Abstract
The compatibility, safety and interaction on antibody induction of a combined vaccine application were assessed. Specific pathogen-free cats were vaccinated with either a modified live virus vaccine containing feline calici- (FCV), herpes- (FHV-1), parvovirus (FPV) and Chlamydophila felis (C. felis), an adjuvanted recombinant feline leukaemia virus (FeLV) vaccine or both vaccines in one syringe. After combined application, FeLV ELISA antibody titres were unaltered, However antibody production based on indirect immunofluorescence assay was remarkably enhanced for FCV and was at selected time points also enhanced for FHV-1 and C. felis but diminished for FPV. The use of these vaccines in combination was safe and will simplify vaccination schedules in veterinary practice.
Keywords: Vaccine combination, Adjuvants, Immunity
1. Introduction
Vaccination has been used in feline medicine since the early 1960s and often represents the safest and most effective way of protecting cats against many of the major feline infectious diseases. Vaccines should, as far as possible be free of adverse side effects and show high antigenicity. For practical reasons, it has become common to combine several feline vaccines within a single syringe, thereby reducing the animal's injection-related stress as well as costs [1]. When different antigens present in a mixture are inoculated simultaneously, competition with respect to stimulation of the immune system may occur [2]. However, combined application could also augment or modulate the quality of the immune response elicited [3]. Therefore, when different immunogens are to be combined in one injection, the immunogenicity of the individual antigens should be evaluated.
In general, vaccines containing living organisms have the potential to raise long-lasting immunity, inducing stronger cellular immune responses than their inactivated counterparts [4]. To improve the antigenicity of inactivated and recombinant vaccines, they are adjuvanted with substances that stimulate early processes in immune recognition, particularly the inflammatory response [5]. The use of adjuvants influences the generation of T helper (Th) lymphocyte subsets and the magnitude of the immune response.
The aim of this study was to assess the antibody induction in kittens after combined administration in a single injection of a multivalent modified live virus (MLV) vaccine against feline calicivirus (FCV), feline herpesvirus-1 (FHV-1), feline parvovirus (FPV) and Chlamydophila felis (C. felis) and an adjuvanted recombinant feline leukaemia virus (FeLV) vaccine. If successful, the results of this study should also be used as part of a submission for regulatory purposes. A preliminary vaccine compatibility test in vitro was performed to evaluate the effects of the aluminium hydroxide and QS-21 adjuvants contained in the FeLV vaccine on the viability of the attenuated live vaccine components. The immunogenicity of the MLV components and C. felis after combination was then tested in vivo by assaying antibodies to the various vaccine antigens. The safety of the vaccines was assessed by observing and recording any systemic or local adverse reaction to the simultaneous injection of both vaccines. The adjuvant included in the recombinant FeLV vaccine is known to stimulate both, the Th1 and Th2 immune response and its presence in any vaccine might have an effect on all components injected together. In order to characterize the T-lymphocyte response in the different groups, the expression of IL-2, IL-4 and IFN-γ mRNA was evaluated in lymphocytes by C. felis-antigen stimulation, by ConA stimulation and by mock-stimulation in vitro. C. felis antigen was selected as model stimulans as it was present in the MLV vaccine and could be handled readily.
2. Materials and methods
2.1. Animals
Thirty specific pathogen-free (SPF), 7- to 8-week-old kittens were provided by Liberty Research Inc. (Waverly, NY, USA). The cats were assigned randomly, regardless of the sex, into three groups of 10 animals each, avoiding familial clustering in any of the groups.
2.2. Vaccines
The vaccines used were commercial batches of Nobivac® Forcat and Nobivac® FeLV (Intervet UK Ltd.). Nobivac® Forcat is a modified live vaccine which contains FCV, FPV, FHV-1 and C. felis without any adjuvant. For this study, the viral and chlamydial doses were adjusted to 105.0 PFU of FCV strain F9, 105.3 PFU of FHV-1 strain G2620A, 104.6 TCID50 of FPV strain MW-1 and 102.8 TCID50 of C. felis Baker strain. The adjustment was done in order to comply with the regulatory requirements, which state that the vaccines at the end of their shelf life must contain the above-mentioned doses. Nobivac® FeLV is a recombinant vaccine containing the p45 FeLV antigen, the non-glycosylated protein fraction of the virus envelope glycoprotein gp70 considered to be responsible for the induction of protective antibodies [6]. Each dose of Nobivac® FeLV contained 100 μg of purified p45 molecule. The antigenic material is adjuvanted with an aluminium hydroxide gel and a derivative of Quil A, a purified derivative of saponin. For the combined application of both vaccines, one dose of lyophilised Nobivac® Forcat was reconstituted in one dose of Nobivac® FeLV.
2.3. In vitro compatibility of the vaccines
The influence of the FeLV vaccine on the viability of the modified live vaccine components was evaluated in vitro. Each live microbial vaccine component (FCV, FHV-1, FPV and C. felis) was individually reconstituted with either FeLV vaccine or solvent and titrated on Crandell feline kidney (CRFK) cells for the viral components, and McCoy cells for C. felis as described [7], [8]. The titrations of all vaccine components took place either immediately after reconstitution or after the vaccine suspensions were held at room temperature for 30 and 60 min, respectively. The titres of each vaccine component were calculated by the method of Reed and Muench [9].
