Highlights
► BRSV-ISCOMs induce strong clinical and virological protection in young conventional calves with BRSV-specific maternal antibodies. ► Protection is associated with local and systemic humoral responses as well as T cell responses dominated by IFNγ production. ► Animals that did not shed virus detectable by PCR or cell culture following challenge possessed particularly high levels of pulmonary IgA.
Keywords: Respiratory syncytial virus, ISCOM, Vaccine, Bovine respiratory disease, Bronchiolitis
Abstract
Bovine respiratory syncytial virus (BRSV) is a major cause of bronchiolitis and pneumonia in cattle and causes yearly outbreaks with high morbidity in Europe. Commercial vaccines against this virus needs improvement of efficacy, especially in calves with BRSV-specific maternally derived antibodies (MDA). We previously reported that an experimental BRSV-ISCOM vaccine, but not a commercial vaccine, induced strong clinical and virological protection in calves with MDA, immunized at 7–15 weeks of age. The aim of the present study was to characterize the immune responses, as well as to investigate the efficacy and safety in younger animals, representing the target population for vaccination. Four groups of five 3–8 week old calves with variable levels of BRSV-specific MDA were immunized s.c. twice at a 3 weeks interval with (i) BRSV immunostimulating complexes (BRSV-ISCOMs), (ii) BRSV-protein, (iii) adjuvant, or (iv) PBS. All calves were challenged with virulent BRSV by aerosol 2 weeks later and euthanized on day 6 after infection. The cellular and humoral responses were monitored as well as the clinical signs, the viral excretion and the pathology following challenge. Despite presence of MDA at the time of the immunization, only a minimum of clinical signs were observed in the BRSV-ISCOM group after challenge. In contrast, in all control groups, clinical signs of disease were observed in most of the animals (respiratory rates up to 76 min−1 and rectal temperatures up to 41 °C). The clinical protection was associated to a highly significant reduction of virus replication in the upper and lower respiratory tract of calves, rapid systemic and local antibody responses and T helper cell responses dominated by IFNγ production. Animals that did not shed virus detectable by PCR or cell culture following challenge possessed particularly high levels of pulmonary IgA. The protective immunological responses to BRSV proteins and the ability to overcome the inhibiting effect of MDA were dependent on ISCOM borne antigen presentation.
1. Introduction
Bovine respiratory syncytial virus (BRSV), a member of the pneumovirus genus in the Paramyxoviridae family, is a major pathogen in the respiratory disease complex of cattle [1], [2], [3]. This virus is cytopathogenic and directly damages the respiratory epithelium in vivo, but also induces host responses that contribute to the development of disease [4], [5]. Synergistically acting co- or super-infections with other respiratory pathogens are common [6], [7], [8]; however, the clinical signs may be severe by infection with BRSV alone [9]. The disease pattern of BRSV in a cattle population varies according to the level of virus circulation and subsequently the herd immunity; in most European countries, clinical disease is highest in calves and is often seen despite the presence of moderate levels of BRSV-specific maternal antibodies [10]. In contrast, high morbidity is observed in animals of all ages in areas where BRSV circulation is low. In these naïve populations, the contagiousness of the virus becomes visible from its rapid spread between herds [11], [12]. Since it is very difficult to control the transmission of BRSV and as seronegative cattle are always at risk for developing severe disease, whether in an endemic situation or not, there is an obvious need for implementation of efficient vaccines.
Commercialized live attenuated and killed vaccines are not fully satisfactory because they do not always prevent clinical signs and virus shedding, and BRSV continues to circulate even in thoroughly vaccinated populations [2], [13], [14]. Furthermore, safety problems have been observed with killed vaccines; such as aggravated disease in vaccinated animals upon BRSV infection [15], [16]. This vaccine-induced pathology is characterized by an influx of eosinophils in the lung, which was also previously observed in children vaccinated against human (H)RSV in the 1960s, with a fatal outcome in two infants [17]. As a consequence of that tragedy, and although HRSV is the most common cause of acute lower respiratory infection in children and a frequent cause of death in developing countries [18], there is still no effective HRSV vaccine available.
Indeed, new vaccines on the bovine or human market must be safe, but should also ideally be designed to induce powerful protective immunity and, in order to have an impact on virus transmission, should completely prevent virus replication upon infection. A major problem to achieve this goal is that maternally derived antibodies suppress active responses to immunization, even at moderate antibody levels that are not per se protecting the young individual against RSV disease [10] and during the age when infections are common. Vaccination in cattle should preferably be performed at a young age; before commingling of calves into large units where BRSV often circulates, at a time when maternal antibody levels differ between calves [19]. To be efficient at the level of a population it is critical that a new vaccine is effective in the face of maternal immunity.
Nasal administration of a live attenuated vaccine has recently been commercialized and is partially protective in this target group of animals [20], [21]. However, this vaccine virus is shed for long periods [22], conferring a risk for passage to sentinel calves, which could potentially increase its virulence. On the other hand, inactivated conventional BRSV vaccines have showed varying efficacy in young calves against BRSV in the field [13], [16]. Furthermore, these vaccines are rarely evaluated in models that fully reproduce clinical signs of BRSV disease in the presence of maternal antibodies, and seldom with molecular diagnostic tools. Nevertheless, reduction of virus shedding has been demonstrated following one or two immunizations and challenge with BRSV inducing mild disease [23], [24], [25], [26].
In previous studies, we used a virulent BRSV model and PCR to compare the efficacy of a conventional inactivated vaccine with an experimental vaccine in 7–15 weeks old calves with maternal antibodies, with exceptional results: the conventional vaccine very poorly protected calves with maternal antibodies, whereas the experimental vaccine, BRSV immunostimulating complexes (BRSV-ISCOMs), conferred strong clinical as well as nearly complete viral protection against a heterologous BRSV strain [27]. The aim of the present study is to characterize in more detail the protective immune responses induced by BRSV-ISCOMs in young animals with maternal antibodies against BRSV, as well as to investigate the efficacy and safety in animals representing the target population for vaccination. On this occasion, the vaccine and challenge virus were homologous, and the time between vaccination and challenge was kept short to approximate the situation of BRSV infection after commingling of young calves. A group of calves immunized with BRSV proteins without adjuvant, qualitatively and quantitatively comparable to those in ISCOMs, was included to further elucidate the adjuvant effect of ISCOM in bovidae.
2. Materials and methods
2.1. Cells and virus
Foetal bovine turbinate (FBT) cells and vero cells were propagated at low passages in Dulbecco's modified Eagle medium (DMEM, Lonza, Belgium), supplemented per litre with 20 ml 1 M Hepes buffer, 10 ml 200 mM l-glutamine and 10 ml 0.9% NaCl solution containing 60 mg benzyl penicillin sodium and 100 mg streptomycin sulfate salt, as well as foetal calf serum (FCS, PAA Laboratories GmbH, Austria) to a final concentration of 5%, 10% or 20% (DMEM + 5–20%). The cells were tested free from bovine viral diarrhoea virus (BVDV), by immunoperoxidase staining using polyclonal antisera (PA0042, VLA, UK).
2.2. Production of BRSV-ISCOMs and controls
A BRSV isolate (no. 9402022, Denmark) was propagated in vero cells for production of ISCOMs. The seventh passage of the same BRSV isolate, propagated in FBT cells, was used for the experimental challenge, as described earlier [27]. Aliquots of the inoculum were titrated before as well as after the challenge of calves and the titers were determined to be 104.5 and 103.75 TCID50 ml−1, respectively. No contaminating mycoplasma could be detected in the virus, as determined by negative culture within the routine diagnostic of the National Veterinary Institute, Sweden (1553 ISO/IEC 17025). The virus was also free from contaminating BVDV, bovine coronavirus (BCoV) and bovine parainfluenza virus 3 (PIV-3), as determined by seronegativity (BVDV and BCoV) or declining antibodies (PIV-3) detected by enzyme-linked immunosorbent assay (ELISA) following two BRSV-ISCOM vaccinations of calves and BRSV challenge. Furthermore, five BALB/c mice remained seronegative to BVDV, BCoV and PIV-3, but not BRSV following two vaccinations with a 5-week interval (data not shown).
Stock solutions for ISCOM formulation were prepared by dissolving cholesterol and phosphatidylcholine (Sigma, USA) to a concentration of 15 mg/ml each, in distilled water containing 20% (w/w) MEGA-10 (Bachem, Bubendorf, Switzerland). Quillaja saponin (QPUF300, Dessert King, Chile) was dissolved in distilled water to a concentration of 100 mg/ml. The stock solutions, kindly provided by Isconova AB (Uppsala, Sweden), were filtered through a 0.2 μm filter, aliquoted and kept at −20 °C until used. BRSV-ISCOMs were prepared from these stocks and from purified, solubilized BRSV. Briefly, sucrose gradient purified BRSV at 2 mg protein/ml was solubilized with 2% (w/v) n-octylglucoside (OG) in PBS (Boehringer Mannheim, GmbH, FRG), for 1 h at 37 °C with agitation. The solubilized virus was applied onto a discontinuous sucrose gradient with 0.5 ml 20% sucrose containing 0.5% OG, over 2.5 ml 50% sucrose. After centrifugation at 160,000 × g (SW55 rotor, Beckman Coulter) for 45 min at 4 °C, the sample volume and the 20% sucrose layer containing viral proteins were collected, and cholesterol, phosphatidylcholine as well as Quillaja saponin were added in proportions of protein:cholesterol:phosphatidycholine:Quillaja saponin = 1:1:1:13, calculated by weight. After 2 h incubation at room temperature, dialysis was performed against 0.15 M ammonium acetate for 72 h at 4 °C, the ISCOMs were purified by centrifugation through 10% sucrose at 240,000 × g (SW41 rotor, Beckman Coulter) for 21 h at 10 °C, and sterile filtrated. The BRSV protein controls were produced according to the same protocol, excluding the addition of cholesterol, phosphatidylcholine and Quillaja saponin before dialysis. The adjuvant control, AbISCO-300® (provided by Isconova AB, Uppsala, Sweden) was made of the same batches of cholesterol, phosphatidylcholine and Quillaja saponin as used in the BRSV-ISCOMs (Dr K. Lövgren Bengtsson, personal communication).
