: receptor binding domain of the Spike glycoprotein; Ad5: adenovirus serotype 5; Ad41: adenovirus serotype 41, MVA: modified vaccinia Ankara virus.
Full-length S used as an immunogen could at least increase the breadth of the antibody response; however, it has been difficult to express, and may require additional work to produce a stable soluble trimer of the S ectodomain. Investigators at the University of Maryland, in collaboration with Novavax, Inc., have overcome this problem through the development of S rosettes that are stable and immunogenic in murine models [33]. Vaccines that mimic natural infection, such as live-attenuated viruses or recombinant viral vectors, may elicit even more robust immunity. Live attenuated viruses have historically been among the most immunogenic platforms available, as they have the capacity to present multiple antigens across the viral life cycle in their native conformations. Although a live-attenuated MERS-CoV has yet to be tested, one has been constructed and has the potential to be protective [34]. However, manufacturing live-attenuated viruses requires containment in a biosafety level 3 or 4 facility. Additionally, live-attenuated viruses carry the hazards of inadequate attenuation or reversion to wild type form and causing disseminated disease, particularly in immunocompromised hosts. Given that moderately immunocompromised adults with co-morbidities such as diabetes mellitus and chronic kidney disease have suffered the most severe MERS-CoV disease, these individuals may comprise a target population for immunization, thus making a live-attenuated virus vaccine a less viable option. Replication competent viral vectors could pose a similar threat for disseminated disease in the immunosuppressed. Replication deficient vectors, however, avoid that risk while maintaining the advantages of native antigen presentation, elicitation of T cell immunity and the ability to express multiple antigens. To date, two recombinant vector platforms—modified vaccinia virus Ankara (MVA) and adenovirus vectors—have been used to express MERS-CoV S glycoprotein. Both viral vectors have been immunogenic and one of the MVA candidates was protective in a mouse model transduced with human DPP4 [35], [36], [37], [38].
Although replication deficient vectors are relatively safe and immunogenic, their ability to deliver genetic material for expression could be impeded by pre-existing or developing immunity to the vector itself. One way to overcome this limitation is by administering different vectors in a so-called prime-boost immunization regimen. As this strategy has been effective for other pathogens, it is likely that the same success could be recapitulated for MERS-CoV. The use of more than one type of platform or antigen in a single vaccine also increases the likelihood of inducing a broad repertoire of antibodies with diverse mechanisms of viral neutralization. One vaccine regimen developed at the US National Institutes of Health is based on full-length S DNA and a truncated S1 subunit glycoprotein and has elicited neutralizing antibodies in mice directed at both the S1—within and outside the RBD—and S2 subunits. Immunization with these constructs also protected NHPs from severe lung disease after intra-tracheal challenge with MERS-CoV [25]. A DNA-only vaccine, expressing multiple antigens, has also been developed by Inovio Pharmaceuticals and GeneOne Life Science Inc. and has been advanced to a Phase I first-in-human trial.
3. Monoclonal antibodies (mAbs) in development
Each of the vaccine candidates that have been mentioned is being developed for prophylactic use. However, as the total number of cases (>1600) [3], [4] and reproductive rate (∼0.7) of MERS-CoV are both relatively low [39], it will be difficult to define the target populations for vaccination that would support the investment in manufacturing and advanced product development. Also, with such low incidence and lack of robust animal models, it would be difficult to achieve a vaccine efficacy result that would be sufficient to support licensure. Human mAbs, on the other hand, could be used without as much discrimination in an outbreak setting for post-exposure prophylaxis and early treatment. The advantages of mAbs over polyclonal antibodies (administered through convalescent plasma or hyperimmune globulin) are their higher potency, greater specificity, more extensive pre-licensing evaluation and consequently improved safety profile. Additionally, mAbs can help define immunogenic epitopes through crystallographic analysis, thereby providing atomic level detail for the design of better immunogens. However, the timeline for mAb development may be longer and potentially cost more than some vaccines.
