Abstract
A recombinant Lactobacillus casei expressing a flagellar antigen from Salmonella enterica serovar Enteritidis was constructed and evaluated as a mucosal vaccine. Intragastric immunization of the recombinant strain conferred protective immunity against Salmonella infection in mice. This immunization did not result in antigen-specific antibody in either feces or sera but induced the release of IFN-γ on restimulation of primed lymphocytes ex vivo. The results suggested that the protective efficacy provided by flagellin-expressing L. casei is mainly attributable to cell-mediated immune responses. In addition, an adjuvant-type effect of the antigen delivery system with L. casei was also observed.
Keywords: Vaccine, Lactobacillus, Salmonella, Flagellin
1. Introduction
Progress in pathogenic bacteriology and genetic engineering has accelerated the development of recombinant oral vaccines composed of protective antigens and antigen delivery vehicles. There are a number of reports of oral vaccine candidates established from genetically modified pathogenic bacteria such as Salmonella and Listeria [1], [2], [3], [4], or commensal bacteria such as Lactococcus lactis and Lactobacillus species. While both pathogenic and commensal bacteria have advantages and disadvantages as an antigen delivery vehicle, lactic acid bacteria (LAB) may be preferable in terms of safety control and minimization of side effects. A series of pioneering studies about LAB expressing tetanus toxin fragment C (TTFC) achieved a highly effective vaccination against tetanus toxin [5], [6], [7]. In addition, there are other studies about LAB-associated model vaccines such as L. lactis expressing the V2-V4 loop from HIV [8], SpaA from Erysipelothrix rhusiopathiae [9], VP7 from rotavirus [10], Lactobacillus casei expressing the N-terminal glycoprotein S from coronavirus [11], the Spike protein segment from severe acute respiratory syndrome (SARS)-associated coronavirus [12], and Lactobacillus plantarum expressing Urease B subunit from Helicobacter pylori [13]. This field of study, however, still needs further research in order to establish practical LAB-associated vaccines. Information about cellular immune responses induced by LAB expressing antigens is especially required because most previous studies concluded that the protective efficacy was due to the production of a neutralizing antibody. There are several lines of evidence that LAB strains promote the production of Th1-type cytokines such as gamma interferon (IFN-γ) and interleukin 12 (IL-12) [14], [15], [16], [17], which suggests that LAB strains have the potential to be an antigen delivery vehicle eliciting cellular immune responses.
In the present study, we generated a L. casei expressing FliC which is the flagellar antigen of Salmonella enterica serovar Enteritidis (SE). SE is a pathogen contaminating eggs and causing diarrhea or severe illness. Since SE is a facultative intracellular bacterium, cell-mediated immune responses are required for its clearance. In fact, a FliC-specific Th1-type response was induced during infections of Salmonella [18], [19]. Thus, recombinant LAB expressing FliC seems to be well suited for investigations into whether LAB-associated vaccines can be used to evoke cellular immune responses. Moreover, the combination of FliC and L. casei is appropriate to evaluate the adjuvant effect of L. casei as an antigen delivery vehicle because protective immunity induced by the oral vaccination of purified flagellin of SE has been already reported [20]. We used C3H/HeJ mice, a lipopolysaccharide (LPS)-hyporesponsive strain, to preclude the effect of LPS and test the FliC-expressing L. casei by oral administration.
2. Materials and methods
2.1. Bacterial strains and growth conditions
A plasmid-free strain of L. casei ATCC 393 was grown in MRS broth (Oxoid) or Lactobacillus-carrying medium (LCM) supplemented with 1% mannitol. Erythromycin was used at a concentration of 5 μg/ml. A human clinical isolate of S. enterica serovar Enteritidis (SE) #40 [21] was cultured in Luria-Bertani (LB) broth (Difco). For the cloning of plasmids, Escherichia coli JM109 was used in this study and grown in LB medium containing 100 μl/ml of ampicillin.