2.4. Cats’ SPF-status verification
Prior to vaccination (day-1) conjunctival, oropharyngeal, nasal and rectal swabs from all cats were collected and tested by polymerase chain reaction (PCR) in order to confirm the SPF-status of the cats used in this study with respect to the following pathogens: FCV, FHV-1, FPV, feline coronavirus (FCoV) and C. felis. Blood samples were taken on the same day in order to detect the possible presence of antibodies to the vaccine antigens and to verify the lack of FeLV p27 antigenaemia.
2.5. Immunisation protocols and post-vaccination clinical monitoring
Cats were vaccinated at the age of 8–9 weeks by subcutaneous injection between the shoulder blades of 1 ml of antigenic material according to their group assignment (day 0): animals of group 1 (n = 10) received Nobivac® Forcat and Nobivac® FeLV applied together in one single syringe, animals of group 2 (n = 10) received Nobivac® Forcat alone and animals of group 3 (n = 10) were administered Nobivac® FeLV. Cats were given a second vaccination after a 3-week interval (day 21), according to the recommended schedule for primary immunisation of kittens [10].
Cats were monitored immediately after injection of the vaccines and daily for local or systemic reactions after vaccination. Rectal temperature was recorded at −24, 0 and 4 h after each inoculation and the injection site was examined for adverse reactions at 0, 4 and 8 h post-vaccination and daily thereafter. The degree of swelling was categorized into one of the four classes: 0 = no swelling observed, 1 = discrete swelling up to 5 mm, significant diffuse thickening, 2 = swelling approximately 6–10 and 3 = swelling over 10 mm. Kittens’ weights were recorded weekly throughout the study.
2.6. Blood collection and processing
Blood specimens were obtained at eight different time points (days 1, 6, 13, 20, 27, 34, 41, 48) by jugular venepuncture using a 2 ml disposable syringe (Omnifix, B. Braun, Germany). An aliquot of the blood (500 μl) was immediately transferred into 1.3 ml K3EDTA micro tubes (Sarstedt, Nümbrecht, Germany) and used for haematological testing. The remaining blood was transferred into evacuated tubes (2.5 ml BD SST glass Vacutainer®, Becton Dickinson AG, Switzerland) and used for serum preparation. The clotted blood samples were centrifuged and an aliquot of serum was taken for clinical biochemical analysis. The remaining serum was stored at −20 °C until required for testing. On day 48, a further 2 ml of blood was collected into evacuated tubes containing heparin (lithium heparin Vacutainer®, Becton Dickinson) in order to characterise the response of T lymphocytes to non-specific and C. felis-antigen stimulation in vitro by measuring their transcription of selected Th1 and Th2 cytokines. The serum samples were tested for the presence of antibodies to FCV, FHV-1, FPV and C. felis by immunofluorescence assay (IFA) and to FeLV p45 by enzyme-linked immunosorbent assay (ELISA), respectively.
2.7. Total nucleic acid extraction and PCR
Total nucleic acids were extracted from swabs (conjunctival, oropharyngeal, nasal and rectal swabs) and blood using MagNA Pure LC Total Nucleic Acid Isolation Kit and MagNA Pure LC Instrument (Roche Diagnostics AG, Rotkreuz, Switzerland) according to the manufacturer's recommendations. Two hundred microliters of PBS (Sigma, St. Louis, MO, USA) were added to the 1.5 ml microcentrifuge tubes containing the swabs and samples were vortexed for 15 s. Samples were incubated at 40 °C for 10 min. After incubation, microcentrifuge tubes were spun down at 8000 × g for 1 min, swabs were inverted and recentrifuged at 8000 × g for 1 min. Swabs were then discarded and the whole eluate was used for extraction of total nucleic acids. The extracted total nucleic acids were analysed by one-tube real-time TaqMan® PCR and reverse transcriptase (RT)-PCR using a sequence detection system (ABI 7700, Applied Biosystems, Rotkreuz, Switzerland). The swabs were tested for the presence of FCV using a modified protocol with the following primers and probe as described by Helps [11]: forward primer: 5′-GTTGGATGAACTACCCGCCAATC-3′; reverse primer: 5′-CATATGCGGCTCTGATGGCTTGAAACTG-3′ (Microsynth AG, Balgach, Switzerland); probe: 5′-6-FAM-TCGGTGTTTGATTTGGCCTG-TAMRA-3′ (Eurogentec, Seraing, Belgium). The detection of FHV-1 [12], FPV [13], FCoV [13], [14] and C. felis [15] was performed using PCR as described elsewhere.
2.8. Detection of FeLV virus protein p27 by enzyme-linked immunosorbent assay (ELISA)
FeLV p27 antigen in the serum was assayed by sandwich ELISA based on monoclonal antibodies as described by Lutz et al. [16].
2.9. Antibody assays
The serum samples were analysed for the presence of antibodies to FCV, FHV-1 and FPV by IFA, as described previously [17]. Semiconfluent CRFK cell monolayers were infected with FCV (F9 strain), FHV-1 (G2620a strain) and FPV (MW-1 strain), respectively. These virus strains were identical to those contained in the combined modified live vaccine. The antigen preparations were tested for absence of contaminating viruses by RT-PCR and PCR for FCV, FHV-1, FPV, FCoV, as described above, FeLV [18] and FIV [19] and were found to be positive only for the antigen intended to be present in the culture. The serum samples were tested at dilutions of 1:10, 1:20, 1:40, 1:80, 1:160, 1:320 and 1:640 for FCV and FHV-1 and of 1:10, 1:20, 1:40, up to 1:10,240 for FPV. The detection of antibodies to C. felis was carried out in 96-well microtitre plates by titration of the serum samples on a substrate consisting of methanol-fixed McCoy cells (2 × 105 cells/ml) infected with C. felis (104 TCID50/ml). After washing unbound antibodies with PBS, antibodies bound to C. felis were detected by incubation of the wells with a goat anti-cat immunoglobulin fluorescein isothiocyanat conjugate (Kirkegaard & Perry Laboratories, Gaithersburg, USA). Subsequently, 50% glycerol in PBS was added to each well and plates were examined under a UV-light microscope for positive fluorescence of chlamydial inclusions. Antibody titres were expressed as the reciprocal of the last serum dilution showing positive fluorescence in the IFA. Antibodies to FeLV p45, the non-glycosylated form of gp70 were measured by ELISA as described elsewhere [20], [21].
