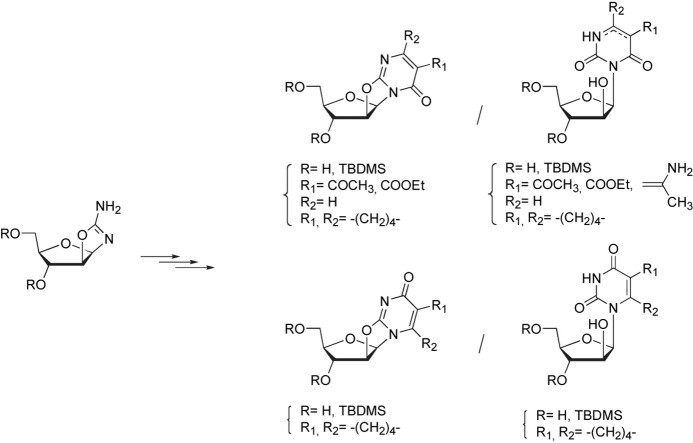
Abstract
A series of nucleoside derivatives was obtained via heteroatom annulation of the amino oxazoline of d-(−)-arabinose. Unequivocal proofs for the stereostructure of some new arabinosyl pyrimidinone derivatives were obtained by X-ray structure analysis. These newly synthesized compounds were then evaluated for their cytostatic activity against murine leukemia (L1210), and human T-lymphocytes (Molt 4/C8 and CEM). Of all the compounds in the series, the protected silylated tricyclic fused pyrimidinone 10 showed the most significant antitumor activity against murine leukemia L1210 (IC50 = 6 μM), and human T-lymphocytes cells Molt 4/C8 (IC50 = 7.9 μM) and CEM/0 cell lines (IC50 = 7.5 μM). None of the compounds exhibited significant antiviral inhibitory activities.
Keywords: Arabinose, Arabinosyl pyrimidinone, Antitumoral activity, X-ray diffraction, Heterocyclisation
Graphical abstract
1. Introduction
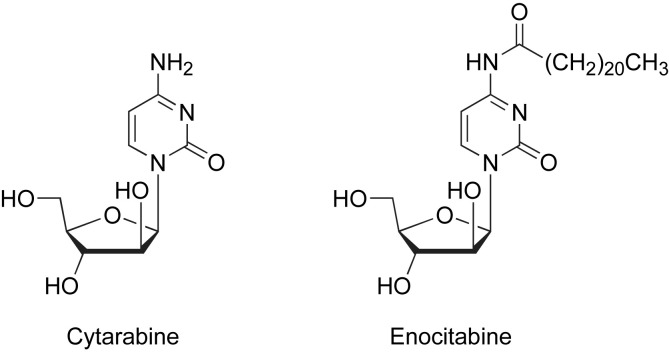
Antimetabolites are widely used cytostatics classified according to their chemical structure and mechanism of action. Amongst them new antimetabolite pyrimidine analogues, i.e. Cytarabine [1], [2], [3], [4], Enocitabine [4], [5], [6], are related to a 1-β-d-arabinofuranoside synthon (Fig. 1 ). As part of our research program, we developed the synthesis of a new series of original 1-β-d-arabino furan[1′,2′:4,5]oxazolo- and arabino-pyrimidinones via 2-amino-2-oxazoline derivatives possessing a 1-β-d-arabinosyl parent. 2-Amino-2-oxazolines are heteroamidine synthons useful for the preparation of various bioactive pyrimidinones through ring annulation, by using the two nucleophilic sites of the amidine moiety. We already developed this approach for obtaining various bicyclic compounds [7], [8], [9].
Fig. 1.
Structures of Cytarabine and Enocitabine.
In this work we reported the synthesis of a series of new 1-β-d-arabino furan[1′,2′:4,5]oxazolo- and arabino-pyrimidinone derivatives through a molecular biodiversity strategy, and the preliminary results of their cytostatic activity against murine leukemia (L1210), and human T-lymphocytes (Molt 4/C8 and CEM).
2. Results and discussion
2.1. Chemistry
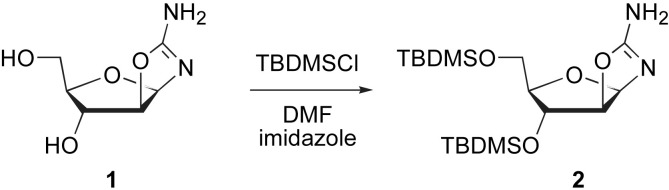
The readily available 2-amino-β-d-arabinofuran[1′,2′:4,5]-2-oxazolines 1–2, either unprotected (compound 1, Scheme 1, Scheme 2 ) or silylated (compound 2, Scheme 1) were prepared according to previously described procedures [10], [11], [12], [13], [14], and used as the starting material for the synthesis of β-d-arabino furan[1′,2′:4,5]oxazolo- and arabino-pyrimidinone derivatives.
Scheme 1.
Synthesis of the silylated 2-amino-D-arabinofuran[1′,2′:4,5]-2-oxazoline.
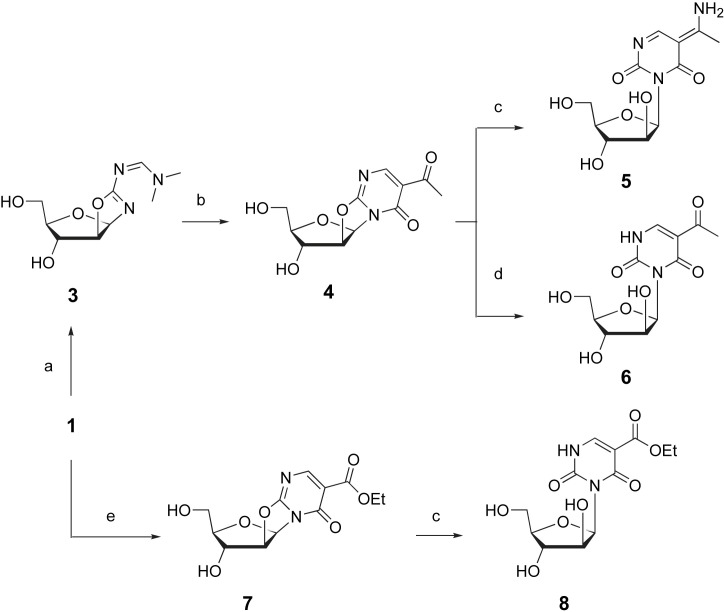
Scheme 2.
(a) DMFDMA, toluene, 50 °C (b) diketene acetone adduct, acetone, r.t. (c) NH4OH, MeOH, r.t. (d) EtONa/EtOH, r.t. (e) DEEM, EtOH 25 °C.
