Abstract
This article represents a review of the current literature on the role of infection in the pathogenesis of chronic obstructive pulmonary disease (COPD), in stable disease, exacerbations, and pneumonia. It outlines the complex interactions between respiratory pathogens and host immune defenses that underlie the clinical manifestations of infection in COPD.
Keywords: Infection in COPD, Airway colonization, Chronic infection, New strain exacerbations, Pneumonia and COPD, Innate immunity in COPD, Virulence factors, Vicious-circle hypothesis
Key points
-
•
Infection and chronic obstructive pulmonary disease (COPD) can be regarded as comorbid conditions, because infections contribute the progression of COPD, and COPD alters the susceptibility and manifestations of lung infections.
-
•
The underlying mechanism of acute exacerbations of COPD is acquisition of new strains of bacteria and viruses. A complex host-pathogen interaction then determines the clinical manifestations and outcomes of such acquisition.
-
•
COPD predisposes to community-acquired pneumonia and alters its cause, treatment, and outcomes.
-
•
Several lines of evidence now suggest that chronic airway infection by bacteria is prevalent in COPD, and by triggering a chronic inflammatory response contributes to progression of disease.
-
•
Lung innate immune defenses are impaired in COPD, making these patients more susceptible to infection. Respiratory pathogens prevalent in COPD use various mechanisms to evade host responses and thereby cause acute and persistent infections.
Introduction
The role of infection in chronic obstructive pulmonary disease (COPD) was first postulated in 1953 by Stuart-Harris and colleagues1 in what is now known as the British hypothesis. They speculated that the decline in the lung function in COPD was the result of mucus hypersecretion and recurrent bacterial infections. In the next 2 decades, several studies were performed to confirm the hypothesis. In some of these studies, sputum microbiology was used to compare the rate of bacterial infection in patients with chronic bronchitis at baseline and during exacerbations, as well as in comparison with individuals without COPD.2, 3, 4, 5, 6, 7 Some differences in bacterial infection related to disease state were found; for example, Smith and colleagues2, 3, 8 found increased colonization with Haemophilus influenzae in patients with severe COPD compared with mild COPD. However, for the most part, differences in the rate of bacterial isolation from sputum at stable state (ie, colonization) versus at acute exacerbation (ie, infection) were not seen in these studies. Advanced molecular biology techniques to differentiate bacterial strains within species had not been developed and were therefore not available to these investigators. Other investigators examined this hypothesis by using serologic studies to determine levels of antibacterial antibodies in patients with chronic bronchitis. These results were also confusing and contradictory and were confounded by the use of laboratory strains as an antigen (discussed in Ref.9). In 1977, Fletcher and colleagues10 published a landmark study that showed that frequency of exacerbations and mucus hypersecretion did not result in faster decline of lung function in patients with COPD. By the early 1980s, because of these observations and the appreciation of the importance of tobacco smoke in COPD pathogenesis, the British hypothesis was rejected, and bacterial infection was relegated to an epiphenomenon in this disease.7
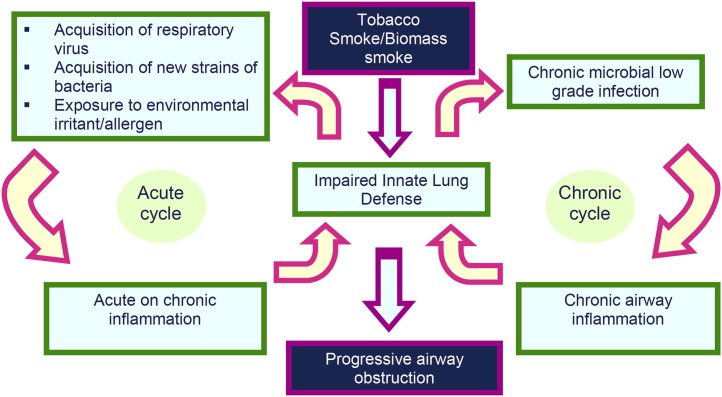
The role of viral infection in COPD exacerbations was also extensively investigated in the 1960s and 1970s with viral cultures and serology at exacerbation.3, 5, 8 Because of the lack of confounding by chronic colonization and serologic cross reactivity, about 30% of exacerbations were confirmed to be of viral origin. Following 20 to –30 years of scant investigation, the role of infection has been revisited in the last 2 decades with new molecular biology, immunology, and microbiology techniques.11 Understanding of infection in COPD, both in the acute and chronic settings, has consequently developed substantially, as discussed later (Fig. 1 ).
Fig. 1.
Acute and chronic infection cycles in the pathogenesis of COPD.
Acute infection
Acute infections in COPD are clinically recognized either as exacerbations or as episodes of pneumonia. The differentiation between the two presentations is based on the presence (pneumonia) or absence (exacerbation) of lung parenchymal involvement, which presents as an infiltrate on chest radiology. Although pneumonia has been always considered to be a more significant acute infection, exacerbations occur with much greater frequency and also have serious consequences in COPD. As the British hypothesis was being largely discredited, the importance of exacerbations in COPD was also minimized. They came to be regarded as self-resolving viral illness of little consequence (chest colds) for which no specific therapy was available and that were part of the natural course of the disease. The last 2 decades have seen considerable revision in this point of view, because data have emerged that exacerbations do contribute to the loss of quality of life and lung function in COPD and account for as much as half the cost of care of COPD. Furthermore, bacterial infection contributes to exacerbations, specific therapies are of benefit, and prevention of exacerbations is possible and is an important therapeutic goal in COPD.
Causes of Exacerbations
Exacerbations of COPD are airway inflammatory events that are induced by infection in most instances. The aggravating infection can be viral, bacterial, or a combination of viral and bacterial infections. Although there are episodes that are induced by poorly understood noninfectious factors, infections likely account for about 80% of exacerbations (Table 1 ).
Table 1.
Microbial pathogens in COPD
| Microbe | Role in Exacerbations | Role in Stable Disease |
|---|---|---|
| Bacteria | ||
| H influenzae | 20%–30% of exacerbations | Major pathogen |
| Streptococcus pneumoniae | 10%–15% of exacerbations | Minor role |
| Moraxella catarrhalis | 10%–15% of exacerbations | Minor role |
| Pseudomonas aeruginosa | 5%–10% of exacerbations, prevalent in advanced disease | Likely important in advanced disease |
| Enterobacteriaceae | Isolated in advanced disease, pathogenic significance undefined | Undefined |
| Haemophilus haemolyticus | Isolated frequently, unlikely cause | Unlikely |
| Haemophilus parainfluenzae | Isolated frequently, unlikely cause | Unlikely |
| Staphylococcus aureus | Isolated infrequently, unlikely cause | Unlikely |
| Viruses | ||
| Rhinovirus | 20%–25% of exacerbations | Unlikely |
| Parainfluenza | 5%–10% of exacerbations | Unlikely |
| Influenza | 5%–10% of exacerbations | Unlikely |
| Respiratory syncytial virus | 5%–10% of exacerbations | Controversial |
| Coronavirus | 5%–10% of exacerbations | Unlikely |
| Adenovirus | 3%–5% of exacerbations | Latent infection seen, pathogenic significance undefined |
| Human metapneumovirus | 3%–5% of exacerbations | Unlikely |
| Atypical Bacteria | ||
| Chlamydophila pneumoniae | 3%–5% of exacerbations | Commonly detected, pathogenic significance undefined |
| Mycoplasma pneumoniae | 1%–2% | Unlikely |
| Fungi | ||
| Pneumocystis jiroveci | Undefined | Commonly detected, pathogenic significance undefined |
From Sethi S, Murphy TF. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med 2008;359:2356; with permission.
