Highlights
-
•
Cell attenuated porcine epidemic diarrhea virus strain Zhejiang08 provides effective immune protection in piglets.
-
•
Zhejiang08 has higher ability to stimulate DCs and activates T-cell proliferation than classical vaccine strain CV777.
-
•
Potential glycosylation site lacking in S protein may cause the stronger immune response.
Keywords: Porcine epidemic diarrhea virus, Immune protective efficiency, Dendritic cells, Glycosylation sites
Abstract
Since 2010, the porcine epidemic diarrhea coronavirus (PEDV) has caused significant damage to the global pork industry. However, classical PEDV vaccine strains only provide limited protection against emerging strains. In this study, we successfully isolated and attenuated the PEDV epidemic strain Zhejiang08, which was characterized by good cell adaptation and high-titer production 48 h post infection in Vero E6 cells. The attenuated virus induced a high level of virus-specific neutralizing antibodies until 120 days after immunization in piglets and provided complete protection when challenged with an emerging virus strain on day 14 post immunization. Moreover, the capability to activate dendritic cells (DCs) of this isolate was identified. Higher expression levels of IL-12 and IFN-γ were recorded in DCs after treatment with Zhejiang08 for 24 h. Furthermore, genome sequencing and phylogenetic analysis revealed high homology between the main antigen epitopes of Zhejiang08 and PEDV pandemic isolates following 2011. Combining the glycosylation site prediction results and their distribution within the spatial structure of the S protein, led to the conclusion that the observed more effective host immune response of Zhejiang08 compared to CV777 was possibly associated with a lack of the potential glycosylation site in the 296 amino acids of the S protein. In summary, we illustrated that the attenuated virus represents a promising vaccine candidate.
1. Introduction
Porcine epidemic diarrhea (PED) is an acute viral enteric disease, characterized by severe villus atrophy, vomiting, and watery diarrhea, and was first reported in the UK in 1971 [1]. Recently, a new wave of PED virus infections has emerged worldwide since the end of 2010, which is characterized by comparatively high mortality rates among suckling piglets, causing significant economic losses to the swine industry (deaths of more than 10% of the US swine population) [2], [3].
Various approaches have been utilized to control disease outbreaks. Many classical PEDV live vaccines, including CV777, Japan 83P-5, and Korea DR13 strains (all without pathogenicity), induced robust immune responses in sows and succeeded to control classical PEDV infections in pig farms for three decades [4], [5], [6]. CV777 for example was isolated from pigs during a PED outbreak in Belgium in 1976 and the attenuated vaccine strain was prepared via serial passaging of the strain in cell cultures. This strain has been widely used in Asian countries, where it provided effective immunological protection in herds for almost two decades [7], [8]. However, these traditional attenuated PEDV vaccines typically displayed lower serum antibody titers against emerging PEDV strains after 2011, suggesting that classical PEDV vaccine strains may only provide partial cross-protection against highly virulent strains. The low immune protective efficiency was attributed to the remarkable antigenic variation of structure proteins and major antigen sites between classical and emerging PEDV isolates [2], [8].
Viruses can co-opt host cellular biosynthetic pathways to modify the proteins that are present on their surface. Several viruses have been reported to utilize the cellular glycosylation pathway during both host cell invasion and immune evasion, including influenza, HIV, hepatitis, and the West Nile virus [9], [10], [11], [12]. The S protein of PEDV is one of the most glycosylated proteins in nature, and many of the glycosylation sites feature high mannose composition. During viral evolution, sites are easily added or deleted and the resulting potential modification diversity increases the complexity of viral glycoproteins [13].
DCs are widely distributed beneath both mucosal surfaces and dermal layers, which are the most powerful antigen-presenting cells within the intestine mucosal immune system [14]. DC-mediated modulation of immune responses is highly dependent on the maturation status of DCs after encountering an exogenous microorganism [15]. Therefore, the capacity of the vaccine to stimulate the maturation of DCs and induce T-cell responses has also been an important criterion for vaccine evaluation [16].
Our research evaluated the potential of vaccination with one PEDV attenuated virus. Combining the sequence character and DCs stimulation ability of this isolate, we further explored the mechanism that accounts for the high immune protective efficiency of this isolate.
2. Method and materials
2.1. Reagents and cell lines
Fluorescent-labeled anti-pig PE-SWC3a, FITC-SWC3a, FITC-CD1a, and the respective isotype and fluorochrome-matched control antibodies mouse IgG1 (SWC3a) and mouse IgG2a (CD1a) were purchased from Abcam (China). FITC-MHCII, PE-CD86, and their isotypes control mouse FITC-IgG1 and PE-IgG2b were purchased from LifeSpan BioSciences (USA) and eBioscience (USA), respectively. The anti-PEDV spike protein monoclonal antibody was purchased from Median Diagnostics (South Korea). Vero E6 cells (ATCC CRL-1586) were provided by the Veterinary Medicine Research Centre of the Da Bei Nong Group.
2.2. Zhejiang08 isolation and cell attenuation
Intestinal and fecal samples were collected from piglets, the displayed clinical features associated with PED. Virus isolation was conducted according to a previously described protocol [17]. After the 105th adapted cell passage, one PEDV strain was successful isolated, which was proven to be free of pathogenicity and thus considered safe for use in neonatal pigs by the Veterinary Medicine Research Centre of the Da Bei Nong Group.
