DEFINITIONS AND OVERVIEW OF EPIDEMIOLOGY
Chronic obstructive pulmonary disease (COPD) is characterized by the presence of airflow obstruction caused by chronic bronchitis or emphysema. This airflow obstruction is generally progressive, may be accompanied by airway hyperreactivity, and often is partially reversible.7 Declining lung function is almost universally caused by decades of tobacco smoke exposure and develops insidiously so that patients often do not complain of exertional dyspnea until their 1-second forced expiratory volume (FEV1) is between 40% and 59% of its predicted value.20 When the FEV1 falls below 1 L, patients are disabled in the activities of daily living and have a 5-year survival of approximately 50%.3 Forced expiratory volume in 1 second declines by about 30 mL/year in healthy nonsmokers, whereas the average decline is approximately 45 mL/year in smokers.20 Approximately 15% of smokers are susceptible to the airway effects of smoking and will develop COPD. These patients show accelerated rates of decline in FEV1 of between 50 and 90 mL/year.24
In 1994, approximately 16 million Americans suffered from COPD, an estimated increase of 60% since 1982.6 It ranks fourth among leading causes of death in North America and is the only leading cause of death that is rising in prevalence.6, 28 According to 1993 estimates made by the National Heart Lung and Blood Institute, the annual total cost arising from COPD was nearly $24 billion dollars. This amount includes almost $15 billion in direct health care expenditures, nearly $5 billion in indirect morbidity costs, and $4.5 billion in indirect mortality costs.6
Periods of relative clinical stability during the course of COPD are interrupted by recurrent exacerbations. The definition of an acute exacerbation of COPD (AECOPD) is imprecise but is generally considered clinically as an episode of increased dyspnea, sputum production, and sputum purulence in a patient with COPD.11 When these symptoms are severe and accompanied by significant hypoxemia or hypercapnia, patients may require hospitalization. This article focuses primarily on the management of hospitalized patients with AECOPD outside of the intensive care unit and reviews the evidence supporting the available therapies for COPD exacerbations.
PATHOPHYSIOLOGY OF EXACERBATIONS
Smoking-related Lung Damage and Pathobiology of Bacterial Colonization
Cigarette smoking is the most important cause of COPD.109 Smoking compromises local airway defense mechanisms by damaging ciliated airway epithelium, increasing mucus viscosity, and slowing mucociliary clearance. These conditions promote bacterial colonization of the lower respiratory tract. The three major bacterial pathogens isolated from patients with COPD during periods of both clinical stability and exacerbation are nontypeable Haemophilus influenzae, Streptococcus pneumoniae, and Moraxella catarrhalis.83 When FEV1 is severely reduced, Enterobacteriaceae and Pseudomonas aeruginosa are also commonly detected.42 These organisms possess a wide array of virulence factors that allow them to evade clearance from the lower airways. Although a detailed discussion of the bacterial mechanisms of colonization and infection is beyond the scope of this article, several concepts are noteworthy.
Smokers prone to acute episodes of bronchitis have a greater degree of bacterial adherence to oropharyngeal airway epithelial cells compared with nonsmokers.101, 123 After adhering to mucus or epithelial cells, pathogenic bacteria elaborate exoproducts that stimulate excess mucous production,1 disorganize and slow ciliary beating,95 damage epithelial cells,94 and impair immune effector-cell function.23 Furthermore, bacterial proteases destroy local immunoglobulins.45 When these bacteria loiter in the airways, a host inflammatory response is stimulated. With the movement of large numbers of neutrophils and their subsequent release of proteases and toxic oxygen radicals, epithelial surface damage may be enhanced. After the inciting impact of smoking, bacterial colonization therefore begets airway damage which, in turn, begets further inflammation and bacterial colonization. This event is the vicious circle hypothesis that has been proposed to explain how the bacteria–host interaction establishes the insidious loss of lung function.83, 135
Mechanisms of Disordered Gas Exchange
Disordered pulmonary gas exchange is characteristic of acute exacerbations of COPD. Patients typically are found to have severe hypoxemia with or without hypercarbia. A variety of infectious and noninfectious insults result in inflammation, bronchospasm, and mucous hypersecretion. These lead to acute airway narrowing that aggravates ventilation-perfusion (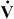 /
/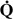 ) mismatching and can worsen existing hyperinflation. Although
) mismatching and can worsen existing hyperinflation. Although 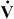 /
/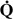 inequality is the most important determinant of hypoxemia, low mixed venous oxygen tension (Pvo
2) is a contributing factor.16 During exacerbations, the work of breathing increases to overcome increased airway resistance and dynamic hyperinflation. Oxygen utilization by the respiratory muscles therefore is markedly increased, resulting in lower Pvo
2. Fortunately, among patients with adequate cardiac reserve, increases in cardiac output partly compensate for diminished Pvo
2 to defend arterial oxygenation.
inequality is the most important determinant of hypoxemia, low mixed venous oxygen tension (Pvo
2) is a contributing factor.16 During exacerbations, the work of breathing increases to overcome increased airway resistance and dynamic hyperinflation. Oxygen utilization by the respiratory muscles therefore is markedly increased, resulting in lower Pvo
2. Fortunately, among patients with adequate cardiac reserve, increases in cardiac output partly compensate for diminished Pvo
2 to defend arterial oxygenation.
Among the mechanisms leading to hypercarbia, 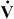 /
/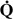 mismatch is probably more important than hypoventilation, at least among patients who recover from their exacerbations without needing mechanical ventilation. This concept is supported by the observation that, during exacerbations, patients are often hypercarbic despite increased minute ventilation.16, 131 Hypoventilation may be an additional mechanism of hypercarbia if respiratory muscle fatigue and acute respiratory failure ensue.
mismatch is probably more important than hypoventilation, at least among patients who recover from their exacerbations without needing mechanical ventilation. This concept is supported by the observation that, during exacerbations, patients are often hypercarbic despite increased minute ventilation.16, 131 Hypoventilation may be an additional mechanism of hypercarbia if respiratory muscle fatigue and acute respiratory failure ensue.
SPECIFIC CAUSES OF EXACERBATIONS
Bacteria
The relationship between bacterial infection and COPD exacerbations is not precisely understood. Several lines of evidence, however, have established an important role for bacterial infection in many exacerbations. High titers of antibody against nontypeable H. influenzae 84, 97, 114 and M. catarrhalis 31 are found following AECOPD. Although bacterial colonization of the distal airways is common in stable COPD, patients with exacerbations often have higher numbers of organisms.44, 80, 115 Table 1 summarizes studies using protected specimen brushes to define the microflora of the distal airways in COPD exacerbations. Monsó et al55 found positive bacterial cultures in 52% of outpatients with AECOPD. Compared with stable patients, exacerbated patients were twice as likely to have positive cultures (i.e., ≥ 1000 CFU/mL) and five times as likely to have bacterial counts greater than 10,000 CFU/mL.80 Likewise, S. pneumoniae is more likely to be found in sputum during exacerbations than remission.
Table 1.
PROTECTED SPECIMEN BRUSH STUDIES OF BACTERIAL INFECTION DURING CHRONIC OBSTRUCTIVE PULMONARY DISEASE EXACERBATIONS
| Monsó et al80Outpatients (n = 29) PSB Cultures | Fagon et al44Ventilated Patients (n = 54) PSB Cultures | Soler et al115Ventilated Patients (n = 50) PSB, TBA, BAL Cultures | |
|---|---|---|---|
| Isolates (n) | |||
| Haemophilus influenzae | 10 | 6 | 11 |
| Streptococcus pneumoniae | 3 | 7 | 4 |
| Moraxella catarrhalis | 2 | 3 | 4 |
| Pseudomonas aeruginosa | – | 3 | 9 |
| Strenotrophomonas maltophilia | – | – | 2 |
| Enterobacteriaceae | – | 5 | 4 |
| Staphylococcus aureus | – | 4 | – |
| Other (nonpathogenic)* | – | 19 (11–H. parainfluenzae) | 30 (13–S. viridans) |
| Patients with positive cultures† | 15/29 | 27/54 | 36/50‡ |
| AECOPD with positive cultures | 52% | 50% | 72% |
| PSB = Protected specimen brush; TBA = tracheobronchial aspirate; BAL = bronchoalveolar lavage fluid; AECOPD = acute exacerbation of COPD. | |||
Streptococcus spp, Corynebacterium spp, H. parainfluenzae, S. epidermidis, Neisseria spp, Candida spp
PSB ≥ 102, BAL ≥ 103, TBA ≥ 105CFU/mL.
Includes patients with positive serology for C. pneumoniae and respiratory viruses.
Fagon44 et al found evidence of bacterial infection in 50% of patients who required mechanical ventilation. Streptococcus pneumoniae, H. influenzae, M. catarrhalis, and enteric gram-negative organisms collectively accounted for 55% of the isolates. The investigators did not attempt to identify M. pneumoniae, C. pneumoniae, or respiratory viruses. Interestingly, gram-negative bacteria accounted for 64% of isolates, and nearly half of these were H. parainfluenzae. Yet, similar studies employing protected brush specimens have not detected H. parainfluenzae among patients with COPD exacerbations.80, 115 Moreover, Smith et al114 were unable to demonstrate rises in antibody against H. parainfluenzae following COPD-related acute respiratory illnesses. It seems justified therefore to consider H. parainfluenzae as generally nonpathogenic.
In contrast to the findings of Fagon et al,115 a more comprehensive microbiologic survey of patients with severe exacerbations found a higher incidence of potential pathogens. Overall, 72% of patients had at least one positive bacterial culture or positive serology for C. pneumoniae or respiratory viruses. All cultures were obtained within 48 hours of admission, thereby reducing the likelihood of nosocomial infection. Streptococcus pneumoniae, H. influenzae, and M. catarrhalis accounted for 56% of potential pathogens. Strikingly, gram-negative enteric bacteria and Pseudomonas or Stenotrophomonas represented 39% of potential pathogens. Positive serology for acute infection with C. pneumoniae or respiratory viruses (almost exclusively influenza) was found in 26% of the patients.115 This study suggests that a broader profile of potential pathogens may be present among patients with COPD with severe exacerbations. Although these findings should be confirmed in a larger study, the choice of empiric antibiotics for patients with AECOPD should be based in part on the degree of exacerbation severity, and broader-spectrum initial coverage may be warranted for patients with AECOPD who present with respiratory failure and require mechanical ventilation.
The source of bacterial infection during a COPD exacerbation may be endogenous or exogenous. In a small group of patients followed for 3 years, exacerbations coincided with reinfection by strains of H. influenzae having either the same (i.e., endogenous) or different (i.e., exogenous) DNA fingerprint. Strains of H. influenzae were shown to persist for several months and antibiotic treatment was not effective in eradicating the bacteria.49
Viruses
Estimates of the proportion of COPD exacerbations associated with viral infection range from 7%117 to 63%.67 This large discrepancy is because of significant differences in study design.25 Several studies lacked adequate control by failing to record the frequency of viral infection during exacerbation-free periods.40, 75 Others, such as those by Sommerville and Stenhouse,116, 118 attempted to detect only selected pathogens. Variability in the definition of an exacerbation is another factor that may affect the percentage of exacerbations caused by viral illness. Finally, different serologic and isolation techniques account for some of the variety in study results.55
The three most rigorous studies are summarized in Table 2 .25, 55, 113 The proportion of exacerbations attributed to viral (or mycoplasma) illness ranges from 18% to 34%. Influenza, parainfluenza, and coronavirus were the most frequent pathogens to be significantly associated with exacerbations.
Table 2.
