Abstract
The abietane-type diterpenoid (+)-ferruginol (1), a bioactive compound isolated from several plants, has attracted much attention as consequence of its pharmacological properties, which includes antibacterial, antifungal, antimicrobial, cardioprotective, anti-oxidative, anti-plasmodial, leishmanicidal, anti-ulcerogenic, anti-inflammatory and antitumor actions. In this study, we report on the antiviral evaluation of ferruginol (1) and several analogues synthesized from commercial (+)-dehydroabietylamine. Thus, the activity against Human Herpesvirus type 1, Human Herpesvirus type 2 and Dengue Virus type 2, was studied. Two ferruginol analogues showed high antiviral selectivity index and reduced viral plaque-size in post-infection stages against both Herpes and Dengue viruses. A promising lead, compound 8, was ten-fold more potent (EC50 = 1.4 μM) than the control ribavirin against Dengue Virus type 2. Our findings suggest that the 12-hydroxyabieta-8,11,13-triene skeleton, which is characteristic of the diterpenoid ferruginol (1), is an interesting molecular scaffold for development of novel antivirals. In addition, the cytotoxic and antifungal activities of the synthesized ferruginol analogues have also been investigated. ©20155 Elsevier Science. All rights reserved.
Keywords: Antiviral, Herpes, Dengue, Abietane, Diterpene, Ferruginol, Dehydroabietylamine
Graphical abstract
Highlights
-
•
Aromatic abietanes are a class of bioactive diterpenoids.
-
•
Article focuses on the antiviral activity of several ferruginol analogues.
-
•
Compound 8 was ten-fold more potent than the reference ribavirin against DENV-2.
1. Introduction
Human Herpesvirus (HHV) types 1 and 2 are enveloped DNA viruses characterized for producing latent infection in sensory neurons and reactivation during periods of severe immunosuppression of the host. Many factors such as stress, fatigue, sexual relations with people who have active lesions, excess exposure to heat or cold, fever, laser treatments, local tissue trauma and nerve damage, can predispose to reactivation, especially in neonates, transplant and immunocompromised patients which tend to present aggressive and recurrent lesions [1]. Also, it has been reported that prolonged therapy with Acyclovir (ACV), and its analogues, has induced the emergence of drug-resistant virus strains. Human Herpesvirus predominantly develops resistance, almost 95%, as a result of mutations in the genes that encode the viral thymidine kinase (TK) [2].
However, mutations in the viral DNA polymerase can also cause this resistance. In immunocompromised patients, the presence of HHV species resistant to ACV, with a prevalence of 4–10%, has complicated their clinical management [3]. Moreover, Dengue virus (DENV) is an enveloped ssRNA virus. To date, there have been described four serotypes established in humans (DENV-1 to DENV-4) and recently, a fifth Dengue subtype that follows the sylvatic cycle has been identified [4]. Dengue is the most important mosquito-borne disease worldwide, since it is distributed over one hundred countries of the tropical belt and the tropics, both the Old and New World [5]. Disease manifestations cover a wide spectrum ranging from dengue with or without warning signs to severe dengue in which plasma leakage, haemorrhage and organ impairment can lead to death. In addition, unusual manifestations such as cardiomyopathy, liver failure and neurological disorders have been reported [6]. The major problem of this pathology is that there are no drugs approved for human treatment or vaccine available for prophylaxis. Also, antiviral drugs addressed to specific genes or proteins of the virus are not a good alternative due to the high mutation rate of RNA viruses, often because viral mutants are selected for drug resistance [7]. During the last years in Colombia, several reports had shown the high rates of evolution of DENV, possibly related with the new Asiatic-American genotypes found in Colombia and Brazil [8].
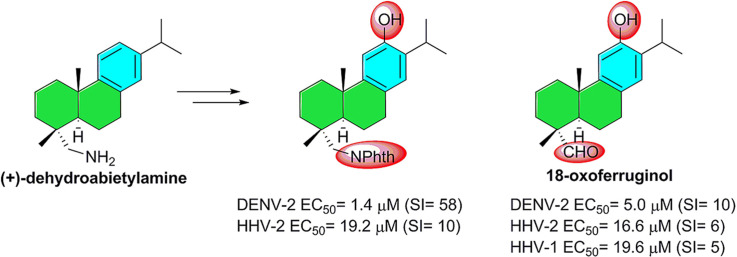
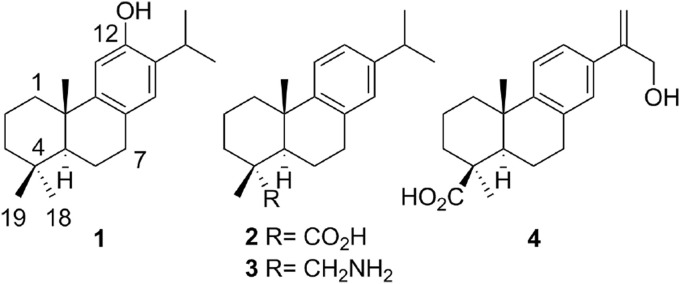
The aromatic abietane-type diterpenoids comprise a large number of natural metabolites of plant origin which possess a wide variety of biological effects [9]. These abietanes have been the target of several synthetic campaigns towards both the natural products and synthetic derivatives with interesting pharmacological properties [10]. Among these known bioactive compounds, (+)-ferruginol (1), some derivatives of (+)-dehydroabietic acid (2) and (+)-dehydroabietylamine (3) as well as (+)-jiadifenoic acid C (4) (Fig. 1 ) have shown promising results, including antiviral properties (b), [11].
Fig. 1.
Examples of bioactive aromatic abietane diterpenoids.
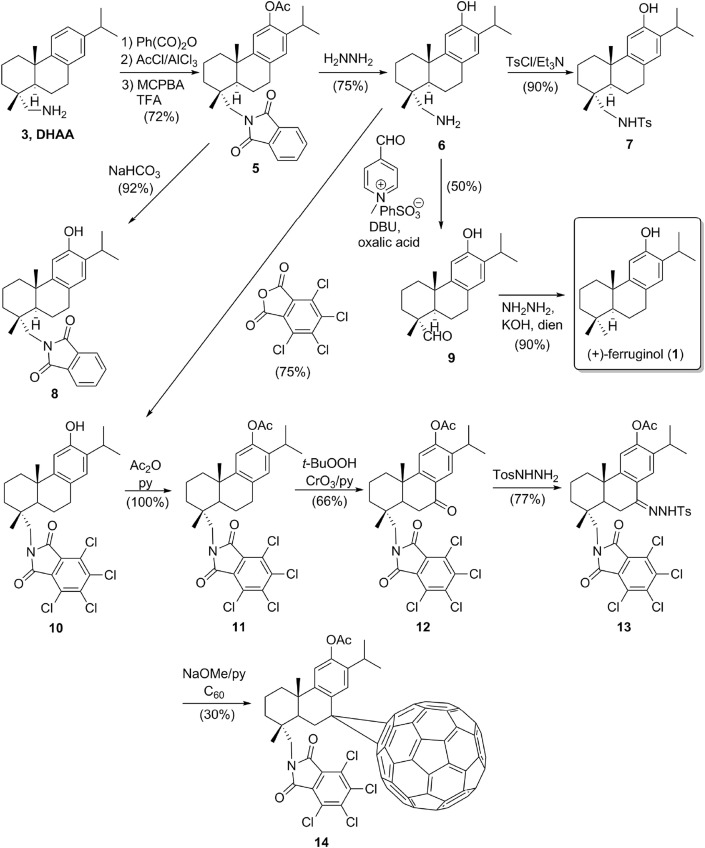
The present study is a continuation of our research programs to discover bioactive diterpenoids [12]. It includes the synthesis of a focused library based on (+)-ferruginol (1) from commercially available (+)-dehydroabietylamine (3), where a series of phthalimides could have potential antiviral activity [13]. A fullerene diterpenoid hybrid was also envisaged as potential inhibitor [14]. Herein, we describe the antiviral activity of ferruginol (1) and some analogues (5–14) (Scheme 1 ) synthesized from dehydroabietylamine (3) against HHV-1 and HHV-2, and DENV-2. Our aim is to identify new antiviral drug candidates which act on cellular and/or molecular targets instead on viral proteins. In this way, the concerns about the viral mutants resistant to the treatments can be avoided. This approach has been recently called host-targeted antiviral in an evolutionary study of resistance of Dengue viruses under selective pressure of several antiviral agents [15].
