Abstract
Recently, canine coronavirus (CCoV) strains with putative recombinant origin with porcine transmissible gastroenteritis virus (TGEV) were shown to be widespread in Europe. In this study, a killed vaccine against TGEV-like CCoV strains, included in the new subtype CCoV-IIb, was developed through inactivation with betapropiolactone and emulsification with MF59™ adjuvant. Safety, immunogenicity and efficacy of the developed vaccine were evaluated in vivo. Five 10-week-old beagle pups were administered (three weeks apart) two vaccine doses, whereas two animals served as unvaccinated controls. The vaccine was shown to be safe as no local neither systemic reactions were observed after first and second dose administration. Serum antibodies against CCoV were detected in vaccinates starting from study day 14 (by enzyme-linked immunosorbent assay) or 28 (by virus neutralisation test). Subsequent challenge with virulent CCoV-IIb resulted in the development of mild gastroenteric disease in control pups, whereas vaccinates did not display clinical signs. Faecal shedding of the challenge virus occurred in both treatment groups, but vaccinated dogs were found to shed very low viral titres in comparison to controls. The developed vaccine may help control the CCoV-IIb-induced disease (and active virus circulation) in environments, such as kennels and shelters, where the pathogenic potential of this virus is greater as a consequence of predisposing factors and concurrent infections.
Keywords: TGEV-like canine coronavirus, Inactivated vaccine, MF59™ adjuvant
1. Introduction
Canine coronavirus (CCoV) is a member of the newly established genus Alphacoronavirus of the family Coronaviridae, order Nidovirales. CCoV is strictly related to feline coronavirus type I (FCoV-I) and type II (FCoV-II), transmissible gastroenteritis virus of swine (TGEV) and its respiratory variant porcine respiratory coronavirus (PRCoV). Based on the similarities in their genomic organisation, all these viruses have been now included in a unique viral species Alphacoronavirus-1 [1]. CCoV has a classical faecal–oral route of transmission and colonises the top of the villi of the enteric tract, being responsible for mild, self-limiting enteritis. Infected pups usually recover spontaneously from CCoV-induced disease [2]. However, hypervirulent CCoV strains have been reported in the last years [3] and a pantropic variant [4] has been associated to systemic, sometimes fatal disease in pups under natural [5] and experimental conditions [6], [7], [8].
Two CCoV genotypes have been identified so far, namely CCoV type I (CCoV-I) and CCoV type II (CCoV-II) [9]. These genotypes are variously distributed worldwide, with a predominance of CCoV-II in Europe [10] and Asia [11], [12]. In addition, CCoVs with a recombinant origin between CCoV-II and TGEV have been identified in the faeces of dogs with diarrhoea and have been found to be widespread in dogs populations. Accordingly, CCoV-II has been further classified into two subtypes, CCoV-IIa and CCoV-IIb, including “classical” CCoVs and TGEV-like strains, respectively [13]. Subtype CCoV-IIb has been reported in several European countries [14], [15], as well as in Japan [12]. Limited antigenic cross-reactivity has been observed between subtype IIa and IIb CCoVs and this has raised some concerns about the real efficacy of the CCoV vaccines available in the market (prepared with subtype IIa) against the TGEV-like strains [13], [15].
The aim of the present study was to develop an inactivated vaccine adjuvanted with MF59™ against CCoV-IIb and to evaluate its safety, immunogenicity and efficacy in beagle pups.
2. Materials and methods
2.1. Cells and viruses
Canine A-72 cells used for virus cultivation were grown in Dulbecco's minimal essential medium (D-MEM) supplemented with 10% foetal calf serum. CCoV-IIb strains 341/05 and 174/06 were isolated from the lungs of an Italian 14-week-old great dane pup and a Hungarian 10-week-old chihuahua pup, respectively [13]. In the present study, virus 341/05 was used for vaccine preparation, whereas the Hungarian strain served as challenge virus. CCoV-IIb strain 341/05 was chosen as vaccine virus since it contains a 154-nucleotide deletion in ORF7b that could be used in the future as vaccine genetic marker, whereas strain174/06 was used as challenge virus since it had a distinct geographical origin. The two strains had been found to be strictly related at the genetic level, displaying a nucleotide identity of more than 96% in the nearly full-length genome [13].
