Abstract
A series of β-amino alcohol tethered 4-aminoquinoline-isatin conjugates were synthesized with the aim of probing their antimalarial structure activity relationship. Two of the most active conjugates (11b and 11f) exhibited antimalarial efficacy comparable to that of chloroquine, with IC50 values of 11.8 and 13.5 nM, respectively against chloroquine resistant W2 strain of Plasmodium falciparum and are devoid of any cytotoxicity.
Keywords: β-amino alcohol, 4-Aminoquinoline-isatin conjugates, Antimalarial activity, Cytotoxicity
Graphical abstract

Highlights
-
•
Synthesis of β-amino alcohol tethered isatin 4-aminoquinoline conjugates.
-
•
Antimalarial evaluation against W2 strains of Plasmodium falciparum.
-
•
Most active and non-cytotoxic conjugate exhibited an IC50 11.7 nM.
1. Introduction
With 207 million cases and 627 thousand deaths in 2012, malaria is one of the world's deadliest diseases, affecting 64% of the global population [1]. Plasmodium falciparum is the most virulent human malaria parasite, and is responsible for most of the malaria-related deaths [2], [3]. Since the discovery of the natural product quinine, many compounds with a quinoline scaffold have displayed good antimalarial activity, leading to the development of effective antimalarial agents, including chloroquine, amodiaquine, piperaquine and mefloquine [4], [5], [6]. Chloroquine has been extensively utilized for decades because of its efficacy, safety and low cost. However, the widespread resistance of P. falciparum to chloroquine [2], [7] has hampered efforts to combat malaria and led to the development of the natural endoperoxide artemisinin and its semisynthetic derivatives (artemether, artesunate, and dihydroartemisinin) as potent and fast acting antimalarials [8]. However, the worldwide deployment of artemisinin based combination therapy is limited by relatively high cost of treatment, safety in pregnancy and [9], [10] early signs of resistance to artemisinin derivatives in southeast Asia [11].
4-Aminoquinoline hybridization is now considered as an attractive and viable strategy for developing new efficacious antimalarials and potentially delaying the emergence of drug resistance [12], [13], [14], [15], [16]. The hybrid drug approach involves the rational design of new chemical entities by covalent fusion of two pharmacophores with complimentary activities and different pharmacological targets. The potential of a quinoline-hybridisation strategy was demonstrated by several active antimalarials that include trioxaferroquines [13], trioxaquines [17], an artemisinin-quinine hybrid [18], 4-aminoquinoline based tetraoxanes [19], clotrimazole-based-4-aminoquinolines [20] and isatin-4-aminoquinoline hybrids [21].
Isatin is one of the most promising heterocyclic scaffolds, with anti-HIV [22], antiviral [23], anti-tumour [24], [25], [26], antifungal [27], [28], anti-angiogenic [29], anticonvulsant [30], and anti-Parkinson’s [31] activity. It is an effective SARS coronavirus 3CL protease inhibitor [32] and is well-tolerated in human subjects [33], [34]. Isatin has major importance in organic synthesis due to a highly reactive C-3 carbonyl group which allows easy transformation into 2-oxoindole compounds upon nucleophilic addition or spiroannulation [35]. 2-oxoindole derivatives such as SU-5416 (semaxanib) and SU-11248 (Sunitinib), a 5-fluoro-3-substituted-2-oxoindole, were reported to have tyrosine kinase inhibitory and anti-angiogenic properties [36], [37]. The encouraging applications of isatin in organic synthesis and its biological potential have prompted efforts towards the synthesis and evaluation of novel isatin-derivatives.
Recently the antimalarial potential of isatins via synthesis of isatin-chalcone and isatin-thiolactone conjugates has been explored [38], [39]. The synthesized thiolactone-isatin conjugates showed superior antimalarial activity; the most active scaffold exhibited an IC50 of 6.92 μM against W2 strain of P. falciparum [39]. In particular, isatin-quinoline hybrids were shown to possess good antiplasmodial profiles as evidenced by isatin-4-aminoquinolines tethered via ethyl linker which exhibited IC50 values in the range of 51–54 nM [21]. These examples suggested that the isatin-quinoline core has an inherent potential as a lead antimalarial agent and the introduction of substituents at appropriate positions and the nature of linker in this structural framework could improve its antimalarial efficacy.
A previous report from our group revealed the synthesis and antimalarial evaluation of 1H-1,2,3-triazole-tethered-7-chloroquinoline-isatin [40], [41] and piperazine-tethered isatin-7-chloroquinoline conjugates [42]. The observed activity profiles among these conjugates were found to depend on the substituent at the C-5 position of isatin and the length of the alkyl chain. Building on our interest in molecular hybridization to develop new antimalarials [43], we report herein the synthesis and biological evaluation of hybrids containing a 4-aminoquinoline tethered to isatin derivatives through an β-amino alcohol functionality. The rationale behind the introduction of a β-amino alcohol linker is its presence in a number of potent antimalarials, as exemplified by mefloquine [44]. The length of the alkyl chain at the C-4 position of the quinoline ring was varied because of its established impact on antimalarial efficacy [45], [46], [47], [48].
2. Results and discussion: synthetic chemistry and pharmacology
2.1. Synthetic chemistry
The synthesis of the target β-amino alcohol tethered isatin-quinoline hybrids involved an initial synthesis of the required precursors viz. 1-oxiranylmethyl isatin 3 and 4-aminoquinolines 9 and 10. The procedure for the synthesis of 1-oxiranylmethyl indole-2,3-dione 3 was based on our recent report involving an initial base promoted allylation of C-5 substituted isatins with its subsequent epoxidation using m-chloroperbenzoic acid (mCPBA) in dry chloroform at 60 °C (Scheme 1 ) [49].
Scheme 1.

Synthesis of 1-oxiranylmethyl indole-2,3diones 3.
The preparation of 4 aminoquinoline-based precursors was realized by heating 4,7-dichloroquinoline with an excess of aliphatic chain/cyclic diaminoalkanes initially at 80 °C and then at 135 °C for 3 h, as shown in Scheme 2 , [43a].
Scheme 2.

Synthesis of 4-aminoquinolines.
The target hybrids 11 were synthesized by refluxing precursors 3 and 9 in dry toluene at 110 °C in the presence of a catalytic amount of p-toluene sulphonic acid for 4–6 h. The conjugates 12 were prepared under similar conditions, however with longer duration of refluxing (Scheme 3 ).
Scheme 3.

Synthesis of isatin-4-aminoquinoline conjugates.
The purification of the reaction mixture via column chromatography resulted in the isolation of desired structural chimeras 11 and 12. The structures were assigned on the basis of spectral data and analytical evidence. The compound 11e, for example, analyzed as C23H23ClN4O3, showed a molecular ion peak at m/z 438.1451 in its high resolution mass spectrum. The salient features of its 1H NMR spectrum included the appearance of a pair of doublet of a doublet at δ 5.23 (J = 1.4 and 10.3 Hz) corresponding to the –NCH2, a multiplet at δ 5.95 corresponding to the methine proton, and the appearance of characteristic quinoline ring protons. The 13C NMR spectrum showed characteristic absorptions at δ 98.1 corresponding to a methine carbon, while absorptions at δ 152.1 and 171.0 corresponded to the presence of isatin carbonyls. The appearance of methylene carbon at δ 115.1, as confirmed by the 13C NMR (DEPT) spectrum along with the requisite number of carbons, further substantiated the assigned structures.
2.2. In vitro antiplasmodial activity
The synthesized compounds were evaluated for their antimalarial profiles against the CQ resistant W2 strain of P. falciparum (Table 1 ). The antimalarial activities depended upon the nature of the substituent at the C-5 position of the isatin ring and upon the alkyl chain length. Activities decreased as the chain length was increased, from ethyl to propyl linker, except in the case of 11b and 11f, which had essentially the same activity. A further increase in chain length via introducing butyl and hexyl linkers did not alter the observed antimalarial efficacy. The nature of the substituent at the C-5 position of the isatin ring also seems to play a crucial role in influencing antimalarial activity. The presence of an inductively electron withdrawing chloro-substituent at C-5 improved activity irrespective of the chain length, with effects more pronounced with shorter (n = 2,3) alkyl chain lengths. The replacement of the chloro substituent with an H, -CH3 or F group decreased activity, although few of these hybrids showed activity comparable with that of chloroquine, while compound 11a (R = H) showed better activity than chloroquine. The replacement of the alkyl chain with a piperazine ring resulted in a complete loss of activity, as evident with compounds 12b–d. Compounds 11b and 11f, with an optimum combination of shorter alkyl chain lengths (n = 2 and 3) and the presence of a chloro-substituent at the C-5 position of th isatin ring, were the most potent of the tested compounds, exhibiting IC50 of 11.8 and 13.5 nM, respectively. The most potent of the tested compounds were then evaluated for their cytotoxic profiles against HCT116 (colon cancer cell line) cells at 0.2, 2.0 and 20 μM (Table 2 ). As evident, the active conjugates were devoid of any cytotoxic activity at low concentration and thus can act as therapeutic templates for the synthesis of new antimalarials.
