Abstract
The use of vaccines is saving millions of lives every year across the globe, but a number of important diseases such as HIV/AIDS, malaria, TB and hepatitis C continue to frustrate attempts to produce effective vaccines against them. Confronting these challenges will require new approaches and increased research efforts by the scientific community. The Sixth Framework Programme (FP6; 2002–2006) of the European Commission (EC) has been an important catalyst in this direction by allocating a financial contribution of more than EUR 210 million to a wide variety of vaccine research activities, ranging from basic vaccinology, translational research to clinical application of vaccines. Taken together, around 581 research groups from 52 countries are participating in the vaccine activities of FP6. This impressive number signals a new spirit of collaborative research, which will facilitate the exploitation of the immense possibilities in modern vaccinology.
Keywords: Vaccines, FP6 research funding, European Commission
1. Introduction
Vaccines work by presenting an antigen to the immune system, which respond by evoking an immune response that ameliorates the effects of an infectious pathogen or a disease process. Vaccines can either be prophylactic or therapeutic, and the antigen material can either be live attenuated pathogens, killed or inactivated forms of these pathogens, or purified or recombinant material such as proteins.
Vaccine research has seen a remarkable renaissance during the last decade. This has partly been catalyzed by scientific breakthroughs such as the full genome sequencing of several infectious pathogens [1], the refinement of knowledge about the human immune response system [2], and new breakthroughs in understanding the host–pathogen interactions [3]. The other major factor has been a significant influx of money from public funding bodies, private charity organisations and commercial enterprises. In the private sector, recent commercial successes such as the human papilloma virus (HPV) vaccine have triggered small and even large pharmaceutical companies to initiate or renew their interest in vaccines. This trend has been further supported by the appearance of novel financing mechanisms such as Product Development Partnerships (PDPs), and the introduction of market-related incentives such as the Vaccine Fund, the Advanced Market Commitment (AMC) and the International Financing Facility for vaccines.
There are several reasons for the public sector to engage actively in vaccine research and to support the development of new vaccines. Firstly, vaccines are one of the most effective ways to protect people against infectious diseases and thereby actively promote better health and quality of life, locally as well as globally [4]. Secondly, vaccines are one of the most cost-effective measures for public health [5]. Safe and effective prophylactic vaccines are significantly more cost-effective than repeated application of drugs or other treatments. Vaccines can therefore release resources, which can be used elsewhere in the health system. Thirdly, the development of vaccines against extraordinary pathogens such as HIV may be so technically and scientifically challenging that it may never occur without sustained support and active contribution from the public sector. Addressing very challenging pathogens may, on the other hand, lead to scientific breakthroughs with a broader impact on technological and economic development. Last but not least, vaccines against pathogens such as dengue or malaria, which are mainly (or only) prevalent in low-income countries, are unlikely to be developed unless the public sector subsidises and supports research. In this respect, international public organisations such as the European Commission (EC) have a particularly important role to play as they can act with a higher emphasis on the global health agenda rather than purely commercial or national research priorities.
2. European support to vaccine research
Europe has a long and successful tradition for vaccine research in both public and private institutions. Two-thirds of global vaccine R&D is conducted by European firms and almost 90% of all vaccine production takes place in Europe [6]. As a region, Europe is therefore well positioned to take on new challenges in vaccine research, and exploit the immense opportunities that are opening up in this field of science. The Sixth Framework Programme for Research (FP6) of the EC, which was adopted in 2002, gave an opportunity to provide further momentum to vaccine research with a European dimension. During the course of FP6 (2002–2006), more than EUR 210 million was allocated by the EC to initiate a total of 66 projects with a focus on vaccines or vaccine-related research (Table 1 ). The projects cover three main categories of research: sixteen projects were funded to develop and mature highly innovative technologies and new vaccinology concepts. Most projects, namely 41, were funded with a view to develop vaccine candidates for a specific disease. Finally, capacity building, clinical investigations and implementation research of existing vaccines received a total funding of nearly EUR 30 million to support 9 projects. In addition, an EC contribution of EUR 200 million was made to the European and Developing Countries Clinical Trials Partnership (EDCTP) initiative, which uses its resources to support capacity building and clinical trials of new vaccines and treatments for HIV/AIDS, malaria and TB in sub-Saharan Africa.