For virus isolation, the lung samples were homogenised (10%, w/v) in D-MEM containing antibiotics (penicillin 5000 IU/mL, streptomycin 2500 μg/mL, amphotericin B 10 μg/mL). Viral growth was monitored constantly by an immunofluorescence (IF) assay using a monoclonal antibody (MAb) that binds the Alphacoronavirus-1 N protein and a goat anti-mouse IgG conjugated with fluorescein isothiocyanate (Sigma Aldrich srl, Milan, Italy). Both viruses induced a cytopathic effect in the inoculated monolayers and tested positive by the IF assay. The cell media of the third serial passage were collected, centrifuged at 3000 × g for 15 min to remove cell debris, aliquoted and stored at −70 °C until their use. Viral titres of isolates 341/05 and 174/06 were 105.75 and 105.50 TCID50 mL−1 of viral suspension, respectively.
2.2. Vaccine preparation
Isolate 341/05 was inactivated with 1:2000 betapropiolacton (0.05%, v/v) and the inactivated suspension, containing a total protein amount of 441 μg mL−1 as determined by spectophometer analysis, was mixed 1:1 with MF59™ adjuvant (Novartis Vaccines and Diagnostics, Siena, Italy). Vaccine stock was aliquoted in 1-mL doses and stored at +4 °C.
2.3. Sterility test
The stock vaccine was tested for sterility from aerobe and anaerobe bacteria, mycoplasmas and mycetes using standardised methods. The presence of contaminant viruses was searched for in the viral suspension prior to adding the adjuvant by means of (RT-)PCR assays for detection of canine parvovirus type 2 (CPV-2) [16], canine distemper virus (CDV) [17], canine adenoviruses (CAdVs) [18], canine herpesvirus 1 [19], rotaviruses [20], reoviruses [21], and caliciviruses [22].
2.4. Experimental study
The experimental study was performed according to the European animal health and well-being regulations and was authorised by the Italian Ministry of Health (authorization no. 81/2010-C). Seven 10-week-old beagle pups were housed at the Infectious Disease Unit of the Animal Hospital, Faculty of Veterinary Medicine of Bari. The dogs had tested negative for CCoV RNA by a real-time RT-PCR assay [23] carried out on the faeces and for CCoV antibodies by an ELISA test [24] carried out on serum samples. All dogs were housed individually in separate boxes, fed twice daily with a commercial dry dog food and provided water ad libitum. After an acclimatization period of one week, five animals (pups #1 to #5) were vaccinated by subcutaneous administration of two doses, three weeks apart (study days 0 and 21), of 1 mL of the experimental vaccine, whereas two dogs (pups #6 and #7) were maintained unvaccinated by receiving subcutaneously two doses of 1 mL of sterile saline solution (placebo). In order to assess injection site reactions after each vaccination, the first vaccine administration was on the right hand side of the interscapular space, whereas the second dose was administered on the left hand side.
On day 35 (two weeks after booster administration), animals were administered a total of 3.0 mL of challenge material (isolate 174/06) with a titre of approximately 105.5 TCID50 mL−1. The challenge dose was established according to previous studies on CCoV vaccination [26], [27]. Approximately 0.5 mL of challenge material was administered per nostril (1.0 mL total), and 2.0 mL was administered orally. Animals were observed for 21 days after challenge for specific clinical signs of CCoV infection. A single veterinarian, who was not aware of the treatment group assignments, was responsible for daily clinical observations in all dogs. At the end of the animal phase (study day 56), animals were kept in the animal facility and tested every five days for CCoV shedding from faecal samples. Animals were assigned to private owners once the laboratory results indicated the animals were not shedding CCoV for three consecutive tests.
2.5. Safety test
Vaccine safety was evaluated by the observation of local and systemic reactions after each vaccination. Qualitative assessment of injection site reaction was made by palpation of the injection site for the occurrence of pain and/or reaction, whereas systemic reactions were assessed by clinical inspection. Examinations for local and systemic reactions were performed twice on the day of vaccination (pre-vaccination and 5 h post-vaccination) and once daily for four days after each vaccine administration.
