Enteric disease in the calf is the second most common cause of loss to the cattle industry, the first being reproductive disease. The United States Department of Agriculture estimates that 8 to 25 per cent of all calves born in the United States die from enteric disease, a loss that is more than that generated by all other diseases combined.
There are many causes of enteric disease in the calf, the major ones being salmonellosis, clostridial enterotoxemia, coccidiosis, and the calf scours syndrome. Salmonellosis, clostridial enterotoxemia, and coccidiosis can usually be differentiated clinically, and the diagnosis confirmed by bacterial cultures or fecal flotations. Calf scours is not an etiologic diagnosis, but instead is a commonly used classification indicating infectious diarrhea that is not caused by the other three specific etiologic agents.
A number of pathogenic agents may be involved either as primary or secondary causes of the calf scours syndrome. Many different bacteria as well as serotypes of the same bacteria, bacterial toxins, chlamydial agents, and a multitude of viruses, including adenovirus, parvovirus, coronavirus, rotavirus (reovirus), enterovirus, bovine virus diarrhea, and infectious bovine rhinotracheitis have been implicated as causes. Escherichia coli is often incriminated; however, many of the strains that are isolated are not pathogenic. In addition, in many cases, the increased number of E. coli in the intestine is secondary to these other factors. Often it is difficult to determine which of these infectious agents are involved, and whether they are primary or secondary. Many noninfectious factors may also play an important role in causing or predisposing calves to the scours syndrome such as: the immunity of the calf, both that passively obtained from the colostrum as well as that actively produced by the calf itself; irregular feeding or overfeeding; the degree of confinement and crowding, which determines the pathogenic challenge to which the calf is exposed; and a multitude of stress factors such as inclement weather and difhcult birth.
Calf scours may affect calves up to two months of age, but it is generally the greatest problem in calves less than two weeks of age, and frequently occurs in those less than one week old. In contrast, coccidiosis is more common in older calves and is generally hemorrhagic. Clostridial enterotoxemia causes sudden death more commonly than it causes diarrhea. Although large numbers of clostridia are present in the small intestine of some calves with diarrhea, they may not be the primary pathogen; therefore, their presence does not confirm clostridial enterotoxemia as the cause of the diarrhea. Salmonellosis occurs most often in calves two weeks to four months of age that are debilitated, stressed, and under close confinement. In some areas it may be endemic and therefore may be the most common cause of diarrhea in those calves. However, in most areas, calf scours is by far the most common cause of diarrhea in the calf. Frequently, the primary etiologic agent is not identified.
The two clinical forms of enteritis in the calf are septicemia endotoxemia and diarrheal dehydration. The septicemic-endotoxemic form is most common in calves that have been deprived of colostrum and occurs most commonly in the first few days of life. This form of the disease has an acute, fatal course. Afflicted calves are often found dead without ever having been observed to be sick, and even before diarrhea has occurred. They account for less than 10 per cent of the cases reported, although in some herds, this form of the disease may be more prevalent.
The most common clinical manifestation of enteritis is diarrhea which results in extensive losses of fluids and electrolytes. These losses and their effects have been described in detail elsewhere6 and are quite similar regardless of the causative agent. Therefore, the treatment of the calf with diarrhea is also quite uniform regardless of the etiology of the disease.
General Principles of Fluid Therapy
The most important aspect in the treatment of the diarrheic calf is the administration of fluids to replace the extensive losses of water and electrolytes that occur and which are responsible for the clinical signs and potential death of the calf. These fluids should also supply energy, particularly in patients requiring more than one or two days of treatment, since anorexia often accompanies the disease. Optimal administration of fluids requires familiarity with several general principles of fluid therapy. In addition, the selection of the route and rate of administration of fluids and the type of fluid used is based upon the acuteness of the illness and the degree of dehydration.
Assessment of the Degree of Dehydration
The degree of dehydration is best estimated from the clinical signs described in Table 1. The amount of fluid needed as well as the condition of the animal can be determined from the degree of dehydration. For example, if a 30 kg calf is 10 per cent dehydrated, the amount of fluid needed to correct this dehydration is 30 kg times 10 per cent, or 3 liters.
Table 1.
