Abstract
Respiratory syncytial virus (RSV) is a major cause of respiratory disease in both cattle and young children. Despite the development of vaccines against bovine (B)RSV, incomplete protection and exacerbation of subsequent RSV disease have occurred. In order to circumvent these problems, calves were vaccinated with the nucleocapsid protein, known to be a major target of CD8+ T cells in cattle. This was performed according to a DNA prime–protein boost strategy. The results showed that DNA vaccination primed a specific T-cell-mediated response, as indicated by both a lymphoproliferative response and IFN-γ production. These responses were enhanced after protein boost. After challenge, mock-vaccinated calves displayed gross pneumonic lesions and viral replication in the lungs. In contrast, calves vaccinated by successive administrations of plasmid DNA and protein exhibited protection against the development of pneumonic lesions and the viral replication in the BAL fluids and the lungs. The protection correlated to the cell-mediated immunity and not to the antibody response.
Keywords: BRSV, Prime–boost, Nucleocapsid gene
1. Introduction
RSV is a major cause of hospitalization of infants less than 2 years of age [1]. Like its human counterpart, bovine respiratory syncytial virus (BRSV) is a major cause of calf morbidity and mortality [2]. Calves less than 1 year of age are particularly susceptible to the disease [3]. Maternal antibodies against respiratory syncytial virus do not prevent infection in calves but antibodies seem to decrease the severity of infection [4], [5], [6].
Furthermore, BRSV is one of the viruses contributing to bovine respiratory disease complex [7]. Protection against BRSV afforded by vaccination has been described and numerous vaccines are licensed and widely used in the field [8], [9], [10]. However, exacerbation of respiratory disease has been induced in calves and in infants vaccinated with formalin-inactivated BRSV vaccine [11], [12]. In mice, enhanced disease has an immunopathological basis, characterized by polarized type 2-helper (TH2) response. Increased IL-4 production has been associated with pulmonary histopathology and eosinophilia [13], [14], [15]. In calves, enhanced pathology has been associated with elevated IgE titers and marked eosinophilia, suggesting a TH2-mediated immune response [16], [17], [18]. In addition, some authors have reported high mortality in calves subsequent to the use of inactivated vaccine in the field [19], [20]. There is thus a need for protective vaccines, able to confer protection in neonates, even in the presence of maternal antibodies and lacking disease exacerbating side effects.
It has previously been shown that the F and G surface glycoproteins, and the N protein, are the major protective antigens of BRSV [21]. Neutralizing antibodies to the F protein can mediate protection [22]. However, serum antibodies provide greater contribution in reducing BRSV induced lower respiratory disease than upper respiratory tract virus shedding [4], [23]. The cell-mediated immunity is also a critical parameter in the outcome of RSV infection [24], [25]. The F, P, G, M, M2 and N proteins are antigenic targets recognized by bovine CD8+ T cells, the latter two proteins being also specific targets for murine (M2 protein) or murine and human (N protein) CD8+ T cells [26], [27], [28], [29]. These cells constitute the major lymphocyte subpopulation in the respiratory tract of calves recovering from BRSV infection and depletion of those cells also induces delayed virus clearance from the lungs and nasopharynges in calves [23], [30], [31]. In mice, diminished CTL activation and migration to the lungs by treatment with anti-LFA-1 delayed viral clearance [32]. Furthermore, priming of the RSV-specific CD8+ response suppressed the eosinophilia induced after challenge of animals vaccinated with the G glycoprotein [33].
DNA vaccination is an effective way of generating humoral and cell-mediated response [34]. Intramuscular vaccination of mice with plasmid DNA expressing the F or G proteins of RSV has shown to induce a strong TH1 response and to reduce the viral load in the lungs after RSV challenge, without inducing enhanced pulmonary inflammatory response or eosinophilia [35], [36]. However, it is generally recognized that DNA vaccines are often less effective in large animals than in mice [37]. Improvement of the immune response can be achieved by boosting animals previously vaccinated with plasmid DNA with inactivated vaccine. This approach has successfully been used to protect calves against bovine herpesvirus-1 (BoHV-1) challenge [38]. This strategy was also successfully used in a previous BRSV experiment. Calves vaccinated with codon-optimized plasmid DNA expressing the F and N BRSV proteins and boosted with an inactivated commercial vaccine were protected against clinical signs, gross pneumonic lesions and virus replication [39]. In that study, the humoral and cell-mediated immune responses were primed by DNA injection and enhanced by the protein boost. They reduced the clinical signs and the virus replication after BRSV challenge. The use of the F protein of RSV in a prime–boost protocol also represents an interesting strategy for early life immunization [40].
The purpose of the work presented here was to investigate the protection of calves against BRSV, by focusing mainly on the stimulation of the BRSV cell-mediated immunity. Therefore, a combination of plasmid and protein vaccination was performed with the N protein as immunogen. The protection conferred by this vaccination scheme was evaluated after BRSV challenge.
2. Materials and methods
2.1. Expression of the N protein
The pcDNA3 plasmid expressing synthetic DNA sequence coding for the ORF of the N protein of BRSV (pNsyn) has been described elsewhere [39]. Large scale plasmid DNA was purified from transformed TOP 10 bacteria cells by affinity chromatography on anion-exchange resin (Plasmid Giga kit, Qiagen).
The purified N protein was obtained from a recombinant baculovirus expressing the coding sequence of the N protein of BRSV strain RB94. Briefly, the N gene was cloned in the pBlueBacHis2A vector (Invitrogen). Recombinant baculovirus expressing the N protein (BacN) was obtained after transfection of Sf9 insect cells with the recombinant plasmid and purification of the β-galactosidase-expressing plaques as recommended by the manufacturer. Sf9 cells were infected with BacN at a moi of 2. Cells were harvested 48–72 h pi, when at least 95% of the cells were lysed, washed with PBS and resuspended in Tris–HCl 20 mM pH 7.9, NaCl 0.5 M, Imidazole 5 mM buffer. The 6His-tagged N protein was purified on Ni-NTA resin (Qiagen) in batch, as recommended by the manufacturer. The protein concentration was determined by BCA protein assay (Pierce) according to the manufacturer's recommendations. As expected, a protein of 43 kDa was purified and analyzed by PAGE (Fig. 1 ) [41]. Prior to immunization, QuilA adjuvant was added to the N protein at a final concentration of 1 mg/ml.
Fig. 1.

Polyacrylamide gel electrophoresis of the N protein, purified from recombinant baculovirus infected Sf9 insect cells (lane 2) and a molecular weight marker (Sigma, lane 1). The proteins were stained with Coomassie blue.
2.2. Cell culture and virus
The BRSV RB90 virus was grown on calf primary kidney cells in Minimal Eagle medium (MEM, GIBCO BRL) supplemented with 10% fetal calf serum, 10 mg/ml gentamycin and 106 IU penicillin.
Challenge inoculum's consisted in lung lavage fluid of a calf collected after intratracheal inoculation with the BRSV strain VRS3761, as described previously [39], [42]. This inoculum contained 103.4TCID50/ml and was free of bovine viral diarrhea virus, bovine herpesvirus-1, bovine parainfluenza virus-3, bovine coronavirus, bovine adenovirus-5, endotoxins, bacteria and mycoplasmas. Back titration confirmed the amount of virus inoculated.
