Abstract
Synthetic analogues of the bradykinin potentiating nonapeptide BPP9α indicate significantly different structural requirements for potentiation of the bradykinin (BK)-induced smooth muscle contraction (GPI) and the inhibition of isolated somatic angiotensin I-converting enzyme (ACE). The results disprove the ACE inhibition as the only single mechanism and also the direct interaction of potentiating peptides with the bradykinin receptors in transfected COS-7 cells as molecular mechanism of potentiation. Our results indicate a stimulation of inositol phosphates (IPn) formation independently from the B2 receptor. Furthermore, the results with La3+ support the role of extracellular Ca2+ and its influx through corresponding channels. The missing effect of calyculin on the GPI disproves the role of phosphatases in the potentiating action. These experimental studies should not only contribute to a better understanding of the potentiating mechanisms but also incorporate a shift in the research towards the immune system, in particular towards the immunocompetent polymorphonuclear leukocytes. The chemotaxis of these cells can be potentiated most likely by exclusive inhibition of the enzymatic degradation of bradykinin. Thus the obtained results give evidence that the potentiation of the bradykinin action can occur by different mechanisms, depending on the system and on the applied potentiating factor.
Abbreviations: AA, arachidonic acid; ABA, 4-azidobenzoic acid; ACE, angiotensin I-converting enzyme; Aloc, allyl oxycarbonyl; ASA, 4-azidosalicylic acid; BK, bradykinin; BKR, bradykinin receptor; BKR-B1, bradykinin B1 receptor; BKR-B2, bradykinin B2 receptor; Boc, tert-butyloxycarbonyl; BPA, p-benzoylphenylalanine; BPP, bradykinin potentiating peptide; BPP9α, bradykinin potentiating peptide 9α (Pyr-Trp-Pro-Arg-Pro-Gln-Ile-Pro-Pro); BOP, benzotriazole-1-yl-oxy-tris (dimethylamino) phosphonium hexafluorophosphate; DCM, dichloromethane; Dde, N-(1-(4,4-dimethyl-2,6-dioxocyclohexylidene)ethyl; Ddz, α,α-dimethyl-3,5-dimethoxy-benzyloxycarbonyl; DEAE, diethylaminoethyl; DIEA, diisopropylethylamine; DIC, diisopropylcarbodiimide; DMEM, Dulbecco's modified Eagle's medium; DMF, N,N-dimethylformamide; DMSO, dimethylsulfoxide; DTE, dithioerithritol; ED, effective dose; Fmoc, 9-fluorenylmethyl oxycarbonyl; ɛAbu(ßPhe), erythro-α-amino-ß-phenyl-butyric acid; FR190997, 8-[2,6-dichloro-3-[N-(E)-4-(N-methylcarbamoyl)cinnamidoacetyl]-N-methylamino]benzyloxy]-2-methyl-4-(2-pyridyl-methoxy)quinoline; GPI, guinea pig ileum; HOAt, 1-hydroxy-7-azabenzotriazole; HBTU, 2-(1H-benzotriazol-1-yl)-1,1,3,3-tetramethylguanidinium hexafluorophosphate; HOBt, 1-hydroxybenzotriazole; HOCr, hydroxycrotonic acid; HYCRAM, hydroxycrotonyl amidomethyl linker; IP3, inositol 1,4,5-trisphosphate; IPn, inositol phosphates; J526, Pyr-Trp-Pro-Lys(ASA)-Pro-Gln-Ile-Pro-Pro; J527, Pro-Trp-Pro-Lys-Pro-Gln-Ile-Pro-Pro; J725, DArg-Arg-Pro-Hyp-Gly-Thi-Ser-Pro-ɛAbu(ßPh)-Arg; MEM, Eagle's minimal essential medium; Mtr, methoxytrimethylbenzene sulphonyl; Pd0, palladium tetrakis triphenylphosphine; PMN, polymorphonuclear leukocytes (neutrophils); Ram, ramiprilat; TBTU, 2-(1H-benzotriazol-1-yl)1,1,3,3-tetramethylguanidinium tetrafluoroborate; TFA, trifluoroacetic acid; Trt, triphenylmethyl
Keywords: Potentiation, Bradykinin, Bradykinin potentiating peptide, Angiotensin I-converting enzyme, Inositol phosphate, Arachidonic acid, Ca2+-influx, Protein phosphatases, Polymorphonuclear leukocytes, Chemotaxis, Smooth muscle contraction, Radioligand binding
1. Introduction
Hormone actions can be potentiated by different factors interacting with the receptor, by enzymatic degradation or by signal pathways. However the entire overall process has been studied in detail for only very few hormones [30], [69], [45].
At least 40 years ago a potentiating action was observed for the nonapeptide hormone bradykinin (BK). Indeed the history of BK isolation and characterization has long been closely related to the use of potentiating factors. Werle and coworkers [74] used snake venoms to trigger the formation of BK from plasma and to describe this tissue hormone functionally. Immediately after the isolation, chemical characterization, synthesis and functional characterization, certain snake venoms were described as bradykinin potentiating compounds. Kato and Suzuki [39], [40], Ferreira et al. [26], [27], and Ondetti et al. [13], [59] isolated different oligopeptides with bradykinin potentiating activity from the venoms of the two snakes Agkistrodon halys blomhoffii and Bothrops jararaca, including the bradykinin potentiating nonapeptide BPP9α (trade name TEPROTIDE).
Bradykinin potentiating peptides have also been isolated from other snakes [8], [9], [23], [24], [25], [35] or other toxins [3], [21], [22], [44], [52], [73] as well. Very recently new potentiating peptides have been isolated from the venom of B. jararaca [36]. Surprisingly peptides with potentiating activity have also been formed by the partial hydrolysis of proteins taken from serum [80], hemoglobin [38], [66], [81], milk [34], [46], or wheat germ [51]. Also degradation fragments of angiotensin such as the heptapeptide 1–7 were found to potentiate the BK action [64]. In addition linear BK analogues, partial sequences [7], certain active and inactive side chain and backbone cyclic agonists are able to potentiate the BK action on GPI [68].
The angiotensin I-converting enzyme (ACE) cleaves dipeptides from the C-terminus of angiotensin I and bradykinin resulting on the one hand in the formation of the highly hypertensive hormone angiotensin II and on the other hand in the inactivation of the hypotensive BK. This enzyme has been extensively studied because of these important functions in the blood pressure regulation. ACE contains a N-terminal as well as a C-terminal catalytic domain, described in most publications as having only slight differences in their structural requirements [14], [79]. More recently Cotton et al. demonstrated in an excellent investigation using domain-specific substrates and inhibitors, affinity differences between N- and C-terminal catalytic domains of about three orders of magnitude [11].
The membrane bound form of this enzyme seems to play an important role in the potentiation of the BK action. Inhibitors of this enzyme are used as drugs for treatment of different forms of hypertension and heart failure. For therapeutically used hormones this knowledge about potentiating compounds and their action mechanisms is very important. This knowledge can help to improve the therapeutic effect, to prevent not only an excessive dose, but also interaction with other drugs and side effects. On the other hand the therapeutic use of potentiating compounds requires the knowledge about the interaction with the hormone action on the molecular basis as well. As shown by Li et al. ACE may also act as a receptor for SARS coronavirus [47].
With extensive therapeutical application of bradykinin potentiating compounds such as captopril [12], enalaprilat [64], ramiprilat [75], quinaprilat [41] and lisinopril [6], studies of the molecular action mechanism have become more and more important. Many other proteases are also able to inactivate BK including the neutral endopeptidase (NEP, Neprolysin; E.C. 3.4.24.11) [42], [71], metalloendopeptidase (E.C. 3.4.25.15/16) [55], [58], aminopeptidase P (E.C. 3.4.11.9) [67], aminopeptidase N (E.C. 3.4.11.2) [63] and carboxypeptidase M (E.C. 3.4.17.12) [72].
Potentiation of BK action has also been studied in in vivo models by pharmacological tests on isolated organs and on the cellular level by biochemical methods. In the in vivo models, potentiation of BK action has been measured on the pressor response to intravenous BK in conscious rabbits [68] or on the hypotensive effect in freely moving Wistar rats [65]. Isolated organs such as the guinea pig ileum (GPI) [54], rat heart [34], rabbit jugular vein [17], [33], cerebral microvasculature (estimation of permeability) [58] and porcine coronary arteries [76] have been used for in vitro tests.
Yet even at the onset of the search for the molecular mechanism of BK potentiation, certain contradictory findings have been observed. The inhibition of ACE by various peptide and nonpeptide compounds did not correlate well with the potentiating activity [5], [10]. Furthermore, the maximum of the BK-induced contraction of guinea pig ileum can be enhanced by potentiating compounds [77]. Also the action evoked by enzymatically stable BK agonists can be potentiated in some test systems [78]. Repeated exposure of porcine coronary arteries to BK has led to receptor desensitization. The addition of the potentiating compounds quinaprilat or angiotensin 1–7 fully restored the relaxant effect at a point when BK alone was no longer able to induce relaxation [76]. At the molecular level the co-immunoprecipitation of ACE and the B2 receptor with an anti receptor antibody clearly indicates an interaction of both partners on the cell membrane [50]. Despite all these contradictory and to some degree confusing findings Regoli and coworkers [29] and Dendorfer et al. [17] have demonstrated that in their test systems (rabbit isolated aorta and venoconstriction) the potentiation by therapeutically used ACE inhibitors results exclusively from the inhibition of enzymatic BK degradation. Nevertheless, the group of Regoli found under influence of ACE inhibitors, a resensitization of the rabbit jugular vein [31] and a change in the density of B2 receptors in rat spinal cord [60].
To understand the molecular basis for these discrepancies many approaches have been undertaken to elucidate the influence of potentiating compounds on the different bradykinin destroying enzymes, on the bradykinin receptors (BKR) and on signal pathways.