HIGHEST-QUALITY STUDIES OF CHRONIC OBSTRUCTIVE PULMONARY DISEASE EXACERBATIONS ASSOCIATED WITH VIRAL AND MYCOPLASMA PNEUMONIAE INFECTION
| Study | Patients(n) | Exacerbations (n) | Viral Exacerbationsn(%) |
Identified Agent (n) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Influenza A & B | Para-influenza 1,2,3 | Corona-virus | Rhino-virus | Adeno-virus | HSV | RSV | Mycoplasma pneumoniae | ||||
| Gump et al55 | 25 | 116 | 39 (34) | 15 | 9 | 6 | 4 | 3 | 11 | 5 | 1 |
| Buscho et al25 | 46 | 166 | 50 (25) | 17 | 10 | 8 | NR | 2 | NR | 0 | 4 |
| Smith et al113 | 150 | 1030 | 186 (18) | 50 | 29 | 17 | 44 | 7 | 21 | 8 | 5 |
| HSV = Herpes simplex virus; RSV = respiratory syncytial virus; NR = not reported. | |||||||||||
More recently, Goh et al48 performed a prospective etiologic study of 90 inpatients with AECOPD. They collected paired sera for influenza A, B, and parainfluenza viruses as well as Legionella, Mycoplasma, and Chlamydia. Positive serology was found in 31 patients (34%), of whom 26 patients (28%) had viral infections. The most common organism was influenza A, with 18 patients demonstrating positive serology (20%). Five patients had positive serology for Legionella, whereas no evidence was found for infections caused by Mycoplasma or Chlamydia.48
Gump et al55 observed 25 patients every 2 weeks for 4 years and documented 116 exacerbations. They derived a striking correlation of infection with exacerbations by interpreting their data in a time-weighted analysis. They found that the incidence of infection was 32% per patient week of exacerbation but only 0.9% per patient week in remission. In their 5-year analysis, Buscho et al25 found that 25% of exacerbations were associated with viral infection. This rate was twice that of viral infections during remission detected as an asymptomatic fourfold rise in antiviral antibody titers. In the largest study, Smith et al113 followed 150 patients over 8 years and analyzed more than 1000 acute respiratory illnesses. They associated nonbacterial infections with approximately 20% of acute respiratory illnesses but only 6% of illness-free periods. In contrast with the work by Buscho and Gump, Smith et al113 noted a high rate of rhinoviral infection. Uncontrolled studies by McNamara and Eadie40, 75 also reported rhinoviral infections to be associated with COPD exacerbations in 43% and 20% of cases, respectively. Common colds may have a deleterious effect upon lung function29 and patients with COPD are more likely to develop increased cough and lower respiratory tract symptoms during rhinoviral infections than healthy subjects.57, 113
Smith et al112 performed a 7-year observational study of 120 patients to assess potential interactions between viral, mycoplasmal, and bacterial infections in patients with COPD. They calculated the ratio of number of observed exacerbations to number of expected viral or bacterial associations. Haemophilus influenzae and S. pneumoniae were isolated more than twice as often as expected following influenza virus infection. Marked rises in titers of antibodies against H. influenzae were associated with preceding viral or Mycoplasma infections, suggesting that viral infection promotes increased invasiveness of H. influenzae and subsequent infection. There are no other rigorously performed clinical studies of the interaction between viral and bacterial infections in AECOPD. Although the concept that viruses promote secondary bacterial infection seems biologically plausible and is supported by animal research, it remains unknown how often bacterial infection in AECOPD follows an inciting viral infection.
Air Pollution
Suspended particulate matter less than 10 μm in diameter (PM10) is produced by vehicle exhaust and many industrial processes. Several epidemiologic studies8, 105, 106 have associated elevated PM10 levels with a wide range of respiratory outcomes, including reduced pulmonary function and increased chronic respiratory symptoms, rates of hospitalization, and mortality. Similar associations exist for other pollutants, notably sulfur dioxide (SO2) and nitrogen dioxide. A 5-year study in Barcelona121 reported that small increases of SO2 and airborne particles produced adjusted increases of 6% in emergency room admissions for COPD in winter and 9% in summer. Similar rates of excess hospitalizations for COPD have been reported from Sydney, Australia (4%); Detroit, Michigan (6%); and Birmingham, Alabama (7%).81, 105, 106 Although small, these effects represent a significant public health concern, particularly because they are demonstrable at pollution levels below current air-quality standards.
In summary, the foregoing studies imply that bacterial pathogens can be identified in approximately half of COPD exacerbations. Viral pathogens are identifiable in about 25% of such episodes. Poor air quality may account for slightly more than 5% of episodes of AECOPD. In many COPD exacerbations, no obvious pathogen or precipitating cause is found. It is not known how frequently other potential factors, such as medication noncompliance or coincidental events such as pulmonary embolism and myocardial infarction, play an inciting role in AECOPD. Indeed, it is often difficult to distinguish clinically between exacerbations with and without an infectious cause. This distinction is discussed subsequently in the section on antibiotic therapy.
PREVENTION
Smoking Prevention, Influenza and Pneumococcal Vaccination, Immunostimulating Medication
Smoking cessation is the most important intervention in the management of patients with COPD. The landmark Lung Health Study confirmed that smoking cessation greatly reduces the rate of FEV1 decline.10 The benefit of smoking cessation is seen even in patients over the age of 60 years.56 Chronic sputum production often clears within 4 weeks of stopping smoking.138 Although nicotine replacement therapy is an effective approach to smoking cessation, counseling by a physician has been shown to be the most potent intervention.124
Each year, influenza and its complications are responsible for hundreds of thousands of excess hospitalizations, tens of thousands of excess deaths, and billions of dollars in health care costs.85, 86 Those with chronic lung disease are at especially high risk for the consequences of influenza. Despite recommendations for annual influenza vaccination, recent studies have documented inadequate vaccination rates in this risk group.91, 92 Among elderly persons, including those with chronic lung disease, Nichol et al86 overwhelmingly demonstrated the efficacy and cost effectiveness of influenza vaccination. In a serial cohort study of more than 25,000 patients,86 vaccination was associated with a 30% to 40% reduction in the rate of hospitalization for all acute and chronic respiratory conditions. Another study by the same authors85 found that influenza vaccination was associated with a 70% reduction in the risk for death from any cause (odds ratio [OR] = 0.3; 95% confidence interval [CI] = 0.21–0.43). A recent meta-analysis of 20 cohort studies concluded that the estimates of vaccine efficacy for preventing respiratory illness, hospitalization, and death were 56%, 50% and 68%, respectively.107 These data clearly affirm that influenza vaccination is an indispensable part of the care of all elderly persons, especially those with COPD.
The value of pneumococcal vaccination for elderly patients with COPD has been controversial. Two randomized controlled trials evaluating the vaccine's efficacy among patients with COPD were unable to show statistically significant protective benefit.36, 69 A recent meta-analysis concluded that the vaccine provides partial protection against bacteremic pneumococcal pneumonia but not against other important outcomes, including bronchitis or mortality caused by pneumococcal infection. This protective benefit was seen only in low-risk groups and not among those with COPD or other high-risk patients.46 Nevertheless, pneumococcal vaccination continues to be strongly recommended for patients with COPD because it is safe and has been found to provide significant benefit for patients in case-control and indirect cohort studies26, 93, 108 as well as in a more recent randomized population-based trial.64
Several additional novel strategies for reducing acute exacerbations of COPD are under investigation. For example, OM-85 BV is an oral immunostimulating agent containing lyophylized fractions of the eight most common respiratory pathogens. Its use was associated with a 40% reduction in the incidence of acute exacerbations of chronic bronchitis and a 28% decrease in antibiotic use among elderly institutionalized patients in one trial, and the same frequency of exacerbations but less than half as many days in hospital as those given placebo in another.32, 90 There was a trend toward fewer hospitalizations for respiratory reasons among those receiving the immunostimulating drug. Although not available in North America, OM-85 BV has been used for many years in Europe. Further trials are required to properly define its role.
Another approach has been the subcutaneous administration of hyaluronic acid (HA), a glycosaminoglycan with neutrophil-regulating functions, to patients with chronic bronchitis. Fewer acute exacerbations of bronchitis and reduced antibiotic use were noted among HA-treated patients in a placebo-controlled crossover study.130 Other investigators found that continous administration of carbocysteine lysine salt monohydrate during winter months was effective in preventing AECOPD and reducing antibiotic consumption in patients with chronic bronchitis.4
Despite these recent intriguing data suggesting that immunomodulatory agents may attenuate the development of AECOPD, randomized, controlled trials are needed to clarify their potential roles in the routine management of patients with COPD.
TREATMENTS
The following sections provide an overview of the merits (or lack thereof) of common therapeutic interventions for AECOPD. Controlled oxygen (O2), bronchodilators, antibiotics, corticosteroids, noninvasive ventilation, nutritional support, and chest physiotherapy are discussed. When available, evidence from randomized controlled trials is presented.
Controlled Oxygen
Hypoxemia is the most immediate threat to life for patients with AECOPD. Hypercapnia is a well-recognized consequence of O2 therapy. In the past, the risk of hypoventilation or even apnea resulting from O2 administration has been vastly overestimated, and the notion that O2 commonly induces clinically important hypercarbia and acidosis has been discredited.12, 13, 39, 104 The traditional concept is that correction of hypoxemia with supplemental O2 removes the hypoxic drive to breathe and leads to a fall in minute ventilation and subsequent carbon dioxide (CO2) retention.47 Aubier et al12 found that although administration of supplemental O2 does reduce minute ventilation and increase arterial partial pressure of carbon dioxide (Paco
2), mouth occlusion pressure (an indicator of central respiratory drive) was significantly higher during acute exacerbations than during stable conditions. The drive to breathe therefore remained very high in spite of oxygen treatment. In a later study by the same authors,13 patients with COPD and acute respiratory failure received 100% O2 for 15 minutes to abolish hypoxic drive. Minute ventilation fell by only 7% and could not account for the entire increase in Paco
2. The authors concluded that the rise in CO2 was caused by increased Vd/Vt) (i.e., increased deadspace ventilation) and that the primary mechanism of O2-induced hypercarbia is 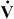 /
/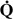 mismatching, perhaps through the loss of hypoxic vasoconstriction. More recent work by Dunn et al39 and Stradling119 has challenged Aubier's conclusions and defended the traditional concept that hypoventilation, rather than
mismatching, perhaps through the loss of hypoxic vasoconstriction. More recent work by Dunn et al39 and Stradling119 has challenged Aubier's conclusions and defended the traditional concept that hypoventilation, rather than 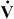 /
/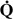 mismatching, is to blame for O2-induced hypercarbia. One further mechanism that appears to contribute, albeit to a minor extent, is the Haldane effect, whereby CO2 is displaced from hemoglobin by O2, causing a rise in Paco
2.119 Nevertheless, the risks for acute hypoxemia far outweigh the risks for severe hypercarbia. As such, supplemental O2 administration is recommended for hypoxemic patients with AECOPD.
mismatching, is to blame for O2-induced hypercarbia. One further mechanism that appears to contribute, albeit to a minor extent, is the Haldane effect, whereby CO2 is displaced from hemoglobin by O2, causing a rise in Paco
2.119 Nevertheless, the risks for acute hypoxemia far outweigh the risks for severe hypercarbia. As such, supplemental O2 administration is recommended for hypoxemic patients with AECOPD.
Oxygen initially should be given to any hypoxemic patient with AECOPD by nasal cannulae or Venturi mask. If nasal cannulae are used, flow rates of 1 to 2 L/minute generally suffice. The inspired concentration of oxygen (Fio 2) usually increases by 3% to 4% for each increase of 1 L/minute in flow but varies according to the patient's own inspiratory flow rate. Venturi masks are more precise and should initially be set to deliver a Fio 2 of 24% to 28%. On average, the Pao 2 increases by 10 mm Hg when the Fio 2 is increased from room air to 24%, and by 20 mm Hg when the Fio 2 is increased to 28%.17 The target Pao 2 is between 60 and 70 mm Hg, corresponding to the Pao 2 at which there is near-complete saturation of hemoglobin with O2. Rarely, overzealous O2 administration produces progressive hypercapnia and respiratory acidosis.20, 34, 74 For this reason, response to O2 must be assessed according to arterial blood gas and pH measurements. These should be obtained at baseline and within 60 minutes of starting or changing the O2 concentration. If Pao 2 remains intractably low or the pH drops as a result of increasing Paco 2, alternate strategies to improve hypoxemia and respiratory acidosis must be devised. These include maximizing bronchodilation and the use of assisted ventilation.
