Abstract
The frequency of moderate to severe adverse reactions associated with smallpox vaccines currently stockpiled in the US, and the continued threat of bioterrorism have prompted the development of effective vaccines with improved safety profiles. LC16m8, an attenuated, replicating smallpox vaccine derived from the Lister strain of vaccinia, is currently licensed in Japan where it was safely used in over 50,000 children in the 1970s. It has been shown to have markedly less neurotoxicity than unattenuated vaccines in nonclinical studies. LC16m8 is immunogenic after a single dose, and recent studies in two different animal models have demonstrated protective efficacy equivalent to that of the only FDA-licensed smallpox vaccine. This article reviews the history and available scientific literature regarding LC16m8 and provides comparisons to other smallpox vaccines.
Keywords: LC16m8, Lister, Smallpox, Vaccinia, Vaccine, Bioterrorism, Orthopoxvirus
1. Historical context of LC16m8 development
As efforts intensified internationally to eradicate smallpox in the 1960s, health officials became increasingly concerned about adverse reactions to vaccinia vaccines. Officials in countries with a low incidence of smallpox were particularly alarmed by the morbidity and mortality associated with postvaccination encephalitis and encephalopathy. While central nervous system (CNS) adverse reactions were generally considered idiosyncratic events, the incidence seemed to vary by strain of vaccinia. This observation was eventually substantiated when the Marennikova group of the Viral Pharmaceutical Institute in Russia published animal data that characterized over 20 commonly used strains of vaccinia and showed a correlation between the degree of pathogenicity and viral replication in CNS and other tissues [1], [2]. According to the 1969 Marennikova report, the Ikeda strain of vaccinia was classified as having high pathogenicity, whereas the Lister strain used by the World Health Organization (WHO) and the UK National Health Service (NHS) was rated as having medium pathogenicity, and the New York City Board of Health strain (NYCBH, also manufactured under the trade name Dryvax®) used in the US was rated as having low pathogenicity.
In response to a growing concern about vaccinia-related adverse events (AEs) in Japan, the Japanese Ministry of Health formed the Smallpox Vaccine Research Group (SVRG) in 1966 [3]. This organization, comprised of both clinical experts and basic scientists, was charged with providing and collecting detailed information on adverse reactions to vaccinia vaccines and advising the Japanese public health authorities.
At the time of the Marennikova report, the strain of vaccinia used for smallpox vaccination in Japan was Ikeda, and the rate of encephalitis in Japan was approximately 20 per million per year [4]. The highest risk of mortality was in primary vaccinees, particularly in infants. As one of its first tasks, the SVRG investigated the CNS toxicity of several strains of vaccinia in mice that were inoculated intracerebrally [5]. The results from the mouse studies confirmed the greater neurotoxicity of the Ikeda strain reported by Marennikova and spawned several clinical trials in children comparing Ikeda with Lister and EM-63, a strain used in Russia.
After collecting data from several thousand primary vaccinees, it was found that all of the studied vaccines induced similar take rates, antibody responses, and febrile reactions, but that Lister had the mildest local reactogenicity. While no direct conclusions could be made regarding the relative neurotoxicity of the studied vaccines, local reactogenicity was at that time regarded as an indicator of overall vaccinia toxicity, and was used to identify Lister as the safest vaccine strain in the trials [5].
In 1970, the SVRG recommended that routine smallpox vaccination in Japan be conducted using Lister instead of the Ikeda strain [2], [3], [5]. To further reduce vaccine-related morbidity and mortality, the SVRG recommended that vaccinations be initiated at 6–24 months of age rather than at 2–12 months, and that only 5–10 punctures be administered with a bifurcated needle [3], [5]. Despite these changes, deaths from vaccine-related encephalitis (Table 1 ) continued in Japan [4], [5].
Table 1.
Estimated occurrence of encephalitis and encephalopathy in Japan by year
| Year | Population slated for vaccination (% actually vaccinated)a | Encephalitis/encephalopathy (deaths) | Incidence per million (deaths) |
|---|---|---|---|
| 1965 | 1125572 (68.0) | 19 (8) | 16.9 (7.1) |
| 1966 | 1070219 (67.3) | 11 (3) | 10.2 (2.8) |
| 1967 | 975315 (60.6) | 17 (7) | 17.7 (7.3) |
| 1968 | 1162468 (70.5) | 16 (5) | 13.7 (4.3) |
| 1969 | 1189549 (64.7) | 15 (3) | 12.6 (2.5) |
| 1970 | 819174 (43.9) | 18 (11) | 21.9 (13.4) |
| 1971 | 937221 (50.3) | 36 (3) | 38.4 (3.2) |
| 1972 | 1151859 (61.2) | 24 (2) | 20.8 (1.7) |
| 1973 | 1355211 (70.9) | 28 (14) | 20.6 (10.3) |
Source: Yamaguchi et al. [4].
Number in parentheses indicates % performed, according to the Ministry of Health and Welfare.
Being at geographic risk of importing smallpox from India, the SVRG was reluctant to discontinue vaccinations altogether as other industrialized nations had done in the early 1970s [3], [5]. Therefore, a decision was made to search for a vaccine that reduced or eliminated CNS risks. The search began with a review of study data from two attenuated smallpox vaccines that had undergone considerable development in other nations.
The modified vaccinia Ankara (MVA) strain had been developed in Germany in the 1960s by passaging vaccinia Ankara in chick embryo explants (CEE) in excess of 516 times [6], [7], [8]. The resulting mutant was dramatically modified, having lost nearly 15% of the original genome [9] as well as the ability to replicate in many mammalian cells [10]. Although clearly not neurotoxic in nonclinical studies and nonreactive in both normal and dermatologically- or immune-compromised hosts, antibody response following single vaccination was poor [2], [11]. Since humoral immune response had become the leading criterion for determining vaccinia protective efficacy, further development of the MVA vaccine in Japan was abandoned.
Another attenuated strain, originally called the First Revived Strain and then later renamed CVI (CV-1) by Parker et al. [12], had been developed in the US in the early 1930s through repeated passage of the NYCBH strain in rabbit testes, CEE, and chorioallantoic membranes (CAM) [2], [13], [14]. The attenuated CVI-78 strain (CVI after 78 passages), developed by Kempe et al. at the Walter Reed Army Institute of Research in the late 1940s, was ultimately selected for development based on the mild dermatologic reactions it produced in rabbit and human studies [14]. Similar results were seen during numerous clinical studies where only mild local reactogenicity and fevers without serious adverse reactions were observed [3], [13], [14]. Additionally, CVI-78 appeared to offer a major advantage for dermatologically compromised individuals as Kempe et al. confirmed the safety of this vaccine in children with eczema [14]. Nevertheless, two critical findings dampened enthusiasm for the CVI-78 vaccine. First, the neurotoxicity of CVI-78 following intracerebral inoculation of monkeys was shown to exceed that of the unattenuated Ikeda, Lister, and NYCBH strains, suggesting a greater human risk for encephalitis with CVI-78 [15]. Second, it was found that the immune response to CVI-78, particularly the neutralizing antibody response, was low when compared to unattenuated Lister or Ikeda strains, calling into question the protective efficacy of this vaccine [2], [16], [17].
The SVRG ultimately decided to sponsor the development of an entirely new attenuated strain. Spearheaded by Dr. Hashizume of the Chiba Serum Institute in Japan, the latest technologies and understanding of vaccinia viruses were coupled to develop a variant of Lister that did not produce neurotoxicity in animal models, yet maintained neutralizing antibody titers comparable to Lister [2], [5], [18]. In addition, cell culture techniques were employed to avoid contamination problems inherent to animal lymph-derived vaccines such as Lister and NYCBH, which may have contributed to toxicity, and to allow for mass production under controlled conditions.
To produce the new strain, Lister was initially passaged in primary rabbit kidney (PRK) cells at low temperature (30 °C) [19]. At the 36th generation, 25 clones were selected and evaluated for their ability to grow in Vero cells. The clone with the lowest titer of growth, designated LC16 (Lister Clone 16), was isolated and evaluated for growth in rabbit skin and the CNS of rabbits and monkeys, where it compared favorably to Lister. A small clinical trial was then conducted, comparing LC16 to Lister in 34 children [19]. The vaccine was well tolerated and had a reduced fever rate compared to Lister. However, a delay in pock formation and resolution was observed in children vaccinated with the attenuated strain.
To lessen the chance of autoinoculation complications due to a prolonged pock reaction, a clone of LC16 that produced small pocks, a feature correlating with diminished growth capacity in mammalian tissues, was selected on CAM [19]. The virus was passaged six more times in PRK at low temperature followed by growth on CAM. Medium-sized (2–3 mm) pocks were isolated and designated LC16mO (Lister Clone 16 medium pock size on CAM original clone). Finally, the LC16mO clones were passaged three more times in PRK cells and grown on CAM where small (0.5–1 mm) pocks were selected. The final attenuated clone was called LC16m8 (clone 8).
Formal in vitro comparisons were subsequently carried out to evaluate the replicative capacity of LC16m8, LC16mO, LC16, Lister, and CVI-78 strains at 37, 40.5, and 41 °C [19]. Whereas all strains were able to grow at 37 °C, LC16m8 was unable to grow at temperatures above 40.5 °C, and LC16 and LC16mO clones were unable to grow at temperatures above 41 °C. In contrast, Lister and CVI-78 grew at all temperatures tested.
The neurotoxic effects of the parent Lister strain and the three attenuated strains (LC16, LC16mO, and LC16m8) were assessed by inoculating rabbits with these strains intracerebrally at a concentration of 106.7 TCID50 of each virus [19]. All of the rabbits inoculated with unattenuated Lister developed encephalitis, whereas none of the rabbits inoculated with the attenuated strains developed any adverse neurological symptoms. Homogenates of the brains of rabbits inoculated with LC16m8 had low levels of detectable virus, whereas brain homogenates of rabbits inoculated with comparators, most notably with the Lister strain, contained higher levels of virus.
A follow-up study was performed in cynomolgus monkeys [19]. In this study, 0.5 mL of 108 pfu of LC16m8 or 108.0 TCID50 of LC16mO was injected intrathalamically, with one monkey in each group euthanized on post-injection Days 6 and 14 to evaluate CNS tissue and virus titers. The growth of LC16m8 in CNS tissues was observed to be profoundly lower. In addition, fewer pathologic changes were observed in the brains and spinal cords of monkeys injected with LC16m8.