2.10. Haematology and clinical biochemistry
Complete haematological analyses were performed by routine procedures using an automatic electronic cell counter (Cell-Dyn 3500; Abbott, Baar, Switzerland).
Bilirubin, glucose, urea, creatinine, protein, albumin, cholesterine, alkaline phosphatase, aspartate aminotransferase, alanine aminotransferase, lipase, sodium, potassium, chloride, calcium and phosphorus were assayed by automated analysis (Cobas-Integra 700; Roche Diagnostics AG) according to standard procedures recommended by the International Federation of Clinical Chemistry [22].
2.11. Culture and stimulation of peripheral blood mononuclear cells (PBMC)
T-lymphocyte responses to different in vitro stimulations were characterized by quantifying various cytokine mRNA transcripts by real-time RT-PCR. A pre-experiment with different stimulation protocols was performed in order to determine the best conditions suitable for the stimulation of feline PBMC. PBMC were incubated with either 10 μg/ml Con A (Type IV-S, Sigma), 5 μg/ml phytohemagglutinin (PHA, M Form, Gibco BRL, Life Technologies, Paisley, Scotland) or 2.5 ng/ml phorbol 12-myristate 13-acetate (PMA, Sigma) and 250 ng/ml Ionomycin (Sigma) under the same conditions as described subsequently.
PBMC were isolated from 2 ml heparinised blood collected at week 4 after the second vaccination by density gradient centrifugation through Ficoll-Hypaque (density 1.077; Sigma) [23]. The mononuclear cell fraction was resuspended in 1.2 ml RPMI 1640 medium (Gibco-BRL) supplemented with 10% fetal calf serum (Gibco), 100 U/ml penicillin, 100 μg/ml streptomycin (Gibco) and 100 U/ml recombinant human IL-2 (Sigma). From each cell suspension, three cell cultures per cat were initiated on 24-well plates at a cell density of 5 × 105 cells/well. Cells were stimulated either with 5 μl of cell culture medium (control), 10 μg of Con A (Sigma, non-specific stimulation) or 5 μl of Chlamydophila-antigen (specific stimulation). The feline Chlamydophila-antigen was a semi-purified preparation and produced as follows: feline Chlamydophila Baker strain material was overlaid on top of a 30% Urograffin 370 gradient (Schering, Burgess Hill, Great Britain). The resulting pellet was resuspended in PBS and subsequently run on a 30–60% Urograffin 370 gradient. Fractions were collected and screened by ELISA for maximum antigen content using standard methods [24]. The antigen used for T-cell stimulation consisted of the fraction that showed the highest activity by ELISA. Cells were then incubated for 16 h at 37 °C in 5% CO2. After incubation, cells were pelleted by centrifugation and lysed directly in the plate by addition of 330 μl of lysis buffer (MagNA Pure LC, mRNA Isolation Kit I, Roche Diagnostics AG). Samples were transferred to 1.5 ml microcentrifuge tubes and stored at −80 °C until use.
2.12. Messenger RNA extraction
mRNA was isolated from the cultured cells by MagNA Pure LC using the mRNA Isolation Kit I (mRNA I Cells protocol) according to the manufacturer's recommendations (Roche Diagnostics AG).
2.13. Quantification of cytokine transcripts by real-time PCR
Transcription of interleukin (IL)-4 and interferon gamma (IFNγ) as well as the housekeeping gene coding for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was quantitated by RT-PCR as described [25]. IL-2 mRNA transcription was detected by RT-PCR using the sense primer 5′-CTCTCCAGGATGCTCACATTTAAA-3′, the antisense primer 5′-TCTTCTACTAGACACTGAAGATGTGTCAAT-3′ (Microsynth AG) and probe 5′-6-FAM-TTTACGTGCCCAAGAAGGCCACAGA-TAMRA-3′ (Eurogentec). Thermal and assay conditions used for measuring IL-2 mRNA transcripts were the same as for the other cytokines tested [25]. The primers and probe concentrations were 300 nM and 200 nM, respectively. Transcription of different cytokines and GAPDH were assayed in the same run for each cat to minimise inter-assay variations. Quantitative analysis of cytokine mRNA concentrations was normalised in relation to feline GAPDH, which was set to a theoretical value of 1,000,000 gene copies.
2.14. Statistical analysis
Statistical analyses of antibody-titres were carried out using Mann–Whitney U-test which was performed using GraphPad Prism version 3.00 for Windows (GraphPad Software, San Diego, CA, USA). A P-value <0.05 was considered to be significant. Haematological and biochemical parameters were analysed for significant differences between the groups (P < 0.05) by the Kruskal–Wallis test (StatView 5.0, SAS institute Inc., Cary, NC, USA). Comparison of the different stimulations in the cytokine assay was performed using the Kruskal–Wallis test followed by Dunn's multiple comparison post-test (GraphPad Prism version 3.00).
