Abstract
Respiratory tract infections caused by viruses, 24, 70 chlamydia, 18, 19, 43, 55, 116 and mycoplasma61 have been implicated in the pathogenesis of asthma. Viruses have been demonstrated to be associated with asthma epidemiologically in at least two ways (Fig. 1). First, during infancy, certain viruses have been implicated as potentially being responsible for the inception of the asthmatic phenotype. Second, in patients, particularly children, with established asthma, viral upper respiratory tract infections play a significant role in producing acute exacerbations of airway obstruction that may result in frequent outpatient visits or hospitalizations. 24, 55, 56, 57 This article reviews these two areas by focusing first on mechanisms by which virus infections may lead to the development of asthma in infants and children and, second, on mechanisms by which virus infections may produce acute asthmatic symptoms in patients who already have established disease.
VIRAL INFECTIONS AND THE INCEPTION OF ASTHMA
Infections with respiratory syncytial virus (RSV) or parainfluenza virus (PIV) have received much attention because of their predilection to produce a pattern of symptoms termed bronchiolitis that parallels many of the features of childhood and adult asthma.67 Respiratory syncytial virus causes about 70% of these episodes and it is estimated that, by age 1 year, 50% to 65% of children will have been infected with this virus77 and 40% of these infections involve the lower respiratory tract.92 By age 2, nearly all children will have been infected with RSV at least once. Children aged 3 to 6 months are most prone to develop lower respiratory tract symptoms, suggesting that a developmental component (e.g., lung or immunologic maturation) may be involved as well. 77, 83
Figure 1.
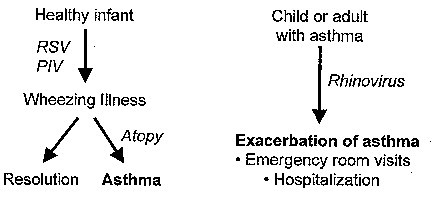
Mechanisms by which viruses may influence either the inception of asthma or exacerbations of the underlying disease process once it has been established. RSV = respiratory syncytial virus; PIV = parainfluenza virus.
The relationship between RSV infections during the first few years of life and the subsequent development of the asthmatic phenotype has been the subject of much interest as well as controversy. Variations in reporting longitudinal outcomes (e.g., recurrent wheezing, measurements of airway hyperresponsiveness, diagnosis of asthma) appear to be influenced mostly by the criteria used to define “bronchiolitis.” These criteria include the type of virus producing the symptoms (in addition to RSV, viruses that may contribute to the development of bronchiolitis in this age group could be PIV, coronavirus, influenzavirus, and rhinovirus33); the age at the time of infection; the nature and severity of symptoms required for inclusion; and, finally, the characteristics of both the study population (community versus hospital-based) and the study design (retrospective versus prospective). A number of long-term prospective studies of children admitted to a hospital with documented RSV-induced bronchiolitis have shown that about 75% experience wheezing in the first 2 years after the initial illness, more than 50% still wheeze 3 years later, and approximately 40% continue to wheeze after 5 years. 42, 49, 73, 91, 117, 121
Additional insight into these areas recently was provided by the results of an 11-year prospective study involving 880 children who were enrolled at birth, followed for the development of lower respiratory tract illnesses (LRIs) in the first 3 years of life, and then evaluated for the presence or absence of physician-diagnosed asthma or a history of current wheezing at ages 6 and 11 years.14 Most importantly, lung function was evaluated in the first few months of life in a subset of these children prior to the development of a documented LRI. During the first 3 years of life, 7.4% had pneumonia documented radiographically and 44.7% had a significant LRI without pneumonia. Respiratory syncytial virus and PIV were identified in 36.4% and 7.3%, respectively, in the subjects with pneumonia, and in 35.6% and 15.2%, respectively, of the subjects with a LRI. At age 6, physician-diagnosed asthma was present in 13.6% (OR = 3.3), 10.2% (OR = 2.4), and 4.6% of the subjects with pneumonia, LRI, and no LRI, respectively. By age 11, these values increased to 25.9% (OR = 2.8), 16.1% (OR = 1.6), and 11%, respectively. Mean maximum volume at functional residual capacity values before any LRI were lower in children with pneumonia and with LRIs than in children with no LRIs. These values continued to be lower at age 6 and by age 11, when forced expiratory volume in 1 second (FEV1) and FEF25–75 were recorded, similar group relationships persisted. Interestingly, despite the persistence of lowered baseline lung function in both the pneumonia and LRI groups, many of these deficits were markedly (but not completely) reduced following administration of albuterol.
In a second report, further follow-up of this large cohort of children demonstrated that the risk for both frequent (more than three episodes of wheezing per year) and infrequent (three episodes of wheezing per year) wheezing in relation to RSV lower respiratory illnesses decreased markedly with age and became nonsignificant by age 13.104 These data suggest that, although RSV infections contribute substantially to the expression of the asthmatic phenotype, other factors (e.g., genetic, environmental, developmental) appear to contribute as well, either in terms of its initial expression or the modification of the phenotype over time.
CONTRIBUTION OF ATOPY
In addition to premorbid lung function, the influence of atopy on the development of the asthmatic phenotype in relationship to viral infections has also been evaluated. Interactions between these two factors appear to be bidirectional and dynamic, in that the atopic state can influence the lower airway response to viral infections, 8, 71 viral infections can influence the development of allergen sensitization, 28, 29, 99 and interactions can occur when individuals are exposed simultaneously to both allergens and viruses. 12, 68, 100
Atopy can be defined as the genetic predisposition to the preferential development of an immunoglobulin (Ig)E antibody response to a variety of environmental allergens. As stated previously, atopy has been considered to be a risk factor for the development of childhood asthma and its influence on the pattern of responses following viral infections has been of interest to many investigative groups. It has also been suggested that atopy could be a significant predisposing factor for the development of acute bronchiolitis during RSV epidemics.64 Although some have found that children most likely to have persistent wheezing were those born to atopic parents, 64, 91, 121 others have not. 14, 73, 84 Some have found that personal atopy is not more prevalent in symptomatic children after bronchiolitis14, 73; others have found that documented RSV bronchiolitis significantly increases a child's chances (32% versus 9% in controls) of subsequently developing IgE antibody99 or lymphocyte proliferative responses75 to both food and aeroallergens.
RESPIRATORY SYNCYTIAL VIRUS AND THE IMMUNE RESPONSE
Respiratory syncytial virus infections may interact with immunoinflammatory mechanisms involved in immediate hypersensitivity responses in a number of ways.18 First, it has been suggested that viruses capable of infecting lower airway epithelium may lead to enhanced absorption of aeroallergens across the airway wall, predisposing to subsequent sensitization. 27, 94 Second, RSV-specific IgE antibody formation may lead to mast-cell–mediator release within the airway, resulting in the development of bronchospasm and the ingress of eosinophils. 32, 60, 85, 115, 119, 120 Third, airway resident and inflammatory cell generation of various cytokines (tumor necrosis factor [TNF], interleukin [IL]-1, IL-6, IL-8), 4, 81, 106, 109 chemokines (MIP-1-, RANTES, MCP-1), 47, 76 leukotrienes, 113 and adhesion molecules (intercellular adhesion molecule)81 may further upregulate the ongoing inflammatory response. Finally, similar to various allergenic proteins, 17 the processing of RSV antigens and their subsequent presentation to lymphocyte subpopulations may provide a unique mechanism of interaction to promote a T-helper 2 (Th2)-like response in a predisposed host.