Scheme 1.
Synthesis of the tested compounds 1 and 5–14.
2. Results and discussion
2.1. Chemistry
The synthesis of (+)-ferruginol (1) and analogues 5–14 from commercial (+)-dehydroabietylamine (3) was performed as outlined in the Scheme 1. Compounds 1 and 5–9 were synthesized as reported in the literature using a Friedel–Crafts acylation and a Baeyer–Villiger oxidation as key steps [16]. Then, compound 6 was used as starting material for the preparation of compounds 10 and 11. Later, compound 11 was converted into compounds 12–14 as described recently in the literature [17]. Thus, the synthesis starts with the introduction of the phthalimide group on (+)-dehydroabietylamine (3), followed by Friedel–Crafts acylation and oxidation under Baeyer-Villiger conditions to afford acetate 5 in 72% overall yield. Hydrolysis of the acetate group in 5 gave phenol 8 in high yield, while overall deprotection of 5 afforded the amino-phenol 6 in 75% yield. Compound 6 was the intermediate of three separate approaches. Firstly, tosylation under standard conditions gave compound 7 in 90% yield. Secondly, oxidative deamination of 6 gave 18-oxoferruginol (9) in moderate yield (50%), which was converted into ferruginol (1) by Wolff-Kishner reduction (90% yield). And finally, the treatment of 6 with tetrachlorophthalic anhydride (TCPA) afforded phenol 10 in 75% yield, which was acetylated with acetic anhydride in pyridine to give acetate 11 in quantitative yield. Acetate 11 was oxidized at C-7 with excess of t-BuOOH as oxidant and CrO3/pyridine mixture as a catalyst in DCM, the yield of ketone 12 was 66%. Subsequently, the reaction of 12 with p-tosylhydrazide yielded the corresponding p-tosylhydrazone 13 (77% yield). The fullerene-terpenoid hybrid 14 was obtained by the treatment with NaOMe of 13 in anhydrous pyridine for 20 min at room temperature followed by addition of a solution of C60 in chlorobenzene and heating at 70 °C for 24 h. This gave compound 14 in ca. 30% yield. All the compounds showed spectroscopic data in agreement with the assigned structures and purity >95%.
2.2. Biological evaluation
The synthesized compounds 1 and 5–14 (Scheme 1) were evaluated for antiviral activity against HHV-1, HHV-2 and DENV-2 (see Table 1 ). Two ferruginol analogues, compounds 8 and 9 (18-oxoferruginol), showed relevant activity.
Table 1.
Reduction of viral titer, inhibition of cytopathic effect and antiviral activity against HHV-1, HHV-2 and DENV-2 of ferruginol analogues 8 and 9.
| Vero cell linea | ||||||
|---|---|---|---|---|---|---|
| Compound | HHV-1 |
HHV-2 |
DENV-2 |
|||
| Rfb | Antiviral activity (μM)c | Rfb | Antiviral activity (μM)c | CPE inhibitiond | Antiviral activity (μM)c | |
| 8 | 102 | 14.5 | 103 | 29.0 | + | 29.0 |
| 9 | NA | NA | 103 | 41.7 | + | 41.7 |
| ACV | 104 | 6.7 | 103 | 6.7 | NT | NT |
| RIBA | NT | NT | NT | NT | + | 123.0 |
HHV-1: Human Herpesvirus type 1 (CDC Atlanta strain). HHV-2: Human Herpesvirus type 2 (VR-734-G strain). DENV-2: Dengue virus (New Guinea strain). NT: not tested. NA: not active.
These values represent the mean of two independent experiments.
Vero Cercopithecus aethiops African green monkey kidney cell line ATCC CCL-81.
Reduction factor of the viral titer.
Maximal non-toxic concentration that showed viral reduction factor.
Cytopathic effect inhibition (+).
In particular, the ferruginol analogue 8 showed moderate activity against HHV-1 (Rf = 1 × 102) at a concentration of 14.5 μM, high activity against HHV-2 (Rf = 1 × 103) and reduction of cytopathic effect during DENV-2 infection, comparable to control of untreated cells, at concentration of 29.0 μM. Compound 9 (18-oxoferruginol) also showed high activity against HHV-2 (Rf = 1 × 103) at a concentration of 41.7 μM, however, its ability to exert reduction of cytopathic effect in the presence of DENV-2 infection was lower than that of compound 8. Moreover, compound 9 did not show activity against HHV-1.
In 2007, Wen and co-workers reported the antiviral potential of certain diterpenoid derivatives, including the abietane-type diterpenoid ferruginol (1), during in vitro coronavirus infection [18]. However, at present, the antiviral activity of the abietane class of compounds has not been much investigated, especially the study of derivatives and analogues; therefore, the activity against HHV-1, HHV-2 and DENV-2 reported in this study is to our knowledge, the first report of antiviral activity of ferruginol analogues. Also, as the compounds 8 and 9 are active against two different viruses, such as herpesvirus and dengue virus, our findings suggest that these molecules probably have a broad-spectrum of antiviral activity against enveloped viruses with DNA and RNA genome.
Later on, to check the specific antiviral activity of these compounds, we carried out the evaluation of other biological activities such as cytotoxic and antifungal, because is well known that ferruginol (1) has promising bioactivities, such as antifungal, antibacterial, miticidal antiplasmodial, antileishmanial, nematicidal, and antitumor, among others [9]. In Table 2 , it is shown the cytotoxic activity on Vero cells as well as on the tumor cells HeLa, Jurkat and U937 of ferruginol 1 and analogues 5–14.
Table 2.
Cytotoxicity (IC50-μM) of ferruginol (1) and analogues 5–14 on HeLa, Jurkat, U937 and Vero cell lines.
| Compound | Cell linesc |
||||||
|---|---|---|---|---|---|---|---|
| HeLa |
Jurkat |
U937 |
Vero |
||||
| IC50a (μM) | SIb | IC50a (μM) | SIb | IC50a (μM) | SIb | IC50a (μM) | |
| 1 | 64.9 | 1.4 | 48.2 | 1.9 | 21.3 | 4.2 | 90.4 |
| 5 | >50 | NA | >50 | NA | >50 | NA | NE |
| 6 | >80 | NA | 49.4 | 0.8 | >80 | NA | 39.8 |
| 7 | >50 | NA | 30.9 | 2.7 | 36.0 | 2.3 | 82.5 |
| 8 | >50 | NA | >50 | NA | >50 | NA | NE |
| 9 | 60.0 | 1.5 | 21.6 | 4.2 | 33.2 | 2.7 | 90.8 |
| 10 | 29.0 | 9.2 | 21.9 | 12.2 | >40 | NA | 266.8 |
| 11 | >40 | NA | >40 | NA | >40 | NA | NE |
| 12 | 7.3 | >43.8 | 12.3 | >26.0 | 8.3 | >38.5 | >320 |
| 13 | >30 | NA | >30 | NA | >30 | NA | NE |
| 14 | >30 | NA | >30 | NA | >30 | NA | NE |
| PXT | 0.099 | NA | 0.0005 | NA | 0.006 | NA | 0.65 |
| DOX | 0.834 | NA | 0.012 | NA | 0.077 | NA | 1.93 |
PXT: Paclitaxel; DOX: Doxorubicine. NA: Not apply; NE: Not evaluated. These values represent the geometric mean of two independent experiments, each one made by quadruplicate.
Inhibitory concentration 50 defined as concentration of compounds that induces 50% of growth inhibition at 48 h.
SI, selectivity index defined as VERO IC50 over either Jurkat, U937 or HeLa IC50.
Jurkat, human acute T cell leukemia ATCC TIB-152; U937, human promonocytic cell line ATCC CRL-1593.2; HeLa, human cervix epitheloid adenocarcinoma cells ATCC CRL-1958; Vero, Cercopithecus aethiops African green monkey kidney cells ATCC CCL-81.
