Abstract
Sewage sludge/biosolids are by-wastes of municipal and industrial wastewater treatment. As sources of nutrients (C, N, P) they are widely used in intensive farming where large supplementation of organic matter to maintain fertility and enhance crop yields is needed. However, according to the report of European Commission published in 2010, only 39% of produced sewage sludge is recycled into agriculture in the European Union. This situation occurs mainly due to the fact, that the sewage sludge may contain a dangerous volume of different contaminants. For over decades, a great deal of attention has been focused on total concentration of few heavy metals and pathogenic bacteria Salmonella and Escherichia coli. The Sewage Sludge Directive (86/278/EEC) regulates the allowable limits of Zn, Cu, Ni, Pb, Cd, Cr and Hg and pathogens and allows for recovery of sludge on land under defined sanitary and environmentally sound conditions. In this paper, a review on quality of sewage sludge based on the publications after 2010 has been presented. Nowadays there are several papers focusing on new serious threats to human health and ecosystem occurring in sewage sludge – both chemicals (such as toxic trace elements – Se, Ag, Ti; nanoparticles; polyaromatic hydrocarbons; polychlorinated biphenyl; perfluorinated surfactants, polycyclic musks, siloxanes, pesticides, phenols, sweeteners, personal care products, pharmaceuticals, benzotriazoles) and biological traits (Legionella, Yersinia, Escherichia coli O157:H7).
Keywords: Sewage sludge, Metallic trace elements, Nanoparticle, Pharmaceutical (PhC), Personal care product (PCP), Pathogen
Highlights
-
•
Sewage sludge contains organic and inorganic contaminants not regulated by law.
-
•
Total content of many compounds (heavy metals) is not a reliable indicator of toxicity.
-
•
Regular monitoring is crucial to limit a release of toxic substances to ecosystem.
1. Introduction
Sewage sludge can be defined as the solid or semi-solid residue left over after the treatment of wastewater. In literature it can be defined as by-product, yet it shall be treated as a waste in the process of wastewater treatment. Sewage sludge may be used as a source of energy (anaerobic digestion, thermal treatment), treated and used on land as a fertilizer and soil conditioner, or may even be used as a source to extract valuable compounds (phosphorous recovery). A significant number of wastewater treatment plants (WWTPs) compost dewatered sewage sludge under aerobic conditions with green wastes or other bulking agents or dry it in heat drying facilities up to 95% dry mass for use as fertilizer or fuel.
In most developed countries particular attention is drawn on proper treatment of sewage sludge to improve the quality and safe use on land. United States Environmental Protection Agency (US EPA) defines biosolids as treated sewage sludge that meets the suitable levels of pollutants or pathogen and is used as fertilizer for landscape application (USEPA, 2009). Wastewater sludge as a complex heterogeneous mixture of micro-organisms, undigested organics as cellulose, plant residues, oils, or fecal material, inorganic material, sand is a resource of organic matter, nitrogen, phosphorous, micronutrients and even heavy metals, bio-fuel, hydrogen, syngas, bio-oil, bio-diesel, bio-plastics, bio-pesticides, proteins, enzymes, bio-fertilizers or voilatile-acids (Tyagi and Lo, 2013). Currently the main trends in development of sustainable human communities include the investigation of the best strategies of the recycling of those precious substances (LeBlanc et al., 2009). However, taking into consideration standards set for waste which are reintroduced in natural systems, precautionary aspects shall be considered, especially on the limit values (quality criteria) for potential contaminants and pollutants dangerous to human health and the environment.
In Europe, the Sewage Sludge Directive (86/278/EEC) (SSD), the one of the oldest obligatory directive was set up to encourage the use of sewage sludge in agriculture and to regulate its use in such a way as to prevent harmful effects on environment by limiting the possible transfer of heavy metals and pathogens. Generally the Directive had the positive effect of improving source control measures in order to ensure a good quality of sludge, though currently it is considered as out-of-date and has been earmarked by the Commission as a candidate for revision for around 10 years (Environment, 2014). According to the report of European Commission published in 2010, only 39% of sewage sludge is recycled into agriculture in the EU due to increasing leaching of contaminants to water and soil, odors and greenhouse gas emissions (CH4 and CO2). Large variations are noted for sludge used on land in the Member States, ranging from none (Nederland, Switzerland) to over 50% (Norway, Great Britain, France). Coalition agreement of the federal government of Germany in November 2013 concluded: “We will face out the direct use of sewage sludge as a fertilizer on land and promote the recycling of phosphorus and other nutrients” (Bergs, 2015). At the other high-income countries, like USA, Canada, Australia, New Zealand treated biosolids are widely used on soils, however incineration has been suggested as a promising alternative of final sewage sludge disposal. Nevertheless, in less developed countries land application of treated sewage sludge is growing alternative for landfilling.