2.3. Study design
Fourteen cross-bred dairy calves between 4 and 6 weeks of age were randomly allocated in 4 experimental groups of 3 or 4 animals and housed in 3 identically conditioned stables. All calves were shown to be seronegative for BRSV, BoHV-1 and Bovine viral diarrhea virus at the start of the experiment.
As shown in Table 1 , 7 calves were vaccinated twice at a 4 weeks interval (day 0 and 29) with 500 μg of pNsyn plasmid DNA in saline. The first vaccine dose was injected intramuscularly and the second dose was administrated intradermally in 4 sites in the neck. Seven other calves were subjected to the same protocol, but with the pcDNA3 control plasmid. Four weeks after the second DNA administration (day 60), respectively, 4 and 3 calves injected with the pNsyn and the control plasmids were subjected to intramuscular injection of 300 μg of purified N protein, adjuvanted with Quil A in a 2 ml volume. The other calves were treated with saline. Three weeks after the third vaccination (day 80), all the calves were challenged with the BRSV 3761 strain by intratracheal (10 ml) injection combined to intranasal nebulization (5 ml). The calves were examined daily after challenge for the following clinical signs: cough, nasal discharge, anorexia, depression, rectal temperature and respiratory rate.
Table 1.
Experimental design
| Group | n | Vaccination |
Challenge | n calves | Euthanized | ||
|---|---|---|---|---|---|---|---|
| Day 0/V1 | Day 29/V2 | Day 60/V3 | Day 80 | Day 86 | Day 92 | ||
| Mock | 4 | pcDNA3 | pcDNA3 | Saline | BRSV | 2 | 2 |
| pNsyn | 3 | pNsyn | pNsyn | Saline | BRSV | 1 | 2 |
| Npur | 3 | pcDNA3 | pcDNA3 | Npur | BRSV | 1 | 2 |
| pNsyn-Npur | 4 | pNsyn | pNsyn | Npur | BRSV | 2 | 2 |
Sera and heparin treated blood were collected prior to each vaccination, on the day of challenge and on days 6 (day 86) and 12 after challenge (day 92) for examination of humoral and cell-mediated responses. Bronchoalveolar lung (BAL) fluids were collected every 2 or 3 days from day 3 before to day 11 after challenge by instillation and aspiration of 50 ml of PBS as described previously [39]. On days 6 and 12 after challenge, one or two calves of each group were euthanized. Lungs were excised immediately after euthanasia. Macroscopic lesions were recorded to score the extent of pneumonic consolidation and seven pieces of tissue from right cranial (cranial and caudal portions), middle and caudal lobes were excised.
2.4. Lymphocyte proliferation assay (LPA)
Heparinised blood samples were ten-fold diluted in RPMI 1640 medium (Gibco, Belgium) supplemented with 2 mM glutamine, 5 × 10−5 M β-2-Mercaptoethanol, 100 U/ml of gentamycin and 2.5 μg/ml fungizone. Two hundred μl of diluted blood samples were incubated during 6 days with 20 μl of BRSV antigen (Sonicated and heat-inactivated culture supernatant of primary calf kidney cells infected with RB90 virus, containing 105TCID50/ml), or 20 μl of control antigen (Sonicated and heat-inactivated culture supernatant of primary mock-infected calf kidney cells). After the 6-days incubation, 0.8 μCi of methyl [3H] thymidine in 25 μl RPMI 1640 medium was added to each well. Cells were collected after a subsequent 18 h incubation using a cell harvester and radioactivity incorporated into the DNA was measured by liquid scintillation counting (Betaplate, Pharmacia, Sweden). The results were expressed as stimulation indexes (SI) corresponding to the number of c.p.m. obtained with the BRSV antigen divided by the number of c.p.m. obtained with the control preparation. The SI cut-off of 2.4 was determined as the mean + 3 standard deviations of the SI values of all animals before vaccination.
2.5. IFN-γ assay
The gamma-interferon (IFN-γ) production was measured after a 24-h in vitro stimulation of 1 ml of heparinised blood with 20 μl of BRSV or control antigen. The amounts of IFN-γ in the plasma were quantified by ELISA with the Bovine γ-interferon EASIA kit (Biosource Europe). The results were expressed as stimulation indexes (SI) corresponding to the OD obtained with the BRSV antigen divided by the OD obtained with the control preparation. The cut-off SI value of 1.6 was calculated as 3 standard deviations above the mean SI value of all calves before vaccination.
2.6. Serological response
Serum samples were collected from all calves before each vaccination, on the day of challenge and on days 6 and 12 after the challenge. They were subjected to the indirect immunofluorescence test as described previously [43]. Briefly, the sera were serially three-fold diluted starting from a 1/15 dilution and they were incubated on both slides covered with BRSV infected and with non-infected primary kidney cells. After a washing step, the cells were incubated with FITC-conjugated rabbit anti-bovine IgG and observed on a fluorescence microscope. Slides were examined without any information on the group assignments.
Local humoral priming was investigated by testing the presence of N-specific IgG and IgA in the BAL after challenge. IgG antibodies were determined in an indirect ELISA, using the purified N protein as coating antigen. After incubation with two-fold dilutions of LBA, starting from 1:10, the test was revealed by using HRP-conjugated rabbit anti-bovine serum and TMB as substrate. N-specific IgA antibodies were determined essentially as described [44]. Briefly, plates were coated with Mab against bovine IgA (Cedi-diagnostics, The Netherlands) and incubated with two-fold dilutions of LBA starting from 1:2. Reaction with the purified N protein was revealed by using biotin-conjugated bovine serum against BRSV, HRP-conjugated streptavidin and TMB.
2.7. Viral replication
The viral load in the BAL fluids and in the lungs was investigated by quantitative real-time RT-PCR. Briefly, for each animal, pieces of approximately 1 cm3 of each lung lobe were mixed together in 10 ml of PBS using an Ultraturrax homogenizer. Two hundred μl of BAL fluid and 200 μl of homogenate extracts were subjected to RNA extraction and cDNA synthesis as described previously [45]. Real-time RT-PCR specific for BRSV and for bovine beta-actin (ACTB) were performed along with the corresponding standard curves allowing quantification. The results were expressed as the number of BRSV RNA copies per 103 ACTB copies in the BAL fluids and per 106 ACTB copies in the lungs.
2.8. Histopathology
A necropsy sample of lesion was taken in each pulmonary lobe. The samples were stored in 10% neutral buffered formalin, embedded in paraffin, sectioned at 5 μm and stained with hematoxylin and eosin for histologic examination. Slides were then examined without any information on the group assignments.
2.9. Statistics
The data collected in the 4 groups were compared by one-way analysis of variance and Tukey's test with a family error rate of 0.05. Logarithmic transformation was applied to the lymphocyte proliferation SI, the IFN-γ SI and the IFI titers in order to fulfil the conditions of variances homogeneity (checked by Bartlett's test) and normality (checked by Ryan and Joiner's test).