Compounds 1, 6, 7, 9, 10 and 12 produced a dose-dependent inhibition on the growth of the three tumor cell lines: Jurkat, U937 and HeLa, as well as the Vero cell line, with R2 (coefficient of linear regression) > 0.8. Most of these compounds showed cytotoxic activity against at least one tumor cell line at concentrations below 30 μM. However, only compound 10 against HeLa and Jurkat cell lines, and compound 12 against the three tumor cell lines, showed a great SI (SI ≥ 5), being compound 12 the most selective agent against tumor cell lines respect the non-tumor cell line (SI > 43.8, >26.0 and > 38.5, for HeLa, Jurkat and U937, respectively). The compounds 5, 8, 11, 13 and 14 were not active against the tumor cell lines at the tested concentrations and hence they were not evaluated on the Vero cell line.
In addition, the antifungal activity of all compounds was studied against two dermatophytes (Trichophyton rubrum and Trichophyton mentagrophytes), and the filamentous fungi Fusarium oxysporum, Aspergillus fumigatus, Aspergillus flavus and Aspergillus terreus. Only one ferruginol analogue, compound 9, showed anti-dermatophyte activity against T. rubrum and T. mentagrophytes with geometric mean of minimal inhibitory concentration (GM-MIC) values of 41.7 μM and 83.3 μM, respectively. None of the compounds showed activity against the filamentous fungus tested (data not shown). Likewise, none of the compounds showed anti-Candida activity against three yeast strains (Candida albicans, Candida parapsilosis and Candida tropicalis) (data not shown).
Although there are several studies showing that ferruginol (1) and some related abietanes exhibit promising antifungal and cytotoxic activity [19], our results reveals that compound 8 lacks of antifungal activity in the fungal models tested and cytotoxicity against HeLa, Jurkat and U937 tumor cells at the evaluated concentrations. Therefore, this compound could be a selective molecule against enveloped viruses.
2.3. Mechanism of antiviral activity of ferruginol analogues 8 and 9
Previous studies suggest that the mechanism by which ferruginol (1) exerts antitumor activity is related to the enhanced expression of BAX proapoptotic protein, inhibition of signaling pathways involved in cell survival and proliferation (Ras /PI3K and Jak/STAT) and reduction of expression of cyclin-dependent kinases [20]. However, the cellular and molecular basis by which these compounds exhibit antiviral activity has not been currently described.
In order to define the stage of viral replication cycle in which the ferruginol analogues 8 and 9 were active, we performed two different conditions of treatment. Firstly, we tested the effect of compounds on early stages of viral replication cycle, such as cell-free viral particle, adhesion and viral entry by treatment before infection (pre-infection treatment). And secondly, we tested the antiviral activity on several steps of viral cycle that includes traffic, replication, assembly, egress of new viral particles and cell-to-cell spread by treatment after infection (post-infection treatment).
As can be seen in Table 3 , compounds 8 and 9 were effective during post-infection treatment and were not active during pre-infection treatment.
Table 3.
Antiviral activity (EC50-μM) on pre and post-infection treatment of ferruginol analogues 8 and 9.
| Compound | IC50 (μM) 72 h |
IC50 (μM) 8 days |
EC50 (μM) (SI) Pre-infection treatment |
EC50 (μM) (SI) Post-infection treatment |
||||
|---|---|---|---|---|---|---|---|---|
| HHV-1 | HHV-2 | DENV-2 | HHV-1 | HHV-2 | DENV-2 | |||
| 8 | 190.7 | 80.8 | NI | NI | NI | NI* | 19.2 (10.0) | 1.4 (57.7)* |
| 9 | 97.8 | 52.2 | NI | NI | NI | 19.6 (4.9) | 16.6 (5.9)* | 5.0 (10.4) |
| DEX-S | >10 | NT | 0.015 | 0.012 | NT | NT | NT | NT |
| ACV | >1700 | NT | NT | NT | NT | 2.2 | 0.27 | NT |
| RIBA | NT | NT | NT | NT | NT | NT | NT | 13.5 |
EC50, 50% antiviral effective concentration; IC50, 50% inhibitory concentration of cell proliferation (μM); SI, Antiviral selectivity index values (IC50/EC50) are presented between parenthesis; DEX-S, dextran sulfate; ACV, acyclovir; RIBA, ribavirin; *Reduction on viral plaque-size. NI, no inhibitory activity; NT, not tested. These values represent the mean of three independent experiments.
With both pre- and post-infection treatments, a plaque reduction assay was performed and the concentration of compounds 8 and 9 that reduced the number of viral plaques in 50% (EC50) was interpolated from the dose–response curves. Furthermore, the antiviral selectivity index (SI) was calculated through the relation between the inhibitory concentration 50 (IC50) in Vero cells and EC50 of each virus (IC50/EC50) (Table 3).
Compound 8 reduced the 50% of plaque forming units at a concentration of 19.2 μM against HHV-2 and showed a high SI equal to 10, whereas compound 9 reduced the number of plaques in 50% at concentrations of 19.6 μM and 16.6 μM, for HHV-1 and HHV-2, respectively. As expected the controls, DEX-S and ACV, did show antiviral activity in pre-treatment and post-treatment, respectively.
These results show that the compounds 8 and 9 have important activity against HHV-2 strain at low concentrations, in comparison to HHV-1. This is possibly due to a smaller number of receptors available in the host cell to carry out the infection with HHV-2, making this virus more sensitive to the action of the compounds [21].
Differences in the activity of these compounds were found against HHV-1 strain. In the end-point titration technique (EPTT) assay, compound 8 showed moderate activity (Table 1, Rf = 1 × 102) and in the plaque forming unit (PFU) assay this compound did not show a dose-dependent effect. However, it showed a significant reduction in viral plaque size (Table 3, highlighted with an asterisk), related among others, with a low rate of viral replication and reduction of cytopathic effect, confirming the antiviral effect of this compound.
On the other hand, compound 9 did not show reduction of viral titer during screening (EPTT) against HHV-1, but inhibits the number of plaque forming units during the PFU assay. This difference can be explained due the amount of virus used in each assay. In the EPTT assay a high amount of virus (10 TCID50) was used and the concentration of compound that showed antiviral activity could not be detected. In the PFU assay, 100 PFU/well was used and the EC50 value was determined, nevertheless, this compound did not show a high SI value (4.9), demonstrating closeness between the cytotoxic concentration and the antiviral concentration.
Likewise, the anti-dengue activity of these ferruginol analogues was evaluated 8 days post-infection (d.p.i), being both compounds 8 and 9 active at EC50 concentrations of 1.4 μM (SI = 57.7) and 5.0 μM (SI = 10.4), respectively.
The antiviral selectivity index is defined as the relative effectiveness of a product to inhibit viral replication compared to the capacity for inducing cell death. This index results useful to make bioactivity comparisons between the compounds and helps in designing more potent compounds. In recent reports, SI values higher than 10 (SI > 10) are considered indicative of a potential therapeutic agent that would merit further biopharmaceutical and pre-clinical studies [22]. Therefore, compound 8 is promissory to continue this type of study.