2.2. Purification of flagellin
Flagellin was isolated from SE #40 based on the method of Ibrahim et al. [22]. Briefly, an overnight culture of SE #40 was centrifuged and cells were resuspended in saline. The pH was then adjusted to 2 with 1 M HCl and the bacteria stored with constant stirring at room temperature. After the bacterial cells were removed by centrifugation, the supernatant was neutralized at pH 7.2 with 1 M NaOH. Dissolved protein was precipitated by addition of ammonium sulfate until a saturated concentration was obtained. The protein suspension was centrifuged and the pellet was redissolved in distilled water. Dialysis against PBS was then carried out. Purity was verified by sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE, 10%) followed by Coomassie brilliant blue staining. The protein concentration was determined by protein assay (BIO-RAD) and the protein solution was stored at −20 °C until used.
2.3. Plasmids and transformation
As the expression vector for cell-surface anchoring of the heterologous antigen, the plasmid pLP401∷FliC was established from pLP401 according to the instructions of Pouwels et al. [23]. DNA fragments of the fliC gene were amplified from SE #40 chromosomal DNA by PCR with a forward primer (IGM200; 5′-GAAAAGGATCCGGCACAAGTCATTAATACAAACAGCCT-3′) and a reverse primer (IGM201; 5′-TCGCCGTCGACACGCAGTAAAGAGAGGACGTT-3′). The PCR fragments were digested with BamHI and SalI, and inserted into the BamHI-XhoI sites of pLP401. The ligated plasmid was then cloned in E. coli JM109. In order to convert it into a mature plasmid, the newly constructed plasmid was treated with NotI and self-ligated before the transformation of lactobacilli. The preparation of competent cells and electroporation of L. casei were carried out according to the method of Pouwels et al. [23]. For the construction of a recombinant L. casei which does not produce FliC as a control strain, a expression-cassette-deleted plasmid, pLPEmpty, was constructed and introduced by electroporation into L. casei.
2.4. Western blotting and flow cytometry
Transformed bacteria were grown overnight in LCM containing 5 μg/ml of erythromycin. Bacterial cells were collected, washed twice with PBS, and disrupted in SDS-PAGE sample buffer by a beads beater (Fast Prep, BIO 101). The cellular debris was removed by centrifugation. Proteins were separated by SDS-PAGE and transferred onto PVDF (Immobilon™-P, Millipore) by electroblotting. The presence of the FliC protein was detected using anti-FliC rabbit antibody and Alexa Fluor™ 488 goat anti-rabbit IgG (Molecular Probes) in PBS supplemented with 1% BSA and 0.05% Tween-20. FliC-specific bands were then visualized with Molecular Imager FX and analyzed with Quantity One (BIO-RAD).
Intact bacterial cells were incubated with anti-FliC rabbit antibody and Alexa Fluor™ 488 goat anti-rabbit IgG in PBS supplemented with 1% BSA and 0.05% Tween-20. The labeled bacterial cells were then analyzed using FACSCalibur and CELLQuest (BD).
2.5. Immunization of mice
Female C3H/HeJ mice (Japan SLC), 8 weeks old, were immunized intragastrically (i.g.) with recombinant strains of L. casei, the purified flagellin solution, or PBS alone. The bacterial cell suspensions for administration were prepared as follows. Bacteria cultured overnight were collected by centrifugation, washed twice with PBS, and resuspended in PBS. The bacterial cell concentrations were adjusted to 5 × 1010 cfu/ml (200 μl/mouse). Three consecutive daily doses were given to the mice three times (week 0, 3, and 6). The care and use of experimental animals complied with local animal welfare laws and guidelines.
2.6. Challenge
Beforehand, several groups of naïve C3H/HeJ mice were inoculated i.g. with different amounts of the SE #40 suspension, and the concentration of bacteria giving a clear infection was selected for subsequent challenges.
Immunized mice werechallenged i.g. with SE #40 (1 × 109 cfu/mouse) 3 weeks after the last booster. The mice received 200 μl of the SE #40 suspension after fasting overnight. Spleens were removed 6 days after the challenge and homogenized in 2 ml of sterile PBS containing 0.5% Triton X-100. The suspensions were properly diluted and spread onto LB plates. The plate cultures were incubated at 37 °C overnight, and the cfu was calculated.
2.7. Sampling
Bloods and fecal samples were collected at 2 weeks after the last boost. Sera were prepared from the blood samples by centrifugation and stored at −20 °C. The feces were suspended in PBS supplemented with 1% BSA, 0.05% Tween 20 and 0.01% NaN3 (1/2 dilution), and cleared extracts were collected by centrifugation and stored at −20 °C.