3. Results
3.1. BRSV-specific cellular response
BRSV-specific lymphoproliferative response was detected in 4 of the 7 calves vaccinated twice with pNsyn plasmid (Fig. 2 ). While injection of the N protein was not able to induce detectable lymphoproliferative response in non-primed animals, this protein boosted the responses in calves previously vaccinated with pNsyn. After the boost, all the calves of the pNsyn–Npur group had a stimulation index above the cut-off and a considerable enhancement of the BRSV-specific proliferative response. At that time, 2 out of the 3 calves of the pNsyn group still had a SI value above the cut-off. On day 80, the mean SI value of the pNsyn–Npur group was significantly different from the mean SI value of the 3 other groups (p < 0.05) and it remained higher than those of both the mock-vaccinated and Npur groups after challenge (p < 0.05). The BRSV challenge did not induce a detectable response in the mock-vaccinated group. In contrast, the lymphoproliferative responses of the calves vaccinated with pNsyn were enhanced and the mean SI value of this group was significantly different from those of the control group and the group vaccinated with the N protein alone (p < 0.05). Twelve days after challenge, the calves of the Npur group also showed low levels of proliferative response.
Fig. 2.

In vitro BRSV-specific lymphoproliferation following vaccination and challenge. Blood samples were tested at the time of each vaccination (day 0, 29 and 60), on the day of challenge (week 80) and on days 6 and 12 after challenge (days 86 and 92). The results are expressed as stimulation index. The bars represent the mean value for each group and the vertical bars represent the standard deviation of the means. The horizontal dashed bar represents the cut-off value. The stars indicate the means that differ significantly from the mean of the mock-vaccinated group (p < 0.05).
After 2 plasmid vaccinations, the IFN-γ SI of all animals remained below the cut-off (Fig. 3 ). However, after the protein boost, the animals subjected to the pNsyn plasmid and the N protein vaccinations displayed significant IFN-γ production compared to the 3 other groups (day 80, p < 0.05). The animals vaccinated with pNsyn alone had also been primed for IFN-γ production, as shown by significant SI values 1 week after the challenge (day 86). On day 86, the IFN-γ SI of the 2 groups vaccinated with pNsyn were significantly different from those of the 2 other groups (p < 0.05). The mock-vaccinated groups remained negative till the end of the experiment, while one animal of the Npur group show a SI above the cut-off on day 92.
Fig. 3.
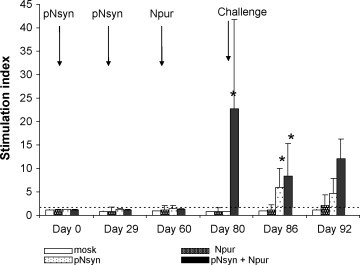
In vitro IFN-γ production after BRSV antigenic stimulation. Blood samples were tested before at the time of each vaccination (days 0, 29 and 60), at challenge (day 80) and at 6 and 12 days after challenge (days 86 and 92). The results are expressed as stimulation index. The bars represent the mean value for each group and the vertical bars represent the standard deviation of the means. The horizontal dashed bar represents the cut-off value. The stars indicate the means that differ significantly from the mean of the mock-vaccinated group.
3.2. BRSV-specific antibody response
BRSV-specific antibody response was measured by indirect immunofluorescence (Fig. 4 ). The pNsyn priming elicited low levels of antibody that increased either after the protein boost in all animals of the pNsyn–Npur group or after the challenge in animals of the pNsyn group. Injection of the N protein alone also induced specific seroconversion. At the time of challenge, the mean antibody titers of the 3 vaccinated groups were not significantly different from each other (p > 0.05). However, the antibody titers of the 3 vaccinated groups were significantly higher than those of the mock-vaccinated group (p < 0.05). Seroconversion of the mock-vaccinated animals was only observed 12 days after challenge, consistent with a priming effect of the challenge.
Fig. 4.
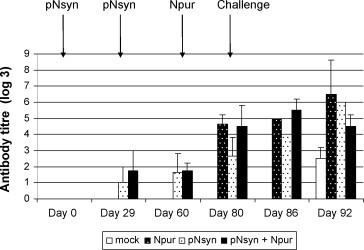
BRSV-specific antibody response. Sera taken at the time of each vaccination (days 0, 29 and 60), on the day of challenge (day 80) and on days 6 and 12 after challenge (days 86 and 92) were analyzed by indirect immunofluorescence. The results are expressed as the log 3 of the last positive dilution and they are presented as the mean value for each group. The vertical bars represent the standard deviation of the means.
The priming of a local response in the different vaccinated groups was investigated by measuring N-specific IgG and IgA in the BAL after challenge. None of the animals exhibited N-specific IgG response 3 days before challenge, and all the animals of the mock- and-pNsyn-vaccinated groups remained negative till the end of the experiment. IgG antibodies were detected in the Npur group from day 5 to 11 after the challenge with a maximum mean titer of 1/20 at D + 5. In the pNsyn–Npur group, positive animals were recorded from 3 to 7 days after the challenge, with a maximum mean titer of 1/30, 5 days after the challenge (data not shown). In contrast, no N-specific IgA response was detected in the BAL, whatever the vaccinated group (data not shown).
3.3. Protection against BRSV challenge
3.3.1. Clinical signs
After challenge, no fever or increase of the respiratory rate were recorded. Three animals of the mock-vaccinated group experienced a mild cough lasting for at least 1 day after challenge, but this was not considered as significant (data not shown).
3.3.2. Viral RNA load in the BAL
The replication of challenged BRSV was followed in the BAL fluids collected every 2 or 3 days by quantitative real-time RT-PCR (Fig. 5 ). As expected, all the calves were negative 3 days before the challenge. Viral RNA was detected in the mock-vaccinated calves from day 3 after the challenge till the end of the experiment. However, at day 11, the infection has almost resolved, as the viral load in the BAL fluids was about 100 copies of viral RNA. Similar kinetic was observed in the calves vaccinated with the N protein, excepted that the virus was cleared on day 11. All animals primed with pNsyn showed lower BRSV RNA copy numbers in their BAL fluids than the 2 other groups. Indeed, for each animal and at each time point, the viral load was below 200 RNA copies/103 ACTB copies. Also, each animal was positive at maximum 2 successive time points and all calves had cleared the virus on day 11 post-challenge. However, the great individual variability and the low number of animals in each group prevented the differences between the 2 groups vaccinated with pNsyn and the 2 other groups to be statistically significant, except on day 5 after challenge (p < 0.05).
Fig. 5.
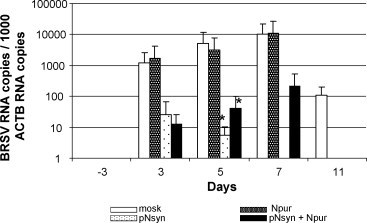
Total RNA was extracted from BAL fluids collected 3 days before and 3, 5, 7 and 11 days after challenge. The viral load was examined by quantitative real-time RT-PCR and expressed as the number of BRSV RNA copies per 103 ACTB RNA copies. The bars represent the mean value for each group and the vertical bars represent the standard deviation of the means. The stars indicate the means that differ significantly from the mean of the control group.
3.3.3. Postmortem examination
One or 2 animals of each group were killed on day 6 after challenge while the 2 remaining animals of each group were killed on day 12.