Despite requirements for greater upfront investment, several groups have developed highly potent mAbs that are currently being advanced through pre-clinical stages of testing (Fig. 3 ). Some have been isolated from immunized animals (mice/humanized mice/NHPs) [25], [40], while others have been identified from either an antibody human phage library [41], [42], [43], [44] or memory B cells of infected and recovered human survivors [45]. Almost all of the mAbs that have been reported target the Spike RBD. It is likely that mAbs directed at other sites on the Spike glycoprotein have been recovered but are not as potent neutralizers. Most of those that have been published bind to recombinant Spike with picomolar affinity and neutralize MERS-CoV pseudovirus at a half maximal inhibitory concentration (IC50) of 0.1 mcg/μl or less. Additionally, some have demonstrated protective efficacy in pre- and post-exposure prophylaxis animal models [44], [45]. The successes thus far in isolating potent and protective mAbs may prove useful for therapeutic development where the target population is well defined. It may prove more challenging to advancing these products to licensure and full-scale production at affordable costs for the purpose of prophylaxis in as of yet undefined populations.
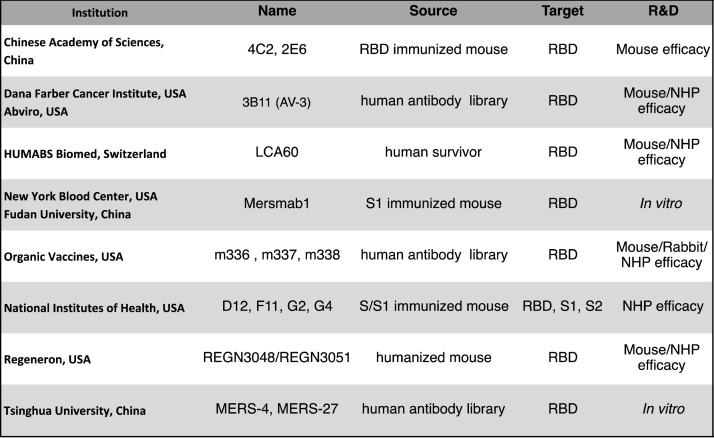
Fig. 3.
Summary of MERS-CoV monoclonal antibodies (mAbs) in development.
Several groups have identified monoclonal antibodies that have at least shown potent neutralization against MERS-CoV and in some cases have protected transgenic mice and non-human primates from MERS-CoV disease after viral challenge. RBD: Receptor Binding Domain; S: Spike glycoprotein; S1: Spike domain containing RBD; S2: Spike domain containing fusion machinery.
4. Target populations for vaccination
The vaguely defined epidemiology of MERS-CoV has complicated the design and implementation of appropriate public health countermeasures. Most transmission events have occurred either in the setting of household clusters or nosocomial outbreaks [46], [47], [48], [49], [50], [51]. It is also likely that the virus has been introduced multiple times into human populations from a large zoonotic reservoir, i.e. dromedary camels. Given the broad distribution and ownership of camels in the Arabian Peninsula where most cases have occurred, a targeted vaccine campaign may prove difficult. As the outbreak in the Republic of Korea revealed, patients and workers in the same healthcare facility as an infected patient are at high risk for secondary acquisition. An optimal strategy may be to use vaccines in conjunction with stringent infection control practices in hospitals where MERS-CoV cases are being treated.
The epidemiologic link of MERS-CoV between bats, camels, and humans presents an opportunity for a veterinary vaccine to interrupt the transmission cycle. A successful precedent for this so-called “OneHealth” approach toward mitigating human disease with a veterinary vaccine exists in the example of Equivac®, a Hendra virus vaccine developed solely for horses [52]. Although Hendra virus is even more rare than MERS-CoV, it is highly fatal with no treatment other than intensive supportive management. In 2012, a protein subunit vaccine was licensed and rolled-out in Australia, where all outbreaks of the virus occurred. Since that time, the incidence in horses has fallen precipitously and no human cases have been detected [53]. A similar strategy may be applicable to MERS-CoV; however, a veterinary vaccination in this context would be deployed solely for the sake of protecting humans, as the virus causes only mild upper respiratory illness in camels. Safety and reduction in viral shedding would have to be demonstrated in immunization, challenge and transmission studies of camel or camelid populations, one of which has shown efficacy [54].
5. Knowledge gaps and ways forward
One of the primary challenges to developing countermeasures to MERS-CoV is the lack of an appropriate animal model that recapitulates the natural history of human disease. Much of the difficulty originates from the absence of the virus's cognate DPP4 receptor. One group approached this problem by successfully transducing mice with an adenoviral vector expressing human DPP4 [55]. Although more relevant than a standard murine model, transient transduction of the desired protein may result in inconsistent tissue expression of adenovirus antigens. Agrawal et al. made an important advance with the development of a transgenic mouse model that demonstrated productive, disseminated MERS-CoV infection [56]. Although rhesus macaques do not manifest full clinical disease, they develop a transient lower respiratory infection that can be quantified and evaluated by computed tomography. Investigators at the NIH Rocky Mountain Laboratories (RML) and Integrated Research Facility (IRF) have also independently been developing potentially lethal marmoset models that could be used for the evaluation of vaccines, mAbs and therapeutics [57].
