Abstract
We're discovering hordes of potentially lethal viruses lurking in bats, which are occasionally jumping into people. Is there anything we can do about it?
PATIENT Zero was a 60-year-old man in Saudi Arabia. He developed a mysterious illness in June 2012 and died 11 days later. His doctor sent off samples for testing, which revealed a new virus related to the one that caused the SARS outbreak a decade ago.
More people soon fell ill with the disease – now called Middle East respiratory syndrome, or MERS – and so far half of all those infected have died. The crucial question is, where is the virus coming from? The pattern of infections suggested it is harboured by some animal living on the Arabian Peninsula, where all the cases to date have originated or been traced to, and occasionally jumps to people. But which animal is it?
Twenty years ago, bats would have been one of the last on the list of suspects. The only serious human disease they were known to carry was rabies. But since then, a whole host of deadly diseases has been linked to bats, from Ebola and hepatitis C to SARS – and now, perhaps, MERS (see graphic) . The MERS virus may have jumped to camels first and then people, but all the evidence so far suggests it is the latest bat virus to infect people. It won't be the last. “Bats will most likely be the source of another new disease outbreak in the next five years,” says Lin-Fa Wang, who helped identify the animals as the source of the 2003 SARS outbreak. “Although bats are obviously not the only source of new viruses, they have been shown again and again in recent times to be one of the most important reservoirs. So we have to be prepared for another potential pandemic of bat origin.”
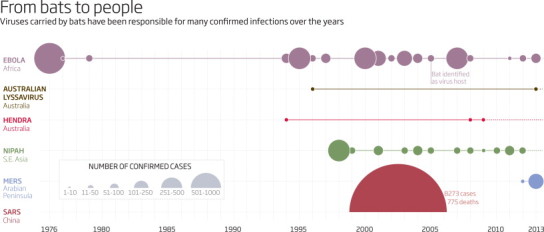
From bats to people
Bats will most likely be the source of another new disease in the next five years
Concern about the spread of viruses from bats to people first arose in 1994 in Australia, after a mysterious virus killed 15 horses in Hendra, a suburb of Brisbane. Two people caught the virus from the horses, possibly through scratches exposed to infected blood, and both eventually died rather horrible deaths. Some animal must have infected the horses, but tests on hundreds of species revealed nothing.
The team widened their search to include bats, just to be sure they weren't missing anything. Over a period of several years, Hume Field, a veterinarian who now works for the non-profit organisation EcoHealth Alliance, trapped and took blood from more than 5000 bats. Antibodies to the Hendra virus were found in the large fruit-eating bats known as flying foxes, making them the prime suspects. In 2000, Field and his colleagues found the virus itself in some individuals, confirming that these bats are the natural reservoir for the disease.
“Hendra virus was the first of the new generation of zoonotic viruses associated with bats,” Field says. Several more were soon discovered in Australia, including a close relative of the rabies virus now called Australian lyssavirus. In 1996, it killed a woman who was bitten by a bat.
Human cases of Hendra and Australian lyssavirus remain very rare. In 1998, however, 229 people in Nipah, Malaysia, fell ill with fever, headache and brain swelling caused by another unheard-of virus. Half of them died.
The Nipah virus turned out to be closely related to Hendra, so it was quickly traced to two species of flying fox found throughout South-east Asia. The virus is present in the saliva of infected bats, so it can spread via partially eaten fruit.
Deadly outbreak
In the 1998 outbreak, pigs had become infected after eating saliva-covered fruit dropped by bats, and then passed the disease to people. In most later outbreaks, which have typically involved dozens of cases, people have been infected directly by, say, drinking juice from date palms contaminated by bats. What's more, in some cases the virus has spread from people who were ill with the virus to those in close physical contact with them.
Nipah remains a bat virus that cannot spread readily between people. But as with bird viruses such as H5N1 flu, the big fear is that a bat virus that is deadly to people will mutate into a still-deadly form that jumps easily from person to person. That's what happened sometime around 2002 in China.