The lungs were examined and the presence of pneumonic consolidation was recorded for each lobe. The number of affected lobes in each group is summarized in Table 2 . In the mock-vaccinated group, all the calves showed lesions in the caudal part of the cranial lobe, and in the middle and accessory lobes. The cranial part of the cranial lobe was affected in 3 calves and the caudal one in 2 calves. In contrast, in the group vaccinated with both pNsyn and the N protein, only one calf showed one spot of consolidation in the cranial lobe (cranial part) and another in the caudal lobe. This group was significantly different from mock-vaccinated group (p < 0.05). The 2 other groups gave intermediate results (8 affected lobes/15), and the 6 animals showed lesions in the cranial part of the cranial lobe. However, the difference with the control group was not statistically supported. These lesions were also less severe in extent in these 2 groups than in the control group with the exception of one animal vaccinated with the N protein. All the lobes of this animal were affected and the extent of the lesions was comparable to those observed in the mock-vaccinated group.
Table 2.
Macroscopic pneumonic lesions
| Mock | Npur | pNsyn | pNsyn–Npur | |
|---|---|---|---|---|
| Cranial: cranial part | 3/4 | 3/3 | 3/3 | 1/4 |
| Cranial: caudal part | 4/4 | 1/3 | 1/3 | 0/4 |
| Middle | 4/4 | 2/3 | 3/3 | 0/4 |
| Caudal | 2/4 | 1/3 | 1/3 | 1/4 |
| Accessory | 4/4 | 1/3 | 0/3 | 0/4 |
| Total | 17/20 | 8/15 | 8/15 | 2/20 |
For each animal, all the pulmonary lobes were examined for the presence of pneumonic consolidation. The ratios of animals presenting lesions are indicated for each lobe. The total number of affected lobes in each group is also presented.
Histological examination of the lungs revealed typical BRSV lesions. Histopathological lesions of the bronchi and bronchioles including lymphoid peribronchial cuffing (Fig. 6 a and b), lymphoid infiltration, hyperplasia and metaplasia of the epithelium with desquamation and the presence of syncytial cells in the lumen (Fig. 6c and d). Infiltration of mononuclear inflammatory cells with consolidation and emphysema were recorded in the alveoli (Fig. 6e and f). However, irrespective of the slaughtering time post-challenge (day 6 or day 12), the lesions were less severe and more limited in the group vaccinated with pNsyn and Npur (Fig. 6a, c and e) than in the 3 other groups (Fig. 6b, d and f).
Fig. 6.

Pulmonary histopathology after BRSV challenge in the pNsyn–Npur vaccinated group (a, c and e) and in the other groups: mock-vaccinated group (b and d), pNsyn-vaccinated group (f). (b) Lymphoid peribronchial cuffing (arrows). (d) Epithelial hyperplasia and metaplasia (arrows) with desquamation of the cells in the lumen (arrowhead). (f) Consolidation of the alveoli with infiltration of mononuclear inflammatory cells. (a) Absence of lymphoid peribronchial cuffing. (c) Absence of epithelial proliferation. (e) Absence of infiltration of mononuclear inflammatory cells.
The viral replication in the lungs was analyzed by quantitative real-time RT-PCR (Table 3 ). No BRSV RNA was detected in the lungs of the calves vaccinated with pNsyn alone and in the calves primed with pNsyn and boosted with the N protein. Two out of the 3 calves vaccinated with the N protein alone were also protected. In contrast, BRSV RNA was detected in the lungs of all the mock-vaccinated animals.
Table 3.
The viral loads in the lungs were examined by conducting quantitative real-time RT-PCR on total RNA extracted from homogenates of pieces from the different pulmonary lobes
| Experimental group | Calf | BRSV RNA copies/106 ACTB RNA copies |
|---|---|---|
| Mock | 6601 | 5.5 × 103 |
| 6604 | 2.0 × 104 | |
| 6621 | 5.2 × 105 | |
| 6628 | 1.2 × 103 | |
| Npur | 6615 | – |
| 6622 | 6.8 × 104 | |
| 6626 | – | |
| pNsyn | 6596 | – |
| 6610 | – | |
| 6624 | – | |
| pNsyn–Npur | 6603 | – |
| 6611 | – | |
| 6612 | – | |
| 6620 | – | |
(–) Not detected.
4. Discussion
Numerous advantages have been described for the use of DNA vaccines. They theoretically combine the efficacy of live vaccines and the safety afforded by the inactivated vaccine [46]. They can circumvent the inhibitory effects of maternal antibodies [47] and they prime the TH1 component of the immune response [48]. This is of particular interest for overcoming the TH2 bias when vaccinating neonates against BRSV [49].
Several studies described the vaccination of mice with plasmids expressing the F or G glycoproteins [35], [50], [51]. These 2 antigens have also been tested as vaccine candidates in calves. A plasmid expressing the G glycoprotein afforded only partial reduction of viral shedding after challenge [52], [53]. More recently, DNA vaccine encoding the BRSV fusion protein induced significant protection against the virus challenge, by reducing the nasopharyngeal excretion of the virus and the extent of gross pneumonic lesions. However, complete protection against BRSV was not conferred [54]. Finally, DNA immunization of infant rhesus monkeys or young calves against both the BRSV F and nucleocapsid (N) proteins stimulated both humoral and cell-mediated immunity against the virus and reduced viral replication, clinical signs and pulmonary lesions after challenge [39], [55]. Reports describing the use of the N protein are rather scarce. However, N was shown to be a major target of the immune response against RSV. Vaccination of mice with recombinant vaccinia virus encoding the (H)RSV N protein induced partial resistance [56], [57]. Similarly, immunization of young calves with a recombinant vaccinia virus expressing the BRSV N protein induced non-neutralizing antibodies and primed the BRSV-specific proliferative response and IFN-γ production that resulted in reduction of viral replication in the upper and lower respiratory tract [21], [28]. Overall, these data suggest that the protection afforded by the N protein might be largely mediated by cellular immunity.
The efficacy of DNA immunization against the N protein alone had never been studied before the present study. The purpose of this work was thus first to stimulate the cellular immune response using a DNA vaccine and then to enhance this response with a proteic boost. Therefore, we used of the nucleocapsid (N) protein of BRSV in a “prime–boost” vaccination strategy and we compare it to the use of DNA or protein vaccination alone.
In the present study, we show that both cellular and humoral arms of the immune response were stimulated by the vaccine combination. Injection of either pNsyn or Npur induced a BRSV-specific humoral response. The neutralizing activity of the antibodies was not investigated here, but antibodies against the N protein were shown non-neutralizing by others [21]. At the time of challenge, however, there was no significant difference between the vaccinated groups. Furthermore, vaccination did not prime for a local N-specific IgA response. This is in accordance with the results published by others. Indeed, vaccination with a recombinant vaccinia virus expressing the N protein induced local IgG1 but not IgA response [21]. A lymphoproliferative response was only induced in calves that received the pNsyn plasmid. This response was increased when calves were boosted with the N protein, resulting in significantly higher stimulation index compared to the 3 other groups. In contrast, only the prime–boost combination induced the production of IFN-γ.
After the challenge, the clinical and virological protections were evaluated. No clinical signs were recorded, even in the mock-vaccinated animals. This is in contrast with the previous experiment, where the same stock of BRSV 3761 was used [39]. The reasons are unclear but could be linked to the age or the genetic background of the animals [58]. However, the virus replicated in the respiratory tract of the mock-vaccinated animals, the highest viral load being observed in the BAL on day 5 post-infection. At autopsy, the viral load in the lungs was as high as previously described [39] and gross pneumonic as well as histopathological lesions were observed. After injection of the N protein, 2 out the 3 calves were protected against BRSV replication in the lungs. However, high viral loads were detected in the BAL fluids, and protection against pneumonic lesions was only partial. DNA vaccination alone was able to protect against viral replication in the lungs and to strongly reduce the viral replication in the BAL but the calves were not totally protected against the development of gross pneumonic lesions. The presence of lung consolidations at autopsy indicates the spread of the virus in the lungs. Indeed, pathological changes have been shown to appear when replication of BRSV spreads to the alveoli and may continue after clearance of the virus [59]. In contrast, protection against both the viral replication in the lungs and the gross and histological pneumonic lesions was afforded by the combination of DNA and protein vaccination, suggesting that infectious virus was cleared before infecting the alveoli.