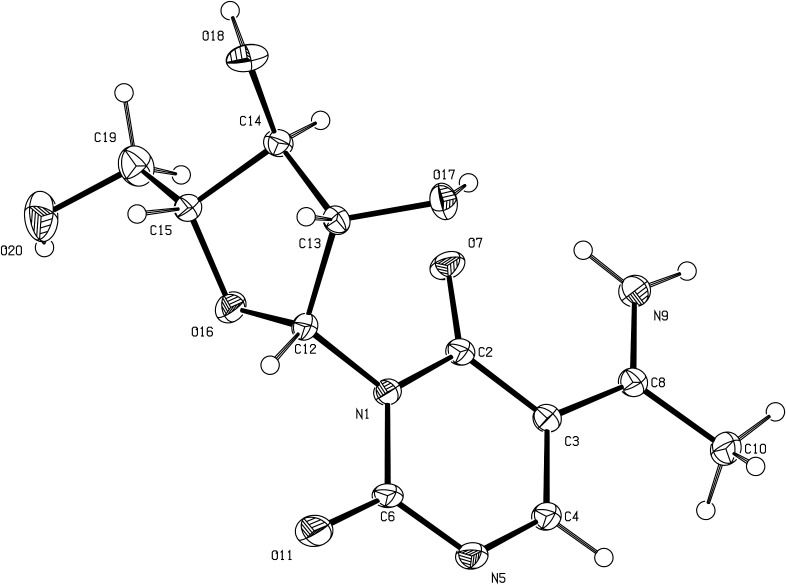
N,N-dimethylformamide dimethylacetal (DMFDMA) reacted with 2-amino-β-d-arabinofuran[1′,2′:4,5]-2-oxazoline 1 to give the expected amidine 3 (Scheme 2). The N,N′-dimethyl-N′-alkylformamide 3 was engaged in an alkylation with diketene acetone adduct, followed by spontaneous elimination of dimethylamine, to give the fused β-d-arabinofuran[1′,2′:4,5]oxazolopyrimidin-6-one 4. The oxazoline ring opening was then conducted in dilute aqueous ammonia [11], [12] to afford the surprising arabinofurano-pyrimidinedione 5 bearing an enamine function. Structural elucidation of 5 was achieved either by 1H and 13C NMR, and by X-ray crystallography (Fig. 2 ). Especially, 1H NMR spectrum of 5 revealed two D2O exchangeable singlets at 9.93 and 10.84 ppm characteristics of the enamine group in position 5. Moreover, the 13C NMR spectrum of 5 showed one signal around 98.4 ppm specific for the enamine alkene bond ( C(Me)NH2). The X-ray crystallography data of 5 unambiguously showed the presence two hydrogen on the N(9), whereas the Csp 2(8)-N(9) bond was found at 1.292(2) Å, as typically observed for C C–NH2 bonds [15]. There is an intramolecular hydrogen bond between N(9)–H(9A) and O(7) where the N(9)–O(7) distance is 2.673(2) &angst. Moreover, N(9) is hydrogen bonded to O(11) of the molecule at (x − 1,−y − 1, +z) with N(9)–H(9B)–O(11) = 2.814(3) Å; other hydrogen bond are found between O(18) and N(5) of the molecule (x, y, z − 1) where the distance is 2.823(2) Å, and O(20) and O(17) of the molecule (x−1, y + 1, z) with O(20)…O(17) = 2.813(2) &angst. The most common type of pucker of sugar rings in nucleosides and nucleotides is with either atom C(14) or atom C(15) displaced from the plane of the other four atoms on the same side as atom C(19), when the pucker is called C(14) or C(15) endo, respectively. In the structure of 5, the pucker is C(14) endo differing from that observed in previously described uracil-β-d-arabinofuranoside [16] where the pucker is C(14) endo making impossible an intramolecular hydrogen bond between O(20) and O(17). Finally, spatial structure of 5 also confirmed that the oxazoline ring opening did not modify the C-1 and C-2 absolute configuration of arabinose. Based on the space group determined from crystallographic data (P1), the absolute configurations at C-1 and C-2 of this pure enantiomer were determined to be R and S, respectively. The formation of 5 was probably due to the action of ammonia on the acetyl substituent leading to an imine function which gave the stable enamine group after alkaline oxazoline ring opening.
Fig. 2.
Perspective view of compound 5 with our atom numbering. Displacement ellipsoids are drawn at the 30% probability level.
On the other hand, treatment of 4 with sodium ethanolate afforded the expected pyrimidinedione 6 (Scheme 2). Diethyl ethoxymethylene malonate (DEEM) is a well-known bis-electrophile reagent used for heterocyclic ring annelation [9], [17]. When reacted at room temperature with 1, only the fused pyrimidinone 7 bearing a β-keto ester moiety was obtained, probably due to the regioselectivity in term of nitrogen reactivity as previously observed in the 2-amino-2-oxazoline series [17]. Oxazoline ring opening was then conducted with ammonium hydroxyde to give the sugar derivative 8 in 63% yield (Scheme 2).
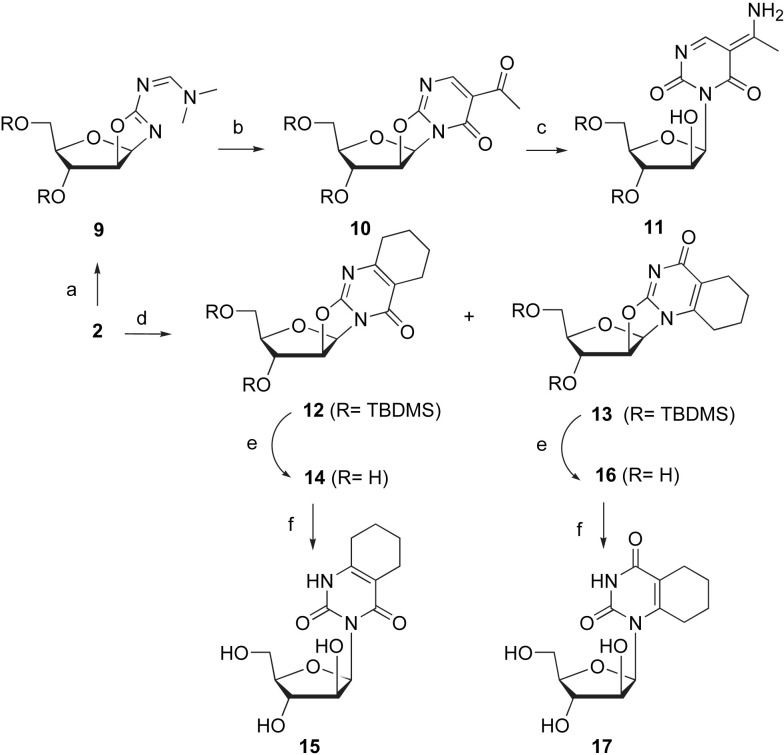
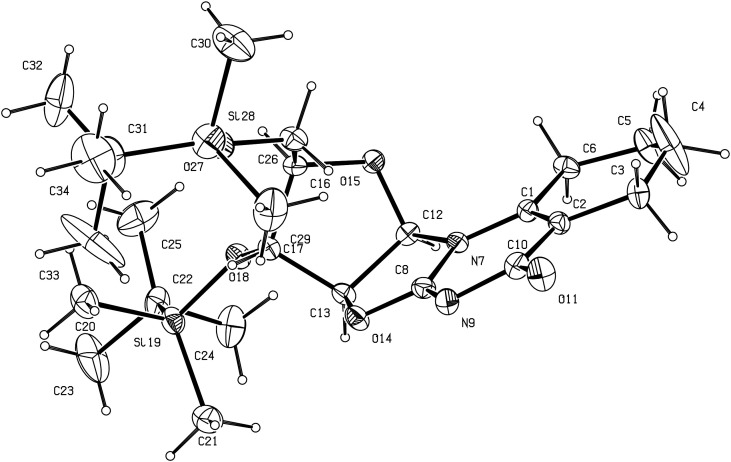
As depicted in Scheme 3 , 2-amino-3′,5′-bis(O-tert-butyldimethylsilyl)-β-d-arabinofuran[1′,2′:4,5]-2-oxazoline 2 was subsequently reacted with DMFDMA to give 9 as described above. Then, 9 was reacted with diketene acetone adduct in acetone to give 10 as previously described for the synthesis of 4 from the unprotected amino oxazoline 1. The 3D spatial determination of 10, established by X-ray crystallography, confirmed the structure in the solid state (Fig. 3 ). Ring opening of 10 was conducted with NH4OH at r.t. to give 11.
Scheme 3.
(a) DMF–DMA, toluene, 50 °C (b) diketene acetone adduct, acetone, r.t. (c) NH4OH, MeOH, r.t. (d) Ethyl-2-oxocyclohexane-carboxylate, xylene, 140 °C (e) TBAF, THF, r.t. (f) EtONa/EtOH, r.t.
Fig. 3.
Perspective view of compound 10 with our atom numbering. Displacement ellipsoids are drawn at the 10% probability level.