To evaluate the ability of the attenuated virus strains to infect tissues beyond the skin, virus titers were assessed in the blood and brain of normal and immunosuppressed (cortisone-treated) mice [19]. Mice were inoculated intraperitoneally (IP) with 107.3 pfu of Lister, LC16, LC16mO, LC16m8, or CVI-78. Five mice in each group were sacrificed daily for evaluation of viremia or CNS viral growth. When inoculated with the Lister or CVI-78 strain, virus was detected in the brain between Days 4 and 7 in both normal and immunosuppressed animals, whereas the LC16 virus was only identified in the brains of immunosuppressed animals. In animals inoculated with either the LC16mO or LC16m8 strain, no virus was detected in the brains of either normal or immunosuppressed animals. However, LC16m8 was detectable in the bloodstream for 3 days in normal mice and 7 days in immunosuppressed mice (Fig. 1 ).
Fig. 1.
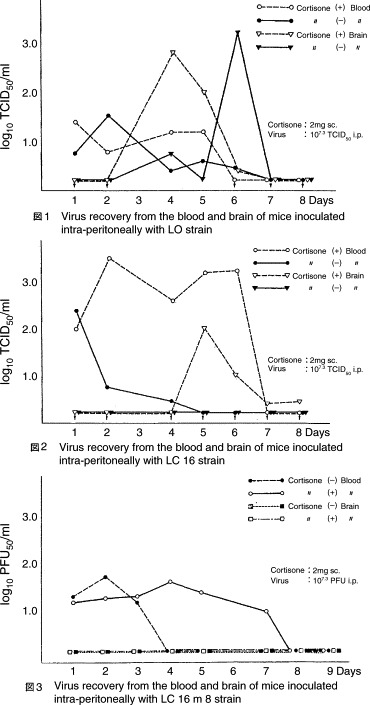
Virus recovery from inoculated mice. Source: Hashizume et al. [19].
Lister, LC16, LC16mO, LC16m8, and CVI-78 were also compared for local reactogenicity by inoculating rabbits intradermally and scoring erythematous responses [19]. The LC16 virus produced the largest erythematous reaction, followed by the Lister strain, LC16mO, CVI-78, and finally LC16m8, which produced the smallest erythematous reaction.
Vaccine protective efficacy was extrapolated from rabbit studies that measured hemagglutination-inhibition (HI) and plaque reduction neutralizing titers (PRNT) [19]. As shown in Table 2 , both HI and PRNT responses to LC16m8 compared favorably with the responses to the Lister strain and supported the clinical evaluation of LC16m8.
Table 2.
Vaccinia antibody levels in rabbits after dermal inoculation with Lister, LC16mO, and LC16m8 vaccinia strains
| Weeks post-inoculation | HI |
PRNT |
||||
|---|---|---|---|---|---|---|
| Lister | LC16mO | LC16m8 | Lister | LC16mO | LC16m8 | |
| 2 | 26.5 | 28.0 | 24.5 | 44.9 | 45.7 | 44.9 |
| 4 | 26.5 | 27.5 | 25.0 | 44.9 | 46.9 | 44.7 |
| 6 | 24.5 | 27.5 | 25.0 | 45.0 | 46.6 | 44.6 |
| 13 | 23.5 | 27.0 | 25.5 | 45.0 | 46.8 | 44.7 |
Lister and LC16mO: inoculated 108 TCID50/animal. LC16m8: inoculated 108 pfu/animal. Note: With respect to the units used to report titers, HI titers were done on two-fold dilutions of test sera, while PRNT titers were done on four-fold dilutions of the test sera. Source: Hashizume et al. [19].
In 1974, sufficient doses of LC16m8 and doses of LC16mO were provided to health officials in 29 prefectures throughout Japan to vaccinate 50,000 and 3000 children, respectively [4]. Practitioners were instructed to use these vaccines or the standard Lister vaccine for routine primary vaccination of children that year (predominantly 2–5 year olds, but some infants were included). Exclusion criteria included known congenital or acquired heart problems, “wet eczema”, known history of severe drug allergies, severe kidney or liver disease, epilepsy, immunocompromise, and a history of convulsion in the past 3 years [20]. All vaccines were administered using an approximately 2 μL dose percutaneously at concentrations of 5.6 × 107 to 1 × 108.1 pfu/mL via bifurcated needles with 5–10 punctures in the upper deltoid region [21].
Within the large cohort of vaccinees, 829 LC16mO- and 10,578 LC16m8-vaccinated children were closely evaluated for local and systemic reactions [4]. Take rates were assessed at 7–14 days, with a physical exam performed 2 and 4 weeks after vaccination [4]. Since a vesicular or pustular reaction following vaccination had been used since the time of Jenner as evidence that a vaccinee has developed protective immunity [22], [23], take rates were defined as a vesicular or pustular reaction at the site of vaccination; local redness or induration alone was recorded as a non-take. Data for local and systemic reactogenicity were derived from surveys provided to the parents or guardians of vaccinees, and clinical assessments (objective and queried) were conducted during postvaccination clinic visits. Endpoints assessed included fever or other systemic symptoms, lymphadenopathy, local redness and induration, take rates, and assessment of adverse reactions [4], [21].
LC16m8 produced take rates equivalent to other smallpox vaccines that had been used in Japan (Table 3 ). As with other vaccinia strains, the maximal adverse effects of LC16m8 were reported around the time of peak pock reactions (7–10 days postvaccination) [4], [21]. Local induration at the site of vaccination, rates of fever, peak temperature, and fever duration were significantly less than with Lister vaccination [4].
Table 3.
Local cutaneous reaction to LC16mO, LC16m8, and conventional vaccinia vaccines (1968–1974)
| Vaccine strain | Year of investigation | No. of persons examined | % Successful vaccinationa | Mean erythema diametera (mm) | Average induration diametera (mm) | Febrile reactionb (%) |
|---|---|---|---|---|---|---|
| Ikeda | 1968–1970 | 1506 | 99.1 | 22.9 | 18.2 | 25.0 |
| Ecuador | 1969–1970 | 1846 | 67.5 | 19.2 | 17.4 | 21.3 |
| Lister | 1968–1971 | 3662 | 93.7 | 17.6 | 15.3 | 26.6 |
| CVI-78 (Japan) | 1971–1973 | 22976 | 92.4 | 21.1 | 16.8 | 8.5 |
| LC16mO | 1973–1974 | 829 | 94.8 | 19.6 | 14.5 | 12.1 |
| LC16m8 | 1973–1974 | 10578 | 95.1 | 18.4 | 6.1 | 7.7 |
Source: Yamaguchi et al. [4].
Take rate, aggregated by highest ratio of successful vaccination or at the time of largest diameter.
Aggregated febrile cases from Days 4 to 14.
In 8544 closely evaluated subjects receiving LC16m8, there were 8 cases of urticaria, 1 case of eczema vaccinatum (EV), 9 cases of autoinoculation, 28 cases of rash localized around the vaccine site, and 3 benign febrile seizures of unclear etiology [4], [21]. Although the case of EV was mild and resolved without sequelae, details regarding any pre-existing skin condition in the vaccinated child are not known.
Several substudies were conducted within this large clinical trial. One involved the removal of excess vaccine at the site of vaccination by alcohol wipes to assess the possible impact on rates of autoinoculation and take rates [4]. Excess vaccine was removed at 1, 3, or 10 min postvaccination. Results showed that vaccination take rates were high (91–99%) and autoinoculation rates were low when wiping the vaccination site within 1 min. In contrast, when the vaccine was allowed to dry for 10 min before wiping, autoinoculation rates were higher (2.3%) [4].
A second substudy examined a Lister challenge vaccine administered at various timepoints, ranging from 10 days to 13 months post-primary vaccination [4], [21]. Japanese public health officials hoped that, by varying the interval between the primary and challenge vaccinations in this substudy and monitoring cutaneous effects, they would be able to gain insight on the speed with which the primary attenuated vaccine elicited an immune response as well as the robustness and longevity of that response. As shown in Table 4 , cross-protective immunity against Lister challenge vaccination was induced early and in the vast majority of vaccinees (∼80%) [4], [21]. Findings with LC16m8 were comparable to Ikeda/Dairen results [4]. Takeuchi reported that when children (1–4 year olds) were vaccinated with LC16m8 and challenge-inoculated with Lister 10 days later, only 1/224 developed a small pustule; the remaining children had either no detectable reaction (54.5%), or redness/induration or scab at the vaccination site [21].
Table 4.
Local cutaneous reaction to revaccination as a measure of protective efficacy of primary LC16m8 vaccination
| Months after initial inoculation | Patients observed | Local reactiona |
|
|---|---|---|---|
| Take (%) | Non-take (%) | ||
| LC16m8 initial vaccination followed by Lister revaccination | |||
| 1 (1–2) | 3 | 0 | 3 (100) |
| 6 (5–7) | 420 | 92 (21.9) | 328 (78.1) |
| 12 (8–13) | 138 | 26 (18.8) | [112 (81.2)]b |
| Ikeda/Dairen initial vaccination followed by Ikeda/Dairen revaccinationc | |||
| Up to 6 | 70 | 6 (8.6) | 64 (91.4) |
| Up to 12 | 714 | 164 (23.0) | 550 (77.0) |
| Up to 24 | 777 | 178 (22.9) | 599 (77.1) |
| Up to 60 | 2024 | 724 (35.8) | 1300 (64.2) |
This set of results were obtained from Numazu, Hyogo Prefecture, Tokyo University Hospital, Sakai University, Kyoto University, and Osaka University.
Error in original Japanese source [4] shows 111 (80.5).
Research relating to vaccine immunogenicity from fiscal year 1964.
These findings suggested that cross-protection against other poxviruses, such as variola, could be mounted quickly following LC16m8 primary vaccination, and be maintained with little variation during the first year post-LC16m8 vaccination (the longest timepoint studied). However, other studies have shown that protection against a primary reaction following revaccination, although proposed historically for this purpose [24], is not a definitive measure of likely protective efficacy [25], [26]. The relevance of a cutaneous reaction upon revaccination is therefore unclear.