Table 1.
Antimalarial activities of tested compounds against CQ resistant W2 strains of P. falciparum.
| Compound | Structure | IC50 (nM) | clogP |
|---|---|---|---|
| 11a |  |
24.9 | 3.6 |
| 11b | 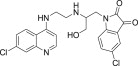 |
11.7 | 4.5 |
| 11c | 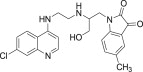 |
ND | 4.1 |
| 11d | 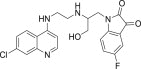 |
488.1 | 3.9 |
| 11e | 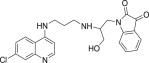 |
75.5 | 3.9 |
| 11f | 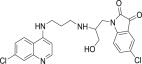 |
13.5 | 4.8 |
| 11g | 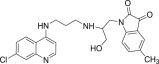 |
302.0 | 4.4 |
| 11h |  |
72.3 | 4.2 |
| 11i |  |
48.7 | 3.1 |
| 11j | 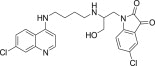 |
101.0 | 4.0 |
| 11k |  |
58.0 | 3.6 |
| 11l | 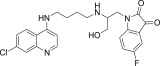 |
45.3 | 3.3 |
| 11m | 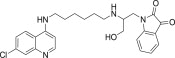 |
62.9 | 4.1 |
| 11n |  |
49.7 | 5.0 |
| 11o | 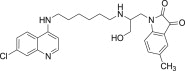 |
55.8 | 4.6 |
| 11p |  |
59.6 | 4.4 |
| 12a |  |
216.0 | 3.0 |
| 12b | 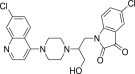 |
1960.0 | 3.8 |
| 12c |  |
1225.0 | 3.4 |
| 12d | 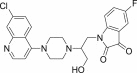 |
1853.5 | 3.3 |
| CQ | 36.37 | ||
| ART | 04.37 |
Table 2.
Cytotoxicity of some active compounds against HCT116 (colon cancer cell line) cells.
| Compound | R | n | Cytotoxicity % inhibition |
||
|---|---|---|---|---|---|
| 0.2 μM | 2.0 μM | 20 μM | |||
| 11a | H | 1 | 0 | 2 | 91 |
| 11b | Cl | 1 | 0 | −11 | 88 |
| 11e | H | 2 | 2 | −12 | 85 |
| 11f | Cl | 2 | 0 | 2 | 46 |
| 11h | F | 2 | −2 | 12 | 66 |
| 11i | H | 3 | 0 | −5 | 37 |
| 11l | F | 3 | −1 | 0 | 83 |
| Doxorubicin | 84 | 79 | 79 | ||
| Staurosporine | 16 | 80 | 90 | ||
In conclusion, the present manuscript describes the synthesis of β-amino alcohol tethered isatin-4-aminoquinoline hybrids and evaluation of their activities against P. falciparum. Our results reveal a clear preference for shorter alkyl chain length (n = 2,3) and the presence of a chloro-substituent at the C-5 position of the isatin ring for optimal antimalarial activity. The most potent and non-cytotoxic conjugates exhibited antimalarial activity comparable to that of chloroquine and artemisinin.
3. Experimental section
Melting points were determined by open capillary using a Veego Precision Digital Melting Point apparatus (MP-D) and are uncorrected. 1H NMR spectra were recorded in deuterochloroform and DMSO-d6 with Jeol 300 (300 MHz) spectrometers using TMS as internal standard. Chemical shift values are expressed as parts per million downfield from TMS, and J values are in hertz. Splitting patterns are indicated as s: singlet, d: doublet, t: triplet, m: multiplet, dd: double doublet, ddd: doublet of a doublet of a doublet, and br: broad peak. 13C NMR spectra were recorded on Jeol 300 (75 MHz) spectrometers in deuterochloroform and DMSO-d6 using TMS as internal standard. High resolution mass spectra were recorded on a Bruker-micrOTOF-Q II spectrometer. Column chromatography was performed on a silica gel (60–120 mesh).
3.1. Procedure for the synthesis of substituted N-allyl isatins 2
Isatin (1 mmol) was added to a stirred suspension of sodium hydride (1.5 mmol) in dry DMF (10 mL) resulting in the formation of purple coloured anionic solution. The solution was allowed to stir at room temperature till the evolution of hydrogen ceases. To this reaction mixture was added drop wise a solution of allyl bromide (1.1 mmol) in dry DMF. The reaction mixture was heated to 60 °C with constant stirring for about 6 h. After the completion of the reaction, as evident from TLC, quenching was done by drop wise addition of water (20 mL) with subsequent extraction with ethyl acetate (3 × 30 mL). The combined organic layers were washed with brine solution, dried over anhydrous Na2SO4 and concentrated under reduced pressure. Purification of the reaction mixture via column chromatography using hexane: ethyl acetate (4:1) mixture furnished the desired N-allyl isatin derivatives in good yields.
3.2. Procedure for the synthesis of 1-oxiranylmethyl isatin 3
N-allyl isatin 2 was added to a stirred suspension of m-chloroperbenzoic acid (m-CPBA) (1.2 mmol) in dry CHCl3 at 0 °C. The reaction mixture was allowed to attain room temperature and subsequently refluxed at 60 °C for 2 h. After the completion of the reaction as evident from TLC, the excess of m-CPBA was quenched by treatment with a saturated solution of K2CO3 and the organic layer was subsequently extracted with chloroform (3 × 30 mL). The combined organic layers were dried over anhydrous Na2SO4 and concentrated under reduced pressure resulting in the isolation of a crude solid which was recrystallized using (9:1) hexane:chloroform mixture.
3.3. General method for the preparation of isatin-4aminoquinoline conjugates 11a–p
To the stirred solution of 1-oxiranylmethyl isatin 3 (1 mmol) and 4-aminoquinoline 9 (1 mmol) in dry toluene was added catalytic amount of p-toluene sulphonic acid. The reaction mixture was refluxed at 110 °C for 6 h and the progress was monitored by TLC. On completion, water (15 mL) was added to the reaction mixture and subsequently extraction was done using chloroform (2 × 50 mL). Combined organic layers were dried over anhydrous sodium sulphate and concentrated under reduce pressure. Purification of reaction mixture via column chromatography using hexane:ethylacetate (3:2) mixture as eluent resulted in the isolation of desired hybrids 11a–p.
3.3.1. 1-{2-[2-(7-Chloro-quinolin-4-ylamino)-ethylamino]-3-hydroxy-propyl}-1H-indole-2,3-dione (11a)
White solid, yield 85%, m.p. 183–184 °C; 1H NMR (300 MHz, CDCl3): δ H 3.48 (s, 2H, -CH2-); 3.80 (s, 4H, 2× –CH2–); 5.16–5.31 (m, 2H, –CH2–); 5.83–5.98 (m, 1H, –CH–); 6.34 (d, J = 5.1 Hz, 1H, H2); 6.56 (d, J = 9.0 Hz, 1H, Ar–H); 7.16–7.31 (m, 3H, H4 + 2Ar–H); 7.53 (s, 1H, Ar–H); 7.89 (s, 1H, H5); 7.96 (d, J = 9.0 Hz, 1H, H3); 8.40 (d, J = 5.1 Hz, 1H, H1); 13C NMR (75 MHz, CDCl3): δ C 37.2, 42.5, 44.1, 99.7, 111.3, 114.3, 114.6, 115.2, 117.5, 123.3, 124.8, 125.7, 127.3, 128.2, 132.3, 134.6, 135.1, 148.9, 151.5, 169.9. HRMS calculated for C22H21ClN4O3 424.1302 [M+] found 424.1309; Anal. Calcd (%) for: C, 62.19; H, 4.98; N, 13.19; found C, 62.26; H, 4.84; N, 13.27.
