Abstract
Several hitherto unknown (E)-but-2-enyl nucleoside phosphonoamidate analogs (ANPs) were prepared directed with nitrogen reagents by cross-metathesis in water-under ultrasound irradiation. Two diastereoisomers were formally identified by X-ray diffraction. These compounds were evaluated against a large spectrum of DNA and RNA viruses. Among them, the phosphonoamidate thymine analogue 19 emerged as the best prodrug against varicella-zoster virus (VZV) with EC50 values of 0.33 and 0.39 μM for wild-type and thymidine kinase deficient strains, respectively, and a selectivity index ≥200 μM. This breakthrough approach paves the way for new purine and pyrimidine (E)-but-2-enyl phosphonoamidate analogs.
Keywords: Ultrasound, Aqueous cross-metathesis, Phosphonoamidate, Acyclic nucleoside phosphonates, DNA viruses
Abbreviations: VZV, varicella zoster virus; VV, vaccinia virus; HSV, herpes simplex virus; VSV, vesicular stomatitis virus; DNA, deoxyribonucleic acid; RNA, ribonucleic acid; CC50, compound concentration affording 50% inhibition of cell growth; EC50, compound concentration affording 50% inhibition of the viral cytopathicity; MCC, minimum cytotoxic concentration required to afford a microscopically detectable alteration of cell morphology; ACN, acetonitrile; DCM, dichloromethane
Graphical abstract
Highlights
-
•
Phosphonoamidate prodrugs acyclic nucleosides were synthesized by convergent approach.
-
•
Metathesis reaction in water was used between pyrimidic bases and a new phosphonoamidate synthons.
-
•
EC50 values of any molecules were in (sub)micromolar range against DNA viruses.
1. Introduction
Modified nucleosides represent a major class of therapeutics for cancer and viral diseases [1]. Among them, acyclic nucleoside phosphonates (ANPs) pioneered with (S)-9-[3-hydroxy-2-(phosphonomethoxy)propyl]adenine ((S)-HPMPA) [2] in 1986 by Antonín Holý and Erik De Clercq, forms a key class of drugs active against various DNA viruses as well as against retroviruses. However, those compounds suffer from limitations such as their reduced cell penetration (the free phosphonic acid form is negatively charged at physiological pH) as well as from nephrotoxicity. This has led to extensive search for new ANPs as well as to the development of prodrug approaches [3,4] for enhanced bioavailability and cell internalisation [[5], [6], [7], [8]]. Several ANP prodrugs were marketed, such as adevofir dipivoxyl (bis-POM PMEA) [9,10] for the treatment of hepatitis B virus (HBV), tenefovir disoproxyl (bis-POC PMPA) [11] or the newly tenofovir alafenamide [[12], [13], [14]] for the treatment of human immunodeficiency virus (HIV) and HBV [15,16].
Over the last decade, our group has developed a new family of ANPs based on a trans-but-2-enyl phosphonate scaffold [17]. Compounds were directly obtained as prodrugs by a highly convergent and modular approach based on the powerful olefin acyclic cross metathesis (CM) between various allylphosphonate synthons bearing biolabile groups and N1- (or N9) crotyl (or allyl) pyrimidines or purines. This approach showed a remarkable breakthrough for the synthesis of nucleoside prodrugs compared to the known linear approaches, which suffer from low yields. It is also clear from the literature that the choice of a prodrug has a direct impact on its targeting and cell release and greatly influences the overall outcome and efficiency of the parent drug [[18], [19], [20]]. Thus, following this synthetic pathway, we have obtained several prodrugs [[21], [22], [23], [24], [25]] including the most commonly used carbonyloxymethyl pronucleotides (pivaloyloxymethyl- or POM, isopropyloxycarbonyloxymethyl- or POC), but also the alkoxyalkyl monoester (hexadecyloxypropyl or HDP, octadecyloxyethyl or ODE) [26]. Several of these (E)-but-2-enyl ANPs prodrugs exhibited remarkable antiviral activity against DNA and RNA viruses in submicromolar concentrations. The bis-(POM)-(E)-TbutP (1) and the prodrugs 2–4 were all very active against several herpesviruses [i.e. herpes simplex virus 1 (HSV-1) and 2 (HSV-2), and varicella-zoster virus (VZV)], representing a new potential antiviral lead.
Despite a significant amount of research and development on aryl phosphoramidate prodrugs reported by McGuigan [27,28], the development of aryl phosphonoamidates, especially in the field of ANPs, has been very sparely investigated [29]. Thus, in this article, we describe the in-water ultrasound promoted synthesis and antiviral evaluation of hitherto unknown (E)-but-2-enyl nucleoside phosphonoamidates with high yields, (Fig. 1 ).
Fig. 1.
(E)-but-2-enyl ANPs prodrugs developed by Agrofoglio et al. and targeted phosphonoamidates.
2. Results and discussion
2.1. Chemistry
Aryl phosphonoamidates are generally obtained by treatment of the parent dimethylphosphonate nucleoside with TMSBr into the corresponding silyl esters, followed by a subsequent treatment with an excess of phenol and l-alanine-O-alkyl ester in the presence of triphenylphosphine and Aldrithiol. The desired products are often isolated with poor yields (<3%) to traces as stated for the preparation of phosphonoamidate prodrug of tenofovir (TAF) [30,31] In order to avoid these low yields, our strategy to the targeted phenyl phosphonoamidate of (E)-but-2-enyl ANPs, was to react the allyl phenylphosphonoamidate with a N1-crotylated nucleobase under acyclic cross metathesis (CM), which is quite challenging taking into account the poisoning effect of nitrogens on CM ruthenium catalysts [32]. Any attempt to obtain the desired allylic phosphonoamidates, by the shortest strategy involving the introduction of allyl group on the phosphorodichloridate 5 to give the desired monoalkenylated compound 6, failed. In our hands as, despite several conditions, only the product of dialkylation was obtained, when observed (Scheme 1 ).
Scheme 1.
Reagents and conditions: (a) allylmagnesium bromide, diethyl ether or THF, −78 °C to RT (failed).
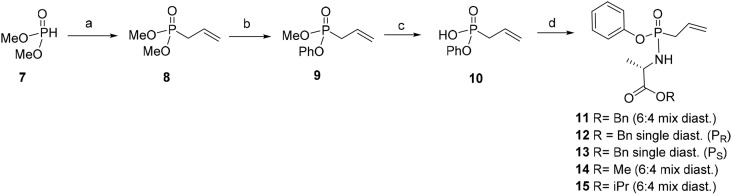
Therefore, we decided to turn our attention to the H-phosphonate chemistry. The dimethylphosphite 7 was reacted with allyl bromide under Michaelis-Becker conditions to give the dimethylallylphosphonate 8 with 78% yield. It is important to quote than this reaction was scaled-up to 50 g. After substitution of a OMe group of 8 by a chlorine in presence of oxalyl chloride, this position was then substituted by a phenolate generated in situ to give 9 (as a mixture of enantiomers) in 65% yield. Compound 9 was treated by bromotrimethylsilane in dichloromethane for 24 h at room temperature, to give the phosphonic acid monoester derivative 10 in excellent 91% yield (Scheme 2 ).
Scheme 2.
Reagents and conditions: (a) Allyl bromide, K2CO3, TBAB, THF, 90 °C, 12h, 78%, (b) 1) (COCl)2, DCM, 50 °C, 24h, 2) Et3N, Phenol, DCM, 50 °C, 48h, 65%, (c) TMSBr, DCM, RT, 24h, 91%, (d) 1) (COCl)2, DMF cat., DCM, RT, 1.5h, 2) Et3N, DCM, l-alanine benzyl ester hydrochloride, RT, 24h, 63% (for 11, 12 and 13). 2) Et3N, DCM, l-alanine methyl ester hydrochloride, RT, 24h, 52% (for 14). 2) Et3N, DCM, l-alanine isopropyl ester hydrochloride, RT, 24h, 57% (for 15).
Following a procedure described by Gajda et al. [33], phosphonate 10 was then converted to various methyl-, isopropyl- and benzyl-l-alanine esters 11–15, in order to compare the influence of the ester group or the phosphorus chirality, on the activity and toxicity of final ANPs. Compounds 11,14 and 15 were obtained as a mixture of diastereomers (from 6:4 to 1:1). Only the diastereomers of compound 11 (R = Bn) were separated by careful column chromatography on silica gel (twice) and compounds 12 and 13 were isolated as single isomers, respectively. 31P NMR spectroscopy confirmed the isolation of each products with the presence of single peak, while a mixture of isomers provide two peaks. The sitting drop crystallization allows to obtain a crystal of both molecules and their structures were unambiguously determined by X-ray to be P(R) for 12 and P(S) for 13, respectively, (Fig. 2 ).