As MERS-CoV vaccines—both active and passive—are developed and tested, not only will more relevant animal models be required, but there will also be a need for a more detailed understanding of the epidemiology, immunology, and pathogenesis of the virus. In the aftermath of the West African Ebola virus epidemic and in the face of the current Zika virus outbreak, the global health community has coalesced around the realization that a multi-faceted plan is required to quickly and efficiently respond to global public health emergencies. The World Health Organization is currently developing a blueprint by which that preparation and response can follow, with MERS-CoV highlighted as a case study. Although MERS-CoV still causes relatively few cases in a limited geographic distribution, its high case fatality and sudden outbreak in in Korea have proven it to be a pathogen of public health concern. The concentration of the epidemic to Saudi Arabia also raises the specter of international spread every year during Hajj, one of the largest mass gathering events in the world. Ultimately, the development of a safe and effective vaccine for MERS-CoV may not yield its greatest benefit for the current epidemic but for the knowledge gained in creating a platform for combating coronaviruses as a whole.
Conflict of interest
The opinions expressed herein are those of the authors and should not be construed as official or representing the views of the US Department of Defense or the Department of the Army.
References
- 1.Zaki A.M., van Boheemen S., Bestebroer T.M., Osterhaus A.D., Fouchier R.A. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367:1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 2.Hijawi B., Abdallat M., Sayaydeh A., Alqasrawi S., Haddadin A., Jaarour N. Novel coronavirus infections in Jordan, April 2012: epidemiological findings from a retrospective investigation. East Mediterr Health J. 2013;19(Suppl 1):S12–S18. [PubMed] [Google Scholar]
- 3.WHO . World Health Organization; Geneva, Switzerland: 2014. Middle East Respiratory Syndrome Coronavirus (MERS-CoV) Summary and Literature Update–as of 11 June 2014. [11 June] [Google Scholar]
- 4.ECDC . European Centre for Disease Prevention and Control; Stockholm, Sweden: 2014. Epidemiological Update: Middle East Respiratory Syndrome Coronavirus (MERS-CoV) [2 July] [Google Scholar]
- 5.Lee S.S., Wong N.S. Probable transmission chains of Middle East respiratory syndrome coronavirus and the multiple generations of secondary infection in South Korea. Int J Infect Dis. 2015;38:65–67. doi: 10.1016/j.ijid.2015.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Graham R.L., Donaldson E.F., Baric R.S. A decade after SARS: strategies for controlling emerging coronaviruses. Nat Rev Microbiol. 2013;11:836–848. doi: 10.1038/nrmicro3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lelli D., Papetti A., Sabelli C., Rosti E., Moreno A., Boniotti M.B. Detection of coronaviruses in bats of various species in Italy. Viruses. 2013;5:2679–2689. doi: 10.3390/v5112679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wacharapluesadee S., Duengkae P., Rodpan A., Kaewpom T., Maneeorn P., Kanchanasaka B. Diversity of coronavirus in bats from Eastern Thailand. Virol J. 2015;12:57. doi: 10.1186/s12985-015-0289-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang L., Wu Z., Ren X., Yang F., Zhang J., He G. MERS-related betacoronavirus in Vespertilio superans bats, China. Emerg Infect Dis. 2014;20:1260–1262. doi: 10.3201/eid2007.140318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alagaili A.N., Briese T., Mishra N., Kapoor V., Sameroff S.C., Burbelo P.D. Middle East respiratory syndrome coronavirus infection in dromedary camels in Saudi Arabia. MBio. 2014;5 doi: 10.1128/mBio.00884-14. e00884-00814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alexandersen S., Kobinger G.P., Soule G., Wernery U. Middle East respiratory syndrome coronavirus antibody reactors among camels in Dubai, United Arab Emirates, in 2005. Transbound Emerg Dis. 2014;61:105–108. doi: 10.1111/tbed.12212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corman V.M., Jores J., Meyer B., Younan M., Liljander