2.6. Immunogenicity test
Vaccine immunogenicity was evaluated by assessing the CCoV-antibody response after each vaccine dose administration. Vaccinated and control dogs were bled for serum collection at days 0 (day of first-dose administration), 7, 14, 21 (day of booster administration), 28 and 35 (day of challenge). Antibody titres were also evaluated after challenge (study days 42, 49 and 56). Serum samples were stored at −20 °C until analysis by using enzyme-linked immunosorbent assay (ELISA) and virus neutralisation (VN) tests, as previously described [6], [7], [23].
For ELISA test, microtitre plates were coated with CCoV antigen and, after treatment with blocking solution and repeated washing, 1:50 dilutions of the plasma samples were added to each well. Plates were incubated for 90 min at 37 °C, washed four times and incubated for 60 min at 37 °C with anti-dog IgG-goat peroxidase conjugate (Sigma–Aldrich srl, Milan, Italy). After another washing cycle, 10 mg of freshly prepared substrate, 2,2′-azino-di-[3-ethylbenzthiazoline sulfonate]diammonium salt (ABTS, Sigma–Aldrich srl) in 50 mL of 0.05 M phosphate citrate buffer (pH 5.0) was added to each well and the optical density at 405 nm (OD405) was determined.
For VN tests, serial two-fold dilutions of heat-inactivated sera were mixed with 100 TCID50 of strain 341/05 (CCoV-IIb) or S378 (CCoV-IIa) in 96-well microtitre plates. After pre-incubation at room temperature for 90 min, 2 × 104 A-72 cells were added to each well. The plates were read after four days of incubation at 37 °C. VN titres were calculated using the Spearman–Karber method and expressed as the highest serum dilution able to neutralise the virus.
2.7. Efficacy test
Vaccine efficacy was evaluated by challenging the vaccinated dogs with virulent TGEV-like strain 174/06, two weeks post-second vaccination, and assessing the prevention or reduction of clinical signs and of viral shedding in comparison to unvaccinated control dogs. Clinical examinations were performed on all dogs, once daily from day 34 (day before challenge) to 56, taking into account the occurrence of abnormal clinical signs, dehydration, lethargy and loss of appetite. General health observations were performed on each animal once daily for the entire observation period (21 days post-challenge). Body weights were recorded on days 34, 38, 40, 42, 49 and 56, whereas rectal temperatures were registered daily from days 34 to 42 and on alternate days from days 43 to 56. The general health of each animal was assessed using a scoring system adapted from previous studies [6], [7] (Table 1 ).
Table 1.
Scoring system for clinical signs after challenge with TGEV-like CCoV 174/06a.
| Parameter | Result | Score |
|---|---|---|
| General appearance | Normal | 0 |
| Depressed state | 2 | |
| Difficulties in breathing | 3 | |
| Lethargy | 3 | |
| Death | 20 | |
| Appetite | Normal; eats all food | 0 |
| Fair; eats more than 1/2 of food | 1 | |
| Poor; eats less than 1/2 of food | 2 | |
| None; eats nothing | 3 | |
| Dehydration | None | 0 |
| Mild | 1 | |
| Moderate | 2 | |
| Severe | 3 | |
| Diarrhoea | None present | 0 |
| Soft | 1 | |
| Liquid | 2 | |
| Bloody | 3 | |
| Abdominal pain | None | 0 |
| Mild | 1 | |
| Moderate | 2 | |
| Severe | 3 |
Faecal swabs were collected daily starting from the day of challenge (study day 35) for the entire observation period (until day 56) and stored at −70 °C until processed. RNA was extracted with commercial kits QIAamp® Viral RNA Mini Kit (Qiagen S.p.A., Milan, Italy) from 140 μL homogenates of faecal swabs and RNA extracts were subjected to real-time RT-PCR for the detection and quantitation of CCoV RNA [23]. Reverse transcription was carried out using GeneAmp® RNA PCR (Applied Biosystems, Applera Italia, Monza, Italy), following the manufacturer's recommendations. Real-time PCR for CCoV RNA detection and quantitation was performed in a 50 μL-reaction mixture containing 25 μL of IQ™ Supermix (Bio-Rad Laboratories Srl), 600 nM of primers CCoV-For (TTGATCGTTTTTATAACGGTTCTACAA) and CCoV-Rev (AATGGGCCATAA TAGCCACATAAT), 200 nM of probe CCoV-Pb (FAM-ACCTCAATTTAGCTGGTTCGTGTATGGCATT-TAMRA) and 20 μL of c-DNA. The thermal cycle protocol was the following: activation of iTaq DNA polymerase at 95 °C for 10 min and 45 cycles consisting of denaturation at 95 °C for 15 s and primer annealing–extension at 60 °C for 1 min.