Clinical Signs Indicating the Percentage of Dehydration in the Diarrheic Calf
| PERCENTAGE OF DECREASE IN BODY WEIGHT SECONDARY TO DEHYDRATION | CLINICAL SIGNS* |
|---|---|
| 4 | None |
| 6 | Loss of skin elasticity, dry mouth and nose |
| 8 | Enophthalmos and cooling of the extremities |
| 10 | Cold legs and oral cavity, generally recumbent |
| 12 | Comatose and in shock |
| 14 | Death |
The more rapid the loss, the more severe the clinical syndrome at that degree of dehydration. The signs given are typical of a 48 hour period of dehydration.
Although the hematocrit and plasma protein concentrations increase linearly as diarrheal dehydration in the calf progresses,6 they are poor indicators of the degree of dehydration. Both parameters may vary widely in normal calves;6 therefore changes from an average value may be meaningless in any individual animal. However, changes in hematocrit and plasma protein concentration as well as in body weight are quite useful in determining whether fluid therapy is correcting the dehydration and compensating for the continuing loss of fluid. Weighing the patient periodically is one of the simplest and most reliable modes of assessment; it is also the most frequently overlooked method.
Temperature of Fluids
All fluids, regardless of the route of administration, should always be administered at body temperature. Warming cold blood to body temperature prior to massive blood transfusion decreased the incidence of cardiac arrest from 58 per cent to 7 per cent in one study in humans.2 Ringer’s lactate solution given intravenously at 5°C to five dogs in hemorrhagic shock resulted in three deaths. However, when five dogs with a similar blood loss were given the same amount of fluid warmed to body temperature prior to administration, no deaths occurred.2 Cold fluids given intravenously have a direct effect on the sino-atrial node, decreasing heart rate, cardiac output, blood pressure, and coronary artery flow, and causing death from arrhythmia and diminished contractility.2 Cold fluids given by nonintravenous routes are absorbed more slowly than are warm fluids.
Routes of Administration
Oral
Oral administration of fluids is always the route of choice in the treatment of the diarrheic calf, unless the condition is so acute that intestinal absorption is not rapid enough to keep up with losses of fluid or unless the animal is too severely dehydrated. If the animal is more than 6 per cent dehydrated or if the disease is fulminating, fluids must be given parenterally (Table 1). In these cases, however, giving fluids by both routes is beneficial and lessens the amount of fluids that must be given parenterally.
Oral fluid therapy has several advantages over the parenteral administration of fluids: the ability to give large volumes rapidly; a sustained input as fluids are absorbed; a lesser expense since the fluids need not be sterile and may be given by the owner; a less critical composition of the fluid compared with fluids given parenterally; and the relative safety of this route.
For best results, fluids administered orally should be formulated to afford the maximal rate of intestinal absorption. This is accomplished by using fluids which contain proteins or amino acids, glucose, sodium, and bicarbonate. These solutes have a synergistic effect on the rate of intestinal absorption of each other and, therefore, of water.7 For example, the rate of intestinal absorption of sodium is greatly increased if glucose is present; conversely, the rate of intestinal absorption of glucose is more than doubled if sodium is present. Of course, through salivary, pancreatic, and hepatic secretion, some sodium is always present in the intestine, but the quantity may not always be sufficient for maximal rates of absorption to occur. Absorption of water is passive; as solutes are absorbed, it is absorbed. Thus, to obtain the maximal rate of water absorption a fluid designed to attain the maximal rate of solute absorption is required. A number of fluids designed for this purpose are available commercially* or can be formulated as shown in Table 2. Oral fluids constitute excellent media for microbial growth and are nonsterile; therefore, after use, the remainder should be refrigerated and, if not used within one to two days, it should be discarded.
Table 2.
Homemade Oral Fluids for Treatment of Diarrhea*
| † Mixture 1 |
| 1 can beef consommé (11 oz) |
| 1 pkg jam and jelly pectin (2 oz)‡ |
| 2 tsp lite salt§ |
| 2 tsp baking soda (NaHCO3) |
| Made up to 2 qt (1892 ml) |
| ‖ Mixture 2 |
| 1 pkg jam and jelly pectin |
| 1 tbsp lite salt |
| 1 tbsp baking soda |
| Made up to 2 qt (1892 ml) |
When level spoonfuls are used, both mixtures contain 2 per cent glucose, 490 to 500 mOsm per liter, and 121 mEq per liter of sodium. All ingredients are available in grocery stores.