3. Results
3.1. In vitro compatibility of the vaccines
The titres of the modified live vaccine components reconstituted with either the FeLV vaccine or solvent did not significantly differ (Table 1 ). Even after incubation for 1 h at room temperature, no significant titre decreases were observed.
Table 1.
Viral titres of the modified live vaccine components reconstituted with either the FeLV vaccine or solvent
| Vaccine components | Time (min) post-reconstitution | Nobivac® Forcat + Nobivac® solvent (titres in log10 TCID50) | Nobivac® Forcat + Nobivac® FeLV (titres in log10 TCID50) |
|---|---|---|---|
| FCV | 0 | 4.9 | 4.8 |
| 30 | 4.7 | 5.1 | |
| 60 | 4.2 | 4.6 | |
| FHV-1 | 0 | 6.8 | 7.4 |
| 30 | 6.9 | 7.2 | |
| 60 | 7.1 | 7.3 | |
| FPV | 0 | 5.4 | 5.3 |
| 30 | 5.2 | 5.2 | |
| 60 | 5.5 | 5.5 | |
| C. felis | 0 | 2.9 | 3.1 |
| 30 | 2.9 | 3.6 | |
| 60 | 3.2 | 3.5 | |
3.2. SPF-status of the cats
No evidence of infection with any of the pathogens assayed (FCV, FHV-1, FPV, FeLV, FCoV, C. felis) was found in any of the samples collected prior to vaccination.
3.3. Clinical aspects
Following vaccination, no systemic reactions or clinical signs were observed in any of the cats in any of the vaccine groups. Rectal temperatures measured at −24, 0 and 4 h after each inoculation and daily thereafter until the end of the study never exceeded the physiological range in any cat (37.8–39.6 °C, data not shown). There were no significant differences in the temperature curves of the three groups (data not shown). The injection site was examined for adverse reactions at 0, 4 and 8 h post-vaccination and daily thereafter. The cats from groups 1 and 3, which had received the FeLV vaccine, over the first 8 h after vaccination, showed a total mean score of 2.6 and 1, respectively, which was significantly higher (P < 0.0001) than the mean score of 0.2 observed in cats of group 2 which were vaccinated by the modified live vaccine alone. The local reaction scores combined for the first 8 h after vaccination did not exceed the score 2 with the exceptions of 2 cats in group 1 which had a cumulated score of 4 and 10, respectively. However, the swelling readily disappeared spontaneously within a few days. The kittens gained weight constantly throughout the study irrespective of the vaccine group.
3.4. Antibody induction
The antibody responses to the different vaccine antigens were measured in all groups at 1-week intervals starting from the day before the first vaccination (day 1) until 4 weeks after completion of the primary immunisation (day 48) (Fig. 1 ). Antibody titres to FCV were significantly higher on days 20–48 in the cats that had received the combined vaccine (group 1) compared to cats vaccinated with only the multivalent live vaccine (group 2) (Fig. 1a, * P < 0.05). Antibody titres to FHV-1 were significantly higher in the cats of group 1 on days 34 and 41 (Fig. 1b). No significant differences were found in antibody titres to FPV between cats of groups 1 and 2, with the exception of days 27 (P = 0.035) and 48 (P = 0.001) where the animals receiving the combined vaccination had lower median titres (Fig. 1c). The cats of group 1 and 2 produced comparable median antibody titres to C. felis except on day 41 (P < 0.05) (Fig. 1d). Vaccinations induced comparable antibody levels against FeLV in groups 1 and 3 with the exception of days 34 and 41 (Fig. 1e).
Fig. 1.
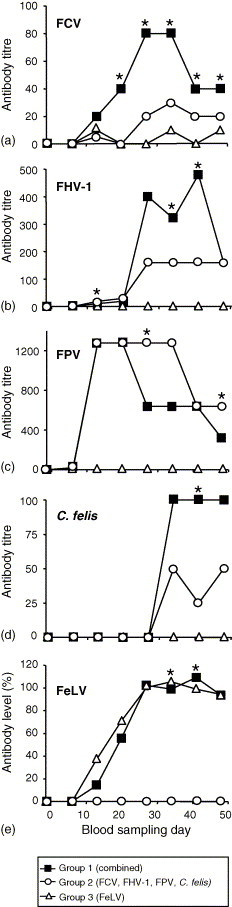
Antibody induction to (a) feline calicivirus (FCV), (b) feline herpesvirus-1 (FHV-1), (c) feline parvovirus (FPV), (d) Chlamydophila felis (C. felis), and (e) feline leukaemia virus (FeLV) in primary immunised kittens with either the combination of multivalent MLV and FeLV vaccine (group 1), only multivalent MLV vaccine (group 2) or only FeLV vaccine (group 3). Vaccinations took place on day 0 and 21. Antibodies to FCV, FHV-1, FPV and C. felis were determined by IFA and median titres are depicted. Antibodies to the recombinant antigen FeLV p45 were determined by ELISA and are given as percentage of a positive control. In (a) to (d), groups 1 and 2, and in (e) groups 1 and 3 were statistically compared for significant differences (*) by the Mann–Whitney U-test (P < 0.05).