The role of the cellular immunity in the protection against RSV and the pathogenesis induced by the virus is controversial. Bovine IFN-γ has been shown to induce a TH1 response, characterized by IgG2 production [60]. On one hand, both CD4+ and CD8+ T cells can eliminate the virus but they also cause immunopathology in mice [61]. IFN-γ was identified as a key molecule involved in both the virus control and the immunopathology induced by CD8+ T cells [62]. In mice, TH1 and TH2 biased T cell responses could either co-exist or down regulate each other [14], [63]. On the other hand, impaired viral clearance and enhanced disease observed after vaccination with FI vaccine have been correlated with decreased IFN-γ production [64]. Formulation of FI-BRSV with CpG ODN resulted in increased IFN-γ secretion and reduction of both gross lung pathology and viral replication in calves and in mice [65], [66]. Furthermore, Modified Live vaccine induced cellular immunity, in particular secretion of IFN-γ by peripheral blood leukocytes, appeared to be a more consistent correlate of protection than pre-challenge serum antibody [67]. Our data are consistent with these results. Indeed, the only animals that were protected against both viral replication in the lungs and the development of macroscopic lung lesions resulting of BRSV challenge were those that had been vaccinated successively with pNsyn and N protein and that exhibited significant IFN-γ production at the time of the challenge.
Injection of the purified N protein, mixed with Quil A did not induce measurable lymphoproliferative response. This was not anticipated because other authors showed that insect cells infected with recombinant baculovirus expressing the F protein and mixed with Quil A stimulate the cellular-mediated immune response in lambs [68]. However, as baculovirus has been shown as stimulator of immune response, this could contribute to the overall effect of the immunogen [69]. In our experiment, the N protein was nevertheless able to prime for a lymphoproliferative response, detected after BRSV challenge, and this might be attributed to a TH1 triggering of the adjuvant. After vaccination with the N protein, 2 out of the 3 calves were protected against viral replication in the lungs. Virus was detected in the lungs of the remaining calf (calf 6622). Interestingly, this calf had also higher viral load in BAL fluids and more extended lesions of the lungs compared to the 2 other calves of this group. The 3 calves showed identical serological responses. However, when the lymphoproliferative response was individually examined on blood collected 6 days after challenge, the SI was below 1 for calf 6622 and of about 3 for the 2 other calves (data not shown). We hypothesize that the development of pulmonary lesions and the viral replication in the lungs were correlated with the absence of cell-mediated immunity.
The prime–boost strategy used here was superior to DNA or protein vaccination alone in term of protection for large animals. This is in accordance with the results published by others. Indeed, this strategy was recently shown to induce stronger humoral immune response and better protection against BVDV in cattle compared to immunization with DNA or protein alone [70]. Boosting DNA vaccinated mice with recombinants vectors can elicit strong CD4 and CD8 immune responses [71]. Furthermore, it has been shown that the longevity of T-cell stimulation was higher after DNA prime–MLV boost than after MLV vaccination alone against BoHV-1 in cattle [72]. Boosting CD8 response with inactivated virus or recombinant proteins is more controversial. While inactivated viral boost was shown to enhance the CD4 and antibody responses but not the CD8 response against severe acute respiratory syndrome coronavirus in mice, other authors described an enhancement of CD8+ CTL and CD4+ T-helper cells against HIV after a protein boost in nonhuman primates [73], [74]. Here also, although the cytotoxic T cell response was not investigated, increased lymphoproliferative response and IFN-γ production were observed, suggesting an enhancement of the cellular response after the protein boost.
In conclusion, the results presented here show protection against BRSV replication in the lung and against lung pathology after nucleocapsid-based DNA prime–protein boost protocol. This vaccination strategy elicited both humoral and cellular immune responses. The observed protection correlated with the lymphoproliferative response and the interferon-γ production. The results also confirm the efficacy of the prime–boost strategy in a large animal model. This strategy is promising for the protection against RSV in cattle and it could also open perspectives for vaccinating young infants as well. Further work should be performed to confirm the present results in neonatal calves harboring maternal antibodies. Finally, further development would combine the nucleocapsid and the fusion proteins in a DNA prime–protein boost strategy in order to stimulate protective cellular immunity and neutralizing antibodies at once.
Acknowledgments
This study was supported by the Federal Public Service Health, Food chain safety and environment (Brussels, Belgium). The authors are grateful to C. Giannoulis for the purification of plasmid DNA, to B. Lambrecht for providing the Sf9 cells, to Stéphanie Durand, Caroline Rodeghiero, P. Van Muylem and Rita Geeroms for their skilled technical assistance and to the staff of the animal facilities of the INRA of Nouzilly for taking care of the animals.