This mixture also contains 48 mEq per liter of bicarbonate and 31 mEq per liter of potassium.
Jam and jelly pectin is greater than 75 per cent glucose.
Lite salt is half sodium chloride and half potassium chloride.
This mixture also contains 72 mEq per liter of bicarbonate and 43 mEq per liter of potassium.
The energy, amino acids, and proteins derived from oral fluids have a dual purpose. They not only enhance intestinal absorption but they also assist in the nutritional maintenance of the animal. Although the energy and protein that most oral fluids provide are certainly of benefit, they are not sufficient to meet the animal’s requirements. Therefore, additional energy must be provided if more than two days of therapy are required. Although the administration of oral fluids may increase the loss of fecal fluid, this does not negate their benefit. Their use will either reduce the net loss of water from the body or will result in a net gain of water. This is demonstrated in the example shown in Table 3, in which, prior to receiving oral fluids, the calf had a net water loss and a decrease in body weight of 1 kg per day. When oral fluids were given, fecal losses doubled, but the calf then had a net water gain and an increase in body weight of 1 kg per day. Weighing the patient eight hours or so after administration of oral fluids will confirm whether or not the fluids given orally or by any other route are being absorbed and retained in quantities adequate to overcome losses.
Table 3.
Example of the Effect of Orally Administered Fluids in the Diarrheic Calf
| INTAKE (LITERS/DAY) | FECAL LOSS (LITERS/DAY) | NET LOSS (−) OR GAIN ( + ) (LITERS/DAY) | BODY CHANGE IN WEIGHT (KG/DAY) | |
|---|---|---|---|---|
| Before giving oral fluids | 0 | 1 | −1 | −1 |
| After giving oral fluids | 3 | 2 | + 1 | + 1 |
In preruminant calves and lambs, suckled fluids will bypass the rumen and reticulum and go directly into the omasum and abomasum. Nursed fluids, therefore, reach the intestine more rapidly than those given by stomach tube and are absorbed more quickly. Thus, the calf should first be encouraged to nurse as much fluid as possible. The remainder of the fluids needed may be given by stomach tube. Most of the fluid administered by stomach tube is deposited in the rumen and takes longer to reach the intestine where absorption occurs. In some cases, this may be of benefit in providing a more prolonged therapeutic delivery.
Subcutaneous
The subcutaneous route of fluid therapy is quite useful in animals other than the horse and pig which lack sufficient subcutaneous space for the administration of significant volumes by this route. Fluids given subcutaneously are absorbed in four to six hours following administration. They provide a slow but sustained delivery of fluid during this period, which may be adequate if losses of fluid from the body are not occurring faster than the rate of absorption. In more severe cases, the subcutaneous administration of fluids in conjunction with intravenous fluids may be quite beneficial and may lessen the amount of fluids that must be given intravenously. If the degree of dehydration is greater than 8 per cent, peripheral circulation is decreased to the extent that fluids given subcutaneously will not be absorbed or will be absorbed more slowly. After correcting dehydration, the continued absorption of additional fluids given subcutaneously can help in replacing continuing losses.
Fluids given subcutaneously should be sterile and warmed to body temperature. They should be from 1 to 1½ times isosmotic. Electrolytes should be ½ to 1 times isosmolality; they may contain from 0 to 2½ per cent glucose and generally contain at least 70 mEq per liter of sodium ( Table 4). If their content varies from this formula, they will draw fluids or electrolytes out of the blood and may worsen the condition, at least initially. Addition of other ingredients to the fluid depends on the specific condition being treated.
Table 4.