Respiratory syncytial virus belongs to the family Paramyxoviridae, the genera Pneumovirus, and can be differentiated into two serologic subgroups, A and B. 44, 77 It has 10 genes, with 12 potential gene products. The G (attachment) and F (fusion) proteins are the major surface glycoproteins against which neutralizing antibody is directed. Interestingly, in both murine2 and human51 in vitro experiments, it has been noted that the G protein elicits a predominant Th2 response, whereas the F protein produces a predominant Th1 response. In mice, to test the activities of T cells recognizing individual RSV proteins in vivo, virus-specific T-cell lines have been produced using recombinant vaccinia viruses that express either the G or F proteins. Following passive transfer of these cell lines to naive recipients and subsequent intranasal inoculation with RSV, mice receiving G-specific cells have more severe illnesses, characterized by lung hemorrhage, pulmonary neutrophil recruitment, and intense pulmonary eosinophilia.1 These experiments are of interest based on the adverse clinical response noted in many infants who received a formalin-inactivated RSV vaccine and subsequently became infected with RSV.77
These intriguing observations regarding RSV and its influence on Th1/Th2 responses have recently been expanded. Roman et al evaluated 15 hospitalized infants (1–15 months) with an acute lower respiratory tract infection caused by RSV. Compared with control infants, peripheral blood cells from infected children had suppressed IFN-γ production ex vivo and, although IL-4 production was also decreased, the IL-4/IFN-γ ratio was significantly increased. Renzi et al88 prospectively followed 26 infants hospitalized with bronchiolitis by obtaining blood samples at the time of illness and 5 months later, and found that immune responses during the acute infection correlated with long-term pulmonary outcomes. Blood lymphocytes, obtained during the time of bronchiolitis, produced less IFN-γ ex vivo in response to IL-2 and more IL-4 in response to D. farinae antigen in children who went on to develop a pattern of recurrent wheezing.88 Finally, lower IFN-γ production at the time of bronchiolitis has been demonstrated to be an indicator of reduced pulmonary function and increased responsiveness to histamine 5 months after bronchiolitis, and was related to the development of asthma after bronchiolitis in infants.87 In contrast, other groups have noted increased levels of IFN-γ respiratory tract secretions during RSV illnesses in infants and children with bronchiolitis and recurrent wheezing compared with those with upper respiratory tract symptoms only.113 Unfortunately, in all of the studies reported thus far, the pattern of cytokine response these infants had prior to infection was not evaluated, begging the question as to which of the observed results may be cause and which effect.
ANIMAL MODELS
To more comprehensively evaluate the relationships among virus infection, atopy (cytokine dysregulation of Th1/Th2 imbalance), and immune system or lung developmental components, a rat model of virus-induced airway dysfunction has been studied extensively.111 In this model, infection with PIV type 1 during a critical developmental time period (when the animals are weaning [3–4 weeks of age] as opposed to when they are neonates [4–5 days] or adults) produces chronic (8–12 weeks following infection), episodic, reversible airway inflammation and remodeling with associated alterations in airway physiology (increased resistance and methacholine responsiveness) that resemble human asthma in high (brown Norway strain) but not low (F344 strain) IgE-antibody producing rats.62 The temporal progression of this asthma-like syndrome is associated with a Th1/Th2 imbalance within the lung, and its development can be significantly attenuated by the exogenous administration of IFN-8 just prior to and during the viral infection in the brown Norway responder strain.102 This model further supports the concept of both genetic (atopy; cytokine dysregulation or imbalance) and environmental factors (virus infection) being important in the inception of the asthmatic phenotype, as well as a developmental component contributing.
EFFECT OF VIRAL INFECTIONS IN PATIENTS WITH ASTHMA
Respiratory viruses are common causes of asthma exacerbations in asthmatic subjects of different age groups. 57, 74, 86 Serology or culture detection methods of viruses initially indicated an association during asthma exacerbations82 despite the fact that these detection methods are relatively insensitive for viruses such as rhinovirus (RV). The use of reverse transcription polymerase chain reaction (RT-PCR) assays that are more sensitive for detection of RV have confirmed and expanded these initial observations.58 Indeed, Johnston et al57 found that 80% to 85% of school-aged children with acute wheezing episodes tested positive for a virus using RT-PCR and other standard virologic techniques. The virus most often detected was RV. Seasonal patterns of upper respiratory virus infections correlate closely with hospital admissions for asthma, particularly in pediatric age groups.55 These studies indicate that RV infections are the most common cause of asthma exacerbations in children, especially during spring and fall. Similar studies, performed in adults, 74 found that about half of asthma exacerbations were associated with RV infection.
As discussed previously, in infancy, atopy may define a susceptibility of the host to wheezing with respiratory infections. Duff et al, 22 for example, studied children who presented to an emergency department with wheezing. Children over 2 years of age were more likely to have respiratory allergies or a confirmed respiratory viral infection compared with children with no wheezing. Children with the highest risk for wheezing were those who had respiratory allergies and respiratory viral infection, implying that respiratory viral infections and respiratory allergies may have synergistic effects on lower airway physiology and enhance the likelihood of wheezing with respiratory infection. In children less than 2 years of age, wheezing was also noted, but risk factors for wheezing were quite different. These infants were not allergic, had RSV as the major viral isolate, and had passive tobacco smoke exposure as a major risk factor for wheezing.
MECHANISMS OF VIRAL-INDUCED AIRWAY OBSTRUCTION AND ASTHMA
Development of Variable Airway Obstruction
Available epidemiologic data in children and adults have shown that episodic drops in peak flow measurements are associated with RV infections. This was found to correlate with an increase in asthma symptoms and nonspecific airway hyperresponsiveness following experimentally infecting asthmatic subjects with RV. 15, 41 Further studies by Grünberg et al40 demonstrated that experimental RV16 infection leads to a transient drop in daily home recordings of FEV1 in subjects with asthma. This variable airway obstruction correlated significantly with cold symptoms, asthma symptoms, and the increase in airway hyperresponsiveness to histamine. Such daily variability in FEV1 reflects the inflammatory changes within the airway wall, which can be induced by the natural RV infection.
Increased Bronchial Hyperresponsiveness
Increased bronchial responsiveness has been found in normal and asthmatic subjects following infections with RV68 and influenza A. 65, 66, 72 In a study by Cheung et al15 14 subjects with mild asthma were inoculated with RV16 or placebo. The maximal contractile response to inhaled methacholine was significantly greater during the RV16 infection and remained elevated for up to 15 days after the acute infection. This study indicates that an upper respiratory viral infection can enhance the reactivity of the lower airway and the magnitude of bronchonstriction changes, which can persist for weeks after the acute infection.
Respiratory viral infections' effect on lower airway responses are also influenced by host factors. In particular, allergic subjects experience greater changes in airway responsiveness after viral infection than nonallergic control subjects. 9, 34 Furthermore, subjects with lower FEV1 values tend to have greater changes in airway responsiveness during viral infection.34 These studies suggest that effects of pre-existing conditions such as allergy and intrinsic lower airway function on caliber are likely to contribute to airway hyperresponsiveness during respiratory viral infection.