3.3.2. 5-Chloro-1-{2-[2-(7-Chloro-quinolin-4-ylamino)-ethylamino]-3-hydroxy-propyl}-1H-indole-2,3-dione (11b)
White solid, yield 86%, m.p. 175–176 °C; 1H NMR (300 MHz, CDCl3): δ H 3.29 (s, 2H, –CH2–); 3.58 (s, 4H, 2x–CH2–); 4.94–5.10 (m, 2H, -CH2-); 5.65–5.76 (m, 1H, -CH-); 6.22 (d, J = 5.1 Hz, 1H, H2); 6.34–6.39 (m, 1H, Ar–H); 6.95–7.15 (m, 3H, H4+ 2Ar–H); 7.24 (s, 1H, –NH exchangeable with D2O); 7.33–7.35 (m, 1H, Ar–H); 7.64–7.70 (m, 2H, H5+ –NH exchangeable with D2O); 7.83 (d, J = 8.8 Hz, 1H, H3); 8.20–8.24 (m, 2H, H1+ –OH exchangeable with D2O); 13C NMR (75 MHz, CDCl3): δ C 37.5, 42.0, 44.7, 98.5, 113.1, 115.4, 115.8, 117.0, 117.8, 123.9, 124.5, 126.0, 127.6, 131.8, 134.2, 134.8, 147.2, 147.7, 150.3, 151.0, 168.5. HRMS calculated for C22H20Cl2N4O3 458.0912 [M+] found 458.0921; Anal. Calcd (%) for: C, 57.53; H, 4.39; N, 12.20; found C, 57.45; H, 4.47; N, 12.28.
3.3.3. 1-{2-[2-(7-Chloro-quinolin-4-ylamino)-ethylamino]-3-hydroxy-propyl}-5methyl-1H-indole-2,3-dione (11c)
White solid, yield 85%, m.p. 169–170 °C; 1H NMR (300 MHz, CDCl3): δ H 2.29 (s, 3H, –CH3); 3.35 (s, 2H, –CH2–); 3.62 (s, 4H, –CH2–); 4.98–5.15 (m, 2H, –CH2–); 5.63–5.71 (m, 1H, –CH–); 6.25 (d, J = 5.1 Hz, 1H, H2); 6.35–6.39 (m, 1H, Ar–H); 7.19 (dd, J = 2.4 Hz, 9.0 Hz, 1H, H4); 7.29–7.30 (m, 1H, Ar–H); 7.53 (s, 1H, Ar–H); 7.67–7.77 (m, 2H, H5+ –NH exchangeable with D2O); 7.93 (d, J = 9.0 Hz, 1H, H3); 8.18–8.21 (m, 2H, H1+ –OH exchangeable with D2O); 13C NMR (75 MHz, CDCl3): δ C 20.1, 37.6, 42.5, 44.9, 99.2, 111.9, 114.3, 114.6, 115.2, 123.9, 124.7, 125.6, 128.1, 132.4, 133.9, 134.6, 135.1, 147.5, 148.8, 150.7, 151.6, 169.8. HRMS calculated for C23H23ClN4O3 438.1459 [M+] found 438.1451; Anal. Calcd (%) for: C, 62.94; H, 5.28; N, 12.77; found C, 62.85; H, 5.16; N, 12.85.
3.3.4. 1-{2-[2-(7-Chloro-quinolin-4-ylamino)-ethylamino]-3-hydroxy-propyl}-5-fluoro-1H-indole-2,3-dione (11d)
White solid, yield 87%, m.p. 180–181 °C; 1H NMR (300 MHz, CDCl3): δ H 3.31 (s, 2H, –CH2–); 3.61 (s, 4H, 2× –CH2–); 4.95–5.15 (m, 2H, –CH2–); 5.61–5.72 (m, 1H, –CH–); 6.27 (d, J = 5.1 Hz, 1H, H2); 6.35 (d, J = 8.2 Hz, 1H, Ar–H); 7.18 (dd, J = 2.4, 8.7 Hz, 1H, H4); 7.27 (d, J = 8.2, 1H, Ar–H); 7.54 (s, 1H, Ar–H); 7.65–7.73 (m, 2H, H5+ –NH exchangeable with D2O); 7.92 (d, J = 9.0 Hz, 1H, H4); 8.32 (d, J = 5.1 Hz, 1H, H1); 13C NMR (75 MHz, CDCl3): δ C 38.1, 42.0, 45.2, 98.7, 113.3, 115.5, 115.6, 117.0, 117.9, 123.9, 124.3, 126.3, 127.6, 131.6, 134.2, 134.9, 147.3, 147.7, 150.4, 151.2, 168.7. HRMS calculated for C22H20ClFN4O3 442.1208 [M+] found 442.1215; Anal. Calcd (%) for: C, 59.66; H, 4.55; N, 12.65; found C, 59.74; H, 4.46; N, 12.57.
3.3.5. 1-{2-[3-(7-Chloro-quinolin-4-ylamino)-propylamino]-3-hydroxy-propyl}-1H-indole-2,3-dione (11e)
White solid, yield 83%, m.p. 158–159 °C; 1H NMR (300 MHz, CDCl3): δ H 1.94–2.01 (m, 2H, –CH2–); 3.48–3.49 (m, 2H, –CH2–); 3.55–3.60 (m, 2H, –CH2–); 3.81 (s, 2H, –CH2–); 5.17 (dd, J = 1.4, 10.3 Hz, 1H, –CH2–); 5.30 (dd, J = 1.4, 10.3 Hz, 1H, –CH2–); 5.91–5.98 (m, 1H, –CH–); 6.38 (d, J = 6.0 Hz, 1H, H2); 6.56 (t, J = 7.2 Hz, 1H, Ar–H); 6.65 (d, J = 8.3 Hz, 1H, Ar–H); 6.96 (t, J = 6.1 Hz, 1H, NH exchangeable with D2O); 7.26–7.32 (m, 2H, H4 + Ar–H); 7.44 (dd, J = 0.9, 7.8 Hz, 1H, Ar–H); 7.58 (s, 1H, NH exchangeable with D2O); 7.72 (s, 1H, OH exchangeable with D2O); 7.90 (d, J = 1.7 Hz, 1H, H5); 8.10 (d, J = 9.0 Hz, 1H, H3); 8.32 (d, J = 6.0 Hz, 1H, H1); 13C NMR (75 MHz, CDCl3): δ C 28.2, 36.6, 39.9, 45.4, 98.1, 112.1, 114.5, 115.1, 116.0, 116.7, 122.9, 124.9, 126.1, 127.6, 133.1, 134.7, 136.6, 148.0, 149.5, 152.1, 171.0. HRMS calculated for C23H23ClN4O3 438.1459 [M+] found 438.1451; Anal. Calcd (%) for: C, 62.94; H, 5.28; N, 12.77; found C, 62.83; H, 5.41; N, 12.68.
3.3.6. 5-Chloro-1-{2-[3-(7-Chloro-quinolin-4-ylamino)-propylamino]-3-hydroxy-propyl}-1H-indole-2,3-dione (11f)
White solid, yield 85%, m.p. 151–152 °C; 1H NMR (300 MHz, CDCl3): δ H 1.93–2.01 (m, 2H, –CH2–); 3.45–3.47 (m, 2H, –CH2–); 3.56–3.60 (m, 2H, –CH2–); 3.79 (s, 2H, –CH2–); 5.19 (dd, J = 1.4, 10.3 Hz, 1H, –CH2–); 5.31 (dd, J = 1.4, 10.3 Hz, 1H, –CH2–); 5.91–5.98 (m, 1H, –CH–); 6.39 (d, J = 6.0 Hz, 1H, H2); 6.71 (d, J = 8.3 Hz, 1H, Ar–H); 6.93 (t, J = 6.1 Hz, 1H, NH exchangeable with D2O); 7.16–7.23 (m, 2H, Ar–H); 7.39 (d, J = 9.0 Hz, 1H, H4); 7.58 (s, 1H, NH exchangeable with D2O); 7.75 (s, 1H, NH exchangeable with D2O); 7.88 (d, J = 9.0 Hz, 1H, H3); 7.99 (s, 1H, H5); 8.66 (d, J = 6.0 Hz, 1H, H1); 13C NMR (75 MHz, CDCl3): δ C 28.9, 35.9, 40.2, 46.1, 99.1, 111.2, 112.8, 115.3, 116.5, 122.4, 123.7, 124.6, 125.7, 127.1, 133.4, 134.1, 135.9, 147.8, 148.9, 150.2, 152.5, 169.3. HRMS calculated for C23H22Cl2N4O3 472.1069 [M+] found 472.1075; Anal. Calcd (%) for: C, 58.36; H, 4.68; N, 11.84; found C, 58.27; H, 4.76; N, 11.93.