Table 1.
Overview of EC funded human vaccine research in FP6.
| Project acronym | EC contribution (EUR) | Partner | Type | Key words | Website |
|---|---|---|---|---|---|
| Basic vaccinology | |||||
| Muvapred | 15,250,000 | 29 | IP | Mucosal | www.mucosalimmunity.org/muvapred |
| Savinmucopath | 1,699,908 | 8 | STREP | Mucosal | www.greenhillsbiotech.com/eu_projects |
| MuNanoVac | 1,505,702 | 8 | STREP | Mucosal | www.munanovac.eu |
| EPI-VAC | 2,400,000 | 7 | STREP | Delivery | NA |
| CompuVac | 7,969,442 | 15 | IP | Genomic | NA |
| BacAbs | 2,269,999 | 9 | STREP | Genomic | www.bacabs.org |
| Microbearray | 1,401,002 | 9 | NoE | Genomic | NA |
| Dec-Vac | 3,400,000 | 10 | IP | Dendritic cells | www.rubr-uni-bochum.de |
| Theravac | 2,267,000 | 7 | STREP | Dendritic cells | NA |
| DC-Vacc | 2,000,000 | 9 | STREP | Dendritic cells | www.biopolo.it |
| Innovac | 2,000,000 | 7 | STREP | Platform | NA |
| VaccTIP | 1,000,000 | 5 | STREP | Platform | www.mtc.ki.se/groups/liljestrom/vacctip/index |
| Mvactor | 1,000,000 | 4 | STREP | Platform | www.pei.de |
| AIDS-CoVAC | 958,000 | 3 | STREP | Platform | www.lfa-sg.ch |
| HIVAB | 950,000 | 7 | STREP | Platform | NA |
| ImmunoGrid | 1,950,000 | 8 | STREP | Virt. immune system | www.immunogrid.org |
| Disease specific projects | |||||
| AVIP | 10,300,000 | 15 | IP | HIV/AIDS | www.avip-eu.org |
| RMVHIV | 5,500,000 | 6 | IP | HIV/AIDS | NA |
| Auto/Allo cell-HIV | 1,700,000 | 6 | STREP | HIV/AIDS | NA |
| Pox-gene | 1,180,000 | 6 | STREP | HIV/AIDS | NA |
| Allomicrovac | 1,100,000 | 7 | STREP | HIV/AIDS | NA |
| VIAV | 1,000,000 | 5 | STREP | HIV/AIDS | NA |
| HIV virosomes | 973,930 | 7 | STREP | HIV/AIDS | NA |
| TIP-VAC | 951,650 | 7 | STREP | HIV/AIDS | www.ruhr-uni-bochum.de/virologie/TIP-VAC/TIP-VAC |
| EPI-VAC | 911,050 | 5 | STREP | HIV/AIDS | www.dbbm.unima.it |
| EMVDA | 13,500,000 | 13 | IP | Malaria | www.emvda.org |
| Pribomal | 2,345,358 | 7 | STREP | Malaria | NA |
| SME-malaria | 1,700,000 | 5 | STREP | Malaria | www.malariastrep.eu |
| Malinv | 587,000 | 5 | STREP | Malaria | NA |
| TB-VAC | 17,000,000 | 33 | IP | Tuberculosis | www.tb-vac.org |
| NEOTIM | 2,000,000 | 8 | STREP | Tuberculosis | www.euprojekt.su.se |
| Vaccines4TB | 1,053,445 | 4 | STREP | Tuberculosis | www.biocompetence.eu |
| Immuno VacTB | 857,298 | 4 | STREP | Tuberculosis | NA |
| Fluvacc | 9,200,000 | 8 | IP | Influenza | www.greenhillsbiotech.com/eu_projects |
| FluVac | 3,500,000 | 6 | STREP | Influenza | www.fluvac-project.eu |
| Panfluvac | 3,334,798 | 9 | STREP | Influenza | www.panfluvac.org |
| Intranasal H5vaccine | 2,680,400 | 6 | STREP | Influenza | www.greenhillsbiotech.com/eu_projects |
| Chimeric vaccines | 1,384,945 | 7 | SME COOP | Influenza | www.greenhillsbiotech.com/eu_projects |
| Universal vaccine | 1,154,717 | 6 | SME COOP | Influenza | www.universalvaccine.org |
| Novaduck | 1,416,380 | 8 | STREP | Avian Flu | www.novaduck.eu |
| AIV Vacc diagnosis | 1,372,890 | 3 | SSA | Avian Flu | www.aiv-vacc-diagnosis.com |
| Hepacivac | 8,800,000 | 12 | IP | Hepatitis C | www.altaweb.eu/hepacivac |
| Dissect | 2,375,892 | 10 | STREP | SARS | www.cnb.uam.es/∼webcoron/EUprojectdissect/ |
| Sarsvac | 1,200,000 | 4 | STREP | SARS | NA |