Virus
The role of viruses in exacerbation was established in older studies (as discussed earlier) by viral culture and serology. Understanding of viral exacerbations has recently been expanded by the use of molecular diagnostic techniques and with the development of a human experimental model of rhinoviral exacerbations. The most common viruses detected in airway secretions at exacerbation are rhinovirus, influenza, respiratory syncytial virus (RSV), parainfluenza, and adenovirus. A recent systematic review found that viruses were detected in 34.1% of exacerbations.12 More recent studies using molecular detection of virus by polymerase chain reaction (PCR) techniques have found viruses in up to half of all exacerbations.13 The human experimental model of rhinoviral exacerbations was described in a study in which 13 subjects with COPD and 13 control subjects were nasally inoculated with a low dose of rhinovirus.14 An increased neutrophilic inflammatory response in the lower airway, and more prominent lower respiratory symptoms and airway obstruction, were found in COPD compared with controls. An impaired interferon response to the infection was seen in patients with COPD. This work confirms the viral causation of exacerbations and has provided insights into susceptibility and pathogenic mechanisms involved in viral exacerbations.
Bacteria
In contrast with the role of viruses, the role of bacteria as a cause of exacerbations has been controversial and was not fully appreciated until recently. At present, pathogens clearly implicated in COPD exacerbations are nontypeable H influenzae, Streptococcus pneumoniae, Moraxella catarrhalis, and Pseudomonas aeruginosa. Whether Staphylococcus aureus and gram-negative enteric bacteria (Enterobacteriaceae), which are frequently isolated from sputum in COPD, are causative for exacerbations or are only capable of airway colonization is unclear at present.
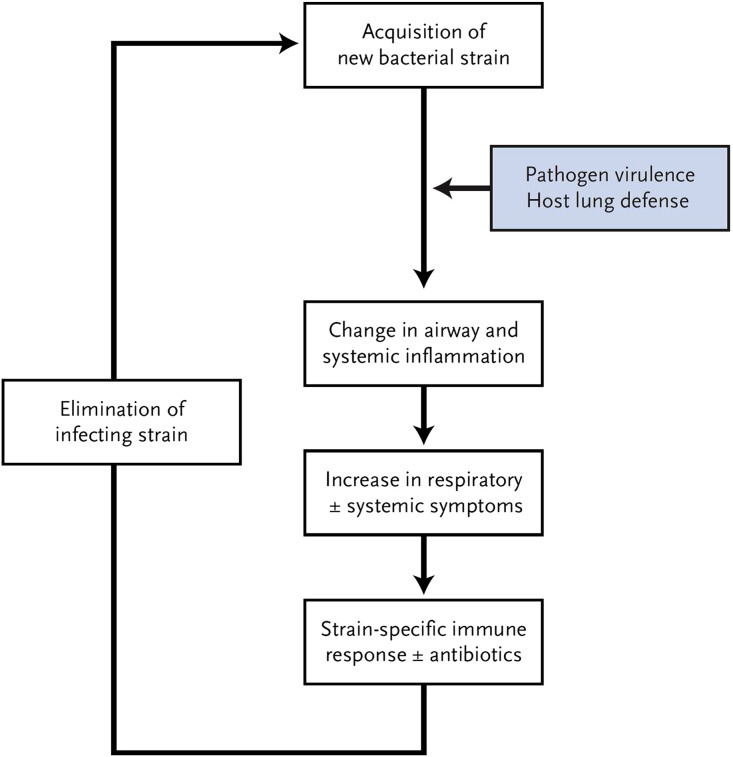
Previous studies that defined bacterial pathogens isolated from sputum only at a species level were unable to fully appreciate the dynamic nature of bacterial infection in COPD. In a longitudinal prospective cohort study in patients with COPD, when bacterial strains in sputum were characterized by molecular techniques, combined with a careful analysis of host immune and immunologic responses, an important mechanism that likely underlies exacerbations caused by the 4 major pathogens listed earlier was found (Fig. 2 ).15 The risk of having an exacerbation was increased by more than 2-fold with respiratory tract acquisition of strains of these bacterial pathogens that were new to the patient. In this initial study, 33% of the visits within a month of new strain acquisition were associated with an exacerbation compared with 15.4% without a new strain.16 Subsequent analyses from this study have now shown that the incidence of exacerbations at a visit with a new strain isolated from sputum is 40% to 50%, and that this holds true for each of the 4 major pathogens (nontypeable H influenzae, S pneumoniae, M catarrhalis, and P aeruginosa).17, 18, 19 Additional support for this mechanism for exacerbations comes from various observations. Exacerbation-associated strains of H influenzae are more inflammatory in in vitro and animal models than strains associated with colonization, showing that clinical implications of bacterial acquisition correlate with strain virulence.20 Strain-specific host immune response and a vigorous neutrophilic inflammatory response distinguish new strain exacerbations from those without new strains.21
Fig. 2.
Proposed mechanism of bacterial exacerbations in COPD.
(From Sethi S, Murphy TF. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med 2008;359:2357; with permission.)
Whether an increase in bacterial concentration (load) in the airway of a preexisting (colonizing) strain can be an additional independent mechanism of exacerbations is controversial. When bacterial sputum concentrations from our longitudinal cohort study were analyzed, either no differences or small differences were found between stable disease and exacerbation, and the small differences were no longer seen once new strain acquisition was taken into account.21 In contrast, Rosell and colleagues22 showed in pooled analysis of their data from bronchoscopic protected brush specimens that 54% of the patients with COPD exacerbation had pathogenic bacteria present in their airway secretions at significant concentrations compared with 29% of the patients with stable COPD. Intracellular H influenzae was found in bronchial mucosal biopsies in 87% of intubated patients with COPD exacerbation, compared with 33% of the patients with stable COPD.23 Garcha and colleagues,24 using quantitative PCR, found higher sputum bacterial loads at exacerbation than at stable state. However, these studies that have shown higher bacterial loads in sputum at exacerbation have not taken into account bacterial strain variation in their specimens.
Coinfection with virus and bacteria
A few recent studies have examined the impact of simultaneous or sequential bacterial and viral infection at exacerbation. Papi and colleagues25 examined 64 patients with COPD exacerbation requiring hospital admission; 25% had combined bacterial and viral infections, and these patients had more severe symptoms and longer hospitalization. Presence of cold symptoms and H influenzae in sputum has also been associated with more symptoms and a larger decrease in lung function than when either is present alone.26 In the rhinoviral human experimental model discussed earlier, as many as 60% of patients with COPD developed a secondary bacterial infection with a greater inflammatory response and duration of symptoms.27 However, the severity of the exacerbations was mild and none of the patients required steroids or antibiotics.
It is likely that viral infection predisposes the susceptible host to bacterial coinfection and vice versa.28, 29 In cultured airway epithelial cells, Sajjan and colleagues29 found that infection with H influenzae increased expression of intercellular adhesion molecule (ICAM)-1 and Toll-like receptor (TLR)-3, receptors for rhinovirus and its double-stranded RNA. In contrast, in another experimental model, Avadhanula and colleagues28 found increased bacterial adhesion to respiratory epithelial cells after viral infection. In the human rhinovirus experimental model, degradation of antimicrobial peptides such as elafin by neutrophil elastase could explain the occurrence of secondary bacterial infection.27
In summary, bacterial and viral infections play a critical role in COPD exacerbations. Application of molecular diagnostic techniques to exacerbations is likely to further enhance understanding of infectious episodes. The role of opportunistic bacterial pathogens in causing exacerbations still needs to be defined.
