Abstract
The burden of pneumonia, including that due to respiratory viruses, is markedly higher in the very young (<5 years) and older adults (≥50 years). Respiratory viruses substantially contribute to pneumonia in both adults and children, and when systematically tested for, are more commonly detected than bacteria in both adults and children. It is difficult to distinguish between viruses by clinical presentation, and the exact clinical implication of viral detections among patients with pneumonia depends on the pathogen detected; however, there is increasing evidence of their importance in pneumonia.
Keywords: Respiratory viruses, Pneumonia, Epidemiology
Key points
-
•
The burden of pneumonia, including that due to respiratory viruses, is markedly higher in the very young (<5 years) and older adults (≥50 years).
-
•
Respiratory viruses substantially contribute to pneumonia in both adults and children, and when systematically tested for, are more commonly detected than bacteria in both adults and children.
-
•
The most commonly detected respiratory viruses in adults and children are adenoviruses, coronaviruses, human metapneumovirus, human rhinoviruses, influenza viruses, parainfluenza viruses, and respiratory syncytial virus.
-
•
It is difficult to distinguish between viruses by clinical presentation, and the exact clinical implication of viral detections among patients with pneumonia depends on the pathogen detected; however, there is increasing evidence of their importance in pneumonia.
-
•
The circulation of respiratory viruses varies from region to region around the world, demonstrating seasonal variation in different parts of the world, which affects the prevalence and incidence of viral pneumonia globally.
Introduction
Worldwide, 900,000 children aged less than 5 years die from pneumonia every year.1 Pneumonia is a leading infectious cause of hospitalization and death among US adults, resulting in more than $10 billion annual expenses.2 Despite advances in clinical diagnostic methods, especially molecular-based methods, a cause is not always ascertained in a patient with pneumonia. Recent prospective pneumonia etiology studies have failed to detect a pathogen in greater than 50% of adults and approximately 20% of children hospitalized with pneumonia.3, 4, 5, 6, 7 In these same studies, viruses were more commonly detected than bacteria in both adults and children, accounting for greater than 25% of detections in adults and greater than 70% in children.3, 7 The exact implications of viral detections among patients with pneumonia depend on the pathogen detected, but there is increasing evidence of their importance in pneumonia.8
US prevalence/incidence
The Etiology of Pneumonia in the Community (EPIC) study was a large prospective multicenter US population-based active surveillance study in which viruses were more commonly detected than bacteria in both adults and children hospitalized with community-acquired pneumonia when systematic testing was used.3, 7 Detailed study details have been previously described,3, 7 but in brief, community-acquired pneumonia was defined as evidence of acute infection, acute respiratory illness, and radiographic evidence of pneumonia; patients with severe immunosuppression and recent hospitalization were excluded. Multiple modalities for pathogen detection of bacteria and viruses were used, including culture, polymerase chain reaction (PCR), serology, and antigen-based diagnostic assays.3, 7
The results of the EPIC study demonstrated that prevalence and incidence of different pathogens varied by age. Among children less than 18 years old enrolled in the EPIC study, 70% of pneumonia hospitalizations occurred among children less than 5 years old.7 Overall annual incidence of community-acquired pneumonia hospitalization in children was 15.7/10,000 children, and incidence was highest in children less than 2 years old (62.2/10,000 children), decreased in children 2 to 4 years old (23.8/10,000), and further decreased with increasing age. These rates were slightly lower than the 2009 national Kids’ Inpatient Database, which reported 22.4 hospitalized pneumonia cases per 10,000 children less than 18 years old.9 There are methodologic differences that likely explain these differences, including nonoverlapping years of analysis, distinctions between the populations studied, and varying case definitions, including exclusion of the severely immunocompromised in the EPIC study. Nonetheless, there were similar trends indicating that pneumonia burden is highest among the youngest children.