| Sars/Flu vaccine | 1,607,500 | 6 | STREP | SARS + FLU | www.greenhillsbiotech.com/eu_projects |
| Scoott | 2,800,000 | 10 | STREP | Helminths | NA |
| Tranchi | 1,950,000 | 7 | STREP | Helminths | www.tranchi.org |
| Bovac | 1,355,443 | 6 | STREP | Lyme disease | www.bovac.org |
| Omvac | 2,320,000 | 7 | STREP | Otitis media | NA |
| Supasalvac | 2,440,670 | 10 | STREP | Diarrhoea | NA |
| Hevar | 1,539,999 | 8 | STREP | Diarrhoea | NA |
| Trypadvac2 | 900,000 | 12 | STREP | Trypanosome | www.trypadvac2.eventos.usb.ve |
| Cancerimmunotherapy | 12,185,102 | 22 | IP | Cancer | www.cancerimmunotherapy.eu |
| Dendritophages | 1,999,900 | 6 | STREP | Cancer | NA |
| Lcvac | 1,231,269 | 5 | SME COOP | Cancer | www.lcvac.org |
| Vital | 2,050,000 | 7 | STREP | Cancer | www.cro.sanita.fvg.it/progetti/vital/index |
| Mimovax | 2,370,155 | 7 | STREP | Alzheimer's | www.mimovax.eu |
| Capacity building and clinical research | |||||
| Europrise | 15,500,000 | 36 | NoE | HIV/AIDS | www.altaweb.it/europrise/neutnet |
| DC-Thera | 7,600,000 | 34 | NoE | Cancer | www.dc-thera.org |
| ENACT | 4,166,513 | 13 | STREP | Cancer | www.enactcancerresearch.org |
| Neutnet | 299,000 | 7 | SSA | HIV/AIDS | NA |
| Eurhavac | 260,000 | 1 | SSA | Malaria | www.emvi.org/eurhavac |
| Pahpv | 132,000 | 3 | SSA | HPV | N/A |
| Advac-EC | 390,000 | 2 | SSA | Training | www.advac.org |
| Coinfect | 290,000 | 4 | SSA | Training | www.coinfect.eu |
| Rebavac | 121,200 | 1 | SSA | Training | www.altaweb.eu/rebavac |
| Total | 210,609,557 | 581 | |||
Projects of different magnitudes have been supported within each category of research. The largest project type, the Integrated Project (IP), has been used to gather multi-disciplinary consortia with sufficient critical mass to translate basic research into applications. Smaller and focused research activities have mostly been supported as Specific Targeted Research Projects (STREP) or in a few cases as Cooperative Research for small- and medium-sized enterprises (COOP). The smaller and more flexible STREP projects are well suited for early stage research, whereas the larger and multidisciplinary IPs may act both to take up new discoveries from STREP projects, while also delivering mature project results for clinical applications or further downstream development by industry organisations, the EDCTP or public–private partnerships. Another project type, the Network of Excellence (NoE) has been applied in a few areas as a special tool to structure and coordinate the European research community around common research agendas. The smallest type of projects, Strategic Support Actions (SSA) has been used to support activities such as harmonization of research standards, conferences, workshops or training schemes.
3. Basic vaccinology
Research in basic vaccinology has been supported with more than EUR 46 million during the course of FP6. The funding has been used to initiate 16 research consortia, all of which are focused on knowledge and technologies with broader relevance for vaccine development. The projects cover a wide range of aspects, but 3 activity areas have received particular attention, namely mucosal vaccinology, post-genomic vaccinology and dendritic cells as vaccine targets.