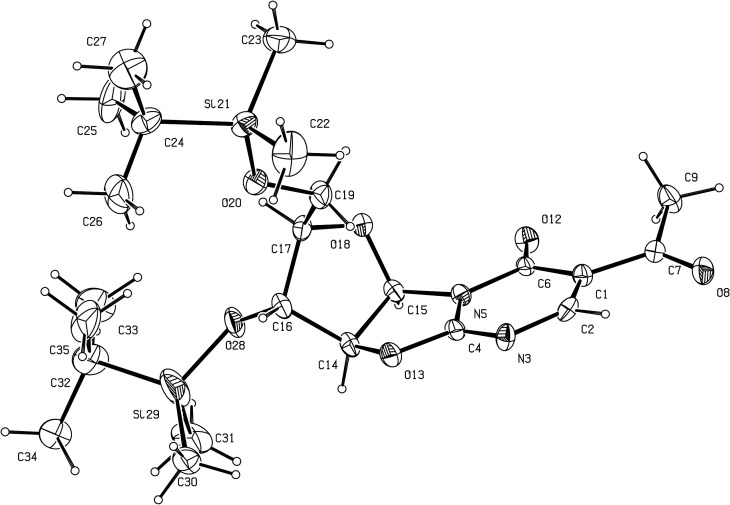
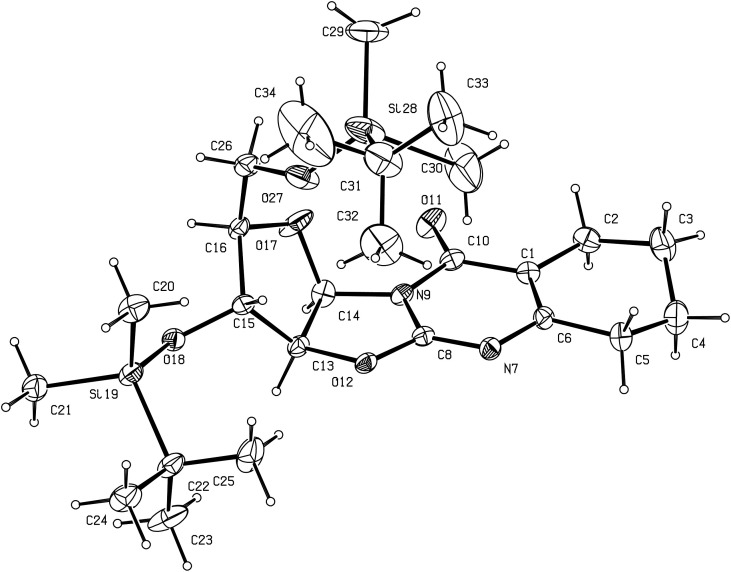
Alternatively, reaction of 2 with ethyl-2-oxocyclohexane-carboxylate in refluxing styrene led to a mixture of isomeric angularly and linearly annelated nitrogen bridgehead compounds 12 and 13, through a one-step ring annulation. This synthesis illustrates the nucleophilic reactivity of both nitrogen atoms of 2-amino-2-oxazolines [9]. Thus, the endocyclic nitrogen atom attacks first the electrophilic carbonyl carbon of ethyl-2-oxocyclohexane-carboxylate, followed by the exocyclic nitrogen attack on the carboxyl carbon (afterwards) to achieve cyclisation. The resulting unstable cyclic β-hydroxyketone is then dehydrated to give 12. On the other hand, compound 13 was obtained after dehydration when the two nitrogen atom attacks order was reversed. After separation these two regioisomers were obtained in 41% and 30% yields, respectively. Each structure has been probed by NMR analysis. Thus, a NOE effect between the H-3′ arabinose proton and one methylene proton of the fused cyclohexyl moiety was observed for compound 13, while no correlation was observed between the two corresponding protons in 12 [9]. For the unequivocal assignment of the two different possible structures, X-ray single crystal analyses were performed with 12 (Fig. 4 ) and 13 (Fig. 5 ). The 3D structures of 12 and 13 confirmed the isomerization, as anticipated on the basis of NOESY data. Both regioisomers 12 and 13 are characterized by a quasi-planar conformation of the oxazolo[3,2-a]pyrimidine moiety with dihedral angle between the mean planes of the pyrimidine base and the sugar ring = 72.6(7)° and 71.4(10)°, respectively. In compound 12, the maximum deviation from planarity for the tricyclic cyclohexa-oxazolo-pyrimidinone moiety is found lying 0.368 Å, while in 13, C(5) deviates by 0.189 Å from the plane in the cyclohexenyl cycle. The C(8)–N(7) and C(1)–C(6) bonds in 12 were 1.277(7) and 1.388(9) Å, respectively, as typically observed for double bonds. In compound 13, the distances in the pyrimidine ring were 1.285(10) Å for C(8)–N(9), and 1.346(11) Å for C(1)–C(2).
Fig. 4.
Perspective view of compound 12 with our atom numbering. Displacement ellipsoids are drawn at the 10% probability level.
Fig. 5.
Perspective view of compound 13 with our atom numbering. Displacement ellipsoids are drawn at the 10% probability level.
Both regioisomers were then desilylated with tetrabutyl ammonium fluoride (TBAF) in THF to give 14 and 16, respectively. Following oxazoline ring opening with EtONa, the arabinose derivatives 15 and 17 were eventually obtained in relatively poor yields (13 and 25%, respectively).
2.2. Biological results
2.2.1. Cytostatic activity
The newly synthesized compounds 3–17 were evaluated for their in vitro inhibitory effects on the proliferation of murine leukemia cells (L1210) and human T-lymphocyte cells (Molt 4/C8 and CEM/0) (Table 1 ). For all compounds, the theoretical calculations of lipophilicity as Clog P were performed using the MarvinSketch program [18].
Table 1.
Cytotoxic activity of compounds 3–17 against murine leukemia cells (L1210/0), and human T-lymphocyte cells (Molt 4/C8 and CEM/0), and theoretically calculated Clog P values.
| Compound | IC50 (μM) |
Clog P | ||
|---|---|---|---|---|
| L1210/0 | Molt 4/C8 | CEM/0 | ||
| Cytarabine | 0.024 ± 0.009 | – | 0.024 ± 0.001 | −1.57 |
| 3 | >500 | >500 | >500 | −0.62 |
| 4 | 312 ± 4 | 380 ± 30 | 254 ± 3 | −0.60 |
| 5 | 211 ± 6 | 299 ± 61 | 217 ± 13 | −3.81 |
| 6 | >500 | >500 | >500 | −2.13 |
| 7 | >500 | >500 | >500 | −0.07 |
| 8 | >500 | >500 | >500 | −1.61 |
| 9 | 254 ± 32 | 226 ± 13 | 215 ± 15 | 5.19 |
| 10 | 6.0 ± 0.1 | 7.9 ± 1.3 | 7.5 ± 0.1 | 5.13 |
| 11 | 30 ± 5 | 18 ± 11 | 17 ± 5 | 1.91 |
| 12 | 201 ± 21 | 129 ± 80 | 126 ± 67 | 5.66 |
| 13 | 229 ± 17 | 185 ± 42 | 212 ± 12 | 5.91 |
| 14 | >500 | >500 | >500 | −0.07 |
| 15 | >500 | >500 | >500 | −1.60 |
| 16 | >500 | >500 | >500 | 0.18 |
| 17 | >500 | >500 | >500 | −1.60 |
As it appears in Table 1 only compounds 4, 5 and 9–13 showed a cytotoxic activity with IC50 < 500 μM, with 9–13 belonging to the silylated series of nucleoside derivatives. Compounds 10 and 11 were found to possess interesting activity against leukemia and human T-lymphocyte cells with IC50 ranging from 6 to 7.9 μM, and 17 to 30 μM, respectively. The fused tricyclic pyrimidinone 10 was found the most potent compound, whereas ring opening of 10 leading to compound 11, provided a lower activity. It must be noted that during this ring opening process, the heterocyclic ring of 11 is structurally rather different from its precursor in compound 10, since an enamine group now replaces the acetyl group. Both groups could be considered as bioisosteres, and may be involved in the biological binding site of compounds 10 and 11. On the other hand, 12 and 13, lacking such reactive functions on the pyrimidine moiety, were found to be less active.