2.8. Enzyme-linked immunosorbent assay (ELISA)
For the titrarion of IgG and IgA, a standard ELISA whose protocol has been described elsewhere was performed. In short, 96-well microplates were coated with 5 μg/ml of flagellin, blocked with 1% BSA, and incubated with serially diluted serum. Antigen-specific antibodies were conjugated with alkaline phosphatase (AP)-labeled anti-mouse IgG (SIGMA), and 4-nitrophenylphosphate (SIGMA) was used for color development. The absorbance was read after 1 h at 405 nm with an EL800 microplate reader (BIO-TEK Instruments). Endpoint titers were defined as the highest dilution that gave an absorbance 0.1 higher than the background.
A sandwich-type ELISA, OptEIA™ mouse IFN-γ ELISA Set (BD Bioscience), was used for detection of IFN-γ in the culture supernatant. The concentration of cytokine was determined according to the manufacturer's directions. The cytokine in the properly diluted samples was captured by anti-mouse IFN-γ IgG and conjugated with HRP-labeled anti-mouse IFN-γ IgG. The ELISA plates were incubated with the HRP-substrate solution kit (BIO-RAD), and the reaction was stopped by adding 2N H2SO4. The absorbance was read at 450 nm with a microplate reader. Concentrations of IFN-γ were calculated by using a standard curve.
2.9. Ex vivo antigen-restimulation of spleen cells and MLN cells
Single cell suspensions from spleens were prepared as follows. The spleen was removed from the immunized mouse, and cells were squeezed out in RPMI-1640 medium. The cells were washed with PBS and then incubated on ice in a sterilized 0.16 M NH4Cl solution. After two washes with RPMI-1640 medium, the cells were resuspended in culture medium (RPMI-1640 including 10% fetal calf serum and penicillin/streptomycin) and enumerated. Two hundred microliters of the cell suspension was dispensed into a 96-well microplate (5 × 105 cells/well) and supplemented with flagellin (10 μg/ml), heat-inactivated L. casei (1 × 107 cfu/ml), concanavalin A (10 μg/ml), or PBS.
The mesenteric lymph nodes (LMNs) were also removed, and a cell suspension was prepared through a cell strainer (BD Falcon). The cells were washed twice with RPMI-1640 medium, resuspended in culture medium, and seeded in a 96-well microplate (1 × 105 cells/well) and supplemented with each stimulants.
Both cultures were incubated at 37 °C in a CO2 incubator. After 72 h incubation, each culture was collected, and a clear supernatant was prepared by centrifugation. The culture supernatants were then stored at −80 °C until the analysis.
2.10. Intracellular cytokine staining
CD4+ cells containing IFN-γ were detected using the protocol recommended by the manufacturer (Cytofix/CytoPerm Plus kit, BD PharMingen). In short, cells were isolated form mouse spleen 2 weeks after the final immunization. A single cell suspension was incubated with 10 μg/ml of flagellin for 24 h at 37 °C, and 1 μg/ml of GolgiPlug was added 4 h before the end of the incubation. The cells were washed with staining buffer (1% FCS, 0.09% NaN3 in PBS), blocked with 10% normal mouse serum, and stained with phycoerythrin (PE)-conjugated anti-mouse CD4 antibody (BD PharMingen). The cells were then treated with Cytoperm solution, washed, stained with FITC-conjugated anti-mouse IFN-γ antibody (BD PharMingen), and subjected to flow cytometric analysis.
2.11. Statistical analysis
Statistical significance was evaluated with Tukey's multiple comparison test and defined as a P-value less than 0.05.
3. Results
3.1. FliC expression by recombinant L. casei
pLP401∷FliC and pLPEmpty were constructed and introduced by electroporation into L. casei. The transformants (LCF, LCN) carrying those plasmids were then analyzed by Western blotting. A specific band corresponding in size to FliC plus the anchor residues (approximately 68 kDa) was detected by anti-FliC antibody in the cell extract of LCF but not LCN (Fig. 1a). The amount of recombinant FliC produced by LCF was also estimated by analyzing the band's density with Quantity One (data not shown). For the gross weight, approximately 12.5–25 ng of FliC was expressed by 5 × 107 cfu of LCF. The surface-associated FliC on the bacterial cell was detected by flow cytometry. As shown in Fig. 1b, LCF showed FliC-specific positive signals from FliC. These results indicated that L. casei carrying pLP401∷FliC produced the SE flagellin and displayed it on the cell surface.