Many infectious diseases are caused by pathogens that enter the human body through mucosal surfaces. This includes serious diseases such as HIV/AIDS, TB, influenza and sexually transmitted diseases. Many of these diseases may better be confronted if the causative pathogen can be stopped at the first port of entry to the human body. This hypothesis is followed by the Muvapred project, which investigates how an effective and lasting immune response can be induced at mucosal surfaces. The Muvapred consortium is coordinated by Novartis Vaccines, and the project comprises 29 research organisations from the public sector in Europe, the biotech industry and African research institutions. The project started in December 2003 and has since explored several new approaches for mucosal vaccination and has provided new solutions to overcome the technical difficulties of sampling the human mucosal immune system. The ethical concerns for undertaking mucosal studies in humans have also been addressed, and two clinical trials involving nasal application have successfully been completed. The Muvapred consortium is complemented by the Savinmucopath project, which aims to develop mucosa specific vaccines against bacterial infections. The Munanovac project explores the use of nanoparticles for mucosal immunization, while the Epivac project is focused on transdermal immunisation. Taken together, these projects cover a wide range of research in delivery of vaccines through new routes of administration. More than 40% of the total EC contribution to basic vaccinology has been allocated to these 4 projects, demonstrating the importance of this area within the EC portfolio of vaccine research.
The use of new genomic data for better selection of candidate antigens is an important aspect of contemporary vaccinology, and this is also reflected in the EC portfolio of projects. The Compuvac project gathers 15 partners in a joint effort to develop a platform for rational design and standardized evaluation of genetic vaccines. The BACABS project aims to build a standard approach for selection of new vaccine candidates on the basis of structural determinants. Together with Microbearray, another research consortium in this category, these projects represent approximately 25% of the total EC funding to basic vaccinology.
The third major activity area for basic vaccinology is the potential use of dendritic cells to facilitate immunization against infectious as well as non-infectious diseases. Most of the current vaccines work by inducing an antibody response, but vaccination against diseases such as AIDS, cancer and auto-immune diseases may also require a concurrent cytotoxic T-cell response. The integrated project Dec-Vac aims to understand the role of dendritic cells in the immune system and use this knowledge to develop a broadly applicable method to enhance antigen uptake and presentation by dendritic cells (DCs). In this effort, Dec-Vac is complemented by DC-Vacc and Theravac, which explore the potential future use of dendritic cells as natural adjuvants and targets for a broad range of vaccines.
Besides the 3 areas above, a number of projects have addressed other areas of basic vaccinology, ranging from the use of new vectors for vaccine development (Mvactor) to the establishment of a virtual immune system (Immunogrid). The projects on basic vaccinology are thus covering a wide range of activities with a common aim of establishing new methods and novel technologies for future vaccine research across a range of diseases.
4. Disease specific vaccine research
Most of the vaccine research activities under FP6 is tightly associated to a single disease or pathogen. This is the case for 42 collaborative projects that have received a total EC contribution of EUR 136 mill to undertake R&D within four different groups of diseases (Fig. 1 ).
Fig. 1.
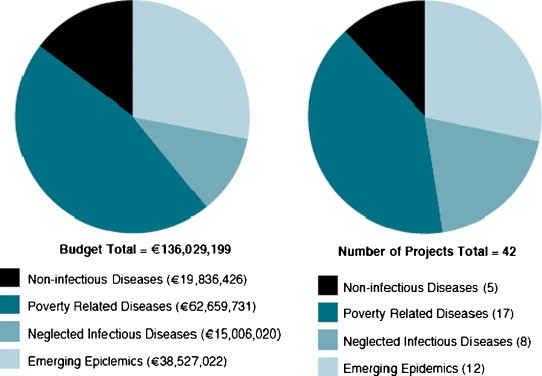
Distribution of projects and EC contribution to disease specific vaccine projects in FP6.