These preliminary results could be related to the lipophilic behaviour of tested compounds, highlighted by the calculated log P parameters (Table 1). As expected the silylated compounds are the most lipophilic ones. This lipophilic character of 9–13 could explain, in a first part, a better permeability, leading to a better cell penetration in vitro. On the other hand, comparable Clog P differences are found between corresponding values calculated for 10 and 4, and 5 and 11, respectively. These desilylated pyrimidinones 4 and 5 showed lower activities with IC50 ranging from 211 to 380 μM on the three cell lines. The tetracylic fused isomeric pyrimidinones 12 and 13 also exhibited moderate antitumor activities, with slightly better IC50s for 12 (IC50 = 201 μM on L1210, IC50 = 129 μM on Molt 4/C8, and IC50 = 126 μM on CEM/0). Finally, the moderate results obtained for compound 9 (IC50 = 215–254 μM) are rather difficult to discuss since this compound appeared in the early stages of the synthesis, and therefore exhibited a quite different chemical structure compared to the other interesting compounds. Nevertheless, it can be compared to its desilylated analog 3 which showed no activity at all, stressing again the important role of the tert-butyldimethylsilyl groups.
2.2.2. Antiviral activity
Compounds 3–17 were also evaluated for their activity against herpes simplex virus type 1 (KOS) and type 2 (G), vaccinia virus and vesicular stomatitis virus in human embryonic lung (HEL) cell cultures, Coxsackie virus B4 and respiratory syncytial virus (RSV) in HeLa cell cultures, parainfluenza-3 virus, reovirus-1, Sindbis virus and Punta Toro virus in Vero cell cultures, Feline corona virus in Crandel feline kidney (CRFK) cell cultures, as well as influenza viruses types A (H1N1 and H3N2 subtypes) and B in MDCK cell cultures. No specific antiviral effects (i.e. minimal antivirally effective concentration > fivefold lower than the minimal cytotoxic concentration) were noted for any of the compounds against any of the viruses evaluated (data not shown). Nevertheless, only the fused pyrimidinone 12 showed an appreciable antiviral activity against vaccinia virus with an effective concentration (EC50) of 45 μM.
3. Conclusion
In summary, we reported here the synthesis of new arabinosyl pyrimidinone derivatives together with their cytostatic activity on L1210, Molt 4/C8 and CEM tumor cell lines. The stereostructure of 5, 10, 12 and 13 was unambiguously confirmed by their X-ray structural analysis. In the series, the lipophilic silylated derivatives 10 and 11 were found to be the most biologically active. They can be used now as lead compounds in the search for more active and potent nucleoside analogues to be applied in cancer chemotherapy.
4. Experimental
4.1. General methods
Commercially reagents were used as received without additional purification. Melting points were determined with an SM–LUX–POL Leitz hot-stage microscope and are uncorrected. IR spectra were recorded on a BRUKER IFS-25 spectrophotometer. NMR spectra were obtained on a Bruker Avance 300 spectrometer (300 MHz for 1H and 75 MHz for 13C). Deuterated dimethylsulfoxide (DMSO-d 6) or chloroform (CDCl3) were used as solvents, with DMSO (δ H 2.5) or DMSO-d 6 (δ C 39.5), CHCl3 (δ H 7.27) or CDCl3 (δ C 77.0) respectively, being employed as internal standards. Silica gel 60 (70–230 mesh) was used for column chromatography. The homogeneity of all the newly synthesized compounds was routinely checked by TLC on silica gel plates (Polygram Sil G/UV254) and visualized by irradiation with a UV lamp. Elemental analyses were conducted by CNRS, Vernaison, France.
4.2. X-ray crystallographic study
The structure of compounds 5, 10, 12 and 13 has been established by X-ray crystallography (Fig. 1, Fig. 2, Fig. 3, Fig. 4). The data were corrected for Lorentz and polarization effects and for empirical absorption correction [19]. The structure was solved by direct methods Shelx 86 [20] and refined using Shelx 97 [21] suite of programs. White single crystal (0.25 × 0.10 × 0.10 mm3) of 5 was obtained by slow evaporation from methanol/chloroform (30/70) solution: triclinic, space group P 1, a = 5.231(1) Å, b = 6.481(1) Å, c = 9.311(2) Å, α = 88.50(1)°, β = 75.39(1)°, γ = 82.16(1)°, V = 302.59(10) Å3, Z = 1, δ(calcd) = 1.595 Mg m−3, FW = 285.26 for C11H15N3O6, F(000) = 150. Yellow single crystal (0.25 × 0.25 × 0.05 mm3) of 10 was obtained by slow evaporation from water/ethanol (50/50) solution: Orthorhombic, space group P 21 21 21, a = 6.916(2) Å, b = 9.3010(10) Å, c = 44.584(4) Å, α = 90°, β = 90°, γ = 90°, V = 2867.9(9) Å3, Z = 4, δ(calcd) = 1.123 Mg m−3, FW = 484.74 for C22H40N2O6Si2, F(000) = 1048. White single crystal (0.30 × 0.15 × 0.07 mm3) of 12 was obtained by slow evaporation from diethyl ether: orthorhombic, space group P 21 21 21, a = 7.3130(10) Å, b = 16.808(2) Å, c = 24.121(2) Å, α = 90°, β = 90°, γ = 90°, V = 2964.9(6) Å3, Z = 4, δ(calcd) = 1.140 Mg m−3, FW = 508.80 for C25H44N2O5Si2, F(000) = 1104. White single crystal (0.30 × 0.15 × 0.10 mm3) of 13 was obtained by slow evaporation from diethyl ether: orthorhombic, space group P 21 21 21, a = 9.391(1) Å, b = 10.506(4) Å, c = 30.001(6) Å, α = 90°, β = 90°, γ = 90°, V = 2960.0(13) Å3, Z = 4, δ(calcd) = 1.142 Mg m−3, FW = 508.80 for C25H44N2O5Si2, F(000) = 1104. The unit cell dimensions was determined using the least-squares fit from 25 reflections (25° < θ < 35°). Intensities were collected with an Enraf–Nonius CAD-4 diffactometer using the CuKα radiation and a graphite monochromator up to θ = 55°. No intensity variation of 2 standard reflections monitored every 90 min was observed. Complete crystallographic data for the structural analysis have been deposited with the Cambridge Crystallographic Data Centre, CCDC no.717109, 717111, 717110 and 717113 for compounds 5, 10, 12 and 13 respectively. Copies of this information may be obtained free of charge from the Director, Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge, CB2 1EZ, UK. (Fax: +44 1223 336033, e-mail: deposit@ccdc.cam.ac.uk or via www.ccdc.cam.ac.uk).
4.3. N′-[6-hydroxy-5-hydroxymethyl)-3a,5,6,6a-tetrahydrofuro[2,3-d][1,3oxazol-2-yl]-N,N-dimethylimidoformamide (3)
Dimethylformamide dimethylacetal (4 mL) was added to a solution of 1 (4.02 g, 0.023 mol) in methanol (100 mL) at 50 °C. After stirring for 2 h at this temperature, the solution was concentrated under reduced pressure. The resulting white visquous residue was chromatographed over silica gel using chloroform–methanol (70/13) as eluant to give 3 (3.16 g, 60%); mp 127 °C; 1H NMR (DMSO-d 6): δ 2.91, 3.08 (2s, 6H, NMe2), 3.17–3.27 (m, 2H, H-5′), 3.65–3.70 (m, 1H, H-4′), 4,05–4,07 (m, 1H, H-3′), 4.55–4.57 (m, 1H, H-2′), 4.72 (t, 1H, J OH,5′ 5.5 Hz, 5′-OH D2O exchangeable), 5.45 (d, 1H, J OH,3′ 4.5 Hz, 3′-OH D2O exchangeable), 5.75 (d, 1H, J 1,2 6.0 Hz, H-1′), 8.30 (s, 1H, CH amidine); 13C NMR (DMSO-d 6): δ 34.74 (NMe2), 61.9, 76.29, 85.42, 87.55, 99.86 (CH arabinose), 160.83 ( CH amidine), 167.83 (C-2 oxazoline). Anal. Calcd for C9H15N3O4: C, 47.16; H, 6.60; N, 18.33. Found: C, 47.33; H, 6.40; N, 18.16.
Anal. Calcd for C9H15N3O4: C, 47.16; H, 6.60; N, 18.33. Found: C, 47.33; H, 6.40; N, 18.16.