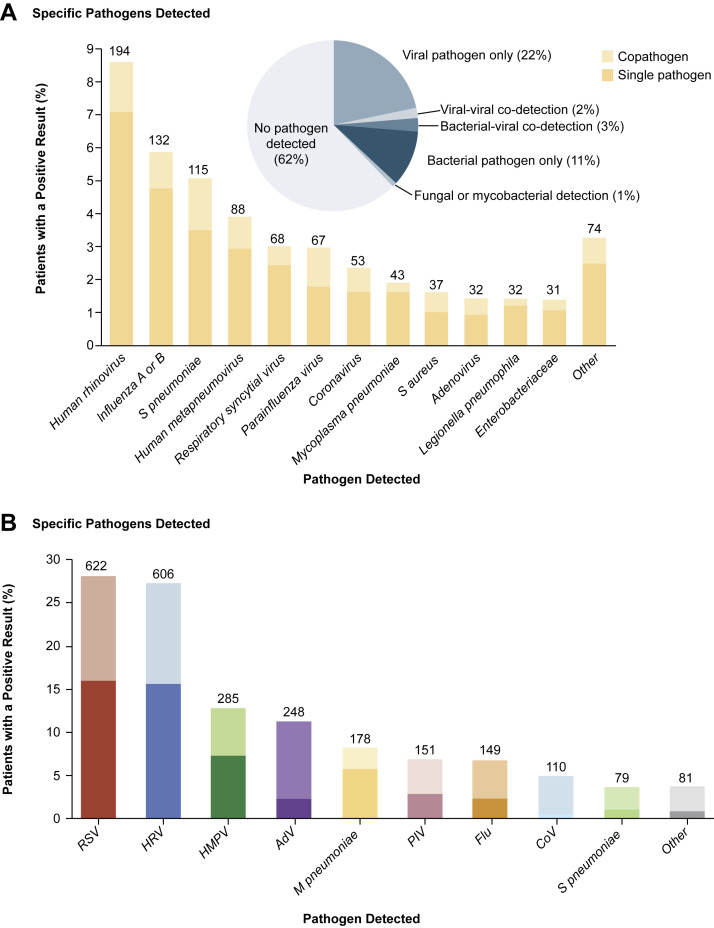
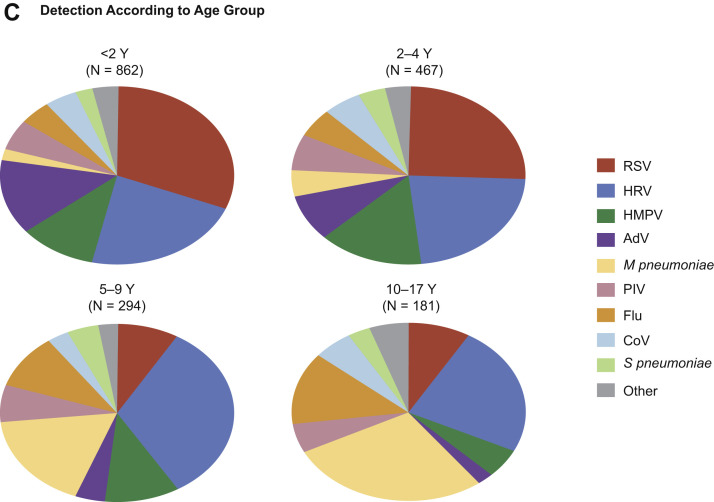
In the EPIC study, among 2222 children with clinical and radiographic pneumonia who had specimens available for bacterial and viral diagnostic testing, a pathogen was detected in 1802 (81%) children with one or more viruses in 1472 (66%), bacteria in 175 (8%), and both bacteria and viruses in 155 (7%). Among these 2222 children, the most commonly detected viruses were respiratory syncytial virus (RSV, 28%), human rhinoviruses (HRV, 27%), human metapneumovirus (HMPV, 13%), adenoviruses (AdV, 11%), parainfluenza 1 to 3 viruses (PIV, 7%), influenza A and B viruses (7%), and coronaviruses (CoV, 5%) (Fig. 1 B, codetections are indicated by the lighter shading).7 Compared with older children, RSV, AdV, and HMPV were all more commonly detected among children less than 5 years old (Fig. 1C). The incidence of RSV, HRV, HMPV, AdV, influenza viruses, PIV, and CoV was all higher among children less than 5 years old than among older children but was highest among children less than 2 years old.7
Fig. 1.
(A) Numbers (above the bars) and percentages of all adults in whom a specific pathogen was detected in the adult component of the EPIC study. The proportions of viral, viral-viral, bacterial-viral, bacterial, fungal or mycobacterial pathogens detected, and no pathogen detected are shown in the pie chart. (B) Numbers (above the bars) and percentages of all children in whom a specific pathogen was detected in the pediatric component of the EPIC study. (C) Proportions of pathogens detected, according to age group in the pediatric component of the EPIC study.
(From [A] Jain S, Self WH, Wunderink RG, et al. Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med 2015;373:420, with permission from Massachusetts Medical Society; and [B, C] Jain S, Williams DJ, Arnold SR, et al. Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med 2015;372:840; with permission from Massachusetts Medical Society.)
In adults enrolled in the EPIC study, overall annual incidence of community-acquired pneumonia hospitalization was 24.8/10,000 adults.3 The overall and pathogen-specific incidences increased with age with rates highest among adults 50 years of age and older. The EPIC study rates and trends are similar to previous pneumonia etiology studies conducted in the 1990s despite methodologic differences,10 but the EPIC study hospitalization rates were lower than more recent estimates based on hospitalization claims data likely due to certain excluded groups in the EPIC study, including those with severe immunosuppression.11
Among the 2259 adults enrolled in the EPIC study with clinical and radiographic pneumonia who had specimens available for bacterial and viral diagnostic testing, a pathogen was detected in 853 (38%) with one or more viruses in 530 (23%), bacteria in 247 (11%), bacteria and viruses in 59 (3%), and a fungal or mycobacterial pathogen in 17 (1%). Among the 2259 adults, the most commonly detected viruses were HRV (9%), influenza A and B viruses (6%), HMPV (4%), RSV (3%), PIV (2%), CoV (2%), and AdV (1%) (Fig. 1A).3 Importantly, the incidence of pneumonia hospitalization with influenza was almost 5 times higher among adults 65 years and older than among younger adults, and the incidence of HRV was almost 10 times as high. Interestingly, the overall incidence of pneumonia hospitalization with influenza (1.5/10,000) was similar to that of pneumococcus (1.2/10,000), a well-known bacterial cause of community-acquired pneumonia.