2.8. Statistical analysis
The data were analyzed using the R software (version 2.8.1). All hypothesis tests were conducted at the 0.05 level of significance (two-sides). The area under curve (AUC) for the faecal shedding of the challenge virus was calculated for each group (controls and vaccinates) and the statistical significance was evaluated using the Mann–Whitney test. Prior to analysis the AUC values were logarithmically transformed. The Mann–Whitney test was also used to compare the clinical scores observed after challenge, whereas the VN antibody titres against CCoV-IIa and CCoV-IIb were analysed by the Wilcoxon test.
3. Results
3.1. Sterility test
No bacterial, fungal or viral contaminants were detected by traditional or molecular methods in the viral suspension used as stock vaccine, thus confirming the sterility of the vaccine batch.
3.2. Safety test
No local (injection site reaction and pain) neither systemic (anaphylaxis, convulsion, depression, diarrhoea, oedema, enlarged lymph nodes, vomiting and wheals) reactions were observed in any vaccinated and control dogs after first and second vaccinations.
3.3. Immunogenicity test
By ELISA test, antibodies against CCoV appeared in vaccinated dogs at study day 14 and increased up to mean OD values of 0.234 at day 28 (Fig. 1 ). VN antibodies against CCoV-IIb were detected only at study day 28 (geometric mean titres of 6.06), reaching maximal pre-challenge titres at day 35. CCoV antibodies were not detected in the control dogs in the pre-challenge phase by either ELISA or VN tests. Challenge administration resulted in a further increase of antibody levels in vaccinated animals and in rapid onset of high antibody titres in control pups. By cross-neutralisation using the heterologous virus (CCoV-IIa), VN antibodies followed a similar kinetics, but the titres were significantly lower (P < 0.05) (Fig. 1).
Fig. 1.
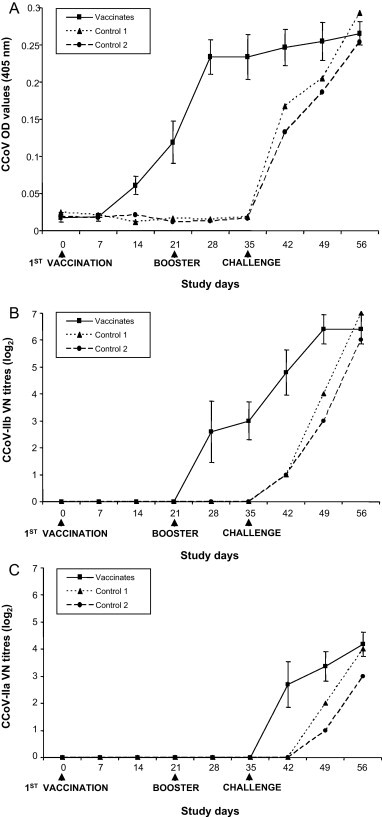
CCoV antibody titres in controls (duplicate values) and vaccinated dogs (means ± SD) after vaccination and challenge. Antibody responses are presented as geometric means of ELISA optical density (OD) values (A) or virus neutralizing (VN) titres against CCoV-IIb (B) and CCoV-IIa (C).
3.4. Efficacy test
Clinical scores and viral shedding in challenged dogs are reported in Fig. 2 . After challenge with virulent CCoV-IIb strain 174/06, vaccinated dogs did not display clinical signs with the exception of soft faeces that were observed in one animal at study day 40 (day 5 post-challenge) and in two animals at day 41. In contrast, challenge administration in unvaccinated dogs resulted in the occurrence of a mild gastroenteritis characterised by liquid diarrhoea or soft faeces for 6–8 days and poor or fair appetite for 4–7 days. Only one control dog showed depression at study day 39 (day 4 post-challenge). All pups gained weight during the post-challenge period and none showed alterations of the rectal temperatures (Table 2 ). The mean clinical score was 0.027 for vaccinated dogs and 0.818 for controls, which was statistically significant (P < 0.01).
