Abstract
We investigated the mechanism by which ALVAC activates innate immunity. Combining ALVAC with protein antigens significantly augmented antigen-specific IgG2a responses; this was dependent on the presence of bioactive interferon (IFN)-γ. Immuno-depletion of NK cells prior to ALVAC immunisation abrogated IFN-γ production indicating that they are the main cellular source of early IFN-γ in vivo. Murine bone-marrow derived dendritic cells (BMDCs) cultured in the presence of ALVAC secreted high levels of the chemokines CXCL10 and CCL2 and up-regulated expression of the maturation markers CD40, CD80 and CD86. Therefore, we conclude that ALVAC acts as an adjuvant through a mechanism requiring NK cell derived IFN-γ, DC activation and chemokine secretion.
Keywords: Th1, Adjuvant, AIDS vaccine, NK cell, Dendritic cell
1. Introduction
Adjuvants improve the adaptive immune response to co-injected antigen by activating innate immunity [1]. In spite of intense research, there is still a limited number of adjuvants available for human use, in particular to enhance the induction of the polarized Th1 response required for optimum protective immunity to viral infection and for cancer therapy [2], [3].
Vaccination strategies based on the Canarypox-virus vector ALVAC have shown potential in both infectious disease [4], [5] and cancer [6]. ALVAC, a large DNA virus whose replication is confined to avian hosts, has a good safety profile in humans [5]. Despite its attenuated nature, ALVAC does cause inflammation at the site of injection as evidenced by infiltration of neutrophils [7]. Furthermore, previous reports show that ALVAC possesses adjuvant activity [7], [8].
In this study, we confirm that ALVAC acts as an adjuvant, enhancing antigen-specific IgG2a to co-injected protein antigen in mice. Intra-muscular (i.m.) injection of ALVAC increases levels of circulating IFN-α and IFN-γ shortly after injection. ALVAC enhanced antigen-specific IgG2a responses to co-injected protein antigen in IFN-α Receptor knockout (IFN-αR−/−) mice but not IFN-γR−/− mice, indicating the importance of bioactive IFN-γ in mediating the adjuvant effect of ALVAC. Immuno-depletion of NK cells dramatically impaired the ability of ALVAC to induce circulating IFN-γ, indicating that these cells are the major producer of this cytokine in vivo. A previous study has shown that ALVAC triggers TNF-α production by human DCs [9]. As the interaction between NK and DCs is important for the induction of the innate immune response [10] we investigated the effect of ALVAC exposure on murine BMDC regarding maturation and cytokine production. We show that under the experimental conditions we used, low levels of apoptosis were detected, and that ALVAC induces the maturation of DCs (up-regulation of CD40, CD80 and CD86 expression) and the production of low levels of inflammatory cytokines (IL-6, TNF-α and IL-12). However, a marked increase in the secretion of the chemokines CXCL10 and CCL2 was observed.
Therefore, this leads us to suggest that ALVAC can act as a Th1 polarizing adjuvant by inducing local inflammation [7], resulting in DC maturation and chemokine production which in turn causes the recruitment of IFN-γ secreting NK cells.
Understanding the mechanism of adjuvant action of ALVAC, will allow more effective targeting of the desired immune response by taking advantage of the fact that ALVAC can be manipulated to encode a variety of immuno-modulatory transgenes, e.g. GM-CSF [11] or IL-12 [12] that can further enhance the activation of NK cells or DCs.
2. Materials and methods
2.1. Mice
Six to eight week old female Balb/c ByJ mice (Charles River, Les Oncins, France) or 129/Sv, IFN-α receptor knockout and IFN-γ receptor knockout (B + K Universal, Hull, UK) were used. Animals were housed and experiments carried out in an accredited facility under the guidelines of Sanofi-Pasteur's local ethics committee.
2.2. Antigens
In this study we used ALVAC-HIV, vCP1452 (Sanofi-Pasteur, Marcy l’Etoile, France) a recombinant Canarypox vector that contains the genes for HIV-1 envelope gp120 (strain MN) linked to the trans-membrane portion of HIV-1 gp41 (strain LAI), the HIV-1 LAI gag gene encoding for the entire gag protein, the sequence of the pol gene encoding the protease, and a synthetic polynucleotide encompassing several known human CTL epitopes from the nef and pol gene products. It also contains sequences encoding the E3L and K3L vaccinia virus proteins in the C6 site. ALVAC Placebo (Sanofi-Pasteur) contains a mixture of 10 mM Tris–HCl buffer pH 9.0, virus stabilizer and freeze-drying medium. Both ALVAC and Placebo were re-constituted in sterile 0.9% saline prior to injection. Recombinant HIV Tat protein from the HIV-1IIIB isolate and glycoprotein B (gB) from cytomegalovirus (CMV) were purified from bacterial culture at Sanofi-Pasteur. All vaccines used in this study were clinical grade and were verified to be free of lipopolysaccharide (LPS) and other impurities.
2.3. The adjuvant effect of ALVAC
Groups of 10 Balb/c, 129/Sv, IFN-αR knockout, or IFN-γR knockout mice were immunised i.m. in the quadriceps following anaesthesia with Imalgene 500 (Merial, Lyon, France) with either gB (2 μg/dose), Tat (20 μg/dose), alone or in combination with Placebo or ALVAC (1/10 human dose). Mice were boosted at day 21 and serum and spleen samples taken at day 35. Anti-Tat and gB antibody titres and specific cellular responses were then analysed as described below.
2.4. Specific anti-Tat and anti-gB antibody titration by ELISA
Maxi-Sorp ELISA plates (Nunc, Wiesbaden, Germany) were coated with 1 μg/ml of either Tat or gB in PBS overnight at 4 °C. Plates were blocked with 1% non-fat milk (Gibco, Paisley, UK) at 37 °C for 1 h, after washing serial dilutions of serum samples and standards were added for 1 h at 37 °C. Plates were then washed and the detection antibody, goat-anti-mouse IgG-HRP (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) was added for a further hour at 37 °C. Plates were washed, and a tetramethlybenzidine substrate solution added (Tebu Bio-Labs, Le Perray-en-Yvelines, France), the enzyme reaction was stopped with the addition of phosphoric acid (1 M), and OD values at 450 nm were determined. Antibody titres were calculated from the linear regression curve of a standard specific serum originating from the same animal species as the samples, present on each ELISA plate (Softmax software™, Molecular Devices, Sunnyvale, CA). The titre of the standard had been previously determined in 15 independent experiments.