3.3.7. 1-{2-[3-(7-Chloro-quinolin-4-ylamino)-propylamino]-3-hydroxy-propyl}-5-methyl-1H-indole-2,3-dione (11g)
White solid, yield 81%, m.p. 157–158 °C; 1H NMR (300 MHz, CDCl3): δ H 1.92–2.00 (m, 2H, –CH2–); 2.29 (s, 3H, –CH3); 3.45–3.46 (m, 2H, –CH2–); 3.56–3.60 (m, 2H, –CH2–); 3.81 (s, 2H, –CH2–); 5.19 (dd, J = 1.4, 10.3 Hz, 1H, –CH2–); 5.30 (dd, J = 1.4, 10.3 Hz, 1H, –CH2–); 5.91–5.97 (m, 1H, –CH–); 6.41 (d, J = 6.0 Hz, 1H, H2); 6.71 (d, J = 8.3 Hz, 1H, Ar–H); 7.16–7.27 (m, 2H, Ar–H); 7.39 (d, J = 9.0 Hz, 1H, H4); 7.56 (s, 1H, NH exchangeable with D2O); 7.73 (s, 1H, NH exchangeable with D2O); 7.89 (d, J = 9.0 Hz, 1H, H3); 7.97 (s, 1H, H5); 8.58 (d, J = 6.0 Hz, 1H, H1); 13C NMR (75 MHz, CDCl3): δ C 20.9, 27.9, 36.3, 39.5, 45.9, 99.7, 111.7, 113.9, 115.7, 116.1, 117.2, 123.2, 123.9, 126.5, 127.1, 133.7, 134.2, 147.5, 147.9, 149.3, 150.9, 151.7, 168.9. HRMS calculated for C24H25ClN4O3 452.1615 [M+] found 452.1608; Anal. Calcd (%) for: C, 63.64; H, 5.56; N, 12.37; found C, 63.75; H, 5.43; N, 12.45.
3.3.8. 1-{2-[3-(7-Chloro-quinolin-4-ylamino)-propylamino]-3-hydroxy-propyl}-5-fluoro-1H-indole-2,3-dione (11h)
White solid, yield 88%, m.p. 148–149 °C; 1H NMR (300 MHz, CDCl3): δ H 1.94–2.00 (m, 2H, –CH2–); 3.45–3.47 (m, 2H, –CH2–); 3.56–3.60 (m, 2H, –CH2–); 3.83 (s, 2H, –CH2–); 5.17 (dd, J = 1.4, 10.3 Hz, 1H, –CH2–); 5.30 (dd, J = 1.4, 10.3 Hz, 1H, –CH2–); 5.92–5.98 (m, 1H, –CH–); 6.37 (d, J = 6.0 Hz, 1H, H2); 6.67 (d, J = 8.3 Hz, 1H, Ar–H); 7.16–7.21 (m, 2H, Ar–H); 7.39 (d, J = 9.0 Hz, 1H, H4); 7.56 (s, 1H, NH exchangeable with D2O); 7.73 (s, 1H, NH exchangeable with D2O); 7.92 (d, J = 9.0 Hz, 1H, H3); 8.12 (s, 1H, H5); 8.58 (d, J = 6.0 Hz, 1H, H1); 13C NMR (75 MHz, CDCl3): δ C 28.5, 36.9, 40.1, 45.7, 98.9, 113.1, 114.6, 115.8, 116.7, 122.1, 123.5, 124.9, 126.4, 127.6, 132.5, 135.1, 136.1, 147.5, 148.5, 150.5, 152.5, 171.3. HRMS calculated for C23H22ClFN4O3 456.1364 [M+] found 456.1373; Anal. Calcd (%) for: C, 60.46; H, 4.85; N, 12.26; found C, 60.59; H, 4.99; N, 12.17.s.
3.3.9. 1-{2-[4-(7-Chloro-quinolin-4-ylamino)-butylamino]-3-hydroxy-propyl}-1H-indole-2,3-dione (11i)
White solid, yield 82%, m.p. 155–156 °C; 1H NMR (300 MHz, CDCl3): δ H 1.63–1.76 (m, 4H, 2× –CH2–); 3.34 (dd, J = 6.04, 12.0 Hz, 4H, 2× –CH2–); 3.77 (s, 2H, –CH2–); 5.11 (dd, J = 1.4, 10.3 Hz, 1H, –CH2–); 5.30 (dd, J = 1.5, 17.2 Hz, 1H, –CH2–); 5.87–5.96 (m, 1H, –CH–); 6.53–6.64 (m, 3H, H2+ 2Ar–H); 7.24 (t, J = 8.2 Hz, 1H, Ar–H); 7.49–7.54 (m, 2H, H4+1Ar–H); 7.83 (d, J = 1.8 Hz, 1H, H5); 7.94 (s, 2H, 2× NH exchangeable with D2O); 8.36–8.42 (m, 3H, H3+H1+OH exchangeable with D2O); 13C NMR (75 MHz, CDCl3): δ C 25.2, 26.6, 38.4, 42.3, 44.6, 98.5, 111.2, 114.2, 115.2, 115.3, 116.9, 124.5, 124.7, 125.1, 128.2, 132.1, 134.6, 135.5, 146.2, 148.9, 149.4, 151.4, 169.1. HRMS calculated for C24H25ClN4O3 452.1615 [M+] found 452.1607; Anal. Calcd (%) for: C, 63.64; H, 5.56; N, 12.37; found C, 63.78; H, 5.69; N, 12.48.
3.3.10. 5-Chloro-1-{2-[4-(7-Chloro-quinolin-4-ylamino)-butylamino]-3-hydroxy-propyl}-1H-indole-2,3-dione (11j)
Pale yellow solid, yield 82%, m.p. 160–161 °C; 1H NMR (300 MHz, CDCl3): δ H 1.65–1.77 (m, 4H, 2x –CH2–); 3.32 (d, J = 5.76 Hz, 2H, –CH2–); 3.35 (d, J = 5.7 Hz, 2H, –CH2–); 3.78 (s, 2H, –CH2–); 5.13 (dd, J = 1.4, 10.3 Hz, 1H, –CH2–); 5.29 (dd, J = 1.5, 17.2 Hz, 1H, –CH2–); 5.84–5.95 (m, 1H, –CH–); 6.51 (d, J = 6.0 Hz, 1H, H2); 6.64 (d, J = 8.3 Hz, 1H, Ar–H); 7.15–7.27 (m, 2H, ArH); 7.35 (d, J = 9.0 Hz, 1H, H4); 7.69 (s, 1H, NH exchangeable with D2O); 7.85 (d, J = 9.0 Hz, 1H, H3); 7.91 (s, 1H, H5); 8.62 (d, J = 6.0 Hz, 1H, H1); 13C NMR (75 MHz, CDCl3): δ C 25.6, 26.9, 37.9, 42.7, 44.1, 99.1, 112.8, 113.7, 114.9, 115.7, 116.1, 124.6, 126.3, 127.3, 128.6, 133.3, 133.9, 135.1, 147.4, 148.5, 150.1, 151.9, 170.1. HRMS calculated for C24H24Cl2N4O3 486.1225 [M+] found 486.1232; Anal. Calcd (%) for: C, 59.14; H, 4.96; N, 11.50; found C, 59.27; H, 4.83; N, 11.43.