Neural Control of the Airways
Potential mechanisms through which viral infections could potentially cause bronchoconstriction and increased airway responsiveness include enhancing parasympathetic bronchoconstrictive responses, stimulation of airway sensory nerves, and interference with the bronchodilatory functions of the nonadrenergic, noncholinergic neurons (Table 1) . Because of difficulties in assessing dysfunction of pulmonary neural regulation in humans, most data that support these proposed mechanisms were derived in animal models of acute respiratory viral infection. Further definition of these pathways in humans will depend upon the development of new experimental techniques or inhibition of specific neural pathways.
Table 1.
NEURAL MECHANISMS IMPLICATED IN VIRUS-INDUCED AIRWAY DYSFUNCTION
| Effect of Virus | Potential Mechanisms | References |
|---|---|---|
| Heightened | • Increased efferent activity of efferent cholinergic nerves | Buckner et al11 |
| parasympathetic | • Viral neuraminidase | Fryer et al30, 31 |
| responses | • Eosinophil cationic protein-induced M2 dysfunction | Jacoby et al52 |
| • M2-independent mechanisms | Sorkness et al101 | |
| Bronchoconstriction | Enhanced contractile responses to neurokinins | Jacoby et al53 |
| secondary to sensory | Ladenius et al63 | |
| C-fibers | Roberts et al89 | |
| Saban et al93 | ||
| Inhibition of nonadrenergic–noncholinergic neurons | Reduced production of nitric oxide | Colasurdo et al16 |
Structural Effects on the Small Airways
Changes in small airways structure and function may also contribute significantly to the severity of hyperinflation and gas exchange abnormalities noted in acute asthma exacerbations. The maximal airway contractile response to methacholine in mild asthmatic subjects is increased during a cold, which is probably secondary to excessive airway narrowing attributable to airway wall thickening, airway parenchymal uncoupling, or abnormalities in smooth muscle contraction.15 Significant changes in airway morphology are noticed in animals with acute viral respiratory illness that leads to marked bronchiolar narrowing and plugging. These changes include bronchiolar airway edema and cell infiltration, epithelial hyperplasia, and folding and sloughing of airway epithelial surfaces. In addition, rats with mild increases in pulmonary resistance and methacholine sensitivity during acute viral respiratory illness have evidence of air trapping and ventilation–perfusion mismatches.101 These latter findings indicate that viruses can induce significant changes in the peripheral airways that have significant functional outcomes in the absence of marked changes in measurements of airway obstruction and hyperresponsiveness.
Effects of Respiratory Viruses on Airway Inflammation
Respiratory viruses can cause inflammation and injury to healthy airways and can worsen injury in airways that are already inflamed, as demonstrable in asthma. Respiratory viruses can induce an inflammatory process by direct cytopathic effects on the airway epithelium (e.g., RSV bronchiolitis) and can induce an immune response to stop viral replication and eradicate the virus. The immune response to viral infection may be a double-edged sword, however, as virus-induced inflammation can also contribute to airway obstruction and respiratory symptoms. Indeed, although many common cold viruses (e.g., RV) do not produce significant cytopathic effects, possibly because few cells are infected, the immunoinflammatory response to the virus is probably the major cause of respiratory symptoms. In this section, the association between virus-induced immune responses and respiratory symptoms is explored.
Role of Epithelial Cells
Respiratory viruses replicate primarily in airway epithelial cells. In addition to serving as host cells, it is now well documented that epithelial cells also initiate the immune response to infections through the secretion of cytokines and chemokines. In vitro studies of epithelial cells or cell lines have demonstrated that respiratory viruses such as RV, RSV, and parainfluenzavirus can induce the secretion of many different proinflammatory cytokines (IL-1, TNF-α, GM-CSF, IL-6, IL-11) and chemokines (RANTES, IL-8, MIP-1α). 10, 20, 23, 96, 98, 105 Epithelial-derived chemokines are likely to be an important signal in initiating antiviral responses through the recruitment of leukocytes to the airway. In support of this concept, IL-8, a potent neutrophil chemoattractant, is found in high levels in nasal secretions of children with virus-induced asthma, and levels of IL-8 correlate with the number of airway neutrophils and neutrophil myeloperoxidase levels (suggesting neutrophil activation).108 There is also evidence, however, that enhanced airway inflammation caused by chemokine secretion may also disturb normal airway physiology. Chemokine levels in nasal secretions correlate closely with cold symptoms, 110 for example, and IL-8 levels correlate with virus-induced changes in airway responsiveness.41 Levels of epithelial-derived cytokines such as IL-6 and IL-11 also correlate with respiratory symptoms, 23 and animal studies indicate that overexpression of IL-11 can cause bronchial hyperresponsiveness. 23, 107
In addition to stimulating cytokine production, RV can upregulate epithelial cell surface expression of intercellular adhesion molecule-1, 79 which, in addition to facilitating cell–cell adhesion, is the receptor for the major group of RV. 38, 103 This enhanced expression of adhesion proteins may contribute to the persistence and severity of inflammation in asthmatic subjects and, possibly, the greater susceptibility of asthmatic children to colds compared with nonasthmatic children.
Mechanisms for the activation of cytokine genes in epithelial cells and adhesion molecules are under investigation. It is known that nuclear factor-κ B activation is important in virus-induced transcriptional regulation of IL-650 and, possibly, for the synthesis of a variety of inflammatory cytokines.7 In addition, nitric oxide may regulate virus-induced chemokine production through a posttranscriptional mechanism and by inhibiting viral replication, 95 although a clinical study did not find a relationship between IL-8 and nitrate levels in nasal secretions.59
Effect on Granulocytes
Granulocyte recruitment and activation seem to have an important role in the pathogenesis of virus-induced asthma exacerbations. Grünberg et al, 41 for example, experimentally inoculated 35 atopic asthma subjects with either RV16 or placebo and found that neutrophil counts in the peripheral blood correlated with the cold and asthma symptom scores and cold-induced changes in airway hyperresponsiveness. In addition, eosinophil granular proteins and leukotriene C4 have been detected in the nasal secretions of infants and children with virus-induced wheezing illnesses. 32, 86, 97, 116 Increased concentrations of sputum eosinophil cationic protein found during the acute phase of RV infection correlated with increases in airway responsiveness in a group of adults with asthma after experimental inoculation with RV16.39 In vitro experiments indicate that RV does not activate eosinophils directly45; it is more likely that inflammatory mediators and cytokines, secreted by virus-activated cells in the lung, contribute to eosinophil activation. Finally, guinea pigs infected with PIV develop airway eosinophils and airway hyperresponsiveness25 and this outcome is blocked if the guinea pigs are pretreated with IL-5–neutralizing antibody.112
Role of Mononuclear Cells
Most respiratory viruses replicate quickly and, within a few days of inoculation, the quantity of viruses and viral proteins is sufficient to activate mononuclear cells in the airway. In vitro infection of human monocytes with respiratory viruses, for example, leads to a potent proinflammatory cytokine response by release of IL-8, IL-1, and TNF-α. 35, 54, 90 Interleukin-1 and TNF-α can increase cell recruitment into the airway by enhancing adhesion molecule expression on endothelial cells. In addition, TNF-α has been associated with wheezing illnesses in infancy6 and the development of late-phase allergic reaction and asthma. 3, 37 Monocytes and macrophages also produce interferon (INF), and its appearance in nasal secretions coincides with the onset of the recovery process. In addition to cytokine production, macrophages incubated with RSV or PIV produce lipid mediators such as prostaglandin E2, platelet-activating factor, and thromboxane B2 48, 78, 114 that can augment airway inflammation.