2.5. IFN-γ ELISPOT
MultiScreen HTS plates (Millipore, Bedford, MA) were coated overnight at 4 °C with anti-IFN-γ capture monoclonal antibody (mAb) (Clone No. R4-6A2, BD Pharmingen, San Diego, CA), washed and blocked. Responder spleen cells were added at a concentration of 2 × 105 cells/well in RPMI 1640 (Gibco) supplemented with 10% FCS (Hyclone, Logan, UT), Penicillin-Streptomycin (Gibco), 200 mM l-glutamine (Gibco), and 50 mM β-mercaptoethanol (Gibco). Cells were stimulated in triplicate with 1 μg/ml of 9 or 15 mer Tat or gag peptides (Neosystem, Strasbourg, France), medium alone as negative control and PMA (Sigma–Aldrich, St. Louis, MO)/anti-CD3 (BD Pharmingen) as positive control. ALVAC specific immune responses were measured by adding P815 cells (Mouse lymphoblast-like mastocytoma cell line) that had been infected with a multiplicity of infection (MOI) of 20 of parental virus (CpPP) overnight. Cultures were incubated overnight at 37 °C/5% CO2. The following day, detection using mAb IFN-γ-biotin (clone No. XMG1.2, BD Pharmingen), followed by Streptavidin-HRP (Southern Biotech, Birmingham, AL) was visualized using 3-amino-9-ethylcarbazole (AEC) substrate (Sigma–Aldrich). IFN-γ producing spots were counted using an automated ELISPOT reader equipped with Spot™ software (MicroVision Instruments, Evry, France).
2.6. In vivo IFN-γ capture assay
ALVAC's ability to induce early circulating IFN-γ following i.m. injection was evaluated using an in vivo IFN-γ detection assay as described by the manufacturer (BD Pharmingen). Briefly, prior to i.m. injection with ALVAC or Placebo groups of Balb/c mice were given an intraperitoneal (i.p.) injection with 10 μg/dose of a biotin-IFN-γ mAb, serum samples were removed at various time-points and frozen at −20 °C until analysis of the IFN-γ mAb complex by ELISA. In some experiments, NK cells were depleted using anti-asialo GM1 polyclonal antibody (Cedarlane Laboratories, Ontario, Canada) i.p. at day −5 and day 0 at 30 μg/dose. Control mice received the same dose of non-specific rabbit IgG (Caltag Laboratories, Burlingame, CA). The capture IFN-γ mAb with either Placebo or ALVAC were injected as described and serum samples were collected at 72 h post-immunisation, and frozen at −20 °C until analysis by ELISA. Spleens were removed from both control and treated animals and the efficacy of the NK cell depletion measured by evaluating the % of spleen cells expressing the NK cell marker CD49b by flow cytometry using a specific mAb, clone no. DX5 (BD Pharmingen).
2.7. Cytokine detection
Secretion of cytokines/chemokines was analyzed by ELISA using commercially available matched pairs of mAbs; murine CXCL10 and CXCL16 (R&D Systems, Minneapolis, MN); murine IL-10, TNF-α, IL-5, IFN-γ, IL-12p40, IL-6, and human IL-10 and IL-12p70 (BD PharMingen, San Diego, CA). The limit of sensitivity for detection was 15 pg/ml for all ELISA assays. Murine IFN-α was detected in serum samples using a commercially available ELISA kit as instructed (Endogen, Rockford, IL). The detection range for this assay was 12.5–500 pg/ml. In some experiments cytokine determinations were performed using the cytometric bead array (CBA) mouse inflammation kit from BD Pharmingen as described by the manufacturer. Samples were acquired on a BD FACscalibur and analysed with Cellquest Pro™ software. The detection range for all cytokines by this method was 39–5000 pg/ml.
2.8. Bone-marrow derived DC generation
Bone-marrow was flushed from the femurs and tibiae of 5 Balb/c ByJ mice using cold complete RPMI. Cells were washed and re-suspended in complete RPMI supplemented with GM-CSF (5 ng/ml, R&D Systems). On day 3 fresh media containing GM-CSF was added to the adherent cells. On day 7, cells were washed, phenotyped by flow cytometry and if >80% of the cells were CD11c+ they were then used for experiments.
2.9. FACs analysis of DC maturation in vitro
BMDCs were cultured at a concentration of 1 × 106 cells/ml in complete RPMI for 24 h in the presence of ALVAC at a MOI of 0.2 and 1, or the equivalent volume of Placebo. Cells cultured in medium alone were used as a negative control and cells cultured with a Toll like receptor (TLR) 4 agonist (Sanofi-Pasteur) at a concentration of 0.1 μg/ml, as a positive control. Cells were washed, re-suspended in PBS with 2% FCS and 0.01% NaN3; labelled with mAbs specific for CD3, CD4, CD8, CD19, CD11c, CD40, CD80, and CD86, with the appropriate isotype controls (all from BD PharMingen). Cells were acquired using a FACScalibur (BD) and results analyzed using CellQuest Pro™ Software (BD).
2.10. Cell death analysis
Cell death analysis of either human peripheral blood mononuclear cells (PBMCs) or murine BMDCs was carried out using the TACS Annexin-V-FITC Apoptosis Detection Kit as described by the manufacturer (R&D Systems).
2.11. Statistical analysis
All results are presented as mean ± S.E.M., unless otherwise indicated. Differences between the means of multiple groups in an experiment were analysed using one-way ANOVA analysis of variance. In the case where only two groups are being considered results were analysed using a two-tailed independent variable Student's T test. All analysis was performed using StatView Software (SAS, Cary, NC). p-Values < 0.05 were considered significant.
