Abstract
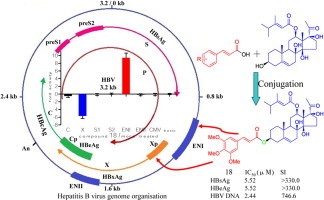
Forty-six conjugated derivatives of caudatin with substituted cinnamic acids were synthesized, and their anti-hepatitis B virus (HBV) activity was evaluated in HepG 2.2.15 cells. Most of the derivatives exhibited potent anti-HBV activity, especially inhibiting the HBV DNA replication with the IC50 values from 2.44 to 22.89 μΜ. Compound 18 showed significant activity against the secretion of HBsAg, HBeAg, and HBV DNA replication with IC50 values of 5.52, 5.52, 2.44 μΜ, respectively, and had good safety (LD50 > 1250 mg/kg) according to the acute toxicity study. Preliminary mechanism investigation suggested that compound 18 exerted antivirus effects via interfering HBV X promoter and enhancer I to influence HBV transcriptions.
Keywords: Synthesis, Molecular hybrids, Caudatin derivatives, Anti-hepatitis B virus, Unique mechanisms
Abbreviations: HBV, hepatitis B virus; HBsAg, hepatitis B surface antigen; HBeAg, hepatitis B e antigen; Xp, HBV X promoter; ENI, enhancer 1; ENII, enhancer 2; HBx, HBV X protein; LD50, 50% lethal dose; CC50, concentration of 50% cytotoxicity; IC50, 50% inhibitory concentration; SI, selectivity index
Graphical abstract
Forty-six conjugated derivatives of caudatin with cinnamic acids bearing various substituents were synthesized, and their anti-hepatitis B virus (HBV) activity was evaluated. Most of the derivatives exhibited potent anti-HBV activity, especially inhibiting the HBV DNA replication with the IC50 values from 2.44 to 22.89 μΜ. Compound 18 could influence HBV transcriptions by inhibiting the activity of HBV X promoter (Xp) and enhancing the activity of HBV enhancer I (ENI).
Highlights
► Conjugated derivatives of caudatin with cinnamic acids were synthesized. ► Most of the derivatives exhibited potent anti-HBV activity. ► The compound 18 exerted antivirus effects by interfering HBV promoters and enhancers. ► The mechanism of compound 18 is different from those of the nucleoside analogs.
1. Introduction
Hepatitis B virus (HBV) infection remains a global health problem, which often leads to severe consequences such as liver failure, fibrosis, cirrhosis, and hepatocellular carcinoma (HCC). There are about 2 billion people who have been infected with HBV, and more than 350 million people are lifelong patients [1], [2], [3]. Immunization therapy is the most effective measure for new infections, but millions of the HBV patients will eventually succumb to the infection sequence due to vaccine failure [4]. Interferon-α and polyethylene glycol interferon-α are used as anti-HBV agents clinically, however, their application is limited for low curing rate and serious side effects (influenza-like symptoms, fatigue, myalgia, nausea, headache, etc.) [5], [6]. Five nucleoside drugs, lamivudine (3TC), adefovir dipivoxil (ADV), entecavir (ETV), telbivudine (LdT) and tenofovir-DF (TDF), have been approved by FDA for HBV treatment. The disadvantages of nucleosides, such as drug resistance and high recurrence, are becoming an important clinical issue because of the single target [6], [7], [8]. New strategies aiming at viral suppression, promoting virologic clearance and preventing drug resistance are fascinating topics [9]. Therefore, novel anti-HBV agents with unique antiviral target and mechanism are still needed.
Natural products and their derivatives by simple functional-group transformations offer many opportunities for finding novel anti-HBV leads or drugs with unique antiviral mechanisms [10], [11], [12]. For example, helioxanthin, initially isolated from the shrub of Taiwania cryptomerioides, had unique mechanisms by diminishing HBV promoter activity and blocking viral gene expression and replication. After chemical modification, its more active derivative 8–1 (Fig. 1 ) was obtained [13], [14], [15], [16], [17]. Oxymatrine (Fig. 1) and its derivatives showed anti-HBV activity through suppressing host heat-stress cognate 70 (Hsc70) expression [18]. In our previous study, alisol A from Alisma orientalis possessed potent anti-HBV activity, and a series of high-activity derivatives were obtained (Fig. 1) after chemical modification [19], [20], [21].
Fig. 1.
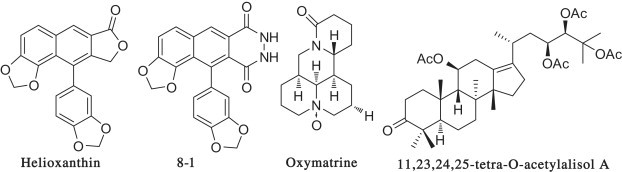
Structures of non-nucleoside HBV inhibitors from natural products and its derivatives.
As an ongoing search for potential anti-HBV inhibitors [22], [23], [24], [25], [26], [27], our latest study revealed that caudatin (1, Fig. 2 ) from Cynanchum auriculatum (Bai-Shou-Wu in Chinese) had activity inhibiting the secretion of HBsAg and HBV DNA replication with the IC50 values of 142.67 μM (SI = 1.7), 40.62 μM (SI = 6.0), respectively. Furthermore, caudatin as a prospective anti-HCC drug with the mechanism of inhibiting cell proliferation and inducing cell apoptosis has been reported [28], [29], [30], [31], [32]. Consequently, it may be interesting for caudatin to be developed as a novel anti-HBV agent by chemical modification.
Fig. 2.
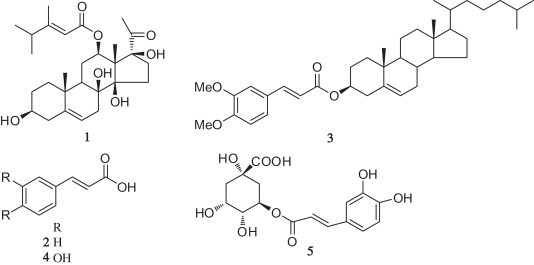
Caudatin, cinnamic acid and its derivatives.
Cinnamic acid analogs (esters, amides and glycosides) have attracted much attention in biology and medicine because of their antiviral [33], [34], antiatherogenic [35], antitumor [36], [37], antituberculosis [38], [39], antioxidant [40], [41], and antibacterial [42], [43] properties. For example, cinnamic acid (2, Fig. 2) could inhibit HIV/SARS-CoV S pseudovirus [33], and the cholesteryl conjugated with the 3, 4-dimethoxy cimmamic acid (3, Fig. 2) could enhance the anti-poliovirus type 1 (PV1) activity [44]. Caffeic acid (3, 4-dihydroxycinnamic acid, 4, Fig. 2) and chlorogenic acid (the ester of 3, 4-dihydroxycinnamic acid with quinic acid, 5, Fig. 2) showed potent anti-HBV activity in vivo and in vitro [45], which suggested that the molecular with cinnamic acid moiety might show potent anti-HBV activity. Meanwhile, cinnamic acid has been widely applied as a food additive with good safety [46].
Molecular hybrids with two pharmacophores often lead to synergistic activity [47], [48]. Thus, the combination of caudatin with cinnamic acids as novel anti-HBV agents was designed, which was anticipated that the hybrids could enhance the anti-HBV activity and decrease the cytotoxicity. Herein, we reported the chemical modification and HBV inhibitory properties of caudatin derivatives designed by molecular hybridization. The most active compound 18 was further investigated for the mechanism of action on HBV promoters (preC⁄pregenomic, S1, S2 and X promoters) and enhancers (ENI and ENII), as well as the acute toxicity in mice.
2. Chemistry
Compounds 6–10 and 12–51 were synthesized by the reaction of corresponding cinnamic acids with caudatin in the presence of N′, N′-dicyclohexylcarbodiimide (DCC) and 4-dimethylaminopyridine (DMAP) (Scheme 1 ). An exception is that compound 11 was obtained starting from caudatin and 3, 4-dihydroxycinnamic acid in the presence of diisopropyl azodicarboxylate (DIAD) and triphenylphosphine (TPP) (Scheme 1) [49]. Generally, the introduction of fluorine is useful to alter the physical properties, binding characteristics, and metabolic disposition in developing drug leads [50], thus various fluorinated cinnamic acids were used to react with caudatin. The structures of the synthesized derivatives (Table 1 ) were identified by spectroscopic means (1H, 13C NMR and MS). The structures of 3, 17-O-dicinnamoyl caudatin derivatives (48–51) were further determined by comparison with caudatin and/or the mono-cinnamoyl analogs.
Scheme 1.
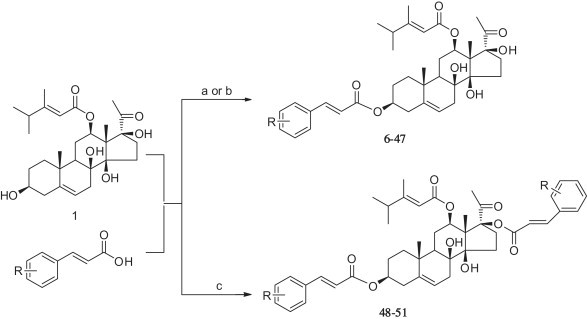
Synthesis of compounds 6–51. Reagents and conditions: (a) DMAP, DCC, CH2Cl2, rt; (b) DIAD, TPP, THF, rt; (c) substituted acids (4 equiv), DMAP, DCC, CH2Cl2, rt.
Table 1.
| Compd | R |
Compd | R |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 5″ | 6″ | 7″ | 8″ | 9″ | 5″ | 6″ | 7″ | 8″ | 9″ | ||
| 6 | H | H | H | H | H | 29 | H | Cl | H | H | H |
| 7 | CH3 | H | H | H | H | 30 | H | H | Cl | H | H |
| 8 | H | CH3 | H | H | H | 31 | H | Br | H | H | H |
| 9 | H | H | CH3 | H | H | 32 | H | H | Br | H | H |
| 10 | H | H | CHO | H | H | 33 | F | F | H | H | H |
| 11 | H | OH | OH | H | H | 34 | H | F | F | H | H |
| 12 | OCH3 | H | H | H | H | 35 | H | F | H | F | H |
| 13 | H | OCH3 | H | H | H | 36 | F | F | F | H | H |
| 14 | H | H | OCH3 | H | H | 37 | F | F | F | F | F |
| 15 | H | OCH3 | OCH3 | H | H | 38 | Cl | H | Cl | H | H |
| 16 | H | OCH3 | H | OCH3 | H | 39 | H | Cl | Cl | H | H |
| 17 | OCH3 | OCH3 | OCH3 | H | H | 40 | CF3 | H | H | H | H |
| 18 | H | OCH3 | OCH3 | OCH3 | H | 41 | H | CF3 | H | H | H |
| 19 | H | OCH2O | H | H | 42 | H | H | CF3 | H | H | |
| 20 | OEt | H | H | H | H | 43 | H | CF3 | H | CF3 | H |
| 21 | H | OEt | H | H | H | 44 | H | CF3 | F | H | H |
| 22 | H | H | AcO | H | H | 45 | NO2 | H | H | H | H |
| 23 | H | OCH3 | AcO | H | H | 46 | H | NO2 | H | H | H |
| 24 | H | OCF3 | H | H | H | 47 | H | NO2 | Cl | H | H |
| 25 | F | H | H | H | H | 48 | H | OCH3 | H | H | H |
| 26 | H | F | H | H | H | 49 | H | OCH3 | OCH3 | H | H |
| 27 | H | H | F | H | H | 50 | H | H | Cl | H | H |
| 28 | Cl | H | H | H | H | 51 | NO2 | H | H | H | H |
3. Results and discussion
3.1. Anti-HBV activity in vitro
All the caudatin derivatives were tested for their anti-HBV activity, namely inhibiting the secretion of HBsAg, HBeAg, and HBV DNA replication in HepG 2.2.15 cells. The data of their anti-HBV activity and cytotoxicity were listed in Table 2 .
Table 2.
Anti-HBV activity and cytotoxicity of caudatin derivatives in Vitro.a
| Compd | CC50b (μM) | HBsAgc |
HBeAgd |
DNA replication |
|||
|---|---|---|---|---|---|---|---|
| IC50e (μM) | SIf | IC50e (μM) | SIf | IC50e (μM) | SIf | ||
| 1 | 244.58 | 142.67 | 1.7 | >183.44 | <1.3 | 40.62 | 6.0 |
| 6 | >1417.59 | 48.33 | >29.3 | 94.24 | >15.0 | 5.91 | >239.9 |
| 7 | 1371.12 | 81.06 | 16.9 | 230.32 | 6.0 | 7.20 | 190.4 |
| 8 | >1390.35 | 25.44 | >54.7 | 132.40 | >10.5 | 7.22 | >192.6 |
| 9 | 549.95 | 108.25 | 5.1 | >549.95 | –g | 11.95 | 46.0 |
| 10 | 40.24 | 23.51 | 1.7 | 19.19 | 2.1 | 6.34 | 6.3 |
| 11 | 8.49 | 16.06 | – | >567.21 | – | 9.10 | – |
| 12 | >2366.24 | 64.04 | >36.9 | 752.90 | >3.1 | 7.30 | >324.1 |
| 13 | >1075.57 | 10.54 | >102.0 | 24.86 | >43.3 | 4.46 | >241.2 |
| 14 | >1382.87 | 107.56 | >12.9 | 568.51 | >2.4 | 10.88 | >127.1 |
| 15 | >1602.23 | 22.05 | >72.7 | 85.25 | >18.8 | 11.82 | >135.6 |
| 16 | >1023.11 | 29.59 | >34.6 | 379.86 | >2.7 | 11.70 | >87.4 |
| 17 | >1548.49 | 12.39 | >125.0 | 27.98 | >55.3 | 21.06 | >73.5 |
| 18 | >1821.75 | 5.52 | >330.0 | 5.52 | >330.0 | 2.44 | >746.6 |
| 19 | >1655.88 | 9.37 | >176.7 | 43.72 | >37.9 | 6.45 | >256.7 |
| 20 | >1726.56 | 40.56 | >42.6 | 403.52 | >4.3 | 12.01 | >143.8 |
| 21 | >2139.74 | 24.92 | >85.9 | 140.57 | >15.2 | 9.30 | >230.1 |
| 22 | <6.96 | <6.96 | – | 230.83 | – | 3.42 | <2.0 |
| 23 | <9.97 | <9.97 | – | 653.10 | – | 4.39 | <2.3 |
| 24 | 584.11 | 235.59 | 2.5 | >584.11 | – | 5.71 | 102.3 |
| 25 | 522.81 | 71.95 | 7.3 | 21.62 | 24.2 | 7.68 | 68.1 |
| 26 | >1064.00 | 8.40 | >126.7 | 12.56 | >84.7 | 4.75 | >224.0 |
| 27 | >1290.15 | 45.62 | >28.3 | 39.54 | >32.6 | 10.41 | >123.9 |
| 28 | >1146.42 | 28.38 | >40.4 | 550.21 | >2.1 | 22.89 | >50.1 |
| 29 | >1318.31 | 18.81 | >70.1 | 359.00 | >3.7 | 12.72 | >103.6 |
| 30 | 701.24 | 119.29 | 5.9 | >701.24 | – | 16.26 | 43.1 |
| 31 | 428.76 | 14.36 | 29.9 | 12.47 | 34.4 | 4.42 | 97.0 |
| 32 | >1291.76 | 36.42 | >35.5 | 484.88 | >2.7 | 16.03 | >80.6 |
| 33 | 117.84 | 13.15 | 9.0 | 16.36 | 7.2 | 2.86 | >41.2 |
| 34 | >1290.96 | 15.61 | >82.7 | 69.18 | >18.7 | 4.66 | >277.0 |
| 35 | >1340.81 | 20.32 | >66.0 | 90.72 | >14.8 | 18.62 | >72.0 |
| 36 | 396.82 | <5.58 | >71.1 | 14.04 | 28.3 | 2.83 | 140.2 |
| 37 | <8.22 | 26.30 | – | 353.14 | – | 9.90 | – |
| 38 | >1894.52 | 215.88 | >8.8 | 1182.02 | >1.6 | 14.54 | >130.3 |
| 39 | >1424.45 | 111.85 | >12.7 | 949.32 | >1.5 | 14.08 | >101.2 |
| 40 | 1764.54 | 46.94 | 37.6 | >1278.48 | <1.4 | 5.00 | 352.9 |
| 41 | 717.98 | 112.33 | 6.4 | >1191.31 | – | 14.88 | 48.3 |
| 42 | >1267.64 | 60.19 | >21.1 | 1267.64 | >1.0 | 10.07 | >125.9 |
| 43 | >1283.31 | 432.57 | >3.0 | 611.68 | >2.1 | >320.83 | – |
| 44 | >1332.81 | 71.58 | >18.6 | 797.70 | >1.7 | 8.93 | >149.2 |
| 45 | >1548.13 | 36.96 | >41.9 | 52.74 | >29.4 | 21.67 | >71.4 |
| 46 | >1593.22 | 50.32 | >31.7 | 206.56 | >7.7 | 56.71 | >28.1 |
| 47 | 450.03 | 9.52 | 47.3 | 79.52 | 5.7 | 6.31 | 71.3 |
| 48 | >1367.03 | >1367.03 | – | >1367.03 | – | >341.76 | – |
| 49 | >1206.31 | 528.48 | >2.3 | 631.88 | >1.9 | >307.12 | – |
| 50 | >1160.94 | >290.24 | – | >290.24 | – | >290.24 | – |
| 51 | >1737.37 | 116.06 | >15.0 | 521.70 | >3.3 | >434.34 | – |
| TFh | >1740.95 | 1450.11 | >1.2 | 1160.23 | >1.5 | 0.68 | >2560.2 |
Values are means determined from at least two experiments.
CC50 is 50% cytotoxicity concentration in HepG 2.2.15 cells.
HBsAg: hepatitis B surface antigen.
HBeAg, hepatitis B e antigen.
IC50 is 50% inhibitory concentration.
SI (selectivity index) = CC50/IC50.
No SI can be obtained.
Tenofovir as the positive control.
Derivative 6 was more effective on inhibiting HBsAg, HBeAg secretion and HBV DNA replication than that of caudatin with the IC50 values of 48.33, 94.24, 5.91 μΜ, and SI values of more than 29.3, 15.0, 239.9, respectively. After methyl group introduced to the cinnamoyl moiety, the anti-HBV activity of compounds 7–9 slightly decreased comparing to compound 6 but still was greater than that of caudatin. The introduction of formyl group into cinnamoyl moiety of compound 10 could increase the anti-HBV activity against secretion of the HBsAg and HBeAg, and HBV DNA replication, but the cytotoxicity (CC50 = 40.24 μΜ) was also increased.
When hydroxyl group was introduced to cinnamoyl, the activity and cytotoxicity of compound 11 were increased. Compounds 12–21 exhibited greater activity and weaker cytotoxicity than those of compound 11 after the hydroxy on the cinnamoyl replaced by alkoxyl groups (MeO–, EtO–, –OCH2O–). 3-O-(3, 4, 5-Trimethoxy) cinnamoyl caudatin (18) possessed the highest activity inhibiting not only the secretion of HBsAg (IC50 = 5.52 μΜ, SI > 330.0), HBeAg (IC50 = 5.52 μΜ, SI > 330.0), but also HBV DNA replication (IC50 = 2.44 μΜ, SI > 746.6). Both the anti-HBV activity and cytotoxicity of the derivatives 22–23 were increased comparing to caudatin when acetoxy group was incorporated into the cinnamic acid moiety.
The introduction of halogen atoms into caudatin derivatives (25–35) could significantly enhance their anti-HBV activity. But, more fluorine atoms in the derivatives led to cytotoxicity (36, CC50 = 396.82 μΜ and 37, CC50 < 8.22 μΜ) increase.
Compounds 24, 40–42, 44 with one CF3 group showed potent activity against HBsAg secretion and HBV-DNA replication but weaker against HBeAg secretion, while the co-existence of 3″- and 5″-CF3 groups on cinnamoyl (43) remarkably decreased the anti-HBV activity. The anti-HBV activity of compounds 45 and 46 with nitro group substituted on the cinnamoyl group was increased, and the cytotoxicity was decreased.