Lymphocytes, including natural killer cells, CD8+ cytotoxic T cells, and CD4+ T cells, are involved in limiting viral replication and viral clearance. To test the possibility that variations in lymphocyte responses might account for variability in the ability to clear viral infections, Parry et al80 measured in vitro lymphocyte responses in a group of allergic subjects who were then inoculated with RV 16. Vigorous virus-induced responses (lymphocyte proliferation or IFN-γ secretion) before inoculation correlated with reduced viral shedding after inoculation. These results suggest that factors related to the host cellular response help determine the degree of viral replication during respiratory viral infections. Further characterization of these host factors may lead to new therapeutic strategies for respiratory infections, a goal that is particularly important for people with asthma.
Several studies have shown that viral infections activate a wide range of T cells. Evidence for this comes from experiments in mice, in which most of the T cells found in the lung after an acute viral infection are not virus-specific, 21 and in vitro studies, in which 25% to 50% of human peripheral blood T cells express the early activation marker CD69 after 24 hours in culture with RV.36 RANTES, induced by respiratory viruses, at high concentrations can also induce antigen-independent T-cell activation.5 These studies suggest that respiratory viruses can induce early, nonspecific T-cell activation and recruitment that could significantly increase the intensity of airway inflammation, resulting in airway dysfunction and respiratory symptoms.
This hypothesis is supported by studies of volunteers infected with rhinovirus. Respiratory virus infections usually cause peripheral lymphopenia and increased numbers of lymphocytes in the upper and lower airways, for example. The degree of peripheral blood lymphopenia and lymphocytic infiltration of the airway epithelium has been correlated with the increases in airway responsiveness. 15, 26
Interactions Between Viral Infections and Responses to Allergen
Although viral infections cause similar upper respiratory symptoms in allergic and nonallergic individuals, 46, 100 there is evidence of interactions between virus- and allergen-induced responses in the lower airway. Lemanske and colleagues, 69 for example, identified 10 patients with allergic rhinitis and experimentally infected them with RV16. The viral infection increased airway reactivity to both inhaled allergen and histamine, and also increased the frequency of a late allergic reaction to inhaled antigens. Moreover, Calhoun and colleagues13 used bronchoscopy to study the inflammatory response to allergen in individual lung segments before, during, and 1 month after RV16 infection. RV infection enhanced the immediate antigen-induced release of histamine, and also increased eosinophil recruitment of eosinophils to the lung.
SUMMARY
Respiratory infections can have dual effects related to asthma. First, there is increasing evidence that severe infections with RSV and PIV in infancy can alter lung development and physiology to increase the risks of subsequent wheezing and asthma. Second, infections with common cold viruses and influenza commonly precipitate wheezing symptoms in children and adults who already have established asthma, and RV appears to be the most important virus in producing exacerbations of the disease. The principal mechanisms by which this occurs appears to be viral replication in epithelial cells, triggering a cascade of inflammation involving granulocytes, macrophages, T cells, and secreted cytokines and mediators. The inflammatory process, although essential to clear the infection, augments pre-existing airway inflammation in asthma, leading to increased airway obstruction and lower respiratory tract symptoms. Greater understanding of virus-induced changes in inflammation and corresponding changes in airway physiology may lead to new therapeutic approaches to the treatment and prevention of virus-induced airway dysfunction.
Footnotes
Address reprint requests to Robert F. Lemanske, Jr, MD, Department of Pediatrics, University of Wisconsin, Children's Hospital, 600 Highland Avenue H4/432, Madison, WI 53792
Supported by NIH Grants Al34891, HL56396, and 1RO1HL61879.
References
- 1.Alwan W.H., Kozlowska W.J., Openshaw P.J.M. Distinct types of lung disease caused by functional subsets of antiviral T cells. J Exp Med. 1994;179:81. doi: 10.1084/jem.179.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alwan W.H., Record F.M., Openshaw P.J.M. Phenotypic and functional characterization of T-cell lines specific for individual respiratory syncytial virus proteins. J Immunol. 1993;150:5211. [PubMed] [Google Scholar]
- 3.Anticevich S.Z., Hughes J.M., Black J.L. Induction of human airway hyperresponsiveness by tumour necrosis factor-α. Eur J Pharmacol. 1995;284:221. doi: 10.1016/0014-2999(95)00463-u. [DOI] [PubMed] [Google Scholar]
- 4.Arnold R., König B., Galatti H. Cytokine (IL-8, IL-6, TNF-α) and soluble TNF receptor-I release from human peripheral blood mononuclear cells after respiratory syncytial virus infection. Immunology. 1995;85:364. [PMC free article] [PubMed] [Google Scholar]
- 5.Bacon K.B., Premack B.A., Gardner P. Activation of dual T-cell signaling pathways by the chemokine RANTES. Science. 1995;269:1727. doi: 10.1126/science.7569902. [DOI] [PubMed] [Google Scholar]
- 6.Balfour-Lynn I.M., Valman H.B., Wellings R. Tumour necrosis factor-α and leukotriene-E4 production in wheezy infants. Clin Exp Allergy. 1994;24:121. doi: 10.1111/j.1365-2222.1994.tb00207.x. [DOI] [PubMed] [Google Scholar]
- 7.Baraniuk J.N., Lundgren J.D., Mizoguchi H. Bradykinin and respiratory mucous membranes. Analysis of bradykinin binding-site distribution and secretory responses in vitro and in vivo. Am Rev Respir Dis. 1990;141:706. doi: 10.1164/ajrccm/141.3.706. [DOI] [PubMed] [Google Scholar]
- 8.Bardin P.G., Fraenkel D.J., Sanderson G. Amplified rhinovirus colds in atopic subjects. Clin Exp Allergy. 1994;24:457. doi: 10.1111/j.1365-2222.1994.tb00934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bardin P.G., Sanderson G., Robinson B.S. Experimental rhinovirus infection in volunteers. Eur Respir J. 1996;9:2250. doi: 10.1183/09031936.96.09112250. [DOI] [PubMed] [Google Scholar]
- 10.Becker S., Koren H.S., Henke D.C. Interleukin-8 expression in normal nasal epithelium and its modulation by infection with respiratory syncytial virus and cytokines tumor necrosis factor, interleukin-1 and interleukin-6. Am J Respir Cell Mol Biol. 1993;8:20. doi: 10.1165/ajrcmb/8.1.20. [DOI] [PubMed] [Google Scholar]