Bronchodilators
The role of bronchodilators in the management of stable COPD is discussed in the article by Ferguson on pharmacologic therapy for COPD. Bronchodilator agents, specifically β2-agonists and ipratropium bromide (IB), also play a central role in the management of patients with AECOPD, with and without respiratory failure. These agents are generally given by inhalation to reduce side-effects and, in the acute setting, nebulizers have been traditionally preferred over metered-dose inhalers (MDIs) for ease of drug administration.68, 74, 127 Although there are few data to support the choice of either β2-agonists or IB as first-line therapy for acute exacerbations, β2-agonists are usually given as the first step, perhaps because of the longer time to peak effect for IB.7, 73 When given in recommended doses (two puffs), IB generally produces greater bronchodilation than β2-agonists.19, 122
Ipratropium bromide and β2-agonists are often used together in the acute setting, despite a lack of evidence from randomized-controlled trials that they are more efficacious in tandem than either agent alone in that setting.79, 89, 96 In a doubleblind, randomized study involving 51 patients with AECOPD, the effects of 0.5 mg of IB, 1.25 mg of fenoterol, or a combination of the two agents were compared. All three regimens resulted in improved spirometric function at 45 and 90 minutes post-treatment, but combination therapy was no better than either agent alone. Similarly, O'Driscoll et al89 compared nebulized salbutamol (10 mg) with and without IB (0.5 mg) in 47 patients with AECOPD. One hour following treatment, peak expiratory flow rates were not significantly different between regimens.89 Others have examined clinical, rather than spirometric, outcomes following combined therapy in AECOPD.79, 110 Patients randomly assigned to a combination of isoetharine and IB were discharged from the emergency department an average of 90 minutes sooner than those who received only isoetharine. The authors attributed this time saving to approximately five puffs of IB. Interestingly, mean discharge FEV1 was not different between the two groups.110 Because these studies followed patients for only 60 to 90 minutes, Moayyedi et al79 recently attempted to capture longer-term benefits of combination therapy. Comparing nebulized treatments of salbutamol with and without IB among inpatients with AECOPD, they found no difference in duration of stay, subjective breathlessness, or spirometric values over a 14-day assessment period.
Considerable effort has been made to establish the most effective method for delivering bronchodilator drugs to patients with acute airflow obstruction. Delivery methods include MDIs with or without a spacer device, nebulizers (hand-held or attached to a face mask), and dry powder inhalers. A recent systematic review of 12 randomized studies comparing MDIs and nebulizers for administration of β2-agonists in acute exacerbations of asthma and COPD found the two methods to be equivalent.128 A reasonable approach advocated by several workers is to begin with nebulized treatments among patients who are too dyspneic to use an MDI and spacer device correctly.74, 111 As early as is feasible patients can then be switched to MDIs, which can result in considerable cost savings.60, 120
Theophylline
Theophylline has been used for decades to ameliorate symptoms in patients with airflow obstruction.129 The use of theophylline in the management of stable COPD is discussed in the article by Ferguson in this issue. Theophylline has now been relegated to having a minor role in the acute setting because of the development of safer and more potent bronchodilators.41, 74 The lack of convincing, well-designed trials showing its efficacy has further contributed to the decline in its use.70, 99, 137 There are only two published studies that relate specifically to the role of theophylline in the treatment of COPD exacerbations. The first, by Rice et al,99 was a small, yet rigorously controlled trial in which patients were randomized to aminophylline infusion or placebo during hospitalization for AECOPD. The drug conferred no incremental benefit over standard care in either subjective (dyspnea scores) or objective (spirometry) outcome measures. Gastrointestinal side effects were more common in the aminophylline group.
An emergency department-based study randomized patients with acute bronchospasm to receive either aminophylline or placebo.137 The trial included patients with asthma and COPD but failed to report the number of each. Unexpectedly, there was a threefold decrease in the hospital admission rate for patients treated with aminophylline (P = 0.016). The authors argued that aminophylline should be considered in selected patients with acute exacerbations of COPD and asthma because reduced hospitalizations would decrease costs. Although their study illustrates a potentially important clinical benefit of theophylline, a cautious approach is necessary. The effect of theophylline on admission rates was not the primary outcome variable and the reduction in admission rates did not quite reach statistical significance after adjusting for multiple comparisons. Even though the magnitude of the clinical benefit was large, theophylline produced no objective improvement in pulmonary function as measured by spirometry. Despite these reservations, similar reports of clinical benefit in the absence of statistically significant spirometric improvement have been found in studies of corticosteroids in acute asthma71 and bronchodilator therapy in acute COPD.110
Antibiotics
Bacterial infections' contribution to exacerbations of COPD has been inferred from studies demonstrating clinical benefit as a result of antibiotic therapy. Antibiotics have been employed for prophylaxis and acute treatment of AECOPD. In the 1950s and 1960s, attention was given to preventing exacerbations with antibiotics. Murphy and Sethi83 reviewed nine prospective, placebocontrolled trials of antibiotic prophylaxis. Of these, five failed to show any reduction in the frequency of exacerbations, although two demonstrated significantly less time lost from work among patients receiving antibiotics. In contrast, compared with placebo, antibiotic prophylaxis significantly reduced the frequency of exacerbations in four studies. In these investigations, prophylaxis seemed to benefit patients suffering the largest annual number of exacerbations. Some authorities therefore have recently suggested that antibiotic prophylaxis may be appropriate for individuals prone to frequent exacerbations,14 although this practice is not recommended as part of the regular care of all patients with COPD.
The prescription of antibiotics to facilitate early recovery in AECOPD has become routine despite unresolved questions about their true benefit.14, 51, 52, 83 Nicotra et al87 randomized 40 inpatients with AECOPD to either tetracycline or placebo for 1 week. At 7 days, there was no difference between groups in terms of oxygenation or lung function. In a similar, but larger, study of outpatients with uncomplicated AECOPD, Jorgensen61 also could not demonstrate a clinically important advantage of amoxicillin over placebo.
These null trials notwithstanding, the highest-quality clinical study to date, by Anthonisen et al,11 concluded that antibiotics improved outcomes. They randomized 173 outpatients to receive either placebo or broad-spectrum antibiotic (amoxicillin, trimethoprim-sulfamethoxazole, or doxycycline) during 362 COPD exacerbations. Exacerbations were classified according to severity. Type 1 (the most severe) was defined as an increase in dyspnea, sputum volume, and sputum purulence. Type 2 involved the presence of only two of the three symptoms, and type 3 was defined as the presence of one of the three symptoms in addition to one other finding (sore throat, rhinorrhea, fever, increased wheeze, or increased cough). Compared with placebo, antibiotics shortened the duration of exacerbations by about 2 days and accelerated recovery of peak expiratory flow rate (P < 0.02 for both). Treatment success, defined as resolution of symptoms within 21 days, occurred in 55% of the placebo group and 68.1% of patients receiving active treatment (P < 0.05). Significantly, among patients presenting with type 1 exacerbations, clinical deterioration was more than twice as common with placebo as with antibiotic. A clinician therefore would need to treat roughly eight exacerbations with antibiotics to achieve one treatment success beyond chance, or two in order to avoid a single deterioration. The authors concluded that avoidance of deleterious outcomes is the strongest reason to offer antibiotics to patients with AECOPD. Furthermore, they stated that antibiotics are clearly indicated in type 1 exacerbations, of no benefit in type 3 exacerbations, and probably justifiable for patients with type 2 presentations.11 Similar findings were reported in another large-scale, randomized trial comparing amoxicillin/clavulanate to placebo.5
Saint and associates103 systematically reviewed the clinical efficacy of antibiotics for AECOPD. They identified nine randomized, placebo-controlled trials of antibiotics in COPD exacerbations in which the patients were followed for at least 5 days. Because no outcome measure was common to all nine studies, the authors derived an overall effect size to quantify the efficacy of antibiotics and concluded that a small but statistically significant improvement could be expected among patients receiving antibiotics. From the six trials that reported peak expiratory flow rates, an overall improvement of 10.75 liters per minute favoring the antibiotic group was noted. Although small, such an effect may be clinically important for patients with severely compromised baseline lung function by preventing respiratory failure and hospital or intensive care unit (ICU) admission.51
To reduce the risk for treatment failure, antibiotics should be selected according to pertinent clinical data and the potential for antimicrobial resistance. Several schemes have been proposed to stratify the patient's risk and select the most appropriate therapy.14, 53, 72, 134 The simplest, and most recent, classification system is presented in Table 3 . Grossman53 has classified acute exacerbations into four groups. Group 1 patients have acute simple bronchitis, likely of viral origin, for which antibiotics are not recommended at the outset. If symptoms persist for longer than 1 week, a macrolide or tetracycline is suggested to treat suspected pathogenic organisms (i.e. M. pneumoniae, C. pneumoniae). Group 2 patients have simple chronic bronchitis with minimally impaired lung function and no additional risk factors for treatment failure. In these patients, amoxicillin, tetracycline, or trimethoprim/sulfamethoxazole are the proposed first-line agents because the consequences of treatment failure in this group are generally few.11 Probable pathogens are H. influenzae, M. catarrhalis, and S. pneumoniae. Group 3 patients have moderate to severe COPD and other risk factors for treatment failure, such as frequent exacerbations and comorbid conditions, including congestive heart failure, diabetes mellitus, chronic renal insufficiency, or chronic liver disease. The probable bacterial pathogens are similar to those in group 2, although gram-negative organisms are more likely in patients with severely impaired lung function. Because the costs of treatment failure are high in this group, and because β-lactamase-producing strains of H. influenzae and M. catarrhalis are increasingly prevalent, fluoroquinolones are the suggested first-line treatment. Group 4 patients have chronic suppurative lung disease, particularly bronchiectasis. Antibiotic therapy is directed at P. aeruginosa and other commonly drug-resistant gram-negative bacteria (see Table 3). As noted, approaches to antibiotic therapy based upon a rational appraisal of patient risk factors and likely pathogens reduce the risk for treatment failure and avoid unnecessary medical and economic expense.
Table 3.
RECOMMENDATIONS FOR CLASSIFICATION AND ANTIBIOTIC TREATMENT
| Group | Clinical State | Risk Factors | Probable Pathogens | First Choice | Alternatives |
|---|---|---|---|---|---|
| I | Acute tracheobronchitis | None | Viral, rarelyM. pneumoniae orC. pneumoniae | No antibiotics | Macrolide or tetracycline (for persistent symptoms) |
| II | Acute exacerbation of chronic bronchitis | None | H. influenzae, Haemophilus spp,M. catarrhalis,S. pneumoniae | Amoxicillin, tetracycline, TMP/SMX | Second generation cephalosporin, second generation macrolide, amoxicillin/clavulanate,fluoroquinolone |
| III | Acute exacerbation of chronic bronchitis with risk factors | Multiple* | Same as above. Also consider gram-negatives especially in patients with severely impaired lung function. | Fluoroquinolone | Amoxicillin/clavulanate, oral second or third generation cephalosporin, or second generation macrolide |
| IV | Chronic suppurative airway disease | Most have bronchiectasis | Same as group IIIplus multiresistantgram-negatives, particularlyP. aeruginosa | Antipseudomonal fluoroquinolone (ciprofloxacin) | Consider parenteral therapy with antipseudomonal agents |
FEV1 < 50% predicted, frequent exacerbations, significant comorbid conditions, malnutrition, chronic steroid use, mucous hypersecretion, duration of COPD >10 years, previous pneumonia. TMP/SMX = Trimethoprim/sulphamethoxazole.