References
- 1.Brandenburg A.H., Neijens H.J., Osterhaus A.D.M.E. Pathogenesis of RSV lower respiratory tract infection: implications for vaccine development. Vaccine. 2001;19:2769–2782. doi: 10.1016/s0264-410x(00)00536-3. [DOI] [PubMed] [Google Scholar]
- 2.Larsen L.E. Bovine respiratory syncytial virus (BRSV): a review. Acta Vet Scand. 2000;41:1–24. doi: 10.1186/BF03549652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van der Poel W.H., Brand A., Kramps J.A., Van Oirschot T.J. Respiratory syncytial virus infections in human beings and in cattle. J Infect. 1994;29:215–228. doi: 10.1016/s0163-4453(94)90866-4. [DOI] [PubMed] [Google Scholar]
- 4.Belknap E.B., Baker J.C., Patterson J.S., Walker R.D., Haines D.M., Clark E.G. The role of passive immunity in bovine respiratory syncytial virus-infected calves. J Infect Dis. 1991;163:470–476. doi: 10.1093/infdis/163.3.470. [DOI] [PubMed] [Google Scholar]
- 5.Kimman T.G., Westenbrink F., Schreuder B.E.C., Straver P.J. Local and systemic antibody response to bovine respiratory syncytial virus infection and reinfection in calves with and without maternal antibodies. J Clin Microbiol. 1987;25:1097–1106. doi: 10.1128/jcm.25.6.1097-1106.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kimman T.G., Zimmer G.M., Westenbrink F., Mars J., van Leeuwen E. Epidemiological study of bovine respiratory syncytial virus infections in calves: Influence of maternal antibodies on the outcome of disease. Vet Rec. 1988;123:104–109. doi: 10.1136/vr.123.4.104. [DOI] [PubMed] [Google Scholar]
- 7.Ellis J.A. The immunology of the bovine respiratory disease complex. Vet Clin North Am Food Anim Pract. 2001;17:535–550. doi: 10.1016/S0749-0720(15)30005-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peters A.R., Thevasagayam S.J., Wieseman A., Salt J.S. Duration of immunity of a quadrivalent vaccine against respiratory diseases caused by BHV-1, PIV3, BVDV, and BRSV in experimentally infected calves. Prev Vet Med. 2004;66:63–77. doi: 10.1016/j.prevetmed.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 9.Patel J.R., Didlick S.A. Evaluation of efficacy of an inactivated vaccine against BRSV in calves with maternal antibodies. Am J Vet Res. 2004;65:417–421. doi: 10.2460/ajvr.2004.65.417. [DOI] [PubMed] [Google Scholar]
- 10.Ellis J., Gow S., West K., Waldner C., Rhodes C., Mutwiri G. Response of calves to challenge exposure with virulent BRSV following intranasal administration of vaccines formulated for parenteral administration. J Am Vet Med Assoc. 2007;15:233–243. doi: 10.2460/javma.230.2.233. [DOI] [PubMed] [Google Scholar]
- 11.Kim H.W., Canchola J.G., Brandt C.D., Pyles G., Chanock R.M., Jensen K. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol. 1969;89:422–434. doi: 10.1093/oxfordjournals.aje.a120955. [DOI] [PubMed] [Google Scholar]
- 12.Gershwin L.J., Schelegle E.S., Gunther R.A., Anderson M.L., Woolums A.R., Larochelle D.R. A bovine model of vaccine enhanced respiratory syncytial virus pathophysiology. Vaccine. 1998;16:1225–1236. doi: 10.1016/s0264-410x(98)80123-0. [DOI] [PubMed] [Google Scholar]
- 13.Hussell T., Baldwin C.J., O’Garra A., Openshaw P.J. CD8+ T cells control Th2-driven pathology during pulmonary respiratory syncytial virus infection. Eur J Immunol. 1997;27:3341–3349. doi: 10.1002/eji.1830271233. [DOI] [PubMed] [Google Scholar]
- 14.Varga S.M., Wang X., Welsh R.M., Braciale T.J. Immunopathology in RSV infection is mediated by a discrete oligoclonal subset of antigen-specific CD4(+) T cells. Immunity. 2001;15:637–646. doi: 10.1016/s1074-7613(01)00209-6. [DOI] [PubMed] [Google Scholar]
- 15.Connors M., Giese N.A., Kulkarni A.B., Firestone C.Y., Morse H.C., III, Murphy B.R. Enhanced pulmonary histopathology induced by respiratory syncytial virus (RSV) challenge of formalin-inactivated RSV-immunized BALB/c mice is abrogated by depletion of interleukin-4 (IL-4) and IL-10. J Virol. 1994;68:5321–5325. doi: 10.1128/jvi.68.8.5321-5325.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Antonis A.F.G., Schrijver R.S., Daus F., Steverink P.J.G.M., Stockhofe N., Hensen E.J. Vaccine-induced immunopathology during bovine respiratory syncytial virus infection: exploring the parameters of pathogenesis. J Virol. 2003;77:12067–12073. doi: 10.1128/JVI.77.22.12067-12073.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gershwin L.J., Gunther R.A., Anderson M.L., Woolums A.R., McArthur- Vaughan K., Randel K.E. Bovine respiratory syncytial virus-specific IgE is associated with interleukin-2 and -4, and interferon-gamma expression in pulmonary lymph of experimentally infected calves. Am J Vet Res. 2000;61:291–298. doi: 10.2460/ajvr.2000.61.291. [DOI] [PubMed] [Google Scholar]
- 18.Kalina W.V., Woolums A.R., Berghaus R.D., Gershwin L.J. Formalin-inactivated bovine RSV vaccine enhances a Th2 mediated immune response in infected cattle. Vaccine. 2004;22:1465–1474. doi: 10.1016/j.vaccine.2003.10.024. [DOI] [PubMed] [Google Scholar]
- 19.Schreiber P., Matheise J.P., Dessy F., Heimann M., Letesson J.-J., Coppe P. High mortality rate associated with bovine respiratory syncytial virus (BRSV) infection in Belgian white blue calves previously vaccinated with an inactivated BRSV vaccine. J Vet Med B Infect Dis Vet Public Health. 2000;47:535–550. doi: 10.1046/j.1439-0450.2000.00380.x. [DOI] [PubMed] [Google Scholar]
- 20.Larsen L.E., Tegtmeier C., Pedersen E. Bovine respiratory syncytial virus (BRSV) pneumonia in beef calf herds despite vaccination. Acta Vet Scand. 2001;42:113–121. doi: 10.1186/1751-0147-42-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taylor G., Thomas L.H., Furze J.M., Cook R.S., Wyld S.G., Lerch R. Recombinant vaccinia viruses expressing the F, G or N, but not the M2, protein of bovine respiratory syncytial virus (BRSV) induce resistance to BRSV challenge in the calf and protect against the development of pneumonic lesions. J Gen Virol. 1997;78:3195–3206. doi: 10.1099/0022-1317-78-12-3195. [DOI] [PubMed] [Google Scholar]
- 22.Thomas L.H., Cook R.S., Wyld S.G., Furze J.M., Taylor G. Passive protection of gnotobiotic caves using monoclonal antibodies directed at different epitopes on the fusion protein of bovine respiratory syncytial virus. J Infect Dis. 1998;177:874–880. doi: 10.1086/515234. [DOI] [PubMed] [Google Scholar]
- 23.Taylor G., Thomas L.H., Wyld S.G., Furze J.M., Sopp P., Howard C.J. Role of T-lymphocyte subsets in recovery from respiratory syncytial virus infection in calves. J Virol. 1995;69:6658–6664. doi: 10.1128/jvi.69.11.6658-6664.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Braciale T.J. Respiratory Syncytial virus and T cells Interplay between the virus and the host adaptive immune system. Proc Am Thorac Soc. 2005;2:141–146. doi: 10.1513/pats.200503-022AW. [DOI] [PubMed] [Google Scholar]
- 25.Openshaw P.J., Tregoning J.S. Immune responses and disease enhancement during respiratory syncytial virus infection. Clin Microbiol Rev. 2005;18:541–555. doi: 10.1128/CMR.18.3.541-555.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bangham C.R., Openshaw P.J., Ball L.A., King A.M., Wertz G.W., Askonas B.A. Human and murine cytotoxic T cells specific to respiratory syncytial virus recognize the viral nucleoprotein (N), but not the major glycoprotein (G), expressed by vaccinia virus recombinants. J Immunol. 1986;137:3973–3977. [PubMed] [Google Scholar]