Solutions Suitable for Subcutaneous Administration
| 1. Isosmotic (290 mOsm) electrolytes |
| 2. Isosmotic electrolyte + isosmotic glucose (5 per cent), mixed in equal quantities |
| 3. Isosmotic electrolytes + 25 grams glucose per liter or 55 ml or 50 per cent glucose per liter; total osmolality, 430 mOsm |
Up to 80 ml of fluid per kg of body weight may be given subcutaneously with a maximum of 10 to 20 ml per kg given in one area. The rate of administration is not important as long as excessive pressure does not cause discomfort to the patient. Fluids given subcutaneously may be administered by gravity flow or they may be given more rapidly under pressure. A three-way stopcock* and syringe may be used (one stopcock attachment going to the needle inserted subcutaneously, one to the fluid bottle, and one to a syringe which is used to draw the fluid from the bottle and inject it into the animal), or a collapsible fluid administration bag that can be squeezed works nicely.
Intravenous
The intravenous route of fluid therapy is the route of choice when the animal is more than 8 per cent dehydrated (Table 1), when the loss of fluids from the body is so rapid that replacement fluids given by other routes may not be absorbed fast enough to compensate for the losses, when shock is present, or when the animal shows clinical signs of altered concentrations of plasma electrolyte or glucose. Intravenous administration of fluids has the advantage that, since absorption is not a factor, the rate at which the fluid enters the body water pool can be controlled. Because of this, the rate at which fluids are given intravenously must be controlled according to the needs of the individual patient, the purpose of therapy, and the composition of the fluids given. Too rapid a rate of administration of any fluid may result in shivering, apprehension, tachycardia, pulmonary edema, or death.
The intravenous administration of more than a small quantity of fluid requires catheterization. A number of commercially available catheters may be used. If a jugular intracath (that is, a catheter on the inside of a needle) is used in a calf, it should not be over 20 cm (8 in) in length. A 30 cm (12 in) catheter may induce ventricular fibrillation and death. A 6 to 12 cm (2.5 to 5 in) 16 or 17 gauge extracath (catheter on the outside of the needle) works well in a calf and does not kink, which may occur with intracaths. To insert the extracath, clip and disinfect the skin over the jugular vein, pinch the skin up and away from the vein, and make a single stab incision with a No. 11 Bard Parker blade. This procedure does not require local anesthesia or suturing after removal of the catheter. Next, place a tab on the hub of the catheter using two pieces of adhesive tape, approximately one half inch by 3 inches in size. The sticky sides should be together with the catheter hub between them. Attach a syringe to the catheter needle and insert the needle and catheter through the skin incision and into the vein, aspirating slightly as you do so. As soon as blood is drawn into the syringe, slide the catheter off the needle and down the vein. Tape the catheter to the calf s neck using adhesive tape. Encircle the calf s neck about 1½ times with the tape which should be firmly stuck to the tab previously attached to the catheter hub. To prevent pulling on the catheter during the administration of fluid, the tubing from the fluid bottle to the catheter should be taped to the calf s forehead with a piece of tape encircling the calf s head just in front of the ears.
Intraperitoneal
The intraperitoneal route of fluid administration is not routinely used. It has no advantage over the subcutaneous route because the absorption of fluids occurs at about the same rate by either route of administration. If fluids are poorly absorbed from the subcutaneous area because of the degree of dehydration, they will also be poorly absorbed from the peritoneal cavity. The intraperitoneal administration of fluids may result in peritonitis, adhesions, or damage by the needle to internal organs.
Fluid Therapy for the Diarrheic Calf
Route and Rate of Administration of Fluids and Amount Needed
If the diarrheic calf is not over 6 per cent dehydrated (Table 1) and if the losses are not so rapid that they exceed absorptive capacity, the administration of oral fluids alone is often adequate. Give enough to rehydrate the calf and to maintain its body weight. This will generally require 1 to 2 liters of the oral fluids previously described two to three times a day. If the calf is over 6 per cent dehydrated or if the fluid losses exceed the intestinal absorptive capacity, as indicated by continued weight loss, increasing hematocrit, or past experience in the herd, fluids must be given parenterally. However, even in these cases the administration of fluids orally is beneficial and lessens the amount of parenteral fluids required. Fluids should be given orally to any calf that is not over 8 per cent dehydrated (Table 1).