Fig. 2.
ORTEP crystal structure for allyl phosphonoamidates 12 (P(R)) and 13 (P(S)).
Next, the silylation of thymine was obtained in 5 min at room temperature in presence of bistrimethylsilylacetamide BSA. The intermediate was directly engaged in nucleophilic substitution reaction with crotyl bromide, chlorotrimethylsilane and sodium iodide [34]. This reaction is performed under ultrasonic activation to afford after seven hours the desired compound 16 in quantitative yield. 16 was then converted to the N3-Boc thymine derivative 17 in quantitative yield, (Scheme 3 ) [35].
Scheme 3.
Reagents and conditions: a) BSA, ACN, RT, 5 min., b) crotyl bromide, TMSCl, NaI))), 55 °C, 7h, > 98% c) Boc2O, DMAP, THF, MW, 70 °C, 10 min, > 98%.
With all partners in hand (16, 17 and 11–15), we turned our attention to the olefin CM reaction using either the 2nd generation Grubbs catalyst [36] (G-II), the more reactive Hoveyda-Grubbs (HG-II) catalyst [37] or its derivative, the Zhan catalyst-1B, (Table 1 ).
Table 1.
Cross-metathesis optimization.
| Entry | Solvent | Equivalents (of 11 and nucleobase) | Catalyst | Activation | R1 | Yield |
|---|---|---|---|---|---|---|
| 1 | DCM | 1–2 | HG-II | Δ, 50 °C, 24h | H | / |
| 2 | DCM | 1.3–1 | HG-II | Δ, 50 °C, 24h | H | / |
| 3 |
DCM |
1–1 |
HG-II, Cy2BCl |
Δ, 50 °C, 24h |
Boc |
/ |
| 4 | DCM | 1.3–1 | G-II | MW, 100 °C, 1h | H | / |
| 5 |
DCM |
1.3–1 |
G-II, CuI |
MW, 100 °C, 1h |
H |
/ |
| 6 | DCM | 1.3–1 | HG-II | ))), 55 °C, 20h | H | / |
| 7 | DCM | 1.3–1 | G-II, CuI | ))), 55 °C, 20h | H | / |
| 8 | DCM | 1.3–1 | G-II, BCl3.SMe2 | ))), 55 °C, 20h | H | / |
| 9 | DCM | 1.3–1 | HG-II | ))), 55 °C, 20h | Boc | / |
| 10 | DCM | 1–2 | Zhan 1B | ))), 55 °C, 20h | Boc | / |
| 11 | H2O | 1–2 | G-IIc | ))), 55 °C, 20h | H | 41% |
| 12 | H2Oa | 1–2 | G-IIc | ))), 55 °C, 20h | H | 40% |
| 13b | H2Oa | 1–2 | G-IIc | ))), 55 °C, 20h | H | 35% |
Bold character represent in the table the best yield obtained in water.
2.5% of Polyoxyethanyl-α-tocopheryl Sebacate PTS.
Sealed tube.
Catalyst introduced in 3 × 6 mol%.
This specific CM reaction with a phosphonoamidate is challenging and needs optimization since, as stated previously, it is well established in the literature that compounds containing basic nitrogen atoms can poison the CM ruthenium catalysts and are thus problematic substrates for olefin metathesis. It was shown that the presence of electron withdrawing groups next to the nitrogen decreases the electron density and the deactivation of the catalyst can be attenuated as well as the use of microwave irradiations [38]. It was shown also that the presence of Lewis acid or Cu(I) salt can improved the yields of RCM of amino acids [39]. The influence of ultrasonication was also tested. For this optimization, we used the allylphosphonoamidate 11 and the crotylthymine 16 or 17, taken as model.
When N1 alkylated thymines 16 or 17 were reacted in DCM with diastereomeric mixture of phosphonoamidate synthon 11 under classical heat activation (Δ) (entries 1–3), no desired compounds were found in all conditions tested (equivalents, substrate, Ru catalyst, co-catalyst (Lewis acid, entry thymine derivatives). The use of microwaves irradiation (MW) (entries 4, 5) and sonication ()))) (entries 6 to 10) failed also. Only the use of water on sonication led the expected phosphonoamidate ANP 18 in moderate 41% yield (entry 11) [40]. The modulation of the conditions in a sealed tube and with an addition of a surfactant (2.5% of polyoxyethanyl-α-tocopheryl sebacate) (entries 12 and 13) does not improve significantly the yield of the reaction [41].
Thanks to this breakthrough approach, some (E)-but-2-enyl C5-substituted thymidine phosphonoamidates 19–22 were obtained in yields ranging from 38% to 44%, (Scheme 4 ). Based on our previous data [22], the 5-fluoro- (23) and 5-chloro- (24) analogs, which are bioisosteres of methyl group, were obtained, in 35% and 36% yield, respectively.
Scheme 4.
Reagents and conditions: (a) N1-crotylated thymine, G-II catalyst (3 × 6 mol%), H2O (2.5% PTS)))), 55 °C, 20h or N1-crotylated 5-fluorouracile, G-II catalyst (3 × 6 mol%), H2O (2.5% PTS)))), 55 °C, 20h or, or N1-crotylated 5-bromouracile G-II catalyst (3 × 6 mol%), H2O (2.5% PTS)))), 55 °C, 20h.
2.2. Biological evaluation
Among all the tested compounds, bearing different biolabile group (POM, POC, HDP, phosphonoamidate), the diastereomeric single phosphonoamidate forms 19 and 20 were the most potent and selective against both wild-type (TK+) and thymidine kinase deficient (TK−) varicella-zoster (VZV) strains with EC50 (50% effective concentration) values in the range of 0.3–0.6 μM (Table 2 ). The cytostatic activity (CC50) decreased by a factor 2 when comparing compound 19 with the bis-(POM)-(E)-TbutP (1) resulting in a selectivity index (SI, ratio CC50 to EC50) superior to 200. The selectivity of 20 against VZV was about half of that calculated for 19. These prodrugs showed also activity against herpes simplex virus 1 (HSV-1), TK− HSV-1 and herpes simplex virus 2 (HSV-2) strains (EC50 in the range of 3–12 μM), which was comparable to the EC50's obtained for cidofovir (Table 3 ). The diastereomeric single phosphonoamidate forms 19 and 20 had weak activity against human cytomegalovirus (HCMV) or no activity at the higher concentration tested (100 μM) against vaccinia virus and adenovirus.
Table 2.
Antiviral properties against HCMV and VZV.
| Compounds | HCMV |
VZV |
Cytotoxicity (μM) |
|||||
|---|---|---|---|---|---|---|---|---|
| EC50a (μM) |
TK+ (OKA) |
TK− (07/1) |
||||||
| (AD-169) | (Davis) | EC50 (μM) | SIb | EC50 (μM) | SI | MCCc | CC50d | |
| 19 | 29.91 | 44.72 | 0.39 ± 0.21 | ≥213 | 0.33 ± 0.05 | ≥252 | >100 | ≥83 ± 24 |
| 20 | 34.2 | 52.53 | 0.59 ± 0.08 | 93 | 0.39 ± 0.14 | 141 | >100 | 55 ± 3 |
| 21 | ≥72 ± 39 | ≥70 ± 42 | 8.13 ± 1.15 | 12 | 1.82 ± 0.33 | 55 | >100 | >100 |
| 22 | 25.9 ± 8.3 | 13.5 ± 2.5 | 4.59 ± 3.18 | 22 | 1.03 ± 0.59 | 97 | >100 | >100 |
| 23 | >20 | >20 | >100 | – | >100 | – | ≥100 | ND |
| 24 | >20 | >20 | 44.72 | – | 20 | – | ≥100 | ND |
| 1 (R1 = Me) | >41 | >41 | 1.91 ± 1.32 | 19 | 0.43 ± 0.21 | 85 | ≥100 | 36.4 ± 2.3 |
| 2 (R1 = Me) | 102 | 83 | 1.26 ± 0.11 | 29 | 0.45 ± 0.32 | 80 | >200 | 36 |
| 03 (R1 = Me) | >6 | >6 | >6 | – | >6 | – | 30 | 14.7 |
| 04 (R1 = Me) | >39 | 13.5 | 19.4 | – | 20.5 | – | 184 | 76 |
| Acyclovir | NDe | ND | 2.93 ± 1.25 | >150 | 54.4 ± 27.8 | >8 | >440 | >440 |
| Brivudine | ND | ND | 0.014 ± 0.012 | >21,429 | ≥93.8 ± 58.0 | >3 | >300 | >300 |
| Ganciclovir | 5.63 ± 3.84 | 4.05 ± 1.35 | ND | – | ND | – | ≥350 | >350 |
| Cidofovir | 0.77 ± 0.41 | 0.90 ± 0.33 | ND | – | ND | – | >300 | >300 |
Effective concentration required to reduce virus plaque formation (VZV) or viral cytopathic effect (HCMV) by 50%.