2.3. Biological and growth characteristics of the isolated Zhejiang08 PEDV strain
After infection of Vero E6 cells, the one-step growth curve of the Zhejing08 strain was confirmed via RT-PCR (Table S1), plaque assays, and an indirect immunofluorescence assay (IFA) subsequent to infection.
2.4. Sequence analysis of the Zhejiang08 PEDV strain
Based on the CV777 PEDV strain sequence (GenBank: AF353511.1), primers (see Table S2) were designed for the amplification of the Zhejaing08 genome. The 5′ and 3′ ends of the Zhejiang08 genome were further validated, using a 3′ and 5′-Full RACE Core Set with PrimeScript (Clontech, Japan). Phylogenetic trees were constructed with the computer program MEGA version 5.0, utilizing the neighbor-joining method. Reference strains used for phylogenetic analysis are listed in Table S3. Potential N-glycan glycosylation sites of the Zhejiang08 spike protein were predicted via NetNGlyc servers (www.cbs.dtu.dk/services/NetNGlyc).
2.5. Spike protein crystal structure analysis and distribution of predicted glycosylation sites of PEDV CV7777, Zhejiang08, and AH2012
The crystal structure of the coronavirus spike protein was applied to the homology model of CV777, Zhejiang08, and AH2012 S protein sequences using the SWISS-MODEL workspace [18]. Based on the previously predicted results of the N-terminal glycosylation sites, we highlighted amino acid sequences of differential glycosylation sites of three strains (Fig. 1 , surface). Informed by the biosynthetic pathway for the generation of N-linked glycosylation and the potential size of glycan structures [13], additional n-glycosylation sites were utilized as the centre of a circle with a radius of 1 nm (Fig. 1, amino acid areas have been marked that have potentially been covered by n-glycosylation sites).
Fig. 1.
Molecular modeling analysis of the S protein of AH2012 (A), CV777 (B), and Zhejiang08 (C). The two and one extra potential glycosylation sites of the aa sequence of AH2012 and CV777 are shown as ribbons; potentially covered regions (glycosylation sites as dot, with a radius of 1 nm) are shown as surfaces; the same site of the Zhejiang08 aa sequence is shown as balls-and-sticks.
2.6. Immune protective efficiency analysis of the Zhejiang08 strain
2.6.1. Design of animal experiments
A total of 30 caesarian-derived and colostrum-deprived 3-day-old piglets (large white) were chosen and serologically confirmed to be negative for PEDV and TGEV antibodies via indirect ELISA. All piglets were randomly assigned to three groups (Control, CV777, and Zhejiang08), were artificially fed with milk, and housed in three separate rooms. Three groups were intramuscularly immunized with 1 mL of CV777 (105.0 TCID50/mL), 1 mL of Zhejiang08 (105.0 TCID50/mL), and the same volume of saline, respectively, all of which were emulsified with complete Freund's adjuvant. Subsequent to immunization, sera samples from piglets of the three different groups were collected at 7 d until 150 d after immunization to explore whether piglets generated PEDV neutralizing antibodies as previously described [19]. On the 14th day after immunization, 18 piglets were chosen, and assigned to groups with six piglets per group. These piglets were divided according to their original group, and each group was housed in a separate room. Then, the piglets of all groups were orally challenged with 1 ml 103 MID/ml of PEDV fourth-generation tissue virulent virus (intestinal tissue from virulent PEDV infected piglets). All animal experiments were approved by the Institutional Animal Care and Use Committee of the Nanjing Agricultural University (Nanjing, China), following the guidelines of the National Institute of Health.
2.6.2. Clinical assessment
Following the challenge, infected piglets were examined for clinical symptoms once per day. In addition, the feces of all piglets were subjectively scored for fecal consistency using the following criteria: (−) normal, (+) semi-liquid without formed consistency, and (++) watery/liquid contents. Fecal scores were recorded daily for six days after the viral challenge.
2.6.3. PEDV fecal shedding analysis
Prior to inoculation, fecal swabs were collected daily and from all piglets. In brief, viral RNA was extracted out of 50 μl of supernatants following centrifugation of the fecal suspensions (2000g for 30 min at 4 °C), using the Ambion1 MagMAXTM viral RNA isolation kit (Life Technologies, Carlsbad, CA) and the KingFisher1 96 magnetic particle processor (Thermo-Fisher Scientific, Waltham, CA) using recommended protocols of the respective manufacturers. Primers and probe were designed according to a previously published method [20].
2.6.4. Necropsy and histopathological examination
Following the challenge, infected piglets were examined daily for symptoms of diarrhea. During this examination period, two piglets from the PBS and CV777 treatment groups that had developed diarrhea on day three were euthanized for a pathological examination on DPI (day post-inoculation) 5. Moreover, two piglets from the Zhejiang08 treatment group (where no piglets developed diarrhea) were randomly selected for comparison. At necropsy, both intestine and other major organs were examined. The jejunum samples (three samples were taken at 40–60 cm intervals) were placed in 4% paraformaldehyde, dehydrated in graded alcohol, embedded in paraffin, and directly cut to 5 μm sections onto microscope slides; four micron sections were cut and stained with hematoxylin and eosin (H&E). The formalin-fixed, paraffin-embedded tissues were prepared and tested via immunofluorescence (IFA) for the detection of PEDV antigens.