- 11.Buckner C.K., Songsiridej V., Dick E.C. In vivo and in vitro studies on the use of the guinea pig as a model for virus-provoked airway hyperreactivity. Am Rev Respir Dis. 1985;132:305. doi: 10.1164/arrd.1985.132.2.305. [DOI] [PubMed] [Google Scholar]
- 12.Calhoun W.J., Dick E.C., Schwartz L.B. A common cold virus, rhinovirus 16, potentiates airway inflammation after segmental antigen bronchoprovocation in allergic subjects. J Clin Invest. 1994;94:2200. doi: 10.1172/JCI117581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calhoun W.J., Dick E.C., Schwartz L.B. A common cold virus, rhinovirus 16, potentiates airway inflammation after segmental antigen bronchoprovocation in allergic subjects. J Clin Invest. 1994;94:2200. doi: 10.1172/JCI117581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castro-Rodriguez J.A., Holberg C.J., Wright A.L. Association of radiologically ascertained pneumonia before age 3 years with asthma-like symptoms and pulmonary function during childhood. A prospective study. Am J Respir Crit Care Med. 1999;159:1891. doi: 10.1164/ajrccm.159.6.9811035. [DOI] [PubMed] [Google Scholar]
- 15.Cheung D., Dick E.C., Timmers M.C. Rhinovirus inhalation causes long-lasting excessive airway narrowing in response to methacholine in asthmatic subjects in vivo. Am J Respir Crit Care Med. 1995;152:1490. doi: 10.1164/ajrccm.152.5.7582282. [DOI] [PubMed] [Google Scholar]
- 16.Colasurdo G.N., Hemming V.G., Prince G.A. Human respiratory syncytial virus affects nonadrenergic noncholinergic inhibition in cotton rat airways. Am J Physiol. 1998;12:L1006–L1011. doi: 10.1152/ajplung.1995.268.6.L1006. [DOI] [PubMed] [Google Scholar]
- 17.Comoy E.E., Pestel J., Duez C. The house-dust-mite allergen, Dermatophagoides pteronyssinus, promotes type 2 responses by modulating the balance between IL-4 and IFN-γ. J Immunol. 1998;160:2456. [PubMed] [Google Scholar]
- 18.Cook P.J., Davies P., Tunnicliffe W. Chlamydia pneumoniae and asthma. Thorax. 1998;53:254. doi: 10.1136/thx.53.4.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cunningham A.F., Johnston S.L., Julious S.A. Chronic Chlamydia pneumoniae infection and asthma exacerbations in children. Eur Respir J. 1998;11:345. doi: 10.1183/09031936.98.11020345. [DOI] [PubMed] [Google Scholar]
- 20.DiCosmo B.F., Geba G.P., Picarella D. Airway epithelial cell expression of interleukin-6 in transgenic mice. Uncoupling of airway inflammation and bronchial hyperreactivity. J Clin Invest. 1994;94:2028. doi: 10.1172/JCI117556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doherty P.C., Hou S., Tripp R.A. CD8+ T-cell memory to viruses. Curr Opin Immunol. 1994;6:545. doi: 10.1016/0952-7915(94)90139-2. [DOI] [PubMed] [Google Scholar]
- 22.Duff A.L., Pomeranz E.S., Gelber L.E. Risk factors for acute wheezing in infants and children: Viruses, passive smoke, and IgE antibodies to inhalant allergens. Pediatr. 1993;92:535. [PubMed] [Google Scholar]
- 23.Einarsson O., Geba G.P., Zhu Z. Interleukin-11: Stimulation in vivo and in vitro by respiratory viruses and induction of airways hyperresponsiveness. J Clin Invest. 1996;4:915. doi: 10.1172/JCI118514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Folkerts G., Busse W.W., Nijkamp F.P. Virus-induced airway hyperresponsiveness and asthma. Am J Respir Crit Care Med. 1998;157:1708. doi: 10.1164/ajrccm.157.6.9707163. [DOI] [PubMed] [Google Scholar]
- 25.Folkerts G., Esch B.V., Janssen M. Virus-induced airway hyperresponsiveness in guinea pigs in vivo: Study of broncho-alveolar cell number and activity. Eur J Pharmacol. 1992;228:219. doi: 10.1016/0926-6917(92)90033-9. [DOI] [PubMed] [Google Scholar]
- 26.Fraenkel D.J., Bardin P.G., Sanderson G. Lower airways inflammation during rhinovirus colds in normal and asthmatic subjects. Am J Respir Crit Care Med. 1995;151:879. doi: 10.1164/ajrccm/151.3_Pt_1.879. [DOI] [PubMed] [Google Scholar]
- 27.Freihorst J., Piedra P.A., Okamoto Y. Effect of respiratory syncytial virus infection on the uptake of and immune response to other inhaled antigens. Proc Soc Exp Biol Med. 1988;188:191. doi: 10.3181/00379727-188-42727. [DOI] [PubMed] [Google Scholar]
- 28.Frick O.L. Effect of respiratory and other virus infections on IgE immunoregulation. J Allergy Clin Immunol. 1986;78:1013. doi: 10.1016/0091-6749(86)90295-2. [DOI] [PubMed] [Google Scholar]
- 29.Frick O.L., German D.F., Mills J. Development of allergen in children. I. Association with virus infections. J Allergy Clin Immunol. 1979;63:228. doi: 10.1016/0091-6749(79)90106-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fryer A.D., el Fakahany E.E., Jacoby D.B. Parainfluenza virus type 1 reduces the affinity of agonists for muscarinic receptors in guinea-pig lung and heart. Eur J Pharmacol. 1990;181:51. doi: 10.1016/0014-2999(90)90244-z. [DOI] [PubMed] [Google Scholar]
- 31.Fryer A.D., Jacoby D.B. Parainfluenza virus infection damages inhibitory M2 muscarinic receptors on pulmonary parasympathetic nerves in the guinea pig. Br J Pharmacol. 1991;102:267. doi: 10.1111/j.1476-5381.1991.tb12164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garofalo R., Kimpen J.L.L., Welliver R.C. Eosinophil degranulation in the respiratory tract during naturally acquired respiratory syncytial virus infection. J Pediatr. 1992;120:28. doi: 10.1016/s0022-3476(05)80592-x. [DOI] [PubMed] [Google Scholar]
- 33.Gern J.E., Busse W.W. Role of T cells in virus-induced asthma. In: Liggett S.B., Meyers D.A., editors. Genetics of Asthma. Marcel Dekker; New York: 1996. p. 39. [Google Scholar]
- 34.Gern J.E., Calhoun W., Swenson C. Rhinovirus infection preferentially increases lower airway responsiveness in allergic subjects. Am J Respir Crit Care Med. 1997;155:1872. doi: 10.1164/ajrccm.155.6.9196088. [DOI] [PubMed] [Google Scholar]
- 35.Gern J.E., Dick E.C., Lee W.M. Rhinovirus enters but does not replicate inside monocytes and airway macrophages. J Immunol. 1996;156:621. [PubMed] [Google Scholar]
- 36.Gern J.E., Vrtis R., Kelly E.A.B. Rhinovirus produces nonspecific activation of lymphocytes through a monocyte-dependent mechanism. J Immunol. 1996;157:1605. [PubMed] [Google Scholar]
- 37.Gosset P., Tsicopoulos A., Wallaert B. Increased secretion of tumor necrosis factor α and interleukin-6 by alveolar macrophages consecutive to the development of the late asthmatic reaction. J Allergy Clin Immunol. 1991;88:561. doi: 10.1016/0091-6749(91)90149-i. [DOI] [PubMed] [Google Scholar]
- 38.Greve J.M., Davis G., Meyer A.M. The major human rhinovirus receptor is ICAM-1. Cell. 1989;56:839. doi: 10.1016/0092-8674(89)90688-0. [DOI] [PubMed] [Google Scholar]