Community-acquired Pneumonia
Epidemiology
Community-acquired pneumonia (CAP) is a major cause of morbidity and mortality worldwide, with incidence of 2.6 to 11 per 1000 adults.30, 31 Mortality can reach 20%, with 14.9% of the mortality risk attributed to smoking, second only to age.32 In a multivariate analysis, COPD was an independent risk factor for developing severe CAP, with an odds ratio (OR) of 1.91.33 Evaluation of COPD subgroups revealed that severe COPD on home oxygen and severe COPD exacerbations requiring hospitalization were independent risk factors for developing CAP.34 Merino-Sanchez and colleagues35 observed a 12.6% incidence of pneumonia in 596 patients with COPD over 3 years, with 55% of the cases with Pneumonia Severity Index (PSI) of 4 and 5. The mortality for a PSI of 5 was 35.7%. In 2 European studies, hospitalized patients with CAP with and without COPD were compared. Although mortality differences between the groups were not seen, patients with COPD experienced more severe pneumonia, higher rates of readmission, and recurrent pneumonia. Lower serum tumor necrosis factor alpha (TNF-α) and interleukin-6 levels were seen in the COPD group, suggesting an impaired inflammatory response in these patients.36, 37 Higher mortality with CAP in COPD has been observed in other studies, reiterating the importance of early recognition and appropriate management in this high-risk population.38, 39, 40
Causes of CAP in COPD
S pneumoniae remains the most common cause of CAP in COPD. However, because of alterations in the lung microbiome in COPD, pathogens such as H influenzae, M catarrhalis, and P aeruginosa may play a larger role in the development of CAP in these patients. Moreover, patients with COPD are exposed to frequent antibiotic courses and they are more likely to be infected with antibiotic-resistant pathogens, making empiric antibiotic choices challenging.41 In a study of hospitalized patients with COPD with CAP, more infections attributable to P aeruginosa were observed.42 However, the use of respiratory specimens to determine the microbiological cause of CAP in COPD is challenging, because chronic colonization with CAP-associated pathogens is common in COPD.
Role of inhaled corticosteroids
Inhaled corticosteroids (ICS), in combination with long acting beta agonists (LABA), are widely used in COPD, and reduce the frequency of exacerbations and daily symptoms in these patients.43 However, the benefits come at a cost of increased risk of pneumonia. This increased risk was originally observed in the TORCH (Toward a Revolution in COPD Health) study, in which the ICS/LABA group had a higher probability (19.6%) of developing pneumonia over the course of 3 years.43 A recent meta-analysis of 24 randomized controlled trials of ICS in COPD confirmed these results with a calculated relative risk of developing pneumonia at 1.56, and a number needed to harm of 60.44, 45, 46 However, mortality was no different from the use of a LABA alone. The association between ICS use and pneumonia should be interpreted with caution. None of these trials were specifically designed to assess the risk of pneumonia, most episodes lack radiological confirmation, and COPD exacerbations may have been misdiagnosed as pneumonia. Mechanisms underlying this association have not been examined, but corticosteroid-induced impairment of local immune response to microbial pathogens is likely responsible.
Antimicrobial therapy in COPD and CAP
In outpatients with CAP, the presence of COPD as a comorbid condition places them in a high-risk group, and treatment with a respiratory fluoroquinolone or a β-lactam plus a macrolide is recommended.47 Monotherapy with a macrolide or doxycycline is not appropriate in these patients. Because antibiotic use is common in these patients, a review of antibiotic use in the previous 3 months should guide empiric choice, and antibiotic classes used in the previous 3 months should be avoided. Among inpatients with CAP and COPD, the same choices are applicable. However, in patients requiring intensive care admission, combination therapy is always recommended, with a β-lactam and a respiratory fluoroquinolone or a macrolide. If Pseudomonas is suspected (previous Pseudomonas isolation, bronchiectasis, malnutrition, recent broad-spectrum antibiotic exposure), an antipseudomonal regimen is recommended.
Chronic infection
In contrast with the (almost) sterile airways of a healthy lung, the lower airway of patients with COPD is frequently colonized with bacteria.48, 49 Although a wide variety of pathogens can be isolated, the two most common are H influenzae and P aeruginosa (see Table 1). Until recently, the presence of these bacteria was regarded as colonization, implying an innocuous process in the airway without sequelae. A growing body of evidence now suggests that this colonization in stable COPD, via complex interactions with the host immune-inflammatory system, could contribute to COPD pathogenesis and progression.
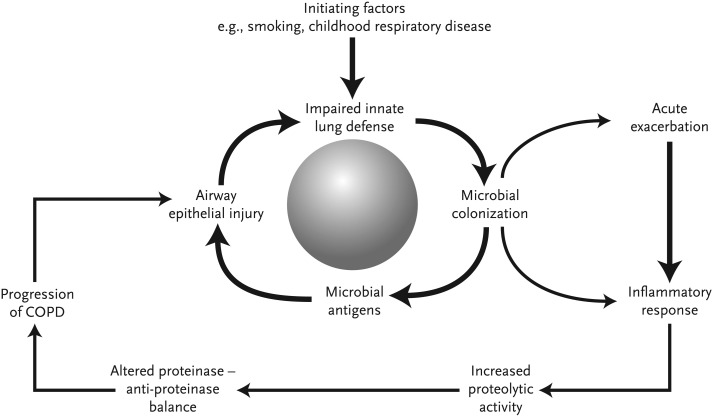
Vicious-circle Hypothesis
Similar to bronchiectasis and cystic fibrosis, the host-pathogen interaction in stable COPD is well described by the vicious-circle hypothesis (Fig. 3 ). Repeated insults to the lung, such as smoking and environmental exposures, lead to impairment of the host immune defenses, thus allowing bacterial colonization. The bacteria cause subclinical inflammatory response in the airway, resulting in further damage to the innate lung defense and persistence of chronic bacterial infection. This process accelerates during acute exacerbations.
Fig. 3.
The vicious-circle hypothesis of infection and inflammation in COPD.
(From Sethi S, Murphy TF. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med 2008;359:2361; with permission.)
Evidence to Support Chronic Infection
Colonization is defined by the absence of damaging effects to the host related to the presence of a pathogen and the absence of a specific immune response. There are several parts of the body where such colonization is seen (eg, skin, colon) and is essential for health. The microbial pathogens that colonize these surfaces constitute a microbiome. Recent advances in research technologies, especially high-throughput genomic characterization, have made it possible to characterize the microbiome. The healthy lung is sterile by standard culture techniques. Even with molecular techniques, the microbiome of the healthy lung is sparse and transient, composed primarily of oral flora that are microaspirated and cleared.50 In contrast, in a third of patients with COPD, potential respiratory pathogens can be retrieved by culture of lower airway samples.49, 51 An abundant microbiome can be found by molecular techniques in COPD lungs.52 Unlike other body surfaces, like the skin and gut mucosa, the lung is not well equipped to handle a microbiome. Therefore, microbial presence in the lower airways in COPD is harmful.
Several studies have described excess inflammation in stable patients with COPD when colonized with bacterial pathogens.51, 53, 54, 55, 56 The airway inflammation associated with bacterial colonization is predominantly neutrophilic. Studies comparing sputum samples from colonized and noncolonized patients have found higher levels of TNF-α, interleukin-8, interleukin-6, leukotriene B4, neutrophil myeloperoxidase, and elastase, and lower levels of the antiprotease secretory leukocyte protease inhibitor.53, 54, 56, 57 Bronchoscopic sampling of the lower airway with bronchoalveolar lavage showed increased levels of neutrophils, TNF-α, interleukin-8, matrix metalloproteinase 9, and endotoxin in association with bacterial colonization.49, 51, 58 Although several mediators contribute to COPD pathogenesis, interleukin-8 in particular has been associated with increased exacerbation frequency, longer recovery periods, worsening airway obstruction, and development of bronchiectasis.58, 59 Bacterial (M catarrhalis) acquisition, even without an increase in symptoms of an exacerbation, has been associated with increases in proteolytic activity and a reduction in antiproteolytic defense, resulting in worsening of the protease/antiprotease imbalance that is thought to cause progressive lung damage in COPD.56 The inflammatory profile seen with bacterial colonization is similar to that seen with bacterial exacerbations, implying that colonization is a low-grade infection.
Following exacerbations of COPD, specific immune responses, both systemic and mucosal, to the infecting strain are often observed. Similar observations have now been described following colonization.18, 19 This active immune response supports the presence of chronic infection in COPD. Furthermore, with M catarrhalis, a differential immune response is seen with colonization, which is accompanied by a stronger mucosal immune response, compared with a stronger systemic immune response accompanying exacerbations.18 Whether the nature of the immune response dictates the clinical expression of infection or vice versa is not clear.