2.7. DC generation
Swine DCs were obtained from bone marrow of piglet femurs according to methods that had been optimized by our laboratory and have previously been published [21].
2.8. Uptake, phenotype, and cytokines assays
DCs were inoculated with both Zhejiang08 and CV777 at a multiplicity of infection (MOI) of 1 for 1 h at 37 °C under 5% CO2. Then the DCs were washed with PBS (pH7.2 at 4 °C) three times to remove unbound virus and maintained in maintenance medium (RPIM1640 supplemented with 2% FBS) at 37 °C. Following culture for 6 h, DCs were stained with anti-S monoclonal antibodies via FACS to investigate virus uptake. Alternatively, DCs were stained after 24 h with various phenotype fluorescent mAbs or isotypes and then investigated via FACS. Furthermore, the transcription levels of IL-12, IFN-γ, and IL-10 were determined via quantitative RT-qPCR analysis, utilizing a StepOnePlus (ABI) instrument (Life Technologies) (see Table S1).
2.9. Mixed lymphocyte reaction
The functional activity of DCs was reflected in a primary allogeneic mixed lymphocyte reaction assay. T-cells were separated from the MLN of allogeneic piglets, using a T-cell isolation kit (Miltenyi, Bergisch Gladbach, Germany) and then labeled with carboxyfluorescein succinimidyl ester (CFSE) (Invitrogen). Subsequently, T-lymphocytes (5 × 105 cell/well) were co-cultured with DCs that had been treated with CV777 (MOI, 1), Zhejiang08 (MOI, 1), LPS (100 ug/ml/well), or Mock-DCs at ratios of 1:1 and 1:5 (DCs: lymphocytes). Co-cultures were incubated at 37 °C in 5% CO2 for 5 d. After five days of culture, cell proliferation was measured via FACS.
2.10. Statistical analysis
Results are expressed as means ± SD. Analysis of variance (ANOVA) or Student’s t test were employed to determine statistical differences.
3. Results
3.1. Biological and growth characteristics of the Zhejiang08 PEDV strain
The Zhejiang08 strain can induce an apparent and classical cytopathic effect (CPE) in Vero E6 cells, including cell fusion and multinucleated giant cell formation after 24 h of infection. The immunofluorescence of the S protein was readily visible 2 h after Zhejing08 infection. Initially, these were distributed in the plasma membrane, followed by distribution in the cytoplasm and a quick increase from 12 to 48 hpi. The fluorescence intensity was proportional to the virus inoculation dose (Fig. 2 A). Growth-kinetic studies showed that Zhejiang08 replicated rapidly and efficiently in Vero E6 cells with an almost logarithmic growth form from 6 h to 48 h, reaching a maximum titer of 106.8 PFU/ml by 48 hpi (Fig. 2B and C).
Fig. 2.
Infectivity of Zhejang08 in Vero E6 cells. (A) CPE of Zhejiang08 in Vero E6 cells. Scale bars represent 50 μm. (B) Immunofluorescence images of PEDV (green) infected Vero E6 cells at 2, 12, 24, and 48 h post-infection (hpi). The scale bars represent 20 μm. (C) Viral plaque assay titer and M gene expression of PEDV infected Vero E6 cells (MOI = 0.1) at different infection times. Data express means ± SD (n = 3). Expression of the M gene was determined via quantitative RT-PCR using GAPDH gene expression for comparison. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.2. Sequence analysis of Zhejiang08
The complete genome of the strain Zhejiang08 had a length of 27,952 nucleotides (nt), excluding the poly (A) tail. The genomic organization of Zhejiang08 was similar to other reported PEDVs with a characteristic gene order. The nt and aa sequence identities of different regions within the Zhejiang08 genome were compared to further 40 PEDV strains (Table S3).
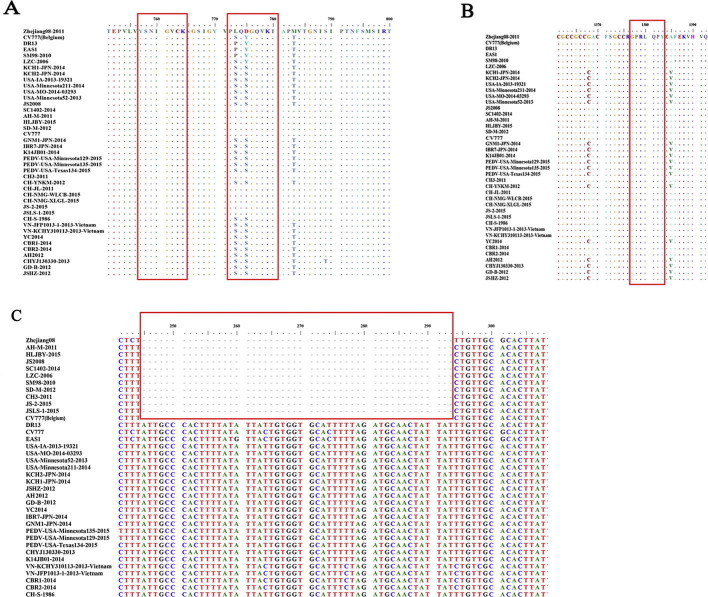
The nucleotide sequence of the S gene of the Zhejiang08 strain was 4149 nt in length, encoding a predicted protein that contained 1382 amino acids (aa). It displayed 92–99.9% aa identity with reference isolates and was most similar to the isolates SD-M-2012, AH-M-2011, SC1402-2014, and SQ2014 (99.6–99%) (Table S3). Phylogenetic analyses based on the S nucleotide and S aa revealed that Zhejiang08 belonged to group IIc, which also contained 12 Chinese domestic pandemic and cell adaption strains that had been reported since 2011 (Fig. 3 A and B). However, further analysis of the COE sequence of all selected strains showed that Zhejiang08 clustered in group Ia, sharing 95.9–100% sequence homology with most of the strains that have been isolated worldwide since the 2011 PEDV pandemic (Fig. 3C). In addition, deduced aa sequences from partial S gene analysis showed that there was no aa change in the known neutralizing epitopes aa 748–755 and 1368–1374 and merely two aa substitutions (S764L and S766D) in neutralizing epitopes aa 764–771 between the strains, regardless of their location in the phylogenetic tree (Fig. S1 A and B). The accessory gene ORF3 was located between the structural genes for S and E, which has been suggested to be a cell marker of PEDV adaptation and attenuation [20], [21]. Zhejiang08, HLJBY, SD-M, and SM98 shared a deletion of 49 nt from nt 245 to 295, causing a shift of the ORF3 reading frame (Fig. S1 C).