The positions of substituents on the cinnamoyl moiety could impact the anti-HBV activity of the 3-O-cinnamoyl caudatin derivatives. When the substitutes were methyl (8 vs 7, 9), alkoxy (13 vs 12, 14; 21 vs 20) or F (26 vs 25, 27), Cl (29 vs 28, 30), Br (31 vs 32), the meta-substituted analogs showed greater activity than those of ortho- and para-substituted. In contrary, the activity of the meta-substituted was weaker than those of ortho- and para-substituted with the substituents of trifluoromethyl (41 vs 40, 42) and nitro (46 vs 45) group. From the above results, it is proposed that small substitutions could affect the steric clash, electron density, or hydrogen-bonding capacity, resulting different anti-HBV activity of caudatin derivatives.
Due to the good activity of the conjugated derivatives of caudatin with one cinnamoyl moiety, 3, 17-O-dicinnamoyl caudatin derivatives (48–51) were further synthesized. Disappointedly, the anti-HBV activity of compounds 48–51 obviously decreased. These results indicate that free hydroxyl group at C-17 of caudatin is essential to the anti-HBV activity, and the steroidal skeleton itself plays a crucial role in maintaining anti-HBV activity of the conjugated esters.
3.2. Preliminary anti-HBV mechanism study
To elucidate the mechanism of action, a luciferase reporter gene assay was used to determine the effects of compound 18 on HBV promoters (preC⁄pregenomic, S1, S2 and X promoters) and enhancers (ENI and ENII). The result of compound 18 (10 μΜ) in the luciferase reporter assay was shown in Fig. 3 (Cells viability was up to 97.4% and 85.6% at 48 h and 72 h, respectively according to the MTT assay). Compound 18 mainly inhibited 5.6 times of the transcript activity of HBV X promoter (Xp) and increased 9.4 times of the HBV enhancer ENI compared with mock-treated control. HBV sequences are transcribed under the control of promoters and enhancers which are important for replication of HBV [51], [52]. The Xp regulates transcription of the small 0.9 kb mRNA sequence encoding HBV X protein (HBx), which plays an important role in stimulating HBV transcription and replication [53], [54]. The HBV ENI element takes a crucial effect in the overall liver-specific regulation of HBV gene expression [55]. Thus, compound 18 might exert anti-HBV activity by interfering promoters and enhancers to influence HBV transcriptions. Considering that there are about 20 nucleotides overlapped between the minimal Xp sequence and the 3′ end of the ENI [56], [57], [58], compound 18 was presumed to simultaneously influence Xp and ENI on the overlapped region. Further studies are needed to clarify how compound 18 takes effect and which region responds exactly.
Fig. 3.
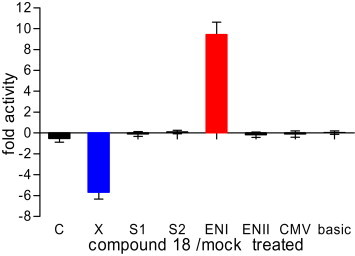
Effects of compound 18 on the activities of HBV promoters and enhancers HepG 2 cells were transiently transfected with a constant amount of pGL3 vector (expressing firefly luciferase) as basic control, and phRL-CMV vector (expressing Renilla luciferase) as an internal control. Firefly and Renilla luciferase activities were assayed using the Dual-Luciferase® reporter assay System. Data represent the average ± standard deviation of triplicated samples. Normalized fold change in activity between test groups: Fold activity = compound 18 (10 μM) treated group promoter activity/mock-treated group promoter activity.
Nucleoside analogs can suppress HBV replication by inhibiting HBV polymerase and terminating premature DNA chain, but do not directly affect the HBV gene expression [59]. Compound 18 exhibits anti-HBV activities by interfering HBV gene expression instead of inhibiting HBV DNA polymerase, which is different from those of the nucleoside analogs.
3.3. Acute toxicity of compound 18in vivo
To evaluate the safety of compound 18, the acute toxicity was tested in mice. Groups of healthy Kunming mice of both genders were orally administrated compound 18 with a single dose at 50, 250, and 1250 mg/kg, respectively. The survival and abnormality of mice were monitored up to 14 days post administration, and no dies and abnormality were observed (including the body weight, Table 3 ) in the mice throughout the observation period. It is demonstrated that compound 18 has good safety in vivo with the LD50 value of more than 1250 mg/kg in oral route.
Table 3.
Acute toxicity of Compound 18 in Mice.a
| Dose (mg/kg) | No. | No. of dead |
Total death/mortality | Body weight (g)c |
|||
|---|---|---|---|---|---|---|---|
| 1–7 day | 8–14 day | 0 day | 7 day | 14 day | |||
| 50 | 10b | 0 | 0 | 0/0 | 22.4 | 26.7 | 28.9 |
| 250 | 10b | 0 | 0 | 0/0 | 22.3 | 27.3 | 29.8 |
| 1250 | 10b | 0 | 0 | 0/0 | 22.6 | 26.5 | 28.8 |
| Blank | 10b | 0 | 0 | 0/0 | 21.9 | 26.3 | 28.5 |
Oral administration.
5 Male plus 5 female.
Body weight was the average weight of the same group.
4. Conclusions
In summary, our design and synthesis have led to a series of non-nucleoside anti-HBV agents by attaching of the cinnamic acids to caudatin. Most of the derivatives displayed potent anti-HBV activity especially inhibited the HBV DNA replication with the IC50 values from 2.44 to 22.89 μΜ. The most active compound 18 inhibited not only the secretion of HBsAg and HBeAg with IC50 values of 5.52 μΜ (SI > 330.0) and 5.52 μΜ (SI > 330.0), but also the HBV DNA replication with IC50 value of 2.44 μΜ (SI > 746.6). Preliminary mechanism study proposed that compound 18 exerted antivirus effects via interfering HBV promoters and enhancers to influence HBV transcriptions. The low cytotoxicity and acute toxicity in mice indicated that compound 18 may be potential novel non-nucleoside anti-HBV drug candidate with unique mechanisms to be further investigated.
5. Experimental
5.1. Chemistry
Nuclear magnetic resonance (NMR) spectra were recorded on Bruker AM 400 (1H/13C, 400 MHz) or DRX-500 (1H/13C, 500 MHz) spectrometers and chemical shifts were given in δ (ppm) with TMS as the internal standard (Bruker, Bremerhaven, Germany); MS and HRMS spectra were determined on an AutoSpec Premier P776 (VG, Manchester, UK) or API QSTAR Pulsar (AB, Foster City, USA) mass spectrometers; column chromatography (CC): silica gel (200–300 mesh; Qingdao Makall Group Co., Ltd; Qingdao; China). All reactions were monitored using thin-layer chromatography (TLC) on silica gel plates. Caudatin was isolated from C. auriculatum (Bai-Shou-Wu) and had the purity of >95.0%. Substituted cinnamic acids were purchased from Alfa Aesar or J&K Scientific Ltd. Organic solvents were analytical reagent grade and purchased from Tianjin Chemical Reagent Co., Ltd.
5.1.1. General procedure for the preparation of derivatives (6–10, 12–47)
A solution of 1 (0.2 mmol), DMAP (0.2 equiv), and the proper cinnamic acid (1.2 equiv) in anhydrous CH2Cl2 (8 mL) was added DCC (1.2 equiv) at 0 °C. The resulting mixture was stirred at room temperature until the starting material was not observed by TLC. The reaction mixture was filtered, and the residue was washed with CH2Cl2 (2 × 10 mL). The CH2Cl2 solution was washed with 5% HCl (3 × 30 mL), saturated NaHCO3 (3 × 30 mL) and saturated NaCl (3 × 30 mL), respectively. The organic layer was dried over anhydrous Na2SO4 and concentrated to dryness under reduced pressure. The residue was chromatographed using a silica gel column to yield the pure target compounds.
5.1.1.1. 3-O-Cinnamoyl caudatin (6)
White amorphous power, yield 64.5% (after chromatography with petroleum ether/acetone, 85:15); 1H NMR (CDCl3, 400 MHz): δ 1.06 (6H, d, J = 6.8 Hz, CH 3-5′, 6′), 1.20 (3H, s, CH 3-19), 1.40 (3H, s, CH 3-18), 1.60 (1H, m, H-9), 1.84–2.00 (9H, overlap, H-1, 2, 11, 15, 16α), 2.13 (3H, s, CH 3-7′), 2.17 (3H, s, CH 3-21), 2.24 (2H, s, H-7), 2.37 (1H, m, H-4′), 2.49 (2H, m, H-4), 2.86 (1H, m, H-16β), 4.59 (1H, t, J = 6.8 Hz, H-12), 4.78 (1H, m, H-3), 5.45 (1H, s, H-6), 5.53 (1H, s, H-2′), 6.42 (1H, d, J = 16.0 Hz, H-2″), 7.38 (3H, overlap, H-6″, 8″, 7″), 7.52 (2H, overlap, H-5″, 9″), 7.68 (1H, d, J = 16.0 Hz, H-3″); 13C NMR (CDCl3, 100 MHz): δ 9.4 (C-18), 16.5 (C-7′), 18.5 (C-19), 20.8 (C-6′), 20.9 (C-5′), 24.2 (C-11), 27.0 (C-2), 27.2 (C-21), 31.7 (C-16), 33.3 (C-7), 34.2 (C-15), 37.0 (C-10), 38.0 (C-1), 38.2 (C-4′), 38.5 (C-4), 43.6 (C-9), 58.0 (C-13), 71.6 (C-12), 73.8 (C-3), 74.2 (C-8), 87.9 (C-14), 91.4 (C-17), 112.9 (C-2′), 118.4 (C-6), 118.8 (C-2″), 128.0 (2C-5″, 9″), 128.9 (2C-6″, 8″), 130.2 (C-7″), 134.4 (C-4″), 139.4 (C-5), 144.7 (C-3″), 166.0 (C-3′), 166.3 (C-1″), 167.0 (C-1′), 208.9 (C-20); ESIMS: m/z 643 [M + Na]+ HRESIMS: calcd for C37H48O8Na [M + Na]+ 643.3246, found 643.3232.
5.1.1.2. 3-O-(2-Methyl)cinnamoyl caudatin (7)
White amorphous power, yield 60.7% (after chromatography with petroleum ether/acetone, 85:15); 1H NMR (CDCl3, 400 MHz): δ 1.06 (6H, d, J = 6.8 Hz, CH 3-5′, 6′), 1.19 (3H, s, CH 3-19), 1.41 (3H, s, CH 3-18), 1.60 (1H, m, H-9), 1.83–2.00 (9H, overlap, H-1, 2, 11, 15, 16α), 2.13 (3H, s, CH 3-7′), 2.17 (3H, s, CH 3-21), 2.22 (2H, s, H-7), 2.36 (1H, m, H-4′), 2.43 (3H, s, CH 3-5″), 2.49 (2H, m, H-4), 2.86 (1H, m, H-16β), 4.57 (1H, dd, J = 5.7, 10.0 Hz, H-12), 4.77 (1H, m, H-3), 5.43 (1H, s, H-6), 5.52 (1H, s, H-2′), 6.33 (1H, d, J = 15.8 Hz, H-2″), 7.18 (2H, m, H-7″, 8″), 7.25 (1H, d, J = 8.8 Hz, H-6″), 7.53 (1H, d, J = 7.3 Hz, H-9″), 7.96 (1H, d, J = 15.8 Hz, H-3″); 13C NMR (CDCl3, 100 MHz): δ 9.4 (C-18), 16.5 (C-7′), 18.4 (C-19), 19.8 (CH3-5″), 20.8 (C-6′), 20.9 (C-5′), 24.2 (C-11), 27.0 (C-2), 27.2 (C-21), 31.8 (C-16), 33.2 (C-7), 34.2 (C-15), 37.0 (C-10), 38.0 (C-1), 38.1 (C-4′), 38.5 (C-4), 43.6 (C-9), 57.9 (C-13), 71.6 (C-12), 73.8 (C-3), 74.2 (C-8), 88.0 (C-14), 91.5 (C-17), 112.9 (C-2′), 118.8 (C-6), 119.3 (C-2″), 126.3 (C-8″), 126.4 (C-9″), 130.0 (C-6″), 130.8 (C-7″), 133.3 (C-5″), 137.6 (C-4″), 139.4 (C-5), 142.4 (C-3″), 166.0 (C-3′), 166.4 (C-1″), 166.9 (C-1′), 208.9 (C-20); ESIMS: m/z 633 [M − H]‾, HRESIMS: calcd for C38H49O8 [M − H]‾ 633.3427, found 633.3413.
5.1.1.3. 3-O-(3-Methyl)cinnamoyl caudatin (8)
White amorphous power, yield 65.5% (after chromatography with petroleum ether/acetone, 85:15); 1H NMR (CDCl3, 400 MHz): δ 1.06 (6H, d, J = 6.8 Hz, CH 3-5′, 6′), 1.19 (3H, s, CH 3-19), 1.41 (3H, s, CH 3-18), 1.72 (1H, m, H-9), 1.84–2.01 (9H, overlap, H-1, 2, 11, 15, 16α), 2.13 (3H, s, CH 3-7′), 2.18 (3H, s, CH 3-21), 2.23 (2H, s, H-7), 2.36 (1H, m, H-4′), 2.37 (3H, s, CH 3-6″), 2.49 (2H, m, H-4), 2.86 (1H, m, H-16β), 4.58 (1H, t, J = 7.0 Hz, H-12), 4.77 (1H, m, H-3), 5.44 (1H, s, H-6), 5.53 (1H, s, H-2′), 6.40 (1H, d, J = 16.0 Hz, H-2″), 7.19 (1H, d, J = 7.4 Hz, H-9″), 7.27 (1H, t, J = 7.8 Hz, H-8″), 7.32 (1H, d, J = 7.2 Hz, H-7″), 7.34 (1H, s, H-5″), 7.65 (1H, d, J = 16.0 Hz, H-3″); 13C NMR (CDCl3, 100 MHz): δ 9.4 (C-18), 16.5 (C-7′), 18.5 (C-19), 20.8 (C-6′), 20.9 (C-5′), 21.3 (CH3-6″), 24.2 (C-11), 27.0 (C-2), 27.2 (C-21), 31.7 (C-16), 33.2 (C-7), 34.2 (C-15), 37.0 (C-10), 38.0 (C-1), 38.2 (C-4′), 38.5 (C-4), 43.6 (C-9), 57.9 (C-13), 71.6 (C-12), 73.7 (C-3), 74.2 (C-8), 87.9 (C-14), 91.4 (C-17), 112.9 (C-2′), 118.1 (C-6), 118.8 (C-2″), 125.2 (C-9″), 128.7 (2C, C-5″, 7″), 131.1 (C-8″), 134.3 (C-4″), 138.5 (C-6″), 139.4 (C-5), 144.8 (C-3″), 166.0 (C-3′), 166.4 (C-1″), 167.0 (C-1′), 208.9 (C-20); ESIMS: m/z 633 [M − H]‾, HRESIMS: calcd for C38H49O8 [M − H]‾ 633.3427, found 633.3427.
5.1.1.4. 3-O-(4-Methyl)cinnamoyl caudatin (9)
White amorphous power, yield 66.5% (after chromatography with petroleum ether/acetone, 85:15); 1H NMR (CDCl3, 400 MHz): δ 1.06 (6H, d, J = 6.8 Hz, CH 3-5′, 6′), 1.18 (3H, s, CH 3-19), 1.40 (3H, s, CH 3-18), 1.72 (1H, m, H-9), 1.83–2.00 (9H, overlap, H-1, 2, 11, 15, 16α), 2.12 (3H, s, CH 3-7′), 2.16 (3H, s, CH 3-21), 2.22 (2H, s, H-7), 2.36 (1H, m, H-4′), 2.37 (3H, s, CH 3-7″), 2.48 (2H, m, H-4), 2.86 (1H, m, H-16β), 4.58 (1H, t, J = 6.0 Hz, H-12), 4.77 (1H, m, H-3), 5.43 (1H, s, H-6), 5.52 (1H, s, H-2′), 6.36 (1H, d, J = 16.0 Hz, H-2″), 7.17 (2H, d, J = 8.1 Hz, H-6″, 8″), 7.41 (2H, d, J = 8.1 Hz, H-5″, 9″), 7.64 (1H, d, J = 16.0 Hz, H-3″); 13C NMR (CDCl3, 100 MHz): δ 9.4 (C-18), 16.5 (C-7′), 18.5 (C-19), 20.8 (C-6′), 20.9 (C-5′), 21.4 (CH3-4″), 24.2 (C-11), 27.0 (C-2), 27.2 (C-21), 31.8 (C-16), 33.2 (C-7), 34.2 (C-15), 37.0 (C-10), 38.0 (C-1), 38.1 (C-4′), 38.5 (C-4), 43.6 (C-9), 57.9 (C-13), 71.6 (C-12), 73.7 (C-3), 74.2 (C-8), 87.9 (C-14), 91.4 (C-17), 112.9 (C-2′), 117.3 (C-2″), 118.8 (C-6), 128.0 (2C-5″, 9″), 129.6 (2C-6″, 8″), 131.7 (C-4″), 139.4 (C-5), 140.6 (C-7″), 144.7 (C-3″), 166.0 (C-3′), 166.5 (C-1″), 166.9 (C-1′), 208.8 (C-20); ESIMS: m/z 633 [M − H]‾, HRESIMS: calcd for C38H49O8 [M − H]‾ 633.3427, found 633.3418.
5.1.1.5. 3-O-(4-Formyl)cinnamoyl caudatin (10)
White amorphous power, yield 72.5% (after chromatography with petroleum ether/acetone, 85:15), 1H NMR (CDCl3, 400 MHz): δ 1.06 (6H, d, J = 6.8 Hz, CH 3-5′, 6′), 1.19 (3H, s, CH 3-19), 1.40 (3H, s, CH 3-18), 1.56 (1H, m, H-9), 1.83–1.99 (9H, overlap, H-1, 2, 11, 15, 16α), 2.12 (3H, s, CH 3-7′), 2.17 (3H, s, CH3-21), 2.22 (2H, s, H-7), 2.36 (1H, m, H-4′), 2.48 (2H, m, H-4), 2.85 (1H, m, H-16β), 4.57 (1H, dd, J = 5.9, 9.8 Hz, H-12), 4.78 (1H, m, H-3), 5.44 (1H, s, H-6), 5.53 (1H, s, H-2′), 6.52 (1H, d, J = 16.0 Hz, H-2″), 7.67 (2H, d, J = 8.2 Hz, H-5″, 9″), 7.69 (1H, J = 16.0 Hz, H-3″), 7.89 (2H, d, J = 8.2 Hz, H-6″, 8″), 10.02 (1H, s, CHO-7″); 13C NMR (CDCl3, 100 MHz): δ 9.4 (C-18), 16.5 (C-7′), 18.4 (C-19), 20.8 (C-6′), 20.9 (C-5′), 24.1 (C-11), 26.9 (C-2), 27.2 (C-21), 31.7 (C-16), 33.3 (C-7), 34.3 (C-15), 37.0 (C-10), 37.9 (C-1), 38.1 (C-4′), 38.4 (C-4), 43.6 (C-9), 57.9 (C-13), 71.6 (C-12), 74.1 (C-3), 74.2 (C-8), 87.9 (C-14), 91.4 (C-17), 112.9 (C-2′), 119.0 (C-6), 121.6 (C-2″), 128.5 (2C, C-5″, 9″), 130.1 (2C, C-6″, 8″), 137.1 (C-7″), 139.2 (C-5), 140.1 (C-4″), 142.9 (C-3″), 166.0 (C-3′), 167.0 (C-1′), 165.7 (C-1″), 191.5 (CHO-7″), 208.9 (C-20); ESIMS: m/z 647 [M − H]‾, HRESIMS: calcd for C38H47O9 [M − H]‾ 647.3220, found 647.3212.