4.1. Poverty-related diseases (HIV/AIDS, malaria and TB)
The combat against the 3 major poverty-related diseases (PRDs) is an established priority within the European Commission [7], and a major component of FP6 has therefore been support to vaccine research in this area. For each of the 3 diseases, a major integrated project has received EC funding of more than EUR 10 million to undertake translational research activities during a 5-year period. More than half of all projects focused on HIV/AIDS, reflecting the huge challenges for vaccine research in this particular area. The Avip consortium comprises 20 research groups from Europe and Africa with an aim to generate novel AIDS vaccine candidates, based on combinations of HIV regulatory (Tat and/or Rev, and/or Nef) and structural (Env and/or Gag/Pol) proteins. The largest malaria project is EMVDA, which aims to select, develop and test candidate vaccines directed against the asexual blood stages of malaria parasites. The project is coordinated by the European Malaria Vaccines Initiative (EMVI), which has access to a number of very different synthetic antigens such as MSP1, MSP2, MSP3, GLURP, EBA 175, AMA1, LSA3, STARP, and Exp-1. The flagship of TB research is the TB-Vac consortium, which joins 33 public and private institutions from 9 European and 4 African countries. The project covers pre-clinical and early clinical development of subunit vaccines such as MVA-Ag85A, Mtb72f and Ag85B-ESAT6, but also new live attenuated vaccines such as rBCG-UreC-Hly and Mtb-PhoP. Collectively, the TB-Vac consortium therefore comprises the world's most comprehensive portfolio of TB vaccine candidates in current development.
4.2. Emerging epidemics
Influenza pandemics such as the Spanish flu of 1918–1919, which killed between 50 and 100 million people around the world, is a reminder that new viral diseases can suddenly appear or re-appear at short notice and with dramatic consequences. A more recent example stems from November 2002, when SARS (Severe Acute Respiratory Syndrome), a hitherto unknown infectious disease, was reported in Guangdong in China, and quickly spread to Hong Kong, Taiwan, Singapore, Vietnam and Canada before it was contained [8]. These events underline the importance of supporting vaccine research for new and re-emerging infectious diseases. While existing seasonal flu vaccines have been highly effective in the management of influenza, there is a thus number of obstacles to the development of safe and effective vaccines for pandemic influenza. Vaccine research for both influenza and SARS has been initiated under FP6, and most of the projects are focused on pandemic influenza and how to increase preparedness for potential future outbreaks. The largest project, FluVacc, is developing an improved technology for quickly producing new live attenuated influenza vaccines based on reverse genetics. With around 170 million carriers and approximately 25 000 newly infected every year, hepatitis C can also be considered an emerging threat to global public health. The Hepacivac project aims to develop a prophylactic and potentially therapeutic vaccine against the hepatitis C virus. The aim of this 5-year project (2007–2011) is to undertake formal pre-clinical testing of the two most promising vaccine candidates, and subsequently progress these to early clinical testing in healthy volunteers as well as chronically infected individuals.
4.3. Neglected infectious diseases
Neglected infectious diseases (NIDs) receive little attention from the media and public in comparison with “high profile” diseases such as HIV/AIDS, malaria and TB, but the effect of some of these diseases can nevertheless be devastating. The group of NIDs is not clearly defined, but broadly comprises infectious diseases, which are often forgotten due to limited incidence or economical incentives to develop prevention or treatments. The NIDs include some of the most common chronic infections among the world's poorest people. The EC has supported vaccine research in neglected infectious diseases since the early 1980s, and 8 projects with a total EC contribution of EUR 15 mill were initiated during FP6. These projects address such diverse diseases as diarrhoeal infections (Hevar), lyme disease (Bovac), otitis media (Omvac), and helminth infections (Tranchi and Scoott). The projects are mostly smaller STREP projects with an EC contribution of EUR 1–3 million, but since they are addressing infectious diseases that are otherwise neglected, the projects can have an impact in terms of societal value that significantly supersedes their modest size.
4.4. Non-infectious diseases
The possibility of creating vaccines for a host of non-infectious diseases such as cancer, neurodegenerative diseases and autoimmune diseases provides one of the most exciting fields for current vaccine research. It holds the potential to meet a huge unmet medical need or provide an alternative to traditional treatments that are costly, only partially effective and associated with adverse effects. With the exception of a vaccine project against Alzheimer's disease (Mimovax), the efforts in this area are concentrated on cancer vaccines and the projects are targeting some of the major cancers, such as lung cancer, melanoma, and carcinoma. The projects are mostly focused on identification of specific tumour antigens that are selectively present in cancer cells, and therefore provide a potential immune target for a vaccine approach. The largest single project, Cancerimmunotherapy, gathers 22 partners for developing a therapeutic vaccine against melanoma and potentially other cancer types. Within this project, different vaccine approaches will be tested and compared, using both peptides and RNA as immunogens and with different types of immunological adjuvants and dendritic cells. The field of vaccines for non-infectious diseases is highly promising, but there are still many fundamental roadblocks to be overcome. As such, some of the EC funded activities in basic vaccinology will undoubtedly also benefit the future advancement of vaccines for non-infectious diseases.