Fig. 3.
Phylogenetic tree of S (nt) (A), S (aa) with corresponding distribution chart of glycosites (B) and COE aa sequence of the S protein (C). Phylogenetic analysis was performed, utilizing the neighbor-joining method and bootstrapping for 1000 replicates with a value >70%, to determine the percentage reliability of each internal node. The sequence of the Zhejiang08 strain was indicated by a red circle. The distribution charts of glycosites were colored according to the potential score of the N-Glyc prediction result, green (+), red (++), and blue (+++) color represent “Potential > 0.5”, “Potential > 0.5 AND Jury agreement (9/9) OR Potential > 0.75”, and “Potential > 0.75 AND Jury agreement”, respectively. Any potential crossing of the default threshold of 0.5 represents a predicted glycosylation site (as long as it occurred within the required sequon Asn-Xaa-Ser/Thr without Proline at Xaa). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.3. N-glycan glycosylation site analysis of PEDV
According to the predicted results, the total number of potential N-glycan glycosylation sites of 41 strains ranged from 19 to 24. Compared to the vaccine strain CV777 and the virulent strain AH2012, one (positions 297) and three (position 297, 378, and 1258) potential glycosylation sites of Zhejiang08 were missing. As previously established, changes in glycosylation can affect the interaction with receptors and cause a virus to become either more or less recognizable by the host immune cells, thus impacting viral replication and infectivity. We modeled the structure of the S protein of PEDV strains, as well as the spatial position of n-glycosylation sites. The additional n-glycosylation sites were used as the centre of a circle with a radius of 1 nm (Fig. 1, amino acid area may be covered by n-glycosylation sites, as marked), which almost located on the aa sequence between positions 249 and 529 of the S protein.
3.4. Zhejiang08 induced antibody neutralization and active immunity effects in swine
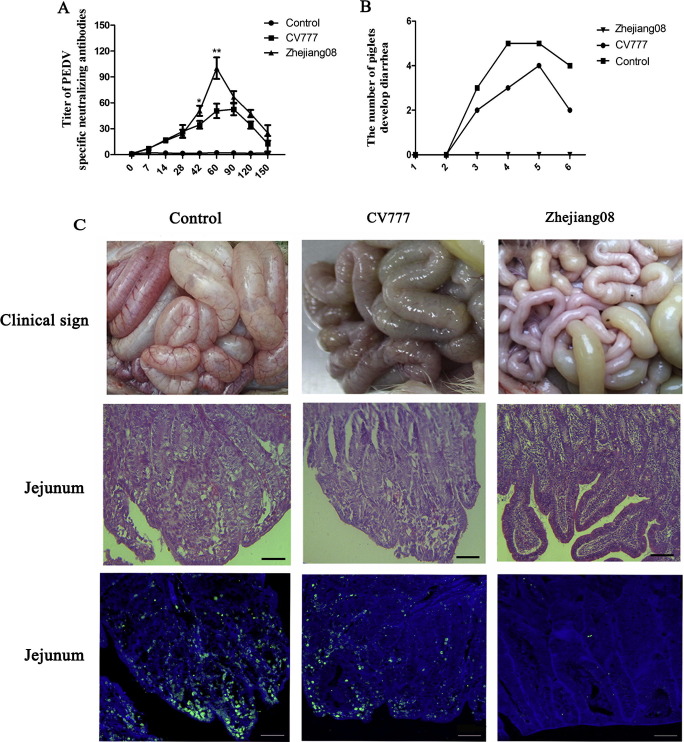
The results of PEDV-neutralizing antibody tests revealed that the antibody neutralizing levels of the Zhejiang08 strain immunized groups increased gradually, beginning at day seven after the first immunization, and still maintaining high levels 120 DPI. The neutralizing antibody titer in serum samples of the group that was immunized with the Zhejiang08 PEDV strain was significantly higher than that of CV777 groups (P < 0.01 or P < 0.05, Fig. 4 A) during the interval from day 42 to day 120. Subsequent to virulent PEDV challenge, diarrhea appeared on day 3 in the PBS pre-treatment group, peaking on days 4 and 5 (when 5 piglets had developed diarrhea). Moreover, the CV777 immunized group showed a decreased number of diarrhea piglets, but failed to provide complete protection. However, no obvious clinical symptoms were detected in the Zhejiang08 immunized groups (Fig. 4B).