- 39.Grünberg K., Kuijpers A.P., de Klerk E.P.A. Effects of experimental rhinovirus 16 infection on airway hyperresponsiveness to bradykinin in asthmatic subjects in vivo. Am J Respir Crit Care Med. 1997;155:833. doi: 10.1164/ajrccm.155.3.9117013. [DOI] [PubMed] [Google Scholar]
- 40.Grünberg K., Timmers M.C., de Klerk E.P.A. Experimental rhinovirus 16 infection causes variable airway obstruction in subjects with atopic asthma. Am J Respir Crit Care Med. 1999;160:1375. doi: 10.1164/ajrccm.160.4.9810083. [DOI] [PubMed] [Google Scholar]
- 41.Grünberg K., Timmers M.C., Smits H.H. Effect of experimental rhinovirus 16 colds on airway hyperresponsiveness to histamine and interleukin-8 in nasal lavage in asthmatic subjects in vivo. Clin Exp Allergy. 1997;27:36. doi: 10.1111/j.1365-2222.1997.tb00670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gurwitz D., Mindorrf C., Levison H. Increased incidence of bronchial reactivity in children with a history of bronchiolitis. J Pediatr. 1981;98:551. doi: 10.1016/s0022-3476(81)80758-5. [DOI] [PubMed] [Google Scholar]
- 43.Hahn D.L., Dodge R.W., Golubjatnikov R. Association of Chlamydia pneumoniae (strain TWAR) infection with wheezing, asthmatic bronchitis, and adult-onset asthma. JAMA. 1991;266:225. [PubMed] [Google Scholar]
- 44.Hall C.B. Respiratory syncytial virus: A continuing culprit and conundrum. J Pediatr. 1999;135:S2–S7. [PubMed] [Google Scholar]
- 45.Handzel Z.T., Busse W.W., Sedgwick J.B. Eosinophils bind rhinovirus and activate virus-specific T cells. J Immunol. 1998;160:1279. [PubMed] [Google Scholar]
- 46.Hansen G., Berry G., DeKruyff R.H. Allergen-specific Th1 cells fail to counterbalance Th2 cell-induced airway hyperreactivity but cause severe airway inflammation. J Clin Invest. 1999;103:175. doi: 10.1172/JCI5155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harrison A.M., Bonville C.A., Rosenberg H.F. Respiratory syncytial virus-induced chemokine expression in the lower airways. Eosinophil recruitment and degranulation. Am J Respir Crit Care Med. 1999;159:1918. doi: 10.1164/ajrccm.159.6.9805083. [DOI] [PubMed] [Google Scholar]
- 48.Henricks P.A.J., Van Esch B., Engels F. Effects of parainfluenza type 3 virus on guinea pig pulmonary alveolar macrophage functions in vitro. Inflammation. 1993;17:663. doi: 10.1007/BF00920472. [DOI] [PubMed] [Google Scholar]
- 49.Henry R.L., Hodges I.G.C., Milner A.D. Respiratory problems 2 years after acute bronchiolitis in infancy. Arch Dis Child. 1983;58:713. doi: 10.1136/adc.58.9.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ichinose M., Barnes P.J. Bradykinin-induced airway microvascular leakage and bronchoconstriction are mediated via a bradykinin β2 receptor. Am Rev Respir Dis. 1990;142:1104. doi: 10.1164/ajrccm/142.5.1104. [DOI] [PubMed] [Google Scholar]
- 51.Jackson M., Scott R. Different patterns of cytokine induction in cultures of respiratory syncytial (RS) virus-specific human Th cell lines following stimulation with RS virus and RS virus proteins. J Med Virol. 1996;49:161. doi: 10.1002/(SICI)1096-9071(199607)49:3<161::AID-JMV2>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 52.Jacoby D.B., Gleich G.J., Fryer A.D. Human eosinophil major basic protein is an endogenous allosteric antagonist at the inhibitory muscarinic M2 receptor. J Clin Invest. 1993;91:1314. doi: 10.1172/JCI116331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jacoby D.B., Tamaoki J., Borson D.B. Influenza infection causes airway hyperresponsiveness by decreasing enkephalinase. J Appl Physiol. 1988;64:2653. doi: 10.1152/jappl.1988.64.6.2653. [DOI] [PubMed] [Google Scholar]
- 54.Johnston S.L., Papi A., Monick M.M. Rhinoviruses induce interleukin-8 mRNA and protein production in human monocytes. J Infect Dis. 1997;175:323. doi: 10.1093/infdis/175.2.323. [DOI] [PubMed] [Google Scholar]
- 55.Johnston S.L., Pattemore P.K., Sanderson G. The relationship between upper respiratory infections and hospital admissions for asthma: A time–trend analysis. Am J Respir Crit Care Med. 1996;154:654. doi: 10.1164/ajrccm.154.3.8810601. [DOI] [PubMed] [Google Scholar]
- 56.Johnston S.L., Pattemore P.K., Sanderson G. Role of virus infection in children with recurrent wheeze or cough [abstr] Thorax Journal. 1993;48:1055–1055. [Google Scholar]
- 57.Johnston S.L., Pattemore P.K., Sanderson G. Community study of role of viral infections in exacerbations of asthma in 9–11 year old children. BMJ. 1995;310:1225. doi: 10.1136/bmj.310.6989.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Johnston S.L., Xie P., Johnson W. Comparison of standard virology and PCR in diagnosis of rhinovirus and respiratory syncytial virus infections in nasal aspirates from children hospitalized with wheezing illness and bronchiolitis [abstr] Am J Respir Crit Care Med. 1996;153:A503. [Google Scholar]
- 59.Kaul P., Singh I., Turner R.B. Effect of nitric oxide on rhinovirus replication and virus-induced interleukin-8 elaboration. Am J Respir Crit Care Med. 1999;159:1193. doi: 10.1164/ajrccm.159.4.9808043. [DOI] [PubMed] [Google Scholar]
- 60.Kimpen J.L.L., Garofalo R., Welliver R.C. Activation of human eosinophils in vitro by respiratory syncytial virus. Pediatr Res. 1992;32:160. doi: 10.1203/00006450-199208000-00007. [DOI] [PubMed] [Google Scholar]
- 61.Kraft M., Cassell G.H., Henson J.E. Detection of Mycoplasma pneumoniae in the airways of adults with chronic asthma. Am J Respir Crit Care Med. 1998;158:998. doi: 10.1164/ajrccm.158.3.9711092. [DOI] [PubMed] [Google Scholar]
- 62.Kumar A., Sorkness R., Kaplan M.R. Chronic, episodic, reversible airway obstruction after viral bronchiolitis in rats. Am J Respir Crit Care Med. 1997;155:130. doi: 10.1164/ajrccm.155.1.9001301. [DOI] [PubMed] [Google Scholar]
- 63.Ladenius A.R.C., Folkerts G., Linde van der H.J. Potentiation by viral respiratory infection of ovalbumin-induced guinea pig tracheal hyperresponsiveness: Role for tachykinins. Br J Pharmacol. 1995;115:1048. doi: 10.1111/j.1476-5381.1995.tb15917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Laing I., Riedel F., Yap P.L. Atopy predisposing to acute bronchiolitis during an epidemic of respiratory syncytial virus. BMJ. 1982;284:1070. doi: 10.1136/bmj.284.6322.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Laitinen L.A., Elkin R.B., Empey D.W. Bronchial hyperresponsiveness in normal subjects during attenuated influenza virus infection. Am Rev Respir Dis. 1991;143:358. doi: 10.1164/ajrccm/143.2.358. [DOI] [PubMed] [Google Scholar]
- 66.Laitinen L.A., Kava T. Bronchial reactivity following uncomplicated influenza A infection in healthy subjects and in asthmatic patients. Eur J Respir Dis. 1980;10:51. [PubMed] [Google Scholar]