- 27.Connors M., Kulkarni A.B., Collins P.L., Firestone C.-Y., Holmes K.L., Morse H.C. Resistance to respiratory syncytial virus (RSV) challenge induced by infection with a vaccinia virus recombinant expressing the RSV M2 protein is mediated by CD8+ T cells, while that induced by Vac-F or Vac-G recombinants is mediated by antibodies. J Virol. 1992;66:1277–1281. doi: 10.1128/jvi.66.2.1277-1281.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gaddum R.M., Cook R.S., Furze J.M., Ellis S.A., Taylor G. Recognition of bovine respiratory syncytial virus proteins by bovine CD8+ T lymphocytes. Immunology. 2003;108:220–229. doi: 10.1046/j.1365-2567.2003.01566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Antonis A.F.G., Claassen E.A.W., Hensen E.J., de Groot R.J., de Groot-Mijnes J.D.F., Schrijver R.S. Kinetics of antiviral CD8 T cell responses during primary and post-vaccination secondary bovine respiratory syncytial virus infection. Vaccine. 2006;24:1551–1561. doi: 10.1016/j.vaccine.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 30.Gaddum R.M., Cook R.S., Thomas L.H., Taylor G. Primary cytotoxic T-cell responses to bovine respiratory syncytial virus in calves. Immunology. 1996;88:421–427. doi: 10.1046/j.1365-2567.1996.d01-667.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McInnes E., Sopp P., Howard C.J., Taylor G. Phenotypic analysis of local cellular responses in calves infected with bovine respiratory syncytial virus. Immunology. 1999;96:396–403. doi: 10.1046/j.1365-2567.1999.00714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rutigliano J.A., Johnson T.R., Hollinger T.N., Fischer J.E., Aung S., Graham B.S. Treatment with anti-LFA-1 delays the CD8+ cytotoxic-T-lymphocyte response and viral clearance in mice with primary respiratory syncytial virus infection. J Virol. 2004;78:3014–3023. doi: 10.1128/JVI.78.6.3014-3023.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Srikiatkhachorn A., Braciale T.J. Virus-specific CD8+ T lymphocytes downregulate T helper cell type 2 cytokine secretion and pulmonary eosinophilia during experimental murine respiratory syncytial virus infection. J Exp Med. 1997;186:421–432. doi: 10.1084/jem.186.3.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robinson H.L., Pertmer T.M. DNA vaccines for viral infections: basic studies and applications. Adv Virus Res. 2000;55:1–74. doi: 10.1016/s0065-3527(00)55001-5. [DOI] [PubMed] [Google Scholar]
- 35.Bembridge G.P., Rodriguez N., Garcia-Beato R., Nicolson C., Melero J.A., Taylor G. DNA encoding the attachment (G) or fusion (F) protein of respiratory syncytial virus induces protection in the absence of pulmonary inflammation. J Gen Virol. 2000;81:2519–2523. doi: 10.1099/0022-1317-81-10-2519. [DOI] [PubMed] [Google Scholar]
- 36.Brady R.P., Topliff C.L., Kelling C.L. In vitro expression of full-length and truncated bovine respiratory syncytial virus G proteins and their antibody responses in BALB/c mice. Vaccine. 2004;22:3762–3768. doi: 10.1016/j.vaccine.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 37.Babiuk L.A., Pontarollo R., Babiuk S., Loehr B., van Drunen Littel-van den Hurk S. Induction of immune response by DNA vaccines in large animals. Vaccine. 2003;21:649–658. doi: 10.1016/s0264-410x(02)00574-1. [DOI] [PubMed] [Google Scholar]
- 38.Toussaint J.F., Letellier C., Paquet D., Dispas M., Kerkhofs P. Prime–boost strategies combining DNA and inactivated vaccines confer high immunity and protection in cattle against bovine herpesvirus-1. Vaccine. 2005;23:5073–5081. doi: 10.1016/j.vaccine.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 39.Boxus M., Tignon M., Toussaint J.-F., Roels S., Benoit M.A., Walravens K. DNA immunization against fusion and nucleocapsid proteins protects calves against BRSV. J Virol. 2007;81:6879–6889. doi: 10.1128/JVI.00502-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martinez X., Li X., Kovarik J., Klein M., Lambert P.-H., Siegrist C.-A. Combining DNA and protein vaccination for early life immunization against RSV in mice. Eur J Immunol. 1999;29:3390–3400. doi: 10.1002/(SICI)1521-4141(199910)29:10<3390::AID-IMMU3390>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 41.Samal S.K., Zamora M., McPhillips T.H., Mohanty S.B. Molecular cloning and sequence analysis of Bovine Respiratory Syncytial Virus mRNA encoding the major nucleocapsid protein. Virology. 1991;180:453–456. doi: 10.1016/0042-6822(91)90057-i. [DOI] [PubMed] [Google Scholar]
- 42.Tjornehoj K., Uttenthal A., Viuff B., Larsen L.E., Rontved C., Ronsholt L. An experimental infection model for reproduction of calf pneumonia with bovine respiratory syncytial virus (BRSV) based on one combined exposure of calves. Res Vet Sci. 2003;74:55–65. doi: 10.1016/S0034-5288(02)00154-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wellemans G., Leunen J. La Rhino-trachéite infectieuse des bovins et sa sérologie. Ann Méd Vét. 1973;117:507518. [Google Scholar]
- 44.Uttenthal A., Larsen L.E., Philipsen J.S., Tjornehoj K., Viuff B., Nielsen K.H. Antibidy dynamics in BRSV-infected Danish dairy herds as determined by isotype-specific immunoglobulins. Vet Microbiol. 2000;76:329–341. doi: 10.1016/S0378-1135(00)00261-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boxus M., Letellier C., Kerkhofs P. Real Time RT-PCR for the detection and quantitation of bovine respiratory syncytial virus. J Virol Methods. 2005;125:125–130. doi: 10.1016/j.jviromet.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 46.Gurunathan S., Klinman D.M., Seder R.A. DNA vaccines: immunology, application, and optimization. Annu Rev Immunol. 2000;18:927–974. doi: 10.1146/annurev.immunol.18.1.927. [DOI] [PubMed] [Google Scholar]
- 47.Van Drunen Little-van den Hurk S., Braun R.P., Lewis P.J., Karvonen B.C., Babiuk L.A., Griebel P.J. Immunization of neonates with DNA encoding a bovine herpesvirus glycoprotein is effective in the presence of maternal antibodies. Viral Immunol. 1999;12:67–77. doi: 10.1089/vim.1999.12.67. [DOI] [PubMed] [Google Scholar]
- 48.Martinez X., Brandt C., Saddallah F., Tougne C., Barrios C., Wild F. DNA immunization circumvents deficient induction of TH1 and cytotoxic T cells lymphocyte responses in neonates and during early life. PNAS. 1997;94:8726–8731. doi: 10.1073/pnas.94.16.8726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morein B., Abusugra I., Blomqvist G. Immunity in neonates. Vet Immunol Immunopathol. 2002;87:207–213. doi: 10.1016/s0165-2427(02)00078-8. [DOI] [PubMed] [Google Scholar]
- 50.Li X., Sambhara S., Li C.X., Ewasyshyn M., Parrington M., Caterini J. Protection against respiratory syncytial virus infection by DNA immunization. J Exp Med. 1998;188:681–688. doi: 10.1084/jem.188.4.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li X., Sambhara S., Li C.X., Ettorre L., Switzer I., Cates G. Plasmid DNA encoding the respiratory syncytial virus G protein is a promising vaccine candidate. Virology. 2000;269:54–65. doi: 10.1006/viro.2000.0186. [DOI] [PubMed] [Google Scholar]