4.4. 7-Acetyl-2,3,3a,9a-tetrahydro-3-hydroxy-2-hydroxymethyl-8H-furo[2′,3′:4,5]oxazolo[3,2-a]pyrimidine-8-one (4)
Diketene acetone adduct (8.38 g, 0.06 mol) was added to a solution of 3 (3 g, 13 mmol) in acetone (100 mL) at 50 °C. After stirring for 2 h at this temperature, the solution was concentrated under reduced pressure. The resulting brown solid residue was applied to a silica gel column eluted with chloroform–methanol (90/10) to give 4 (0.6 g, 17%); mp 142 °C; 1H NMR DMSO-d 6: δ 2.49 (s, 3H, CH3), 3.35–3.37 (m, 2H, H-5′), 4.11–4.12 (m, 1H, H-4′), 4.42–4.44 (m, 1H, H-3′), 4.96 (t, 1H, J OH,5′ 5.1 Hz, 5′-OH D2O exchangeable), 5.27 (d, 1H, J 1′,2′ 6.0, H-2′), 5.93 (d, 1H, J OH,3′ 4.4 Hz, 3′-OH D2O exchangeable), 6.48 (d, 1H, H-1′), 8.39 (s, 1H, CH pyrimidinone); 13C NMR (DMSO-d 6): δ 30.49 (CH3), 60.74, 74.66, 87.93, 89.45, 90.10 (CH arabinose), 117.82 (C-5 pyrimidinone), 157.95 (C-2 pyrimidinone), 161.86 (C-6 pyrimidinone), 162.0 (C O pyrimidinone), 194.32 (C O acetyl). Anal. Calcd for C11H12N2O6: C, 49.26; H, 4.51; N, 10.44. Found: C, 49.38; H, 4.59; N, 10.63.
4.5. (5Z)-5-(1-Aminoethylidene)-3-[3,4-dihydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl]pyrimidine-2,4(3H,5H)-dione (5)
To a solution of 4 (1.0 g, 3.7 mmol) in methanol (20 mL) at r.t. was rapidily added ammonium hydroxyde (32% soln., 4 mL). After stirring for 2 h at r.t., the solution was concentrated under reduced pressure and the residue purified over silica gel using chloroform–methanol (70/30) as eluant to give 5 (0.69 g, 65%); mp 270 °C; 1H NMR (DMSO-d 6): δ 2.43 (s, 3H, CH3), 3.51–3.59 (m, 1H, H-4′), 3.58–3.68 (m, 2H, H-5′), 4.15–4.28 (m, 2H, H-3′ and H-2′), 4.5, 5.12, 5.14 (3 m, 3H, 5′-OH, 4′-OH, 3′-OH), 6.52 (d, 1H, J 1′,2′ 8.0 Hz, H-1′), 8.57 (s, 1H, H-6 pyrimidine), 9.93, 10.84 (2s, 2H, NH2); 13C NMR (DMSO-d 6): δ 18.80 (CH3), 61.90, 76.06, 77.13, 80.50, 82.74 (CH arabinose), 98.39 ( C(Me)NH2), 157.78 (C-5 pyrimidine), 165.24 (C-6 pyrimidine), 165.62 (2-CO pyrimidine), 173.58 (4-CO pyrimidine). Anal. Calcd for C11H15N3O6: C, 46.32; H, 5.30; N, 14.73. Found: C, 46.44; H, 5.18; N, 14.92.
4.6. 5-Acetyl-3-[3,4-dihydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl]pyrimidine-2,4(1H,3H)-dione (6)
A solution of compound 4 (2.68 g, 0.01 mol) in ethanol (150 mL), was quickly added to sodium ethanolate (2.04 g, 0.03 mol) at room temperature. The yellow solution was stirred for 4 h at r.t. After concentration under reduced pressure, the residue was poured into water (40 mL) and acidified to pH 1 with aqueous 5% HCl. Water was removed under vacuum, and absolute alcohol (50 mL) was added to the residue. After filtration (NaCl removal) the filtrate was concentrated and the resulting solid recrystallized in methanol to give 6 (0.71 g, 25%); mp 205 °C; 1H NMR (DMSO-d 6): δ 2.44 (s, 3H, CH3), 3.58–3.66 (m, 3H, H-5′ and H-4′), 4.17 (t, 1H, J 2′,3′ 7.5 Hz, H-3′), 4.26 (t, 1H, H-2′), 4.48–4.53 (large s, 3H, 2′-OH, 3′-OH, 5′-OH), 6.48 (d, 1H, J 1′,2′ 8.1 Hz, H-1′), 8.07 (d, 1H, J 6,NH 6.6 Hz, CH pyrimidine), 11.81 (d, 1H, NH); 13C NMR (DMSO-d 6): δ 30.02 (CH3), 61.91, 75.93, 76.64, 80.80, 83.00 (CH arabinose), 110.66 (C-5 pyrimidine), 146.99 (C-6 pyrimidine), 149.92 (2-CO pyrimidine), 161.37 (4-CO pyrimidine), 193.51 (CO acetyl). Anal. Calcd for C11H14N2O7: C, 46.16; H, 4.93; N, 9.79. Found: C, 45.96; H, 5.02; N, 9.94.
4.7. 7-Ethylcarboxylate-2,3,3a,9a-tetrahydro-3-hydroxy-2-hydroxymethyl-8H-furo[2′,3′:4,5] oxazolo[3,2-a]pyrimidine-8-one (7)
To a solution of 1 (6.96 g, 0.04 mol) in a minimum amount of absolute ethanol, DEEM (8.64 g, 0.04 mol) was added under stirring at room temperature. Stirring was continued for 12 h, the solution was concentrated under reduced pressure, and the resulting residue was applied to a column of silica gel eluted with chloroform–methanol (90/10) to give 7 (5.1 g, 43%); mp 165 °C; IR ν cm−1 (potassium bromide), 3428 (OH), 1734 (C O), 1660–1600 (C N, C C); 1H NMR (DMSO-d 6) δ: 1.25 (t, 3H, J CH2,CH3 7.2 Hz, OCH2CH 3), 3.26–3.39 (m, 2H, H-5′), 4.09–4.12 (m, 1H, H-4′), 4.20 (q, 2H, OCH 2CH3), 4.41–4,43 (m, 1H, H-3′), 4.93 (large s, 1H, 5′-OH D2O exchangeable), 5.25 (d, 1H, J 1′,2′ 6.0, H-2′), 5.90 (large s, 1H, 3′-OH D2O exchangeable), 6.43 (d, 1H, H-1′), 8. 43 (s, 1H, = CH pyrimidine); 13C NMR (DMSO-d 6): δ 14.2 (OCH2 CH3), 60.2 (OCH2CH3), 60.8, 74.7, 87.9, 89.4, 89.9 (CH arabinose), 110.7 (C-5 pyrimidine), 155.8 (C-2 pyrimidine), 161.8 (C-6 pyrimidine), 163.1 (4-CO pyrimidine), 194.3 (CO2Et). Anal. Calcd for C12H14N2O7: C, 48.33; H, 4.73; N, 9.39. Found: C, 48.14; H, 4.66; N, 9.45.
4.8. Ethyl 3-[3,4-dihydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl]-2,4-dioxo-1,2,3,4-tetra hydropyrimidine-5-carboxylate (8)
To a solution of 7 (2.68 g, 0.01 mol) in methanol (150 mL) at r.t. was rapidily added ammonium hydroxyde (32% soln., 10 mL). After stirring for 4 h at r.t., the solution was concentrated under reduced pressure and the residue purified over silica gel using chloroform–methanol (70/30) as eluant to give 8 (1.51 g, 48%); mp 103 °C; 1H NMR (DMSO-d 6): δ 1.25 (t, 3H, J CH2,CH3 7.1, OCH2CH 3), 3.17–3.75 (m, 3H, H-5′ and H-4′), 4.09–4.23 (m, 4H, H-3′ and H-2′), 4.42 (large s, 1H, 5′-OH D2O exchangeable), 5.21, 5.38 (2 large s, 2H, 3′-OH and 2′-OH), 6.44 (d, 1H, J 1′,2′ 8.1 Hz, H-1′), 8.16 (s, 1H, H-6 pyrimidine), 11.72 (large s, 1H, NH D2O exchangeable); 13C NMR (DMSO-d 6): δ 14.22 (OCH2 CH3), 60.16 (OCH2CH3), 62.03, 75.99, 76.65, 80.80, 83.09 (CH arabinose), 102.64 (C-5 pyrimidine), 146.25 (2-CO pyrimidine), 150.21 (C-6 pyrimidine), 159.32 (4-CO pyrimidine), 162.55 (CO2Et). Anal. Calcd for C12H16N2O8: C, 45.57; H, 5.10; N, 8.86. Found: C, 45.70; H, 4.89; N, 8.97.