The potential contribution of viral and atypical pathogens to chronic infection in COPD has been controversial. Latent adenoviral infection of the lungs, in the form of integration of portions of adenoviral DNA into cellular DNA, was found to enhance the inflammatory response to tobacco smoke, and thereby was thought to contribute to COPD pathogenesis.60 Initial studies showed that such adenoviral integration was more common in COPD than in controls; however, subsequent studies have not supported this observation. Latent RSV infection has been described in COPD by one group of investigators, but has not been found by others.61, 62 The presence and contribution of chronic chlamydial lung infection in COPD remains similarly controversial, with inconsistent observations from various investigators.
Besides the direct microbiological evidence of infection and its consequences, other indirect lines of evidence of chronic infection in COPD have emerged from radiological and pathologic studies. In a pathologic study of small airways of patients with COPD, the extent of formation of lymphoid aggregates predominantly composed of B cells had the best correlation with the degree of airflow obstruction.63 It is likely that these aggregates represent a local host immune response to chronic microbial infection. Furthermore, this pathologic finding was replicated in a mouse model of chronic inflammation in the lungs induced by repeated instillation of nontypeable H influenzae lysate.64
Widespread use of high resolution computed tomography scans has revealed that bronchiectasis develops in a substantial proportion of patients with COPD. In a comprehensive study of 92 patients with stable moderate or severe disease, 57.5% had bronchiectasis, and its presence was related to worse lung function, hospital admission in the past year, and chronic bronchitic symptoms.65 Repeated sputum cultures in these patients linked chronic colonization with potential bacterial pathogens (predominantly nontypeable H influenzae and P aeruginosa) with the presence of bronchiectasis.65
In summary, these various lines of investigation support the paradigm of a vicious circle of infection and inflammation in COPD. However, COPD is a heterogeneous disease and it is likely that in 30% to 50% of these patients chronic infection plays a prominent role, these being the ones with chronic colonization, bronchiectasis, and/or chronic bronchitis. Future longitudinal natural history studies or studies with interventions that decrease bacterial colonization and measure disease progression are needed to prove the vicious-circle hypothesis.
Mechanism of Increased Susceptibility to Infection in COPD
Although infection is a comorbid condition in COPD and much has been learned about the incidence and consequences of infection in this disease, understanding of the mechanisms underlying increased susceptibility to infection seen in COPD is still in its early stages. Alterations in both the host and pathogen can contribute to establishment of acute and chronic infections, and both play a role in COPD. Disruptions in the host that increase susceptibility to infection can be categorized into changes in innate or adaptive lung defense. Pathogen alterations include host defense evasion mechanisms.
Host Defects: Innate Immunity
The healthy lung possesses a multilayered, redundant, and highly efficient innate defense system that allows it to maintain an almost pathogen-free environment in spite of being constantly exposed to a variety of microbes through inhalation and microaspiration. This innate immune system of the lung has 3 components: mechanical barrier, humoral, and cellular response systems. This nonspecific immunity is the first line of defense against viruses, bacteria, and other particulates. It recognizes antigenic ligands entering the airway via pattern-recognition receptors and triggers series of responses resulting in complement activation and phagocytosis. The end result is elimination of the antigen or its presentation on the surface of the macrophages and activation of the adaptive immunity. In patients with COPD, several innate responses are impaired, leading to increased susceptibility to infection.
Mucociliary clearance
The mucociliary clearance is the first barrier to noxious agents, by effectively trapping and clearing inhaled and microaspirated microbial pathogens. Both normal mucus and a normal ciliary apparatus are required for effective mucociliary clearance. Abnormal mucus (such as in cystic fibrosis) and a dysfunctional ciliary apparatus (such as in ciliary dyskinesia) are associated with acute and chronic bronchial infection. Augmented mucus production can be regarded as a defensive response to particulate or microbial exposure. However, when the exposure is chronic, and inflammation and ciliary dysfunction are also present, it could worsen mucociliary clearance.
Smoking disrupts mucociliary clearance, not only by augmenting mucus production but also by inducing structural abnormalities in the ciliary apparatus.66 Studies in moderate to heavy smokers have shown longer lung clearance times, although the degree of impairment is variable.67, 68 Further deterioration in mucociliary clearance is seen with development of chronic bronchitis and airway obstruction in smokers.69, 70, 71 Patients with COPD have hypertrophy and hyperplasia of their airway goblet cells and increased mucus stores.72, 73 In tissue and animal models, exposure to S pneumoniae and H influenzae results in further upregulation of mucin production.74, 75 Neutrophilic inflammation also worsens mucociliary function, mediated by increased mucus production, reduced ciliary beating, and altered viscoelastic properties of mucus.
Immunoglobulin A
Immunoglobulin (Ig) A, especially polymeric secretory IgA, plays an important role in innate defense by coating the bacterial pathogen, thereby interfering with its ability to interact with the mucosal surface (immune exclusion). IgA can also neutralize infectious agents and could act as an opsonin assisting in pathogen elimination. Localized areas of IgA deficiency in the large and small airways are seen in COPD that were associated with squamous metaplasia. Polymeric IgG receptor expression, a receptor required for transcytosis of the IgA molecule from the basolateral to the apical surface of the epithelial cell, was reduced in these areas.76 These changes in IgA could be an important mechanism of infection susceptibility in COPD.
Antimicrobial peptides
Antimicrobial polypeptides abundant in the airway surface lining fluid have antimicrobial and immunoregulatory functions. One major group, the cationic polypeptides, includes lysozyme, lactoferrin, defensins, the cathelicidins (LL-37), and secretory leukocyte protease inhibitor (SLPI).77, 78, 79, 80, 81, 82 Another important group, the collectins, include surfactant protein-A (SP-A), surfactant protein-D (SP-D), and mannose-binding lectin.83 Complex and dynamic alterations in various antimicrobial polypeptides have been described in COPD, both in the stable state and during exacerbations.
Deficiencies of SLPI and lysozyme in the stable state have been associated with more frequent exacerbations.84, 85 Decreased serum mannose-binding lectin has been linked with exacerbation frequency in COPD, but this has not been a consistent observation.86 Lower airway concentrations of SP-A and SP-D are seen in smokers, with further decreases in association with emphysema.87, 88 Lower levels of beta-defensin 2 and Clara Cell Protein 16 (CC16) and increased levels of elafin and SLPI in sputum supernatants in stable COPD have been observed.89 Decreased levels of beta-defensin 2 in the central airways, but not in the distal airways, of smokers with COPD were found in a study of resected lung specimens.90
Dynamic changes in antimicrobial peptides have also been described with exacerbations of COPD. SLPI levels decrease significantly at the time of such exacerbations, which return to baseline after resolution.78 Lysozyme and lactoferrin levels decrease and LL-37 levels increase with both colonization and infective exacerbations with H influenzae and M catarrhalis.78 In a human model of rhinoviral infection, impaired elafin and SLPI responses following rhinoviral infection were associated with secondary bacterial infection.27
Macrophage function
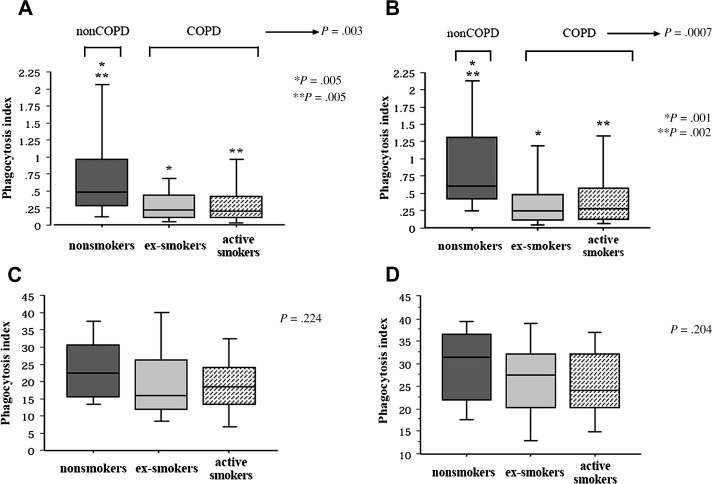
Key cellular components on innate lung defense are alveolar macrophages and airway epithelial cells. Phagocytic and cytokine responses of alveolar macrophages to bacterial pathogens are crucial for dealing with small pathogen inocula, without invoking potentially damaging inflammatory and adaptive immune responses. Alveolar macrophages from patients with COPD show impaired ability to phagocytose H influenzae and M catarrhalis, but not S pneumoniae or inert microspheres. This impairment is correlated with worsening lung function (Fig. 4 ).91 Alveolar macrophages from patients with COPD also have a less robust cytokine response to bacterial proteins, specifically outer membrane protein P6 and lipo-oligosaccharide (endotoxin) of H influenzae.92, 93, 94 Following exposure to rhinovirus, alveolar macrophages showed decreased cytokine responses to bacterial lipopolysaccharide and lipoteichoic acid,95 which could explain the increased susceptibility to bacterial infection after viral infection in COPD.