Worldwide/regional prevalence, incidence, and mortalities
It is well known that respiratory viruses contribute to acute respiratory infections, including those involving the lower respiratory tract and leading to bronchiolitis, pneumonia, and other complications. Although some global estimates of respiratory virus burden have been derived, including some from low- and middle-income countries, these data remain sparse because little surveillance for respiratory viruses is systematically carried out in many countries. In addition, most surveillance and thus estimates are not specific to pneumonia, and definitions of pneumonia vary widely between studies, making comparisons difficult.
In a 2005 study, RSV was associated with 22% of acute lower respiratory infections in children less than 5 years old worldwide with 3.4 (2.8–4.3) million hospitalizations and 66,000 to 199,000 deaths; 99% of deaths occurred in developing countries.12 Data from this same analysis demonstrated that most RSV deaths in high-income countries were in children less than 1 year old, whereas in low- and middle-income countries, these deaths extended into the second year of life.
Similar analyses have been done for the burden of influenza virus infection, again not necessarily limited to pneumonia. According to the World Health Organization (WHO), influenza occurs globally with an annual attack rate estimated at 5% to 10% in adults and 20% to 30% in children.13 Worldwide, these annual epidemics are estimated to result in about 3 to 5 million cases of severe illness, and about 250,000 to 500,000 deaths. Although hospitalizations and deaths occur in healthy people, certain groups are at higher risk for complications, and thus, influenza vaccines are targeted for these high-risk groups, including children 6 months to 5 years old, elderly 65 years and older, people with chronic medical conditions, pregnant women, and health care workers.
As part of the Pneumonia Research for Child Health (PERCH) project, 7 low- and middle-income countries have conducted research on pneumonia etiology among children less than 5 years old. In the PERCH project, the pneumonia case-definition per the WHO definitions was of severe (lower chest-wall indrawing in a child with history of cough or difficulty breathing) or very severe (cyanosis, oxygen saturation <90%, inability to feed, head nodding, or impaired consciousness in a child with history of cough or difficulty in breathing) pneumonia. Two types of outpatient controls without pneumonia included asymptomatic children and children with upper respiratory tract infection. In a preliminary analysis from one study site in rural Kenya, respiratory viruses were detected in most (60%) children less than 5 years old with pneumonia but also in controls (47%).14 Of the viruses detected, RSV was the most commonly detected virus in case-patients but not controls with a statistically significant association between virus detection and pneumonia hospitalization.14
In other pneumonia studies conducted in high-, low-, and middle-income countries, although the prevalence of specific viruses varies greatly, viruses are more commonly detected than bacteria, particularly in children. Prevalence of viruses can vary by geography and other factors, such as immunization coverage, as well as study design, including case definitions, specimen collection methods, and diagnostic tools applied; however, in most of these studies, certain viruses predominate, including RSV but also HMPV, AdV, and PIV. In a study of severe and very severe pneumonia conducted in Kenya and using multiplex PCR, a virus was detected in 56% of children less than 12 years old; RSV was most common and detected in 34% of children.15 In a different study conducted in Mozambique, viruses were detected in 49% of children with severe pneumonia, and in this case, HRV (41%), AdV (21%), and RSV (11%) were the most common.16 In similar studies, HMPV, AdV, PIV, and CoV combined account for 25% to 40% of pathogens detected in children when using PCR methods.17, 18 In many studies, codetections (viral and bacterial) in children with pneumonia have been demonstrated in more than one-quarter of cases.7, 14, 19
The role of viruses in adults has had increasing attention because viruses like RSV and HMPV have been commonly detected in systematic studies of hospitalized adults.20, 21 Although the same viruses that circulate in children also affect adults, the prevalence of the viruses differs between children and adults with pneumonia and also compared with data from controls. For example, HRV has been commonly detected in adult pneumonia patients, including from sterile lower respiratory tract specimens; in contrast with children, HRV is not commonly detected among adult asymptomatic controls and is often detected as the sole pathogen in adults, whereas, in children, it is often codetected.3, 4, 8, 22, 23, 24, 25 Influenza viruses are a known contributor to viral pneumonia,25 as well as a precursor to bacterial pneumonia, and are a common cause of pneumonia among persons 65 years and older. The range of other virus detections, including HMPV, RSV, PIV, CoV, and AdV, in adults with pneumonia is broad, ranging from 11% to 28% depending on the study location, design, and diagnostic tools.4, 5, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28 Bacterial or viral codetections are less frequently detected in adults than in children.3
Worldwide/regional seasonality and circulation patterns
The circulation of respiratory viruses varies from region to region around the world and demonstrates seasonal variation in different parts of the world, which affects the prevalence and incidence of viral pneumonia globally. In the United States, similar to other Northern Hemisphere countries, there are distinct peaks and troughs for different viruses. Generally, except for the 2009 H1N1 pandemic period from 2008 to 2009, there have been very clear peaks of influenza virus circulation annually in the United States that occur in the late fall or early winter, although it is never predictable exactly when the influenza season will start, and there is also regional variation. US influenza virus circulation data are reported weekly at the national and regional level on the Centers for Disease Control and Prevention (CDC) FluView Web site: http://www.cdc.gov/flu/weekly/fluactivitysurv.htm. Similarly, influenza circulation varies worldwide and surveillance methods vary from nation to nation, but data on global influenza circulation can be accessed on the WHO FluNet Web site: http://www.who.int/influenza/gisrs_laboratory/flunet/en/. Weekly global update reports are also available here: http://www.who.int/influenza/surveillance_monitoring/updates/latest_update_GIP_surveillance/en/.
