Abstract
Influenza vaccination coverage among health-care workers (HCWs) remains the lowest compared with other priority groups for immunization. Little is known about the acceptability and compliance with the pandemic (H1N1) 2009 influenza vaccine among HCWs during the current campaign. Between 23 December 2009 and 13 January 2010, once the workplace vaccination program was over, we conducted a cross-sectional, questionnaire-based survey at the University Hospital 12 de Octubre (Madrid, Spain). Five hundred twenty-seven HCWs were asked about their influenza immunization history during the 2009–2010 season, as well as the reasons for accepting or declining either the seasonal or pandemic vaccines. Multiple logistic-regression analysis was preformed to identify variables associated with immunization acceptance. A total of 262 HCWs (49.7%) reported having received the seasonal vaccine, while only 87 (16.5%) affirmed having received the pandemic influenza (H1N1) 2009 vaccine. “Self-protection” and “protection of the patient” were the most frequently adduced reasons for acceptance of the pandemic vaccination, whereas the existence of “doubts about vaccine efficacy” and “fear of adverse reactions” were the main arguments for refusal. Simultaneous receipt of the seasonal vaccine (odds ratio [OR]: 0.27; 95% confidence interval [95% CI]: 0.14–0.52) and being a staff (OR: 0.08; 95% CI: 0.04–0.19) or a resident physician (OR: 0.16; 95% CI: 0.05–0.50) emerged as independent predictors for pandemic vaccine acceptance, whereas self-reported membership of a priority group was associated with refusal (OR: 5.98; 95% CI: 1.35–26.5). The pandemic (H1N1) 2009 influenza vaccination coverage among the HCWs in our institution was very low (16.5%), suggesting the role of specific attitudinal barriers and misconceptions about immunization in a global pandemic scenario.
Keywords: Attitudes, Health-care workers, Pandemic A (H1N1) 2009 influenza, Seasonal influenza, Vaccination
1. Introduction
Influenza is a major health problem that poses a significant clinical and socioeconomic burden in all age groups [1]. In comparison to the general population, influenza may lead to more severe and life-threatening complications among hospitalized patients with underlying conditions [2]. It is a well-established fact that the health-care workers (HCWs) are at risk of occupational exposure, contraction of seasonal influenza and subsequent transmission to inpatients [3]. The most effective method of preventing these annual outbreaks and resulting morbidity and mortality is by influenza vaccination [4], [5], [6]. Vaccine has been reported to prevent influenza-related respiratory tract infection by 56% and overall mortality by 68% [5]. In addition, generalized influenza vaccination of HCWs has been demonstrated to positively impact on absenteeism rates and economic burden associated with seasonal epidemics [6]. In light of these evidences, the Advisory Committee on Immunization Practices (ACIP) of the US Public Health Service has suggested since 1981 vaccination for groups at risk of influenza-related complications, as well as for HCWs who care for patients susceptible to significant morbidity following influenza infection [7]. However, influenza vaccine acceptance by HCW has been consistently one of the lowest among groups for which immunization is recommended [8], [9], [10], [11]. The overall vaccination coverage in a previous survey performed at our institution over three consecutive campaigns ranged from 16% to 40%, far below the 60% established by the World Health Organization (WHO) for high-risk groups [9]. The reluctance of HCWs to accept influenza vaccination appears to be associated in the literature with lack of knowledge of influenza and its complications, lack of availability, or perception of low personal susceptibility, among others factors [10].
On 11 June 2009, only 2 months after the first human infections with a new influenza A (H1N1) virus of swine origin were reported from Mexico and the USA, the WHO declared the first influenza pandemic of this century [12]. As a result of the strain's novelty, most people lack innate immunity against this agent. An early case report from Mexico reported that 12% of HCWs caring for influenza cases developed respiratory symptoms [13], and nosocomial outbreaks of pandemic (H1N1) 2009 influenza have been recently documented [14]. On 29 July 2009 the ACIP recommended the vaccination efforts focus on five key populations, including health-care and emergency medical services personnel [15]. To date, the majority of studies examining vaccination attitudes and compliance among HCWs have been centered on seasonal influenza [8], [9], [10], [11], [16]. In our knowledge, very few reports have specifically assessed the barriers to and facilitators of pandemic vaccine receipt in this population, and all of them were performed before the beginning of the current immunization campaign [17], [18], [19]. Thus, the aim of our study was to evaluate and compare the actual vaccination rates as well as the conceptions and attitudes towards both seasonal and pandemic influenza vaccine among HCWs in a tertiary-care university hospital.
2. Methods
2.1. Study design and setting
We conducted a cross-sectional, observational study at the University Hospital 12 de Octubre, a 1300-bed tertiary-care centre in central Spain with a patient population over 750,000 inhabitants in 2003. According to data from the Department of Human Resources, in 2007 the hospital workforce consisted of 7396 employees (excluding directive and teaching personnel): 1232 staff physicians, 477 resident physicians, 2169 nurses, 1385 nursing assistants, and 2170 medical technicians and other ancillary staff.