3.5. Haematology and clinical biochemistry
No changes related to vaccination in the measured haematological and biochemical parameters were observed (data not shown), except in the white blood cells (WBC) counts. Cats of group 1 and 2, which were vaccinated with the multivalent MLV vaccine, showed a significantly decreased number of WBC 1 week after the first vaccination (day 6, P = 0.0007). Differential blood counts revealed that the reduction of WBC in groups 1 and 2 was mostly due to a transient lymphopenia (mean lymphocyte counts in group 1 = 1200/μl, in group 2 = 1230/μl and in group 3 = 4830/μl, P between groups 1 and 3, 2 and 3 <0.01) and to a lesser degree to decreased neutrophil counts (mean neutrophil counts in group 1 = 3780/μl, in group 2 = 3560/μl and in group 3 = 7120/μl, P between groups 1 and 3, 2 and 3 <0.01), monocytes and eosinophils (data not shown).
3.6. Cytokine mRNA transcription after in vitro stimulation with Chlamydophila-antigen
PBMC were isolated from all cats and were tested for mRNA levels to the Th1 cytokines IFNγ and IL-2 and the Th2 cytokine IL-4 in response to Chlamydophila-antigen. The purpose of these experiments was to characterise the type of immune response induced by the C. felis vaccine antigen (Fig. 2 ). PBMC collected from cats of group 1 (combined vaccine) produced higher levels of IL-2 specific mRNA after Con A and C. felis-antigen stimulation compared to cats of groups 2 and 3 (P < 0.05). However, a significant difference in IL-2 expression was also observed in unstimulated lymphocytes (P < 0.01). It is interesting to note that expression levels of mean IL-2 values in lymphocytes from cats of groups 1 and 2 stimulated by C. felis antigen and in non-stimulated lymphocytes did not differ (Fig. 2a, middle and right graph, P > 0.05). However, the levels of IL-2 mRNA expression in lymphocytes from group 3 were higher after C. felis antigen stimulation than in unstimulated lymphocytes of group 3 cats, P = 0.0355. The pattern of IL-4 expression was similar to that of IL-2 mRNA synthesis, but here, no difference was found between IL-4 mRNA levels of lymphocytes stimulated by C. felis and non-stimulated lymphocytes (P > 0.05). No difference in IFNγ transcription was observed among the groups. An increase in IL-4 expression was found after C. felis-stimulation in the cats of group 1 (P < 0.005). However, a similar increase was also seen in the unstimulated lymphocytes (P < 0.005).
Fig. 2.
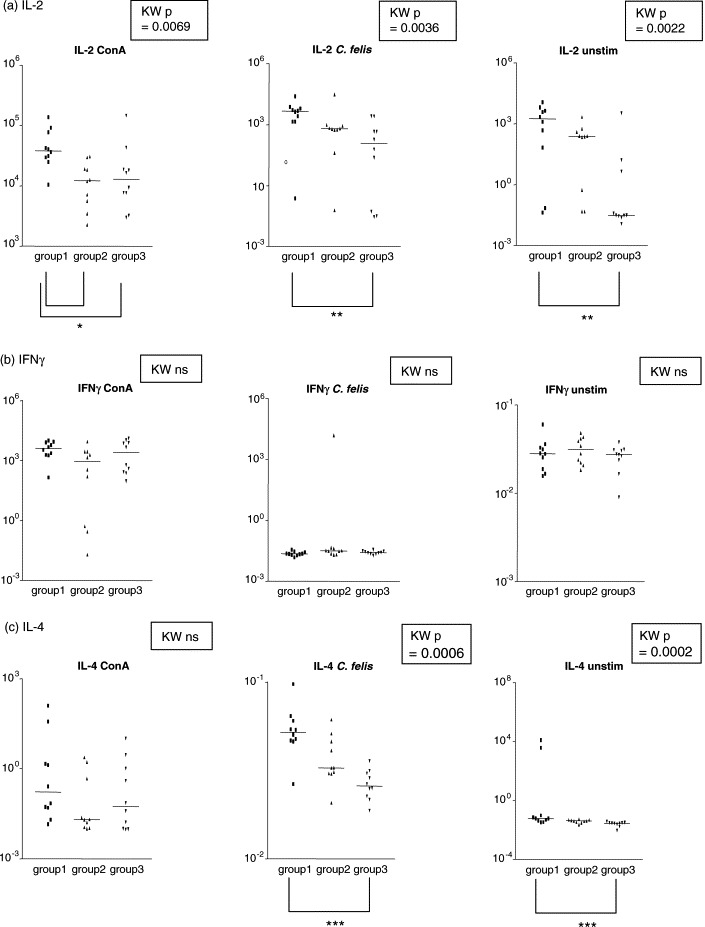
Expression of mRNA of (a) IL-2, (b) IFNγ and (c) IL-4 in Con A stimulated, C. felis-antigen stimulated and unstimulated PBMC cultures. The cytokine expression was normalised to GAPDH, which was set to a theoretical value of 1,000,000 gene copies. Statistical analysis was performed using Kruskal–Wallis and Dunn's multiple comparison test (*, P < 0.05; **, P < 0.01; ***, P < 0.005; ns = not significantly different).