Fig. 1.
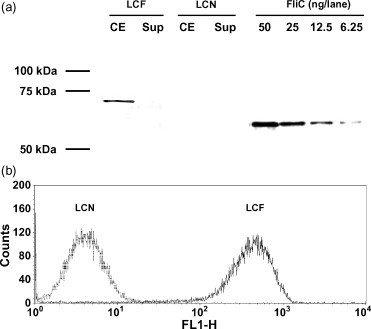
Detection of FliC production and surface-display by L. casei transformants. (a) Immunoblotting of total cell extract (CE) and culture supernatant (Sup) of L. casei carrying pLP401∷FliC (LCF) and pLPEmpty (LCN). An amount equivalent to 5 × 107 cfu of bacteria was loaded into each lane, and 50, 25, 12.5, and 6.25 ng of purified FliC were included as a standard. The sizes of the molecular mass markers are shown in the left margin. (b) Flow cytometric analysis of the L. casei transformants. Ten thousand particles were analyzed and fluorescence levels (FL1-H) from bacterial cells are shown in histogram form.
3.2. Protective immunity using LCF
Mice were immunized with LCF and challenged i.g. with SE #40. As shown in Fig. 2 , immunization with LCF resulted in a significant level of protective immunity. Since immunization with LCN did not confer any protection, the effect of LCF was specific for the antigen. The SE #40 count (cfu) in the spleen from LCF-immunized mice was about 10−1 less than that from LCN-immunized or non-immunized mice. As reported [20], a high dose of purified flagellin also induced protective immunity against SE-infection, and there was no significant difference in the level of protection between immunization with LCF and with free flagellin although the amount of FliC carried by LCF was at least 1/10 less than free flagellin.
Fig. 2.
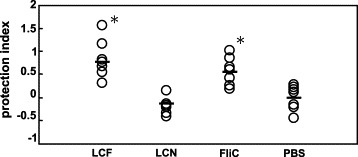
Protective efficacy conferred by i.g. immunization of FliC-expressing L. casei. The level of protection was compared to that provided by immunization with the same amount (1010 cfu) of LCN, 50 μg of purified FliC, and PBS. A protection index was calculated as (mean value of log 10 SE cfu from non-immunized (PBS) group) − (log10 SE cfu in each individual). Bars represent the mean value of each group (n = 7). *P < 0.05 (vs. LCN or PBS).
3.3. FliC-specific antibody production
FliC-specific IgG in serum and the specific IgA in feces were detected by ELISA. The group immunized with a high dose (50 μg) of flagellin showed relatively high levels of FliC-specific IgG production in sera while the other groups exhibited little specific IgG production (Fig. 3 ). No FliC-specific IgA was detected in the feces from immunized or non-immunized mice except for very low levels in the two mice immunized with the highest dose of flagellin (data not shown). These results might suggest that a large amount of flagellin was required to induce production of the specific antibody.
Fig. 3.
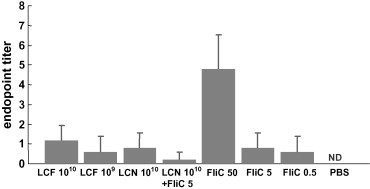
Detection of FliC-specific serum IgG by ELISA (n = 6). Antibody titers are given as − log2 (dilution × 10) + standard deviation (S.D.). Clear signals from the non-immunized group were not detected (ND). The types of immunization are shown in the bottom margin. 1010:1010 cfu, 109:109 cfu, 50:50 μg, 5:5 μg, 0.5:0.5 μg.
3.4. IFN-γ production by splenic cells and MLN cells
Splenic cells and MLN cells were isolated from immunized mice and stimulated with flagellin. The amount of IFN-γ released into the culture supernatant was measured (Fig. 4a). High levels of IFN-γ were detected in the splenic cells isolated from mice immunized with LCF or purified flagellin, and a dose-dependent increase in the release of the cytokine was also observed. Immunization with 1010 cfu of LCF induced more IFN-γ production than 1010 cfu of LC plus 5 μg of flagellin or 5 μg of flagellin alone despite that these three doses contained almost the same amount of flagellin. This result indicates that the anchoring of the antigen to the cell surface enhances the efficiency of the immunization. A low level of IFN-γ was detected even in the stimulated culture of cells from non-immunized mice. This reaction might be generated through non-specific responses such as innate immune responses involving Toll-like receptor (TLR) 5. Similar results were obtained with MLN cells although individual differences were relatively high (data not shown).