2.2. Vaccination program
Since October 2009, the Department of Preventive Medicine developed a passive communication strategy consisting of informative posters distributed through the hospital, staff meetings, and information sheets sent to heads of medical departments and nursing supervisors. These posters provided information on the disease, immunization recommendations, and on timing and sites of vaccination sessions. Seasonal and pandemic (H1N1) 2009 influenza vaccines were offered free of charge to all active HCWs between 16 September and 13 November, and 16 November and 15 December 2009, respectively. A team composed of a physician and a nurse form de Department of Preventive Medicine visited all hospital wards offering vaccination at the workplace in the case of seasonal influenza, or at six specific points distributed through the entire hospital in the case of pandemic (H1N1) 2009 influenza.
2.3. Study design
Based on a previous study performed at our institution [9], we expected an overall vaccination coverage among HCWs close to 40%. Assuming a confidence level of 95% and a maximum error of 5%, the sample size was calculated to be at least 352 HCWs to estimate the main objective of the study. Given an expected high rate of non-response [20], we randomly contacted 900 HCWs. However, the response rate was higher than initially expected, so the sample of obtained questionnaires was stratified according HCWs categories via fixed minimum quota responded.
2.4. Data collection
Staff roster was used as sampling frame. A systematic random sample was undertaken to obtain the study population. After receiving a brief oral description of the aim of the study, all of the participants received a standardized, anonymous, self-administered questionnaire. Participation was voluntary and questionnaires were completed privately. The survey was performed in four different days over the time period from 23 December 2009 through 13 January 2010, once the immunization campaign was over. The questionnaire was designed on the basis of existing literature [9], [10], [11] and consisted of 14 items grouped in six sections: (a) demographics (gender and age), professional category and patient contact; (b) history of seasonal influenza vaccination in the 2008–2009 immunization campaign; (c) history of both seasonal and pandemic (H1N1) 2009 influenza vaccination in the current 2009–2010 campaign; (d) being in a priority group for seasonal or pandemic (H1N1) 2009 influenza vaccination due to medical conditions; (e) reasons for accepting or declining the seasonal influenza vaccination, selected from a structured repertoire with 6 and 8 possible answers, respectively; (f) and reasons for accepting or declining the pandemic (H1N1) 2009 influenza vaccination, also selected from a structured repertoire similar to that for seasonal influenza. Only one response was allowed for each of these last two items, including an open-ended question in which responders were asked to freely describe their personal reasons to accept or decline vaccination.
2.5. Statistical analysis
Descriptive statistics of the responses, expressed as absolute and relative frequencies, were generated. Categorical data were analyzed by χ 2 test or Fisher's exact test, as appropriate, whereas Student's t-test for independent samples was applied for continuous variables. We used bivariate analysis to evaluate the effect of each independent variable on the likehood of receiving either the seasonal or pandemic (H1N1) 2009 influenza vaccine. The variables found to be statistically significant in the bivariate analysis were included in a multivariate logistic-regression analysis to evaluate independent predictors of refusal of immunization. Associations are given as odds ratios (OR). All the analysis were two-tailed, and differences were considered to be significant at a P-value < 0.05. Statistical analysis was performed by software package SPSS, version 15.0 (SPSS Inc., Chicago, IL).
3. Results
The questionnaire was completed by 527 HCWs, 401 (76.1%) of them were females and 142 (26.9%) were aged over 50 years. Characteristics of the surveyed participants are summarized in Table 1 . A total of 262 HCWs (49.7%) reported having received the seasonal influenza vaccine during the current immunization campaign, while only 87 of them (16.5%) affirmed having received the pandemic influenza (H1N1) 2009 vaccine. Seventy-two responders (13.7%) reported having undergone immunization for both seasonal and pandemic influenza. Receipt of seasonal influenza vaccine was significantly more likely among males (P < 0.001), HCWs with history of previous seasonal vaccination in the 2008–2009 campaign or pandemic influenza vaccination during the current campaign (P < 0.001 in both cases), resident and staff physicians (P < 0.001 in both cases), and being in a priority group for seasonal influenza immunization (P < 0.001). Conversely, HCWs who reported regular contact with patients (P = 0.003) as well as the nursing assistants (P = 0.002) and nurses (P < 0.001) had lower coverage rates for seasonal vaccination (Table 2 ). For pandemic (H1N1) 2009 influenza, the factors showing a significant association with receipt of vaccination were male gender (P < 0.001), a history of seasonal vaccination either in the previous or current campaign (P < 0.001 for both), being in a priority group for seasonal (P = 0.01) or pandemic influenza immunization (P < 0.001), and being a resident or staff physician (P <0.001 in both cases). Nursing assistants (P = 0.015) and nurses (P < 0.001) were again more likely to refuse pandemic vaccine, as summarized in Table 2.
Table 1.