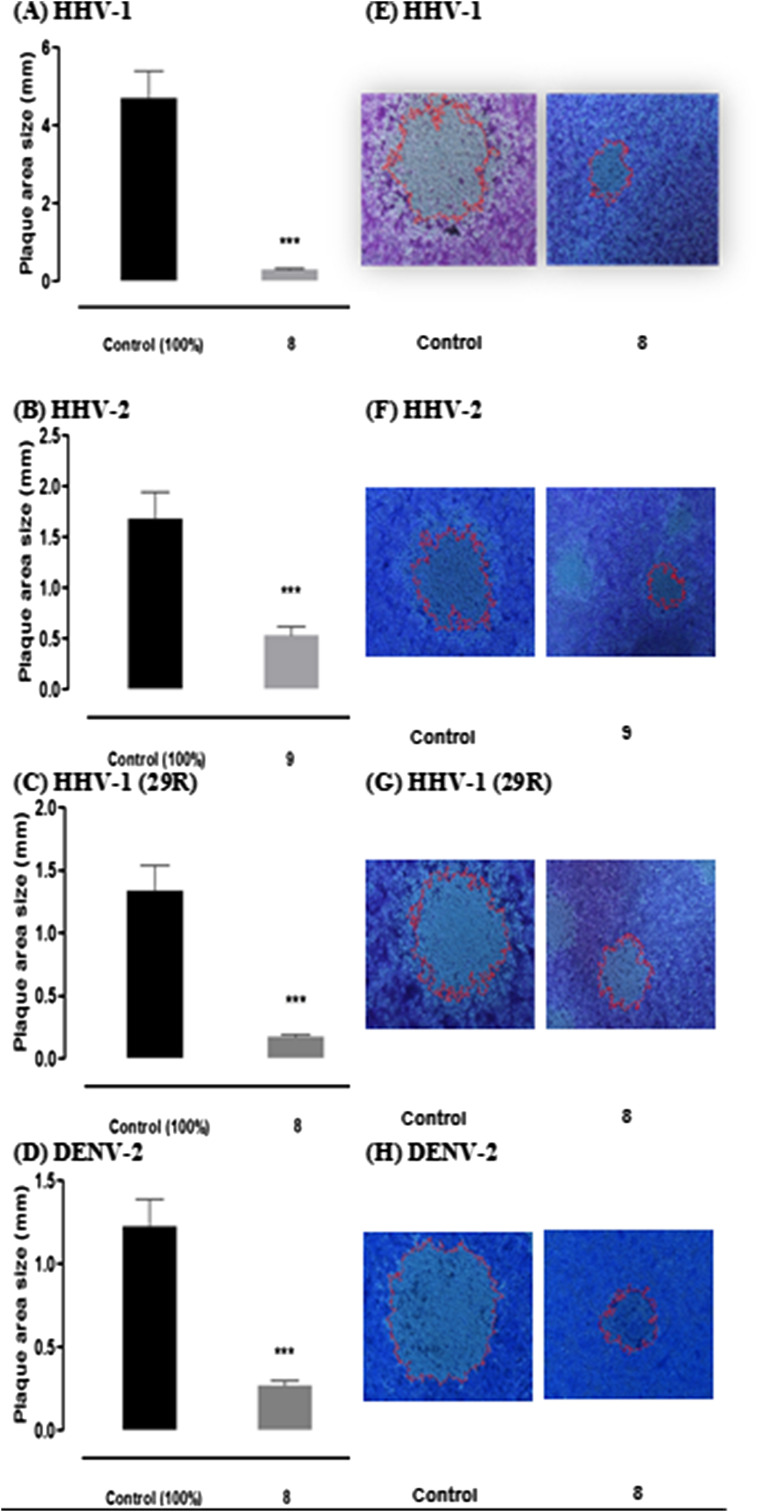
Additionally to the dose-dependent effect previously described during post-infection treatment, we found that compounds 8 and 9 induced a reduction on viral plaque size. To determine the average of reduction of viral plaque size during compound-treatment relative to mock-treated control, we performed an image analysis using Image-Pro Plus 6.0 software (Fig. 2 ). The area of plaques was measured in millimeters and statistically significant differences (P value < 0.001) were found in all cases. Compound 8 showed a dramatic reduction on viral plaque size (93.7%) at a concentration of 29.0 μM against HHV-1 (CDC-Atlanta acyclovir-sensitive strain) (Fig. 2A). Respect to HHV-2, compound 9 reduced the size of viral plaques (68.2%) at a concentration of 20.8 μM (Fig. 2B).
Fig. 2.
Effect of compounds 8 and 9 on HHV-1, HHV-2 and DENV-2 plaque-size during Vero cell infection. Effect on viral plaque-size of compounds 8 and 9 in presence of 100 PFU/mL of HHV-1 (2A), HHV-2 (2B), HHV-1 29R strain (2C) and DENV-2 (2D). Results were expressed as an average of plaque size area (mm) of 80 viral plaques developed in compound-treated cells relative to mock-treated controls. Statistically significant differences were found in all cases with p values < 0.001 (∗∗∗). Parallel, the image analysis using Image-Pro Plus 6.0 software represents the reduction of viral plaque size during treatment with compounds 8 and 9 of HHV-1 (2E), HHV-2 (2F), HHV-1 29R strain (2G) and DENV-2 (2H). Two separate experiments were carried out for each compound.
Furthermore, during treatment with compound 8 at a concentration of 29.0 μM (Fig. 2C) on cell infection by HHV-1 acyclovir-resistant strain 29R (thymidine kinase mutant), we found a similar effect to that showed against the acyclovir-sensitive strain, reducing the viral plaque size in 87.1%. This finding may suggest that the phosphorylation by viral thymidine kinase it is not required for the effect exerted by this compound, thus, is possible that anti-herpetic mechanism of action of ferruginol analogue 8 differs to acyclovir and other nucleoside analogues.
Likewise, reduction on viral plaque-size (77.9%) was observed when cells infected with DENV-2 were treated with compound 8 at concentration of 3.6 μM (Fig. 2D). The reduction percentages reported for all viruses indicate the reduction of viral plaque size during compound-treatment relative to mock-treated control (100%). Representations of plaque morphology of HHV-1(CDC Atlanta), HHV-1 (29R) and DENV-2 after the treatment with compound 8 and mock-treated control, are shown in Figures 2E, F and 2H, respectively. Fig. 2G shows the effect after treatment with compound 9 against HHV-2 and mock-treated control.
The reduction on viral plaque size of HHV strains and DENV-2 in the presence of compounds 8 and 9 suggests that these ferruginol analogues may prevent the first-round of viral replication, release and/or cell-to-cell spread, being this last stage an important factor in infectivity, virulence, establishment of latency and immune response evasion during Herpesvirus infections [23]. Therefore, inhibition of viral intercellular diffusion is an attractive target in the search for new antiviral drugs, mainly to treat the latent infections and drug-resistant strains.
In recent years, several compounds have shown effects on virus cell-to-cell spreading during Herpesvirus infection. For example, Lin and co-workers reported that chebulagic acid and punicalagin, two hydrolyzable tannins isolated from of Terminalia chebula (Combretaceae), inhibit the HHV-1 cell-to-cell spread. This fact was verified 24 h post-treatment using a fluorescent plaque assay in the presence of HHV-1 neutralizing antibodies to guarantee cell-to-cell transmission via intercellular junctions between infected and uninfected cells [24]. Meanwhile, Nyberg and Ekblad and co-workers demonstrated that the low molecular weight heparin sulfate-mimic, PI-88, which is a mixture of highly sulfated mannose containing oligosaccharides, reduced the area of viral plaques with IC50 values of 2 μg/mL and 0.7 μg/mL for HHV-1 and HHV-2, respectively [25]. Nevertheless, to date it has not been reported a similar effect on viral plaque size of HHV and other viruses from abietane diterpenoids, among these, ferruginol (1) and their derivatives.
Several molecular and cellular components involved in Herpesvirus cell-to-cell spread have been previously reported. For example, viral glycoproteins such as E and M play a relevant role in normal viral plaque phenotype of HHV-1, because the plaque size of the double-deletion mutant (ΔgEgM) reduced over 5-fold less than wild type strain [26]. In polarized epithelial cells, Johnson and co-workers demonstrated by electron microscopy, that the gE/gI glycoproteins complex is necessary to accumulate virions at cell junctions, since mutants that not express gE glycoprotein leads to particles accumulation more frequently on apical surfaces. Thus, they concluded that the gE/gI complex is required to sort selectively HHV-1 particles to lateral surfaces and specifically to cell junctions [27]. On the other hand, structures containing actin and tubulin during Herpesviruses infection have the ability to project virions towards adjacent cells. Additionally, both the membrane composition and cytoskeletal structures of egress sites are modified during infection in non-polarized cells [28]. According to the findings of Dixit and co-workers, HHV-1 also promotes cytoskeletal rearrangements that facilitate viral spread in vitro, including dramatic increases of filopodia in cultured neurons [29]. Multiple and convergent ways by which several viruses such as Vaccinia, Hepatitis B and C viruses, Human Immunodeficiency virus, Human T-lymphotropic virus, Murine leukemia virus and HHV cause subversion of cytoskeleton for different objectives in viral cycle are summarized by Taylor and co-workers [30]. The actin cytoskeleton is reorganized by these viruses affecting every stage of the viral life cycle, from entry through assembly to egress. Using immunochemical assays, drug inhibition assays and protein interaction profiling methods, Wang and co-workers identified the ways in which DENV-2 interacts with actin cytoskeleton. This study showed that dynamic treadmilling of actin is necessary for Dengue virus entry, production and release, indicating a direct effect of viral E protein on the structural modifications of actin cytoskeleton [31].