4.9. N′-[6-(O-tert-Buthyldimethylsilyl)-5-(O-tert-buthyldimethylsilyl-methyl)-3a,5,6,6a-tetrahydrofuro[2,3-d][1,3]oxazol-2-yl]-N,N-dimethylimidoformamide (9)
To a stirred solution of 2 (3.68 g, 9.20 mmol) in toluene (100 mL) at 50 °C, DMF–DMA (1.19 g, 0.01 mol) was added and heating was continued for 5 h. After concentration under reduced pressure, the resulting white visquous residue was chromatographed over silica gel with chloroform–methanol (90/10) as eluant to give 9 (1.26 g, 30%); mp 126 °C; 1H NMR (CDCl3): δ 0.01, 0.02, 0.09, 0.12 (4s, 12H, SiMe), 0.86 (s, 18H, 2 tBuSi), 3.02, 3.07 (2s, 6H, NMe2), 3.34–3.62 (m, 2H, H-5′), 3.87–3.91 (m, 1H, H-4′), 4.40 (s, 1H, H-3′), 4.64 (t, 1H, J 1′,2′ 4.7 Hz, H-2′), 5.95 (d, 1H, H-1′), 8.39 (s, 1H, CH); 13C NMR (CDCl3): δ −5.41, −4.91 (SiMe2), 17.97, 18.29 ((CH3)3 C), 25.89 ((CH3)3C), 34.90, 41.06 (NMe2), 63.10, 77.31, 86.0, 90.0, 99.9 (CH arabinose), 160.82 (CH amidine), 162.35, (C-2 oxazoline). Anal. Calcd for C21H43N3O4Si2: C, 55.10; H, 9.47; N, 9.18. Found: C, 55.31; H, 9.40; N, 9.42.
4.10. 7-Acetyl-2,3,3a,9a-tetrahydro-3-(O-tert-buthyldimethylsilyl)-2-(O-tert-buthyldimethyl silyl-methyl)-8H-furo[2′,3′:4,5] oxazolo[3,2-a]pyrimidine-8-one (10)
To a stirred solution of 9 (4.12 g, 9.00 mmol) in acetone (150 mL) at 50 °C, diketene acetone adduct (6.39 g, 45.00 mmol) was added dropwise After stirring for 4 h, the solution was concentrated under reduced pressure and the resulting visquous residue applied over silica gel with chloroform–methanol (90/10) as eluant. The title compound was isolated as a fluorescent solid which was recrystallized in aqueous ethanol (50/50) to yield pure 10 (1.74 g, 40%); mp 78 °C; 1H NMR (CDCl3): δ −0.03, 0.03, 0.16, 0.18 (4s, 12H, 2 SiMe2), 0.81, 0.93 (2s, 18H, 2 tBuSi), 2.64 (s, 3H, CH3), 3.47–3.70 (m, 2H, H-5′), 4.17–4.19 (m, 1H, H-4′), 4.73–4.74 (m, 1H, H-3′), 5.20 (d, 1H, J 1′,2′ 5.9 Hz, H-2′), 6.57 (d, 1H, H-1′), 8.59 (s, 1H, CH pyrimidine); 13C NMR (CDCl3): δ −5.48, −4.90 (2 SiMe2), 17.97, 18.33 ((CH3)3 C), 25.44, 25.77 ((CH3)3C), 30.93 (CH3), 61.60, 75.71, 87.20, 88.65, 90.59 (CH arabinose), 118.86 (C-5 pyrimidine), 158.27 (C-2 pyrimidine), 161.02 (C-6 pyrimidine), 162.60 (CO pyrimidine), 195.04 (COCH3). Anal. Calcd for C23H40N2O6Si2: C, 55.61; H, 8.12; N, 5.64. Found: C, 55.93; H, 7.98; N, 5.53.
4.11. (5Z)-5-(1-Aminoethylidene)-3-[3-hydroxy-4-(O-tert-buthyldimethylsilyl)-5-(O-tert-buthyldimethyl silyl-methyl)tetrahydrofuran-2-yl]pyrimidine-2,4(3H,5H)-dione (11)
To a solution of 10 (0.56 g, 1.13 mol) in THF (4 mL) at r.t. was rapidily added ammonium hydroxyde (32% soln., 0.1 mL). After stirring for 24 h at r.t., the solution was concentrated under reduced pressure and the resulting yellow residue purified over silica gel using chloroform–methanol (90/10) as eluant to give 11 (0.32 g, 56%); mp 207 °C; 1H NMR (DMSO-d6): δ −0.03, −0.02, 0.04, 0.06 (4s, 12H, 2 SiMe2), 0.81, 0.84 (2s, 18H, 2 tBuSi), 2.44 (s, 3H, CH3), 3.55–3.73 (m, 2H, H-5′), 3.85–3.91 (m, 1H, H-4′), 4.33–4.38 (m, 1H, H-3′), 5.15 (d, 1H, J 1′,2′ 7.1 Hz, H-2′), 6.50 (d, 1H, H-1′), 8.55 (s, 1H, = CH pyrimidine), 9.94, 10.81 (2s, 2H, NH2); 13C NMR (DMSO-d6): δ −5.30, −5.14, −4.83, −3.95 (SiMe), 17.64, 18.10 ((CH3)3 C), 20.92, 21.36 ((CH3)3C), 25.54 (CH3), 64.23, 76.85, 77.92, 80.57, 82.89 (CH arabinose), 105.38 (C(Me)NH2), 147.96 (C-5 pyrimidine), 150.47 (C-6 pyrimidine), 163.36 (2-CO pyrimidine), 173.21 (4-CO). Anal. Calcd for C23H43N3O6Si2: C, 53.77; H, 8.44; N, 8.18. Found: C, 53.71; H, 8.57; N, 8.05.
4.12. Cyclohexa[1,2-d]-2,3,3a,11a-tetrahydro-3-(O-tert-buthyldimethylsilyl)-2- (O-tert-buthyldimethylsilyl-methyl)-10H-furo[2′,3′:4,5]oxazolo[3,2-a]pyrimidine-10-one (12) and cyclohexa[1,2-e]-2,3,3a,11a-tetrahydro-3- (O-tert-buthyldimethylsilyl)-2- (O-tert-buthyldimethylsilyl-methyl)-6H-furo[2′,3′:4,5]oxazolo[3,2-a]pyrimidine-6-one (13)
To a stirred solution of 2 (3.0 g, 7.5 mmol) in xylene (50 mL) was added ethyl-2-oxocyclohexane-carboxylate (1.27 g, 7.50 mmol). The solution was heated to reflux for 5 h and the water formed was continuously extracted by means of a Dean–Stark apparatus. After the reaction mixture was cooled, the solvent was removed under reduced pressure. Silica gel column chromatography of the oily residue with CHCl3 afforded 12, Rf 0.91 (CHCl3) followed by 13, Rf 0.74 (CHCl3); both compounds were recrystallized in diethyl ether.
