Abstract
The recombinant nucleocapsid (rN) protein of the coronavirus (CoV) responsible for severe acute respiratory syndrome (SARS) was cloned and expressed in Escherichia coli, extracted from cell lysates containing 6 M urea, then purified by Ni2+-affinity chromatography. In animal immunogenicity studies, we found that most anti-rN protein antibodies were IgG2a in BALB/c mice vaccinated with rN emulsified in Montanide ISA-51 containing the synthetic oligodeoxynucleotide, CpG. In contrast, anti-rN protein antibodies of mice immunized with rN protein in PBS were found to mainly be IgG1. These results indicated that ISA-51/CpG-formulated rN protein was dramatically biased toward a Th1 immune response. To identify the B-cell immunodominant epitopes of the rN protein in the mouse and monkey, the reactivities of antisera raised against purified rN proteins formulated in ISA-51/CpG were tested with a panel of overlapping synthetic peptides covering the entire N protein sequence. Three immunodominant linear B-cell epitope regions were mapped to residues 166–180, 356–375, and 396–410 of the rN protein. When the reactivities of these peptides were screened with human sera from five SARS patients, peptides corresponding to residues 156–175 reacted strongly with sera from two of the SARS patients. These results indicated that the region around residues 156–175 of the N protein is immunogenic in the mouse, monkey, and human. We found that peptides corresponding to residues 1–30, 86–100, 306–320, and 351–365 contained murine immunodominant T-cell epitopes. To identify functional CTL epitopes of the N protein, BALB/c mice were immunized with peptides containing the H-2Kd CTL motif emulsified in adjuvant ISA-51/CpG. Using an IFN-γ secretion cell assay and analysis by flow cytometry, peptides containing residues 81–95 were found to be capable of stimulating both CD4+ and CD8+ cell proliferation in vitro. We also only observed that peptides corresponding to residues 336–350 were capable of stimulating IFN-γ production in T-cell cultures derived from peripheral blood mononuclear cells (PBMCs) of macaques immunized with the rN protein emulsified in ISA/CpG adjuvant. Our current results together with those of others suggest that some immunodominant B-cell and T-cell epitopes are conserved in the mouse, monkey, and human. This information is very important for the development SARS diagnostic kits and a vaccine.
Abbreviations: SARS CoV, severe acute respiratory syndrome-associated coronavirus; rN, recombinant nucleocapsid; CTL, cytotoxic T lymphocyte; IFA, incomplete Freund's adjuvant; PBMCs, peripheral blood mononuclear cells
Keywords: SARS CoV, Epitopes, Vaccine, Nucleocapsid protein
1. Introduction
A newly emerging infectious disease caused by the severe acute respiratory syndrome-associated coronavirus (SARS CoV) had significant economic impacts in countries affected by the disease outbreak in 2003–2004. The lack of efficient treatments and prevention means the possibility exists for SARS to return in the future. In addition, high-risk populations such as laboratory and hospital workers need effective SARS vaccines for protection. Unfortunately, recent reports have shown that inactivated coronavirus vaccines in a cat model might worsen the disease rather than prevent it [1]. Furthermore, the SARS virus may cause mild liver inflammation in ferrets, and the damage was much more serious if animals were first given a candidate SARS vaccine based on the vaccinia virus [2]. Yang et al. [3] also found that antibodies against SARS CoV S protein enhanced the entry of virus in in vitro cell culture studies. Therefore, in order to generate safe and effective SARS vaccines, we urgently need greater understanding of the immunological responses of vaccine candidates in different animal models.
The N protein of the feline infectious peritonitis virus (FIPV) has been used as subunit vaccines to induce protective immunity and to prevent the progression of diseases in a cat model [4]. The literature indicates that immunization of DNA plasmids that encode the N protein of the Ebola virus and influenza virus elicits protective antigen-specific cytotoxic T lymphocyte (CTL) responses [5], [6], [7]. In avian coronaviruses, N protein-specific T-cell responses were shown to generate protective immunity [8]. These results indicate that a good N protein vaccine candidate should elicit strong cellular immune responses. The N protein of SARS CoV is 422 amino acids long and highly conserved (99%) within different isolates, but it shares only 20–30% amino acid homology with the N proteins of other coronaviruses [9]. In fact, the DNA vaccine candidate encoding the N protein of the SARS CoV fused with calreticulin (CRT) was shown to induce potent humoral and cellular immune responses in a mouse model, and inhibited replication of the recombinant vaccinia virus that expressed the N protein in vivo [10]. We therefore focused on the N protein of the SARS CoV as the target antigen for subunit vaccine development.
With an eye toward clinical applications, we selected two potential adjuvants in clinical used that have been shown to be strong cellular immune response inducers: Montanide ISA-51 and the immune-stimulating oligonucleotide, CpG [11]. ISA-51 and CpG were used to emulsify the rN protein as vaccine candidates for mouse and macaque immunogenicity studies. In the present study, we report that (1) ISA-51/CpG-formulated rN protein was dramatically biased toward the Th1 immune response; (2) the immunodominant B-and T-cell epitopes of the N protein were present in both BALB/c mice and macaques; (3) conserved immunodominant B-cell epitopes were identified in mice, macaques, and SARS patients; (4) N protein-specific CD8+ cells could be induced by ISA-51/CpG formulated with either the rN protein or synthetic peptides containing the H-2Kd CTL epitope motif.