Considering that cytoskeletal components such as microtubules and actin microfilaments are required for both Herpesvirus and Flavivirus replication cycle (mainly involved in the entry, traffic, release and cell-to-cell spread), is possible that ferruginol analogues 8 and 9 cause disruption of these cellular pathways which may be involved in viral spread. Nonetheless, other experiments are required to confirm this hypothesis.
Although it is very risky assure that some viral or cellular component are involved in our findings, the study of ferruginol analogues will be an important tool from medicinal chemistry for dissecting these complicated cellular and molecular processes of the viral infections. In the future, this research will be routed to understanding of some cellular and molecular mechanisms where, two very different viruses (Herpesvirus and Dengue), are converging in a conserved evolutionary pathway during cell infection, affecting cytoskeleton elements, endomembrane system and signaling pathways involved in viral egress from the host-cells and spreading infection toward neighbor cells.
3. Conclusions
In summary, we have discovered important antiviral properties in the molecular scaffold 12-hydroxyabieta-8,11,13-triene functionalized at C-18, which is reminiscent of the abietane diterpenoid ferruginol (1). Two ferruginol analogues, compounds 8 and 9, synthesized from readily available (+)-dehydroabietylamine (3) were found to consistently inhibit Human Herpesvirus (type 1 and 2) and Dengue virus type 2 at low micromolar concentrations. These compounds showed antiviral activity specifically in post-infective stages and significantly reduced the viral plaque-size during infection. The presence of a phthalimide moiety in the molecule, compound 8, resulted in the highest antiviral selectivity index against the viruses tested and a ten-fold more potency (EC50 = 1.4 μM) than the control ribavirin against Dengue Virus type 2.
With regard to the cytotoxic properties of the tested compounds, it should be noted that the chlorinated-phthalimide analogue 12 exhibited moderate cytotoxicity of broad spectrum, without toxicity towards the non-tumor cell line (Vero). Additionally, the tested compounds did not show relevant antifungal activity.
Based on the above, the results here reported are relevant for further research of possible cellular and molecular mechanisms involved in the effect of reduction on viral plaque size. Also, these findings are important to promote the realization of biopharmaceutical testing and finding new highly selective antiviral drugs mainly directed towards cellular targets.
4. Experimental
4.1. Chemistry
4.1.1. General procedures
Optical rotations were measured using a 5 cm cell in a Schmidt-Haensch Polartronic-D polarimeter. NMR spectra were recorded on a 300 MHz spectrometer. All spectra were recorded in CDCl3 as solvent unless otherwise stated. Complete assignments of 13C NMR multiplicities were made on the basis of DEPT experiments. J values are given in Hz. MS data were acquired on a QTOF spectrometer. Reactions were monitored by TLC using Merck silica gel 60 F-254 in 0.25 mm-thick plates. Compounds on TLC plates were detected under UV light at 254 nm and visualized by immersion in a 10% sulfuric acid solution and heating with a heat gun. Purifications were performed by flash chromatography on Merck silica gel (230–400 mesh). Commercial reagent grade solvents and chemicals were used as purchased unless otherwise noted. Combined organic extracts were washed with brine, dried over anhydrous NaSO4, filtered, and concentrated under reduced pressure.
4.1.2. Materials
Compounds 1 and 5–9 have been prepared according to known procedures [16]. Compound 6 was used as starting material for the preparation of compounds 10 and 11. Later, compound 11 was converted into compounds 12–14 as described recently in the literature [17]. All compounds prepared in this work exhibit spectroscopic data in agreement with the proposed structures. Purity of all final compounds was 95% or higher.
4.1.2.1. 12-Hydroxy-N,N- (tetrachlorophthaloyl)dehydroabietylamine (10)
A mixture of the tetrachlorophthalic anhydride (640 mg, 2.14 mmol) and 12-hydroxydehydroabietylamine 6 [16a] (647 mg, 2.14 mmol) in acetic acid (7.5 mL) was heated at reflux for 2 h, then cooled to rt and 50 mL of water was added. The resulting solid was filtered off, washed with water, dried under vacuum and purified by column chromatography eluting with toluene to give compound 10 (913 mg, 75%) as a yellow solid: [α]D 20 −10.0 (c 1.0, CHCl3); 1H NMR (300 MHz) δ 6.87 (1H, s), 6.60 (1H, s), 3.67 (1H, d, J = 15.0), 3.54 (1H, d, J = 15.0), 3.10 (1H, m), 2.92 (2H, m), 2.15 (2H, m), 1.24 (3H, d, J = 6.0), 1.22 (3H, d, J = 6.0), 1.22 (3H, s), 1.04 (3H, s); 13C NMR (75 MHz) δ C 2 × 164.5 (s), 150.6 (s), 148.1 (s), 2 × 140.1 (s), 131.6 (s), 2 × 129.5 (s), 2 × 127.4 (s), 127.1 (s), 126.8 (d), 110.5 (d), 49.8 (t), 45.2 (d), 39.5 (s), 38.0 (t), 37.5 (s), 37.1 (t), 29.3 (t), 26.7 (d), 25.7 (q), 22.7 (q), 22.5 (q), 19.5 (t), 19.0 (q), 18.4 (t). HRMS (ESI) m/z 568.0986 [M+1]+, calcd for C28H30Cl4NO3: 568.0980.
4.1.2.2. 12-Acetoxy-N,N-(tetrachlorophthaloyl)dehydroabietylamine (11)
A solution of phenol 10 (656 mg, 1.15 mmol) in a 1:1 mixture (14 mL) of acetic anhydride and pyridine was stirred at rt for one day. Then, 150 mL of water were added and the mixture was extracted with ethyl acetate (3 × 25 mL). The combined organic extracts were washed with 6% NaHCO3 (2 × 50 mL), water and brine. The resulting organic extract was dried over sodium sulfate, filtered and concentrated. The residue was chromatographed on silica eluting with chloroform to afford acetate 11 (704 mg, 100%) as a white solid with identical spectroscopic data to the reported in the literature [17].
4.3. Biology
4.3.1. Reagents and compounds
Dulbecco's Modified Eagle's Medium (DMEM) and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) were obtained from Sigma–Aldrich Chemical Co. (St. Louis, MO, USA). Fetal bovine serum (FBS) and penicillin/streptomycin were purchased from Invitrogen Life Technologies (Carlsbad, CA, USA). Acyclovir was obtained from Biogen Laboratory. Ribavirin was obtained from Calbiochem (La Jolla, CA, USA). Paclitaxel, Doxorubicine, Amphotericine B and Itraconazole were purchased from Sigma–Aldrich Chemical Co. Terbinafine was obtained from Recalcine Laboratories (Santiago de Chile, Chile). Dimethyl sulfoxide (DMSO) was purchased from Merck KGaA (Darmstadt, Germany).
Stock solutions of compounds were prepared in DMSO and frozen at −70 °C. The concentration of DMSO in biological assays was of 0.05%. Cell controls with DMSO at 0.05% were used.
4.3.2. Cell culture and viruses
Human Herpesvirus type 1 (HHV-1 CDC Atlanta acyclovir-sensitive strain) was obtained from the Center for Disease Control (Atlanta, GA, USA); HHV-1 29R (acyclovir-resistant strain) was donated by Prof. Claudia Oliveira (University of Santacatarina-Brazil) and Human Herpesvirus type 2 (HHV-2 VR-734-G acyclovir-sensitive strain) was obtained from the Center for Disease Control. All of them were amplified in Vero cells (African green monkey kidney-Cercopithecus aethiops, ATCC: CCL 81 line). Virus stocks were titrated in Vero cells by plaque assay and expressed as plaque forming units (PFU/mL). Dengue virus type 2 (DENV-2 New Guinea strain) was donated by Maria Elena Peñaranda and Eva Harris (Sustainable Sciences Institute and the University of California at Berkeley) was amplified in C6/36HT of Aedes albopictus cells, from ATCC, and titrated in Vero cells following our laboratory conditions [32].