In November that year, people in Guangdong province began dying of a severe form of pneumonia. The outbreak hit the headlines in February 2003 when an American businessman flying from China fell ill. The plane landed in Vietnam, where the man died in hospital, as did several of the health workers who treated him. Within weeks the disease, dubbed severe acute respiratory syndrome or SARS, was spreading in nearly 20 countries.
Governments were forced to take drastic action. People with SARS were isolated and all their contacts traced. Take-your-temperature campaigns were launched in affected cities to detect the high fever characteristic of SARS, and passengers were screened before flights. Beijing built a 1000-bed hospital in a week. Fortunately, because the symptoms of SARS appear before people become infectious, these measures worked. The SARS outbreak was rapidly contained, but not before it had infected more than 8000 people, killing 775.
SARS was caused by a coronavirus, but human coronaviruses usually cause nothing more than a cold. At first it was suspected that SARS had jumped to people from civets being sold in the markets of Guangdong. But the virus has not been found in any civets, wild or otherwise, since 2003. Also, while viruses usually cause only mild disease in their host species, civets infected with SARS in the lab became seriously ill.
This made Lin-Fa Wang, an infectious disease specialist at Duke-NUS Graduate Medical School in Singapore and a member of the team that discovered the source of Hendra, suspect that civets were just an intermediate host. Sure enough, in 2004 his team found antibodies against SARS-like coronaviruses in three species of bat. Bats infected with SARS in the lab also showed no sign of illness.
What may have happened is that a few wild civets were infected by bats, caught and taken to market, where the virus jumped to humans, Wang suggested in a 2005 paper. At some point in the process, the virus mutated in a way that allowed it to spread between people. Confirmation that bats were the source of the outbreak came last year when a team including Wang found viruses almost identical to SARS in Chinese horseshoe bats.
In the same year, another particularly nasty virus was linked to bats. Ebola causes bleeding from all orifices and is usually fatal. The first known outbreak in people was in southern Sudan in 1976. Its animal host had long remained elusive, but in 2005 Ebola RNA was found in three species of fruit bat in the Republic of Congo and Gabon. Worryingly, the virus may not be limited to central Africa. Last year, a team including virologist Ian Lipkin of Columbia University reported finding antibodies to Ebola in fruit bats in Bangladesh.
Marburg, another hemorrhagic virus closely related to Ebola, also appears to come from bats. In 2007 and again in 2008, live Marburg virus was found in Egyptian fruit bats roosting in Kitaka cave in Uganda, the site of a 2007 outbreak.
The linking of SARS to bats in 2005 was a wake-up call, says virologist Charles Calisher of Colorado State University. At the time, only 66 viruses were known to infect bats. That figure has jumped to nearly 150 now that many groups have actively begun hunting for bat viruses, and there are surely many more out there. Lipkin recently estimated that mammals play host to at least 300,000 viruses, most of which remain undiscovered.
What has been discovered is far from reassuring. Take coronaviruses, for instance. While all kinds of animals play host to them, bats seem especially prone. Kathryn Holmes of the University of Colorado recently found 10 per cent of big brown bats in the state were infected with an alpha-coronavirus. In the Philippines, a third of bats tested were infected with a beta-coronavirus. And in Spain, 14 different coronaviruses were found in nine species of bat.
The diversity and the high prevalence of coronaviruses in bats create more chances for these viruses to spill over into humans, Holmes says. “There are lots and lots of coronaviruses in many different species. Most of them preferentially infect only one species, but with several mutations, they can jump to a new host species.”
The big fear is that a virus could not only jump to people but also acquire the ability to spread readily among them, causing another big outbreak like SARS or worse. Fortunately, the MERS virus has not done this – there have been fewer than 200 confirmed cases so far. But with every new case, there is a risk that the MERS virus will evolve the ability to spread more readily among people.
Another kind of virus that may have jumped from bats to humans has learned to spread well, with devastating effect. Last year, Lipkin's team reported that bats are probably the source of the hepatitis C virus, a liver-destroying, cancer-causing virus that now infects about 3 per cent of the world's population. It has been described as a silent plague – many are unaware they are infected until they develop serious symptoms.
