Abstract
The efficient synthesis of a new series of acyclonucleotide analogues with a 1,2,3-triazole linker is described starting from diethyl azidomethyl-, 2-azidoethyl-, 3-azidopropyl-, 4-azidobutyl-, 2-azido-1-hydroxyethyl-, 3-azido-2-hydroxypropyl- and 3-azido-1-hydroxypropylphosphonates and selected alkynes under microwave irradiation. Several O,O-diethylphosphonate acyclonucleotides were transformed into the respective phosphonic acids. All compounds were evaluated in vitro for activity against a broad variety of DNA and RNA viruses and cytostatic activity against murine leukaemia L1210, human T-lymphocyte CEM and human cervix carcinoma HeLa cells. Acyclonucleotide 22e exhibited activity against both herpes simplex viruses (HSV-1, HSV-2) in HEL cell cultures (EC50 = 17 μM) and feline herpes virus (EC50 = 24 μM) in CRFK cell cultures, while compounds 20k, 21k, 22k and 23k preferentially inhibited proliferation of human T-lymphocyte CEM cells at IC50 in the 2.8–12 μM range.
Keywords: Aziodophosphonates; Acyclonucleotides; 1,2,3-Triazoles; Synthesis; Antiviral; Cytostatic
Graphical abstract
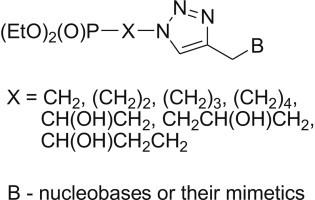
The 1,2,3-triazoloacyclonucleotides were evaluated in vitro for activity against a broad variety of DNA and RNA viruses and cytostatic activity against murine leukaemia L1210, human T-lymphocyte CEM and human cervix carcinoma HeLa cells. Diethyl 3-{4-[(3-benzoyl-2,4-dioxoquinazolin-1-yl)methyl]-1H-1,2,3-triazol-1-yl}propylphosphonate exhibited activity against both herpes simplex viruses (HSV-1, HSV-2) in HEL cell cultures (EC50 = 17 μM) and feline herpes virus (EC50 = 24 μM) in CRFK cell cultures. Several compounds preferentially inhibited proliferation of human T-lymphocyte CEM cells at IC50 in the 2.8–12 μM range.
Highlights
-
•
Nucleotide analogues with aliphatic linker between phosphorus and 1,2,3-triazole.
-
•
Efficient synthesis of 1,2,3-triazole analogues of nucleotides.
-
•
Antiviral activity and inhibitory effect on the proliferation of CEM cells.
1. Introduction
Several acyclic nucleosides and nucleotides exhibit antiviral or anticancer activities. However, in most cases, the clinical use of antiviral nucleosides is hampered by the drug resistance and/or toxicity problems. Under these circumstances intensive search for new drugs effective in the chemotherapy of viruses such as HIV, herpes virus, hepatitis viruses and cytomegalovirus has been conducted by many laboratories. A large number of modifications has been introduced to both the nucleobase and the sugar moieties of natural nucleosides. Consequently, several analogues like adefovir [1], [2], tenofovir [3] and cidofovir [4] (Fig. 1 ) were synthesised in which the furanose ring and the readily hydrolysable phosphate ester linkage present in natural nucleotides have been replaced by an acyclic chain and phosphonate moiety, respectively.
Fig. 1.
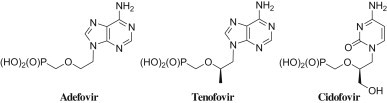
Acyclic nucleoside phosphonates used in the treatment of viral infections.
In drug discovery replacement of canonical nucleobases [5] by substituted five-membered heterocyclic rings such as imidazole or triazole has been particularly successful. Ribavirin [6], [7], [8], [9], [10], [11], [12], AICA [13], [14], Bredinin [15], [16] and TSAO analogues [17], [18] are the best known examples among these analogues (Fig. 2 ).
Fig. 2.
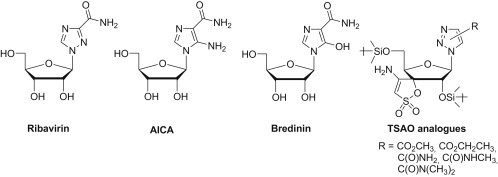
Ribavirin, AICA, Bredinin and TSAO analogues.
Further efforts in this field led to the synthesis of several analogues containing both modified nucleobases and 1,2,3-triazole moieties [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37]. Among them compounds 1–11 [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37] in which nucleobases or their mimetics and substituted triazoles are linked by the methylene group are of special interest (Fig. 3 ).
Fig. 3.
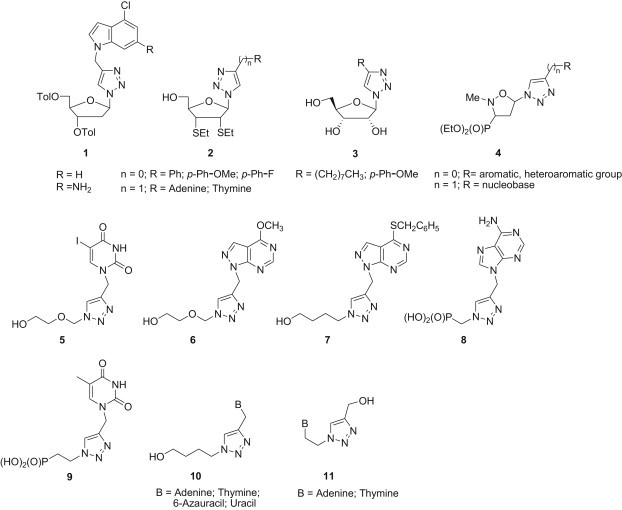
Nucleoside analogues having natural nucleobases connected to the 1,2,3-triazole ring by a methylene linker.
Moreover, several compounds possessing the 1,2,3-triazole ring show cytostatic [38], [39], [40], [41], [42], [43], [44], [45], antiviral [46], [47], [48], [49], antibacterial [50], [51], [52], [53], [54], [55], [56] or antifungal activities [57], [58], [59], [60]. The conventional synthesis of 1,2,3-triazoles relies on the Hüisgen [3 + 2] cycloaddition between alkynes and organic azides and usually provides a mixture of 1,4- and 1,5-disubstitued regioisomers [61], [62]. Recent discovery of copper(I) as an efficient and regiospecific catalyst for this transformation [63], [64] provides a general and mild approach for the preparation of 1,4-disubstituted 1,2,3-triazole derivatives.
In continuation of our studies on nucleotide analogues [65], [66], [67], [68], [69], [70] a new series of modified phosphonylated 1,2,3-triazoloacyclonucleosides bearing selected nucleobases or their mimetics at C-4 of the 1,2,3-triazole moiety (Scheme 1 ) has been synthesised and subjected to biological evaluation.
Scheme 1.
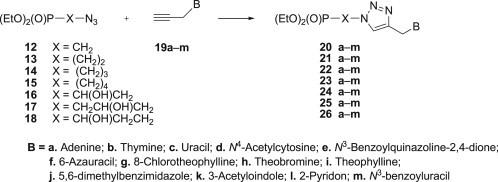
Synthesis of phosphonylated 1,2,3-triazoloacyclonucleosides 20–26 a–m.
2. Results and discussion
2.1. Chemistry
Compounds 20–26 a–l were obtained by the 1,3-dipolar cycloaddition of azidoalkylphosphonates 12–18 with propargylated nucleobases 19a–l: N 9-propargyladenine 19a [27], N 1-propargylthymine 19b [27], N 1-propargyluracil 19c [71] and N 4-acetyl-N 1-propargylcytosine 19d [71] and mimetics of nucleobases: N 3-benzoyl-N 1-propargylquinazoline-2,4-dione 19e, N 1-propargyl-6-azauracil 19f [30], 8-chloro-N 7-propargyltheophylline 19g [72], N 1-propargyltheobromine 19h [73], [74], N 7-propargyltheophylline 19i [75], 5,6-dimethyl-N 1-propargylbenzimidazole 19j [76], 3-acetyl-N-propargylindole 19k [77], N-propargyl-2-pyridon 19l [76]. The required azidoalkylphosphonates 12–18 were synthesised according to the procedures described previously [65], [66], [69], [78], [79].
Except for N 3-benzoyl-N 1-propargylquinazoline-2,4-dione 19e all alkynes used in this paper have already been described in the literature. N 3-Benzoyl-N 1-propargylquinazoline-2,4-dione 19e was synthesised in 19% overall yield in three steps from quinazoline-2,4-dione 27 beginning with bis-N 1,N 3-benzoylation to 28 followed by the selective N 1-debenzoylation to 29 and propargylation (Scheme 2 ). The N 3-benzoylquinazoline-2,4-dione 29 was previously obtained from 2-benzoylaminobenzoxazinone in less than 5% yield [80].
Scheme 2.
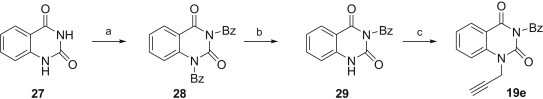
Reagents and conditions: a. benzoyl chloride (2.2 equiv.), pyridine, CH3CN, r.t, 12 h, 87%; b. 1 N K2CO3 aq., dioxane, r.t., 24 h, 23%; c. propargyl bromide (1.2 equiv.), K2CO3 (1.1 equiv.), DMF, r.t., 24 h, 97%.
The structure of N 3-benzoyl-N 1-propargylquinazoline-2,4-dione 19e was confirmed on the basis of 1H, 13C NMR and IR spectral data and finally proved by 2D COSY and NOESY experiments (Fig. 4 ).
Fig. 4.
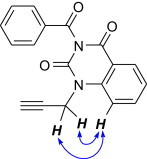
NOESY correlations in 19e.
All 1,2,3-triazoles 20–26 were obtained in good yields in 1,3-dipolar cycloadditions which were carried out in a microwave oven at 40–45 °C. This approach caused significant improvements in purity of the final products and allows to shorten the reaction time from 48 h at room temperature to 10 min of irradiation. Since syntheses of phosphonylated 1,2,3-triazoles 20a–d, 21a–d, 22a–d, 24a–d and 25a–d have already been described and their biological activity evaluated [32], [69], N 9-propargyladenine 19a, N 1-propargylthymine 19b, N 1-propargyluracil 19c and N 4-acetyl-N 1-propargylcytosine 19d were reacted with two azidophosphonates 15 and 18, only. However, mimetics of nucleobases 19e–l were applied in cycloadditions with all seven azidophosphonates 12–18 (Schemes 3 and 4 ).
Scheme 3.
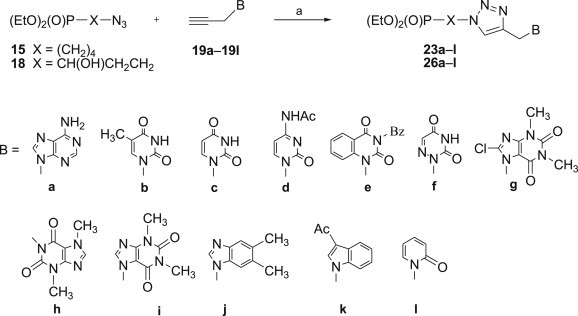
Reagents and conditions: a. CuSO4 × 5H2O (0.05 equiv.), sodium ascorbate (0.1 equiv.), H2O–EtOH (1:1), MW, 40–45 °C, 10 min.
Scheme 4.
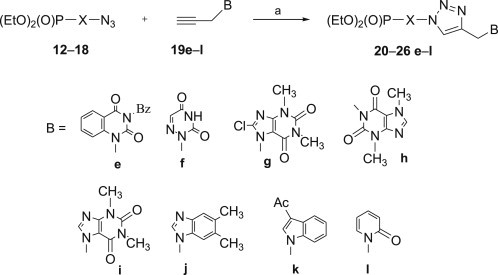
Reagents and conditions: a. CuSO4 × 5H2O (0.05 equiv.), sodium ascorbate (0.1 equiv.), H2O–EtOH (1:1), MW, 40–45 °C, 10 min.
Since the preliminary experiments have demonstrated antiviral activity of compound 22e against herpes simplex viruses (HSV-1, HSV-2) in HEL cell cultures (EC50 = 17 μM) and feline herpes virus (EC50 = 24 μM) in CRFK cell cultures, phosphonate 22m was synthesised in order to establish the influence of the fused phenyl ring in 22e on the observed activity (Scheme 5 ).
Scheme 5.
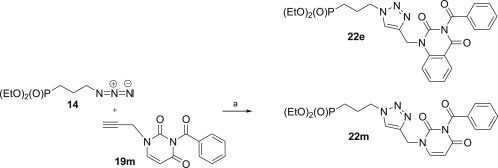
Reagents and conditions: a. CuSO4 × 5H2O (0.05 equiv.), sodium ascorbate (0.1 equiv.), H2O–EtOH (1:1), MW, 40–45 °C, 10 min.
Our synthesis of N 3-benzoyl-N 1-propargyluracil 19m by propargylation of N 3-benzoyluracil with propargyl bromide (Scheme 6 ) appeared more efficient (95%) in comparison to the previously described approach from N 3-benzoyluracil and propargyl alcohol via the Mitsunobu reaction (76% yield) [81].
Scheme 6.
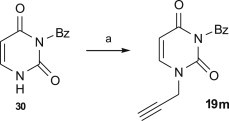
Reagents and conditions: a. propargyl bromide (1.2 equiv.), K2CO3 (1.1 equiv.), DMF, r.t., 24 h, (95%).
Finally, using bromotrimethylsilane [82] selected diethyl phosphonates 22e, 22g, 22j, 22m, 23a–c and 24i were transformed into the respective phosphonic acids in good yields (Scheme 7 ).
Scheme 7.
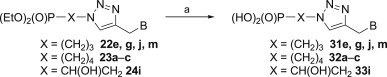
Reagents and conditions: a. TMSBr, CH2Cl2, r.t., 24 h.
2.2. Conformational analysis
Conformational preferences of phosphonates described in this paper were ascertained by analyses of 1H, 1H{31P} and 13C NMR spectra. Since all vicinal proton–proton couplings within the H2C–CH2 fragment in phosphonates 21e–l were observed around 7 Hz, it was concluded that the rotation around the PCH2–CH2N bond is fully unrestricted. On the other hand, based on the values of 3 J H1–H2a = 10.0 Hz, 3 J H1–H2b = 2.9 Hz [83] and small values of 3 J P–H2a and 3 J P–H2b (ca. 5.5 Hz) [84], [85] one may deduce that phosphonates 24e–l exist in the antiperiplanar conformation 34 which is probably stabilised by the intramolecular hydrogen bond (Fig. 5 ). Conformational behaviour of the three-carbon linkers between the diethoxyphosphoryl and substituted 1,2,3-triazole groups in phosphonates 22e–l, 25e–l and 26e–l is primarily governed by the presence of the hydroxy groups. Thus, values of vicinal proton–proton couplings within PCH2–CH2 and CH2–CH2N subunits in 22e–l (ca. 7 Hz) can only be observed when free rotation around these bonds is allowed. 2-Hydroxyphosphonates 25e–l adopt the H-bond stabilised antiperiplanar conformation 35 along the PCH2–CH bond [3 J H1a–H2 = 3.9 Hz, 3 J H1b–H2 = 8.6 Hz, 3 J P–H2 = 12.0 and 3 J P–C3 = 14–18 Hz [85], [86], [87], while the rotation around the CH–CH2N bond is unrestricted [3 J H2–H3a = 3.0 Hz, 3 J H2–H3b = 7.0 Hz]. To establish a conformational behaviour of 1-hydroxyphosphonates 26a–l the extensive NMR studies on 1-hydroxyphosphonate 26i were conducted at 600 MHz. From the values of 3 J H1–H2a = 3.3 Hz, 3 J H1–H2b = 10.9 Hz as well as 3 J P–H2a = 6.1 and 3 J P–H2b = 6.4, 3 J P–C3 = 15–18 Hz, it is evident that along the PCH–CH2 bond, this phosphonate exists in the antiperiplanar conformation 36, while based on the values of 3 J H2a–H3a = 3 J H2a–H3b = 8.0 Hz, 3 J H2b–H3a = 5.9 Hz and 3 J H2b–H3b = 5.9 Hz one may suggest that along the CH2–CH2N bond in 26i the rotation is almost free.
Fig. 5.
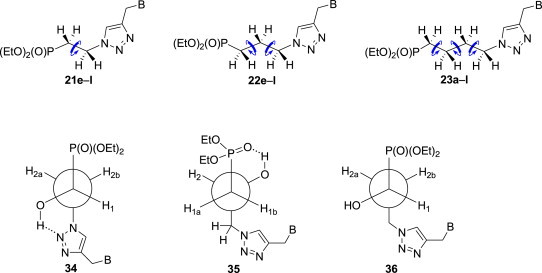
Preferred conformations of phosphonates investigated in this paper.
The anticipated full conformational freedom along the tetramethylene linker in the butylphosphonates 23a–l is confirmed by the values of 3 J H–H = 7.1 Hz between all methylene groups (Fig. 5).
2.3. Antiviral activity and cytotoxicity evaluation
All the synthesised compounds 20–26 a–m, 31e, 31g, 31j, 31m, 32a–c and 33i were evaluated for their antiviral activities against a wide variety of DNA and RNA viruses, using the following cell-based assays: (a) human embryonic lung (HEL) cells: herpes simplex virus-1 (KOS), herpes simplex virus-2 (G), herpes simplex virus-1 (TK− ACVr KOS), vaccinia virus and vesicular stomatitis virus; (b) CEM cell cultures: human immunodeficiency virus-1 [HIV-1] and HIV-2; (c): Vero cell cultures: para-influenza-3 virus, reovirus-1, Sindbis virus, Coxsackie virus B4, Punta Toro virus; (d): HeLa cell cultures: vesicular stomatitis virus, Coxsackie virus B4 and respiratory syncytial virus; (e): Crandell-Rees Feline Kidney (CRFK) cell cultures: feline corona virus (FIPV) and feline herpes virus (FHV); (f): Madin Darby Canine Kidney (MDCK) cell cultures: influenza A virus H1N1 subtype (A/PR/8), influenza A virus H3N2 subtype (A/HK/7/87) and influenza B virus (B/HK/5/72). Ganciclovir, cidofovir, acyclovir, brivudin, (S)-9-(2,3-dihydroxypropyl)adenine [(S)-DHPA], Hippeastrum hybrid agglutinin (HHA), Urtica dioica agglutinin (UDA), dextran sulphate (molecular weight 5000, DS-5000), ribavirin, oseltamivir carboxylate, amantadine and rimantadine were used as the reference compounds. The antiviral activity was expressed as the EC50: the compound concentration required to reduce virus plaque formation (VZV) by 50% or to reduce virus-induced cytopathogenicity by 50% (other viruses).
The cytotoxicity of the tested compounds toward the uninfected host cells was defined as the minimum cytotoxic concentration (MCC) that causes a microscopically detectable alteration of normal cell morphology. The 50% cytotoxic concentration (CC50), causing a 50% decrease in cell viability was determined using a colorimetric 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay system.
Among all the 1,2,3-triazole derivatives tested, compound 22e having a three carbon fragment between the phosphorus atom and the 1,2,3-triazole ring substituted at C4′ with N 3-benzoyl-N 1-methylquinazoline-2,4-dione showed a moderate activity against both herpes simplex viruses (HSV-1, HSV-2) (EC50 = 17 μM) in HEL cell cultures, and feline herpes virus (EC50 = 24 μM) in CRFK cell cultures.
2.4. Evaluation of cytostatic activity
The cytostatic activity of the tested compounds was defined as the 50% cytostatic inhibitory concentration (IC50), causing a 50% decrease in cell proliferation was determined against murine leukaemia L1210, human lymphocyte CEM and human cervix carcinoma HeLa cells. Most compounds were not cytostatic at 200 μM.
Several compounds showed very moderate inhibitory against the proliferation of tumour cell lines (Table 1 ), although in a few cases, the compounds seemed to be preferentially cytostatic against human tumour cell lines (esp. lymphocyte CEM cells) than murine (L1210) cells. In particular, compound 20k, 21k, 22k and 23k were antiproliferative at IC50 values ranging between 2.8 and 12 μM.
Table 1.
Inhibitory effect of tested compounds against the proliferation of murine leukaemia (L1210), human T-lymphocyte (CEM) and human cervix carcinoma cells (HeLa).
| Compound | B | IC50a (μM) |
||
|---|---|---|---|---|
| CEM | L1210 | HeLa | ||
| 20e | N3-Benzoylquinazoline-2,4-dione | ≥72 | >200 | ≥89 |
| 20f | 6-Azauracil | >200 | >200 | >200 |
| 20g | 8-Chlorotheophylline | >200 | >200 | >200 |
| 20h | Theobromine | >200 | >200 | ≥149 |
| 20i | Theophylline | >200 | >200 | >200 |
| 20j | 5,6-Dimethylbenzimidazole | 97 ± 35 | >200 | 42 ± 16 |
| 20k | 3-Acetylindole | 2.78 ± 1.4 | 72 ± 19 | 11 ± 7.6 |
| 20l | 2-Pyridon | >200 | >200 | >200 |
| 21f | 6-Azauracil | >200 | >200 | >200 |
| 21g | 8-Chlorotheophylline | >200 | >200 | 200 |
| 21h | Theobromine | >200 | >200 | 159 ± 59 |
| 21i | Theophylline | >200 | >200 | >200 |
| 21j | 5,6-Dimethylbenzimidazole | 23.4 ± 7.9 | ≥200 | >200 |
| 21k | 3-Acetylindole | 11.4 ± 2.2 | 197 ± 4.2 | 18 ± 3.5 |
| 21l | 2-Pyridon | >200 | >200 | >200 |
| 22e | N3-Benzoylquinazoline-2,4-dione | 44 ± 42 | >200 | 129 ± 78 |
| 22f | 6-Azauracil | >200 | >200 | >200 |
| 22g | 8-Chlorotheophylline | >200 | >200 | >200 |
| 22h | Theobromine | >200 | >200 | >200 |
| 22i | Theophylline | >200 | >200 | >200 |
| 22j | 5,6-Dimethylbenzimidazole | 14 ± 8.3 | 189 ± 16 | 101 ± 23 |
| 22k | 3-Acetylindole | 4.54 ± 1.24 | 164 ± 52 | 19 ± 13 |
| 22l | 2-Pyridon | >200 | >200 | >200 |
| 22m | N3-Benzoyluracil | >200 | >200 | >200 |
| 23a | Adenine | >200 | >200 | >200 |
| 23b | Thymine | >200 | >200 | >200 |
| 23c | Uracil | >200 | >200 | >200 |
| 23d | N4-Acetylcytosine | >200 | >200 | >200 |
| 23f | 6-Azauracil | >200 | >200 | >200 |
| 23g | 8-Chlorotheophylline | >200 | >200 | >200 |
| 23h | Theobromine | >200 | >200 | >200 |
| 23i | Theophylline | >200 | >200 | >200 |
| 23j | 5,6-Dimethylbenzimidazole | 24.7 ± 8.1 | >200 | 172 ± 40 |
| 23k | 3-Acetylindole | 12 ± 3.5 | 200 | >200 |
| 23l | 2-Pyridon | >200 | >200 | >200 |
| 24e | N3-Benzoylquinazoline-2,4-dione | ≥77 | >200 | >200 |
| 24f | 6-Azauracil | >200 | >200 | >200 |
| 24g | 8-Chlorotheophylline | >200 | >200 | >200 |
| 24h | Theobromine | >200 | >200 | >200 |
| 24i | Theophylline | >200 | >200 | >200 |
| 24j | 5,6-Dimethylbenzimidazole | >200 | >200 | >200 |
| 24k | 3-Acetylindole | 21 ± 0.14 | >200 | >200 |
| 24l | 2-Pyridon | >200 | >200 | >200 |
| 25e | N3-Benzoylquinazoline-2,4-dione | 72 ± 45 | >200 | >200 |
| 25f | 6-Azauracil | >200 | >200 | >200 |
| 25g | 8-Chlorotheophylline | >200 | >200 | >200 |
| 25h | Theobromine | >200 | >200 | >200 |
| 25i | Theophylline | >200 | >200 | >200 |
| 25j | 5,6-Dimethylbenzimidazole | 78 ± 26 | >200 | ≥150 |
| 25k | 3-Acetylindole | 43 ± 3.5 | >200 | 97 ± 80 |
| 25l | 2-Pyridon | >200 | >200 | >200 |
| 26b | Thymine | >200 | >200 | >200 |
| 26c | Uracil | >200 | >200 | >200 |
| 26d | N4-Acetylcytosine | >200 | >200 | >200 |
| 26e | N3-Benzoylquinazoline-2,4-dione | 70 ± 18 | >200 | >200 |
| 26f | 6-Azauracil | >200 | >200 | >200 |
| 26g | 8-Chlorotheophylline | >200 | >200 | >200 |
| 26h | Theobromine | >200 | >200 | >200 |
| 26i | Theophylline | >200 | >200 | >200 |
| 26j | 5,6-Dimethylbenzimidazole | 163 ± 53 | >200 | >200 |
| 26k | 3-Acetylindole | 100 ± 2.8 | 124 ± 62 | >200 |
| 26l | 2-Pyridon | >200 | >200 | >200 |
| 31e | N3-Benzoylquinazoline-2,4-dione | >200 | >200 | >200 |
| 31g | 8-Chlorotheophylline | >200 | >200 | >200 |
| 31j | 5,6-Dimethylbenzimidazole | >200 | >200 | >200 |
| 31m | N3-Benzoyluracil | >200 | >200 | >200 |
| 32a | Adenine | >200 | >200 | >200 |
| 32b | Thymine | >200 | >200 | >200 |
| 32c | Uracil | >200 | >200 | >200 |
| 33i | Theophylline | >200 | >200 | >200 |
50% Inhibitory concentration or compound concentration required to inhibit tumour cell proliferation by 50%.
2.5. Structure–activity relationship
Structure–activity relationship studies on a series of 1,2,3-triazoloacyclonucleotides 20–26 a–m and 30e, g, j, m, 31a–c and 32i revealed cytostatic activity of compounds substituted at C4′ of the 1,2,3-triazole ring with 3-acetylindole, N 3-benzoylquinazoline-2,4-dione and 5,6-dimethylbenzimidazole. Regardless of the length of the linker (1–4 carbon atoms) all derivatives of the 1,2,3-triazoles with 3-acetylindole were the most active towards CEM cell lines (e.g. IC50 = 2.78 ± 1.4 μM for 20k) and also some of them against HeLa cells. Furthermore, compounds 21j, 22e, 23k, 24k, 25e and 26e selectively inhibited the proliferation of human T-lymphocyte (CEM).
As could be expected the phosphonic acids were found inactive in all biological tests including these acids for which the corresponding diethyl esters displayed significant activity (22e vs. 31e and 22j vs. 31j). This is in full agreement with observation that lipophilic phosphonate esters better penetrate through membranes in comparison with phosphonic acids and thus the sufficient concentration of the ester is achieved in cells to undergo further reactions.