Humoral immune responses at 1–6 months post-LC16m8 vaccination were compared to data generated following vaccination with LC16mO, CVI-78, or Lister vaccines [4]. HI titers induced by the four vaccines did not differ significantly (Table 5 ). Although the numbers of LC16m8 recipients assessed for PRNT antibody generation were less than those sampled for HI (97 versus 513), titers following LC16m8 vaccination appeared comparable to those generated following Lister vaccination and superior to titers achieved with CVI-78 vaccination.
Table 5.
Antibody response to LC16m8 and conventional smallpox vaccines
| Strain | Time post-vaccination (in months) | Cases observed | HI antigenic value |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| <2–4 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | Average | |||
| LC16m8 | 1–1.5 | 513 | 18 | 12 | 88 | 161 | 155 | 72 | 6 | 1 | 23.3 |
| 3–6 | 19 | 1 | 3 | 3 | 3a | 3a | 6 | 23.2 | |||
| LC16mO | 1–1.5 | 47 | 4 | 23 | 16 | 4 | 24.4 | ||||
| CVI-78 | 1–1.5 | 26 | 1 | 7 | 11 | 7 | 23.0 | ||||
| 3–6 | 25 | 2 | 10 | 11 | 1 | 1 | 23.6 | ||||
| Lister | 1–1.5 | 7 | 5 | 2 | 24.3 | ||||||
| 3–6 | 19 | 4 | 5 | 8 | 2 | 23.4 | |||||
| Strain | Monthly progress | Cases observed | PRNT antigenic value |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| <40.9 | 41.0 | 41.5 | 42.0 | 42.5 | 43.0 | 43.5 | 44.0 | Average | |||
| LC16m8 | 1–1.5 | 97 | 5 | 10 | 25 | 37 | 18 | 2 | 42.5 | ||
| LC16mO | 1–1.5 | 15 | 6 | 9 | 43.0 | ||||||
| CVI-78 | 1–1.5 | 6 | 1 | 2 | 2 | 1 | 41.9 | ||||
| 3–6 | 11 | 1 | 1 | 4 | 2 | 3 | 42.0 | ||||
| Lister | 1–1.5 | 5 | 3 | 2 | 42.4 | ||||||
| 3–6 | 12 | 1 | 2 | 2 | 7 | 42.2 | |||||
Note: PRNT titers were done on four-fold dilutions of the test sera.
Error in original Japanese source [4] shows 2.
Although encephalitis had been rarely observed in subjects vaccinated with different strains of vaccinia, the investigators believed that strains with the potential to induce overt neurotoxicity might also produce transient brainwave anomalies. While the use of an electroencephalogram (EEG) to predict postvaccination encephalitis has never been validated, EEGs were performed to document possible subclinical neurotoxicity in a cohort of 56 children (aged 6–8) vaccinated with LC16m8 [2]. Testing was done prior to vaccination, and 1, 4, and 8 weeks postvaccination. Findings were then compared to historical EEG data from children vaccinated with Lister and CVI-78 (Table 6 ) [4].
Table 6.
Post-vaccinia vaccination EEG test results
| Vaccine | No. of subjects | % With transient EEG abnormalities |
|---|---|---|
| Lister | 19 | 26.3 |
| CVI-78 | 30 | 3.3 |
| LC16m8 | 56 | 0 |
Source: Yamaguchi et al. [4].
The combined clinical and immunological data convinced the SVRG that LC16m8 was a safe and effective vaccine. Ninety-thousand doses of LC16m8 vaccine were distributed for use in 1974 and 1975, with no reports of severe AEs [19]. Nevertheless, it is unclear how many of these doses were actually administered as the rapid eradication of smallpox in India led to the discontinuation of routine smallpox vaccination in Japan in 1976. Shortly thereafter, the last known naturally acquired case of smallpox occurred in Africa in 1977.
2. LC16m8 molecular characterization
The LC16m8 strain differs from the parent Lister strain in a number of biological characteristics as summarized in Table 7 .
Table 7.
Characteristics of vaccinia from LC16 lineage
| Lister | LC16 | LC16mO | LC16m8 | |
|---|---|---|---|---|
| Ceiling temperature (RK cells) (°C) | >41 | 41 | 41 | 40.5 |
| Plaque size (RK cells) | L | M | M | M |
| Pock size (CAM cells) | L | L | M | S |
| Proliferation ability in each cell type | ||||
| CAM | +++ | +++ | +++ | +++ |
| Vero cells | +++ | +++ | +++ | + |
| RK cells | +++ | +++ | +++ | +++ |
| CEF cells | ++ | ++ | ++ | ++ |
| Central nervous system pathology | ||||
| Proliferation | ||||
| Rabbit | ++ | + | + | ± |
| Monkey | +++ | + | + | + |
| Mouse | + | + | nd | nd |
| Invasion | ||||
| Mouse | +++ | + | – | – |
| Skin proliferation | ||||
| Rabbit | +++ | +++ | ++ | + |
| Human | ++ | +++ | ++ | + |
| Antibody production ability | ||||
| Rabbit | ++ | +++ | +++ | ++ |
| Human | ++ | +++ | +++ | ++ |
nd = not done. Source: Hashizume [18].
The temperature sensitivity of LC16m8 is due to restricted replication at temperatures above 40.5 °C, rather than thermal lability. The stability of the LC16m8 phenotype on CAM (small pock size), growth kinetics, and temperature sensitivity have been tested and confirmed by repeated passaging of virus in PRK cells [27]. With regards to replication kinetics, Lister and LC16m8 viruses are essentially the same in single-step growth curves [28], suggesting that the small pock size of LC16m8 on CAM is not due to restricted intracellular replication but rather to the relative inability of the LC16m8 virus to spread cell-to-cell.
Analysis of Lister and LC16m8 genomes by restriction endonucleases and cross-hybridization has revealed that LC16m8 contains a new restriction site (XhoI), located in a HindIII D fragment near the 3′ terminus of the genome [29]. Recombinant studies, in which the HindIII D fragment of Lister has been introduced into the LC16m8 strain, have shown that a part of the genome containing the extra XhoI is responsible for small plaque and pock size, and restricted growth in Vero cells [30]. The gene in this fragment, originally called ps/hr for plaque size/host range, and now identified as B5R, is related to the regulator of complement activation (RCA) gene family in mammals that contain the characteristic short consensus repeat (SCR) domains (Fig. 2 ) [30], [31].
Fig. 2.
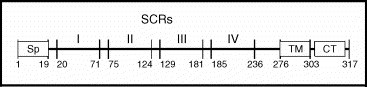
The B5R protein produced by vaccinia viruses with SCR domains annotated. SCR, short consensus repeat; Sp, signal peptide; TM, transmembrane domain; CT, cytoplasmic tail. Source: Adapted from figure presented in Herrera et al. [32].
Recent analysis of the full sequence of the Lister, LC16mO, and LC16m8 genomes confirmed the 1-base deletion in the B5R gene of LC16m8, which introduces a stop codon within the gene [33]. As a result, while the full-length B5R gene encodes for a 317-residue protein, the altered gene in LC16m8 theoretically would encode for a 92-residue protein that, if processed correctly, would be secreted from infected cells as a 73-residue protein.
All orthopoxviruses produce four forms of virus particles: the intracellular mature virus (IMV), the intracellular enveloped virus (IEV), cell-associated enveloped virus (CEV), and the extracellular enveloped virus (EEV) [34]. Deletion of B5R generally results in decreased production of EEV [34], which is critical for cell-to-cell transmission of virus within the infected host and plays an important role in disease pathogenesis [34], [35]. B5R is also a target for neutralizing antibodies [36], and anti-B5R antibodies have been shown to protect mice against lethal infection [37]. Yet recent lethal poxvirus challenge studies in rabbits and mice [33], [38] demonstrated that a deletion in the B5R gene does not diminish LC16m8's efficacy or its ability to induce EEV antibodies. A primate study [39] also suggested that the B5R protein is not critical for smallpox vaccine efficacy. Hooper et al. were able to show that a Dryvax-vaccinated monkey with no detectable B5R-specific neutralizing antibodies could survive a lethal monkeypox virus challenge. The serum of this particular monkey was able to neutralize monkeypox and the vv-Connaught vaccine strain (derived from the NYCBH strain) in PRNT assays. It is possible that the protection conferred by LC16m8 is B5R-independent and other EEV surface proteins may serve as epitopes for neutralizing antibodies [37], [39], [40]. There is also evidence that smallpox vaccines induce cell-mediated immunity (CMI) [41], [42], [43], [44], [45], [46]. Hence it is possible that neutralizing epitopes on the truncated B5R protein might contribute to the overall protective immune response without being the primary mediator. LC16m8-induced CMI responses are currently being investigated and the results should provide important information regarding the extent and longevity of immune responses to LC16m8 compared with that of other vaccines.
Recently it was shown that with prolonged passaging in cell culture, LC16m8 undergoes a phenotypic reversion characterized by the production of plaques that are of intermediate size between wild-type LC16m8 and the precursor virus, LC16mO. This plaque-size heterogeneity can be mapped to the B5R gene where some variants exhibit point mutations upstream of the known B5R single-base deletion. These compensatory mutations correct the observed frameshift and lead to the reconstitution of a full-length B5R gene. Western blot analysis has confirmed a restored ability of these variants to produce a full-length B5R protein. In a severe combined immunodeficiency (SCID) mouse LD50 study, the pathogenicity of two revertant viruses and the LC16mO precursor strain was similar. In addition, plaque-purified LC16m8 and a construct of LC16m8 lacking the B5R gene were shown to have safety profiles comparable to that of MVA in the same animal models and to confer protective immunity in a mouse/intranasal vaccinia (Western Reserve [WR] strain) challenge study [47].
The specific clinical consequences that correlate with deleted or mutated genes in attenuated vaccinia strains are only just beginning to be studied. Orthopoxviruses are known to use a wide array of immunomodulatory strategies to establish a rapid and ongoing infection within the host [48], [49]. These mechanisms target the innate, humoral, and cell-mediated immune pathways, using mechanisms as diverse as functional mimicry of host proteins, masking, and avoidance of innate antiviral pathways [48], [50].
It is not yet known what immunomodulatory mechanisms are used or altered by the attenuation of Lister to LC16m8. The complete genome sequences of the LC16m8, LC16mO, and Lister viruses have been published recently [33] and studies are underway to compare the genomic and proteomic profiles of this group with profiles of other orthopoxviruses.