In the United States, RSV circulation usually starts in late October and lasts until late January but can shift, starting in late January and lasting until early April depending on the year and circulation of other respiratory pathogens. Like influenza viruses, there is also regional variation with an earlier season starting in Florida and lasting longer than other US regions. National and regional RSV surveillance trends can be monitored at the CDC National Respiratory and Enteric Virus Surveillance System (NREVSS) Web site: http://www.cdc.gov/surveillance/nrevss/rsv/natl-trend.html. Through NREVSS, national and regional data are available for HMPV (http://www.cdc.gov/surveillance/nrevss/hmpv/natl-trend.html) and national data are also available for AdV (http://www.cdc.gov/surveillance/nrevss/adeno/natl-trend.html) and PIV (http://www.cdc.gov/surveillance/nrevss/human-paraflu/natl-trend.html), although specimens are not routinely tested for these viruses.
Globally, RSV surveillance efforts and methods vary from country to country. Data from 7 countries (Bangladesh, China, Egypt, Guatemala, Kenya, South Africa, and Thailand) over 8 years (2004–2012) demonstrated that RSV infection had 1 to 2 epidemic periods each year in each country; in general, seasonality patterns were similar within country but differed between countries from year to year. Likely factors affecting circulation were weather, geography, precipitation, and temperature.29 Similar reports from tropical and subtropical areas of southern and southeastern Asia have also shown that influenza virus circulation is highly dependent on weather patterns, especially rainfall and monsoons, and affects the seasonality of influenza virus circulation regionally, which has direct implications for influenza vaccination timing in these regions.30, 31
Clinical correlation and risk factors
Many pneumonia etiology studies rely on naso/oropharyngeal specimens for pathogen detection in addition to blood, serum, sputum, and urine (only in adults) because lower respiratory specimens are not practical or possible unless clinically necessary. Because most of these specimens do not come not directly from the lung, it is difficult to discern the association of a pathogen detection with pneumonia. Thus, many studies have enrolled asymptomatic controls along with pneumonia cases to help determine the possible contribution of respiratory viruses (attributable risk) to pneumonia at a population level. However, studies show variable prevalence of viruses among controls, possibly due to differences in definitions and methods for ascertainment of controls.32 Different strategies for control enrollment may be applicable to different study objectives and settings.32
In the EPIC study, all pathogens except for HRV were detected in 3% or less of asymptomatic controls; HRV was detected in 17% of pediatric controls compared with 22% of children with pneumonia (P = .04).7, 8 In asymptomatic adult controls, only 2% had any pathogen detection, compared with 27% among the patients hospitalized with pneumonia; interestingly, HRV was rarely detected in adult controls (1%).3, 8 In the EPIC study, the attributable fraction (AF) was calculated by comparing prevalence of viruses in pneumonia cases with asymptomatic controls, and adjusting for age, enrollment month, and enrollment city. In this analysis, the AF indicated that the detection of influenza, RSV, and HMPV among all patients, both children and adults, indicated an etiologic role. However, the detections of PIV, CoV, and AdV, particularly in children, did not demonstrate high AF for pneumonia. HRV was associated with pneumonia in adults but not children.8 The exact role of HRV in pneumonia remains unclear and controversial, even when detected as a single pathogen, particularly because HRV can shed for greater than 2 weeks after a primary infection, making its detection at time of pneumonia challenging to interpret with respect to the current clinical illness.22, 23, 24
Data from the EPIC study are similar to other US data from Singleton and colleagues,33 which compared asymptomatic Alaskan children less than 3 years old from the community with children hospitalized with respiratory infections. In this study, RSV, PIV, HMPV, and influenza viruses were all significantly more common in hospitalized children than controls, but HRV, AdV, and CoV were not; interestingly, children with RSV only or HMPV only had more severe illness compared with children with the other viruses.