3. Results
3.1. ALVAC enhances antigen-specific Th1 responses to co-injected protein antigen
Groups of Balb/c mice were immunised i.m. with either antigen alone (Tat or gB) or in combination with Placebo or ALVAC. Mice received a booster immunisation after 3 weeks, and were sacrificed 2 weeks later in order to investigate antigen-specific immune responses. Titres of antigen-specific IgG and IgG1/IgG2a were determined by ELISA. The levels of gB specific IgG observed in the group who received gB and ALVAC were higher than both the group that received gB alone, and the group immunised with gB and Placebo (Fig. 1A). A similar increase in antigen-specific IgG was observed when ALVAC was co-injected with another antigen, Tat (Fig. 1B). Looking at the particular subclasses of IgG, the addition of ALVAC to the antigens Tat or gB did not cause an increase in the levels of antigen-specific IgG1 (Fig. 1C and D). However, a 2-fold increase was observed in the levels of anti-gB IgG2a in mice that received gB and ALVAC compared to mice that received gB alone (Fig. 1E). A similar augmentation of Tat-specific IgG2a was observed in the groups of mice that received Tat and ALVAC compared to those that received either Tat alone or Tat and Placebo (Fig. 1F). These results clearly show that ALVAC can act as an adjuvant augmenting antigen-specific IgG2a responses to co-injected antigen.
Fig. 1.
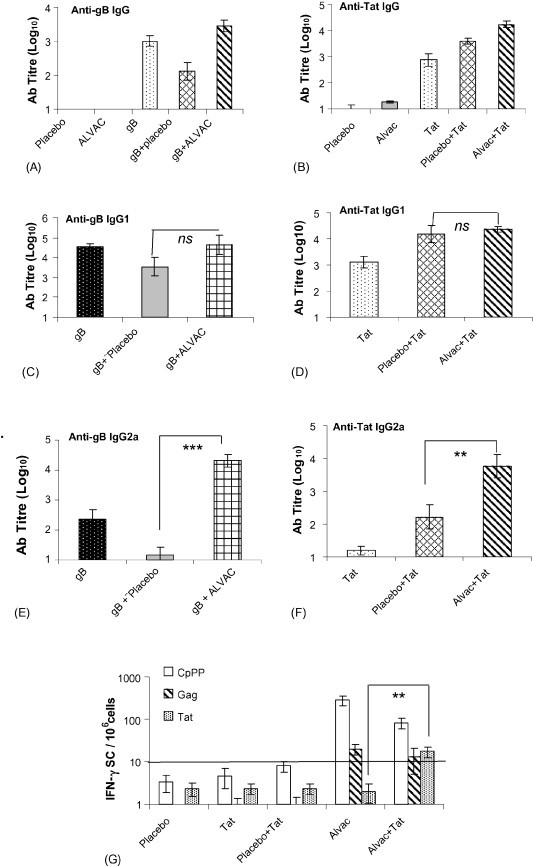
Co-immunisation of ALVAC with either Tat or gB antigens augments the induction of either Tat or gB specific IgG2a. Five groups of Balb/c mice (10 per group) were immunised i.m. with gB (2 μg/dose), ALVAC (1/10 human dose), Placebo and either ALVAC or Placebo in combination with gB. The mice were boosted at day 21 and at day 35 serum samples were taken. Titres of antigen-specific IgG (A), IgG1 (C) and IgG2a (D) values were determined by ELISA. In a separate experiment 5 groups of Balb/c mice (10 per group) were immunised i.m. with Tat (20 μg/dose), ALVAC (1/10 human dose), Placebo and either ALVAC or Placebo in combination with Tat. The mice were boosted at day 21 and at day 35 serum samples were taken. Titres of antigen-specific IgG (B), IgG1 (D) and IgG2a (F) values were determined by ELISA Values shown represent the mean endpoint titre ± standard error of 10 mice per group (A–F). Spleens were removed from the mice that received Tat (20 μg/dose), ALVAC (1/10 human dose), Placebo and either ALVAC or Placebo in combination with Tat and the number of gag, CpPP and Tat-specific cells secreting IFN-γ were determined by ELISPOT. (G) Values are represented as the mean ± standard error of 5 mice per group, *p < 0.05, **p < 0.01, and ***p < 0.001, ns = not significant.
Next, we investigated the effect of ALVAC on the cellular immune response to co-injected antigen. Groups of 10 Balb/c mice were immunised with Tat alone, Tat and Placebo or Tat and ALVAC and 2 weeks after a booster immunisation the mice were sacrificed and their spleen removed to analyse the cellular immune responses. The spleen cells were re-stimulated in vitro and the numbers of antigen-specific IFN-γ secreting cells were determined by ELISPOT (Fig. 1G). The number of Tat-specific IFN-γ secreting cells in the groups of mice that received Tat alone or Tat and Placebo were not significantly different from background levels (Fig. 1G). However, Tat-specific IFN-γ secreting cells were detectable in the group that received Tat together with ALVAC. IFN-γ secreting cells specific for the vector itself (CpPP) and for gag (encoded by ALVAC) were detected in all groups that received ALVAC (Fig. 1G). These data show that ALVAC augments both humoral and cellular type 1 responses to co-injected antigen.
3.2. Intra-muscular injection of ALVAC induces circulating IFN-α and IFN-γ but with differing kinetics
Two major signalling pathways participate in the innate immune response to viral DNA, namely, the nuclear factor-κB (NF-κB)-dependent pathway leading to inflammatory cytokine secretion (including IL-12 and IFN-γ) and the type I IFN-dependent pathway inducing IFN-α and IFN-regulated genes [13]. Therefore, we wished to determine if IFN-α and/or IFN-γ were produced in vivo following an injection of ALVAC. Groups of Balb/c mice were immunised simultaneously by the i.m. route with 1/10th of the human dose of ALVAC and i.p. with a biotin-conjugated anti-IFN-γ mAb, and subsequently the mice were bled at various time-points after immunisation. Levels of the IFN-γ:mAb complex were determined by ELISA. Significant quantities of IFN-γ were detectable in the circulation of mice 15 h after immunisation (Fig. 2A). The IFN-γ:mAb complex continued to accumulate in the circulation of the ALVAC immunised mice up to 72 h post-immunisation (Fig. 2A). The anti-IFN-γ antibody is stable for up to 72 h in vivo. Using 1/50th of the human dose resulted in less circulating IFN-γ than 1/10th of the human dose, illustrating the effect is dose-dependent (Fig. 2A). In order to detect circulating IFN-α groups of Balb/c mice were immunised i.m. with 1/10th of the human dose of ALVAC and then bled at various time-points, and the levels of IFN-α in the serum were determined by ELISA. A low and transient amount of IFN-α could be detected at 2 h in the serum of ALVAC immunised mice (Fig. 2B). In summary, ALVAC immunisation induces a low amount of IFN-α and significant amounts of IFN-γ with very different kinetics. IFN-α being early and transient and IFN-γ appearing later but more sustained. However, as we used an in vivo capture assay to determine the circulating amounts of IFN-γ (stable IFN-γ:mAb complex) and a straightforward ELISA approach to detect the relatively labile IFN-α, we cannot draw any conclusions about the relative importance of the two cytokines in the adjuvanticity of ALVAC from these data.