Fig. 4.
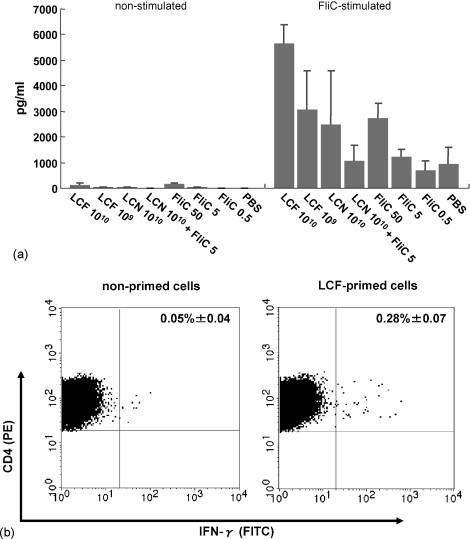
(a) Amount of IFN-γ released from primed cells by ex vivo FliC-restimulation (n = 3). Spleen cells from immunized mice were incubated with or without FliC and the amount of IFN-γ released was measured by ELISA. Values are given as means + S.D. (b) Intracellular cytokine (IFN-γ) staining of primed cells (n = 3). Ten thousand events were acquired and the percentage of CD4+ cells containing IFN-γ was analyzed. The figures represent one mouse of each group, and the values are given as the mean ± S.D.
The CD4+ lymphocytes producing IFN-γ, which is a typical phenotype of Th1, were detected by intracellular cytokine staining (Fig. 4b). The splenic cells primed with 1010 cfu of LCF were restimulated with flagellin, prepared and stained properly, and analyzed by flow cytometry. The number of IFN-γ-producing FliC-specific CD4+ cells was greater in LCF-immunized mice than in the controls. These results suggested that Th1-type immune responses were induced by the immunization of mice with recombinant L. casei expressing FliC.
4. Discussion
This study demonstrates that the oral administration of recombinant L. casei producing FliC on the cell surface can confer protective immunity against SE infection. Although the level of protection provided by 1010 cfu of recombinant L. casei was equivalent to that provided by 50 μg of purified flagellin, these two kinds of immunization induced different types of immunity. In particular, immunization with the soluble antigen mainly elicited humoral immune responses, and recombinant L. casei provided cellular immunity instead.
The presence of FliC-specific antibody, especially s-IgA in the gastrointestinal tract, would be beneficial for host defense if such antibodies interfered with the motility of SE at the intestinal mucosa. A high dose (50 μg) of purified flagellin induced humoral immune responses as already reported [20], but lower (5 and 0.5 μg) doses did not elicit specific antibody production. This result suggests that a large amount of soluble flagellin has to be administered to induce humoral immune responses. In this concept, a possible explanation of why FliC-expressing L. casei lacks the ability to induce antigen-specific antibody production is the absence of the antigen because 1 × 1010 cfu of the recombinant strain constructed in the present study expresses less than 5 μg of FliC.
Nevertheless, immunization with L. casei expressing FliC was still effective for protection against SE-infection despite a lack of anti-FliC antibody. This phenomenon indicates that other immune systems such as cell-mediated immunity were involved. In fact, the Th1-type immune response, associated with cellular immunity, is known to be an important component of the protection against Salmonella. Regarding this point, we determined whether the lymphocytes primed with FliC-expressing lactobacilli had the ability to induce IFN-γ production as a benchmark of Th1-type immune responses. A high level of IFN-γ production was elicited from the primed lymphocytes by stimulation with flagellin ex vivo. Moreover, the ability was much higher than that of soluble flagellin even though the amount of antigen for the priming was about 10-fold lower. CD4+ cells producing IFN-γ in response to the stimulation were also detected by flow cytometry. These results suggest that FliC-specific cellular immunity can be induced by immunization with L. casei producing FliC and be enhanced in combination with the bacterial cells. The production level of the cytokine correlated with the efficacy of protective immunity conferred by the recombinant L. casei, hence this immune response probably contributes to the protection against SE.