Fig. 4.
Comparison of phagocytosis of nontypeable H influenzae (A), Moraxella catarrhalis (B), Streptococcus pneumoniae (C), and latex microspheres (D) by human alveolar macrophages from healthy controls, current smokers with COPD, and ex-smokers with COPD.
(Modified from Berenson CS, Kruzel RL, Eberhardt E, et al. Phagocytic dysfunction of human alveolar macrophages and severity of chronic obstructive pulmonary disease. J Infect Dis 2013; with permission.)
These decrements in macrophage function are likely secondary to several mechanisms, including reduction in pattern-recognition receptors such as Toll-like receptors TLR2 and TLR4, reduction in scavenger receptors such as macrophage receptor with collagenous structure (MARCO), or alteration in subpopulations of macrophages in the airway.96, 97, 98 TLR2 has been found to be downregulated in smokers, patients with COPD, and farmers exposed to organic dust.96, 99, 100 TLR4 downregulation is associated with the development of emphysema and worse airflow limitation in smokers.101 Polymorphism T399I of the TLR4 gene has been associated with development of COPD phenotype in smokers.102
Pathogen Mechanisms
Tissue invasion
H influenzae was traditionally regarded as an extracellular pathogen. However, molecular detection techniques have shown this pathogen in the bronchial epithelium and inside subepithelial macrophages in COPD.23, 48 These tissue bacteria could be shielded from the actions of antibiotics and antibodies, and therefore could be more resistant to eradication. Molecular detection often detects H influenzae in airway secretions and lung samples when cultures are negative, which could be explained by such tissue invasion.
Biofilm formation
Biofilms are bacteria encased with an extracellular matrix, which is usually composed of polysaccharides produced and secreted by bacteria. Bacteria in the core of the film, which is predominantly anaerobic, are in a low metabolic state. Antibiotic penetration into biofilms is limited, requiring up to 1000 times higher concentrations to achieve eradication.103 Parts of the biofilm can detach and cause distant infection. Pathogens common in COPD, including H influenzae, M catarrhalis, and P aeruginosa, are capable of biofilm formation. Furthermore, smoke exposure has been shown to increase biofilm formation.104 P aeruginosa in cystic fibrosis is the prototypical example of biofilm formation as a mechanism of persistence in the lung. Whether bacterial biofilms are present in COPD airways is not yet known.105 Mucoid P aeruginosa and some strains of H influenzae persist clinically for long periods in spite of repeated antibiotic exposure, which is reminiscent of cystic fibrosis.
Antigenic alteration
Pathogens can evade the host immune response by alteration of their surface proteins, which are targets of the host immune system. The P2 outer membrane protein of H influenzae, which is a major target of bactericidal antibodies in COPD, shows extensive antigenic variation among strains of this pathogen.106 Serial persistent isolates of H influenzae in COPD show diminution of high-molecular-weight adhesin expression, which could represent another immune evasion mechanism.107
Future directions
The role of infection in COPD is an evolving topic with extensive ongoing research trying to better understand host-pathogen interactions and find suitable targets for intervention. Although exacerbation pathogenesis is better understood now, much still needs to be learned about pathogen virulence and causal overlap. The vicious-circle hypothesis exposes the complex interactions between smoking, innate immunity, and respiratory pathogens. Augmentation and modulation of the innate and adaptive host immunity as well as formulation of novel antibacterial agents and vaccines are paramount in future research and development in COPD.
Footnotes
Funding Sources: S. Sethi, supported by VA Merit Review and NHLBI. K. Rangelov, Nil.
Conflict of Interest: The authors declare no conflict of interest.
References
- 1.Stuart-Harris C.H., Pownall M., Scothorne C.M. The factor of infection in chronic bronchitis. Q J Med. 1953;22:121–132. [PubMed] [Google Scholar]
- 2.Smith C.B., Golden C.A., Kanner R.E. Haemophilus influenzae and Haemophilus parainfluenzae in chronic obstructive pulmonary disease. Lancet. 1976;1:1253–1255. doi: 10.1016/s0140-6736(76)91733-5. [DOI] [PubMed] [Google Scholar]
- 3.Smith C.B., Golden C., Klauber M.R. Interactions between viruses and bacteria in patients with chronic bronchitis. J Infect Dis. 1976;134:552–561. doi: 10.1093/infdis/134.6.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gump D.W., Phillips C.A., Forsyth B.R. Role of infection in chronic bronchitis. Am Rev Respir Dis. 1976;113:465–473. doi: 10.1164/arrd.1976.113.4.465. [DOI] [PubMed] [Google Scholar]
- 5.McHardy V.U., Inglis J.M., Calder M.A. A study of infective and other factors in exacerbations of chronic bronchitis. Br J Dis Chest. 1980;74:228–238. doi: 10.1016/0007-0971(80)90048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fagon J.Y., Chastre J. Severe exacerbations of COPD patients: the role of pulmonary infections. Semin Respir Infect. 1996;11:109–118. [PubMed] [Google Scholar]
- 7.Tager I., Speizer F.E. Role of infection in chronic bronchitis. N Engl J Med. 1975;292:563–571. doi: 10.1056/NEJM197503132921105. [DOI] [PubMed] [Google Scholar]
- 8.Smith C.B., Golden C., Kanner R. Association of viral and Mycoplasma pneumoniae infections with acute respiratory illness in patients with chronic obstructive pulmonary diseases. Am Rev Respir Dis. 1980;121:225–232. doi: 10.1164/arrd.1980.121.2.225. [DOI] [PubMed] [Google Scholar]
- 9.Murphy T.F., Sethi S. Bacterial infection in chronic obstructive pulmonary disease. Am Rev Respir Dis. 1992;146:1067–1083. doi: 10.1164/ajrccm/146.4.1067. [DOI] [PubMed] [Google Scholar]
- 10.Fletcher F., Peto R. The natural history of chronic airflow obstruction. Br Med J. 1977;1:1645–1648. doi: 10.1136/bmj.1.6077.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sethi S., Murphy T.F. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med. 2008;359:2355–2365. doi: 10.1056/NEJMra0800353. [DOI] [PubMed] [Google Scholar]
- 12.Mohan A., Chandra S., Agarwal D. Prevalence of viral infection detected by PCR and RT-PCR in patients with acute exacerbation of COPD: a systematic review. Respirology. 2010;15:536–542. doi: 10.1111/j.1440-1843.2010.01722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kherad O., Kaiser L., Bridevaux P.O. Upper-respiratory viral infection, biomarkers, and COPD exacerbations. Chest. 2010;138:896–904. doi: 10.1378/chest.09-2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mallia P., Message S.D., Gielen V. Experimental rhinovirus infection as a human model of chronic obstructive pulmonary disease exacerbation. Am J Respir Crit Care Med. 2011;183:734–742. doi: 10.1164/rccm.201006-0833OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sethi S., Sethi R., Eschberger K. Airway bacterial concentrations and exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;176(4):356–361. doi: 10.1164/rccm.200703-417OC. [DOI] [PubMed] [Google Scholar]
- 16.Sethi S., Evans N., Grant B.J. New strains of bacteria and exacerbations of chronic obstructive pulmonary disease. N Engl J Med. 2002;347:465–471. doi: 10.1056/NEJMoa012561. [DOI] [PubMed] [Google Scholar]