5.1.1.6. 3-O-(2-Methoxy)cinnamoyl caudatin (12)
White amorphous power, yield 74.6% (after chromatography with petroleum ether/acetone, 85:15), 1H NMR (CDCl3, 400 MHz): δ 1.06 (6H, d, J = 6.8 Hz, CH 3-5′, 6′), 1.19 (3H, s, CH 3-19), 1.44 (3H, s, CH 3-18), 1.59 (1H, m, H-9), 1.83–1.96 (9H, overlap, H-1, 2, 11, 15, 16α), 2.13 (3H, s, CH 3-7′), 2.18 (3H, s, CH 3-21), 2.21 (2H, s, H-7), 2.36 (1H, m, H-4′), 2.48 (2H, m, H-4), 2.87 (1H, m, H-16β), 3.88 (3H, s, OCH 3-5″), 4.57 (1H, dd, J = 5.2, 10.2 Hz, H-12), 4.76 (1H, m, H-3), 5.42 (1H, s, H-6), 5.53 (1H, s, H-2′), 6.50 (1H, d, J = 16.1 Hz, H-2″), 6.90 (1H, d, J = 7.8 Hz, H-6″), 6.95 (1H, t, J = 7.8 Hz, H-8″), 7.33 (1H, t, J = 7.8 Hz, H-7″), 7.49 (1H, d, J = 7.8 Hz, H-9″), 7.97 (1H, d, J = 16.1 Hz, H-3″); 13C NMR (CDCl3, 100 MHz): δ 9.5 (C-18), 16.5 (C-7′), 18.4 (C-19), 20.8 (C-6′), 20.9 (C-5′), 24.2 (C-11), 27.0 (C-2), 27.1 (C-21), 31.9 (C-16), 33.2 (C-7), 34.2 (C-15), 37.0 (C-10), 38.0 (C-1), 38.1 (C-4′), 38.5 (C-4), 43.6 (C-9), 55.4 (OCH3-5″), 57.8 (C-13), 71.5 (C-12), 73.6 (C-3), 74.1 (C-8), 88.1 (C-14), 91.5 (C-17), 111.1 (C-6″), 112.9 (C-2′), 118.7 (2C-6, 2″), 120.6 (C-8″), 123.3 (C-4″), 128.9 (C-7″), 131.4 (C-9″), 139.4 (2C-5, 3″), 140.1 (C-3″), 158.3 (C-5″), 165.9 (C-3′), 166.7 (C-1″), 166.9 (C-1′), 208.9 (C-20); EIMS: m/z 650, HREIMS: calcd for C38H50O9 650.3455, found 650.3433.
5.1.1.7. 3-O-(3-Methoxy)cinnamoyl caudatin (13)
White amorphous power, yield 70.8% (after chromatography with petroleum ether/acetone, 85:15), 1H NMR (CDCl3, 400 MHz): δ 1.06 (6H, d, J = 6.8 Hz, CH 3-5′, 6′), 1.20 (3H, s, CH 3-19), 1.40 (3H, s, CH 3-18), 1.60 (1H, m, H-9), 1.84–2.00 (9H, overlap, H-1, 2, 11, 15, 16α), 2.13 (3H, s, CH 3-7′), 2.17 (3H, s, CH 3-21), 2.24 (2H, s, H-7), 2.35 (1H, m, H-4′), 2.49 (2H, m, H-4), 2.86 (1H, m, H-16β), 3.83 (3H, s, OCH 3-6″), 4.58 (1H, t, J = 6.8 Hz, H-12), 4.78 (1H, m, H-3), 5.44 (1H, s, H-6), 5.53 (1H, s, H-2′), 6.40 (1H, d, J = 15.8 Hz, H-2″), 6.93 (1H, dd, J = 2.0, 8.2 Hz, H-7″), 7.04 (1H, s, H-5″), 7.12 (1H, d, J = 7.6 Hz, H-9″) 7.30 (1H, t, J = 7.9 Hz, H-8″), 7.64 (1H, d, J = 15.8 Hz, H-3″); 13C NMR (CDCl3, 100 MHz): δ 9.4 (C-18), 16.5 (C-7′), 18.5 (C-19), 20.8 (C-6′), 20.9 (C-5′), 24.2 (C-11), 27.0 (C-2), 27.2 (C-21), 31.7 (C-16), 33.3 (C-7), 34.3 (C-15), 37.0 (C-10), 38.0 (C-1), 38.2 (C-4′), 38.5 (C-4), 43.6 (C-9), 55.3 (OCH3-6″), 58.0 (C-13), 71.6 (C-12), 73.8 (C-3), 74.2 (C-8), 87.9 (C-14), 91.4 (C-17), 112.8 (C-7″), 112.9 (C-2′), 116.1 (C-5″), 118.7 (C-6), 118.8 (C-2″), 120.8 (C-9″), 129.8 (C-8″), 135.7 (C-4″), 139.4 (C-5), 144.6 (C-3″), 159.8 (C-6″), 166.0 (C-3′), 166.2 (C-1″), 167.0 (C-1′), 208.9 (C-20); ESIMS: m/z 673 [M + Na]+, HRESIMS: calcd for C38H50O9Na [M + Na]+ 673.3352, found 673.3369.
5.1.1.8. 3-O-(4-Methoxy)cinnamoyl caudatin (14)
White amorphous power, yield 51.5% (after chromatography with petroleum ether/acetone, 85:15), 1H NMR (CDCl3, 400 MHz): δ 1.06 (6H, d, J = 6.8 Hz, CH 3-5′, 6′), 1.19 (3H, s, CH 3-19), 1.43 (3H, s, CH 3-18), 1.59 (1H, m, H-9), 1.83–2.00 (9H, overlap, H-1, 2, 11, 15, 16α), 2.13 (3H, s, CH 3-7′), 2.18 (3H, s, CH 3-21), 2.22 (2H, s, H-7), 2.36 (1H, m, H-4′), 2.48 (2H, m, H-4), 2.87 (1H, m, H-16β), 3.82 (3H, s, OCH 3-7″), 4.57 (1H, dd, J = 5.6, 10.4 Hz, H-12), 4.74 (1H, m, H-3), 5.42 (1H, s, H-6), 5.53 (1H, s, H-2′), 6.28 (1H, d, J = 15.9 Hz, H-2″), 6.89 (2H, d, J = 8.8 Hz, H-6″, 8″), 7.47 (2H, d, J = 8.8 Hz, H-5″, 9″), 7.62 (1H, d, J = 15.9 Hz, H-3″); 13C NMR (CDCl3, 100 MHz): δ 9.4 (C-18), 16.4 (C-7′), 18.3 (C-19), 20.7 (C-6′), 20.8 (C-5′), 24.1 (C-11), 26.9 (C-2), 27.1 (C-21), 31.7 (C-16), 33.1 (C-7), 34.1 (C-15), 36.9 (C-10), 37.9 (C-1), 38.0 (C-4′), 38.4 (C-4), 43.5 (C-9), 55.2 (OCH3-7″), 57.8 (C-13), 71.5 (C-12), 73.5 (C-3), 74.1 (C-8), 88.0 (C-14), 91.4 (C-17), 112.8 (C-2′), 114.2 (2C-6″, 8″), 115.7 (C-2″), 118.7 (C-6), 127.0 (C-4″), 161.2 (C-7″), 129.6 (2C-5″, 9″), 139.3 (C-5), 144.3 (C-3″), 165.8 (C-3′), 166.6 (C-1″), 166.7 (C-1′), 208.8 (C-20); ESIMS: m/z 673 [M + Na]+, HRESIMS: calcd for C38H50O9Na [M + Na]+ 673.3352, found 673.3357.
5.1.1.9. 3-O-(3, 4-Dimethoxy)cinnamoyl caudatin (15)
White amorphous power, yield 57.5% (after chromatography with petroleum ether/acetone, 85:15), 1H NMR (CDCl3, 400 MHz): δ 1.03 (6H, d, J = 6.8 Hz, CH 3-5′, 6′), 1.16 (3H, s, CH 3-19), 1.39 (3H, s, CH 3-18), 1.58 (1H, m, H-9), 1.83–1.98 (9H, overlap, H-1, 2, 11, 15, 16α), 2.10 (3H, s, CH 3-7′), 2.15 (3H, s, CH 3-21), 2.19 (2H, s, H-7), 2.33 (1H, m, H-4′), 2.44 (2H, m, H-4), 2.83 (1H, m, H-16β), 3.87 (2 × 3H, s, OCH 3-6″, 7″), 4.54 (1H, dd, J = 5.6, 10.2 Hz, H-12), 4.73 (1H, m, H-3), 5.40 (1H, s, H-6), 5.50 (1H, s, H-2′), 6.26 (1H, d, J = 15.9 Hz, H-2″), 6.83 (1H, d, J = 8.3 Hz, H-8″), 7.02 (1H, s, H-5″), 7.07 (1H, d, J = 8.3 Hz, H-9″), 7.58 (1H, d, J = 15.9 Hz, H-3″); 13C NMR (CDCl3, 100 MHz): δ 9.4 (C-18), 16.5 (C-7′), 18.4 (C-19), 20.8 (C-6′), 20.9 (C-5′), 24.1 (C-11), 27.0 (C-2), 27.1 (C-21), 31.8 (C-16), 33.2 (C-7), 34.2 (C-15), 37.0 (C-10), 38.0 (C-1), 38.1 (C-4′), 38.4 (C-4), 43.6 (C-9), 55.8 (OCH3-7″), 55.9 (OCH3-6″), 57.8 (C-13), 71.6 (C-12), 73.6 (C-3), 74.1 (C-8), 88.0 (C-14), 91.5 (C-17), 109.5 (C-8″), 111.0 (C-5″), 112.9 (C-2′), 116.0 (C-2″), 118.8 (C-6), 122.6 (C-9″), 127.3 (C-4″), 139.3 (C-5), 144.6 (C-3″), 149.1 (C-7″), 151.0 (C-6″), 165.9 (C-3′), 166.5 (C-1″), 166.8 (C-1′), 208.9 (C-20); EIMS: m/z 680, HREIMS: calcd for C39H52O10 680.3560, found 680.3523.
5.1.1.10. 3-O-(3, 5-Dimethoxy)cinnamoyl caudatin (16)
White amorphous power, yield 74.6% (after chromatography with petroleum ether/acetone, 85:15), 1H NMR (CDCl3, 400 MHz): δ 1.02 (6H, d, J = 6.8 Hz, CH 3-5′, 6′), 1.15 (3H, s, CH 3-19), 1.40 (3H, s, CH 3-18), 1.54 (1H, m, H-9), 1.79–1.96 (9H, overlap, H-1, 2, 11, 15, 16α), 2.09 (3H, s, CH 3-7′), 2.13 (2H, s, H-7), 2.18 (3H, s, CH 3-21), 2.33 (1H, m, H-4′), 2.44 (2H, m, H-4), 2.84 (1H, m, H-16β), 3.76 (2 × 3H, s, OCH 3-6″, 8″), 4.53 (1H, dd, J = 4.9, 10.6 Hz, H-12), 4.72 (1H, m, H-3), 5.38 (1H, s, H-6), 5.49 (1H, s, H-2′), 6.34 (1H, d, J = 15.9 Hz, H-2″), 6.44 (1H, s, H-7″), 6.62 (2H, s, H-5″, 9″), 7.54 (1H, d, J = 15.9 Hz, H-3″); 13C NMR (CDCl3, 100 MHz): δ 9.5 (C-18), 16.5 (C-7′), 18.3 (C-19), 20.8 (C-6′), 20.9 (C-5′), 24.1 (C-11), 27.0 (C-2), 27.2 (C-21), 31.8 (C-16), 33.2 (C-7), 34.2 (C-15), 36.9 (C-10), 37.9 (C-1), 38.1 (C-4′), 38.4 (C-4), 43.5 (C-9), 55.3 (2C, OCH3-6″, 8″), 57.8 (C-13), 71.5 (C-12), 73.8 (C-3), 74.1 (C-8), 88.1 (C-14), 91.5 (C-17), 102.5 (C-7″), 105.8 (2C-5″, 9″), 112.9 (C-2′), 118.8 (C-6), 118.9 (C-2″), 136.2 (C-4″), 139.1 (C-6), 144.7 (C-3″), 160.9 (2C-6″, 8″), 165.9 (C-3′), 166.2 (C-1″), 166.8 (C-1′), 209.0 (C-20); ESIMS: m/z 679 [M − H]‾, HRESIMS: calcd for C39H51O10 [M − H]‾ 679.3482, found 679.3473.
5.1.1.11. 3-O-(2, 3, 4-Trimethoxy)cinnamoyl caudatin (17)
White amorphous power, yield 64.1% (after chromatography with petroleum ether/acetone, 85:15), 1H NMR (CDCl3, 400 MHz): δ 1.06 (6H, d, J = 6.8 Hz, CH 3-5′, 6′), 1.18 (3H, s, CH 3-19), 1.41 (3H, s, CH 3-18), 1.55 (1H, m, H-9), 1.83–2.00 (9H, overlap, H-1, 2, 11, 15, 16α), 2.08 (3H, s, CH 3-7′), 2.17 (3H, s, CH 3-21), 2.22 (2H, s, H-7), 2.36 (1H, m, H-4′), 2.47 (2H, m, H-4), 2.86 (1H, m, H-16β), 3.86 (3H, s, OCH 3-5″), 3.88 (3H, s, OCH 3-7″), 3.91(3H, s, OCH 3-6″), 4.57 (1H, t, J = 6.8 Hz, H-12), 4.76 (1H, m, H-3), 5.43 (1H, s, H-6), 5.52 (1H, s, H-2′), 6.38 (1H, d, J = 16.1 Hz, H-2″), 6.68 (1H, d, J = 8.8 Hz, H-8″), 7.25 (1H, d, J = 8.8 Hz, H-9″), 7.85 (1H, d, J = 16.1 Hz, H-3″); 13C NMR (CDCl3, 100 MHz): δ 9.4 (C-18), 16.5 (C-7′), 18.5 (C-19), 20.8 (C-6′), 20.9 (C-5′), 24.2 (C-11), 27.0 (C-2), 27.2 (C-21), 31.8 (C-16), 33.2 (C-7), 34.2 (C-15), 37.0 (C-10), 38.0 (C-1), 38.1 (C-4′), 38.5 (C-4), 43.6 (C-9), 56.0 (OCH3-6″), 57.9 (C-13), 60.9 (OCH3-7″), 61.4 (OCH3-5″), 71.6 (C-12), 73.5 (C-3), 74.2 (C-8), 87.9 (C-14), 91.4 (C-17), 107.5 (C-8″), 112.9 (C-2′), 117.2 (C-2″), 118.7 (C-6), 121.4 (C-4″), 123.2 (C-9″), 139.5 (C-5), 139.6 (C-3″), 142.3 (C-5″), 153.2 (C-6″), 155.4 (C-7″), 166.0 (C-3′), 166.9 (2C-1′, 1″), 208.9 (C-20); ESIMS: m/z 709 [M − H]‾, HRESIMS: calcd for C40H53O11 [M − H]‾ 709.3587, found 709.3583.
5.1.1.12. 3-O-(3, 4, 5-Trimethoxy)cinnamoyl caudatin (18)
White amorphous power, yield 77.4% (after chromatography with petroleum ether/acetone, 85:15), 1H NMR (CDCl3, 400 MHz): δ 1.03 (6H, d, J = 6.8 Hz, CH 3-5′, 6′), 1.16 (3H, s, CH 3-19), 1.38 (3H, s, CH 3-18), 1.55 (1H, m, H-9), 1.80–1.98 (9H, overlap, H-1, 2, 11, 15, 16α), 2.10 (3H, s, CH 3-7′), 2.14 (3H, s, CH 3-21), 2.19 (2H, s, H-7), 2.33 (1H, m, H-4′), 2.44 (2H, m, H-4), 2.84 (1H, m, H-16β), 3.84 (2 × 3H, s, OCH 3-6″, 8″), 3.85 (3H, s, OCH 3-7″), 4.54 (1H, dd, J = 5.7, 10.1 Hz, H-12), 4.74 (1H, m, H-3), 5.40 (1H, s, H-6), 5.50 (1H, s, H-2′), 6.29 (1H, d, J = 15.8 Hz, H-2″), 6.72 (2H, s, H-5″, 9″), 7.55 (1H, d, J = 15.8 Hz, H-3″); 13C NMR (CDCl3, 100 MHz): δ 9.4 (C-18), 16.4 (C-7′), 18.4 (C-19), 20.8 (C-6′), 20.9 (C-5′), 24.1 (C-11), 27.0 (C-2), 27.1 (C-21), 31.7 (C-16), 33.2 (C-7), 34.2 (C-15), 36.9 (C-10), 37.9 (C-1), 38.1 (C-4′), 38.4 (C-4), 43.5 (C-9), 56.0 (2C, OCH3-6″, 8″), 57.8 (C-13), 60.9 (OCH3-7″), 71.5 (C-12), 73.7 (C-3), 74.1 (C-8), 87.9 (C-14), 91.4 (C-17), 105.0 (2C-5″, 9″), 112.9 (C-2′), 117.6 (C-2″), 118.9 (C-6), 129.8 (C-4″), 139.2 (C-7″), 139.9 (C-5), 144.6 (C-3″), 153.3 (2C-6″, 8″), 165.9 (C-3′), 166.2 (C-1″), 166.9 (C-1′), 208.9 (C-20); ESIMS: m/z 709 [M − H]‾, HRESIMS: calcd for C40H53O11 [M − H]‾ 709.3587, found 709.3578.
5.1.1.13. 3-O-(3, 4-Methylenedioxy)cinnamoyl caudatin (19)
White amorphous power, yield 60.6% (after chromatography with petroleum ether/acetone, 85:15), 1H NMR (CDCl3, 400 MHz): δ 1.04 (6H, d, J = 6.8 Hz, CH 3-5′, 6′), 1.17 (3H, s, CH 3-19), 1.40 (3H, s, CH 3-18), 1.59 (1H, m, H-9), 1.81–1.98 (9H, overlap, H-1, 2, 11, 15, 16α), 2.11 (3H, s, CH 3-7′), 2.16 (3H, s, CH 3-21), 2.20 (2H, s, H-7), 2.34 (1H, m, H-4′), 2.44 (2H, m, H-4), 2.85 (1H, m, H-16β), 4.55 (1H, dd, J = 5.8, 9.9 Hz, H-12), 4.73 (1H, m, H-3), 5.41 (1H, s, H-6), 5.51 (1H, s, H-2′), 5.98 (2H, s, -OCH 2O-6″, 7″), 6.21 (1H, d, J = 15.9 Hz, H-2″), 6.78 (1H, d, J = 8.0 Hz, H-8″), 6.97 (1H, d, J = 8.0 Hz, H-9″), 7.00 (1H, s, H-5″), 7.55 (1H, d, J = 15.9 Hz, H-3″); 13C NMR (CDCl3, 100 MHz): δ 9.4 (C-18), 16.5 (C-7′), 18.4 (C-19), 20.8 (C-6′), 20.9 (C-5′), 24.2 (C-11), 27.0 (C-2), 27.1 (C-21), 31.8 (C-16), 33.2 (C-7), 34.2 (C-15), 37.0 (C-10), 38.0 (C-1), 38.1 (C-4′), 38.4 (C-4), 43.6 (C-9), 57.9 (C-13), 71.6 (C-12), 73.7 (C-3), 74.2 (C-8), 88.0 (C-14), 91.4 (C-17), 101.5 (OCH2O-6″, 7″), 106.4 (C-5″), 108.5 (C-8″), 112.9 (C-2′), 116.3 (C-2″), 118.8 (C-6), 124.4 (C-9″), 128.8 (C-4″), 139.4 (C-5), 144.4 (C-3″), 148.3 (C-7″), 149.5 (C-6″), 165.9 (C-3′), 166.5 (C-1″), 166.9 (C-1′), 208.9 (C-20); EIMS: m/z 664, HREIMS: calcd for C38H48O10 664.3247, found 664.3251.