If the calf is 6 to 8 per cent dehydrated (Table 1), in addition to giving fluids orally, give 2 liters subcutaneously in four or five locations under the loose skin on each side of the neck, shoulders, and back. Do not give over 500 ml in any one area. If the state of hydration is greater than 8 per cent, or if losses occur more rapidly than oral and subcutaneous fluids can be absorbed, fluids must be given intravenously. In this instance a minimum of three liters of fluid is needed initially by the 30 to 40 kg calf. Administer 1 liter intravenously over a period of about one hour, that is, at a rate of 30 to 40 ml. per kg per hr. The additional 2 liters may also be given intravenously at a rate no faster than half of this rate, that is, 500 ml per hr per calf. If it is not possible to give all fluids slowly over this prolonged period of time, give the first liter intravenously and the additional fluid needed may be given more rapidly subcutaneously. A second treatment, administered 8 to 12 hours later, if necessary, again may be either by the intravenous or subcutaneous routes or by a combination of both.
In summary, at 6 per cent dehydration or less, administer 1 to 2 liters of fluid orally two to three times a day. If the state of dehydration is 6 to 8 per cent, give fluids orally and 2 liters subcutaneously. At greater than 8 per cent dehydration, 3 liters of fluid should be administered. Give 1 liter intravenously over a period of one hour; the remaining 2 liters should be given intravenously over a period of not less than 4 hours, or the remaining 2 liters may be given subcutaneously. In acute cases, it may be necessary to administer fluids intravenously, regardless of the degree of dehydration present when therapy is instituted, in order to compensate for diarrheal fluid losses.
Types of Parenteral Fluids
Even though certain fluid and electrolyte compositions may be more effective than others, the administration of nearly any fluid to the diarrheic calf will be beneficial since the most important ingredient is water. However, to be of maximal benefit, therapy should be aimed at not only correcting dehydration and vascular imbalances, but at correcting whole body imbalances. The plasma potassium concentration of a severely diarrheic calf is nearly doubled. This fact may be very misleading since diarrhea causes extensive loss of potassium from the body, resulting in a total body deficit of potassium and a decrease in the intracellular concentration of potassium.6 Potassium should be given to correct the total body deficit; it must pass into body cells to correct the extracellular increase and intracellular decrease in concentration of potassium. Glucose and bicarbonate are both beneficial in this regard.
Glucose is needed for the following reasons: it enhances the movement of potassium into cells; hypoglycemia is often present in diarrheic calves;6 and it serves as a source of energy. Bicarbonate is needed to treat metabolic acidosis. Acidosis, which is both intracellular and extracellular, causes an increase in the efflux of potassium from cells.6 Correcting acidosis reverses this effect, causing movement of hydrogen ions out of and movement of potassium into cells.
Ringer’s lactate solution is often used to treat acidosis; the lactate must be metabolized to be effective, however. In the diarrheic calf, more lactate is present than can be metabolized as indicated by an increase in plasma lactate levels.6 In addition, half of the lactate in lactated solutions is the D-isomer. Only the L-isomer is normally metabolized in the body. The D-isomer is poorly metabolized even by healthy animals. Thus, it is better if a lactate solution is not used for the treatment of the diarrheic calf. Based on existing data and clinical results, Ringer’s bicarbonate is recommended instead. However, if Ringer’s bicarbonate is not available, Ringer’s lactate, acetate, or gluconate may be used. The following should be added to any of these fluids used:
For the calf that is dehydrated 8 per cent or more (Table 1), 20 mEq of potassium chloride, 50 mEq of sodium bicarbonate, and 120 ml of 50 per cent glucose or dextrose solution should be added to the first liter of fluids given intravenously.
To any additional fluids given intravenously, to all fluids given subcutaneously, and for the calf that is less than 8 per cent dehydrated, 10 mEq of potassium chloride, 25 mEq of sodium bicarbonate, and 50 ml of 50 per cent glucose or dextrose solution should be added to each liter.
If Ringer’s bicarbonate, acetate, gluconate, or lactate are not available, mix equal parts of a 5 per cent glucose solution either with isotonic saline or Ringer’s solution. Then add the same amount of potassium chloride, 25 mEq per liter more sodium bicarbonate than that given in either case mentioned above, and omit the glucose.
The easiest way to add the potassium chloride and sodium bicarbonate is to inject the correct number of milliliters of a concentrated solution of these substances into the fluid used. Ringer’s lactate solutions often contain calcium, and therefore should be administered immediately after adding sodium bicarbonate. If this is not done, the calcium will combine with the bicarbonate to form a precipitate. It takes two to three hours for the precipitate to be seen.