- 17.Murphy T.F., Brauer A.L., Sethi S. Haemophilus haemolyticus: a human respiratory tract commensal to be distinguished from Haemophilus influenzae. J Infect Dis. 2007;195:81–89. doi: 10.1086/509824. [DOI] [PubMed] [Google Scholar]
- 18.Murphy T.F., Brauer A.L., Grant B.J. Moraxella catarrhalis in chronic obstructive pulmonary disease: burden of disease and immune response. Am J Respir Crit Care Med. 2005;172:195–199. doi: 10.1164/rccm.200412-1747OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murphy T.F., Brauer A.L., Eschberger K. Pseudomonas aeruginosa in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;177:853–860. doi: 10.1164/rccm.200709-1413OC. [DOI] [PubMed] [Google Scholar]
- 20.Chin C.L., Manzel L.J., Lehman E.E. Haemophilus influenzae from patients with chronic obstructive pulmonary disease exacerbation induce more inflammation than colonizers. Am J Respir Crit Care Med. 2005;172:85–91. doi: 10.1164/rccm.200412-1687OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sethi S., Wrona C., Eschberger K. Inflammatory profile of new bacterial strain exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;177:491–497. doi: 10.1164/rccm.200708-1234OC. [DOI] [PubMed] [Google Scholar]
- 22.Rosell A., Monso E., Soler N. Microbiologic determinants of exacerbation in chronic obstructive pulmonary disease. Arch Intern Med. 2005;165:891–897. doi: 10.1001/archinte.165.8.891. [DOI] [PubMed] [Google Scholar]
- 23.Bandi V., Apicella M.A., Mason E. Nontypeable Haemophilus influenzae in the lower respiratory tract of patients with chronic bronchitis. Am J Respir Crit Care Med. 2001;164:2114–2119. doi: 10.1164/ajrccm.164.11.2104093. [DOI] [PubMed] [Google Scholar]
- 24.Garcha D.S., Thurston S.J., Patel A.R. Changes in prevalence and load of airway bacteria using quantitative PCR in stable and exacerbated COPD. Thorax. 2012;67:1075–1080. doi: 10.1136/thoraxjnl-2012-201924. [DOI] [PubMed] [Google Scholar]
- 25.Papi A., Bellettato C.M., Braccioni F. Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbations. Am J Respir Crit Care Med. 2006;173:1114–1121. doi: 10.1164/rccm.200506-859OC. [DOI] [PubMed] [Google Scholar]
- 26.Wilkinson T.M., Hurst J.R., Perera W.R. Effect of interactions between lower airway bacterial and rhinoviral infection in exacerbations of COPD. Chest. 2006;129:317–324. doi: 10.1378/chest.129.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mallia P., Footitt J., Sotero R. Rhinovirus infection induces degradation of antimicrobial peptides and secondary bacterial infection in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;186:1117–1124. doi: 10.1164/rccm.201205-0806OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Avadhanula V., Rodriguez C.A., Devincenzo J.P. Respiratory viruses augment the adhesion of bacterial pathogens to respiratory epithelium in a viral species- and cell type-dependent manner. J Virol. 2006;80:1629–1636. doi: 10.1128/JVI.80.4.1629-1636.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sajjan U.S., Jia Y., Newcomb D.C. H. influenzae potentiates airway epithelial cell responses to rhinovirus by increasing ICAM-1 and TLR3 expression. FASEB J. 2006;20:2121–2123. doi: 10.1096/fj.06-5806fje. [DOI] [PubMed] [Google Scholar]
- 30.Marston B.J., Plouffe J.F., File T.M., Jr. Incidence of community-acquired pneumonia requiring hospitalization. Results of a population-based active surveillance Study in Ohio. The Community-Based Pneumonia Incidence Study Group. Arch Intern Med. 1997;157:1709–1718. [PubMed] [Google Scholar]
- 31.Vinogradova Y., Hippisley-Cox J., Coupland C. Identification of new risk factors for pneumonia: population-based case-control study. Br J Gen Pract. 2009;59:e329–e338. doi: 10.3399/bjgp09X472629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Naucler P., Darenberg J., Morfeldt E. Contribution of host, bacterial factors and antibiotic treatment to mortality in adult patients with bacteraemic pneumococcal pneumonia. Thorax. 2013;68:571–579. doi: 10.1136/thoraxjnl-2012-203106. [DOI] [PubMed] [Google Scholar]
- 33.Ishiguro T., Takayanagi N., Yamaguchi S. Etiology and factors contributing to the severity and mortality of community-acquired pneumonia. Intern Med. 2013;52:317–324. doi: 10.2169/internalmedicine.52.8830. [DOI] [PubMed] [Google Scholar]
- 34.Mullerova H., Chigbo C., Hagan G.W. The natural history of community-acquired pneumonia in COPD patients: a population database analysis. Respir Med. 2012;106:1124–1133. doi: 10.1016/j.rmed.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 35.Merino-Sanchez M., Alfageme-Michavila I., Reyes-Nunez N. Prognosis in patients with pneumonia and chronic obstructive pulmonary disease. Arch Bronconeumol. 2005;41:607–611. doi: 10.1016/s1579-2129(06)60294-2. [in Spanish] [DOI] [PubMed] [Google Scholar]
- 36.Crisafulli E., Menendez R., Huerta A. Systemic inflammatory pattern of patients with community-acquired pneumonia with and without COPD. Chest. 2013;143:1009–1017. doi: 10.1378/chest.12-1684. [DOI] [PubMed] [Google Scholar]
- 37.Liapikou A., Polverino E., Ewig S. Severity and outcomes of hospitalised community-acquired pneumonia in COPD patients. Eur Respir J. 2012;39:855–861. doi: 10.1183/09031936.00067111. [DOI] [PubMed] [Google Scholar]
- 38.Arancibia F., Bauer T.T., Ewig S. Community-acquired pneumonia due to gram-negative bacteria and Pseudomonas aeruginosa: incidence, risk, and prognosis. Arch Intern Med. 2002;162:1849–1858. doi: 10.1001/archinte.162.16.1849. [DOI] [PubMed] [Google Scholar]
- 39.Torres A., Dorca J., Zalacain R. Community-acquired pneumonia in chronic obstructive pulmonary disease: a Spanish multicenter study. Am J Respir Crit Care Med. 1996;154:1456–1461. doi: 10.1164/ajrccm.154.5.8912764. [DOI] [PubMed] [Google Scholar]
- 40.Rello J., Rodriguez A., Torres A. Implications of COPD in patients admitted to the intensive care unit by community-acquired pneumonia. Eur Respir J. 2006;27:1210–1216. doi: 10.1183/09031936.06.00139305. [DOI] [PubMed] [Google Scholar]
- 41.Desai H., Richter S., Doern G. Antibiotic resistance in sputum isolates of Streptococcus pneumoniae in chronic obstructive pulmonary disease is related to antibiotic exposure. COPD. 2010;7:337–344. doi: 10.3109/15412555.2010.510162. [DOI] [PubMed] [Google Scholar]
- 42.Restrepo M.I., Mortensen E.M., Pugh J.A. COPD is associated with increased mortality in patients with community-acquired pneumonia. Eur Respir J. 2006;28:346–351. doi: 10.1183/09031936.06.00131905. [DOI] [PubMed] [Google Scholar]
- 43.Calverley P.M., Anderson J.A., Celli B. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356:775–789. doi: 10.1056/NEJMoa063070. [DOI] [PubMed] [Google Scholar]
- 44.Singh S., Loke Y.K. An overview of the benefits and drawbacks of inhaled corticosteroids in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2010;5:189–195. doi: 10.2147/copd.s6942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Singh S., Amin A.V., Loke Y.K. Long-term use of inhaled corticosteroids and the risk of pneumonia in chronic obstructive pulmonary disease: a meta-analysis. Arch Intern Med. 2009;169:219–229. doi: 10.1001/archinternmed.2008.550. [DOI] [PubMed] [Google Scholar]