2. Materials and methods
2.1. Production of rN protein
Recombinant SARS CoV N protein (rN) containing a N-terminal His tag gene was cloned into the pRSETA vector (Invitrogen, Carlsbad, CA, USA) for protein expression. The plasmid was transformed into an Escherichia coli BL21(DE3)Gold (Stratagene, Cedar Creek, TX, USA) host strain and incubated at 32 °C overnight. The expression of rN was induced with 0.5 mM of isopropyl β-d-thiogalactoside (IPTG) for 3 h, then cells were harvested by centrifugation (8000 × g for 20 min), and cell pellets were re-suspended in 100 ml of homogenate buffer (20 mM Tris–Cl (pH 8.0), 500 mM NaCl, 10% glycerol, 50 mM sucrose, and 10 mM imidazole). After disruption using a French Press (Constant Systems, Daventry, UK) at 186 Mpa, cell lysates were clarified by centrifugation (80,000 × g for 60 min). Most of the target protein was extracted from the pellet with 8 M urea in homogenate buffer and loaded into 1 ml of Ni-NTA resin (Qiagen, San Diego, CA, USA). The resin was washed with the same buffer, and the target protein was purified by changing the pH value. After further washing with pH 6.3 and 5.9 homogenate buffers and 8 M urea, the rN was eluted from the resin using pH 4.5 homogenate buffer and 8 M urea, then the purified rN was refolded by dialyzation against phosphate-buffered saline (PBS). The purified rN was stored at −20 °C in PBS with 10% glycerol for further studies.
2.2. SDS–PAGE and Western blot analysis
When analyzing samples by SDS–PAGE, 10 μl of samples were mixed with an equal volume of the sample buffer (63 mM Tris–HCl (pH 6.8), 2% SDS, 5% 2-mercaptoethanol, 10% glycerol, and 0.002% bromophenol blue) and heated in boiling water for 3 min. The samples (5–50 μg protein per lane) were separated on a 10% SDS–PAGE, then electrophoretically transferred from the gel to a polyvinylidene difluoride (PVDF) membrane (Millipore, Billerica, MA, USA) at 392 mA for 40 min. The membrane was blocked overnight with 5% non-fat milk in PBS containing 0.05% Tween-20 (PBST) at 4 °C. After washing with PBST, the blot was incubated at room temperature for 1 h with a mouse anti-His antibody (1:1500 dilution, Amersham Biosciences, New Territories, HK). After extensive washing with PBST then incubation for 1 h with horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG (1:5000, Bethyl Laboratories, Montgomery, TX, USA), the blot was developed with 0.2 mM DAB (3,3′-diaminobenzidine tetrahydrochloride, Sigma, St. Louis, MO, USA). After development for 2–3 min, the blot was washed with distilled water to stop the reaction.
2.3. Animals for immunogenicity studies
Six-to eight-week old female BALB/c mice were obtained from the National Laboratory Animal Breeding and Research Center (Taipei, Taiwan). All mice were housed at the Animal Technology Institute Taiwan (ATIT, Miaoli, Taiwan) animal facility. Monkeys (Formosan macaques, Macaca cyclopis) were maintained in accordance with institutional animal care protocols of the Center of Disease Control, Taipei, Taiwan. All of the animal studies were approved by the Animal Committee of the National Health Research Institutes, Taipei, Taiwan.
2.4. Peptide synthesis
Peptides derived from the SARS CoV N protein sequence were synthesized as 15-mer fragments with 10-amino-acid overlap that covered the entire N protein sequence of the Urbani strain (Kelowna, Taipei, Taiwan); peptides were dissolved in 5% DMSO at 2 mg/ml. Two CTL epitopes, QFKDNVILL and VWVATEGAL, produced from the N protein with the BABL/c (H-2Kd) mice class I binding motif, were predicted by a computer-based program on the web site of the BioInformatics and Molecular Analysis Section (Bethesda, MD, USA) [12].
2.5. Production of N protein-specific antisera
Mice were immunized subcutaneously with 50 μg of rN protein emulsified in incomplete Freund's adjuvant (IFA) or Montanide ISA-51 (Seppic, Paris, France) with 50 μg of the CpG oligonucleotide (TCG TCG TTT TGT CGT TTT GTC GTT TTG TCG TT). Mice received two booster doses of the immunogen, one each at 2 and 4 weeks later. Blood samples were collected every 2 weeks post-immunization. For T-cell analysis, mice were sacrificed at 7 days after the final immunization. In the pooled peptides groups, all peptides (Table 2) containing H-2Kd-restricted epitopes were emulsified in IFA or ISA-51 with the CpG oligonucleotide (ISA/CpG), and immunized subcutaneously. For the monkey experiments, the 200 μg of rN was emulsified in 0.5 ml of ISA/CpG, and multiple injections were given muscularly at 0, 4, and 8 weeks. Sera were collected at specified time points. Sera were obtained from clotted blood samples by centrifugation and were heat-inactivated at 56 °C for 30 min.
Table 2.
Amino acid sequences of the pooled peptides
| Peptide no. | Sequencea | Start position | MHC I peptide binding scoreb |
|---|---|---|---|
| 9 mer-1 | QFKDNVILL | 346 | 1382 |
| 9 mer-2 | VWVATEGAL | 132 | 576 |
| 17 | PDDQIGYYRRATRRV | 81 | 500 |
| 66 | TPSGTWLTYHGAIKL | 326 | 400 |
| 25 | SLPYGANKEGIVWVA | 121 | 345 |
| 63 | SASAFFGMSRIGMEV | 311 | 120 |
| 23 | FYYLGTGPEASLPYG | 111 | 120 |
| 32 | AATVLQLPQGTTLPK | 156 | 115 |
The bold letters represent the computer-predicted H-2Kd-restricted peptide on SARS CoV N protein.
The score of MHC class I binding peptides were predicted by a computer-based program on the web site of the BioInformatics and Molecular Analysis Section [12].
2.6. Serum specimens from SARS patients
Sera from five convalescent-phase humans were obtained from the Center of Disease Control, Taipei, Taiwan. Sera were collected 30–60 days after recovery from the disease based on the clinical diagnosis. All sera were inactivated following government guidelines before transfer to our laboratory.