Fig. 4.
Detection of neutralizing antibodies and immune protective efficiency in piglets during virus exposure. (A) Serum collected from piglets 0, 7, 14, 28, 42, 60, 120, and 150 days after first immunization were analyzed with PEDV-specific neutralizing antibodies via plaque assay. Error bars represent standard deviations. (B) Number of piglets treated with PBS, CV777, and Zhejiang08 that developed diarrhea from 1 to 6 days after high virulent PEDV challenging. (C) Clinical sign, hematoxylin-eosin, and immunofluorescence staining of small intestines in all three groups. The scale bar represents 50 μm.
Clinical assessment showed that piglets remained active and fleshy prior to inoculation with normal fecal consistency on DPI 1. Two PBS treated piglets displayed mild lethargy and anorexia starting on DPI 2. Then, the next day, half of the pigs in the PBS treatment group and a third of the CV777 treatment group developed watery feces. Lethargy, anorexia, and a progression to watery diarrhea were most severe on DPIs 4 and 5 with a gradual decline thereafter. However, the Zhejiang08 treatment group did not show any clinical symptoms or diarrhea during the entire experiment. Table 1 summarizes the progression of clinical signs throughout the study. PEDV-associated mortality did not occur in this study.
Table 1.
Summary of clinical signs and fecal consistency per group by days post-inoculation(DPI)in piglets of each group.
| DPI | Clinical observations |
Fecal consistency |
||||
|---|---|---|---|---|---|---|
| PBS | CV777 | Zhejiang08 | PBS | CV777 | Zhejiang08 | |
| 1 | 6a (Normal)b | 6 (Normal) | 6 (Normal) | 6a (−)c | 6 (−) | 6 (−) |
| 2 | 2 (Lethargy and anorexia); 4 (Normal) | 1 (Anorexia); 5 (Normal) | 6 (Normal) | 3 (+); 3 (−) | 1 (+); 5 (−) | 6 (−) |
| 3 | 5 (Lethargy, anorexia and gaunt); 1 (Normal) | 3 (Lethargy, anorexia and gaunt); 3 (Normal) | 6 (Normal) | 3 (++); 2 (+); 1(−) | 2 (++); 2 (+); 2 (−) | 6 (−) |
| 4 | 6 (Lethargy, anorexia and gaunt) | 3 (Lethargy, anorexia and gaunt); 3 (Normal) | 6 (Normal) | 5 (++); 1 (+) | 3 (++); 3 (+) | 2 (+); 4 (−) |
| 5 | 5 (Lethargy, anorexia and gaunt); 1 (Normal) | 4 (Lethargy, anorexia and gaunt); 2 (Normal) | 6 (Normal) | 5 (++); 1 (+) | 4 (++); 2 (+) | 1 (+); 5 (−) |
| 6 | 4 (Lethargy, anorexia and gaunt); 2 (Normal) | 2 (Lethargy, anorexia and gaunt); 4 (Normal) | 6 (Normal) | 4 (++); 2(+) | 2 (++); 2 (+) | 6 (−) |
The number of piglets.
Clinical signs.
Fecal score.
Fecal shedding of PEDV was not detected in rectal swabs of all tested piglets prior to virulent PEDV inoculation. All piglets in the PBS treated group tested positive for PEDV RNA from DPI 3 to 6 with a maximum mean CT value of 15.8 on DPI 5. PEDV RNA was detected in fecal swab samples of four piglets in the CV777 immunized group and was lower than in piglets from the PBS treated group. However, PEDV RNA was sporadically detected and presented extremely low levels of RNA levels in the Zhejian08 treated group (Table S4). After sacrificing all piglets, small intestine samples from both the PBS and CV777 treatment groups revealed prominent changes, characterized by thinner intestine mucosa with increasing transparency, mucosa swelling, and congesting. Microscopic examinations of the jejunum of diarrhea piglets from the PBS and CV777 treatment groups revealed multifocal to diffuse villous atrophy, swelling, sloughing, attenuation villous, and fusion of numerous villi. Furthermore, such pathological changes were not observed during the examination of Zhejiang08 treatment group samples. A large amount of immunofluorescence-stained PEDV positive cells was detected in the jejunum of diarrhea piglets from both PBS and CV777 treatment groups. PEDV antigens were mainly observed in the cytoplasm of villi epithelial cells (Fig. 4C).
3.5. Analysis of maturation alteration of DCs treated via CV777 and Zhejiang08 in vitro
As shown in Fig. 5 , immature DCs were either semi-suspended or suspended with a round appearance and dendritic processes (Fig. 5A left). Immature DCs that had been stimulated with LPS for 24 h typically expressed a DC-like morphology, including a significantly larger cell body and irregular shape (Fig. 5A right). Flow cytometry analysis revealed that Zhejiang08 uptake by DCs was higher compared to that of the CV777 group (Fig. 5B). Then, we assessed the phenotypic maturation marker of DCs via flow cytometry. Expressions of CD1a, SWC3a, and CD86 were remarkably up-regulated in the Zhejiang08 group compared to both CV777 and control (Fig. 5C). Mixed leukocyte reaction was used to determine whether the ability of PEDV-treated DCs to stimulate T-cell proliferation had been influenced in vitro. Our study revealed that Zhejiang08-treated DCs had a stronger ability to activate T-cells at a DC/T cell ratio of 1:1 (Fig. 5D). In addition, our results showed that DCs treated with Zhejiang08 had significant levels of mRNA expression of IL-12 and IFN-γ compared to both the CV777 and the control group, while no change could be detected in IL-10 secretion (Fig. 5E).
