Abstract
We have investigated to develop novel vaccines against SARS CoV using cDNA constructs encoding the structural antigen; spike protein (S), membrane protein (M), envelope protein (E), or nucleocapsid (N) protein, derived from SARS CoV. Mice vaccinated with SARS-N or -M DNA using pcDNA 3.1(+) plasmid vector showed T cell immune responses (CTL induction and proliferation) against N or M protein, respectively. CTL responses were also detected to SARS DNA-transfected type II alveolar epithelial cells (T7 cell clone), which are thought to be initial target cells for SARS virus infection in human. To determine whether these DNA vaccines could induce T cell immune responses in humans as well as in mice, SCID-PBL/hu mice was immunized with these DNA vaccines. As expected, virus-specific CTL responses and T cell proliferation were induced from human T cells. SARS-N and SARS-M DNA vaccines and SCID-PBL/hu mouse model will be important in the development of protective vaccines.
Keywords: SARS DNA vaccine, SCID-PBL/hu, Human CTL
1. Introduction
The causative agent of severe acute respiratory syndrome (SARS) has been identified as a new type of corona virus, SARS corona virus (SARS CoV) [1], [2], [3]. SARS has infected more than 8400 patients in about 7 months in over 30 countries and caused more than 800 deaths. The deadly epidemic has had significant impacts on many health, social, economic and political aspects. SARS is assumed to resurge in the near future. However, no SARS vaccine is currently available for clinical use. Therefore, we have developed novel vaccine candidates against SARS CoV using cDNA constructs encoding the structural antigens; S, M, E, or N protein. In immunized mice, neutralizing antibodies against the virus and T cell immunity against virus-infected-cells were studied, since these immunities play important roles in protection against many virus infections. In particular, CD8+ CTL plays an important role in T cell immunity dependent protection against virus infections and the eradication of murine and human cancers [4], [5]. In the present study, a type II alveolar epithelial cell clone, T7, was used for analyzing precise mechanism of CTL against SARS CoV membrane antigens, as the SARS-CoV infects alveolar epithelial cell in the lungs [6]. Furthermore, the SCID-PBL/hu model, which is capable of analyzing in vivo human immune response, was also used because it is a more relevant translational model for human cases [4].
2. Materials and methods
Three kinds of SARS CoV strains: HKU39849(1), TW-1 and FFM-1(2) and their cDNAs were used. S, M, N or E cDNA was transferred into pcDNA 3.1(+) vector and pcDNA 3.1(+)/vs-His Topo (QIAGEN K K, Tokyo, Japan). These genes were expressed in eukaryotic cells and Escherichia coli. pcDNA 3.1(+) vector, 50 μg each, containing SARS S, M, N, or E DNA was injected i.m. (M.tibia anterior) into C57BL/6 mice (female, 8 weeks CLEA Japan Inc, Japan) and BALB/c mice (female, 8 weeks) three times, at an interval of 7 days. Neutralizing antibodies against SARS CoV in the serum from the mice immunized with SARS S, M, N or -E DNA vaccines were assayed by use of Vero-E6 cell. CTL activity against SARS CoV was studied using human type II alveolar epithelial cells, T7, expressing SARS antigens [6]. PBL from healthy human volunteers were administered i.p. into IL-2 receptor γ-chain disrupted NOD SCID mice [IL-2R(−/−) NOD-SCID], and SCID-PBL/hu mice were constructed [4]. SARS DNA vaccines at 50 μg were injected i.m. into the SCID-PBL/hu mice. CTL activity of human CD8-positive lymphocytes in the spleen from SCID-PBL/hu was assessed using IFN-γ production and 51Cr-release assay [4], [5].
3. Results
3.1. Induction of CTL against SARS CoV by SARS (N) DNA and SARS (M) DNA vaccine
Spleen cells from C57BL/6 mice immunized with SARS-S, -M, -N or -E DNA vaccine were cultured with syngeneic T7 lung cells transfected with S, M, N or E cDNA. pcDNA 3.1(+) SARS (N) DNA vaccine induced significantly CTL activity (IFN-γ production) against N cDNA transfected T7 cells (Fig. 1A). Similarly, SARS M DNA vaccine induced SARS antigen M-specific CTL against T7 cells transfected with SARS M DNA (data not shown).
Fig. 1.