2.7. Immunoassay
The presence of N protein-or peptide-specific antibodies in the sera was determined by ELISA. In briefly, 50 μl (10 μg/ml) of purified rN or peptides were coated in 96-well microtiter plates with 0.1 M carbonate buffer (pH 9.6) by overnight incubation at 4 °C. Coated plates were washed twice with PBST and then blocked with 5% non-fat milk in PBS at room temperature for 2 h. Diluted sera from immunized animals were applied to wells at room temperature for 2 h. Followed by HRP-conjugated goat anti-mouse IgG or HRP-conjugated goat anti-monkey IgG (Sigma, St. Louis, MO, USA), the assay was developed with 3,3′,5,5′-tetramethylbenzidine (TMB), and the reaction was stopped by adding 100 μl of 1 M H2SO4 per well. Plates were read at 450 nm using an ELISA plate reader (Molecular Devices, Sunnyvale, CA, USA). For antibody isotype analysis, biotin-conjugated rat anti-mouse IgG1 and IgG2a (BD Pharmingen, San Diego, CA, USA) were added to the wells for sera binding, then HRP-conjugated streptavidin was added and developed with the TMB substrate. The antibody titer was defined as the reciprocal of the highest dilution that produced an OD450-nm value of two-fold higher than the pre-immune sera.
2.8. IFN-γ Secretion cell staining and flow cytometric analysis
On day 7 after the final immunization, mice were sacrificed to harvest their spleens. Erythrocyte-depleted splenocytes (1.5 × 106 cells/well) in 96-well plates were cultured in vitro with 5 μg/ml of pooled peptide or individual peptide that covered all of the N protein amino acid plates for 5 h. IFN-γ-secreting cells were detected with a Mouse IFN-γ Secretion Assay Detection Kit (Miltenyi Biotec., Gladbach, Germany). In brief, 1 × 106 splenocytes were harvested, and labeled with an IFN-γ capture antibody for 5 min at 4 °C. Afterwards, cells were transferred into 37 °C warm medium for 45 min, then washed twice, and stained with phycoerythrin (PE)-conjugated IFN-γ detection reagent, and fluorescein isothiocyanate (FITC)-conjugated anti-CD8 or anti-CD4 antibodies. After washing, cells were collected and analyzed on a FACSCalibur instrument with CellQuest software. Dead cells were gated by 1 μg/ml propidium iodide.
2.9. Cytokine production assay
T-cell epitope mapping was performed in mice and monkeys immunized with purified rN (50 and 200 μg, respectively) emulsified in ISA-51 plus 50 μg of the CpG oligonucleotide. Seven days after the second boost, splenocytes from mice or peripheral blood mononuclear cells (PBMCs) from monkeys (4 × 105 cells/well) were cultured in RPMI 1640 (GIBCO, San Diego, CA, USA) supplemented with 10% heat-inactivated fetal calf serum, 100 U/ml of penicillin, 100 μg/ml of streptomycin, and 50 μM 2-mercaptoethanol with 5 μg/ml of pooled or individual peptides at 37 °C for 5 days. Concanavalin A (Con A) or medium alone served as the positive and background controls, respectively. The supernatant was harvested and assayed for cytokine production. Mouse IFN-γ was quantitated by ELISA using the matching anti-IFN-γ antibody set (R&D Systems, Minneapolis, MN, USA) in accordance with the manufacturer's directions. Monkey IFN-γ was measured using an ELISA kit from Biosource International (Camarillo, CA, USA) according to the manufacturer's protocol.
2.10. Statistical analysis
Data were evaluated using Student's t-test analysis of variance (ANOVA) to determine the statistical significance of differences between samples. p < 0.05 was considered statistically significant.
3. Results and discussion
3.1. N Protein as the target of the SARS candidate vaccine
The ability of certain individuals to overcome infection without treatment led to the hypothesis that administration of vaccines against SARS may provide effective protection against the disease. Like other viruses with spike proteins on the viral surface, coronaviruses have been shown to elicit enhancement of disease through non-neutralizing spike protein-specific antibodies [3], so vaccines that elicit T-cell immunity against SARS proteins are likely to receive more-favorable clinical attention. In animal coronavirus vaccine development, the N protein has been widely tested as an immunogen for protection against coronavirus infections. Inoculation of chickens with plasmid DNA encoding the infectious bronchitis virus (IBV) carboxyl terminus of the N protein led to the induction of a specific cytotoxic T lymphocyte population capable of recognizing two distinct IBV strains. In addition, the adoptive transfer of T cells from animals inoculated with IBV to naïve chicks provided CD8+ αβ specific protection [13]. A Th1 T-cell line resistant to the murine hepatitis virus (MHV) showed marked increases in IFN-γ expression with a decrease in IL-4 production when incubated with MHV-infected cells [14]. Infusion of this resistant cell line into susceptible mice led to complete protection against MHV infection. The immunity against MHV was demonstrated to be a process dependent upon CD8+, perforin, and IFN-γ in the absence of CD4+ T cells [15]. The generation of IgG2a antibodies following DNA immunization of N protein suggested that there was a significant Th1-type response [16]. With the strong potential protective immunity of N protein-based vaccines, we hypothesized that the SARS CoV N protein would elicit effective immune responses against SARS diseases.