4. Discussion
In this study, the question was evaluated whether a MLV vaccine could be injected together in one syringe with a recombinant FeLV vaccine without loss of antigenicity compared with the single immunization of cats by each of the MLV and the FeLV vaccines. To this end, three groups of cats were vaccinated. It would have been interesting to include a forth group in which the cats would have been vaccinated with the MLV and the FeLV vaccine on separate injection sites. As veterinarians are focussing on the use of a single injection and we did not have the means to include this forth group, the question on how the immune response had been in this forth group cannot be answered. Safety-related assessments of a vaccine and its adjuvants should include serum biochemistry, haematology and injection site observation. The combined administration of the multivalent MLV vaccine and FeLV vaccine caused no noticeable increase in side effects as compared to the administration of the separate components and did not induce any undesirable systemic reactions such as fever, depression or loss of appetite. A local reaction to the vaccination was observed in cats of groups 1 and 3. This local inflammatory reaction was almost certainly caused by the FeLV vaccine and was probably related to its aluminium and Quil A adjuvant. However, this side effect is of little clinical relevance and the swelling at the injection site which was comparable with that induced by the single application of the FeLV vaccine disappeared without any treatment within a few days. Aluminium hydroxide is a depot-forming adjuvant that induces the formation of a macrophage-rich granuloma at the vaccine injection site, which prolongs the antigenic stimulus [1]. The adjuvant of the FeLV vaccine component is a non-toxic fraction of Quil A (QS 21) derived from the bark of the South American tree Quillaja saponaria which has significant adjuvant activity [26]. No haematological nor biochemical changes were observed after vaccination with the exception of a transient leukopenia 1 week after the first vaccination (day 6) in both groups vaccinated with the multivalent MLV vaccine. This transient leukopenia was most probably caused by the FPV component of the vaccine and has also been observed by others [27]. It may correspond to the transient leukopenia observed during infection with virulent FPV under field conditions. Both vaccines were thus well tolerated by the cats in the combined application as well as in the single application.
The efficacy of antibody induction after combined vaccine application was compared with that following vaccination with either vaccine alone. Remarkably, the antibody production against FCV and at selected time points against FHV-1 and C. felis induced by the vaccination was significantly higher after the combined vaccination than after the single vaccination with the multivalent MLV vaccine. In group 1 cats, the antibody titres against FCV, FHV-1 and FPV somewhat declined versus the end of the observation period, especially against FPV after the second vaccination. While no firm explanation for this decrease can be offered, it can be speculated that it represents a natural decline after exposure to the antigen had peaked to high levels. In the cats of group 3 (vaccinated solely with the FeLV vaccine), low titres against FCV were observed on days 14, 34 and 48. As these groups were completely separated from each other, these low titres most likely do not indicate that the cats became infected by FCV inadvertently but rather a non-specific immunofluorescence reaction observed at low serum dilutions. No information is available with respect to the correlation between the antibody titres found in our cats and protection against field virus infection. The observation that the combined vaccines in group 1 induced antibody titres similar or even higher than those of cats in groups 2 and 3, respectively, suggests that a similar degree of protection under field conditions can be expected.
It might have been expected that the combination of live attenuated viruses with the Quil A fraction QS 21 would result in inactivation of the enveloped FHV-1 and possibly also of the non-enveloped FCV and FPV because of its detergent properties. However, the in vitro compatibility study showed that the viability of the four live components of the multivalent vaccine was not impaired when combined with the FeLV vaccine. Interestingly, in vivo, antibody titres to FPV were somewhat reduced in group 1 on two occasions (days 27 and 48). No explanation can be offered for this observation; it is well known that parvoviruses are extremely resistant to detergents and that it is difficult to imagine that the adjuvants that did not alter the viability of the FCV and FHV-1 should have partially inactivated the parvoviruses. The antibody titres against FeLV were similar in cats receiving combined and single vaccine regimens, respectively. The observation that cats of groups 1 and 3 showed identical antibody kinetics and titres against FeLV is not surprising as in both groups the nature and amount of adjuvants were identical. The cats produced antibodies against all vaccine antigens. Although this seroconversion is no proof that the viruses did in fact replicate, replication must be postulated for the three MLV components. If the FCV, FHV-1 and FPV had been inactivated, the titres would have undoubtedly been much lower as the antigenic mass of these viruses probably were too small for antibody induction.
Protection against intracellular bacteria such as C. felis is mediated by macrophage activation and cytotoxic T cells, i.e., cell-mediated immunity. It has previously been observed that only vaccines containing live organisms are able to induce a protective immunity against intracellular bacteria [28]. In the PBMC cultures, the T lymphocytes were stimulated to produce IL-2 and IL-4 particularly in group 1 while IFNγ expression was equal in all groups, independent of the mode of stimulation. Interestingly, the increase of IL-4 expression in the lymphocytes of group 1 observed after C. felis-stimulation was paralleled by an increased IL-4 expression in unstimulated lymphocytes. When a ratio of IL-2 and IL-4 expression in lymphocytes with C. felis-stimulation and no stimulation was made in order to eliminate the influence of in vivo T cell stimulation, no difference between the groups in the degree of IL-2 and IL-4 expression after C. felis-stimulation was found. This observation suggests that lymphocytes of cats of group 1 had already been stimulated in vivo possibly due to the combination of the MLV components with the adjuvant of the FeLV vaccine. This adjuvant contains QS-21, an immunomodulator fraction of adjuvant Quil A which has been shown to enhance significantly both antibody (Th1) and cell-mediated (Th2) immune responses [29], [30], [31]. The aluminium hydroxide adjuvant promotes primarily a humoral, Th2 biased immune response. Interestingly, lymphocytes of group 3 cats stimulated with C. felis expressed significantly higher IL-2 levels than unstimulated lymphocytes which also suggests that the adjuvants of the FeLV vaccine had induced an in vivo activation of lymphocytes to readily induce IL-2 but not IL-4.