2.3. Animals and experimental design
Twenty conventionally-reared calves of Swedish red and white breed and Swedish Holstein breed (13 male, 7 female, aged 2–7 weeks) were obtained from a closed dairy herd, certified as free from BVDV and bovine leucosis. Natural BRSV infections before experimental BRSV challenge were ruled out by regular monitoring of the calves as well as five seronegative 5–6-month-old sentinel animals in the herd for BRSV-specific serum antibodies by ELISA. After arrival at the Department of Clinical Sciences, Swedish University of Agriculture, Uppsala, all calves were treated with 15 mg toltrazuril/kg once per oral route as well as 20 mg/kg procain benzylpenicillin intramuscularly, once daily for five days, to minimize the effect of concurrent infections. The calves were matched into four groups according to age and BRSV-specific serum IgG levels; they were housed in four pens and were fed milk replacer as well as calf pellets twice daily, in addition to hay and water ad libitum. All calves were left unweaned throughout the study. The experiment was approved by the Ethical Committee of the University of Uppsala, Sweden (Ref. no. C68/10).
At the age of 3–8 weeks, the calves were immunized subcutaneously (s.c.) with either (i) BRSV-ISCOMs (containing 188 μg total protein and diluted in 2 ml PBS, n = 5); (ii) BRSV-protein (188 μg total protein diluted in 2 ml PBS, n = 5); (iii) 390 μg AbISCO-300® diluted in 2 ml PBS (hereafter called adjuvant, n = 5); or (iv) 2 ml PBS (n = 5), twice with an interval of 3 weeks. The mean age at first vaccination was 47, 48, 47, and 46 days in the BRSV-ISCOM, BRSV protein, adjuvant and PBS groups, respectively.
Two weeks after the second vaccination, all calves were challenged with 3 ml of BRSV by aerosol inhalation (Super Dandy Inhailerboy, SveVet Piab, Sweden). The challenge procedure was carried out as described by Tjornehoj et al. [9], with the exception that intra-tracheal injections were not included. The order in which the groups of calves were challenged was as follows: BRSV-ISCOMs, BRSV protein, adjuvant and PBS.
A veterinarian observed the calves for general and local adverse clinical reactions on a daily basis after immunizations and performed daily clinical examinations of the calves from post infection day (PID) −1 through PID 6. Prior to all procedures on PID −1 and PID 6, each calf's pulmonary function was measured by the forced oscillation technique at 3, 5, 7 and 10 Hz (EEMS Hants UK; Pringle et al., in preparation [28]). The clinical signs of disease were scored as presented in Table 1 (modified after West et al. [29]). Nasal swabs (Virocult®, MedicalWire and Equipment Co. Ltd., UK) were obtained on indicated days (Table 2 ) and stored at −70 °C until analysed for BRSV-RNA by real-time quantitative RT-PCR (RT-qPCR). Nasal secretions were obtained using tampons left in one nostril for 5 min (o.b. ProComfort™, mini, Sweden) on PID −1 and PID 5. Upon addition of 3 ml PBS to each tampon and recovery of fluids by a syringe, samples were stored at −70 °C until analysed by ELISA for antibody. Serum was obtained from blood collected on selected days throughout the experiment, and peripheral blood mononuclear cells (PBMCs), used for lymphoproliferation and cytokine assays, were extracted from heparinized blood collected on PID −3 or −2 as well as PID 4 or 5 (two or three calves per group and day before and after challenge), as described previously [30].
Table 1.
Clinical scoring protocol of calves.
| Score | Fever (°C) | Cough | Auscultation noise | Nasal discharge | Respiratory rate (breaths/min) |
|---|---|---|---|---|---|
| 0 | ≤39.5 | No cough observed | No abnormal sounds noticed upon lung auscultation | Normal nasal discharge | <49 |
| 1 | 39.6–39.9 | Only cough on compression of trachea | Wheezing sounds noticed upon lung auscultation | Serous and/or very little mucopurulent nasal discharge | 50–54 |
| 2 | 40–40.4 | Spontaneous cough during 20 min observation | Moderate mucopurulent nasal discharge | 55–64 | |
| 3 | 40.5–40.9 | Marked mucopurulent nasal discharge | 65–74 | ||
| 4 | >40.9 | 75–85 |
Table 2.
Effect of vaccination on BRSV infection. BRSV-specific maternally derived antibodies and detection of BRSV in calves (identified as calf ‘a’ through ‘t’), immunized with BRSV-ISCOMs (n = 5), BRSV protein (n = 5) adjuvant (n = 5) or PBS (n = 5) on post infection days (PID) −35 and −14 and challenged with BRSV on PID 0.
| Groupa | Calf id | IgGb | Agec | BRSV real-time PCR (units correlating to TCID50)d |
BRSV virus isolatione |
Lung lesion (%)f |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nasal secretions |
BAL |
Lung |
|||||||||||
| PID −1 | 1 | 2 | 3 | 4 | 5 | 6 | 6 | 6 | 6 | ||||
| BRSV- ISCOM | a | 85 | 42 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | – | – | 7 |
| b | 25 | 62 | 0 | 0 | 0 | 0 | 0 | 0.3 | 0 | – | – | 6 | |
| c | 74 | 56 | 0 | 0 | 0 | 0 | 0.2 | 0.2 | 1 | + P2 | – | 1 | |
| d | 111 | 49 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | – | – | 1 | |
| e | 208 | 24 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | – | – | 4 | |
| BRSV protein | f | 25 | 58 | 0 | 0 | 0 | 0.2 | 17 | 11 | 419 | + P1 | + P1 | 17 |
| g | 228 | 40 | 0 | 0 | 0 | 8 | 128 | 620 | 2181 | + P1 | – | 6 | |
| h | 100 | 40 | 0 | 0 | 0 | 1 | 23 | 16 | 150 | + P1 | + P1 | 1 | |
| i | 80 | 61 | 0 | 0 | 0 | 1 | 37 | 202 | 422 | + P1 | + P1 | 6 | |
| j | 100 | 40 | 0 | 0 | 0 | 0 | 1 | 12 | 57 | + P1 | + P1 | 8 | |
| Adjuvant | k | 126 | 55 | 0 | 0 | 0 | 6 | 156 | 203 | 352 | + P1 | + P1 | 25 |
| l | 100 | 42 | 0 | 0 | 0 | 0 | 41 | 15 | 291 | + P1 | – | 8 | |
| m | 50 | 54 | 0 | 0 | 0 | 1 | 23 | 18 | 123 | + P1 | – | 2 | |
| n | 50 | 47 | 0 | 0 | 0 | 0 | 1 | 2.5 | 173 | + P1 | + P1 | 7 | |
| o | 164 | 39 | 0 | 0 | 0 | 3 | 10 | 512 | 956 | + P1 | + P1 | 37 | |
| PBS | p | 111 | 62 | 0 | 0 | 0 | 0.1 | 7 | 153 | 142 | + P1 | – | 15 |
| q | 143 | 55 | 0 | 0 | 0 | 9 | 99 | 1812 | 2212 | + P1 | + P1 | 29 | |
| r | 110 | 49 | 0 | 0 | 0 | 1 | 49 | 366 | 540 | + P1 | + P1 | 10 | |
| s | <25 | 32 | 0 | 0 | 0 | 0 | 0.4 | 9 | 34 | + P1 | + P2 | 20 | |
| t | 98 | 31 | 0 | 0 | 0 | 0.2 | 73 | 368 | 484 | + P1 | + P1 | 7 | |
Calves were immunised with BRSV-ISCOMs, BRSV proteins, adjuvant or PBS and challenged with BRSV on day 0.
BRSV-specific maternally derived IgG titer, nine days before first vaccination.
Calf age at first vaccination, expressed as days.
Nasal swabs were collected on indicated post infection days (PID) and analysed for BRSV RNA by real-time PCR. RNA was quantified by using a dilution series of BRSV with a known TCID50 as standards included in each run.
BAL cells and inflamed lung tissue were obtained on day 6 for virus isolation, determined as visible CPE in turbinate cells after one or two passages in 25 cm2 tissue flasks (+P1 or +P2). No visible CPE after two passages (–).
Macroscopic pathologic lung lesions on day 6, expressed as lesion percentage.
On PID 6, all animals were euthanized under general anesthesia overdose (5 mg/kg ketamine followed by 15 mg/kg pentobarbital sodium and exsanguination) and lungs were excised. Visible macroscopic lesions were photographed and together with palpable lesions were recorded on a standard lung diagram. These lung diagrams were scanned and converted to monochromatic representations of normal and lesioned lung tissue (Adobe Photoshop for Mac) and, using a completely filled in lung diagram for reference, a lesion percentage was calculated by digital counting of colored and uncolored pixels (unpublished software, Borland Delphi 7 source code can be requested from the author). A broncho-alveolar lavage (BAL) was performed post mortem, and prepared as previously described [31]. BAL cells and supernatants were stored at −70 °C until processed for virus isolation, RT-qPCR and antibody analysis, respectively. Tissue samples from three sites of each of the three lobes of the right lung and one site of the accessory lobe were fixed in 4% neutral-buffered formalin. Areas of pneumonic consolidation if present were always chosen for sampling. Moreover, an additional such sample was homogenized in DMEM + 20% and stored at −70 °C prior to virus isolation attempt.