Selectivity index: ratio CC50 to EC50.
Minimum cytotoxic concentration that causes a microscopically detectable alteration of cell morphology.
Cytostatic concentration required to reduce cell growth by 50%.
Not done.
Table 3.
Antiviral properties against HSV, vaccinia virus and adenovirus.
| Compounds | EC50a (μM) |
Cytotoxicity (μM) |
|||||
|---|---|---|---|---|---|---|---|
| HSV-1 |
HSV-2 (G) | Vaccinia virus | Human Coronavirus (229E) | MCCb | CC50c | ||
| (KOS) | (TK-KOS ACVr) | ||||||
| 19 | 9.6 ± 4.7 | 3.2 ± 3.2 | 5.3 ± 4.2 | >100 | 24.6 ± 18.5 | >100 | >83 ± 24 |
| 20 | 11.5 ± 4.7 | 4.1 ± 5.1 | 2.9 ± 3.4 | >100 | 60 ± 35 | >100 | 55 ± 3 |
| 21 | 59.0 ± 19.8 | 23.5 ± 16.3 | 31.0 ± 4.1 | >100 | >100 | >100 | >100 |
| 22 | >100 | >100 | >100 | >100 | >100 | >100 | >100 |
| 23 | >100 | 79.0 ± 29.7 | >100 | >100 | 45 ± 0 | >100 | ND |
| 24 | 43 ± 21 | 24 ± 6 | 40 ± 8 | >100 | 8.9 ± 0 | >100 | ND |
| 1 (R1 = Me) | 3.1 ± 1.4 | 9.2 ± 7.2 | 6.5 ± 3.4 | 30.7 ± 14.5 | NDd | ≥100 | 36.4 ± 2.3 |
| 2 (R1 = Me) | 4.2 ± 0.7 | 2.8 ± 1.2 | 6.1 ± 2.9 | >200 | ND | >200 | 36 |
| 3 (R1 = Me) ( | >152 | >152 | >152 | ND | ND | >152 | 14.7 |
| 4 (R1 = Me) | >37 | >37 | >37 | >37 | ND | 184 | 76 |
| Acyclovir | 1.2 ± 1.1 | 94 ± 25 | 0.7 ± 0.7 | >250 | – | >440 | >440 |
| Brivudine | 0.081 ± 0.034 | 420 ± 280 | 169 ± 116 | 10.8 9.4 | – | >300 | >300 |
| Cidofovir | 6.5 ± 3.7 | 4.6 ± 2.7 | 4.1 ± 2.7 | 21.3 ± 3.8 | – | >300 | >300 |
| UDA | – | – | – | – | 5.4 ± 5.0 μg/mL | >100 | – |
Effective concentration required to viral cytopathic effect by 50%.
Minimum cytotoxic concentration that causes a microscopically detectable alteration of cell morphology.
Cytostatic concentration required to reduce cell growth by 50%.
Not done.
The (E)-but-2-enyl C5-substituted pyrimidine phosphonoamidates 21 and 22 inhibited VZV replication with EC50's in the range of 1–8 μM and did not affect cell growth or morphology at the highest tested concentration (100 μM). In contrast to 22, compound 21 had some anti-HSV activity while both were able to reduce HCMV multiplication.
The introduction of these biolabile prodrugs revealed the potential of our ANPs to inhibit HCMV replication (EC50's in the range of 13–70 μM for compounds 19, 20, 21, 22), hitherto undetected under other pronucleotide forms. Several hypotheses can support these results, as a better bioavailability of these molecules under the phosphonoamidate form compared to the other prodrug forms. The activity can also be increased by a better half-life and less toxic side-products. However, the newly synthesized prodrugs did not showed activity against vaccinia virus in contrast to bis-(POM)-(E)-TbutP (1).
The phosphonamidate ANPs 23 and 24 were not active against various viral strains; only the chlorine analog 24 shown a moderate activity against human coronavirus (EC50 8.9 μM).
The compounds were also evaluated against different RNA viruses, but no activity was found.
3. Conclusion
We have described herein the synthesis of (E)-but-2-enyl nucleoside phosphonoamidates using the cross-metathesis in water-under ultrasound irradiation. The overall yield obtained from commercial dimethylallylphosphonate is >15%, well above the datas reported in the literature for the preparation of phosphonoamidates (∼3%). Two diastereoisomers were formally identified by X-ray diffraction. All those compounds were evaluated against various DNA viruses for their antiviral properties. Among them, the thymine analogue 19 showed to be the best prodrug tested against VZV with an EC50 = 0.33–0.39 μM and a selectivity index increased up to ≥200, compared to its other prodrugs 1–4. This breakthrough approach paves the way for new purine and pyrimidine (E)-but-2-enyl phosphonoamidates.
4. Experimental section
4.1. Chemistry
General.
Commercially available chemicals were of reagent grade and used as received. All reactions requiring anhydrous conditions were carried out using oven-dried glassware and under an atmosphere of dry Ar or N2. All reactions under microwave irradiation were performed using the Microwave Biotage Initiator in 2–5 mL.sealed tubes. The reactions under ultrasound were carried out with Elmasonic P30H apparatus with a frequency of 80 kHz and effective power of 100 W. The reactions were monitored by thin layer chromatography (TLC) analysis using silica gel plates (Kieselgel 60F254, E. Merck). Column chromatography was performed on Silica Gel 60 M (0.040–0.063 mm, E. Merck). The 1H and 13C NMR spectra were recorded on Bruker Avance DPX 250 or Bruker Avance 400 Spectrometers using deuterated solvents as internal standard. Chemical shifts are given in ppm and multiplicities are reported as s (singlet), d (doublet), t (triplet), q (quartet), bs (broad signal) and m (multiplet). High Resolution Mass spectra were performed on a Bruker Q-TOF MaXis mass spectrometer by the “Fédération de Recherche” ICOA/CBM (FR2708) platform. LC-MS data was acquired on a Thermo-Fisher UHPLC-MSQ system equipped with an electron spray ionization source (ESI). The temperature of the source was maintained at 350 °C. Initially, the cone voltage was set at 35 V and after 5 min was increased to 75V. In full scan mode, data was acquired between 100 and 1000 m/z in the positive mode with a 1.00 S scan time. In addition a UV detection was performed with a Diode array detector at three wavelengths 273, 254 and 290 nm, respectively. A water/methanol (70%/30%) solution mixture with 0.1% formic acid was used as mobile phase. The composition of the mobile phase was increased to 100% methanol with 0.1% formic acid with a 7% ramp. The flow rate was set at 0.300 mL.min-1. Samples diluted in the mobile phase were injected (3 μL) on a C18 column (X-terra, Waters), 2.1 mm internal diameter, 100 mm length placed into an oven at 40 °C. The conversion of compound 11 is equal to 67%. LC-MS analysis of allylphosphonoamidate 11 was optimized in terms of separation and identification. The reaction with crotylthymine was followed by LC-MS in Electron Spray Ionization (ESI), in positive mode. After 20h, three compounds were observed the allylphosphonoamidate 11, ((M + H)+,360), its homodimer form ((M + H)+,690), and phosphonoamidate ANP 18 product ((M + H)+,498). Electronic extraction of ions was performed and the subsequent areas under the corresponding chromatographic peaks determined. The conversion yield was determined as the ratio of the concentration of the allylphosphonoamidate 11 transformed to its initial concentration.
4.1.1. Dimethyl allylphosphonate (8)
Under inert atmosphere, allyl bromide (51 mL, 1.25 eq.,0.57 mol) was dissolved in THF (400 mL). To this mixture potassium carbonate (94 g, 1.5 eq., 0.68mol), tert-butylammonium bromide (2.9 g, 2 mol%, 9.1 mmol) and finally dimethylphosphite 7 (41.2 mL, 1 eq., 0.45 mol) were added. The resulting solution was stirred for 36 h at room temperature, followed by the filtration of all solids present in the flask. The filtrate was then evaporated under reduced pressure, and the crude product was then distilled at 130 °C under 40 mm/Hg. After collection of the different fractions, the clean product 8 was obtained as a colorless liquid. (53 g, 75%). 1H NMR (250 MHz, CDCl3) δ 5.80 (m, 1H, CH=CH2), 5.23 (m, 2H, CH2=CH), 3.77 (s, 3H, OMe), 3.72 (s, 3H, OMe), 2.62 (ddt, J = 22.0, 7.4, 1.3 Hz, 2H, CH2-P). CAS # 757-54-0.