12 (1.58 g, 41%); mp 117 °C. 1H NMR (CDCl3): δ −0.05, −0.01, 0.12, 0.15 (4s, 12H, 2 SiMe2), 0.81, 0.90 (2s, 18H, 2 tBuSi), 1.67–1.75 (m, 4H, 2 CH2 cyclohexyl), 2.43–2.51 (m, 4H, 2 CH2 cyclohexyl), 3.42–3.64 (m, 2H, H-5′), 4.07–4.11 (m, 1H, H-4′), 4.73–4.75 (m, 1H, H-3′), 5.0 (d, 1H, J 1′,2′ 5.9 Hz, H-2′), 6.45 (d, 1H, H-1′); 13C NMR (CDCl3): δ −5.63, −5.54 (2 SiMe2), 17.91, 18.21 ((CH3)3 C), 25.72 ((CH3)3C), 21.75, 21.84, 22.19, 32.01 (CH2 cyclohexyl), 61.70, 75.93, 86.89, 88.16, 88.8 (CH arabinose), 115.22 (C-5 pyrimidine), 155.32 (C-6 pyrimidine), 160.40 (C-2 pyrimidine), 161.83 (C O). Anal. Calcd for C25H44N2O5Si2: C, 59.02; H, 8.72; N, 5.51. Found: C, 58.85; H, 8.77; N, 5.69.
13 (1.14 g, 30%); mp 239 °C. α = −82.9 ± 1; 1H NMR (CDCl3): δ −0.02, −0.12, 0.14, 0.16 (4s, 12H, 2 SiMe2), 0.83, 0.91 (2s, 18H, 2 tBuSi), 1.70–1.83 (m, 4H, 2 CH2 cyclohexyl), 2.37–2.74 (m, 4H, 2 CH2 cyclohexyl), 3.40–3.57 (m, 2H, H-5′), 4.07–4.12 (m, 1H, H-4′), 4.58–4.59 (m, 1H, H-3′), 5.07–5.09 (m, 1H, H-2′), 6.24 (d, 1H, J 1′,2′ 5.8, H-1′); 13C NMR (CDCl3): δ −5.50, −5.48, −4.94, −4.84 (SiCH3), 17.92, 18.19 ((CH3)3 C), 21.24, 21.43, 22.14, 23.95 (CH2 cyclohexyl), 25.63, 25.71 ((CH3)3C), 61.77, 75.90, 88.15 (C arabinose), 88.41 (C-1′ and C-2′ arabinose), 116.16, 142.75 (C-5, C-6 cyclohexyl), 158.58 (C-2 pyrimidine), 171.90 (C O). Anal. Calcd for C25H44N2O5Si2: C, 59.02; H, 8.72; N, 5.51. Found: C, 58.98; H, 8.43; N, 5.32.
4.13. Cyclohexa[1,2-d]-2,3,3a,11a-tetrahydro-3-hydroxy-2-hydroxymethyl-10H-furo[2′,3′:4,5] oxazolo[3,2-a]pyrimidine-10-one (14)
To a stirred solution of 12 (1.016 g, 0.002 mol) in THF (10 mL) was added tetra-n-butylammonium fluoride (1 M solution in THF, 10 mL). Reaction mixture was stirred at room temperature for 20 min and then the solvent was evaporated. The crude product was submitted to column chromatography with acetone–methanol (80/20) as eluant, yielding pure 14 (0.45 g, 80%); mp 246 °C; 1H NMR (DMSO-d 6): δ 1.56–1.72 (m, 4H, 2 CH2 cyclohexyl), 2.24–2.32 and 2.38–2.46 (2 m, 4H, 2 CH2 cyclohexyl), 3.17–3.30 (m, 2H, H-5′), 3.98–4.03 (m, 1H, H-4′), 4.32–4.36 (m, 1H, H-3′), 4.90 (t, 1H, J OH,5′ 5.4 Hz, 5′-OH D2O exchangeable), 5.12 (d, 1H, J 1′,2′ 6.1 Hz, H-2′), 5.83 (d, 1H, J OH,3′ 4.5 Hz, 3′-OH D2O exchangeable), 6.33 (d, 1H, H-1′); 13C NMR (DMSO-d 6): δ 21.50, 21.69, 21.86, 31.51 (CH2 cyclohexyl), 60.72, 74.20, 79.22, 87.07, 88.31 (CH arabinose), 113.56 (C-5 pyrimidine), 155.89 (C-6 pyrimidine), 159.76 (C-2 pyrimidine), 161.21 (C O). Anal. Calcd for C13H16N2O5: C, 55.71; H, 5.75; N, 9.99. Found: C, 55.97; H, 6.02; N, 10.13.
4.14. 3-[3,4-dihydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl]-5,6,7,8-tetrahydroquina zoline-2,4(1H,3H)-dione (15)
To a stirred solution of 14 (0.12 g, 0.43 mmol) in ethanol (20 mL) was added sodium ethylate (0.013 mol) at room temperature. The mixture was then heated at 80 °C and then stirred for 6 h at room temperature. After concentration under reduced pressure, the residue was taken up in water (20 mL) and acidified with 5% aqueous HCl until pH 1. After concentration to dryness, absolute ethanol (50 mL) was added, the mixture was filtered and the filtrate evaporated. The crude product was submitted to column chromatography eluted with chloroform–methanol (70/30) to give pure 15 (16.5 mg, 13%); mp 98 °C; UV λ max(MeOH)/nm 293 (ɛ 26,243); 1H NMR (DMSO-d 6): δ 1.60–1.62 (m, 4H, 2 CH2 cyclohexyl), 2.13–2.14 and 2.29–2.30 (2 m, 4H, 2 CH2 cyclohexyl), 3.53–3.55 (m, 1H, H-4′), 3.59–3.61 (m, 2H, H-5′), 4.18–4.22 (m, 2H, H-3′ and H-2′), 4.46 (t, 1H, J OH,5′ 5.0 Hz, 5′-OH D2O exchangeable), 5.15 (d, 1H, J 4.6 Hz, 3′-OH or 2′-OH D2O exchangeable), 5.24 (d, 1H, J 5.0 Hz, 2′-OH or 3′-OH D2O exchangeable), 6.44 (d, 1H, J 1′,2′ 7.2 Hz, H-1′), 10.77 (s, 1H, NH); 13C NMR (DMSO-d 6): δ 20.82, 20.95, 21.27, 25.61 (CH2 cyclohexyl), 61.63, 75.66, 76.87, 80.57, 82.61 (CH arabinose), 105.42 and 150.57 (Cq pyrimidine), 147.90 and 163.42 (C O). Anal. Calcd for C13H18N2O6: C, 52.35; H, 6.08; N, 9.39. Found: C, 52.29; H, 6.14; N, 9.31.
4.15. Cyclohexa[1,2-e]-2,3,3a,11a-tetrahydro-3-hydroxy-2-hydroxymethyl-6H-furo[2′,3′:4,5] oxazolo[3,2-a]pyrimidine-6-one (16)
To a stirred solution of 13 (0.4 g, 0.78 mmol) in THF (5 mL) was added tetra-n-butylammonium fluoride (1 M solution in THF, 4 mL). Reaction mixture was stirred at room temperature for 20 min and then the solvent was evaporated. The crude product was submitted to column chromatography with acetone–methanol (80/20) as eluant, yielding pure 16 (0.21 g, 100%); mp 102 °C; 1H NMR (DMSO-d 6): δ 1.63–1.76 (m, 4H, 2 CH2 cyclohexyl), 2.49–2.51 and 2.59–2.61 (2 m, 4H, 2 CH2 cyclohexyl), 3.17–3.42 (m, 2H, H-5′), 4.02–4.05 (m, 1H, H-4′), 4.36–4.38 (m, 1H, H-3′), 4.95 (large s, 1H, 5′-OH D2O exchangeable), 5.14 (d, 1H, J 1′,2′ 5.7 Hz, H-2′), 5.86 (large s, 1H, 3′-OH D2O exchangeable), 6.45 (d, 1H, H-1′); 13C NMR (DMSO-d 6): δ 21.24, 21.62, 22.39, 23.72 (CH2 cyclohexyl), 61.27, 75.01, 88.27, 89.05, 89.25 (CH arabinose), 114.59, 143.84, 159.18 (Cq pyrimidine), 171.52 (C O). Anal. Calcd for C13H16N2O5: C, 55.71; H, 5.75; N, 9.99. Found: C, 55.85; H, 5.58; N, 10.07.