Direct interaction of the potentiating compounds was postulated with the B1 receptor [37]. Phosphorylation of the receptor by protein kinase C leads to internalization [19] (desensitization) and dephosphorylation by phosphatase SHP-2 to resensitization [18], [49]. Bradykinin potentiating factors can influence both desensitization and resensitization, as well as the hetero-oligomerization of the B2 receptor including the interaction with ACE [54] and other degrading enzymes [15]. At the level of signal pathways, a crosstalk with other pathways induced by other hormones [43] or nonreceptor-mediated intracellular reactions [28] have been observed.
As initial experimentation on the potentiation of the bradykinin action was primarily performed on isolated smooth muscle organs, in the last decade the potentiating activity was mainly investigated on the affinity and density of the receptor [54], the intracellular mobilization of Ca2+ [49], [54], the release of arachidonic acid [49], [54], of inositol phosphates [54], and of nitric oxide [37]. Later studies on the molecular basis of bradykinin-evoked actions have been performed more and more on cell cultures instead of smooth muscles. These studies have used primarily Chinese hamster ovary cells (CHO cells) cotransfected with the B2 receptor and ACE, ACE-mutants or neutral endopeptidase (NEP) [15]. Endothelial cells have been used because they constitutively express the B2 receptor and ACE [49].
The aim of our experiments was to contribute to a better understanding of the potentiating mechanisms, and to prove or disprove postulated action mechanisms. For these studies we used three different systems: the smooth muscle contraction, transiently transfected COS-7 cells and polymorphonuclear leucocytes. We began to find differences between ACE inhibition and potentiation of the BK action. We were interested in determining the role of Ca2+-uptake from extracellular sources and its release from sarcoplasmatic reticulum for the potentiating action. We tested the direct interaction with the B2 receptor in COS-7 cells and checked the influence of potentiating peptides on receptor dephosphorylation and selected signal transduction reactions.
Since BK plays an important role in inflammatory processes we studied the potentiation on immunocompetent polymorphonuclear leucocytes. However it was not only our aim to verify or negate the contradictory opinions regarding the mechanism of bradykinin potentiation, we would also like to show that there simultaneously exist different mechanisms, depending on the assay and on the potentiating factors used.
2. Methods
2.1. Synthesis of peptides
Boc-, Fmoc- and Ddz-amino acids were purchased from BACHEM (Switzerland) and ORPEGEN-Pharma (Germany). Boc-Pro-OCr-OH was synthesized according to the general procedure published by Gothe et al. [32]. To form the HYCRAM™-linker the hydroxycrotonic ester was coupled to the aminomethyl resin Tentagel-S-NH2. The used resins were purchased from the following companies: amino methyl polystyrene, Tentagel-S-NH2 and Wang resin from RAPP Polymere (Germany), and chlorotrityl resin from NOVA-Biochem (Germany). The peptides were synthesized on a PSS-80 automatic synthesizer (Applied Protein Technologies, USA) or on the semiautomatic synthesizer SP-650 (BACHEM, Switzerland). Each step was monitored by the Kaiser test. Couplings were carried out after neutralization by repeated washings (5–10 times) of the resin with 5% DIEA in DCM in a two-fold excess of Nα-protected amino acid and diisopropylcarbodiimide (DIC) in DCM for 4 h; 2-(1H-benzotriazol-1-yl) 1,1,3,3-tetramethylguanidinium tetrafluoroborate (TBTU)/1-hydroxybenzotriazole (HOBt): Boc- or Fmoc- protected amino acids were used in a four-fold excess, solved in DMF or DCM/ DMF 1:1. TBTU and HOBt were applied in the same excess, diisopropylethylamine (DIEA) in a six-fold excess. The reaction time was between 4 and 12 h.
Deprotecting procedures: Boc-deprotection was performed with TFA/DCM 1:1 without any scavenger in two steps, 5 min treatment followed by washing with DCM and second treatment for 20 min; Ddz-deprotection was carried out with 5% TFA in DCM in two steps, 5 and 20 min; Fmoc-deprotection was achieved with 20% piperidine in DMF in two steps, 5 and 20 min.
2.2. Potentiation of the BK-induced contraction of GPI
The ileum (1.5 cm) of the guinea pigs was suspended in a 5 ml organ bath containing Tyrode solution at 37 °C. Isotonic contractions were recorded under a resting tension of 500 mg. BK was applied in a concentration of 1 × 10−8 M that corresponded to an ED25 to ED30 of BK under these experimental conditions. Generally this effect was increased twice by BPP9α (20 nM) that was used as standard. The bradykinin potentiating peptides or the ACE inhibitors were given 5 min before application of BK. The potentiating effects of the analogues were calculated as per cent of the BK-induced contraction and compared with the effect caused by BPP9α (=1.0 or 100%) in the same experiment.
2.3. Ca2+-dependence of the potentiation of the BK-induced contraction of GPI
To investigate the influence of Ca2+ on the BK-potentiating action of BPP9α (20 nM BK, 20 nM BPP9α), La3+ (1.8 mM) was used as an inhibitor of the Ca2+ influx in Ca2+-free or Ca2+-containing Tyrode solution. LaCl3 was always given 1 min before or after the BK-induced contraction and before the application of BPP9α (see Fig. 3).
Fig. 3.
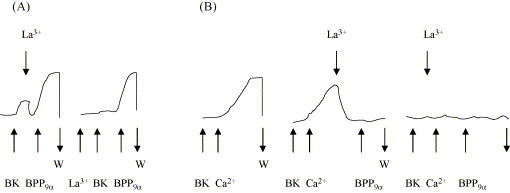
Effect of La3+ (1.8 mM) on the potentiation of a BK-induced (20 nM) contraction of the guinea pig ileum by BPP9α (20 nM) in Tyrode-solution with (A) and without [Ca2+] (B). The arrows indicate the addition of BK, BPP9α, lanthanium or calcium ions. W = wash period. Curves are examples of five separate experiments.
2.4. Inhibition of phosphatase activity
Calyculin (100 nM) was used to estimate the involvement of BK-induced (20 nM BK) and potentiated (20 nM BK, 20 nM BPP9α) contraction of the guinea pig ileum. Calyculin was applied before BPP9α was given at the maximum of the BK-induced contraction or 1 min before the BK- and BPP-induced effect was elicited (see Fig. 6).
Fig. 6.
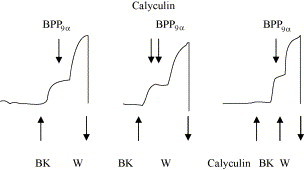
Influence of calyculin (100 nM) on the potentiation of a BK-induced (20 nM) contraction of the guinea pig ileum by BPP9α (20 nM). The arrows indicate the addition of BK, BPP9α and calyculin. W = wash period. Curves are examples of four to six separated experiments.
2.5. Inhibition of isolated angiotensin I-converting enzyme
Inhibitory activities were determined with an enzyme preparation from the guinea pig lung, using Benzoyl-Gly-His-Leu as the substrate. This method is described elsewhere [70].
2.6. Cell culture and transfection of COS-7 cells
Cell culture and transfection of COS-7 cells have been described in detail [2]. COS-7 cells (ATCC) were routinely grown in Dulbecco's modified Eagle's medium (DMEM) containing 10% (v/v) fetal calf serum and antibiotics, and were kept in a humidified 5% CO2, 95% air atmosphere. Subconfluent cells were transfected in 24-well plates with BKR-B2-cDNA (1 μg cDNA/well) by the DEAE-dextran technique, and cells were used 48–72 h after transfection. Preparation of COS-7 membranes was done as described previously [57].
2.7. Receptor binding assay
Competition binding studies were performed using [3H]BK (102 Ci/mmol, NEN Life Science Products, USA) and intact COS-7 cells (initial density (1–2) × 105 cells/well) in 24-well plates expressing the BKR-B2 [56]. Cells were incubated in binding buffer containing 0.8 nM of [3H]BK with different concentrations of BK in the absence or the presence of J526 (0.1 μM) or J527 (0.1 μM), or in increasing concentrations of J527 alone for 90 min at 4 °C. Non-specific binding was measured in the presence of 1 μM unlabeled BK. To estimate binding properties at 37 °C membranes of COS-7 cells were used. Membranes (0.15 mg/tube) were incubated with 0.8 nM of [3H]BK or with 0.8 nM of [3H]BK and 100 nM J526 for 20 min at 37 °C in binding buffer in the absence (total binding) or in the presence of 1 μM BK (non-specific binding). Thereafter, the samples were filtered through Whatman GF/B glass fiber filters pretreated with 0.1% (w/v) aqueous polyethylenimine. The filters were washed three times with 5 ml of ice-cold binding buffer, transferred into scintillation vials, dried and analyzed for bound radioactivity by scintillation counting.
2.8. Phosphatidylinositol turnover
COS-7 cells expressing the BKR were grown in 24-well plates (5 × 104 to 105 cells/well) and labeled with 4 μCi/ml myo-[3H]inositol (10–25 Ci/mmol, NEN Life Science Products, USA) for 24 h. Cells were stimulated in DMEM without captopril with 3 × 10−8 M BK for 5 min at 37 °C, or with 3 × 10−8 M BK in the presence of J526 (0.1 μM), or with 3 × 10−8 M BK in the presence of J527 (0.1 μM). Basal IPn was measured with J526 or J527 (0.1 μM), too. Preparation of stimulation and determination of the levels of inositol phosphates by anion exchange chromatography using AG-X8 as a resin were performed as recently described in detail [2].
2.9. Arachidonic acid release
COS-7 cells were transfected and labeled with 0.4 μCi/ml [3H]arachidonic acid (60–100 Ci/mmol, NEN Life Science Products, USA) for 24 h as described previously [57]. The cells were washed three times with label-free DMEM containing 0.5% (v/v) fetal calf serum and then stimulated in 0.5 ml in the same medium with 3 × 10−8 M BK for 30 min in the absence or the presence of J526 (0.1 μM) or J527 (0.1 μM) at 37 °C. Assays were terminated by removing the medium from the cells. The medium was measured for [3H]arachidonic acid release by scintillation counting.