Fig. 5.
Expression of surface markers and cytokine production in DCs treated with different PEDV strains. (A) Morphology of immature (left) and mature Mo-DCs (right) analyzed via microscopy. The scale bar represents 50 μm. (B) PEDV samples of DCs were determined via FACS. (C) Differently treated DCs were stained for indicated surface molecules and analyzed via FACS. The mean fluorescence intensity (MFI) values of indicated surface molecules are shown as mean ± SD values. (D) In mixed lymphocyte reaction (MLR) experiments, Zhejiang08 or CV777 treated DCs were co-cultured with CFSE-labeled T lymphocytes (at ratios 1:1 and 1:5). DCs were stimulated with LPS or remained unstimulated, and were then co-cultured with T lymphocytes either as positive or control. After five days, proliferation was detected via FACS. (E) Cytokine expression levels of DCs after PEDV exposure at 24 hpi. The mRNA expression levels of IL-10, IL-12, and IFN-γ were detected via real-time quantitative PCR (RT-qPCR). All data shown are means ± SD of three samples. *P < 0.05; **P < 0.01. The error bars represent standard deviations.
4. Discussion
The isolation and attenuation of pandemic PEDV strains has been an urgent issue for PEDV control. In this study, we successfully isolated Zhejiang08 after 105 passages in Vero E6 cells and the virus was successfully attenuated through piglet challenge experiments (data not shown). Growth characteristics, including the cytopathic effect, infectious titer, and growth kinetics, indicated that the isolate possessed typical characteristics of PEDV.
Subsequently, we evaluated the immune protective potential of these attenuated strains. In our study, Zhejiang08 elicited stronger specific immunization and production of neutralizing antibodies than the CV777 vaccine. Moreover, Zhejiang08 isolates were capable of protecting piglets against diarrhea and severe disease processes following viral challenge, which also revealed higher immune protection efficiency compared to the CV777 vaccinated group.
The ability of promoting DC function for cellular maturation, induce lymphocyte cell proliferation, pro-inflammatory cytokine secretion, and antigen presentation is necessary to assess the potential of antigen initiation for both innate and adaptive immune responses [22], [23], [24], [25]. We demonstrated that the Zhejiang08 strain was a stronger and more potent inducer of DC maturation and mRNA expression of IL-12 and IFN-γ in DCs than CV777. IL-12 plays an important role in the differentiation of naive CD4+ T-cells into IFN-γ-producing TH1-type cells. IFN-γ helps to direct the immune response and to regulate the immune network, causing an antiviral effect [26], [27], [28]. Furthermore, Zhejiang08 treated DCs also exhibited a stronger stimulating effect on T lymphocyte cells. Finally, it is worth noting that the Zhejiang08 treatment group showed a higher virus load than the CV777 group despite identical MOI, illustrating that the Zhejiang08 virus was easier identified and captured by DCs.
The S protein of PEDV is closely associated with receptor binding and neutralizing antibodies, induced in the natural host [29]. Phylogenetic analysis of all 40 isolates based on S-gene nucleotides and deduced aa indicated that Zhejiang08 isolates clustered with several of the PEDV epidemic strains of recent years and most of attenuated vaccine isolates; however, the neutralizing epitopes in the S protein of the strains were highly homologous compared to all selected isolates. In addition, it is worth noting that the high homology of the COE epitope between Zhejiang08 and most of the prevailing strains isolated since the 2011 PEDV pandemic were clustered into one independent big group, thus revealing Zhejiang08 as a good candidate for vaccination.
As the most important post-translational modification of the PEDV spike protein, the main feature of N-linked glycosylation was the attachment of a high mannose core to the amide nitrogen of asparagine in the context of the conserved Asn-X-Ser/Thr motif [13]. Via comparison and analysis of the NetNGlyc predicted results of the S protein and compared to Zhejiang08, we found that three and one additional glycosylation sites may have formed in the S proteins of AH2012 and CV777, respectively. Our results revealed that glycan has the ability to form a barrier, thus shielding the antigen of a potential virus and neutralizing epitopes, and influencing the identification and immune response of downstream elements [9]. To further analyze the potential influence on the immunogenicity of the S protein by glycosylation sites, we compared the spatial position of n-glycosylation sites via the homology modeling method. However, to our surprise, we found that two extra aa n-glycosylation sites ASN300 and ASN381 completely covered the sequence between positions 249 and 529 of the AH2012 S protein. Furthermore, the site ASN296 in the S protein of CV777 also proved to be partially hidden in this region. A previous study has reported a potential porcine aminopeptidase N (pAPN) binding site in this region of the PEDV S protein [30], [31], [32]. It has been reported that PEDV uses porcine APN as its receptor, consequently enabling the virus to enter into host cells and to recognize immune cells, which is initiated via binding of the S protein to this cellular receptor [33]. Therefore, we suggest that the difference of the immune efficiency between CV777 and Zhejiang08 could partly be attributed to epitope exposure and cell binding, influenced by additional glycosylation sites. Moreover, in AH2012 and other highly virulent viruses that have emerged since 2011, the two additional glycosylation sites were likely acquired by selective pressure, thus providing a more complete shielding to this region, which may be associated with viral escape from the immune system. However, future studies could involve the generation of specific mutants, using reverse genetics to validate our suggestion.