3. LC16mO and LC16m8 as vector systems
In 1982, two groups independently showed that vaccinia viruses could be modified to serve as cloning and expression vectors by inserting foreign DNA into non-essential regions of the genome [51], [52]. Since then, numerous studies have demonstrated the utility of recombinant vaccinia viruses (rVVs) as effective vector systems due to their ability to produce robust cellular and humoral immune responses. With a carrying capacity for large DNA fragments that rivals other vector systems (e.g., lentivirus, alphavirus, AAV, adenovirus), rVVs may be a preferred strategy for the delivery of foreign antigens.
Both replication-deficient (e.g., MVA) and replication-competent vector strategies have been employed, with safety being a major distinction between the two approaches. Since fever, encephalitis, progressive vaccinia, and myopericarditis sometimes accompany vaccination with replication-competent vaccines, a replicating rVV vector system that is both safe and capable of eliciting a strong immune response is highly desirable. LC16mO and LC16m8 are attractive options for live rVVs because they are characterized by temperature sensitivity, restricted host range, and infrequent low-grade adverse events. Moreover, LC16mO has been found to be more immunogenic than its unattenuated parent, Lister, and LC16m8, so it has generally been the preferred vector system [53]. Table 8 highlights many of the antigen delivery and protein expression applications that have used either LC16mO or LC16m8 as a vector system.
Table 8.
History of LC16mO- and LC16m8-based rVVs as vectors for antigen delivery and protein expression (modified and updated from [53])
| Parental vector | rVV nomenclature | Foreign antigen(s) and virus | Insertion site in rVV | Promoter | References |
|---|---|---|---|---|---|
| LC16mO | m0HB9-1 | HBsAg (HBV) | TK | TK | Morita et al. [56] |
| LC16m8 | m8HB20-3 | HBsAg (HBV) | TK | TK | |
| LC16mO | pProHBm0143 | HBsAg (HBV) | TK | 7.5 kDa | Watanabe et al. [27], [57] |
| LC16m8 | pProHBm839 | HBsAg (HBV) | TK | 7.5 kDa | |
| LC16mO | m0-proenv | Env (HTLV-1) | HA | 7.5 kDa | Shida et al. [58] |
| LC16mO | m0J6 | PreM & E glycoproteins (JEV) | TK | 7.5 kDa | Yasuda et al. [59] |
| LC16mO | m0-HA/ATI | Env (BLV) | HA | ATI | Ohishi et al. [60] |
| m0-HA/7.5kD | Env (BLV) | HA | 7.5 kDa | ||
| LC16mO | mp7.5/RVV | Hemagglutinin (RPV) | HA | 7.5 kDa | Asano et al. [61] |
| LC16mO | p7.5/RVV | Hemagglutinin (RPV) | HA | 7.5 kDa | Yamanouchi et al. [62] |
| LC16mO | VgB, VgC, VgD | gB, gC, gD (CHV) | TK | 7.5 kDa | Xuan et al. [63] |
| LC16mO | rRV | Hemagglutinin (RPV) | HA | 7.5 kDa | Ohishi et al. [64] |
| LC16mO | m0TDH/HisX1, m0TDH/HisC2, m0TDF1 | Hemagglutinin (MV) F protein (MV) | HA | pSFJ1-10 and pSFJ2-16 (ATI + tandem repeats of p7.5 kDa) | Kidokoro et al. [55] |
| LC16m8 | SARS CoV-S rVV | Spike protein (SARS coronavirus) | HA | pSFJ1-10 | Kitabatake et al. [54] |
ATI = A-type inclusion body; BLV = bovine leukemia virus; CHV = canine herpesvirus; HA = hemagglutinin; HBV = hepatitis B virus; HTLV-1 = human T-cell leukemia virus type 1; JEV = Japanese encephalitis virus; MV = measles virus; RPV = rinderpest virus; SARS = severe acute respiratory syndrome; TK = thymidine kinase.
A recent abstract [54] showed particularly promising results for an LC16m8-vectored SARS coronavirus (SARS-CoV) vaccine. It was observed that neutralizing antibody titers to the SARS-CoV spike protein increased 100-fold 1 week after vaccination and increased an additional 10-fold 2 weeks after a booster injection. Interestingly, rabbits with pre-existing antibodies to the vector (after being pre-immunized with LC16m8) still generated an antibody response, suggesting that this vector system might produce vaccines suitable for populations that have undergone prior immunization with smallpox vaccine(s).
Work by Kidokoro et al. [55] demonstrated that LC16mO-based vectors provide a robust protein expression system that preserves the functional integrity of the expressed protein. These investigators were able to obtain yields of up to 42 mg/L and purity of 94–98% for the glycosylated measles virus hemagglutinin (H) protein. Based on these promising results, further studies to determine the general utility of this LC16mO vector for large-scale expression of other proteins are warranted.
4. Recent animal data demonstrating efficacy of LC16m8
Since animal challenge studies were not conducted during the development of LC16m8 in the 1970s, alternative measures of immunity that had been used to characterize other smallpox vaccines [65], [66] were used to evaluate the efficacy of LC16m8 in early clinical trials in Japan. These included basic antibody and T-cell assays and monitoring of cutaneous responses to primary vaccination with LC16m8 [4]. However, current standards for determining vaccinia efficacy rely on more sophisticated measurements of humoral and CMI responses as well as lethal poxvirus challenges in vaccinated animal models [41], [42], [67], [68]. One example of the latter is the lethal rabbitpox virus (RPXV) in rabbits, which mimics smallpox infection in humans [69] since it is rapidly and widely disseminated, is highly infectious, and produces high levels of EEV. The latter appears to play a prominent role in the pathogenesis of both RPXV and variola [34], [70], making the RPXV challenge model particularly suitable for testing the EEV-neutralizing capability of LC16m8. Hence, the protective efficacy of LC16m8 was recently evaluated in the rabbit model using a RPXV challenge [38].
In this comparative study, 20 rabbits per group were vaccinated by scarification with approximately 2 × 105 pfu of LC16m8 or Dryvax, or with phosphate-buffered saline (PBS) as placebo. After 28 days, the groups were divided in two, challenged with low or high intradermal doses of RPXV (200 and 1000 pfu, respectively), and monitored for 10 days. At 10 days post-challenge, all vaccinated animals had survived, whereas 9/10 (low-dose challenge) and 10/10 (high-dose challenge) placebo (PBS) animals had died (Fig. 3 ; only 1000 pfu dose challenge data shown). There were no differences in postvaccination and post-challenge clinical symptoms between the LC16m8- and Dryvax-vaccinated groups.
Fig. 3.
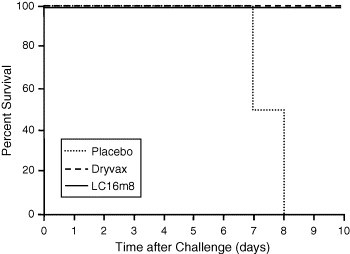
Rabbits immunized with LC16m8 protected against lethal RPXV infection. Rabbits immunized with approximately 2 × 105 pfu of LC16m8 or Dryvax, or with placebo (PBS) (n = 20 per group) were challenged 28 days later with either 200 or 1000 pfu of RPV via the intradermal route. Survival was determined 10 days after challenge. At Day 10, surviving rabbits were euthanized. Only high-dose challenge data are shown.
The antibody response was characterized at Days 0, 14, and 28 postvaccination and measured for the ability to bind IMV as well as the ability to neutralize both IMV and EEV of RPXV in PRNT assays. Antibodies from the LC16m8-vaccinated rabbits had a greater capacity to bind RPXV in an ELISA (data not shown) as well as neutralize RPXV IMV when compared to antibodies from Dryvax-vaccinated rabbits (Fig. 4 ). Interestingly, antibodies from LC16m8- and Dryvax-vaccinated animals showed comparable abilities to neutralize RPXV EEV.
Fig. 4.
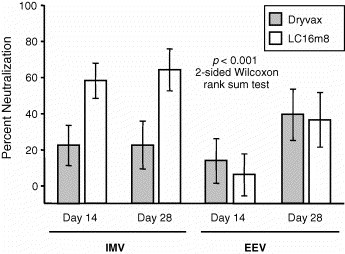
Antibody responses to LC16m8 and Dryvax in rabbits. Sera obtained from rabbits 14 and 28 days after immunization were tested at a 1:10 dilution for neutralization of IMV and EEV forms of RPV by plaque-reduction assays. Each data point represents the mean of 20 serum samples. Error bars indicate 95% confidence intervals. IMV neutralization titers elicited by LC16m8 were significantly greater than those elicited by Dryvax (p < 0.001, two-sided Wilcoxon rank sum test). Sera obtained from PBS-immunized animals demonstrated no neutralization. These data are not shown.
Finally, a plaquing virus assay used to measure virus in different tissues showed there was no detectable RPXV in the lung, liver, or spleen of both groups of vaccinated animals, whereas placebo animals had high titers of virus in these organs.
In a second animal model, LC16m8 was compared to Dryvax for its ability to protect A/NCR mice from aerosolized ectromelia virus (ECTV), the causative agent of mousepox [38]. This model was chosen since it closely mimics the route in which smallpox might be delivered during a bioterrorist attack [71]. Ten mice per group were vaccinated by scarification with approximately 2 × 105 pfu LC16m8 or Dryvax, or with PBS as placebo. Forty-nine (49) days later, all animals were challenged with a dose approximately 1–2 times the Lethal Dose 50 (LD50) of aerosolized ECTV. At 25 days following challenge, all vaccinated animals had survived, whereas 90% of the placebo mice had died (Fig. 5 ).
Fig. 5.
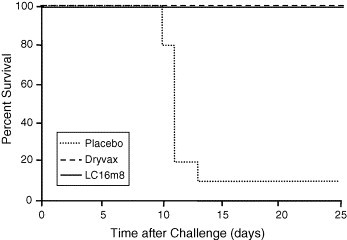
Mice immunized with LC16m8 protected against lethal ECTV infection. Mice immunized with approximately 2 × 105 pfu of LC16m8 (n = 9) or Dryvax (n = 10) or with placebo (PBS) (n = 10) were challenged 49 days later with approximately 1–2× LD50 of ECTV by aerosol. At Day 25, surviving mice were euthanized. Survival was determined 25 days after challenge, at which point, surviving rabbits were euthanized for further analysis.