In conclusion, our results indicate that the use of a multivalent MLV vaccine with a chlamydophilal component in combination with recombinant FeLV vaccine is safe. No loss of antigenicity was observed and the combined application even resulted in a higher antibody response to the FCV, FHV-1 and C. felis components. The latter observation can be explained by the immunostimulating effects of the adjuvants contained in the FeLV vaccine [32].
Acknowledgements
Laboratory work was performed using the logistics of the Center for Clinical Studies at the Vetsuisse Faculty of the University of Zurich. We are grateful to E. Gönczi, V. Fornera, T. Meili Prodan, E. Rogg, E. Rhiner, Y. Bosshart, C. Brönnimann, U. Egger, E. Grässli, M. Huder, B. Lange and J. Wälchli for excellent laboratory assistance. R.H.L. is the recipient of a professorship by the Swiss National Science Foundation (PP00B-102866).
Contributor Information
Chantal Brunner, Email: cbrunner@rocketmail.com.
Theo Kanellos, Email: theo.kanellos@intervet.com.
Marina L. Meli, Email: mmeli@vetclinics.unizh.ch.
David J. Sutton, Email: david.sutton@intervet.com.
Ricarda Gisler, Email: ricarda.gisler@veterinaria.ch.
Maria Alice Gomes-Keller, Email: mgomes@vetclinics.unizh.ch.
Regina Hofmann-Lehmann, Email: rhofmann@vetclinics.unizh.ch.
Hans Lutz, Email: hlutz@vetclinics.unizh.ch.
References
- 1.Tizzard I.R., editor. Veterinary immunology: an introduction. 6th ed. W.B. Saunders Company; Philadelphia: 2000. [Google Scholar]
- 2.Manoj S., Babiuk L., van Drunen Littel-van den Hurk S. Immunization with a dicistronic plasmid expressing a truncated form of bovine herpesvirus-1 glycoprotein D and the amino-terminal subunit of glycoprotein B results in reduced gB-specific immune responses. Virology. 2003;313(1):296–307. doi: 10.1016/s0042-6822(03)00325-8. [DOI] [PubMed] [Google Scholar]
- 3.Kang S.M., Guo L., Yao Q., Skountzou I., Compans R.W. Intranasal immunization with inactivated influenza virus enhances immune responses to coadministered simian-human immunodeficiency virus-like particle antigens. J Virol. 2004;78(18):9624–9632. doi: 10.1128/JVI.78.18.9624-9632.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alexandersen S. Advantages and disadvantages of using live vaccines risks and control measures. Acta Vet Scand Suppl. 1996;90:89–100. [PubMed] [Google Scholar]
- 5.Kensil C.R., Mo A.X., Truneh A. Current vaccine adjuvants: an overview of a diverse class. Front Biosci. 2004;9:2972–2988. doi: 10.2741/1452. [DOI] [PubMed] [Google Scholar]
- 6.Marciani D.J., Kensil C.R., Beltz G.A., Hung C.H., Cronier J., Aubert A. Genetically-engineered subunit vaccine against feline leukaemia virus: protective immune response in cats. Vaccine. 1991;9(2):89–96. doi: 10.1016/0264-410x(91)90262-5. [DOI] [PubMed] [Google Scholar]
- 7.Coutts A.J., Dawson S., Willoughby K., Gaskell R.M. Isolation of feline respiratory viruses from clinically healthy cats at UK cat shows. Vet Rec. 1994;135(23):555–556. [PubMed] [Google Scholar]
- 8.Chalmers W.S., Truyen U., Greenwood N.M., Baxendale W. Efficacy of feline panleucopenia vaccine to prevent infection with an isolate of CPV2b obtained from a cat. Vet Microbiol. 1999;69(1/2):41–45. doi: 10.1016/s0378-1135(99)00085-1. [DOI] [PubMed] [Google Scholar]
- 9.Reed L.J., Meunch H. A simple method of estimating fifty percent endpoints. Am J Hyg. 1938;(27):493–497. [Google Scholar]
- 10.American Association of Feline Practitioners and Academy of Feline Medicine feline vaccine panel. Recommended guidelines for vaccination of cats. Hillsborough NJ, USA, 2001.