3.3.11. 1-{2-[4-(7-Chloro-quinolin-4-ylamino)-butylamino]-3-hydroxy-propyl}-5-methyl-1H-indole-2,3-dione (11k)
White solid, yield 83%, m.p. 145–146 °C; 1H NMR (300 MHz, CDCl3): δ H 1.62–1.73 (m, 4H, 2x –CH2–); 2.29 (s, 3H, –CH3); 2.51–2.56 (m, 2H, –CH2–); 3.33 (d, J = 5.7 Hz, 2H, –CH2–); 3.34 (d, J = 5.7 Hz, 2H, –CH2–); 3.76 (s, 2H, –CH2–); 5.15 (dd, J = 1.4, 10.3 Hz, 1H, –CH2–); 5.27 (dd, J = 1.4, 10.3 Hz, 1H, –CH2–); 5.84–5.93 (m, 1H, –CH–); 6.51 (d, J = 6.0 Hz, 1H, H2); 6.59 (d, J = 8.3 Hz, 1H, Ar–H); 7.13–7.24 (m, 2H, Ar–H); 7.39 (d, J = 9.0 Hz, 1H, H4); 7.63 (s, 1H, NH exchangeable with D2O); 7.81 (d, J = 9.0 Hz, 1H, H3); 7.91 (s, 1H, H5); 8.67 (d, J = 6.0 Hz, 1H, H1); 13C NMR (75 MHz, CDCl3): δ C 20.9, 25.9, 27.1, 38.8, 42.4, 45.1, 98.9, 111.9, 114.5, 115.2, 115.8, 116.5, 123.8, 124.9, 125.5, 127.6, 132.5, 134.1, 135.4, 146.7, 148.8, 149.7, 151.1, 169.7. HRMS calculated for C25H27ClN4O3 466.1772 [M+] found 466.1766; Anal. Calcd (%) for: C, 64.30; H, 5.83; N, 12.00; found C, 64.43; H, 5.91; N, 12.11.
3.3.12. 1-{2-[4-(7-Chloro-quinolin-4-ylamino)-butylamino]-3-hydroxy-propyl}-5-fluoro-1H-indole-2,3-dione (11l)
White solid, yield 82%, m.p. 148–149 °C; 1H NMR (300 MHz, CDCl3): δ H 1.67–1.75 (m, 4H, 2x –CH2–); 2.53–2.57 (m, 2H, –CH2–); 3.32 (d, J = 5.7 Hz, 2H, –CH2–); 3.34 (d, J = 5.7 Hz, 2H, –CH2–); 3.75 (s, 2H, –CH2–); 5.14 (dd, J = 1.4, 10.3 Hz, 1H, –CH2–); 5.23 (dd, J = 1.5, 17.2 Hz, 1H, –CH2–); 5.82–5.93 (m, 1H, –CH–); 6.51–6.60 (m, 3H, H2+ 2Ar–H); 7.33 (d, J = 9.0 Hz, 1H, H4); 7.49 (dd, J = 0.9, 7.8 Hz, 1H, Ar–H); 7.88 (d, J = 1.8 Hz, 1H, H5); 7.95 (s, 2H, H3+ NH exchangeable with D2O); 8.65 (d, J = 6.0 Hz, 1H, H1); 13C NMR (75 MHz, CDCl3): δ C 25.2, 26.7, 38.5, 41.9, 44.2, 98.4, 113.1, 114.5, 115.1, 115.3, 116.7, 124.5, 124.6, 125.3, 128.3, 132.5, 134.6, 135.5, 146.1, 148.8, 149.2, 151.3, 169.1. HRMS calculated for C24H24ClFN4O3 470.1521 [M+] found 470.1529; Anal. Calcd (%) for: C, 61.21; H, 5.14; N, 11.90; found C, 61.36; H, 5.29; N, 11.81.
3.3.13. 1-{2-[6-(7-Chloro-quinolin-4-ylamino)-hexylamino]-3-hydroxy-propyl}-1H-indole-2,3-dione (11m)
White solid, yield 79%, m.p. 165–166 °C; 1H NMR (300 MHz, CDCl3): δ H 1.27–1.31 (m, 4H, –CH2–); 1.91–1.97 (m, 2H, –CH2–); 2.52–2.55 (m, 2H, –CH2–); 3.31 (dd, J = 6.1, 12.1 Hz, 2H, –CH2–); 3.35 (dd, J = 6.3, 12.1 Hz, 2H, –CH2–); 3.75 (s, 2H, –CH2–); 5.12 (dd, J = 1.4, 10.3 Hz, 1H, –CH2–); 5.31 (dd, J = 1.5, 17.2 Hz, 1H, –CH2–); 5.85–5.94 (m, 1H, –CH–); 6.36 (d, J = 6.0 Hz, 1H, H2); 6.63 (d, J = 8.3 Hz, 1H, Ar–H); 6.96 (t, J = 8.1 Hz, 1H, Ar–H); 7.23–7.29 (m, 2H, H4 + Ar–H); 7.48 (dd, J = 0.9, 7.8 Hz, 1H, Ar–H); 7.55 (s, 1H, NH exchangeable with D2O); 7.74 (s, 1H, NH exchangeable with D2O); 7.91 (d, J = 1.7 Hz, 1H, H5); 8.10 (d, J = 9.0 Hz, 1H, H3); 8.31 (d, J = 6.0 Hz, 1H, H1); 13C NMR (75 MHz, CDCl3): δ C 28.2, 28.5, 31.9, 32.7, 36.6, 39.9, 45.5, 98.7, 112.5, 114.1, 115.8, 116.1, 116.3, 122.9, 123.4, 126.2, 127.5, 133.2, 134.7, 136.6, 148.1, 149.5, 152.1, 171.1. HRMS calculated for C26H29ClN4O3 480.1928 [M+] found 480.1935; Anal. Calcd (%) for: C, 64.92; H, 6.08; N, 11.65; found C, 64.86; H, 6.19; N, 11.53.
3.3.14. 5-Chloro-1-{2-[6-(7-Chloro-quinolin-4-ylamino)-hexylamino]-3-hydroxy-propyl}-1H-indole-2,3-dione (11n)
White solid, yield 80%, m.p. 153–154 °C; 1H NMR (300 MHz, CDCl3): δ H 1.28–1.32 (m, 4H, –CH2–); 1.93–1.97 (m, 2H, –CH2–); 2.52–2.56 (m, 2H, –CH2–); 3.34 (dd, J = 5.7 Hz, 12.0 Hz, 2H, –CH2–); 3.36 (dd, J = 5.7, 12.1 Hz, 2H, –CH2–); 3.75 (s, 2H, –CH2–); 5.13 (dd, J = 1.4, 10.3 Hz, 1H, –CH2–); 5.31 (dd, J = 1.5, 17.2 Hz, 1H, –CH2–); 5.83–5.95 (m, 1H, –CH–); 6.35 (d, J = 6.0 Hz, 1H, H2); 6.63 (d, J = 8.3 Hz, 1H, Ar–H); 7.37 (d, J = 9.0 Hz, 1H, H4); 7.43–7.49 (m, 2H, 2Ar–H); 7.53 (s, 1H, NH exchangeable with D2O); 7.74 (s, 1H, NH exchangeable with D2O); 7.92 (d, J = 1.76 Hz, 1H, H5); 8.10 (d, J = 9.04 Hz, 1H, H3); 8.32 (d, J = 6.0 Hz, 1H, H1); 13C NMR (75 MHz, CDCl3): δ C 28.4, 29.1, 31.5, 33.1, 36.8, 39.4, 45.7, 99.1, 111.5, 113.7, 115.3, 116.5, 117.2, 117.8, 122.5, 123.7, 126.7, 127.8, 133.5, 134.1, 147.3, 148.2, 150.3, 152.5, 169.3. HRMS calculated for C26H28Cl2N4O3 514.1538 [M+] found 514.1545; Anal. Calcd (%) for: C, 60.59; H, 5.48; N, 10.87; found C, 60.43; H, 5.59; N, 10.74.
3.3.15. 1-{2-[6-(7-Chloro-quinolin-4-ylamino)-hexylamino]-3-hydroxy-propyl}-5-methyl-1H-indole-2,3-dione (11o)
White solid, yield 85%, m.p. 155–156 °C; 1H NMR (300 MHz, CDCl3): δ H 1.25–1.30 (m, 4H, 2× –CH2); 1.87–1.95 (m, 2H, –CH2–); 2.28 (s, 3H, –CH3); 2.58–2.63 (m, 2H, –CH2–); 3.31 (dd, J = 5.8, 12.1 Hz, 2H, –CH2–); 3.35 (dd, J = 5.7, 12.1 Hz, 2H, –CH2–); 3.87 (s, 2H, –CH2–); 5.07 (dd, J = 1.4, 10.3 Hz, 1H, –CH2–); 5.28 (dd, J = 1.5, 17.2 Hz, 1H, –CH2–); 5.65–5.76 (m, 1H, –CH–); 6.25 (d, J = 6.0 Hz, 1H, H2); 6.54 (d, J = 8.3 Hz, 1H, Ar–H); 6.98 (d, J = 9.0 Hz, 1H, H4); 7.37–7.41 (m, 2H, 2Ar–H); 7.40 (s, 1H, NH exchangeable with D2O); 7.81 (s, 1H, NH exchangeable with D2O); 7.89 (d, J = 1.7 Hz, 1H, H5); 8.13 (d, J = 9.0 Hz, 1H, H3); 8.32 (d, J = 6.0 Hz, 1H, H1); 13C NMR (75 MHz, CDCl3): δ C 21.5, 27.9, 28.6, 31.1, 33.3, 36.2, 40.0, 46.0, 98.9, 112.3, 114.9, 115.7, 116.4, 117.3, 122.1, 124.5, 125.8, 127.1, 133.9, 135.2, 136.9, 147.2, 148.7, 151.3, 152.1, 170.0. HRMS calculated for C27H31ClN4O3 494.2085 [M+] found 494.2077; Anal. Calcd (%) for: C, 65.51; H, 6.31; N, 11.32; found C, 65.67; H, 6.42; N, 11.43.