No significant clinical symptoms or weight differences were noted between the LC16m8- and Dryvax-vaccinated mice post-challenge. Serum samples were taken from all mice 41 days after vaccination. Each sample was assessed for recognition of orthopoxvirus using an ELISA with vaccinia Western Reserve (WR) as the antigen. As was seen in the rabbit study, sera from LC16m8-vaccinated mice generated higher vaccinia-specific ELISA titers than sera from Dryvax-vaccinated mice (Fig. 6 ).
Fig. 6.
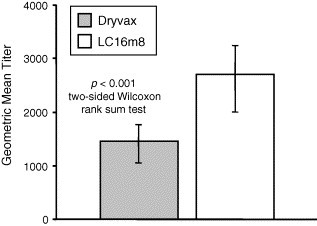
Geometric mean titer of vaccinia virus-specific antibodies in mice vaccinated with Dryvax or LC16m8. Sera obtained from mice 41 days after immunization were tested for vaccinia virus-specific antibodies by ELISA. The bars represent the geometric mean titers from 29 Dryvax-immunized animals and 28 LC16m8-immunized animals. Although 30 mice were vaccinated with Dryvax or LC16m8, serum volumes from some animals were insufficient for analysis. Error bars indicate 95% confidence intervals. The geometric mean titer elicited by LC16m8 were significantly greater than those elicited by Dryvax (p < 0.001, two-sided Wilcoxon rank sum test). Sera obtained from PBS-immunized animals did not contain detectable vaccinia virus-specific antibodies. These data are not shown.
The rabbit and mouse data from the preceding studies are in agreement with recent work in a mouse-vaccinia WR intranasal challenge model by Kidokoro et al. who reported that B5R-deficient LC16m8 was able to induce PRNT antibodies, and that protection against lethal challenge was equivalent to that of the B5R-containing Dryvax [47]. In the same report, these authors determined that, similar to MVA and in contrast to Dryvax, LC16m8 did not induce mortality in SCID mice, despite being given at doses 1000-fold higher than the protective dose in this particular mouse model.
Further confirmation of the protective efficacy of LC16m8 in another mouse-vaccinia WR challenge model was reported by Morikawa et al., who demonstrated that protection was equivalent to that of the B5R-containing Lister and LC16mO vaccine strains [33]. Finally, it has recently been reported by Saijo et al. that LC16m8 and Lister provided protection from disease in monkeys challenged with severe intranasal monkeypox [72].
5. Recently published LC16m8 clinical data
Japan has retained a national stockpile of LC16m8 since conditionally licensing it in 1975 (unconditional licensure was granted in 1980). The government decided to increase the volume of its LC16m8 stockpile in 2000 in response to a resurgence of monkeypox in Africa, and again in early 2002 in response to terrorist threats in the US and other regions of the world. In anticipation of increased LC16m8 production, 58 employees of the Chiba Serum Institute (manufacturers of LC16m8 until mid-2002) volunteered for vaccinations in November 2001 and were followed for safety and immunogenicity [73]. Fifty-six (56) of these were revaccinees (the strain and date of previous vaccination unknown) and 2 were primary vaccinees. LC16m8 was administered using a five-puncture bifurcated needle inoculation technique, and symptom diaries were kept for 2 weeks. The vaccination site was inspected by medical personnel 6–10 days postvaccination. Clinical observation records for 48 of the vaccinated workers are summarized in Table 9 . Vaccination site symptoms were mild (mostly pruritis) and resolved within a week.
Table 9.
Clinical manifestations following vaccination with LC16m8 vaccine in adults
| Age (years) | Sex |
Fever (≥37 °C) | Vesicle (take reaction) | Redness (mm) | |
|---|---|---|---|---|---|
| Male (n = 9) | Female (n = 39) | ||||
| 20 | 1 | 2 | 0/3 | 3/3 (5.5 mm) | 8.2 |
| 30 | 1 | 4 | 0/5 | 5/5 (4.6 mm) | 10.2 |
| 40 | 5 | 17 | 1/22a | 21/22 (6.2 mm) | 12.3 |
| 50 | 0 | 14 | 0/14 | 14/14 (5.4 mm) | 11.4 |
| 60 | 2 | 2 | 0/4 | 4/4 (6.2 mm) | 9.6 |
| Total | 9 | 39 | 1/48 (2%) | 47/48 (98%) 5.6 mm mean size | 10.3 mm mean size |
Source: Horiuchi et al. [73].
Headache.
A Phase I/II clinical trial of LC16m8 is presently underway in the US to evaluate the safety and immunogenicity of LC16m8. Preliminary data was recently presented and will be formally published shortly [74].
6. Discussion
The current US stockpile of smallpox vaccines is comprised of vaccines using the NYCBH vaccinia strain, primarily the calf lymph derivatives (APSV “Wetvax” and Dryvax manufactured in the 1950s and early 1980s, respectively) and one of the more recently manufactured cell culture derivatives (ACAM2000) [75], [76]. Although well proven with regard to protective efficacy against smallpox, a first-generation vaccine such as Dryvax may produce rates of local and systemic adverse reactions that are unacceptable in a post-endemic smallpox era, particularly given the higher rates of immunosuppression and skin disease in today's population. Common reactions to Dryvax include fever, lymphadenopathy, and lymphadenitis, while more serious adverse events include auto/contact inoculation, generalized vaccinia, eczema vaccinatum, progressive vaccinia, and encephalitis [77]. Concern has also been expressed about the possible presence of adventitious agents in stockpiled Dryvax and APSV since calf lymph-perpetuated smallpox vaccines were not screened for mycoplasma, viruses, or prions during manufacturing [76].
Deaths from Dryvax vaccination in the US prior to the eradication of smallpox were frequently due to encephalitis, which occurred at a rate of approximately one per million primary vaccinees [77]. In recent clinical trials evaluating Dryvax, myopericarditis, a serious adverse event rarely reported in the past, was found to occur at high rates among primary vaccinees [78], [79]. Through careful evaluation of vaccinees for both clinical and subclinical symptoms, this reaction was identified in up to 1/145 primary vaccinees, prompting the addition of a black box warning to the package insert of Dryvax in 2004 [80]. This discovery has further fueled the drive to identify smallpox vaccines with improved safety profiles.
Initial attempts to improve vaccine safety focused on modernizing the manufacture of Dryvax by growing the virus in cell culture and cloning single subpopulation strains of NYCBH that had been characterized by protective efficacy and low neurovirulence in animal studies [75], [81], [82]. Clinical trials with the Acambis-produced second-generation NYCBH vaccines, ACAM1000 and ACAM2000 (derived from ACAM1000 and grown in Vero cells rather than MRC-5 cells), confirmed high take rates (∼99%) and immunogenicity [75]. The quality, progression, and timing of pock reactions were similar to those seen with Dryvax controls, as were most local and systemic reactions. Apart from local reactions at the injection site, the most common adverse reaction to ACAM1000 or ACAM2000 was lymph node or axillary pain (seen in 67–73% of primary vaccinees) [75]. Unfortunately, subclinical or overt myopericarditis was also found to occur at high rates in both the ACAM2000 and Dryvax control groups [79], [80]. In addition, one vaccinee given the experimental vaccine experienced a new onset seizure 8 days postvaccination [75]. In another recent Phase II study, myopericarditis developed in one subject 10 days after vaccination with 6.8 × 107 pfu/mL of ACAM2000 [83].
CCSV (cell-cultured smallpox vaccine), a version of the NYCBH vaccinia strain grown in MRC-5 cells by DynPort Vaccine Company (DVC), was recently evaluated in a double-blinded Dryvax-controlled clinical trial conducted in 350 vaccinia naive and non-naive volunteers [82]. As with the Acambis products, rates of adverse reactions to CCSV were similar to Dryvax, however, no serious adverse reactions or myopericarditis were reported during the trial. CCSV-induced PRNT titers and seroconversion rates were generally lower than those induced by Dryvax.
Given the disappointing safety findings of cell culture-derived second-generation vaccines, focus has once again shifted to third-generation vaccines such as LC16m8 and MVA (IMVAMUNE, Bavarian Nordic; and MVA3000, Acambis) as a potential means of replenishing national stockpiles. The Japanese government recently announced that it will significantly expand its national stockpile of LC16m8, affording protection for 56 million people in the event of a bioterrorist attack [84].
MVA, with its extensive history of safety in humans (>100,000 people have been vaccinated in Germany although some with only a single dose of approximately 105 pfu), is presently being reassessed in various clinical and nonclinical studies [8], [85], [86], [87], [88], [89], [90], [91]. Although the rationale for using this non-replicating vaccine in high-risk populations such as those with immunosuppression or skin disease is compelling [88], its efficacy against smallpox is unknown. Most nonclinical studies evaluating the protective efficacy of MVA indicate that multiple-dose regimens using higher concentrations of approximately 108 pfu are required [8], [85], [86], [87], [88], [89], [90], [91]. In one study of mice challenged intradermally or intranasally with replicating vaccinia, protective efficacy was observed only after two or three doses of MVA had been administered [89].
LC16m8 is currently the only smallpox vaccine under investigation that is both attenuated and able to replicate in humans, potentially offering protection after a single dose. The available clinical and nonclinical data suggest that LC16m8 merits further study as a potential vaccine for use in the future. However, development of any new smallpox vaccine is complicated by the evolving nature of the field.
It is clear that historical data on protective antibody titers from the smallpox eradication era cannot be relied upon. With smallpox no longer an endemic disease, vaccine efficacy can only be inferred from animal challenge studies and corresponding immune responses in humans. The US FDA has published specific requirements for proof of efficacy in animal models that every new smallpox vaccine must meet (the so-called “animal rule”) [68]. Given the multifactorial nature of the immune response to smallpox vaccines, various markers for both humoral and cellular immunity are being studied with the aim of identifying objective measurements of efficacy [41], [42], [43], [92], [93], [94].
Correlates of protection in humans have yet to be defined unequivocally, but may rely in part on the elicitation of neutralizing antibody responses that correspond to those demonstrated to be efficacious in animal models. Although the threshold titer of neutralizing antibodies needed for protection is unknown [23], [87], it was recently shown that human vaccinia-specific antibodies passively transferred to non-immunized macaques offered protection against a lethal monkeypox challenge [95].