Antimicrobial Drugs
Oral Antimicrobial Drugs
In the treatment of severe, acute diarrhea, orally administered antimicrobial drugs are often of questionable value. Controlled studies have failed to show benefit or harm from the oral administration of a number of different antimicrobial drugs in the treatment of acute undifferentiated diarrhea in the calf.8 In some bacterial cases of diarrhea, normal intestinal flora have been shown to be instrumental in clearing enteropathogenic organisms from the intestinal tract. Therefore, antibacterial suppression of the normal flora may succeed only in prolonging the disease without affecting the signs.
It is also difficult to determine to which antibacterial drug the actual enteropathogenic agent is sensitive. If the diarrhea is virally induced, as many are, none of the antibacterial agents is effective, but they may be effective against secondary opportunistic bacteria such as E. coli. In vitro determinations of antibacterial sensitivities to fecal swabs may show little or no correlation to the actual sensitivity in the calf. If the calf is still getting milk, tetracyclines will be bound to calcium, decreasing their effectiveness despite the fact that the causative agent may be sensitive to tetracycline. If given in conjunction with intestinal adsorbents, antimicrobial drugs may be bound to the adsorbent with subsequent decreased effectiveness.
However, the clinical impressions of many have indicated that oral antibacterial drugs are of benefit in the treatment of acute, severe diarrhea in the calf. If used at all, they should be restricted to acute, severe cases, and they should be given only at the proper dosage for an interval of two to three days. The antibacterials generally thought to be the most clinically beneficial in these cases in our area are the nitrofurans, gentamiein, chloramphenicol, and polymyxin B ( Fig. 1). The dosages used are: nitrofurans (Enterfur* ), two boluses initially, followed by one twice a day per calf; chloramphenicol, 100 mg per kg initially, followed by 50 mg per kg two to three times a day; gentamicin, 2 to 3 mg per kg twice a day; and polymyxin B, 4 to 6 mg per kg twice a day. These drugs were the only ones found by the Kirby-Bauer technique to effectively inhibit in vitro the majority of E. coli isolated from 75 diarrheic calves over a two year period.
Figure 1.
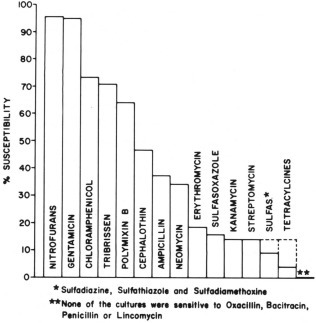
The relative susceptibility of enteropathogenic agents to various antibacterial drugs.
It must be remembered, however, that chloramphenicol is not approved for use in food-producing animals. The nitrofurans are currently being carefully scrutinized by the Food and Drug Administration; they are suspected to be potential carcinogens and may be removed from the market in the near future. In addition, because of manufacturing problems, Enterfur is not presently available. Gentamicin is approved for use in food-producing animals, but is extremely expensive.
Systemic Antimicrobial Drugs
Regardless of whether antimicrobial drugs are given orally, for severe cases of diarrhea in the calf they are indicated systemically to prevent or treat respiratory disease. Pneumonia commonly occurs in conjunction with or secondary to diarrhea in the calf. In one study involving 19 held cases of acute, severe diarrhea in which the calves were given extensive fluid therapy intravenously but were given no antibacterial drugs systemically, 10 died. All deaths occurred after the diarrhea had resolved and when the calves were well hydrated with normal blood electrolytes, glucose, and acid-base values. Based upon evaluation at necropsy, the death of each of these calves was a result of pneumonia.
Systemic antimicrobial drugs may be given parenterally or, in the case of those such as chloramphenicol which are readily absorbed from the intestinal tract, they may be given orally. Nitrofurans and polymyxin B are poorly absorbed following oral administration. Systemic administration of nitrofuran is not recommended.
Drugs that Affect the Intestinal Tract
The best method of treatment for any disease for which prevention has failed is to correct the primary alteration which has caused the disease. For diarrhea, this means the correction of the intestinal dysfunction. Currently, knowledge of the intestinal defects responsible for diarrhea is minimal, which makes rational treatment difficult or impossible. In addition, there have been few controlled trials of therapy to correct intestinal dysfunctions responsible for diarrhea in the calf. This includes the treatments given below.