Fig. 2.
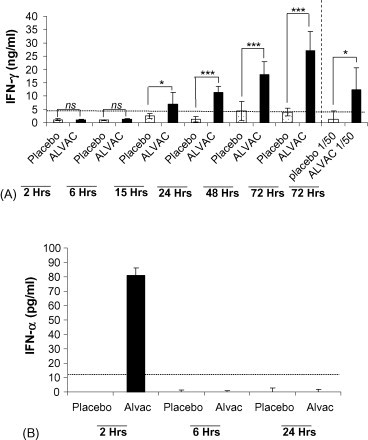
i.m. Injection of Balb/c mice with ALVAC induces circulatory IFN-α and IFN-γ. (A) At time 0, groups of mice (5–6 per group) were immunised i.p. with 10 μg of a biotinylated anti-IFN-γ-mAb (no azide/low endotoxin) followed by i.m. immunisation of either placebo or ALVAC at 1/10 human dose. At various time points serum samples were prepared from these mice and frozen at −20 °C until analysis. Levels of the complex of mAb + IFNγ were determined by an ELISA using a standard curve generated using recombinant IFN-γ that had been pre-incubated with biotinylated mAb. Results are expresses as mean cytokine concentration ± standard deviation. *p < 0.05, ***p < 0.001 and ns = not significant. (B) In a separate experiment groups of Balb/c mice (5 per group) were immunised with Placebo or ALVAC (1/10 human dose) at time 0. Serum samples were removed at various time-points and frozen at −20 °C until analysis. Levels of serum IFN-α were determined by ELISA in triplicate on serum pools from each group. Results are expressed as the mean cytokine concentration ± standard error.
3.3. The ability of ALVAC to enhance antigen-specific IgG2a to co-injected antigen depends on bioactive IFN-γ not IFN-α
As we demonstrated that ALVAC could induce both innate IFN-α and IFN-γ (albeit in different amounts and with varied kinetics), we wished to address the question whether the induction of one or both of these cytokines were crucial for the adjuvant activity of ALVAC. In order to do this we immunised groups of wild type (129/Sv), IFN-αR−/− and IFN-γR−/− mice with either gB and Placebo or gB and ALVAC and then evaluated the gB specific immune responses. This approach enabled us to observe if ALVAC maintained its adjuvant properties in the absence of bioactive IFN-α or IFN-γ. Anti-gB specific IgG levels were similar in all groups of mice irrespective of mouse genotype or vaccine (Fig. 3A). Anti-gB specific IgG1 levels were marginally reduced in the groups that received gB and ALVAC compared with those that received gB and Placebo in each of the genotypes (Fig. 3B). However, a clear augmentation of gB specific IgG2a was observed in both 129/Sv and IFN-αR−/− mice that received gB and ALVAC compared to gB and Placebo. In contrast, no significant increase in anti-gB IgG2a was observed in IFN-γR−/− mice upon the addition of ALVAC to the protein antigen (Fig. 3C). These data indicates that although ALVAC induces both innate IFN-α and IFN-γ production in vivo, IFN-α is dispensable for the augmentation of specific IgG2a to co-injected antigen.
Fig. 3.
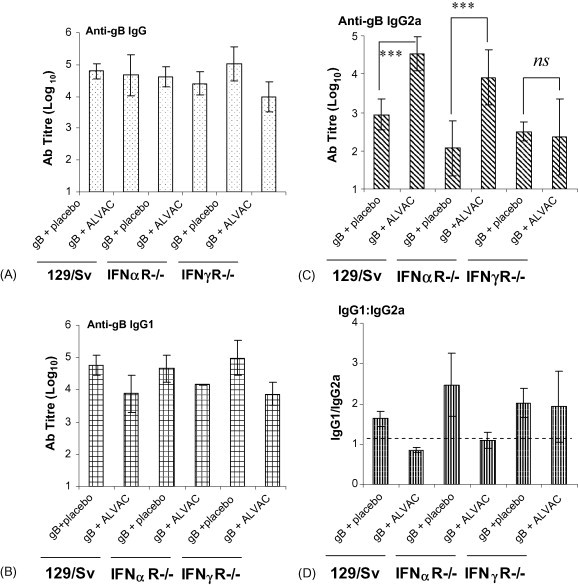
The ability of ALVAC to enhance gB specific IgG2a depends on bioactive IFN-γ. Groups of wild type (129/Sv), IFN-α receptor knockout mice (IFN-αR−/−), and IFN-γ receptor knockout mice (IFN-γR−/−) were immunised i.m. with gB (2 μg/dose) in combination with either placebo or ALVAC (1/10 human dose). The animals were boosted at day 21 and serum samples taken at day 35. Anti-gB specific IgG (A), IgG1 (B) and IgG2a (C) titres were determined by ELISA. Results represented are the mean ± standard deviation of 5 mice per group, ***p < 0.001 and ns = not significant. The anti-gB specific IgG1:IgG2a ratio was determined (D). A value of 1 indicates that equal amounts of antigen-specific IgG1 and IgG2a was produced, values greater than 1 indicate that more anti-specific IgG1 than IgG2a, while values less than one signify that more IgG2a than IgG1.