3. Detection of BRSV
3.1. Detection of BRSV RNA
BRSV RNA was analysed by RT-qPCR detecting the BRSV F gene, according to the certifications SS-EN ISO 9001:2008 and SS-EN ISO 14001:2004, within the routine diagnostic of the National Veterinary Institute, Sweden. Briefly, RNA was extracted from 90 μl nasal swab medium, using an extraction robot (Magnatrix™ 8000plus and Vet Viral NA kit, Nordiag, Sweden). The RNA was dissolved in 75 μl RNase-free water and qPCR including reverse transcription of 2 μl RNA was carried out in Applied Biosystems 7500 real-time PCR, using AgPath-ID™ One-Step RT-PCR Kit (Applied Biosystems, Sweden), with previously published primers and probe [32]. An RNA dilution series from an extracted cell culture containing 100,000 TCID50 BRSV ml−1 was included as standard in each PCR run. The BRSV standard samples (n = 5, 10-fold dilutions) were each assigned a value correlating to TCID50 in accordance with their dilution, and the starting quantities of the unknown samples were expressed as units correlating to TCID50, according to the standard curve.
3.2. Isolation of BRSV
Virus isolations were attempted from homogenized lung tissue and BAL cells inoculated onto confluent FBT cells in 25 cm2 tissue culture flasks, as described earlier, but without previous sterile filtration [27]. Daily examinations of the cell cultures for visible cytopathic effect were made in a blind manner during six days of incubation and negative samples were passed a second time in a new set of 25 cm2 tissue culture flasks.
3.3. Serology
IgG antibodies to BRSV in sera, nasal secretions and BAL as well as BVDV, BCoV and PIV3-specific IgG in sera were analysed using commercial indirect ELISAs in samples diluted 1:25 (Svanovir®, Svanova Biotech, Sweden), according to the manufacturers’ instructions. Additionally, sera collected before vaccination were serially diluted and endpoint titers of BRSV-specific maternally derived IgG antibodies were calculated by linear regression. The endpoint titers are given as reciprocals of the dilutions. The cut-off corrected OD (COD) value was set to two times the mean COD of a negative control serum provided in the kit.
For BRSV-specific IgG1 and IgG2 antibodies, the conjugated antibody in the same kit was changed to the following monoclonal antibodies: mouse anti-bovine-IgG2 (K1924F10) followed by rat anti-mouse-IgG1: HRP (LO-MG1-2) for detection of BRSV-specific IgG2 or mouse anti-bovine-IgG1: HRP (IL-A60) for detection of BRSV-specific IgG1. Each antibody solution was added in a volume of 100 μl per well and incubated for 1 h at 37 °C, prior to three washes with 0.05% PBS-Tween solution
A capture ELISA was used for detection of BRSV-specific IgA, according to Uttenthal et al. [33], but with the following modifications: ELISA plates (MaxiSorp, Nunc™, Denmark) were coated 18 h at 4 °C with mouse anti-bovine-IgA (IL-A71) and blocked for 1 h at 25 °C with PBS containing 2% (w/v) BSA, prior to addition of samples diluted 1:25, BRSV antigen or cell control, mouse anti-BRSV N protein (Mab 6 [34]), rat anti-mouse-IgG1:HRP (LO-MG1-2), TMB substrate and H2O2. Each antibody or antigen solution was added in a volume of 100 μl per well and incubated for 1 h at 37 °C, prior to three washes with 0.05% PBS-Tween solution. COD values were calculated by subtracting absorbance values at 450 nm of wells containing control antigen from wells containing BRSV antigen and data were expressed as percentage of the COD of positive control sera. All monoclonal antibodies specific for bovine Ig isotypes were obtained from AbDSerotec, UK.
3.4. BRSV-specific lymphocyte proliferation assays
PBMCs obtained from all animals (described in Section 2.3) were restimulated, in triplicate wells, with BRSV antigen prepared from frozen and thawed FBT cells infected with BRSV (no. 9402022, Denmark) and identical uninfected cell lysates, as described previously [35]. COD values were calculated between BRSV and control stimulated PBMCs, after six days of incubation at 37 °C and addition of Alamar Blue® reagent (Invitrogen, Sweden) 6 h prior to measurement of absorbance by spectrophotometry, according to the manufacturers’ instructions. This method of counting viable cells has previously been shown to be as reliable and sensitive as radioactive methods [36].
3.5. Detection of cytokine mRNA in PBMC
PBMCs obtained from all animals (described in Section 2.3) were screened for the presence of IFNγ and IL-4 mRNA as well as 28 rRNA by RT-qPCR. After BRSV stimulation of 800,000 PBMC per calf for 24 h at 37 °C, cells were centrifuged at 200 × g, supernatants removed, and total RNA was extracted from the cells using RNeasy Mini Kit (Qiagen, Sweden). The RNA purity was verified using spectrophotometry (Picodrop Ltd, UK), at OD 280/260. The RNA samples were stored at −70 °C until analysed by RT-qPCR using iScript one-step RT-PCR kit for probes (Bio-Rad, Sweden) in an IQ5 qPCR machine (Bio-Rad, Sweden) with previously published standards, primers and probes, designed to exclude amplification of genomic DNA [37]. All samples were analysed in duplicate (28S rRNA) or triplicate (IFNγ and IL-4 mRNA). IFNγ and IL-4 mRNA results are given in relation to 28S rRNA.
3.6. Histology
After fixation, the 10 lung tissue samples from each calf were paraffin wax embedded, sectioned at 3–5 μm and stained with haematoxylin/eosin and according to Luna's method for detection of eosinophil granules [38]. The sections were assessed without prior knowledge of the animal's treatment. The inflammatory changes in each lung lobe were scored as normal or mild (1), moderate (2) or severe (3).
3.7. Data analysis
Data were analysed using Minitab® 16.1.1 statistical software using ANOVA followed by Dunnett's test on square root values, when not otherwise specified. The statistical significance was set as 0.01 < p ≤ 0.05 (*); 0.001 < p ≤ 0.01 (**); p ≤ 0.001 (***).
4. Results
4.1. BRSV-ISCOM vaccination protects against clinical signs of disease
In contrast to BRSV proteins alone, adjuvant alone or PBS, the experimental BRSV-ISCOM vaccine used to immunize cattle induced clinical protection against a virulent BRSV challenge (Fig. 1 ). There was a significant reduction in the mean clinical scores of animals immunized with BRSV-ISCOMs vs. those immunized with adjuvant alone on PID 4 and 6 (p < 0.05). The clinical respiratory signs, observed in the majority of animals which received adjuvant or PBS, were characterized by moderate to marked mucopurulent nasal discharge, wheezing sounds on lung auscultation, tachypnea, and spontaneous coughing. However, only one calf, in the adjuvant group, had a markedly affected general condition, on the day of experiment termination (calf ‘o’, PID 6). On this day, the maximum rectal temperatures and respiratory rates per minute in each group were 38.9 °C, 39.3 °C, 40.4 °C and 41.0 °C and 52, 52, 76 and 76 for animals immunized with BRSV-ISCOMs, BRSV proteins, adjuvant or PBS, respectively. Pulmonary function data using the forced oscillation technique showed no changes indicative respiratory function impairment in the BRSV-ISCOM calves. All other calf groups had significantly increased resistance and/or decreased reactance at selected frequencies on PID 6, indicating measurable pulmonary impairment and consistent with their increased severity of clinical score (Pringle et al., in preparation [28]).
Fig. 1.
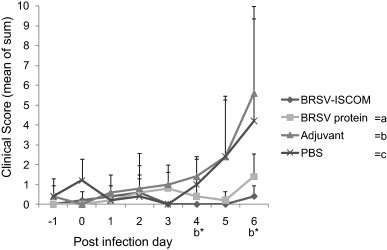
Clinical scores of vaccinated calves following challenge with BRSV. Calves were immunized with BRSV-ISCOMs (n = 5), BRSV protein (n = 5), adjuvant (n = 5) or PBS (n = 5) on post infection days (PID) −35 and −14 and challenged with BRSV on PID 0. Fever, cough, auscultation noise, nasal discharge and tachypnea were scored as shown in Table 1. Data represent group means of the sum of the assigned scores per calf on indicated day, from PID −1 to PID 6. Standard deviations are shown by upward deflection lines. Stars indicate a statistically significant difference between the BRSV-ISCOM and adjuvant (b) group, p ≤ 0.05 (*).
4.2. BRSV-ISCOM vaccination reduced macroscopic and microscopic lung lesions
In agreement with the clinical signs, animals immunized with BRSV-ISCOMs had on average less extensive lung lesions after BRSV challenge compared to controls (Fig. 2 , Table 2). The mean area of macroscopic lung lesions was significantly less in animals immunized with BRSV-ISCOMs vs. those immunized with PBS (p < 0.01), and a similar tendency was observed vs. the adjuvant group (p = 0.09), when analysed by Student's t-test (two-tailed, with assumed equal variance), but not by ANOVA. Animals immunized with BRSV proteins alone also tended to have less gross pathological lesions when compared with calves immunized with PBS, but the difference was not statistically significant (p = 0.09, Student's t-test). A correlation was found between clinical signs and gross pathology in animals in the BRSV-ISCOM, adjuvant and PBS groups when comparing the individual percentage of macroscopic pathologic lung lesions with the sum of clinical scores on PID −1 to 6 (Pearson's r = 0.81, −0.24, 0.84 and 0.87 for calves immunized with BRSV-ISCOM, BRSV protein, adjuvant and PBS, respectively).
Fig. 2.
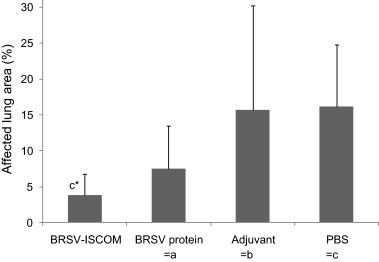
Macroscopic pathologic lung lesions in vaccinated calves following challenge with BRSV. Calves were immunized as described in Fig. 1 and macroscopic lung lesions determined on PID 6. Visible macroscopic lesions were photographed and together with palpable lesions were recorded on a standard lung diagram. These lung diagrams were scanned and a lesion percentage was calculated by digital counting. Standard deviations are shown by upward deflection lines. The star indicates a statistically significant difference between the BRSV-ISCOM and the PBS (c) group, p ≤ 0.05 (*).