5.1.1.14. 3-O-(2-Ethoxy)cinnamoyl caudatin (20)
White amorphous power, yield 68.7% (after chromatography with petroleum ether/acetone, 90:10), 1H NMR (CDCl3, 500 MHz): δ 1.05 (6H, d, J = 6.8 Hz, CH 3-5′, 6′), 1.18 (3H, s, CH 3-19), 1.39 (3H, s, CH 3-18), 1.46 (3H, t, J = 6.9 Hz, OCH2CH 3-2″), 1.56 (1H, m, H-9), 1.83–2.03 (9H, overlap, H-1, 2, 11, 15, 16α), 2.12 (3H, s, CH 3-7′), 2.16 (3H, s, CH 3-21), 2.21 (2H, s, H-7), 2.33 (1H, m, H-4′), 2.47 (1H, m, H-4), 2.86 (1H, m, H-16β), 4.09 (2H, q, 6.9 Hz, OCH 2–CH3–2″), 4.56 (1H, dd, J = 5.9, 10.2 Hz, H-12), 4.75 (1H, m, H-3), 5.42 (1H, s, H-6), 5.52 (1H, s, H-2′), 6.49 (1H, d, J = 16.1 Hz, H-2″), 6.87 (1H, d, J = 7.8 Hz, H-6″), 6.92 (1H, t, J = 7.8 Hz, H-8″), 7.30 (1H, t, J = 7.5 Hz, H-7″), 7.48 (1H, d, J = 8.2 Hz, H-9″), 8.00 (1H, d, J = 16.1 Hz, H-3″); 13C NMR (CDCl3, 100 MHz): δ 9.8 (C-18), 15.2 (OCH2 CH3-2″), 16.9 (C-7′), 18.9 (C-19), 21.3 (C-6′), 21.4 (C-5′), 24.6 (C-11), 27.4 (C-2), 27.6 (C-21), 32.2 (C-16), 33.6 (C-7), 34.7 (C-15), 37.4 (C-10), 38.4 (C-1), 38.6 (C-4′), 38.9 (C-4), 44.0 (C-9), 58.3 (C-13), 64.4 (OCH2-2″), 72.0 (C-12), 74.0 (C-3), 74.6 (C-8), 88.4 (C-14), 91.9 (C-17), 112.4 (C-2′), 113.4 (C-6″), 119.1 (2C-6, 2″), 120.9 (C-8″), 123.8 (C-4″), 129.3 (C-7″), 131.8 (C-9″), 140.0 (C-5), 140.8 (C-3″), 158.1 (C-5″), 166.4 (C-3′), 167.3 (C-1″), 167.4 (C-1′), 209.4 (C-20); ESIMS: m/z 663 [M − H]‾, HRESIMS: calcd for C39H51O9 [M − H]‾ 663.3533, found 663.3532.
5.1.1.15. 3-O-(3-Ethoxy)cinnamoyl caudatin (21)
White amorphous power, yield 64.7% (after chromatography with petroleum ether/acetone, 95:5), 1H NMR (CDCl3, 400 MHz): δ 1.05 (6H, d, J = 6.8 Hz, CH 3-5′, 6′), 1.18 (3H, s, CH 3-19), 1.41 (3H, t, J = 7.0 Hz, OCH2CH 3-3″), 1.56 (1H, m, H-9), 1.82–1.99 (9H, overlap, H-1, 2, 11, 15, 16α), 2.12 (3H, s, CH 3-7′), 2.16 (3H, s, CH 3-21), 2.21 (2H, s, H-7), 2.35 (1H, m, H-4′), 2.46 (2H, m, H-4), 2.86 (1H, m, H-16β), 4.02 (2H, q, J = 7.0 Hz, OCH 2-3″), 4.56 (1H, dd, J = 5.7, 10.1 Hz, H-12), 4.74 (1H, m, H-3), 5.42 (1H, s, H-6), 5.52 (1H, s, H-2′), 6.38 (1H, d, J = 16.0 Hz, H-2″), 6.90 (1H, d, J = 8.2 Hz, H-7″), 7.02 (1H, s, H-5″), 7.08 (1H, d, J = 7.7 Hz, H-9″), 7.27 (1H, t, J = 7.9 Hz, H-8″), 7.61 (1H, d, J = 16.0 Hz, H-3″); 13C NMR (CDCl3, 100 MHz): δ 9.4 (C-18), 14.8 (OCH2 CH3-3″), 16.5 (C-7′), 18.4 (C-19), 20.8 (C-6′), 20.9 (C-5′), 24.2 (C-11), 27.0 (C-2), 27.2 (C-21), 31.8 (C-16), 33.2 (C-7), 34.2 (C-15), 37.0 (C-10), 38.0 (C-1). 38.1 (C-4′), 38.4 (C-4), 43.6 (C-9), 57.9 (C-13), 63.5 (OCH2-3″), 71.6 (C-12), 73.8 (C-3), 74.1 (C-8), 88.0 (C-14), 91.5 (C-17), 112.9 (C-2′), 113.4 (C-5″), 116.7 (C-7″), 118.5 (C-6), 118.8 (C-2″), 120.6 (C-9″), 129.8 (C-8″), 135.7 (C-4″), 139.3 (C-5), 144.7 (C-3″), 159.2 (C-6″), 165.9 (C-3′), 166.3 (C-1″), 166.9 (C-1′), 208.9 (C-20); ESIMS: m/z 663 [M − H]‾, HRESIMS: calcd for C39H51O9 [M − H]‾ 663.3533, found 663.3538.
5.1.1.16. 3-O-(4-Acetoxy)cinnamoyl caudatin (22)
White amorphous power, yield 63.8% (after chromatography with petroleum ether/acetone, 85:15), 1H NMR (CDCl3, 400 MHz): δ 1.06 (6H, d, J = 6.8 Hz, CH3-5′, 6′), 1.19 (3H, s, CH3-19), 1.41 (3H, s, CH 3-18), 1.58 (1H, m, H-9), 1.83–2.00 (9H, overlap, H-1, 2, 11, 15, 16α), 2.13 (3H, s, CH 3-7′), 2.17 (3H, s, CH 3-21), 2.22 (2H, s, H-7), 2.30 (3H, s, COCH 3-7″), 2.36 (1H, m, H-4′), 2.48 (2H, m, H-4), 2.86 (1H, m, H-16β), 4.58 (1H, t, J = 6.3 Hz, H-12), 4.76 (1H, m, H-3), 5.43 (1H, s, H-6), 5.53 (1H, s, H-2′), 6.36 (1H, d, J = 16.0 Hz, H-2″), 7.11 (2H, d, J = 8.6 Hz, H-6″, 8″), 7.53 (2H, d, J = 8.6 Hz, H-5″, 9″), 7.64 (1H, d, J = 16.0 Hz, H-3″); 13C NMR (CDCl3, 100 MHz): δ 9.4 (C-18), 16.5 (C-7′), 18.5 (C-19), 20.8 (C-6′), 20.9 (C-5′), 21.1 (COCH3-7″), 24.2 (C-11), 27.0 (C-2), 27.2 (C-21), 31.8 (C-16), 33.2 (C-7), 34.2 (C-15), 37.0 (C-10), 38.0 (C-1), 38.1 (C-4′), 38.4 (C-4), 43.6 (C-9), 57.9 (C-13), 71.6 (C-12), 73.9 (C-3), 74.2 (C-8), 87.9 (C-14), 91.4 (C-17), 112.9 (C-2′), 118.5 (C-6), 118.9 (C-2″), 122.1 (2C, C-6″, 8″), 129.2 (2C, C-5″, 9″), 132.1 (C-4″), 139.2 (C-5), 143.5 (C-3″), 152.0 (C-7″), 166.0 (C-3′), 166.2 (C-1″), 167.0 (C-1′), 169.2 (C-7″-COCH3), 208.9 (C-20); ESIMS: m/z 677 [M − H]‾, HRESIMS: calcd for C39H49O10 [M − H]‾ 677.3325, found 677.3324.
5.1.1.17. 3-O-(3-Methoxy-4-acetoxy)cinnamoyl caudatin (23)
White amorphous power, yield 55.2% (after chromatography with petroleum ether/acetone, 80:20), 1H NMR (CDCl3, 400 MHz): δ 1.05 (6H, d, J = 6.8 Hz, CH3-5′, 6′), 1.17 (3H, s, CH3-19), 1.40 (3H, s, CH 3-18), 1.57 (1H, m, H-9), 1.82–1.98 (9H, overlap, H-1, 2, 11, 15, 16α), 2.11 (3H, s, CH 3-7′), 2.16 (3H, s, CH 3-21), 2.22 (2H, s, H-7), 2.31 (3H, s, COCH 3-7″), 2.36 (1H, m, H-4′), 2.47 (2H, m, H-4), 2.85 (1H, m, H-16β), 3.83 (3H, s, OCH 3-3″), 4.55 (1H, dd, J = 5.5, 11.4 Hz, H-12), 4.75 (1H, m, H-3), 5.42 (1H, s, H-6), 5.51 (1H, s, H-2′), 6.35 (1H, d, J = 16.0 Hz, H-2″), 7.03 (1H, d, J = 7.7 Hz, H-8″), 7.08–7.11 (2H, overlap, H-5″, 9″), 7.61 (1H, d, J = 16.0 Hz, H-3″); 13C NMR (CDCl3, 100 MHz): δ 9.4 (C-18), 16.5 (C-7′), 18.4 (C-19), 20.6 (COCH3-7″), 20.8 (C-6′), 20.9 (C-5′), 24.2 (C-11), 27.0 (C-2), 27.2 (C-21), 31.8 (C-16), 33.2 (C-7), 34.2 (C-15), 37.0 (C-10), 38.0 (C-1), 38.1 (C-4′), 38.4 (C-4), 43.6 (C-9), 55.8 (OCH3-6″), 57.9 (C-13), 71.6 (C-12), 73.9 (C-3), 74.1 (C-8), 88.0 (C-14), 91.4 (C-17), 111.1 (C-5″), 112.9 (C-2′), 118.6 (C-2″), 118.9 (C-6), 121.2 (C-9″), 123.2 (C-8″), 133.3 (C-4″), 139.2 (C-5), 141.3 (C-7″), 143.9 (C-3″), 151.3 (C-6″), 165.9 (C-3′), 166.1 (C-1″), 166.9 (C-1′), 168.8 (COCH3-7″), 208.9 (C-20); ESIMS: m/z 707 [M − H]‾, HRESIMS: calcd for C40H51O11 [M − H]‾ 707.3431, found 707.3434.
5.1.1.18. 3-O-(3-Trifluoromethoxy)cinnamoyl caudatin (24)
White amorphous power, yield 64.7% (after chromatography with petroleum ether/acetone, 85:15), 1H NMR (CDCl3, 400 MHz): δ 1.05 (6H, d, J = 6.8 Hz, CH3-5′, 6′), 1.19 (3H, s, CH3-19), 1.41 (3H, s, CH 3-18), 1.57 (1H, m, H-9), 1.83–1.97 (9H, overlap, H-1, 2, 11, 15, 16α), 2.12 (3H, s, CH 3-7′), 2.17 (3H, s, CH 3-21), 2.22 (2H, s, H-7), m, 2.36 (1H, m, H-4′), 2.47 (2H, m, H-4), 2.85 (1H, m, H-16β), 4.56 (1H, dd, J = 5.7, 10.2 Hz, H-12), 4.77 (1H, m, H-3), 5.43 (1H, s, H-6), 5.52 (1H, s, H-2′), 6.42 (1H, d, J = 16.0 Hz, H-2″), 7.22 (1H, d, J = 7.1 Hz, H-7″), 7.35 (1H, s, H-5″), 7.38–7.44 (2H, overlap, H-8″, 9″), 7.62 (1H, d, J = 16.0 Hz, H-3″); 13C NMR (CDCl3, 100 MHz): δ 9.4 (C-18), 16.5 (C-7′), 18.4 (C-19), 20.8 (C-6′), 20.9 (C-5′), 24.1 (C-11), 26.9 (C-2), 27.2 (C-21), 31.7 (C-16), 33.2 (C-7), 34.2 (C-15), 37.0 (C-10), 37.9 (C-1), 38.1 (C-4′), 38.4 (C-4), 43.6 (C-9), 57.9 (C-13), 71.6 (C-12), 74.0 (C-3), 74.1 (C-8), 88.0 (C-14), 91.4 (C-17), 112.9 (C-2′), 119.0 (C-6), 120.0 (C-5″), 120.2 (C-7″), 121.6 (OCF3, q, J C–F = 256.1 Hz), 122.4 (C-2″), 126.4 (C-9″), 130.3 (C-8″), 136.5 (C-4″), 139.2 (C-5), 142.8 (C-3″), 149.6 (C-6″), 165.8 (C-3′), 166.0 (C-1″), 167.0(C-1′), 208.9 (C-20); ESIMS: m/z 703 [M − H]‾, HRESIMS: calcd for C38H46O9F3 [M − H]‾ 703.3093, found 703.3084.
5.1.1.19. 3-O-(2-Fluoro) cinnamoyl caudatin (25)
White amorphous power, yield 67.4% (after chromatography with petroleum ether/acetone, 9:1), 1H NMR (CDCl3, 500 MHz): δ 1.05 (6H, d, J = 6.8 Hz, CH3-5′, 6′), 1.19 (3H, s, CH3-19), 1.40 (3H, s, CH 3-18), 1.57 (1H, m, H-9), 1.84–2.00 (9H, overlap, H-1, 2, 11, 15, 16α), 2.12 (3H, s, CH 3-7′), 2.16 (3H, s, CH 3-21), 2.22 (2H, s, H-7), 2.36 (1H, m, H-4′), 2.49 (2H, m, H-4), 2.85 (1H, m, H-16β), 4.58 (1H, t, J = 6.4 Hz, H-12), 4.76 (1H, m, H-3), 5.43 (1H, s, H-6), 5.52 (1H, s, H-2′), 6.50 (1H, d, J = 16.2 Hz, H-2″), 7.09 (1H, t, J = 9.4 Hz, H-8″), 7.15 (1H, t, J = 7.6 Hz, H-9″), 7.34 (1H, m, H-6″), 7.52 (1H, t, J = 7.0 Hz, H-7″), 7.79 (1H, d, J = 16.2 Hz, H-3″); 13C NMR (CDCl3, 100 MHz): δ 9.4 (C-18), 16.5 (C-7′), 18.5 (C-19), 20.8 (C-6′), 20.9 (C-5′), 24.2 (C-11), 27.0 (C-2), 27.2 (C-21), 31.8 (C-16), 33.3 (C-7), 34.3 (C-15), 37.0 (C-10), 38.0 (C-1), 38.2 (C-4′), 38.5 (C-4), 43.7 (C-9), 58.0 (C-13), 71.6 (C-12), 73.9 (C-3), 74.2 (C-8), 87.9 (C-14), 91.4 (C-17), 113.0 (C-2′), 116.2 (C-6″, d, J C–F = 21.7 Hz), 118.8 (C-6), 121.0 (C-2″), 122.5 (C-4″, d, J C–F = 11.7 Hz), 124.4 (C-8″), 129.1 (C-9″), 131.6 (C-7″, d, J C–F = 8.6 Hz), 137.2 (C-3″), 139.4 (C-5), 166.0 (C-3′), 161.3 (C-5″, d, J C–F = 252.4 Hz), 166.1 (C-1″), 166.9 (C-1′), 208.8 (C-20); ESIMS: m/z 637 [M − H]‾, HRESIMS: calcd for C37H46O8F [M − H]‾ 637.3176, found 637.3163.
5.1.1.20. 3-O-(3-Fluoro)cinnamoyl caudatin (26)
White amorphous power, yield 90.2% (after chromatography with petroleum ether/acetone, 9:1), 1H NMR (CDCl3, 400 MHz): δ 1.06 (6H, d, J = 6.8 Hz, CH3-5′, 6′), 1.20 (3H, s, CH3-19), 1.40 (3H, s, CH 3-18), 1.59 (1H, m, H-9), 1.84–1.99 (9H, overlap, H-1, 2, 11, 15, 16α), 2.13 (3H, s, CH 3-7′), 2.17 (3H, s, CH 3-21), 2.24 (2H, s, H-7), 2.37 (1H, m, H-4′), 2.48 (2H, m, H-4), 2.84 (1H, m, H-16β), 4.59 (1H, t, J = 6.8 Hz, H-12), 4.77 (1H, m, H-3), 5.44 (1H, s, H-6), 5.53 (1H, s, H-2′), 6.40 (1H, d, J = 16.0 Hz, H-2″), 7.07 (1H, m, H-5″), 7.22 (1H, m, H-7″), 7.28 (1H, m, H-8″), 7.35 (1H, m, H-9″), 7.62 (1H, d, J = 16.0 Hz, H-3″); 13C NMR (CDCl3, 100 MHz): δ 9.4 (C-18), 16.5 (C-7′), 18.5 (C-19), 20.8 (C-6′), 20.9 (C-5′), 24.1 (C-11), 26.9 (C-2), 27.2 (C-21), 31.7 (C-16), 33.3 (C-7), 34.3 (C-15), 37.0 (C-10), 37.9 (C-1), 38.2 (C-4′), 38.4 (C-4), 43.6 (C-9), 58.0 (C-13), 71.6 (C-12), 74.0 (C-3), 74.2 (C-8), 87.9 (C-14), 91.4 (C-17), 112.9 (C-2′), 114.2 (C-5″, d, J C–F = 21.9 Hz), 117.1 (C-7″, d, J C–F = 21.0 Hz), 118.9 (C-2″), 119.9 (C-6), 124.0 (C-9″), 130.4 (C-8″, d, J C–F = 8.0 Hz), 136.6 (C-4″, d, J C–F = 7.1 Hz), 139.3 (C-5), 143.2 (C-3″), 164.2 (C-6″, d, J C–F = 245.4 Hz), 166.0 (2C-3′, 1″), 167.1 (C-1′), 208.9 (C-20); ESIMS: m/z 661 [M + Na]+, HRESIMS: calcd for C37H47O8FNa [M + Na]+ 661.3152, found 661.3142.
5.1.1.21. 3-O-(4-Fluoro) cinnamoyl caudatin (27)
White amorphous power, yield 70.5% (after chromatography with Petroleum ether/Acetone, 9:1), 1H NMR (CDCl3, 500 MHz): δ 1.06 (6H, d, J = 6.8 Hz, CH3-5′, 6′), 1.18 (3H, s, CH3-19), 1.40 (3H, s, CH 3-18), 1.53 (1H, m, H-9), 1.84–2.00 (9H, overlap, H-1, 2, 11, 15, 16α), 2.12 (3H, s, CH 3-7′), 2.16 (3H, s, CH 3-21), 2.22 (2H, s, H-7), 2.36 (1H, m, H-4′), 2.48 (2H, m, H-4), 2.85 (1H, m, H-16β), 4.58 (1H, dd, J = 6.0, 9.3 Hz, H-12), 4.76 (1H, m, H-3), 5.43 (1H, s, H-6), 5.52 (1H, s, H-2′), 6.32 (1H, d, J = 16.0 Hz, H-2″), 7.06 (2H, t, J = 8.5 Hz, H-6″, 8″), 7.50 (2H, dd, J = 5.5, 8.3 Hz, H-5″, 9″), 7.62 (1H, d, J = 16.0 Hz, H-3″); 13C NMR (CDCl3, 125 MHz): δ 9.4 (C-18), 16.5 (C-7′), 18.5 (C-19), 20.8 (C-6′), 20.9 (C-5′), 24.2 (C-11), 27.0 (C-2), 27.2 (C-21), 31.7 (C-16), 33.3 (C-7), 34.3 (C-15), 37.0 (C-10), 38.0 (C-1), 38.1 (C-4′), 38.4 (C-4), 43.6 (C-9), 58.0 (C-13), 71.6 (C-12), 73.8 (C-3), 74.2 (C-8), 87.9 (C-14), 91.4 (C-17), 112.9 (C-2′), 116.0 (2C-6″, 8″, d, J C–F = 22.0 Hz), 118.2 (C-6), 118.9 (C-2″), 129.9 (2C-5″, 9″, d, J C–F = 8.4 Hz), 130.6 (C-4″), 139.4 (C-5), 143.3 (C-3″), 162.8 (C-7″, d, J C–F = 249.4 Hz), 166.0 (C-3′), 166.2 (C-1″), 166.9 (C-1′), 208.8 (C-20); ESIMS: m/z 637 [M − H]‾, HRESIMS: calcd for C37H46O8F [M − H]‾ 637.3176, found 637.3177.