For compound 22e containing a three methylene linker between the phosphorus atom and the 1,2,3-triazole ring substituted at C4′ with N 3-benzoyl-N 1-methylquinazoline-2,4-dione activity against both herpes simplex viruses (HSV-1, HSV-2) (EC50 = 17 μM) in HEL cell cultures, and feline herpes virus (EC50 = 24 μM) in CRFK cell cultures was established. The presence of the quinazoline ring appeared necessary for 22e to display antiviral activity, since its structural analogue lacking the condensed phenyl ring (compound 22m) was found inactive EC50 > 200 μM.
3. Conclusion
A new series of 1,2,3-triazoloacyclonucleotides 20–26 a–m has been efficiently obtained from diethyl azidomethyl-, 2-azidoethyl-, 3-azidopropyl-, 4-azidobutyl-, 2-azido-1-hydroxyethyl-, 3-azido-2-hydroxypropyl- and 3-azido-1-hydroxypropylphosphonates and selected N-propargyl alkynes including N-propargyl nucleobases (N 9-propargyladenine 19a, N 1-propargylthymine 19b, N 1-propargyluracil 19c and N 4-acetyl-N 1-propargylcytosine 19d) and several mimetics (N 3-benzoyl-N 1-propargylquinazoline-2,4-dione 19e, N 1-propargyl-6-azauracil 19f, 8-chloro-N 7-propargyltheophylline 19g, N 1-propargyltheobromine 19h, N 7-propargyltheophylline 19i, 5,6-dimethyl-N 1-propargylbenzimidazole 19j, 3-acetyl-N-propargylindole 19k, N-propargyl-2-pyridon 19l and N 3-benzoyl-N 1-propargyluracil 19m) via 1,3-dipolar cycloadditions carried out under microwave irradiation.
The N 3-benzoyl-N 1-propargylquinazoline-2,4-dione 19e was synthesised from quinazoline-2,4-dione 27 in the sequence of reactions including bis-N 1,N 3-benzoylation followed by the selective N 1-debenzoylation and propargylation.
Under standard conditions (TMSBr, ethanol) several O,O-diethyl phosphonates 22e, g, j, m, 23a–c and 24i were transformed into their respective phosphonic acid.
All synthesised compounds were evaluated against a variety of DNA and RNA viruses. Compound 22e containing a three carbon linker between the phosphorus atom and the 1,2,3-triazole ring substituted at C4′ with N 3-benzoyl-N 1-methylquinazoline-2,4-dione showed a moderate activity (EC50 = 17 μM) against both herpes simplex viruses (HSV-1, HSV-2) in HEL cell cultures and feline herpes virus (EC50 = 24 μM) in CRFK cell cultures.
All synthesised compounds were also evaluated for their anti-proliferative activity against three tumour cell lines (L1210, CEM and HeLa) and several compounds, i.e. 20k, 21j–k, 22j–k, 23j–k and 24k were found to be the most active (and preferentially inhibitory) towards T-lymphocyte CEM cell proliferation.
4. Experimental
4.1. Chemistry
1H NMR were taken in CDCl3, CD3OD or D2O on the following spectrometers: Varian Mercury-300 and Bruker Avance III (600 MHz) with TMS as an internal standard; chemical shifts δ in ppm with respect to TMS; coupling constants J in Hz. 13C NMR spectra were recorded for CDCl3, CD3OD, DMSO-d 6 or D2O solutions on a Varian Mercury-300 and Bruker Avance III (600 MHz) spectrometer at 75.5 and 150.5 MHz, respectively. 31P NMR spectra were taken in CDCl3, CD3OD or D2O on Varian Mercury-300 at 121.5 MHz.
IR spectral data were measured on an Infinity MI-60 FT-IR spectrometer. Melting points were determined on a Boetius apparatus and are uncorrected. Elemental analyses were performed by the Microanalytical Laboratory of this Faculty on a Perkin Elmer PE 2400 CHNS analyzer.
The following adsorbents were used: column chromatography, Merck silica gel 60 (70–230 mesh); analytical TLC, Merck TLC plastic sheets silica gel 60 F254. TLC plates were developed in chloroform–methanol solvent systems. Visualisation of spots was effected with iodine vapours. All solvents were purified by methods described in the literature.
All microwave irradiation experiments were carried out in microwave reactor Plazmartonika RM 800. The reaction carried out in 50 mL glass vial.
4.1.1. Synthesis of 1,3-dibenzoylquinazoline-2,4-dione 28
The benzoyl chloride (6.48 mL, 0.056 mol) was added to a stirred suspension of quinazoline-2,4-dione (4.00 g, 0.025 mol) in dry acetonitrile (25 mL) containing dry pyridine (10 mL) at room temperature. After 24 h the products were concentrated under reduced pressure. The residue was partitioned between dichloromethane (100 mL) and water (100 mL). The organic layer was dried (MgSO4), concentrated in vacuo and the residue was crystallised from ethanol to give 1,3-dibenzoylquinazoline-2,4-dione 28 (7.985 g, 87%) as a white powder; m.p.: 159–160 °C; IR (KBr): ν = 3040, 1753, 1723; 1674, 1605, 1472, 974; 866; 753, 688 cm−1; 1H NMR (300 MHz, CDCl3): δ = 8.28 (dd, J = 7.9 Hz, J = 1.5 Hz, 1H, H5); 8.02–7.98 (m, 4H, 4× o-CH); 7.73–7.63 (m, 3H, 2× p-CH, H7); 7.56–7.48 (m, 4H, 4× m-CH); 7.36 (dt, J = 7.9 Hz, J = 0.7 Hz, 1H, H6); 7.10 (d, J = 8.4 Hz, 1H, H8); 13C NMR (151 MHz, CDCl3): δ = 169.2; 167.8; 160.9; 147.9; 138.6; 136.1; 135.7; 135.3; 131.8; 131.4; 130.6; 130.5; 129.5; 129.3; 129.1; 124.8; 115.2; 114.9; Anal. Calcd. for C22H14N2O4: C, 71.35; H, 3.81; N, 7.56. Found: C, 71.49; H, 3.76; N, 7.44.
4.1.2. Synthesis of N3-benzoylquinazoline-2,4-dione 29
A mixture of 1,3-dibenzoylquinazoline-2,4-dione 28 (1.73 g, 4.67 mmol), dioxane (50 mL) and 1 N K2CO3 aq. (25 mL) was stirred at room temperature. After 24 h glacial acetic acid was added to pH 5. The products were concentrated under reduced pressure and the residue was stirred with saturated aqueous sodium bicarbonate (200 mL) at room temperature for 2 h. The solid was filtered off, washed with cold water and dried on air. The crude product was chromatographed on a silica gel column with chloroform–methanol (200:1, 100:1, v/v) and crystallised from ethyl acetate–petroleum ether to give the compound 29 (0.255 g, 23%) as white needles; m.p.: 209–211 °C; IR (KBr): ν = 3436, 3063, 2937, 1753, 1707, 1668, 1400, 760, 687 cm−1; 1H NMR (300 MHz, CDCl3): δ = 9.34 (brs, 1H, NH); 8.13 (dd, J = 7.9 Hz, J = 1.5 Hz, 1H); 8.03–7.99 (m, 2H, 2× o-CH); 7.71–7.62 (m, 2H); 7.55–7.49 (m, 2H); 7.29 (dd, J = 7.9 Hz, J = 0.9 Hz, 1H); 7.31 (ddd, J = 8.2 Hz, J = 0.9 Hz, J = 0.5 Hz, 1H); 13C NMR (151 MHz, DMSO-d 6): δ = 170.3; 162.3; 149.3; 140.9; 136.4; 135.9; 132.0; 130.9; 129.9; 127.7; 123.6; 116.4; 114.3; Anal. Calcd. for C15H10N2O3: C, 67.67; H, 3.79; N, 10.52. Found: C, 67.48; H, 3.91; N, 10.45.
4.1.3. Synthesis of N3-benzoyl-N1-propargylquinazoline-2,4-dione 19e
A suspension of N 3-benzoylquinazoline-2,4-dione 29 (0.239 g, 0.898 mmol), potassium carbonate (0.135 g, 0.978 mmol) and propargyl bromide (0.081 mL, 1.08 mmol) in DMF (3 mL) was stirred at room temperature for 24 h. The mixture was co-evaporated with toluene (5 × 10 mL). The residue was dissolved in chloroform (10 mL) and washed with brine (2 × 5 mL). The organic phase was dried over MgSO4, concentrated in vacuo and the residue was crystallised from methanol–diethyl ether to give compound 19e (0.265 g, 97%) as a white powder; m.p.: 180–182 °C; IR (KBr): ν = 3256, 3002, 2925, 1751, 1697, 1659, 1482, 756, 684 cm−1; 1H NMR (600 MHz, CDCl3): δ = 8.26 (dd, J = 7.9 Hz, J = 1.3 Hz, 1H, H5); 8.12–7.99 (m, 2H, 2× o-CH); 7.83 (dt, J = 7.3 Hz, J = 1.4 Hz, 1H, H7); 7.69–7.67 (m, 1H, p-CH); 7.54–7.50 (m, 3H, 2× m-CH, H8); 7.31 (brt, J = 7.6 Hz, 1H); 4.96 (d, J = 2.5 Hz, 2H, CH CCH 2); 3.74 (t, J = 2.5 Hz, 1H, CH CCH2); 13C NMR (151 MHz, CDCl3): δ = 168.4 (s, C O); 160.9 (s, C O); 148.8 (s, C O); 139.6; 136.1; 135.1; 131.7; 130.5; 129.2; 129.2; 123.9; 115.8; 114.7; 73.8; 32.8; Anal. Calcd. for C18H12N2O3: C, 71.05; H, 3.97; N, 9.21. Found: C, 70.92; H, 4.05; N, 9.14.
4.1.4. N3-Benzoyl-N1-propargyluracil 19m
A suspension of N 3-benzoyluracil 30 (0.506 g, 0.234 mmol), potassium carbonate (0.356 g, 0.257 mmol) and propargyl bromide (0.211 mL, 0.281 mmol) in DMF (4 mL) was stirred at room temperature for 24 h. The mixture was co-evaporated with toluene (5 × 10 mL). The residue was dissolved in chloroform (10 mL) and washed with brine (2 × 5 mL). The organic phase was dried over MgSO4, concentrated in vacuo and crystallised from methanol–diethyl ether to give compound 19m (0.562 g, 95%) as a white solid; m.p.: 139–140 °C; 1H NMR (600 MHz, CDCl3): 7.95–7.92 (m, 2H, 2× o-CH); 7.69–7.63 (m, 1H, p-CH); 7.58 (d, J = 8.1 Hz, 1H, HC CH); 7.55–7.48 (m, 2H, m-CH); 5.89 (d, J = 8.1 Hz, 1H, HC CH); 4.59 (d, J = 2.6 Hz, 2H, CH CCH 2); 2.55 (t, J = 2.6 Hz, 1H, CH CCH2).
4.1.5. General procedure for the preparation of 1,2,3-triazoles
To a solution of azidoalkylphosphonate (1.00 mmol) in EtOH (1 mL) and H2O (1 mL) were added CuSO4 × 5H2O (0.05 mmol), sodium ascorbate (0.10 mmol) and alkynes (1.00 mmol). The suspension was microwave irradiated in the microwave reactor (Plazmatronika RM 800, 800 W) at 40–45 °C for 10 min. After cooling the solvent was removed by vacuum evaporation. The residue was suspended in dry chloroform (5 mL) and filtered through a layer of Celite. The solution was concentrated in vacuo and the crude product was purified on a silica gel column with chloroform–methanol mixtures (50:1, 20:1 or 10:1, v/v) to give the appropriate 1,2,3-triazoles.
4.1.5.1. Diethyl {4-[(3-benzoyl-2,4-dioxoquinazolin-1-yl)methyl]-1H-1,2,3-triazol-1-yl}methylphosphonate 20e
From azide 12 (0.095 g, 0.492 mmol) and N 3-benzoyl-N 1-propargylquinazoline-2,4-dione 19e (0.150 g, 0.492 mmol) the phosphonate 20e (0.199 g, 81%) was obtained as a colourless oil after purification on a silica gel column with chloroform–methanol (50:1, v/v). IR (film): ν = 3030, 2982, 1750, 1700, 1662, 1021, 757, 671 cm−1; 1H NMR (600 MHz, CDCl3): δ = 8.20 (dd, J = 7.9 Hz, J = 1.6 Hz, 1H, H5); 7.99–7.95 (m, 2H, 2× o-CH); 7.88 (brd, J = 8.5 Hz, 1H, H8); 7.86 (s, 1H, HC5′); 7.76 (ddd, J = 8.5 Hz, J = 7.9 Hz, J = 1.6 Hz, 1H, H7); 7.68–7.64 (m, 1H, p-CH); 7.52–7.49 (m, 2H, 2× m-CH); 7.31 (dt, J = 7.9 Hz, J = 0.6 Hz, 1H, H6); 5.42 (s, 2H, CH2); 4.73 (d, J = 13.1 Hz, 2H, PCH 2); 4.17–4.06 (m, 4H, 2× POCH 2CH3); 1.25 (t, J = 7.2 Hz, 3H, POCH2CH 3); 1.24 (t, J = 7.2 Hz, 3H, POCH2CH 3); 13C NMR (75.5 MHz, CDCl3): δ = 168.6 (s, C O); 161.1 (s, C O); 149.5 (s, C O); 142.8 (s, HC C); 140.2; 136.2; 135.2; 131.6; 130.6; 129.4; 129.0; 124.8 (s, HC C); 123.9; 116.0; 115.3; 63.7 (d, J = 6.5 Hz, POC); 46.1 (d, J = 154.9 Hz, PC); 38.9; 16.5 (d, J = 5.7 Hz, POCC); 31P NMR (121.5 MHz, CDCl3): δ = 16.49 ppm. Anal. Calcd. for C23H24N5O6P: C, 55.53; H, 4.86; N, 14.08. Found: C, 55.24; H, 4.73; N, 13.86.
4.1.5.2. Diethyl {4-[(3,5-dioxo-1,2,4-triazin-2-yl)methyl]-1H-1,2,3-triazol-1-yl}methylphosphonate 20f
From azide 12 (0.132 g, 0.683 mmol) and N 1-propargyl-6-azauracil 19f (0.103 g, 0.683 mmol) the phosphonate 20f (0.184 g, 78%) was obtained as a white solid after purification on silica gel with chloroform–methanol (50:1, v/v); m.p.: 139–140 °C; IR (KBr): ν = 3344, 2988, 1697, 1668, 1235, 1025 cm−1; 1H NMR (300 MHz, CDCl3): δ = 10.6 (s, 1H, NH); 7.94 (s, 1H); 7.40 (s, 1H); 5.22 (s, 2H, CH2); 4.76 (d, J = 13.3 Hz, 2H, PCH 2); 4.18–4.07 (m, 4H, 2× POCH 2CH3); 1.30 (t, J = 6.9 Hz, 3H, POCH2CH 3); 1.29 (t, J = 6.9 Hz, 3H, POCH2CH 3); 13C NMR (75.5 MHz, CDCl3): δ = 155.9 (s, C O); 149.1 (C O); 142.2 (s, HC C); 134.8 (s, HC N); 125.1 (s, HC C); 66.9 (d, J = 6.6 Hz, POC); 46.0 (d, J = 155.5 Hz, PC); 34.7; 16.5 (d, J = 5.8 Hz, POCC); 31P NMR (121.5 MHz, CDCl3): δ = 16.83 ppm. Anal. Calcd. for C11H17N6O5P: C, 38.38; H, 4.98; N, 24.41. Found: C, 38.50; H, 4.80; N, 24.55.
4.1.5.3. Diethyl {4-[(8-chloro-1,3-dimethyl-2,6-dioxopurin-7-yl)methyl]-1H-1,2,3-triazol-1-yl}methylphosphonate 20g
From azide 12 (0.100 g, 0.518 mmol) and 8-chloro-N 7-propargyltheophylline 19g (0.131 g, 0.518 mmol) the phosphonate 20g (0.210 g, 91%) was obtained as a white solid after purification on silica gel with chloroform–methanol (50:1, v/v); m.p.: 156–157 °C; IR (KBr): ν = 2996, 2955, 1707, 1667, 1251, 1025, 757 cm−1; 1H NMR (300 MHz, CDCl3): δ = 7.98 (s, 1H, HC5′); 5.65 (s, 2H, CH2); 4.74 (d, J = 13.1 Hz, 2H, PCH 2); 4.18–4.07 (m, 4H, 2× POCH 2CH3); 3.54 (s, 3H, CH3); 3.40 (s, 3H, CH3); 1.30 (t, J = 7.2 Hz, 3H, POCH2CH 3); 1.28 (t, J = 7.2 Hz, 3H, POCH2CH 3); 13C NMR (75.5 MHz, CDCl3): δ = 154.3 (s, C O); 151.1 (s, C O); 147.3; 142.0; 138.9; 124.5; 107.5; 63.7 (d, J = 6.5 Hz, POC); 46.0 (d, J = 154.9 Hz, PC); 41.0; 30.0; 28.1; 16.4 (d, J = 5.7 Hz, POCC); 31P NMR (121.5 MHz, CDCl3): δ = 16.48 ppm. Anal. Calcd. for C15H21ClN7O5P: C, 40.41; H, 4.75; N, 21.99. Found: C, 40.53; H, 4.60; N, 21.80.
4.1.5.4. Diethyl {4-[(3,7-dimethyl-2,6-dioxopurin-1-yl)methyl]-1H-1,2,3-triazol-1-yl}methylphosphonate 20h
From azide 12 (0.116 g, 0.601 mmol) and N 1-propargyltheobromine 19h (0.131 g, 0.601 mmol) the phosphonate 20h (0.194 g, 79%) was obtained as a white solid after purification on silica gel with chloroform–methanol (50:1, v/v); m.p.: 139–140 °C; IR (KBr): ν = 2984, 2944, 2830, 1706, 1663, 1237, 1028 cm−1; 1H NMR (300 MHz, CDCl3): δ = 7.83 (s, 1H); 7.52 (s, 1H); 5.33 (s, 2H, CH2); 4.73 (d, J = 13.1 Hz, 2H, PCH 2); 4.17–4.06 (m, 4H, 2× POCH 2CH3); 3.99 (s, 3H, CH3); 3.56 (s, 3H, CH3); 1.29 (t, J = 7.0 Hz, 3H, POCH2CH 3); 1.28 (t, J = 7.0 Hz, 3H, POCH2CH 3); 13C NMR (75.5 MHz, CDCl3): δ = 154.7 (s, C O); 151.2 (s, C O); 148.9; 144.0; 141.7; 124.3; 107.7; 63.6 (d, J = 6.6 Hz, POC); 45.9 (d, J = 154.9 Hz, PC); 36.1; 33.8; 29.9; 16.5 (d, J = 5.7 Hz, POCC); 31P NMR (121.5 MHz, CDCl3): δ = 16.84 ppm. Anal. Calcd. for C15H22N7O5P: C, 43.80; H, 5.39; N, 23.84. Found: C, 43.69; H, 5.21; N, 23.66.
4.1.5.5. Diethyl {4-[(1,3-dimethyl-2,6-dioxopurin-7-yl)methyl]-1H-1,2,3-triazol-1-yl}methylphosphonate 20i
From azide 12 (0.136 g, 0.704 mmol) and N 7-propargyltheophylline 19i (0.154 g, 0.704 mmol) the phosphonate 20i (0.254 g, 88%) was obtained as a white solid after purification on a silica gel column with chloroform–methanol (50:1, v/v); m.p.: 105–108 °C; IR (KBr): ν = 2994, 2945, 1705, 1660, 1244, 1026 cm−1; 1H NMR (300 MHz, CDCl3): δ = 8.02 (s, 1H); 7.80 (s, 1H); 5.60 (s, 2H, CH2); 4.74 (d, J = 13.1 Hz, 2H, PCH 2); 4.18–4.08 (m, 4H, 2× POCH 2CH3); 3.57 (s, 3H, CH3); 3.40 (s, 3H, CH3); 1.29 (t, J = 6.9 Hz, 3H, POCH2CH 3); 1.28 (t, J = 6.9 Hz, 3H, POCH2CH 3); 13C NMR (75.5 MHz, CDCl3): δ = 155.3 (s, C O); 151.6 (s, C O); 149.0; 142.6; 141.3; 124.7; 106.5; 63.7 (d, J = 6.6 Hz, POC); 46.2 (d, J = 154.9 Hz, PC); 41.7; 30.0; 28.2; 16.5 (d, J = 5.7 Hz, POCC); 31P NMR (121.5 MHz, CDCl3): δ = 16.50 ppm. Anal. Calcd. for C15H22N7O5P: C, 43.80; H, 5.39; N, 23.84. Found: C, 43.88; H, 5.45; N, 23.69.
4.1.5.6. Diethyl {4-[(5,6-dimethylbenzimidazol-1-yl)methyl]-1H-1,2,3-triazol-1-yl}methylphosphonate 20j
From azide 12 (0.091 g, 0.471 mmol) and 5,6-dimethyl-N 1-propargylbenzimidazole 19j (0.087 g, 0.471 mmol) the phosphonate 20j (0.121 g, 68%) was obtained as a white powder after purification on silica gel with chloroform–methanol (50:1, v/v); m.p.: 100–102 °C; IR (KBr): ν = 3004, 2960, 2945, 1025, 846, 757 cm−1; 1H NMR (300 MHz, CDCl3): δ = 7.93 (s, 1H); 7.52 (s, 1H); 7.56 (s, 1H); 7.18 (s, 1H); 5.46 (s, 2H, CH2); 4.69 (d, J = 13.3 Hz, 2H, PCH 2); 4.11–4.01 (m, 4H, 2× POCH 2CH3); 3.36 (s, 6H, 2× CH3); 1.22 (t, J = 7.2 Hz, 6H, 2× POCH2CH 3); 13C NMR (75.5 MHz, CDCl3): δ = 143.5; 142.1; 141.9; 132.4; 131.8; 131.3; 123.3; 120.1; 110.0; 63.6 (d, J = 6.6 Hz, POC); 46.0 (d, J = 154.9 Hz, PC); 40.5; 20.6; 20.3; 16.3 (d, J = 5.8 Hz, POCC); 31P NMR (121.5 MHz, CDCl3): δ = 16.52 ppm. Anal. Calcd. for C17H24N5O3P: C, 54.11; H, 6.41; N, 18.56. Found: C, 53.97; H, 6.38; N, 18.44.
4.1.5.7. Diethyl {4-[(3-acetylindol-1-yl)methyl]-1H-1,2,3-triazol-1-yl}methylphosphonate 20k
From azide 12 (0.109 g, 0.564 mmol) and 3-acetyl-N-propargylindole 19k (0.111 g, 0.564 mmol) the phosphonate 20k (0.164 g, 75%) was obtained as a white solid after chromatography on a silica gel column with chloroform–methanol (50:1, v/v); m.p.: 128–129 °C; IR (KBr): ν = 3004, 2960, 2945, 1025, 846, 757 cm−1; 1H NMR (300 MHz, CDCl3): δ = 8.42–8.34 (m, 1H); 7.86 (s, 1H, HC5′); 7.62 (s, 1H); 7.45–7.36 (m, 1H); 7.33–7.24 (m, 2H); 5.48 (s, 2H, CH2); 4.70 (d, J = 13.3 Hz, 2H, PCH 2); 4.10–4.00 (m, 4H, 2× POCH 2CH3); 2.25 (s, 3H, CH3); 1.21 (t, J = 6.9 Hz, 6H, 2× POCH2CH 3); 13C NMR (75.5 MHz, CDCl3): δ = 192.9 (s, C O); 143.6; 136.6; 134.6; 126.6; 123.7; 123.3; 123.0; 122.9; 117.9; 109.9; 63.8 (d, J = 6.5 Hz, POC); 46.3 (d, J = 155.4 Hz, PC); 42.7; 27.9 (s, CH3); 16.5 (d, J = 5.7 Hz, POCC); 31P NMR (121.5 MHz, CDCl3): δ = 16.51 ppm. Anal. Calcd. for C18H23N4O4P: C, 55.38; H, 5.94; N, 14.35. Found: C, 55.35; H, 6.03; N, 14.21.
4.1.5.8. Diethyl {4-[(2-oxopyridin-1-yl)methyl]-1H-1,2,3-triazol-1-yl}methylphosphonate 20l
From azide 12 (0.120 g, 0.621 mmol) and N-propargyl-2-pyridon 19l (0.083 g, 0.621 mmol) the phosphonate 20l (0.178 g, 88%) was obtained as a brown solid after chromatography on a silica gel column with chloroform–methanol (50:1, v/v); m.p.: 82–85 °C; IR (KBr): ν = 3080, 2985, 2935, 1660, 1025, 978 cm−1; 1H NMR (300 MHz, CDCl3): δ = 7.93 (s, 1H); 7.56 (dd, J = 6.7 Hz, J = 1.9 Hz, 1H); 7.31 (ddd, J = 9.2 Hz, J = 6.7 Hz, J = 1.9 Hz, 1H); 6.59 (d, J = 9.2 Hz, 1H); 6.17 (dt, J = 6.7 Hz, J = 1.2 Hz, 1H); 5.20 (s, 2H, CH2); 4.73 (d, J = 13.1 Hz, 2H, PCH 2); 4.18–4.07 (m, 4H, 2× POCH 2CH3); 1.29 (t, J = 6.9 Hz, 3H, POCH2CH 3); 1.28 (t, J = 6.9 Hz, 3H, POCH2CH 3); 13C NMR (75.5 MHz, CDCl3): δ = 162.1 (s, C O); 142.9 (s, HC C); 139.9; 137.6; 124.9 (s, HC C); 106.4; 63.5 (d, J = 6.7 Hz, POC); 45.8 (d, J = 154.5 Hz, PC); 44.4; 16.3 (d, J = 5.7 Hz, POCC); 31P NMR (121.5 MHz, CDCl3): δ = 16.62 ppm. Anal. Calcd. for C13H19N4O4P: C, 47.85; H, 5.87; N, 17.17. Found: C, 48.01; H, 6.00; N, 17.25.
4.1.5.9. Diethyl 2-{4-[(3,5-dioxo-1,2,4-triazin-2-yl)methyl]-1H-1,2,3-triazol-1-yl}ethylphosphonate 21f
From azide 13 (0.147 g, 0.710 mmol) and N 1-propargyl-6-azauracil 19f (0.107 g, 0.710 mmol) the phosphonate 21f (0.227 g, 89%) was obtained as a white solid after purification on silica gel with chloroform–methanol (50:1, v/v); m.p.: 119–121 °C; IR (KBr): ν = 3301, 2999, 2985, 1688, 1220, 1045 cm−1; 1H NMR (300 MHz, CDCl3): δ = 11.80 (s, 1H, NH); 7.90 (s, 1H); 7.41 (s, 1H); 5.22 (s, 2H, CH2); 4.68–4.50 (m, 2H, PCH 2); 4.16–4.04 (m, 4H, 2× POCH 2CH3); 2.78–2.62 (m, 2H, PCCH 2); 1.31 (t, J = 7.1 Hz, 6H, 2× POCH2CH 3); 13C NMR (75.5 MHz, CDCl3): δ = 155.9 (s, C O); 149.1 (C O); 141.6 (s, HC C); 134.7 (s, HC N); 124.4 (s, HC C); 62.5 (d, J = 6.6 Hz, POC); 44.5; 34.6; 27.2 (d, J = 140.9 Hz, PC); 16.4 (d, J = 6.0 Hz, POCC); 31P NMR (121.5 MHz, CDCl3): δ = 27.15 ppm. Anal. Calcd. for C12H19N6O5P: C, 40.23; H, 5.35; N, 23.46. Found: C, 40.28; H, 5.29; N, 23.52.