Characteristics of surveyed HCWs and vaccination rates for seasonal and pandemic (H1N1) 2009 influenza according to demographics and professional variables.
| Variable (%) | Total (n = 527) | Seasonal influenza |
Pandemic (H1N1) 2009 influenza |
||
|---|---|---|---|---|---|
| Vaccination | No vaccination | Vaccination | No vaccination | ||
| Overall | 262 (49.7) | 265 (50.3) | 87 (16.5) | 440 (83.5) | |
| Age group (years) | |||||
| <30 | 76 (14.4) | 37 (48.7) | 39 (51.3) | 17 (22.3) | 59 (77.7) |
| 31–40 | 144 (27.3) | 63 (43.7) | 81 (56.3) | 26 (18.0) | 118 (82.0) |
| 41–50 | 165 (31.3) | 79 (47.9) | 86 (52.1) | 20 (12.1) | 145 (87.9) |
| >50 | 142 (26.9) | 83 (58.4) | 59 (41.6) | 24 (16.9) | 118 (83.1) |
| Gender* | |||||
| Male | 126 (23.9) | 80 (63.5) | 46 (36.5) | 35 (27.8) | 91 (72.2) |
| Female | 401 (76.1) | 182 (45.4) | 219 (54.6) | 52 (12.9) | 349 (87.1) |
| Regular patient contact* | 422 (80.1) | 196 (46.4) | 226 (53.6) | 70 (16.6) | 352 (83.4) |
| History of previous vaccinationa,* | 244 (46.3) | 203 (83.2) | 41 (16.8) | 63 (25.8) | 181 (74.2) |
| Professional category* | |||||
| Nursing assistant | 99 (18.8) | 35 (35.3) | 64 (64.7) | 8 (8.1) | 91 (91.9) |
| Nurse | 154 (29.2) | 55 (35.7) | 99 (64.3) | 6 (3.9) | 148 (96.1) |
| Staff physician | 88 (16.7) | 59 (67.1) | 29 (32.9) | 43 (48.9) | 45 (51.1) |
| Resident physician | 32 (6.1) | 31 (96.9) | 1 (3.1) | 14 (43.7) | 18 (56.3) |
| Ancillary staff | 154 (29.2) | 82 (53.2) | 72 (46.8) | 16 (10.4) | 138 (89.6) |
| Priority group for vaccination* | |||||
| Seasonal influenza | 84 (15.9) | 59 (70.2) | 25 (29.8) | 22 (26.2) | 62 (73.8) |
| Pandemic influenza | 74 (14.0) | 51 (68.9) | 23 (31.1) | 23 (31.1) | 51 (68.9) |
Refers to receipt of seasonal influenza vaccine during the 2008–2009 immunization campaign.
P-Value < 0.05 with the χ2 test.
Table 2.
Factors associated with the refusal of both seasonal and pandemic (H1N1) 2009 influenza vaccine: bivariate and multivariate logistic models (n = 527).
| Predictor variable | Bivariate analysis |
Multivariate analysis |
||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P-Value | Adjusted ORa | 95% CI | P-Value | |
| Seasonal influenza vaccination | ||||||
| Male gender | 0.48 | 0.32–0.72 | <0.001 | – | – | – |
| Regular patient contact | 1.95 | 1.26–3.03 | 0.003 | – | – | – |
| History of previous vaccinationb | 0.05 | 0.03–0.08 | <0.001 | 0.05 | 0.03–0.09 | <0.001 |
| Receipt of pandemic vaccinec | 0.16 | 0.09–0.28 | <0.001 | 0.32 | 0.14–0.75 | 0.008 |
| Professional category | ||||||
| Nursing assistant | 2.06 | 1.31–3.25 | 0.002 | – | – | – |
| Nurse | 2.24 | 1.52–3.31 | <0.001 | – | – | – |
| Staff physician | 0.42 | 0.26–0.68 | <0.001 | – | – | – |
| Resident physician | 0.03 | 0.00–0.21 | <0.001 | 0.01 | 0.00–0.08 | <0.001 |
| Priority group for seasonal vaccination | 0.36 | 0.22–0.59 | <0.001 | – | – | – |
| Pandemic (H1N1) 2009 influenza vaccination | ||||||
| Male gender | 0.39 | 0.24–0.63 | <0.001 | – | – | – |
| History of previous vaccinationb | 0.27 | 0.16–0.44 | <0.001 | – | – | – |
| Receipt of seasonal vaccined | 0.16 | 0.09–0.28 | <0.001 | 0.27 | 0.14–0.52 | <0.001 |
| Professional category | ||||||
| Nursing assistant | 2.57 | 1.20–5.52 | 0.015 | – | – | – |
| Nurse | 6.84 | 2.92–16.05 | <0.001 | – | – | – |
| Staff physician | 0.12 | 0.07–0.19 | <0.001 | 0.08 | 0.04–0.19 | <0.001 |
| Resident physician | 0.22 | 0.11–0.47 | <0.001 | 0.16 | 0.05–0.50 | 0.002 |
| Priority group for seasonal vaccination | 0.48 | 0.28–0.84 | 0.01 | – | – | – |
| Priority group for pandemic vaccination | 0.36 | 0.21–0.64 | <0.001 | 5.98 | 1.35–26.5 | 0.019 |
CI: confidence interval; OR: odds ratio.
Adjusted odds ratio by age and gender.
Receipt of seasonal influenza vaccine during the 2008–2009 campaign.
Receipt of pandemic (H1N1) 2009 influenza vaccine during the current campaign.
Receipt of seasonal influenza vaccine during the current campaign.