Cells were maintained in Dulbecco's Modified Eagle Medium (DMEM) supplemented with 5% of inactivated FBS to Vero cells and 10% of inactivated FBS to C6/36HT cells, 100 units/mL of penicillin, 100 μg/mL of streptomycin, 100 μg/mL of l-glutamine, 0.14% NaHCO3, and 1% of each non-essential amino acids and minimum essential medium vitamin solution (choline chloride, D-calcium pantothenate, folic acid, nicotinamide, pyridoxal hydrochloride, riboflavin, thiamine hydrochloride and i-inositol). Vero cells were incubated at 37 °C in humidified 5% CO2 atmosphere and C6/36 HT at 34 °C in humidified 5% CO2 atmosphere.
To determine the cytotoxic activity, cell lines of human cervix epitheloid adenocarcinoma cells (HeLa, ATCC CCL-2), acute T cell leukemia (Jurkat, ATCC TIB-152) and human promonocytic cell line (U937, ATCC CRL-1593.2) were used, as well as, the non-tumor cell line Vero. Vero and HeLa cells were grown in DMEM supplemented. Jurkat and U937 cells were maintained in RPMI-1640 medium (supplemented with 10% FBS), 100 units/mL of penicillin, 100 μg/mL of streptomycin, and 100 μg/mL of neomycin and maintained at 37 °C in humidified 5% CO2 atmosphere.
4.4. Antiviral activity
4.4.1. Screening against HHV-1 and HHV-2
The anti-herpetic activity of ferruginol analogues on Vero cells against 10 TCID50 of HHV-1 and HHV-2, were carried out using the end-point titration technique (EPTT). Vero cells were harvested in 96-well plates at a density of 2.0 × 104 cells/well at 37 °C in humidified 5% CO2 atmosphere until constituted ≥80% of the cell monolayer. Viral suspension/compound mixture were performed in DMEM with 2% FBS supplemented containing carboxymethylcellulose (CMC) to 1% and 0.5% for HHV-1 and HHV-2, respectively and incubated for 15 min at room temperature. Compounds were evaluated at concentrations of 6.25–50 μg/mL. After 48 h (HHV-1) and 72 h (HHV-2) of incubation at 37 °C in a humidified 5% CO2 atmosphere, the cell monolayers were stained with a solution of 3.5% formaldehyde with 0.2% crystal violet and the plaques were counted. Two independent experiments by quadruplicate were carried out for each viral serotype and each compound. Controls were included untreated cells, cells treated with compounds and cells infected with HHV-1 or HHV-2. Positive control included in this assay was Acyclovir. According to the parameters established by Vlietinck et al. [33] the relevant or moderate antiviral activity of a purified natural product, is one whose reduction factor (Rf) of viral titer is respectively of ≥ 1 × 103 or 1 × 102. This parameter was used in our study.
4.4.2. Screening against DENV-2
To determine the anti-DENV activity we carried out the protocol previously described by Tang et al. [34] with some modifications. Briefly, Vero cells were harvested in 96-well plates at a density of 2.0 × 104 cells/well at 37 °C in humidified 5% CO2 atmosphere. After 24 h, medium was removed and two-fold serial dilutions of DENV-2 at 10TCID50 and non-cytotoxic concentrations of compounds were incubated for 10 min at room temperature. Then, 50 μL of each dilution (compound/DENV-2) was added and the plates were incubated at 37 °C for 3 days, time at which the cytopathic effect (CPE) in control (infected cells) was observed in 100% of cell monolayer. The cell monolayers were stained with a solution of 3.5% formaldehyde with 0.2% crystal violet and CPE was observed under inverted microscope. Controls were included untreated cells, cells treated with compounds and cells infected with DENV-2. As positive control we used Ribavirin. Compounds were considered active against DENV-2 if has the ability to reduce or avoid the cytopathic effect during viral infection. This assay was conducted by quadruplicate and repeated twice for each compound.
4.4.3. Assays for pre- and post-infective stages of HHV-1, HHV-2 and DENV-2
Subsequently, the potential antiviral of active compounds was evaluated by the plaque reduction assay as previously described [35]. Vero cell monolayers grown in 24-well plates (2.5 × 104 cells/well) were infected with 100 PFU per well of each virus. Treatments were performed by adding compounds either simultaneously with virus (pre-infection treatment) or after viral infection (post-infection treatment). For pre-infection treatment, a virus/compound mixture was added to cell monolayers and was incubated for 1 h at 37 °C (5% CO2). After incubation, washing was performed with PBS (pH = 7.0) and added CMC 1%, 0.75% and 2% for HHV-1, HHV-2 and DENV-2, respectively. In post-infection treatment, virus was added on cell monolayer and incubated for 1 h at 37 °C (5% CO2). After, washing was performed with PBS (pH = 7.0) and compounds previously prepared in CMC 1%, 0.75% and 2% for HHV-1, HHV-2 and DENV-2 respectively, were added. In both treatments, plates allowed to incubate for 72 h (HHV-1 and HHV-2) and 8 days (DENV-2). Cells were then fixed and stained with a solution of 3.5% formaldehyde with 0.2% crystal violet and viral plaques were counted. Dextran sulfate was employed as positive control in pre-infection assays. Ayclovir (ACV) and Ribavirin (RIBA) were used as positive controls in post-infection stages to HHV strains and DENV-2, respectively. The fifty effective concentration (EC50) was defined as the concentration that reduces the 50% of plaque forming units. EC50 for each compound were obtained from dose–effect curves for linear regression methods using the statistical GraphPad Prisma 5.0 and EC50 values are expressed as the mean of at least four dilutions by quadruplicate. To define which compounds were more selective to infected cells than to non-infected cells, the antiviral selectivity index (SI) was calculated and defined as the ratio between the inhibitory concentration 50 (IC50) in Vero cells and the EC50 for each virus on Vero cells.
4.4.4. Plaque size-reduction assay
For this, a PFU assay was carried out on Vero cells (2.5 × 104 cells/well) and compounds previously prepared in CMC, were added 2 h after infection with 100 PFU/well of virus [25b]. Compound 8 was added at concentrations from 1.56 μg/mL to 12.5 μg/mL and compound 9 at concentrations from 0.78 μg/mL to 6.25 μg/mL, against both HHV-1 (CDC-Atlanta and 29R) and HHV-2. To determine the effect against DENV-2, compounds 8 and 9 were added in a concentration range of 0.05 μg/mL to 1.56 μg/mL. Finally, the plates were incubated for 72 h (HHV strains) and 8 days (DENV-2), fixed and stained with a solution of 3.5% formaldehyde/0.2% crystal violet. The images of 20 plaques for each compound concentration and each well were captured using a Nikon digital camera view DS-L1 attached to a Nikon inverted microscope (Tokyo, Japan) [25a]. The area of each plaque in millimeters was determined using the Image-Pro Plus 6.0 software®. Two independent experiments were performed by duplicate.
4.4.5. Cytotoxic activity
Cell growth inhibition and/or cytotoxicity on Vero, HeLa, Jurkat and U937 cells were measured using the technique MTT. The MTT 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (Sigma, New Jersey, USA) assay, according to the protocol previously described [12c] with few modifications, was used. Briefly, Vero and HeLa cells were plated at 2.0 × 104 cells per well in a 96-well flat-bottomed plates and incubated for 24 h at 37 °C. Moreover, Jurkat and U937 cells were plated at 3.0 × 104 cells per well in a 96-well round-bottomed plate. Then each diluted compound was added to the appropriate wells and the plates were incubated for further 48 h at 37 °C in a humidified incubator with 5% CO2. Finally, spectrophotometric reading was carried out at 570 nm to determine the inhibitory concentration 50 (IC50), defined as the concentration for each compound that inhibits 50% of cell growth. The IC50 values for each compound were obtained by linear regression analysis of the dose–response curves generated from the absorbance data with the statistical GraphPad Prisma 5.0. IC50 values were expressed as the geometric mean of two independent experiments done in quadruplicate. To define which compounds were more selective to cancerous cells than to non-cancerous cells, it was calculated selectivity index (SI) defined as Vero IC50 over HeLa, Jurkat or U937 IC50. A compound with an IC50 < 30 μM was considered active and with a SI ≥ 5 was considered selective. Paclitaxel and Doxorubicine were used as positive controls.