3.2. Expression and purification of the recombinant nucleocapsid protein
To aid the downstream purification process, the C-terminal end of the N protein-coding region was tagged with six histidine residues. Expression of rN protein could be induced by adding 1 mM of isopropyl β-d-thiogalactoside (IPTG) into the E. coli cell culture, and the level of expression was observed using an SDS–PAGE analysis (Fig. 1 A, lanes 1 and 2). The electrophoretic position of the rN protein corresponded to the predicted size of 46 kDa. rN proteins were collected as pellets from cell lysates using centrifugation. rN proteins were purified using an IMAC (immobilized metal affinity chromatography) column and by a pH stepwise gradient containing 8 M urea (Fig. 1A, lanes 3–9). Purified rN proteins were refolded by dialysis against PBS and could be detected by blotting with an anti-His antibody (Fig. 1B). We obtained 4–5 mg of purified rN protein from 1 L of cell culture, and it represented a 20% overall yield.
Fig. 1.
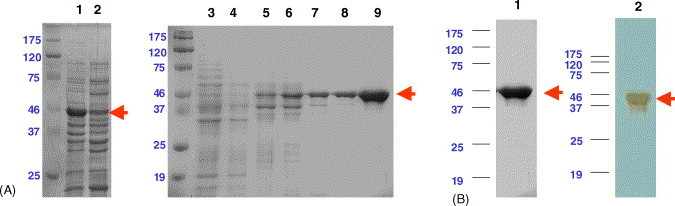
Expression and purification of the rN protein using SDS–PAGE and immunoblot analysis. (A) Coomassie blue-stained 10% reduced SDS–PAGE showing the rN protein purification process. Lane 1, cell lysates after IPTG induction; lane 2, cell lysate before IPTG induction; lane 3, flow-through fraction of crude extracts from the inclusion body fraction; lane 4, fraction washed at pH 8.0 with 8 M urea in homogenate buffer (see Section 2); lane 5, fraction washed at pH 6.3; lanes 6 and 7, fractions washed at pH 5.9; lanes 8 and 9, rN protein eluted at pH 4.5 with 8 M urea in homogenate buffer. (B) Lane 1, purified rN protein stained with Coomassie blue; lane 2, purified rN protein detected by blotting with anti-His antibody. The arrows indicate the electrophoretic mobility of the rN protein.
3.3. Immunogenicity of rN protein in mice
As mentioned above, a strong adjuvant that elicits strong cellular immune responses is necessary for the N protein to be effective as a vaccine candidate. Different adjuvant formulations induced different immune responses in immunized animals. To assess the immune responses elicited by the rN protein in our BALB/c mouse model, rN protein was formulated with either incomplete Freund's adjuvant (IFA), a potent adjuvant complex, ISA/CpG, or PBS alone. After three immunizations (50 μg of rN protein per dose), the N protein-specific antibody responses were measured using ELISA, and results are shown in Fig. 2 A. The antibody titers were found to be 8 × 10−8, 5 × 10−7, and 5 × 10−6 in the ISA/CpG, IFA, and PBS groups, respectively. To test whether the strong immune response elicited by the rN protein formulated with ISA/CpG was useful in clinical application, we analyzed the subtypes of reactive antibodies from all groups. The isotypes generated in different groups are shown in Fig. 2B; most of the antibodies were IgG1 found in the PBS group (1.0 × 10−5), with less IgG2a (1.0 × 10−4). There were similar IgG1 titers found in both the IFA and ISA/CpG groups (3 × 10−6). Not surprisingly, the ISA/CpG group generated strong and dominant IgG2a antibodies (1 × 10−6). But the IFA group produced less IgG2a (6 × 10−5). This result indicated that ISA/CpG could drive the immune response toward Th1, and is consistent with previous results which showed that the synthetic oligodeoxynucleotide CpG motif could skew the host's immune response toward Th1 [17] and the combination of CpG and ISA-51 also showed the ability to enhance the protective efficacy of a subunit malaria vaccine candidate [11].
Fig. 2.
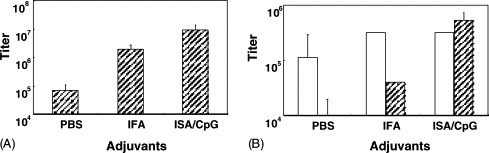
Effects of adjuvant on anti-N protein antibody titers. Mice were immunized with 50 μg of the rN protein in PBS, IFA, or ISA/CpG (see Section 2) subcutaneously. Serum of each mouse was collected at 2 weeks after the second boost and measured with ELISA for detection of N protein-specific antibody titers. (A) Total IgG antibody titers and (B) the IgG2a/IgG1 ratio in each group. Pre-immunization titers were subtracted from the post-immunization titers. Data are presented as the mean ± S.D. from seven mice in each group.
3.4. Identification of the immunodominant linear B-cell epitopes of NP
Identification of the immunodominant B cell epitopes could be useful for future immunological investigation. To identify B-cell epitopes of the N protein, mice were immunized with rN protein formulated with either IFA or ISA/CpG. To systemically analyze the B-cell epitopes, each of the peptide pools (NP1–NP8), consisting of 10 consecutive 15-mer overlapping peptides of the N protein, was used to screen against anti-N antisera (see Table 1 ). In this peptide-ELISA assay, we used the rN protein as the positive control and internal standard to normalize the reactivity. Since we desired to determine the immunodominant epitopes, mouse sera were diluted to 1:5000 for epitope screening. When mouse antisera were generated from the group immunized with rN protein formulated with IFA, we found two major reactive peptides pools to be NP1 and NP8. Further individual peptide reactivity analysis showed that peptides no. 8, 72, 73, 78, and 80 contained immunodominant epitopes (Fig. 3 A). In the ISA/CpG-immunized mouse group, NP4 and NP8 were the two major reactive peptide pools. With further individual peptide screening, we found the major reactive peptides to be nos. 34, 72, 73, 78, and 80 (Fig. 3A). Regardless of which adjuvants were used, the results showed that BALB/c mouse immunodominant B-cell epitopes were located at the C-terminal regions (peptides no. 72, 73, 78, and 80). However, sera from BALB/c mice immunized with β-propiolactone-inactivated SARS virus recognized the N-terminal region (residues 76–101) of the N protein [18]. These different results could have been due to the nature of the antigens (refolded rN protein versus the inactivated SARS virus) and adjuvants used.