4.1.2. Methoxyphenoxy allylphosphonate (9)
To a mixture of dimethylallylphosphonate 8 (4.9 g, 1 eq., 32.5 mmol) and DCM (150 mL), oxalyl chloride (8.6 mL, 3 eq., 97.5 mmol) was added. The reaction was stirred 24 h at reflux, followed by the removal of the volatiles in vacuo to obtain the methyl allylphosphonochloridate. In another flask, a solution of phenol (6.12 g, 2 eq., 65 mmol), triethylamine (8.8 mL, 2 eq., 65 mmol) and DCM (0.2 M) was stirred at room temperature. The phosphonate residue was then dissolved in DCM (0.2 M), and slowly added to this solution, and refluxed during 48h. After evaporation of all volatiles, the residue was purified by silica gel column chromatography, eluting Petroleum ether/Ethyl acetate 8/2, to afford 9 as a colorless oil. (4.5 g, 65%). 1H NMR (400 MHz, CDCl3) δ 7.29 (m, 2H, Aromatic H), 7.15 (m, 3H, Aromatic H), 5.80 (m, 1H, CH=CH2), 5.24 (m, 2H, CH2=CH), 3.76 (d, J = 11.1 Hz, 3H, OMe), 2.73 (dd, J = 22.0, 7.3 Hz, 2H, CH2-P). 13C NMR (101 MHz, CDCl3) δ 150.46 (d, J = 8.5 Hz), 129.72 (Aromatic C), 126.59 (d, J = 11.6 Hz, CH=CH2), 124.95 (d, J = 1.3 Hz, Aromatic C), 120.67 (d, J = 14.8 Hz, CH2=CH), 120.42 (d, J = 4.4 Hz, Aromatic C), 53.16 (d, J = 5.1 Hz, OMe), 31.21 (d, J = 139.9 Hz, CH2-P). 31P NMR (162 MHz, CDCl3) δ 25.06. HRMS (ESI) m/z [M+H]+ calcd for C10H14O3P: 213.0681, found: 213.0675.
4.1.3. Phenyloxy allylphosphonic acid (10)
Bromotrimethylsilane (8.9 mL, 6 eq.,61.8 mmol) was slowly added to a solution of 9 (2.2 g, 1 eq., 10.3 mmol) in DCM (110 mL). After 24 h at room temperature and evaporation of all volatiles, the crude product was co-evaporated 5 times with methanol (5 × 15 mL). The residue was then purified by flash column chromatography (DCM/MeOH 95/5) to obtain desired product 10 as an amorphous white solid. (1.85 g, 91%). 1H NMR (400 MHz, CDCl3) δ 8.12 (s, 1H, OH), 7.28 (m, 2H, Aromatic H), 7.14 (m, 3H, Aromatic H), 5.79 (m, 1H, CH=CH2), 5.22 (m, 2H, CH2=CH), 2.67 (dd, J = 22.5, 7.3 Hz, 2H, CH2-P). 13C NMR (101 MHz, CDCl3) δ 150.18 (d, J = 8.9 Hz), 129.61 (Aromatic C), 126.65 (d, J = 11.4 Hz, CH=CH2), 124.92 (Aromatic C), 120.79 (d, J = 4.4 Hz, Aromatic C), 120.66 (d, J = 14.9 Hz,CH2=CH), 31.55 (d, J = 141.5 Hz, CH2-P). 31P NMR (162 MHz, CDCl3) δ 26.26. HRMS (ESI) m/z [M+H]+ calcd for C9H12O3P: 199.0525, found: 199.0518.
4.2. General procedure for the synthesis of allylphosphonoamidates 11-15
Under inert atmosphere, phenyloxy allylphosphonic acid (10) (1 eq.) was dissolved in DCM. A catalytical amount of DMF (0.3 eq.) was then introduced, followed by the addition of oxalyl chloride (2 eq.). After 30 min. at 0 °C and 1.5 h at room temperature, the volatiles were removed, and the residue diluted with 20 mL of DCM to afford solution A. A second solution was prepared with a l-alanine ester chlorhydrate (1.2 eq.), freshly distilled triethylamine (8 eq.) and DCM. To this mixture, the solution A was slowly added at 0 °C, and stirred 24 h at room temperature. For 1 g of starting phosphonate, the solution was successively washed with 10 mL of water, 10 mL NaOH 1 M, 10 mL water, 10 mL HCl 10%, 10 mL NaHCO3 and 10 mL water. After this work-up, the aqueous phase was extracted with 4 × 10 mL with ethyl acetate, the organic phases were washed with brine (20 mL), dried over MgSO4, filtered and evaporated. The residue was purified twice by flash column chromatography to afford the desired allylphosphonoamidates 11, 14 and 15 as diastereomeric mixture. Diastereomers of compound 11 were separated as single diastereomers 12 and 13, respectively.
4.2.1. Benzyl 2-[(S)-[allyl(phenoxy)phosphoryl]amino] propanoate (11)
Titled compound was obtained following general procedure starting from compound 10 (1 g, 1 eq., 5.05 mmol). The residue was purified twice by flash column chromatography, first eluting PE/EA (7/3) and then pentane/diethyl ether (55/45); the 6:4 diastereomeric mixture of 11 was obtained as a white powder (1.14 g, 63%). Each diastereoisomers were crystallized from a toluene/pentane mixture, as colorless needles, respectively, and their structures were established by X-ray.
Diastereoisomer 1: Benzyl 2-[(S)-[(R)-allyl(phenoxy)phosphoryl]amino] propanoate (12): 1H NMR (400 MHz, (CD3)2CO) δ 7.37 (m, 7H, Aromatic H), 7.24 (m, 2H, Aromatic H), 7.14 (t, J = 7.4 Hz, 1H, Aromatic H), 5.89 (m, 1H, CH=CH2), 5.18 (m, 4H, CH2=CH, CH2-O), 4.60 (t, J = 11.2 Hz, 1H, NH), 4.16 (m, 1H, CH-NH), 2.76 (ddd, J = 20.7, 7.1, 2.5 Hz, 2H, CH2-P), 1.22 (d, J = 7.2 Hz, 3H, CH3). 13C NMR (101 MHz, (CD3)2CO) δ 174.42 (d, J = 4.8 Hz, C=O), 151.94 (d, J = 9.1 Hz), 137.12, 130.19 (Aromatic C), 129.46 (d, J = 11.0 Hz, CH=CH2), 129.28 (Aromatic C), 128.92 (Aromatic C), 128.88 (Aromatic C), 125.09 (d, J = 1.1 Hz, Aromatic C), 121.82 (d, J = 4.6 Hz, Aromatic C), 119.86 (d, J = 14.3 Hz, CH2=CH), 67.09 (CH2-O), 50.47 (CH-N), 35.03 (d, J = 128.7 Hz, CH2-P), 20.88 (d, J = 4.8 Hz, CH3). 31P NMR (162 MHz, (CD3)2CO) δ 26.70. HRMS (ESI): m/z [M+H]+ calcd for C19H23O4NP: 360.1365, found: 360.1355.
Diastereoisomer 2: Benzyl 2-[(S)-[(S)-allyl(phenoxy)phosphoryl]amino] propanoate (13): 1H NMR (400 MHz, CDCl3) δ 7.19 (m, 10H, Aromatic H), 5.79 (m, 1H, CH=CH2), 5.17 (m, 2H, CH2=CH), 5.04 (s, 2H, CH2-O), 4.06 (m, 1H, CH-NH), 3.49 (t, J = 10.6 Hz, 1H, NH), 2.69 (dd, J = 21.5, 7.4 Hz, 2H, CH2-P), 1.27 (d, J = 7.1 Hz, 3H, CH3). 13C NMR (101 MHz, CDCl3) δ 173.56 (d, J = 5.3 Hz, C=O), 150.52 (d, J = 9.0 Hz), 135.25, 129.65 (Aromatic C), 128.62 (Aromatic C), 128.48 (Aromatic C), 128.21 (Aromatic C), 127.45 (d, J = 11.2 Hz, CH=CH2), 124.67 (Aromatic C), 120.60 (d, J = 14.3 Hz, CH2=CH), 120.56 (d, J = 4.7 Hz, Aromatic C), 67.12 (CH2-O), 49.61 (CH-NH), 34.25 (d, J = 128.8 Hz, CH2-P), 21.57 (d, J = 4.3 Hz, CH3). 31P NMR (162 MHz, CDCl3) δ 26.47. HRMS (ESI): m/z [M+H]+ calcd for C19H23O4NP: 360.1365, found: 360.1354.