3.3.16. 1-{2-[6-(7-Chloro-quinolin-4-ylamino)-hexylamino]-3-hydroxy-propyl}-5-fluoro-1H-indole-2,3-dione (11p)
White solid, yield 87%, m.p. 152–153 °C; 1H NMR (300 MHz, CDCl3): δ H 1.28–1.34 (m, 4H, 2× –CH2–); 1.91–1.95 (m, 2H, –CH2–); 2.48–2.52 (m, 2H, –CH2–); 3.41 (dd, J = 5.8, 12.1 Hz, 2H, –CH2–); 3.47 (dd, J = 5.8, 12.1 Hz, 2H, –CH2–); 3.78 (s, 2H, –CH2–); 5.11–5.32 (m, 2H, –CH2–); 5.91–5.98 (m, 1H, –CH–); 6.41 (d, J = 6.0 Hz, 1H, H2); 6.69 (d, J = 8.3 Hz, 1H, Ar–H); 7.31 (d, J = 9.0 Hz, 1H, H4); 7.38–7.43 (m, 2H, 2Ar–H); 7.52 (s, 1H, -NH exchangeable with D2O); 7.67 (s, 1H, -NH exchangeable with D2O); 7.98 (d, J = 1.8 Hz, 1H, H5); 8.17 (d, J = 9.0 Hz, 1H, H3); 8.33 (d, J = 6.0 Hz, 1H, H1); 13C NMR (75 MHz, CDCl3): δ C 28.3, 28.5, 31.7, 32.6, 36.9, 40.1, 45.1, 98.1, 112.1, 114.3, 115.7, 116.1, 116.9, 122.4, 124.3, 126.8, 127.9, 133.2, 134.5, 136.6, 148.4, 149.6, 150.6, 152.2, 171.2. HRMS calculated for C26H28ClFN4O3 498.1834 [M+] found 498.1829; Anal. Calcd (%) for: C, 62.58; H, 5.66; N, 11.23; found C, 62.67; H, 5.75; N, 11.34.
3.4. General method for the preparation of isatin-4-piperazin-1-yl-quinoline conjugates 12a–d
The stirred solution of 1-Oxiranylmethyl isatin 3 (1 mmol), 4-piperazin-1-yl-quinoline 10 (1 mmol) and catalytic amount of p-toluene sulphonic acid in dry toluene was refluxed at 110 °C for 12 h. On completion of the reaction, as monitored by TLC, water (15 mL) was added to the reaction mixture and extracted with chloroform (2 × 30 mL). Combined organic layers were dried over anhydrous sodium sulphate and concentrated under reduce pressure. Purification of reaction mixture thus obtained via column chromatography using hexane:ethylacetate (3:2) mixture as eluent resulted in the isolation of desired chimeras 12a–d.
3.4.1. 1-{2-[4-(7-Chloro-quinolin-4-yl)-piperazin-1-yl]-3-hydroxy-propyl}-1H-indole-2,3-dione (12a)
White solid, yield 87%, m.p. 121–122 °C; 1H NMR (300 MHz, CDCl3): δ H 2.75 (s, 4H, 2x –CH2–); 3.18 (s, 4H, 2x –CH2–); 3.28 (s, 2H, –CH2–); 4.95–5.13 (m, 2H, –CH2–); 5.85–5.97 (m, 1H, –CH–); 6.35 (d, J = 5.1 Hz, 1H, H2); 6.53–6.59 (m, 1H, Ar–H); 7.17 (dd, J = 2.4 Hz, 8.7 Hz, 1H, H4); 7.28–7.30 (m, 1H, Ar–H); 7.47–7.53 (m, 1H, Ar–H); 7.89 (d, J = 2.4 Hz, 1H, H5); 7.96 (d, J = 9.0 Hz, 1H, H3); 8.41 (d, J = 5.1 Hz, 1H, H1); 13C NMR (75 MHz, CDCl3): δ C 45.7, 53.1, 58.9, 98.1, 112.5, 114.4, 115.6, 116.2, 116.9, 122.8, 124.9, 126.2, 127.6, 133.2, 134.6, 136.5, 148.2, 149.6, 152.2, 171.1. HRMS calculated for C24H23ClN4O3 450.1459 [M+] found 450.1465; Anal. Calcd (%) for: C, 63.93; H, 5.14; N, 12.43; found C, 63.87; H, 5.27; N, 12.51.
3.4.2. 5-Chloro-1-{2-[4-(7-Chloro-quinolin-4-yl)-piperazin-1-yl]-3-hydroxy-propyl}-1H-indole-2,3-dione (12b)
Pale yellow solid, yield 82%, m.p. 117–118 °C; 1H NMR (300 MHz, CDCl3): δ H 2.76 (s, 4H, 2x –CH2–); 3.15 (s, 4H, 2x –CH2–); 3.30 (s, 2H, –CH2–); 4.96–5.15 (m, 2H, –CH2–); 5.87–5.99 (m, 1H, –CH–); 6.25 (d, J = 5.1 Hz, 1H, H2); 6.32–6.37 (m, 1H, Ar–H); 7.13–7.24 (m, 2H, 2Ar–H); 7.34 (d, J = 9.0 Hz, 1H, H4); 7.64–7.73 (m, 2H, H5+ –OH exchangeable with D2O); 7.82 (d, J = 8.8 Hz, 1H, H3); 8.17–8.25 (m, 2H, H1+ –OH exchangeable with D2O); 13C NMR (75 MHz, CDCl3): δ C 45.5, 54.1, 58.2, 99.1, 111.7, 113.2, 114.8, 115.1, 116.5, 117.7, 123.6, 124.3, 125.4, 127.3, 133.7, 136.1, 147.5, 148.7, 150.1, 152.5, 169.1. HRMS calculated for C24H22Cl2N4O3 484.1069 [M+] found 484.1060; Anal. Calcd (%) for: C, 59.39; H, 4.57; N, 11.54; found C, 59.47; H, 4.69; N, 11.42.
3.4.3. 1-{2-[4-(7-Chloro-quinolin-4-yl)-piperazin-1-yl]-3-hydroxy-propyl}-5-methyl-1H-indole-2,3-dione (12c)
Pale yellow solid, yield 82%, m.p. 119–120 °C; 1H NMR (300 MHz, CDCl3): δ H 2.29 (s, 3H, –CH3); 2.76 (s, 4H, 2x –CH2–); 3.18 (s, 4H, 2x –CH2–); 3.29 (s, 2H, –CH2–); 4.95–5.15 (m, 2H, –CH2–); 5.85–5.96 (m, 1H, –CH–); 6.22 (d, J = 5.1 Hz, 1H, H2); 6.34–6.37 (m, 1H, Ar–H); 7.11–7.16 (m, 1H, Ar–H); 7.25 (s, 1H, Ar–H); 7.39 (d, J = 9.0 Hz, 1H, H4); 7.98 (s, 1H, H5); 7.82 (d, J = 9.0 Hz, 1H, H3); 8.19–8.22 (m, 2H, H1+ –OH exchangeable with D2O); 13C NMR (75 MHz, CDCl3): δ C 19.8, 46.1, 53.8, 57.4, 98.7, 112.8, 114.3, 116.5, 122.8, 124.9, 125.2, 125.9, 127.7, 132.8, 134.1, 135.8, 147.9, 148.2, 149.2, 151.7, 152.1, 171.2. HRMS calculated for C25H25ClN4O3 464.1615 [M+] found 464.1608; Anal. Calcd (%) for: C, 64.58; H, 5.42; N, 12.05; found C, 64.65; H, 5.59; N, 12.18.