There are outcome data to suggest this approach leads to improved clinical outcomes, with reduced overall costs.37 A retrospective study by Destache and colleagues37 demonstrated that, compared with the usual first-line antibiotics in the treatment of acute exacerbations of chronic bronchitis, the use of newer antibiotics reduced both the hospitalization rate and failure rate. Although the acquisition cost of newer antibiotics (cephalosporins, macrolides and fluoroquinolones) was higher, the overall costs of the treated patients given these drugs were lower. In particular, the group receiving amoxicillin/clavulanate, azithromycin, or ciprofloxacin had the lowest hospitalization rate, clinical failure rate, and costs compared with cephalosporins or first-line therapy.
The hypothesis that aggressive antibiotic therapy should be offered to high-risk patients was tested in a recent, prospective, health economic study.54 Patients with at least three treated exacerbations of chronic bronchitis in the past year were randomized to receive either ciprofloxacin or any nonquinolone-based therapy for their next acute exacerbation. Clinical endpoints (days of illness, hospitalizations, time to next exacerbation) were blended with quality-of-life measurements (Nottingham Health Profile, St. George's Hospital Respiratory Questionnaire, Health Utility Index), and total respiratory costs from a societal perspective. Although the overall results indicated no advantage for either treatment arm, in patients with risk factors (severe underlying lung disease, more than four exacerbations per year, duration of bronchitis greater than 10 years, elderly, significant comorbid illness) the use of ciprofloxacin led to improved clinical outcome, higher quality of life, and fewer costs. The results of this study would suggest that aggressive antimicrobial therapy directed especially toward resistant organisms in high-risk patients is a more effective strategy than no therapy or therapy with older antimicrobials that would not be effective against the usual target organisms, particularly β-lactamase-producing H. influenzae.
Further studies are needed to clarify the optimal antibiotic treatment regimens for subgroups of patients with AECOPD.
Corticosteroids
Randomized, controlled trials of the efficacy of corticosteroids for acute exacerbations of COPD are summarized in Table 4 . Their role in outpatient exacerbations has been evaluated in only one small study.125 Compared with placebo, oral prednisone (60 mg tapered to 0 mg over 9 days) significantly improved airflow and oxygenation and resulted in fewer treatment failures. FEV1 improved on average by only 50 mL per day among patients receiving prednisone.99 These findings support earlier retrospective data that suggest that, among patients with AECOPD presenting to an emergency department (ED), the incidence of revisit to the ED within 48 hours is significantly reduced if corticosteroids are prescribed.82
Table 4.
SUMMARY OF RANDOMIZED CONTROLLED TRIALS OF CORTICOSTEROIDS FOR ACUTE EXACERBATIONS OF CHRONIC OBSTRUCTIVE PULMONARY DISEASE
| Study | Population | Intervention | n | Outcome | P | |
|---|---|---|---|---|---|---|
| Niewoehner et al88 | 1999 | Inpatients | Steroid × 8 wks | 80 | Faster recovery of FEV1 | <0.05 |
| FEV1 < 0.8 L | Steroid × 2 wks | 80 | Fewer treatment failures | <0.05 | ||
| Placebo | 111 | No difference between 2 and 8 wks | ns | |||
| Davies et al (A)35 | 1997 | Inpatients | Prednisolone × 2 wks | 28 | Faster recovery of FEV1 | <0.05 |
| FEV1 = 0.61 L | Placebo | 22 | ||||
| Thompson et al125 | 1996 | Outpatients | Prednisone × 9 d | 13 | Improved FEV1 and oxygenation | <0.05 |
| FEV1 = 1.3 L | Placebo | 14 | Fewer treatment failures | <0.05 | ||
| Bullard et al22 | 1996 | ER patients | Steroid × 5 d | 60 | Faster recovery of FEV1 by 6 hr | <0.05 |
| FEV1 < 0.55 L | Placebo | 52 | ||||
| Rostom et al (A)102 | 1994 | Inpatients | Steroid × 30 d | Total | No difference in FEV1 at 30 d | ns |
| Placebo | 30 | 20% dropout rate | ||||
| Emerman et al43 | 1989 | ER patients | MPS 100 mg IV × 1 | 52 | No difference in FEV1 | ns |
| FEV1 < 30% | Placebo | 44 | No difference in admission rates | |||
| Albert et al3 | 1980 | Inpatients | MPS × 3 d | 22 | Faster recovery of FEV1 | <0.01 |
| FEV1 < 0.64 | Placebo | 22 | No difference in ABGs | ns | ||
| FEV1 = Forced expiratory volume in one second; A = published in abstract form only; ER = emergency room; MPS = methylprednisolone; ABG = arterial blood gas; IV = intravenously; ns = not significant. | ||||||
Corticosteroids are often given initially by ED physicians and several trials have examined this practice.22, 43 Emerman et al43 studied the effect of a single dose of methylprednisolone (100 mg intravenously [IV]) upon pulmonary function and hospitalization rates for 98 patients in the ED with COPD exacerbations. They failed to show any improvement in spirometry or decrease in the rate of hospitalization. Patients were treated for approximately 4.5 hours and the single steroid dose and short period of observation, however, have been postulated to account for the apparent lack of efficacy of steroids in this study.74 More recently, Bullard et al22 demonstrated a beneficial effect of steroids upon flow rates as early as 6 hours after initiation of treatment.
In 1980, Albert3 provided the initial justification for the routine use of systemic steroids in the care of hospitalized patients with COPD exacerbations. Forty-four patients with COPD and acute respiratory insufficiency were randomized to placebo or methylprednisolone 0.5 mg/kg intravenously every 6 hours for 3 days. Those treated with steroids were significantly more likely to achieve a 40% or greater increase in FEV1 over their baseline. This effect was observed by 12 hours following the start of treatment and persisted for 72 hours. Corticosteroids improved postbronchodilator lung function more than placebo but had minimal effect upon total symptoms in another small trial in which hospitalized patients were randomized to receive placebo or 30 mg of oral prednisolone for 14 days.35
Although the studies described have established that corticosteroids significantly increase FEV1 over the short term, no study was explicitly designed to capture longer-term endpoints or the adverse consequences associated with steroid therapy.88, 102 Rostom102 studied hospitalized patients with AECOPD given placebo or methylprednisolone (40 mg tapered to 0 mg over 1 month) and followed for 1 month after discharge. Mean FEV1 and FVC values were no different between the treatment groups at the end of the study. More recently, Niewoehner et al88 published the important Systemic Corticosteroids in Chronic Obstructive Pulmonary Disease Exacerbations trial. They performed a three-way randomization whereby 80 patients received an 8-week course of glucocorticoids, 80 received a 2-week course, and 111 received placebo. Steroids resulted in faster recovery of FEV1 and shortened hospital stay by 1 day (P < 0.05). At both 30 and 90 days, steroid therapy reduced treatment failures (defined as death from any cause, need for intubation, readmission, or intensification of drug therapy) by approximately 10%. There was no difference between 2 and 8 weeks of treatment with respect to spirometry or treatment failure rates, however. The dose of methylprednisolone was high (125 mg every 6 hours for 3 days) and resulted in significantly more hyperglycemia and, possibly, increased secondary infection rates.106
In summary, the evidence from randomized, controlled trials supports the conclusion that among patients with acute exacerbations, oral or intravenous corticosteroids significantly increase the FEV1 for up to 72 hours and likely reduce the risk for treatment failure. There is no proved benefit for treatment longer than 2 weeks. Hyperglycemia is the most common short-term complication of steroid treatment. As further studies become available, it will be possible to better understand the risk–benefit ratio for corticosteroids and, through meta-analysis, to better define the optimum dose and duration of therapy.136 It is also important to investigate the long-term risk for adverse effects of intermittent corticosteroids in patients who require them for recurrent exacerbations over many years time.
Noninvasive Positive-Pressure Ventilation
Noninvasive positive-pressure ventilation (NPPV) is arguably the most significant recent advance in the care of patients with COPD with acute respiratory failure. It avoids the complications of endotracheal intubation and preserves airway defense mechanisms while allowing patients to eat, speak, and expectorate secretions. Acute respiratory failure in COPD is often characterized by a vicious circle wherein the respiratory muscles must meet ever-increasing ventilatory demands under conditions of worsening hypoxemia, hypercapnia, and acidosis. When the increased metabolic requirements of the respiratory muscles cannot be matched by a commensurate rise in the cardiac output, further acidosis and muscle fatigue ensue. By allowing the muscles to rest, NPPV interrupts this process, thereby preventing respiratory arrest and death.77 Table 5 summarizes the randomized, controlled trials of NPPV in AECOPD.
Table 5.
SUMMARY OF RANDOMIZED CONTROLLED TRIALS OF NONINVASIVE POSITIVE-PRESSURE VENTILATION FOR ACUTE EXACERBATIONS OF CHRONIC OBSTRUCTIVE PULMONARY DISEASE
| Rights were not granted to include this data in electronic media. Please refer to the printed journal. |
Bott et al18 randomized 60 patients with COPD and hypoxemic–hypercarbic respiratory failure to either conventional treatment or volume-cycled nasal NPPV. Patients in both groups had similar pretreatment blood gas and spirometric abnormalities. At 1 hour, there was a significant rise in pH and drop in Paco 2 in the NPPV group compared with conventional treatment. NPPV also resulted in significantly less breathlessness by day 3. Most importantly, however, intention-to-treat analysis revealed a trend toward lower 30-day mortality in the NPPV group (3/30 versus 9/30, relative risk = 0.33, 95% CI 0.1–1.11, P = ns). This effect became significant after excluding the four patients randomized to NPPV who did not receive it (two were confused, one was unable to breathe through his nose, one had all active treatment withdrawn). None of the patients randomized to NPPV required intubation and no serious complications of NPPV were reported. The study has been criticized for the lack of standardized treatment in both groups and for the unusually high mortality in the control group.76
Kramer et al65 investigated the impact of NPPV on need for intubation among 31 patients with severe hypercarbic respiratory failure, most with COPD exacerbations. Sixteen patients (11 with COPD) were randomized to pressure-limited nasal NPPV in addition to standard care and 15 patients (12 with COPD) received standard care alone. Clear a priori indications for intubation were given. Significantly, only five patients (31%) in the NPPV group required intubation, compared with 11 (73%) in the standard therapy arm. Furthermore, maximal inspiratory pressures increased significantly in the NPPV arm over 6 hours, indicating a rapid reversal of diaphragmatic fatigue. In contrast to work by Bott,18 however, there were no significant differences in Paco 2 between the treatment groups at any time over the first 24 hours. The study was underpowered to detect differences in mortality.
A more recent European multicenter trial21 randomized 85 patients to standard therapy or pressure-limited NPPV by face mask. All patients required admission to an intensive care unit and were followed until death or hospital discharge. Noninvasive ventilation markedly reduced the need for intubation (controls 74% versus NPPV 26%). Compared with standard care, NPPV also significantly reduced mortality (9% versus 29%), complication rates (16% versus 48%), and mean duration of hospital stay (23 days versus 35 days) (all P < 0.05).21
More recently, Çelikel et al30 compared pressure-limited NPPV by face mask with usual care among patients with moderately severe hypercarbic acute respiratory failure and COPD. Noninvasive positive-pressure ventilation resulted in significantly fewer treatment failures, defined as need for intubation in the NPPV group and need for NPPV or intubation in the control group. Hospital stays were significantly shorter in the NPPV group. In contrast, Barbé et al,15 however, were unable to demonstrate any statistically significant benefit of nasal NPPV over conventional treatment in terms of duration of hospitalization, dyspnea scores, arterial blood gas measurements, or maximal inspiratory pressures. Patients in this trial, however, were clearly not as ill as those in other studies. Indeed, no patient in either group required intubation.