4.1.5.10. Diethyl 2-{4-[(8-chloro-1,3-dimethyl-2,6-dioxopurin-7-yl)methyl]-1H-1,2,3-triazol-1-yl}ethylphosphonate 21g
From azide 13 (0.130 g, 0.628 mmol) and 8-chloro-N 7-propargylthephylline 19g (0.159 g, 0.628 mmol) the phosphonate 21g (0.254 g, 88%) was obtained as a white solid after chromatography on a silica gel column with chloroform–methanol (50:1, v/v); m.p.: 101–103 °C; IR (KBr): ν = 3426, 3139, 2953, 2903, 1703, 1661, 1250, 1043 cm−1; 1H NMR (600 MHz, CDCl3): δ = 7.85 (s, 1H, HC5′); 5.65 (s, 2H, CH2); 4.63–4.56 (m, 2H, PCH 2); 4.13–4.05 (m, 4H, 2× POCH 2CH3); 3.56 (s, 3H, CH3); 3.43 (s, 3H, CH3); 2.45–2.39 (m, 2H, PCCH 2); 1.32 (t, J = 7.0 Hz, 6H, 2× POCH2CH 3); 13C NMR (151 MHz, CDCl3): δ = 155.4 (s, C O); 151.2 (s, C O); 147.4; 141.8; 139.0; 123.9; 107.3; 62.2 (d, J = 6.0 Hz, POC); 44.7; 40.9; 29.8; 28.0; 27.2 (d, J = 141.9 Hz, PC); 16.3 (d, J = 5.7 Hz, POCC); 31P NMR (243 MHz, CDCl3): δ = 25.20 ppm. Anal. Calcd. for C16H23ClN7O5P: C, 41.79; H, 5.04; N, 21.32. Found: C, 41.85; H, 4.94; N, 21.43.
4.1.5.11. Diethyl 2-{4-[(3,7-dimethyl-2,6-dioxopurin-1-yl)methyl]-1H-1,2,3-triazol-1-yl}ethylphosphonate 21h
From azide 13 (0.130 g, 0.628 mmol) and N 1-propargyltheobromine 19h (0.137 g, 0.628 mmol) the phosphonate 21h (0.219 g, 82%) was obtained as a white solid after chromatography on a silica gel column with chloroform–methanol (50:1, v/v); m.p.: 100–102 °C; IR (KBr): ν = 3133, 3087, 2989, 2830, 1701, 1665, 1233, 1023 cm−1; 1H NMR (300 MHz, CDCl3): δ = 7.67 (s, 1H); 7.52 (d, J = 0.6 Hz, 1H, HC5′); 5.32 (s, 2H, CH2); 4.62–4.52 (m, 2H, PCH 2); 4.14–4.00 (m, 4H, 2× POCH 2CH3); 3.99 (d, J = 0.6 Hz, 3H, CH3); 3.57 (s, 3H, CH3); 2.46–2.34 (m, 2H, PCCH 2); 1.29 (t, J = 7.0 Hz, 6H, 2× POCH2CH 3); 13C NMR (75.5 MHz, CDCl3): δ = 154.7 (s, C O); 151.2 (s, C O); 148.9; 143.6; 141.7; 123.5; 107.6; 62.2 (d, J = 6.3 Hz, POC); 52.4; 44.6; 36.1; 31.9 (d, J = 293.7 Hz, PC); 28.3; 26.5; 16.5 (d, J = 6.0 Hz, POCC); 31P NMR (121.5 MHz, CDCl3): δ = 26.59 ppm. Anal. Calcd. for C16H24N7O5P: C, 45.18; H, 5.69; N, 23.05. Found: C, 45.00; H, 5.56; N, 22.96.
4.1.5.12. Diethyl 2-{4-[(1,3-dimethyl-2,6-dioxopurin-7-yl)methyl]-1H-1,2,3-triazol-1-yl}ethylphosphonate 21i
From azide 13 (0.130 g, 0.628 mmol) and N 7-propargylthephylline 19i (0.137 g, 0.628 mmol) the phosphonate 21i (0.219 g, 82%) was obtained as a colourless oil after purification on silica gel with chloroform–methanol (50:1, v/v); IR (film): ν = 3033, 2987, 2889, 2830, 1703, 1666, 1230, 1023 cm−1; 1H NMR (600 MHz, CDCl3): δ = 7.89 (s, 1H); 7.82 (s, 1H, HC5′); 5.60 (s, 2H, CH2); 4.63–4.55 (m, 2H, PCH 2); 4.13–4.08 (m, 4H, 2× POCH 2CH3); 3.59 (s, 3H, CH3); 3.43 (s, 3H, CH3); 2.45–2.39 (m, 2H, PCCH 2); 1.31 (t, J = 7.0 Hz, 6H, 2× POCH2CH 3); 13C NMR (151 MHz, CDCl3): δ = 155.4 (s, C O); 151.6 (s, C O); 149.0; 142.2; 141.4; 123.9; 106.5; 62.2 (d, J = 5.8 Hz, POC); 44.7; 41.4; 29.7; 28.0; 27.2 (d, J = 141.9 Hz, PC); 16.3 (d, J = 6.0 Hz, POCC); 31P NMR (243 MHz, CDCl3): δ = 25.15 ppm. Anal. Calcd. for C16H24N7O5P: C, 45.18; H, 5.69; N, 23.05. Found: C, 45.30; H, 5.77; N, 23.17.
4.1.5.13. Diethyl 2-{4-[(5,6-dimethylbenzimidazol-1-yl)methyl]-1H-1,2,3-triazol-1-yl}ethylphosphonate 21j
From azide 13 (0.139 g, 0.671 mmol) and 5,6-dimethyl-N-propargylbenzimidazole 19j (0.124 g, 0.671 mmol) the phosphonate 21j (0.196 g, 75%) was obtained as a colourless oil after chromatography on a silica gel column with chloroform–methanol (50:1, v/v); IR (film): ν = 3014, 2950, 2895, 1045, 856, 759 cm−1; 1H NMR (600 MHz, CDCl3): δ = 7.93 (s, 1H); 7.58 (s, 1H); 7.43 (s, 1H); 7.23 (s, 1H); 5.45 (s, 2H, CH2); 4.54 (dt, J = 12.7 Hz, J = 7.7 Hz, 2H, PCH 2); 4.06–4.00 (m, 4H, 2× POCH 2CH3); 2.38 (dt, J = 18.5 Hz, J = 7.7 Hz, 2H, PCCH 2); 2.39 (s, 3H, CH3); 2.38 (s, 3H, CH3); 1.26 (t, J = 7.0 Hz, 6H, 2× POCH2CH 3); 13C NMR (151 MHz, CDCl3): δ = 143.3; 142.4; 142.0; 132.5; 132.0; 131.4; 122.5; 120.3; 109.9; 62.2 (d, J = 6.5 Hz, POC); 44.7 (d, J = 1.7 Hz, PCC); 40.4; 27.0 (d, J = 142.0 Hz, PC); 20.5; 20.2; 16.2 (d, J = 6.4 Hz, POCC); 31P NMR (243 MHz, CDCl3): δ = 25.27 ppm. Anal. Calcd. for C18H26N5O3P: C, 55.24; H, 6.70; N, 17.89. Found: C, 55.08; H, 6.84; N, 17.72.
4.1.5.14. Diethyl 2-{4-[(3-acetylindol-1-yl)methyl]-1H-1,2,3-triazol-1-yl}ethylphosphonate 21k
From azide 13 (0.100 g, 0.483 mmol) and 3-acetyl-N-propargylindole 19k (0.095 g, 0.483 mmol) the phosphonate 21k (0.144 g, 74%) was obtained as a white solid after purification on silica gel with chloroform–methanol (50:1, v/v); m.p.: 83–84 °C; IR (KBr): ν = 3430, 3110, 2989, 1642, 1528, 1390, 1026, 753 cm−1; 1H NMR (300 MHz, CDCl3): δ = 8.44–8.32 (m, 1H); 7.87 (s, 1H, HC5′); 7.47 (s, 1H); 7.45–7.42 (m, 1H); 7.32–7.29 (m, 2H); 5.47 (s, 2H, CH2); 4.61–4.52 (m, 2H, PCH 2); 4.05–3.94 (m, 4H, 2× POCH 2CH3); 2.55 (s, 3H, CH3); 2.42–2.31 (m, 2H, PCCH 2); 1.22 (t, J = 6.8 Hz, 6H, 2× POCH2CH 3); 13C NMR (75.5 MHz, CDCl3): δ = 192.9 (s, C O); 142.8; 136.4; 134.8; 126.3; 123.5; 122.7; 122.7; 122.6; 117.4; 109.8; 62.2 (d, J = 6.6 Hz, POC); 44.7 (d, J = 2.0 Hz, PCC); 42.3; 27.7 (s, CH3); 27.1 (d, J = 141.4 Hz, PC); 16.4 (d, J = 5.7 Hz, POCC); 31P NMR (121.5 MHz, CDCl3): δ = 26.39 ppm. Anal. Calcd. for C19H25N4O4P: C, 56.43; H, 6.23; N, 13.85. Found: C, 56.54; H, 6.14; N, 13.72.
4.1.5.15. Diethyl 2-{4-[(2-oxopyridin-1-yl)methyl]-1H-1,2,3-triazol-1-yl}ethylphosphonate 21l
From azide 13 (0.147 g, 0.710 mmol) and N-propargyl-2-pyridon 19l (0.095 g, 0.710 mmol) the phosphonate 21l (0.214 g, 89%) was obtained as a brown oil after chromatography on a silica gel column with chloroform–methanol (50:1, v/v); IR (film): ν = 3110, 2976, 2875, 1668, 1035, 988 cm−1; 1H NMR (300 MHz, CDCl3): δ = 7.84 (s, 1H); 7.59 (dd, J = 6.8 Hz, J = 1.6 Hz, 1H); 7.33 (ddd, J = 9.2 Hz, J = 6.8 Hz, J = 2.0 Hz, 1H); 6.55 (dd, J = 9.2 Hz, J = 0.5 Hz, 1H); 6.19 (dt, J = 6.8 Hz, J = 1.6 Hz, 1H); 5.18 (s, 2H, CH2); 4.62–4.52 (m, 2H, PCH 2); 4.13–4.03 (m, 4H, 2× POCH 2CH3); 2.46–2.35 (m, 2H, PCCH 2); 1.29 (t, J = 7.0 Hz, 3H, POCH2CH 3); 1.28 (t, J = 6.9 Hz, 3H, POCH2CH 3); 13C NMR (75.5 MHz, CDCl3): δ = 162.2 (s, C O); 142.5 (s, HC C); 140.0; 137.7; 124.3 (s, HC C); 120.6; 106.5; 62.2 (d, J = 6.3 Hz, POC); 44.6 (d, J = 2.8 Hz, PCC); 27.1 (d, J = 141.2 Hz, PC); 16.4 (d, J = 6.0 Hz, POCC); 31P NMR (121.5 MHz, CDCl3): δ = 26.37 ppm. Anal. Calcd. for C14H21N4O4P: C, 49.41; H, 6.22; N, 16.46. Found: C, 49.24; H, 6.09; N, 16.28.
4.1.5.16. Diethyl 3-{4-[(3-benzoyl-2,4-dioxoquinazolin-1-yl)methyl]-1H-1,2,3-triazol-1-yl}propylphosphonate 22e
From azide 14 (0.100 g, 0.452 mmol) and N 3-benzoyl-N 1-propargylquinazoline-2,4-dione 19e (0.138 g, 0.452 mmol) the phosphonate 22e (0.198 g, 83%) was obtained as a colourless oil after purification on silica gel with chloroform–methanol (50:1, v/v); IR (film): ν = 3141, 3064, 2939, 1799, 1606, 1481; 1220, 1025 cm−1; 1H NMR (300 MHz, CDCl3): δ = 8.20 (dd, J = 7.9 Hz, J = 1.6 Hz, 1H); 8.00–7.95 (m, 2H, 2× o-CH); 7.91 (d, J = 8.5 Hz, 1H); 7.78 (ddd, J = 8.5 Hz, J = 7.9 Hz, J = 1.6 Hz, 1H); 7.71 (s, 1H, HC5′); 7.70–7.62 (m, 1H, p-CH); 7.54–7.48 (m, 2H, 2× m-CH); 7.32 (dt, J = 7.9 Hz, J = 0.8 Hz, 1H); 5.40 (s, 2H, CH2); 4.41 (t, J = 7.0 Hz, 2H, PCCCH 2); 4.16–3.99 (m, 4H, 2× POCH 2CH3); 2.20 (dqu, J = 14.5 Hz, J = 7.0 Hz, 2H, PCCH 2); 1.71 (dt, J = 18.7 Hz, J = 7.0 Hz, 2H, PCH 2); 1.30 (t, J = 7.1 Hz, 6H, 2× POCH2CH 3); 13C NMR (75.5 MHz, CDCl3): δ = 168.6 (s, C O); 161.0 (s, C O); 149.5 (s, C O); 142.4 (s, HC C); 140.2; 136.2; 135.2; 131.5; 130.5; 129.2; 128.9; 123.9 (s, HC C); 123.8; 115.5; 115.3; 61.8 (d, J = 6.7 Hz, POC); 50.3 (d, J = 15.7 Hz, PCCC); 38.9; 23.7 (d, J = 4.9 Hz, PCC); 22.8 (d, J = 142.9 Hz, PC); 16.6 (d, J = 6.0 Hz, POCC); 31P NMR (121 MHz, CDCl3): δ = 30.82 ppm. Anal. Calcd. for C25H28N5O6P: C, 57.14; H, 5.37; N, 13.33. Found: C, 57.27; H, 5.49; N, 13.40.
4.1.5.17. Diethyl 3-{4-[(3,5-dioxo-1,2,4-triazin-2-yl)methyl]-1H-1,2,3-triazol-1-yl}propylphosphonate 22f
From azide 14 (0.154 g, 0.697 mmol) and N 1-propargyl-6-azauracil 19f (0.105 g, 0.697 mmol) the phosphonate 22f (0.215 g, 83%) was obtained as a white solid after chromatography on a silica gel column with chloroform–methanol (50:1, v/v); m.p.: 96–97 °C; IR (KBr): ν = 3384, 3232, 3138, 2984, 2908, 1730, 1677, 1217, 1025 cm−1; 1H NMR (300 MHz, CDCl3): δ = 11.51 (s, 1H, NH); 7.77 (s, 1H, HC5′); 7.40 (s, 1H); 5.22 (s, 2H, CH2); 4.44 (t, J = 7.0 Hz, 2H, PCCCH 2); 4.16–4.03 (m, 4H, 2× POCH 2CH3); 2.21 (dqu, J = 14.9 Hz, J = 7.0 Hz, 2H, PCCH 2); 1.75 (dt, J = 19.0 Hz, J = 7.0 Hz, 2H, PCH 2); 1.32 (t, J = 7.0 Hz, 6H, 2× POCH2CH 3); 13C NMR (75.5 MHz, CDCl3): δ = 155.8 (s, C O); 148.9 (C O); 141.3 (s, HC C); 134.5 (s, HC N); 124.2 (s, HC C); 62.0 (d, J = 6.4 Hz, POC); 50.0 (d, J = 15.1 Hz, PCCC); 34.5; 23.4 (d, J = 4.3 Hz, PCC); 22.2 (d, J = 143.0 Hz, PC); 16.4 (d, J = 6.0 Hz, POCC); 31P NMR (121.5 MHz, CDCl3): δ = 31.41 ppm. Anal. Calcd. for C13H21N6O5P: C, 41.94; H, 5.69; N, 22.57. Found: C, 41.92; H, 5.52; N, 22.41.
4.1.5.18. Diethyl 3-{4-[(8-chloro-1,3-dimethyl-2,6-dioxopurin-7-yl)methyl]-1H-1,2,3-triazol-1-yl}propylphosphonate 22g
From azide 14 (0.160 g, 0.723 mmol) and 8-chloro-N 7-propargyltheophylline 19g (0.183 g, 0.723 mmol) the phosphonate 22g (0.298 g, 84%) was obtained as a white solid after purification on silica gel with chloroform–methanol (50:1, v/v); m.p.: 127–128 °C; IR (KBr): ν = 3362, 3101, 2981, 2935, 1707, 1679, 1224, 1020, 956 cm−1; 1H NMR (600 MHz, CDCl3): δ = 7.83 (s, 1H, HC5′); 5.66 (s, 2H, CH2); 4.46 (t, J = 7.0 Hz, 2H, PCCCH 2); 4.15–4.05 (m, 4H, 2× POCH 2CH3); 3.57 (s, 3H, CH3); 3.44 (s, 3H, CH3); 2.23 (dqu, J = 14.7 Hz, J = 7.0 Hz, 2H, PCCH 2); 1.74 (dt, J = 18.7 Hz, J = 7.0 Hz, 2H, PCH 2); 1.34 (t, J = 7.1 Hz, 6H, 2× POCH2CH 3); 13C NMR (151 MHz, CDCl3): δ = 154.5 (s, C O); 151.2 (s, C O); 147.4; 141.8; 139.0; 123.7; 107.4; 61.8 (d, J = 6.4 Hz, POC); 50.1 (d, J = 15.2 Hz, PCCC); 41.0; 29.8; 27.9; 23.6 (d, J = 4.8 Hz, PCC); 22.6 (d, J = 142.3 Hz, PC); 16.4 (d, J = 6.1 Hz, POCC); 31P NMR (243 MHz, CDCl3): δ = 29.80 ppm. Anal. Calcd. for C17H25ClN7O5P: C, 43.09; H, 5.32; N, 20.69. Found: C, 42.88; H, 5.44; N, 20.71.
4.1.5.19. Diethyl 3-{4-[(3,7-dimethyl-2,6-dioxopurin-1-yl)methyl]-1H-1,2,3-triazol-1-yl}propylphosphonate 22h
From azide 14 (0.160 g, 0.723 mmol) and N 1-propargyltheobromine 19h (0.158 g, 0.723 mmol) the phosphonate 22h (0.270 g, 85%) was obtained as a white powder after crystallisation from diethyl ether; m.p.: 175–176 °C; IR (KBr): ν = 3444, 3001, 2984, 1704, 1668, 1221, 1020 cm−1; 1H NMR (600 MHz, CDCl3): δ = 7.76 (s, 1H); 7.53 (s, 1H, HC5′); 5.35 (s, 2H, CH2); 4.42 (t, J = 7.0 Hz, 2H, PCCCH 2); 4.15–4.05 (m, 4H, 2× POCH 2CH3); 4.02 (s, 3H, CH3); 3.60 (s, 3H, CH3); 2.22 (dqu, J = 14.2 Hz, J = 7.0 Hz, 2H, PCCH 2); 1.74 (dt, J = 18.7 Hz, J = 7.0 Hz, 2H, PCH 2); 1.34 (t, J = 7.0 Hz, 6H, 2× POCH2CH 3); 13C NMR (151 MHz, CDCl3): δ = 154.8 (s, C O); 151.4 (s, C O); 148.9; 143.7; 141.6; 123.4; 107.7; 61.7 (d, J = 6.5 Hz, POC); 50.0 (d, J = 16.1 Hz, PCCC); 36.0; 33.6; 29.7; 23.6 (d, J = 4.5 Hz, PCC); 22.7 (d, J = 143.1 Hz, PC); 16.4 (d, J = 5.8 Hz, POCC); 31P NMR (243 MHz, CDCl3): δ = 30.03 ppm. Anal. Calcd. for C17H26N7O5P: C, 46.47; H, 5.96; N, 22.31. Found: C, 46.36; H, 5.90; N, 22.06.
4.1.5.20. Diethyl 3-{4-[(1,3-dimethyl-2,6-dioxopurin-7-yl)methyl]-1H-1,2,3-triazol-1-yl}propylphosphonate 22i
From azide 14 (0.091 g, 0.412 mmol) and N 7-propargyltheophylline 19i (0.090 g, 0.412 mmol) the phosphonate 22i (0.181 g, 74%) was obtained as a white solid after chromatography on a silica gel column with chloroform–methanol (50:1, v/v); m.p.: 187–190 °C; IR (KBr): ν = 3440, 2996, 2984, 1704, 1668, 1225, 1018 cm−1; 1H NMR (600 MHz, CDCl3): δ = 7.87 (s, 1H); 7.83 (s, 1H, HC5′); 5.61 (s, 2H, CH2); 4.46 (t, J = 7.0 Hz, 2H, PCCCH 2); 4.16–4.05 (m, 4H, 2× POCH 2CH3); 3.60 (s, 3H, CH3); 3.44 (s, 3H, CH3); 2.24 (dqu, J = 14.6 Hz, J = 7.0 Hz, 2H, PCCH 2); 1.74 (dt, J = 18.7 Hz, J = 7.0 Hz, 2H, PCH 2); 1.34 (t, J = 7.0 Hz, 6H, 2× POCH2CH 3); 13C NMR (151 MHz, CDCl3): δ = 155.4 (s, C O); 151.5 (s, C O); 149.0; 142.2; 141.4; 123.8; 106.4; 61.8 (d, J = 6.5 Hz, POC); 50.1 (d, J = 15.3 Hz, PCCC); 41.4; 29.7; 27.9; 23.6 (d, J = 4.8 Hz, PCC); 22.6 (d, J = 142.4 Hz, PC); 16.4 (d, J = 5.8 Hz, POCC); 31P NMR (243 MHz, CDCl3): δ = 29.75 ppm. Anal. Calcd. for C17H26N7O5P: C, 46.47; H, 5.96; N, 22.31. Found: C, 46.59; H, 6.11; N, 22.45.
4.1.5.21. Diethyl 3-{4-[(5,6-dimethylbenzimidazol-1-yl)methyl]-1H-1,2,3-triazol-1-yl}propylphosphonate 22j
From azide 14 (0.160 g, 0.723 mmol) and 5,6-dimethyl-N 1-propargylbenzimidazole 19j (0.133 g, 0.723 mmol) the phosphonate 22j (0.221 g, 76%) as a colourless oil after purification on silica gel with chloroform–methanol (50:1, v/v); IR (film): ν = 3446, 2990, 2938, 1498, 1444, 1224, 1050, 965 cm−1; 1H NMR (600 MHz, CDCl3): δ = 8.33 (s, 1H); 7.61 (s, 1H); 7.54 (s, 1H); 7.32 (s, 1H); 5.55 (s, 2H, CH2); 4.43 (t, J = 7.0 Hz, 2H, PCCCH 2); 4.15–4.05 (m, 4H, 2× POCH 2CH3); 2.41 (s, 3H, CH3); 2.40 (s, 3H, CH3); 2.21 (dqu, J = 14.6 Hz, J = 7.0 Hz, 2H, PCCH 2); 1.70 (dt, J = 19.2 Hz, J = 7.0 Hz, 2H, PCH 2); 1.34 (t, J = 7.1 Hz, 6H, 2× POCH2CH 3); 13C NMR (151 MHz, CDCl3): δ = 143.4; 142.3; 142.0; 132.4; 131.4; 122.4; 120.3; 109.9; 61.8 (d, J = 6.5 Hz, POC); 50.0 (d, J = 14.5 Hz, PCCC); 40.5; 23.5 (d, J = 4.5 Hz, PCC); 22.4 (d, J = 142.3 Hz, PC); 20.5; 20.1; 16.4 (d, J = 5.8 Hz, POCC); 31P NMR (243 MHz, CDCl3): δ = 29.68 ppm. Anal. Calcd. for C19H28N5O3P: C, 56.29; H, 6.96; N, 17.27. Found: C, 56.07; H, 7.14; N, 17.10.
4.1.5.22. Diethyl 3-{4-[(3-acetylindol-1-yl)methyl]-1H-1,2,3-triazol-1-yl}propylphosphonate 22k
From azide 14 (0.110 g, 0.497 mmol) and 3-acetyl-N-propargylindole 19k (0.098 g, 0.497 mmol) the phosphonate 22k (0.202 g, 97%) was obtained as a colourless oil after chromatography on a silica gel column with chloroform–methanol (50:1, v/v); IR (film): ν = 3394, 3110, 2941, 2825, 1648, 1229, 1029 cm−1; 1H NMR (300 MHz, CDCl3): δ = 8.43–8.38 (m, 1H); 7.88 (s, 1H, HC5′); 7.43–7.38 (m, 2H); 7.36–7.27 (m, 2H); 5.48 (s, 2H, CH2); 4.44 (t, J = 7.0 Hz, 2H, PCCCH 2); 4.10–4.01 (m, 4H, 2× POCH 2CH3); 2.53 (s. 3H, CH3); 2.20 (dqu, J = 14.7 Hz, J = 7.0 Hz, 2H, PCCH 2); 1.65 (dt, J = 18.4 Hz, J = 7.0 Hz, 2H, PCH 2); 1.29 (t, J = 7.1 Hz, 6H, 2× POCH2CH 3); 13C NMR (75.5 MHz, CDCl3): δ = 192.9 (s, C O); 142.9; 136.4; 134.7; 126.3; 123.5; 122.7; 122.6; 122.5; 117.5; 109.9; 61.9 (d, J = 6.3 Hz, POC); 50.0 (d, J = 14.9 Hz, PCCC); 42.4; 27.8; 23.6 (d, J = 4.9 Hz, PCC); 22.1 (d, J = 142.8 Hz, PC); 16.4 (d, J = 6.1 Hz, POCC); 31P NMR (121.5 MHz, CDCl3): δ = 30.85 ppm. Anal. Calcd. for C20H27N4O4P: C, 57.41; H, 6.50; N, 13.39. Found: C, 57.60; H, 6.73; N, 13.50.