3.4.4. 1-{2-[4-(7-Chloro-quinolin-4-yl)-piperazin-1-yl]-3-hydroxy-propyl}-5-fluoro-1H-indole-2,3-dione (12d)
Pale yellow solid, yield 79%, m.p. 131–132 °C; 1H NMR (300 MHz, CDCl3): δ H 2.75 (s, 4H, 2x –CH2–); 3.17 (s, 4H, 2x–CH2–); 3.29 (s, 2H, –CH2–); 4.96–5.14 (m, 2H, –CH2–); 5.87–5.97 (m, 1H, –CH–); 6.29 (d, J = 5.1 Hz, 1H, H2); 6.43–6.50 (m, 1H, Ar–H); 7.19–7.21 (m, 1H, Ar–H); 7.24–7.31 (m, 1H, Ar–H); 7.41 (d, J = 9.0 Hz, 1H, H4); 7.64 (s, 1H, H5); 7.93 (d, J = 8.8 Hz, 1H, H3); 8.35–8.40 (m, 2H, H1+ –OH exchangeable with D2O); 13C NMR (75 MHz, CDCl3): δ C 45.2, 53.8, 58.3, 99.3, 113.1, 114.2, 115.9, 116.5, 117.1, 123.8, 124.7, 126.9, 127.1, 133.6, 134.9, 136.1, 147.3, 148.4, 150.6, 152.8, 169.3. HRMS calculated for C24H22ClFN4O3 468.1364 [M+] found 468.1372; Anal. Calcd (%) for: C, 61.47; H, 4.73; N, 11.95; found C, 61.58; H, 4.65; N, 11.83.
3.5. Methods for assessment of antimalarial activity of test compounds
The W2 strain of P. falciparum was cultured in RPMI-1640 medium with 10% human serum, following standard methods, and parasites were synchronized with 5% D-sorbitol [50]. Beginning at the ring stage, microwell cultures were incubated with different concentrations of compounds for 48 h. The compounds were added from DMSO stocks; the maximum concentration of DMSO used was 0.1%. Controls without inhibitors included 0.1% DMSO. After 48 h, when control cultures had progressed to new rings, the culture medium was removed, and cultures were incubated for 48 h with 1% formaldehyde in PBS, pH 7.4, at room temperature. Fixed parasites were then transferred to 0.1% Triton X-100 in PBS containing 1 nM YOYO-1 dye (Molecular Probes). Parasitaemia was determined from dot plots (forward scatter vs. fluorescence) acquired on a FACSort flow cytometer using Cell Quest software (Beckton Dickinson). IC50 values for growth inhibition were determined from plots of percent control parasitaemia over inhibitor concentration using the Prism 3.0 program, (GraphPad Software), with data from duplicate experiments fitted by nonlinear regression [51].
3.6. In vitro cytotoxic studies
HCT116 (colon cancer cell line) cells were cultured in DMEM supplemented with 10% FBS. Cells were plated in 96 well tissue culture plates and treated with compound at three concentrations- 20 μM, 2 μM, 0.2 μM for 48 h 10 μl MTT 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide 5 mg/ml in PBS was added to the wells. Plates were incubated for 4 h. Plates were centrifuged at 1000 rpm for 5 min. Supernatant was removed. 100 μl DMSO was added to each well and mixed on shaker for 30 min. Absorbance was measured at 550 nm in Envision Multilabel Plate Reader (Perkin Elmer). Staurosporine and Doxorubicin were used as positive control. Percentage inhibition was calculated from absorbance using DMSO control as 100%.
Acknowledgements
Financial assistance from the University Grants Comission (UGC) under a Rajiv Gandhi fellowship for SC/ST students (Nisha) is gratefully acknowledged.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.ejmech.2014.07.064.
Appendix A. Supplementary data
References
- 1.WHO . 2013. World Malaria Report.http://www.who.int/malaria/publications/world malaria report 2012/en/index.html [Google Scholar]
- 2.Ridley R.G. Nature. 2002;415:686–693. doi: 10.1038/415686a. [DOI] [PubMed] [Google Scholar]
- 3.Kumar N., Singh R., Rawat D.S. Med. Res. Rev. 2012;32:581–610. [PubMed] [Google Scholar]
- 4.Gelb M.H. Curr. Opin. Chem. Biol. 2007;11:440–445. doi: 10.1016/j.cbpa.2007.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wiesner J., Ortmann R., Jomaa H., Schlitzer M. Angew. Chem. Int. Ed. 2003;42:5274–5293. doi: 10.1002/anie.200200569. [DOI] [PubMed] [Google Scholar]
- 6.Biagini G.A., O’Neill P.M., Bray P.G., Ward S.A. Curr. Opin. Pharmacol. 2005;5:473–478. doi: 10.1016/j.coph.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 7.Hyde J.E. Parasitol. Today. 1989;5:252–255. doi: 10.1016/0169-4758(89)90257-3. [DOI] [PubMed] [Google Scholar]
- 8.O’Neill P.M., Posner G.H.A. J. Med. Chem. 2004;47:2945–2964. doi: 10.1021/jm030571c. [DOI] [PubMed] [Google Scholar]
- 9.Bloland P.B., Ettling M., Meek S. Bull. World Health Org. 2000;78:1378–1388. [PMC free article] [PubMed] [Google Scholar]
- 10.Mutabingwa T.K. Acta Trop. 2005;95:305–315. doi: 10.1016/j.actatropica.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 11.Jambou R., Legrand E., Niang M., Khim N., Lim P., Volney B., Ekala M.T., Bouchier C., Esterre P., Fandeur T., Mercereau-Puijalon O. Lancet. 2005;366:1960–1963. doi: 10.1016/S0140-6736(05)67787-2. [DOI] [PubMed] [Google Scholar]
- 12.Dechy-Cabaret O., Benoit-Vical F., Loup C., Robert A., Gornitzka H., Bonhoure A., Vial H., Magnaval J.F., Seguela J.P., Meunier B. Chem. Eur. J. 2004;10:1625–1636. doi: 10.1002/chem.200305576. [DOI] [PubMed] [Google Scholar]
- 13.Bellot F., Cosledan F., Vendier L., Brocard J., Meunier B., Robert A. J. Med. Chem. 2010;53:4103–4109. doi: 10.1021/jm100117e. [DOI] [PubMed] [Google Scholar]
- 14.Kumar A., Srivastava K., Kumar S.R., Siddiqi M.I., Puri S.K., Saxena J.K., Chauhan P.M.S. Eur. J. Med. Chem. 2011;46:676–690. doi: 10.1016/j.ejmech.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 15.Manohar S., Rajesh U.C., Khan S.I., Tekwani B.L., Rawat D.S. ACS Med. Chem. Lett. 2012;3:555–559. doi: 10.1021/ml3000808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guantai E.M., Ncokazi K., Egan T.J., Gut J., Rosenthal P.J., Bhampidipati R., Kopinathan A., Smith P.J., Chibale K. J. Med. Chem. 2011;54:3637–3649. doi: 10.1021/jm200149e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.(a) Robert A., Dechy-Cabaret O., Cazelles J., Meunier B. Acc. Chem. Res. 2002;35:167–174. doi: 10.1021/ar990164o. [DOI] [PubMed] [Google Scholar]; (b) Benoit-Vical F., Lelievre J., Berry A., Deymier C., Dechy-Cabaret O., Cazelles J., Loup C., Robert A., Magnaval J.F., Meunier B. Antimicrob. Agents Chemother. 2007;51:1463–1472. doi: 10.1128/AAC.00967-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walsh J.J., Coughlan D., Heneghan N., Gaynor C., Bell A. Bioorg. Med. Chem. Lett. 2007;17:3599–3602. doi: 10.1016/j.bmcl.2007.04.054. [DOI] [PubMed] [Google Scholar]
- 19.Opsenica I., Opsenica D., Lanteri C.A., Anova L., Milhous W.K., Smith K.S., Salaja B.A. J. Med. Chem. 2008;51:6216–6219. doi: 10.1021/jm8006905. [DOI] [PubMed] [Google Scholar]