Randomized, controlled trials of NPPV for the treatment of AECOPD with hypercarbic respiratory failure recently were reviewed systematically.63 The pooled odds ratio for intubation following NPPV is 0.12 (95% CI, 0.05–0.29). More importantly, however, the trials that included mortality as an outcome collectively demonstrate a strong survival benefit for NPPV.2, 18, 21 The pooled odds ratio for death is 0.22 (95% CI, 0.09–0.54). Therefore, at worst, NPPV increases the patient's chance of surviving by nearly 50%; at best, the chance of survival is 90% better than that of a similar patient not receiving NPPV. Improvements in pH and Paco 2 within 1 hour of initiating NPPV and good level of consciousness at the beginning of NPPV are associated with successful responses to NPPV in patients with AECOPD and respiratory acidosis.9
Nutritional Support and Physiotherapy
Malnutrition is common among patients with COPD and increases the morbidity and mortality associated with the disease. The Veterans Administration Cooperative Study of Pulmonary Function98 showed that patients with FEV1 less than or equal to 0.5 L weighed less than 82% of their ideal body weight (IBW), compared with near normal body weight in less severely impaired patients. In another study,133 43% of patients with emphysema were found to weigh less than 90% of their IBW. Furthermore, Hunter58 reported that more than 70% of hospitalized patients with COPD had evidence of weight loss. Pingleton91 found that, among ventilated patients, mortality was significantly higher in those who were poorly nourished than in those with better nutritional status. Poorly nourished patients also had a significantly higher frequency of hypercapnia.
The principal effects of malnutrition upon the respiratory system are thought to be worsened respiratory muscle function, impairment of ventilatory drive, and immune dysfunction. Malnutrition impairs muscle function by reducing the availability of energy substrates such as glycogen and phosphate and by altering the structure of muscle fibers. When combined with intercurrent airway infection and the mechanical disadvantage of the diaphragm in COPD, malnutrition may have a profound effect on respiratory muscle mechanics. Experimental evidence for blunted hypoxic drive in response to semistarvation suggests another mechanism by which patients with COPD may be predisposed to respiratory failure.91, 133
No randomized, controlled trial has demonstrated reduced morbidity and mortality as a result of nutritional support during acute respiratory failure. Nevertheless, clinicians should be able to identify malnourished patients and understand the goals of nutritional therapy. Particular attention should be paid to patients with hypercatabolic states that increase the risk for protein-calorie malnutrition. The Subjective Global Assessment and the Harris-Benedict equation are two valid clinical instruments for assessing malnutrition and planning nutritional therapy. They are reviewed elsewhere.38, 91 In general, the goals of nutritional supplementation among patients with acute respiratory failure consist of maintaining body weight and preventing protein breakdown. The effects of malnutrition and nutritional supplementation in patients with COPD are discussed in more detail in the article by Schols and Wouters in this issue.
The value of chest physiotherapy (postural drainage with or without chest percussion) in AECOPD has not been demonstrated. Studies of patients with AECOPD have failed to demonstrate a beneficial effect of chest physiotherapy upon sputum volume, gas exchange, or spirometry.66 One trial27 documented a transient but significant decrease in FEV1 as a result of bronchoconstriction following chest percussion and vibration. Some evidence suggests that patients with larger volumes of airway secretions (>25 mL/d), particularly those with bronchiectasis, may benefit. In some guidelines, therefore, chest physiotherapy has been advocated in this situation.7 In general, however, it is not recommended in the routine management of AECOPD.20
OUTCOMES
Although outcome data are important, caution is required in their interpretation. Because data are generated by observing large numbers of patients, typically in tertiary referral centers, the pertinence of these data to individual patients may be limited, especially outside of major centers where most COPD exacerbations are treated.33 Studies of prognosis in patients with COPD and acute respiratory failure performed during previous decades are less relevant by today's standards. Current outcomes appear to be better than those of the past, in part because of the widespread use of controlled O2 therapy, corticosteroids and IB, availability of better β2-agonists, reduced use of methylxanthines, and increased use of noninvasive ventilatory support.33 Indeed, there is evidence to support a trend toward improved survival for hypercapnic respiratory failure over the past 20 years. The mortality rates in studies of survival of acute respiratory failure in COPD conducted from 1968 to 1973 ranged from 22% to 34%, with an overall mortality of 26%. For similar studies between 1978 and 1992, the range is 6% to 12%, with an overall mortality of 10%.132
The most recent and comprehensive evaluation of outcomes following AECOPD was published by Connors et al as a component of the landmark Study to Understand Prognoses for Outcomes and Risks of Treatment (SUPPORT) trial.33 They prospectively studied more than 1000 patients admitted to five US tertiary care hospitals with severe hypercarbic COPD exacerbations (initial Paco 2≥50 mm Hg). Baseline FEV1 was not available for most patients. Half the patients required intensive care unit admission and 35% required mechanical ventilation. Hospital mortality was 11%. More striking, however, was the finding that following discharge, one third of the patients died in within 6 months and one half within 2 years. Not surprisingly, patients who survived hospitalization had a substantial risk of discharge to a facility other than their home (20%) and of readmission to acute care over the ensuing 6 months (50%). Higher acute physiology score (Acute Physiology and Chronic Health Evaluation; APACHE III), older age, and poor functional status prior to admission independently increased risk of death. Improved survival was predicted by greater BMI and albumin level, higher Pao 2/Fio 2, and, surprisingly, the presence of cor pulmonale and congestive heart failure as the cause of the exacerbation. The latter findings may be explained by the good response of these two disorders to acute therapy.
A study by Dewan and colleagues38a also supports the finding that host factors are principal determinants of outcomes of AECOPD. In their retrospective analysis of 232 exacerbations in 107 patients with COPD, severity of airflow obstruction, use of home O2, frequency of exacerbation, history of previous pneumonia or sinusitis, and use of maintenance corticosteroids each were independently associated with treatment failure. Surprisingly, age, choice of antibiotics, and presence of comorbid conditions did not affect the treatment outcome in that study.
Several investigators62, 78, 100, 107 have evaluated the prognosis of patients with COPD who require ICU admission for acute exacerbations. The results have been somewhat contradictory. Kaelin et al,62 for example, using several easily obtainable indices, were unable to discriminate between patients surviving more or less than 6 months following intubation. In their analysis of 39 consecutive acute COPD exacerbations, neither age nor spirometric, blood gas, or nutritional indices predicted survival. In contrast, Menzies et al78 reported that, among their 95 patients, higher baseline FEV1 and serum albumin were significantly associated with improved 1-year survival following mechanical ventilation. These contradictory findings are especially curious given that both studies had similar inclusion and exclusion criteria and periods of observation. Moreover, their populations both had a mean percent-predicted FEV1 of 35% and nearly identical baseline values for serum albumin.
More recently, Rieves et al100 tried to identify clinically useful variables that predict successful weaning from mechanical ventilation and short-term survival in patients with COPD with acute respiratory failure. They observed episodes of acute respiratory failure in 19 and 33 patients with baseline FEV1 of greater and less than 1 L respectively. Only 56% of the cohort with severe COPD survived weaning and spontaneous breathing for 72 hours. Furthermore, in the same group, 1-year survival was only 27%. Absence of infiltrates on chest radiograph was the most influential predictor of survival in patients with severe COPD. Pneumonia accounted for most of the infiltrates that were seen in this group. Baseline FEV1 obtained during a period of clinical stability prior to the episode of acute respiratory failure was available for all patients. The extent of baseline obstruction alone was not statistically correlated with short-term survival in either group, but the combination of severe baseline obstruction and pulmonary infiltrates markedly increased the risk for death. Outcomes following AECOPD associated with ICU admission and respiratory failure are discussed further in the article by Sethi and Siegel in this issue.
Seneff et al107 recently refined the discussion over the relative prognostic value of different clinical variables following COPD exacerbation. They analyzed 362 admissions for acute COPD exacerbation from the APACHE III database. Hospital mortality was 24%. For patients aged 65 and older, hospital and 1-year mortality rates were 30% and 59% respectively. Their report emphasizes that individual clinical variables have different value for predicting short- and long-term survival. Patient age, for example, was a statistically significant determinant of 6-month survival but not influential for hospital mortality after accounting for nonrespiratory organ dysfunction. Similarly, the presence of hypercarbia was of no value in predicting hospital mortality but became important over the long term; 1-year mortality rates for patients with admission Paco 2 of less than 50 mm Hg versus greater than 50 mm Hg were 54% and 70% respectively. The most significant predictors of short and long-term mortality are development and severity of multiple organ dysfunction syndrome. Respiratory dysfunction is more important over the longer term. As the authors state: “In most cases, the acute, life-threatening components of the exacerbations can be reversed and short-term death avoided by mechanical ventilation and other appropriate treatments. However, because abnormalities in respiratory physiology reflect underlying severity of lung disease, patients with greater abnormality who survive hospitalization are at greater risk of subsequent death.”107
SUMMARY
Chronic obstructive pulmonary disease is the only leading cause of death with a rising prevalence. The medical and economic costs arising from acute exacerbations of COPD are therefore expected to increase over the coming years. Although exacerbations may be initiated by multiple factors, the most common identifiable associations are with bacterial and viral infections. These are associated with approximately 50% to 70% and 20% to 30% of COPD exacerbations, respectively. In addition to smoking cessation, annual influenza vaccination is the most important method for preventing exacerbations. Controlled O2 is the most important intervention for patients with acute hypoxic respiratory failue. Evidence from randomized, controlled trials justifies the use of corticosteroids, bronchodilators (but not theophylline), noninvasive positive-pressure ventilation (in selected patients), and antibiotics, particularly for severe exacerbations. Antibiotics should be chosen according to the patient's risk for treatment failure and the potential for antibiotic resistance. In the acute setting, combined treatment with β-agonist and anticholinergic bronchodilators is reasonable but not supported by randomized controlled studies. Physicians should identify and, when possible, correct malnutrition. Chest physiotherapy has no proven role in the management of acute exacerbations.