4.1.5.23. Diethyl 3-{4-[(2-oxopyridin-1-yl)methyl]-1H-1,2,3-triazol-1-yl}propylphosphonate 22l
From azide 14 (0.154 g, 0.696 mmol) and N-propargyl-2-pyridon 19l (0.093 g, 0.696 mmol) the phosphonate 22l (0.206 g, 83%) was obtained as a brown oil after purification on silica gel with chloroform–methanol (50:1, v/v); IR (film): ν = 3426, 3144, 2986, 1657, 1226; 1026, 968 cm−1; 1H NMR (300 MHz, CDCl3): δ = 7.81 (s, 1H, HC5′); 7.60 (ddd, J = 6.8 Hz, J = 2.1 Hz, J = 0.7 Hz, 1H); 7.32 (ddd, J = 9.2 Hz, J = 6.6 Hz, J = 2.1 Hz, 1H); 6.56 (ddd, J = 9.2 Hz, J = 1.3 Hz, J = 0.7 Hz, 1H); 6.20 (dt, J = 6.8 Hz, J = 1.3 Hz, 1H); 5.19 (s, 2H, CH2); 4.41 (t, J = 7.1 Hz, 2H, PCCCH 2); 4.15–4.03 (m, 4H, 2× POCH 2CH3); 2.22 (dqu, J = 14.9 Hz, J = 7.1 Hz, 2H, PCCH 2); 1.66 (dt, J = 18.2 Hz, J = 7.1 Hz, 2H, PCH 2); 1.34 (t, J = 6.9 Hz, 3H, POCH2CH 3); 1.33 (t, J = 6.9 Hz, 3H, POCH2CH 3); 13C NMR (75.5 MHz, CDCl3): δ = 162.1 (s, C O); 142.5 (s, HC C); 139.9; 137.6; 124.0 (s, HC C); 120.4; 106.4; 61.8 (d, J = 6.4 Hz, POC); 50.0 (d, J = 16.1 Hz, PCCC); 44.5; 23.5 (d, J = 4.4 Hz, PCC); 22.1 (d, J = 147.1 Hz, PC); 16.4 (d, J = 6.0 Hz, POCC); 31P NMR (121.5 MHz, CDCl3): δ = 31.05 ppm. Anal. Calcd. for C15H23N4O4P: C, 50.84; H, 6.54; N, 15.81. Found: C, 50.61; H, 6.39; N, 15.64.
4.1.5.24. Diethyl 3-(4-{[3-benzoyl-2,4-dioxopyrimidin-1-yl]methyl}-1H-1,2,3-triazol-1-yl)propylphosphonate 22m
From azide 14 (0.201 g, 0.909 mmol) and N 3-benzoyl-N 1-propargylquinazoline-2,4-dione 19m (0.230 g, 0.909 mmol) the phosphonate 22m (0.399 g, 93%) was obtained as a colourless oil after chromatography on a silica gel column with chloroform–methanol (100:1; 50:1, 20:1 v/v); IR (film): ν = 3020, 3005, 2963, 2899, 1669, 1664, 1220, 1020, 772, 689 cm−1; 1H NMR (300 MHz, CDCl3): δ = 7.94–7.90 (m, 2H, 2× o-CH); 7.71 (s, 1H, HC5′); 7.69–7.63 (m, 1H, p-CH); 7.64 (d, J = 8.0 Hz, 1H, HC CH); 7.53–7.47 (m, 2H, 2× m-CH); 5.84 (d, J = 8.0 Hz, 1H, HC CH); 5.02 (s, 2H, CH2); 4.46 (t, J = 7.3 Hz, 2H, PCCCH 2); 4.18–4.01 (m, 4H, 2× POCH 2CH3); 2.23 (dqu, J = 14.7 Hz, J = 7.3 Hz, 2H, PCCH 2); 1.73 (dt, J = 18.7 Hz, J = 7.3 Hz, 2H, PCH 2); 1.31 (t, J = 7.1 Hz, 6H, 2× POCH2CH 3); 13C NMR (151 MHz, CDCl3): δ = 168.7 (s, C O); 162.2 (s, C O); 144.2 (s, C O); 141.3 (s, HC C); 135.2; 131.3; 130.4; 129.2; 124.1 (s, HC C); 102.5; 61.9 (d, J = 6.6 Hz, POC); 50.2 (d, J = 15.4 Hz, PCCC); 43.5; 23.7 (d, J = 4.7 Hz, PCC); 22.7 (d, J = 143.0 Hz, PC); 16.6 (d, J = 6.0 Hz, POCC); 31P NMR (121 MHz, CDCl3): δ = 30.12 ppm. Anal. Calcd. for C21H26N5O6P: C, 53.05; H, 5.51; N, 14.73. Found: C, 52.89; H, 5.33; N, 14.58.
4.1.5.25. Diethyl 4-(4-{[6-aminopurin-9-yl]methyl}-1H-1,2,3-triazol-1-yl)butylphosphonate 23a
From azide 15 (0.061 g, 0.259 mmol) and N 9-propargyladenine 19a (0.045 g, 0.259 mmol) the phosphonate 23a (0.097 g, 92%) was obtained as a white powder after chromatography on a silica gel column with chloroform–methanol (10:1, v/v); m.p.: 119–120 °C; IR (KBr): ν = 3462, 3306, 3140, 2984, 2912, 2870, 1662, 1597, 1244, 1033 cm−1; 1H NMR (600 MHz, CDCl3): δ = 8.41 (s, 1H); 8.02 (s, 1H); 7.68 (s, 1H); 5.59 (brs, 2H, NH2); 5.51 (s, 2H, CH2); 4.36 (t, J = 7.1 Hz, 2H, PCCCCH 2); 4.14–4.04 (m, 4H, 2× POCH 2CH3); 2.03 (qu, J = 7.1 Hz, 2H, PCCCH 2); 1.80–1.73 (m, 2H, PCH 2); 1.65 (dqu, J = 14.1 Hz, J = 7.1 Hz, 2H, PCCH 2); 1.32 (t, J = 7.0 Hz, 6H, 2× POCH2CH 3); 13C NMR (151 MHz, CDCl3): δ = 155.6; 153.1; 149.8; 142.4; 140.4; 122.8; 119.5; 61.6 (d, J = 6.6 Hz, POC); 49.9; 38.6; 30.6 (d, J = 15.1 Hz, PCCC); 24.9 (d, J = 142.1 Hz, PC); 19.6 (d, J = 5.0 Hz, PCC); 16.4 (d, J = 6.2 Hz, POCC); 31P NMR (243 MHz, CDCl3): δ = 30.73 ppm. Anal. Calcd. for C16H25N8O3P: C, 47.06; H, 6.17; N, 27.44. Found: C, 46.88; H, 6.02; N, 27.29.
4.1.5.26. Diethyl 4-(4-{[5-methyl-2,4-dioxopyrimidin-1-yl]methyl}-1H-1,2,3-triazol-1-yl)butylphosphonate 23b
From azide 15 (0.100 g, 0.425 mmol) and N 1-propargylthymine 19b (0.070 g, 0.425 mmol) the phosphonate 23b (0.165 g, 97%) was obtained as a white powder after crystallisation from ethyl acetate–petroleum ether mixtures; m.p.: 63–65 °C; IR (KBr): ν = 3425, 3132, 2986, 2912, 2827, 1688, 1219, 1027 cm−1; 1H NMR (600 MHz, CDCl3): δ = 8.69 (brs, 1H, NH); 7.72 (s, 1H, HC5′); 7.36 (d, J = 1.0 Hz, 1H, HC CCH3); 4.97 (s, 2H, CH2); 4.38 (t, J = 7.1 Hz, 2H, PCCCCH 2); 4.16–4.06 (m, 4H, 2× POCH 2CH3); 2.06 (qu, J = 7.1 Hz, 2H, PCCCH 2); 1.93 (d, J = 1.0 Hz, 3H, HC CCH 3); 1.78 (dt, J = 15.7 Hz, J = 7.1 Hz, 2H, PCH 2); 1.69–1.64 (m, 2H, PCCH 2); 1.33 (t, J = 7.0 Hz, 6H, 2× POCH2CH 3); 13C NMR (151 MHz, CDCl3): δ = 164.2 (s, C O); 151.2 (s, C O); 142.1; 140.2; 123.7; 111.3; 61.6 (d, J = 6.6 Hz, POC); 49.9; 43.0; 30.6 (d, J = 15.2 Hz, PCCC); 24.9 (d, J = 142.0 Hz, PC); 19.7 (d, J = 5.2 Hz, PCC); 16.4 (d, J = 6.2 Hz, POCC); 12.2 (s, CH3); 31P NMR (243 MHz, CDCl3): δ = 30.84 ppm. Anal. Calcd. for C16H26N5O5P: C, 48.12; H, 6.56; N, 17.54. Found: C, 47.90; H, 6.33; N, 17.41.
4.1.5.27. Diethyl 4-(4-{[2,4-dioxopyrimidin-1-yl]methyl}-1H-1,2,3-triazol-1-yl)butylphosphonate 23c
From azide 15 (0.100 g, 0.425 mmol) and N 1-propargyluracil 19c (0.064 g, 0.425 mmol) the phosphonate 23c (0.133 g, 81%) was obtained as a white powder after crystallisation from ethyl acetate–petroleum ether mixtures; m.p.: 124–125 °C; IR (KBr): ν = 3435, 3142, 2994, 2952, 2867, 1648, 1229, 1023 cm−1; 1H NMR (600 MHz, CDCl3): δ = 8.80 (brs, 1H, NH); 7.72 (s, 1H, HC5′); 7.53 (d, J = 8.0 Hz, 1H, HC CH); 5.73 (d, J = 8.0 Hz, 1H, HC CH); 5.00 (s, 2H, CH2); 4.39 (t, J = 7.1 Hz, 2H, PCCCCH 2); 4.16–4.06 (m, 4H, 2× POCH 2CH3); 2.06 (qu, J = 7.1 Hz, 2H, PCCCH 2); 1.82–1.76 (m, 2H, PCCH 2); 1.71–1.64 (dqu, J = 14.3 Hz, J = 7.1 Hz, 2H, PCH 2); 1.34 (t, J = 7.0 Hz, 6H, 2× POCH2CH 3); 13C NMR (151 MHz, CDCl3): δ = 163.7 (s, C O); 151.1 (s, C O); 144.3; 141.9; 123.7; 102.7; 61.6 (d, J = 6.6 Hz, POC); 49.9; 43.2; 30.6 (d, J = 15.2 Hz, PCCC); 25.0 (d, J = 142.0 Hz, PC); 19.7 (d, J = 5.3 Hz, PCC); 16.5 (d, J = 6.0 Hz, POCC); 31P NMR (243 MHz, CDCl3): δ = 30.77 ppm. Anal. Calcd. for C15H24N5O5P: C, 46.75; H, 6.28; N, 18.17. Found: C, 46.84; H, 6.36; N, 18.00.
4.1.5.28. Diethyl 4-(4-{[N4-acetylamino-2-oxopyrimidin-1-yl]methyl}-1H-1,2,3-triazol-1-yl)butylphosphonate 23d
From azide 15 (0.060 g, 0.259 mmol) and N 4-acetyl-N 1-propargylcytosine 19d (0.050 g, 0.259 mmol) the phosphonate 23d (0.078 g, 72%) was obtained as a white powder after purification on silica gel with chloroform–methanol (20:1, v/v); m.p.: 159–161 °C; IR (KBr): ν = 3217, 3133, 3084, 2982, 1707, 1650, 1217, 1025 cm−1; 1H NMR (600 MHz, CDCl3): δ = 8.83 (brs, 1H, NH); 7.96 (d, J = 7.3 Hz, 1H, HC CH); 7.84 (s, 1H, HC5′); 7.40 (d, J = 7.3 Hz, 1H, HC CH); 5.16 (s, 2H, CH2); 4.37 (t, J = 7.1 Hz, 2H, PCCCCH 2); 4.16–4.04 (m, 4H, 2× POCH 2CH3); 2.25 (s, 3H, CH3); 2.05 (qv, J = 7.1 Hz, 2H, PCCCH 2); 1.79 (dt, J = 15.5 Hz, J = 7.1 Hz, 2H, PCH 2); 1.71–1.63 (m, 2H, PCCH 2); 1.33 (t, J = 7.0 Hz, 6H, 2× POCH2CH 3); 13C NMR (151 MHz, CD3OD): δ = 163.1 (s, C O); 157.0 (s, C O); 149.4; 142.1; 124.1; 96.9; 61.8 (d, J = 6.6 Hz, POC); 49.3; 44.9; 30.2 (d, J = 16.2 Hz, PCCC); 23.7 (d, J = 140.4 Hz, PC); 23.2; 19.1 (d, J = 5.2 Hz, POCC); 15.4; 31P NMR (243 MHz, CDCl3): δ = 30.90 ppm. Anal. Calcd. for C17H27N6O5P: C, 47.88; H, 6.38; N, 19.71. Found: C, 47.63; H, 6.41; N, 19.52.
4.1.5.29. Diethyl 4-{4-[(3,5-dioxo-1,2,4-triazin-2-yl)methyl]-1H-1,2,3-triazol-1-yl}butylphosphonate 23f
From azide 15 (0.151 g, 0.642 mmol) and N 1-propargyl-6-azauracil 19f (0.097 g, 0.642 mmol) the phosphonate 23f (0.240 g, 97%) was obtained as a colourless oil after chromatography on a silica gel column with chloroform–methanol (50:1, v/v); IR (film): ν = 3439, 3231, 3141, 3012, 2909, 1730, 1676, 1216, 1027, 754 cm−1; 1H NMR (300 MHz, CDCl3): δ = 11.58 (brs, 1H, NH); 7.70 (s, 1H, HC5′); 7.39 (s, 1H, HC N); 5.20 (s, 2H, CH2); 4.33 (t, J = 7.2 Hz, 2H, PCCCCH 2); 4.15–4.03 (m, 4H, 2× POCH 2CH3); 2.01 (qu, J = 7.1 Hz, 2H, PCCCH 2); 1.83–1.56 (m, 4H, PCH 2CH 2); 1.31 (t, J = 7.0 Hz, 6H, 2× POCH2CH 3); 13C NMR (75.5 MHz, CDCl3): δ = 155.9 (s, C O); 148.9 (s, C O); 141.5; 134.6; 123.8; 61.8 (d, J = 6.6 Hz, POC); 49.7; 34.5; 30.6 (d, J = 15.5 Hz, PCCC); 24.7 (d, J = 141.4 Hz, PC); 19.5 (d, J = 4.9 Hz, PCC); 16.4 (d, J = 6.1 Hz, POCC); 31P NMR (121.5 MHz, CDCl3): δ = 32.44 ppm. Anal. Calcd. for C14H23N6O5P: C, 43.52; H, 6.00; N, 21.75. Found: C, 43.65; H, 5.87; N, 21.69.
4.1.5.30. Diethyl 4-{4-[(8-chloro-1,3-dimethyl-2,6-dioxopurin-7-yl)methyl]-1H-1,2,3-triazol-1-yl}butylphosphonate 23g
From azide 15 (0.129 g, 0.473 mmol) and 8-chloro-N 7-propargyltheophylline 19g (0.119 g, 0.473 mmol) the phosphonate 23g (0.242 g, 98%) was obtained as a white solid after chromatography on a silica gel column with chloroform–methanol (20:1, v/v); m.p.: 69–70 °C; IR (KBr): ν = 3013, 2988, 2962, 1707, 1668, 1225, 1015 cm−1; 1H NMR (600 MHz, CDCl3): δ = 7.79 (s, 1H, HC5′); 5.62 (s, 2H, CH2); 4.35 (t, J = 7.3 Hz, 2H, PCCCCH 2); 4.14–4.04 (m, 4H, 2× POCH 2CH3); 3.55 (s, 3H, CH3); 3.42 (s, 3H, CH3); 2.04 (qu, J = 7.3 Hz, 2H, PCCCH 2); 1.77 (dt, J = 14.8 Hz, J = 7.3 Hz, 2H, PCH 2); 1.69–1.61 (m, 2H, PCCH 2); 1.32 (t, J = 7.0 Hz, 6H, 2× POCH2CH 3); 13C NMR (151 MHz, CDCl3): δ = 154.5 (s, C O); 151.2 (s, C O); 147.4; 141.7; 139.1; 123.5; 107.3; 61.6 (d, J = 6.5 Hz, POC); 49.8; 41.0; 30.6 (d, J = 15.1 Hz, PCCC); 29.8; 28.0; 24.9 (d, J = 142.1 Hz, PC); 19.6 (d, J = 4.8 Hz, PCC); 16.4 (d, J = 5.9 Hz, POCC); 31P NMR (243 MHz, CDCl3): δ = 30.49 ppm. Anal. Calcd. for C18H27ClN7O5P: C, 44.31; H, 5.58; N, 20.10. Found: C, 44.56; H, 5.44; N, 20.35.
4.1.5.31. Diethyl 4-{4-[(3,7-dimethyl-2,6-dioxopurin-1-yl)methyl]-1H-1,2,3-triazol-1-yl}butylphosphonate 23h
From azide 15 (0.135 g, 0.573 mmol) and N 1-propargyltheobromine 19h (0.125 g, 0.573 mmol) the phosphonate 23h (0.224 g, 88%) was obtained as a white solid after purification on silica gel with chloroform–methanol (20:1, v/v); m.p.: 59–61 °C; IR (KBr): ν = 3432, 3115, 2983, 2952, 1708, 1662, 1235, 1025 cm−1; 1H NMR (600 MHz, CDCl3): δ = 7.62 (s, 1H); 7.51 (s, 1H); 5.33 (s, 2H, CH2); 4.33 (t, J = 7.3 Hz, 2H, PCCCCH 2); 4.13–4.05 (m, 4H, 2× POCH 2CH3); 4.01 (s, 3H, CH3); 3.59 (s, 3H, CH3); 2.02 (qu, J = 7.3 Hz, 2H, PCCCH 2); 1.73 (dt, J = 18.0 Hz, J = 7.3 Hz, 2H, PCH 2); 1.63 (dqu, J = 14.0 Hz, J = 7.3 Hz, 2H, PCCH 2); 1.32 (t, J = 7.0 Hz, 6H, 2× POCH2CH 3); 13C NMR (151 MHz, CDCl3): δ = 154.8 (s, C O); 151.3 (s, C O); 148.9; 143.6; 141.7; 123.1; 107.6; 61.6 (d, J = 6.5 Hz, POC); 49.6; 36.0; 33.5; 30.7 (d, J = 15.3 Hz, PCCC); 29.7; 25.0 (d, J = 144.9 Hz, PC); 19.6 (d, J = 4.7 Hz, PCC); 16.4 (d, J = 6.1 Hz, POCC); 31P NMR (243 MHz, CDCl3): δ = 30.91 ppm. Anal. Calcd. for C18H28N7O5P: C, 47.68; H, 6.22; N, 21.62. Found: C, 47.74; H, 6.03; N, 21.71.
4.1.5.32. Diethyl 4-{4-[(1,3-dimethyl-2,6-dioxopurin-7-yl)methyl]-1H-1,2,3-triazol-1-yl}butylphosphonate 23i
From azide 15 (0.146 g, 0.535 mmol) and N 7-propargyltheophylline 19i (0.117 g, 0.535 mmol) the phosphonate 23i (0.189 g, 72%) was obtained as a white solid after chromatography on a silica gel column with chloroform–methanol (20:1, v/v); m.p.: 58–59 °C; IR (KBr): ν = 3432, 3115, 2983, 2952, 1708, 1662, 1235, 1025 cm−1; 1H NMR (600 MHz, CDCl3): δ = 7.84 (s, 1H); 7.83 (s, 1H); 5.60 (s, 2H, CH2); 4.37 (t, J = 7.1 Hz, 2H, PCCCCH 2); 4.15–4.05 (m, 4H, 2× POCH 2CH3); 3.60 (s, 3H, CH3); 3.44 (s, 3H, CH3); 2.06 (qu, J = 7.1 Hz, 2H, PCCCH 2); 1.80–1.74 (m, 2H, PCH 2); 1.69–1.62 (m, 2H, PCCH 2); 1.33 (t, J = 7.4 Hz, 6H, 2× POCH2CH 3); 13C NMR (151 MHz, CDCl3): δ = 155.4 (s, C O); 151.6 (s, C O); 149.0; 142.2; 141.4; 123.5; 106.5; 61.6 (d, J = 6.4 Hz, POC); 49.9; 41.5; 30.6 (d, J = 14.7 Hz, PCCC); 29.8; 25.0 (d, J = 142.2 Hz, PC); 19.6 (d, J = 4.5 Hz, PCC); 16.4 (d, J = 6.4 Hz, POCC); 31P NMR (243 MHz, CDCl3): δ = 30.79 ppm. Anal. Calcd. for C18H28N7O5P: C, 47.68; H, 6.22; N, 21.62. Found: C, 47.80; H, 6.00; N, 21.74.
4.1.5.33. Diethyl 4-{4-[(5,6-dimethylbenzimidazol-1-yl)methyl]-1H-1,2,3-triazol-1-yl}butylphosphonate 23j
From azide 15 (0.150 g, 0.549 mmol) and 5,6-dimethyl-N 1-propargylbenzimidazole 19j (0.100 g, 0.549 mmol) the phosphonate 23j (0.122 g, 74%) was obtained as a yellow oil after purification on silica gel with chloroform–methanol (50:1, v/v); IR (film): ν = 3303, 3102, 2982, 1219, 1027 cm−1; 1H NMR (600 MHz, CDCl3): δ = 8.30 (s, 1H); 7.60 (s, 1H); 7.30 (s, 1H); 7.32 (s, 1H); 5.48 (s, 2H, CH2); 4.38 (t, J = 7.4 Hz, 2H, PCCCCH 2); 4.16–4.04 (m, 4H, 2× POCH 2CH3); 2.40 (s, 3H, CH3); 2.39 (s, 3H, CH3); 1.99 (qu, J = 7.4 Hz, 2H, PCCCH 2); 1.75 (dt, J = 18.2 Hz, J = 7.4 Hz, 2H, PCH 2); 1.63 (dq, J = 15.4 Hz, J = 7.4 Hz, 2H, PCCH 2); 1.31 (t, J = 7.1 Hz, 6H, 2× POCH2CH 3);13C NMR (151 MHz, CDCl3): δ = 143.0; 132.7; 130.8; 122.1; 120.3; 61.6 (d, J = 6.6 Hz, POC); 49.8; 30.5 (d, J = 15.1 Hz, PCCC); 24.9 (d, J = 142.1 Hz, PC); 20.4; 20.2; 19.5 (d, J = 4.7 Hz, PCC); 16.4 (d, J = 5.8 Hz, POCC); 31P NMR (243 MHz, CDCl3): δ = 30.84 ppm. Anal. Calcd. for C20H30N5O3P: C, 57.27; H, 7.21; N, 16.70. Found: C, 57.10; H, 7.08; N, 16.79.
4.1.5.34. Diethyl 4-{4-[(3-acetylindol-1-yl)methyl]-1H-1,2,3-triazol-1-yl}butylphosphonate 23k
From azide 15 (0.114 g, 0.485 mmol) and 3-acetyl-N-propargylindole 19k (0.096 g, 0.485 mmol) the phosphonate 23k (0.187 g, 89%) was obtained as a colourless oil after chromatography on a silica gel column with chloroform–methanol (20:1, v/v); IR (film): ν = 3283, 3110, 2983, 2872, 1797, 1231, 1045, 750 cm−1; 1H NMR (300 MHz, CDCl3): δ = 8.45–8.38 (m, 1H); 7.76 (s, 1H, HC5′); 7.43–7.39 (m, 1H); 7.36–7.27 (m, 3H); 5.42 (s, 2H, CH2); 4.37 (t, J = 7.0 Hz, 2H, PCCCCH 2); 4.10–4.00 (m, 4H, 2× POCH 2CH3); 2.53 (s, 3H, CH3); 1.98 (qu, J = 7.0 Hz, 2H, PCCCH 2); 1.85–1.50 (m, 4H, PCH 2CH 2); 1.28 (t, J = 7.0 Hz, 6H, 2× POCH2CH 3); 13C NMR (75.5 MHz, CDCl3): δ = 193.0 (s, C O); 143.0; 136.5; 134.8; 123.5; 122.7; 122.7; 122.1; 117.5; 109.9; 61.7 (d, J = 6.4 Hz, POC); 50.0; 42.5; 30.8 (d, J = 15.2 Hz, PCCC); 27.8; 24.9 (d, J = 141.7 Hz, PC); 19.7 (d, J = 4.9 Hz, PCC); 16.6 (d, J = 6.0 Hz, POCC); 31P NMR (121.5 MHz, CDCl3): δ = 31.92 ppm. Anal. Calcd. for C21H29N4O4P: C, 58.32; H, 6.76; N, 12.96. Found: C, 58.48; H, 6.81; N, 13.10.
4.1.5.35. Diethyl 4-{4-[(2-oxopyridin-1-yl)methyl]-1H-1,2,3-triazol-1-yl}butylphosphonate 23l
From azide 15 (0.123 g, 0.523 mmol) and N-propargyl-2-pyridon 19l (0.070 g, 0.523 mmol) the phosphonate 23l (0.182 g, 94%) was obtained as a brown oil after purification on silica gel with chloroform–methanol (20:1, v/v); IR (film): ν = 3134, 2996, 2935, 1659, 1222; 1020, 968 cm−1; 1H NMR (300 MHz, CDCl3): δ = 7.78 (s, 1H, HC5′); 7.60 (dd, J = 6.7 Hz, J = 2.2 Hz, 1H); 7.38 (ddd, J = 9.1 Hz, J = 6.7 Hz, J = 2.2 Hz, 1H); 6.54 (d, J = 9.1 Hz, 1H); 6.19 (dt, J = 6.7 Hz, J = 1.5 Hz, 1H); 5.18 (s, 2H, CH2); 4.33 (t, J = 7.2 Hz, 2H, PCCCCH 2); 4.17–4.00 (m, 4H, 2× POCH 2CH3); 2.22–1.96 (m, 2H, PCCCH 2); 1.82–1.60 (m, 4H, PCH 2CH 2); 1.31 (t, J = 6.9 Hz, 6H, 2× POCH2CH 3); 13C NMR (75.5 MHz, CDCl3): δ = 162.3 (s, C O); 142.7 (s, HC C); 140.0; 137.8; 123.9 (s, HC C); 120.8; 106.6; 61.7 (d, J = 6.5 Hz, POC); 49.9; 44.7; 30.8 (d, J = 15.5 Hz, PCCC); 25.1 (d, J = 141.7 Hz, PC); 19.8 (d, J = 5.2 Hz, PCC); 16.6 (d, J = 6.0 Hz, POCC); 12.2 (s, CH3); 31P NMR (121.5 MHz, CDCl3): δ = 32.08 ppm. Anal. Calcd. for C16H25N4O4P: C, 52.17; H, 6.84; N, 15.21. Found: C, 51.90; H, 6.78; N, 15.11.