A., Said M.Y. Antibodies against MERS coronavirus in dromedary camels, Kenya, 1992–2013. Emerg Infect Dis. 2014;20:1319–1322. doi: 10.3201/eid2008.140596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hemida M.G., Chu D.K., Poon L.L., Perera R.A., Alhammadi M.A., Ng H.Y. MERS coronavirus in dromedary camel herd, Saudi Arabia. Emerg Infect Dis. 2014;20:1231–1234. doi: 10.3201/eid2007.140571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Azhar E.I., El-Kafrawy S.A., Farraj S.A., Hassan A.M., Al-Saeed M.S., Hashem A.M. Evidence for camel-to-human transmission of MERS coronavirus. N Engl J Med. 2014;370:2499–2505. doi: 10.1056/NEJMoa1401505. [DOI] [PubMed] [Google Scholar]
- 15.Raj V.S., Osterhaus A.D., Fouchier R.A., Haagmans B.L. MERS: emergence of a novel human coronavirus. Curr Opin Virol. 2014;5:58–62. doi: 10.1016/j.coviro.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu G., Hu Y., Wang Q., Qi J., Gao F., Li Y. Molecular basis of binding between novel human coronavirus MERS-CoV and its receptor CD26. Nature. 2013;500:227–231. doi: 10.1038/nature12328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ohnuma K., Haagmans B.L., Hatano R., Raj V.S., Mou H., Iwata S. Inhibition of Middle East respiratory syndrome coronavirus infection by anti-CD26 monoclonal antibody. J Virol. 2013;87:13892–13899. doi: 10.1128/JVI.02448-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention Update: severe respiratory illness associated with Middle East respiratory syndrome coronavirus (MERS-CoV)—worldwide, 2012–2013. MMWR Morb Mortal Wkly Rep. 2013;62:480–483. [PMC free article] [PubMed] [Google Scholar]
- 19.de Wit E., Rasmussen A.L., Falzarano D., Bushmaker T., Feldmann F., Brining D.L. Middle East respiratory syndrome coronavirus (MERS-CoV) causes transient lower respiratory tract infection in rhesus macaques. Proc Natl Acad Sci U S A. 2013;110:16598–16603. doi: 10.1073/pnas.1310744110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Al-Tawfiq J.A., Assiri A., Memish Z.A. Middle East respiratory syndrome novel corona MERS-CoV infection. Epidemiology and outcome update. Saudi Med J. 2013;34:991–994. [PubMed] [Google Scholar]
- 21.Assiri A., Al-Tawfiq J.A., Al-Rabeeah A.A., Al-Rabiah F.A., Al-Hajjar S., Al-Barrak A. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect Dis. 2013;13:752–761. doi: 10.1016/S1473-3099(13)70204-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Falzarano D., de Wit E., Rasmussen A.L., Feldmann F., Okumura A., Scott D.P. Treatment with interferon-alpha2b and ribavirin improves outcome in MERS-CoV-infected rhesus macaques. Nat Med. 2013;19:1313–1317. doi: 10.1038/nm.3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mair-Jenkins J., Saavedra-Campos M., Baillie J.K., Cleary P., Khaw F.M., Lim W.S. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta-analysis. J Infect Dis. 2015;211:80–90. doi: 10.1093/infdis/jiu396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumar V., Jung Y.S., Liang P.H. Anti-SARS coronavirus agents: a patent review (2008—present) Expert Opin Ther Pat. 2013;23:1337–1348. doi: 10.1517/13543776.2013.823159. [DOI] [PubMed] [Google Scholar]
- 25.Wang L., Shi W., Joyce M.G., Modjarrad K., Zhang Y., Leung K. Evaluation of candidate vaccine approaches for MERS-CoV. Nat Commun. 2015;6:7712. doi: 10.1038/ncomms8712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Du L., Kou Z., Ma C., Tao X., Wang L., Zhao G. A truncated receptor-binding domain of MERS-CoV spike protein potently inhibits MERS-CoV infection and induces strong neutralizing antibody responses: implication for developing therapeutics and vaccines. PLoS One. 2013;8:e81587. doi: 10.1371/journal.pone.0081587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma C., Li Y., Wang L., Zhao G., Tao X., Tseng C.T. Intranasal vaccination with recombinant receptor-binding domain of MERS-CoV spike protein induces much stronger local mucosal immune responses than subcutaneous immunization: Implication for designing novel mucosal MERS vaccines. Vaccine. 2014;32:2100–2108. doi: 10.1016/j.vaccine.