Data from the recent US studies, including the EPIC study, are somewhat in contrast to data from the PERCH project site in Kenya, which tested for multiple pathogens (bacteria and viruses) in children less than 5 years old with pneumonia and in comparison to community controls (who could have had an upper respiratory infection). In the Kenya site for the PERCH project, compared with controls, RSV was the only virus determined to have a statistically significant association with hospitalization.14 The differences between the study results are likely due to variations in case and control definitions, prevalence of pathogens in the different studies, geography, and sociocultural characteristics that determine access to health care, threshold for hospitalization, and vaccination coverage for available immunizations.
In addition to nuances around etiology, the clinical spectrum of illness due to respiratory viruses is broad and encompasses asymptomatic infection, upper respiratory infection, and lower respiratory tract infection, resulting in pneumonia as well as other complications (eg, acute respiratory distress or secondary bacterial infection). Clinically, symptoms due to any specific virus infection, including in relation to pneumonia, largely overlap; thus, there are few clinical clues to distinguish between illnesses due to different pathogens. Some epidemiologic clues such as age and seasonality may be helpful. In addition, greater than 25% of children and less than 5% of adults are found to have multiple pathogens, potentially including both viruses and bacteria, and on this basis alone could be expected to have a mixed clinical presentation.3, 7
There are some data from comparisons of patients with illness of varying severity that suggest that bacterial pneumonia contributes to more severe illness. For example, in the EPIC study, Streptococcus pneumoniae, Staphylococcus aureus, and Enterobacteriaceae combined accounted for 16% of detected pathogens among adults admitted to the intensive care unit (ICU) as compared with 6% among adults not admitted to the ICU.3 Thus, when bacteria were detected, it was more likely in a severely ill patient. However, it is important to note that viruses were detected in 22% of adults admitted to the ICU compared with 24% not admitted to the ICU; so although there were no statistically significant differences between the severe and nonsevere groups, viral pneumonia contributed to ICU admissions, mechanical ventilation, and also death.
Influenza viruses are some of the more commonly recognized viruses that cause pneumonia and can lead to a primary viral pneumonia, secondary bacterial pneumonia, or mixed viral-bacterial pneumonia.25, 34, 35 Much of the influenza-associated pneumonia literature has focused on pandemics,36, 37 including the most recent 2009 H1N1 pandemic.38, 39, 40 During interpandemic years, influenza virus circulation varies, and thus, rates of influenza-associated pneumonia vary from year to year. Data from these reports indicate that in comparison to patients with influenza virus infection without pneumonia, patients with influenza virus infection and radiographic evidence of pneumonia have a more severe course of illness in terms of longer length of stay, ICU admission, mechanical ventilation, acute respiratory distress syndrome, sepsis, and death.40
Although RSV is commonly associated with bronchiolitis, it is also a well-known cause of the clinically and radiographically defined pneumonia, with RSV burden highest among children less than 2 years old. Most children are infected with RSV by 2 years of age, and the first infection, which may not confer immunity, is usually most severe. Prematurity and young age have been shown to be independent risk factors for hospitalization.41 In countries with a high HIV prevalence, RSV-associated acute lower respiratory tract infection has led to an increased risk of hospitalization and death, and longer hospital stay in children with HIV infection compared with children without HIV infection.42 RSV has also been shown to lead to acute respiratory infection in adults, including hospitalization, with the highest burden among adults greater than 50 years old.20 In addition to older age, other risk factors for RSV infection in adults include immunocompromised states and chronic lung conditions.43
Pneumonia was reported in almost half of the children who were hospitalized with HMPV infection in one multisite study of acute respiratory illness conducted in the United States.44 Similar to RSV, prematurity and asthma have been shown to be more frequent among hospitalized children with HMPV infection than children without HMPV infection.44 Risk factors for HMPV infection and subsequent complications, including pneumonia and hospitalization in adults, include older age, and underlying conditions, including asthma, cancer, and chronic obstructive pulmonary disease.21
In addition to annual epidemics of respiratory viruses, it is important to be vigilant for new and emerging viruses that can lead to severe illness including pneumonia, hospitalization, and death. Recent examples include Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV)45 and Middle-East Respiratory Syndrome Coronavirus (MERS-CoV).46 SARS-CoV was first recognized in China in 2002 and caused a worldwide outbreak with 8098 probable cases and 774 deaths from 2002 to 2003; however, no cases have been reported since 2004. MERS-CoV was first reported in 2012 in Saudi Arabia and has since caused outbreaks in multiple countries in the region. Most MERS cases have been in older men and those with chronic medical conditions, but all ages have been affected, including those in contact with a person who had MERS, especially health care workers.47
Avian influenza viruses are circulating in birds, both wild and domesticated, throughout the world. Although many viruses are considered low pathogenic avian influenza A viruses, there are highly pathogenic virus strains as well that have been detected in humans. Illness can range from mild to severe, including pneumonia, and cases are often sporadic and after contact with infected birds or their fluids; closing of live bird markets has been shown to reduce transmission. From 2003 to 2016, there have been 854 infections of avian influenza A (H5N1) virus reported to WHO, among which there were 450 (53%) deaths.48 Human infections with avian influenza A (H7N9) virus were first reported in China in 2013.49 Since then, there have been 798 laboratory-confirmed infections in China; most infections are sporadic with a few clusters of infection. There is no evidence of sustained human-to-human transmission.50 Since 2014, there have also been outbreaks in North America and elsewhere among wild and domesticated birds with influenza A (H5Nx and H7N8), which are considered highly pathogenic viruses in birds, but thus far, there have not been any human cases (http://www.oie.int/en/animal-health-in-the-world/update-on-avian-influenza/2016/).