Intestinal Protectants
Intestinal protectants, such as kaolin and pectin products, are of doubtful value in severe diarrhea. They have not been shown to alter losses of fluids and electrolytes. Although they may bind toxins and protect the mucosal wall, they have not been shown to be of any definitive benefit or harm.
Drugs that Affect Intestinal Motility
It is not known whether the drugs that alter intestinal motility are beneficial or detrimental. Anticholinergic agents decrease intestinal peristalsis, segmentation, and sphincter tone. A decrease in all of these parameters may allow an increase in bacterial migration or multiplication of bacteria in the more cranial aspects of the small intestine. Although some of these organisms may not be pathogenic when restricted to the large intestine, in the small intestine they may produce a number of detrimental effects which will cause diarrhea.1., 3. In addition, a decrease in segmentation and sphincter tone allows a more rapid passage of fluid down the intestinal tract, particularly the colon, which could worsen diarrheal fluid losses. For these reasons, anticholinergics are not recommended in the treatment of the diarrheic calf.
Narcotic analgesics such as paragoric may, in contrast, be beneficial. These drugs decrease peristalsis and stimulate segmentation and sphincter tone which increase resistance to the flow of ingesta down the tract. These effects may decrease diarrheal fluid losses and aboral bacterial migration. However, these beneficial effects from narcotic analgesics have not been documented in the diarrheic calf.
Drugs Affecting Intestinal Secretion
Anticholinergics decrease intestinal secretion, an action that would be expected to be of benefit in the treatment of diarrhea. However, because of their effect on intestinal motility, their use is not recommended. Salicylates, such as are contained in Pepto Bismol and aspirin, may be beneficial in diarrheal diseases associated with T. coli or Clostridium perfringens, whether these organisms are primary or secondary factors. These organisms induce diarrhea by secreting an enterotoxin which stimulates secretion from the intestinal epithelial cells. Salicylates inhibit prostaglandin synthesis which decreases enterotoxin-induced intestinal secretion of water and electrolytes. However, the role of prostaglandins, if any, in bacterial or virus-induced diarrhea is not known at this time. In the rabbit, Pepto Bismol has been shown to bind Vibrio cholerae and E. coli enterotoxins which were not previously bound to the intestinal wall, and to decrease intestinal secretion stimulated by these toxins by 91 and 78 per cent, respectively.4
Corticosteroids
There is no indication for the use of either corticosteroids or antihistamines in the treatment of diarrhea. The diarrheic calf already has an increased circulating level of endogenously produced corticosteroids.6 Supplementation of this natural level of corticosteroids has not been shown to be of benefit. Massive doses of corticosteroids (5 mg per kg of dexamethasone) have been shown to be beneficial when given early in the treatment of hemorrhagic shock in the dog.5 In addition, prolonged glucocorticoid therapy is beneficial in endotoxic shock, which can be a sequela of diarrhea. However, a more beneficial response may be obtained from the diarrheic calf in hypovolemic shock by correcting the hypovolemia with proper fluid therapy. Therefore, corticosteroids, as well as drugs such as antihistamines and stimulants, are of doubtful value and are not recommended in the general treatment of the diarrheic calf.
Care and Timing
Regardless of all other treatments given the diarrheic calf, the most important aspects are fluid therapy, care, and timing. The earlier in the course of the disease that treatment is initiated, the less therapy required and the greater the chance of recovery. Treatment is not usually indicated in the lively, nursing calf with a loose, pasty stool unless prior experience in that herd indicates that this may lead to a more severe diarrhea. Watery, voluminous feces, however, indicate the need for immediate therapy, even if the calf is not dehydrated, appears alert, and is nursing. The calf generally will not become depressed or decrease its food intake until the state of dehydration is greater than 8 per cent (Table 1). Do not allow this to occur, since treatment becomes much more extensive with progressive disease. The owner should begin giving oral fluids as soon as diarrhea occurs. However, it should be emphasized that if the rate of absorption of oral fluids does not compensate for the rate of loss of fluids from diarrhea, fluids must be given either subcutaneously, intravenously, or both. The calf should not be allowed to become severely dehydrated before fluids are given parenterally.