We identified three variables independently associated with the acceptance of seasonal influenza vaccine: history of previous seasonal vaccination (OR: 0.05; 95% confidence interval [95% CI]: 0.03–0.09; P < 0.001), simultaneous pandemic vaccination during the current campaign (OR: 0.32; 95% CI: 0.14–0.75; P = 0.008), and being a resident physician (OR: 0.01; 95% CI: 0.00–0.08; P < 0.001). We also identified three variables associated with the acceptance of the pandemic (H1N1) 2009 influenza: simultaneous receipt of seasonal vaccine (OR: 0.27; 95% CI: 0.14–0.52; P < 0.001), being a staff (OR: 0.08; 95% CI: 0.04–0.19; P < 0.001) or a resident physician (OR: 0.16; 95% CI: 0.05–0.50; P = 0.002). Self-reported membership of a priority group for pandemic vaccination was associated with refusal (OR: 5.98; 95% CI: 1.35–26.5; P = 0.019).
As shown in Fig. 1 , the most frequent reasons for accepting seasonal influenza vaccination were “self-protection” (51.9%), “protection of the patient” (23.7%), and “being in a priority group for immunization” (12.2%). Fig. 2 summarized the reasons adduced for refusal of seasonal influenza vaccination, with “doubts about vaccine efficacy” (20.8%), “fear of adverse reactions” (14.0%), and “lack of concern” (13.2%) as the most commonly reported. With regards to the pandemic (H1N1) 2009 influenza vaccination, “self-protection” (33.3%), “protection of the patient” (31.0%) and “protection of own family and colleagues” (21.8%) were the most common reasons for acceptance (Fig. 1), whereas the existence of “doubts about vaccine efficacy” (30.0%), “fear of adverse reactions” (16.6%), and “lack of concern” (9.1%) were the main reasons adduced for refusal of such a measure (Fig. 2). In most of the cases, the main arguments either to accept or to refuse the pandemic influenza vaccine significantly differed to those reported for seasonal influenza. When specifically analyzed the reasons for refusal of pandemic (H1N1) 2009 influenza immunization among the 190 HCWs (36% of all responders) that reported having received the seasonal influenza vaccine but not the pandemic, the presence of “doubts about vaccine efficacy” (38.1%), “fear of adverse reactions” (19.4%) and “lack of concern” (9.0%) again emerged as the ones most commonly adduced. Finally, among the 15 HCWs (2.8% of the total sample) vaccinated against pandemic (H1N1) 2009 influenza but not against seasonal influenza, the most frequent reasons given for the refusal of the latter were “lack of time availability” (7 responders) and the “perception of not being at risk for acquiring the disease” (2 responders).
Fig. 1.
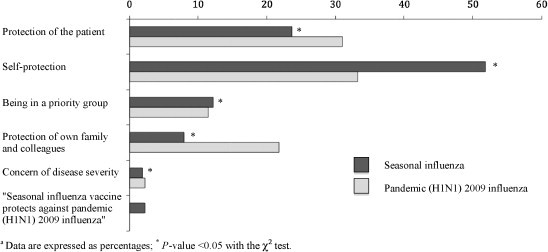
Self-reported reasons adduced for receipt of seasonal and pandemic (H1N1) 2009 influenza vaccine (data are expressed as percentages; *P-value < 0.05 with the χ2 test).
Fig. 2.
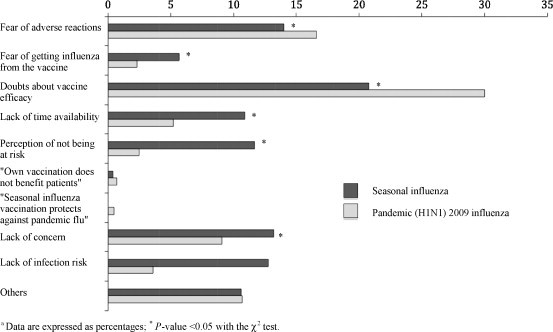
Self-reported reasons adduced for non-receipt of seasonal and pandemic (H1N1) 2009 influenza vaccine (data are expressed as percentages; *P-value < 0.05 with the χ2 test).
A total of 84 surveyed HCWs (15.9%) declared to be included in a priority group for the seasonal influenza vaccination because of their medical conditions. Most of them were females (77.4%) and medical technicians (33.3%) with habitual patient contact (76.2%). The most frequent reasons for non-receipt of seasonal influenza vaccine in this subgroup were the existence of “doubts about vaccine efficacy” (36.0%) or “fear of adverse reactions” (20.0%). In the same way, among the 74 HCWs (14.0%) that reported being in a priority group for the pandemic influenza vaccination the arguments for refusal were “doubts about vaccine efficacy” (30.4%) and “fear of adverse reactions” (17.4%).
4. Discussion
To the best of our knowledge this is the first study to specifically assess not only attitudes or willingness to accept pandemic (H1N1) 2009 influenza vaccine, but also the actual behaviour regarding both seasonal and pandemic immunization among HCWs during the current campaign. We have found an acceptable coverage rate for seasonal vaccine (49.7%), slightly above that achieved at our center during the 2003–2004 campaign (40%) and considerably higher compared with the 2001–2002 season (15.9%) [9], [21]. In contrast, the overall coverage rate for pandemic (H1N1) 2009 influenza vaccination was as low as 16.5%, in spite of the additional education efforts regarding immunization among priority or high-risk groups and the media and public attention focused on the new influenza pandemic during the last months [22]. Such a meaningful difference may be explained by the existence of specific attitudinal barriers and misconceptions about the vaccine, as well as the lack of a single, coherent communication policy shared by the health authorities and the media since the WHO declaration of pandemic alert level 6 in June 2009.