- 67.Landau L.I. Bronchiolitis and asthma: Are they related? Thorax. 1994;49:293. doi: 10.1136/thx.49.4.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lemanske R.F., Jr, Dick E.C., Swenson C.A. Rhinovirus upper respiratory infection increases airway hyperreactivity and late asthmatic reactions. J Clin Invest. 1989;83:1. doi: 10.1172/JCI113843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lemanske R.F., Jr, Dick E.C., Swenson C.A. Rhinovirus upper respiratory infection increases airway hyperreactivity and late asthmatic reactions. J Clin Invest. 1989;83:1. doi: 10.1172/JCI113843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lemanske R.F., Jr, Lemen R.J., Gern J.E. Infections in childhood. In: Barnes P.J., Grunstein M.M., Leff A.R., editors. Asthma. Lippincott-Raven; Philadelphia: 1997. p. 1207. [Google Scholar]
- 71.Martinez F.D., Wright A.L., Taussig L.M. Asthma and wheezing in the first 6 years of life. N Engl J Med. 1995;332:133. doi: 10.1056/NEJM199501193320301. [DOI] [PubMed] [Google Scholar]
- 72.Minor T.E., Dick E.C., Baker J.W. Rhinovirus and influenza type A infections as precipitants of asthma. Am Rev Respir Dis. 1976;113:149. doi: 10.1164/arrd.1976.113.2.149. [DOI] [PubMed] [Google Scholar]
- 73.Murray M., Webb M.S., O'Callaghan C. Respiratory status and allergy after bronchiolitis. Arch Dis Child. 1992;67:482. doi: 10.1136/adc.67.4.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nicholson K.G., Kent J.K., Ireland D.C. Respiratory viruses and exacerbations of asthma in adults. BMJ. 1993;307:982. doi: 10.1136/bmj.307.6910.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Noma T., Yoshizawa I. Induction of allergen-specific IL-2 responsiveness of lymphocytes after respiratory syncytial virus infection and prediction of onset of recurrent wheezing and bronchial asthma. J Allergy Clin Immunol. 1997;98:816. doi: 10.1016/s0091-6749(96)70131-8. [DOI] [PubMed] [Google Scholar]
- 76.Olszewska-Pazdrak B., Casola A., Saito T. Cell-specific expression of RANTES, MCP-1, and MIP-1α by lower airway epithelial cells and eosinophils infected with respiratory syncytial virus. J Virol. 1998;72:4756. doi: 10.1128/jvi.72.6.4756-4764.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Openshaw P.J.M. Immunopathological mechanisms in respiratory syncytial virus disease. Springer Seminars in Immunopathology. 1995;17:187. doi: 10.1007/BF00196165. [DOI] [PubMed] [Google Scholar]
- 78.Panuska J.R., Midulla F., Cirino N.M. Virus-induced alterations in macrophage production of tumor necrosis factor and prostaglandin-E2. Am J Physiol. 1990;259:L396–L402. doi: 10.1152/ajplung.1990.259.6.L396. [DOI] [PubMed] [Google Scholar]
- 79.Papi A., Johnston S.L. Rhinovirus infection induces expression of its own receptor intercellular adhesion molecule 1 (ICAM-1) via increased NF-kappaB-mediated transcription. J Biol Chem. 1999;274:9707. doi: 10.1074/jbc.274.14.9707. [DOI] [PubMed] [Google Scholar]
- 80.Parry DE, Busse WW, Sukow KA, et al: Rhinovirus-induced peripheral blood mononuclear cell responses and outcome of experimental infection in allergic subjects. J Allergy Clin Immunol, in press [DOI] [PubMed]
- 81.Patel J.A., Kunimoto M., Sim T.C. Interleukin-1α mediates the enhanced expression of intercellular adhesion molecule-1 in pulmonary epithelial cells infected with respiratory syncytial virus. Am J Respir Cell Mol Biol. 1995;13:602. doi: 10.1165/ajrcmb.13.5.7576697. [DOI] [PubMed] [Google Scholar]
- 82.Pattemore P.K., Johnston S.L., Bardin P.G. Viruses as precipitants of asthma symptoms. I. Epidemiology. Clin Exp Allergy. 1992;22:325. doi: 10.1111/j.1365-2222.1992.tb03094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Prescott S.L., Macaubas C., Smallacombe T. Development of allergen-specific T-cell memory in atopic and normal children. Lancet. 1999;353:196. doi: 10.1016/S0140-6736(98)05104-6. [DOI] [PubMed] [Google Scholar]
- 84.Pullan C.R., Hey E.N. Wheezing, asthma, and pulmonary dysfunction 10 years after infection with respiratory syncytial virus in infancy. BMJ. 1982;284:1665. doi: 10.1136/bmj.284.6330.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rabatic S., Gagro A., Lokar-Kolbas R. Increase in CD23+ cells in infants with bronchiolitis is accompanied by appearance of IgE and IgG4 antibodies specific for respiratory syncytial virus. J Infect Dis. 1997;175:32. doi: 10.1093/infdis/175.1.32. [DOI] [PubMed] [Google Scholar]
- 86.Rakes C.P., Arruda E., Ingram J.M. Rhinovirus and respiratory syncytial virus in wheezing children requiring emergency care—IgE and eosinophil analyses. Am J Respir Crit Care Med. 1999;159:785. doi: 10.1164/ajrccm.159.3.9801052. [DOI] [PubMed] [Google Scholar]
- 87.Renzi P.M., Turgeon J.P., Marcotte J.E. Reduced interferon-γ production in infants with bronchiolitis and asthma. Am J Respir Crit Care Med. 1999;159:1417. doi: 10.1164/ajrccm.159.5.9805080. [DOI] [PubMed] [Google Scholar]
- 88.Renzi P.M., Turgeon J.P., Yang J.P. Cellular immunity is activated and a TH-2 response is associated with early wheezing in infants after bronchiolitis. J Pediatr. 1997;130:584. doi: 10.1016/s0022-3476(97)70243-9. [DOI] [PubMed] [Google Scholar]
- 89.Roberts N.J. Different effects of influenza virus, respiratory syncytial virus, and sendai virus on human lymphocytes and macrophages. Infect Immun. 1982;35:1142. doi: 10.1128/iai.35.3.1142-1146.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Roberts N.J., Prill A.H., Mann T.N. Interleukin-1 and interleukin-1 inhibitor production by human macrophages exposed to influenza virus or respiratory syncytial virus. J Exp Med. 1986;163:511. doi: 10.1084/jem.163.3.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rooney J.C., Williams H.E. The relationship between proved viral bronchiolitis and subsequent wheezing. J Pediatr. 1971;79:744. doi: 10.1016/s0022-3476(71)80385-2. [DOI] [PubMed] [Google Scholar]
- 92.Ruuskanen O., Ogra P.L. Respiratory syncytial virus. Curr Probl Pediatr. 1993;2:50. doi: 10.1016/0045-9380(93)90003-U. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Saban R., Dick E.C., Fishleder R.I. Enhancement by parainfluenza 3 infection of contractile responses to substance P and capsaicin in airway smooth muscle from the guinea pig. Am Rev Respir Dis. 1987;136:586. doi: 10.1164/ajrccm/136.3.586. [DOI] [PubMed] [Google Scholar]
- 94.Sakamoto M., Ida S., Takishima T. Effect of influenza virus infection on allergic sensitization to aerosolized ovalbumin in mice. J Immunol. 1984;132:2614. [PubMed] [Google Scholar]