4.2.2. Methyl 2-[(S)-[allyl(phenoxy)phosphoryl]amino] propanoate (14)
Titled compound was obtained following general procedure 1, starting from compound 10 (1.3 g, 1 eq., 6.56 mmol). The obtained residue was purified twice by flash column chromatography, eluting PE/EA (55:45 to 5:5) to afford a non-separable 6:4 mixture of diastereoisomers 14 as a colorless oil, (960 mg, 52%). 1H NMR (400 MHz, CDCl3) δ 7.30 (m, 2H, Aromatic H), 7.18 (m, 3H, Aromatic H), 5.87 (m, 1H, CH=CH2), 5.27 (m, 2H, CH2=CH), 4.08 (m, 1H, CH-NH), 3.66 (d, J = 10.6 Hz, 3H, OMe), 3.32 (2 × t, J = 10.1 Hz, 1H, NH), 2.77 (m, 2H, CH2-P), 1.28 (2 × d, J = 7.2 Hz, 3H, CH3-CH).13C NMR (101 MHz, CDCl3) δ 174.53 (d, J = 6.1 Hz, C=O), 174.20 (d, J = 5.3 Hz, C=O), 150.53 (d, J = 9.5 Hz), 150.45 (d, J = 9.9 Hz), 129.65 (Aromatic C), 129.60 (Aromatic C), 127.68 (d, J = 11.4 Hz, CH=CH2), 127.52 (d, J = 11.2 Hz, CH=CH2), 124.72 (d, J = 1.2 Hz, Aromatic C), 124.68 (d, J = 1.1 Hz, Aromatic C), 120.70 (d, J = 4.8 Hz, Aromatic C), 120.60 (d, J = 14.4 Hz, CH2=CH), 120.57 (d, J = 4.8 Hz, Aromatic C), 52.35 (CH3-O), 49.62 (CH-NH), 49.45 (CH-NH), 34.32 (d, J = 128.3 Hz, CH2-P), 34.30 (d, J = 128.3 Hz, CH2-P), 21.60 (d, J = 4.3 Hz, CH3), 21.46 (d, J = 3.8 Hz, CH3).31P NMR (162 MHz, CDCl3) δ26.78, 26.34.HRMS (ESI) m/z [M+H]+ calcd for C13H19NO4P: 284.1052, found: 284.1045.
4.2.3. Isopropyl 2-[(S)-[allyl(phenoxy)phosphoryl]amino] propanoate (15)
Titled compound was obtained following general procedure 1, starting from compound 10 (1.3 g, 1 eq., 6.56 mmol). The obtained residue was purified by flash column chromatography, eluting PE/EA (65:35) to afford a non-separable 6:4 mixture of two diastereoisomers 15 as a colorless oil. (1.16 g, 57%). 1H NMR (400 MHz, CDCl3) δ 7.32 (m, 2H, Aromatic H), 7.19 (m, 3H, Aromatic H), 5.92 (m, 1H, CH=CH2), 5.28 (m, 2H, CH2=CH), 4.99 (pd, J = 6.3, 4.9 Hz, 1H, CH-iPr), 4.04 (m, 1H, CH-NH), 3.38 (2 × t, J = 10.1 Hz, 1H, NH), 2.78 (m, 2H, CH2-P), 1.26 (m, 9H, CH3-CH-NH, CH3 iPr).13C NMR (101 MHz, CDCl3) δ 173.24 (d, J = 5.7 Hz, C=O), 150.58 (d, J = 9.5 Hz), 150.45 (d, J = 9.9 Hz), 129.65 (Aromatic C), 129.59 (Aromatic C), 127.71 (d, J = 11.4 Hz, C2′), 127.53 (d, J = 11.4 Hz, C2′), 124.69 (Aromatic C), 124.65 (Aromatic C), 120.75 (C3'/Aromatic C), 120.70 (C3'/Aromatic C), 120.68 (C3'/Aromatic C), 120.64 (C3'/Aromatic C), 120.59 (C3'/Aromatic C), 120.55 (C3'/Aromatic C), 120.50 (C3'/Aromatic C), 69.09 (CH iPr), 69.07 (CH iPr), 49.77 (CH-NH), 49.62 (CH-NH), 34.30 (d, J = 129.3 Hz, CH2-P), 21.71 (CH3), 21.70 (CH3), 21.67 (CH3), 21.62 (CH3), 21.60 (CH3), 21.57 (CH3). 31P NMR (162 MHz, CDCl3) δ 26.77, 26.35. HRMS (ESI) m/z [M+H]+ calcd for C15H23NO4P: 312.1365, found: 312.1359.
4.3. General procedure for in water-ultrasound-promoted convergent synthesis of (E)-but-2-enyl nucleoside phosphonoamidates
To a 2.5% Polyoxyethanyl-α-tocopheryl Sebacate (PTS) solution of water (0.5 M) of phosphonoamidate (1 eq.) and crotylnucleobase (2 eq.), second generation Grubbs catalyst (6 mol%) was added. The reaction was irradiated under ultrasound activation (55 °C, 80 KHz) (two additional portions of 6 mol% Grubbs catalyst were respectively added at 2h and 4h). The progress of the reaction was followed by LC/MS and reaction was stopped after 20 h of ultrasouns activation. A maximum of 67% of phosphonoamidate conversion was observed. Volatiles were removed under reduced pressure, and the residue was submitted twice to flash chromatography purification, firstly DCM/MeOH 95/5 followed by a second one eluting toluene/acetone 6/4.
4.3.1. Benzyl 2-[(S)-[(R)-[(E)-4-(thymin-1-yl)but-2-enyl]-phenoxyphosphoryl]amino] propanoate (19)
Titled compound was obtained following general procedure, starting from single diastereomer phosphonoamidate 12 (68 mg, 1 eq., 0.19 mmol) and crotylthymine (68 mg, 0.38 mmol, 2 eq.). The obtained residue was purified by twice flash chromatography (DCM/MeOH 95/5 then toluene/acetone 6/4), to afford 19 as a white solid (36 mg, 38%). 1H NMR (400 MHz, CDCl3) δ 8.35 (s, 1H, NH), 7.32 (m, 7H, Aromatic H), 7.15 (m, 3H, Aromatic H), 6.97 (d, J = 1.3 Hz, 1H, H6), 5.78 (dt, J = 13.7, 7.3 Hz, 1H, H3′), 5.67 (dt, J = 15.5, 4.8 Hz, 1H, H2′), 5.10 (d, AB system, J = 13.0 Hz, 2H, CH2-O), 4.27 (t, J = 4.8 Hz, 2H, H1′), 4.15 (dq, J = 9.9, 7.3 Hz, 1H, CH-NH), 3.38 (d, J = 10.9 Hz, 1H, NH), 2.76 (dt, J = 21.0, 7.3 Hz, 2H, CH2-P), 1.89 (d, J = 1.3 Hz, 3H, CH3-C), 1.25 (s, 3H, CH3-CH). 13C NMR (101 MHz, CDCl3) δ 173.86 (d, J = 5.9 Hz, C=O), 163.77 (C=O), 150.59 (C=O), 150.24 (d, J = 9.1 Hz), 139.73 (C6), 135.28, 129.68 (Aromatic C), 129.07 (d, J = 14.0 Hz, C2′), 128.64 (Aromatic C), 128.49 (Aromatic C), 128.18 (Aromatic C), 125.32 (d, J = 11.5 Hz, C3′), 124.90 (Aromatic C), 120.4 (d, J = 4.6 Hz, Aromatic C), 110.97 (C5), 67.19 (CH2-O), 49.75 (CH-NH), 49.53 (d, J = 2.2 Hz, C1′), 32.88 (d, J = 128.9 Hz, CH2-P), 21.31 (d, J = 4.0 Hz, CH3-CH), 12.27 (CH3-C). 31P NMR (162 MHz, CDCl3) δ 26.00. HRMS (ESI) m/z [M+H]+ calcd for C25H29N3O6P:498.1794, found: 498.1786.
4.3.2. Benzyl 2-[(S)-[(S)-[(E)-4-(thymin-1-yl)but-2-enyl]-phenoxyphosphoryl]amino] propanoate (20)
Titled compound was obtained following general procedure, starting from phosphonoamidate 13 (125 mg, 1 eq., 0.35 mmol) and crotylthymine (125 mg, 2 eq., 0.70 mmol). The obtained residue was purified by twice flash chromatography (DCM/MeOH 95/5 and toluene/acetone 6/4), to afford 20 as a white solid (70 mg, 41%). 1H NMR (400 MHz, CDCl3) δ 8.79 (bs, 1H, NH), 7.27 (m, 7H, Aromatic H), 7.12 (m, 3H, Aromatic H), 6.94 (d, J = 1.1 Hz, 1H), 5.67 (m, 2H, H2′, H3′), 5.07 (s, 2H), 4.23 (t, J = 4.9 Hz, 2H, CH2-O), 4.03 (dq, J = 9.6, 7.6 Hz, 1H, CH-NH), 3.38 (d, J = 10.3 Hz, 1H, NH), 2.70 (dt, J = 20.3, 5.7 Hz, 2H), 1.84 (d, J = 1.1 Hz, 3H), 1.27 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 173.46 (d, J = 5.3 Hz, C=O), 163.95 (C=O), 150.68 (C=O), 150.40 (d, J = 9.1 Hz), 139.57 (C6), 135.19, 129.74 (Aromatic C), 129.17 (d, J = 14.4 Hz, C2′), 128.66 (Aromatic C), 128.56 (Aromatic C), 128.25 (Aromatic C), 125.08 (d, J = 11.1 Hz, C3′), 124.84 (Aromatic C), 120.37 (d, J = 4.9 Hz, Aromatic C), 111.00 (C5), 67.25 (CH2-O), 49.67 (CH-NH), 49.17 (d, J = 2.2 Hz, C1′), 32.73 (d, J = 128.2 Hz), 21.46 (d, J = 4.3 Hz, CH3-CH), 12.27 (CH3-C) 31P NMR (162 MHz, CDCl3) δ 25.60. HRMS (ESI) m/z [M+H]+ calcd for C25H29N3O6P: 498.1794, found: 498.1789.