2.10. Preparation of human polymorphonuclear leukocytes (PMN) [62]
Heparinized venous blood from healthy volunteers was mixed with 1% dextran sulphate in 0.9% NaCl at a ratio of 1:1 (v/v) and left for 50 min at 37 °C for erythrocyte sedimentation to occur. The leukocyte-rich plasma was layered over an equal volume of Histopaque-1077, and the gradient was centrifuged for 30 min at 700 × g. Contaminating erythrocytes were eliminated by lysis with ammonium chloride (0.98%), and the pure PMN were washed twice with Eagle‘s minimal essential medium (MEM) and resuspended (1 × 106 PMN/ml).
2.11. Chemotaxis assay
Chemotaxis was quantified using the Boyden chamber technique as described previously [61]. PMN were suspended in Eagle's MEM containing 20 mM HEPES (pH 7.3) and 5 × 105/0.5 ml, pipetted into the top of Boyden chamber which were separated by a 3 μm pore filter (Sartorius SM 11302) from the lower part. Peptides were put into the lower stimulus compartment. Inhibitors or potentiating peptides were applied to the PMN for 15 min at 37 °C and then to the upper compartment of the chamber. After an incubation period of 3 h at 37 °C in an atmosphere of 5% CO2, 95% air at high humidity, the filters were fixed and stained with methylene blue. Using an image analyzer (Chemotaxis Analyzer HCA-100, HA-SOTEC, Rostock, Germany), the PMN were counted at every 10 μm interval from the original monolayer to the distal surface in 10 high power fields of each duplicate filter. An average locomotion index (LIm+) was quantified and expressed as the migration index in comparison to the control (control = 1.0).
2.12. Statistical analysis
Mostly the values are presented as means ± S.E.M. Statistical analysis was evaluated with the Student's t-test for independent samples or for paired samples, depending on the experimental protocol. Details are described in the legends for each figure.
3. Results
3.1. Synthesis of peptides
BPP9α and a variety of analogues were synthesized according to the three different strategies in Scheme 1 on the solid phase. To prevent dioxopiperazine formation, chlorotrityl resin or HYCRAM™ resin was used. In the case of Cl-trityl resin, cyclization of the C-terminal dipeptide was avoided by sterical hindrancy at the resin. Using the Boc-strategy with the HYCRAM™ resin prevents ring formation and thereby loss of loading. Syntheses on Wang resin provided very low yields. After removal from the resins all the peptides were purified by semipreparative HPLC and chemically characterized. Some of the analogues are prepared for photoaffinity labeling of the binding protein.
Scheme 1.

Strategies for synthesis of BPP9α analogues using different linkers and resins (Wang resin, chlorotrityl resin, HYCRAM™ resin) and different protecting group combinations.
3.2. Inhibition of the isolated angiotensin I-converting enzyme
The inhibitory activities of all analogues listed in Table 1 were determined on isolated ACE from guinea pig lungs. As most organs, particularly guinea pig ileum, contain different BK degrading enzymes, we did not use an organ homogenate to estimate the inhibition of BK degradation. We used the isolated ACE to test the inhibition of hydrolysis of a synthetic tripeptide substrate by the potentiating peptides. As shown in Table 1, distinct differences exist between potentiation of the BK-induced contraction of the guinea pig ileum and the inhibition of the isolated ACE.
Table 1.
Analogues of the bradykinin potentiating peptide BPP9α (TEPROTIDE) with distinct differences between potentiation of the BK-induced contraction of the isolated guinea pig ileum (GPI) and inhibition of the isolated angiotensin I-converting enzyme (ACE)
| Compound | Aminoacid sequence | Potentiation (%) | ACE-inhibition: IC50 (M) | Quotient |
|---|---|---|---|---|
| BPP9α | Pyr-Trp-Pro-Arg-Pro-Gln-Ile-Pro-Pro | 100 | 3 × 10−9 | 7.0 |
| Pro-Trp-Pro-Phe-Pro-Gln-Tyr-Pro-Pro | 100 | 8 × 10−9 | 19 | |
| Pro-Trp-Pro-Leu-Pro-Lys-Tyr-Pro-Pro | 100 | 6 × 10−8 | 0.1 × 103 | |
| Pro-Trp-Pro-Leu-Pro-Gln-Ile-Pro-Pro | 90 | 1 × 10−5 | 2.5 × 104 | |
| [1-Pro]-BPP9α | Pro-Trp-Pro-Arg-Pro-Gln-Ile-Pro-Pro | 115 | 5 × 10−10 | 1 |
| Pro-Trp-Pro-Arg-Pro-Gln-Ile-Ala-Pro | 70 | 3 × 10−7 | 1 × 103 | |
| Pro-Trp-Ala-Arg-Pro-Gln-Ile-Pro-Pro | 75 | 3 × 10−8 | 93 | |
| Pro-Trp-Pro-Leu-Pro-Leu-Ile-Pro-Pro | 50 | 1 × 10−7 | 0.5 × 103 | |
| Pro-Trp-Pro-Phe-Pro-Gln-Ile-Pro-Pro | 90 | 5 × 10−8 | 0.1 × 103 | |
| J527 | Pro-Trp-Pro-Lys-Pro-Gln-Ile-Pro-Pro | 70 | 3 × 10−8 | 0.1 × 103 |
| Pro-Trp-Pro-Lys-Pro-Lys-Ile-Pro-Pro | 75 | 2 × 10−8 | 63 | |
| Pro-Trp-Pro-Lys-Pro-Lys-Tyr-Pro-Pro | 90 | 5 × 10−5 | 1.3 × 105 | |
| Pro-Trp-Pro-Leu-Pro-Lys-Ile-Pro-Pro | 85 | 3 × 10−8 | 82 | |
| Pro-Trp-Pro-Phe-Pro-Gln-Ile-Ala-Pro | 70 | 3 × 10−6 | 1.0 × 104 | |
| Pro-Trp-Pro-Phe-Pro-Gln-Tyr-Ala-Pro | 58 | 3 × 10−8 | 0.1 × 103 | |
| Pro-Trp-Pro-Arg-Pro-Gln-Tyr-Pro-Pro | 76 | 2 × 10−8 | 61 | |
| Pyr-Trp-Pro-Lys-Pro-Gln-Ile-Pro-Pro | 65 | 2 × 10−8 | 72 | |
| Pro-Trp-Pro-Arg-Pro-Lys-Ile-Pro-Pro | 50 | 7 × 10−8 | 0.3 × 103 | |
| Trp-Pro-Arg-Pro-Gln-Ile-Pro-Pro | 65 | 2 × 10−8 | 71 | |
| Leu-Pro-Gln-Ile-Pro-Pro | 40 | 7 × 10−6 | 4.0 × 104 | |
| Arg-Pro-Gln-Ile-Pro-Pro | 55 | 7 × 10−6 | 3.0 × 104 | |
| Analogues and partialsequences with the photolabels ASAa and ABAb | ||||
| ABA-Pro-Trp-Pro-Phe-Pro-Gln-Tyr-Pro-Pro | 93 | 1 × 10−7 | 0.3 × 103 | |
| ASA-Pro-Trp-Pro-Leu-Pro-Gln-Ile-Pro-Pro | 110 | 3 × 10−7 | 0.7 × 103 | |
| ASA-Pro-Trp-Pro-Arg-Pro-Gln-Ile-Pro-Pro | 90 | 6 × 10−9 | 16 | |
| Pro-Trp-Pro-Lys(ASA)-Pro-Lys-Ile-Pro-Pro | 99 | 1 × 10−8 | 23 | |
| Pro-Trp-Pro-Lys-Pro-Lys(ASA)-Ile-Pro-Pro | 103 | 8 × 10−8 | 0.2 × 103 | |
| Pro-Trp-Pro-Lys(ABA)-Pro-Lys-Tyr-Pro-Pro | 88 | 3 × 10−8 | 79 | |
| Pro-Trp-Pro-Leu-Pro-Lys(ASA)-Ile-Pro-Pro | 86 | 5 × 10−7 | 1.4 × 103 | |
| ABA-Pro-Trp-Pro-Phe-Pro-Gln-Tyr-Ala-Pro | 20 | 1 × 10−5 | 1.1 × 105 | |
| J526 | Pyr-Trp-Pro-Lys(ASA)-Pro-Gln-Ile-Pro-Pro | 160 | 7 × 10−5 | 1.0 × 105 |
| ASA-Trp-Pro-Arg-Pro-Gln-Ile-Pro-Pro | 110 | 6 × 10−8 | 0.1 × 103 | |
| ASA-Leu-Pro-Gln-Ile-Pro-Pro | 70 | 8 × 10−6 | 2.6 × 104 | |
| ASA-Arg-Pro-Gln-Ile-Pro-Pro | 40 | 4 × 10−5 | 2.3 × 105 | |
| Analogues with the photolabel Bpac | ||||
| Bpa-Pro-Trp-Pro-Lys-Pro-Lys-Tyr-Pro-Pro | 70 | 7 × 10−5 | 2.3 × 105 | |
| Bpa-Pro-Trp-Pro-Lys-Pro-Lys-Tyr(I)-Pro-Pro | 75 | 5 × 10−8 | 0.2 × 103 | |
| Bpa-Pro-Trp-Pro-Phe-Pro-Gln-Tyr-Ala-Pro | 20 | 1 × 10−6 | 1.1 × 104 | |
| Bpa-Pro-Trp-Pro-Arg-Pro-Gln-Tyr-Pro-Pro | 72 | 2 × 10−8 | 65 | |
| Pro-Bpa-Pro-Arg-Pro-Gln-Tyr-Pro-Pro | 63 | 2 × 10−8 | 74 | |
| Pro-Trp-Pro-Bpa-Pro-Gln-Tyr-Pro-Pro | 90 | 3 × 10−8 | 77 | |
To quantify the difference between potentiation and inhibition we calculated a quotient from both activities. For the analogue with the highest ACE-inhibitory activity [1-Pro]-BPP9α the quotient was accounted to 1.