- 46.Spencer S., Karner C., Cates C.J. Inhaled corticosteroids versus long-acting beta(2)-agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2011;(12) doi: 10.1002/14651858.CD007033.pub3. CD007033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mandell L.A., Wunderink R.G., Anzueto A. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(Suppl 2):S27–S72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Murphy T.F., Brauer A.L., Schiffmacher A.T. Persistent colonization by Haemophilus influenzae in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2004;170:266–272. doi: 10.1164/rccm.200403-354OC. [DOI] [PubMed] [Google Scholar]
- 49.Sethi S., Maloney J., Grove L. Airway inflammation and bronchial bacterial colonization in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2006;173:991–998. doi: 10.1164/rccm.200509-1525OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Charlson E.S., Bittinger K., Haas A.R. Topographical continuity of bacterial populations in the healthy human respiratory tract. Am J Respir Crit Care Med. 2011;184:957–963. doi: 10.1164/rccm.201104-0655OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Soler N., Ewig S., Torres A. Airway inflammation and bronchial microbial patterns in patients with stable chronic obstructive pulmonary disease. Eur Respir J. 1999;14:1015–1022. doi: 10.1183/09031936.99.14510159. [DOI] [PubMed] [Google Scholar]
- 52.Cabrera-Rubio R., Garcia-Nunez M., Seto L. Microbiome diversity in the bronchial tracts of patients with chronic obstructive pulmonary disease. J Clin Microbiol. 2012;50:3562–3568. doi: 10.1128/JCM.00767-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bresser P., Out T.A., van Alphen L. Airway inflammation in nonobstructive and obstructive chronic bronchitis with chronic Haemophilus influenzae airway infection. Comparison with noninfected patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;162:947–952. doi: 10.1164/ajrccm.162.3.9908103. [DOI] [PubMed] [Google Scholar]
- 54.Banerjee D., Khair O.A., Honeybourne D. Impact of sputum bacteria on airway inflammation and health status in clinical stable COPD. Eur Respir J. 2004;23:685–691. doi: 10.1183/09031936.04.00056804. [DOI] [PubMed] [Google Scholar]
- 55.Hill A.T., Campbell E.J., Hill S.L. Association between airway bacterial load and markers of airway inflammation in patients with stable chronic bronchitis. Am J Med. 2000;109:288–295. doi: 10.1016/s0002-9343(00)00507-6. [DOI] [PubMed] [Google Scholar]
- 56.Parameswaran G.I., Wrona C.T., Murphy T.F. Moraxella catarrhalis acquisition, airway inflammation and protease-antiprotease balance in chronic obstructive pulmonary disease. BMC Infect Dis. 2009;9:178. doi: 10.1186/1471-2334-9-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang M., Li Q., Zhang X.Y. Relevance of lower airway bacterial colonization, airway inflammation, and pulmonary function in the stable stage of chronic obstructive pulmonary disease. Eur J Clin Microbiol Infect Dis. 2010;29:1487–1493. doi: 10.1007/s10096-010-1027-7. [DOI] [PubMed] [Google Scholar]
- 58.Tumkaya M., Atis S., Ozge C. Relationship between airway colonization, inflammation and exacerbation frequency in COPD. Respir Med. 2007;101:729–737. doi: 10.1016/j.rmed.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 59.Patel I.S., Vlahos I., Wilkinson T.M. Bronchiectasis, exacerbation indices, and inflammation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2004;170:400–407. doi: 10.1164/rccm.200305-648OC. [DOI] [PubMed] [Google Scholar]
- 60.Retamales I., Elliott W.M., Meshi B. Amplification of inflammation in emphysema and its association with latent adenoviral infection. Am J Respir Crit Care Med. 2001;164:469–473. doi: 10.1164/ajrccm.164.3.2007149. [DOI] [PubMed] [Google Scholar]
- 61.Falsey A.R., Formica M.A., Hennessey P.A. Detection of respiratory syncytial virus in adults with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2006;173:639–643. doi: 10.1164/rccm.200510-1681OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wilkinson T.M., Donaldson G.C., Johnston S.L. Respiratory syncytial virus, airway inflammation, and FEV1 decline in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2006;173:871–876. doi: 10.1164/rccm.200509-1489OC. [DOI] [PubMed] [Google Scholar]
- 63.Hogg J.C., Chu F., Utokaparch S. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:2645–2653. doi: 10.1056/NEJMoa032158. [DOI] [PubMed] [Google Scholar]
- 64.Moghaddam S.J., Clement C.G., De la Garza M.M. Haemophilus influenzae lysate induces aspects of the chronic obstructive pulmonary disease phenotype. Am J Respir Cell Mol Biol. 2008;38:629–638. doi: 10.1165/rcmb.2007-0366OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Martinez-Garcia M.A., Soler-Cataluna J.J., Donat-Sanz Y. Factors associated with bronchiectasis in chronic obstructive pulmonary disease patients. Chest. 2011;140(5):1130–1137. doi: 10.1378/chest.10-1758. [DOI] [PubMed] [Google Scholar]
- 66.Verra F., Escudier E., Lebargy F. Ciliary abnormalities in bronchial epithelium of smokers, ex-smokers, and nonsmokers. Am J Respir Crit Care Med. 1995;151:630–634. doi: 10.1164/ajrccm/151.3_Pt_1.630. [DOI] [PubMed] [Google Scholar]
- 67.Foster W.M., Langenback E.G., Bergofsky E.H. Disassociation in the mucociliary function of central and peripheral airways of asymptomatic smokers. Am Rev Respir Dis. 1985;132:633–639. doi: 10.1164/arrd.1985.132.3.633. [DOI] [PubMed] [Google Scholar]
- 68.Koblizek V., Tomsova M., Cermakova E. Impairment of nasal mucociliary clearance in former smokers with stable chronic obstructive pulmonary disease relates to the presence of a chronic bronchitis phenotype. Rhinology. 2011;49:397–406. doi: 10.4193/Rhino11.051. [DOI] [PubMed] [Google Scholar]
- 69.Smaldone G.C., Foster W.M., O'Riordan T.G. Regional impairment of mucociliary clearance in chronic obstructive pulmonary disease. Chest. 1993;103:1390–1396. doi: 10.1378/chest.103.5.1390. [DOI] [PubMed] [Google Scholar]
- 70.Vastag E., Matthys H., Zsamboki G. Mucociliary clearance in smokers. Eur J Respir Dis. 1986;68:107–113. [PubMed] [Google Scholar]
- 71.Wanner A., Salathe M., O'Riordan T.G. Mucociliary clearance in the airways. Am J Respir Crit Care Med. 1996;154:1868–1902. doi: 10.1164/ajrccm.154.6.8970383. [DOI] [PubMed] [Google Scholar]
- 72.Innes A.L., Woodruff P.G., Ferrando R.E. Epithelial mucin stores are increased in the large airways of smokers with airflow obstruction. Chest. 2006;130:1102–1108. doi: 10.1378/chest.130.4.1102. [DOI] [PubMed] [Google Scholar]
- 73.Ma R., Wang Y., Cheng G. MUC5AC expression up-regulation goblet cell hyperplasia in the airway of patients with chronic obstructive pulmonary disease. Chin Med Sci J. 2005;20:181–184. [PubMed] [Google Scholar]
- 74.Ha U., Lim J.H., Jono H. A novel role for IkappaB kinase (IKK) alpha and IKKbeta in ERK-dependent up-regulation of MUC5AC mucin transcription by Streptococcus pneumoniae. J Immunol. 2007;178:1736–1747. doi: 10.4049/jimmunol.178.3.1736. [DOI] [PubMed] [Google Scholar]
- 75.Chen R., Lim J.H., Jono H. Nontypeable Haemophilus influenzae lipoprotein P6 induces MUC5AC mucin transcription via TLR2-TAK1-dependent p38 MAPK-AP1 and IKKbeta-IkappaBalpha-NF-kappaB signaling pathways. Biochem Biophys Res Commun. 2004;324:1087–1094. doi: 10.1016/j.bbrc.2004.09.157. [DOI] [PubMed] [Google Scholar]