4.3.3. Methyl 2-[(S)-[[(E)-4-(thymin-1-yl)but-2-enyl]-phenoxyphosphoryl]amino] propanoate (21)
Titled compound was obtained following general procedure, starting from phosphonoamidate 14 (50 mg, 1 eq., 0.18 mmol) and crotylthymine (64 mg, 2 eq., 0.35 mmol). The obtained residue was purified by twice flash chromatography (DCM/MeOH 95/5 and toluene/acetone 6/4), to afford 21 as a white solid (30 mg, 41%). 1H NMR (400 MHz, CDCl3) δ 8.06 (bs, 1H, NH), 7.27 (m, 2H, Aromatic H), 7.15 (m, 3H, Aromatic H), 6.97 (bs, 1H, H6), 5.73 (m, 2H, CH=CH), 4.28 (t, J = 4.9 Hz, 2H, CH2-N), 4.00 (m, 1H, CH-NH), 3.72–3.59 (m, 3H), 3.42 (m, 1H, NH), 2.74 (dd, J = 20.9, 6.8 Hz, 2H, CH2-P), 1.88 (m, 3H, CH3 thym), 1.26 (m, 3H, CH3-CH). 31P NMR (162 MHz, CDCl3) δ 26.01, 25.51. HRMS (ESI): m/z [M+H]+ calcd for C19H25N3O6P: 422.1481 found: 422.1480.
4.3.4. Isopropyl 2-[(S)-[[(E)-4-(thymin-1-yl)but-2-enyl]-phenoxyphosphoryl]amino] propanoate (22)
Titled compound was obtained following general procedure, starting from phosphonoamidate 15 (50 mg, 1 eq., 0.16 mmol) and crotylthymine (58 mg, 2 eq., 0.32 mmol). The obtained residue was purified by twice flash chromatography (DCM/MeOH 95/5 and toluene/acetone 6/4), to afford 22 as a white solid (32 mg, 44%). 1H NMR (400 MHz, CDCl3) δ 8.38 (bs, 1H, NH), 7.30 (m, 2H, Aromatic H), 7.16 (m, 3H, Aromatic H), 7.00 (s, 1H, H6), 5.75 (m, 2H, CH=CH), 4.96 (d sept., J = 6.3, 2.1 Hz, 1H, CH-iPr), 4.31 (t, J = 4.7 Hz, 1H, H1′), 3.99 (m, 1H, CH-NH), 3.45 (2 × t, J = 10.8 Hz, 1H, NH), 2.77 (m, 2H, CH2-P), 1.88 (s, 3H, CH3-C) 1.26 (m, 9H, CH3-CH-NH, CH3 iPr). 13C NMR (101 MHz, CDCl3) δ 173.17 (C=O), 163.76 (C=O), 150.60 (C=O), 150.54, 139.69, 139.59 (C6), 129.74 (Aromatic C), 129.67 (Aromatic C), 129.12 (d, J = 14.0 Hz, C2′), 125.20 (d, J = 11.1 Hz, C3′), 124.85 (Aromatic C), 120.65 (Aromatic C), 120.61 (Aromatic C), 120.44 (Aromatic C), 120.40 (Aromatic C), 111.03 (CH-iPr), 69.09 (CH iPr), 69.07 (CH iPr), 49.77 (CH-NH), 49.64 (CH-NH), 49.19 (CH2-O), 32.70 (d, J = 129.4 Hz, CH2-P), 21.68 (CH3), 21.60 (CH3), 21.58 (CH3), 21.54 (CH3), 21.57 (CH3-C). 31P NMR (162 MHz, CDCl3) δ 26.02, 25.61. HRMS (ESI) m/z [M+H]+ calcd for C21H29N3O6P: 450.1795, found: 450.1789.
4.3.5. Benzyl 2-[(S)-[(E)-4-(5-fluorouridin-1-yl)but-2-enyl]-phenoxyphosphoryl]amino] propanoate (23)
Titled compound was obtained following general procedure, starting from phosphonoamidate 11 (64 mg, 1 eq., 0.18 mmol) and N1-crotyl-5-fluorouracile (66 mg, 2 eq., 0.36 mmol). The obtained residue was purified by twice flash chromatography (DCM/MeOH 95/5 and toluene/acetone 6/4), to afford 23 as a white solid (32 mg, 36%). 1H NMR (400 MHz, CDCl3) δ 9.26 (bs, 1H, NH), 7.34 (m, 7H, Aromatic H), 7.17 (m, 3H, Aromatic H),5.75 (m, 2H, CH=CH), 5.13 (s, 1H, CH2 benzylic), 5.09 (t, J = 5.0 Hz, 1H, H1′), 3.99 (2 × dq, J = 9.3, 6.9 Hz, 1H, CH-NH), 3.72 (t, J = 10.8 Hz, 1H, major NH-P), 3.45 (t, J = 10.8 Hz, 1H, minor NH-P), 2.77 (m, 2H, CH2-P), 1.29 (s, 3H, CH3-CH). 13C NMR (101 MHz, CDCl3) δ 173.49 (d, J = 5.3 Hz, C=O), 159.14 (C=O), 150.38 (C=O), 150.27 (d, J = 9.1 Hz, minor C), 149.75 (d, J = 9.1 Hz, major C), 140.57, 135.21 (C6), 129.79 (Aromatic C), 129.72 (Aromatic C), 128.68 (Aromatic C), 128.62 (d, J = 4.7 Hz, Aromatic C), 128.49 (Aromatic C), 128.30 (d, J = 13.9 Hz, C3′), 128.28 (Aromatic C), 128.18 (Aromatic C), 126.50 (d, J = 10.1 Hz, minor C2′), 126.28 (d, J = 10.1 Hz, major C2′), 124.93 (Aromatic C), 120.66 (d, J = 4.6 Hz, minor Aromatic C), 120.38 (d, J = 4.6 Hz, major Aromatic C), 67.30 (CH2-O), 49.73 (CH-NH), 49.19 (CH2-O), 32.51 (d, J = 128.8 Hz, CH2-P), 21.36 (d, J = 4.6 Hz, major CH3), 21.28 (d, J = 4.6 Hz, minor CH3). 31P NMR (162 MHz, CDCl3) δ 25.95, 25.54. HRMS (ESI) m/z [M+H]+ calcd for C24H26FN3O6P: 502.1544, found: 502.1537.
4.3.6. Benzyl 2-[(S)-[[(E)-4-(5-chlorouridin-1-yl)but-2-enyl]-phenoxyphosphoryl]amino] propanoate (24)
Titled compound was obtained following general procedure 2, starting from phosphonoamidate 13* (50 mg, 1 eq., 0.14 mmol) and N1-crotyl-5-chlorouracile (56 mg, 2 eq., 0.28 mmol). The obtained residue was purified by twice flash chromatography (DCM/MeOH 95/5 and toluene/acetone 6/4), to afford compound 24* as a white solid (33 mg, 35%). 1H NMR (400 MHz, CDCl3) δ 9.26 (bs, 1H, NH), 7.33 (m, 7H, Aromatic H), 7.18 (m, 3H, Aromatic H), 5.74 (m, 2H, CH=CH), 5.13 (s, 1H, CH2 benzylic), 5.09 (s, 1H, H1′), 4.07 (2 × dq, J = 8.8, 7.0 Hz, 1H, CH-NH), 3.68 (t, J = 10.8 Hz, 1H, major NH-P), 2.77 (m, 2H, CH2-P), 1.29 (s, 3H, CH3-CH). 13C NMR (101 MHz, CDCl3) δ 173.47 (d, J = 5.2 Hz, C=O), 159.14 (C=O), 150.34 (d, J = 9.1 Hz), 149.12 (C=O), 141.69, 139.33, 135.28, 135.21 (C6), 129.79 (Aromatic C), 128.68 (Aromatic C), 128.60 (Aromatic C), 128.49 (Aromatic C), 128.28 (d, J = 20.6 Hz, C3′), 128.28 (Aromatic C), 128.17 (Aromatic C), 127.62 (Aromatic C),126.23 (d, J = 11.0 Hz, C2′), 124.94 (Aromatic C),120.35 (d, J = 4.8 Hz, Aromatic C), 67.31 (CH2-O), 49.73 (CH-NH), 49.71 (CH2-O), 32.48 (d, J = 128.9 Hz, CH2-P), 21.35 (d, J = 4.6 Hz, CH3). 31P NMR (162 MHz, CDCl3) δ 25.91, 25.53. HRMS (ESI) m/z [M+H]+ calcd for C24H26ClN3O6P: 518.1248, found: 518.1242.