Three previous surveys conducted through 2009, before the start of national immunization programs in the Northern hemisphere, have focused on the knowledge and attitudes towards pandemic influenza vaccination among specific groups of HCWs [17], [18], [19]. In a study performed at 31 hospital departments in Hong Kong, Chor et al. evaluated the acceptability of pre-pandemic vaccination against both influenza A subtypes H5N1 and H1N1 among 2255 HCWs [18]. The survey was conducted during two different periods: January 2009 to March 2009, when the WHO pandemic alert level assigned to H5N1 influenza was phase 3, and May 2009, when the WHO pandemic influenza alert level for (H1N1) 2009 influenza reached phase 5. The authors found a consistently low overall willingness to accept vaccination against either H5N1 (28.4% for the first survey, increased to 34.8% in the second one) or H1N1 influenza (47.9%). The most common reasons cited for refusal of immunization were similar to those found in our study, and included “worry about side effects” and “query on the efficacy of the vaccine”. No significant differences were found in the level of acceptance for pre-pandemic H5N1 influenza vaccine along the two study periods, despite the escalation to WHO alert phase 5 [18]. Schwarzinger et al. conducted a cross-sectional survey among 1434 French general practitioners (GPs) between June 16 and September 22, 2009 [17]. Over 60% of respondents declared their willingness to accept the pandemic influenza (H1N1) 2009 vaccination as soon as it became available; GPs working part-time in long term care facilities, being on call for emergencies, and having the highest workload in practice were more likely to express their willingness to accept immunization. A history of seasonal influenza vaccination in the prior years emerged as the strongest predictive factor associated with acceptance [17]. Finally, a recent questionnaire survey on the attitude towards pandemic influenza vaccination of 441 HCWs at five Greek hospitals in November 2009 has showed a low acceptability rate (17%), which is in line with that of our study [19].
It should be highlighted the notable differences between the self-reported acceptability of pandemic (H1N1) 2009 influenza vaccine in some of these surveys [17], [18], ranging from about 50% to about 60%, and the vaccination coverage rate observed at our institution (16.5%). Various explanations could be considered. Among the most obvious is that such studies only documented the willingness to accept immunization, which may not necessarily reflect the actual behaviour of the surveyed worker or the extent of the “emotional epidemiology” regarding pandemic influenza vaccination, as it has been recently termed by Ofri [24]. Comparison of these results should also take into consideration the role of workplace cultures or local factors, such as the 2002–2003 severe acute respiratory syndrome (SARS) outbreak in Hong Kong, where 27% of cases were HCWs [25]. Finally, the discrepancy we observed could be consistent with other preliminary reports. Schwarzinger et al. pointed out that 1 month after the pandemic influenza vaccination campaign started in France, uptake rates remained below 10% among HCWs regardless of the positive attitudes reported by GPs surveyed in their study [17]. With regards to the general population, Lau et al. reported that 45% of participants in a cross-sectional survey in Hong Kong would be highly likely to take up pandemic influenza vaccine [26], and 67% of responders in another study among adult Australians indicated their willingness to receive such a measure [27]. However, and despite these relatively high reported acceptance rates for pandemic vaccination, the actual coverage rates among the general adult population in France and the United States were as low as 7.9% and 20% by the end of January 2010, respectively [28], [29].
In accordance to literature [9], [10], [16], [18], both resident and staff physicians in our institution were found to be more receptive to either seasonal or pandemic vaccination than HCWs belonging to other groups. A higher professional category may be associated with a better knowledge about the indications and safety of vaccines, or could act as a surrogate for salary level, a variable that has been demonstrated to predict the acceptability of immunization [23]. On the opposite, nurses and nursing assistants have the lowest coverage rates for pandemic vaccination (3.9% and 8.1%, respectively), a finding that is consistent with previous experience on seasonal influenza [9], [16], [20]. In the present study, as well as in others [9], [10], [21], a history of previous seasonal influenza vaccination has been identified as a strong predictor of receipt of immunization in the following year. However, this factor did not retain significance in our multivariate model for pandemic vaccination, which is clearly opposite to the survey by Schwarzinger et al. [17]. Regardless of the potential impact of variations in attitudes toward vaccination between different professional categories, these results suggest the role of specific barriers among HCWs that might have limited the acceptability in a global scenario of pandemic alert. In the bivariate analysis, a history of regular patient contact was related with a lower seasonal influenza vaccination coverage, with the difference not reaching statistical significance in the multivariate model. We hypothesized that this subgroup of HCWs may have developed some degree of “emotional tolerance” towards the disease. The massive application of additional measures (i.e., protective facial mask or hand washing) imposed during the current pandemic could have favoured the development of a subjective sense of protection against the risks associated with the seasonal influenza. Specific educational interventions should be aimed at reducing such a misperception.