3.4. NK cells produce the innate IFN-γ induced by ALVAC
NK cells are a major cellular source of the IFN-γ produced during the first phase of the immune response to infection [14]. In order to determine their role in the innate immune response to ALVAC we depleted NK cells from Balb/c mice and then measured the ability of these mice to produce IFN-γ in response to an i.m. injection of ALVAC. NK cell depletion was achieved using two doses of a polyclonal anti-asialo-GM1 Ab, one given 5 days before and the second together with the ALVAC injection. The efficacy of this depletion is illustrated by the lack of CD49b (a NK cell specific marker in Balb/c mice) expressing cells in the spleens of anti-asislo-GM1 treated mice compared with mice treated with an isotype control antibody (Fig. 4B). Groups of mice, either NK cell depleted or not, were immunised with Placebo or ALVAC and were given a simultaneous i.p. injection of biotin-anti-IFN-γ. After 3 days, the mice were sacrificed and their serum analysed for the levels of circulating IFN-γ:mAb complex by ELISA. We found that the induction of circulating IFN-γ by ALVAC was significantly reduced following NK cell depletion (Fig. 4A). This indicates that NK cells are the primary source of the IFN-γ secreted in response to ALVAC within the first 72 h of immunisation.
Fig. 4.
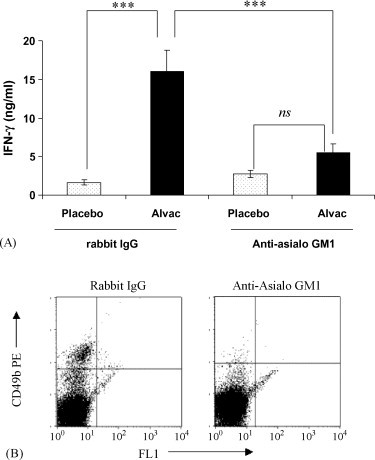
ALVAC induction of circulating innate IFN-γ that is dependent upon the presence of CD49b+ NK cells. (A) NK cells were depleted from Balb/c mice using polyclonal anti-asialo GM1 Antibody. Mice were injected i.p. with 30 μg of antibody at day −5 and day 0, rabbit IgG was used as an isotype control. On day 0 biotinylated anti-IFN-γ at 10 μg/dose was given i.p. to all animals, and then the various groups were immunised i.m. with either placebo or ALVAC at 1/10 human dose. Serum samples were prepared at day 3 and the levels of the circulating IFN-γ:antibody complex were determined by ELISA. Values shown are the mean ± standard deviation of 5 mice per group, ***p < 0.001 and ns = not significant. (B) Treatment with Anti-asialo-GM1 Ab reduced the % CD49b+ cells in the spleens of the treated mice from 3.381% (±0.38) in the control treated group to 1.129% (±0.17) in the anti-asialoGM1 treated group (the average of 10 mice/treatment group ± standard deviation). Spleen cells were isolated from 10 mice per group and the expression of CD49b determined by flow cytometry using a PE-labelled specific mAb. Quadrants were determined by the staining of the isotype control antibodies; representative FACs plots of 10 per treatment are shown.
3.5. Exposure to ALVAC up-regulates the expression of co-stimulatory molecules by dendritic cells (DCs)
Activated DCs augment IFN-γ production by NK cells [10]. Therefore, we wished to investigate if the exposure to ALVAC activates DCs. In order for ALVAC to act as an effective vaccine vector it must infect the host cells in order to produce the antigens which are integrated in its genome. However, here as we are examining the adjuvant effect of ALVAC we are interested in investigating the immuno-modulatory effects of exposing cells to ALVAC without them necessarily becoming infected. In addition in vitro infection protocols using low or no serum conditions and a high MOI have been shown to induce apoptosis in the target cells [9]. Preliminary experiments showed that a MOI of 0.2 to 1 did not induce significant cell death in murine bone-marrow derived DCs (BMDCs) over a culture period of 24 h (data not shown). Murine BMDCs were cultured for 24 h at a concentration of 106 cells/ml in complete RPMI supplemented with 10% FCS alone or with the addition of ALVAC with a MOI of 0.2, the equivalent volume of Placebo or with a TLR4 agonist as a positive control. Cells were then stained with fluorescently labelled mAbs specific for the co-stimulatory molecules CD40, CD80 and CD86 and their expression levels were analysed by flow cytometry. The percentage of BMDCs expressing co-stimulatory molecules (CD40, CD80 and CD86) was significantly increased following exposure to ALVAC compared to medium alone or Placebo (Fig. 5A–C). However, the enhancement of co-stimulatory molecule expression induced by ALVAC was weaker than that observed following the stimulation of BMDCs with a TLR4 agonist (Fig. 5A–C). Nonetheless, these data illustrate that exposure to ALVAC activates DCs.
Fig. 5.
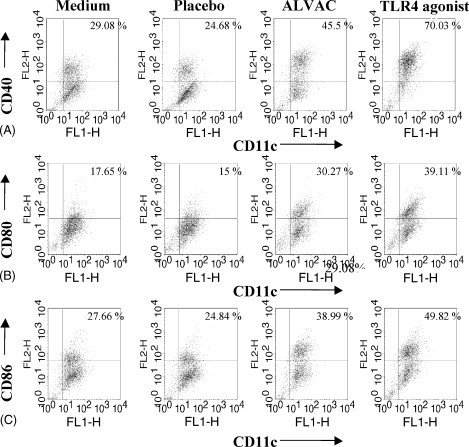
ALVAC exposure triggers DC maturation. Murine bone-marrow derived DCs were incubated for 24 h with either medium alone, Placebo (equivalent volume to ALVAC) both to serve as negative controls; ALVAC at an MOI of 0.2 or a TLR4 agonist at a concentration of 0.1 μg/ml. Cells were harvested, washed, and labelled with anti-CD11c-FITC and anti-CD40-PE (A), anti-CD80-PE (B), or anti-CD86-PE (C), or the appropriate isotype control mAb. Representative FACs plots of one of three experiments are shown.
3.6. Incubation of BMDCs with ALVAC in vitro up-regulates secretion of the chemokines CXCL10 and CCL2
Activated DCs secrete inflammatory cytokines and chemokines to recruit and activate the cells of both the innate and adaptive immune response. In response to ALVAC low amounts of the pro-inflammatory cytokines IL-6, TNF-α and IL-12p40 are produced by DCs, and in general the amounts are 10-fold less than those induced by the TLR4 agonist used as a positive control in these experiments (Fig. 6D–F). However, the levels of the chemokines CXCL10 and CCL2 produced by BMDCs following incubation with ALVAC exceeded or were equivalent to those induced by the TLR4 agonist (Fig. 6A and B). CXCL16 secretion by BMDC was not detected in response to ALVAC (Fig. 6C).