Gaps in knowledge about the epidemiology of respiratory virus–associated pneumonia
Although active and systematic surveillance for all respiratory viruses has increased globally and is strongest for influenza viruses, it is still lacking throughout much of the world.51 Many questions remain about the seasonality of influenza and other respiratory viruses in tropical countries where there is increasing evidence of year-round circulation of influenza in warmer, more humid climates.29, 30, 31, 52 Country-specific studies to better understand the contribution of viruses to pneumonia are still required, especially from low- and middle-income countries. The PERCH project will further the understanding of pneumonia in children less than 5 years old,14 but data on older adults and pneumonia remain scant.
Country-specific data on pneumonia, from high, low, and middle income are continually needed because the prevalence of respiratory viruses varies due to seasonal and geographic differences. Incidence also varies, including by age but also due to socioeconomic and sociocultural factors. In addition, access to prevention and control methods that may be in place in some but not all countries (ie, vaccination coverage for S pneumoniae or Haemophilus influenzae, antibiotics, or antivirals) can affect the epidemiology of viral pneumonia. Risk factors for pneumonia, such as malnutrition, air pollution, tuberculosis, or coexisting malarial infection, may be more relevant in low- and middle-income countries than in more developed countries, where underlying conditions or tobacco exposure may be more prevalent among patients with pneumonia.34, 35
In addition, most pneumonia studies have been performed in hospital settings. However, in many parts of the world, including developed and developing nations, viral pneumonia does not always result in hospitalization; deaths are missed because they occur at home.53 Thus, more studies conducted in the community and also outpatient settings are needed to more fully understand the burden and epidemiology of respiratory viral pneumonia.
Summary
The burden of pneumonia, including that due to respiratory viruses, is markedly higher in the very young (<5 years) and older adults (≥50 years). Viruses are commonly detected, and clinically, it is difficult to distinguish between viral and bacterial pneumonia for most patients at presentation. Further development of new rapid diagnostic tests that can accurately distinguish among potential pathogens is urgently needed to better inform clinical care and public health practice.54 Treatment and vaccination are only currently available for influenza despite the high burden of RSV, HMPV, and other viruses. Development of effective vaccines and treatments for these viruses of importance could reduce the burden of pneumonia and their complications in both children and adults around the world.
Footnotes
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
References
- 1.Liu L., Oza S., Hogan D. Global, regional, and national causes of child mortality in 2000–13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet. 2015;385:430–440. doi: 10.1016/S0140-6736(14)61698-6. [DOI] [PubMed] [Google Scholar]
- 2.Pfunter A., Wier L.M., Steiner C. Agency for Healthcare Research and Quality, Healthcare Cost and Utilization Project; Rockville (MD): 2013. Costs for hospital stays in the United States, 2011 (Statistical Brief No. 168) [Google Scholar]
- 3.Jain S., Self W.H., Wunderink R. Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med. 2015;373:415–427. doi: 10.1056/NEJMoa1500245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jennings L.C., Anderson T.P., Beynon K.A. Incidence and characteristics of viral community-acquired pneumonia in adults. Thorax. 2008;63:42–48. doi: 10.1136/thx.2006.075077. [DOI] [PubMed] [Google Scholar]
- 5.Johnstone J., Majumdar S.R., Fox J.D. Viral infection in adults hospitalized with community-acquired pneumonia: prevalence, pathogens, and presentation. Chest. 2008;134:1141–1148. doi: 10.1378/chest.08-0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Charles P.G.P., Whitby M., Fuller A.J. The etiology of community-acquired pneumonia in Australia: why penicillin plus doxycycline or a macrolide is the most appropriate therapy. Clin Infect Dis. 2008;46:1513–1521. doi: 10.1086/586749. [DOI] [PubMed] [Google Scholar]
- 7.Jain S., Williams D.J., Arnold S.R. Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med. 2015;372:835–845. doi: 10.1056/NEJMoa1405870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Self W.H., Williams D.J., Zhu Y. Respiratory viral detection in children and adults: comparing asymptomatic controls and patients with community-acquired pneumonia. J Infect Dis. 2016;213:584–591. doi: 10.1093/infdis/jiv323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee G.E., Lorch S.A., Sheffler-Collins S. National hospitalization trends for pediatric pneumonia and associated complications. Pediatrics. 2010;126:204–213. doi: 10.1542/peds.2009-3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marston B.J., Plouffe J.F., File T.M. Incidence of community-acquired pneumonia requiring hospitalization. Arch Intern Med. 1997;157:1709–1718. [PubMed] [Google Scholar]
- 11.Griffin M.R., Zhu Y., Moore M.R. U.S. hospitalization for pneumonia after a decade of pneumococcal vaccination. N Engl J Med. 2013;369:155–163. doi: 10.1056/NEJMoa1209165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nair H., Nokes D.J., Gessner B.D. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet. 2010;375:1545–1555. doi: 10.1016/S0140-6736(10)60206-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization. Influenza (Seasonal) Fact Sheet Number 211. 2014. Available at: http://www.who.int/mediacentre/factsheets/fs211/en/. Accessed September 19, 2016.