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang J., Zhang N., Tao X., Zhao G., Guo Y., Tseng C.T. Optimization of antigen dose for a receptor-binding domain-based subunit vaccine against MERS coronavirus. Hum Vaccin Immunother. 2015;11:1244–1250. doi: 10.1080/21645515.2015.1021527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang N., Tang J., Lu L., Jiang S., Du L. Receptor-binding domain-based subunit vaccines against MERS-CoV. Virus Res. 2015;202:151–159. doi: 10.1016/j.virusres.2014.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen Y., Rajashankar K.R., Yang Y., Agnihothram S.S., Liu C., Lin Y.L. Crystal structure of the receptor-binding domain from newly emerged Middle East respiratory syndrome coronavirus. J Virol. 2013;87:10777–10783. doi: 10.1128/JVI.01756-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang N., Shi X., Jiang L., Zhang S., Wang D., Tong P. Structure of MERS-CoV spike receptor-binding domain complexed with human receptor DPP4. Cell Res. 2013;23:986–993. doi: 10.1038/cr.2013.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sui J., Deming M., Rockx B., Liddington R.C., Zhu Q.K., Baric R.S. Effects of human anti-spike protein receptor binding domain antibodies on severe acute respiratory syndrome coronavirus neutralization escape and fitness. J Virol. 2014;88:13769–13780. doi: 10.1128/JVI.02232-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coleman C.M., Liu Y.V., Mu H., Taylor J.K., Massare M., Flyer D.C. Purified coronavirus spike protein nanoparticles induce coronavirus neutralizing antibodies in mice. Vaccine. 2014;32:3169–3174. doi: 10.1016/j.vaccine.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Almazan F., DeDiego M.L., Sola I., Zuniga S., Nieto-Torres J.L., Marquez-Jurado S. Engineering a replication-competent, propagation-defective Middle East respiratory syndrome coronavirus as a vaccine candidate. MBio. 2013;4 doi: 10.1128/mBio.00650-13. e00650-00613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Song F., Fux R., Provacia L.B., Volz A., Eickmann M., Becker S. Middle East respiratory syndrome coronavirus spike protein delivered by modified vaccinia virus Ankara efficiently induces virus-neutralizing antibodies. J Virol. 2013;87:11950–11954. doi: 10.1128/JVI.01672-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Volz A., Kupke A., Song F., Jany S., Fux R., Shams-Eldin H. Protective efficacy of recombinant modified vaccinia virus Ankara delivering Middle East respiratory syndrome coronavirus spike glycoprotein. J Virol. 2015;89:8651–8656. doi: 10.1128/JVI.00614-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guo X., Deng Y., Chen H., Lan J., Wang W., Zou X. Systemic and mucosal immunity in mice elicited by a single immunization with human adenovirus type 5 or 41 vector-based vaccines carrying the spike protein of Middle East respiratory syndrome coronavirus. Immunology. 2015;145:476–484. doi: 10.1111/imm.12462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim E., Okada K., Kenniston T., Raj V.S., AlHajri M.M., Farag E.A. Immunogenicity of an adenoviral-based Middle East respiratory syndrome coronavirus vaccine in BALB/c mice. Vaccine. 2014;32:5975–5982. doi: 10.1016/j.vaccine.2014.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Majumder M.S., Rivers C., Lofgren E., Fisman D. Estimation of MERS-coronavirus reproductive number and case fatality rate for the spring 2014 Saudi Arabia outbreak: insights from publicly available data. PLoS Curr. 2014;6 doi: 10.1371/currents.outbreaks.98d2f8f3382d84f390736cd5f5fe133c. pii: ecurrents.outbreaks.98d2f8f3382d84f390736cd5f5fe133c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Faure E., Poissy J., Goffard A., Fournier C., Kipnis E., Titecat M. Distinct immune response in two MERS-CoV-infected patients: can we go from bench to bedside? PLoS One. 2014;9:e88716. doi: 10.1371/journal.pone.0088716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jiang L., Wang N., Zuo T., Shi X., Poon K.M., Wu Y. Potent neutralization of MERS-CoV by human neutralizing monoclonal antibodies to the viral spike glycoprotein. Sci Transl Med. 2014;6(234):234ra59. doi: 10.1126/scitranslmed.3008140. [DOI] [PubMed] [Google Scholar]