Upon histological examination most of the calves showed signs of bronchointerstitial pneumonia in one or several tissue sections (Fig. 3 ). The inflammatory pattern in the animals varied between very mild nonspecific inflammatory changes to severe bronchointerstitial pneumonia with consolidation of the parenchyma and marked lymphoid peribronchial cuffing as well as presence of neutrophils and cellular debri in bronchi/bronchioli and in alveoli. The severity of the inflammatory responses in the ten sampled areas per calf were lowest in the BRSV-ISCOM group, with a mean rank of 5.6 compared with 9.2, 11.8 and 15.4 in the BRSV protein, adjuvant and PBS group, respectively (p = 0.055, Kruskal–Wallis test) and this difference was significant for the cranial lobe (p < 0.05, Kruskal–Wallis test). In 13 animals the neutrophilic component of the inflammation was prominent in at least one tissue section, of those one animal belonged to the BRSV-ISCOM group (calf ‘e’, which had an extent of macroscopic lesions of 3.7%), three to the BRSV protein group, five to the adjuvant group and four to the PBS group. The calf with the worst clinical signs also had the most severe histopathological changes and a marked neutrophilic response (calf ‘o’, adjuvant group). Though eosinophils sometimes were present in the inflamed tissue they did not constitute the major inflammatory cell type, and no differences in eosinophilia were detected between the different groups.
Fig. 3.
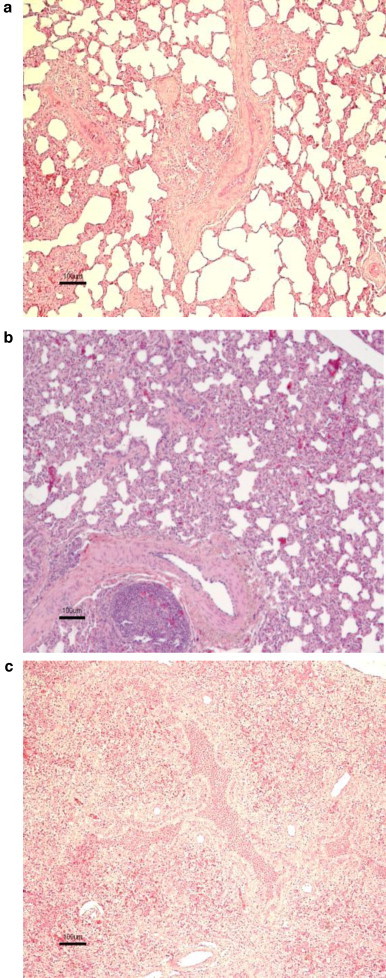
Scoring of pulmonary histopathology in vaccinated calves following challenge with BRSV. Calves were immunized as described in Fig. 1 and microscopic lung lesions determined on PID 6. (a) Few peribronchiolar lymphocytes and absence of bronchiolitis and alveolitis (calf ‘a’, BRSV-ISCOM group, scored as mild). (b) Moderate infiltration of inflammatory cells in alveolar walls and septa. Small amounts of cellular debri present in bronchioli. Focal hyperplasia in peribronchiolar lymph aggregates (calf ‘p’, PBS group, scored as moderate). (c) Bronchointerstitial pneumonia with consolidation of the parenchyma, marked inflammatory peribronchial cuffing and presence of neutrophils and cellular debri in bronchi/bronchioli and in alveoli (calf ‘k’, adjuvant group, scored as severe).
4.3. BRSV-ISCOM vaccination reduced BRSV excretion
A strong reduction in nasopharyngeal viral excretion was observed in calves immunized with BRSV-ISCOMs when compared with all other groups of calves (Table 2). Remarkably, viral RNA was detected by RT-qPCR on nasal swabs only in two out of five animals immunized with BRSV-ISCOMs (calves b and c), whereas it was detected in all controls, including those immunized with BRSV proteins alone, from PID 3 or 4 and until the end of the experiment, on PID 6 (the expected day of peak virus shedding). The reduction of nasopharyngeal viral excretion in animals immunized with BRSV-ISCOMs was statistically significantly different from those immunized with BRSV protein (p < 0.05, PID 6), adjuvant (p < 0.05, PID 4) or PBS (p < 0.05, PID 4–6, all by Kruskal–Wallis test). The mean quantities of viral RNA detected on PID 6, expressed as TCID50 equivalent units ± SD, were 0.2 (±0.45), 646 (±873), 379 (±335) and 682 (±882) for animals immunized with BRSV-ISCOMs, BRSV proteins, adjuvant or PBS, respectively. Surprisingly, those calves in the control groups which shed the highest quantities of virus also had the highest levels of BRSV-specific, maternally derived, IgG antibodies at challenge (calves g, o and q; Table 2, Fig. 4 ). The mean quantities of BRSV RNA detected in BAL cells on PID 6 were 0.3, 4.34, 4.52 and 4.99 log10 TCID50 equivalent units/106 28S copies for animals in the BRSV-ISCOM, BRSV protein, adjuvant and PBS groups, respectively. One sample for each of the protein and adjuvant group (calves j and n) was not quantified, however, due to insufficient RNA material for the 28S analysis.
Fig. 4.
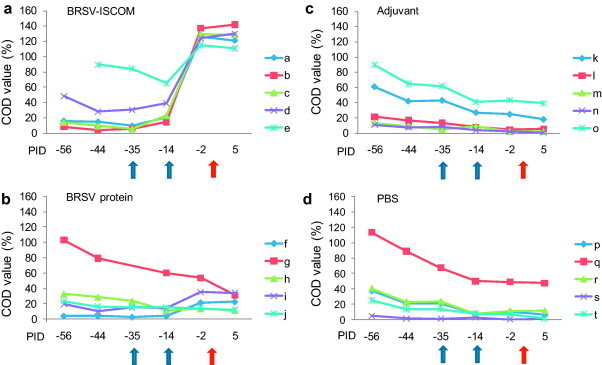
BRSV-specific IgG serum antibody responses in vaccinated calves. Calves (identified as calf ‘a’ through ‘t’) were immunized as described in Fig. 1 (blue arrows) and challenged with BRSV on PID 0 (red arrow). Sera were obtained on days indicated, diluted 1:25 and analysed by ELISA. Corrected OD (COD) values are presented as percent of positive reference sera. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
In agreement with the RT-qPCR data, animals immunized with BRSV-ISCOMs had strongly reduced quantities of live virus in the lung, as determined by virus isolation attempts on BAL and lung tissue from PID 6 (Table 2). BRSV was isolated only from one out of five animals immunized with BRSV-ISCOMs: from BAL cells on the second virus passage (calf ‘c’), whereas isolation was possible from BAL cells of all controls and from lung tissue of 10 out of 15 controls, on the first virus passage.
4.4. BRSV-ISCOM vaccination induces BRSV-specific immune responses
A decline of BRSV-specific, maternally derived, IgG antibodies was observed from first sampling until vaccination in the calves immunized with BRSV-ISCOMs and BRSV proteins and until the end of study in the adjuvant and PBS controls, demonstrating the absence of natural BRSV-infections (Fig. 4). All calves but one (calf ‘s’) had BRSV-specific, maternally derived, IgG antibodies before vaccination, with titers varying between 1/25 and 1/228 (Table 2).
No adverse immunological reactions were observed after the first vaccination, despite careful clinical examination. Conversely, 12 h after the second vaccination, all animals immunized with BRSV-ISCOMs or adjuvant alone developed local reactions (flat swellings, up to hand palm size) and increased rectal temperatures (40.0–40.3 °C), which both remained up to 48 h. The animals continued to have a good appetite for milk and pellets during this period. No reactions were observed in animals immunized with BRSV proteins or PBS, and their rectal temperatures remained normal. There was no significant difference in calf bodyweights at first vaccination, nor in daily weight gains between first vaccination and challenge between the different immunization groups (neither with ANOVA, nor with Student's t-test, data not shown).
Formulation of BRSV antigens into BRSV-ISCOMs was required for induction of serum IgG responses after a single injection (Fig. 4). Indeed, BRSV-specific serum IgG tended to increase after priming in four out of five animals immunized by BRSV-ISCOMs, but in none of the animals immunized with BRSV protein. Furthermore, strong IgG responses were induced in all animals immunized with BRSV-ISCOMs rapidly after the boost, contrasting with weak responses induced and only in two out of five animals immunized with BRSV proteins alone. Sera from PID −2 and PID 5 were further verified by BRSV-specific IgA, IgG1 and IgG2 ELISAs (Fig. 5a–c). Significantly higher IgA, IgG1 and IgG2 responses were detected in serum from the animals immunized with BRSV-ISCOMs compared to all controls (IgA PID 5 p < 0.05, IgG1 and IgG2 PID −2 and 5, p < 0.0001). Within the BRSV-ISCOM group, calf ‘a’ showed the weakest serum IgG2 responses, although this individual had high serum levels of IgG1 and high BRSV-specific lymphocyte proliferation responses both before and after challenge (Fig. 5, Fig. 7). This calf was the only one in its group that developed fever after challenge (40.5 °C, PID 2) but did not shed detectable virus (Table 2).
Fig. 5.