Table 1.
Amino acid sequence of overlapping 15-mer peptides of the N protein of the SARS CoV
| Peptide number | 15-mer Amino acid sequence | Pool | Start position |
|---|---|---|---|
| 1 | MSDNGPQSNQRSAPR | 1 | 1 |
| 2 | PQSNQRSAPRITFGG | 1 | 6 |
| 3 | RSAPRITFGGPTDST | 1 | 11 |
| 4 | ITFGGPTDSTDNNQN | 1 | 16 |
| 5 | PTDSTDNNQNGGRNG | 1 | 21 |
| 6 | DNNQNGGRNGARPKQ | 1 | 26 |
| 7 | GGRNGARPKQRRPQG | 1 | 31 |
| 8 | ARPKQRRPQGLPNNT | 1 | 36 |
| 9 | RRPQGLPNNTASWFT | 1 | 41 |
| 10 | LPNNTASWFTALTQH | 1 | 46 |
| 11 | ASWFTALTQHGKEEL | 2 | 51 |
| 12 | ALTQHGKEELRFPRG | 2 | 56 |
| 13 | GKEELRFPRGQGVPI | 2 | 61 |
| 14 | RFPRGQGVPINTNSG | 2 | 66 |
| 15 | QGVPINTNSGPDDQI | 2 | 71 |
| 16 | NTNSGPDDQIGYYRR | 2 | 76 |
| 17 | PDDQIGYYRRATRRV | 2 | 81 |
| 18 | GYYRRATRRVRGGDG | 2 | 86 |
| 19 | ATRRVRGGDGKMKEL | 2 | 91 |
| 20 | RGGDGKMKELSPRWY | 2 | 96 |
| 21 | KMKELSPRWYFYYLG | 3 | 101 |
| 22 | SPRWYFYYLGTGPEA | 3 | 106 |
| 23 | FYYLGTGPEASLPYG | 3 | 111 |
| 24 | TGPEASLPYGANKEG | 3 | 116 |
| 25 | SLPYGANKEGIVWVA | 3 | 121 |
| 26 | ANKEGIVWVATEGAL | 3 | 126 |
| 27 | IVWVATEGALNTPKD | 3 | 131 |
| 28 | TEGALNTPKDHIGTR | 3 | 136 |
| 29 | NTPKDHIGTRNPNNN | 3 | 141 |
| 30 | HIGTRNPNNNAATVL | 3 | 146 |
| 31 | NPNNNAATVLQLPQG | 4 | 151 |
| 32 | AATVLQLPQGTTLPK | 4 | 156 |
| 33 | QLPQGTTLPKGFYAE | 4 | 161 |
| 34 | TTLPKGFYAEGSRGG | 4 | 166 |
| 35 | GFYAEGSRGGSQASS | 4 | 171 |
| 36 | GSRGGSQASSRSSSR | 4 | 176 |
| 37 | SQASSRSSSRSRGNS | 4 | 181 |
| 38 | RSSSRSRGNSRNSTP | 4 | 186 |
| 39 | SRGNSRNSTPGSSRG | 4 | 191 |
| 40 | RNSTPGSSRGNSPAR | 4 | 196 |
| 41 | GSSRGNSPARMASGG | 5 | 201 |
| 42 | NSPARMASGGGETAL | 5 | 206 |
| 43 | MASGGGETALALLLL | 5 | 211 |
| 44 | GETALALLLLDRLNQ | 5 | 216 |
| 45 | ALLLLDRLNQLESKV | 5 | 221 |
| 46 | DRLNQLESKVSGKGQ | 5 | 226 |
| 47 | LESKVSGKGQQQQGQ | 5 | 231 |
| 48 | SGKGQQQQGQTVTKK | 5 | 236 |
| 49 | QQQGQTVTKKSAAEA | 5 | 241 |
| 50 | TVTKKSAAEASKKPR | 5 | 246 |
| 51 | SAAEASKKPRQKRTA | 6 | 251 |
| 52 | SKKPRQKRTATKQYN | 6 | 256 |
| 53 | QKRTATKQYNVTQAF | 6 | 261 |
| 54 | TKQYNVTQAFGRRGP | 6 | 266 |
| 55 | VTQAFGRRGPEQTQG | 6 | 271 |
| 56 | GRRGPEQTQGNFGDQ | 6 | 276 |
| 57 | EQTQGNFGDQDLIRQ | 6 | 281 |
| 58 | NFGDQDLIRQGTDYK | 6 | 286 |
| 59 | DLIRQGTDYKHWPQI | 6 | 291 |
| 60 | GTDYKHWPQIAQFAP | 6 | 296 |
| 61 | HWPQIAQFAPSASAF | 7 | 301 |
| 62 | AQFAPSASAFFGMSR | 7 | 306 |
| 63 | SASAFFGMSRIGMEV | 7 | 311 |
| 64 | FGMSRIGMEVTPSGT | 7 | 316 |
| 65 | IGMEVTPSGTWLTYH | 7 | 321 |
| 66 | TPSGTWLTYHGAIKL | 7 | 326 |
| 67 | WLTYHGAIKLDDKDP | 7 | 331 |
| 68 | GAIKLDDKDPQFKDN | 7 | 336 |
| 69 | DDKDPQFKDNVILLN | 7 | 341 |
| 70 | QFKDNVILLNKHIDA | 7 | 346 |
| 71 | VILLNKHIDAYKTFP | 8 | 351 |
| 72 | KHIDAYKTFPPTEPK | 8 | 356 |
| 73 | YKTFPPTEPKKDKKK | 8 | 361 |
| 74 | PTEPKKDKKKKTDEA | 8 | 366 |
| 75 | KDKKKKTDEAQPLPQ | 8 | 371 |
| 76 | KTDEAQPLPQRQKKQ | 8 | 376 |
| 77 | QPLPQRQKKQPTVTL | 8 | 381 |
| 78 | RQKKQPTVTLLPAAD | 8 | 386 |
| 79 | PTVTLLPAADMDDFS | 8 | 391 |
| 80 | LPAADMDDFSRQLQN | 8 | 396 |
Fig. 3.