5. Clinical research and capacity building
Vaccines can contribute enormously to public health if they are used appropriately. The implementation of new vaccines in the public health sector, and the improved use of existing vaccines are both critical factors for optimizing the impact of vaccinations. To achieve this, a detailed understanding of disease epidemiology and vaccine protection is necessary. Furthermore, a thorough understanding of the socio-economic benefits of individual vaccines must support decisions about vaccine campaigns. Finally, researchers and health personnel must receive appropriate and sufficient training to obtain a better understanding of vaccinology and the impact of different vaccines in the field. The largest single initiative in clinical vaccine research under FP6 is the Europrise network-of-excellence. The aim of Europrise is to better synergise European research on new preventive technologies for HIV/AIDS, including both microbicides and vaccine research. The Europrise network creates links between about 15 ongoing research projects, which are either funded by the European Commission or by other funding bodies, including the Bill and Melinda Gates Foundation. In total, this network comprises representatives from more than 132 institutions in 22 countries. Another NoE, DC-Thera, is focused on the clinical application of vaccines based on dendritic cells. This network integrates the activities of 26 groups of scientists, clinicians and SMEs in order to translate genomic, proteomic and bioinformatic information into useful endpoints for clinical trials of DC-based therapies for cancer and HIV. The EC has also supported a number of projects with focus on networking of activities, harmonization of clinical activities (e.g. Eurhavac) and standardization of research methods (e.g. the Neutnet project). Most of these activities have a relatively small budget, but they play an important role in filling the gaps in many research activities or in building bridges within the scientific community. Finally, the important aspect of human capacity building through training of scientists from developing countries has been supported in various ways. Dedicated projects such as ADVAC-EC and Coinfect provide theoretical and field training, respectively, to scientists from developing countries. Furthermore, several of the larger projects, e.g. Muvapred and EMVDA, have integrated training programmes and fellowship that are specifically earmarked for training of scientists from developing countries.
6. Concluding remarks
The great success of vaccines in the past and present should be used as inspiration to intensify research in this area of life science even further. A number of important infectious diseases such as HIV/AIDS, malaria and hepatitis C continue to escape attempts to produce effective vaccines against them. Vaccines against other infectious diseases such as TB exist, but are only partially effective and need dramatic improvement to respond to the real public health needs. To confront these challenges in vaccine research, the creation and strengthening of partnerships between multiple stakeholders is necessary.
One of the characterizing features of the EC Framework programme is the strong focus on cooperation between researchers in different countries. Around 581 research groups from 52 countries are thus participating in the ongoing EC supported vaccine research projects (Fig. 2 ). The framework programmes have historically been focused on integration of research within the EU, and the majority of participants in FP6 are located in EU Member States (84%). However, increasing globalization in health science means that more and more participants are coming from other countries, either neighbouring countries in Europe (7%) or non-European countries (9%). This trend is expected to become significantly more pronounced in Framework Programme 7 (2007–2013).
Fig. 2.
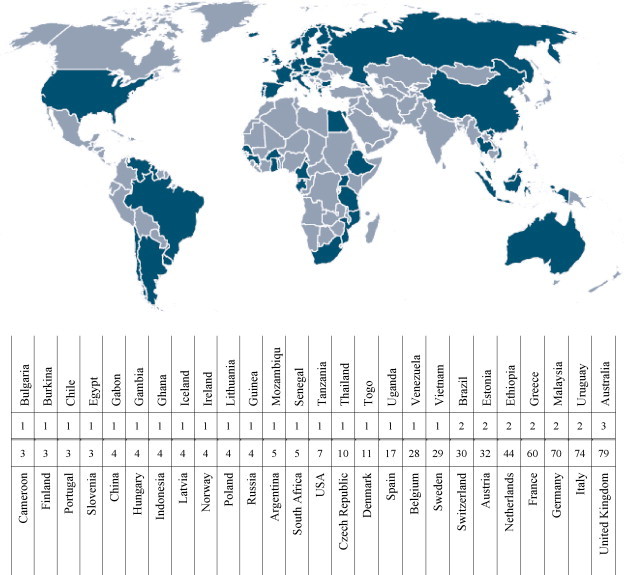
Participants in EC funded human vaccine research in FP6 come from a large number of different countries. Participating countries are marked on the map, and numbers of participating research groups from each country are indicated in the table.