4.4.6. Antifungal activity
The in vitro antifungal activity of compounds were evaluated following the Clinical and Laboratory Standards Institute M38-A protocol for filamentous fungi [36] with a few modifications and the standard method proposed by the Antifungal Susceptibility Testing Subcommittee of the European Committee on Antibiotic Susceptibility Testing (AFST-EUCAST) for yeasts [37]. The filamentous fungi F. oxysporum (ATCC 48112), A. fumigatus (ATCC 204305), A. flavus (ATCC 204304), A. terreus (CDC 317) and dermatophytes, T. rubrum (ATCC 28188) and T. mentagrophytes (ATCC 24198) were used to evaluate antifungal activity at inoculum size of 0.2–2.5 × 105 CFU/mL. The yeasts C. albicans (ATCC 1023), C. parapsilosis (ATCC 22019) and C. tropicalis (ATCC 200956) were used to evaluate antifungal activity at inoculum size of 1–5 × 105 CFU/mL. The dilutions of compounds were dispensed into 96-well flat-bottom microdilution plates in duplicate at final concentrations between 50 μg/mL and 3.125 μg/mL. The compounds were considered active when they exhibited MIC values ≤ 50 μg/mL. For the CLSI M38-A method, the MIC were defined as the lowest dilution that resulted in an 80% of inhibition of visible growth after incubation at 28 °C to 48 h to F. oxysporum and 6 days to dermatophytes. Amphotericine B was evaluated, at a range of 0.031–16 μg/mL, with the strains A. fumigatus (ATCC 204305) and A. flavus (ATCC 204304) and Terbinafine was used as positive control at a range of 0.0078–4 μg/mL on both dermatophytes. For the AFST-EUCAST method, the MIC was determined after 24 h of incubation at 35 °C and defined as the lowest concentration that resulted in 90% of reduction of growth. Itraconazole was used as positive control at final concentrations of 0.031–16 μg/mL. A negative control (inoculum without treatment) was also included. MIC values were expressed as geometric mean (GM-MIC) of tests performed in duplicate in three different assays against each fungi species.
Competing interests
One patent application based on these results has been submitted by M.A.G. and L.A.B.-G. as inventors.
Acknowledgments
Financial support from COLCIENCIAS-Grant RC-366-2011 and 111554531592 (Patrimonio Autónomo del Fondo Nacional de Financiamiento para la Ciencia, la Tecnología y la Innovación, Francisco José de Caldas) is gratefully acknowledged. V. C. R.-L., L. S. A.-G. and V. T.-C. thank COLCIENCIAS Programa Jóvenes Investigadores for their fellowships.
References
- 1.Richman D.D., Whitley R.J., Hayden F.G. third ed. ASM Press; Washington, DC: 2009. Clinical Virology. [Google Scholar]
- 2.Piret J., Boivin G. Antiviral drug resistance in herpesviruses other than cytomegalovirus. Rev. Med. Virol. 2014;24:186–218. doi: 10.1002/rmv.1787. [DOI] [PubMed] [Google Scholar]
- 3.Piret J., Boivin G. Resistance of herpes simplex viruses to nucleoside analogues: mechanisms, prevalence, and management. Antimicrob. Agents Chemother. 2011;55:459–472. doi: 10.1128/AAC.00615-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mustafa M.S., Rasotgi V., Jain S., Gupta V. Discovery of fifth serotype of dengue virus (DENV-5): a new public health dilemma in dengue control. Med. J. Armed Forces India. 2015;71:67–70. doi: 10.1016/j.mjafi.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hanley K.A., Weaver S.C. Caister Academic Press; Norfolk: 2010. Frontiers in Dengue Virus Research. [Google Scholar]
- 6.TDR/WHO . World Health Organization; Geneva: 2009. Dengue: guidelines for diagnosis, treatment, Prevention and Control. [PubMed] [Google Scholar]
- 7.De Wispelaere M., LaCroix A.J., Yang P.L. The small molecules AZD0530 and dasatinib inhibit dengue virus RNA replication via fyn kinase. J. Virol. 2013;87:7367–7381. doi: 10.1128/JVI.00632-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.(a) Usme-Ciro J.A., Mendez J.A., Tenorio A., Rey G.J., Domingo C., Gallego-Gomez J.C. Simultaneous circulation of genotypes I and III of dengue virus 3 in Colombia. Virol. J. 2008;5:101. doi: 10.1186/1743-422X-5-101. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Mendez J.A., Usme-Ciro J.A., Domingo C., Rey G.J., Sanchez J.A., Tenorio A., Gallego-Gomez J.C. Phylogenetic history demonstrates two different lineages of dengue type 1 virus in Colombia. Virol. J. 2010;7:226. doi: 10.1186/1743-422X-7-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.González M.A. Aromatic abietane diterpenoids: their biological activity and synthesis. Nat. Prod. Rep. 2015;32:684–704. doi: 10.1039/c4np00110a. [DOI] [PubMed] [Google Scholar]
- 10.(a) González M.A. Aromatic abietane diterpenoids: total syntheses and synthetic studies. Tetrahedron. 2015;71:1883–1908. [Google Scholar]; (b) González M.A. Synthetic derivatives of aromatic abietane diterpenoids and their biological activities. Eur. J. Med. Chem. 2014;87:834–842. doi: 10.1016/j.ejmech.2014.10.023. [DOI] [PubMed] [Google Scholar]
- 11.Zhang G.-J., Li Y.-H., Jiang J.-D., Yu S.-S., Qu J., Ma S.-G., Liu Y.-B., Yu D.-Q. Anti-Coxsackie virus B diterpenes from the roots of Illicium jiadifengpi. Tetrahedron. 2013;69:1017–1023. [Google Scholar]
- 12.(a) Betancur-Galvis L., Zuluaga C., Arnó M., González M.A., Zaragozá R.J. Structure-activity relationship of in vitro antiviral and cytotoxic activity of semisynthetic analogues of scopadulane diterpenes. J. Nat. Prod. 2001;64:1318–1321. doi: 10.1021/np010207l. [DOI] [PubMed] [Google Scholar]; (b) González M.A. Spongiane diterpenoids. Curr. Bioact. Comp. 2007;3:1–36. [Google Scholar]; (c) Zapata B., Rojas M., Betancur-Galvis L., Mesa-Arango A.C., Pérez-Guaita D., González M.A. Cytotoxic, immunomodulatory, antimycotic, and antiviral activities of semisynthetic 14-hydroxyabietane derivatives and triptoquinone C-4 epimers. Med. Chem. Comm. 2013;4:1239–1246. [Google Scholar]
- 13.Balzarini J., De Clercq E., Kaminska B., Orzeszko A. Synthesis and antiviral activity of some 5′-N-phthaloyl-3′-azido-2′,3′-dideoxythymidine analogues. Antivir. Chem. Chemother. 2003;14:139–144. doi: 10.1177/095632020301400303. [DOI] [PubMed] [Google Scholar]
- 14.Bakry R., Vallant R.M., Najam-ul-Haq M., Rainer M., Szabo Z., Huck C.W., Bonn G.K. Medicinal applications of fullerenes. Int. J. Nanomed. 2007;2:639–649. [PMC free article] [PubMed] [Google Scholar]
- 15.Plummer E., Buck M.D., Sanchez M., Greenbaum J.A., Turner J., Grewal R., Klose B., Sampath A., Warfield K.L., Peters B., Ramstedt U., Shresta S. Dengue virus evolution under a host-targeted antiviral. J. Virol. 2015;89:5592–5601. doi: 10.1128/JVI.00028-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.(a) González M.A., Pérez-Guaita D. Short syntheses of (+)-ferruginol from (+)-dehydroabietylamine. Tetrahedron. 2012;68:9612–9615. [Google Scholar]; (b) Malkowsky I.M., Nieger M., Kataeva O., Waldvogel S.R. Synthesis and properties of optically pure phenols derived from (+)-dehydroabietylamine. Synthesis. 2007;5:773–778. [Google Scholar]