4-Azidosalicylic acid.
4-Azidobenzoic acid.
p-Benzoylphenylalanine.
To quantify this difference we calculated a quotient from both activities. For the analogue [1-Pro]-BPP9α this quotient was accounted to 1. Most distinct differences were found by nonapeptide analogues and partial sequences labeled with azidosalicylic acid (ASA). Some quotients reach 105. Thus the compound J526 shows about 160% potentiation compared to BPP9α, but to enrich the same inhibition of ACE as the 1-Pro analogue a 105 higher concentration is required, resulting in a quotient of 105.
3.3. Potentiation of the BK-induced contraction of the isolated guinea pig ileum
One of the earliest pharmacological tests used for bradykinin was the contraction of the isolated guinea pig ileum (GPI). In our test the contraction of GPI can be potentiated by BPP9α and by the ACE inhibitor ramiprilat as well. Both potentiators enhance the isotonic measured contraction after 5 min preincubation (Fig. 1 ). The pD2-values for BK were shifted in the presence of 20 nM BPP9α from 7.15 ± 0.11 (n = 8) to 7.84 ± 0.19 (n = 4) and by ramiprilat (20 nM) to 7.87 ± 0.22 (n = 4). Both potentiators are also able to shift the cumulative GPI contraction curves for the enzymatically more stable bradykinin analogue DArg-Arg-Pro-Hyp-Gly-Thi-Ser-Pro-ɛAbu(ßPh)-Arg [16] to higher affinities. The pD2-value of this BK analogue was shifted by BPP9α (20 nM) from 6.65 ± 0.14 (n = 6) to 7.15 ± 0.18 (n = 3) and by ramiprilat to 7.16 ± 0.15 (n = 3).
Fig. 1.
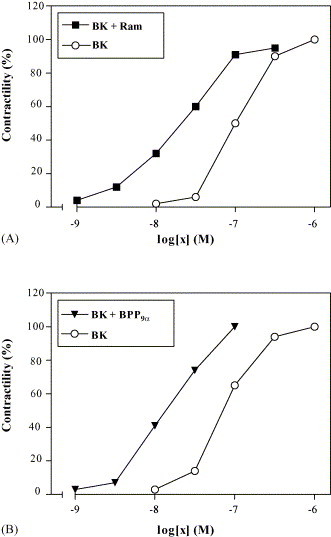
Concentration–response curves to bradykinin on the guinea pig ileum without and after preincubation (5 min) of 20 nM ramiprilat (A) or 20 nM BPP9α (B). Contractions are expressed as a percentage of the maximum BK-induced response (10−8 M BK) before addition of ramiprilat or bradykinin potentiating peptides. The values are accounted from one representative experiment, which was repeated three to eight times.
3.4. Influence on the bradykinin B2 receptor
To study the influence of bradykinin potentiating peptides on the bradykinin B2 receptor COS-7 cells were transfected with the gene of the human receptor. Expression, chemical and functional characterization of the expressed receptor is described in a preceding publication [56]. Radioligand binding studies with tritium labeled bradykinin [3H]BK were performed on intact cells. Fig. 2 shows the displacement curves of [3H]BK by BK alone and in the presence of the both potentiating nonapeptide analogues J526 and J527. Both peptides were used in such a concentration (0.1 μM) as necessary for the maximum potentiating effect on the isolated smooth muscle organ GPI. The potentiating peptides have no effect on the binding of bradykinin, indicating that the peptides used are neither able to enhance the affinity nor the number of active receptors. An unexpected finding is that analogue J527 is able to displace slightly [3H]BK in concentrations of 0.1 μM (20% displacement) to 1 μM (40% displacement) (not shown).
Fig. 2.
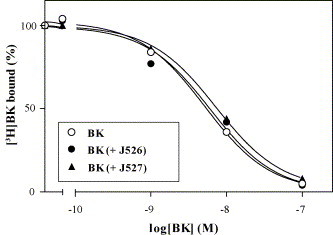
Competition curves of bradykinin (BK), BK with J526 (0.1 μM), or BK with J527 (0.1 μM) for specific binding of [3H]BK to COS-7 cells expressing the bradykinin B2 receptor. Shown are representative experiments performed in quadruplicate determinations and repeated once with the same result.
3.5. Influence of bradykinin potentiating peptides on signal pathways
3.5.1. Influence on Calcium uptake
Lanthanium ions (La3+) act as inhibitor of the uptake of Ca2+ from extracellular space. We used La3+ to estimate the influence of BPP9α on Ca2+ uptake. BK induces the contraction of the GPI only in the presence of extracellular calcium ions. The lowest extracellular concentration of Ca2+ necessary for a detectable contraction is 1.8 mM. In a Ca2+ containing Tyrode buffer, BK induces the contraction of the isolated GPI, which can also be potentiated in the presence of 1.8 mM La3+ (Fig. 3 ). Bradykinin alone is unable to contract the GPI in the presence of La3+. The ileum contracts only after addition of BPP9α, as shown in part A of Fig. 3. Part B of this figure, reflecting the results in the Ca2+ free Tyrode buffer, clearly underlines the important role of extracellular Ca2+ for the BK-induced smooth muscle contraction. Part A also shows evidence that mobilization of intracellular Ca2+ is necessary for the potentiation by the peptides.
3.5.2. Influence on the phosphatidyinositol turnover
Bradykinin induces the formation of inositol phosphates in COS-7 cells transiently expressing the BK receptor. Both analogues of the potentiating nonapeptide are not able to augment the BK-induced intracellular concentration of inositol phosphates. Rather it seems that J527 reduces the BK-induced IPn formation. The most striking result from Fig. 4 is the significant enhancement of the basal level by both potentiating peptides in the absence of bradykinin, possibly indicating a nonreceptor-mediated pathway.
Fig. 4.
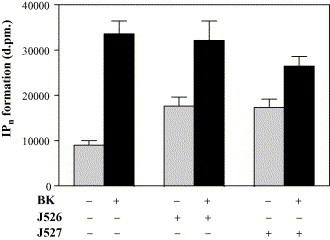
Effect of BPP9α analogues J526 and J527 on basal level of inositol phosphate production in COS-7 cells transiently transfected with BKR-B2 cDNA. Cells were prelabeled with 4 μCi/ml myo[3H]-inositol for 24 h and than stimulated with 3 × 10−8 M bradykinin for 5 min in absence or in presence of J526 (0.1 μM) or J527 (0.1 μM). Inositol phosphate formation was determined in quadruplicates. Shown are the mean ± S.E.M. of four independent comparative experiments.
3.5.3. Influence on release of arachidonic acid
Bradykinin triggers the release of [3H]-labeled arachidonic acid from labeled phospholipids, presumably through activation of phospholipase A2 by a Gα-protein. The level of arachidonic acid is significantly enhanced by BK as shown in Fig. 5 . Both potentiating peptides J526 and J527 slightly, but significantly, enhance the BK-mediated release of labeled arachidonic acid. Without BK both peptides have no significant influence on the basal level.
Fig. 5.
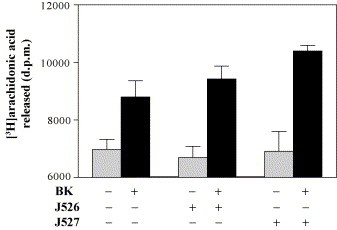
Influence of BPP9α analogues J526 and J527 on the BK-induced increase in arachidonic acid production in COS-7 cells which have transiently expressed the bradykinin B2 receptor. Cells were prelabeled with 0.4 μCi/ml [3H]arachidonic acid for 24 h and stimulated with 3 × 10−8 M BK for 30 min in absence or in presence of J526 (0.1 μM) or J527 (0.1 μM). Arachidonic acid release was six-fold determined. Results are expressed as the mean ± S.E.M.
3.5.4. Influence on protein phosphatases
Desensitization and resensitization are processes at the receptor level elicited by cytosolic receptor phosphorylation, followed by internalization or dephosphorylation, which is followed by reintegration into the cell membrane. Dephosphorylation of cytosolic Ser- and Thr-residues of the BK receptor results from activated protein phosphatases. Calyculin is known as a potent inhibitor of the protein phosphatases 1 and 2A. We used this inhibitor to check the involvement of these phosphatases in the potentiation of bradykinin action. As Fig. 6 illustrates calyculin has no influence on the potentiation of the BK-induced contraction. Application of calyculin, neither before nor after BK administration, changes the potentiating effects of BPP9α or ramiprilat.
3.6. Influence of potentiating factor on the migration of polymorphonuclear leukocytes (PMN)
Bradykinin stimulates the migration of PMN corresponding to its concentration gradient. This effect could be characterized as true chemotaxis. These cells contain both types of BK receptors, BKR-B1 and BRK-B2, as demonstrated using BK agonists and antagonists in the migration assay [62]. They also contain the complete system for synthesis and release of bioactive kinins. Degradation of BK proceeds in PMN mainly by the neutral endopeptidase NEP (E.C. 3.4.24.11). The BK-induced accelerated migration of PMN can be potentiated after preincubation (5 min) of the cells with the NEP inhibitor phosphoramidon (10−7 M). The migration index (MI = 1.12 ± 0.03) of BK (10−8 M) is significantly (p < 0.5; t-test for paired samples; n = 12) enhanced in the presence of phosphoramidon (MI = 1.19 ± 0.03) (not shown).
The migratory capacity of BK for PMN can also be potentiated by the ACE inhibitor ramiprilat. The migation index of BK (10−7 M) without ramiprilat is MI = 1.30 ± 0.06, in the presence of ramiprilat (10 nM) MI = 1.69 ± 0.26, measured in six experiments (not shown).