As a somewhat unexpected result, self-reported membership to a priority group for pandemic influenza immunization emerged as an independent predictor of non-receipt of vaccine in our multivariate model. It may be hypothesized a higher reluctance to accept immunization in this subgroup of HCWs based on particular concerns about vaccine efficacy or safety, as demonstrated by the proportion of responders reporting “fear of adverse effects” as the main reason for refusal (17.4%). Numerous studies suggested that non-recipients might not actually realize their own risk for getting seasonal influenza, as Hollmeyer et al. highlighted [10]. Accordingly, immunization coverage rate was lower among those physicians assigned to certain hospital departments in our institution (i.e., emergency or intensive care medicine) with specific risk of exposure to the pandemic (H1N1) 2009 influenza virus (data not shown).
Our results showed that for most of HCWs self-protection constitutes a more important reason for the acceptance of either seasonal or pandemic influenza vaccine than the concern about the risk of nosocomial transmission to high-risk inpatients. Perceived risk of contracting the infection was one of the factors showing the strongest association with intention to accept pandemic vaccination in the study by Chor et al. [18]. Although these results agree with those reported by previous studies focused on seasonal influenza [10], [11], [16], we found statistically significant differences in the relative distribution of both arguments according to the type of immunization (Fig. 1). “Self-protection” was more frequently argued among HCWs that reported having received the seasonal influenza vaccine (51.9%) as compared to those that accepted pandemic influenza vaccination (33.3%); conversely, “protection of the patient” was cited as the main argument by 23.7% and 31.0% of responders who received seasonal and pandemic influenza vaccine, respectively (P-value < 0.05 for both comparisons). Most educational programs for HCWs have focused on diminishing the risk of nosocomial influenza transmission to high-risk patients, and it may be assumed that this message has been strengthened in view of the initial uncertainty regarding the novel virus. Interestingly, those surveyed who received the pandemic vaccine were also more likely to argue the “protection of own family and colleagues” as the main reason for acceptance, despite the lack of a professional or contractual relationship between HCWs and their relatives.
The inappropriate assessment of vaccine safety data could severely undermine the acceptability and therefore effectiveness of mass campaigns against pandemic (H1N1) 2009 influenza [30]. In the current study, the proportion of non-recipients expressing “fear of adverse reactions” as the main reason for refusal significantly differs between seasonal and pandemic vaccination (Fig. 2). The coincident temporal association between some life-threatening conditions (i.e., Guillain–Barré syndrome) and previous pandemic vaccination programs, as observed after the swine influenza virus outbreak in 1976, may have raised unfounded concerns among the general population [31]. It is reasonable to assume that HCWs share to some extent a similar view about the vaccine, despite of their training and the communication efforts by the health authorities [22], [32]. In addition, some media reports have expressed concerns about the safety of the new pandemic vaccine and its adjuvants, or the reliability of the expedited authorization process, thus magnifying those unfounded perceptions [33]. Conversely, the low compliance with immunization schedules by HCWs as covered by the media may pose an additional barrier against the acceptability of pandemic vaccine among the general population [34].
There are a number of limitations of our study to be considered. The random sampling was conducted initially, but subsequent selection by quotas may enter a potential selection bias of unknown magnitude or direction. Most of the questionnaires used for the analysis were obtained in the first two waves of the survey reflecting HCWs with similar attitudes. We exclusively relied on self-reports of immunization status or perceived membership in a high-risk group, and these data were not verified through medical or institutional records. Reporting may be subjected to workers’ own knowledge or attitudes towards immunization programs. The survey design prevented us from indentifying any non-responder bias, although similar studies have failed to find relevant differences between responders and non-responders [35]. We did not evaluate the role of specific conditions in the perception of risk among HCWs falling into a priority group for immunization (i.e., chronic lung diseases, pregnancy or obesity). Among the strengths of our study is that it is the first to evaluate not only the anticipated willingness previous to the implementation of immunization schedules, but also the actual uptake of pandemic vaccine in a group of HCWs. The survey was performed very close to the end of the immunization campaign with the aim of minimizing the risk of recall-bias. Finally, our results are consistent in general with those previously focused on seasonal influenza vaccination, including those performed in our setting [9], [21].
To conclude, our study is the first to assess rates of both seasonal and pandemic (H1N1) 2009 influenza vaccination among HCWs during the current campaign, as well as the attitudinal and professional predictors of acceptability. The overall coverage of pandemic immunization among the HCWs in our institution (16.5%) was clearly susceptible of improvement. In our opinion, the findings presented herein could provide valuable implications for health policy decisions in anticipation of possible future pandemic waves, and support the need for continuous education efforts to reduce barriers and unfounded misconceptions in relation to pandemic influenza vaccination.
Acknowledgement
Funding: Francisco López-Medrano has received a grant from Fundación Mutua Madrileña (FMM). Purificación Magán-Tapia has received a grant from the European Regional Development Fund (ERDF).