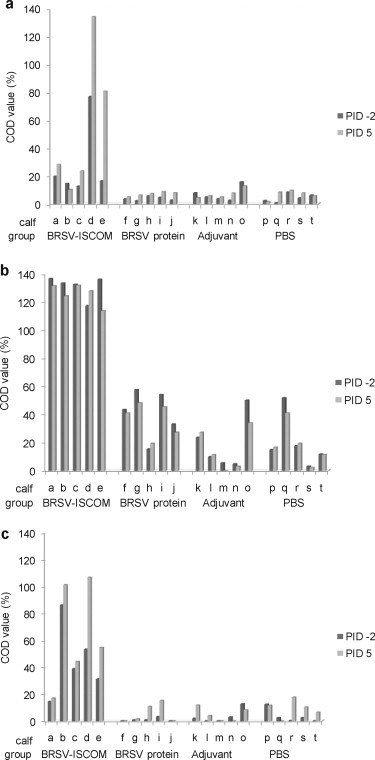
Isotype of the BRSV-specific serum antibody responses in vaccinated calves. Calves (identified as calf ‘a’ through ‘t’) were immunized as described in Fig. 1 and challenged with BRSV on PID 0. Sera obtained on PID −2 (dark grey bars) and PID 5 (light grey bars), were diluted 1:25 and analysed by ELISA for IgA (a), IgG1 (b) and IgG2 (c). Corrected OD (COD) values are presented as percent of positive reference sera.
Fig. 7.
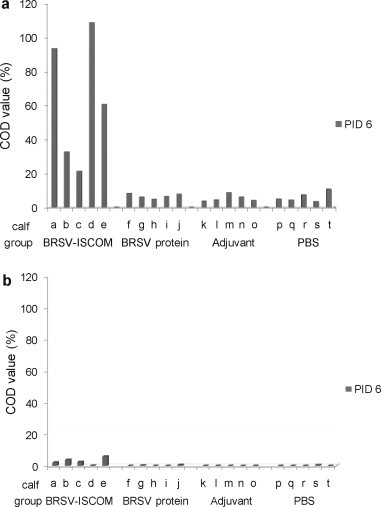
BRSV-specific antibodies in broncho-alveolar lavages of vaccinated calves. Calves (identified as calf ‘a’ through ‘t’) were immunized as described in Fig. 1 and challenged with BRSV on PID 0. Broncho-alveolar lavages obtained on PID 6 were centrifuged, and the supernatants diluted 1:25 before analysis by ELISA for IgA (a) and IgG (b). Corrected OD (COD) values are presented as percent of positive reference sera.
Significantly higher levels of BRSV-specific IgA and IgG were detected in nasal fluids of animals immunized with BRSV-ISCOMs compared to all control groups (IgA PID 5, p < 0.005, IgG PID −1 and 5, p < 0.001, Fig. 6 ). Those calves immunized with BRSV-ISCOMs that shed detectable virus had the largest decrease in nasal IgG after challenge (calves b and c, Fig. 6). BRSV-specific IgA and IgG were furthermore detected in the lungs on PID 6 (Fig. 7 ), and levels were significantly greater in animals immunized with BRSV-ISCOMs (IgA and IgG, p < 0.01). In the BRSV-ISCOM group the mean ratio of IgA to IgG in BAL was 121 (range 8–536) whereas the opposite was found in the nose with a mean ratio of 0 and 1 on PID −1 and 5, respectively (PID −1, range 0.06–0.13; PID 5 range 0.37–1.34). The three calves with the highest levels of IgA in BAL were the only calves in which no virus was detected in nasal swabs (calf ‘a’, ‘d’ and ‘e’, Fig. 7a, Table 2).
Fig. 6.
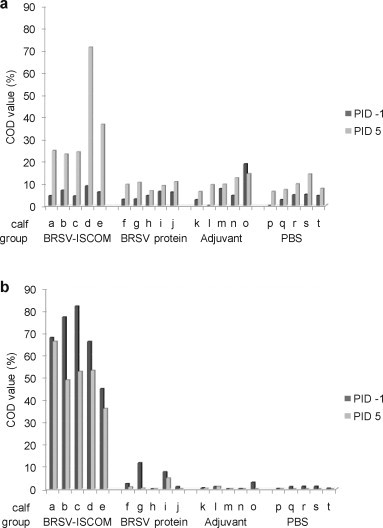
BRSV-specific antibody responses in nasal fluids of vaccinated calves. Calves (identified as calf ‘a’ through ‘t’) were immunized as described in Fig. 1 and challenged with BRSV on PID 0. Nasal fluids were obtained by tampon on PID −1 (dark grey bars) and PID 5 (light grey bars). Tampons were washed with 3 ml PBS and samples were then diluted 1:25 before analysis by ELISA for IgA (a) and IgG (b). Corrected OD (COD) values are presented as percent of positive reference sera.
The complete ISCOM formulation was also required for the induction of BRSV-specific lymphocyte proliferative responses before and after challenge (Fig. 8 ). Two days before challenge, BRSV-specific T-cell proliferation was detected in two out of five calves immunized with BRSV-ISCOMs, which was more than in control groups but not significantly different (Fig. 8). However, four days after challenge, lymphocytes from four out of five animals in the BRSV-ISCOM group proliferated after restimulation, in vitro, with BRSV antigen. In contrast, a BRSV-specific lymphocyte proliferative response was not detected in any of the control calves or in calves immunized with BRSV proteins alone. These results were reproducible using two additional dilutions of BRSV antigen for the restimulation, all tested in triplicate for each sample (data not shown). Lymphocytes from all animals at all sample occasions proliferated when stimulated with Concanavalin A, demonstrating cell viability (COD ranging between 0.2 and 0.6).
Fig. 8.
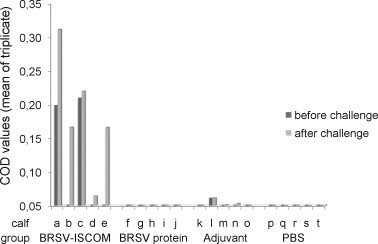
BRSV-specific lymphocyte proliferative responses in vaccinated calves. Calves (identified as calf ‘a’ through ‘t’) were immunized as described in Fig. 1 and challenged with BRSV on PID 0. Peripheral blood mononuclear cells were obtained on PID −3 or −2 (before challenge, dark grey bars) and PID 4 or 5 (after challenge, light grey bars), and were stimulated with BRSV or control antigen. Proliferation is expressed as corrected OD values (mean of triplicate) after six days of stimulation and addition of Alamar Blue® reagent.
Increased IFNγ and IL-4 mRNA expression was detected in BRSV-stimulated PBMC obtained from calves immunized with complete ISCOMs (Fig. 9 ). After 24 h stimulation with BRSV antigen, PBMC collected after challenge from BRSV-ISCOM immunized animals contained significantly more IFNγ and IL-4 mRNA per reference gene compared with those collected from animals immunized with adjuvant or PBS (IFNγ p < 0.05, IL-4 p < 0.01). There was also a significantly stronger induction of IL-4 mRNA in PBMC from animals immunized with BRSV-ISCOMs compared with those immunized with BRSV proteins (p < 0.05). The IFNγ mRNA levels were on average 46 times higher than those for IL-4 among animals immunized with BRSV-ISCOMs, which however did not significantly differ from the ratio among controls (average 22, 14 and 18 for BRSV-protein, adjuvant and PBS). Similar patterns were observed in PBMC collected after boost but before challenge. Additionally, within the BRSV-ISCOM group, the individual levels of IFNγ and IL-4 mRNA correlated perfectly to the degree of virological protection, when not standardized against 28S (data not shown).
Fig. 9.
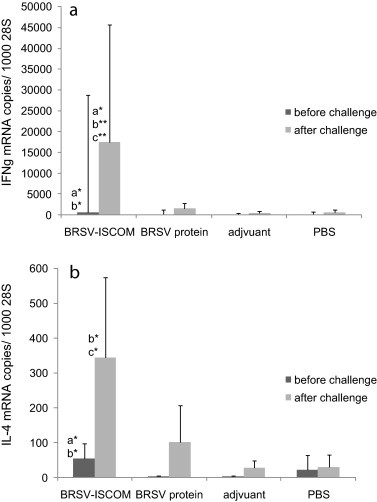
BRSV-specific cytokine mRNA production by peripheral blood mononuclear cells (PBMCs) from vaccinated calves. Calves were immunized as described in Fig. 1 and were challenged with BRSV on PID 0. Group means of IFNγ (a) and IL-4 (b) mRNA copies per 1000 28S rRNA copies, quantified in PBMCs obtained on PID −3 or −2 (before challenge, dark grey bars) and PID 4 or 5 (after challenge, light grey bars) and stimulated 24 h with BRSV in vitro. Standard deviations are shown by upward deflection lines. Stars indicate statistically significant difference between the BRSV-ISCOM and BRSV protein (a), adjuvant (b) or PBS (c) group, p ≤ 0.05 (*); p ≤ 0.01 (**). Please note that the diagrams show different scales.
5. Discussion
In this paper we demonstrate highly significant protection induced by BRSV-ISCOMs despite the presence of BRSV-specific maternal antibodies at the time of vaccination. We thus extend the previous finding in 7–15-week-old calves [27], to 3–8-week old calves, representing a more relevant target population for BRSV vaccination. Supporting earlier data, BRSV-ISCOMs provided clinical protection and a highly significant reduction in virus excretion from the upper respiratory tract of calves, associated with rapid systemic and nasal antibody responses. Additionally, virological protection of the lower respiratory tract was associated with local and systemic humoral responses as well as T cell responses dominated by IFNγ production. Moreover, we study the safety of this experimental vaccine through clinical and pathological investigations. The absence of clinical signs and the character of pulmonary cellular responses upon succeeding virulent BRSV infection, without excess of neutrophils or eosinophils, indicated good safety of the BRSV-ISCOM vaccine, although this should be further confirmed. By including a group of animals immunized with BRSV proteins alone, we showed that the protective immunological response to these proteins was dependent on ISCOM borne antigen presentation.
BRSV-ISCOMs induced a strong protection of calves at an age when they are likely to be susceptible to BRSV infection and during which protection is difficult to achieve. The age of animal at first immunization was extended from 7–15 weeks [27] to 3–8 weeks in the present study.