Footnotes
Address reprint requests to Ronald F. Grossman, MD, Division of Respiratory Medicine, Department of Medicine, Mount Sinai Hospital, Suite 640, 600 University Avenue, Toronto, Ontario, Canada M5G 1X5, e-mail: ronaldf.grossman@utoronto.ca
References
- 1.Adler K.B., Hendley D.D., Davies G.S. Bacteria associated with obstructive pulmonary disease elaborate extracellular products that stimulate mucin secretion by explants of guinea pig airways. Am J Pathol. 1986;125:501–514. [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmed A., Fenwick L., Angus R.M. Nasal ventilation vs. doxapram in the treatment of type II respiratory failure complicating chronic airflow obstruction. Thorax. 1992;47:858. [Google Scholar]
- 3.Albert R.K., Martin T.R., Lewis S.W. Controlled clinical trial of methylprednisolone in patients with chronic bronchitis and acute respiratory insufficiency. Ann Intern Med. 1980;92:753–758. doi: 10.7326/0003-4819-92-6-753. [DOI] [PubMed] [Google Scholar]
- 4.Allegra L., Cordaro C.I., Grassi C. Prevention of acute exacerbations of chronic obstructive bronchitis with carbocysteine lysine salt monohydrate: A multicentre, double-blind, placebo-controlled trial. Respiration. 1996;63:174–180. doi: 10.1159/000196540. [DOI] [PubMed] [Google Scholar]
- 5.Allega L., Grassi C., Grossi E. Ruolo degli antibiotici nel trattamento delle riacutizza della bronchite cronica. Ital J Chest Dis. 1991;45:138–148. [Google Scholar]
- 6.American Lung Association: Trends in chronic bronchitis and emphysema: morbidity and mortality.www.lungusa.org
- 7.American Thoracic Society Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1995;152:S77–120. [PubMed] [Google Scholar]
- 8.American Thoracic Society Health effects of outdoor air pollution (Part 1) Am J Respir Crit Care Med. 1996;153:3–50. doi: 10.1164/ajrccm.153.1.8542133. [DOI] [PubMed] [Google Scholar]
- 9.Anton A., Guell R., Gomez J. Predicting the results of non-invasive ventilation in severe acute exacerbations of patients with chronic airflow limitation. Chest. 2000;117:828–833. doi: 10.1378/chest.117.3.828. [DOI] [PubMed] [Google Scholar]
- 10.Anthonisen N.R., Connett J.E., Kiley J.P. Effects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1. The Lung Health Study. JAMA. 1994;272:1497–1505. [PubMed] [Google Scholar]
- 11.Anthonisen N.R., Manfreda J., Warren C.P.W. Antibiotic therapy of acute exacerbations of chronic obstructive pulmonary disease. Ann Intern Med. 1987;106:196–204. doi: 10.7326/0003-4819-106-2-196. [DOI] [PubMed] [Google Scholar]
- 12.Aubier M., Murciano D., Fournier M. Central respiratory drive in acute respiratory failure of patients with chronic obstructive pulmonary disease. Am Rev Respir Dis. 1980;122:191–199. doi: 10.1164/arrd.1980.122.2.191. [DOI] [PubMed] [Google Scholar]
- 13.Aubier M., Murciano D., Milic-Emili J. Effects of the administration of O2 on ventilation and blood gasses in patients with chronic obstructive disease during acute respiratory failure. Am Rev Respir Dis. 1980;122:747–754. doi: 10.1164/arrd.1980.122.5.747. [DOI] [PubMed] [Google Scholar]
- 14.Balter M.S., Hyland R.H., Low D.E. Recommendations on the management of chronic bronchitis. CMAJ. 1994;151:8–23. [Google Scholar]
- 15.Barbé F., Togores B., Rubi M. Noninvasive ventilatery support does not facilitate recovery from acute respiratory failure in chronic obstructive pulmonary disease. Eur Respir J. 1996;9:1240–1245. doi: 10.1183/09031936.96.09061240. [DOI] [PubMed] [Google Scholar]
- 16.Barbera J.A., Roca J., Ferrer A. Mechanisms of worsening gas exchange during acute exacerbations of chronic obstructive pulmonary disease. Eur Respir J. 1996;10:1285–1291. doi: 10.1183/09031936.97.10061285. [DOI] [PubMed] [Google Scholar]
- 17.Bone R.C., Pierce A.K., Johnson R.L. Controlled oxygen administration in acute respiratory failure in chronic obstructive pulmonary disease. A reappraisal. Am J Med. 1978;65:896–902. doi: 10.1016/0002-9343(78)90740-4. [DOI] [PubMed] [Google Scholar]
- 18.Bott J., Carroll M.P., Conway J.H. Randomised controlled trial of nasal ventilation in acute ventilatory failure due to chronic obstructive airways disease. Lancet. 1993;341:1555–1557. doi: 10.1016/0140-6736(93)90696-e. [DOI] [PubMed] [Google Scholar]
- 19.Braun S.R., McKenzie W.N., Copeland C. A comparison of the effect of ipratropium bromide and albuterol in the treatment of chronic obstructive pulmonary disease. Arch Intern-Med. 1989;149:544–547. [PubMed] [Google Scholar]
- 20.British Thoracic Society BTS guidelines for the management of chronic obstructive pulmonary disease. Thorax. 1997;52(suppl 5):1–28. [PMC free article] [PubMed] [Google Scholar]
- 21.Brochard L., Mancebo J., Wysocki M. Noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease. N Engl J Med. 1995;333:817–822. doi: 10.1056/NEJM199509283331301. [DOI] [PubMed] [Google Scholar]
- 22.Bullard M.J., Liaw S.J., Tsai Y.H. Early corticosteroid use in acute exacerbations of chronic airflow obstruction. Am J Emerg Med. 1996;14:139–143. doi: 10.1016/S0735-6757(96)90120-5. [DOI] [PubMed] [Google Scholar]
- 23.Buret A., Cripps A.W. The immunoevasive activities of Pseudomonas aeruginosa. Am Rev Respir Dis. 1993;148:793–805. doi: 10.1164/ajrccm/148.3.793. [DOI] [PubMed] [Google Scholar]
- 24.Burrows B. Predictors of loss of lung function and mortality in obstructive lung disease. Eur Respir Rev. 1991;1:340–345. [Google Scholar]
- 25.Buscho L.D., Saxtan D., Schultz P.S. Infections with viruses and Mycoplasma pneumoniae during exacerbations of chronic bronchitis. J Infect Dis. 1978;137:377–383. doi: 10.1093/infdis/137.4.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Butler J.C., Breiman R.F., Campbell J.F. Pneumococcal polysaccharide vaccine efficacy: An evaluation of current recommendations. JAMA. 1993;270:1826–1831. [PubMed] [Google Scholar]
- 27.Campbell A., O'Connell J., Wilson F. The effect of chest physiotherapy upon the FEV1 in chronic bronchitis. Med J Aust. 1975;1:33–35. doi: 10.5694/j.1326-5377.1975.tb111210.x. [DOI] [PubMed] [Google Scholar]
- 28.Canadian Lung Association: The impact of COPD. www.lung.ca/copd/intro/impact.html
- 29.Cate T.R., Roberts J.S., Russ M.A. Effects of common colds on pulmonary function. Am Rev Respir Dis. 1973;108:858–865. doi: 10.1164/arrd.1973.108.4.858. [DOI] [PubMed] [Google Scholar]
- 30.Çelikel T., Sungur M., Ceyhan B. Comparison of noninvasive positive pressure ventilation with standard medical therapy in hypercapnic acute respiratory failure. Chest. 1998;114:1636–1642. doi: 10.1378/chest.114.6.1636. [DOI] [PubMed] [Google Scholar]
- 31.Chapman A.J., Musher D.M., Jonsson S. Development of bacterididal antibody during Branhamella catarrhalis infection. J Infect Dis. 1985;151:878–882. doi: 10.1093/infdis/151.5.878. [DOI] [PubMed] [Google Scholar]
- 32.Collet J.P., Shapiro S., Ernst P. Effects of an immunostimulating agent on acute exacerbations and hospitalizations in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1997;156:1719–1724. doi: 10.1164/ajrccm.156.6.9612096. [DOI] [PubMed] [Google Scholar]
- 33.Connors A.F., Dawson N.V., Thomas C. Outcomes following acute exacerbation of severe chronic obstructive lung disease. Am J Respir Crit Care Med. 1996;154:959–967. doi: 10.1164/ajrccm.154.4.8887592. [DOI] [PubMed] [Google Scholar]
- 34.Curtis J.R., Hudson L.D. Emergency assessment and management of acute respiratory failure in COPD. Clin Chest Med. 1994;15:481–500. [PubMed] [Google Scholar]
- 35.Davies L., Angus R., Calverley P. A prospective randomised double-blind placebo controlled study of oral corticosteroids in patients admitted with normocapnic exacerbations of chronic obstructive pulmonary disease (COPD) Thorax. 1997;53(suppl 6):A5. [Google Scholar]
- 36.Davis A.L., Aranda C.P., Schiffman G. Pneumococcal infection and immunologic response to pneumococcal vaccine in chronic obstructive pulmonary disease: A pilot study. Chest. 1987;92:204–212. doi: 10.1378/chest.92.2.204. [DOI] [PubMed] [Google Scholar]
- 37.Destache C.J., Dewan N., O'Donohue W.J. Clinical and economic considerations in the treatment of acute exacerbations of chronic bronchitis. J Antimicrob Chemother. 1999;43(suppl A):107–113. doi: 10.1093/jac/43.suppl_1.107. [DOI] [PubMed] [Google Scholar]
- 38.Detsky A.S., Smalley P.S., Chang J. Is this patient malnourished? JAMA. 1994;271:54–58. doi: 10.1001/jama.271.1.54. [DOI] [PubMed] [Google Scholar]
- Dewan N.A., Rafique S., Kanwar B. Acute exacerbation of COPD: Factors associated with poor treatment outcome. Chest. 2000;117:662–671. doi: 10.1378/chest.117.3.662. [DOI] [PubMed] [Google Scholar]
- 39.Dunn W.F., Nelson S.B., Hubmayr R.D. Oxygeninduced hypercarbia in obstructive pulmonary disease. Am Rev Respir Dis. 1991;144:526–530. doi: 10.1164/ajrccm/144.3_Pt_1.526. [DOI] [PubMed] [Google Scholar]
- 40.Eadie M.B., Stott E.J., Grist N.R. Virological studies in chronic bronchitis. BMJ. 1966;2:671–673. doi: 10.1136/bmj.2.5515.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eason J., Markowe H.J. Aminophylline toxicity—how many hospital asthma deaths does it cause? Respir Med. 1989;83:219–226. doi: 10.1016/s0954-6111(89)80035-6. [DOI] [PubMed] [Google Scholar]
- 42.Eller J., Ede J., Schaberg I. Infective exacerbations of chronic brochitis. Relation between bacteriologic eitiology and long-term function. Chest. 1998;113:1542–1548. doi: 10.1378/chest.113.6.1542. [DOI] [PubMed] [Google Scholar]
- 43.Emerman C.L., Connors A.F., Lukens T.W. A randomized controlled trial of methylprednisolone in the emergency treatment of acute exacerbations of COPD. Chest. 1989;95:563–567. doi: 10.1378/chest.95.3.563. [DOI] [PubMed] [Google Scholar]
- 44.Fagon J.Y., Chastre J., Trouillet J.L. Characterization of distal bronchial microflora during acute exacerbation of chronic bronchitis. Am Rev Respir Dis. 1990;142:1004–1008. doi: 10.1164/ajrccm/142.5.1004. [DOI] [PubMed] [Google Scholar]
- 45.Farley M.M., Stephens D.S., Mulks M.H. Pathogenesis of IgA1 protease-producing and nonproducing Hemophilus influenzae in human nasopharyngeal organ cultures. J Infect Dis. 1986;154:752–759. doi: 10.1093/infdis/154.5.752. [DOI] [PubMed] [Google Scholar]