As shown in Fig. 7 BPP9α can enhance the migration of PMN induced as well by BK and by the enzymatically stable BK agonist D-Arg-Arg-Pro-Hyp-Gly-Thi-Ser-Pro-ɛAbu(βPh)-Arg [16], but is unable to enhance the migratory activity of the nonpeptide agonist FR190997 [4] and the BKR-B1 agonist desArg9-BK. BPP9α (10 nM) is able to potentiate the dose–response curve of the BK-induced migration of the PMN (Fig. 8 A). It potentiates the BK-induced effect in a concentration-dependent way (Fig. 8B).
Fig. 7.
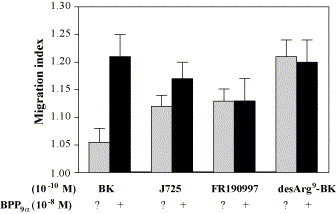
Influence of BPP9α on BKR-B2 and BKR-B1 agonists-induced migration of polymorphonuclear leukocytes (PMN). Cells in the upper compartment of the chamber were preincubated (15 min) with BPP9α (10−8 M). Bradykinin, the nonpeptide agonist FR190997 or desArg9-BK (10−10 M) were given into the lower compartment. Results are expressed as migration index (control = 1.0) and represent mean ± S.E.M. of five to seven experiments.
Fig. 8.
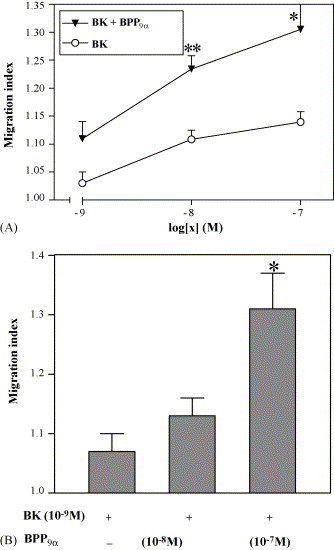
Effect of BPP9α on the BK-induced migration of human polymorphonuclear leukocytes (PMN) in Boyden chambers. (A) Concentration–response curves of BK without and in presence of 10 nM BPP9α. Cells in the upper compartment of the chamber were preincubated (15 min) with BPP9α and BK was given into the lower compartment. (B): The BK-induced (10−9 M) migration is dose-dependently potentiated by BPP9α in concentrations of 10−8 and 10−7 M. Results are expressed as migration index (control = 1.0) and represent mean ± S.E.M. of 3–11 experiments. Values are significantly different from control values without BPP9α: **p < 0.01, *p < 0.05 (Student's t-test).
4. Discussion
The potentiating action, despite many long years of experimental research and investigation, remains a phenomenon not fully understood. Beginning with studies on the potentiation of smooth muscle contraction more than 30 years ago, the search has been extended to other organs such as the vascular system, to different bradykinin degrading enzymes, to immunocompetent cells, and in the last decade to molecular mechanisms at the level of the bradykinin receptors and signal pathways, primarily studied on cell cultures. Other potentiating peptides and peptidomimetics besides the oligopeptides isolated from snake venoms have been used in these studies.
Generally, some of the contradictory findings and therefore explanations in the literature seem to result not only from the complexity of the system, but also from: the use of enzymatically not fully stable bradykinin agonists e.g. [Hyp3,Tyr(Me)8]-BK [31], [53], the species dependency of the bradykinin receptor, the very different protease compositions of the tissues and cell lines used in the different studies, and the different structural requirements for both catalytic centers of ACE. Furthermore, we have to keep in mind the different density of the BK receptors, their localization in microdomains in the plasma membrane, the presence of certain other hormone receptors, the different signal pathways in the used tissues and cells, a possible influence on G-protein independent signal transduction, on G-protein trafficking pattern in the cells and on activators or regulators of G-protein signaling [48]. Additionally the potentiating effect can be differentiated into an unspecific and specific part. Consequently, the search for potentiation of hormone actions is strongly related to the very recent research on multifunctional signal proteins.
4.1. Distinct differences between potentiation of the BK-induced contraction of the isolated guinea pig ileum and the inhibition of the isolated angiotensin I-converting enzyme
The isolation, chemical and functional characterization of oligopeptides taken from the venoms of different snakes provided lead structures for a number of potentiating peptides. Long ago these oligopeptides gave some evidence of different structural requirements for potentiation of smooth muscle contraction and inhibition of ACE. Our synthesized analogues and fragments of the highly active nonapeptide BPP9α show in some cases differences between potentiation and enzyme inhibition of more than five orders of magnitude. This result indicates strongly different structural requirements for both biological activities, and is inconsistent with the exclusive reduction of potentiating action to the inhibition of ACE, as discussed by Regoli and his coworkers [31] and Dendorfer et al. [17]. We might draw this conclusion despite the different sources for the isolated organs and the ACE. Since the BK receptor is species dependent, the human BKR-B2 shares about 80% sequence homology with that of the guinea pig [20], whereas the ACE seems to be nearly species independent. Interestingly, the potentiating activity is much less influenced by amino acid replacements or modifications of the lead peptide BPP9α than the inhibition of ACE.
From the analogues listed in Table 1, it is clearly evident that the potentiation of GPI contraction is less sensitive to amino acid replacements and modifications in the peptide sequence than the inhibition of ACE. Thus potentiating activities vary between 160% and 20%, whereas the IC50 values for ACE inhibition vary between 5 × 10−10 and 7 × 10−5 M. Both activities show no correlation. The estimated values indicate that the potentiating activity allows beside amino acid replacements, a shortening of the sequence as well as substitutions of the N-terminus or side chains by aromatic moieties. The inhibitory activity seems to depend on the whole sequence. The replacement of the basic amino acid Arg at position 4 by neutral aliphatic or aromatic amino acids influences the ACE inhibition only slightly, on the other hand the acylation of the Lys side chain at the same position by ASA reduces the IC50 value by four orders of magnitude. According to Cotton et al. [11] position 4 seems to be crucial for inhibitory activity. But, our results did not provide clear structure activity relationships.
Discussing the potentiation of the BK-induced contraction of GPI we have to keep in mind that this tissue also contains other proteases than ACE involved in BK degradation. But this finding is not able to explain the strong differences between both activities as expressed in the ratio in Table 1.
4.2. Direct interaction with the bradykinin receptors
It is reasonable to assume an interaction of the potentiating peptides with the BK receptor. For this interaction certain possible mechanisms exist. At first, the potentiating factors could act like allosteric effectors stabilizing the active receptor conformation. Secondly, these factors could influence the phosphorylation and dephosphorylation of cytosolic parts of the receptor, resulting in desensitization and resenzitization of the receptor. A third possible mechanism consists in the influence of potentiating factors on receptor hetero-oligomerization. AbdAlla et al. [1] found that both the active high affinity angiotensin receptor AT1 and the BKR-B2 are able to form heteromeric complexes in smooth muscle cells. Most importantly, this heterodimerization evokes a signal enhancement, a further possible useful explanation of the potentiating effect.
For checking these possibilities we used COS-7 cells transiently transfected with the human BKR-B2. The displacement curves show no differences between the displacement of [3H]-BK by BK alone nor in the presence of potentiating peptides. This finding clearly demonstrates that the analogues of BPP9α used in this study do not act as allosteric effectors stabilizing the active receptor conformation. Because COS-7 cells do not contain ACE, a heterodimerization of the B2 receptor with somatic ACE as demonstrated by Erdos and Marcic [19] is impossible in our system. Our radioligand binding curves show no influence of the potentiating peptides on the receptor capacity, indicating that under the test conditions the number of intramembranal receptors was not changed. This result excludes an internalization or reintegration of the receptor under the influence of potentiating peptides. But, we would not exclude these processes after heterodimerization with ACE or with the angiotensin receptor.
4.3. Influence on signal pathways
The contraction of smooth muscles requires calcium ions. Consequently the potentiation of BK-induced contraction of GPI depends on extracellular and intracellular Ca2+. In a variety of publications the enhancement of the intracellular Ca2+-level is used as a qualitative or quantitative proof of the potentiating action. Our results with the inhibition of Ca2+-uptake from extracellular sources by La3+ indicates that the potentiation of BK-action requires mobilization of Ca2+ from intracellular stores. On the other hand, in the beginning phase of the BK-evoked GPI contraction extracellular calcium is needed. Thus, our results with La3+ agree well with the finding of Marcic et al. [49], who described the inhibition of resensitization of the BK receptor in CHO cells by La3+.
The inositol phosphates (IPn) formed in transiently with the B2 receptor transfected COS-7 cells opens the Ca2+ channels of the endoplasmatic reticulum leading to an increase of cytosolic Ca2+. But, both potentiating nonapeptides J526 and J527 are unable to enhance the BK-induced formation of IPn. Surprisingly, both potentiating peptides increase the basal level of IPn in the absence of BK. Possibly this may be due to an unspecific, nonreceptor-mediated part of the potentiating action.
Contrary to the absent effect of both potentiating peptides on the BK-induced enhancement of inositol phosphates, the BK-induced release of arachidonic acid is significantly increased. Furthermore, contrary to the IPn formation, no influence of either potentiating peptides on the basal level could be found. We continue to be unable to explain the potentiation of arachidonic acid release because no effect on the receptor has been detected. The missing effect of calyculin on the potentiation might indicate that the inhibited phosphatases are not involved into resensitization.