Fig. 2.
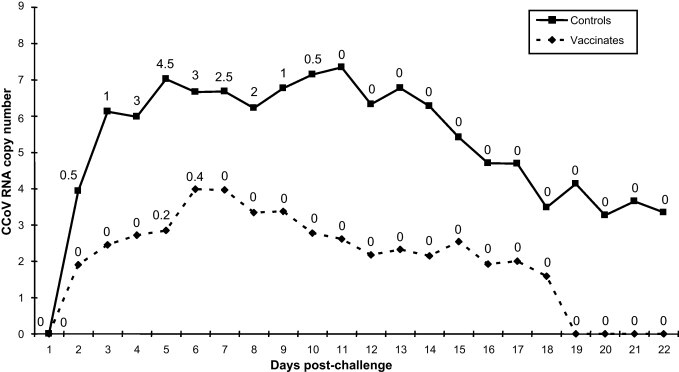
CCoV faecal shedding and clinical scores in control and vaccinated dogs after challenge. Viral RNA titres as determined by real-time RT-PCR are expressed as log10 copy numbers per μl of template. Clinical scores were calculated as shown in Table 1 and are reported for each day in correspondence of the faecal shedding curves.
Table 2.
Summary of clinical signs observed on study days (D) in control and vaccinated dogs after challenge with CCoV-IIb strain 174/06.
| Treatment group | Dog ID | General appearancea | Appetiteb | Diarrhoeac | Dehydration | Fever | Body weight |
|---|---|---|---|---|---|---|---|
| Vaccinates | 1 | Normal | Normal | D5, D6 (S) | NO | NO | Gain |
| 2 | Normal | Normal | NO | NO | NO | Gain | |
| 3 | Normal | Normal | NO | NO | NO | Gain | |
| 4 | Normal | Normal | NO | NO | NO | Gain | |
| 5 | Normal | Normal | D6 (S) | NO | NO | Gain | |
| Controls | 6 | D4 (D) | D1–D2, D6 (F); D3–D5, D7 (P) | D2, D6–D9 (S); D3–D5 (L) | NO | NO | Gain |
| 7 | Normal | D3–D5 (F); D6 (P) | D3, D5, D7, D8 (S); D4, D6 (L) | NO | NO | Gain | |
NO: not observed.
D: depression.
F: fair; P: poor; N: none.
S: soft; L: liquid.
Challenge virus was detected in the faeces of vaccinated dogs for 15 mean days, from study day 36 to 52, with very low viral titres that reached maximal values at study day 40 (mean viral RNA load of 9.27 × 103 copies μl−1 of template). Controls shed CCoV for the entire post-challenge observation period (21 days) and viral titres were generally high, peaking at study day 10 (mean viral titre of 2.23 × 107 copies μl−1 of template). The mean values for the area under the curve of real time RT-PCR results from faecal swabs for the post-challenge period were 76, 613, 957 and 27, 245.7 for vaccinated and unvaccinated dogs, respectively (P < 0.001).
4. Discussion
A CCoV strain antigenically similar to TGEV was first isolated in California in the 1970s from an outbreak of gastroenteritis in dogs [25]. Analogous CCoV strains, TGEV-like, have been identified about 40 years later in dead pups raised in Italy or imported from eastern Europe [13]. Upon genomic characterization, the virus was found to have a chimerical S gene, with the 5′ end of the S gene being acquired from TGEV via recombination. Also, the virus was associated with a form of mild gastroenteritis in infected pups and it was named CCoV-IIb, in order to distinguish it from classical CCoV-II viruses (CCoV-IIa). Subsequent studies showed that CCoV-IIb is widespread in Europe with highest frequency of detection in eastern countries [15]. Limited antigenic cross-reactivity has been demonstrated between CCoV-IIa and CCoV-IIb and this has been hypothesised to account for a possible decreased efficacy of the current vaccines, based on inactivated CCoV-IIa strains, against TGEV-like CCoV strains [13], [15].