- 46.Fine M.J., Smith M.A., Carson C.A. Efficacy of pneumococcal vaccination in adults: A meta-analysis of randomized controlled trials. Arch Intern Med. 1994;154:2666–2677. doi: 10.1001/archinte.1994.00420230051007. [DOI] [PubMed] [Google Scholar]
- 47.Gelb A.F., Klein E., Schiffman P. Ventilatory response and drive in acute and chronic obstructive pulmonary disease. Am Rev Respir Dis. 1977;16:9–16. doi: 10.1164/arrd.1977.116.1.9. [DOI] [PubMed] [Google Scholar]
- 48.Goh S.K., Johan A., Cheong T.H. A prospective study of infections with atypical pneumonia organisms in acute exacerbations of chronic bronchits. Ann Acad Med Singapore. 1999;28:476–480. [PubMed] [Google Scholar]
- 49.Groeneveld K., van Alphen L., Eijk P.P. Endogenous and exogenous reinfections by Haemophilus influenzae in patients with chronic obstructive pulmonary disease: The effect of antibiotic treatment on persistence. J Infect Dis. 1990;161:512–517. doi: 10.1093/infdis/161.3.512. [DOI] [PubMed] [Google Scholar]
- 50.Gross P.A., Hermogenes A.W., Sacks H.S. The efficacy of influenza vaccine in elderly persons. A meta-analysis and review of the literature. Ann Intern Med. 1995;123:518–527. doi: 10.7326/0003-4819-123-7-199510010-00008. [DOI] [PubMed] [Google Scholar]
- 51.Grossman R.F. The value of antibiotics and the outcomes of antibiotic therapy in exacerbations of COPD. Chest. 1998;113(suppl):249–255. doi: 10.1378/chest.113.4_supplement.249s. [DOI] [PubMed] [Google Scholar]
- 52.Grossman R.F. Management of acute exacerbation of chronic bronchitis. Can Respir J. 1999;6(suppl):40A–45A. [PubMed] [Google Scholar]
- 53.Grossman R.F. Use of guidelines and risk stratification in acute exacerbations of chronic bronchitis. Semin Respir Crit Care. 2000;21:113–122. doi: 10.1055/s-2000-9932. [DOI] [PubMed] [Google Scholar]
- 54.Grossman R.F., Mukerjee J., Vaughan D. A 1-year community-based health economic study of ciprofloxacin vs. usual antibiotic treatment in acute exacerbations of chronic bronchitis. Chest. 1998;113:131–141. doi: 10.1378/chest.113.1.131. [DOI] [PubMed] [Google Scholar]
- 55.Gump D.W., Phillips C.A., Forsyth B.R. Role of infection in chronic bronchitis. Am Rev Respir Dis. 1976;113:465–474. doi: 10.1164/arrd.1976.113.4.465. [DOI] [PubMed] [Google Scholar]
- 56.Higgins M.W., Enright P.L., Kronmal R.A. Smoking and lung function in elderly men and women. The Cardiovascular Health Study. JAMA. 1993;269:2741–2748. [PubMed] [Google Scholar]
- 57.Horn M., Gregg I. Role of viral infection and host factors in acute episodes of asthma and chronic bronchitis. Chest. 1973;63:445–485. doi: 10.1378/chest.63.4_supplement.44s-a. [DOI] [PubMed] [Google Scholar]
- 58.Hunter A.M.B., Carey M.A., Larsh H.U. The nutritional status of patients with chronic obstructive pulmonary disease. Am Rev Respir Dis. 1981;124:376–381. doi: 10.1164/arrd.1981.124.4.376. [DOI] [PubMed] [Google Scholar]
- 59.Influenza and pneumococcal vaccination levels among adults aged ≥ 65 years—United States. MMWR Morb Mort Wkly Rep. 1998;47:797–802. [PubMed] [Google Scholar]
- 60.Jasper A.C., Moshenifar Z., Kahan S. Cost–benefit comparison of aerosol bronchodilator delivery methods in hospitalized patients. Chest. 1987;91:614–618. doi: 10.1378/chest.91.4.614. [DOI] [PubMed] [Google Scholar]
- 61.Jorgensen A.F., Coolidge J., Pedersen P.A. Amoxicillin in treatment of acute uncomplicated exacerbations of chronic bronchitis. Scand J Primary Health Care. 1992;10:7–11. doi: 10.3109/02813439209014027. [DOI] [PubMed] [Google Scholar]
- 62.Kaelin R.M., Assimacopoulos A., Chevrolet J.C. Failure to predict 6-month survival of patients with COPD requiring mechanical ventilation by analysis of simple indices—a prospective study. Chest. 1987;92:971–978. doi: 10.1378/chest.92.6.971. [DOI] [PubMed] [Google Scholar]
- 63.Keenan S.P., Kernerman P.D., Cook D.J. Effect of non-invasive positive pressure ventilation on mortality in patients admitted with acute respiratory failure: A meta-analysis. Crit Care Med. 1997;25:1685–1692. doi: 10.1097/00003246-199710000-00018. [DOI] [PubMed] [Google Scholar]
- 64.Koivula I., Sten M., Leinonen M. Clinical efficacy of pneumococcal vaccine in the elderly: A randomized, single-blind population-based trial. Am J Med. 1997;103:281–290. doi: 10.1016/s0002-9343(97)00149-6. [DOI] [PubMed] [Google Scholar]
- 65.Kramer N., Meyer T.J., Meharg J. Randomized, prospective trial of noninvasive positive-pressure ventilation in acute respiratory failure. Am J Respir Crit Care Med. 1995;151:1799–1806. doi: 10.1164/ajrccm.151.6.7767523. [DOI] [PubMed] [Google Scholar]
- 66.Krilloff L.H., Owens G.R., Rogers R.M. Does chest physical therapy work? Chest. 1985;88:436–444. doi: 10.1378/chest.88.3.436. [DOI] [PubMed] [Google Scholar]
- 67.Lamy M.E., Pouthier-Simon F., Debacker-Williame E. Respiratory viral infections in hospital patients with chronic bronchitis. Chest. 1973;63:336–341. doi: 10.1378/chest.63.3.336. [DOI] [PubMed] [Google Scholar]
- 68.Larsson S., Svedmyr N. Bronchodilating effect and side effects of β2 adrenoreceptor stimulants by different modes of administration. Am Rev Respir Dis. 1977;116:861–869. doi: 10.1164/arrd.1977.116.5.861. [DOI] [PubMed] [Google Scholar]
- 69.Leech J.A., Gervais A., Ruben F.L. Efficacy of pneumococcal vaccine in severe chronic obstructive pulmonary disease. Can Med Assoc J. 1987;136:361–365. [PMC free article] [PubMed] [Google Scholar]
- 70.Littenberg B. Aminophylline treatment in severe acute asthma. A meta-analysis. JAMA. 1988;259:1678–1684. [PubMed] [Google Scholar]
- 71.Littenberg B., Gluck E.H. A controlled trial of methylprednisolone in the emergency treatment of acute asthma. N Engl J Med. 1986;314:150–152. doi: 10.1056/NEJM198601163140304. [DOI] [PubMed] [Google Scholar]
- 72.Lode H. Respiratory tract infections: When is antibiotic therapy indicated? Clin Ther. 1991;13:149–156. [PubMed] [Google Scholar]
- 73.Madison J.M., Irwin R.S. Chronic obstructive pulmonary disease. Lancet. 1998;352:467–473. doi: 10.1016/S0140-6736(97)11081-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mador M.J., Tobin M.J. Acute respiratory failure. In: Caverley P., Pride N., editors. Chronic Obstructive Pulmonary Disease. Chapman & Hall; London: 1995. p. 469. [Google Scholar]
- 75.McNamara M.J., Phillips I.A., Williams O.B. Viral and Mycoplasma pneumoniae infections in exacerbations of chronic lung disease. Am Rev Respir Dis. 1969;100:19–24. doi: 10.1164/arrd.1969.100.1.19. [DOI] [PubMed] [Google Scholar]
- 76.Mehta S. Noninvasive positive pressure ventilation in acute respiratory failure. Intensive Care Med. 1998;24:1113–1114. doi: 10.1007/s001340050726. [DOI] [PubMed] [Google Scholar]
- 77.Mehta S., Hill N. Noninvasive ventilation in acute respiratory failure. Respir Care Clin North Am. 1996;2:267–292. [PubMed] [Google Scholar]
- 78.Menzies R., Gibbons W., Goldberg P. Determinants of weaning and survival among patients with COPD who require mechanical ventilation for acute respiratory failure. Chest. 1989;95:398–405. doi: 10.1378/chest.95.2.398. [DOI] [PubMed] [Google Scholar]
- 79.Moayyedi P., Congleton J., Page R.L. Comparison of nebulised salbutamol and ipratropium bromide with salbutamol alone in the treatment of chronic obstructive pulmonary disease. Thorax. 1995;50:834–837. doi: 10.1136/thx.50.8.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Monsó E., Ruiz J., Rosell A. Bacterial infection in chronic obstructive pulmonary disease (a study of stable and exacerbated outpatients using the protected specimen brush) Am J Respir Crit Care Med. 1995;152:1316–1320. doi: 10.1164/ajrccm.152.4.7551388. [DOI] [PubMed] [Google Scholar]
- 81.Morgan G., Corbett S., Wlodarczyk J. Air pollution and hospital admissions in Sydney, Australia, 1990 to 1994. Am J Public Health. 1998;88:1761–1766. doi: 10.2105/ajph.88.12.1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Murata G.H., Gorby M.S., Chick T.W. Intravenous and oral corticosteroids for the prevention of relapse after treatment of decompensated COPD. Chest. 1990;98:845–849. doi: 10.1378/chest.98.4.845. [DOI] [PubMed] [Google Scholar]
- 83.Murphy T.M., Sethi S. Bacterial infection in chronic obstructive pulmonary disease. Am Rev Respir Dis. 1992;146:1067–1083. doi: 10.1164/ajrccm/146.4.1067. [DOI] [PubMed] [Google Scholar]
- 84.Musher D.M., Kubitschek K.R., Crennan J. Pneumonia and acute febrile tracheobronchitis due to Haemophilus influenzae. Ann Intern Med. 1983;99:444–450. doi: 10.7326/0003-4819-99-4-444. [DOI] [PubMed] [Google Scholar]
- 85.Nichol K.L., Baken L., Nelson A. Relation between influenza vaccination and outpatient visits, hospitalization and mortality in elderly persons with chronic lung disease. Ann Intern Med. 1999;130:397–403. doi: 10.7326/0003-4819-130-5-199903020-00003. [DOI] [PubMed] [Google Scholar]
- 86.Nichol K.L., Margolis K.L., Wuorenma J. The efficacy and cost effectiveness of vaccination against influenza among elderly persons living in the community. N Engl J Med. 1994;331:778–784. doi: 10.1056/NEJM199409223311206. [DOI] [PubMed] [Google Scholar]
- 87.Nicotra M.B., Rivera M., Awe R. Antibiotic therapy of acute exacerbations of chronic bronchitis. Ann Intern Med. 1982;97:18–21. doi: 10.7326/0003-4819-97-1-18. [DOI] [PubMed] [Google Scholar]
- 88.Niewoehner D.E., Erbland M.L., Deupree R.H. Effect of systemic glucocorticoids on exacerbations of chronic obstructive pulmonary disease. N Engl J Med. 1999;340:1941–1947. doi: 10.1056/NEJM199906243402502. [DOI] [PubMed] [Google Scholar]
- 89.O'Driscoll B.R., Taylor R.J., Horsley M.G. Nebulized salbutamol with and without ipratropium bromide in acute airflow obstruction. Lancet. 1989;I:1418–1420. doi: 10.1016/s0140-6736(89)90126-8. [DOI] [PubMed] [Google Scholar]
- 90.Orcel B., Delclaux B., Baud M. Oral immunization with bacterial extracts for protection against acute bronchitis in elderly institutionalized patients with chronic bronchits. Eur Respir J. 1994;7:446–452. doi: 10.1183/09031936.94.07030446. [DOI] [PubMed] [Google Scholar]
- 91.Pingleton S.K. Nutrition in acute respiratory failure. Lung. 1986;164:127–137. doi: 10.1007/BF02713637. [DOI] [PubMed] [Google Scholar]
- 92.Prevention and control of influenza: recommendations of the advisory committee on immunization practices (ACIP) MMWR Morb Mortal Wkly Rep. 1998;47(RR–6):1–26. [PubMed] [Google Scholar]