- 20.Gemma S., Campiani G., Butini S., Joshi B.P., Kukreja G., Coccone S.S., Burrutti M., Persico M., Nacci V., Fiorini I., Novellino E., Taramerlli D., Banilico N., Parapini S., Yardley V., Croft S., Maerk S.K., Rottman M., Brun R., Coletta M., Marini S., Guiso G., Caccia S., Fattorusso C. J. Med. Chem. 2009;52:502–513. doi: 10.1021/jm801352s. [DOI] [PubMed] [Google Scholar]
- 21.Chiyanzu I., Clarkson C., Smith P.J., Gut J., Rosenthal P.J., Chibale K. Bioorg. Med. Chem. 2005;13:3249–3261. doi: 10.1016/j.bmc.2005.02.037. [DOI] [PubMed] [Google Scholar]
- 22.Ratan B.T., Anand B., Yogeeswari P., Sriram Dh. Bioorg. Med. Chem. Lett. 2005;15:4451–4455. doi: 10.1016/j.bmcl.2005.07.046. [DOI] [PubMed] [Google Scholar]
- 23.Jiang T., Kuhen K.L., Wolff K., Yin H., Bieza K., Caldwell J., Bursulaya B., Tuntland T., Zhang K., Karanewsky D., He Y. Bioorg. Med. Chem. Lett. 2006;16:2109–2112. doi: 10.1016/j.bmcl.2006.01.066. [DOI] [PubMed] [Google Scholar]
- 24.Tripathy R., Reiboldt A., Messina P.A., Iqbal M., Singh J., Bacon E.R., Angeles Th S., Yang Sh X., Albom M.S., Robinson C., Chang H., Ruggeri B.A., Mallamo J.P. Bioorg. Med. Chem. Lett. 2006;16:2158–2168. doi: 10.1016/j.bmcl.2006.01.063. [DOI] [PubMed] [Google Scholar]
- 25.Cane A., Tournaire M.C., Barritault D., Crumeyrolle-Arias M. Biochem. Biophys. Res. Commun. 2000;276:379–384. doi: 10.1006/bbrc.2000.3477. [DOI] [PubMed] [Google Scholar]
- 26.Silveira V. Ch, Luz J.S., Oliveira C.C., Graziani I., Ciriolo M.R., Costa-Ferreira A.M. J. Inorg. Biochem. 2008;102:1090–1103. doi: 10.1016/j.jinorgbio.2007.12.033. [DOI] [PubMed] [Google Scholar]
- 27.Amal R.A., Raghunathan R., Sridevikumaria M.R., Raman N. Bioorg. Med. Chem. 2003;11:407–419. doi: 10.1016/s0968-0896(02)00439-x. [DOI] [PubMed] [Google Scholar]
- 28.Rodriguez-Arguelles M.C., Mosquera-Vazaquez S., Touron-Touceda P., Sanmartin-Matalobos J., Garcia-Deibe A.M., Belicchi-Ferraris M., Pelosi G., Pelizzi C., Zani F. J. Inorg. Biochem. 2007;101:138–147. doi: 10.1016/j.jinorgbio.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 29.Maskell L., Blanche E.A., Colucci M.A., Whatmore J.L., Moody Ch J. Bioorg. Med. Chem. Lett. 2007;17:1575–1578. doi: 10.1016/j.bmcl.2006.12.108. [DOI] [PubMed] [Google Scholar]
- 30.Verma P.S., Nath, Nand S.K., Stables J.P. Acta Pharm. 2004;54:49–56. [PubMed] [Google Scholar]
- 31.Igosheva N., Lorz C., O’Conner E., Glover V., Mehmet H. Neurochem. Int. 2005;47:216–224. doi: 10.1016/j.neuint.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 32.Chen L.R., Wang Y. Ch, Lin Y.W., Chou Sh Y., Chen Sh F., Liu L.T., Wu Y.T., Kuo Ch J., Chen T. Sh Sh, Juang Sh H. Bioorg. Med. Chem. Lett. 2005;15:3058–3062. doi: 10.1016/j.bmcl.2005.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pandeya S.N., Smitha S., Jyoti M., Sridhar S.K. Acta Pharm. 2005;55:27–46. [PubMed] [Google Scholar]
- 34.Vine K.L., Matesic L., Locke J.M., Ranson M., Skropeta D. Anti-cancer Agents Med. Chem. 2009;9:397–414. doi: 10.2174/1871520610909040397. [DOI] [PubMed] [Google Scholar]
- 35.Singh G.S., Desta Z.Y. Chem. Rev. 2012;112:6104–6155. doi: 10.1021/cr300135y. [DOI] [PubMed] [Google Scholar]
- 36.Ma J., Li S., Reed K., Guo P., Gallo J.M. Ther. J. Pharmacol. Exp. 2003;305:833–839. doi: 10.1124/jpet.102.048587. [DOI] [PubMed] [Google Scholar]
- 37.Prenen H., Cools J., Mentens N., Folens C., Sciot R., Schoffski P., Van Oosterom A., Marynen P., Debiec-Rychter M. Clin. Cancer Res. 2006;8:2622–2627. doi: 10.1158/1078-0432.CCR-05-2275. [DOI] [PubMed] [Google Scholar]
- 38.Hans R.H., Gut J., Rosenthal P.J., Chibale K. Bioorg. Med. Chem. Lett. 2010;20:2234–2237. doi: 10.1016/j.bmcl.2010.02.017. [DOI] [PubMed] [Google Scholar]
- 39.Hans R.H., Wiid I.J.F., Helden P.D., Wan B., Franzblau S.G., Gut J., Rosenthal P.J., Chibale K. Bioorg. Med. Chem. Lett. 2011;21:2055–2058. doi: 10.1016/j.bmcl.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 40.Raj R., Singh P., Singh P., Gut J., Rosenthal P.J., Kumar V. Eur. J. Med. Chem. 2013;62:590–596. doi: 10.1016/j.ejmech.2013.01.032. [DOI] [PubMed] [Google Scholar]
- 41.Raj R., Singh P., Singh P., Gut J., Rosenthal P.J., Kumar V. Bioorg. Med. Chem. Lett. 2014;24:756–759. doi: 10.1016/j.bmcl.2013.12.109. [DOI] [PubMed] [Google Scholar]
- 42.Raj R., Biot C., Carrère-Kremer S., Kremer L., Guérardel Y., Gut J., Rosenthal P.J., Forge D., Kumar V. Chem. Biol. Drug. Des. 2014;83:622–629. doi: 10.1111/cbdd.12273. [DOI] [PubMed] [Google Scholar]
- 43.(a) Singh P., Raj R., Singh P., Gut J., Rosenthal P.J., Kumar V. Eur. J. Med. Chem. 2014;71:128–134. doi: 10.1016/j.ejmech.2013.10.079. [DOI] [PubMed] [Google Scholar]; (b) Kumar K., Carrère-Kremer S., Kremer L., Guérardel Y., Biot C., Kumar V. Organometallics. 2013;32:5713–5719. [Google Scholar]; (c) Nisha, Kumar K., Bhargava G., Land K.M., Chang K.-H., Arora R., Sen S., Kumar V. Eur. J. Med. Chem. 2014;74:657. doi: 10.1016/j.ejmech.2014.01.015. P. Schlagenhauf, M. Adamcova, L. Regep, M. T Schaerer, H.-G. Rhein, Malaria Journal 9 (2010) 357–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.De D., Krogstad F.M., Cogswell F.B., Krogstad D.J. Am. J. Trop. Med. Hyg. 1996;55:579–583. doi: 10.4269/ajtmh.1996.55.579. [DOI] [PubMed] [Google Scholar]
- 45.De D., Krogstad F.M., Byers L.D., Krogstad D.J. J. Med. Chem. 1998;41:4918–4926. doi: 10.1021/jm980146x. [DOI] [PubMed] [Google Scholar]
- 46.Stocks P.A., Raynes K.J., Bray P.G., Park B.K., O Neill P.M., Ward S.A. J. Med. Chem. 2002;45:4975–4983. doi: 10.1021/jm0108707. [DOI] [PubMed] [Google Scholar]
- 47.Ridley R.G., Hofheinz W., Matile H., Jaquet C., Dorn A., Masciadri R., Jolidon S., Richter W.F., Guenzi A., Girometta M.A., Urwyler H., Huber W., Thaithong S., Peters W. Antimicrob. Agents Chemother. 1996;40:1846–1854. doi: 10.1128/aac.40.8.1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nisha N., Mehra V., Hopper M., Patel N., Land K.M., Kumar V. Med. Chem. Commun. 2013;4:1018–1024. [Google Scholar]
- 49.Jensen J.B. In vitro culture of plasmodium parasites. In: Doolan D.L., editor. Malaria Methods and Protocols. Humana; Totowa, NJ: 2002. pp. 477–488. [Google Scholar]
- 50.Singh A., Rosenthal P.J. Antimicrob. Agents Chemother. 2001;45:949–951. doi: 10.1128/AAC.45.3.949-951.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