5.1.1.22. 3-O-(2-Chloro) cinnamoyl caudatin (28)
White amorphous power, yield 76.7% (after chromatography with petroleum ether/acetone, 85:15), 1H NMR (CDCl3, 400 MHz): δ 1.06 (6H, d, J = 6.8 Hz, CH3-5′, 6′), 1.19 (3H, s, CH3-19), 1.40 (3H, s, CH 3-18), 1.60 (1H, m, H-9), 1.84–2.09 (9H, overlap, H-1, 2, 11, 15, 16α), 2.12 (3H, s, CH 3-7′), 2.17 (3H, s, CH 3-21), 2.23 (2H, s, H-7), 2.35 (1H, m, H-4′), 2.49 (2H, m, H-4), 2.86 (1H, m, H-16β), 4.58 (1H, t, J = 6.8 Hz, H-12), 4.78 (1H, m, H-3), 5.44 (1H, s, H-6), 5.53 (1H, s, H-2′), 6.40 (1H, d, J = 16.0 Hz, H-2″), 7.28 (2H, m, H-7″, 8″), 7.42 (1H, d, J = 7.5 Hz, H-9″), 7.60 (1H, d, J = 7.4 Hz, H-6″), 8.07 (1H, d, J = 16.0 Hz, H-3″); 13C NMR (CDCl3, 100 MHz): δ 9.4 (C-18), 16.5 (C-7′), 18.5 (C-19), 20.8 (C-6′), 20.9 (C-5′), 24.2 (C-11), 26.9 (C-2), 27.2 (C-21), 31.7 (C-16), 33.2 (C-7), 34.2 (C-15), 37.0 (C-10), 37.9 (C-1), 38.1 (C-4′), 38.4 (C-4), 43.6 (C-9), 58.0 (C-13), 71.6 (C-12), 74.0 (C-3), 74.2 (C-8), 87.9 (C-14), 91.4 (C-17), 112.9 (C-2′), 118.9 (C-6), 121.0 (C-2″), 127.0 (C-8″), 127.6 (C-9″), 130.1 (C-6″), 131.0 (C-7″), 132.7 (C-5″), 134.9 (C-4″), 139.3 (C-5), 140.4 (C-3″), 165.8 (C-1″), 166.0 (C-3′), 167.0 (C-1′), 208.9 (C-20); ESIMS: m/z 677 [M + Na]+, HRESIMS: calcd for C37H47O8ClNa [M + Na]+ 677.2850, found 677.2857.
5.1.1.23. 3-O-(3-Chloro)cinnamoyl caudatin (29)
White amorphous power, yield 61.1% (after chromatography with petroleum ether/acetone, 85:15), 1H NMR (CDCl3, 400 MHz): δ 1.04 (6H, d, J = 6.8 Hz, CH3-5′, 6′), 1.17 (3H, s, CH3-19), 1.39 (3H, s, CH 3-18), 1.57 (1H, m, H-9), 1.82–1.99 (9H, overlap, H-1, 2, 11, 15, 16α), 2.11 (3H, s, CH 3-7′), 2.15(3H, s, CH 3-21), 2.20 (2H, s, H-7), 2.34 (1H, m, H-4′), 2.46 (2H, m, H-4), 2.84 (1H, m, H-16β), 4.56 (1H, dd, J = 5.9, 10.2 Hz, H-12), 4.75 (1H, m, H-3), 5.42 (1H, s, H-6), 5.50 (1H, s, H-2′), 6.39 (1H, d, J = 16.0 Hz, H-2″), 7.29–7.37 (3H, overlap, H-7″, 8″, 9″), 7.48 (1H, s, H-5″), 7.57 (1H, d, J = 16.0 Hz, H-3″); 13C NMR (CDCl3, 100 MHz): δ 9.4 (C-18), 16.5 (C-7′), 18.4 (C-19), 20.8 (C-6′), 20.9 (C-5′), 24.1 (C-11), 26.9 (C-2), 27.1 (C-21), 31.7 (C-16), 33.2 (C-7), 34.2 (C-15), 36.9 (C-10), 37.9 (C-1), 38.1 (C-4′), 38.4 (C-4), 43.5 (C-9), 57.9 (C-13), 71.5 (C-12), 74.0 (C-3), 74.1 (C-8), 87.9 (C-14), 91.4 (C-17), 112.9 (C-2′), 118.9 (C-6), 119.8 (C-2″), 126.2 (2C-5″, 9″), 127.7 (C-7″), 130.1 (C-8″), 134.8 (C-6″), 136.2 (C-4″), 139.2 (C-5), 143.0 (C-3″), 165.8 (C-3′), 165.9 (C-1″), 166.9 (C-1′), 208.9 (C-20); ESIMS: m/z 653 [M − H]‾, HRESIMS: calcd for C37H46O8Cl [M − H]‾ 653.2881, found 653.2866.
5.1.1.24. 3-O-(4-Chloro) cinnamoyl caudatin (30)
White amorphous power, yield 64.5% (after chromatography with petroleum ether/acetone, 85:15), 1H NMR (CDCl3, 400 MHz): δ 1.06 (6H, d, J = 6.8 Hz, CH3-5′, 6′), 1.18 (3H, s, CH3-19), 1.40 (3H, s, CH3-18), 1.53 (1H, m, H-9), 1.83–2.00 (9H, overlap, H-1, 2, 11, 15, 16α), 2.12 (3H, s, CH3-7′), 2.16 (3H, s, CH3-21), 2.22 (2H, s, H-7), 2.36 (1H, m, H-4′), 2.48 (2H, m, H-4), 2.85 (1H, m, H-16β), 4.58 (1H, t, J = 7.8 Hz, H-12), 4.76 (1H, m, H-3), 5.43 (1H, s, H-6), 5.52 (1H, s, H-2′), 6.39 (1H, d, J = 16.0 Hz, H-2″), 7.36 (2H, d, J = 8.5 Hz, H-6″, 8″), 7.44 (2H, d, J = 8.5 Hz, H-5″, 9″), 7.61 (1H, d, J = 16.0 Hz, H-3″); 13C NMR (CDCl3, 100 MHz): δ 9.4 (C-18), 16.5 (C-7′), 18.5 (C-19), 20.8 (C-6′), 20.9 (C-5′), 24.2 (C-11), 27.0 (C-2), 27.2 (C-21), 31.7 (C-16), 33.3 (C-7), 34.3 (C-15), 37.0 (C-10), 37.9 (C-1), 38.2 (C-4′), 38.4 (C-4), 43.6 (C-9), 57.9 (C-13), 71.6 (C-12), 73.9 (C-3), 74.2 (C-8), 87.9 (C-14), 91.4 (C-17), 112.9 (C-2′), 118.9 (C-6), 119.0 (C-2″), 129.1 (2C-6″, 8″), 129.2 (2C-5″, 9″), 132.9 (C-4″), 136.1 (C-7″) 139.3 (C-5), 143.2 (C-3″), 166.0 (C-3′), 166.1 (C-1″), 167.0 (C-1′), 208.9 (C-20); EIMS: m/z 654, HREIMS: calcd for C37H47O8Cl 654.2959, found 654.2943.
5.1.1.25. 3-O-(3-Bromo)cinnamoyl caudatin (31)
White amorphous power, yield 65.4% (after chromatography with petroleum ether/acetone, 85:15), 1H NMR (CDCl3, 400 MHz): δ 1.06 (6H, d, J = 6.8 Hz, CH3-5′, 6′), 1.19 (3H, s, CH3-19), 1.40 (3H, s, CH3-18), 1.60 (1H, m, H-9), 1.84–2.04 (9H, overlap, H-1, 2, 11, 15, 16α), 2.13 (3H, s, CH3-7′), 2.17 (3H, s, CH3-21), 2.23 (2H, s, H-7), 2.36 (1H, m, H-4′), 2.48 (2H, m, H-4), 2.85 (1H, m, H-16β), 4.58 (1H, t, J = 6.8 Hz, H-12), 4.77 (1H, m, H-3), 5.44 (1H, s, H-6), 5.53 (1H, s, H-2′), 6.41 (1H, d, J = 16.0 Hz, H-2″), 7.25 (1H, t, J = 7.8 Hz, H-8″), 7.42 (1H, d, J = 7.8 Hz, H-7″), 7.49 (1H, d, J = 7.7 Hz, H-9″), 7.58 (1H, d, J = 16.0 Hz, H-3″), 7.66 (1H, s, H-5″); 13C NMR (CDCl3, 100 MHz): δ 9.4 (C-18), 16.5 (C-7′), 18.5 (C-19), 20.8 (C-6′), 20.9 (C-5′), 24.2 (C-11), 27.0 (C-2), 27.2 (C-21), 31.7 (C-16), 33.3 (C-7), 34.2 (C-15), 37.0 (C-10), 38.0 (C-1), 38.2 (C-4′), 38.4 (C-4), 43.6 (C-9), 58.0 (C-13), 71.6 (C-12), 74.0 (C-3), 74.2 (C-8), 87.9 (C-14), 91.4 (C-17), 112.9 (C-2′), 118.9 (C-2″), 119.9 (C-6), 123.0 (C-6″), 126.6 (C-9″), 130.4 (C-5″), 130.7 (C-7″), 133.0 (C-8″), 136.5 (C-4″), 139.3 (C-5), 142.9 (C-3″), 165.9 (C-1″), 166.0 (C-3′), 167.1 (C-1′), 208.9 (C-20); ESIMS: m/z 721 [M + Na]+, HRESIMS: calcd for C37H47O8BrNa [M + Na]+ 721.2351, found 721.2355.
5.1.1.26. 3-O-(4-Bromo)cinnamoyl caudatin (32)
White amorphous power, yield 57.7% (after chromatography with petroleum ether/acetone, 85:15), 1H NMR (CDCl3, 400 MHz): δ 1.06 (6H, d, J = 6.8 Hz, CH3-5′, 6′), 1.18 (3H, s, CH3-19), 1.40 (3H, s, CH3-18), 1.55 (1H, m, H-9), 1.83–2.00 (9H, overlap, H-1, 2, 11, 15, 16α), 2.12 (3H, s, CH3-7′), 2.17 (3H, s, CH3-21), 2.22 (2H, s, H-7), 2.36 (1H, m, H-4′), 2.47 (2H, m, H-4), 2.85 (1H, m, H-16β), 4.57 (1H, dd, J = 6.0, 9.5 Hz, H-12), 4.76 (1H, m, H-3), 5.43 (1H, s, H-6), 5.52 (1H, s, H-2′), 6.39 (1H, d, J = 16.0 Hz, H-2″), 7.37 (2H, d, J = 8.5 Hz, H-6″, 8″), 7.50 (2H, d, J = 8.5 Hz, H-5″, 9″), 7.58 (1H, d, J = 16.0 Hz, H-3″); 13C NMR (CDCl3, 100 MHz): δ 9.4 (C-18), 16.5 (C-7′), 18.4 (C-19), 20.8 (C-6′), 20.9 (C-5′), 24.1 (C-11), 27.0 (C-2), 27.2 (C-21), 31.7 (C-16), 33.2 (C-7), 34.2 (C-15), 37.0 (C-10), 37.9 (C-1), 38.2 (C-4′), 38.4 (C-4), 43.6 (C-9), 57.9 (C-13), 71.6 (C-12), 73.9 (C-3), 74.1 (C-8), 87.9 (C-14), 91.4 (C-17), 112.9 (C-2′), 118.9 (C-6), 119.1 (C-2″), 124.5 (C-7″), 129.4 (2C-5″, 9″), 132.1 (2C-6″, 8″), 133.3 (C-4″), 139.2 (C-5), 143.3 (C-3″), 166.0 (C-3′), 166.1 (C-1″), 167.0 (C-1′), 208.9 (C-20); ESIMS: m/z 697 [M − H]‾, HRESIMS: calcd for C37H46O8Br [M − H]‾ 697.2376, found 697.2360.
5.1.1.27. 3-O-(2, 3-Difluoro)cinnamoyl caudatin (33)
White amorphous power, yield 56.5% (after chromatography with petroleum ether/acetone, 85:15), 1H NMR (CDCl3, 500 MHz): δ 1.06 (6H, d, J = 6.8 Hz, CH3-5′, 6′), 1.19 (3H, s, CH3-19), 1.40 (3H, s, CH3-18), 1.56 (1H, m, H-9), 1.83–2.00 (9H, overlap, H-1, 2, 11, 15, 16α), 2.12 (3H, s, CH3-7′), 2.16 (3H, s, CH3-21), 2.22 (2H, s, H-7), 2.36 (1H, m, H-4′), 2.47 (2H, m, H-4), 2.85 (1H, m, H-16β), 4.57 (1H, dd, J = 5.7, 10.1 Hz, H-12), 4.77 (1H, m, H-3), 5.44 (1H, s, H-6), 5.52 (1H, s, H-2′), 6.50 (1H, d, J = 16.2 Hz, H-2″), 7.08 (1H, m, H-8″), 7.17 (1H, m, H-7″), 7.28 (1H, d, J = 7.1 Hz, H-9″), 7.74 (1H, d, J = 16.2 Hz, H-3″); 13C NMR (CDCl3, 100 MHz): δ 9.4 (C-18), 16.5 (C-7′), 18.5 (C-19), 20.8 (C-6′), 20.9 (C-5′), 24.2 (C-11), 26.9 (C-2), 27.2 (C-21), 31.7 (C-16), 33.3 (C-7), 34.3 (C-15), 37.0 (C-10), 37.9 (C-1), 38.2 (C-4′), 38.4 (C-4), 43.6 (C-9), 57.9 (C-13), 71.6 (C-12), 74.1 (C-3), 74.2 (C-8), 87.9 (C-14), 91.4 (C-17), 112.9 (C-2′), 118.4 (C-2″), 118.9 (C-6), 122.3 (C-7″, d, J C–F = 6.5 Hz), 123.7 (C-9″, broad), 124.3 (C-8″, dd, J C–F = 6.8, 4.8 Hz), 124.6 (C-4″, d, J C–F = 8.6 Hz), 136.1 (C-3″), 139.3 (C-5), 149.3 (C-5″, dd, J C–F = 12.7, 254.1 Hz), 150.9 (C-6″, dd, J C–F = 12.7, 247.3 Hz), 165.8 (C-3′), 166.0 (C-1″), 167.0 (C-1′), 208.9 (C-20); ESIMS: m/z 655 [M − H]‾, HRESIMS: calcd for C37H45O8F2 [M − H]‾ 655.3082, found 655.3071.
5.1.1.28. 3-O-(3, 4-Difluoro)cinnamoyl caudatin (34)
White amorphous power, yield 61.7% (after chromatography with petroleum ether/acetone, 85:15), 1H NMR (CDCl3, 400 MHz): δ 1.06 (6H, d, J = 6.8 Hz, CH3-5′, 6′), 1.19 (3H, s, CH3-19), 1.41 (3H, s, CH3-18), 1.58 (1H, m, H-9), 1.84–2.01 (9H, overlap, H-1, 2, 11, 15, 16α), 2.13 (3H, s, CH3-7′), 2.17 (3H, s, CH3-21), 2.23 (2H, s, H-7), 2.36 (1H, m, H-4′), 2.47 (2H, m, H-4), 2.85 (1H, m, H-16β), 4.58 (1H, dd, J = 6.1, 9.0 Hz, H-12), 4.77 (1H, m, H-3), 5.44 (1H, s, H-6), 5.53 (1H, s, H-2′), 6.33 (1H, d, J = 15.9 Hz, H-2″), 7.14–7.24 (2H, m, H-5″, 8″), 7.34 (1H, m, H-9″), 7.56 (1H, d, J = 15.9 Hz, H-3″); 13C NMR (CDCl3, 100 MHz): δ 9.4 (C-18), 16.5 (C-7′), 18.4 (C-19), 20.8 (C-6′), 20.9 (C-5′), 24.1 (C-11), 27.0 (C-2), 27.2 (C-21), 31.7 (C-16), 33.5 (C-7), 34.3 (C-15), 37.0 (C-10), 37.9 (C-1), 38.2 (C-4′), 38.4 (C-4), 43.6 (C-9), 58.0 (C-13), 71.6 (C-12), 74.0 (C-3), 74.1 (C-8), 87.9 (C-14), 91.4 (C-17), 112.9 (C-2′), 116.2 (C-5″, d, J C–F = 17.5 Hz), 117.8 (C-8″, d, J C–F = 17.5 Hz), 118.9 (C-2″), 119.6 (C-6), 124.8 (C-9″, dd, J C–F = 3.2, 6.3 Hz), 131.6 (C-4″, pseudo t, J C–F = 4.9 Hz), 139.2 (C-5), 142.2 (C-3″), 150.5 (C-7″, dd, J C–F = 12.9, 247.9 Hz), 151.4 (C-6″, dd, J C–F = 12.9, 251.7 Hz), 165.8 (C-1″), 166.0 (C-3′), 167.0 (C-1′), 208.9 (C-20); ESIMS: m/z 655 [M − H]‾, HRESIMS: calcd for C37H45O8F2 [M − H]‾ 655.3082, found 655.3066.
5.1.1.29. 3-O-(3, 5-Difluoro)cinnamoyl caudatin (35)
White amorphous power, yield 60.3% (after chromatography with petroleum ether/acetone, 85:15), 1H NMR (CDCl3, 400 MHz): δ 1.05 (6H, d, J = 6.8 Hz, CH3-5′, 6′), 1.18 (3H, s, CH3-19), 1.40 (3H, s, CH3-18), 1.57 (1H, m, H-9), 1.83–2.00 (9H, overlap, H-1, 2, 11, 15, 16α), 2.12 (3H, s, CH3-7′), 2.16 (3H, s, CH3-21), 2.22 (2H, s, H-7), 2.35 (1H, m, H-4′), 2.47 (2H, m, H-4), 2.85 (1H, m, H-16β), 4.56 (1H, dd, J = 5.7, 9.9 Hz, H-12), 4.76 (1H, m, H-3), 5.43 (1H, s, H-6), 5.52 (1H, s, H-2′), 6.38 (1H, d, J = 16.0 Hz, H-2″), 6.81 (1H, t, J = 8.6 Hz, H-7″), 7.01 (2H, d, J = 6.0 Hz, H-5″, 9″), 7.54 (1H, d, J = 16.0 Hz, H-3″); 13C NMR (CDCl3, 100 MHz): δ 9.4 (C-18), 16.5 (C-7′), 18.4 (C-19), 20.8 (C-6′), 20.9 (C-5′), 24.1 (C-11), 26.9 (C-2), 27.2 (C-21), 31.7 (C-16), 33.3 (C-7), 34.2 (C-15), 36.9 (C-10), 37.9 (C-1), 38.1 (C-4′), 38.4 (C-4), 43.6 (C-9), 57.9 (C-13), 71.6 (C-12), 74.1 (C-3), 74.2 (C-8), 87.9 (C-14), 91.4 (C-17), 105.3 (C-7″, t, J C–F = 25.2 Hz), 110.6 (2C-5″, 9″, dd, J C–F = 7.0, 18.8 Hz), 112.9 (C-2′), 119.0 (C-6), 121.1 (C-2″), 137.6 (C-4″, t, J C–F = 9.5 Hz), 139.1 (C-5), 142.0 (C-3″), 163.1 (2C-6″, 8″, dd, J C–F = 12.5, 247.7 Hz), 165.5 (C-1″), 166.0 (C-3′), 167.0 (C-1′), 208.9 (C-20); ESIMS: m/z 655 [M − H]‾, HRESIMS: calcd for C37H45O8F2 [M − H]‾ 655.3082, found 655.3083.
5.1.1.30. 3-O-(2, 3, 4-Trifluoro)cinnamoyl caudatin (36)
White amorphous power, yield 57.8% (after chromatography with petroleum ether/acetone, 85:15), 1H NMR (CDCl3, 400 MHz): δ 1.06 (6H, d, J = 6.8 Hz, CH3-5′, 6′), 1.19 (3H, s, CH3-19), 1.40 (3H, s, CH3-18), 1.57 (1H, m, H-9), 1.84–2.02 (9H, overlap, H-1, 2, 11, 15, 16α), 2.13 (3H, s, CH3-7′), 2.17 (3H, s, CH3-21), 2.23 (2H, s, H-7), 2.37 (1H, m, H-4′), 2.47 (2H, m, H-4), 2.85 (1H, m, H-16β), 4.58 (1H, t, J = 7.8 Hz, H-12), 4.78 (1H, m, H-3), 5.45 (1H, s, H-6), 5.53 (1H, s, H-2′), 6.48 (1H, d, J = 16.2 Hz, H-2″), 7.01 (1H, m, H-8″), 7.25 (1H, m, H-9″), 7.68 (1H, d, J = 16.2 Hz, H-3″); 13C NMR (CDCl3, 100 MHz): δ 9.4 (C-18), 16.5 (C-7′), 18.4 (C-19), 20.8 (C-6′), 20.9 (C-5′), 24.1 (C-11), 26.9 (C-2), 27.2 (C-21), 31.7 (C-16), 33.2 (C-7), 34.2 (C-15), 36.9 (C-10), 37.9 (C-1), 38.1 (C-4′), 38.4 (C-4), 43.6 (C-9), 57.9 (C-13), 71.6 (C-12), 74.1 (C-3), 74.2 (C-8), 87.9 (C-14), 91.4 (C-17), 112.6 (C-8″, dd, J C–F = 2.7, 17.8 Hz), 112.9 (C-2′), 119.0 (C-6), 120.3 (C-4″, dd, J C–F = 3.8, 9.0 Hz), 121.9 (C-9″, dd, J C–F = 2.0, 6.6 Hz), 122.8 (C-2″), 135.4 (C-3″), 139.2 (C-5), 140.2 (C-6″, t, d, J C–F = 15.2, 250.7 Hz), 150.3 (C-5″, ddd, J C–F = 3.2, 3.1, 255.7 Hz), 151.9 (C-7″, ddd, J C–F = 3.3, 3.4, 253.2 Hz), 165.6 (C-1″), 166.0 (C-3′), 167.0 (C-1′), 208.9 (C-20); ESIMS: m/z 673 [M − H]‾, HRESIMS: calcd for C37H44O8F3 [M − H]‾ 673.2988, found 673.2972.