4.4. Influence on migration of polymorphonuclear leukocytes
Besides GPI and COS-7 cells the PMN represent our third system for studies about the potentiating mechanism. These cells constitutively express B1 as well as B2 receptors and have a cell specific set of proteases involved in BK degradation. In this system the potentiating nonapeptide BPP9α and ramiprilat accelerate the BK-induced migration. In contrast to Ignjatovic et al. [37], who found on other cells a concentration-dependent direct activation of the B1 receptor in the absence of kinins, we could not detect any activity of the potentiating nonapeptide without administration of B1 or B2 agonists. Though the migratory activity of the proteolytic stabilized BK analogue J725 [16] is potentiated, the action of the nonpeptide agonist FR190997 [4] could not be accelerated by BPP9α and ramiprilat. It is difficult to explain this finding. In agreement with the weaker acceleration of the stabilized BK analogue, the effect of the NEP inhibitor phosphoramidon and the concentration-dependent potentiation induced by BPP9α, we might suppose that in this test system the potentiation occurs possibly exclusively by inhibition of the enzymatic BK degradation. But, we also have to consider a different mechanism for the peptide and nonpeptide agonist. This assumption is supported in that test by the strongly reduced intrinsic activity of the nonpeptide agonist FR190997 compared to BK itself.
5. Conclusions
During the last three decades many different and controversial explanations on the potentiating mechanism have been derived from experiments performed in vivo, in vitro and at the cellular level.
The distinct and in some cases very significant differences between the inhibitory and potentiating activity of the synthesized BPP9α analogues clearly indicate, in our opinion, that on guinea pig ileum this mechanism cannot be exclusively reduced to enzyme inhibition. The observed differences even exceed the different structural requirements for N- and C-terminal catalytic centers of ACE. The experiments with La3+ gave evidence that the potentiation of smooth muscle contraction requires extracellular Ca2+ which is taken up through opened channels. The increase of the intracellular level of inositol phosphates, triggered by potentiating peptides, leads to an additional enhancement of the intracellular Ca2+ concentration. Application of potentiating peptides to COS-7 cells which were transiently transfected with the B2 receptor and did not contain endogenous ACE, shows no increase in receptor affinity or density. These results disprove the described suggestion of direct interaction of BPP with the BK receptor.
The described receptor resensitization by potentiating peptides could result from dephosphorylation of the activated receptor. Our results obtained with the phosphatase inhibitor calyculin disprove the influence of BPP9α on the calyculin sensitive phosphatase. No difference between potentiation neither in the absence nor in the presence of the inhibitor could be observed.
The release of arachidonic acid is one of the prerequisites for inflammatory processes. The potentiating peptides have no effect on the basal level but they significantly increase the BK-induced release in COS-7 cells. This result indicates the possible involvement of potentiating peptides in inflammatory processes without affecting ACE.
Contrary to that mechanism the potentiation of the migratory activity of polymorphonuclear leucocytes seems to be nearly exclusively evoked by the ACE inhibition. On these B1 and B2 receptor containing cells no direct interaction with the B1 receptor could be observed.
Our results together with those from the literature may lead to the general conclusion that different potentiating factors can act on different organs, tissues and cells by different mechanisms. Furthermore, BPP can evoke both receptor-mediated and nonreceptor-mediated intracellular reactions. In our opinion the contradictory explanations describing the potentiation mechanism result on the one hand from the very high complexity of the kallikrein–kinin and renin–angiotensin systems and on the other hand from the different in vivo and in vitro tests used.
Acknowledgements
We thank I. Agricola and H. Rohde for skilful technical assistance. This work was supported by the Deutsche Forschungsgemeinschaft (Re 853/2-1 and Collaborative Research Center 436, Jena).
References
- 1.AbdAlla S., Lother H., Quitterer U. AT1-receptor heterodimers show enhanced G-protein activation and altered receptor sequestration. Nature. 2000;407(6800):94–98. doi: 10.1038/35024095. [DOI] [PubMed] [Google Scholar]
- 2.Adomeit A., Graness A., Gross S., Seedorf K., Wetzker R., Liebmann C. Bradykinin B(2) receptor-mediated mitogen-activated protein kinase activation in COS-7 cells requires dual signaling via both protein kinase C pathway and epidermal growth factor receptor transactivation. Mol Cell Biol. 1999;19(8):5289–5297. doi: 10.1128/mcb.19.8.5289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akchunov A.A., Golubenko Z., Sosnina N. Isolation and characterization of biological properties of inhibitors angiotensin-1-converting enzyme from the spider venom Latrodectus tredecimguttatus. Agents Actions Suppl. 1992;38(Pt 1):469–474. doi: 10.1007/978-3-0348-7321-5_59. [DOI] [PubMed] [Google Scholar]
- 4.Asano M., Hatori C., Sawai H., Johki S., Inamura N., Kayakiri H. Pharmacological characterization of a nonpeptide bradykinin B2 receptor antagonist, FR165649, and agonist FR190997. Br J Pharmacol. 1998;124(3):441–446. doi: 10.1038/sj.bjp.0701813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Auerswald W., Doleschel W. On the potentiation of kinins by sulfhydrylic compounds. Arch Int Pharmacodyn Ther. 1967;168(1):188–198. [PubMed] [Google Scholar]
- 6.Brunner D.B., Desponds G., Biollaz J., Keller I., Ferber F., Gavras H. Effect of a new angiotensin converting enzyme inhibitor MK 421 and its lysine analogue on the components of the renin system in healthy subjects. Br J Clin Pharmacol. 1981;11(5):461–467. doi: 10.1111/j.1365-2125.1981.tb01151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaturvedi D., Huelar E., Gunthorpe M., Gofman M., Krapf D.S., Apostol E. Bradykinin analogs as inhibitors of angiotensin-converting enzyme. Pept Res. 1993;6(6):308–312. [PubMed] [Google Scholar]
- 8.Chi C.W., Wang S.Z., Xu L.G., Wang M.Y., Lo S.S., Huang W.D. Structure–function studies on the bradykinin potentiating peptide from Chinese snake venom (Agkistrodon halys Pallas) Peptides. 1985;6(Suppl 3):339–342. [PubMed] [Google Scholar]
- 9.Cintra A.C., Vieira C.A., Giglio J.R. Primary structure and biological activity of bradykinin potentiating peptides from Bothrops insularis snake venom. J Protein Chem. 1990;9(2):221–227. doi: 10.1007/BF01025312. [DOI] [PubMed] [Google Scholar]
- 10.Cirstea M. Potentiation of some bradykinin effects by thiol compounds. Br J Pharmacol. 1965;25(2):405–410. doi: 10.1111/j.1476-5381.1965.tb02059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cotton J., Hayashi M.A., Cuniasse P., Vazeux G., Ianzer D., De Camargo A.C. Selective inhibition of the C-domain of angiotensin I converting enzyme by bradykinin potentiating peptides. Biochemistry. 2002;41(19):6065–6071. doi: 10.1021/bi012121x. [DOI] [PubMed] [Google Scholar]
- 12.Cushman D.W., Cheung H.S., Sabo E.F., Ondetti M.A. Design of potent competitive inhibitors of angiotensin-converting enzyme. Carboxyalkanoyl and mercaptoalkanoyl amino acids. Biochemistry. 1977;16(25):5484–5491. doi: 10.1021/bi00644a014. [DOI] [PubMed] [Google Scholar]
- 13.Cushman D.W., Pluscec J., Williams N.J., Weaver E.R., Sabo E.F., Kocy O. Inhibition of angiotensin-coverting enzyme by analogs of peptides from Bothrops jararaca venom. Experientia. 1973;29(8):1032–1035. doi: 10.1007/BF01930447. [DOI] [PubMed] [Google Scholar]
- 14.Deddish P.A., Marcic B., Jackman H.L., Wang H.Z., Skidgel R.A., Erdos E.G. N-domain-specific substrate and C-domain inhibitors of angiotensin-converting enzyme: angiotensin-(1-7) and keto-ACE. Hypertension. 1998;31(4):912–9177. doi: 10.1161/01.hyp.31.4.912. [DOI] [PubMed] [Google Scholar]
- 15.Deddish P.A., Marcic B.M., Tan F., Jackman H.L., Chen Z., Erdos E.G. Neprilysin inhibitors potentiate effects of bradykinin on b2 receptor. Hypertension. 2002;39(2 Pt 2):619–623. doi: 10.1161/hy0202.103298. [DOI] [PubMed] [Google Scholar]