- 42.Ying T., Du L., Ju T.W., Prabakaran P., Lau C.C., Lu L. Exceptionally potent neutralization of Middle East respiratory syndrome coronavirus by human monoclonal antibodies. J Virol. 2014;88:7796–7805. doi: 10.1128/JVI.00912-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tang X.C., Agnihothram S.S., Jiao Y., Stanhope J., Graham R.L., Peterson E.C. Identification of human neutralizing antibodies against MERS-CoV and their role in virus adaptive evolution. Proc Natl Acad Sci U S A. 2014;111:E2018–E2026. doi: 10.1073/pnas.1402074111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pascal K.E., Coleman C.M., Mujica A.O., Kamat V., Badithe A., Fairhurst J. Pre- and postexposure efficacy of fully human antibodies against spike protein in a novel humanized mouse model of MERS-CoV infection. Proc Natl Acad Sci U S A. 2015;112:8738–8743. doi: 10.1073/pnas.1510830112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Corti D., Zhao J., Pedotti M., Simonelli L., Agnihothram S., Fett C. Prophylactic and postexposure efficacy of a potent human monoclonal antibody against MERS coronavirus. Proc Natl Acad Sci U S A. 2015;112(33):10473–10478. doi: 10.1073/pnas.1510199112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abroug F., Slim A., Ouanes-Besbes L., Hadj Kacem M.A., Dachraoui F., Ouanes I. Family cluster of Middle East respiratory syndrome coronavirus infections, Tunisia, 2013. Emerg Infect Dis. 2014;20:1527–1530. doi: 10.3201/eid2009.140378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Al-Abdallat M.M., Payne D.C., Alqasrawi S., Rha B., Tohme R.A., Abedi G.R. Hospital-associated outbreak of Middle East respiratory syndrome coronavirus: a serologic, epidemiologic, and clinical description. Clin Infect Dis. 2014;59:1225–1233. doi: 10.1093/cid/ciu359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Al-Tawfiq J.A., Hinedi K., Ghandour J., Khairalla H., Musleh S., Ujayli A. Middle East respiratory syndrome coronavirus: a case-control study of hospitalized patients. Clin Infect Dis. 2014;59:160–165. doi: 10.1093/cid/ciu226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Assiri A., McGeer A., Perl T.M., Price C.S., Al Rabeeah A.A., Cummings D.A. Hospital outbreak of Middle East respiratory syndrome coronavirus. N Engl J Med. 2013;369:407–416. doi: 10.1056/NEJMoa1306742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harriman K., Brosseau L., Trivedi K. Hospital-associated Middle East respiratory syndrome coronavirus infections. N Engl J Med. 2013;369:1761. doi: 10.1056/NEJMc1311004. [DOI] [PubMed] [Google Scholar]
- 51.Memish Z.A., Al-Tawfiq J.A., Assiri A. Hospital-associated Middle East respiratory syndrome coronavirus infections. N Engl J Med. 2013;369:1761–1762. doi: 10.1056/NEJMc1311004. [DOI] [PubMed] [Google Scholar]
- 52.Middleton D., Pallister J., Klein R., Feng Y.R., Haining J., Arkinstall R. Hendra virus vaccine, a one health approach to protecting horse, human, and environmental health. Emerg Infect Dis. 2014;20:372–379. doi: 10.3201/eid2003.131159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Middleton D. Hendra virus. Vet Clin N Am Equine Pract. 2014;30:579–589. doi: 10.1016/j.cveq.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Haagmans B.L., van den Brand J.M., Raj V.S., Volz A., Wohlsein P., Smits S.L. An orthopoxvirus-based vaccine reduces virus excretion after MERS-CoV infection in dromedary camels. Science. 2015;351(6268):77–81. doi: 10.1126/science.aad1283. [DOI] [PubMed] [Google Scholar]
- 55.Zhao J., Li K., Wohlford-Lenane C., Agnihothram S.S., Fett C., Zhao J. Rapid generation of a mouse model for Middle East respiratory syndrome. Proc Natl Acad Sci U S A. 2014;111:4970–4975. doi: 10.1073/pnas.1323279111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Agrawal A.S., Garron T., Tao X., Peng B.H., Wakamiya M., Chan T.S. Generation of a transgenic mouse model of Middle East respiratory syndrome coronavirus infection and disease. J Virol. 2015;89:3659–3670. doi: 10.1128/JVI.03427-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van Doremalen N., Munster V.J. Animal models of Middle East respiratory syndrome coronavirus infection. Antivir Res. 2015;122:28–38. doi: 10.1016/j.antiviral.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]