5.1.1.31. 3-O-(2, 3, 4, 5, 6-Pentafluoro)cinnamoyl caudatin (37)
White amorphous power, yield 63.4% (after chromatography with petroleum ether/acetone, 85:15), 1H NMR (CDCl3, 400 MHz): δ 1.06 (6H, d, J = 6.8 Hz, CH3-5′, 6′), 1.19 (3H, s, CH3-19), 1.40 (3H, s, CH3-18), 1.58 (1H, m, H-9), 1.84–2.00 (9H, overlap, H-1, 2, 11, 15, 16α), 2.13 (3H, s, CH3-7′), 2.17 (3H, s, CH3-21), 2.23 (2H, s, H-7), 2.37 (1H, m, H-4′), 2.47 (2H, m, H–4H), 2.84 (1H, m, H-16β), 4.58 (1H, dd, J = 6.2, 9.1 Hz, H-12), 4.77 (1H, m, H-3), 5.45 (1H, s, H-6), 5.53 (1H, s, H-2′), 6.71 (1H, d, J = 16.4 Hz, H-2″), 7.63 (1H, d, J = 16.4 Hz, H-3″); 13C NMR (CDCl3, 100 MHz): δ 9.4 (C-18), 16.5 (C-7′), 18.4 (C-19), 20.8 (C-6′), 20.9 (C-5′), 24.1 (C-11), 26.9 (C-2), 27.1 (C-21), 31.8 (C-16), 33.2 (C-7), 34.2 (C-15), 36.9 (C-10), 37.8 (C-1), 38.1 (C-4′), 38.4 (C-4), 43.6 (C-9), 57.8 (C-13), 71.6 (C-12), 74.1 (C-3), 74.5 (C-8), 88.0 (C-14), 91.5 (C-17), 109.8 (C-4″, dt, J C–F = 4.0, 15.5 Hz), 112.9 (C-2′), 119.1 (C-6), 126.4 (C-2″), 128.1 (C-3″), 137.7 (2C-6″, 8″, md, J C–F = 255.4 Hz), 139.0 (C-5), 141.6 (C-7″, md, J C–F = 257.0 Hz), 145.6 (2C-5″, 9″, md, J C–F = 253.2 Hz), 165.3 (C-1″), 166.0 (C-3′), 166.9 (C-1′), 209.0 (C-20); EIMS: m/z 710, HREIMS: calcd for C37H43O8F5 710.2878, found 710.2850.
5.1.1.32. 3-O-(2, 4-dichloro) cinnamoyl caudatin (38)
White amorphous power, yield 68.8% (after chromatography with petroleum ether/acetone, 85:15), 1H NMR (CDCl3, 400 MHz): δ 1.04 (6H, d, J = 6.8 Hz, CH3-5′, 6′), 1.17 (3H, s, CH3-19), 1.39 (3H, s, CH3-18), 1.57 (1H, m, H-9), 1.82–1.99 (9H, overlap, H-1, 2, 11, 15, 16α), 2.11 (3H, s, CH3-7′), 2.15 (3H, s, CH3-21), 2.21 (2H, s, H-7), 2.34 (1H, m, H-4′), 2.47 (2H, m, H-4), 2.84 (1H, m, H-16β), 4.55 (1H, dd, J = 6.1, 10.1 Hz, H-12), 4.76 (1H, m, H-3), 5.42 (1H, s, H-6), 5.51 (1H, s, H-2′), 6.37 (1H, d, J = 16.0 Hz, H-2″), 7.23 (1H, d, J = 8.5 Hz, H-8″), 7.41 (1H, s, H-6″), 7.52 (1H, d, J = 8.5 Hz, H-9″), 7.96 (1H, d, J = 16.0 Hz, H-3″); 13C NMR (CDCl3, 100 MHz): δ 9.4 (C-18), 16.5 (C-7′), 18.4 (C-19), 20.8 (C-6′), 20.9 (C-5′), 24.1 (C-11), 26.9 (C-2), 27.1 (C-21), 31.7 (C-16), 33.2 (C-7), 34.2 (C-15), 36.9 (C-10), 37.9 (C-1), 38.1 (C-4′), 38.4 (C-4), 43.5 (C-9), 57.9 (C-13), 71.5 (C-12), 74.1 (2C-3, 8), 87.9 (C-14), 91.4 (C-17), 112.9 (C-2′), 118.9 (C-6), 121.4 (C-2″), 127.5 (C-8″), 128.3 (C-6″), 129.9 (C-9″), 131.2 (C-7″), 135.4 (C-4″), 136.2 (C-5″), 139.2 (2C-5, 3″), 165.6 (C-3′), 165.9 (C-1″), 167.0 (C-1′), 208.9 (C-20); ESIMS: m/z 687 [M − H]‾, HRESIMS: calcd for C37H45O8Cl2 [M − H]‾ 687.2491, found 687.2499.
5.1.1.33. 3-O-(3, 4-dichloro) cinnamoyl caudatin (39)
White amorphous power, yield 67.6% (after chromatography with petroleum ether/acetone, 85:15), 1H NMR (CDCl3, 500 MHz): δ 1.04 (6H, d, J = 6.8 Hz, CH3-5′, 6′), 1.16 (3H, s, CH3-19), 1.39 (3H, s, CH3-18), 1.52 (1H, m, H-9), 1.81–1.99 (9H, overlap, H-1, 2, 11, 15, 16α), 2.10 (3H, s, CH3-7′), 2.15 (3H, s, CH3-21), 2.20 (2H, s, H-7), 2.34 (1H, m, H-4′), 2.44 (2H, m, H-4), 2.84 (1H, m, H-16β), 4.55 (1H, dd, J = 5.7, 10.2 Hz, H-12), 4.73 (1H, m, H-3), 5.41 (1H, s, H-6), 5.51 (1H, s, H-2′), 6.37 (1H, d, J = 16.0 Hz, H-2″), 7.31 (1H, d, J = 8.4 Hz, H-9″), 7.43 (1H, d, J = 8.4 Hz, H-8″), 7.52 (1H, d, J = 16.0 Hz, H-3″), 7.57 (1H, s, H-5″); 13C NMR (CDCl3, 100 MHz): δ 9.4 (C-18), 16.5 (C-7′), 18.4 (C-19), 20.8 (C-6′), 20.9 (C-5′), 24.1 (C-11), 26.9 (C-2), 27.1 (C-21), 31.7 (C-16), 33.2 (C-7), 34.2 (C-15), 36.9 (C-10), 37.9 (C-1), 38.1 (C-4′), 38.4 (C-4), 43.5 (C-9), 57.9 (C-13), 71.5 (C-12), 74.0 (C-3), 74.1 (C-8), 87.9 (C-14), 91.4 (C-17), 112.9 (C-2′), 118.9 (C-6), 120.2 (C-2″), 126.9 (C-9″), 129.5 (C-5″), 130.8 (C-8″), 133.1 (C-7″), 134.0 (C-6″), 134.4 (C-4″), 139.1 (C-5), 141.8 (C-3″), 165.6 (C-1″), 165.9 (C-3′), 167.0 (C-1′), 208.9 (C-20); ESIMS: m/z 687 [M − H]‾, HRESIMS: calcd for C37H45O8Cl2 [M − H]‾ 687.2491, found 687.2485.
5.1.1.34. 3-O-(2-Trifluoromethyl)cinnamoyl caudatin (40)
White amorphous power, yield 68.3% (after chromatography with petroleum ether/acetone, 9:1), 1H NMR (CDCl3, 500 MHz): δ 1.04 (6H, d, J = 6.8 Hz, CH3-5′, 6′), 1.18 (3H, s, CH3-19), 1.40 (3H, s, CH3-18), 1.57 (1H, m, H-9), 1.83–1.99 (9H, overlap, H-1, 2, 11, 15, 16α), 2.12 (3H, s, CH3-7′), 2.16 (3H, s, CH3-21), 2.21 (2H, s, H-7), 2.34 (1H, m, H-4′), 2.47 (2H, m, H-4), 2.85 (1H, m, H-16β), 4.56 (1H, dd, J = 5.2, 8.2 Hz, H-12), 4.76 (1H, m, H-3), 5.42 (1H, s, H-6), 5.52 (1H, s, H-2′), 6.37 (1H, d, J = 15.8 Hz, H-2″), 7.46 (1H, t, J = 7.4 Hz, H-7″), 7.55 (1H, t, J = 7.5 Hz, H-8″), 7.68 (2H, d, J = 7.7 Hz, H-6″, 9″), 8.02 (1H, d, J = 15.8 Hz, H-3″); 13C NMR (CDCl3, 100 MHz): δ 8.3 (C-18), 15.4 (C-7′), 17.4 (C-19), 19.7 (C-6′), 19.8 (C-5′), 23.1 (C-11), 25.8 (C-2), 26.1 (C-21), 30.7 (C-16), 32.1 (C-7), 33.2 (C-15), 35.9 (C-10), 36.8 (C-1), 37.0 (C-4′), 37.3 (C-4), 42.5 (C-9), 56.8 (C-13), 70.5 (C-12), 73.1 (2C-3, 8), 86.9 (C-14), 90.4 (C-17), 111.8 (C-2′), 117.8 (C-6), 121.6 (C-2″), 122.8 (C–CF3-5″, q, J C–F = 272.4 Hz), 125.0 (C-9″, q, J C–F = 5.6 Hz), 126.8 (C-6″), 127.6 (C-5″, q, J C–F = 30.2 Hz), 128.4 (C-7″), 131.0 (C-8″), 132.2 (C-4″), 138.2 (C-5), 139.0 (C-3″), 164.4 (C-3′), 164.9 (C-1″), 165.8 (C-1′), 207.8 (C-20); ESIMS: m/z 687 [M − H]‾, HRESIMS: calcd for C38H46O8F3 [M − H]‾ 687.3144, found 687.3146.
5.1.1.35. 3-O-(3-Trifluoromethyl) cinnamoyl caudatin (41)
White amorphous power, yield 64.8% (after chromatography with petroleum ether/acetone, 9:1), 1H NMR (CDCl3, 400 MHz): δ 1.07 (6H, d, J = 6.8 Hz, CH3-5′, 6′), 1.20 (3H, s, CH3-19), 1.41 (3H, s, CH3-18), 1.60 (1H, m, H-9), 1.85–2.02 (9H, overlap, H-1, 2, 11, 15, 16α), 2.14 (3H, s, CH3-7′), 2.18 (3H, s, CH3-21), 2.24 (2H, s, H-7), 2.37 (1H, m, H-4′), 2.48 (2H, m, H-4), 2.87 (1H, m, H-16β), 4.59 (1H, t, J = 7.8 Hz, H-12), 4.79 (1H, m, H-3), 5.46 (1H, s, H-6), 5.54 (1H, s, H-2′), 6.48 (1H, d, J = 16.0 Hz, H-2″), 7.52 (1H, t, J = 7.8 Hz, H-8″), 7.62–7.70 (3H, overlap, H-5″, 7″, 9″), 7.74 (1H, d, J = 16.0 Hz, H-3″); 13C NMR (CDCl3, 100 MHz): δ 9.3 (C-18), 16.5 (C-7′), 18.5 (C-19), 20.8 (C-6′), 20.9 (C-5′), 24.2 (C-11), 26.9 (C-2), 27.2 (C-21), 31.7 (C-16), 33.3 (C-7), 34.3 (C-15), 37.0 (C-10), 37.9 (C-1), 38.2 (C-4′), 38.4 (C-4), 43.6 (C-9), 58.0 (C-13), 71.6 (C-12), 74.0 (C-3), 74.2 (C-8), 87.9 (C-14), 91.4 (C-17), 112.9 (C-2′), 119.0 (C-6), 120.4 (C-2″), 123.0 (CF3-6″, q, J C–F = 271.6 Hz), 124.5 (C-7″, q, J C–F = 3.8 Hz), 126.6 (C-5″), 129.4 (C-8″), 131.0 (C-9″), 131.1 (C-6″, q, J C–F = 32.0 Hz), 135.2 (C-4″), 139.3 (C-5), 142.8 (C-3″), 165.7 (C-1″), 166.0 (C-3′), 167.1 (C-1′), 208.9 (C-20); ESIMS: m/z 687 [M − H]‾, HRESIMS: calcd for C38H46O8F3 [M − H]‾ 687.3144, found 687.3128.
5.1.1.36. 3-O-(4-Trifluoromethyl)cinnamoyl caudatin (42)
White amorphous power, yield 64.7% (after chromatography with petroleum ether/acetone, 9:1), 1H NMR (CDCl3, 400 MHz): δ 1.05 (6H, d, J = 6.8 Hz, CH3-5′, 6′), 1.18 (3H, s, CH3-19), 1.40 (3H, s, CH3-18), 1.56 (1H, m, H-9), 1.82–1.98 (9H, overlap, H-1, 2, 11, 15, 16α), 2.11 (3H, s, CH3-7′), 2.16 (3H, s, CH3-21), 2.21 (2H, s, H-7), 2.34 (1H, m, H-4′), 2.47 (2H, m, H-4), 2.87 (1H, m, H-16β), 4.56 (1H, dd, J = 5.8, 10.3 Hz, H-12), 4.76 (1H, m, H-3), 5.43 (1H, s, H-6), 5.52 (1H, s, H-2′), 6.47 (1H, d, J = 16.0 Hz, H-2″), 7.59–7.62 (4H, overlap, H-5″, 6″, 7″, 8″), 7.66 (1H, d, J = 16.0 Hz, H-3″); 13C NMR (CDCl3, 100 MHz): δ 9.4 (C-18), 16.4 (C-7′), 18.4 (C-19), 20.8 (C-6′), 20.9 (C-5′), 24.1 (C-11), 26.9 (C-2), 27.1 (C-21), 31.7 (C-16), 33.2 (C-7), 34.2 (C-15), 36.9 (C-10), 38.1 (2C-1, 4′), 38.4 (C-4), 43.5 (C-9), 57.9 (C-13), 71.6 (C-12), 74.1 (2C-3, 8), 87.9 (C-14), 91.4 (C-17), 112.9 (C-2′), 119.0 (C-6), 120.9 (C-2″), 122.7 (CF3-7″, q, J C–F = 272.0 Hz), 125.8 (3C-6″, 8″, q, J C–F = 3.7 Hz), 128.1 (2C-5″, 9″), 131.6 (C-7″, q, J C–F = 31.7 Hz), 137.7 (C-4″), 139.1 (C-5), 142.7 (C-3″), 165.7 (C-1″), 166.0 (C-3′), 167.0 (C-1′), 208.9 (C-20); ESIMS: m/z 687 [M − H]‾, HRESIMS: calcd for C38H46O8F3 [M − H]‾ 687.3144, found 687.3134.
5.1.1.37. 3-O-(3, 5-Bis(trifluoromethyl)) cinnamoyl caudatin (43)
White amorphous power, yield 69.4% (after chromatography with petroleum ether/acetone, 85:15), 1H NMR (CDCl3, 400 MHz): δ 1.05 (6H, d, J = 6.8 Hz, CH3-5′, 6′), 1.19 (3H, s, CH3-19), 1.41 (3H, s, CH3-18), 1.57 (1H, m, H-9), 1.83–2.00 (9H, overlap, H-1, 2, 11, 15, 16α), 2.12 (3H, s, CH3-7′), 2.17 (3H, s, CH3-21), 2.21 (2H, s, H-7), 2.36 (1H, m, H-4′), 2.47 (2H, m, H-4), 2.85 (1H, m, H-16β), 4.57 (1H, dd, J = 5.5, 10.3 Hz, H-12), 4.76 (1H, m, H-3), 5.44 (1H, s, H-6), 5.52 (1H, s, H-2′), 6.55 (1H, d, J = 16.0 Hz, H-2″), 7.70 (1H, d, J = 16.0 Hz, H-3″), 7.86 (2H, s, H-5″, 9″), 7.93 (1H, s, H-7″); 13C NMR (CDCl3, 100 MHz): δ 9.8 (C-18), 16.9 (C-7′), 18.8 (C-19), 21.2 (C-6′), 21.3 (C-5′), 24.6 (C-11), 27.4 (C-2), 27.6 (C-21), 32.2 (C-16), 33.7 (C-7), 34.7 (C-15), 37.4 (C-10), 38.3 (C-1), 38.6 (C-4′), 38.8 (C-4), 44.0 (C-9), 58.4 (C-13), 72.0 (C-12), 74.6 (C-3), 74.8 (C-8), 88.4 (C-14), 91.9 (C-17), 113.3 (C-2′), 119.6 (C-6), 122.9 (C-2″), 123.7 (C-7″, t, J C–F = 3.5 Hz), 123.4 (2C–CF3-6″, 8″, q, J C–F = 271.2 Hz), 128.0 (2C-5″, 9″, d, J C–F = 2.8 Hz), 132.7 (2C-6″, 8″, q, J C–F = 33.5 Hz), 136.9 (C-4″), 139.5 (C-5), 141.4 (C-3″), 165.6 (C-1″), 166.4 (C-3′), 167.4 (C-1′), 209.4 (C-20); ESIMS: m/z 755 [M − H]‾, HRESIMS: calcd for C39H45O8F6 [M − H]‾ 755.3018, found 755.3003.
5.1.1.38. 3-O-(4-Fluoro-3-trifluoromethyl)cinnamoyl caudatin (44)
White amorphous power, yield 65.6% (after chromatography with petroleum ether/acetone, 85:15), 1H NMR (CDCl3, 500 MHz): δ 1.06 (6H, d, J = 6.8 Hz, CH3-5′, 6′), 1.19 (3H, s, CH3-19), 1.40 (3H, s, CH3-18), 1.57 (1H, m, H-9), 1.84–2.01 (9H, overlap, H-1, 2, 11, 15, 16α), 2.12 (3H, s, CH3-7′), 2.16 (3H, s, CH3-21), 2.22 (2H, s, H-7), 2.36 (1H, m, H-4′), 2.47 (2H, m, H-4), 2.85 (1H, m, H-16β), 4.57 (1H, dd, J = 6.0, 9.9 Hz, H-12), 4.77 (1H, m, H-3), 5.44 (1H, s, H-6), 5.53 (1H, s, H-2′), 6.40 (1H, d, J = 16.0 Hz, H-2″), 7.23 (1H, t, J = 9.2 Hz, H-8″), 7.62 (1H, d, J = 16.0 Hz, H-3″), 7.69 (1H, m, H-5″), 7.75 (1H, d, J = 6.5 Hz, H-9″); 13C NMR (CDCl3, 100 MHz): δ 9.4 (C-18), 16.5 (C-7′), 18.4 (C-19), 20.8 (C-6′), 20.9 (C-5′), 24.1 (C-11), 26.9 (C-2), 27.2 (C-21), 31.7 (C-16), 33.3 (C-7), 34.3 (C-15), 37.0 (C-10), 37.9 (C-1), 38.2 (C-4′), 38.4 (C-4), 43.6 (C-9), 58.0 (C-13), 71.6 (C-12), 74.1 (2C-3, 8), 87.9 (C-14), 91.4 (C-17), 112.9 (C-2′), 117.5 (C-8″, d, J C–F = 21.2 Hz), 120.1 (C-2″), 119.0 (C-6), 122.1 (CF3-6″, q, J C–F = 271.6 Hz), 126.6 (C-6″), 126.7 (C-5″), 130.9 (C-4″), 133.1 (C-9″, d, J C–F = 8.7 Hz), 139.2 (C-5), 141.6 (C-3″), 160.4 (C-7″, d, J C–F = 259.8 Hz), 165.6 (C-1″), 166.0 (C-3′), 167.1 (C-1′), 208.9 (C-20); ESIMS: m/z 705 [M − H]‾, HRESIMS: calcd for C38H45O8F4 [M − H]‾ 705.3050, found 705.3039.