In order to overcome the possible limitations of the existing CCoV formulations, we have developed an inactivated, MF59™-adjuvanted vaccine prepared with a CCoV-IIb strain. This vaccine was found to be safe as neither local nor systemic reactions were induced in subcutaneously injected beagle pups. The CCoV-IIb vaccine was immunogenic, eliciting seroconversion in inoculated pups, and effective, as it was able to prevent CCoV-induced disease. Mild gastroenteritis occurred in all unvaccinated pups after infection with the challenge virus, as observed in previous challenge studies [13].
The inactivated CCoV vaccines available in the market have been shown to protect dogs from the disease but not from the infection [26]. By converse, an experimental modified-live virus (MLV) vaccine administered oronasally appeared to be able to prevent both CCoV disease and infection [27]. In this study, the inactivated CCoV-IIb vaccine, albeit not completely protective against the infection, was able to decrease markedly shedding of the challenge-virus, thus preventing the spread of virulent strains in the environment. A possible explanation for the good vaccine efficacy may rely on the MF59™ adjuvant used in the formulation. MF59™ is an oil-in-water formulation that contains surfactants (Tween 80 and Span 85), along with squalene emulsified under high-pressure conditions [29]. This adjuvant is satisfactorily included in the vaccines commonly used for prevention of seasonal and pandemic influenza [28]. The immune responses elicited by the MF59™-adjuvanted CCoV-IIb vaccine, in terms of ELISA OD values and homologous VN antibody titres, were greater than those observed with an aluminium hydroxide-adjuvanted CCoV-IIa formulation [26] and comparable to experimental MLV vaccines [27]. The higher efficacy of the vaccines based on MF59™ adjuvant is still largely unclear. Enhancement of the interaction between the antigen and the dendritic cell seems to be involved [28]. However, considering that the efficacy of commercially available CCoV vaccines was not evaluated in the present study, the claimed higher efficacy of the MF59™-adjuvanted formulation may be biased by different ages and/or genetic backgrounds of the pups employed for the vaccination trials.
In dogs CCoV generally causes mild, self-limiting infections restricted to the gastrointestinal tract. Accordingly, CCoV vaccines are not considered as core vaccines and their use is discretional. The World Small Animal Veterinary Association currently does not recommend CCoV vaccination as the prevalence of CCoV-confirmed clinical cases is rather low [30]. However, the prevalence of CCoV-induced enteric disease in dogs is likely largely underrated, as diagnosis of CCoV infection requires specific tests that are not available in all the laboratories [2]. In addition, fatal diseases may occur as a consequence of mixed infections by CCoV and other canine pathogens, such as CPV-2, CAdV-1, or CDV [2]. Although it has never been proved that vaccination against CCoV reduces the severity of mixed infections, CCoV vaccination should be considered a priority in some settings, such as kennels and animal shelters, where CCoV is expected to circulate largely, mixed infections are common and animals are subjected to stress conditions, that may trigger the development of severe clinical forms. To date, there is still limited information on CCoV-IIb infection in dogs, but our findings seem to suggest that the pathogenetic patterns of this new CCoV subtype are similar to those observed for CCoV-IIa [13], [15]. Accordingly, similar prophylaxis strategies could be enacted to prevent and control CCoV-IIb-associated disease in dogs. The one-way antigenic cross-reactivity, already shown by neutralisation in vitro between CCoV-IIb and CCoV-IIa [13], was confirmed by the present study, as antibodies elicited against recombinant CCoV were able to cross-neutralise only partially the heterologous virus (CCoV-IIa). Based on these findings, as well as on the poor efficacy of aluminium hydroxide-adjuvanted formulations even against CCoV-IIa, it could be speculated that commercially available vaccines are not protective against CCoV-IIb. Therefore, it will be pivotal to assess whether and to which extent cross-protection occurs in vivo and whether CCoV-IIb (TGEV-like)-based vaccines may be developed for prevention of CCoV disease induced by both CCoV subtypes in dogs.
Acknowledgements
The authors thank Carlo Armenise, Arturo Gentile and Donato Narcisi for their excellent technical support. This work was supported by grants from the Italian Ministry of Education, University and Research to Canio Buonavoglia (PRIN 2008, project “Evoluzione genetica e patogenetica dei coronavirus: il modello coronavirus del cane”) and from the Italian Ministry of Health to Nicola Decaro (Ricerca finalizzata 2007, project “Mammalian coronaviruses: molecular epidemiology, vaccine development and implications for animal and human health”).