Another key priority has been partnerships between scientists in different sectors. Significant efforts were therefore made to identify research areas with appeal to both the public and private sector. The result has been that more industry partners have joined vaccine research projects in FP6 than in any previous framework programme. The industry partners include a wide range of companies, ranging from small and medium-sized enterprises (SMEs) in the biotech sector to some of the largest vaccine manufacturers in the world. In total, more than 20% of all partners in the vaccine projects under FP6 came from the private industry sector.
The FP6 vaccine projects were all initiated between 2003 and 2007, and with a typical duration of 3–5 years. Most of them are therefore still in progress, but impressive results have already been achieved by some of the projects. Many more results can be expected as the various projects evolve during the coming years. At the beginning of 2007, FP6 was followed up by the introduction of the Seventh Framework Programme (FP7), in which Health research plays an equally important role as in FP6, and with an even higher average annual budget. FP7 runs from 2007 to 2013, and has a budget allocation of EUR 6 billion to cooperative Health research, corresponding to an annual average of approximately EUR 900 million. Activities in poverty-related diseases (HIV/AIDS, malaria, TB) remain a key activity in FP7, while the activity areas for Emerging Infectious Epidemics and Neglected Infectious Diseases have been strengthened. During FP7, the area of Emerging Infectious Epidemics will fund research on upcoming threats to European health from viral infections, while activities in Neglected Infectious Diseases will address a range of protozoal, bacterial and helminth infections of significant importance to global public health. FP7 will also continue to support research in clinical applications and basic vaccinology with a broad applicability. Last but not least, FP7 will also provide continued support to research in a range of non-infectious diseases, where vaccine approaches are becoming increasingly relevant. FP7 is thus a potential source of sustained support to a wide range of vaccine research activities. Furthermore, an overall focus on translational research in the health theme of FP7 makes it feasible that many of the promising results in vaccine research obtained during the course of FP6 can be accelerated and translated into application.
References
- 1.Mora M., Donati C., Medini D., Covacci A., Rappuoli R. Microbial genomes and vaccine design: refinements to the classical reverse vaccinology approach. Curr Opin Microbiol. 2006;5:532–536. doi: 10.1016/j.mib.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 2.Plotkin S.A. Vaccines: correlates of vaccine-induced immunity. Clin Infect Dis. 2008;47:401–409. doi: 10.1086/589862. [DOI] [PubMed] [Google Scholar]
- 3.Demuth A., Aharonowitz Y., Bachmann T.T., Blum-Oehler G., Buchrieser C., Covacci A. Pathogenomics: an updated European Research Agenda. Infect Genet Evol. 2008;8(3):386–393. doi: 10.1016/j.meegid.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 4.Schuchat A., Bell B.P. Monitoring the impact of vaccines postlicensure: new challenges, new opportunities. Expert Rev Vaccines. 2008;7(4):437–456. doi: 10.1586/14760584.7.4.437. [DOI] [PubMed] [Google Scholar]
- 5.Rappuoli Rino, Miller Henry I., Falkow Stanley. The intangible value of vaccination. Science. 2002;297:937–939. doi: 10.1126/science.1075173. [DOI] [PubMed] [Google Scholar]
- 6.Galambos Louis. What are the prospects for a new golden era in vaccines? Eurohealth. 2008;14:12–14. [Google Scholar]
- 7.COM(2005) 179 of 27.04.2005. From the commission to the council and the European parliament: a European programme for action to confront HIV/AIDS, malaria and tuberculosis through external action; 2007–2011.
- 8.Shaw K. The 2003 SARS outbreak and its impact on infection control practices. Public Health. 2006;120(1):8–14. doi: 10.1016/j.puhe.2005.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]