- 14.Hammitt L.L., Kazungu S., Morpeth S.C. A preliminary study of pneumonia etiology among hospitalized children in Kenya. Clin Infect Dis. 2012;54(S2):S190–S199. doi: 10.1093/cid/cir1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berkley J.A., Munywoki P., Ngama M. Viral etiology of severe pneumonia among Kenyan infants and children. JAMA. 2010;303:2051–2057. doi: 10.1001/jama.2010.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Callaghan-Gordo C., Bassat Q., Morais L. Etiology and epidemiology of viral pneumonia among hospitalized children in rural Mozambique: a malaria endemic area with high prevalence of human immunodeficiency virus. Pediatr Infect Dis J. 2011;30:39–44. doi: 10.1097/INF.0b013e3181f232fe. [DOI] [PubMed] [Google Scholar]
- 17.García- García M.L., Calvo C., Pozo F. Spectrum of respiratory viruses in children with community-acquired pneumonia. Pediatr Infect Dis J. 2012;31:808–813. doi: 10.1097/INF.0b013e3182568c67. [DOI] [PubMed] [Google Scholar]
- 18.Pavia A.T. Viral infections of the lower respiratory tract: old viruses, new viruses, and the role of diagnosis. Clin Infect Dis. 2011;52(Suppl 4):S284–S289. doi: 10.1093/cid/cir043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Michelow I.C., Olsen K., Lozano J. Epidemiology and clinical characteristics of community-acquired pneumonia in hospitalized children. Pediatrics. 2004;113:701–707. doi: 10.1542/peds.113.4.701. [DOI] [PubMed] [Google Scholar]
- 20.Falsey A.R., Hennessey P.A., Formica M.A. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med. 2005;352:1749–1759. doi: 10.1056/NEJMoa043951. [DOI] [PubMed] [Google Scholar]
- 21.Walsh E.E., Peterson D.R., Falsey A.R. Human metapneumovirus infections in adults: another piece of the puzzle. Arch Intern Med. 2008;168:2489–2496. doi: 10.1001/archinte.168.22.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fry A.M., Lu X., Olsen S.J. Human rhinovirus infections in rural Thailand: epidemiological evidence for rhinovirus as both pathogen and bystander. PLoS One. 2011;6(3):e17780. doi: 10.1371/journal.pone.0017780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karhu J., Ala-Kokko T.I., Vuorinen T. Lower respiratory tract virus findings in mechanically ventilated patients with severe community-acquired pneumonia. Clin Infect Dis. 2014;59:62–70. doi: 10.1093/cid/ciu237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruuskanen O., Järvinen A. What is the real role of respiratory viruses in severe community-acquired pneumonia? Clin Infect Dis. 2014;59:71–73. doi: 10.1093/cid/ciu242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brundage J.F. Interactions between influenza and bacterial respiratory pathogens: implications for pandemic preparedness. Lancet Infect Dis. 2006;6:303–312. doi: 10.1016/S1473-3099(06)70466-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johansson N., Kalin M., Tivelhung-Lin-dell A. Etiology of community-acquired pneumonia: increased microbiological yield with new diagnostic methods. Clin Infect Dis. 2010;50:202–209. doi: 10.1086/648678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lieberman D., Shimoni A., Shemer-Avni Y. Respiratory viruses in adults with community-acquired pneumonia. Chest. 2010;138:811–816. doi: 10.1378/chest.09-2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Templeton K.E., Scheltinga S.A., van den Beden W.C. Improved diagnosis of the etiology of community-acquired pneumonia with real-time polymerase chain reaction. Clin Infect Dis. 2005;41:345–351. doi: 10.1086/431588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haynes A.K., Manangan A.P., Iwane M.K. RSV Circulation in 7 countries with GDD regional centers. J Infect Dis. 2013;208(S3):S246–S254. doi: 10.1093/infdis/jit515. [DOI] [PubMed] [Google Scholar]
- 30.Saha S., Chadha M., Al Mamun A. Influenza seasonality and vaccination timing in tropical and subtropical areas of southern and south-eastern Asia. Bull World Health Organ. 2014;92:318–330. doi: 10.2471/BLT.13.124412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chadha M.S., Potdar V.A., Saha S. Dynamics of influenza seasonality at sub-regional levels in India and implications for vaccination timing. PLoS One. 2015;10:e0124122. doi: 10.1371/journal.pone.0124122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deloria-Knoll M., Feikin D.R., Scott A.G. Identification and selection of cases and controls in the pneumonia etiology research for child health project. Clin Infect Dis. 2012;54(S2):S117–S123. doi: 10.1093/cid/cir1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singleton R.J., Bulkow L.R., Miernyk K. Viral respiratory infections in hospitalized and community control children in Alaska. J Med Virol. 