References
- 1.Carstens E.B. Ratification vote on taxonomic proposals to the International Committee on Taxonomy of Viruses (2009) Arch Virol. 2010;155:133–146. doi: 10.1007/s00705-009-0547-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Decaro N., Buonavoglia C. An update on canine coronaviruses: viral evolution and pathobiology. Vet Microbiol. 2008;132:221–234. doi: 10.1016/j.vetmic.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Evermann J.F., Abbott J.R., Han S. Canine coronavirus-associated puppy mortality without evidence of concurrent canine parvovirus infection. J Vet Diagn Invest. 2005;17:610–614. doi: 10.1177/104063870501700618. [DOI] [PubMed] [Google Scholar]
- 4.Buonavoglia C., Decaro N., Martella V., Elia G., Campolo M., Desario C., et al. Canine coronavirus highly pathogenic for dogs. Emerg Infect Dis. 2006;12:492–494. doi: 10.3201/eid1203.050839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Decaro N., Martella V., Elia G., Campolo M., Desario C., Cirone F., et al. Molecular characterisation of the virulent canine coronavirus CB/05 strain. Virus Res. 2007;125:54–60. doi: 10.1016/j.virusres.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Decaro N., Campolo M., Lorusso A., Desario C., Mari V., Colaianni M.L., et al. Experimental infection of dogs with a novel strain of canine coronavirus causing systemic disease and lymphopenia. Vet Microbiol. 2008;128:253–260. doi: 10.1016/j.vetmic.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Decaro N., Elia G., Martella V., Campolo M., Mari V., Desario C., et al. Immunity after natural exposure to enteric canine coronavirus does not provide complete protection against infection with the new pantropic CB/05 strain. Vaccine. 2010;28:724–729. doi: 10.1016/j.vaccine.2009.10.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marinaro M., Mari V., Bellacicco A.L., Tarsitano E., Elia G., Losurdo M., et al. Prolonged depletion of circulating CD4+ T lymphocytes and acute monocytosis after pantropic canine coronavirus infection in dogs. Virus Res. 2010;152:73–78. doi: 10.1016/j.virusres.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Decaro N., Martella V., Ricci D., Elia G., Desario C., Campolo M., Cavaliere N., et al. Genotype-specific fluorogenic RT-PCR assays for the detection and quantitation of canine coronavirus type I and type II RNA in faecal samples of dogs. J Virol Methods. 2005;130:72–78. doi: 10.1016/j.jviromet.2005.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Decaro N., Desario C., Billi M., Mari V., Elia G., Cavalli A., et al. Western European epidemiological survey for parvovirus and coronavirus infections in dogs. Vet J. 2009 doi: 10.1016/j.tvjl.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Y., Ma G., Lu C., Wen H. Detection of canine coronaviruses genotype I and II in raised Canidae animals in China. Berl Munch Tierarztl Wochenschr. 2006;119:35–39. [PubMed] [Google Scholar]
- 12.Soma T., Ohinata T., Ishii H., Takahashi T., Taharaguchi S., Hara M. Detection and genotyping of canine coronavirus RNA in diarrheic dogs in Japan. Res Vet Sci. 2010 doi: 10.1016/j.rvsc.2010.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Decaro N., Mari V., Campolo M., Lorusso A., Camero M., Elia G., et al. Recombinant canine coronaviruses related to transmissible gastroenteritis virus of swine are circulating in dogs. J Virol. 2009;83:1532–1537. doi: 10.1128/JVI.01937-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Erles K., Brownlie J. Sequence analysis of divergent canine coronavirus strains present in a UK dog population. Virus Res. 2009;141:21–25. doi: 10.1016/j.virusres.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Decaro N., Mari V., Elia G., Addie D.D., Camero M., Lucente M.S., et al. Recombinant canine coronaviruses in dogs, Europe. Emerg Infect Dis. 2010;16:41–47. doi: 10.3201/eid1601.090726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Decaro N., Elia G., Martella V., Desario C., Campolo M., Di Trani L., et al. A real-time PCR assay for rapid detection and quantitation of canine parvovirus type 2 DNA in the feces of dogs. Vet Microbiol. 