A lack of published data and the varying reagents used in plaquing virus assays make comparison of absolute PRNT titers elicited by different smallpox vaccines difficult. However, published results suggest that cell-cultured NYCBH vaccines produce immune responses similar to Dryvax [75], [83]. No recent data on PRNT levels elicited by MVA or LC16m8 in humans have been published, but findings should be forthcoming from ongoing clinical trials.
The duration of humoral and cell-mediated protective immunity afforded by smallpox vaccination remains unknown, yet there is both empirical [96], [97] and experimental [43], [98], [99], [100] evidence that some degree of immunity to smallpox persists for several decades postvaccination. In addition, Hammarlund and colleagues recently reported that smallpox vaccination as much as 48 years pre-exposure conferred immunity to humans exposed to monkeypox [101]. No data on the duration of long-term immunity to LC16m8 are currently available, however, immunity duration will be an important consideration for all new smallpox vaccine candidates.
While the biodefense-focused quest for a safe and effective smallpox vaccine is urgent, only the eventual outcomes of controlled clinical and nonclinical studies of new smallpox vaccine candidates will ensure their suitability for use in humans should smallpox recur in the future.
Acknowledgements
We would like to thank the following individuals for their contributions to this manuscript: Hiroyuki Yokote, Munehiro Hirayama, and So Hashizume for permission to reproduce figures and tables from original LC16m8 research reports; Kouji Matsushima for helpful discussions concerning the use of LC16m8 as a vector system; Catherine Bolger for editorial assistance; and Timothy Corrigan for document translation support. In addition, we would like to express our gratitude to clinical investigators Karl Beutner (Solano Clinical Research), Cornelia Dekker (Stanford University Medical Center), Kathryn Edwards (Vanderbilt University Medical Center), Sharon Frey (St. Louis University Health Sciences), and Richard Greenberg (University of Kentucky Medical Center) as well as the many volunteers who have participated in the ongoing Phase I/II trial.
References
- 1.Marennikova S.S., Chimishkyan K.L., Maltseva N.N., Shelukhina E.M., Fedorov V.V. Characteristics of virus strains for production of smallpox vaccines. In: Gusic B., editor. Proceedings of the symposium on smallpox. Yugoslav Academy of Sciences and Arts; 1969. pp. 65–79. [Google Scholar]
- 2.Kitamura T. Effort to improve smallpox vaccine. Rinsho to Uirusu [Clin Virus] 1996;24:41–47. [Google Scholar]
- 3.Hirayama M. In search of attenuated vaccine. Clinical special edition: vaccination in future—everything about attenuated vaccine. Rinsho to Uirusu [Clin Virus] 1975;3:225–228. [Google Scholar]
- 4.Yamaguchi M., Kimura M., Hirayama M. Vaccination research group research report: Ministry of Health and Welfare special research: postvaccination side effects and research regarding treatment of complications. Rinsho to Uirusu [Clin Virus] 1975;3:269–279. [Google Scholar]
- 5.Kimura M., Sakai H. Vaccination in Japan. Rinsho to Uirusu [Clin Virus] 1996;24:30–40. [Google Scholar]
- 6.Meiser A., Boulanger D., Sutter G., Krijnse Locker J. Comparison of virus production in chicken embryo fibroblasts infected with the WR, IHD-J and MVA strains of vaccinia virus: IHD-J is most efficient in trans-Golgi network wrapping and extracellular enveloped virus release. J Gen Virol. 2003;84:1383–1392. doi: 10.1099/vir.0.19016-0. [DOI] [PubMed] [Google Scholar]
- 7.Hochstein-Mintzel V., Hanichen T., Huber H.C., Stickl H. An attenuated strain of vaccinia virus (MVA). Successful intramuscular immunization against vaccinia and variola (author's translation) Zentralbl Bakteriol. 1975;230:283–297. [PubMed] [Google Scholar]
- 8.McCurdy L.H., Larkin B.D., Martin J.E., Graham B.S. Modified vaccinia Ankara: potential as an alternative smallpox vaccine. Clin Infect Dis. 2004;38:1749–1753. doi: 10.1086/421266. [DOI] [PubMed] [Google Scholar]
- 9.Meyer H., Sutter G., Mayr A. Mapping of deletions in the genome of the highly attenuated vaccinia virus MVA and their influence on virulence. J Gen Virol. 1991;72:1031–1038. doi: 10.1099/0022-1317-72-5-1031. [DOI] [PubMed] [Google Scholar]
- 10.Blanchard T.J., Alcami A., Andrea P., Smith G.L. Modified vaccinia virus Ankara undergoes limited replication in human cells and lacks several immunomodulatory proteins: implications for use as a human vaccine. J Gen Virol. 1998;79:1159–1167. doi: 10.1099/0022-1317-79-5-1159. [DOI] [PubMed] [Google Scholar]
- 11.Hochstein-Mintzel V., Huber H.C., Stickl H. Virulence and immunogenicity of a modified vaccinia virus (MVA) Z Immun Exp Klin Immunol. 1972;144:140–156. [PubMed] [Google Scholar]
- 12.Parker R.F., Bronson L.H., Green R.H. Further studies of the infectious unit of vaccinia. J Exp Med. 1941;74(3):263–281. doi: 10.1084/jem.74.3.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kempe C.H., Fulginiti V., Minamitani M., Shinefield H. Smallpox vaccination of eczema patients with a strain of attenuated live vaccinia (CVI-78) Pediatrics. 1968;42:980–989. [PubMed] [Google Scholar]
- 14.Kempe C.H. Smallpox vaccination of eczema patients with attenuated live vaccinia virus. Yale J Biol Med. 1968;41:1–12. [PMC free article] [PubMed] [Google Scholar]
- 15.Hashizume S., Morita M., Yoshizawa H., Suzuki K., Arita M., Komatsu T. Proceedings of the international symposium on smallpox vaccine, Bilthoven, symp. series immunobiol. standard, vol. 19. 1972. Intracerebral inoculation of monkeys with several vaccinia strains: an approach to the comparison of different strains; pp. 325–331. [Google Scholar]
- 16.van der Noordaa J., Dekking F., Posthuma J., Beunders B.J. Primary vaccination with an attenuated strain of vaccinia virus. Arch Gesamte Virusforsch. 1967;22(1):210–214. doi: 10.1007/BF01240515. [DOI] [PubMed] [Google Scholar]
- 17.Tint H. Proceedings of the symposia series in immunobiological standardization, vol. 19. 1973. The rationale for elective prevaccination with attenuated vaccinia (CVI-78) in preventing some vaccination complications; pp. 281–292. [Google Scholar]
- 18.Hashizume S. Chiba Serum Institute. Special edition future of vaccination: everything about attenuated vaccines. Basics of new attenuated vaccine strain LC16m8. Clin Virus. 1975;3:229–235. [Google Scholar]
- 19.Hashizume S., Yoshizawa N., Morita M., Suzuki K. Properties of attenuated mutant of vaccinia virus, LC16m8, derived from Lister strain. In: Quinnan, editor. Vaccinia viruses as vectors for vaccine antigens. Elsevier; 1985. [Google Scholar]
- 20.KAKETSUKEN (Chiba Serum Institute) documents on file.