4.16. 1-[3,4-dihydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl]-5,6,7,8-tetrahydroquinazoline-2,4(1H,3H)-dione (17)
To a stirred solution of 16 (2.80 g, 0.01 mol) in ethanol (30 mL) was added sodium ethylate (0.03 mol) at room temperature. The mixture was then heated at 80 °C and then stirred for 6 h at room temperature. After concentration under reduced pressure, the residue was taken up in water (20 mL) and acidified with 5% aqueous HCl until pH 1. After concentration to dryness, absolute ethanol (50 mL) was added, the mixture was filtered and the filtrate evaporated. The crude product was submitted to column chromatography eluted with chloroform–methanol (70/30) to give pure 17 (0.74 g, 25%); mp 93 °C; UV λ max (MeOH)/nm 294 (ɛ 54,200); 1H NMR (DMSO-d 6): δ 1.50–1.57 (m, 4H, 2 CH2 cyclohexyl), 2.20–2.22, 2.70–2.72 (2 m, 4H, 2 CH2 cyclohexyl), 3.45–3.48 (m, 1H, H-4′), 3.57–3.63 (m, 2H, H-5′), 4.00–4.03 (m, 1H, H-3′), 4.13–4.15 (m, 1H, H-2′), 4.68 (t, 1H, J OH,5′ 5.5 Hz, 5′-OH D2O exchangeable), 5.31 (t, 1H, J OH,3′ 5.7 Hz, 3′-OH D2O exchangeable), 5.48 (t, 1H, J OH,2′ 5.1 Hz, 3′-OH D2O exchangeable), 6.12 (d, 1H, J 1′,2′ 7.2 Hz, H-1′); 13C NMR (DMSO-d 6): δ 20.63, 21.83, 22.10, 26.70 (CH2 cyclohexyl), 60.89, 75.92, 76.94, 82.20, 84.51 (CH arabinose), 108.27, 150.71 (Cq pyrimidine), 151.00, 163.11 (C O). Anal. Calcd for C13H18N2O6: C, 52.35; H, 6.08; N, 9.39. Found: C, 52.43; H, 6.27; N, 9.22.
4.17. Cytotoxicity evaluations
Murine leukemia L1210, human T-lymphocyte Molt 4/C8, and CEM cells were suspended at 300,000–500,000 cells/mL of culture medium, and an amount of 100 μL of these cell suspensions was added to 200 μL microtiter plate wells containing 100 μL of an appropriate dilution of the test compounds. After 2 days (L1210) or 3 days (Molt 4/C8 and CEM) of incubation at 37 °C, the cell number was determined using a Coulter counter. The 50% cytostatic concentration (IC50) was defined as the compound concentration required to inhibit cell proliferation by 50% [22].
4.18. Antiviral activity assays
The antiviral activity of the compounds was determined as described [23], [24]. The assays were based on inhibition of virus-induced cytopathicity in HEL [herpes simplex virus type 1 (HSV-1) (KOS), HSV-2 (G), vaccinia virus and vesicular stomatitis virus], Vero (parainfluenza-3, reovirus-1, Sindbis, Coxsackie B4, and Punta Toro virus), HeLa (vesicular stomatitis virus, Coxsackie virus B4, and respiratory syncytial virus) or MDCK (influenza A (H1N1; H3N2); and influenza B) cell cultures. Confluent cell cultures in microtiter 96-well plates were inoculated with 100 CCID50 of virus (CCID50 being the virus dose to infect 50% of the cell cultures). After a 1 h virus adsorption period, residual virus was removed, and the cell cultures were incubated in the presence of varying concentrations (200, 40, 8 μM) of the test compounds. Viral cytopathicity was recorded as soon as it reached completion in the control virus-infected cell cultures that were not treated with the test compounds.
References
- 1.Gahrton G. Adv. Cancer Res. 1983;40:255–329. doi: 10.1016/s0065-230x(08)60682-x. [DOI] [PubMed] [Google Scholar]
- 2.Donehower R.C., Karp J.E., Burke P.J. Cancer Treat. Rep. 1986;70:1059–1063. [PubMed] [Google Scholar]
- 3.Kovalova L., McArdell C.S., Hollender J. J. Chromatogr. A. 2009;1216:1100–1108. doi: 10.1016/j.chroma.2008.12.028. [DOI] [PubMed] [Google Scholar]
- 4.Hamada A., Kawaguchi T., Nakano M. Clin. Pharmacokinet. 2002;41:705–718. doi: 10.2165/00003088-200241100-00002. [DOI] [PubMed] [Google Scholar]
- 5.Aoshima M., Tsukagoshi S., Sakurai Y., Oh-ishi J.-I., Ishida T., Kobayas H. Cancer Res. 1976;36:2726–2732. [PubMed] [Google Scholar]
- 6.Kataoka T., Sakurai Y. Recent Results Cancer Res. 1980;70:147–151. doi: 10.1007/978-3-642-81392-4_15. [DOI] [PubMed] [Google Scholar]
- 7.Forfar I., Jarry C., Laguerre M., Léger J.-M., Pianet I. Tetrahedron. 1999;55:12819–12828. [Google Scholar]
- 8.Jarry C., Forfar I., Thomas J., Léger J.-M., Laguerre M. Heterocycles. 1993;36:2465–2473. [Google Scholar]
- 9.Adetchessi O.-S., Léger J.-M., Guillon J., Forfar-Bares I., Bosc J.-J., Jarry C. Tetrahedron. 2005;61:4453–4460. [Google Scholar]
- 10.Wierenga W., Loughman B.E., Gibbons A.J., Renis H.E. J. Med. Chem. 1978;21:558–562. doi: 10.1021/jm00204a011. [DOI] [PubMed] [Google Scholar]
- 11.Wierenga W., Woltersom J.A. J. Org. Chem. 1978;43:529–535. [Google Scholar]
- 12.Schroeder A.C., Srikrishnan T., Parthasarathy R., Bloch A. J. Heterocycl. Chem. 1981;18:571–574. [Google Scholar]
- 13.Purkayastha S., Panzica R.P. J. Heterocycl. Chem. 1990;27:743–751. [Google Scholar]
- 14.Ingar A.A., Luke R.W.A., Hayter B.R., Sutherland J.D. ChemBioChem. 2003;4:504–507. doi: 10.1002/cbic.200300554. [DOI] [PubMed] [Google Scholar]
- 15.Allen F.H., Kennard O., Watson D.G., Brammer L., Orpen A.G., Taylor R. J. Chem. Soc. Perkin Trans. 1987:S1–S18. [Google Scholar]
- 16.Tollin P., Wilson H.R., Young D.W. Acta Crystallogr. 1973;B29:1641–1647. [Google Scholar]
- 17.Chaimbault C., Bosc J.-J., Jarry C., Daulouede S., Vincendeau P. Pharm. Pharmacol. Commun. 2000;6:101–105. [Google Scholar]
- 18.MarvinSketch 4.1.13, ChemAxon Ltd 2007. http://www.chemaxon.com/marvin
- 19.North A.C.T., Phillips D.C., Mathews F.S. Acta Crystallogr. A. 1968;24:351–359. [Google Scholar]
- 20.Sheldrick G.M. University of Göttingen; Germany: 1997. SHELXS 97, Program for Crystal Structure Refinement. [Google Scholar]
- 21.Sheldrick G.M. University of Göttingen; Germany: 1997. SHELXS 97, Program Crystal Structure Solution. [Google Scholar]
- 22.De Clercq E., Balzarini J., Torrence P.F., Mertes M.P., Schmidt C.L., Shugar D., Barr P.J., Jones A.S., Verhelst G., Walker R. Mol. Pharmacol. 1981;19:321–330. [PubMed] [Google Scholar]
- 23.De Clercq E., Holý A., Rosenberg I., Sakuma T., Balzarini J., Maudgal P.C. Nature. 1986;323:464–467. doi: 10.1038/323464a0. [DOI] [PubMed] [Google Scholar]
- 24.Balzarini J., Naesens L., Slachmuylders J., Niphuis H., Rosenberg I., Holý A., Schellekens H., De Clercq E. AIDS. 1991;5:21–28. doi: 10.1097/00002030-199101000-00003. [DOI] [PubMed] [Google Scholar]