In this study, we also evaluated the adjuvant effect of the antigen delivery vehicle. The levels of IFN-γ produced by primed and flagellin-restimulated lymphocytes were compared between three forms of immunizing agent, i.e. recombinant L. casei exposing FliC on the cell surface, a mixture of purified flagellin and normal L. casei, and purified flagellin alone. The result, that immunization with recombinant lactobacilli elicits IFN-γ production more efficiently than that with the free-form of flagellin with or without normal L. casei, indicates that this Lactobacillus strain functions as an adjuvant if FliC is exposed on the cell-surface. Every Lactobacillus strain has several components which elicit innate immune responses through molecular pattern-recognition receptors of mammalian cells such as peptidoglycan, lipoteichoic acids (LTA), and bacterial olygodeoxynucleotides (ODNs) [24], [25], [26], hence these bacteria may be a potential adjuvant for vaccination. In fact, there is evidence that orally administered L. casei strains show adjuvant effects [27], [28]. In the present study, however, simple mixing of the bacterial cells with the antigen was not enough to bring about adjuvanticity. This result suggests that the location of the antigen is critical to the adjuvant effect of the antigen delivery vehicle. However, there is evidence that flagellin conjugated to polyacryl starch microparticles was less immunogenic than the free form of flagellin [20], which indicates that the location of the antigen is not the single factor increasing immunogenicity. Therefore, the adjuvant effect of the recombinant L. casei expressing FliC is probably due to a combination of the immune-stimulating activity of the bacterial cell components and the antigen's physical location.
Several Lactobacillus strains have been applied so far for vaccine delivery. Shaw et al. and Grangette et al. reported that L. plantarum is a better agent for vaccination with TTFC than L. casei or L. lactis, and an intracellular antigen is more effective than cell-surface expression [5], [29]. In this context, we constructed L. plantarum NCIMB 8826 expressing FliC on the cell surface and L. casei producing FliC intracellularly to compare to the strain in this study. Contrary to our expectation, neither provided clear protection against SE. The recombinant L. plantarum produced a higher amount of FliC than L. casei, but the frequency of the cell-surface antigen was lower than L. casei (unpublished data). The location of FliC is a critical factor to confer cellular immunity against SE, as mentioned above, which may be why these two recombinant strains failed to provide protection. Moreover, there is a possibility that L. casei is a preferable agent for the induction of cellular immunity.
Oral vaccines derived from lactic acid bacteria are potentially safe and cost-effective. However, there have been few successful studies on the oral immunization of LAB-based vaccines, and immune responses other than antibody production were poorly determined. The current study provides evidence that FliC-expressing L. casei elicits antibody-independent protective immunity.
Acknowledgement
This work was supported by a grant from the Ministry of Health, Labour and Welfare (Research on Food Safety).
References
- 1.Anderson R., Dougan G., Roberts M. Delivery of the Pertactin/P.69 polypeptide of Bordetella pertussis using an attenuated Salmonella typhimurium vaccine strain: expression levels and immune responses. Vaccine. 1996;14:1384–1390. doi: 10.1016/s0264-410x(96)00036-9. [DOI] [PubMed] [Google Scholar]