- 11.Helps C., Lait P., Tasker S., Harbour D. Melting curve analysis of feline calicivirus isolates detected by real-time reverse transcription PCR. J Virol Methods. 2002;106(2):241–244. doi: 10.1016/s0166-0934(02)00167-2. [DOI] [PubMed] [Google Scholar]
- 12.Vogtlin A., Fraefel C., Albini S., Leutenegger C.M., Schraner E., Spiess B. Quantification of feline herpesvirus 1 DNA in ocular fluid samples of clinically diseased cats by real-time TaqMan PCR. J Clin Microbiol. 2002;40(2):519–523. doi: 10.1128/JCM.40.2.519-523.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meli M., Kipar A., Muller C., Jenal K., Gönczi E., Borel N. High viral loads despite absence of clinical and pathological findings in cats experimentally infected with feline coronavirus (FCoV) type I and in naturally FCoV-infected cats. J Feline Med Surg. 2004;6(2):69–81. doi: 10.1016/j.jfms.2003.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gut M., Leutenegger C.M., Huder J.B., Pedersen N.C., Lutz H. One-tube fluorogenic reverse transcription-polymerase chain reaction for the quantitation of feline coronaviruses. J Virol Methods. 1999;77(1):37–46. doi: 10.1016/S0166-0934(98)00129-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Helps C., Reeves N., Egan K., Howard P., Harbour D. Detection of Chlamydophila felis and feline herpesvirus by multiplex real-time PCR analysis. J Clin Microbiol. 2003;41(6):2734–2736. doi: 10.1128/JCM.41.6.2734-2736.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lutz H., Pedersen N.C., Durbin R., Theilen G.H. Monoclonal antibodies to three epitopic regions of feline leukemia virus p27 and their use in enzyme-linked immunosorbent assay of p27. J Immunol Methods. 1983;56(2):209–220. doi: 10.1016/0022-1759(83)90413-1. [DOI] [PubMed] [Google Scholar]
- 17.Hofmann-Lehmann R., Fehr D., Grob M., Elgizoli M., Packer C., Martenson J.S. Prevalence of antibodies to feline parvovirus, calicivirus, herpesvirus, coronavirus, and immunodeficiency virus and of feline leukemia virus antigen and the interrelationship of these viral infections in free-ranging lions in east Africa. Clin Diagn Lab Immunol. 1996;3(5):554–562. doi: 10.1128/cdli.3.5.554-562.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hofmann-Lehmann R., Huder J.B., Gruber S., Boretti F., Sigrist B., Lutz H. Feline leukaemia provirus load during the course of experimental infection and in naturally infected cats. J Gen Virol. 2001;82(Pt 7):1589–1596. doi: 10.1099/0022-1317-82-7-1589. [DOI] [PubMed] [Google Scholar]
- 19.Leutenegger C.M., Klein D., Hofmann-Lehmann R., Mislin C., Hummel U., Boni J. Rapid feline immunodeficiency virus provirus quantitation by polymerase chain reaction using the TaqMan fluorogenic real-time detection system. J Virol Methods. 1999;78(1/2):105–116. doi: 10.1016/s0166-0934(98)00166-9. [DOI] [PubMed] [Google Scholar]
- 20.Lutz H., Pedersen N., Higgins J., Hubscher U., Troy F.A., Theilen G.H. Humoral immune reactivity to feline leukemia virus and associated antigens in cats naturally infected with feline leukemia virus. Cancer Res. 1980;40(10):3642–3651. [PubMed] [Google Scholar]
- 21.Lehmann R., Franchini M., Aubert A., Wolfensberger C., Cronier J., Lutz H. Vaccination of cats experimentally infected with feline immunodeficiency virus, using a recombinant feline leukemia virus vaccine. J Am Vet Med Assoc. 1991;199(10):1446–1452. [PubMed] [Google Scholar]
- 22.Tieze N.W. W.B. Saunders Company; Philadelphia: 1995. Clinical guide to laboratory tests. [Google Scholar]
- 23.Kipar A., Leutenegger C.M., Hetzel U., Akens M.K., Mislin C.N., Reinacher M. Cytokine mRNA levels in isolated feline monocytes. Vet Immunol Immunopathol. 2001;78(3/4):305–315. doi: 10.1016/s0165-2427(01)00240-9. [DOI] [PubMed] [Google Scholar]
- 24.Crowther J.R. Humana Press; Totowa, NJ: 2001. The ELISA guidebook. [Google Scholar]
- 25.Leutenegger C.M., Mislin C.N., Sigrist B., Ehrengruber M.U., Hofmann-Lehmann R., Lutz H. Quantitative real-time PCR for the measurement of feline cytokine mRNA. Vet Immunol Immunopathol. 1999;71(3/4):291–305. doi: 10.1016/S0165-2427(99)00100-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kensil C.R., Patel U., Lennick M., Marciani D. Separation and characterization of saponins with adjuvant activity from Quillaja saponaria Molina cortex. J Immunol. 1991;146(2):431–437. [PubMed] [Google Scholar]
- 27.Buonavoglia C., Marsilio F., Tempesta M., Buonavoglia D., Tiscar P.J., Cavalli A. Use of a feline panleukopenia modified live virus vaccine in cats in the primary-stage of feline immunodeficiency virus infection. Zentralbl Veterinarmed B. 1993;40(5):343–346. doi: 10.1111/j.1439-0450.1993.tb00148.x. [DOI] [PubMed] [Google Scholar]
- 28.Kaufmann S.H. Immunity to bacteria. Curr Opin Immunol. 1989;2(3):353–359. doi: 10.1016/0952-7915(89)90141-6. [DOI] [PubMed] [Google Scholar]
- 29.Newman M.J., Wu J.Y., Gardner B.H., Munroe K.J., Leombruno D., Recchia J. Saponin adjuvant induction of ovalbumin-specific CD8+ cytotoxic T lymphocyte responses. J Immunol. 1992;148(8):2357–2362. [PubMed] [Google Scholar]
- 30.Sjölander A., van’t Land B., Lovgren Bengtsson K. Iscoms containing purified Quillaja saponins upregulate both Th1-like and Th2-like immune responses. Cell Immunol. 1997;177(1):69–76. doi: 10.1006/cimm.1997.1088. [DOI] [PubMed] [Google Scholar]
- 31.Kim S.K., Ragupathi G., Musselli C., Choi S.J., Park Y.S., Livingston P.O. Comparison of the effect of different immunological adjuvants on the antibody and T-cell response to immunization with MUC1-KLH and GD3-KLH conjugate cancer vaccines. Vaccine. 1999;18(7/8):597–603. doi: 10.1016/s0264-410x(99)00316-3. [DOI] [PubMed] [Google Scholar]
- 32.Kensil C.R., Newman M.J., Coughlin R.T., Soltysik S., Bedore D., Recchia J. The use of stimulon adjuvant to boost vaccine response. Vaccine Res. 1993;2(4):273–281. [Google Scholar]