4.1.5.36. Diethyl 2-(4-{[3-benzoyl-2,4-dioxopyrimidin-1-yl]methyl}-1H-1,2,3-triazol-1-yl)-1-hydroxyethylphosphonate 24e
From azide 16 (0.146 g, 0.654 mmol) and N 3-benzoyl-N 1-propargylquinazoline-2,4-dione 19e (0.199 g, 0.654 mmol) the phosphonate 24e (0.340 g, 98%) was obtained as a colourless oil after purification on silica gel with chloroform–methanol (50:1, v/v); IR (film): ν = 3356, 2982, 2831, 1750, 1702, 1668, 1234, 1027, 785, 688 cm−1; 1H NMR (600 MHz, CDCl3): δ = 8.16 (dd, J = 7.9 Hz, J = 1.4 Hz, 1H); 7.97–7.94 (m, 2H, 2× o-CH); 7.92 (s, 1H, HC5′); 7.88 (brd, J = 8.3 Hz, 1H); 7.75 (ddd, J = 8.3 Hz, J = 7.9 Hz, J = 1.4 Hz, 1H); 7.68–7.62 (m, 1H, p-CH); 7.52–7.46 (m, 2H, 2× m-CH); 7.31 (dt, J = 7.9 Hz, J = 0.6 Hz, 1H); 5.44 (AB, J = 15.8 Hz, 1H, CH aHb); 5.42 (AB, J = 15.8 Hz, 1H, CHa H b); 4.77 (ddd, J = 14.3 Hz, J = 5.3 Hz, J = 2.8 Hz, 1H, PCCH aHb); 4.48 (ddd, J = 14.3 Hz, J = 10.0 Hz, J = 5.8 Hz, 1H, PCCHa H b); 4.23 (ddd, J = 10.3 Hz, J = 7.9 Hz, J = 2.8 Hz, 1H, PCH(OH)); 4.16–4.04 (m, 4H, 2× POCH 2CH3); 1.27 (t, J = 7.0 Hz, 3H, POCH2CH 3); 1.26 (t, J = 7.0 Hz, 3H, POCH2CH 3); 13C NMR (75.5 MHz, CDCl3): δ = 168.6 (s, C O); 161.0 (s, C O); 149.4 (s, C O); 142.0 (s, HC C); 140.2; 136.2; 135.2; 131.6; 130.5; 129.3; 128.8; 125.6 (s, HC C); 123.8; 115.5; 115.4; 67.0 (d, J = 163.2 Hz, PC); 63.8 (d, J = 7.5 Hz, POC); 63.6 (d, J = 7.5 Hz, POC); 51.6 (d, J = 10.0 Hz, PCC); 39.0; 16.6 (d, J = 5.3 Hz, POCC); 16.5 (d, J = 5.3 Hz, POCC); 31P NMR (121.5 MHz, CDCl3): δ = 21.21 ppm. Anal. Calcd. for C24H26N5O7P: C, 54.65; H, 4.97; N, 13.28. Found: C, 54.47; H, 5.11; N, 13.12.
4.1.5.37. Diethyl 2-{4-[(3,5-dioxo-1,2,4-triazin-2-yl)methyl]-1H-1,2,3-triazol-1-yl}-1-hydroxyethylphosphonate 24f
From azide 16 (0.138 g, 0.618 mmol) and N 1-propargylazauracil 19f (0.093 g, 0.618 mmol) the phosphonate 24f (0.161 g, 70%) was obtained as a white solid after chromatography on a silica gel column with chloroform–methanol (50:1, v/v); m.p.: 145–147 °C; IR (KBr): ν = 3300, 2913, 2837, 1729, 1674, 1023 cm−1; 1H NMR (300 MHz, CDCl3): δ = 12.07 (s, 1H, NH); 7.92 (s, 1H); 7.41 (s, 1H); 5.30 (brs, 1H, OH); 5.15 (s, 2H, CH2); 4.82 (ddd, J = 14.0 Hz, J = 4.6 Hz, J = 2.2 Hz, 1H, PCCH aHb); 4.52–4.34 (m, 2H, PCH(OH), PCCHa H b); 4.15–4.02 (m, 4H, 2× POCH 2CH3); 1.36 (t, J = 6.9 Hz, 3H, POCH2CH 3); 1.34 (t, J = 6.9 Hz, 3H, POCH2CH 3); 13C NMR (75.5 MHz, CDCl3): δ = 155.9 (s, C O); 149.5 (C O); 141.2 (s, HC C); 135.1 (s, HC N); 125.7 (s, HC C); 66.8 (d, J = 144.0 Hz, PC); 63.8 (d, J = 6.6 Hz, POC); 63.7 (d, J = 6.6 Hz, POC); 51.7 (d, J = 10.6 Hz); 34.7; 16.6 (d, J = 5.5 Hz, POCC); 31P NMR (121.5 MHz, CDCl3): δ = 21.60 ppm. Anal. Calcd. for C12H19N6O6P: C, 38.51; H, 5.12; N, 22.45. Found: C, 38.27; H, 5.02; N, 22.55.
4.1.5.38. Diethyl 2-{4-[(8-chloro-1,3-dimethyl-2,6-dioxopurin-7-yl)methyl]-1H-1,2,3-triazol-1-yl}-1-hydroxyethylphosphonate 24g
From azide 16 (0.100 g, 0.448 mmol) and 8-chloro-N 7-propargyltheophylline 19g (0.113 g, 0.448 mmol) the phosphonate 24g (0.185 g, 87%) was obtained as a white solid after chromatography on a silica gel column with chloroform–methanol (50:1, v/v); m.p.: 183–184 °C; IR (KBr): ν = 3281, 3057, 2986, 1707, 1665, 1216, 1047 cm−1; 1H NMR (300 MHz, CDCl3): δ = 7.95 (s, 1H, HC5′); 5.61 (s, 2H, CH2); 5.08 (t, J = 5.7 Hz, 1H, OH); 4.75 (ddd, J = 14.2 Hz, J = 5.1 Hz, J = 2.6 Hz, 1H, PCCH aHb); 4.44 (ddd, J = 14.2 Hz, J = 10.0 Hz, J = 5.6 Hz, 1H, PCCHa H b); 4.28 (dddd, J = 10.0 Hz, J = 8.0 Hz, J = 5.7 Hz, J = 5.1 Hz, 1H, PCH(OH)); 4.21–4.10 (m, 4H, 2× POCH 2CH3); 3.51 (s, 3H, CH3); 3.37 (s, 3H, CH3); 1.33 (t, J = 7.0 Hz, 3H, POCH2CH 3); 1.32 (t, J = 7.0 Hz, 3H, POCH2CH 3); 13C NMR (75.5 MHz, CDCl3): δ = 154.4 (s, C O); 151.1 (s, C O); 147.3; 139.0; 125.2; 107.4; 67.0 (d, J = 163.8 Hz, PC); 63.7 (d, J = 7.2 Hz, POC); 63.5 (d, J = 7.2 Hz, POC); 51.7 (d, J = 9.7 Hz, PCC); 41.1; 30.0; 28.2; 16.6 (d, J = 5.5 Hz, POCC); 31P NMR (121.5 MHz, CDCl3): δ = 20.42 ppm. Anal. Calcd. for C16H23ClN7O6P: C, 40.39; H, 4.87; N, 20.61. Found: C, 40.55; H, 4.87; N, 20.47.
4.1.5.39. Diethyl 2-{4-[(3,7-dimethyl-2,6-dioxopurin-1-yl)methyl]-1H-1,2,3-triazol-1-yl}-1-hydroxyethylphosphonate 24h
From azide 16 (0.100 g, 0.448 mmol) and N 1-propargyltheobromine 19h (0.098 g, 0.448 mmol) the phosphonate 24h (0.190 g, 96%) was obtained as a white powder after purification on silica gel with chloroform–methanol (50:1, v/v); m.p.: 166–168 °C; IR (KBr): ν = 3237, 2989, 1708, 1663, 1235, 1023 cm−1; 1H NMR (600 MHz, CDCl3): δ = 7.80 (s, 1H); 7.53 (s, 1H, HC5′); 5.35 (AB, J = 14.6 Hz, 1H, CH aHb); 5.30 (AB, J = 14.6 Hz, 1H, CHa H b); 4.79 (ddd, J = 14.2 Hz, J = 6.0 Hz, J = 2.5 Hz, 1H, PCCH aHb); 4.47 (ddd, J = 14.2 Hz, J = 9.7 Hz, J = 5.2 Hz, 1H, PCCHa H b); 4.40–4.34 (m, 1H, PCH(OH)); 4.27–4.15 (m, 4H, 2× POCH 2CH3); 4.06 (dd, J = 9.4 Hz, J = 5.8 Hz, 1H); 4.01 (s, 3H, CH3); 3.60 (s, 3H, CH3); 1.38 (t, J = 7.0 Hz, 3H, POCH2CH 3); 1.36 (t, J = 7.0 Hz, 3H, POCH2CH 3); 13C NMR (151.5 MHz, CDCl3): δ = 154.7 (s, C O); 151.2 (s, C O); 148.8; 143.2; 141.9; 124.9; 107.6; 67.0 (d, J = 165.1 Hz, PC); 64.4 (d, J = 6.9 Hz, POC); 63.2 (d, J = 6.9 Hz, POC); 51.7 (d, J = 9.6 Hz, PCC); 36.0; 33.6; 29.7; 16.4 (d, J = 5.3 Hz, POCC); 31P NMR (243 MHz, CDCl3): δ = 19.86 ppm. Anal. Calcd. for C16H24N7O6P: C, 43.54; H, 5.48; N, 22.21. Found: C, 43.67; H, 5.28; N, 22.30.
4.1.5.40. Diethyl 2-{4-[(1,3-dimethyl-2,6-dioxopurin-7-yl)methyl]-1H-1,2,3-triazol-1-yl}-1-hydroxyethylphosphonate 24i
From azide 16 (0.100 g, 0.448 mmol) and N 7-propargyltheophylline 19i (0.098 g, 0.448 mmol) the phosphonate 24i (0.145 g, 73%) was obtained as a white powder after chromatography on a silica gel column with chloroform–methanol (50:1, v/v); m.p.: 164–165 °C; IR (KBr): ν = 3264, 3152, 2990, 1705, 1660, 1224, 1025 cm−1; 1H NMR (600 MHz, CDCl3): δ = 8.00 (s, 1H); 7.84 (s, 1H, HC5′); 5.62 (AB, J = 15.0 Hz, 1H, CH aHb); 5.58 (AB, J = 15.0 Hz, 1H, CHa H b); 4.80 (ddd, J = 14.3 Hz, J = 5.2 Hz, J = 2.7 Hz, 1H, PCCH aHb); 4.78 (dd, J = 13.3 Hz, J = 5.9 Hz, 1H); 4.49 (ddd, J = 14.3 Hz, J = 10.0 Hz, J = 5.6 Hz, 1H, PCCHa H b); 4.36–4.28 (m, 1H, PCH(OH)); 4.27–4.16 (m, 4H, 2× POCH 2CH3); 3.57 (s, 3H, CH3); 3.41 (s, 3H, CH3); 1.37 (t, J = 7.1 Hz, 3H, POCH2CH 3); 1.36 (t, J = 7.1 Hz, 3H, POCH2CH 3); 13C NMR (151 MHz, CDCl3): δ = 155.4 (s, C O); 151.6 (s, C O); 148.9; 141.8; 141.5; 125.4; 106.5; 67.0 (d, J = 164.6 Hz, PC); 63.6 (d, J = 7.4 Hz, POC); 63.4 (d, J = 7.4 Hz, POC); 51.7 (d, J = 9.6 Hz, PCC); 41.4; 29.8; 27.9; 16.4 (d, J = 5.9 Hz, POCC); 31P NMR (243 MHz, CDCl3): δ = 19.90 ppm. Anal. Calcd. for C16H24N7O6P: C, 43.54; H, 5.48; N, 22.21. Found: C, 43.38; H, 5.55; N, 22.30.
4.1.5.41. Diethyl 2-{4-[(5,6-dimethylbenzoimidazol-1-yl)methyl]-1H-1,2,3-triazol-1-yl}-1-hydroxyethylphosphonate 24j
From azide 16 (0.100 g, 0.448 mmol) and 5,6-dimethyl-N-propargylbenzimidazole 19j (0.083 g, 0.448 mmol) the phosphonate 24j (0.118 g, 65%) was obtained as a yellow oil after purification on silica gel with chloroform–methanol (50:1, v/v); IR (film): ν = 3131, 2990, 2945, 1217, 1048, 757 cm−1; 1H NMR (600 MHz, CDCl3): δ = 7.91 (s, 1H); 7.76 (s, 1H); 7.43 (s, 1H); 7.23 (s, 1H); 5.38 (AB, J = 15.7 Hz, 1H, CH aHb); 5.34 (AB, J = 15.7 Hz, 1H, CHa H b); 4.82 (ddd, J = 14.2 Hz, J = 4.9 Hz, J = 2.5 Hz, 1H, PCCH aHb); 4.46 (ddd, J = 14.2 Hz, J = 9.9 Hz, J = 5.3 Hz, 1H, PCCHa H b); 4.31 (dt, J = 9.9 Hz, J = 5.2 Hz, 1H, PCH(OH)); 4.12–4.06 (m, 4H, 2× POCH 2CH3); 2.34 (s, 3H, CH3); 2.33 (s, 3H, CH3); 1.32 (t, J = 7.0 Hz, 3H, POCH2CH 3); 1.30 (t, J = 7.0 Hz, 3H, POCH2CH 3); 13C NMR (151 MHz, CDCl3): δ = 142.1; 141.5; 141.2; 132.7; 131.7; 131.6; 124.5; 119.6; 110.1; 66.6 (d, J = 166.1 Hz, PC); 63.4 (d, J = 7.1 Hz, POC); 63.2 (d, J = 7.1 Hz, POC); 51.7 (d, J = 9.6 Hz, PCC); 40.2; 20.7; 20.4; 16.7 (d, J = 5.4 Hz, POCC); 31P NMR (243 MHz, CDCl3): δ = 21.28 ppm. Anal. Calcd. for C18H26N5O4P: C, 53.07; H, 6.43; N, 17.19. Found: C, 52.88; H, 6.17; N, 17.05.
4.1.5.42. Diethyl 2-{4-[(3-acetylindol-1-yl)methyl]-1H-1,2,3-triazol-1-yl}-1-hydroxyethylphosphonate 24k
From azide 16 (0.142 g, 0.636 mmol) and 3-acetyl-N-propargylindole 19k (0.125 g, 0.636 mmol) the phosphonate 24k (0.196 g, 73%) was obtained as a colourless oil after purification on silica gel with chloroform–methanol (50:1, v/v); IR (film): ν = 3266, 2959, 2911, 1642, 1528, 1390, 1217, 1024, 754 cm−1; 1H NMR (300 MHz, CDCl3): δ = 8.36–8.26 (m, 1H); 7.84 (s, 1H, HC5′); 7.62 (s, 1H); 7.43–7.35 (m, 1H); 7.30–7.23 (m, 2H); 5.46 (AB, J = 15.4 Hz, 1H, CH aHb); 5.44 (AB, J = 15.4 Hz, 1H, CHa H b); 4.77 (ddd, J = 14.3 Hz, J = 6.0 Hz, J = 2.6 Hz, 1H, PCCH aHb); 4.44 (ddd, J = 14.3 Hz, J = 10.0 Hz, J = 5.6 Hz, 1H, PCCHa H b); 4.21 (ddd, J = 10.0 Hz, J = 7.9 Hz, J = 2.6 Hz, 1H, PCH(OH)); 4.14–4.06 (m, 4H, 2× POCH 2CH3); 3.85 (brs, 1H, OH); 2.51 (s, 3H, CH3); 1.29 (t, J = 6.8 Hz, 6H, 2× POCH2CH 3); 13C NMR (75.5 MHz, CDCl3): δ = 194.0 (s, C O); 142.6; 136.6; 135.0; 126.4; 124.2; 123.6; 122.8; 122.7; 117.5; 110.0; 66.2 (d, J = 159.3 Hz, PC); 63.4 (d, J = 7.0 Hz, POC); 63.3 (d, J = 7.0 Hz, POC); 51.9 (d, J = 9.7 Hz, PCC); 42.4; 27.7 (s, CH3); 16.6 (d, J = 5.4 Hz, POCC); 31P NMR (121.5 MHz, CDCl3): δ = 21.03 ppm. Anal. Calcd. for C19H25N4O5P: C, 54.28; H, 5.99; N, 13.33. Found: C, 54.10; H, 6.12; N, 13.20.
4.1.5.43. Diethyl 1-hydroxy-2-{4-[(2-oxopyridin-1-yl)methyl]-1H-1,2,3-triazol-1-yl}ethylphosphonate 24l
From azide 16 (0.134 g, 0.600 mmol) and N-propargy-2-pyridon 19l (0.080 g, 0.600 mmol) the phosphonate 24l (0.172 g, 80%) was obtained as a brown oil after chromatography on a silica gel column with chloroform–methanol (50:1, v/v); IR (film): ν = 3274, 2984, 2831, 1673, 1027 cm−1; 1H NMR (300 MHz, CDCl3): δ = 8.03 (s, 1H); 7.64 (ddd, J = 6.7 Hz, J = 2.0 Hz, J = 0.6 Hz, 1H); 7.36 (ddd, J = 9.2 Hz, J = 6.7 Hz, J = 2.0 Hz, 1H); 6.52 (dd, J = 9.2 Hz, J = 0.6 Hz, 1H); 6.22 (dt, J = 6.7 Hz, J = 1.3 Hz, 1H); 5.20 (AB, J = 14.3 Hz, 1H, CH aHb); 5.12 (AB, J = 14.3 Hz, 1H, CHa H b); 4.79 (ddd, J = 14.2 Hz, J = 5.0 Hz, J = 2.6 Hz, 1H, PCCH aHb); 4.51 (ddd, J = 14.2 Hz, J = 10.0 Hz, J = 5.0 Hz, 1H, PCCHa H b); 4.36 (ddd, J = 10.0 Hz, J = 8.9 Hz, J = 2.6 Hz, 1H, PCH(OH)); 4.26–4.14 (m, 4H, 2× POCH 2CH3); 2.56 (brs, 1H, OH); 1.35 (t, J = 7.0 Hz, 3H, POCH2CH 3); 1.33 (t, J = 6.9 Hz, 3H, POCH2CH 3); 13C NMR (75.5 MHz, CDCl3): δ = 162.3 (s, C O); 141.9 (s, HC C); 140.2; 137.8; 125.6 (s, HC C); 120.2; 106.9; 66.7 (d, J = 164.9 Hz, PC); 63.3 (d, J = 7.1 Hz, POC); 63.2 (d, J = 7.1 Hz, POC); 51.8 (d, J = 10.4 Hz, PCC); 44.5; 16.5 (d, J = 5.2 Hz, POCC); 31P NMR (121.5 MHz, CDCl3): δ = 21.29 ppm. Anal. Calcd. for C14H21N4O5P: C, 47.19; H, 5.94; N, 15.72. Found: C, 47.01; H, 6.10; N, 15.80.
4.1.5.44. Diethyl 3-(4-{[3-benzoyl-2,4-dioxopyrimidin-1-yl]methyl}-1H-1,2,3-triazol-1-yl)-2-hydroxyethylphosphonate 25e
From azide 17 (0.115 g, 0.485 mmol) and N 3-benzoyl-N 1-propargylquinazoline-2,4-dione 19e (0.148 g, 0.485 mmol) the phosphonate 25e (0.235 g, 89%) was obtained as a white solid after purification on silica gel with chloroform–methanol (50:1, v/v); m.p.: 75–77 °C; IR (KBr): ν = 3386, 3054, 2988, 2851, 1754, 1709, 1658, 1224, 1025, 795, 694 cm−1; 1H NMR (300 MHz, CDCl3): δ = 8.17 (dd, J = 7.9 Hz, J = 1.5 Hz, 1H); 7.97–7.93 (m, 2H, 2× o-CH); 7.87 (brd, J = 8.4 Hz, 1H); 7.85 (s, 1H, HC5′); 7.75 (ddd, J = 8.4 Hz, J = 7.9 Hz, J = 1.5 Hz, 1H); 7.67–7.61 (m, 1H, p-CH); 7.51–7.45 (m, 2H, 2× m-CH); 7.29 (dt, J = 7.9 Hz, J = 0.5 Hz, 1H); 5.44 (AB, J = 14.2 Hz, 1H, CH aHb); 5.36 (AB, J = 14.2 Hz, 1H, CHa H b); 4.45 (dd, J = 15.4 Hz, J = 6.5 Hz, 1H, PCCCH aHb); 4.44–4.32 (m, 2H, PCCHCHa H b); 4.14–4.01 (m, 4H, 2× POCH 2CH3); 3.40 (brs, 1H, OH); 1.96 (ddd, J = 19.2 Hz, J = 15.1 Hz, J = 3.3 Hz, 1H, PCH aHb); 1.73 (ddd, J = 16.4 Hz, J = 15.1 Hz, J = 9.1 Hz, 1H, PCHa H b); 1.30 (t, J = 7.0 Hz, 3H, POCH2CH 3); 1.27 (t, J = 7.0 Hz, 3H, POCH2CH 3); 13C NMR (75.5 MHz, CDCl3): δ = 168.6 (s, C O); 161.0 (s, C O); 149.5 (s, C O); 142.3 (s, HC C); 140.3; 136.3; 135.2; 131.6; 130.6; 129.3; 128.9; 125.4 (s, HC C); 123.8; 115.6; 115.4; 65.5 (d, J = 4.0 Hz, PCC); 62.5 (d, J = 5.8 Hz, POC); 62.4 (d, J = 5.8 Hz, POC); 52.4 (d, J = 14.1 Hz, PCCC); 39.0; 30.0 (d, J = 140.4 Hz, PC); 16.6 (d, J = 5.2 Hz, POCC); 31P NMR (121.5 MHz, CDCl3): δ = 29.27 ppm. Anal. Calcd. for C25H28N5O7P: C, 55.45; H, 5.21; N, 12.93. Found: C, 55.28; H, 5.15; N, 13.11.
4.1.5.45. Diethyl 3-{4-[(3,5-dioxo-1,2,4-triazin-2-yl)methyl]-1H-1,2,3-triazol-1-yl}-2-hydroxypropylphosphonate 25f
From azide 17 (0.151 g, 0.637 mmol) and N-propargyl-6-azauracil 19f (0.096 g, 0.637 mmol) the phosphonate 25f (0.188 g, 76%) was obtained as a colourless oil after chromatography on a silica gel column with chloroform–methanol (50:1, v/v); IR (film): ν = 3302, 2986, 2913, 2833, 1730, 1673, 1028, 970 cm−1; 1H NMR (600 MHz, CDCl3): δ = 11.80 (s, 1H, NH); 7.89 (s, 1H); 7.46 (s, 1H); 5.25 (AB, J = 15.6 Hz, 1H, CH aHb); 5.19 (AB, J = 15.6 Hz, 1H, CHa H b); 4.55 (dd, J = 13.7 Hz, J = 3.0 Hz, 1H, PCCCH aHb); 4.43 (ddddd, J = 8.6 Hz, J = 7.0 Hz, J = 3.9 Hz, J = 3.0 Hz, 1H, PCCH(OH)); 4.38 (dd, J = 13.7 Hz, J = 7.0 Hz, 1H, PCCCHa H b); 4.19–4.02 (m, 4H, 2× POCH 2CH3); 2.05 (ddd, 2H, J = 19.2 Hz, J = 15.2 Hz, J = 3.9 Hz, PCH aHb); 1.97 (ddd, 2H, J = 17.6 Hz, J = 15.2 Hz, J = 8.6 Hz, PCHa H b); 1.31 (t, J = 7.2 Hz, 3H, POCH2CH 3); 1.30 (t, J = 7.2 Hz, 3H, POCH2CH 3); 13C NMR (75.5 MHz, CDCl3): δ = 156.0 (s, C O); 149.4 (C O); 141.4 (s, HC C); 135.1 (s, HC N); 125.7 (s, HC C); 65.6 (s, PCC); 62.7 (d, J = 6.3 Hz, POC); 62.4 (d, J = 6.3 Hz, POC); 56.2 (d, J = 16.6 Hz, PCCC); 34.8; 31.0 (d, J = 140.9 Hz, PC); 16.6 (d, J = 6.0 Hz, POCC); 31P NMR (243 MHz, CDCl3): δ = 29.38 ppm. Anal. Calcd. for C13H21N6O6P: C, 40.21; H, 5.45; N, 21.64. Found: C, 40.08; H, 5.59; N, 21.72.
4.1.5.46. Diethyl 3-{4-[(8-chloro-1,3-dimethyl-2,6-dioxopurin-7-yl)methyl]-1H-1,2,3-triazol-1-yl}-2-hydroxypropylphosphonate 25g
From azide 17 (0.125 g, 0.527 mmol) and 8-chloro-N 7-propargyltheophylline 19g (0.133 g, 0.527 mmol) the phosphonate 25g (0.211 g, 82%) was obtained as a white solid after chromatography on a silica gel column with chloroform–methanol (50:1, v/v); m.p.: 137–139 °C; IR (KBr): ν = 3354, 3151, 2983, 2928, 1702, 1675, 1221, 1027 cm−1; 1H NMR (300 MHz, CDCl3): δ = 7.93 (s, 1H, HC5′); 5.63 (s, 2H, CH2); 4.53–4.33 (m, 3H, PCCHCH 2); 4.17–4.03 (m, 4H, 2× POCH 2CH3); 3.52 (s, 3H, CH3); 3.38 (s, 3H, CH3); 1.98 (ddd, J = 19.0 Hz, J = 15.3 Hz, J = 3.1 Hz, 1H, PCH aHb); 1.77 (ddd, J = 16.8 Hz, J = 15.3 Hz, J = 9.2 Hz, 1H, PCHa H b); 1.32 (t, J = 7.0 Hz, 3H, POCH2CH 3); 1.31 (t, J = 7.0 Hz, 3H, POCH2CH 3); 13C NMR (75.5 MHz, CDCl3): δ = 154.3 (s, C O); 151.1 (s, C O); 147.2; 141.4; 138.9; 125.2; 107.4; 65.4 (d, J = 3.7 Hz, PCC); 62.4 (d, J = 6.5 Hz, POC); 62.3 (d, J = 6.5 Hz, POC); 56.0 (d, J = 18.4 Hz, PCCC); 41.1; 30.8 (d, J = 135.9 Hz, PC); 30.0; 28.2; 16.6 (d, J = 5.7 Hz, POCC); 16.5 (d, J = 5.7 Hz, POCC); 31P NMR (121.5 MHz, CDCl3): δ = 28.72 ppm. Anal. Calcd. for C17H25ClN7O6P: C, 41.68; H, 5.14; N, 20.02. Found: C, 41.70; H, 4.97; N, 19.90.
4.1.5.47. Diethyl 3-{4-[(3,7-dimethyl-2,6-dioxopurin-1-yl)methyl]-1H-1,2,3-triazol-1-yl}-2-hydroxypropylphosphonate 25h
From azide 17 (0.108 g, 0.455 mmol) and N 1-propargyltheobromine 19h (0.099 g, 0.455 mmol) the phosphonate 25h (0.192 g, 93%) was obtained as a white powder after chromatography on a silica gel column with chloroform–methanol (50:1, v/v); m.p.: 148–150 °C; IR (KBr): ν = 3445, 2984, 2924, 1707, 1664, 1231, 1025 cm−1; 1H NMR (300 MHz, CDCl3): δ = 7.78 (s, 1H); 7.51 (d, J = 0.6 Hz, 1H, HC5′); 5.30 (s, 2H, CH2); 4.52–4.32 (m, 3H, PCCHCH 2); 4.17–4.03 (m, 4H, 2× POCH 2CH3); 3.98 (d, J = 0.6 Hz, 3H, CH3); 3.55 (s, 3H, CH3); 3.02 (brs, 1H, OH); 2.46–2.34 (m, 2H, PCH 2); 1.30 (t, J = 7.0 Hz, 3H, POCH2CH 3); 1.28 (t, J = 7.0 Hz, 3H, POCH2CH 3);13C NMR (75.5 MHz, CDCl3): δ = 154.8 (s, C O); 151.3 (s, C O); 148.9; 143.5; 141.7; 124.9; 107.7; 65.6 (d, J = 3.8 Hz, PCC); 62.5 (d, J = 6.4 Hz, POC); 62.4 (d, J = 6.4 Hz, POC); 55.8 (d, J = 15.3 Hz, PCCC); 36.2; 33.9; 32.0 (d, J = 292.9 Hz, PC); 31.7; 30.0; 16.6 (d, J = 5.6 Hz, POCC); 16.5 (d, J = 5.6 Hz, POCC); 31P NMR (121.5 MHz, CDCl3): δ = 29.53 ppm. Anal. Calcd. for C17H26N7O6P: C, 44.84; H, 5.75; N, 21.53. Found: C, 44.70; H, 5.59; N, 21.60.