- 2.Ascon M.A., Hone D.M., Walters N., Pascual D.W. Oral immunization with a Salmonella typhimurium vaccine vector expressing recombinant enterotoxigenic Escherichia coli K99 fimbriae elicits elevated antibody titers for protective immunity. Infect Immun. 1998;66:5470–5476. doi: 10.1128/iai.66.11.5470-5476.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kohler J.J., Pathangey L., Hasona A., Progulske-Fox A., Brown T.A. Long-term immunological memory induced by recombinant oral Salmonella vaccine vectors. Infect Immun. 2000;68:4370–4373. doi: 10.1128/iai.68.7.4370-4373.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peters C., Peng X., Douven D., Pan Z.K., Paterson Y. The induction of HIV Gag-specific CD8+ T cells in the spleen and gut-associated lymphoid tissue by parenteral or mucosal immunization with recombinant Listeria monocytogenes HIV Gag. J Immunol. 2003;170:5176–5187. doi: 10.4049/jimmunol.170.10.5176. [DOI] [PubMed] [Google Scholar]
- 5.Shaw D.M., Gaerthe B., Leer R.J., van der Stap J.G.M.M., Smittenaar C., Heijne den Bak-Glashouwer M.J. Engineering the microflora to vaccinate the mucosa: serum immunoglobulin G responses and activated draining cervical lymph nodes following mucosal application of tetanus toxin fragment C-expressing lactobacilli. Immunology. 2000;100:510–518. doi: 10.1046/j.1365-2567.2000.00069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reveneau N., Geoffroy M.C., Locht C., Chagnaud P., Mercenier A. Comparison of the immune responses induced by local immunizations with recombinant Lactobacillus plantarum producing tetanus toxin fragment C in different cellular locations. Vaccine. 2002;20:1769–1777. doi: 10.1016/s0264-410x(02)00027-0. [DOI] [PubMed] [Google Scholar]
- 7.Grangette C., Müller-Alouf H., Goudercourt D., Geoffroy M., Turneer M., Mercenier A. Enhanced mucosal delivery of antigen with cell wall mutants of lactic acid bacteria. Infect Immun. 2004;72:2731–2737. doi: 10.1128/IAI.72.5.2731-2737.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xin K., Hoshino Y., Toda Y., Igimi S., Kojima Y., Jounai N. Immunogenicity and protective efficacy of orally administered recombinant Lactococcus lactis expressing surface-bound HIV Env. Blood. 2003;102:223–228. doi: 10.1182/blood-2003-01-0110. [DOI] [PubMed] [Google Scholar]
- 9.Cheun H.I., Kawamoto K., Hiramatsu M., Tamaoki H., Shirahata T., Igimi S. Protective immunity of SpaA-antigen producing Lactococcus lactis against Erysipelothrix rhusiopathiae infection. J Appl Microbiol. 2004;96:1347–1353. doi: 10.1111/j.1365-2672.2004.02283.x. [DOI] [PubMed] [Google Scholar]
- 10.Perez C.A., Eichwald C., Burrone O., Mendoza D. Rotavirus vp7 antigen produced by Lactococcus lactis induces neutralizing antibodies in mice. J Appl Microbiol. 2005;99:1158–1164. doi: 10.1111/j.1365-2672.2005.02709.x. [DOI] [PubMed] [Google Scholar]
- 11.Ho P.S., Kwang J., Lee Y.K. Intragastric administration of Lactobacillus casei expressing transmissible gastroenteritis coronavirus spike glycoprotein induced specific antibody production. Vaccine. 2005;23:1335–1342. doi: 10.1016/j.vaccine.2004.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee J., Poo H., Han D.P., Hong S.P., Kim K., Cho M.W. Mucosal immunization with surface-displayed severe acute respiratory syndrome coronavirus spike protein on Lactobacillus casei induces neutralizing antibodies in mice. J Virol. 2006;80:4079–4087. doi: 10.1128/JVI.80.8.4079-4087.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Corthesy B., Boris S., Lsler P., Grangette C., Mercenier A. Oral immunization of mice with lactic acid bacteria producing Helicobacter pylori Urease B subunit partially protects against challenge with Helicobacter felis. J Infect Dis. 2005;192:1141–1149. doi: 10.1086/444425. [DOI] [PubMed] [Google Scholar]
- 14.Hessle C., Hanson L.A., Wold A.E. Lactobacilli from human gastrointestinal mucosa are strong stimulators of IL-12 production. Clin Exp Immunol. 1999;116:276–282. doi: 10.1046/j.1365-2249.1999.00885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hessle C., Andersson B., Wold A.E. Gram-positive bacteria are potent inducers of monocytic interleukin-12 (IL-12) wile Gram-negative bacteria preferentially stimulate IL-10 production. Infect Immun. 2000;68:3581–3586. doi: 10.1128/iai.68.6.3581-3586.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haller D., Blum S., Bode C., Hammes W.P., Schiffrin E.J. Activation of human peripheral blood mononuclear cell by nonpathogenic bacteria in vitro: evidence of NK cells as primary targets. Infect Immun. 