5.1.1.39. 3-O-(2-Nitro)cinnamoyl caudatin (45)
Pale yellow amorphous power, yield 65.6% (after chromatography with petroleum ether/acetone, 85:15), 1H NMR (CDCl3, 400 MHz): δ 1.06 (6H, d, J = 6.8 Hz, CH3-5′, 6′), 1.19 (3H, s, CH3-19), 1.40 (3H, s, CH3-18), 1.57 (1H, m, H-9), 1.83–2.01 (9H, overlap, H-1, 2, 11, 15, 16α), 2.12 (3H, s, CH3-7′), 2.17 (3H, s, CH3-21), 2.22 (2H, s, H-7), 2.36 (1H, m, H-4′), 2.48 (2H, m, H-4), 2.86 (1H, m, H-16β), 4.58 (1H, t, J = 7.8 Hz, H-12), 4.79 (1H, m, H-3), 5.44 (1H, s, H-6), 5.53 (1H, s, H-2′), 6.34 (1H, d, J = 15.8 Hz, H-2″), 7.54 (1H, m, H-8″), 7.62–7.67 (2H, overlap, H-7″, 9″), 8.03 (1H, d, J = 8.0 Hz, H-6″), 8.09 (1H, d, J = 15.8 Hz, 3″-H); 13C NMR (CDCl3, 100 MHz): δ 9.4 (C-18), 16.5 (C-7′), 18.5 (C-19), 20.8 (C-6′), 20.9 (C-5′), 24.2 (C-11), 26.9 (C-2), 27.2 (C-21), 31.7 (C-16), 33.2 (C-7), 34.2 (C-15), 37.0 (C-10), 37.9 (C-1), 38.1 (C-4′), 38.4 (C-4), 43.6 (C-9), 57.9 (C-13), 71.6 (C-12), 74.2 (C-3), 74.3 (C-8), 87.9 (C-14), 91.4 (C-17), 112.9 (C-2′), 119.0 (C-6), 123.5 (C-2″), 124.9 (C-6″), 129.1 (C-9″), 130.2 (C-7″), 130.6 (C-4″), 133.5 (C-8″), 139.2 (C-5), 139.9 (C-3″), 148.3 (C-5″), 165.1 (C-1″), 166.0 (C-3′), 167.0 (C-1′), 208.9 (C-20); ESIMS: m/z 664 [M − H]‾, HRESIMS: calcd for C37H46NO10 [M − H]‾ 664.3121, found 664.3119.
5.1.1.40. 3-O-(3-Nitro)cinnamoyl caudatin (46)
Pale yellow amorphous power, yield 64.5% (after chromatography with petroleum ether/acetone, 85:15), 1H NMR (CDCl3, 400 MHz): δ 1.05 (6H, d, J = 6.8 Hz, CH3-5′, 6′), 1.18 (3H, s, CH3-19), 1.40 (3H, s, CH3-18), 1.57 (1H, m, H-9), 1.82–2.00 (9H, overlap, H-1, 2, 11, 15, 16α), 2.11 (3H, s, CH3-7′), 2.16 (3H, s, CH3-21), 2.21 (2H, s, H-7), 2.35 (1H, m, H-4′), 2.48 (2H, m, H-4), 2.84 (1H, m, H-16β), 4.56(1H, dd, J = 5.6, 9.9 Hz, H-12), 4.77 (1H, m, H-3), 5.43 (1H, s, H-6), 5.52 (1H, s, H-2′), 6.53 (1H, d, J = 16.0 Hz, H-2″), 7.57 (1H, t, J = 8.0 Hz, H-8″), 7.68 (1H, d, J = 16.0 Hz, H-3″), 7.81 (1H, d, J = 7.8 Hz, H-9″), 8.36 (1H, s, H-5″), 8.21 (1H, d, J = 8.2 Hz, 7″-H); 13C NMR (CDCl3, 100 MHz): δ 9.4 (C-18), 16.5 (C-7′), 18.4 (C-19), 20.8 (C-6′), 20.9 (C-5′), 24.1 (C-11), 26.9 (C-2), 27.2 (C-21), 31.7 (C-16), 33.3 (C-7), 34.2 (C-15), 36.9 (C-10), 37.9 (C-1), 38.1 (C-4′), 38.4 (C-4), 43.6 (C-9), 57.9 (C-13), 71.6 (C-12), 74.1 (C-3), 74.3 (C-8), 87.9 (C-14), 91.4 (C-17), 112.9 (C-2′), 119.0 (C-6), 121.6 (C-2″), 122.3 (C-5″), 124.5 (C-7″), 129.9 (C-8″), 133.6 (C-9″), 136.1 (C-4″), 139.1 (C-5), 141.7 (C-3″), 148.6 (C-6″), 165.4 (C-1″), 166.0 (C-3′), 167.0 (C-1′), 208.9 (C-20); ESIMS: m/z 664 [M − H]‾, HRESIMS: calcd for C37H46NO10 [M − H]‾ 664.3121, found 664.3108.
5.1.1.41. 3-O-(4-chloro-3-nitro)cinnamoyl caudatin (47)
White amorphous power, yield 83.4% (after chromatography with petroleum ether/acetone, 85:15), 1H NMR (CDCl3, 400 MHz): δ 1.06 (6H, d, J = 6.8 Hz, CH3-5′, 6′), 1.19 (3H, s, CH3-19), 1.40 (3H, s, CH3-18), 1.60 (1H, m, H-9), 1.85–2.02 (9H, overlap, H-1, 2, 11, 15, 16α), 2.13 (3H, s, CH3-7′), 2.17 (3H, s, CH3-21), 2.23 (2H, s, H-7), 2.37 (1H, m, H-4′), 2.49 (2H, m, H-4), 2.85 (1H, m, H-16β), 4.59 (1H, t, J = 7.8 Hz, H-12), 4.78 (1H, m, H-3), 5.45 (1H, s, H-6), 5.54 (1H, s, H-2′), 6.48 (1H, d, J = 16.0 Hz, H-2″), 7.52–7.66 (3H, overlap, H-3″, 8″, 9″), 8.01 (1H, s, H-5″); 13C NMR (CDCl3, 100 MHz): δ 9.3 (C-18), 16.5 (C-7′), 18.4 (C-19), 20.8 (C-6′), 20.9 (C-5′), 24.1 (C-11), 26.9 (C-2), 27.2 (C-21), 31.7 (C-16), 33.3 (C-7), 34.3 (C-15), 37.0 (C-10), 37.9 (C-1), 38.2 (C-4′), 38.4 (C-4), 43.6 (C-9), 58.0 (C-13), 71.6 (C-12), 74.1 (C-3), 74.4 (C-8), 87.8 (C-14), 91.4 (C-17), 112.9 (C-2′), 119.1 (C-6), 122.0 (C-2″), 124.5 (C-5″), 128.2 (C-7″), 131.8 (C-8″), 132.5 (C-9″), 134.5 (C-4″), 139.1 (C-5), 140.5 (C-3″), 148.2 (C-6″), 165.2 (C-1″), 166.0 (C-3′), 167.1 (C-1′), 208.8 (C-20); ESIMS: m/z 698 [M − H]‾, HRESIMS: calcd for C37H45NO10Cl [M − H]‾ 698.2731, found 698.2733.
5.1.2. 3-O-(3, 4-Dihydroxy)cinnamoyl caudatin (11)
A solution of 1 (0.2 mmol), the 3, 4-dihydroxycinnamic acid (1.5 equiv) in dry THF were added TPP (1.5 equiv) at 0 °C. The reaction mixture was stirred at room temperature until the starting material disappeared by the TLC. Then the reaction was worked up by removal of the solvent and redissolved on EtOAc. The organic solution was washed with saturated NaCl (3 × 30 mL), dried over anhydrous Na2SO4, and concentrated to dryness under reduced pressure. The residue was chromatographed on a silica gel column with petroleum ether/acetone (85:15) and then CHCl3/CH3OH (98:2) to afford 11 (white amorphous power, yield 55.1%). 1H NMR (CDCl3, 500 MHz): δ 1.06 (6H, d, J = 6.8 Hz, CH3-5′, 6′), 1.17 (3H, s, CH3-19), 1.42 (3H, s, CH3-18), 1.62 (1H, m, H-9), 1.82–1.98 (9H, overlap, H-1, 2, 11, 15, 16α), 2.12 (3H, s, CH3-7′), 2.19 (3H, s, CH3-21), 2.20 (2H, s, H-7), 2.36 (1H, m, H-4′), 2.46 (2H, m, H-4), 2.86 (1H, m, H-16β), 4.58 (1H, dd, J = 5.2, 10.2 Hz, H-12), 4.73 (1H, m, H-3), 5.39 (1H, s, H-6), 5.54 (1H, s, H-2′), 6.21 (1H, d, J = 16.0 Hz, H-2″), 6.87 (1H, d, J = 8.2 Hz, H-9″), 6.98 (1H, d, J = 8.2 Hz, H-8″), 7.10 (1H, s, H-5″), 7.56 (1H, d, J = 16.0 Hz, H-3″); 13C NMR (CDCl3, 100 MHz): δ 9.4 (C-18), 16.6 (C-7′), 18.5 (C-19), 20.8 (C-6′), 20.9 (C-5′), 24.2 (C-11), 27.0 (C-2), 27.2 (C-21), 31.9 (C-16), 33.0 (C-7), 34.2 (C-15), 36.9 (C-10), 38.0 (C-1), 38.2 (C-4′), 38.5 (C-4), 43.6 (C-9), 57.9 (C-13), 71.7 (C-12), 73.8 (C-3), 74.2 (C-8), 88.0 (C-14), 91.5 (C-17), 112.8 (C-2′), 114.3 (C-2″), 115.4 (C-5″), 115.6 (C-8″), 118.7 (C-6), 122.3 (C-9″), 139.4 (C-5), 127.3 (C-4″), 144.0 (C-3″), 145.0 (C-6″), 146.5 (C-7″), 166.2 (C-3′), 167.1 (C-1″), 167.4 (C-1′), 209.4 (C-20); ESIMS: m/z 651 [M − H]‾, HRESIMS: calcd for C37H47O10 [M − H]‾ 651.3169, found 651.3179.
5.1.3. General procedure for the preparation of derivatives (48–51)
A solution of 1 (0.2 mmol), DMAP (0.4 equiv), and substituted cinnamic acids (4 equiv) in anhydrous CH2Cl2 (8 mL) was added DCC (4 equiv) at 0 °C. The resulting mixture was stirred at room temperature until the starting material could not defected by TLC. The reaction mixture was filtered, and the residue was washed with CH2Cl2 (2 × 10 mL). The CH2Cl2 solution was washed with 5% HCl (3 × 30 mL), saturated NaHCO3 (3 × 30 mL) and saturated NaCl (3 × 30 mL), respectively. The organic layer was dried over anhydrous Na2SO4 and concentrated to dryness under reduced pressure. The residue was subjected to a silica gel column with petroleum ether/acetone and then CHCl3/CH3OH to afford 48–51.
5.1.3.1. 3, 17-O-Di[(3-methoxy) cinnamoyl] caudatin (48)
White amorphous power, yield 31.6% (after chromatography with petroleum ether/acetone, 85:15; and then CHCl3/CH3OH, 99:1), 1H NMR (CDCl3, 400 MHz): δ 1.06 (6H, d, J = 6.8 Hz, CH3-5′, 6′), 1.21 (3H, s, CH3-19), 1.58 (1H, m, H-9), 1.63 (3H, s, CH3-18), 1.87–1.96 (9H, overlap, H-1, 2, 11, 15, 16α), 2.08 (3H, s, CH3-7′), 2.15 (3H, s, CH3-21), 2.22 (2H, s, H-7), 2.36 (1H, m, H-4′), 2.47 (2H, m, H-4), 3.18 (1H, m, H-16β), 3.82 (3H, s, OCH 3-6″), 3.84 (3H, s, OCH 3-6″), 4.61 (1H, dd, J = 4.2, 5.7 Hz, H-12), 4.76 (1H, m, H-3), 5.44 (1H, s, H-6), 5.55 (1H, s, H-2′), 6.38–6.48 (2 × 1H, m, H-2″), 6.94–6.98 (2 × 1H, m, H-7″), 7.03–7.05 (2 × 1H, s, H-5″), 7.10–7.15 (2 × 1H, overlap, H-9″) 7.26–7.32 (2 × 1H, overlap, H-8″), 7.61–7.71 (2 × 1H, overlap, H-3″); 13C NMR (CDCl3, 100 MHz): δ 10.6 (C-18), 16.6 (C-7′), 18.3 (C-19), 20.8 (C-6′), 20.9 (C-5′), 24.0 (C-11), 27.0 (C-2), 29.8 (2C-21, 16), 33.9 (C-7), 34.6 (C-15), 36.9 (C-10), 38.0 (C-1), 38.1 (C-4′), 38.4 (C-4), 43.4 (C-9), 55.3 (2 × C, OCH3-6″), 58.2 (C-13), 70.9 (C-12), 73.8 (C-3), 74.4 (C-8), 88.2 (C-14), 97.8 (C-17), 112.8 (2 × C-7″), 113.0 (C-2′), 116.1 (C-5″), 116.8 (C-5″), 117.4 (C-2″), 119.0 (C-6), 118.7 (C-2″), 120.8 (C-9″), 121.1 (C-9″), 129.8 (C-8″), 130.0 (C-8″), 135.1 (C-4″), 135.7 (C-4″), 138.9 (C-5), 144.5 (C-3″), 146.7 (C-3″), 159.8 (C-6″), 159.9 (C-6″), 166.3 (C-3′), 165.3 (C-1″), 165.6 (C-1″), 167.0 (C-1′), 203.0 (C-20); EIMS: m/z 810, HREIMS: calcd for C48H58O11 810.3979, found 810.3981.
5.1.3.2. 3, 17-O-Di[(3, 4-dimethoxy)cinnamoyl] caudatin (49)
White amorphous power, yield 36.8% (after chromatography with petroleum ether/acetone, 85:15; and then CHCl3/CH3OH, 99:1), 1H NMR (CDCl3, 400 MHz): δ 1.06 (6H, d, J = 6.8 Hz, CH3-5′, 6′), 1.21 (3H, s, CH3-19), 1.64 (3H, s, CH3-18), 1.54 (1H, m, H-9), 1.86–1.96 (9H, overlap, H-1, 2, 11, 15, 16α), 2.08 (3H, s, CH3-7′), 2.17 (3H, s, CH3-21), 2.22 (2H, s, H-7), 2.36 (1H, m, H-4′), 2.47 (2H, m, H-4), 3.17 (1H, m, H-16β), 3.86–3.93 (2 × 6H, overlap, OCH 3-6″, 7″), 4.61 (1H, t, J = 5.6 Hz, H-12), 4.77 (1H, m, H-3), 5.43 (1H, s, H-6), 5.54 (1H, s, H-2′), 6.27–6.35 (2 × 1H, overlap, H-2″), 6.85–6.89 (2 × 1H, overlap, H-8″), 7.04–7.14 (2 × 2H, overlap, H-5″, 9″), 7.59–7.68 (2 × 1H, overlap, H-3″); 13C NMR (CDCl3, 100 MHz): δ 10.6 (C-18), 16.6 (C-7′), 18.2 (C-19), 20.8 (C-6′), 20.9 (C-5′), 24.0 (C-11), 27.1 (C-2), 29.2 (C-21), 29.6 (C-16), 34.0 (C-7), 34.7 (C-15), 36.9 (C-10), 38.0 (C-1), 38.1 (C-4′), 38.5 (C-4), 43.4 (C-9), 55.8 (2 × C, OCH3-7″), 55.9 (2 × C, OCH3-6″), 58.1 (C-13), 70.9 (C-12), 73.7 (C-3), 74.3 (C-8), 88.3 (C-14), 97.9 (C-17), 109.4 (2 × C-8″), 110.9 (2 × C-5″), 112.8 (C-2′), 114.6(C-2″), 116.1 (C-2″), 119.0 (C-6), 122.6 (C-9″), 123.4 (C-9″), 126.7 (C-4″), 127.4 (C-4″), 138.9 (C-5), 144.5 (C-3″), 146.8 (C-3″), 149.1 (C-7″), 149.3 (C-7″), 151.0 (C-6″), 151.7 (C-6″), 165.8 (C-3′), 166.5 (2 × C-1″), 167.0 (C-1′), 202.9 (C-20); EIMS: m/z 870, HREIMS: calcd for C50H62O13 870.4190, found 870.4194.
5.1.3.3. 3, 17-O-Di[(4-chloro)cinnamoyl] caudatin (50)
White amorphous power, yield 24.5% (after chromatography with petroleum ether/acetone, 85:15; and then CHCl3/CH3OH, 99:1), 1H NMR (CDCl3, 400 MHz): δ 1.05 (6H, d, J = 6.8 Hz, CH3-5′, 6′), 1.20 (3H, s, CH3-19), 1.53 (1H, m, H-9), 1.62 (3H, s, CH3-18), 1.87–1.96 (9H, overlap, H-1, 2, 11, 15, 16α), 2.08 (3H, s, CH3-7′), 2.16 (3H, s, CH3-21), 2.22 (2H, s, H-7), 2.36 (1H, m, H-4′), 2.48 (2H, m, H-4), 3.18 (1H, m, H-16β), 4.61 (1H, dd, J = 4.4, 5.7 Hz, H-12), 4.77 (1H, m, H-3), 5.44 (1H, s, H-6), 5.54 (1H, s, H-2′), 6.37–6.47 (2 × 1H, overlap, H-2″), 7.34–7.39 (2 × 2H, overlap, H-6″, 8″), 7.44–7.50 (2 × 2H, overlap, H-5″, 9″), 7.60–7.70 (2 × 1H, overlap, H-3″); 13C NMR (CDCl3, 100 MHz): δ 10.6 (C-18), 16.6 (C-7′), 18.3 (C-19), 20.8 (C-6′), 20.9 (C-5′), 24.0 (C-11), 27.0 (C-2), 29.2 (C-21), 29.9 (C-16), 33.9 (C-7), 34.6 (C-15), 37.0 (C-10), 37.9 (C-1), 38.1 (C-4′), 38.4 (C-4), 43.4 (C-9), 58.2 (C-13), 70.9 (C-12), 73.9 (C-3), 74.4 (C-8), 88.1 (C-14), 98.0 (C-17), 112.8 (C-2′), 117.7 (C-6), 119.0 (2 × 1C-2″), 129.1(2C, C-6″, 8″), 129.2 (2C, C-6″, 8″), 129.3 (2C, C-5″, 9″), 129.5 (2C, C-5″, 9″), 132.3 (C-4″), 132.9 (C-4″), 136.1 (C-7″), 136.8 (C-7″), 138.9 (C-5), 143.2 (C-3″), 145.2 (C-3″), 165.4 (C-3′), 166.1 (2 × C-1″), 167.1 (C-1′), 202.9 (C-20); EIMS: m/z 818, HREIMS: calcd for C46H52O9Cl2 818.2988, found 818.2950.