4.1.5.48. Diethyl 3-{4-[(1,3-dimethyl-2,6-dioxopurin-7-yl)methyl]-1H-1,2,3-triazol-1-yl}-2-hydroxypropylphosphonate 25i
From azide 17 (0.105 g, 0.443 mmol) and N 7-propargyltheophylline 19i (0.097 g, 0.443 mmol) the phosphonate 25i (0.182 g, 90%) was obtained as a white solid after chromatography on a silica gel column with chloroform–methanol (50:1, v/v); m.p.: 132–133 °C; IR (KBr): ν = 2994, 2989, 2930, 1701, 1663, 1245, 1033 cm−1; 1H NMR (300 MHz, CDCl3): δ = 7.97 (s, 1H); 7.81 (s, 1H, HC5′); 5.58 (s, 2H, CH2); 4.55–4.39 (m, 3H, PCCHCH 2); 4.18–4.04 (m, 4H, 2× POCH 2CH3); 3.55 (s, 3H, CH3); 3.38 (s, 3H, CH3); 2.46–2.34 (m, 2H, PCH 2); 1.31 (t, J = 7.0 Hz, 3H, POCH2CH 3); 1.28 (t, J = 7.0 Hz, 3H, POCH2CH 3); 13C NMR (75.5 MHz, CDCl3): δ = 155.2 (s, C O); 151.5 (s, C O); 148.8; 141.9; 141.4; 125.3; 106.5; 65.5 (d, J = 3.8 Hz); 62.4 (d, J = 6.6 Hz, POC); 62.2 (d, J = 6.6 Hz, POC); 56.1 (d, J = 17.2 Hz, PCCC); 41.6; 31.8; 29.0 (d, J = 136.6 Hz, PC); 16.6 (d, J = 6.0 Hz, POCC); 16.5 (d, J = 6.0 Hz, POCC); 31P NMR (121.5 MHz, CDCl3): δ = 29.34 ppm. Anal. Calcd. for C17H26N7O6P: C, 44.84; H, 5.75; N, 21.53. Found: C, 44.77; H, 5.67; N, 21.32.
4.1.5.49. Diethyl 3-{4-[(5,6-dimethylbenzoimidazol-1-yl)methyl]-1H-1,2,3-triazol-1-yl}-2-hydroxypropylphosphonate 25j
From azide 17 (0.101 g, 0.427 mmol) and 5,6-dimethyl-N-propargylbenzimidazole 19j (0.078 g, 0.427 mmol) the phosphonate 25j (0.146 g, 82%) was obtained as a yellow oil after purification on silica gel with chloroform–methanol (50:1, v/v); IR (film): ν = 3339, 3140, 2982, 2935, 1222, 1048, 965, 838 cm−1; 1H NMR (300 MHz, CDCl3): δ = 7.83 (s, 1H); 7.61 (s, 1H); 7.49 (s, 1H); 7.20 (s, 1H); 5.36 (s, 2H, CH2); 4.53–4.47 (m, 1H, PCCCH aHb); 4.42–4.28 (m, 2H, PCCHCHa H b); 4.15–4.05 (m, 4H, 2× POCH 2CH3); 3.63 (brs, 1H, OH); 2.34 (s, 3H, CH3); 2.33 (s, 3H, CH3); 2.04–1.75 (m, 2H, PCH 2); 1.30 (t, J = 7.0 Hz, 3H, POCH2CH 3); 1.28 (t, J = 7.0 Hz, 3H, POCH2CH 3); 13C NMR (75.5 MHz, CDCl3): δ = 142.5; 141.9; 141.5; 132.7; 131.9; 131.6; 124.3; 119.8; 110.2; 65.3 (d, J = 3.4 Hz, PCC); 62.4 (d, J = 6.3 Hz, POC); 62.3 (d, J = 6.3 Hz, POC); 56.3 (d, J = 16.0 Hz, PCCC); 40.5; 31.1 (d, J = 139.8 Hz, PC); 20.7; 20.4; 16.6 (d, J = 6.3 Hz, POCC); 16.5 (d, J = 6.3 Hz, POCC); 31P NMR (121.5 MHz, CDCl3): δ = 28.53 ppm. Anal. Calcd. for C19H28N5O4P: C, 54.15; H, 6.70; N, 16.62. Found: C, 53.98; H, 6.64; N, 16.56.
4.1.5.50. Diethyl 3-{4-[(3-acetylindol-1-yl)methyl]-1H-1,2,3-triazol-1-yl}-2-hydroxypropylphosphonate 25k
From azide 17 (0.105 g, 0.443 mmol) and 3-acetyl-N-propargylindole 19k (0.087 g, 0.443 mmol) the phosphonate 25k (0.186 g, 97%) was obtained as a colourless oil after purification on silica gel with chloroform–methanol (50:1, v/v); IR (film): ν = 3352, 2984, 2924, 1799, 1528, 1391, 1025 cm−1; 1H NMR (600 MHz, CDCl3): δ = 8.40–8.34 (m, 1H); 7.88 (s, 1H, HC5′); 7.65 (s, 1H); 7.47–7.41 (m, 1H); 7.33–7.28 (m, 2H); 5.46 (s, 2H, CH2); 4.54–4.47 (m, 1H, PCCCH aHb); 4.40–4.32 (m, 2H, PCCHCHa H b); 4.17–4.01 (m, 4H, 2× POCH 2CH3); 2.49 (s, 3H, CH3); 1.97 (ddd, J = 19.3 Hz, J = 15.2 Hz, J = 3.2 Hz, 1H, PCH aHb); 1.77 (ddd, J = 16.6 Hz, J = 15.2 Hz, J = 9.0 Hz, 1H, PCHa H b); 1.32 (t, J = 6.9 Hz, 3H, POCH2CH 3); 1.30 (t, J = 6.9 Hz, 3H, POCH2CH 3); 13C NMR (75.5 MHz, CDCl3): δ = 193.2 (s, C O); 142.6; 136.5; 135.0; 126.3; 124.1; 123.5; 122.7; 122.6; 117.4; 109.9; 65.4 (d, J = 3.7 Hz, PCC); 62.4 (d, J = 6.4 Hz, POC); 62.3 (d, J = 6.4 Hz, POC); 56.1 (d, J = 17.2 Hz, PCCC); 42.4; 30.8 (d, J = 140.0 Hz, PC); 27.7 (s, CH3); 16.6 (d, J = 6.0 Hz, POCC); 16.5 (d, J = 6.0 Hz, POCC); 31P NMR (243 MHz, CDCl3): δ = 28.12 ppm. Anal. Calcd. for C20H27N4O5P: C, 55.29; H, 6.26; N, 12.90. Found: C, 55.04; H, 6.14; N, 13.06.
4.1.5.51. Diethyl 2-hydroxy-3-{4-[(2-oxopyridin-1-yl)methyl]-1H-1,2,3-triazol-1-yl}propylphosphonate 25l
From azide 17 (0.151 g, 0.637 mmol) and N-propargyl-2-pyridon 19l (0.085 g, 0.637 mmol) the phosphonate 25l (0.218 g, 92%) was obtained as a brown oil after purification on silica gel with chloroform–methanol (50:1, v/v); IR (film): ν = 3307, 2988, 2909, 1658, 1226; 1048, 776 cm−1; 1H NMR (300 MHz, CDCl3): δ = 7.95 (s, 1H); 7.60 (dd, J = 6.6 Hz, J = 3.0 Hz, 1H); 7.33 (ddd, J = 9.3 Hz, J = 6.3 Hz, J = 1.8 Hz, 1H); 6.53 (d, J = 9.0 Hz, 1H); 6.20 (dt, J = 6.6 Hz, J = 0.9 Hz, 1H); 5.18 (AB, J = 14.4 Hz, 1H, CH aHb); 5.16 (AB, J = 14.4 Hz, 1H, CHa H b); 4.53 (dd, J = 15.6 Hz, J = 6.6 Hz, 1H, PCCCH aHb); 4.56–4.34 (m, 2H, PCCHCHa H b); 4.18–4.03 (m, 4H, 2× POCH 2CH3); 2.95 (brs, 1H, OH); 2.07–1.80 (m, 2H, PCH2); 1.33 (t, J = 6.9 Hz, 3H, POCH2CH 3); 1.32 (t, J = 6.9 Hz, 3H, POCH2CH 3); 13C NMR (75.5 MHz, CDCl3): δ = 162.2 (s, C O); 141.8 (s, HC C); 140.1; 137.8; 125.4 (s, HC C); 120.2; 106.7; 65.2 (d, J = 2.6 Hz, PCC); 62.2 (d, J = 6.6 Hz, POC); 61.9 (d, J = 6.6 Hz, POC); 55.9 (d, J = 14.9 Hz, PCCC); 44.4; 31.0 (d, J = 139.7 Hz, PC); 16.3 (d, J = 6.0 Hz, POCC); 31P NMR (121.5 MHz, CDCl3): δ = 29.28 ppm. Anal. Calcd. for C15H23N4O5P: C, 48.65; H, 6.26; N, 15.13. Found: C, 48.88; H, 6.34; N, 15.00.
4.1.5.52. Diethyl 1-hydroxy-3-(4-{[5-methyl-2,4-dioxopyrimidin-1(2H)-yl]methyl}-1H-1,2,3-triazol-1-yl)propylphosphonate 26b
From azide 18 (0.105 g, 0.443 mmol) and N 1-propargylthymine 19b (0.073 g, 0.443 mmol) the phosphonate 26b (0.150 g, 84%) was obtained as a white powder after chromatography on a silica gel column with chloroform–methanol (50:1, v/v); m.p.: 169–171 °C; IR (KBr): ν = 3410, 2989, 2938, 1682, 1225, 1022 cm−1; 1H NMR (300 MHz, CDCl3): δ = 9.89 (brs, 1H, NH); 7.87 (s, 1H, HC5′); 7.37 (d, J = 1.0 Hz, 1H, HC CH); 4.95 (AB, J = 14.9 Hz, 1H, CH aHb); 4.92 (AB, J = 14.9 Hz, 1H, CHa H b); 4.63–4.55 (m, 2H, PCCCH 2); 4.23–4.09 (m, 4H, 2× POCH 2CH3); 3.80 (ddd, J = 10.7 Hz, J = 6.3 Hz, J = 3.3 Hz, 1H, PCH(OH)); 2.40–2.17 (m, 3H, PCCH 2, OH); 1.91 (d, J = 1.0 Hz, 3H, CH3); 1.33 (t, J = 6.9 Hz, 3H, POCH2CH 3); 1.30 (t, J = 6.9 Hz, 3H, POCH2CH 3); 13C NMR (75.5 MHz, CD3OD): δ = 166.7 (s, C O); 152.6 (s, C O); 142.6; 125.5; 111.6; 65.1 (d, J = 167.8 Hz, PC); 64.4 (d, J = 7.5 Hz, POC); 64.2 (d, J = 7.5 Hz, POC); 47.7 (d, J = 16.1 Hz, PCCC); 43.9; 33.2 (d, J = 4.0 Hz, PCC); 17.0 (d, J = 4.9 Hz, POCC); 16.9 (d, J = 4.9 Hz, POCC); 12.5; 31P NMR (121.5 MHz, CDCl3): δ = 24.76 ppm. Anal. Calcd. for C15H24N5O6P: C, 44.89; H, 6.03; N, 17.45. Found: C, 44.90; H, 6.16; N, 17.50.
4.1.5.53. Diethyl 3-(4-{[2,4-dioxopyrimidin-1-yl]methyl}-1H-1,2,3-triazol-1-yl)-1-hydroxypropylphosphonate 26c
From azide 18 (0.108 g, 0.455 mmol) and N 1-propargyluracil 19c (0.068 g, 0.455 mmol) the phosphonate 26c (0.143 g, 81%) was obtained as a white solid after chromatography on a silica gel column with chloroform–methanol (50:1, v/v); m.p.: 136–138 °C; IR (KBr): ν = 3405, 2984, 2932, 1680, 1227, 1025 cm−1; 1H NMR (300 MHz, CDCl3): δ = 10.49 (brs, 1H, NH); 7.90 (s, 1H, HC5′); 7.59 (d, J = 7.9 Hz, 1H, HC CH); 5.72 (d, J = 7.9 Hz, 1H, HC CH); 5.01 (AB, J = 15.5 Hz, 1H, CH aHb); 4.97 (AB, J = 15.5 Hz, 1H, CHa H b); 4.61 (brt, J = 6.4 Hz, 2H, PCCCH 2); 4.23–4.10 (m, 4H, 2× POCH 2CH3); 3.86–3.74 (m, 1H, PCH(OH)); 2.44–2.17 (m, 2H, PCCH 2); 1.32 (t, J = 7.0 Hz, 3H, POCH2CH 3); 1.30 (t, J = 7.0 Hz, 3H, POCH2CH 3); 13C NMR (75.5 MHz, CDCl3): δ = 164.5 (s, C O); 151.4 (s, C O); 144.9; 141.7; 124.9; 102.6; 64.0 (d, J = 166.0 Hz, PC); 63.3 (d, J = 7.0 Hz, POC); 63.1 (d, J = 7.0 Hz, POC); 46.7 (d, J = 17.2 Hz, PCCC); 43.3; 31.9; 16.6 (d, J = 5.4 Hz, POCC); 16.5 (d, J = 5.4 Hz, POCC); 31P NMR (121.5 MHz, CDCl3): δ = 24.84 ppm. Anal. Calcd. for C14H22N5O6P: C, 43.41; H, 5.73; N, 18.08. Found: C, 43.57; H, 5.61; N, 17.89.
4.1.5.54. Diethyl 3-(4-{[N4-acetylamino-2-oxopyrimidin-1-yl]methyl}-1H-1,2,3-triazol-1-yl)-1-hydroxypropylphosphonate 26d
From azide 18 (0.130 g, 0.548 mmol) and N 4-acetyl-N 1-propargylcytosine 19d (0.105 g, 0.548 mmol) the phosphonate 26d (0.186 g, 79%) was obtained as a white powder after chromatography on a silica gel column with chloroform–methanol (50:1, v/v); m.p.: 175–177 °C; IR (KBr): ν = 3406, 3124, 2930, 2873, 1706, 1654, 1221, 1021 cm−1; 1H NMR (300 MHz, CDCl3): δ = 10.96 (brs, 1H, NH); 8.71 (s, 1H, HC5′); 7.93 (d, J = 7.4 Hz, 1H, HC CH); 7.44 (d, J = 7.4 Hz, 1H, HC CH); 5.32 (d, J = 14.6 Hz, 1H, CH aHb); 5.05 (d, J = 14.6 Hz, 1H, CHa H b); 4.91–4.83 (m, 1H, PCCCH aHb); 4.75–4.65 (m, 1H, PCCCHa H b); 4.25–4.14 (m, 2H, POCH 2CH3); 4.13–4.03 (m, 2H, POCH 2CH3); 3.87–3.81 (m, 1H, PCH(OH)); 2.32–2.24 (m, 3H, PCCH 2, OH); 2.24 (s, 3H, CH3); 1.35 (t, J = 7.1 Hz, 3H, POCH2CH 3); 1.27 (t, J = 7.1 Hz, 3H, POCH2CH 3); 13C NMR (75.5 MHz, CD3OD): δ = 172.8; 164.4; 158.2; 150.7; 143.2; 125.9; 98.4; 65.0 (d, J = 168.0 Hz, PC); 64.4 (d, J = 7.5 Hz Hz, POC); 64.1 (d, J = 7.5 Hz, POC); 47.7 (d, J = 16.3 Hz, PCCC); 46.4; 33.2 (s, PCC); 24.7; 16.9 (d, J = 4.9 Hz, POCC); 31P NMR (121.5 MHz, CDCl3): δ = 25.96 ppm. Anal. Calcd. for C16H25N6O6P: C, 44.86; H, 5.88; N, 19.62. Found: C, 45.10; H, 6.00; N, 19.74.
4.1.5.55. Diethyl 3-(4-{[3-benzoyl-2,4-dioxopyrimidin-1-yl]methyl}-1H-1,2,3-triazol-1-yl)-1-hydroxyethylphosphonate 26e
From azide 18 (0.102 g, 0.430 mmol) and N 3-benzoyl-N 1-propargylquinazoline-2,4-dione 19e (0.131 g, 0.430 mmol) the phosphonate 26e (0.213 g, 91%) was obtained as a white powder after chromatography on a silica gel column with chloroform–methanol (50:1, v/v); m.p.: 82–84 °C; IR (KBr): ν = 3299, 2988, 1746, 1702, 1664, 1233, 1027, 757, 674 cm−1; 1H NMR (300 MHz, CDCl3): δ = 8.20 (dd, J = 7.9 Hz, J = 1.6 Hz, 1H); 7.99–7.95 (m, 2H, 2× o-CH); 7.93 (brd, J = 8.5 Hz, 1H); 7.78 (ddd, J = 8.5 Hz, J = 7.9 Hz, J = 1.6 Hz, 1H); 7.74 (s, 1H, HC5′); 7.70–7.64 (m, 1H, p-CH); 7.54–7.46 (m, 2H, 2× m-CH); 7.29 (dt, J = 7.9 Hz, J = 0.8 Hz, 1H); 5.42 (AB, J = 15.7 Hz, 1H, CH aHb); 5.36 (AB, J = 15.7 Hz, 1H, CHa H b); 4.66–4.54 (m, 2H, PCCCH 2); 4.18–4.04 (m, 4H, 2× POCH 2CH3); 3.78 (ddd, J = 10.7 Hz, J = 6.2 Hz, J = 3.4 Hz, 1H, PCH(OH)); 2.38–2.10 (m, 2H, PCCH 2); 1.30 (t, J = 7.0 Hz, 3H, POCH2CH 3); 1.28 (t, J = 7.0 Hz, 3H, POCH2CH 3); 13C NMR (75.5 MHz, CDCl3): δ = 168.7 (s, C O); 161.1 (s, C O); 149.6 (s, C O); 142.3 (s, HC C); 140.3; 136.3; 135.2; 131.6; 130.7; 129.3; 128.9; 124.4 (s, HC C); 123.9; 115.6; 115.4; 64.4 (d, J = 166.4 Hz, PC); 63.3 (d, J = 6.8 Hz, POC); 63.0 (d, J = 6.8 Hz, POC); 48.6 (d, J = 16.1 Hz, PCCC); 39.0; 31.9, 16.7 (d, J = 5.2 Hz, POCC); 16.6 (d, J = 5.2 Hz, POCC); 31P NMR (121.5 MHz, CDCl3): δ = 24.57 ppm. Anal. Calcd. for C25H28N5O7P: C, 55.45; H, 5.21; N, 12.93. Found: C, 55.60; H, 5.34; N, 13.09.
4.1.5.56. Diethyl 1-hydroxy-3-{4-[(3,5-dioxo-1,2,4-triazin-2-yl)methyl]-1H-1,2,3-triazol-1-yl}-1-hydroxypropylphosphonate 26f
From azide 18 (0.131 g, 0.552 mmol) and N 1-propargyl-6-azauracil 19f (0.083 g, 0.552 mmol) the phosphonate 26f (0.193 g, 90%) was obtained as a yellow solid after chromatography on a silica gel column with chloroform–methanol (50:1, v/v); m.p.: 116–118 °C; IR (KBr): ν = 3284, 3152, 2988, 2912, 1731, 1658, 1050 cm−1; 1H NMR (300 MHz, CDCl3): δ = 12.1 (s, 1H, NH); 7.85 (s, 1H); 7.40 (s, 1H); 5.17 (s, 1H, CH2); 4.60–4.51 (m, 2H, PCCCH 2); 4.21–4.09 (m, 4H, 2× POCH 2CH3); 3.87 (ddd, J = 10.1 Hz, J = 6.1 Hz, J = 2.9 Hz, 1H, PCH(OH)); 2.41–2.16 (m, 3H, PCCH 2, OH); 1.31 (t, J = 7.0 Hz, 3H, POCH2CH 3); 1.29 (t, J = 7.0 Hz, 3H, POCH2CH 3); 13C NMR (75.5 MHz, CDCl3): δ = 155.9 (s, C O); 149.4 (C O); 141.4 (s, HC C); 135.0 (s, HC N); 124.8 (s, HC C); 64.1 (d, J = 166.3 Hz, PC); 63.3 (d, J = 7.2 Hz, POC); 63.2 (d, J = 7.2 Hz, POC); 46.6 (d, J = 17.2 Hz, PCCC); 34.7; 31.9; 16.6 (d, J = 5.6 Hz, POCC); 16.5 (d, J = 5.6 Hz, POCC); 31P NMR (121.5 MHz, CDCl3): δ = 25.12 ppm. Anal. Calcd. for C13H21N6O6P: C, 40.21; H, 5.45; N, 21.64. Found: C, 40.05; H, 5.61; N, 21.77.
4.1.5.57. Diethyl 3-{4-[(8-chloro-1,3-dimethyl-2,6-dioxopurin-7-yl)methyl]-1H-1,2,3-triazol-1-yl}-1-hydroxypropylphosphonate 26g
From azide 18 (0.109 g, 0.460 mmol) and 8-chloro-N 7-propargyltheophylline 19g (0.116 g, 0.460 mmol) the phosphonate 26g (0.187 g, 83%) was obtained as a white powder after chromatography on a silica gel column with chloroform–methanol (50:1, v/v); m.p.: 164–165 °C; IR (KBr): ν = 3300, 2993, 1706, 1663, 1217, 1027 cm−1; 1H NMR (300 MHz, CDCl3): δ = 7.84 (s, 1H); 5.64 (AB, J = 14.8 Hz, 1H, CH aHb); 5.61 (AB, J = 14.8 Hz, 1H, CHa H b); 4.60–4.50 (m, 2H, PCCCH 2); 4.21–4.09 (m, 4H, 2× POCH 2CH3); 3.80 (ddd, J = 10.7 Hz, J = 6.3 Hz, J = 3.4 Hz, 1H, PCH(OH)); 3.54 (s, 3H, CH3); 3.40 (s, 3H, CH3); 3.25 (brs, 1H, OH); 2.37–2.20 (m, 2H, PCCH2); 1.33 (t, J = 6.9 Hz, 3H, POCH2CH 3); 1.31 (t, J = 6.9 Hz, 3H, POCH2CH 3); 13C NMR (75.5 MHz, CDCl3): δ = 154.3 (s, C O); 151.0 (s, C O); 147.2; 141.4; 138.9; 124.1; 107.3; 64.1 (d, J = 165.7 Hz, PC); 63.1 (d, J = 7.1 Hz, POC); 62.9 (d, J = 7.1 Hz, POC); 46.6 (d, J = 15.8 Hz, PCCC); 41.0; 31.9 (d, J = 2.6 Hz, PCC); 29.9; 28.1; 16.6 (d, J = 5.2 Hz, POCC); 16.5 (d, J = 5.2 Hz, POCC); 31P NMR (121.5 MHz, CDCl3): δ = 24.61 ppm. Anal. Calcd. for C17H25ClN7O6P: C, 41.68; H, 5.14; N, 20.02. Found: C, 41.79; H, 5.08; N, 20.10.
4.1.5.58. Diethyl 3-{4-[(3,7-dimethyl-2,6-dioxopurin-1-yl)methyl]-1H-1,2,3-triazol-1-yl}-1-hydroxypropylphosphonate 26h
From azide 18 (0.110 g, 0.464 mmol) and N 1-propargyltheobromine 19h (0.101 g, 0.464 mmol) the phosphonate 26h (0.167 g, 79%) was obtained as a white powder after chromatography on a silica gel column with chloroform–methanol (50:1, v/v); m.p.: 129–130 °C; IR (KBr): ν = 3288, 2984, 1707, 1661, 1233, 1049 cm−1; 1H NMR (300 MHz, CDCl3): δ = 7.69 (s, 1H); 7.54 (s, 1H); 5.33 (AB, J = 14.5 Hz, 1H, CH aHb); 5.28 (AB, J = 14.5 Hz, 1H, CHa H b); 4.65–4.45 (m, 2H, PCCCH 2); 4.21–4.09 (m, 4H, 2× POCH 2CH3); 4.00 (s, 3H, CH3); 3.85–3.76 (m, 1H, PCH(OH)); 3.57 (s, 3H, CH3); 2.33–2.16 (m, 3H, PCH2, OH); 1.32 (t, J = 6.4 Hz, 3H, POCH2CH 3); 1.31 (t, J = 6.4 Hz, 3H, POCH2CH 3); 13C NMR (75.5 MHz, CDCl3): δ = 154.5 (s, C O); 151.0 (s, C O); 148.6; 143.2; 141.8; 123.7; 107.4; 64.4 (d, J = 166.3 Hz, PC); 62.8 (d, J = 7.2 Hz, POC); 62.7 (d, J = 7.2 Hz, POC); 46.3 (d, J = 16.0 Hz, PCCC); 35.9; 33.6; 31.9; 29.7; 16.4 (d, J = 5.5 Hz, POCC); 16.2 (d, J = 5.5 Hz, POCC); 31P NMR (121.5 MHz, CDCl3): δ = 24.85 ppm. Anal. Calcd. for C17H26N7O6P: C, 44.84; H, 5.75; N, 21.53. Found: C, 44.62; H, 5.66; N, 21.46.