In addition to fluid therapy, tender loving care is indicated. Make sure that the calf is dry and warm. A heat lamp or electric blanket works nicely. Also, do remember to consider the recumbent side of the calf. Extensive loss of heat may occur as a result of an uninsulated cold floor or ground. Bare concrete is particularly detrimental. Plenty of bedding should be placed underneath the calf or, preferably, something such as an electrically warmed pad similar to that used for baby pigs.*
Following recovery, care must be taken to ensure that the calf does not overeat. If the calf is with the cow or will be returned to her, the cow should be milked out first. If this is not possible, the calf should be given several liters of the oral replacement fluid just prior to returning the calf to the cow. If this is not done, frequently the calf will ingest a large quantity of milk which may result either in a relapse or in sudden death from septicemia or endotoxemia. If the calf is to be fed by hand, dilute the milk by half with water or oral replacement fluid for the first one to two days.
Summary and Conclusions
Many calves are lost as a result of diarrhea during the first few weeks of life. Prevention is the most important goal. When prevention fails, proper therapy will save many affected calves. The most important aspects of therapy are the administration of fluids to replace the extensive losses of water and electrolytes which have occurred, and tender loving care. If treatment is begun early, fluids may be given orally. This reduces the need for more intensive therapy that must be administered either subcutaneously or intravenously. Although oral antimicrobial drugs are often given, there is little evidence to indicate that they are of benefit. However, systemic antimicrobial drugs are recommended in all severe cases of diarrhea to treat or prevent respiratory diseases. Corticosteroids, antihistamines, stimulants, and anticholinergic drugs are not recommended.
Footnotes
Life Guard Oral-Norden Labs, Lincoln, Nebraska; Resorb-Beecham-Massengil, Bristol, Tennessee.
Becton-Dickinson Co., Rutherford, New Jersey.
Norden Labs, Lincoln, Nebraska.
Protein Plus Labs, Box 190, Colfax, Illinois; or Osborne Industry Inc., Box 294, Osborne, Kansas.
References
- 1.Acres S.D., Saunders J.R., Radostits O.M. Acute undifferentiated neonatal diarrhea of beef calves: The prevalence of enterotoxigenic E. coli, Reo-like (ROTA) virus, and other enteropathogens in cow-calf herds. Canad. Vet. J. 1977;18:113. [PMC free article] [PubMed] [Google Scholar]
- 2.Copping J.W., Jr., Mather G.G., Winkler J.M. Physiological responses to the administration of cold, room temperature, and warm balanced salt solutions in hemorrhagic shock in dogs. Surgery. 1972;71:206. [PubMed] [Google Scholar]
- 3.Donaldson R.M., Jr. Role of enteric microorganisms in malabsorption. Fed. Proc. 1967;26:1426. [PubMed] [Google Scholar]
- 4.Ericsson C.D., Evans D.G., DuPont H.L. Bismuth subsalicylate inhibits activity of crude toxins of Escherichia coli and Vibrio chlorae. J. Infect. Dis. 1977;136:693. doi: 10.1093/infdis/136.5.693. [DOI] [PubMed] [Google Scholar]
- 5.Ferguson J.L., Roesel O.F., Bottoms G.D. Dexamethasone treatment during hemorrhagic shock: Blood pressure, tissue perfusion, and plasma enzymes. Am. T. Vet. Res. 1978;39:817. [PubMed] [Google Scholar]
- 6.Lewis L.D., Phillips R.W. Pathophysiologic changes due to coronavirus-induced diarrhea in the calf. J. Am. Vet. Med. Assoc. 1978;173:636. [PubMed] [Google Scholar]
- 7.Phillips R.W., Lewis L.D. Edition 4. Iowa State University Press; Ames, Iowa: 1977. Veterinary Pharmacology and Therapeutics. Ch. 34. [Google Scholar]
- 8.Radostits O.M., Rhodes C.S., Mitchell M.E. A clinical evaluation of antimicrobial agents and temporary starvation in the treatment of acute undifferentiated diarrhea in newborn calves. Canad. Vet. J. 1975;16:220. [PMC free article] [PubMed] [Google Scholar]