- 21.Takeuchi K., Kawakami K., Akagi N., Kawanishi K. Results of experimental inoculation of attenuated LC16m8 reducing effect of LC16m8 strain pre-treatment. Shonika Shinryo [Pediatr Diagn] 1976;39:1208–1219. [translated from Japanese] [Google Scholar]
- 22.Fenner F., Henderson D.A., Arita I., Jezek Z., Ladnyi I.D. World Health Organization; Geneva: 1988. Smallpox and its eradication. p. 289–96. [Google Scholar]
- 23.McClain D.J., Harrison S., Yeager C.L., Cruz J., Ennis A.F., Gibbs P. Immunologic responses to vaccinia vaccines administered by different parenteral routes. J Infect Dis. 1997;175:756–763. doi: 10.1086/513968. [DOI] [PubMed] [Google Scholar]
- 24.Cockburn W.C., Cross R.M., Downie A.W., Dumbell K.R., Kaplan C., McClean D. Laboratory and vaccination studies with dried smallpox vaccines. Bull World Health Organ. 1957;16:63–77. [PMC free article] [PubMed] [Google Scholar]
- 25.Cross R.M., Kaplan C., McClean D. Studies with dried and glycerinated smallpox vaccines of full and diminished potencies. Bull World Health Organ. 1958;19:123–128. [PMC free article] [PubMed] [Google Scholar]
- 26.Kaplan C., Benson P.F., Butler N.R. Immunogenicity of ultraviolet-irradiated, non-infectious, vaccinia-virus vaccine in infants and young children. Lancet. 1965;191:573–574. doi: 10.1016/s0140-6736(65)91146-3. [DOI] [PubMed] [Google Scholar]
- 27.Watanabe K., Morita M., Kojima A. Stability of recombinant vaccinia virus LC16mO or LC16m8: preserved laboratory attenuation markers and conserved expression of HBsAg gene. Vaccine. 1989;7:499–502. doi: 10.1016/0264-410x(89)90272-7. [DOI] [PubMed] [Google Scholar]
- 28.Takahashi-Nishimaki F., Suzuki K., Morita M., Maruyama T., Miki K., Hashizume S. Genetic analysis of vaccinia virus Lister strain and its attenuated mutant LC16m8: production of intermediate variants by homologous recombination. J Gen Virol. 1987;68:2705–2710. doi: 10.1099/0022-1317-68-10-2705. [DOI] [PubMed] [Google Scholar]
- 29.Sugimoto M., Yasuda A., Miki K., Morita M., Suzuki K., Uchida N. Gene structures of low-neurovirulent vaccinia virus LC16mO, LC16m8, and their Lister original (LO) strains. Microbiol Immunol. 1985;29:421–428. doi: 10.1111/j.1348-0421.1985.tb00843.x. [DOI] [PubMed] [Google Scholar]
- 30.Takahashi-Nishimaki F., Funahashi S.-I., Miki K., Hashizume S., Sugimoto M. Regulation of plaque size and host range by a vaccinia virus gene related to complement system proteins. Virology. 1991;181:158–164. doi: 10.1016/0042-6822(91)90480-y. [DOI] [PubMed] [Google Scholar]
- 31.Engelstad M., Howard S.T., Smith G.L. A constitutively expressed vaccinia gene encodes a 42-kDa glycoprotein related to complement control factors that forms part of the extracellular virus envelope. Virology. 1992;188:801–810. doi: 10.1016/0042-6822(92)90535-w. [DOI] [PubMed] [Google Scholar]
- 32.Herrera E., Lorenzo M.M., Blasco R., Isaacs S.N. Functional analysis of vaccinia virus B5R protein: essential role in virus envelopment is independent of a large portion of the extracellular domain. J Virol. 1998;72:294–302. doi: 10.1128/jvi.72.1.294-302.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morikawa S., Sakiyama T., Hasegawa H., Saijo M., Maeda A., Kurane I. An attenuated LC16m8 smallpox vaccine: analysis of full-genome sequence and induction of immune protection. J Virol. 2005;79:11873–11891. doi: 10.1128/JVI.79.18.11873-11891.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith G.L., Vanderplasschen A., Law M. The formation and function of extracellular enveloped vaccinia virus. J Gen Virol. 2002;83:2915–2931. doi: 10.1099/0022-1317-83-12-2915. [DOI] [PubMed] [Google Scholar]
- 35.Payne L.G. Significance of extracellular enveloped virus in the in vitro and in vivo dissemination of vaccinia. J Gen Virol. 1980;50:89–100. doi: 10.1099/0022-1317-50-1-89. [DOI] [PubMed] [Google Scholar]
- 36.Bell E., Shamim M., Whitbeck J.C., Sfyroera G., Lambris J.D., Isaacs S.N. Antibodies against the extracellular enveloped virus B5R protein are mainly responsible for the EEV neutralizing capacity of vaccinia immune globulin. Virology. 2004;325:425–431. doi: 10.1016/j.virol.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 37.Galmiche M.C., Goenaga J., Wittek R., Rindisbacher L. Neutralizing and protective antibodies directed against vaccinia virus envelope antigens. Virology. 1999;254:71–80. doi: 10.1006/viro.1998.9516. [DOI] [PubMed] [Google Scholar]
- 38.Empig C., Kenner J., Perret-Gentil M., Youree B.E., Bell E., Chen A. Highly attenuated smallpox vaccine protects rabbits and mice against pathogenic orthopoxvirus challenge. Vaccine. 2006;24:3686–3694. doi: 10.1016/j.vaccine.2005.03.029. [DOI] [PubMed] [Google Scholar]
- 39.Hooper J.W., Thompson E., Wilhelmsen C., Zimmerman M., Ichou M.A., Steffen S.E. Smallpox DNA vaccine protects nonhuman primates against lethal monkeypox. J Virol. 2004;78:4433–4443. doi: 10.1128/JVI.78.9.4433-4443.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fogg C., Lustig S., Whitbeck J.C., Eisenberg R.J., Cohen G.H., Moss B. Protective immunity to vaccinia virus induced by vaccination with multiple recombinant outer membrane proteins of intracellular and extracellular virions. J Virol. 2004;78:10230–10237. doi: 10.1128/JVI.78.19.10230-10237.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Demkowicz W.E., Ennis F.A. Vaccinia virus-specific CD8+ cytotoxic T lymphocytes in humans. J Virol. 1993;67(3):1538–1544. doi: 10.1128/jvi.67.3.1538-1544.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kennedy J.S., Frey S.E., Yan L., Rothman A.L., Cruz J., Newman F.K. Induction of human T cell-mediated immune responses after primary and secondary smallpox vaccination. J Infect Dis. 2004;190:1286–1294. doi: 10.1086/423848. [DOI] [PubMed] [Google Scholar]
- 43.Crotty S., Felgner P., Davies H., Glidewell J., Villarreal L., Ahmed R. Cutting edge: long-term B cell memory in humans after smallpox vaccination. J Immunol. 2003;171:4969–4973. doi: 10.4049/jimmunol.171.10.4969. [DOI] [PubMed] [Google Scholar]
- 44.Hammarlund E., Lewis M.W., Hansen S.G., Strelow L.I., Nelson J.A., Sexton G.J. Duration of antiviral immunity after smallpox vaccination. Nat Med. 2003;9:1131–1137. doi: 10.1038/nm917. [DOI] [PubMed] [Google Scholar]
- 45.Rock M.T., Yoder S.M., Wright P.F., Talbot T.R., Edwards K.M., Crowe J.E., Jr. Differential regulation of granzyme and perforin in effector and memory T cells following smallpox immunization. J Immunol. 2005;174:3757–3764. doi: 10.4049/jimmunol.174.6.3757. [DOI] [PubMed] [Google Scholar]
- 46.Amara R.R., Nigam P., Sharma S., Liu J., Bostik V. Long-lived poxvirus immunity, robust CD4 help, and better persistence of CD4 than CD8 T cells. J Virol. 2004;78:3811–3816. doi: 10.1128/JVI.78.8.3811-3816.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kidokoro M., Tashiro M., Shida H. Genetically stable and fully effective smallpox vaccine strain constructed from highly attenuated vaccinia LC16m8. Proc Natl Acad Sci USA. 2005;102:4152–4157. doi: 10.1073/pnas.0406671102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Johnston J.B., McFadden G. Poxvirus immunomodulatory strategies: current perspectives. J Virol. 2003;77:6093–6100. doi: 10.1128/JVI.77.11.6093-6100.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Johnston J.B., McFadden G. Technical knockout: understanding poxvirus pathogenesis by selectively deleting viral immunomodulatory genes. Cell Microbiol. 2004;6:695–705. doi: 10.1111/j.1462-5822.2004.00423.x. [DOI] [PubMed] [Google Scholar]
- 50.Shchelkunov S.N. Immunomodulatory proteins of orthopoxviruses. Mol Biol. 2003;37:37–48. [PubMed] [Google Scholar]
- 51.Mackett M., Smith G.L., Moss B. Vaccinia virus: a selectable eukaryotic cloning and expression vector. Proc Natl Acad Sci USA. 1982;79:7415–7419. doi: 10.1073/pnas.79.23.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Panicali D., Paoletti E. Construction of poxviruses as cloning vectors: insertion of the thymidine kinase gene from herpes simplex virus into the DNA of infectious vaccinia virus. Proc Natl Acad Sci USA. 1982;79:4927–4931. doi: 10.1073/pnas.79.16.4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sugimoto M., Yamanouchi K. Characteristics of an attenuated vaccinia virus strain, LC16mO, and its recombinant virus vaccines. Vaccine. 1994;12:675–681. doi: 10.1016/0264-410x(94)90215-1. [DOI] [PubMed] [Google Scholar]
- 54.Kitabatake M., Yasui F., Inoue S., Morita K., Murai F., Kidokoro M. Proceedings of the eighth annual conference on vaccine research. 2005. Development of SARS vaccine using recombinant vaccinia virus derived from LC16m8. Abstract P65. [Google Scholar]
- 55.Kidokoro M., Aoki A., Horiuchi K., Shida H. Large-scale preparation of biologically active measles virus haemagglutinin expressed by attenuated vaccinia virus vectors. Microb Infect. 2002;4:1035–1044. doi: 10.1016/s1286-4579(02)01627-1. [DOI] [PubMed] [Google Scholar]
- 56.Morita M., Suzuki K., Yasuda A., Kojima A., Sugimoto M., Watanabe K. Recombinant vaccinia virus LC16mO or LC16m8 that expresses hepatitis B surface antigen while preserving the attenuation of the parental virus strain. Vaccine. 1987;5:65–70. doi: 10.1016/0264-410x(87)90012-0. [DOI] [PubMed] [Google Scholar]
- 57.Watanabe K., Kobayashi H., Kajiyama K., Morita M., Yasuda A., Gotoh H. Improved recombinant LC16mO or LC16m8 vaccinia virus successfully expressing hepatitis B surface antigen. Vaccine. 1989;7:53–59. doi: 10.1016/0264-410x(89)90011-x. [DOI] [PubMed] [Google Scholar]
- 58.Shida H., Hinuma Y., Hatanaka M., Morita M., Kidokoro M., Suzuki K. Effects and virulences of recombinant vaccinia viruses derived from attenuated strains that express the human T-cell leukemia virus type 1 envelope gene. J Virol. 1988;62:4474–4480. doi: 10.1128/jvi.62.12.4474-4480.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yasuda A., Kimura-Kuroda J., Ogimoto M., Miyamoto M., Sata T., Sato T. Induction of protective immunity in animals vaccinated with recombinant vaccinia viruses that express PreM and E glycoproteins of Japanese encephalitis virus. J Virol. 1990;64:2788–2795. doi: 10.1128/jvi.64.6.2788-2795.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ohishi K., Suzuki H., Maruyama T., Yamamoto T., Funahashi S., Miki K. Induction of neutralizing antibodies against bovine leucosis virus in rabbits by vaccination with recombinant vaccinia virus expressing bovine leucosis virus envelope glycoprotein. Am J Vet Res. 1990;51:1170–1173. [PubMed] [Google Scholar]
- 61.Asano K., Tsukiyama T., Shibata S., Yamaguchi K., Momoki T., Maruyama T. Immunological and virological characterization of improved construction of recombinant vaccinia virus expressing rinderpest virus hemagglutinin. Arch Virol. 1991;116:81–90. doi: 10.1007/BF01319233. [DOI] [PubMed] [Google Scholar]
- 62.Yamanouchi K., Inui K., Sugimoto M., Asano K., Nishimaki F., Kitching R.P. Immunisation of cattle with a recombinant vaccinia vector expressing the haemagglutinin gene of rinderpest virus. Vet Rec. 1993;132:152–156. doi: 10.1136/vr.132.7.152. [DOI] [PubMed] [Google Scholar]
- 63.Xuan X., Kojima A., Murata T., Mikami T., Otsuka H. Analysis of canine herpesvirus gB, gC, and gD expressed by a recombinant vaccinia virus. Arch Virol. 1997;142:1003–1010. doi: 10.1007/s007050050135. [DOI] [PubMed] [Google Scholar]
- 64.Ohishi K., Inui K., Barrett T., Yamanouchi K. Long-term protective immunity to rinderpest in cattle following a single vaccination with a recombinant vaccinia virus expressing the virus haemagglutinin protein. J Gen Virol. 2000;81:1439–1446. doi: 10.1099/0022-1317-81-6-1439. [DOI] [PubMed] [Google Scholar]
- 65.Cherry J.D., Rolfe U.T., Dudley J.P., Garakian A.J., Murphy M. Clinical and immunological study of percutaneous revaccination in children who originally received smallpox vaccine subcutaneously. J Clin Microbiol. 1978;7(2):158–164. doi: 10.1128/jcm.7.2.158-164.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McIntosh K., Cherry J.D., Benenson A.S., Connor J.D., Alling D.W., Rolfe U.T. Standard percutaneous revaccination of children who receive primary percutaneous vaccination. J Infect Dis. 1977;135(1):155–166. doi: 10.1093/infdis/135.1.155. [DOI] [PubMed] [Google Scholar]
- 67.Rosenthal S.R., Merchlinsky M., Kleppinger C., Goldenthal K.L. Developing new smallpox vaccines. Emerg Infect Dis. 2001;7:920–962. doi: 10.3201/eid0706.010602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.21 CFR Section 601.90: approval of biological products when human efficacy studies are not ethical or feasible.