- 93.Prevention and control of pneumoccocal disease Recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Morb Mortal Wkly Rep. 1997;46(RR–8):1–24. [PubMed] [Google Scholar]
- 94.Rayner C.F.J., Jackson A.D., Rutman A. The interaction of pneumolysin sufficient and deficient isogenic variants of Streptococcus pneumoniae with human respiratory mucosa. Infect Immun. 1995;63:442–447. doi: 10.1128/iai.63.2.442-447.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Read R.C., Roberts P., Munro N. Effect of Pseudomonas aeruginosa rhamnolipids on mucociliary transport and ciliary beating. J Appl Physiol. 1992;72:2271–2277. doi: 10.1152/jappl.1992.72.6.2271. [DOI] [PubMed] [Google Scholar]
- 96.Rebuck A.S., Chapman K.R., Abboud R. Nebulized anticholinergic and sympathomimetic treatment of asthma and chronic obstructive airways disease in the emergency room. Am J Med. 1987;82:59–64. doi: 10.1016/0002-9343(87)90378-0. [DOI] [PubMed] [Google Scholar]
- 97.Reichek N., Lewin E.B., Rhoden D.L. Antibody responses to bacterial antigens during exacerbations of chronic bronchitis. Am Rev Respir Dis. 1970;101:238–244. doi: 10.1164/arrd.1970.101.2.238. [DOI] [PubMed] [Google Scholar]
- 98.Renzetti A.D., McClement J.H., Litt B.D. The Veterans Administration cooperative study of pulmonary function-mortality in relation to respiratory function in chronic obstructive pulmonary disease. Am J Med. 1966;41:115–129. doi: 10.1016/0002-9343(66)90009-x. [DOI] [PubMed] [Google Scholar]
- 99.Rice K.L., Leatherman J.W., Duane P.G. Aminophylline for acute exacerbations of chronic obstructive pulmonary disease. Ann Intern Med. 1987;107:305–309. doi: 10.7326/0003-4819-107-2-305. [DOI] [PubMed] [Google Scholar]
- 100.Rieves R.D., Bass D., Carter R.R. Severe COPD and acute respiratory failure. Correlates for survival at the time of tracheal intubation. Chest. 1993;104:854–860. doi: 10.1378/chest.104.3.854. [DOI] [PubMed] [Google Scholar]
- 101.Riise G.C., Larsson S., Andersson B.A. Bacterial adhesion to oropharyngeal and chronchial epithelial cells in smokers with chronic bronchitis and in healthy nonsmokers. Eur Respir J. 1994;7:1759–1764. doi: 10.1183/09031936.94.07101759. [DOI] [PubMed] [Google Scholar]
- 102.Rostom R., Mink S., Hebert P. The long-term efficacy of methylprednisolone in the treatment of acute exacerbation of COPD. Chest. 1994;106(suppl):161. [Google Scholar]
- 103.Saint S., Bent S., Vittinghoff E. Antibiotics in chronic obstructive pulmonary disease exacerbations. A meta-analysis. JAMA. 1995;273:957–960. [PubMed] [Google Scholar]
- 104.Schmidt G.A., Hall J.B. Acute on chronic respiratory failure—assessment and management of patients with COPD in the emergent setting. JAMA. 1989;261:3444–3453. doi: 10.1001/jama.261.23.3444. [DOI] [PubMed] [Google Scholar]
- 105.Schwartz J. Air pollution and hospital admissions for the elderly in Detroit, Michigan. Am J Respir Crit Care Med. 1994;150:648–655. doi: 10.1164/ajrccm.150.3.8087333. [DOI] [PubMed] [Google Scholar]
- 106.Schwartz J. PM10, ozone, and hospital admissions for the elderly in Minneapolis–St. Paul, Minnesota. Arch Environ Health. 1994;49:366–374. doi: 10.1080/00039896.1994.9954989. [DOI] [PubMed] [Google Scholar]
- 107.Seneff M.G., Wagner D.P., Wagner R.P. Hospital and 1-year survival of patients admitted to intensive care units with acute exacerbation of chronic obstructive pulmonary disease. JAMA. 1995;274:1852–1857. [PubMed] [Google Scholar]
- 108.Shapiro E.D., Berg A.T., Austrian R. The protective efficacy of polyvalent pneumoccocal polysaccharide vaccine. N Engl J Med. 1991;325:1453–1460. doi: 10.1056/NEJM199111213252101. [DOI] [PubMed] [Google Scholar]
- 109.Sherrill D.L., Lebowitz M.D., Burrows B. Epidemiology of chronic obstructive pulmonary disease. Clin Chest Med. 1990;11:375–387. [PubMed] [Google Scholar]
- 110.Shrestha M., O'Brien T., Haddox R. Decreased duration of emergency department treatment of chronic obstructive pulmonary disease exacerbations with the addition of ipratropium bromide to β-agonist therapy. Ann Emerg Med. 1991;120:1206–1209. doi: 10.1016/s0196-0644(05)81472-6. [DOI] [PubMed] [Google Scholar]
- 111.Siefkin A.D. Optimal pharmacologic treatment of the critically ill patient with obstructive airways disease. Am J Med. 1996;100(suppl 1A):54–61. doi: 10.1016/s0002-9343(96)80097-0. [DOI] [PubMed] [Google Scholar]
- 112.Smith C.B., Golden C.A., Klauber M.R. Interactions between viruses and bacteria in patients with chronic bronchitis. J Infect Dis. 1976;134:552–561. doi: 10.1093/infdis/134.6.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Smith C.B., Golden C.A., Kanner R.E. Association of viral and Mycoplasma pneumoniae infections with acute respiratory illness in patients with chronic obstructive pulmonary disease. Am Rev Respir Dis. 1980;121:225–232. doi: 10.1164/arrd.1980.121.2.225. [DOI] [PubMed] [Google Scholar]
- 114.Smith C.B., Kanner R.E., Golden C.A. Haemophilus influenzae and Haemophilus parainfluenzae in chronic obstructive pulmonary disease. Lancet. 1976;1:1253–1255. doi: 10.1016/s0140-6736(76)91733-5. [DOI] [PubMed] [Google Scholar]
- 115.Soler N., Torres A., Ewig S. Bronchial microbial patterns in severe exacerbations of chronic obstructive pulmonary disease (COPD) requiring mechanical ventilation. Am J Respir Crit Care Med. 1998;157:1498–1505. doi: 10.1164/ajrccm.157.5.9711044. [DOI] [PubMed] [Google Scholar]
- 116.Sommerville R.G. Respiratory syncytial virus in acute exacerbations of chronic bronchitis. Lancet. 1963;2:1247–1248. doi: 10.1016/s0140-6736(63)90894-8. [DOI] [PubMed] [Google Scholar]
- 117.Stark J.E., Heath R.B., Curwen M.P. Infection with influenza and parainfluenza viruses in chronic bronchitis. Thorax. 1965;20:124–127. doi: 10.1136/thx.20.2.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Stenhouse A.C. Rhinovirus infection in acute exacerbations of chronic bronchitis: A controlled prospective study. BMJ. 1969;3:461–463. doi: 10.1136/bmj.3.5563.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Stradling J.R. Hypercapnia during oxygen therapy in airways obstruction: A reappraisal. Thorax. 1986;41:897–902. doi: 10.1136/thx.41.12.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Summer W., Elston R., Tharpe L. Aerosol bronchodilator delivery methods: Relative impact on pulmonary function and cost of respiratory care. Arch Intern Med. 1989;149:618–623. doi: 10.1001/archinte.149.3.618. [DOI] [PubMed] [Google Scholar]
- 121.Sunyer J., Saez M., Murillo C. Air pollution and emergency room admissions for chronic obstructive pulmonary disease: A 5-year study. Am J Epidemiol. 1993;137:701–705. doi: 10.1093/oxfordjournals.aje.a116730. [DOI] [PubMed] [Google Scholar]
- 122.Tashkin D.P., Ashutosh K., Blacker E.R. Comparison of anticholinergic bronchodilator ipratropium bromide with metaproterenol in chronic obstructive pulmonary disease: A 90-day multicentre study. Am J Med. 1986;81:59–68. doi: 10.1016/0002-9343(86)90468-7. [DOI] [PubMed] [Google Scholar]
- 123.Taylor D.C., Clancy R.L., Cripps A.W. An alteration in the host-parasite relationship in subjects with chronic bronchitis prone to recurrent episodes of acute bronchitis. Immunol Cell Biol. 1994;72:143–151. doi: 10.1038/icb.1994.22. [DOI] [PubMed] [Google Scholar]
- 124.The Smoking Cessation Clinical Practice Guideline Panel and Staff The Agency for Health Care Policy and Research Smoking Cessation clinical practice guideline. JAMA. 1996;275:1270–1280. [PubMed] [Google Scholar]
- 125.Thompson W.H., Nielson C.P., Carvalho P. Controlled trial of oral prednisone in outpatients with acute COPD exacerbation. Am J Respir Crit Care Med. 1996;154:407–412. doi: 10.1164/ajrccm.154.2.8756814. [DOI] [PubMed] [Google Scholar]
- 126.Traver G.A., Cline M.G., Burrows B. Predictors of mortality in chronic obstructive pulmonary disease: A 15-year follow-up study. Am Rev Respir Dis. 1979;119:895–902. doi: 10.1164/arrd.1979.119.6.895. [DOI] [PubMed] [Google Scholar]
- 127.Turner M.O., Gafni A., Swan D. A review and economic evaluation of bronchodilator delivery methods in hospitalized patients. Arch Intern Med. 1996;156:2113–2118. [PubMed] [Google Scholar]
- 128.Turner M.O., Patel A., Ginsburg S. Bronchodilator delivery in acute airflow obstruction—a meta-analysis. Arch Intern Med. 1997;157:1736–1744. [PubMed] [Google Scholar]
- 129.Vassallo R., Lipsky J.J. Theophylline: Recent advances in the understanding of its mode of action and uses in clinical practice. Mayo Clin Proc. 1998;73:346–354. doi: 10.1016/S0025-6196(11)63701-4. [DOI] [PubMed] [Google Scholar]
- 130.Venge P., Pedersen B., Hakansson L. Subcutaneous administration of hyaluronan reduces the number of infectious exacerbations in patients with chronic bronchitis. Am J Respir Crit Care Med. 1996;153:312–316. doi: 10.1164/ajrccm.153.1.8542136. [DOI] [PubMed] [Google Scholar]
- 131.Weinberger S.E., Schwartzstein R.M., Weiss J.W. Hypercapnia. N Engl J Med. 1989;321:1223–1231. doi: 10.1056/NEJM198911023211804. [DOI] [PubMed] [Google Scholar]
- 132.Weiss S.M., Hudson L.D. Outcome from respiratory failure. Crit Care Clin. 1994;10:197–215. [PubMed] [Google Scholar]
- 133.Wilson D.O., Rogers M., Openbrier D. Nutritional aspects of chronic obstructive pulmonary disease. Clin Chest Med. 1986;7:643–656. [PubMed] [Google Scholar]
- 134.Wilson R. Outcome predictors in bronchitis. Chest. 1995;108(suppl):53–57. doi: 10.1378/chest.108.2_supplement.53s. [DOI] [PubMed] [Google Scholar]
- 135.Wilson R., Dowling R.B., Jackson A.D. The biology of bacterial colonization and invasion of the respiratory mucosa. Eur Resp, J. 1996;9:1523–1530. doi: 10.1183/09031936.96.09071523. [DOI] [PubMed] [Google Scholar]
- 136.Wood-Baker R., Walters E.H. The Cochrane Library, Issue 4. Update Software; Oxford: 1998. The role of corticosteroids in acute exacerbations of chronic obstructive pulmonary disease (Cochrane review) [Google Scholar]
- 137.Wrenn K., Slovis C.M., Murphy F. Aminophylline therapy for acute bronchospastic disease in the emergency room. Ann Intern Med. 1991;115:241–247. doi: 10.7326/0003-4819-115-4-241. [DOI] [PubMed] [Google Scholar]
- 138.Wynder E.L., Kaufman P.L., Lesser R.L. A short-term follow-up study on ex-cigarette smokers: With special emphasis on persistent cough and weight gain. Am Rev Respir Dis. 1967;96:645–655. doi: 10.1164/arrd.1967.96.4.645. [DOI] [PubMed] [Google Scholar]