References
- 1.Molinari N.A., Ortega-Sanchez I.R., Messonnier M.L., Thompson W.W., Wortley P.M., Weintraub E. The annual impact of seasonal influenza in the US: measuring disease burden and costs. Vaccine. 2007;25(27):5086–5096. doi: 10.1016/j.vaccine.2007.03.046. [DOI] [PubMed] [Google Scholar]
- 2.Rothberg M.B., Haessler S.D., Brown R.B. Complications of viral influenza. Am J Med. 2008;121(4):258–264. doi: 10.1016/j.amjmed.2007.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Talbot T.R., Bradley S.E., Cosgrove S.E., Ruef C., Siegel J.D., Weber D.J. Influenza vaccination of healthcare workers and vaccine allocation for healthcare workers during vaccine shortages. Infect Control Hosp Epidemiol. 2005;26(11):882–890. doi: 10.1086/502512. [DOI] [PubMed] [Google Scholar]
- 4.Poland G.A., Tosh P., Jacobson R.M. Requiring influenza vaccination for health care workers: seven truths we must accept. Vaccine. 2005;23(17/18):2251–2255. doi: 10.1016/j.vaccine.2005.01.043. [DOI] [PubMed] [Google Scholar]
- 5.Orenstein W.A., Wharton M., Bart K.J., Hinman A.R. Immunization. In: Mandell G.L., Bennett J.E., Dolin R., editors. Mandell, Douglas and Bennett's principles and practice of infectious diseases. 6th ed. Elsevier Churchill Livingston; Philadelphia, PA: 2005. pp. 3557–3589. [Google Scholar]
- 6.Wilde J.A., McMillan J.A., Serwint J., Butta J., O’Riordan M.A., Steinhoff M.C. Effectiveness of influenza vaccine in health care professionals: a randomized trial. JAMA. 1999;281(10):908–913. doi: 10.1001/jama.281.10.908. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention Recommendation of the Public Health Service Immunization Practices Advisory Committee: influenza vaccine 1981–82. MMWR Morb Mortal Wkly Rep. 1981;30:279–287. [Google Scholar]
- 8.Mereckiene J., Cotter S., Nicoll A., Levy-Bruhl D., Ferro A., Tridente G. National seasonal influenza vaccination survey in Europe, 2008. Euro Surveill. 2008;13(43):19017. doi: 10.2807/ese.13.43.19017-en. [DOI] [PubMed] [Google Scholar]
- 9.de Juanes J.R., García de Codes A., Arrazola M.P., Jaén F., Sanz M.I., González A. Influenza vaccination coverage among hospital personnel over three consecutive vaccination campaigns (2001–2002 to 2003–2004) Vaccine. 2007;25(1):201–204. doi: 10.1016/j.vaccine.2005.10.057. [DOI] [PubMed] [Google Scholar]
- 10.Hollmeyer H.G., Hayden F., Poland G., Buchholz U. Influenza vaccination of health care workers in hospitals: a review of studies on attitudes and predictors. Vaccine. 2009;27(30):3935–3944. doi: 10.1016/j.vaccine.2009.03.056. [DOI] [PubMed] [Google Scholar]
- 11.Wicker S., Rabenau H.F., Doerr H.W., Allwinn R. Influenza vaccination compliance among health care workers in a German university hospital. Infection. 2009;37(3):197–202. doi: 10.1007/s15010-008-8200-2. [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization. Transcript of statement by Margaret Chan, Director-General, June 11; 2009. http://www.who.int/mediacentre/influenzaAH1N1_presstranscript_20090611.pdf.
- 13.Perez-Padilla R., de la Rosa-Zamboni D., Ponce de Leon S., Hernandez M., Quiñones-Falconi F., Bautista E. Pneumonia and respiratory failure from swine-origin influenza A (H1N1) in Mexico. N Engl J Med. 2009;361(7):680–689. doi: 10.1056/NEJMoa0904252. [DOI] [PubMed] [Google Scholar]
- 14.Chironna M., Tafuri S., Santoro N., Prato R., Quarto M., Germinario C.A. A nosocomial outbreak of 2009 pandemic influenza A(H1N1) in a paediatric oncology ward in Italy, October–November 2009. Euro Surveill. 2010;15(1) doi: 10.2807/ese.15.01.19454-en. pii: 19454. [DOI] [PubMed] [Google Scholar]
- 15.Centers for Disease Control advisors make recommendations for use of vaccine against novel H1N1. http://www.cdc.gov/media/pressrel/2009/r090729b.htm.