- 95.Sanders S.P., Siekierski E.S., Porter J.D. Nitric oxide inhibits rhinovirus-induced cytokine production and viral replication in a human respiratory epithelial cell line. J Virol. 1998;72:934. doi: 10.1128/jvi.72.2.934-942.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schroth M.K., Grimm E., Frindt P. Rhinovirus replication causes RANTES production in primary bronchial epithelial cells. Am J Respir Cell Mol Biol. 1999;20:1220. doi: 10.1165/ajrcmb.20.6.3261. [DOI] [PubMed] [Google Scholar]
- 97.Seminario M.-C., Squillace D., Bardin P.G. Increased levels of eosinophil major basic protein in nasal secretions in rhinovirus infection [abstract] J Allergy Clin Immunol. 1995;95:259. [Google Scholar]
- 98.Siddiqi A., Peeples M., Brees B. Respiratory syncytial virus-induced release of RANTES and MIP-1 by bronchial epithelial and peripheral mononuclear cells. J Allergy Clin Immunol. 1996;37:305. [Google Scholar]
- 99.Sigurs N., Bjarnason R., Sigurbergsson F. Asthma and immunoglobulin-E antibodies after respiratory syncytial virus bronchiolitis: A prospective cohort study with matched controls. Pediatric. 1995;95:500. [PubMed] [Google Scholar]
- 100.Skoner D.P., Doyle W.J., Seroky J. Lower airway responses to influenza A virus in healthy allergic and nonallergic subjects. Am J Respir Crit Care Med. 1996;154:661. doi: 10.1164/ajrccm.154.3.8810602. [DOI] [PubMed] [Google Scholar]
- 101.Sorkness R., Clough J.J., Castleman W.L. Virus-induced airway obstruction and parasympathetic hyperresponsiveness in adult rats. Am J Respir Crit Care Med. 1994;150:28. doi: 10.1164/ajrccm.150.1.8025764. [DOI] [PubMed] [Google Scholar]
- 102.Sorkness R.L., Castleman W.L., Kumar A. Prevention of chronic post-bronchiolitis airway sequelae with interferon-γ treatment in rats. Am J Respir Crit Care Med. 1999;160:705. doi: 10.1164/ajrccm.160.2.9810002. [DOI] [PubMed] [Google Scholar]
- 103.Staunton D.E., Merluzzi V.J., Rothlein R. A cell adhesion molecule, ICAM-1, is the major surface receptor for rhinoviruses. Cell. 1989;56:849. doi: 10.1016/0092-8674(89)90689-2. [DOI] [PubMed] [Google Scholar]
- 104.Stein R.T., Sherrill D., Morgan W.J. Respiratory syncytial virus in early life and risk of wheeze and allergy by age 13 years. Lancet. 1999;354:541. doi: 10.1016/S0140-6736(98)10321-5. [DOI] [PubMed] [Google Scholar]
- 105.Subauste M.C., Jacoby D.B., Richards S. Infection of a human respiratory epithelial cell line with rhinovirus. Induction of cytokine release and modulation of susceptibility to infection by cytokine exposure. J Clin Invest. 1995;96:549. doi: 10.1172/JCI118067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Takeuchi R., Tsutsumi H., Osaki M. Respiratory syncytial virus infection of neonatal monocytes stimulates synthesis of interferon regulatory factor-1 and interleukin-1β (IL-1β)–converting enzyme and secretion of IL-1β. J Virol. 1998;72:837. doi: 10.1128/jvi.72.1.837-840.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tang W., Geba G.P., Zheng T. Targeted expression of IL-11 in the murine airway causes lymphocytic inflammation, bronchial remodelling, and airways obstruction. J Clin Invest. 1996;98:2845. doi: 10.1172/JCI119113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Teran L.M., Johnston S.L., Schroder J.-M. Role of nasal interleukin-8 in neutrophil recruitment and activation in children with virus-induced asthma. Am J Respir Crit Care Med. 1997;155:1362. doi: 10.1164/ajrccm.155.4.9105080. [DOI] [PubMed] [Google Scholar]
- 109.Tsutsumi H., Matsuda K., Sone S. Respiratory syncytial virus-induced cytokine production by neonatal macrophages. Clin Exp Immunol. 1996;106:442. doi: 10.1046/j.1365-2249.1996.d01-874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Turner R.B., Weingand K.W., Yeh C.H. Association between interleukin-8 concentration in nasal secretions and severity of symptoms of experimental rhinovirus colds [see comments] Clin Infect Dis. 1998;26:840. doi: 10.1086/513922. [DOI] [PubMed] [Google Scholar]
- 111.Uhl E.W., Castleman W.L., Sorkness R.L. Parainfluenza virus-induced persistence of airway inflammation, fibrosis, and dysfunction associated with TGF-β1 expression in brown Norway rats. Am J Respir Crit Care Med. 1996;154:1834. doi: 10.1164/ajrccm.154.6.8970378. [DOI] [PubMed] [Google Scholar]
- 112.Van Oosterhout A.J.M., Van Ark I., Folkerts G. Antibody to interleukin-5 inhibits virus-induced airway hyperresponsiveness to histamine in guinea pigs. Am J Respir Crit Care Med. 1995;151:177. doi: 10.1164/ajrccm.151.1.7812550. [DOI] [PubMed] [Google Scholar]
- 113.van Schaik S.M., Tristram D.A., Nagpal I.S. Increased production of IFN-γ and cysteinyl leukotrienes in virus-induced wheezing. J Allergy Clin Immunol. 1999;103:630. doi: 10.1016/s0091-6749(99)70235-6. [DOI] [PubMed] [Google Scholar]
- 114.Villani A., Cirino N.M., Baldi E. Respiratory syncytial virus infection of human mononuclear phagocytes stimulates synthesis of platelet-activating factor. J Biol Chem. 1991;266:5472. [PubMed] [Google Scholar]
- 115.Volvovitz B., Welliver R.C., De Castro G. The release of leukotrienes in the respiratory tract during infection with respiratory syncytial virus: Role in obstructive airway disease. Pediatr Res. 1988;24:504. doi: 10.1203/00006450-198810000-00018. [DOI] [PubMed] [Google Scholar]
- 116.Von Hertzen L., Töyrylä M., Gimishanov A. Asthma, atopy and Chlamydia pneumoniae antibodies in adults. Clin Exp Allergy. 1999;29:522. doi: 10.1046/j.1365-2222.1999.00504.x. [DOI] [PubMed] [Google Scholar]
- 117.Webb M.S.C., Henry R.L., Milner A.D. Continuing respiratory problems three and a half years after acute viral bronchiolitis. Arch Dis Child. 1985;60:1064. doi: 10.1136/adc.60.11.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Welliver R.C. Immunologic mechanisms of virus-induced wheezing and asthma. J Pediatr. 1999;135:S14–S20. [PubMed] [Google Scholar]
- 119.Welliver R.C., Sun M., Rinaldo D. Predictive value of respiratory syncytial virus-specific IgE responses for recurrent wheezing following bronchiolitis. J Pediatr. 1986;109:776. doi: 10.1016/s0022-3476(86)80692-8. [DOI] [PubMed] [Google Scholar]
- 120.Welliver R.C., Wong D.T., Sun M. The development of respiratory syncytial virus-specific IgE and the release of histamine in nasopharyngeal secretions after infection. N Engl J Med. 1981;305:841. doi: 10.1056/NEJM198110083051501. [DOI] [PubMed] [Google Scholar]
- 121.Zweiman B., Schoenwetter W.F., Pappano J.E. Patterns of allergic respiratory disease in children with a past history of bronchiolitis. J Allergy Clin Immunol. 1971;48:283. doi: 10.1016/0091-6749(71)90029-7. [DOI] [PubMed] [Google Scholar]