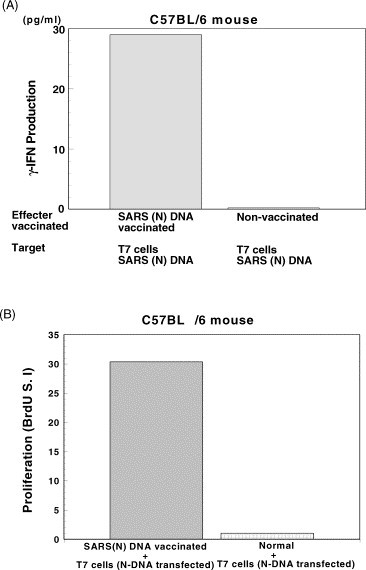
Induction of CTL and T cell proliferation against SARS (N). (A) Induction of CTL against SARS (N) antigen in the spleen cells from C57BL/6 mice immunized with SARS (N) DNA vaccine. SARS (N) DNA using pcDNA3.1(+) vector was injected i.m. into C57BL/6 mice three times, at an interval of 7 days. CTL activity was assessed by IFN-γ production in the culture of 1 × 106 spleen cells and 1 × 104 T7 lung alveolar type II epithelial cells transfected with SARS (N) DNA at the E/T ratio of 100:1. IFN-γ production was assessed by ELISA assay. (B) Augmentation of lymphocyte proliferation specific for SARS (N) DNA vaccine. 1 × 105 responder cells from vaccinated mice were cultured with Mitomycin C treated 1 × 104 T7 cells transfected with SARS (N) DNA for 48 h and then Bromodeoxy Uridine (BrdU) was added. Proliferative responses were assessed by BrdU assay.
3.2. Augmentation of lymphocyte proliferation specific for SARS CoV antigens by the immunization with SARS (M) DNA and SARS (N) DNA vaccine
The proliferation of splenic T cells stimulated by co-culture either with T7 cells transfected with M DNA or SARS M peptide (TW1 M102-116) was strongly augmented by M DNA vaccine (data not shown). SARS N DNA vaccine also induced proliferation of splenic T cells in the presence of recombinant N protein as well as N DNA-transfected T7 cells (Fig. 1B). Thus, both SARS N DNA vaccine and M DNA vaccine were shown to induce T cell immune responses against the relevant SARS CoV antigens.
3.3. SARS M DNA and N DNA vaccines induced human T cell immune responses (CTL and proliferation) in SCID-PBL/hu model
The M DNA vaccine enhanced the CTL activity and proliferation in the presence of M peptide in SCID-PBL/hu mice (Fig. 2 ). Furthermore, the SARS N DNA vaccine induced CTL activity (IFN-γ production by recombinant N protein or N protein pulsed-autologous B blast cells) and proliferation of spleen cells in SCID-PBL/hu mice (Fig. 3 ). From these results, it was demonstrated that SARS M DNA vaccine and N DNA vaccine induced human CTL and human T cell proliferative responses.
Fig. 2.
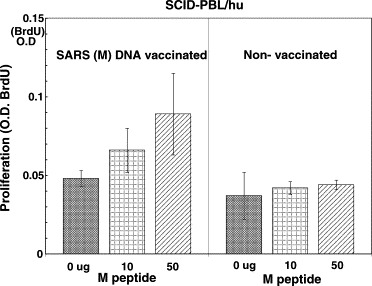
SARS (M) DNA vaccine induces in vivo human T cell proliferation against SARS CoV in the SCID-PBL/hu human immune systems. 4 × 107 PBL from healthy human volunteers were administered i.p. into IL-2 receptor γ-chain disrupted NOD SCID mice [IL-2R (−/−) NOD-SCID], and SCID-PBL/hu mice were constructed. Fifty micrograms of SARS DNA vaccine was injected i.m. into these SCID-PBL/hu mice. 1 × 105 spleen cells from these vaccinated mice were cultured with 10∼50 μg of SARS M peptide for 3 days. Proliferation was assayed by BrdU.
Fig. 3.
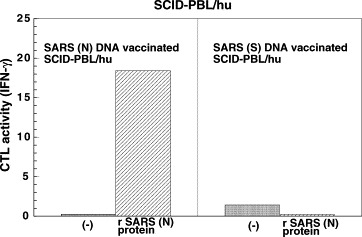
SARS (N) DNA vaccine induces in vivo human CTL against SARS CoV in the SCID-PBL/hu human immune systems. 4 × 107 PBL from healthy human volunteers were administered i.p. into IL-2 receptor γ-chain disrupted NOD SCID mice [IL-2R (−/−) NOD-SCID], and SCID-PBL/hu mice were constructed 50 μg of SARS (N) DNA vaccine or 50 μg of SARS (S) DNA vaccine. 1 × 105 spleen cells from SCID-PBL/hu were cultured with 10 μg of recombinant SARS (N) protein for 72 h. IFN-γ production in the culture supernatant was assayed using ELISA.