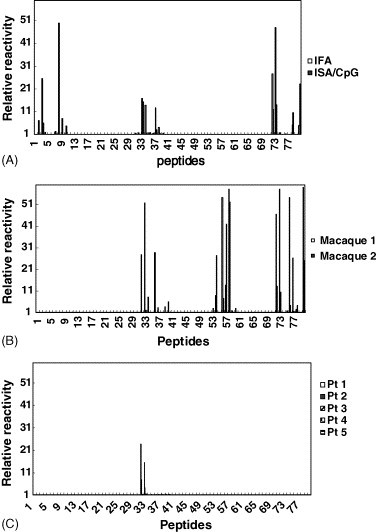
Immunodominant B-cell epitopes of the SARS CoV N protein in sera from mice, macaques, and SARS patients. (A) Mice; (B) macaques; (C) SARS patients. The eighty 15-mer overlapping peptides that cover the entire sequence of the SARS CoV N protein were used in an ELISA to measure the immunodominant linear B-cell epitopes. The “relative reactivity” of each peptide with the tested sera was calculated using the mean OD of each peptide divided by the mean OD of peptide no. 1. Samples with ≥5 times the “relative reactivity” were considered to be positive.
Since the rN protein formulated in ISA/CpG provided the best immune responses in the mouse model, we selected this adjuvant for the macaque immunogenicity studies. Sera from two immunized macaques both had about a 10−6 reactivity titer against the rN protein according to ELISA (data not shown). For immunodominant B-cell epitope identification, macaque sera were diluted 1:200 to screen the reactive peptide pools. Serum from monkey no. 1 recognized the peptide pools NP4, NP6, and NP8. Serum from monkey no. 2 reacted with peptide pools NP6 and NP8. To further identify the reactive peptides, we found that peptides no. 54 and 58 contained the immunodominant B-cell epitopes in peptide pool NP6, since they were recognized by the antisera from both macaques. Also peptides no. 72, 73, and 80 in NP8 were found to contain macaque immunodominant B-cell epitopes. Peptides no. 32–34 and 36 in peptide pool NP4 were shown to be most reactive with serum from monkey no. 1 (Fig. 3B). From the results described above, the conserved immunodominant B-cell epitopes in mice and monkeys were located within peptides no. 34 (residues 166–180), 72 (residues 351–365), 73 (residues 356–370), and 80 (residues 396–410).
We further studied the B-cell epitopes of the N protein which recognized SARS patients’ sera. When patients’ sera were diluted at 1:500 and used to screen the reactivity with the peptide pools, we found that only two patients’ sera reacted with peptide pool NP4 and purified NP (data not shown) out of five randomly selected patients’ sera. With further individual peptide screening, the major reactive peptides were found to be peptides no. 32 and 33 that corresponded to residues 156–175 (Fig. 3C). These results were consistent with previous reports by Shichijo et al. [19], He et al. [18], and Guo et al. [20] who also found that the region around residues 156–175 reacted with sera from more than 50% of SARS patients.
We summarized all these data in Fig. 4 , which shows that immunodominant B-cell epitopes corresponding to residues 356–375 and 396–410 were conserved in mice and macaques. Interestingly, the region corresponding to residues 156–175 was found to contain B-cell epitopes in all three species. This region (residues 156–175) will be useful for diagnostic kit development and as a biomarker for vaccine development as well.
Fig. 4.
Diagrammatic representation of the immunodominant linear B-cell and T-cell epitopes of the N protein in mice, macaques, and humans.
3.5. Identification of immunodominant T-cell epitopes
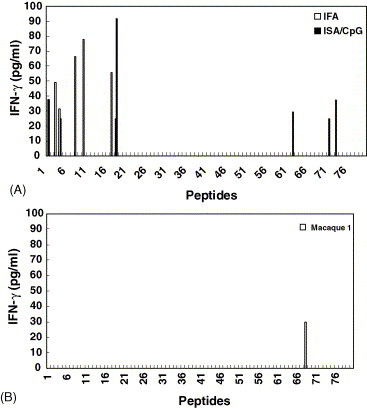
Murine T-cell epitopes were identified using the in vitro stimulatory effects of overlapping peptides on T cells derived from splenocytes of BALB/c mice immunized with the rN protein formulated with either IFA or ISA/CpG adjuvants. The amounts of secreted IFN-γ in culture supernatants were determined by IFN-γ-specific ELISA kits. No IFN-γ was detected in PBS, which served as the control group (data not shown). As shown in Fig. 5 A, IFN-γ production was observed in both IFA-and ISA/CpG-immunized groups, and several different peptides were found to be capable of stimulating IFN-γ secretion. The potential T-cell epitopes were found in the IFA group to correspond to residues 11–30, 36–60, 81–100, and 361–375. In the ISA/CpG group, the T-cell-reactive regions were identified to be residues 1–30, 86–110, 306–320, and 351–365. Only peptides no. 4 (residues 16–40) and 18 (residues 86–100) were found to stimulate IFN-γ production in both immunized groups. Although we could not determine the H-2Kd-restricted CTL epitope from natural SARS CoV infections, it seemed likely that residues 81–95 can be used as an indicator to study the T-cell response of N protein subunit vaccines. Interestingly, peptide no. 18 contains a potential BALB/c mouse CTL epitope, YYRRATRRV, identified by epitope prediction algorithms.