4.4. Antiviral activity assays
The antiviral assays, other than the anti-HIV assays, were based on inhibition of virus-induced cytopathicity or plaque formation in HEL [herpes simplex virus 1 (HSV-1) (KOS), HSV-2 (G), vaccinia virus, vesicular stomatitis virus, human cytomegalovirus (HCMV), varicella-zoster virus (VZV) and Human Coronavirus (229E)], Vero (parainfluenza-3, reovirus-1, Sindbis virus and Coxsackie B4), HeLa (vesicular stomatitis virus, Coxsackie virus B4, and respiratory syncytial virus) or MDCK [influenza A (H1N1; H3N2) and influenza B] cell cultures. Confluent cell cultures (or nearly confluent for MDCK cells) in microtiter 96-well plates were inoculated with 100 CCID50 of virus (1 CCID50 being the virus dose to infect 50% of the cell cultures) or with 20 plaque forming units (PFU) (for VZV) in the presence of varying concentrations (100, 20, …μM) of the test compounds. Viral cytopathic effect (CPE) or plaque formation (VZV) was recorded as soon as it reached completion in the control virus-infected cell cultures that were not treated with the test compounds. Antiviral activity was expressed as the EC50 or compound concentration required reducing virus-induced CPE or viral plaque (VZV) plaque formation by 50%. The minimal cytotoxic concentration (MCC) of the compounds was defined as the compound concentration that caused a microscopically visible alteration of cell morphology. Alternatively, the cytostatic activity of the test compounds was measured based on inhibition of cell growth. HEL cells were seeded at a rate of 5 × 103 cells/well into 96-well microtiter plates and allowed to proliferate for 24 h. Then, medium containing different concentrations of the test compounds was added. After 3 days of incubation at 37 °C, the cell number was determined with a Coulter counter. The cytostatic concentration was calculated as the CC50, or the compound concentration required to reduce cell proliferation by 50% relative to the number of cells in the untreated controls.
The antiviral activity of the compounds was evaluated against HIV-1 in activated primary human PBM cells [42]. Cytotoxicity was evaluated in normal PBM cells, along with CEM and Vero cells [43].
Acknowledgment
MB is grateful to MESR for a PhD scholarship. We thank the LABEX SynOrg (ANR-11-LABX-0029) for VH PhD fellowship and partial financial support.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ejmech.2018.01.086.
Contributor Information
Vincent Roy, Email: vincent.roy@univ-orleans.fr.
Luigi A. Agrofoglio, Email: luigi.agrofoglio@univ-orleans.fr.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Jordheim L.P., Durantel D., Zoulim F., Dumontet C. Advances in the development of nucleoside and nucleotide analogues for cancer and viral diseases. Nat. Rev. Drug Discov. 2013;12:447–464. doi: 10.1038/nrd4010. [DOI] [PubMed] [Google Scholar]
- 2.De Clercq E., Hóly A., Rosenberg I., Sakuma T., Balzarini J., Maudgal P.C. A novel selective broad-spectrum anti-DNA virus agent. Nature. 1986;323:464–467. doi: 10.1038/323464a0. [DOI] [PubMed] [Google Scholar]
- 3.Pertusati F., Serpi M., McGuigan C. Medicinal chemistry of nucleoside phosphonate prodrugs for antiviral therapy. Antivir. Chem. Chemother. 2012;22:181–203. doi: 10.3851/IMP2012. [DOI] [PubMed] [Google Scholar]
- 4.Baszczynski O., Janeba Z. Medicinal chemistry of fluorinated cyclic and acyclic nucleoside phosphonates. Med. Res. Rev. 2013;33:1304–1344. doi: 10.1002/med.21296. [DOI] [PubMed] [Google Scholar]
- 5.De Clercq E., Field H.J. Antiviral prodrugs - the development of successful prodrug strategies for antiviral chemotherapy. Br. J. Pharmacol. 2006;147:1–11. doi: 10.1038/sj.bjp.0706446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pradere U., Garnier-Amblard E.C., Coats S.J., Amblard F., Schinazi R.F. Synthesis of nucleoside phosphate and phosphonate prodrugs. Chem. Rev. 2015;114:9154–9218. doi: 10.1021/cr5002035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thornton P.J., Kadri H., Niccoli A., Mehellou Y. Nucleoside phosphate and phosphonate prodrug clinical candidates. J. Med. Chem. 2016;59:10400–10410. doi: 10.1021/acs.jmedchem.6b00523. [DOI] [PubMed] [Google Scholar]
- 8.Rautio J., Kumpulainen H., Heimbach T., Oliyai R., Oh D., Järvinen T., Savolainen J. Prodrugs: design and clinical applications. Nat. Rev. Drug Discov. 2008;7:255–270. doi: 10.1038/nrd2468. [DOI] [PubMed] [Google Scholar]
- 9.Starrett J.E., Tortolani D.R., Hitchcock M.J.M., Martin J.C., Mansuri M.M. Synthesis and in vitro evaluation of a phosphonate prodrug: bis(pivaloyloxymethyl) 9-(2-phosphonylmethoxyethyl)adenine. Antivir. Res. 1992;19:267–273. doi: 10.1016/0166-3542(92)90084-i. [DOI] [PubMed] [Google Scholar]
- 10.Naesens L., Balzarini J., Bischofberger N., De Clercq E. Antiretroviral activity and pharmacokinetics in mice of oral bis(pivaloyloxymethyl)-9-(2-phosphonylmethoxyethyl)adenine, the bis(pivaloyloxymethyl) ester prodrug of 9-(2-phosphonylmethoxyethyl)adenine. Antimicrob. Agents Chemother. 1996;40:22–28. doi: 10.1128/aac.40.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Naesens L., Bischofberger N., Augustijns P., Annaert P., Van den Mooter G., Arimilli M.N., Kim C.U., De Clercq E. Antiretroviral efficacy and pharmacokinetics of oral bis(isopropyloxycarbonyloxymethyl)9-(2-phosphonylmethoxypropyl)adenine in mice. Antimicrob. Agents Chemother. 1998;42:1568–1573. doi: 10.1128/aac.42.7.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ray A.S., Fordvce M.W., Hitchcock M.J. Tenofovir alafenamide: a novel prodrug of tenofovir for treatment of Human Immunodeficiency Virus. Antivir. Res. 2016;125:63–70. doi: 10.1016/j.antiviral.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 13.Gibson A.K., Shah B.M., Nambiar P.H., Schafer J.J. Tenofovir alafenamide. Ann. Pharmacother. 2016;50:942–952. doi: 10.1177/1060028016660812. [DOI] [PubMed] [Google Scholar]
- 14.Lee W.A., He G.-X., Eisenberg E., Cihlar T., Swaminathan S., Mulato A., Cundy K.C. Selective intracellular activation of a novel prodrug of the human immunodeficiency virus reverse transcriptase inhibitor tenofovir leads to preferential distribution and accumulation in lymphatic tissue. Antimicrob. Agents Chemother. 2005;49:1898–1906. doi: 10.1128/AAC.49.5.1898-1906.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Clercq E., Holý A. Acyclic nucleoside phosphonates: a key class of antiviral drugs. Nat. Rev. Drug Discov. 2005;4:928–940. doi: 10.1038/nrd1877. [DOI] [PubMed] [Google Scholar]
- 16.De Clercq E. Acyclic nucleoside phosphonates: past, present and future: bridging chemistry to HIV, HBV, HCV, HPV, adeno-, herpes-, and poxvirus infections: the phosphonate bridge. Biochem. Pharmacol. 2007;73:911–922. doi: 10.1016/j.bcp.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 17.Topalis D., Pradère U., Roy V., Caillat C., Azzouzi A., Broggi J., Snoeck R., Andrei G., Lin J., Eriksson S., Alexandre J.A.C., El Amri C., Deville-Bonne D., Meyer P., Balzarini J., Agrofoglio L.A. Novel antiviral C5-substituted pyrimidine acyclic nucleoside phosphonates selected as human thymidylate kinase substrates. J. Med. Chem. 2011;54:222–232. doi: 10.1021/jm1011462. [DOI] [PubMed] [Google Scholar]