5.1.3.4. 3, 17-O-Di[(2-nitro) cinnamoyl] caudatin (51)
Pale yellow amorphous power, yield 45.6% (after chromatography with petroleum ether/acetone, 85:15; and then CHCl3/CH3OH, 100:1), 1H NMR (CDCl3, 400 MHz): δ 1.05 (6H, d, J = 6.8 Hz, CH3-5′, 6′), 1.20 (3H, s, CH3-19), 1.57 (1H, m, H-9), 1.63 (3H, s, CH3-18), 1.87–1.95 (9H, overlap, H-1, 2, 11, 15, 16α), 2.10 (3H, s, CH3-7′), 2.15 (3H, s, CH3-21), 2.23 (2H, s, H-7), 2.36 (1H, m, H-4′), 2.48 (2H, m, H-4), 3.17 (1H, m, H-16β), 4.61 (1H, dd, J = 4.4, 5.6 Hz, H-12), 4.78 (1H, m, H-3), 5.43 (1H, s, H-6), 5.55 (1H, s, H-2′), 6.32–6.41 (2 × 1H, overlap, H-2″), 7.54–7.69 (2 × 3H, overlap, H-7″, 8″, 9″), 8.02–8.07 (2 × 1H, overlap, H-6″), 8.09 (1H, d, J = 15.8 Hz, H-3″), 8.28 (1H, d, J = 15.8 Hz, H-3″); 13C NMR (CDCl3, 100 MHz): δ 10.5 (C-18), 16.6 (C-7′), 18.4 (C-19), 20.8 (C-6′), 20.9 (C-5′), 24.0 (C-11), 26.9 (2C-2, 21), 30.1 (C-16), 33.2 (C-7), 34.6 (C-15), 37.0 (C-10), 37.9 (C-1), 38.1 (C-4′), 38.4 (C-4), 43.4 (C-9), 58.6 (C-13), 70.9 (C-12), 74.3 (C-3), 74.5 (C-8), 87.8 (C-14), 97.9 (C-17), 112.8 (C-2′), 119.0 (C-6), 122.6 (C-2″), 123.5 (C-2″), 124.9 (C-6″), 125.1 (C-6″), 129.1 (2 × 1C-9″), 130.2 (2 × 1C-7″), 130.6 (C-4″), 130.7 (C-4″), 133.5 (C-8″), 133.7 (C-8″), 139.0 (C-5), 139.8 (C-3″), 141.7 (C-3″), 148.1 (C-5″), 148.3 (C-5″), 164.7 (C-1″), 165.1 (C-1″), 165.4 (C-3′), 167.0 (C-1′), 203.2 (C-20); ESIMS: m/z 875 [M − H]‾, HRESIMS: calcd for C46H52N2O13 [M − H]‾ 875.3157, found 875.3152.
5.2. Biology
5.2.1. In vitro anti-HBV assay
The assay was determined according to our previous description [57]. The anti-HBV activities and cytotoxicity of compounds 1, 6–51 were evaluated on the Hep G 2.2.15 cell line. The anti-HBV antigen secretion activities were assayed by enzyme-linked immunosorbent assay (ELISA; Autobio Diagnostics Co., Ltd, China). For detection of HBV DNA, a real-time PCR assay was used. Briefly, 10 μL of DNA sample was amplified in a 25 μL mixture containing 2 × SYBR Green PCR Master Mix (Applied Biosystems) and 2 primers specific for HBV: a forward primer (HBV-t1: 5-CAA GGA ACC TCT ATG TAT CCC TCC-3) and reverse primer (HBV-t2: 5-TCC GTC CGA AGG TTT GGT AC-3) covering the 50–base pair insertion from 541 bp to 591 bp. Cytotoxicity was assayed with a modified 3-(4, 5-dimethylthiazole-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) method (Gibco Invitrogen, Carlsbad, CA, USA). All the compounds were dissolved in DMSO (Gibco; solvent control) for the anti-HBV activity and cytotoxicity assays. The concentration of DMSO in the culture was kept below 2.5 μL/mL to ensure that the growth of the cells was not affected. An antiviral agent, tenofovir (Jiangxi Chenyang Pharmaceutical Co. Ltd, China) was used as a positive control.
5.2.2. HBV promoter luciferase reporter assay [60], [61], [62], [63]
The promoter regions of HBV core [nucleotides (nt) 1501–1822], X (nt 831–1280), preS (nt 2461–2820), S (nt 2871–30), ENI (1070–1234) and the ENII (1627–1774) were cloned upstream of the luciferase gene of pGL3-Basic (Promega), respectively. HepG 2 was transiently transfected with the reporter vector in a 24-well dish by using Lipofectamine™ 2000 according to the manufacturer's instructions (Invitrogen). HepG 2 cells were transiently transfected with a constant amount of pGL3 Vector (expressing firefly luciferase) as Basic Control, phRL-CMV vector (expressing Renilla luciferase) as an internal control. Cells were split into 24-well plates for 24 h after transfection and continuously cultured for 3 days. Compound was then added to the medium for 3 days (similar to an antiviral assay). Data represent the average ± standard deviation of triplicated samples. Transcriptional activity was determined by measuring luciferase activity in a multiwell plate luminometer (MDS Analytical Technologies, US) using the Luciferase Reporter Assay System (Promega).
5.2.3. Cell line and cell culture
Hep G 2.2.15 cells were cultured in RPMI-1640 medium (Gibco) supplemented with 10% fetal calf serum (Gibco), 100 μg/mL G148 (Gibco), 100 IU/mL penicillin (Gibco), and 100 IU/mL streptomycin (Gibco), and maintained at 37 °C in a moist atmosphere containing 5% CO2. Cells were subcultured once a week, and fresh medium was added every other day.
5.2.4. Acute toxicity
Male and female Kunming mice (21 ± 2 g) were purchased from Chengdu Dashuo Biotechnology Co., Ltd. (Chengdu, China) with animal certificate No. 0012039 and license No. SCXK (Chuan) 2008–24. They were randomly divided into 4 groups with 10 mice each (5 male plus 5 female). Groups of mice were orally administrated compound 18 at a single dose 0 (blank group, the same volume of physiological saline), 50, 250, and 1250 mg/kg, respectively. Mice were housed with free access to water and food in stainless cages in a room with controlled temperature (25 ± 1 °C) and a 12-h light/dark cycle. The mouse survival and body weight were monitored and recorded up to 14 days posttreatment. The protocol complied with the guidelines of Kunming City Laboratory Animal Administration Committee of China for the care and use of laboratory animals.
Acknowledgment
The work was supported by the National Natural Science Foundation of China for Distinguished Young Scholars (No. 81025023).
References
- 1.Rizzetto M., Ciancio A. Mol. Aspects Med. 2008;29:72–84. doi: 10.1016/j.mam.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 2.Neuveut C., Wei Y., Buendia M.A. J. Hepatol. 2010;52:594–604. doi: 10.1016/j.jhep.2009.10.033. [DOI] [PubMed] [Google Scholar]
- 3.Chemin I., Zoulim F. Cancer Lett. 2009;286:52–59. doi: 10.1016/j.canlet.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 4.Chang M.H., Chen T.H.H., Hsu H.M., Wu T.C., Kong M.S., Liang D.C., Ni Y.H., Chen C.J., Chen D.S. Clin. Cancer Res. 2005;11:7953–7957. doi: 10.1158/1078-0432.CCR-05-1095. [DOI] [PubMed] [Google Scholar]
- 5.Wong D.K.H., Cheung A.M., O′Rourke K., Naylor C.D., Detsky A.S., Heathcote J. Ann. Intern. Med. 1993;119:312–323. doi: 10.7326/0003-4819-119-4-199308150-00011. [DOI] [PubMed] [Google Scholar]
- 6.Sato K., Mori M. Mini. Rev. Med. Chem. 2010;10:20–31. doi: 10.2174/138955710791112613. [DOI] [PubMed] [Google Scholar]
- 7.Wu G.Y., Chen H.S. World J. Gastroenterol. 2007;6:830–836. doi: 10.3748/wjg.v13.i6.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Locarnini S., Mason W.S. J. Hepatol. 2006;44:422–431. doi: 10.1016/j.jhep.2005.11.036. [DOI] [PubMed] [Google Scholar]
- 9.Zoulim F., Locarnini S. Gastroenterology. 2009;137:1593–1608. doi: 10.1053/j.gastro.2009.08.063. [DOI] [PubMed] [Google Scholar]
- 10.Newman D.J. J. Med. Chem. 2008;51:2589–2599. doi: 10.1021/jm0704090. [DOI] [PubMed] [Google Scholar]
- 11.Cragg G.M., Grothaus P.G., Newman D.J. Chem. Rev. 2009;109:3012–3043. doi: 10.1021/cr900019j. [DOI] [PubMed] [Google Scholar]
- 12.Zhan P., Jiang X.M., Liu X.Y. Mini. Rev. Med. Chem. 2010;10:162–171. doi: 10.2174/138955710791185118. [DOI] [PubMed] [Google Scholar]
- 13.Cheng Y.C., Ying C.X., Leung C.H., Li Y. J. Clin. Virol. 2005;34(S1):S147–S150. doi: 10.1016/s1386-6532(05)80026-5. [DOI] [PubMed] [Google Scholar]
- 14.Ying C.X., Li Y., Leung C.H., Robek M.D., Cheng Y.C. Proc. Natl. Acad. Sci. U.S.A. 2007;20:8526–8531. doi: 10.1073/pnas.0609883104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yeo H., Li Y., Fu L., Zhu J.L., Gullen E.A., Dutschman G.E., Lee Y., Chung R., Huang E.S., Austin D.J., Cheng Y.C. J. Med. Chem. 2005;48:534–546. doi: 10.1021/jm034265a. [DOI] [PubMed] [Google Scholar]
- 16.Ying C.X., Tan S.L., Yung Y.C. Antivir. Chem. Chemother. 2010;21:97–103. doi: 10.3851/IMP1686. [DOI] [PubMed] [Google Scholar]
- 17.Tseng Y.P., Kuo Y.H., Hu C.P., Jeng K.S., Janmanchi D., Lin C.H., Chou C.K., Yeh S.F. Antivir. Res. 2008;77 doi: 10.1016/j.antiviral.2007.12.011. 206–214. [DOI] [PubMed] [Google Scholar]
- 18.Gao L.M., Han Y.X., Wang Y.P., Li Y.H., Shan Y.Q., Li X., Peng Z.G., Bi C.W., Zhang T., Du N.N., Jiang J.D., Song D.Q. J. Med. Chem. 2011;54:869–876. doi: 10.1021/jm101325h. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Q., Jiang Z.Y., Luo J., Cheng P., Ma Y.B., Zhang X.M., Zhang F.X., Zhou J., Chen J.J. Bioorg. Med. Chem. Lett. 2008;18:4647–4650. doi: 10.1016/j.bmcl.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Q., Jiang Z.Y., Luo J., Liu J.F., Ma Y.B., Guo R.H., Zhang X.M., Zhou J., Chen J.J. Bioorg. Med. Chem. Lett. 2009;19:2148–2153. doi: 10.1016/j.bmcl.2009.02.122. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Q., Jiang Z.Y., Luo J., Cheng P., Ma Y.B., Zhang X.M., Zhang F.X., Zhou J., Niu W., Du F.F., Li L., Li C., Chen J.J. Bioorg. Med. Chem. Lett. 2009;19:6659–6665. doi: 10.1016/j.bmcl.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 22.Guo R.H., Zhang Q., Ma Y.B., Luo J., Geng C.A., Wang L.J., Zhang X.M., Zhou J., Jiang Z.Y., Chen J.J. Eur. J. Med. Chem. 2011;46:307–319. doi: 10.1016/j.ejmech.2010.11.019. [DOI] [PubMed] [Google Scholar]
- 23.Guo R.H., Zhang Q., Ma Y.B., Huang X.Y., Luo J., Wang L.J., Geng C.A., Zhang X.M., Zhou J., Jiang Z.Y., Chen J.J. Bioorg. Med. Chem. 2011;19:1400–1408. doi: 10.1016/j.bmc.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 24.Geng C.A., Zhang X.M., Shen Y., Zuo A.X., Liu J.F., Ma Y.B., Luo J., Zhou J., Jiang Z.Y., Chen J.J. Org. Lett. 2009;11:4838–4841. doi: 10.1021/ol901881w. [DOI] [PubMed] [Google Scholar]
- 25.Geng C.A., Jiang Z.Y., Ma Y.B., Luo J., Zhang X.M., Wang H.L., Shen Y., Zuo A.X., Zhou J., Chen J.J. Org. Lett. 2009;11:4120–4123. doi: 10.1021/ol901592f. [DOI] [PubMed] [Google Scholar]
- 26.Geng C.A., Zhang X.M., Ma Y.B., Jiang Z.Y., Luo J., Zhou J., Wang H.L., Chen J.J. Tetrahedron Lett. 2010;51:2483–2485. [Google Scholar]
- 27.Yan M.H., Cheng P., Jiang Z.Y., Ma Y.B., Zhang X.M., Zhang F.X., Yang L.M., Zheng Y.T., Chen J.J. J. Nat. Prod. 2008;71:760–763. doi: 10.1021/np070479+. [DOI] [PubMed] [Google Scholar]
- 28.Chen J.J., Zhang Z.X., Zhou J. Acta Bot. Yunnan. 1989;11:358–360. [Google Scholar]
- 29.Peng Y.R., Li Y.B., Liu X.D., Zhang J.F., Duan J.A. Phytomedicine. 2008;15:1016–1020. doi: 10.1016/j.phymed.2008.02.021. [DOI] [PubMed] [Google Scholar]
- 30.Peng Y.R., Li Y.B., Liu X.D., Zhang J.F., Duan J.A. Chin. J. Nat. Med. 2008;6:210–213. [Google Scholar]
- 31.Wang D.M., Zhang H.Q., Li X. Acta Pharm. Sin. 2007;42:366–370. [Google Scholar]
- 32.Peng Y.R., Wang D.W., Ding Y.F., Luo Y.H., Li Y.B., Liu X.D. Chin. J. Nat. Med. 2010;8:471–473. [Google Scholar]
- 33.Zhuang M., Jiang H., Suzuki Y., Li X.G., Xiao P., Tanaka T., Ling H., Yang B.F., Saitoh H., Zhang L.F., Qin C.A., Sugamura K., Hattori T. Antivir. Res. 2009;82:73–81. doi: 10.1016/j.antiviral.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee S.U., Shin C.-G., Lee C.K., Lee Y.S. Eur. J. Med. Chem. 2007;42:1309–1315. doi: 10.1016/j.ejmech.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 35.Lapeyre C., Delomenede M., Bedos-Belval F., Duran H., Negre-Salvayre A., Baltas M. J. Med. Chem. 2005;48:8115–8124. doi: 10.1021/jm050454c. [DOI] [PubMed] [Google Scholar]
- 36.Hedvati L., Nudelman A., Falb E., Kraiz B., Zhuk R., Sprecher M. Eur. J. Med. Chem. 2002;37:607–616. doi: 10.1016/s0223-5234(02)01375-2. [DOI] [PubMed] [Google Scholar]
- 37.Qian Y., Zhang H.J., Zhang H., Xu C., Zhao J., Zhu H.L. Bioorg. Med. Chem. 2010;18:4991–4996. doi: 10.1016/j.bmc.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 38.De P., Yoya G.K., Constant P., Bedos-Belval F., Duran H., Saffon N., Daffé M., Baltas M. J. Med. Chem. 2011;54:1449–1461. doi: 10.1021/jm101510d. [DOI] [PubMed] [Google Scholar]
- 39.Bairwa R., Kakwani M., Tawari N.R., Lalchandani J., Ray M.K., Rajan M.G.R., Degani M.S. Bioorg. Med. Chem. Lett. 2010;20:1623–1625. doi: 10.1016/j.bmcl.2010.01.031. [DOI] [PubMed] [Google Scholar]
- 40.Gaspar A., Garrido E.M., Esteves M., Quezada E., Milhazes N., Garrido J., Borges F. Eur. J. Med. Chem. 2009;44:2092–2099. doi: 10.1016/j.ejmech.2008.10.027. [DOI] [PubMed] [Google Scholar]
- 41.Menezes J.C.J.M.D.S., Kamat S.P., Cavaleiro J.A.S., Gaspar A., Garrido J., Borges F. Eur. J. Med. Chem. 2011;46:773–777. doi: 10.1016/j.ejmech.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 42.Narasimhan B., Belsare D., Pharande D., Mourya V., Dhake A. Eur. J. Med. Chem. 2004;39:827–834. doi: 10.1016/j.ejmech.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 43.Fu J., Cheng K., Zhang Z.M., Fang R.Q., Zhu H.L. Eur. J. Med. Chem. 2010;45:2638–2643. doi: 10.1016/j.ejmech.2010.01.066. [DOI] [PubMed] [Google Scholar]
- 44.Galabov A.S., Nikolaeva L., Todorova D., Milkova T. Z. Naturforsch. C: Biosci. 1998;53:883–887. doi: 10.1515/znc-1998-9-1017. [DOI] [PubMed] [Google Scholar]
- 45.Wang G.F., Shi L.P., Ren Y.D., Liu Q.F., Liu H.F., Zhang R.J., Li Z., Zhu F.H., He P.L., Tang W., Tao P.Z., Li C., Zhao W.M., Zuo J.P. Antivir. Res. 2009;83:186–190. doi: 10.1016/j.antiviral.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 46.Letizia C.S., Cocchiara J., Lapczynski A., Lalko J., Api A.M. Food Chem. Toxicol. 2005;43:925–943. doi: 10.1016/j.fct.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 47.Zawidlak-Wegrzyńska B., Kawalec M., Bosek I., Łuczyk-Juzwa M., Adamus G., Rusin A., Filipczak P., Glowala-Kosińska M., Wolańska K., Krawczyk Z., Kurcok P. Eur. J. Med. Chem. 2010;45:1833–1842. doi: 10.1016/j.ejmech.2010.01.020. [DOI] [PubMed] [Google Scholar]
- 48.Krajacic M.B., Novak P., Cindric M., Brajsa K., Dumic M., Kujundzic N. Eur. J. Med. Chem. 2007;42:138–145. doi: 10.1016/j.ejmech.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 49.Appendino G., Minassi A., Daddario N., Bianchi F., Tron G.C. Org. Lett. 2002;4:3839–3841. doi: 10.1021/ol0266471. [DOI] [PubMed] [Google Scholar]
- 50.Hagmann W.K. J. Med. Chem. 2008;51:4359–4369. doi: 10.1021/jm800219f. [DOI] [PubMed] [Google Scholar]
- 51.Hu K.Q., Siddiqui A. Virology. 1991;181:721–726. doi: 10.1016/0042-6822(91)90906-r. [DOI] [PubMed] [Google Scholar]
- 52.Choi B.H., Park G.T., Rho H.M. J. Biol. Chem. 1999;274:2858–2865. doi: 10.1074/jbc.274.5.2858. [DOI] [PubMed] [Google Scholar]
- 53.Zheng Y.W., Riegler J., Wu J., Yen T.S. J. Biol. Chem. 1994;269:22593–22598. [PubMed] [Google Scholar]
- 54.Tang H., Delgermaa L., Huang F.J., Oishi N., Liu L., He F., Zhao L.S., Murakami S. J. Virol. 2005;79:5548–5556. doi: 10.1128/JVI.79.9.5548-5556.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kosovsky M.J., Huan B.F., Siddiqui A. J. Biol. Chem. 1996;271:21859–21869. doi: 10.1074/jbc.271.36.21859. [DOI] [PubMed] [Google Scholar]
- 56.Zhang P., Raney A.K., McLachlan A. Virology. 1992;191:31–41. doi: 10.1016/0042-6822(92)90163-j. [DOI] [PubMed] [Google Scholar]
- 57.Treinin M., Laub O. Mol. Cell. Biol. 1987;7:545–548. doi: 10.1128/mcb.7.1.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guo W.T., Bell K.D., Ou J.H. J. Virol. 1991;65:6686–6692. doi: 10.1128/jvi.65.12.6686-6692.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gonzalez S.A., Keeffe E.B. Future Virol. 2009;4:437–452. [Google Scholar]
- 60.Geng C.A., Wang L.J., Zhang X.M., Ma Y.B., Huang X.Y., Luo J., Guo R.H., Zhou J., Shen Y., Zuo A.X., Jiang Z.Y., Chen J.J. Chem-Eur J. 2011;17:3893–3903. doi: 10.1002/chem.201003180. [DOI] [PubMed] [Google Scholar]
- 61.López-Cabrera M., Letovsky J., Hu K.Q., Siddiqui A. Proc. Natl. Acad. Sci. U.S.A. 1990;87:5069–5073. doi: 10.1073/pnas.87.13.5069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Su H., Yee J.K. Proc. Natl. Acad. Sci. U.S.A. 1992;89:2708–2712. doi: 10.1073/pnas.89.7.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.He X.X., Lin J.S., Chang Y., Zhang Y.H., Li Y., Wang X.Y., Xu D., Cheng X.M. World J. Gastroenterol. 2008;14:1836–1841. doi: 10.3748/wjg.14.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]