In summary, our study revealed that the cell attenuated PEDV virus Zhejiang08 displayed effective immune protection in piglets, which could be attribute to its strong ability to stimulate DCs and activate T-cell proliferation. The strain is a promising vaccine candidate and can assist in the development of specific and directed vaccine protection strategies against PEDV in pig farms.
Acknowledgments
We very much appreciate the support of the Veterinary Medicine Research Centre of the Beijing Da Bei Nong Group. This work was supported by grant # 31372465 from the National Science Grant of China and PAPD from the support program of Jiangsu province.
Acknowledgments
Competing financial interests
The authors declare no competing financial interests.
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.vaccine.2017.10.052.
Appendix A. Supplementary material
Supplementary Fig. S1.
Alignment of the aa sequences of the Zhejiang08 S protein and the non-structural gene ORF3 with other strains in our study. Red boxes indicate neutralizing epitopes (A, 748–755; SS2, 764-771; SS6) and (B, 1368-1374; 2C10). (C) A 49 nt deletion between nt 245 and 295 of ORF3 was found in Zhejiang08 as well as in several other relevant strains.
References
- 1.Chasey D., Cartwright S.F. Virus-like particles associated with porcine epidemic diarrhoea. Res Veter Sci. 1978;25:255–256. doi: 10.1016/S0034-5288(18)32994-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sun D., Wang X., Wei S., Chen J., Feng L. Epidemiology and vaccine of porcine epidemic diarrhea virus in China: a mini-review. J Veter Med Sci/Jpn Soc Veter Sci. 2016;78:355–363. doi: 10.1292/jvms.15-0446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vlasova A.N., Marthaler D., Wang Q., Culhane M.R., Rossow K.D., Rovira A. Distinct characteristics and complex evolution of PEDV strains, North America, May 2013-February 2014. Emerg Infect Dis. 2014;20:1620–1628. doi: 10.3201/eid2010.140491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Song D.S., Oh J.S., Kang B.K., Yang J.S., Moon H.J., Yoo H.S. Oral efficacy of Vero cell attenuated porcine epidemic diarrhea virus DR13 strain. Res Veter Sci. 2007;82:134–140. doi: 10.1016/j.rvsc.2006.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sato T., Takeyama N., Katsumata A., Tuchiya K., Kodama T., Kusanagi K. Mutations in the spike gene of porcine epidemic diarrhea virus associated with growth adaptation in vitro and attenuation of virulence in vivo. Virus Genes. 2011;43:72–78. doi: 10.1007/s11262-011-0617-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park S.J., Kim H.K., Song D.S., An D.J., Park B.K. Complete genome sequences of a Korean virulent porcine epidemic diarrhea virus and its attenuated counterpart. J Virol. 2012;86:5964. doi: 10.1128/JVI.00557-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li W., Li H., Liu Y., Pan Y., Deng F., Song Y. New variants of porcine epidemic diarrhea virus, China, 2011. Emerg Infect Dis. 2012;18:1350–1353. doi: 10.3201/eid1808.120002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song D., Moon H., Kang B. Porcine epidemic diarrhea: a review of current epidemiology and available vaccines. Clin Exp Vaccine Res. 2015;4:166–176. doi: 10.7774/cevr.2015.4.2.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abe Y., Takashita E., Sugawara K., Matsuzaki Y., Muraki Y., Hongo S. Effect of the addition of oligosaccharides on the biological activities and antigenicity of influenza A/H3N2 virus hemagglutinin. J Virol. 2004;78:9605–9611. doi: 10.1128/JVI.78.18.9605-9611.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goffard A., Callens N., Bartosch B., Wychowski C., Cosset F.L., Montpellier C. Role of N-linked glycans in the functions of hepatitis C virus envelope glycoproteins. J Virol. 2005;79:8400–8409. doi: 10.1128/JVI.79.13.8400-8409.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li J., Bhuvanakantham R., Howe J., Ng M.L. The glycosylation site in the envelope protein of West Nile virus (Sarafend) plays an important role in replication and maturation processes. J Gen Virol. 2006;87:613–622. doi: 10.1099/vir.0.81320-0. [DOI] [PubMed] [Google Scholar]
- 12.Sagar M., Wu X., Lee S., Overbaugh J. Human immunodeficiency virus type 1 V1–V2 envelope loop sequences expand and add glycosylation sites over the course of infection, and these modifications affect antibody neutralization sensitivity. J Virol. 2006;80:9586–9598. doi: 10.1128/JVI.00141-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vigerust D.J., Shepherd V.L. Virus glycosylation: role in virulence and immune interactions. Trends Microbiol. 2007;15:211–218. doi: 10.1016/j.tim.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haverson K., Singha S., Stokes C.R., Bailey M. Professional and non-professional antigen-presenting cells in the porcine small intestine. Immunology. 2000;101:492–500. doi: 10.1046/j.1365-2567.2000.00128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Banchereau J., Steinman R.M. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 16.Subramaniam S., Cao D., Tian D., Cao Q.M., Overend C., Yugo D.M. Efficient priming of CD4 T cells by Langerin-expressing dendritic cells targeted with porcine epidemic diarrhea virus spike protein domains in pigs. Virus Res. 2017;227:212–219. doi: 10.1016/j.virusres.2016.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kusanagi K., Kuwahara H., Katoh T., Nunoya T., Ishikawa Y., Samejima T. Isolation and serial propagation of porcine epidemic diarrhea virus in cell cultures and partial characterization of the isolate. J Veter Med Sci/Jpn Soc Veter Sci. 1992;54:313–318. doi: 10.1292/jvms.54.313. [DOI] [PubMed] [Google Scholar]