Fig. 6.
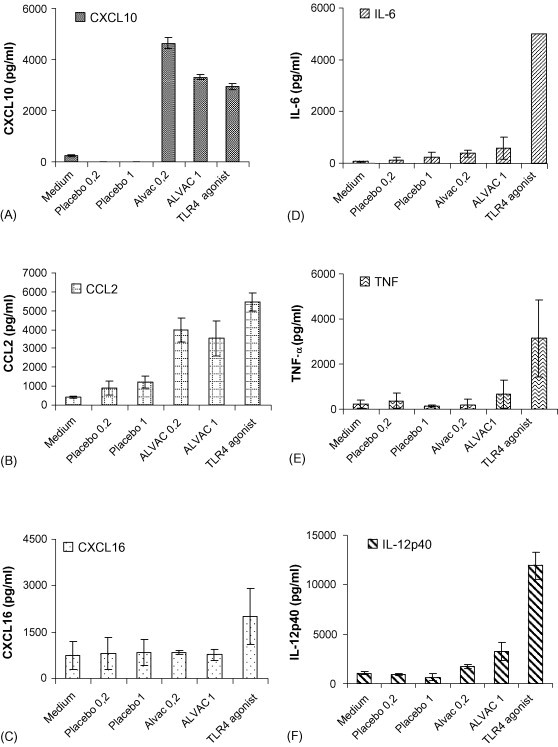
Exposure of Murine BMDC to ALVAC enhances CXCL10 and CCL2 secretion. Murine bone-marrow derived DCs were incubated for 24 h with either medium alone, Placebo (equivalent volume to ALVAC) both to serve as negative controls; ALVAC at an MOI of 0.2 or a TLR4 agonist at a concentration of 0.1 μg/ml. Supernatant was removed and analysed for CXCL10 (A), CCL2 (B), CXCL16 (C), IL-6 (D), TNF-α (E), and IL-12p40 (F) by ELISA. Results are expressed as mean concentration ± standard deviation and are representative of three independent experiments.
4. Discussion
In order to reap the optimum benefit from therapeutic immunisation strategies in cancer or chronic viral infections, vaccines will need to be formulated in such a way as to augment the Th1 effector arm of the immune response. In this report, we show that ALVAC can act as a Th1 polarizing adjuvant in a mouse model (Fig. 1G). We show that bioactive IFN-γ but not IFN-α is crucial for this adjuvant effect, as antigen-specific IgG2a responses to co-injected protein are enhanced in IFN-αR−/− mice but not IFN-γR−/− mice (Fig. 3C). Immuno-depletion of NK cells revealed that they are an important source of the innate IFN-γ produced in response to ALVAC injection in vivo (Fig. 4A). Furthermore, we show that stimulation of murine BMDCs with ALVAC in vitro can induce DC maturation (Fig. 5A–C) and chemokine secretion (particularly CXCL10 and CCL2, Fig. 6A and B). Additionally, ALVAC injection resulted in an enlargement of the local draining lymph node (popliteal) following i.m. injection (data not shown). Therefore, we can conclude that ALVAC injection activates the innate immune response enhancing the induction of Th1 responses to co-injected antigen.
The recombinant ALVAC (vCP1452) that we use in this study has been shown to be well tolerated in clinical trials [15]. Previous reports have shown that ALVAC can act as an adjuvant [7], [8]. In particular, Boudet et al. [7] showed that purified ALVAC in either an infectious or non-infectious form had an adjuvant effect when co-administered with the recombinant HIV antigen, gp160; resulting in an increase of antigen-specific IgG in both mice and guinea pigs. Hutchings et al. [8] furthered this by showing that this adjuvant activity was common to other poxviruses (NYVAC, fowlpox (FP9), as well as ALVAC). The poxviruses: NYVAC, fowlpox (FP9), ALVAC and to a lesser extent, adenovirus all enhanced the immune response to co-injected recombinant Hepatitis B surface antigen (HBsAg) [8]. Interestingly, the combination of the non-recombinant ALVAC vector with HBsAg induced the strongest antigen-specific cellular response in the draining lymph node compared to the responses resulting from including the other poxvirus vectors investigated [8].
Two major TLR mediated signalling pathways participate in the response to pathogens, the nuclear-factor κB (NF-κB)-dependent pathway leading to the secretion of IL-12 and other inflammatory cytokines and the type I IFN pathway which synergises with the NF-κB pathway for optimal IL-12p70 secretion [13]. We show that injection of ALVAC results in an increase in the levels of both circulating IFN-α and IFN-γ (Fig. 2A and B). In order to determine if the induction of one or both of these cytokines was crucial for the adjuvant effect of ALVAC, we immunised wild type (129/Sv), IFN-αR−/− and IFN-γR−/− mice with gB (CMV) either with or without ALVAC. Both the 129/Sv and IFN-αR−/− mice that received gB and ALVAC produced higher titres of gB specific IgG2a when compared to the groups that received gB and Placebo (Fig. 3), indicating that bioactive IFN-α is dispensable for the adjuvant effect of ALVAC. A study investigating the immunogenicity of adenovirus vectors demonstrated that although type I IFN signalling is an important component of adenovirus-mediated DC maturation, it was dispensable during the generation of transgene product specific CD8+ T cell responses [16]. Therefore, it appears that although IFN-α is induced by ALVAC and may well play a role in augmenting the innate immune response, it is not required for its’ adjuvant effect.
Activated NK cells can secrete large amounts of IFN-γ in response to pathogens or activated DCs [17]. In order to determine if the IFN-γ produced in response to immunisation with ALVAC (Fig. 2A) was produced by NK cells, we immunised NK cell depleted mice with ALVAC and then determined the levels of circulating IFN-γ (Fig. 4A). The IFN-γ produced in response to ALVAC in the NK cell-depleted mice was significantly lower than that observed in mice treated with the isotype control indicating that NK cells are a significant source of the IFN-γ produced. It has been recently reported that ALVAC preferentially infects human cells of the monocytic lineage and was infact unable to infect human NK cells in vitro [18]. Our data indicates that in a mouse model, NK cells do play a crucial role in mediating the inflammatory response to ALVAC. Further studies with human cells in vitro or ex vivo are required to determine the role of NK cells in the innate response to ALVAC following vaccination in man.