Fig. 5.
Immunodominant T-cell epitopes of the SARS CoV N protein in mice and macaques. Immunodominant T-cell epitopes were measured by an IFN-γ secretion assay. The isolated splenocytes from vaccinated mice (A) or peripheral blood mononuclear cells from a vaccinated macaque (B) were cultured with 15-mer individual peptides (5 μg/ml) at 37 °C for 5 days. Levels of IFN-γ in the supernatants were determined by an ELISA assay.
Macaque immunodominant T-cell epitope mapping was also performed with PBMCs isolated from two macaques (M1 and M2) vaccinated twice with the rN protein formulated in ISA/CpG. We found IFN-γ production only in the T-cell culture derived from the PBMCs of M1 (Fig. 5B). In further studies, we observed that only peptide no. 68 (residues 336–350, GAIKLDDKDPQFKDN) was capable of stimulating IFN-γ production in T-cell culture derived from PBMCs of monkey M1. This result could be important information for further SARS vaccine evaluation, especially in a macaque infection model. In our human immunodominant T-cell epitope identification studies (Des et al. published elsewhere), we also found that peptide no. 68 elicited responses from CD4- and CD8-positive cells derived from PBMCs of two SARS patients with the HLA-A11 class I haplotype. Our findings suggest that the rN protein formulated in ISA/CpG adjuvant can elicit T-cell immune responses, which may be important in the protective immunity against SARS diseases.
3.6. Identification of functional CD8+ T-cell epitopes of N protein
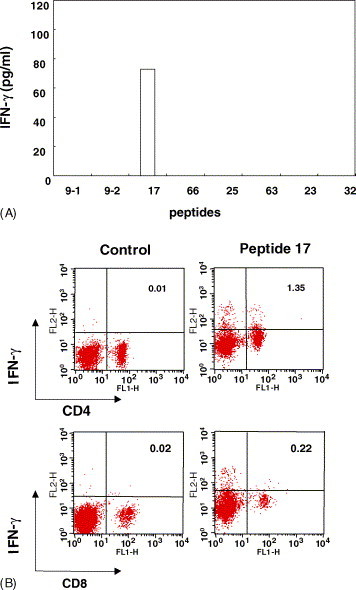
In addition to the B-cell and Th1-cell responses, the CTL may play an important role in virus-infected cell clearance. A computer-based program, BIMAS HLA Peptide Binding Prediction program [12], was used to predict candidate CTL peptides of N protein. Two 9-mer peptides (QFKDNVILL and VWVATEGAL) were identified and synthesized based on the highest score values (Table 2 ). These two peptides were mixed and formulated in ISA/CpG adjuvant with six other 15-mer peptides of N protein that were identified to consist of H2-Kd CTL-binding epitopes. At 7 days after the third immunizations, splenocytes of BALB/c mice were harvested, and various peptides were added to the splenocyte cultures for in vitro stimulation. After 5 days, cytokine secretions of IL-4 and IFN-γ were measured using a cytokine-specific ELISA. Only peptide no. 17 (PDDQIGYYRRATRRV) was observed to be capable of stimulating IFN-γ production (73 pg/ml) (Fig. 6 A). This result and the T-cell epitope mapping described above confirmed that YYRRATRRV is a very potent functional T-cell epitope.
Fig. 6.

Peptide-specific IFN-γ release in the peptide mixture of vaccinated BALB/c mice. (A) The isolated splenocytes at 1 × 106 in 24-well plates were stimulated with 5 μg/ml of each peptide. The numbers 9-1 and 9-2 represent the top two ranking nonamer peptides, respectively (see Table 2); the peptide order was based on the predicted score. (B) Positive wells cultured with corresponding peptides (2.5 μg/ml) at 37 °C for 7 days in the presence of 50 U/ml IL-2. Cultured cells were re-stimulated with the corresponding peptide at 37 °C for 4 h. IFN-γ-secreting CD4+ and CD8+ cells were analyzed by flow cytometry.
In order to analyze the reactive T-cell subsets, we re-stimulated splenocytes with peptide no. 17 in the presence of 50 U/ml recombinant IL-2. After 5 h, two-color flow cytometric analysis of T-cell subsets and IFN-γ staining revealed that the T-cell population was only reactive to peptide no. 17. Concentrations of CD4+/IFN-γ+ and CD8+/IFN-γ+ cells were found to be 1.35 and 0.22%, respectively (Fig. 6B). The lower CD8+/IFN-γ+ cell population was perhaps due to peptide 17 being a 15-mer peptide that might not have the best match to the MHC class I preferred peptide length (8–10-mer peptides).
In conclusion, we have demonstrated in mouse and macaque models that the rN protein formulated with potential clinically useful adjuvants, ISA-5 and CpG, elicited strong Th1 immune responses and may be excellent SARS vaccine candidates. Some of human B- and T-cell immunodominant epitopes of the N protein were also found in mice and macaques immunized with the rN protein formulated in ISA/CpG adjuvants. The conserved immunodominant epitopes identified in these three species provide very important information for further vaccine design.
Acknowledgment
The financial support from National Science Council of Taiwan (grant # SVAC-12-06) for this study is gratefully acknowledged.