In accordance with previous experiments using the same experimental model [9], [27], the clinical signs observed in controls mimicked those observed after natural BRSV infection in the field, including fever and tachypnea in individuals with maternal antibodies at challenge. As previously reported after natural and experimental BRSV infection [3], [9], [39], the clinical signs of disease varied within the control groups. Standard deviations were used to illustrate this variation, instead of standard error of the mean that is often misused for this purpose [40]. The variations in clinical signs and pathology might have been due to the short time between challenge and euthanasia and being the result of the variation in the time needed to develop disease, but were most probably influenced by other factors. The three control calves with most severe tachypnea and gross pathology, also had the highest levels of BRSV-specific maternal IgG at challenge and the highest viral loads in nasal secretions, within their groups, and were among the oldest and youngest. This supports the suggestion that maternal antibodies are not fully protective and that T-cell and/or mucosal antibody responses play an important role in protection. Hypothetically, the severe disease in these three control calves could be linked to individual genetic variation in the response to BRSV infection, as has been suggested in the case of HRSV [41] and other animal viral infections [42].
In contrast to controls, the animals immunized with BRSV-ISCOMs were protected against clinical signs of disease. The clinical scorings were further supported by lung function measurements by forced oscillation. This method, originally evaluated in calves using broncho-modulatory treatments [43], could potentially be used to improve the objectiveness of clinical assessments in any respiratory infection model of calves, as will be discussed elsewhere (Pringle et al., in preparation).
The ability of BRSV-ISCOMs to induce efficient clinical protection following BRSV challenge was associated with a highly significant virological protection in both the upper and lower respiratory tract. Several animals possessing BRSV-specific maternal antibodies at the time of first immunization appeared to be completely protected since no nasal virus shedding was detected by RT-qPCR. This technique was chosen to increase the virus detection sensitivity compared to other vaccine evaluations in BRSV seropositive calves, which used virus isolation alone [21], [24], [25], [26]. Indeed, high quantities of viral RNA were found in respiratory samples from all controls.
The repeated demonstration of limited or no virus shedding in BRSV-ISCOM immunized animals inevitably led to the question whether viral replication is completely prevented in certain animals after challenge. From the data presented herein it is evident that replication did occur, although only very low levels of viral RNA were detected in BAL of BRSV-ISCOM immunized animals, six days after inoculation. Moreover, one such animal harbored live virus in the lung, although, in contrast to all controls, this was not detectable at first virus passage. The ease of virus isolation from controls highlights the virulence of the challenge and it remains to be investigated if the low amounts of virus detected in the BRSV-ISCOM group would transmit to sentinels. Our data suggest that BRSV-ISCOMs would have an impact on BRSV transmission in the field.
Not only must a new BRSV vaccine candidate be efficient, but also safe. The adverse reactions in the BRSV-ISCOM and adjuvant group upon boosting were of similar extent as can be observed with commercial cattle vaccines. These reactions were probably linked to the Quillaja saponin component that was increased from 5 to 13 times that of the lipids and proteins compared to previously [27]. Besides not causing direct toxicity and acute hypersensitivity reactions, BRSV vaccines must not induce exaggerated inflammatory responses upon natural infection. Indeed, this has been a problem both for vaccines against BRSV in cattle and against the closely related HRSV in man. In the 1960s a formalin inactivated (FI)-HRSV vaccine induced severe pulmonary neutrophilia in children, with the presence of eosinophils in fatal cases [17]. The vaccine induced enhanced disease has been reproduced experimentally in mice and cattle, with reduced IFNγ production and even more prominent pulmonary eosinophilia [44]. In small animal models FI-HRSV induces Th2-skewed immune responses that may be responsible for bronchoconstriction and inflammation following RSV infection (reviewed by Castilow and Varga and Meyer et al. [45], [46]).
Although no or only very mild clinical signs of disease have been recorded in ISCOM immunized calves after BRSV challenge, it is necessary to characterize the pulmonary inflammatory response providing the strong limitation of viral replication. This was further highlighted by Chen et al. [47], who observed virological protection associated with peribronchiolary inflammation and increased eosinophils in BAL of HRSV-ISCOM immunized mice after HRSV challenge. No clinical signs of disease were reported in that study. Our vaccination protocol and challenge model did induce influx of leucocytes into limited areas of the lungs after virulent BRSV challenge, however, no increase in numbers of cells was observed compared to controls. A deeper characterization of the pulmonary cellular response to infection in ISCOM- immunized animals is presently ongoing.
The immunological response was investigated herein by several other approaches. As in the previous experiment [27], not only were the systemic and local humoral responses very rapid and strong after boosting; four out of five animals showed an antibody response after a single injection. Furthermore, as the limited virus detection in ISCOM immunized animals was not associated with pre-vaccination passive antibody status, ISCOMs capacity to overcome maternal immunity was confirmed.
Although being one of the major problems in vaccinology and relevant for a broad range of pathogens, the exact mechanisms responsible for the inhibitory effects of maternally derived antibodies on active immunization are still not fully understood. It is clear, however, that this inhibition is determinant-specific and occurs primarily on B-cells, probably by antibody masking of epitopes on vaccine antigens and preventing the binding to these cells. There is possibly also interference with antigen uptake and presentation, although cellular responses are much less affected than humoral responses [48]. Notably, even if a BRSV specific T-cell memory can be induced in the presence of maternal antibodies in calves [49], the antibody responses, induced by commercial vaccines in previous studies, were commonly absent [26] and the induced clinical protection was poor in a model reproducing the clinical signs of disease observed in the field [27].
The mechanisms by which ISCOMs overcome the inhibiting effect of maternal antibodies is unknown. One possibility is that the effect is due to the antigen dose in relation to the level of maternal antibodies [50]. However, as will be described in detail elsewhere (Hägglund et al., in preparation [51]), one dose of BRSV-ISCOMs contained less than 100 μg BRSV proteins, which is 3–20 times less than that in other subunit BRSV vaccines administered to calves [52], [53]. Nevertheless, given the weak responses induced by BRSV proteins alone, the presence of antigens in the form of ISCOM particles, and/or the effect of adjuvant component, was essential. We are presently investigating whether the incorporation of BRSV-proteins into ISCOMs is required or alternatively whether similar immunogenicity can be obtained with the antigens mixed with commercially available, pre-formed empty ISCOMs or another adjuvant.
The capacity of ISCOMs to prime for rapid IgA production after challenge was confirmed, as previously shown in serum [27], and now in BAL and nasal fluids of immunized and challenged animals. Similarly, long lasting pulmonary IgA was induced after intranasal BRSV-ISCOM immunization of mice [54]. In contrast, low levels of pulmonary IgG were detected in BAL, probably due to high dilution of samples, which further suggests that the IgA was actively transported into the lung and present in very high amounts. The two calves in the ISCOM group that shed detectable amounts of virus had the lowest levels of pulmonary BRSV-specific IgA within their group.
Although IgA has been related to Th2 cytokines in mice, the presence of ISCOMs in the vaccine skewed the immune responses of calves more towards Th1, confirming earlier publications [55]. Thus, in contrast to calves immunized with BRSV protein alone, the animals immunized with BRSV-ISCOMs rapidly acquired serum IgG2, an immunoglobulin associated with Th1 cytokines. Furthermore, a similar pattern was seen for the induction of IgG2a in mice (data not shown). Since the IgG1 and IgG2 assays were normalized against different positive control sera, it is not possible to compare the concentrations of these parameters. However, bearing in mind that at four months of age, calves have normally similar levels of total IgG1 as adult cattle, whereas total IgG2 then is only half of adult levels [56], and that IgG2 is complement activating [57], [58]; the presence of this antibody isotype might be linked to protection or associated with a strong T-cell response.
The powerful antibody responses were probably associated with the ability of ISCOMs to induce strong and balanced T-helper cell responses, as assessed by restimulation of PBMCs with killed antigens ex vivo, for antigen presentation on MHC II to memory T helper cells. After stimulation, PBMCs from BRSV-ISCOM immunized animals showed significantly higher levels of IFNγ and IL-4 mRNA per cell than those from controls, with about 10–160 times higher levels of IFNγ than IL-4 mRNA. Additionally, significantly higher lymphocyte proliferative responses were induced in animals immunized with BRSV-ISCOMs, indicating that their lymphocytes would both undergo efficient clonal expansion and produce more cytokines per cell, upon BRSV infection. This remains to be verified in vivo. Bovine IL-4 is generally associated with enhanced B-cell survival and IgG1 antibody production in cattle, whereas IFNγ is normally linked to a Th1 type of response with production of IgG2 and activation of macrophages as well as cytotoxic lymphocytes (CTL, reviewed by Estes and Brown [57]). ISCOMs have previously shown an exceptional capacity to induce CTL responses, possibly by enabling B-cell cross-presentation of killed antigens to naïve CD8+ lymphocytes [58], and this will be investigated for BRSV in the future. Supposedly, the marked IFNγ and the moderate IL-4 mRNA inductions were promoted by ISCOM induction of type I interferons, which in bovine in vitro systems has been shown to induce not only IgG2, but also IgA production [59]. IFNγ additionally upregulates the production of IgA secretory component and IgA transcytosis by human bronchial epithelial cells in vitro [60].
In summary, BRSV-ISCOMs is an efficient inactivated vaccine that mimics a natural BRSV infection in that it strongly primes for production of IFNγ mRNA from lymphocytes and local as well as systemic immunity, with particularly strong IgA pulmonary responses, without disease enhancing effects.
Acknowledgement
This work was supported by the Swedish Farmers’ Foundation for Agricultural Research (grant H0750358). We thank Mr B. Norén with staff, SLU, Sweden for rearing and maintenance of the experimental animals, Prof L. E. Larsen, DTU, Denmark for generously sharing the BRSV isolate no. 9402022, Prof B. Morein and Dr K. Lövgren Bengtsson, Isconova AB, Sweden, for supply of AbISCO-300®, technical advice and material for ISCOM production. Furthermore, Mrs C. Vernersson, Mrs I. Dahlén and Dr L. Treiberg-Berndtsson, SVA, is thanked for FBT cells, vero cells and providing facilities for virus production. We would also like to present our gratitude to Mrs L. Fuxler, BVF, SLU and Dr R. Kruse, KV, SLU as well as Dr M. Juremalm, Dr M. Hakhverdyan and Dr A. Yacoub, SVA, for support during laboratory analyses.