Recently, it has become clear that there is an important bi-directional dialogue between DC and NK cells (reviewed by [10]). TLR-dependent microbial stimuli typically associated with Th1 responses confer on DCs the ability to activate NK cells [19] to secrete IFN-γ directly or induce NK cells the ability to lyse immature DCs [20]. The discovery that human NK cells express TLR3 and TLR9 suggested the existence of TLR-based NK cell-DC crosstalk [21]. This might be relevant in explaining how NK cells and DCs can be activated simultaneously by the same pathogen and build an efficient innate immune response, which amplifies the downstream adaptive Th1 response [10].
A previous report showed that both mature and immature human DCs could be successfully infected with ALVAC [9]. Many ALVAC infected immature DCs rapidly underwent apoptosis, and the endocytosis of infected, dead or dying DCs by uninfected immature DCs was observed. Concurrently, a sub-population of ALVAC-exposed DCs matured. Maturation was driven by the TNF-α secreted following exposure to ALVAC and partially by the ingestion of infected cell debris [9].
We investigated the effect of exposing murine BMDCs to ALVAC in vitro and found that exposure for 24 h to a MOI of 0.2 (conditions that did not induce a significant amount of apoptosis) did activate DCs as evidenced by their up-regulation of the co-stimulatory molecules CD40, CD80 and CD86 (Fig. 5). Additionally, exposure to ALVAC induced murine BMDCs to secrete significant quantities of the chemokines CXCL10 and CCL2 (Fig. 6). CXCL10 (IP-10) is chemotactic for T cells [22] and NK cells [23] expressing CXCR3. While CCL2 (MCP-1) plays a role in the recruitment of monocytes/macrophages by interacting with CCR2. Recent studies with CXCL10 neutralising antibodies or CXCL10−/− mice demonstrate that the absence of functional CXCL10 results in increased mortality and reduced T cell infiltration within the brains of mice infected intracerebrally with a murine coronavirus [22], [24], illustrating the importance of this chemokine in co-ordinating a protective immune response against a viral pathogen. CCL2's interaction with CCR2 is an important factor promoting early inflammatory responses and effective local antiviral defense [25]. The ability of ALVAC to induce DCs to secrete chemokines, such as CXCL10 may provide the signal required for NK cells to migrate to DC-rich areas bringing them in close proximity enabling direct cell–cell contact, thereby resulting in downstream Th1 responses.
We found that there was a significant increase in both size and cellularity in the local draining lymph node following injection with ALVAC (data not shown). Using flow cytometric analysis of the lymph node cells we could not determine a change in the % of any cell type (data not shown). These data in conjunction with previous work by Boudet et al. demonstrate that ALVAC can trigger an inflammatory response, induce the migration of neutrophils to the site of inoculation within 6 h of administration [7] and enlargement of the local draining lymph node.
Therefore, we now propose the following mechanism for the adjuvanticity of ALVAC: ALVAC causes inflammation, and PMN recruitment to the site of injection [7]. This, in turn, causes the activation of local DCs (as demonstrated by increased co-stimulatory molecule expression). These cells may then migrate to the local draining lymph node. Here, DC secretion of chemokines including CCL2 and CXCL10 cause an influx of cells including NK and T cells. The interaction of the DC and NK cells in the local lymph node triggers IFN-γ secretion by the NK cells that results in an increase in Th1 cells specific for the co-administered protein. When these cells re-circulate to the B cell rich areas of the spleen they enhance isotype switching to IgG2a.
Acknowledgement
E.J.R. and A.H. were funded by a Marie-Curie Industry Host Fellowship from the European Union.
References
- 1.Medzhitov R., Janeway C.A., Jr. Innate immune induction of the adaptive immune response. Cold Spring Harb Symp Quant Biol. 1999;64:429–435. doi: 10.1101/sqb.1999.64.429. [DOI] [PubMed] [Google Scholar]
- 2.Moingeon P., Haensler J., Lindberg A. Towards the rational design of Th1 adjuvants. Vaccine. 2001;19(31):4363–4372. doi: 10.1016/s0264-410x(01)00193-1. [DOI] [PubMed] [Google Scholar]
- 3.Burdin N., Guy B., Moingeon P. Immunological foundations to the quest for new vaccine adjuvants. BioDrugs. 2004;18(2):79–93. doi: 10.2165/00063030-200418020-00002. [DOI] [PubMed] [Google Scholar]
- 4.Gupta K., Hudgens M., Corey L., McElrath M.J., Weinhold K., Montefiori D.C. Safety and immunogenicity of a high-titered canarypox vaccine in combination with rgp120 in a diverse population of HIV-1-uninfected adults: AIDS Vaccine Evaluation Group Protocol 022A. J Acquir Immune Defic Syndr. 2002;29(3):254–261. doi: 10.1097/00126334-200203010-00005. [DOI] [PubMed] [Google Scholar]
- 5.Nitayaphan S., Pitisuttithum P., Karnasuta C., Eamsila C., de Souza M., Morgan P. Safety and immunogenicity of an HIV subtype B and E prime-boost vaccine combination in HIV-negative Thai adults. J Infect Dis. 2004;190(4):702–706. doi: 10.1086/422258. [DOI] [PubMed] [Google Scholar]
- 6.Bonnet M.C., Tartaglia J., Verdier F., Kourilsky P., Lindberg A., Klein M. Recombinant viruses as a tool for therapeutic vaccination against human cancers. Immunol Lett. 2000;74(1):11–25. doi: 10.1016/s0165-2478(00)00244-3. [DOI] [PubMed] [Google Scholar]
- 7.Boudet F., Chevalier M., Jourdier T.M., Tartaglia J., Moste C. Modulation of the antibody response to the HIV envelope subunit by co-administration of infectious or heat-inactivated canarypoxvirus (ALVAC) preparations. Vaccine. 2001;19(30):4267–4275. doi: 10.1016/s0264-410x(01)00150-5. [DOI] [PubMed] [Google Scholar]