- 16.Dendorfer A., Wagemann M., Reissmann S., Dominiak P. Structural requirements for B2-agonists with improved degradation stability. Immunopharmacology. 1999;45(1–3):199–205. doi: 10.1016/s0162-3109(99)00078-8. [DOI] [PubMed] [Google Scholar]
- 17.Dendorfer A., Reissmann S., Wolfrum S., Raasch W., Dominiak P. Potentiation of kinin analogues by ramiprilat is exclusively related to their degradation. Hypertension. 2001;38(1):142–146. doi: 10.1161/01.hyp.38.1.142. [DOI] [PubMed] [Google Scholar]
- 18.Duchene J., Schanstra J.P., Pecher C., Pizard A., Susini C., Esteve J.P. A novel protein–protein interaction between a G protein-coupled receptor and the phosphatase SHP-2 is involved in bradykinin-induced inhibition of cell proliferation. J Biol Chem. 2002;277(43):40375–40383. doi: 10.1074/jbc.M202744200. [DOI] [PubMed] [Google Scholar]
- 19.Erdos E.G., Marcic B.M. Kinins, receptors, kininases and inhibitors—where did they lead us? Biol Chem. 2001;382(1):43–47. doi: 10.1515/BC.2001.007. [DOI] [PubMed] [Google Scholar]
- 20.Farmer S.G., Powell S.J., Wilkins D.E., Graham A. Cloning, sequencing and functional expression of a guinea pig lung bradykinin B2 receptor. Eur J Pharmacol. 1998;346(2/3):291–298. doi: 10.1016/s0014-2999(98)00024-7. [DOI] [PubMed] [Google Scholar]
- 21.Ferreira L.A., Alves E.W., Henriques O.B. Peptide T, a novel bradykinin potentiator isolated from Tityus serrulatus scorpion venom. Toxicon. 1993;31(8):941–947. doi: 10.1016/0041-0101(93)90253-f. [DOI] [PubMed] [Google Scholar]
- 22.Ferreira L.A., Alves W.E., Lucas M.S., Habermehl G.G. Isolation and characterization of a bradykinin potentiating peptide (BPP-S) isolated from Scaptocosa raptoria venom. Toxicon. 1996;34(5):599–603. doi: 10.1016/0041-0101(96)00010-4. [DOI] [PubMed] [Google Scholar]
- 23.Ferreira L.A., Galle A., Raida M., Schrader M., Lebrun I., Habermehl G. Isolation: analysis and properties of three bradykinin-potentiating peptides (BPP-II, BPP-III, and BPP-V) from Bothrops neuwiedi venom. J Protein Chem. 1998;17(3):285–289. doi: 10.1023/a:1022545020764. [DOI] [PubMed] [Google Scholar]
- 24.Ferreira L.A., Henriques O.B., Lebrun I., Batista M.B., Prezoto B.C., Andreoni A.S. A new bradykinin-potentiating peptide (peptide P) isolated from the venom of Bothrops jararacussu (jararacucu tapete, urutu dourado) Toxicon. 1992;30(1):33–40. doi: 10.1016/0041-0101(92)90499-u. [DOI] [PubMed] [Google Scholar]
- 25.Ferreira L.A., Mollring T., Lebrun F.L., Raida M., Znottka R., Habermehl G.G. Structure and effects of a kinin potentiating fraction F (AppF) isolated from Agkistrodon piscivorus piscivorus venom. Toxicon. 1995;33(10):1313–1319. doi: 10.1016/0041-0101(95)00071-s. [DOI] [PubMed] [Google Scholar]
- 26.Ferreira S.H. A bradykinin-potentiating factor (Bpf) present in the venom of Bothrops jararaca. Br J Pharmacol. 1965;24:163–169. doi: 10.1111/j.1476-5381.1965.tb02091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferreira S.H., Bartelt D.C., Greene L.J. Isolation of bradykinin-potentiating peptides from Bothrops jararaca venom. Biochemistry. 1970;9(13):2583–2593. doi: 10.1021/bi00815a005. [DOI] [PubMed] [Google Scholar]
- 28.Ferry X., Brehin S., Kamel R., Landry Y. G protein-dependent activation of mast cell by peptides and basic secretagogues. Peptides. 2002;23(8):1507–1515. doi: 10.1016/s0196-9781(02)00090-6. [DOI] [PubMed] [Google Scholar]
- 29.Fortin J.P., Gobeil F., Jr., Adam A., Regoli D., Marceau F. Do angiotensin-converting enzyme inhibitors directly stimulate the kinin B1 receptor? Am J Physiol Heart Circ Physiol. 2003;285(1):H277–H282. doi: 10.1152/ajpheart.01124.2002. [DOI] [PubMed] [Google Scholar]
- 30.Genin M.J., Mishra R.K., Johnson R.L. Dopamine receptor modulation by a highly rigid spiro bicyclic peptidomimetic of Pro-Leu-Gly-NH2. J Med Chem. 1993;36(22):3481–3483. doi: 10.1021/jm00074a032. [DOI] [PubMed] [Google Scholar]
- 31.Gobeil F., Jr., Halle S., Blais P.A., Regoli D. Studies on the angiotensin-converting enzyme and the kinin B2 receptor in the rabbit jugular vein: modulation of contractile response to bradykinin. Can J Physiol Pharmacol. 2002;80(2):153–163. doi: 10.1139/y02-014. [DOI] [PubMed] [Google Scholar]
- 32.Gothe R., Seyfahrt L., Schuhmann C., Agricola I., Reissmann S., Lifferth A. Combination of allyl protection and HYCRAM™-linker technology for the synthesis of peptides with problematical amino acids and sequences. J Prakt Chem. 1999;341:369–377. [Google Scholar]
- 33.Hecker M., Bara A.T., Busse R. Potentiation of the biological efficacy of bradykinin by ACE inhibitors: a shift in the affinity of the B2 receptor? Immunopharmacology. 1996;33(1–3):93–94. doi: 10.1016/0162-3109(96)00018-5. [DOI] [PubMed] [Google Scholar]
- 34.Henriques O.B., de Deus R.B., Santos R.A. Bradykinin potentiating peptides isolated from alpha-casein tryptic hydrolysate. Biochem Pharmacol. 1987;36(1):182–184. doi: 10.1016/0006-2952(87)90398-4. [DOI] [PubMed] [Google Scholar]
- 35.Higuchi S., Murayama N., Saguchi K., Ohi H., Fujita Y., Camargo A.C. Bradykinin-potentiating peptides and C-type natriuretic peptides from snake venom. Immunopharmacology. 1999;44(1/2):129–135. doi: 10.1016/s0162-3109(99)00119-8. [DOI] [PubMed] [Google Scholar]
- 36.Ianzer D., Konno K., Marques-Porto R., Vieira Portaro F.C., Stöcklin R., Martins de Camargo A.C. Identification of five new bradykinin potentiating peptides (BPPs) from Bothrops jararaca crude venom by using electrospray ionization tandem mass spectrometry after a two-step liquid chromatography. Peptides. 2004;25(7):1085–1092. doi: 10.1016/j.peptides.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 37.Ignjatovic T., Tan F., Brovkovych V., Skidgel R.A., Erdos E.G. Activation of bradykinin B1 receptor by ACE inhibitors. Int Immunopharmacol. 2002;2(13/14):1787–1793. doi: 10.1016/s1567-5769(02)00146-7. [DOI] [PubMed] [Google Scholar]
- 38.Ivanov V.T., Karelin A.A., Philippova M.M., Nazimov I.V., Pletnev V.Z. Hemoglobin as a source of endogenous bioactive peptides: the concept of tissue-specific peptide pool. Biopolymers. 1997;43:171–188. doi: 10.1002/(SICI)1097-0282(1997)43:2<171::AID-BIP10>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 39.Kato H., Suzuki T. Bradykinin-potentiating peptides from the venom of Agkistrodon halys blomhoffii. Experientia. 1969;25(7):694–695. doi: 10.1007/BF01897565. [DOI] [PubMed] [Google Scholar]
- 40.Kato H., Suzuki T. Bradykinin-potentiating peptides from the venom of Agkistrodon halys blomhoffi. Isolation of five bradykinin potentiators and the amino acid sequences of two of them, potentiators B and C. Biochemistry. 1971;10(6):972–980. doi: 10.1021/bi00782a007. [DOI] [PubMed] [Google Scholar]
- 41.Klutchko S., Blankley C.J., Fleming R.W., Hinkley J.M., Werner A.E., Nordin I. Synthesis of novel angiotensin converting enzyme inhibitor quinapril and related compounds. A divergence of structure–activity relationships for non-sulfhydryl and sulfhydryl types. J Med Chem. 1986;29(10):1953–1961. doi: 10.1021/jm00160a026. [DOI] [PubMed] [Google Scholar]
- 42.Koehne P., Schaper C., Graf K., Kunkel G. Neutral endopeptidase 24.11: its physiologic and possibly pathophysiologic role in inflammation with special effect on respiratory inflammation. Allergy. 1998;53(11):1023–1042. doi: 10.1111/j.1398-9995.1998.tb03812.x. [DOI] [PubMed] [Google Scholar]
- 43.Kudoh A., Kudoh E., Katagai H., Takazawa T. Insulin potentiates bradykinin-induced inositol 1,4,5-triphosphate in neonatal rat cardiomyocytes. J Cardiovasc Pharmacol. 2002;39(5):621–627. doi: 10.1097/00005344-200205000-00001. [DOI] [PubMed] [Google Scholar]
- 44.L‘vov V.M., Yukel‘son L.Y. Bradykinin-potentiating peptides from Echis multisquamatus venom. Khim Prir Soedin. 1995;1:435–440. [Google Scholar]
- 45.Ladram A., Montagne J.J., Bulant M., Nicolas P. Analysis of structural requirements for TRH-potentiating peptide receptor binding by analogue design. Peptides. 1994;15(3):429–433. doi: 10.1016/0196-9781(94)90200-3. [DOI] [PubMed] [Google Scholar]
- 46.Lebrun I., Lebrun F.L., Henriques O.B., Carmona A.K., Juliano L., Camargo A.C. Isolation and characterization of a new bradykinin potentiating octapeptide from gamma-casein. Can J Physiol Pharmacol. 1995;73(1):85–91. doi: 10.1139/y95-012. [DOI] [PubMed] [Google Scholar]
- 47.Li W., Moore M.J., Vasilieva N., Sui J., Wong S.K., Berne M.A. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426(6965):450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liebmann C. G protein-coupled receptors and their signaling pathways: classical therapeutical targets susceptible to novel therapeutic concepts. Curr Pharm Des. 2004;10(16):1937–1958. doi: 10.2174/1381612043384367. [DOI] [PubMed] [Google Scholar]