4. Discussion
We have demonstrated that SARS (M) DNA and (N) DNA vaccines induce virus-specific immune responses (CTL and T cell proliferation) in the mouse systems using type II lung alveolar T cell lines in clone target models [6]. These DNA vaccines induced SARS-CoV-specific CTL and T cell proliferation in vivo human immune systems using SCID-PBL/hu. Gao et al. developed adenovirus based a SARS DNA vaccine encoding S1 polypeptide was capable of inducing neutralizing antibody, while another SARS DNA vaccine encoding N protein generated IFN-γ producing T cells in rhesus monkeys [7]. SARS S DNA vaccine which elicits effective neutralizing antibody responses that generate protective immunity in a mouse model [8]. However its immunogenicity in humans has yet to be established. Therefore, it is very important to evaluate the efficacy of SARS DNA vaccine in a SCID-PBL/hu mice, which is a highly relevant translational model for demonstrating human immune responsiveness. Recently, SARS DNA vaccines capable of inducing human neutralizing antibodies against SARS CoV have been established by our SCID-PBL/hu model. It has been demonstrated that Angiotensin-converting enzyme 2 (ACE2) is a functional receptor for the SARS CoV [9]. A transgenic mouse with human ACE-2 may be useful as an animal model of SARS. Furthermore, ACE-2 transgenic SCID mice should be useful as a human model for pre-clinical trial for SARS vaccines, since ACE-transgenic SCID-PBL/hu model could analyze the human immune responses against SARS infection in vivo. The effect of combination immunization with such SARS vaccines and neutralizing antibody dependent DNA vaccine is now being studied. These DNA vaccines should provide a useful tool for development of protective vaccines.
Acknowledgements
This study was supported by Grant-in-Aid for the science and technology and Grant-in-Aid for Scientific Research on Priority Areas from the Ministry of Education Culture Sports, Science and Technology, Japan. This study was also supported by a Heath and Labour Science Research Grant from the Ministry of Health, Labour, and Welfare, Japan.
References
- 1.Peiris J.S., Lai S.T., Poon L.L., Guan Y., Yam L.Y., Lim W. SARS study group. Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet. 2003;361(9366):1319–1325. doi: 10.1016/S0140-6736(03)13077-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drosten C., Gunther S., Preiser W., van der Werf S., Brodt H.R., Becker S. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med. 2003;348(20):1967–1976. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- 3.Peiris J.S., Yuen K.Y., Osterhaus A.D., Stohr K. The severe acute respiratory syndrome. N Engl J Med. 2003;349(25):2431–2441. doi: 10.1056/NEJMra032498. [DOI] [PubMed] [Google Scholar]
- 4.Tanaka F., Abe M., Akiyoshi T., Nomura T., Sugimachi K., Kishimoto T. The anti-human tumor effect and generation of human cytotoxic T cells in SCID mice given human peripheral blood lymphocytes by the in vivo transfer of the Interleukin-6 gene using adenovirus vector. Cancer Res. 1997;57(7):1335–1343. [PubMed] [Google Scholar]
- 5.Okada M., Yoshimura N., Kaieda T., Yamamura Y., Kishimoto T. Establishment and characterization of human T hybrid cells secreting immunoregulatory molecules. Proc Natl Acad Sci USA. 1981;78(12):7717–7721. doi: 10.1073/pnas.78.12.7717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.deMello D.E., Mahmoud S., Padfield P.J., Hoffmann J.W. Generation of an immortal differentiated lung type-II epithelial cell line from the adult H-2K(b)tsA58 transgenic mouse. In Vitro Cell Dev Biol Anim. 2000;36(6):374–382. doi: 10.1290/1071-2690(2000)036<0374:GOAIDL>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gao W., Tamin A., Soloff A., D’Aiuto L., Nwanegbo E., Robbins P.D. Effects of a SARS-associated coronavirus vaccine in monkeys. Lancet. 2003;362(9399):1895–1896. doi: 10.1016/S0140-6736(03)14962-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang Z.Y., Kong W.P., Huang Y., Roberts A., Murphy B.R., Subbarao K. A DNA vaccine induces SARS coronavirus neutralization and protective immunity in mice. Nature. 2004;428(6982):561–564. doi: 10.1038/nature02463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li W., Moore M.J., Vasilieva N., Sui J., Wong S.K., Berne M.A. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426(6965):450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]