2005;105:19–28. doi: 10.1016/j.vetmic.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 17.Elia G., Decaro N., Martella V., Cirone F., Lucente M.S., Lorusso E., et al. Detection of canine distemper virus in dogs by real-time RT-PCR. J Virol Methods. 2006;136:171–176. doi: 10.1016/j.jviromet.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 18.Hu R.L., Huang G., Qiu W., Zhong Z.H., Xia X.Z., Yin Z. Detection and differentiation of CAV-1 and CAV-2 by polymerase chain reaction. Vet Res Commun. 2001;25:77–84. doi: 10.1023/a:1006417203856. [DOI] [PubMed] [Google Scholar]
- 19.Decaro N., Amorisco F., Desario C., Lorusso E., Camero M., Bellacicco A.L., et al. Development and validation of a real-time PCR assay for specific and sensitive detection of canid herpesvirus 1. J Virol Methods. 2010;169:176–180. doi: 10.1016/j.jviromet.2010.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gouvea V., Santos N., Timenetsky Mdo C. Identification of bovine and porcine rotavirus G types by PCR. J Clin Microbiol. 1994;32:1338–1340. doi: 10.1128/jcm.32.5.1338-1340.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Decaro N., Campolo M., Desario C., Ricci D., Camero M., Lorusso E., et al. Virological and molecular characterization of a mammalian orthoreovirus type 3 strain isolated from a dog in Italy. Vet Microbiol. 2005;109:19–27. doi: 10.1016/j.vetmic.2005.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang X., Huang P.W., Zhong W.M., Farkas T., Cubitt D.W., Matson D.O. Design and evaluation of a primer pair that detects both Norwalk- and Sapporo-like caliciviruses by RT-PCR. J Virol Methods. 1999;83:145–154. doi: 10.1016/s0166-0934(99)00114-7. [DOI] [PubMed] [Google Scholar]
- 23.Decaro N., Pratelli A., Campolo M., Elia G., Martella V., Tempesta M., et al. Quantitation of canine coronavirus RNA in the faeces of dogs by TaqMan RT-PCR. J Virol Methods. 2004;119:145–150. doi: 10.1016/j.jviromet.2004.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pratelli A., Elia G., Martella V., Palmieri A., Cirone F., Tinelli A., et al. Prevalence of canine coronavirus antibodies by an enzyme-linked immunosorbent assay in dogs in the south of Italy. J Virol Methods. 2002;102:67–71. doi: 10.1016/S0166-0934(01)00450-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wesley R.D. The S gene of canine coronavirus, strain UCD-1, is more closely related to the S gene of transmissible gastroenteritis virus than to that of feline infectious peritonitis virus. Virus Res. 1999;61:145–152. doi: 10.1016/S0168-1702(99)00032-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pratelli A., Tinelli A., Decaro N., Cirone F., Elia G., Roperto S., et al. Efficacy of an inactivated canine coronavirus vaccine in pups. New Microbiol. 2003;26:151–155. [PubMed] [Google Scholar]
- 27.Pratelli A., Tinelli A., Decaro N., Martella V., Camero M., Tempesta M., et al. Safety and efficacy of a modified-live canine coronavirus vaccine in dogs. Vet Microbiol. 2004;99:43–49. doi: 10.1016/j.vetmic.2003.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.El Sahly H MF59™ as a vaccine adjuvant: a review of safety and immunogenicity. Expert Rev Vaccines. 2010;9:1135–1141. doi: 10.1586/erv.10.111. [DOI] [PubMed] [Google Scholar]
- 29.Ott G., Barchfeld G.L., Chernoff D., Radhakrishnan R., van Hoogevest P., Van Nest G. MF59. Design and evaluation of a safe and potent adjuvant for human vaccines. Pharm Biotechnol. 1995;6:277–296. doi: 10.1007/978-1-4615-1823-5_10. [DOI] [PubMed] [Google Scholar]
- 30.Day M.J., Horzinek M.C., Schultz R.D., Vaccination Guidelines Group (VGG) of the World Small Animal Veterinary Association (WSAVA) Guidelines for the vaccination of dogs and cats. Compiled by the Vaccination Guidelines Group (VGG) of the World Small Animal Veterinary Association (WSAVA) J Small Anim Pract. 2007;48:528–541. doi: 10.1111/j.1748-5827.2007.00462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