- 49.Marcic B., Deddish P.A., Jackman H.L., Erdos E.G. Enhancement of bradykinin and resensitization of its B2 receptor. Hypertension. 1999;33(3):835–843. doi: 10.1161/01.hyp.33.3.835. [DOI] [PubMed] [Google Scholar]
- 50.Marcic B., Deddish P.A., Skidgel R.A., Erdos E.G., Minshall R.D., Tan F. Replacement of the transmembrane anchor in angiotensin I-converting enzyme (ACE) with a glycosylphosphatidylinositol tail affects activation of the B2 bradykinin receptor by ACE inhibitors. J Biol Chem. 2000;275(21):16110–16118. doi: 10.1074/jbc.M909490199. [DOI] [PubMed] [Google Scholar]
- 51.Matsui T., Li C.H., Osajima Y. Preparation and characterization of novel bioactive peptides responsible for angiotensin I-converting enzyme inhibition from wheat germ. J Pept Sci. 1999;5(7):289–297. doi: 10.1002/(SICI)1099-1387(199907)5:7<289::AID-PSC196>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 52.Meki A.R., Nassar A.Y., Rochat H. A bradykinin-potentiating peptide (peptide K12) isolated from the venom of Egyptian scorpion Buthus occitanus. Peptides. 1995;16(8):1359–1365. doi: 10.1016/0196-9781(95)02036-5. [DOI] [PubMed] [Google Scholar]
- 53.Minshall R.D., Nedumgottil S.J., Igic R., Erdos E.G., Rabito S.F. Potentiation of the effects of bradykinin on its receptor in the isolated guinea pig ileum. Peptides. 2000;21(8):1257–1264. doi: 10.1016/s0196-9781(00)00267-9. [DOI] [PubMed] [Google Scholar]
- 54.Minshall R.D., Tan F., Nakamura F., Rabito S.F., Becker R.P., Marcic B. Potentiation of the actions of bradykinin by angiotensin I-converting enzyme inhibitors. The role of expressed human bradykinin B2 receptors and angiotensin I-converting enzyme in CHO cells. Circ Res. 1997;81(5):848–856. doi: 10.1161/01.res.81.5.848. [DOI] [PubMed] [Google Scholar]
- 55.Molina H.M., Carmona A.K., Kouyoumdjian M., Borges D.R., Juliano L. Liver bradykinin-inactivating-endopeptidase is similar to the metalloendopeptidase (EC 3.4.24.15) Immunopharmacology. 1996;32(1–3):176–179. doi: 10.1016/0162-3109(95)00086-0. [DOI] [PubMed] [Google Scholar]
- 56.Muller S., Adomeit A., Kaufmann R., Appelhans H., Passow H., Reissmann S. Expression and functional characterization of a pHis-tagged human bradykinin B2 receptor in COS-7 cells. Biol Chem. 2000;381(4):343–347. doi: 10.1515/BC.2000.045. [DOI] [PubMed] [Google Scholar]
- 57.Mueller S., Liebmann C., Reissmann S. Intramolecular signal transduction by the bradykinin B2 receptor. Int Immunopharmacol. 2002;2(13/14):1763–1770. doi: 10.1016/s1567-5769(02)00167-4. [DOI] [PubMed] [Google Scholar]
- 58.Norman M.U., Lew R.A., Smith A.I., Hickey M.J. Metalloendopeptidases EC 3.4.24.15/16 regulate bradykinin activity in the cerebral microvasculature. Am J Physiol Heart Circ Physiol. 2003;284(6):1942–1948. doi: 10.1152/ajpheart.00948.2002. [DOI] [PubMed] [Google Scholar]
- 59.Ondetti M.A., Williams N.J., Sabo E.F., Pluscec J., Weaver E.R., Kocy O. Angiotensin-converting enzyme inhibitors from the venom of Bothrops jararaca. Isolation, elucidation of structure, and synthesis. Biochemistry. 1971;10(22):4033–4039. doi: 10.1021/bi00798a004. [DOI] [PubMed] [Google Scholar]
- 60.Ongali B., Buck Hde S., Cloutier F., Legault F., Regoli D., Lambert C. Chronic effects of angiotensin-converting enzyme inhibition on kinin receptor binding sites in the rat spinal cord. Am J Physiol Heart Circ Physiol. 2003;284(6):H1949–H1958. doi: 10.1152/ajpheart.01113.2002. [DOI] [PubMed] [Google Scholar]
- 61.Paegelow I. Gustav Fischer Verlag; Jena: 1991. Nachweis chemotaktischer Faktoren. [Google Scholar]
- 62.Paegelow I., Trzeczak S., Bockmann S., Vietinghoff G. Migratory responses of polymorphonuclear leukocytes to kinin peptides. Pharmacology. 2002;66(3):153–161. doi: 10.1159/000063797. [DOI] [PubMed] [Google Scholar]
- 63.Papapetropoulos A., Ryan J.W., Antonov A., Virmani R., Kolodgie F.D., Gerrity R.G. Human aortic endothelial cell aminopeptidase N. Immunopharmacology. 1996;32(1–3):153–156. doi: 10.1016/0162-3109(95)00079-8. [DOI] [PubMed] [Google Scholar]
- 64.Patchett A.A., Harris E., Tristram E.W., Wyvratt M.J., Wu M.T., Taub D. A new class of angiotensin-converting enzyme inhibitors. Nature. 1980;288(5788):280–283. doi: 10.1038/288280a0. [DOI] [PubMed] [Google Scholar]
- 65.Paula R.D., Lima C.V., Britto R.R., Campagnole-Santos M.J., Khosla M.C., Santos R.A. Potentiation of the hypotensive effect of bradykinin by angiotensin-(1–7)-related peptides. Peptides. 1999;20(4):493–500. doi: 10.1016/s0196-9781(99)00031-5. [DOI] [PubMed] [Google Scholar]
- 66.Piot J.M., Zhao Q., Guillochon D., Ricart G., Thomas D. Isolation and characterization of a bradykinin-potentiating peptide from a bovine peptic hemoglobin hydrolysate. FEBS Lett. 1992;299(1):75–79. doi: 10.1016/0014-5793(92)80104-o. [DOI] [PubMed] [Google Scholar]
- 67.Ryan J.W., Papapetropoulos A., Ju H., Denslow N.D., Antonov A., Virmani R. Aminopeptidase P is disposed on human endothelial cells. Immunopharmacology. 1996;32(1–3):149–152. doi: 10.1016/0162-3109(95)00078-x. [DOI] [PubMed] [Google Scholar]
- 68.Schumann C., Seyfarth L., Greiner G., Paegelow I., Reissmann S. Synthesis and biological activities of new side chain and backbone cyclic bradykinin analogues. J Pept Res. 2002;60(2):128–140. doi: 10.1034/j.1399-3011.2002.02986.x. [DOI] [PubMed] [Google Scholar]
- 69.Sellitti D.F., Doi S.Q. C-type natriuretic peptide (CNP) increases [125I]ANF binding to FRTL-5 rat thyroid cells by increasing ANF receptor affinity. Peptides. 1994;15(7):1249–1253. doi: 10.1016/0196-9781(94)90149-x. [DOI] [PubMed] [Google Scholar]
- 70.Siems W.E., Heder G., Komissarowa N.W. Procedure for the determination of angiotensin converting enzyme. Z Med Lab Diagn. 1985;26(4):232–234. [PubMed] [Google Scholar]
- 71.Skidgel R.A., Erdos E.G. Angiotensin converting enzyme (ACE) and neprilysin hydrolyze neuropeptides: a brief history, the beginning and follow-ups to early studies. Peptides. 2004;25(3):521–525. doi: 10.1016/j.peptides.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 72.Skidgel R.A., McGwire G.B., Li X.Y. Membrane anchoring and release of carboxypeptidase M: implications for extracellular hydrolysis of peptide hormones. Immunopharmacology. 1996;32(1–3):48–52. doi: 10.1016/0162-3109(96)00008-2. [DOI] [PubMed] [Google Scholar]
- 73.Sosnina N.A., Golubenko Z., Akhunov A.A., Kugaevskaia E.V., Eliseeva Iu E., Orekhovich V.N. Bradykinin-potentiating peptides from the spider Latrodectus tredecimguttatus—inhibitors of carboxycathepsin and of a preparation of karakurt venom kininase. Dokl Akad Nauk SSSR. 1990;315(1):236–239. [PubMed] [Google Scholar]
- 74.Steinmann W. The E.K. Frey–E. Werle Foundation of the Henning L. Voigt Family Award ceremony. Braz J Med Biol Res. 1994;27(8):1687–1692. [PubMed] [Google Scholar]
- 75.Teetz V., Geiger R., Henning R., Urbach H. Synthesis of a highly active angiotensin converting enzyme inhibitor: 2-[N-[(S)-1-ethoxycarbonyl-3-phenylpropyl]-l-alanyl]-(1S,3S,5S)-2-azabicyclo[3.3.0]octane-3-carboxylic acid (Hoe 498) Arzneimittelforschung. 1984;34(10B):1399–1401. [PubMed] [Google Scholar]
- 76.Tom B., de Vries R., Saxena P.R., Danser A.H. Bradykinin potentiation by angiotensin-(1–7) and ACE inhibitors correlates with ACE C- and N-domain blockade. Hypertension. 2001;38(1):95–99. doi: 10.1161/01.hyp.38.1.95. [DOI] [PubMed] [Google Scholar]
- 77.Ufkes J.G., Aarsen P.N., van der Meer C. The mechanism of action of two bradykinin-potentiating peptides on isolated smooth muscle. Eur J Pharmacol. 1977;44(2):89–97. doi: 10.1016/0014-2999(77)90094-2. [DOI] [PubMed] [Google Scholar]
- 78.Ufkes J.G., Visser B.J., Heuver G., Van der Meer C. Structure–activity relationships of bradykinin potentiating peptides. Eur J Pharmacol. 1978;50(2):119–122. doi: 10.1016/0014-2999(78)90006-7. [DOI] [PubMed] [Google Scholar]
- 79.Wei L., Alhenc-Gelas F., Corvol P., Clauser E. The two homologous domains of human angiotensin I-converting enzyme are both catalytically active. J Biol Chem. 1991;266(14):9002–9008. [PubMed] [Google Scholar]
- 80.Yamafuji K., Taniguchi Y., Sakamoto E. The thiol enzyme from rat spleen that produces bradykinin potentiating peptide from rat plasma. Immunopharmacology. 1996;32(1–3):157–159. doi: 10.1016/0162-3109(95)00080-1. [DOI] [PubMed] [Google Scholar]
- 81.Zhao Q., Garreau I., Sannier F., Piot J.M. Opioid peptides derived from hemoglobin: hemorphins. Biopolymers. 1997;43(2):75–98. doi: 10.1002/(SICI)1097-0282(1997)43:2<75::AID-BIP2>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]