References
- 1.Marshall E., Enserink M. Caution urged on SARS vaccines. Science. 2004;303(5660):944–946. doi: 10.1126/science.303.5660.944. [DOI] [PubMed] [Google Scholar]
- 2.Enserink M. One year after outbreak, SARS virus yields some secrets. Science. 2004;304(5674):1097. doi: 10.1126/science.304.5674.1097. [DOI] [PubMed] [Google Scholar]
- 3.Yang Z.Y., Werner H.C., Kong W.P., Leung K., Traggiai E., Lanzavecchia A. Evasion of antibody neutralization in emerging severe acute respiratory syndrome coronaviruses. PNAS. 2005;102(3):797–801. doi: 10.1073/pnas.0409065102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hohdatsu T., Yamato H., Ohkawa T., Kaneko M., Motokawa K., Kusuhara H. Vaccine efficacy of a cell lysate with recombinant baculovirus-expressed feline infectious peritonitis (FIP) virus nucleocapsid protein against progression of FIP. Vet Microbiol. 2003;97(1/2):31–44. doi: 10.1016/j.vetmic.2003.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilson J.A., Hart M.K. Protection from Ebola virus mediated by cytotoxic T lymphocytes specific for the viral nucleoprotein. J Virol. 2001;75(6):2660–2664. doi: 10.1128/JVI.75.6.2660-2664.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vanderzanden L., Bray M., Fuller D., Roberts T., Custer D., Spik K. DNA vaccines expressing either the GP or NP genes of Ebola virus protect mice from lethal challenge. Virology. 1998;246(1):134–144. doi: 10.1006/viro.1998.9176. [DOI] [PubMed] [Google Scholar]
- 7.Fu T.-M., Guan L., Friedman A., Schofield T.L., Ulmer J.B., Liu M.A. Dose dependence of CTL precursor frequency induced by a DNA vaccine and correlation with protective immunity against Influenza virus challenge. J Immunol. 1999;162(7):4163–4170. [PubMed] [Google Scholar]
- 8.Collisson E.W., Pei J., Dzielawa J., Seo S.H. Cytotoxic T lymphocytes are critical in the control of infectious bronchitis virus in poultry. Dev Comp Immunol. 2000;24(2/3):187–200. doi: 10.1016/s0145-305x(99)00072-5. [DOI] [PubMed] [Google Scholar]
- 9.Rota P.A., Oberste M.S., Monroe S.S., Nix W.A., Campagnoli R., Icenogle J.P. Characterization of a novel coronavirus associated with severe acute respiratory syndrome. Science. 2003;300(5624):1394–1399. doi: 10.1126/science.1085952. [DOI] [PubMed] [Google Scholar]
- 10.Kim T.W., Lee J.H., Hung C.F., Peng S., Roden R., Wang M.-C. Generation and characterization of DNA vaccines targeting the nucleocapsid protein of severe acute respiratory syndrome coronavirus. J Virol. 2004;78(9):4638–4645. doi: 10.1128/JVI.78.9.4638-4645.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumar S., Jones T.R., Oakley M.S., Zheng H., Kuppusamy S.P., Taye A. CpG oligodeoxynucleotide and montanide ISA 51 adjuvant combination enhanced the protective efficacy of a subunit malaria vaccine. Infect Immun. 2004;72(2):949–957. doi: 10.1128/IAI.72.2.949-957.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parker K.C., Bednarek M.A., Coligan J.E. Scheme for ranking potential HLA-A2 binding peptides based on independent binding of individual peptide side-chains. J Immunol. 1994;152(1):163–175. [PubMed] [Google Scholar]
- 13.Seo S.H., Pei J., Briles W.E., Dzielawa J., Collisson E.W. Adoptive transfer of infectious bronchitis virus primed [alpha][beta] T cells bearing CD8 antigen protects chicks from acute infection. Virology. 2000;269(1):183–189. doi: 10.1006/viro.2000.0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu M.F., Ning Q., Pope M., Mosmann T., Leibowitz J., Ding J.W. Resistance of naive mice to murine hepatitis virus strain 3 requires development of a Th1, but not a Th2, response, whereas pre-existing antibody partially protects against primary infection. Adv Exp Med Biol. 1998;440:415–423. doi: 10.1007/978-1-4615-5331-1_52. [DOI] [PubMed] [Google Scholar]
- 15.Marten N.W., Stohlman S.A., Bergmann C.C. MHV infection of the CNS: mechanisms of immune-mediated control. Viral Immunol. 2001;14(1):1–18. doi: 10.1089/08828240151061329. [DOI] [PubMed] [Google Scholar]
- 16.Hayashi M., Okada F., Ishida K., Maeda A., Kon Y., Mizutani T. Cytolytic activity induced by intramuscular injection of plasmid DNA expressing the nucleocapsid protein of the JHM strain of mouse hepatitis virus into C57BL/6 mice. J Vet Med Sci. 1996;58(8):731–735. doi: 10.1292/jvms.58.731. [DOI] [PubMed] [Google Scholar]
- 17.Klinman D.M. Immunotherapeutic uses of CpG oligonucleotides. Nat Rev Immunol. 2004;4(4):249–259. doi: 10.1038/nri1329. [DOI] [PubMed] [Google Scholar]
- 18.He Y., Zhou Y., Wu H., Kou Z., Liu S., Jiang S. Mapping of antigenic sites on the nucleocapsid protein of the severe acute respiratory syndrome coronavirus. J Clin Microbiol. 2004;42(11):5309–5314. doi: 10.1128/JCM.42.11.5309-5314.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shichijo S., Keicho N., Long H.T., Quy T., Phi N.C., Ha L.D. Assessment of synthetic peptides of severe acute respiratory syndrome coronavirus recognized by long-lasting immunity. Tissue Antigens. 2004;64(5):600–607. doi: 10.1111/j.1399-0039.2004.00314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo J.P., Petric M., Campbell W., McGeer P.L. SARS corona virus peptides recognized by antibodies in the sera of convalescent cases. Virology. 2004;324(2):251–256. doi: 10.1016/j.virol.2004.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]