Bats may be the source of hepatitis C, the liver-damaging virus that infects 3 per cent of people
So what is it about bats that makes them such good virus carriers? Are they especially likely to play host to viruses that can cause nasty diseases in people? Calisher, Field and Holmes think they might be.
For one thing, they can fly and thus carry viruses long distances. They also tend to roost in huge numbers in close proximity, so infected animals are more likely to infect others. Birds, of course, are similar in these respects, and birds carry many varieties of flu around the world, including the H5N1 flu that can be fatal. Because bats are more closely related to us than birds, though, it might be easier for bat viruses to infect us.
What's more, on cold nights and over winter, many bats enter a state called torpor during which they let their body temperature drop. Infected animals normally soon clear viruses from their bodies, but bats may act like fridges, allowing viruses to survive in them longer – even over an entire winter.
A 2012 study by a team including Wang also proposed that bats have particularly good immune systems, making them more able to carry viruses without succumbing or becoming too ill to fly. Flying takes a lot of energy and generates many DNA-damaging free radicals. Bats had to ramp up their defences against these destructive chemicals as they evolved, and genetic studies suggest this had a side effect of boosting their immune system's ability to contain viruses.
Yet even if bats are especially good at harbouring and spreading viruses, blaming them misses the point, Lipkin says. The emergence of an infectious disease requires a perfect storm of factors, only one of which is the animal species in which the virus originates. Human factors play a major role in why these diseases move from bats to people. Deforestation, habitat fragmentation, climate change, urban sprawl and international travel are all involved. “Our world is getting smaller and we're encroaching on areas to which we weren't previously exposed,” Lipkin says.
Take the first Nipah outbreak. In 1998, deforestation and drought combined to create a crisis for bats. So, to find food, the animals turned to the newly planted orchards that had replaced their rainforest habitat. Pigs kept nearby ate bats' leftovers and passed the virus to people. “The natural landscape where flying foxes used to forage has been fractured and fragmented,” Field says. “You've also got oases of food resources scattered throughout the countryside, very often near humans.”
So, really, we're to blame. But given that habitat loss and climate change are set to continue, should we kill the bats before their diseases kill us? Before answering, imagine your family was at risk. In Australia last year, after 8-year-old Lincoln Boucher died of a lyssavirus infection after being scratched by a bat, many called for a cull. Conservationists were outraged. “It's a knee-jerk reaction,” says Louise Saunders of local charity Bat Conservation and Rescue in Queensland. “The politicians don't seem to be listening to scientific advice that says these don't work.”
Certainly culling has not worked in South America, where there has been widespread poisoning of vampire bats since the 1970s to try to prevent rabies outbreaks. According to a three-year study of 20 bat colonies in Peru led by Sonia Altizer, an ecologist at the University of Georgia in the US, if anything it can make things worse.
The problem is that killing off every single individual is next to impossible, and with less competition for food, bat populations rebound rapidly. What's more, culling may boost the spread of rabies by increasing movement between colonies – and rebounding populations have a higher proportion of younger bats with no immunity. “You pretty much have to scorch the earth and kill everything for culling to be effective,” Altizer says.
With bats making up nearly a quarter of all mammal species, they also have tremendous value from a biodiversity perspective. And while vampire bats have few fans, their insect-eating relatives help us directly by gobbling up billions of pests. A 2011 study estimated that their loss in the US would cost farmers between $4 billion and $50 billion a year.
There are plenty of other things we can do besides culling. Vigilance is vital, from monitoring emerging diseases such as MERS to finding out what viruses are lurking in bats well before they affect people. Vaccines are already being developed against many viruses, from Ebola to Nipah. In Australia, horses are now being vaccinated against Hendra.
Warning people about the dangers posed by bats can make a difference, too. What makes the death of Lincoln Boucher all the more tragic is that he never knew to tell his parents he'd been scratched by a bat. They found out only two months later when he was already seriously ill. If they had known earlier, his life could have been saved.
This article appeared in print under the headline “CONTAGION”