- 8.Hutchings C.L., Gilbert S.C., Hill A.V., Moore A.C. Novel protein and poxvirus-based vaccine combinations for simultaneous induction of humoral and cell-mediated immunity. J Immunol. 2005;175(1):599–606. doi: 10.4049/jimmunol.175.1.599. [DOI] [PubMed] [Google Scholar]
- 9.Ignatius R., Marovich M., Mehlhop E., Villamide L., Mahnke K., Cox W.I. Canarypox virus-induced maturation of dendritic cells is mediated by apoptotic cell death and tumor necrosis factor alpha secretion. J Virol. 2000;74(23):11329–11338. doi: 10.1128/jvi.74.23.11329-11338.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moretta A. The dialogue between human natural killer cells and dendritic cells. Curr Opin Immunol. 2005;17(3):306–311. doi: 10.1016/j.coi.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 11.Reali E., Canter D., Zeytin H., Schlom J., Greiner J.W. Comparative studies of Avipox-GM-CSF versus recombinant GM-CSF protein as immune adjuvants with different vaccine platforms. Vaccine. 2005;23(22):2909–2921. doi: 10.1016/j.vaccine.2004.11.060. [DOI] [PubMed] [Google Scholar]
- 12.Triozzi P.L., Strong T.V., Bucy R.P., Allen K.O., Carlisle R.R., Moore S.E. Intratumoral administration of a recombinant canarypox virus expressing interleukin 12 in patients with metastatic melanoma. Hum Gene Ther. 2005;16(1):91–100. doi: 10.1089/hum.2005.16.91. [DOI] [PubMed] [Google Scholar]
- 13.Gautier G., Humbert M., Deauvieau F., Scuiller M., Hiscott J., Bates E.E. A type I interferon autocrine-paracrine loop is involved in Toll-like receptor-induced interleukin-12p70 secretion by dendritic cells. J Exp Med. 2005;201(9):1435–1446. doi: 10.1084/jem.20041964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malmgaard L. Induction and regulation of IFNs during viral infections. J Interferon Cytokine Res. 2004;24(8):439–454. doi: 10.1089/1079990041689665. [DOI] [PubMed] [Google Scholar]
- 15.Jin X., Ramanathan M., Jr., Barsoum S., Deschenes G.R., Ba L., Binley J. Safety and immunogenicity of ALVAC vCP1452 and recombinant gp160 in newly human immunodeficiency virus type 1-infected patients treated with prolonged highly active antiretroviral therapy. J Virol. 2002;76(5):2206–2216. doi: 10.1128/jvi.76.5.2206-2216.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hensley S.E., Giles-Davis W., McCoy K.C., Weninger W., Ertl H.C. Dendritic cell maturation, but not CD8 + T cell induction, is dependent on type I IFN signaling during vaccination with adenovirus vectors. J Immunol. 2005;175(9):6032–6041. doi: 10.4049/jimmunol.175.9.6032. [DOI] [PubMed] [Google Scholar]
- 17.Vitale M., Della Chiesa M., Carlomagno S., Romagnani C., Thiel A., Moretta L. The small subset of CD56brightCD16- natural killer cells is selectively responsible for both cell proliferation and interferon-gamma production upon interaction with dendritic cells. Eur J Immunol. 2004;34(6):1715–1722. doi: 10.1002/eji.200425100. [DOI] [PubMed] [Google Scholar]
- 18.Yu Q., Jones B., Hu N., Chang H., Ahmad S., Liu J. Comparative analysis of tropism between canarypox (ALVAC) and vaccinia viruses reveals a more restricted and preferential tropism of ALVAC for human cells of the monocytic lineage. Vaccine. 2006;24(40–41):6376–6391. doi: 10.1016/j.vaccine.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 19.Zanoni I., Foti M., Ricciardi-Castagnoli P., Granucci F. TLR-dependent activation stimuli associated with Th1 responses confer NK cell stimulatory capacity to mouse dendritic cells. J Immunol. 2005;175(1):286–292. doi: 10.4049/jimmunol.175.1.286. [DOI] [PubMed] [Google Scholar]
- 20.Sivori S., Falco M., Della Chiesa M., Carlomagno S., Vitale M., Moretta L. CpG and double-stranded RNA trigger human NK cells by Toll-like receptors: induction of cytokine release and cytotoxicity against tumors and dendritic cells. Proc Natl Acad Sci USA. 2004;101(27):10116–10121. doi: 10.1073/pnas.0403744101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hart O.M., Athie-Morales V., O’Connor G.M., Gardiner C.M. TLR7/8-mediated activation of human NK cells results in accessory cell-dependent IFN-gamma production. J Immunol. 2005;175(3):1636–1642. doi: 10.4049/jimmunol.175.3.1636. [DOI] [PubMed] [Google Scholar]
- 22.Liu M.T., Keirstead H.S., Lane T.E. Neutralization of the chemokine CXCL10 reduces inflammatory cell invasion and demyelination and improves neurological function in a viral model of multiple sclerosis. J Immunol. 2001;167(7):4091–4097. doi: 10.4049/jimmunol.167.7.4091. [DOI] [PubMed] [Google Scholar]
- 23.Trifilo M.J., Montalto-Morrison C., Stiles L.N., Hurst K.R., Hardison J.L., Manning J.E. CXC chemokine ligand 10 controls viral infection in the central nervous system: evidence for a role in innate immune response through recruitment and activation of natural killer cells. J Virol. 2004;78(2):585–594. doi: 10.1128/JVI.78.2.585-594.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dufour J.H., Dziejman M., Liu M.T., Leung J.H., Lane T.E., Luster A.D. IFN-gamma-inducible protein 10 (IP-10; CXCL10)-deficient mice reveal a role for IP-10 in effector T cell generation and trafficking. J Immunol. 2002;168(7):3195–3204. doi: 10.4049/jimmunol.168.7.3195. [DOI] [PubMed] [Google Scholar]
- 25.Hokeness K.L., Kuziel W.A., Biron C.A., Salazar-Mather T.P. Monocyte chemoattractant protein-1 and CCR2 interactions are required for IFN-alpha/beta-induced inflammatory responses and antiviral defense in liver. J Immunol. 2005;174(3):1549–1556. doi: 10.4049/jimmunol.174.3.1549. [DOI] [PubMed] [Google Scholar]