- 16.Galicia-García M.D., González-Torga A., García-González C., Fuster-Pérez M., Garrigós-Gordo I., López-Fresneña N. Influenza vaccination in healthcare workers. Why are some vaccinated whereas others are not. Enferm Infect Microbiol Clin. 2006;24(7):413–417. doi: 10.1157/13091777. [DOI] [PubMed] [Google Scholar]
- 17.Schwarzinger M., Verger P., Guerville M.A., Aubry C., Rolland S., Obadia Y. Positive attitudes of French general practitioners towards A/H1N1 influenza-pandemic vaccination: a missed opportunity to increase vaccination uptakes in the general public? Vaccine. 2010 doi: 10.1016/j.vaccine.2010.01.027. January 28 [Epub ahead of print], doi:10.1016/j.vaccine.2010.01.027. [DOI] [PubMed] [Google Scholar]
- 18.Chor J.S., Ngai K.L., Goggins W.B., Wong M.C., Wong S.Y., Lee N. Willingness of Hong Kong healthcare workers to accept pre-pandemic influenza vaccination at different WHO alert levels: two questionnaire surveys. BMJ. 2009;339:b3391. doi: 10.1136/bmj.b3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rachiotis G., Mouchtouri V., Kremastinou J., Gourgoulianis K., Hadjichristodoulou C. Low acceptance of vaccination against the 2009 pandemic influenza A (H1N1) among healthcare workers in Greece. Euro Surveill. 2010;15(6) pii: 19486. [PubMed] [Google Scholar]
- 20.Lester R.T., McGeer A., Tomlinson G., Detsky A.S. Use of, effectiveness of, and attitudes regarding influenza vaccine among house staff. Infect Control Hosp Epidemiol. 2003;24(11):839–844. doi: 10.1086/502146. [DOI] [PubMed] [Google Scholar]
- 21.García de Codes Ilario A., Arrazola Martínez M.P., de Juanes Pardo J.R., Sanz Gallardo M.I., Jaén Herreros F., Lago López E. Influenza vaccination in healthcare workers. Strategies to achieve compliance in a general hospital. Med Clin (Barc) 2004;123(14):532–534. doi: 10.1016/s0025-7753(04)74586-8. [DOI] [PubMed] [Google Scholar]
- 22.Duncan B. How the media reported the first days of the pandemic (H1N1) 2009: results of EU-wide media analysis. Euro Surveill. 2009;14:19286. doi: 10.2807/ese.14.30.19286-en. [DOI] [PubMed] [Google Scholar]
- 23.Doebbeling B.N., Edmond M.B., Davis C.S., Woodin J.R., Zeitler R.R. Influenza vaccination of health care workers: evaluation of factors that are important in acceptance. Prev Med. 1997;26(1):68–77. doi: 10.1006/pmed.1996.9991. [DOI] [PubMed] [Google Scholar]
- 24.Ofri D. The emotional epidemiology of H1N1 influenza vaccination. N Engl J Med. 2009;361(27):2594–2595. doi: 10.1056/NEJMp0911047. [DOI] [PubMed] [Google Scholar]
- 25.World Health Organization. Consensus document on the epidemiology of severe acute respiratory syndrome (SARS). http://www.who.int/csr/sars/en/WHOconsensus.pdf.
- 26.Lau J.T., Yeung N.C., Choi K.C., Cheng M.Y., Tsui H.Y., Griffiths S. Acceptability of A/H1N1 vaccination during pandemic phase of influenza A/H1N1 in Hong Kong: population based cross sectional survey. BMJ. 2009;339:b4164. doi: 10.1136/bmj.b4164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eastwood K., Durrheim D.N., Jones A., Butler M. Acceptance of pandemic (H1N1) 2009 influenza vaccination by the Australian public. Med J Aust. 2010;192(1):33–36. doi: 10.5694/j.1326-5377.2010.tb03399.x. [DOI] [PubMed] [Google Scholar]
- 28.Ministère de l’intérieur, de l’outre-mer et des collectivités territoriales. Campagne de vaccination contre la grippe A (H1N1). Communiqué du 4 Janvier 2010. http://www.interieur.gouv.fr/sections/a_la_une/toute_l_actualite/grippea-h1n1/vaccination-semaine-04-01/view.
- 29.Centers for Disease Control and Prevention (CDC). Interim results: state-specific influenza A (H1N1) 2009 monovalent vaccination coverage—United States, October 2009-January 2010. MMWR Morb Mortal Wkly Rep 2010; 59(12):363–8. [PubMed]
- 30.Black S., Eskola J., Siegrist C.A., Halsey N., Macdonald N., Law B. Importance of background rates of disease in assessment of vaccine safety during mass immunisation with pandemic H1N1 influenza vaccines. Lancet. 2009;374(9707):2115–2122. doi: 10.1016/S0140-6736(09)61877-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sencer D.J., Millar J.D. Reflections on the 1976 swine flu vaccination program. Emerg Infect Dis. 2006;12(1):29–33. doi: 10.3201/eid1201.051007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.World Health Organization. Safety of pandemic vaccines. Pandemic (H1N1) 2009 briefing note 6. http://www.who.int/csr/disease/swineflu/notes/h1n1_safety_vaccines_20090805/en/index.html.
- 33.Benítez de Lugo MT. [La vacuna de la gripe A puede ser insegura por su acelerado desarrollo.] El Mundo August 7; 2009. http://www.elmundo.es/papel/2009/08/07/ciencia/18351121.html.
- 34.Valeiro M. [La mayoría de los sanitarios no quieren vacunarse de la gripe A.] El Mundo August 29; 2009. http://www.elmundo.es/papel/2009/08/26/ciencia/18995656.html.
- 35.Yassi A., Kettner J., Hammond G., Cheang M., McGill M. Effectiveness and cost-benefit of an influenza vaccination program among health-care workers. Can J Infect Dis. 1991;2:101–108. doi: 10.1155/1991/376502. [DOI] [PMC free article] [PubMed] [Google Scholar]