- 17.Zhou Z., Lin Z.-X., Liang D., Hu J.-Q. First synthesis of ring-B C60-substituted derivatives of N,N-(tetrachlorophthaloyl)dehydroabietylamine. Tetrahedron. 2013;69:43–49. [Google Scholar]
- 18.Wen C.-C., Kuo Y.-H., Jan J.-T., Liang P.-H., Wang S.-Y., Liu H.-G., Lee C.-K., Chang S.-T., Kuo C.-J., Lee S.-S., Hou C.-C., Hsiao P.-W., Chien S.-C., Shyur L.-F., Yang N.-S. Specific plant terpenoids and lignoids possess potent antiviral activities against severe acute respiratory syndrome coronavirus. J. Med. Chem. 2007;50:4087–4095. doi: 10.1021/jm070295s. [DOI] [PubMed] [Google Scholar]
- 19.(a) Becerra J., Flores C., Mena J., Aqueveque P., Alarcón J., Bittner M., Hernández V., Hoeneisen M., Ruiz E., Silva M. Antifungal and antibacterial activity of diterpenes isolated from wood extractables of Chilean podocarpaceae. Bol. Soc. Chil. Quim. 2002;47:151–157. [Google Scholar]; (b) Fronza M., Murillo R., Slusarczyk S., Adams M., Hamburguer M., Heinzmann B., Laufer S., Merfort I. In vitro cytotoxic activity of abietane diterpenes from peltodon longipes as well as salvia miltiorrhiza and salvia sahendica. Bioorg. Med. Chem. 2011;19:4876–4881. doi: 10.1016/j.bmc.2011.06.067. [DOI] [PubMed] [Google Scholar]
- 20.Bispo de Jesus M., Zambuzzi W.F., Ruela de Sousa R.R., Areche C., Santos de Souza A.C., Aoyama H., Schmeda-Hirschmann G., Rodriguez J.A., de Souza Brito A.R.M., Peppelenbosch M.P., den Hertog J., de Paula E., Ferreira C.V. Ferruginol suppresses survival signaling pathways in androgen-independent human prostate cancer cells. Biochimie. 2008;90:843–854. doi: 10.1016/j.biochi.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 21.Krummenacher C., Carfí A., Eisenberg R.J., Cohen G.H. Entry of herpesviruses into cells: the enigma variations. Adv. Exp. Med. Biol. 2013;790:178–195. doi: 10.1007/978-1-4614-7651-1_10. [DOI] [PubMed] [Google Scholar]
- 22.Chattopadhyay D., Sarkar M.C., Chatterjee T., Sharma Dey R., Bag P., Chakraborti S., Khan M.T. Recent advancements for the evaluation of anti-viral activities of natural products. N. Biotechnol. 2009;25:347–368. doi: 10.1016/j.nbt.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhong P., Agosto L.M., Munro J.B., Mothes W. Cell-to-cell transmission of viruses. Curr. Opin. Virol. 2013;3:44–50. doi: 10.1016/j.coviro.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin L.T., Chen T.Y., Chung C.Y., Noyce R.S., Grindley T.B., McCormick C., Lin T.C., Wang G.H., Lin C.C., Richardson C.D. Hydrolyzable Tannins (Chebulagic Acid and Punicalagin) Target Viral glycoprotein-glycosaminoglycan interactions to inhibit herpes simplex virus 1 entry and cell-to-cell spread. J. Virol. 2011;85:4386–4398. doi: 10.1128/JVI.01492-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.(a) Nyberg K., Ekblad M., Bergström T., Freeman C., Parish C.R., Ferro V., Trybala E. The low molecular weight heparan sulfate-mimetic, PI-88, inhibits cell-to-cell spread of herpes simplex virus. Antivir. Res. 2004;63:15–24. doi: 10.1016/j.antiviral.2004.01.001. [DOI] [PubMed] [Google Scholar]; (b) Ekblad M., Adamiak B., Bergstrom T., Johnstone K.D., Karoli T., Liu L., Ferro V., Trybala E. A highly lipophilic sulfated tetrasaccharide glycoside related to muparfostat (PI-88) exhibits virucidal activity against herpes simplex virus. Antivir. Res. 2010;86:196–203. doi: 10.1016/j.antiviral.2010.02.318. [DOI] [PubMed] [Google Scholar]
- 26.Maringer K., Stylianou J., Elliott G. A network of protein interactions around the herpes simplex virus tegument protein VP22. J. Virol. 2012;86:12971–12982. doi: 10.1128/JVI.01913-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson D.C., Webb M., Wisner T.W., Brunetti C. Herpes simplex virus gE/gI sorts nascent virions to epithelial cell junctions, promoting virus spread. J. Virol. 2001;75:821–833. doi: 10.1128/JVI.75.2.821-833.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mingo R.M., Han J., Newcomb W.W., Brown J.C. Replication of herpes simplex virus: egress of progeny virus at specialized cell membrane sites. J. Virol. 2012;86:7084–7097. doi: 10.1128/JVI.00463-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dixit R., Tiwari V., Shukla D. Herpes simplex virus type 1 induces filopodia in differentiated P19 neural cells to facilitate viral spread. Neurosci. Lett. 2008;440:113–118. doi: 10.1016/j.neulet.2008.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taylor M.P., Koyuncu O.O., Enquist L.W. Subversion of the actin cytoskeleton during viral infection. Nat. Rev. Microbiol. 2011;9:427–439. doi: 10.1038/nrmicro2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang J.-L., Zhang J.-L., Chen W., Xu X.-F., Gao N., Fan D.-Y., An J. Roles of small GTPase Rac1 in the regulation of actin cytoskeleton during dengue virus infection. PLoS Negl. Trop. Dis. 2010;4:e809. doi: 10.1371/journal.pntd.0000809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martínez-Gutierrez M., Castellanos J.E., Gallego-Gómez J.C. Statins reduce dengue virus production via decreased virion assembly. Intervirology. 2011;54:202–216. doi: 10.1159/000321892. [DOI] [PubMed] [Google Scholar]
- 33.Vlietinck A.J., Van Hoof L., Totté J., Lasure A., Vanden Berghe D., Rwangabo P.C., Myukiyumwami J. Screening of hundred Rwandese medicinal plants for antimicrobial and antiviral properties. J. Ethnopharm. 1995;46:31–47. doi: 10.1016/0378-8741(95)01226-4. [DOI] [PubMed] [Google Scholar]
- 34.Tang L.I.C., Ling A.P.K., Koh R.Y., Chye S.M., Voon K.G.L. Screening of anti-dengue activity in methanolic extracts of medicinal plants. BMC Complementary Altern. Med. 2012;12(3) doi: 10.1186/1472-6882-12-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cardozo F.T., Camelini C.M., Mascarello A., Rossi M.J., Nunes R.J., Barardi C.R., de Mendonça M.M., Simões C.M. Antiherpetic activity of a sulfated polysaccharide from agaricus brasiliensis mycelia. Antivir. Res. 2011;92:108–114. doi: 10.1016/j.antiviral.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 36.NCCLS . National committee for clinical Laboratory Standards; Wayne: 2002. Reference method for broth dilution antifungal susceptibility testing of conidial forming filamentous fungi. Approved standard NCCLS M38-A. [Google Scholar]
- 37.Cuenca-Estrella M., Moore C.B., Barchiesi F., Bille J., Chryssanthou E., Denning D.W., Donnelly J.P., Dromer F., Dupont B., Rex J.H., Richardson M.D., Sancak B., Verweij P.E., Rodríguez-Tudela J.L. Multicenter evaluation of the reproducibility of the proposed antifungal susceptibility testing method for fermentative yeasts of the antifungal susceptibility testing subcommittee of the European committee on antimicrobial susceptibility testing (AFST-EUCAST) Clin. Microbiol. Infect. 2003;9:467–474. doi: 10.1046/j.1469-0691.2003.00592.x. [DOI] [PubMed] [Google Scholar]