- 52.Schrijver R.S., Langedijk J.P., Keil G.M., Middel W.G.J., Maris-Veldhuis M., Van Oirschot J.T. Immunization of cattle with a BHV1 vector vaccine or a DNA vaccine both coding for the G protein of BRSV. Vaccine. 1997;15:1908–1916. doi: 10.1016/s0264-410x(97)00129-1. [DOI] [PubMed] [Google Scholar]
- 53.Schrijver R.S., Langedijk J.P., Keil G.M., Middel W.G.J., Maris-Veldhuis M., Van Oirschot J.T. Comparison of DNA application methods to reduce BRSV shedding in cattle. Vaccine. 1998;16:130–134. doi: 10.1016/s0264-410x(97)00198-9. [DOI] [PubMed] [Google Scholar]
- 54.Taylor G., Bruce C., Barbet A.F., Wyld S.G., Thomas L.H. DNA vaccination against respiratory syncytial virus in young calves. Vaccine. 2005;23:1242–1250. doi: 10.1016/j.vaccine.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 55.Vaughan K., Rhodes G.H., Gershwin L.J. DNA immunization against respiratory syncytial virus (RSV) in infant rhesus monkeys. Vaccine. 2005;23:2928–2942. doi: 10.1016/j.vaccine.2004.10.046. [DOI] [PubMed] [Google Scholar]
- 56.King A.M.Q., Stott E.J., Langer S.J., Young K.K.-Y., Ball L.A., Wertz G.W. Recombinant vaccinia virus carrying the N gene of Human respiratory syncytial virus: studies of gene expression in cell culture and immune response in mice. J Virol. 1987;61:2885–2890. doi: 10.1128/jvi.61.9.2885-2890.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Connors M., Collins P.L., Firestone C.-Y., Murphy B. Respiratory syncytial virus (RSV) F, G, M2, and N proteins each induce resistance to RSV challenge, but resistance induced by M2 and N proteins is relatively short-lived. J Virol. 1991;65:1634–1637. doi: 10.1128/jvi.65.3.1634-1637.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Grell S.N., Riber U., Tjornehoj K., Larsen L.E., Heegaard P.M.H. Age-dependent differences in cytokine and antibody responses after experimental RSV infection in a bovine model. Vaccine. 2005;23:3412–3423. doi: 10.1016/j.vaccine.2005.01.094. [DOI] [PubMed] [Google Scholar]
- 59.Viuff B., Tjornehoj K., Larsen L.E., Rontved C., Uttenthal A., Ronsholt L. Replication and clearance of respiratory syncytial virus apoptosis is an important pathway of virus clearance after experimental infection with BRSV. Am J Pathology. 2002;161:2195–2207. doi: 10.1016/S0002-9440(10)64496-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Estes D.M., Brown W.C. Type 1 and type 2 responses in regulation of Ig isotype expression in cattle. Vet Immunol Immunopathol. 2002;90:1–10. doi: 10.1016/s0165-2427(02)00201-5. [DOI] [PubMed] [Google Scholar]
- 61.Cannon M.J., Openshaw P.J., Askonas B.A. Cytotoxic T cells clear virus but augment lung pathology in mice infected with respiratory syncytial virus. J Exp Med. 1988;168:1163–1168. doi: 10.1084/jem.168.3.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ostler T., Davidson W., Ehl S. Virus clearance and immunopathology by CD8(+) T cells during infection with respiratory syncytial virus are mediated by IFN-gamma. Eur J Immunol. 2002;32:2117–2123. doi: 10.1002/1521-4141(200208)32:8<2117::AID-IMMU2117>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 63.Aung S., Tang Y.W., Graham B.S. Interleukin-4 diminishes CD8(+) respiratory syncytial virus-specific cytotoxic T lymphocyte activity in vivo. J Virol. 1999;73:8944–8949. doi: 10.1128/jvi.73.11.8944-8949.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Woolums A.R., Singer R.S., Boyle G.A., Gershwin L.J. Interferon gamma production during bovine respiratory syncytial virus (BRSV) infection is diminished in calves vaccinated with formalin-inactivated BRSV. Vaccine. 1999;17:1293–1297. doi: 10.1016/s0264-410x(98)00379-x. [DOI] [PubMed] [Google Scholar]
- 65.Oumouna M., Mapletoft J.W., Karvonen B.C., Babiuk L.A., van Drunen Littel-van den Hurk S. Formulation with CpG oligodeoxynucleotides prevents induction of pulmonary immunopathology following priming with formalin-inactivated or commercial killed bovine respiratory syncytial virus vaccine. J Virol. 2005;79:2024–2032. doi: 10.1128/JVI.79.4.2024-2032.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mapletoft J.W., Oumouna M., Townsend H.G., Gomis S., Babiuk L.A., van Drunen Littel-van den Hurk S. Formulation with CpG oligodeoxynucleotides increases cellular immunity and protection induced by vaccination of calves with formalin-inactivated bovine respiratory syncytial virus. Virology. 2006;353:316–323. doi: 10.1016/j.virol.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 67.West K., Petrie L., Konoby C., Haines D.M., Cortese V., Ellis J.A. The efficacy of modified-live bovine respiratory syncytial virus vaccines in experimentally infected calves. Vaccine. 2000;18:907–919. doi: 10.1016/s0264-410x(99)00324-2. [DOI] [PubMed] [Google Scholar]
- 68.Sharma A.K., Woldehiwet Z., Walravens K., Letesson J. Immune response of lambs to the fusion glycoprotein of BRSV expressed on insect cells infected with a recombinant baculovirus. Vaccine. 1996;14:773–779. doi: 10.1016/0264-410x(95)00248-y. [DOI] [PubMed] [Google Scholar]
- 69.Abe T., Takahashi H., Hamazaki H., Miyano-kurosaki N., Matsuura Y., Takaku H. Baculovirus induces an innate immune response and confers protection from lethal Influenza virus infection in mice. J Immunol. 2003;171:1133–1139. doi: 10.4049/jimmunol.171.3.1133. [DOI] [PubMed] [Google Scholar]
- 70.Liang R., van den Hurk J.V., Babiuk L.A., van Drunen Littel-van den Hurk S. Priming with DNA encoding E2 and boosting with E2 protein formulated with CpG oligodeoxynucleotides induces strong immune responses and protection from Bovine viral diarrhea virus in cattle. J Gen Virol. 2006;87:2971–2982. doi: 10.1099/vir.0.81737-0. [DOI] [PubMed] [Google Scholar]
- 71.Estcourt M.J., Ramsay A.J., Brooks A., Thomson S.A., Medveckzy C.J., Ramshaw I.A. Prime–boost immunization generates a high frequency, high-avidity CD8(+) cytotoxic T lymphocyte population. Int Immunol. 2002;14:31–37. doi: 10.1093/intimm/14.1.31. [DOI] [PubMed] [Google Scholar]
- 72.Loehr B.I., Pontarollo R., Rankin R., Latimer L., Willson P., Babiuk L.A. Priming by DNA immunization augments T-cell responses induced by modified live bovine herpesvirus vaccine. J Gen Virol. 2001;82:3035–3043. doi: 10.1099/0022-1317-82-12-3035. [DOI] [PubMed] [Google Scholar]
- 73.Kong W.-p., Xu l., Stadler K., Ulmer J.B., Abrignani S., Rappuoli R. Modulation of the immune response to the severe acute respiratory syndrome spike glycoprotein by gene-based and inactivated virus immunization. J Virol. 2005;79:13915–13923. doi: 10.1128/JVI.79.22.13915-13923.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cristillo A.D., Wang S., Caskey M.S., Unangst T., Hocker L., He L. Preclinical evaluation of cellular immune responses elicited by a polyvalent DNA prime/protein boost HIV-1 vaccine. Virology. 2006;346:151–168. doi: 10.1016/j.virol.2005.10.038. [DOI] [PubMed] [Google Scholar]