References
- 1.Valarcher J.F., Taylor G. Bovine respiratory syncytial virus infection. Vet Res. 2007;38(March–April (2)):153–180. doi: 10.1051/vetres:2006053. [DOI] [PubMed] [Google Scholar]
- 2.Assie S., Seegers H., Makoschey B., Desire-Bousquie L., Bareille N. Exposure to pathogens and incidence of respiratory disease in young bulls on their arrival at fattening operations in France. Vet Rec. 2009;165(August (7)):195–199. doi: 10.1136/vr.165.7.195. [DOI] [PubMed] [Google Scholar]
- 3.Verhoeff J., van Nieuwstadt A.P. BRS virus, PI3 virus and BHV1 infections of young stock on self-contained dairy farms: epidemiological and clinical findings. Vet Rec. 1984;114(March (12)):288–293. doi: 10.1136/vr.114.12.288. [DOI] [PubMed] [Google Scholar]
- 4.Viuff B., Tjornehoj K., Larsen L.E., Rontved C.M., Uttenthal A., Ronsholt L. Replication and clearance of respiratory syncytial virus: apoptosis is an important pathway of virus clearance after experimental infection with bovine respiratory syncytial virus. Am J Pathol. 2002;161(December (6)):2195–2207. doi: 10.1016/S0002-9440(10)64496-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Viuff B., Uttenthal A., Tegtmeier C., Alexandersen S. Sites of replication of bovine respiratory syncytial virus in naturally infected calves as determined by in situ hybridization. Vet Pathol. 1996;33(July (4)):383–390. doi: 10.1177/030098589603300403. [DOI] [PubMed] [Google Scholar]
- 6.Liu L., Lehmkuhl H.D., Kaeberle M.L. Synergistic effects of bovine respiratory syncytial virus and non-cytopathic bovine viral diarrhea virus infection on selected bovine alveolar macrophage functions. Can J Vet Res. 1999;63(January (1)):41–48. [PMC free article] [PubMed] [Google Scholar]
- 7.Gershwin L.J., Berghaus L.J., Arnold K., Anderson M.L., Corbeil L.B. Immune mechanisms of pathogenetic synergy in concurrent bovine pulmonary infection with Haemophilus somnus and bovine respiratory syncytial virus. Vet Immunol Immunopathol. 2005;107(August (1–2)):119–130. doi: 10.1016/j.vetimm.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 8.Babiuk L.A., Lawman M.J., Ohmann H.B. Viral-bacterial synergistic interaction in respiratory disease. Adv Virus Res. 1988;35:219–249. doi: 10.1016/S0065-3527(08)60713-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tjornehoj K., Uttenthal A., Viuff B., Larsen L.E., Rontved C., Ronsholt L. An experimental infection model for reproduction of calf pneumonia with bovine respiratory syncytial virus (BRSV) based on one combined exposure of calves. Res Vet Sci. 2003;74(February (1)):55–65. doi: 10.1016/S0034-5288(02)00154-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kimman T.G., Zimmer G.M., Westenbrink F., Mars J., van Leeuwen E. Epidemiological study of bovine respiratory syncytial virus infections in calves: influence of maternal antibodies on the outcome of disease. Vet Rec. 1988;123(July (4)):104–109. doi: 10.1136/vr.123.4.104. [DOI] [PubMed] [Google Scholar]
- 11.Inaba Y., Tanaka Y., Sato K., Omori T., Matumoto M. Bovine respiratory syncytial virus. Studies on an outbreak in Japan, 1968–1969. Jpn J Microbiol. 1972;16(September (5)):373–383. [PubMed] [Google Scholar]
- 12.Elvander M. Severe respiratory disease in dairy cows caused by infection with bovine respiratory syncytial virus. Vet Rec. 1996;138(February (5)):101–105. doi: 10.1136/vr.138.5.101. [DOI] [PubMed] [Google Scholar]
- 13.Larsen L.E., Tegtmeier C., Pedersen E. Bovine respiratory syncytial virus (BRSV) pneumonia in beef calf herds despite vaccination. Acta Vet Scand. 2001;42(1):113–121. doi: 10.1186/1751-0147-42-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Valarcher J.F., Schelcher F., Bourhy H. Evolution of bovine respiratory syncytial virus. J Virol. 2000;74(November (22)):10714–10728. doi: 10.1128/jvi.74.22.10714-10728.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gershwin L.J., Schelegle E.S., Gunther R.A., Anderson M.L., Woolums A.R., Larochelle D.R. A bovine model of vaccine enhanced respiratory syncytial virus pathophysiology. Vaccine. 1998;16(July (11–12)):1225–1236. doi: 10.1016/s0264-410x(98)80123-0. [DOI] [PubMed] [Google Scholar]
- 16.Schreiber P., Matheise J.P., Dessy F., Heimann M., Letesson J.J., Coppe P. High mortality rate associated with bovine respiratory syncytial virus (BRSV) infection in Belgian white blue calves previously vaccinated with an inactivated BRSV vaccine. J Vet Med B, Infect Dis Vet Public Health. 2000;47(September (7)):535–550. doi: 10.1046/j.1439-0450.2000.00380.x. [DOI] [PubMed] [Google Scholar]
- 17.Kim H.W., Canchola J.G., Brandt C.D., Pyles G., Chanock R.M., Jensen K. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol. 1969;89(April (4)):422–434. doi: 10.1093/oxfordjournals.aje.a120955. [DOI] [PubMed] [Google Scholar]
- 18.Nair H., Nokes D.J., Gessner B.D., Dherani M., Madhi S.A., Singleton R.J. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet. 2010;375(May (9725)):1545–1555. doi: 10.1016/S0140-6736(10)60206-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fulton R.W., Briggs R.E., Payton M.E., Confer A.W., Saliki J.T., Ridpath J.F. Maternally derived humoral immunity to bovine viral diarrhea virus (BVDV) 1a, BVDV1b, BVDV2, bovine herpesvirus-1, parainfluenza-3 virus bovine respiratory syncytial virus, Mannheimia haemolytica and Pasteurella multocida in beef calves, antibody decline by half-life studies and effect on response to vaccination. Vaccine. 2004;22(January (5–6)):643–649. doi: 10.1016/j.vaccine.2003.08.033. [DOI] [PubMed] [Google Scholar]
- 20.Vangeel I., Antonis A.F., Fluess M., Riegler L., Peters A.R., Harmeyer S.S. Efficacy of a modified live intranasal bovine respiratory syncytial virus vaccine in 3-week-old calves experimentally challenged with BRSV. Vet J. 2007;174(November (3)):627–635. doi: 10.1016/j.tvjl.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 21.Ellis J.A., Gow S.P., Goji N. Response to experimentally induced infection with bovine respiratory syncytial virus following intranasal vaccination of seropositive and seronegative calves. J Am Vet Med Assoc. 2010;236(May (9)):991–999. doi: 10.2460/javma.236.9.991. [DOI] [PubMed] [Google Scholar]
- 22.Timsit E., Le Drean E., Maingourd C., Belloc C., Guatteo R., Bareille N. Detection by real-time RT-PCR of a bovine respiratory syncytial virus vaccine in calves vaccinated intranasally. Vet Rec. 2009;165(August (8)):230–233. doi: 10.1136/vr.165.8.230. [DOI] [PubMed] [Google Scholar]
- 23.Stott E.J., Thomas L.H., Taylor G. Development of a potent inactivated vaccine against respiratory syncytial virus infection of calves. In: Hartigan P.J., Monaghan M.L., editors. 14th World Conference on Diseases of Cattle. 1986. pp. 669–673. [Google Scholar]
- 24.Patel J.R., Didlick S.A. Evaluation of efficacy of an inactivated vaccine against bovine respiratory syncytial virus in calves with maternal antibodies. Am J Vet Res. 2004;65:417–421. doi: 10.2460/ajvr.2004.65.417. [DOI] [PubMed] [Google Scholar]
- 25.Mawhinney I.C., Burrows M.R., Straub O.C., Lees P., Higgins A.J., Reid D.S. Protection against bovine respiratory syncytial virus challenge following a single dose of vaccine in young calves with maternal antibody. Vet Rec. 2005;156:139–143. doi: 10.1136/vr.156.5.139. [DOI] [PubMed] [Google Scholar]
- 26.van der Sluijs M.T., Kuhn E.M., Makoschey B. A single vaccination with an inactivated bovine respiratory syncytial virus vaccine primes the cellular immune response in calves with maternal antibody. BMC Vet Res. 2010;6:2. doi: 10.1186/1746-6148-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hägglund S., Hu K.F., Larsen L.E., Hakhverdyan M., Valarcher J.F., Morein B. Bovine respiratory syncytial virus ISCOMs - protection in the presence of maternal antibodies. Vaccine. 2004;23:646–655. doi: 10.1016/j.vaccine.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 28.Pringle J, Valarcher JF, Hu KF, Blodörn K, Hägglund S. Forced oscillation technique measurements of pulmonary function in calves undergoing experimental BRSV infection. Manuscript. (in preparation).