4.1.5.59. Diethyl 3-{4-[(1,3-dimethyl-2,6-dioxopurin-7-yl)methyl]-1H-1,2,3-triazol-1-yl}-1-hydroxypropylphosphonate 26i
From azide 18 (0.111 g, 0.468 mmol) and N 7-propargyltheophylline 19i (0.102 g, 0.468 mmol) the phosphonate 26i (0.183 g, 86%) was obtained as a white powder after chromatography on a silica gel column with chloroform–methanol (50:1, v/v); m.p.: 123–125 °C; IR (KBr): ν = 3365, 2991, 1704, 1658, 1222, 1050 cm−1; 1H NMR (600 MHz, CDCl3): δ = 7.89 (s, 1H); 7.87 (s, 1H); 5.62 (AB, J = 14.9 Hz, 1H, CH aHb); 5.59 (AB, J = 14.9 Hz, 1H, CHa H b); 4.63–4.55 (m, 2H, PCCCH 2); 4.22–4.09 (m, 4H, 2× POCH 2CH3); 3.80 (ddd, J = 10.9 Hz, J = 6.2 Hz, J = 3.3 Hz, 1H, PCH(OH)); 3.59 (s, 3H, CH3); 3.43 (s, 3H, CH3); 2.37 (ddddd, J = 14.4 Hz, J = 8.0 Hz, J = 8.0 Hz, J = 6.1 Hz, J = 3.3 Hz, 1H, PCCH aHb); 2.26 (ddddd, J = 14.4 Hz, J = 10.9 Hz, J = 6.4 Hz, J = 5.6 Hz, J = 5.6 Hz, 1H, PCCHa H b); 1.35 (t, J = 6.9 Hz, 3H, POCH2CH 3); 1.33 (t, J = 6.9 Hz, 3H, POCH2CH 3); 13C NMR (75.5 MHz, CDCl3): δ = 155.2 (s, C O); 151.4 (s, C O); 148.7; 141.9; 141.6; 124.3; 106.3; 64.0 (d, J = 166.1 Hz, PC); 63.1 (d, J = 7.1 Hz, POC); 62.9 (d, J = 7.1 Hz, POC); 46.6 (d, J = 15.8 Hz, PCCC); 41.3; 31.9 (d, J = 2.8 Hz, PCC); 29.9; 28.1; 16.6 (d, J = 5.4 Hz, POCC); 16.5 (d, J = 5.4 Hz, POCC); 31P NMR (243 MHz, CDCl3): δ = 23.44 ppm. Anal. Calcd. for C17H26N7O6P: C, 44.84; H, 5.75; N, 21.53. Found: C, 45.00; H, 5.90; N, 21.40.
4.1.5.60. Diethyl 3-{4-[(5,6-dimethylbenzoimidazol-1-yl)methyl]-1H-1,2,3-triazol-1-yl}-1-hydroxypropylphosphonate 26j
From azide 18 (0.103 g, 0.434 mmol) and 5,6-dimethyl-N 1-propargylbenzimidazole 19j (0.080 g, 0.434 mmol) the phosphonate 26j (0.146 g, 80%) was obtained as a yellow oil after purification on silica gel with chloroform–methanol (50:1, v/v); IR (film): ν = 3339, 3140, 2982, 2935, 1222, 1048, 965, 838 cm−1; 1H NMR (300 MHz, CDCl3): δ = 8.08 (s, 1H); 7.56 (s, 1H); 7.53 (s, 1H); 7.25 (s, 1H); 5.45 (s, 2H, CH2); 4.64–4.54 (m, 2H, PCCCH 2); 4.18–4.05 (m, 4H, 2× POCH 2CH3); 3.75 (ddd, J = 10.5 Hz, J = 6.5 Hz, J = 3.2 Hz, 1H, PCH(OH)); 3.40 (brs, 1H, OH); 2.37 (s, 3H, CH3); 2.35 (s, 3H, CH3); 2.34–2.14 (m, 2H, PCCH 2); 1.29 (t, J = 7.0 Hz, 3H, POCH2CH 3); 1.26 (t, J = 7.0 Hz, 3H, POCH2CH 3); 13C NMR (75.5 MHz, CDCl3): δ = 142.7; 142.0; 141.4; 132.6; 131.8; 131.6; 123.1; 119.7; 110.2; 64.0 (d, J = 151.2 Hz, PC); 62.8 (d, J = 7.1 Hz, POC); 62.8 (d, J = 7.1 Hz, POC); 46.7 (d, J = 15.7 Hz, PCCC); 40.5; 32.0; 20.7; 20.4; 16.6 (d, J = 6.3 Hz, POCC); 16.6 (d, J = 6.3 Hz, POCC); 31P NMR (121.5 MHz, CDCl3): δ = 24.71 ppm. Anal. Calcd. for C19H28N5O4P: C, 54.15; H, 6.20; N, 16.62. Found: C, 54.00; H, 6.89; N, 16.70.
4.1.5.61. Diethyl 3-{4-[(3-acetylindol-1-yl)methyl]-1H-1,2,3-triazol-1-yl}-1-hydroxypropylphosphonate 26k
From azide 18 (0.107 g, 0.451 mmol) and 3-acetyl-N-propargylindole 19k (0.089 g, 0.451 mmol) the phosphonate 26k (0.182 g, 93%) was obtained as a colourless oil after chromatography on a silica gel column with chloroform–methanol (50:1, v/v); IR (film): ν = 3330, 3140, 2984, 1799, 1527, 1389, 1223, 1022 cm−1; 1H NMR (300 MHz, CDCl3): δ = 8.38–8.33 (m, 1H); 7.86 (s, 1H, HC5′); 7.47 (s, 1H); 7.43–7.38 (m, 1H); 7.31–7.25 (m, 2H); 5.41 (s, 2H, CH2); 4.60–4.45 (m, 2H, PCCCH 2); 4.16–4.03 (m, 4H, 2× POCH 2CH3); 3.72 (ddd, J = 10.8 Hz, J = 6.4 Hz, J = 3.5 Hz, 1H, PCH(OH)); 2.49 (s, 3H, CH3); 2.36–2.11 (m, 2H, PCCH 2); 1.29 (t, J = 7.0 Hz, 3H, POCH2CH 3); 1.26 (t, J = 7.0 Hz, 3H, POCH2CH 3); 13C NMR (75.5 MHz, CDCl3): δ = 193.3 (s, C O); 142.8; 136.5; 135.0; 126.4; 123.6; 123.0; 122.8; 122.7; 117.5; 109.9; 64.2 (d, J = 165.9 Hz, PC); 63.2 (d, J = 6.3 Hz, POC); 62.9 (d, J = 6.3 Hz, POC); 46.7 (d, J = 15.6 Hz, PCCC); 42.4; 31.9 (d, J = 2.9 Hz, PCC); 27.8 (s, CH3); 16.7 (d, J = 6.0 Hz, POCC); 16.6 (d, J = 6.0 Hz, POCC); 31P NMR (121.5 MHz, CDCl3): δ = 24.60 ppm. Anal. Calcd. for C20H27N4O5P: C, 55.29; H, 6.26; N, 12.90. Found: C, 55.40; H, 6.10; N, 13.03.
4.1.5.62. Diethyl 1-hydroxy-3-{4-[(2-oxopyridin-1-yl)methyl]-1H-1,2,3-triazol-1-yl}propylphosphonate 26l
From azide 18 (0.131 g, 0.552 mmol) and N-propargyl-2-pyridon 19l (0.074 g, 0.552 mmol) the phosphonate 26l (0.168 g, 82%) was obtained as a brown oil after chromatography on a silica gel column with chloroform–methanol (50:1, v/v); IR (film): ν = 3401, 2986, 2912, 1656, 1224; 1025 cm−1; 1H NMR (300 MHz, CDCl3): δ = 7.90 (s, 1H, HC5′); 7.63 (ddd, J = 6.8 Hz, J = 2.0 Hz, J = 0.6 Hz, 1H); 7.34 (ddd, J = 9.1 Hz, J = 6.8 Hz, J = 2.0 Hz, 1H); 6.55 (ddd, J = 9.1 Hz, J = 1.3 Hz, J = 0.6 Hz, 1H); 6.21 (dt, J = 6.8 Hz, J = 1.3 Hz, 1H); 5.22 (AB, J = 14.3 Hz, 1H, CH aHb); 5.16 (AB, J = 14.3 Hz, 1H, CHa H b); 4.86 (s, brs, 1H, OH); 4.60–4.45 (m, 2H, PCCCH 2); 4.22–4.09 (m, 4H, 2× POCH 2CH3); 3.79 (ddd, J = 10.8 Hz, J = 6.2 Hz, J = 3.2 Hz, 1H, PCH(OH)); 2.41–2.16 (m, 2H, PCCH 2); 1.33 (t, J = 6.9 Hz, 3H, POCH2CH 3); 1.30 (t, J = 6.9 Hz, 3H, POCH2CH 3); 13C NMR (75.5 MHz, CDCl3): δ = 162.4 (s, C O); 142.3 (s, HC C); 140.3; 137.9; 124.9 (s, HC C); 120.6; 106.9; 64.0 (d, J = 165.8 Hz, PC); 63.1 (d, J = 7.1 Hz, POC); 62.9 (d, J = 7.1 Hz, POC); 46.6 (d, J = 16.0 Hz, PCCC); 44.8; 31.9 (d, J = 3.1 Hz, PCC); 16.7 (d, J = 5.2 Hz, POCC); 16.6 (d, J = 5.2 Hz, POCC); 31P NMR (121.5 MHz, CDCl3): δ = 24.85 ppm. Anal. Calcd. for C15H23N4O5P: C, 48.65; H, 6.26; N, 15.13. Found: C, 48.76; H, 6.12; N, 15.03.
4.1.6. General procedure for the synthesis of phosphonic acids
Solutions of diethyl phosphonates 22e, 22g, 22j, 22m, 23a–c or 24i (1.00 mmol) in CH2Cl2 (3 mL) were treated with bromotrimethylsilane (10.0 mmol) at room temperature under argon atmosphere. The reaction mixture was protected from light and stirred at room temperature for 24 h. After concentration to dryness the residue was co-evaporated with dichloromethane (5 mL) and ethanol (3 × 3 mL) to afford crude phosphonic acids 31e, 31g, 31j, 31m, 32a–c or 33i which was purified by crystallisation from methanol–diethyl ether mixtures.
4.1.6.1. 3-(4-{[3-Benzoyl-2,4-dioxopyrimidin-1-yl]methyl}-1H-1,2,3-triazol-1-yl)propylphosphonic acid 31e
From 22e (0.165 g, 0.314 mmol) phosphonic acid 31e (0.118 g, 80%) was obtained as a white powder; m.p.: 130–133 °C; IR (KBr): ν = 3341, 3014, 2939, 1746, 1699, 1662; 1478; 1240, 987; 756; 674 cm−1; 1H NMR (300 MHz, CD3OD): δ = 8.16 (dd, J = 7.9 Hz, J = 1.6 Hz, 1H); 8.07 (s, 1H, HC5′); 8.06–8.03 (m, 2H, 2× o-CH); 7.82 (ddd, J = 8.6 Hz, J = 7.9 Hz, J = 1.6 Hz, 1H); 7.77–7.68 (m, 2H, p-CH, H8); 7.60–7.54 (m, 2H, 2× m-CH); 7.32 (dt, J = 7.9 Hz, J = 0.9 Hz, 1H); 5.49 (s, 2H, CH2); 4.49 (t, J = 7.0 Hz, 2H, PCCCH 2); 2.29–2.10 (m, 2H, PCCH 2); 1.73–1.67 (m, 2H, PCH 2); 13C NMR (151 MHz, CD3OD): δ = 168.6 (s, C O); 161.4 (s, C O); 149.6 (s, C O); 141.2 (s, HC C); 140.2; 136.2; 135.1; 131.6; 130.3; 129.1; 128.2; 125.3 (s, HC C); 123.7; 115.5; 114.9; 51.2 (d, J = 17.8 Hz, PCCC); 37.7; 23.4 (d, J = 3.7 Hz, PCC); 23.4 (d, J = 140.7 Hz, PC); 31P NMR (121 MHz, CD3OD): δ = 29.05 ppm. Anal. Calcd. for C21H20N5O6P × H2O: C, 51.75; H, 4.55; N, 14.37. Found: C, 51.55; H, 4.32; N, 14.83.
4.1.6.2. 3-{4-[(8-Chloro-1,3-dimethyl-2,6-dioxopurin-7-yl)methyl]-1H-1,2,3-triazol-1-yl}propylphosphonic acid 31g
From 22g (0.088 g, 0.180 mmol) phosphonic acid 31g (0.061 g, 79%) was obtained as a white powder; m.p.: 216–220 °C; solubility of 31g in methanol or water was insufficient to measure the 13C NMR spectrum; IR (KBr): ν = 3124, 2998, 2978, 1608, 1463, 1220, 1028, 968 cm−1; 1H NMR (600 MHz, CD3OD): δ = 8.09 (s, 1H); 5.70 (s, 2H, CH2); 4.51 (t, J = 7.0 Hz, 2H, PCCCH 2); 3.37 (s, 3H, CH3); 3.34 (s, 3H, CH3); 2.17 (dqv, J = 14.0 Hz, J = 7.0 Hz, 2H, PCCH 2); 1.70 (dt, J = 19.2 Hz, J = 7.0 Hz, 2H, PCH 2); 31P NMR (243 MHz, CD3OD): δ = 27.81 ppm. Anal. Calcd. for C13H17ClN5O5P × H2O: C, 35.83; H, 4.39; N, 22.50. Found: C, 35.60; H, 4.32; N, 22.33.
4.1.6.3. 3-{4-[(5,6-Dimethyl-benzimidazol-1-yl)methyl]-1H-1,2,3-triazol-1-yl}propylphosphonic acid 31j
From 22j (0.065 g, 0.160 mmol) phosphonic acid 31j (0.046 g, 83%) was obtained as a white powder; m.p.: 148–151 °C; IR (KBr): ν = 3100, 2999, 2948, 2889, 1244, 1040, 965 cm−1; 1H NMR (600 MHz, CD3OD): δ = 9.44 (s, 1H); 8.31 (s, 1H); 7.78 (s, 1H); 7.63 (s, 1H); 5.86 (s, 2H, CH2); 4.55 (t, J = 7.0 Hz, 2H, PCCCH 2); 2.50 (s, 3H, CH3); 2.47 (s, 3H, CH3); 2.22 (dqv, J = 14.3 Hz, J = 7.0 Hz, 2H, PCCH 2); 1.70 (dt, J = 18.7 Hz, J = 7.0 Hz, 2H, PCH 2); 13C NMR (151 MHz, CD3OD): δ = 140.1; 139.6; 137.4; 137.1; 129.5; 129.4; 125.1; 114.0; 112.7; 50.4 (d, J = 18.1 Hz, PCCC); 41.6; 23.6 (d, J = 4.0 Hz, PCC); 23.5 (d, J = 139.2 Hz, PC); 19.3; 19.1; 31P NMR (243 MHz, CD3OD): δ = 28.08 ppm. Anal. Calcd. for C15H20N5O3P: C, 51.57; H, 5.77; N, 20.05. Found: C, 51.80; H, 5.52; N, 19.92.
4.1.6.4. 3-(4-{[3-Benzoyl-2,4-dioxopyrimidin-1-yl]methyl}-1H-1,2,3-triazol-1-yl)propylphosphonic acid 31m
From 22m (0.130 g, 0.274 mmol) phosphonic acid 31m (0.093 g, 81%) was obtained as a colourless oil; IR (film): ν = 3396, 3010, 2983, 2967, 1665, 1654, 1436; 1237, 978, 782, 701 cm−1; 1H NMR (300 MHz, CD3OD): δ = 8.30 (s, 1H, HC5′); 7.98–7.94 (m, 2H, H aromat); 7.91 (d, J = 8.0 Hz, 1H, HC CH); 7.75–7.69 (m, 1H, H aromat); 7.58–7.52 (m, 2H, H aromat); 5.86 (d, J = 8.0 Hz, 1H, HC CH); 5.15 (s, 2H, CH2); 4.60 (t, J = 7.1 Hz, 2H, PCCCH 2); 2.29–2.18 (m, 2H, PCCH 2); 1.83–1.71 (m, 2H, PCH 2); 13C NMR (75.5 MHz, CD3OD): δ = 168.9 (s, C O); 163.1 (s, C O); 150.0 (s, C O); 145.8; 140.4; 135.2; 131.4; 130.2; 129.2; 129.1; 128.3; 126.3; 101.6; 101.6; 51.5 (d, J = 17.9 Hz, PCCC); 42.4; 23.3 (s, J = 1.4 Hz, PCC); 23.4 (d, J = 146.8 Hz, PC); 31P NMR (121 MHz, CD3OD): δ = 29.63 ppm. Anal. Calcd. for C15H18N5O6P × H2O: C, 46.69; H, 4.61; N, 16.01. Found: C, 46.74; H, 4.70; N, 15.94.
4.1.6.5. 4-(4-{[6-Aminopurin-9-yl]methyl}-1H-1,2,3-triazol-1-yl)butylphosphonic acid 32a
From 23a (0.063 g, 0.154 mmol) phosphonic acid 32a (0.050 g, 93%) was obtained as a white powder; m.p: 217–220 °C; solubility of 32a in methanol or water was insufficient to measure the 13C NMR spectrum; IR (KBr): ν = 3460, 3300, 3100, 2981, 2910, 2880, 1660, 1647, 1240, 1023 cm−1; 1H NMR (600 MHz, CD3OD): δ = 8.45 (s, 1H); 8.42 (s, 1H); 8.15 (s, 1H); 5.65 (s, 2H, CH2); 4.46 (t, J = 7.0 Hz, 2H, PCCCCH 2); 2.03 (qv, J = 7.0 Hz, 2H, PCCCH 2); 1.77–1.72 (m, 2H, PCH 2); 1.64–1.57 (m, 2H, PCCH 2); 31P NMR (243 MHz, CD3OD): δ = 29.02 ppm. Anal. Calcd. for C12H17N8O3P × H2O: C, 38.92; H, 5.17; N, 30.26. Found: C, 39.09; H, 4.99; N, 30.49.
4.1.6.6. 4-(4-{[6-Aminopurin-9-yl]methyl}-1H-1,2,3-triazol-1-yl)butylphosphonic acid 32b
From 23b (0.060 g, 0.150 mmol) phosphonic acid 32b (0.042 g, 80%) was obtained as an amorphous solid; m.p.: 224–226 °C; solubility of 32b in methanol or water was insufficient to measure the 13C NMR spectrum; IR (KBr): ν = 3445, 3102, 2980, 2910, 1668, 1223, 1025 cm−1; 1H NMR (600 MHz, CD3OD): δ = 8.30 (s, 1H, HC5′); 7.60 (d, J = 1.0 Hz, 1H, HC CCH3); 5.08 (s, 2H, CH2); 4.55 (t, J = 7.1 Hz, 2H, PCCCCH 2); 2.09 (qv, J = 7.0 Hz, 2H, PCCCH 2); 1.90 (d, J = 1.0 Hz, 3H, HC CCH 3); 1.84–1.79 (m, 2H, PCH 2); 1.70–1.63 (m, 2H, PCCH 2); 31P NMR (243 MHz, CD3OD): δ = 29.86 ppm. Anal. Calcd. for C12H18N5O5P × H2O: C, 39.89; H, 5.58; N, 19.38. Found: C, 40.10; H, 5.43; N, 19.24.
4.1.6.7. 4-(4-{[2,4-Dioxopyrimidin-1-yl]methyl}-1H-1,2,3-triazol-1-yl)butylphosphonic acid 32c
From 23c (0.072 g, 0.187 mmol) phosphonic acid 32c (0.052 g, 84%) was obtained as an amorphous solid; m.p.: 204–206 °C; IR (KBr): ν = 3440, 3112, 2980, 2942, 1667, 1219, 1020 cm−1; 1H NMR (600 MHz, CD3OD): δ = 8.27 (s, 1H, HC5′); 7.74 (d, J = 7.9 Hz, 1H, HC CH); 5.71 (d, J = 7.9 Hz, 1H, HC CH); 5.01 (s, 2H, CH2); 4.39 (t, J = 7.0 Hz, 2H, PCCCCH 2); 2.06 (qv, J = 7.3 Hz, 2H, PCCCH 2); 1.84–1.78 (m, 2H, PCCH 2); 1.70–1.62 (m, 2H, PCH 2); 13C NMR (151 MHz, CD3OD): δ = 166.5 (s, C O); 151.9 (s, C O); 146.6; 141.5; 125.5; 102.1; 50.5; 43.0; 30.6 (d, J = 16.7 Hz, PCCC); 25.5 (d, J = 135.3 Hz, PC); 19.0 (d, J = 4.5 Hz, PCC); 31P NMR (243 MHz, CD3OD): δ = 29.81 ppm. Anal. Calcd. for C11H16N5O5P × H2O: C, 38.05; H, 5.22; N, 20.17. Found: C, 38.17; H, 5.48; N, 20.02.
4.1.6.8. 3-{4-[(1,3-Dimethyl-2,6-dioxopurin-7-yl)methyl]-1H-1,2,3-triazol-1-yl}-1-hydroxyethyphosphonic acid 33i
From 24i (0.116 g, 0.263 mmol) phosphonic acid 33i (0.071 g, 70%) was obtained as a white powder; m.p.: <244 °C; solubility of 33i in methanol or water was insufficient to measure the 13C NMR spectrum; IR (KBr): ν = 3344, 3102, 2986, 1699, 1672, 1220, 1015 cm−1; 1H NMR (300 MHz, CD3OD): δ = 8.19 (s, 1H); 8.17 (s, 1H); 5.69 (s, 2H, CH2); 4.80 (ddd, J = 14.2 Hz, J = 4.0 Hz, J = 2.7 Hz, 1H, PCCH aHb); 4.51 (ddd, J = 14.2 Hz, J = 10.3 Hz, J = 5.8 Hz, 1H, PCCHa H b); 4.17 (dt, J = 10.3 Hz, J = 2.7 Hz, 1H, PCH(OH)); 3.53 (s, 3H, CH3); 3.35 (s, 3H, CH3); 31P NMR (121 MHz, CD3OD): δ = 19.61 ppm. Anal. Calcd. for C12H16N7O6P: C, 37.41; H, 4.19; N, 25.45. Found: C, 37.56; H, 4.28; N, 25.30.
4.2. Antiviral activity assays
The antiviral assays were based on inhibition of virus-induced cytopathicity in HEL [herpes simplex virus type 1 (HSV-1), HSV-2 (G), vaccinia virus and vesicular stomatitis virus], Vero (parainfluenza-3, reovirus-1, Sindbis, Coxsackie B4, and Punta Toro virus), HeLa (vesicular stomatitis virus, Coxsackie virus B4, and respiratory syncytial virus), MDCK (influenza A (H1N1 and H3N1) and influenza B virus) or CRFK (feline herpes virus; feline corona virus (FIPV)) cell cultures. Confluent cell cultures in microtiter 96-well plates were inoculated with 100 cell culture inhibitory dose-50 (CCID50) of virus (1 CCID50 being the virus dose to infect 50% of the cell cultures) in the presence of varying concentrations (100, 20, 4, … μM) of the test compounds. Viral cytopathicity was recorded as soon as it reached completion in the control virus-infected cell cultures that were not treated with the test compounds. The antiviral concentration was expressed as the EC50 or 50%-effective compound concentration required to inhibit virus-induced cytopathicity by 50%.
4.3. Cytotoxicity and cytostatic assays
The cytotoxicity of the test compounds was monitored as a microscopically visible alteration of cell morphology, and expressed as the minimal cytotoxic concentration (MCC) or compound concentration required to afford a microscopically detectable alteration of cell culture morphology.
The cytostatic activity of the test compounds was determined as the 50% cytostatic concentration (IC50) or compound concentration required to inhibit cell proliferation by 50%. For this purpose, cells were seeded in 200 μl-wells of 96-well microtiter plates and allowed to proliferate for 2 (murine leukaemia L1210) to 3 (human T-lymphocyte CEM, human cervix carcinoma HeLa) days in the absence or presence of different serial concentrations of the test compounds. At the end of the exponential proliferation phase, the cells were counted by an automated Coulter ZI particle counter (Analis, Ghent, Belgium).
Acknowledgements
The authors wish to express their gratitude to Mrs. Małgorzata Pluskota, Mrs. Leentje Persoons, Mrs. Frieda De Meyer and Mrs. Lizette van Berckelaer for excellent technical assistance. This work was supported by the National Science Centre under Decision UMO-2011/01/D/NZ4/01276. The virological part of this work was supported by the K.U. Leuven (GOA no. 10/014).
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.ejmech.2013.10.057.