- 18.Peterson L.W., McKenna C.E. Prodrug approaches to improving the oral absorption of antiviral nucleotide analogues. Expert Opin. Drug Deliv. 2009;6:405–420. doi: 10.1517/17425240902824808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hecker S.J., Erion M.D. Prodrugs of phosphates and phosphonates. J. Med. Chem. 2008;51:2328–2345. doi: 10.1021/jm701260b. [DOI] [PubMed] [Google Scholar]
- 20.Wagner C.R., Lyer V.V., McIntee E.J. Pronucleotides: toward the in vivo delivery of antiviral and anticancer nucleotides. Med. Res. Rev. 2000;20:417–451. doi: 10.1002/1098-1128(200011)20:6<417::aid-med1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 21.(a) Montagu A., Pradére U., Roy V., Nolan S.P., Agrofoglio L.A. Expeditious convergent procedure for the preparation of bis(POC) prodrugs of new (E)-4-phosphono-but-2-en-1-yl nucleosides. Tetrahedron. 2011;67:5319–5328. [Google Scholar]
- 22.Pradère U., Clavier H., Roy V., Nolan S.P., Agrofoglio L.A. The Shortest strategy for generating phosphonate prodrugs by olefin cross-metathesis - application to acyclonucleoside phosphonates. Eur. J. Org Chem. 2011;2011:7324–7330. [Google Scholar]
- 23.Pradère U., Roy V., Montagu A., Sari O., Hamada M., Balzarini J., Snoeck R., Andrei G., Agrofoglio L.A. Synthesis and antiviral evaluation of bis(POM) prodrugs of (E)-[4’-phosphono-but-2’-en-1’-yl]purine nucleosides. Eur. J. Med. Chem. 2012;57:126–133. doi: 10.1016/j.ejmech.2012.08.042. [DOI] [PubMed] [Google Scholar]
- 24.Sari O., Hamada M., Roy V., Nolan S.P., Agrofoglio L.A. The preparation of trisubstituted alkenyl nucleoside phosphonates under ultrasound-assisted olefin cross-metathesis. Org. Lett. 2013;15:4390–4393. doi: 10.1021/ol401922r. [DOI] [PubMed] [Google Scholar]
- 25.Bessières M., Sari O., Roy V., Warszycki D., Bojarski A.J., Nolan S.P., Snoeck R., Andrei G., Schinazi R.F., Agrofoglio L.A. Sonication-assisted synthesis of (E)-2-methyl-but-2-enyl nucleoside phosphonate prodrugs. ChemistrySelect. 2016;1:3108–3113. [Google Scholar]
- 26.Hostetler K.Y. Alkoxyalkyl prodrugs of acyclic nucleoside phosphonates enhance oral antiviral activity and reduce toxicity: current state of the art. Antivir. Res. 2009;82:84–98. doi: 10.1016/j.antiviral.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.(a) Mehellou Y., Balzarini J., McGuigan C. Aryloxy phosphoramidate triesters: a technology for delivering monophosphorylated nucleosides and sugars into cells. ChemMedChem. 2009;4:1779–1791. doi: 10.1002/cmdc.200900289. [DOI] [PubMed] [Google Scholar]
- 28.Cahard D. Aryloxy phosphoramidate triesters as Pro-Tides. Mini Rev. Med. Chem. 2004;4:371–381. doi: 10.2174/1389557043403936. [DOI] [PubMed] [Google Scholar]
- 29.Eisenberg E.J., He G.-X., Lee W.A. Metabolism of GS-7340, a novel phenyl monophosphoramidate intracellular prodrug of PMPA in blood. Nucleos Nucleot. Nucleic Acids. 2001;20:1091–1098. doi: 10.1081/NCN-100002496. [DOI] [PubMed] [Google Scholar]
- 30.Cihlar T., Ray A.S., Boojamra C.G., Zhang L., Hui H., Laflamme G., Vela J.E., Grant D., Chen J., Myrick F., White K.L., Gao Y., Lin K.-Y., Douglas J.L., Parkin N.T., Carey A., Pakdaman R., Mackman R.L. Design and profiling of GS-9148, a novel nucleotide analog active against nucleoside-resistant variants of human immunodeficiency virus type 1, and its orally bioavailable phosphonoamidate prodrug, GS-9131. Antimicrob. Agents Chemother. 2008;52:655–665. doi: 10.1128/AAC.01215-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chapman H., Kernan M., Prisbe E., Rohloff J., Sparacino M., Terhorst T., Yu R. Practical synthesis, separation, and stereochemical assignment of the PMPA prodrug GS-7340. Nucleos Nucleot. Nucleic Acids. 2001;20:621–628. doi: 10.1081/NCN-100002338. [DOI] [PubMed] [Google Scholar]
- 32.Wilson G.O., Porter K.A., Weissman H., White S.R., Sottos N.R., Moore J.S. Stability of second generation Grubbs' alkylidenes to primary amines: formation of novel ruthenium-amine complexes. Adv. Synth. Catal. 2009;351:1817–1825. [Google Scholar]
- 33.Sikora D., Nonas T., Gajda T. O-Ethyl 1-azidoalkylphosphonic acids versatile reagents for the synthesis of protected phosphonoamidate peptides. Tetrahedron. 2001;57:1619–1625. [Google Scholar]
- 34.Hernandez-Reyes C.X., Angeles-Beltran D., Lomas-Romero L., Gonzalez-Zamora E., Gaviao R., Cardenas J., Morales-Serna J.A., Negron-Silva G.E. Synthesis of azanucleosides through regioselective ring-opening of epoxides catalyzed by sulphated zirconia under microwave and solvent-free conditions. Molecules. 2012;17:3359–3369. doi: 10.3390/molecules17033359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bessières M., Roy V., Agrofoglio L.A. A convenient, highly selective and eco-friendly N-Boc protection of pyrimidines under microwave irradiation. RSC Adv. 2014;4:59747–59749. [Google Scholar]
- 36.Scholl M., Ding S., Lee C.W., Grubbs R.H. Synthesis and activity of a new generation of ruthenium-based olefin metathesis catalysts coordinated with 1,3-dimesityl-4,5-dihydroimidazol-2-ylidene ligands. Org. Lett. 1999;1:953–956. doi: 10.1021/ol990909q. [DOI] [PubMed] [Google Scholar]
- 37.Garber S.B., Kingsbury J.S., Gray B.L., Hoveyda A.H. Efficient and recyclable monomeric and dendritic Ru-based metathesis catalysts. J. Am. Chem. Soc. 2000;122:8168–8179. [Google Scholar]
- 38.Ettari R., Micale N., Schirmeister T., Gelhaus C., Leippe M., Nizi E., Di Francesco M.E., Grasso S., Zappala M. Novel peptidomimetics containing a vinyl ester moiety as highly potent and selective Falcipain-2 inhibitors. J. Med. Chem. 2009;52:2157–2160. doi: 10.1021/jm900047j. [DOI] [PubMed] [Google Scholar]
- 39.Yang Q., Xiao W.-J., Yu Z. Lewis acid assisted ring-closing metathesis of chiral diallylamines: an efficient approach to enantiopure pyrrolidine derivatives. Org. Lett. 2005;7:871–874. doi: 10.1021/ol047356q. [DOI] [PubMed] [Google Scholar]
- 40.Gułajski Ł., Śledź P., Lupa A., Grela K. Olefin metathesis in water using acoustic emulsification. Green Chem. 2008;10:271–274. [Google Scholar]
- 41.Lipshutz B.H., Ghorai S. Olefin Metathesis. John Wiley & Sons, Inc.; Hoboken, NJ, USA: 2014. Olefin metathesis in water and aqueous media; pp. 515–521. [Google Scholar]
- 42.Schinazi R.F., Sommadossi J.P., Saalman V., Cannon D.L., Xie M.W., Hart G.C., Hahn E.F. Activities of 3'-azido-3'-deoxythymidine nucleotide dimers in primary lymphocytes infected with human immunodeficiency virus type 1. Antimicrob. Agents Chemother. 1990;34:1061–1067. doi: 10.1128/aac.34.6.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stuyver L.J., Lostia S., Adams M., Mathew J., Pai B.S., Grier J., Tharnish P., Choi Y., Chong Y., Choo H., Chu C.K., Otto M.J., Schinazi R.F. Antiviral activities and cellular toxicities of modified 2′,3′-dideoxy-2′,3′-didehydrocytidine analogues. Antimicrob. Agents Chemother. 2002;46:3854–3860. doi: 10.1128/AAC.46.12.3854-3860.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.