- 29.West K., Petrie L., Konoby C., Haines D.M., Cortese V., Ellis J.A. The efficacy of modified-live bovine respiratory syncytial virus vaccines in experimentally infected calves. Vaccine. 1999;18(December (9–10)):907–919. doi: 10.1016/s0264-410x(99)00324-2. [DOI] [PubMed] [Google Scholar]
- 30.Taylor G., Thomas L.H., Wyld S.G., Furze J., Sopp P., Howard C.J. Role of T-lymphocyte subsets in recovery from respiratory syncytial virus infection in calves. J Virol. 1995;69(November (11)):6658–6664. doi: 10.1128/jvi.69.11.6658-6664.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taylor G., Bruce C., Barbet A.F., Wyld S.G., Thomas L.H. DNA vaccination against respiratory syncytial virus in young calves. Vaccine. 2005;23(January (10)):1242–1250. doi: 10.1016/j.vaccine.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 32.Hakhverdyan M., Hägglund S., Larsen L.E., Belak S. Evaluation of a single-tube fluorogenic RT-PCR assay for detection of bovine respiratory syncytial virus in clinical samples. J Virol Methods. 2005;123(February (2)):195–202. doi: 10.1016/j.jviromet.2004.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Uttenthal A., Larsen L.E., Philipsen J.S., Tjornehoj K., Viuff B., Nielsen K.H. Antibody dynamics in BRSV-infected Danish dairy herds as determined by isotype-specific immunoglobulins. Vet Microbiol. 2000;76(October (4)):329–341. doi: 10.1016/S0378-1135(00)00261-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taylor G., Stott E.J., Bew M., Fernie B.F., Cote P.J., Collins A.P. Monoclonal antibodies protect against respiratory syncytial virus infection in mice. Immunology. 1984;52(May (1)):137–142. [PMC free article] [PubMed] [Google Scholar]
- 35.Taylor G., Thomas L.H., Furze J.M., Cook R.S., Wyld S.G., Lerch R. Recombinant vaccinia viruses expressing the F, G or N, but not the M2, protein of bovine respiratory syncytial virus (BRSV) induce resistance to BRSV challenge in the calf and protect against the development of pneumonic lesions. J Gen Virol. 1997;78(December (12)):3195–3206. doi: 10.1099/0022-1317-78-12-3195. [DOI] [PubMed] [Google Scholar]
- 36.Ahmed S.A., Gogal R.M., Jr., Walsh J.E. A new rapid and simple non-radioactive assay to monitor and determine the proliferation of lymphocytes: an alternative to [3H]thymidine incorporation assay. J Immunol Methods. 1994;170(April (2)):211–224. doi: 10.1016/0022-1759(94)90396-4. [DOI] [PubMed] [Google Scholar]
- 37.Rosbottom A., Guy C.S., Gibney E.H., Smith R.F., Valarcher J.F., Taylor G. Peripheral immune responses in pregnant cattle following Neospora caninum infection. Parasite Immunol. 2007;29(April (4)):219–228. doi: 10.1111/j.1365-3024.2007.00936.x. [DOI] [PubMed] [Google Scholar]
- 38.Rotting A.K., Freeman D.E., Constable P.D., Eurell J.A., Wallig M.A. Mucosal distribution of eosinophilic granulocytes within the gastrointestinal tract of horses. Am J Vet Res. 2008;69(July (7)):874–879. doi: 10.2460/ajvr.69.7.874. [DOI] [PubMed] [Google Scholar]
- 39.Bryson D.G., McFerran J.B., Ball H.J., Neill S.D. Observations on outbreaks of respiratory disease in housed calves – (1) epidemiological, clinical and microbiological findings. Vet Rec. 1978;103(November (22)):485–489. doi: 10.1136/vr.103.22.485. [DOI] [PubMed] [Google Scholar]
- 40.Nagele P. Misuse of standard error of the mean (SEM) when reporting variability of a sample. A critical evaluation of four anaesthesia journals. Br J Anaesth. 2003;90(April (4)):514–516. doi: 10.1093/bja/aeg087. [DOI] [PubMed] [Google Scholar]
- 41.Ramet M., Korppi M., Hallman M. Pattern recognition receptors and genetic risk for RSV infection: value for clinical decision-making? Pediatr Pulmonol. 2011;46(February (2)):101–110. doi: 10.1002/ppul.21348. [DOI] [PubMed] [Google Scholar]
- 42.Brostrom H., Fahlbrink E., Dubath M.L., Lazary S. Association between equine leucocyte antigens (ELA) and equine sarcoid tumors in the population of Swedish halfbreds and some of their families. Vet Immunol Immunopathol. 1988;19(October (3–4)):215–223. doi: 10.1016/0165-2427(88)90109-2. [DOI] [PubMed] [Google Scholar]
- 43.Reinhold P., Macleod D., Lekeux P. Comparative evaluation of impulse oscillometry and a monofrequency forced oscillation technique in clinically healthy calves undergoing bronchochallenges. Res Vet Sci. 1996;61(November (3)):206–213. doi: 10.1016/s0034-5288(96)90064-8. [DOI] [PubMed] [Google Scholar]
- 44.Antonis A.F., Schrijver R.S., Daus F., Steverink P.J., Stockhofe N., Hensen E.J. Vaccine-induced immunopathology during bovine respiratory syncytial virus infection: exploring the parameters of pathogenesis. J Virol. 2003;77(November (22)):12067–12073. doi: 10.1128/JVI.77.22.12067-12073.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Castilow E.M., Varga S.M. Overcoming T cell-mediated immunopathology to achieve safe RSV vaccination. Future Virol. 2008;3(5):445–454. doi: 10.2217/17460794.3.5.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meyer G., Deplanche M., Schelcher F. Human and bovine respiratory syncytial virus vaccine research and development. Comp Immunol Microbiol Infect Dis. 2008;31(March (2–3)):191–225. doi: 10.1016/j.cimid.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 47.Chen M., Hu K.F., Rozell B., Orvell C., Morein B., Liljestrom P. Vaccination with recombinant alphavirus or immune-stimulating complex antigen against respiratory syncytial virus. J Immunol. 2002;169(September (6)):3208–3216. doi: 10.4049/jimmunol.169.6.3208. [DOI] [PubMed] [Google Scholar]
- 48.Getahun A., Heyman B. Studies on the mechanism by which antigen-specific IgG suppresses primary antibody responses: evidence for epitope masking and decreased localization of antigen in the spleen. Scand J Immunol. 2009;70(September (3)):277–287. doi: 10.1111/j.1365-3083.2009.02298.x. [DOI] [PubMed] [Google Scholar]
- 49.Sandbulte M.R., Roth J.A. T-cell populations responsive to bovine respiratory syncytial virus in seronegative calves. Vet Immunol Immunopathol. 2002;84(January (1–2)):111–123. doi: 10.1016/s0165-2427(01)00393-2. [DOI] [PubMed] [Google Scholar]
- 50.Crowe J.E., Jr. Influence of maternal antibodies on neonatal immunization against respiratory viruses. Clin Infect Dis. 2001;33(November (10)):1720–1727. doi: 10.1086/322971. [DOI] [PubMed] [Google Scholar]
- 51.Hägglund S, Hu KF, Eléouët JF, Ellencrona K, Lövgren-Bengtsson K, Riffault S, et al. Detailed characterization of ISCOMs against bovine respiratory syncytial virus. Manuscript. (in preparation).
- 52.Letellier C., Boxus M., Rosar L., Toussaint J.F., Walravens K., Roels S. Vaccination of calves using the BRSV nucleocapsid protein in a DNA prime-protein boost strategy stimulates cell-mediated immunity and protects the lungs against BRSV replication and pathology. Vaccine. 2008;26(September (37)):4840–4848. doi: 10.1016/j.vaccine.2008.06.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Riffault S., Meyer G., Deplanche M., Dubuquoy C., Durand G., Soulestin M. A new subunit vaccine based on nucleoprotein nanoparticles confers partial clinical and virological protection in calves against bovine respiratory syncytial virus. Vaccine. 2010;28(May (21)):3722–3734. doi: 10.1016/j.vaccine.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hu K.F., Elvander M., Merza M., Akerblom L., Brandenburg A., Morein B. The immunostimulating complex (ISCOM) is an efficient mucosal delivery system for respiratory syncytial virus (RSV) envelope antigens inducing high local and systemic antibody responses. Clin Exp Immunol. 1998;113(August (2)):235–243. doi: 10.1046/j.1365-2249.1998.00650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hu K.F., Regner M., Siegrist C.A., Lambert P., Chen M., Bengtsson K.L. The immunomodulating properties of human respiratory syncytial virus and immunostimulating complexes containing Quillaja saponin components QH-A, QH-C and ISCOPREP703. FEMS Immunol Med Microbiol. 2005;43(February (2)):269–276. doi: 10.1016/j.femsim.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 56.Chase C.C., Hurley D.J., Reber A.J. Neonatal immune development in the calf and its impact on vaccine response. Vet Clin North Am Food Anim Pract. 2008;24(March (1)):87–104. doi: 10.1016/j.cvfa.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bastida-Corcuera F.D., Butler J.E., Yahiro S., Corbeil L.B. Differential complement activation by bovine IgG2 allotypes. Vet Immunol Immunopathol. 1999;71(October (2)):115–123. doi: 10.1016/s0165-2427(99)00095-1. [DOI] [PubMed] [Google Scholar]
- 58.Robson N.C., Donachie A.M., Mowat A.M. Simultaneous presentation and cross-presentation of immune-stimulating complex-associated cognate antigen by antigen-specific B cells. Eur J Immunol. 2008;38(May (5)):1238–1246. doi: 10.1002/eji.200737758. [DOI] [PubMed] [Google Scholar]
- 59.Estes D.M., Brown W.C. Type 1 and type 2 responses in regulation of Ig isotype expression in cattle. Vet Immunol Immunopathol. 2002;90(November (1–2)):1–10. doi: 10.1016/s0165-2427(02)00201-5. [DOI] [PubMed] [Google Scholar]
- 60.Godding V., Sibille Y., Massion P.P., Delos M., Sibille C., Thurion P. Secretory component production by human bronchial epithelial cells is upregulated by interferon gamma. Eur Respir J. 1998;11(May (5)):1043–1052. doi: 10.1183/09031936.98.11051043. [DOI] [PubMed] [Google Scholar]