- 18.Walls A.C., Tortorici M.A., Frenz B., Snijder J., Li W., Rey F.A. Glycan shield and epitope masking of a coronavirus spike protein observed by cryo-electron microscopy. Nat Struct Mol Biol. 2016;23:899–905. doi: 10.1038/nsmb.3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chua A.J., Vituret C., Tan M.L., Gonzalez G., Boulanger P., Ng M.L. A novel platform for virus-like particle-display of flaviviral envelope domain III: induction of Dengue and West Nile virus neutralizing antibodies. Virol J. 2013;10:129. doi: 10.1186/1743-422X-10-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Madson D.M., Magstadt D.R., Arruda P.H., Hoang H., Sun D., Bower L.P. Pathogenesis of porcine epidemic diarrhea virus isolate (US/Iowa/18984/2013) in 3-week-old weaned pigs. Veter Microbiol. 2014;174:60–68. doi: 10.1016/j.vetmic.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 21.Mou C., Zhu L., Xing X., Lin J., Yang Q. Immune responses induced by recombinant Bacillus subtilis expressing the spike protein of transmissible gastroenteritis virus in pigs. Antivir Res. 2016;131:74–84. doi: 10.1016/j.antiviral.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 22.Zhou W., Peng Q., Li K., Sacks S.H. Role of dendritic cell synthesis of complement in the allospecific T cell response. Mol Immunol. 2007;44:57–63. doi: 10.1016/j.molimm.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 23.Varol C., Vallon-Eberhard A., Elinav E., Aychek T., Shapira Y., Luche H. Intestinal lamina propria dendritic cell subsets have different origin and functions. Immunity. 2009;31:502–512. doi: 10.1016/j.immuni.2009.06.025. [DOI] [PubMed] [Google Scholar]
- 24.Carrasco C.P., Rigden R.C., Vincent I.E., Balmelli C., Ceppi M., Bauhofer O. Interaction of classical swine fever virus with dendritic cells. J Gen Virol. 2004;85:1633–1641. doi: 10.1099/vir.0.19716-0. [DOI] [PubMed] [Google Scholar]
- 25.Zhao S., Gao Q., Qin T., Yin Y., Lin J., Yu Q. Effects of virulent and attenuated transmissible gastroenteritis virus on the ability of porcine dendritic cells to sample and present antigen. Veter Microbiol. 2014;171:74–86. doi: 10.1016/j.vetmic.2014.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shi X.J., Wang B., Zhang C., Wang M. Expressions of Bovine IFN-gamma and foot-and-mouth disease VP1 antigen in P. pastoris and their effects on mouse immune response to FMD antigens. Vaccine. 2006;24:82–89. doi: 10.1016/j.vaccine.2005.07.051. [DOI] [PubMed] [Google Scholar]
- 27.Tian J., Ma J., Ma K., Ma B., Tang X., Baidoo S.E. Up-regulation of GITRL on dendritic cells by WGP improves anti-tumor immunity in murine Lewis lung carcinoma. PloS One. 2012;7:e46936. doi: 10.1371/journal.pone.0046936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Y.P., Liu D., Guo L.J., Tang Q.H., Wei Y.W., Wu H.L. Enhanced protective immune response to PCV2 subunit vaccine by co-administration of recombinant porcine IFN-gamma in mice. Vaccine. 2013;31:833–838. doi: 10.1016/j.vaccine.2012.11.062. [DOI] [PubMed] [Google Scholar]
- 29.Duarte M., Laude H. Sequence of the spike protein of the porcine epidemic diarrhoea virus. J Gen Virol. 1994;75(Pt 5):1195–1200. doi: 10.1099/0022-1317-75-5-1195. [DOI] [PubMed] [Google Scholar]
- 30.Deng F., Ye G., Liu Q., Navid M.T., Zhong X., Li Y. Identification and comparison of receptor binding characteristics of the spike protein of two porcine epidemic diarrhea virus strains. Viruses. 2016;8:55. doi: 10.3390/v8030055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee DK. Kyungpook National University, Daegu, Republic of Korea. The N-terminal region of the porcine epidemic diarrhea virus spike protein is important for the receptor binding. Korean J Microbiol Biotechnol; 2011.
- 32.Sun D.B., Chen J.F., Shi H.Y., Shen S.C., Mao-Jie L.V., Fan X.P. Analysis of receptor binding domain on the s protein of porcine epidemic diarrhea virus. Chinese J Anim Veter Sci. 2009;40:528–532. [Google Scholar]
- 33.Kamau A.N., Park J.E., Park E.S., Yu J.E., Rho J., Shin H.J. Porcine amino peptidase N domain VII has critical role in binding and entry of porcine epidemic diarrhea virus. Virus Res. 2017;227:150–157. doi: 10.1016/j.virusres.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.