- 69.Bedson H.S., Duckworth M.J. Rabbit pox: an experimental study of the pathways of infection in rabbits. J Pathol Bacteriol. 1963;85:1–20. [PubMed] [Google Scholar]
- 70.Appleyard G., Hapel A.J., Boulter E.A. An antigenic difference between intracellular and extracellular rabbitpox virus. J Gen Virol. 1971;13:9–17. doi: 10.1099/0022-1317-13-1-9. [DOI] [PubMed] [Google Scholar]
- 71.Buller R.M., Owens G., Schriewer J., Melman L., Beadle J.R., Hostetler K.Y. Efficacy of oral active ether lipid analogs of cidofovir in a lethal mousepox model. Virology. 2004;318:474–481. doi: 10.1016/j.virol.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 72.Saijo M., Ami Y., Suzaki Y., Nagata N., Iwata N., Hasegawa H. LC16m8, a highly attenuated vaccinia virus vaccine lacking expression of the membrane protein B5R, protects monkeys from monkeypox. J Virol. 2006;80:5179–5188. doi: 10.1128/JVI.02642-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Horiuchi K, Otani S, Tsuchiya H, Tanaka Y, Miwa Y, Kubotani H, et al. Reintroduction of smallpox vaccine with attenuated vaccine LC16m8 timeline of arriving at manufacturing, clinical studies, and investigation of immunity; 2002.
- 74.Kenner JK, Gurwith M, Luck A, Empig C, Greenberg RN, Edwards K, et al. Safety and immunogenicity of attenuated smallpox vaccine, LC16m8: results from a phase 2 study [poster 206E]. In: Proceedings of the 4th Annual American Society for Microbiology Biodefense Research Meeting; 2006 Feb 17; Washington, DC.
- 75.Monath T.P., Caldwell J.R., Mundt W., Fusco J., Johnson C.S., Buller M. ACAM 2000 clonal Vero cell culture vaccinia virus (New York City Board of Health Strain)—a second-generation smallpox vaccine for biological defense. Int J Inf Dis. 2004;8:s31–s44. doi: 10.1016/j.ijid.2004.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Murphy F.A., Osburn B.I. Adventitious agents and smallpox vaccine in strategic national stockpile. CDC Emerg Infect Dis. 2005;11(7) doi: 10.3201/eid1107.050277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lane J.M., Ruben F.L., Abrutyn E., Millar J.D. Deaths attributable to smallpox vaccination 1959 to 1966 and 1968. JAMA. 1970;212:441–444. [PubMed] [Google Scholar]
- 78.Arness M.K., Eckart R.E., Love S.S., Atwood J.E., Wells T.S., Engler R.J. Myopericarditis following smallpox vaccination. Am J Epidemiol. 2004;160:642–651. doi: 10.1093/aje/kwh269. [DOI] [PubMed] [Google Scholar]
- 79.Enserink M. Smallpox vaccines: looking beyond the next generation. Science. 2004;304:809. doi: 10.1126/science.304.5672.809a. [DOI] [PubMed] [Google Scholar]
- 80.Dryvax [package insert]. Collegeville, PA: Wyeth Pharmaceuticals Inc.; 2004.
- 81.Weltzin R., Liu J., Pugachev K.V., Myers G.A., Coughlin B., Blum P.S. Clonal vaccinia virus grown in cell culture as a new smallpox vaccine. Nat Med. 2003;9:1125–1130. doi: 10.1038/nm916. [DOI] [PubMed] [Google Scholar]
- 82.Greenberg R.N., Kennedy J.S., Clanton D.J., Plummer E.A., Hague L., Cruz J. Safety and immunogenicity of new cell-cultured smallpox vaccine compared with calf-lymph derived vaccine: a blind, single-centre, randomised controlled trial. Lancet. 2005;365:398–409. doi: 10.1016/S0140-6736(05)17827-1. [DOI] [PubMed] [Google Scholar]
- 83.Artenstein A.W., Johnson C., Marbury T.C., Morrison D., Blum P.S., Kemp T. A novel, cell culture-derived smallpox vaccine in vaccinia-naive adults. Vaccine. 2005;23:3301–3309. doi: 10.1016/j.vaccine.2005.01.079. [DOI] [PubMed] [Google Scholar]
- 84.Government urged to stockpile smallpox vaccine. The Daily Yomiuri. Tokyo:July 12, 2005.
- 85.Wyatt L.S., Earl P.L., Eller L.A., Moss B. Highly attenuated smallpox vaccine protects mice with and without immune deficiencies against pathogenic vaccinia virus challenge. Proc Natl Acad Sci USA. 2004;101:4590–4595. doi: 10.1073/pnas.0401165101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Earl P.L., Americo J.L., Wyatt L.S., Eller L., Whitbeck J.C., Cohen G.H. Immunogenicity of a highly attenuated MVA smallpox vaccine and protection against monkeypox. Nature. 2004;428:182–185. doi: 10.1038/nature02331. [DOI] [PubMed] [Google Scholar]
- 87.Slifka M.K. The future of smallpox vaccination: is MVA the key? Med Immunol. 2005;4:2. doi: 10.1186/1476-9433-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Engler R.J., Kenner J.R., Leung D.Y. Smallpox vaccination: risk considerations for patients with atopic dermatitis. J Allergy Clin Immunol. 2002;110:357–365. doi: 10.1067/mai.2002.128052. [DOI] [PubMed] [Google Scholar]
- 89.McCurdy L.H., Rutigliano J.A., Johnson T.R., Chen M., Graham B.S. Modified vaccinia virus Ankara immunization protects against lethal challenge with recombinant vaccinia virus expressing murine interleukin-4. J Virol. 2004;78:12471–12479. doi: 10.1128/JVI.78.22.12471-12479.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Edghill-Smith Y., Bray M., Whitehouse C.A., Miller D., Mucker E., Manischewitz J. Smallpox vaccine does not protect macaques with AIDS from a lethal monkeypox virus challenge. J Infect Dis. 2005;191:372–381. doi: 10.1086/427265. [DOI] [PubMed] [Google Scholar]
- 91.Stittelaar K.J., van Amerongen G., Kondova I., Kuiken T., van Lavieren R.F., Pistoor F.H. Modified vaccinia virus Ankara protects macaques against respiratory challenge with monkeypox virus. J Virol. 2005;79:7845–7851. doi: 10.1128/JVI.79.12.7845-7851.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tscharke D.C., Karupiah G., Zhou J., Palmore T., Irvine K.R., Haeryfar S.M. Identification of poxvirus CD8+ T cell determinants to enable rational design and characterization of smallpox vaccines. J Exp Med. 2005;201(1):95–104. doi: 10.1084/jem.20041912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mathew A., Terajima M., West K., Green S., Rothman A.L., Ennis F.A. Identification of murine poxvirus-specific CD8+ CTL epitopes with distinct functional profiles. J Immunol. 2005;174:2212–2219. doi: 10.4049/jimmunol.174.4.2212. [DOI] [PubMed] [Google Scholar]
- 94.Davies D.H., Liang X., Hernandez J.E., Randall A., Hirst S., Mu Y. Profiling the humoral immune response to infection by using proteome microarrays: high-throughput vaccine and diagnostic antigen discovery. Proc Natl Acad Sci USA. 2005;102:547–552. doi: 10.1073/pnas.0408782102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Edghill-Smith Y., Golding H., Manischewitz J., King L.R., Scott D., Bray M. Smallpox vaccine-induced antibodies are necessary and sufficient for protection against monkeypox virus. Nat Med. 2005;11:740–747. doi: 10.1038/nm1261. [DOI] [PubMed] [Google Scholar]
- 96.Cohen J. Bioterrorism. smallpox vaccinations: how much protection remains? Science. 2001;294:985. doi: 10.1126/science.294.5544.985. [DOI] [PubMed] [Google Scholar]
- 97.Mack T.M. Smallpox in Europe, 1950–1971. J Infect Dis. 1972;125:161–169. doi: 10.1093/infdis/125.2.161. [DOI] [PubMed] [Google Scholar]
- 98.el-Ad B., Roth Y., Winder A., Tochner Z., Lublin-Tennenbaum T., Katz E. The persistence of neutralizing antibodies after revaccination against smallpox. J Infect Dis. 1990;161:446–448. doi: 10.1093/infdis/161.3.446. [DOI] [PubMed] [Google Scholar]
- 99.Demkowicz W.E., Jr., Littaua R.A., Wang J., Ennis F.A. Human cytotoxic T-cell memory: long-lived responses to vaccinia virus. J Virol. 1996;70:2627–2631. doi: 10.1128/jvi.70.4.2627-2631.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Frelinger J.A., Garba M.L. Responses to smallpox vaccine. N Engl J Med. 2002;347:689–690. [PubMed] [Google Scholar]
- 101.Hammarlund E., Lewis M.W., Carter S.V., Amanna I., Hansen S.G., Strelow L.I. Multiple diagnostic techniques identify previously vaccinated individuals with protective immunity against monkeypox. Nat Med. 2005;11:1005–1011. doi: 10.1038/nm1273. [DOI] [PubMed] [Google Scholar]