2010;82:1282–1290. doi: 10.1002/jmv.21790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mandell L.A., Wunderink R.G., Anzueto A. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44:S27–S72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bradley J.S., Byington C.L., Shah S.S. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53(7):e25–e76. doi: 10.1093/cid/cir531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Louria D.B., Blumenfield H.L., Ellis J.T. Studies on influenza in the pandemic of 1957-1958. II. Pulmonary complications of influenza. J Clin Invest. 1959;38:213–265. doi: 10.1172/JCI103791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwarzmann S.W., Adler J.L., Sullivan R.J. Arch Intern Med. 1971;127:1037–1041. [PubMed] [Google Scholar]
- 38.Perez-Padilla R., de la Rosa-Zamboni D., Ponce de Leon S. Pneumonia and respiratory failure from swine-origin influenza A (H1N1) in Mexico. N Engl J Med. 2009;361:680–689. doi: 10.1056/NEJMoa0904252. [DOI] [PubMed] [Google Scholar]
- 39.Reyes S., Montull B., Martinez R. Risk factors of A/H1N1 etiology in pneumonia and its impact on mortality. Respir Med. 2011;105:1404–1411. doi: 10.1016/j.rmed.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 40.Jain S., Benoit S.R., Skarbinski J. Influenza-associated pneumonia among hospitalized patients with 2009 pandemic influenza A (H1N1) virus—United States, 2009. Clin Infect Dis. 2012;54(9):1221–1229. doi: 10.1093/cid/cis197. [DOI] [PubMed] [Google Scholar]
- 41.Hall C.B., Weinberg G.A., Iwane M.K. The burden of RSV infection in young children. N Engl J Med. 2009;360:588–598. doi: 10.1056/NEJMoa0804877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moyes J., Cohen C., Pretorius M. Epidemiology of RSV-associated ALRTI hospitalizations among HIV-infected and HIV-uninfected south African children, 2010-2011. J Infect Dis. 2013;208(S3):S217–S226. doi: 10.1093/infdis/jit479. [DOI] [PubMed] [Google Scholar]
- 43.Dowell S.F., Anderson L.J., Gary H.E., Jr. RSV is an important cause of community-acquired lower respiratory infection among hospitalized adults. J Infect Dis. 1996;176:456–462. doi: 10.1093/infdis/174.3.456. [DOI] [PubMed] [Google Scholar]
- 44.Edwards K.M., Zhu Y., Griffin M.R. Burden of human metapneumovirus infection in young children. N Engl J Med. 2013;368:633–643. doi: 10.1056/NEJMoa1204630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee N., Hui D., Wu A. A major outbreak of a severe acute respiratory syndrome in Hong Kong. N Engl J Med. 2003;348:1986–1994. doi: 10.1056/NEJMoa030685. [DOI] [PubMed] [Google Scholar]
- 46.Zaki A.M., van Boheemen S., Bestebroer T.M. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367:1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 47.Assiri A., McGeer A., Perl T.M. Hospital outbreak of Middle East respiratory syndrome coronavirus. N Engl J Med. 2013;369(5):407–416. doi: 10.1056/NEJMoa1306742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.World Health Organization. Cumulative number of confirmed human cases for avian influenza A(H5N1) reported to WHO. 2003–2016. Available at: http://www.who.int/influenza/human_animal_interface/2016_07_19_tableH5N1.pdf?ua=1. Accessed September 21, 2016.
- 49.Gao H., Lu H.Z., Cao B. Clinical findings in 111 cases of influenza A(H7N9) virus infection. N Engl J Med. 2013;368:2277–2285. doi: 10.1056/NEJMoa1305584. [DOI] [PubMed] [Google Scholar]
- 50.World Health Organization. Human infection with avian influenza A(H7N9) virus—China. Available at: http://www.who.int/csr/don/17-august-2016-ah7n9-china/en/. Accessed September 21, 2016.
- 51.Polansky L.S., Outin-Blenman S., Moen A.C. Improved global capacity for influenza surveillance. Emerg Infect Dis. 2016;22:993–1001. doi: 10.3201/eid2206.151521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hirve S., Newman L.P., Paget J. Influenza seasonality in the tropics and subtropics—when to vaccinate? PLoS One. 2016;11(4):e0153003. doi: 10.1371/journal.pone.0153003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nasreen S., Luby S.P., Brooks W.A. Population-based incidence of severe acute respiratory virus infections among children aged <5 years in rural Bangladesh, June-October 2010. PLoS One. 2014;9(2):e89978. doi: 10.1371/journal.pone.0089978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Caliendo A.M., Gilbert D.N., Ginocchio C.G. Better tests, better care: improved diagnostics for infectious diseases. Clin Infect Dis. 2013;59:S139–S170. doi: 10.1093/cid/cit578. [DOI] [PMC free article] [PubMed] [Google Scholar]