Appendix A. Supplementary data
References
- 1.Naesens L., Snoeck R., Andrei G., Balzarini J., Neyts J., De Clercq E. HPMPC (cidofovir), PMEA (adefovir) and related acyclic nucleoside phosphonate analogues: a review of their pharmacology and clinical potential in the treatment of viral infections. Antivir. Chem. Chemother. 1997;8:1–23. [Google Scholar]
- 2.Starrett J.E., Jr., Tortolani D.R., Hitchcock M.J., Martin J.C., Mansuri M.M. Synthesis and in vitro evaluation of a phosphonate prodrug: bis(pivaloyloxymethyl) 9-(2-phosphonylmethoxyethyl)adenine. Antivir. Res. 1992;19:267–273. doi: 10.1016/0166-3542(92)90084-i. [DOI] [PubMed] [Google Scholar]
- 3.Srinivas R.V., Fridland A. Antiviral activities of 9-R-2-phosphonomethoxypropyl adenine (PMPA) and bis(isopropyloxymethylcarbonyl)PMPA against various drug-resistant human immunodeficiency virus strains. Antimicrob. Agents Chemother. 1998;42:1484–1487. doi: 10.1128/aac.42.6.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Clercq E. Towards an effective chemotherapy of virus infections: therapeutic potential of cidofovir [(S)-1-[3-hydroxy-2-(phosphonomethoxy)propyl]cytosine, HPMPC] for the treatment of DNA virus infections. Collect. Czech. Chem. Commun. 1998;63:480–506. [Google Scholar]
- 5.Carell T., Brandmayr C., Hienzsch A., Müller M., Pearson D., Reiter V., Thoma I., Thumbs P., Wagner M. Structure and function of noncanonical nucleobases. Angew. Chem. Int. Ed. 2012;51:7110–7131. doi: 10.1002/anie.201201193. [DOI] [PubMed] [Google Scholar]
- 6.Gish R.G. Treating HCV with ribavirin analogues and ribavirin-like molecules. J. Antimicrob. Chemother. 2006;57:8–13. doi: 10.1093/jac/dki405. [DOI] [PubMed] [Google Scholar]
- 7.Graci J.D., Cameron C.E. Mechanism of action of ribavirin against distinct viruses. Rev. Med. Virol. 2006;16:37–48. doi: 10.1002/rmv.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moriyama K., Suzuki T., Negishi K., Graci J.D., Thompson C.N., Cameron C.E., Watanabe M. Effect of introduction of hydrophobic group on ribavirin base on mutation induction and Anti-RNA viral activity. J. Med. Chem. 2008;51:159–166. doi: 10.1021/jm7009952. [DOI] [PubMed] [Google Scholar]
- 9.Brochot E., Castelain S., Duverlie G., Carpon D., Nguyen-Khac E., François C. Ribavirin monitoring in chronic hepatitis C therapy: anemia versus efficacy. Antivir. Ther. 2010;15:687–695. doi: 10.3851/IMP1609. [DOI] [PubMed] [Google Scholar]
- 10.Parker B.W. Metabolism and antiviral activity of ribavirin. Virus Res. 2005;101:165–171. doi: 10.1016/j.virusres.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 11.Witkowski J.T., Robins R.K., Khare G.P., Sidwell R.W. Synthesis and antiviral activity of 1,2,4-triazole-3-thiocarboxamide and 1,2,4-triazole-3-carboxamidine ribonucleosides. J. Med. Chem. 1973;16:935–937. doi: 10.1021/jm00266a014. [DOI] [PubMed] [Google Scholar]
- 12.Allen L.B., Boswell K.H., Khwaja T.A., Meyer R.B., Jr., Sidwell R.W., Witkowski J.T., Christensen L.F., Robins R.K. Synthesis and antiviral activity of some phosphate of the broad-spectrum antiviral nucleoside, 1-β-d-ribofuranosyl-1,2,4-triazole-3-carboxamide (ribavirin) J. Med. Chem. 1978;21:742–746. doi: 10.1021/jm00206a005. [DOI] [PubMed] [Google Scholar]
- 13.Guigas B., Sakamoto K., Taleux N., Reyna S.M., Musi N., Viollet B., Hue L. Beyond AICA riboside: in search of new specific AMP-activated protein kinase activators. IUBMB Life. 2009;61:18–26. doi: 10.1002/iub.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Acadesine AICA riboside, ARA 100, arasine, GP 1–110. Drugs R & D. 2008;9:169–175. doi: 10.2165/00126839-200809030-00004. [DOI] [PubMed] [Google Scholar]
- 15.Firestine S.M., Wu W., Youn H., Davisson V.J. Interrogating the mechanism of a tight binding inhibitor of AIR carboxylase. Bioorg. Med. Chem. 2009;17:794–803. doi: 10.1016/j.bmc.2008.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferris J.P., Devadas B., Huang Ch.-H., Ren W.-Y. Nucleosides from carbohydrate adducts of diaminomaleonitrile. A novel synthesis of 5-amino-1-(β-d-ribopyranosyl)imidazole-4-carboxamide. J. Org. Chem. 1985;50:747–754. [Google Scholar]
- 17.Alvarez R., Velázquez S., San-Félix A., Aquaro S., De Clercq E., Perno C.-F., Karlsson A., Balzarini J., Camarasa M.J. 1,2,3-Triazole-[2,5-bis-O-(tert-butyldimethylsilyl)-beta-d-ribofuranosyl]-3′-spiro-5′-(4′-amino-1′,2′-oxathiole 2′,2′-dioxide) (TSAO) analogs: synthesis and anti-HIV-1 activity. J. Med. Chem. 1994;37:4185–4194. doi: 10.1021/jm00050a015. [DOI] [PubMed] [Google Scholar]
- 18.San-Félix A., Alvarez R., Velázquez S., De Clercq E., Balzarini J., Camarasa M.J. Synthesis and anti-HIV-1 activity of 4- and 5-substituted 1,2,3-triazole-TSAO derivatives. Nucleosides & Nucleotides. 1995;14:595–598. [Google Scholar]
- 19.Agalave S.G., Maujan S.R., Pore V.S. Click chemistry: 1,2,3-triazoles as pharmacophores. Chem. Asian J. 2011;6:2696–2718. doi: 10.1002/asia.201100432. [DOI] [PubMed] [Google Scholar]
- 20.Koszytkowska-Stawińska M., Mironiuk-Puchalska E., Rowicki T. Synthesis of 1,2,3-triazolo-nucleosides via the post-triazole N-alkylation. Tetrahedron. 2012;68:214–225. [Google Scholar]
- 21.Kaczmarek O., Scheidt H.A., Bunge A., Föse D., Karsten S., Arbuzova A., Huster D., Liebscher J. 2′-Linking of lipids and other functions to uridine trough 1,2,3-triazoles and membrane anchoring of the amphiphilic products. Eur. J. Org. Chem. 2010:1579–1586. [Google Scholar]
- 22.Ganesan M., Muraleedharan K.M. Synthesis of β-hydroxyphosphonate and 1,2,-dihydroxyacyclic nucleoside analogs via 1,3-dipolar cycloaddition strategy. Nucleosides, Nucleotides and Nucleic Acids. 2010;29:91–96. doi: 10.1080/15257771003597709. [DOI] [PubMed] [Google Scholar]
- 23.Park S.M., Yang H., Park S.-K., Kim H.M., Kim B.H. Design, synthesis, and anticancer activities of novel perfluoroalkyltriazole-appended 2′-deoxyuridines. Bioorg. Med. Chem. Lett. 2010;20:5831–5834. doi: 10.1016/j.bmcl.2010.07.126. [DOI] [PubMed] [Google Scholar]
- 24.Chaudhary P.M., Chavan S.R., Shirazi F., Razdan M., Nimkar P., Maybhate S.P., Likhite A.P., Gonnade R., Hazara B.G., Deshpande M.V., Deshpande S.R. Exploration of click reaction for the synthesis of modified nucleosides as chitin synthase inhibitors. Bioorg. Med. Chem. 2009;17:2433–2440. doi: 10.1016/j.bmc.2009.02.019. [DOI] [PubMed] [Google Scholar]
- 25.Fujino T., Yamazaki N., Isobe H. Convergent synthesis of oligomers of triazole-linked DNA analogue (TLDNA) in solution phase. Tetrahedron Lett. 2009;50:4101–4103. [Google Scholar]
- 26.Lee Y.-S., Park S.M., Kim H.M., Park S.-K., Lee K., Lee Ch. W., Kim B.H. C5-Modified nucleosides exhibiting anticancer activity. Bioorg. Med. Chem. Lett. 2009;19:4688–4691. doi: 10.1016/j.bmcl.2009.06.072. [DOI] [PubMed] [Google Scholar]
- 27.Lazrek H.B., Taourirte M., Oulih T., Barascut J.L., Imbach J.L., Pannecouque C., Witvrouw M., De Clercq E. Synthesis and anti-HIV activity of new modified 1,2,3-triazole acyclonucleosides. Nucleosides, Nucleotides and Nucleic Acids. 2001;20:1949–1960. doi: 10.1081/NCN-100108325. [DOI] [PubMed] [Google Scholar]
- 28.Moukha-Chafiq O., Taha M.L., Lazrek H.B., Pannecouque C., Witvrouw M., De Clercq E. Synthesis and biological evaluation of some 4-substituted 1-[1-(4-hydroxybutyl)-1,2,3-triazol-(4 & 5)-ylmethyl]-1H-pyrazolo[3,4-d]pyrimidines. Nucleosides, Nucleotides and Nucleic Acids. 2001;20:1811–1821. doi: 10.1081/NCN-100107192. [DOI] [PubMed] [Google Scholar]
- 29.Moukha-Chafiq O., Taha M.L., Lazrek H.B., Vasseur J.J., Pannecouque C., Witvrouw M., De Clercq E., Barascut J.L., Imbach J.L. Synthesis and biological activity of 4-substituted 1-[1-(2-hydroxyethoxy)-methyl-1,2,3-triazol-(4 & 5)-ylmethyl]-1H-pyrazolo[3,4-d]pyrimidines. Nucleosides, Nucleotides and Nucleic Acids. 2001;20:1797–1810. doi: 10.1081/NCN-100107191. [DOI] [PubMed] [Google Scholar]
- 30.Krim J., Taourirte M., Engels J.W. Synthesis of 1,4-disubstituted mono and bis-triazolocarboacyclonucleoside analogues of 9-(4-hydroxybutyl)guanine by Cu(I)-catalyzed click azide–alkyne cycloaddition. Molecules. 2012;17:179–190. doi: 10.3390/molecules17010179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trakossas S., Coutouli-Argyopoulou E., Hadjipavlou-Litina D. Synthesis of modified triazole nucleosides possessing one or two base moieties via a click chemistry approach. Tetrahedron Lett. 2011;14:1673–1676. [Google Scholar]
- 32.Elayadi H., Smietana M., Pannecouque Ch., Leyssen P., Neyts J., Vasseur J.-J., Lazrek H.B. Straightforward synthesis of triazoloacyclonucleotide phosphonates as potential HCV inhibitors. Bioorg. Med. Chem. Lett. 2010;20:7365–7368. doi: 10.1016/j.bmcl.2010.10.046. [DOI] [PubMed] [Google Scholar]
- 33.Lazrek H.B., Taourirte M., Oulih T., Kabbaj Y., Barascut J.L., Imbach J.L., Almasoudi N.A., Pfleiderer W. Nucleosides & Nucleotides. 1997;16:1073–1077. [Google Scholar]
- 34.Chittepu P., Sirivolu V.R., Seela F. Nucleosides and oligonucleotides containing 1,2,3-triazole residues with nucleobase tethers: synthesis via the azide–alkyne “click” reaction. Bioorg. Med. Chem. 2008;16:8427–8439. doi: 10.1016/j.bmc.2008.08.026. [DOI] [PubMed] [Google Scholar]
- 35.Yu J.-L., Wu Q.-P., Zhang Q.-S., Liu Y.-H., Li Y.-Z., Zhou Z.-M. Synthesis and antitumor activity of novel 2′,3′-dideoxy-2′,3′-diethanethionucleosides bearing 1,2,3-triazole residue. Bioorg. Med. Chem. Lett. 2010;20:240–243. doi: 10.1016/j.bmcl.2009.10.127. [DOI] [PubMed] [Google Scholar]
- 36.Piotrowska D.G., Balzarini J., Głowacka I.E. Design, synthesis, antiviral and cytostatic evaluation of novel isoxazolidine nucleotide analogues with 1,2,3-triazole linker. Eur. J. Med. Chem. 2012;47:501–509. doi: 10.1016/j.ejmech.2011.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu J.-L., Wu Q.-P., Zhang Q.-S., Liu Y.-H., Li Y.-Z., Zhou Z.-M. Synthesis and antitumor activity of novel 2′,3′-dideoxy-2′,3′-diethane thionucleosides bearing 1,2,3-triazole residues. Bioorg. Med. Chem. Lett. 2010;20:240–243. doi: 10.1016/j.bmcl.2009.10.127. [DOI] [PubMed] [Google Scholar]
- 38.Kamal A., Shankaraiah N., Devaiah V., Reddy K.L., Juvekar A., Sen S., Kurian N., Zingde S. Synthesis of 1,2,3-triazole-linked pyrrolobenzodiazepine conjugates employing ‘click’ chemistry: DNA-binding affinity and anticancer activity. Bioorg. Med. Chem. Lett. 2008;18:1468–1473. doi: 10.1016/j.bmcl.2007.12.063. [DOI] [PubMed] [Google Scholar]
- 39.Chen Y., Lopez-Sanchez M., Savoy D.N., Billadeau D.D., Dow G.S., Kozikowski A.P. A series of potent and selective, triazolylphenyl-based histone deacetylases inhibitors with activity against pancreatic cancer cells and Plasmodium falciparum. J. Med. Chem. 2008;51:3437–3448. doi: 10.1021/jm701606b. [DOI] [PubMed] [Google Scholar]
- 40.Vantikommu J., Palle S., Reddy P.S., Ramanatham V., Khagga M., Pallapothula V.R. Synthesis and cytotoxicity evaluation of novel 1,4-disubstituted 1,2,3-triazoles via CuI catalysed 1,3-dipolar cycloaddition. Eur. J. Med. Chem. 2010;45:5044–5050. doi: 10.1016/j.ejmech.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 41.Akri K.E., Bougrin K., Balzarini J., Faraj A., Benhida R. Efficient synthesis and in vitro cytostatic activity of 4-substituted triazoyl-nucleosides. Bioorg. Med. Chem. Lett. 2007;16:6656–6659. doi: 10.1016/j.bmcl.2007.08.077. [DOI] [PubMed] [Google Scholar]
- 42.Yan S.-J., Liu Y.-J., Chen Y.-L., Liu L., Lin J. An efficient one pot synthesis of heterocycle-fused 1,2,3-triazole derivatives as anti-cancer agents. Bioorg. Med. Chem. Lett. 2010;20:5225–5228. doi: 10.1016/j.bmcl.2010.06.141. [DOI] [PubMed] [Google Scholar]
- 43.Elanki A.R., Islam A., Rama V.S., Pulipati R.P., Rambadu D., Krishna G.R., Reddy C.M., Mukkanti K., Vanaja G.R., Kalle A.M., Kumar K.S., Pal M. Solvent effect on copper-catalyzed azide–alkyne cycloaddition (CuAAC): synthesis of novel triazolyl substituted quinazolines as potential anticancer agents. Bioorg. Med. Chem. Lett. 2012;22:3455–3459. doi: 10.1016/j.bmcl.2012.03.091. [DOI] [PubMed] [Google Scholar]
- 44.Singh P., Raj R., Kumar V., Mahajan M.P., Bedi P.M.S., Kaur T., Saxena A.K. 1,2,3-Triazole tethered β-lactam-chalkone bifunctional hybrids: synthesis and anticancer evaluation. Eur. J. Med. Chem. 2012;47:594–600. doi: 10.1016/j.ejmech.2011.10.033. [DOI] [PubMed] [Google Scholar]
- 45.Zhou L., Amer A., Korn M., Burda R., Balzarini J., De Clercq E., Kern E.R., Torrence P.F. Synthesis and antiviral activities of 1,2,3-triazole functionalized thymidines: 1,3-dipolar cycloaddition for efficient regioselective diversity generation. Antivir. Chem. Chemother. 2005;16:375–383. doi: 10.1177/095632020501600604. [DOI] [PubMed] [Google Scholar]
- 46.Mohan S., McAtamney S., Haselhorst T., von Itzstein M., Pinto B.M. Carbocycles related to oseltamivir as influenza virus group-1-specific neuraminidase inhibitors. Binding to N1 enzymes in the context of virus-like particles. J. Med. Chem. 2010;53:7377–7391. doi: 10.1021/jm100822f. [DOI] [PubMed] [Google Scholar]
- 47.Miyakoshi H., Miyahara S., Yokogawa T., Endoh K., Muto T., Yano W., Wakas T., Ueno H., Chong K.T., Tagichi J., Nomura M., Tako Y., Fujioka A., Hashimoto A., Itou K., Yamamura K., Shoto S., Nagasawa H., Fukuoka M. 1,2,3-Triazole-containing uracil derivatives with excellent pharmacokinetics as novel class of potent human deoxyuridine triphosphate inhibitors. J. Med. Chem. 2012;55:6427–6437. doi: 10.1021/jm3004174. [DOI] [PubMed] [Google Scholar]
- 48.Cheng H., Wan J., Lin M.-I., Lu X., Liu J., Xu Y., Chen J., Tu Z., Cheng Y.-S.E., Ding K. Design, synthesis, and in vitro biological evaluation of 1H-1,2,3-triazole-4-carboxyamide derivatives as new anti-influenza A agents. J. Med. Chem. 2012;55:2144–2153. doi: 10.1021/jm2013503. [DOI] [PubMed] [Google Scholar]
- 49.Aufort M., Herscovici J., Bouhours P., Moreau N., Girard C. Synthesis and antibiotic activity of a small molecules library of 1,2,3-triazole derivatives. Bioorg. Med. Chem. Lett. 2008;18:1195–1198. doi: 10.1016/j.bmcl.2007.11.111. [DOI] [PubMed] [Google Scholar]
- 50.Demaray J.A., Thuener J.E., Dawson M.N., Sucheck S.J. Synthesis of triazole-oxazolidinones via a one-pot reaction and evaluation of their antimicrobial activity. Bioorg. Med. Chem. Lett. 2008;18:4868–4871. doi: 10.1016/j.bmcl.2008.07.087. [DOI] [PubMed] [Google Scholar]
- 51.Wang X.-L., Wan K., Zhou Ch.-H. Synthesis of novel sulfanilamide-derived 1,2,3-triazoles and their evaluation for antibacterial and antifungal activities. Eur. J. Med. Chem. 2010;45:4631–4639. doi: 10.1016/j.ejmech.2010.07.031. [DOI] [PubMed] [Google Scholar]
- 52.Valverde I.E., Lecaille F., Lalmonach G., Aucagne V., Delmas A.F. Synthesis of a biologically active triazole-containing analogue of cystatin A through successive peptidomimetic alkyne-azide ligations. Angew. Chem. Int. Ed. 2012;51:718–722. doi: 10.1002/anie.201107222. [DOI] [PubMed] [Google Scholar]
- 53.Lampo V.L., Sesti-Costa R., Carneiro Z.A., Silva J.S., Schenkman S., Carvalho I. Design, synthesis and the effect of 1,2,3-triazole sialylmimetic neoglycoconiugates on Trypanosoma cruzi and its cell surface trans-sialidase. Bioorg. Med. Chem. 2012;20:145–156. doi: 10.1016/j.bmc.2011.11.022. [DOI] [PubMed] [Google Scholar]
- 54.Akselsen Ø.W., Odlo K., Cheng J.-J., Maccari G., Botta M., Hansen T.V. Synthesis, biological evaluation and molecular modeling of 1,2,3-triazole analogs of combretastatin A-1. Bioorg. Med. Chem. 2012;20:234–242. doi: 10.1016/j.bmc.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 55.Singh O., Singh P., Kumar M., Gut J., Rosenthal P.J., Kumar K., Kumar V., Mahajan M.P., Bisetty K. Synthesis, docking and in vitro antimalarian evaluation of bifunctional hybrids derived from β-lactams and 7-chloroquinoline using click chemistry. Bioorg. Med. Chem. Lett. 2012;22:57–61. doi: 10.1016/j.bmcl.2011.11.082. [DOI] [PubMed] [Google Scholar]
- 56.Chan D.C.M., Laughton C.A., Queener S.F., Stevens M.F.G. Structural studies on bioactive compounds. part 36: design, synthesis and biological evaluation of pyrimethamine-based antifolates against Pneumocystis carinii. Bioorg. Med. Chem. 2002;10:3001–3010. doi: 10.1016/s0968-0896(02)00128-1. [DOI] [PubMed] [Google Scholar]
- 57.Jordan D.B., Basarab G.S., Liao D.-I., Johnson W.M.P., Winzenberg K.N., Winkler D.A. Structure-based design of inhibitors of the rice blast fungal enzyme trihydroxynaphtalene reductase. J. Mol. Graph. Modell. 2001;19:434–447. doi: 10.1016/s1093-3263(00)00103-0. [DOI] [PubMed] [Google Scholar]
- 58.Reddy L.V.R., Reddy P.V., Mishra N.N., Shukla P.K., Yadav G., Srivastava R., Shaw A.K. Synthesis and biological evaluation of glycal-derived novel tetrahydrofuran 1,2,3-triazoles by ‘click’ chemistry. Carbohydr. Res. 2010;345:1515–1521. doi: 10.1016/j.carres.2010.03.031. [DOI] [PubMed] [Google Scholar]
- 59.McNulty J., Nair J.J., Vurgun N., DiFrancesco B.R., Brown C.E., Tsoi B., Crankshaw D.J., Holloway A.C. Discovery of novel class of aldol-derived 1,2,3-triazoles: potent and selective inhibitors of human cytochrome P450 !9A1 (aromatase) Bioorg. Med. Chem. Lett. 2012;22:718–722. doi: 10.1016/j.bmcl.2011.10.039. [DOI] [PubMed] [Google Scholar]
- 60.Zou Y., Zhao Q., Liao J., Hu H., Yu S., Chai X., Xu M. New triazole derivatives as antifungal agents: synthesis via click reaction, in vitro evaluation and molecular docking studies. Bioorg. Med. Chem. Lett. 2012;22:2959–2962. doi: 10.1016/j.bmcl.2012.02.042. [DOI] [PubMed] [Google Scholar]
- 61.Norris P., Horton D., Levine B.R. Cycloaddition of acetylenes with 5-azido-5-deoxy-d-aldopentose derivatives: synthesis of triazole reversed nucleoside analogs. Heterocycles. 1996;43:2643–2655. [Google Scholar]
- 62.Talekar R.R., Wightman R.H. Synthesis of same pyrrolo[2,3-d]pyrimidine and 1,2,3-triazole isonucleosides. Tetrahedron. 1997;53:3831–3842. [Google Scholar]
- 63.Tornøe C.W., Christensen C., Meldal M. Peptidotriazoles on solid phase: [1,2,3]-triazoles by regiospecific copper(I)-catalyzed 1,3-dioplar cycloadditions of terminal alkynes to azides. J. Org. Chem. 2002;67:3057–3064. doi: 10.1021/jo011148j. [DOI] [PubMed] [Google Scholar]
- 64.Rostovtsev V.V., Green L.G., Fokin V.V., Sharpless K.B. A stepwise Huisgen cycloaddition process: copper(I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew. Chem. Int. Ed. 2002;41:2596–2599. doi: 10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 65.Głowacka I.E. Synthesis of enantiomerically pure diethyl (R)- and (S)-2-hydroxy-3-(1,2,3-triazol-1-yl)propylphosphonates. Tetrahedron: Asymmetry. 2009;20:2270–2278. [Google Scholar]
- 66.Głowacka I.E., Cieślak M., Piotrowska D.G. Synthesis of novel 1-hydroxy-2-(1,2,3-triazol-1-yl)ethylphosphonates and 2-hydroxy-3-(1,2,3-triazol-1-yl)propylphosphonates. Phosphorus, Sulfur and Silicon. 2011;186:431–449. [Google Scholar]
- 67.Wróblewski A.E., Głowacka I.E. Synthesis of (1R,2S)- and (1S,2S)-3-(4-carbamoyl-1,2,3-triazol-1-yl)-1,2-dihydroxypropylphosphonates. Tetrahedron: Asymmetry. 2004;15:1457–1464. [Google Scholar]
- 68.Wróblewski A.E., Głowacka I.E. Synthesis of four enantiomerically pure 4-(4-carbamoyl-1,2,3-triazol-1-yl)-2,3-dihydroxy-1-methoxybutylphosphonic acids. Tetrahedron: Asymmetry. 2005;16:4056–4064. [Google Scholar]
- 69.Głowacka I.E., Balzarini J., Wróblewski A.E. Design, synthesis, antiviral, and cytotoxic evaluation of novel phosphonylated 1,2,3-triazoles as acyclic nucleotide analogues. Nucleosides, Nucleotides and Nucleic Acids. 2012;31:293–318. doi: 10.1080/15257770.2012.662611. [DOI] [PubMed] [Google Scholar]
- 70.Głowacka I.E., Balzarini J., Wróblewski A.E. Synthesis and biological evaluation of novel 1,2,3-triazolonucleotides. Arch. Pharm. Chem. Life Sci. 2013;346:278–291. doi: 10.1002/ardp.201200421. [DOI] [PubMed] [Google Scholar]
- 71.Lindsell W.E., Murray Ch., Preston P.N., Woodman T.A.J. Synthesis of 1,3-diynes in the purine, pyrimidine, 1,3,5-triazine and acridine series. Tetrahedron. 2000;56:1233–1245. [Google Scholar]
- 72.F. Himmelsbacg, M. Mark, M. Eckhardt, E. Langkopf, R. Maier, Xanthine derivatives, the preparation thereof and use as pharmaceutical compositions, US2002/198205, 2002 (A1).
- 73.M.J.A. Waer, P.A.M.M. Herdewijn, W.E. Pfleiderer, Immunosupressive effects of 8-substituted xanthine derivatives, US7,253,176 B1, 2007.
- 74.Casaschi A., Grigg R., Sansano J.M. Palladium catalysed tandem cyclization-anion capture. Part 6:1 synthesis of sugar. Nucleoside, purine, benzodiazepinone and β-lactam analogues via capture of in situ generated vinylstannanes. Tetrahedron. 2000;56:7553–7560. [Google Scholar]
- 75.Daly J.W., Padgett W.L., Shamim M.T. Analogues of caffeine and theophylline: effect of structural alterations on affinity at adenosine receptors. J. Med. Chem. 1986;29:1305–1308. doi: 10.1021/jm00157a035. [DOI] [PubMed] [Google Scholar]
- 76.Casaschi A., Grigg R., Sansano J.M. Palladium catalysed tandem cyclization-anion capture. Part 7:1 synthesis of α-amino esters, nitrogen heterocycles and β-aryl/hetroaryl ethylamines via in situgenerated vinylstannanes. Tetrahedron. 2001;57:607–615. [Google Scholar]
- 77.Pérez-Serrano L., Casarrubios L., Dominguez G., González-Pérez P., Pérez-Castells J. Synthesis of enynoindoles via vinyl and ethynyl indoles. Synthesis. 2002;13:1810–1812. [Google Scholar]
- 78.Bankowska E., Balzarini J., Głowacka I.E., Wróblewski A.E. Design, synthesis and antiviral and cytotoxic evaluation of novel acyclic phosphonate nucleotide analogues with a 5,6-dihydro-1H-[1,2,3]triazolo[4,5-d]pyridazine-4,7-dione system. Monatsh. Chem. 2013 doi: 10.1007/s00706-013-1137-x. under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Głowacka I.E., Balzarini J., Wróblewski A.E. Synthesis of a new series of phosphonylated 1,2,3-triazoles as acyclic analogues of ribavirin. Arch. Pharm. Chem. Life Sci. 2013;346:677–687. doi: 10.1002/ardp.201300156. [DOI] [PubMed] [Google Scholar]
- 80.Neumann U., Gütschow M. 3,1-Benzothiazin-4-ones and 3,1-benzoxazin-4-ones: highly different activities in chymotrypsin inactivation. Bioorg. Chem. 1995;32:72–88. [Google Scholar]
- 81.Lee Y.-S., Kim B.H. Heterocyclic nucleoside analogues: design and synthesis of antiviral, modified nucleosides containing isoxazole heterocycles. Bioorg. Med. Chem. Lett. 2002;12:1395–1397. doi: 10.1016/s0960-894x(02)00182-8. [DOI] [PubMed] [Google Scholar]
- 82.Saito Y., Escuret V., Durantel D., Zoulim F., Schinazi R.F., Agrofoglio L.A. Synthesis of 1,2,3-triazolo-carbonucleoside analogues of ribavirin targeting an HCV in replication. Bioorg. Med. Chem. 2003;11:3633–3639. doi: 10.1016/s0968-0896(03)00349-3. [DOI] [PubMed] [Google Scholar]
- 83.Karplus M. Vicinal proton coupling in nuclear magnetic resonance. J. Am. Chem. Soc. 1963;85:2870–2871. [Google Scholar]
- 84.Benezra C. The variation of vicinal 31P–C–C–1H couplings with dihedral angle in phosphonates. J. Am. Chem. Soc. 1973;95:6890–6894. [Google Scholar]
- 85.Neeser J.-R., Tronchet J.M.J., Charollais E.J. Structural analysis of 3-C-phosphonates, -phosphinates, and -phosphine oxides of branched-chain sugars. Can. J. Chem. 1983;61:2112–2120. [Google Scholar]
- 86.Adiwidjaja G., Meyer B., Thiem J. Synthesis and crystal structure of endo-2-dimethylphosphono-exo-2-hydroxy-(−)-camphane for the determination of 3J(CCCP) vicinal coupling constants. Z. Naturforsch. 1979;34b:1547–1551. [Google Scholar]
- 87.Buchanan G.W., Bourque K., Seeley A. 13C and 31P NMR spectra of 1-diethylphosphono-1-hydroxycycloalkanes. Magn. Res. Chem. 1986;24:360–367. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