- 76.Polosukhin V.V., Cates J.M., Lawson W.E. Bronchial secretory immunoglobulin a deficiency correlates with airway inflammation and progression of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2011;184:317–327. doi: 10.1164/rccm.201010-1629OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ganz T. Defensins: antimicrobial peptides of vertebrates. C R Biol. 2004;327:539–549. doi: 10.1016/j.crvi.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 78.Parameswaran G.I., Sethi S., Murphy T.F. Effects of bacterial infection on airway antimicrobial peptides and proteins in chronic obstructive pulmonary disease. Chest. 2011;140(3):611–617. doi: 10.1378/chest.10-2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tjabringa G.S., Rabe K.F., Hiemstra P.S. The human cathelicidin LL-37: a multifunctional peptide involved in infection and inflammation in the lung. Pulm Pharmacol Ther. 2005;18:321–327. doi: 10.1016/j.pupt.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 80.Dajani R., Zhang Y., Taft P.J. Lysozyme secretion by submucosal glands protects the airway from bacterial infection. Am J Respir Cell Mol Biol. 2005;32:548–552. doi: 10.1165/rcmb.2005-0059OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ellison R.T., 3rd, Giehl T.J. Killing of gram-negative bacteria by lactoferrin and lysozyme. J Clin Invest. 1991;88:1080–1091. doi: 10.1172/JCI115407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fitch P.M., Roghanian A., Howie S.E. Human neutrophil elastase inhibitors in innate and adaptive immunity. Biochem Soc Trans. 2006;34:279–282. doi: 10.1042/BST20060279. [DOI] [PubMed] [Google Scholar]
- 83.Crouch E.C. Structure, biologic properties, and expression of surfactant protein D (SP-D) Biochim Biophys Acta. 1998;1408:278–289. doi: 10.1016/s0925-4439(98)00073-8. [DOI] [PubMed] [Google Scholar]
- 84.Taylor D., Cripps A., Clancy R. A possible role for lysozyme in determining acute exacerbation in chronic bronchitis. Clin Exp Immunol. 1995;102:406–416. doi: 10.1111/j.1365-2249.1995.tb03798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gompertz S., Bayley D.L., Hill S.L. Relationship between airway inflammation and the frequency of exacerbations in patients with smoking related COPD. Thorax. 2001;56:36–41. doi: 10.1136/thorax.56.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yang I.A., Seeney S.L., Wolter J.M. Mannose-binding lectin gene polymorphism predicts hospital admissions for COPD infections. Genes Immun. 2003;4:269–274. doi: 10.1038/sj.gene.6363961. [DOI] [PubMed] [Google Scholar]
- 87.Betsuyaku T., Kuroki Y., Nagai K. Effects of ageing and smoking on SP-A and SP-D levels in bronchoalveolar lavage fluid. Eur Respir J. 2004;24:964–970. doi: 10.1183/09031936.04.00064004. [DOI] [PubMed] [Google Scholar]
- 88.Honda Y., Takahashi H., Kuroki Y. Decreased contents of surfactant proteins A and D in BAL fluids of healthy smokers. Chest. 1996;109:1006–1009. doi: 10.1378/chest.109.4.1006. [DOI] [PubMed] [Google Scholar]
- 89.Tsoumakidou M., Bouloukaki I., Thimaki K. Innate immunity proteins in chronic obstructive pulmonary disease and idiopathic pulmonary fibrosis. Exp Lung Res. 2010;36:373–380. doi: 10.3109/01902141003690389. [DOI] [PubMed] [Google Scholar]
- 90.Pace E., Ferraro M., Minervini M.I. Beta defensin-2 is reduced in central but not in distal airways of smoker COPD patients. PLoS One. 2012;7:e33601. doi: 10.1371/journal.pone.0033601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Berenson C.S., Kruzel R.L., Eberhardt E. Phagocytic dysfunction of human alveolar macrophages and severity of chronic obstructive pulmonary disease. J Infect Dis. 2013 doi: 10.1093/infdis/jit400. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hodge S., Hodge G., Ahern J. Smoking alters alveolar macrophage recognition and phagocytic ability: implications in chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 2007;37:748–755. doi: 10.1165/rcmb.2007-0025OC. [DOI] [PubMed] [Google Scholar]
- 93.Berenson C.S., Garlipp M.A., Grove L.J. Impaired phagocytosis of nontypeable Haemophilus influenzae by human alveolar macrophages in chronic obstructive pulmonary disease. J Infect Dis. 2006;194:1375–1384. doi: 10.1086/508428. [DOI] [PubMed] [Google Scholar]
- 94.Berenson C.S., Wrona C.T., Grove L.J. Impaired alveolar macrophage response to Haemophilus antigens in chronic obstructive lung disease. Am J Respir Crit Care Med. 2006;174:31–40. doi: 10.1164/rccm.200509-1461OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Oliver B.G., Lim S., Wark P. Rhinovirus exposure impairs immune responses to bacterial products in human alveolar macrophages. Thorax. 2008;63:519–525. doi: 10.1136/thx.2007.081752. [DOI] [PubMed] [Google Scholar]
- 96.Droemann D., Goldmann T., Tiedje T. Toll-like receptor 2 expression is decreased on alveolar macrophages in cigarette smokers and COPD patients. Respir Res. 2005;6:68. doi: 10.1186/1465-9921-6-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Harvey C.J., Thimmulappa R.K., Sethi S. Targeting Nrf2 signaling improves bacterial clearance by alveolar macrophages in patients with COPD and in a mouse model. Sci Transl Med. 2011;3:78ra32. doi: 10.1126/scitranslmed.3002042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kunz L.I., Lapperre T.S., Snoeck-Stroband J.B. Smoking status and anti-inflammatory macrophages in bronchoalveolar lavage and induced sputum in COPD. Respir Res. 2011;12:34. doi: 10.1186/1465-9921-12-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Macredmond R.E., Greene C.M., Dorscheid D.R. Epithelial expression of TLR4 is modulated in COPD and by steroids, salmeterol and cigarette smoke. Respir Res. 2007;8:84. doi: 10.1186/1465-9921-8-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sahlander K., Larsson K., Palmberg L. Daily exposure to dust alters innate immunity. PLoS One. 2012;7:e31646. doi: 10.1371/journal.pone.0031646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lee S.W., Kim D.R., Kim T.J. The association of down-regulated toll-like receptor 4 expression with airflow limitation and emphysema in smokers. Respir Res. 2012;13:106. doi: 10.1186/1465-9921-13-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Speletas M., Merentiti V., Kostikas K. Association of TLR4-T399I polymorphism with chronic obstructive pulmonary disease in smokers. Clin Dev Immunol. 2009;2009:260286. doi: 10.1155/2009/260286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hoiby N., Ciofu O., Johansen H.K. The clinical impact of bacterial biofilms. Int J Oral Sci. 2011;3:55–65. doi: 10.4248/IJOS11026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Goldstein-Daruech N., Cope E.K., Zhao K.Q. Tobacco smoke mediated induction of sinonasal microbial biofilms. PLoS One. 2011;6:e15700. doi: 10.1371/journal.pone.0015700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Starner T.D., Zhang N., Kim G. Haemophilus influenzae forms biofilms on airway epithelia: implications in cystic fibrosis. Am J Respir Crit Care Med. 2006;174:213–220. doi: 10.1164/rccm.200509-1459OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hiltke T.J., Sethi S., Murphy T.F. Sequence stability of the gene encoding outer membrane protein P2 of nontypeable Haemophilus influenzae in the human respiratory tract. J Infect Dis. 2002;185:627–631. doi: 10.1086/339362. [DOI] [PubMed] [Google Scholar]
- 107.Cholon D.M., Cutter D., Richardson S.K. Serial isolates of persistent Haemophilus influenzae in patients with chronic obstructive pulmonary disease express diminishing quantities of the HMW1 and HMW2 adhesins. Infect Immun. 2008;76(10):4463–4468. doi: 10.1128/IAI.00499-08. [DOI] [PMC free article] [PubMed] [Google Scholar]