2000;68:752–759. doi: 10.1128/iai.68.2.752-759.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mohamadzadeh M., Olson S., Kalina W.V., Ruthel G., Demmin G.L., Warfield K.L. Lactobacilli activate human dendritic cells that skew T cells toward T helper 1 polarization. Proc Natl Acad Sci. 2005;102:2880–2885. doi: 10.1073/pnas.0500098102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McSorley S.J., Cookson B.T., Jenkins M.K. Characterization of CD4+ T cell responses during natural infection with Salmonella typhimurium. J Immunol. 2000;164:986–993. doi: 10.4049/jimmunol.164.2.986. [DOI] [PubMed] [Google Scholar]
- 19.Cunningham A.F., Khan M., Ball J., Toellner K.M., Serre K., Mohr E. Responses to the soluble flagellar protein FliC are Th2, while those to FliC on Salmonella are Th1. Eur J Immunol. 2004;34:2986–2995. doi: 10.1002/eji.200425403. [DOI] [PubMed] [Google Scholar]
- 20.Strindelius L., Filler M., Sjöholm I. Mucosal immunization with purified flagellin from Salmonella induces systemic and mucosal immune responses in C3H/HeJ mice. Vaccine. 2004;22:3797–3808. doi: 10.1016/j.vaccine.2003.12.035. [DOI] [PubMed] [Google Scholar]
- 21.Amano F., Karahashi H., Ishii Y., Igimi S. Different susceptibility between Salmonella Enteritidis in logarithmic and stationary phases to an anti-Salmonella neutralizing antibody that blocks binding and infection to a human colon epithelial cell line, T-84. Bacterial Adherence Biofilm. 2000;14:71–75. [Google Scholar]
- 22.Ibrahim G.F., Fleet G.H., Lyons M.J., Walker R.A. Method for the isolation of highly purified Salmonella flagellins. J Clin Microbiol. 1985;22:1040–1044. doi: 10.1128/jcm.22.6.1040-1044.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pouwels P.H., Vriesema A., Martinez B., Tielen F.J., Seegers J.F.M.L., Leer R.J. Lactobacilli as vehicles for targeting antigens to mucosal tissues by surface exposition of foreign antigens. Methods Enzymol. 2001;336:369–389. doi: 10.1016/s0076-6879(01)36602-8. [DOI] [PubMed] [Google Scholar]
- 24.Kim Y.G., Ohta T., Takahashi T., Kushiro A., Nomoto K., Yokokura T. Probiotic Lactobacillus casei activates innate immunity via NF-kappaB and p38 MAP kinase signaling pathways. Microbes Infect. 2006;8:994–1005. doi: 10.1016/j.micinf.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 25.Matsuguchi T., Takagi A., Matsuzaki T., Nagaoka M., Ishikawa K., Yokokura T. Lipoteichoic acids from Lactobacillus strains elicit strong tumor necrosis factor alpha-inducing activities in macrophages through Toll-like receptor 2. Clin Diagn Lab Immunol. 2003;10:259–266. doi: 10.1128/CDLI.10.2.259-266.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shimosato T., Kitazawa H., Katoh S., Tohno M., Iliev I.D., Nagasawa C. Augmentation of T(H)-1 type response by immunoactive AT oligonucleotide from lactic acid bacteria via Toll-like receptor 9 signaling. Biochem Biophys Res Commun. 2005;326:782–787. doi: 10.1016/j.bbrc.2004.11.119. [DOI] [PubMed] [Google Scholar]
- 27.Pouwels P.H., Leer R.J., Boersma W.J.A. The potential of Lactobacillus as a carrier for oral immunization: development and preliminary characterization of vector systems for targeted delivery of antigens. J Biotechnol. 1996;44:183–192. doi: 10.1016/0168-1656(95)00140-9. [DOI] [PubMed] [Google Scholar]
- 28.de Waard R., Garssen J., Snel J., Bokken G.C., Sako T., Veld J.H. Enhanced antigen-specific delayed-type hypersensitivity and immunoglobulin G2b responses after oral administration of viable Lactobacillus casei YIT9029 in Wistar and Brown Norway rats. Clin Diagn Lab Immunol. 2001;8:762–767. doi: 10.1128/CDLI.8.4.762-767.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grangette C., Müller-Alouf H., Geoffroy M., Goudercourt D., Turneer M., Mercenier A. Protection against tetanus toxin after intragastric administration of two recombinant lactic acid bacteria: impact of strain viability and in vivo persistence. Vaccine. 2002;20:3304–3309. doi: 10.1016/s0264-410x(02)00301-8. [DOI] [PubMed] [Google Scholar]