Hence the major question is what kind of contaminants can be found nowadays in sewage sludge? There are several papers focusing on new serious threats to human health and ecosystem occurring in sewage sludge – both chemicals (polyaromatic hydrocarbons (PAH), hydrocarbons; polychlorinated biphenyl (PCB), Perfluorinated Surfactants (PFCs), Personal Care Products (PCPs), Pharmaceuticals (PhCs), Benzotriazoles) and biologicals (Legionella, Yersinia, Escherichia coli O157:H7). However only some countries, e.g. Sweden initiated a program to systematically sample, analyze and bank sewage sludge (Olofsson et al., 2012). In the present paper we clearly demonstrate the necessity of introduction of monitoring program for emerging pollutants. Therefore a review on quality of sewage sludge based on the publications after 2010 has been presented.
2. Possible strategies for sewage sludge management
Sewage sludge as a waste (by-product) from the wastewater treatment process for many years has been mainly utilized by landfarming or/and landfilling. Due to the increasing content of toxic pollutants in many countries the sewage sludge has been found as hazardous waste and often incinerated. Sewage sludge (after meet the requirements of SSD in terms of limit values) is widely used as a fertilizer. However, the Member States significantly vary in the amount of generated sludge used in agriculture ranging from none to well over 50%.
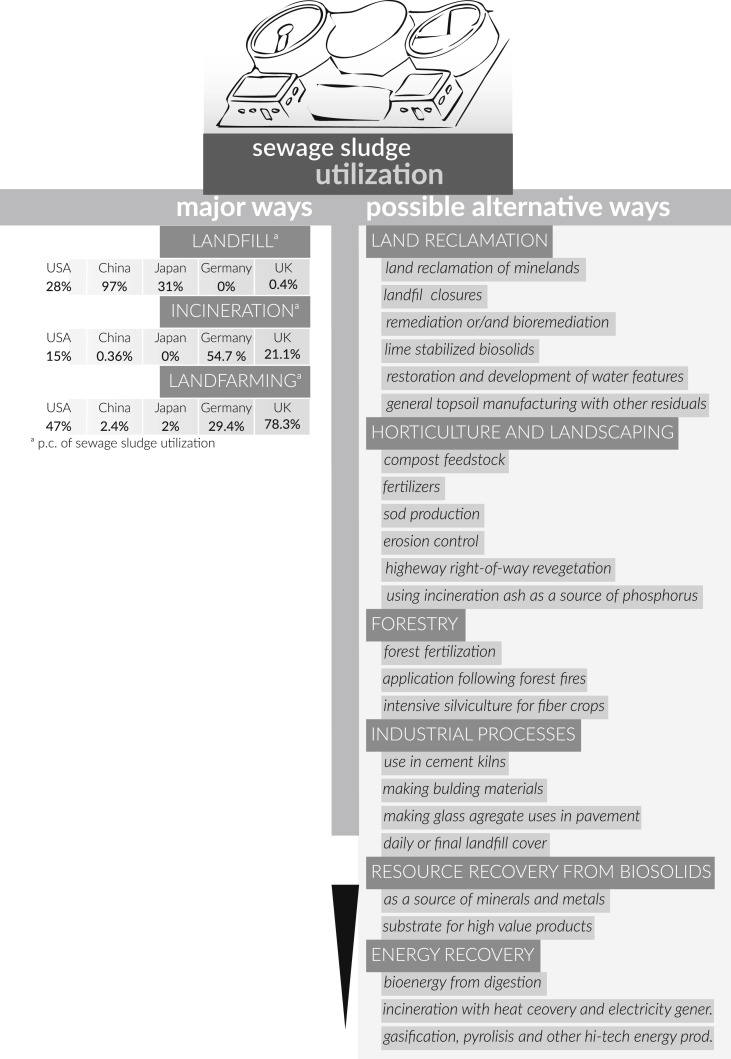
From environmental point of view, agricultural use of sewage sludge is preferable because the organic and inorganic nutrients are recovered. Yet, nowadays, alternative treatment and disposal processes are selected and proposed (Fig. 1 ). As a result of conversion, sewage sludge loses its original properties and then becomes useful in the form of other products. Hence, a by-product can be obtained from biowastes. However, the change of waste into a product is also connected with a change of legislation. For instance, compost produced from sludge must meet the legal requirements for organic fertilizer.
Fig. 1.
Current and proposed ways of sewage sludge utilization (LeBlanc et al., 2009, Zhen et al., 2017).
Moreover, new technologies often require introduction of eco-innovative solution and holistic approach. OECD defined innovation as “the implementation of a new or significantly improved product (good or service), or process, a new marketing method, or a new organizational method in business practices, workplace organization or external relations” (Marchal et al., 2011).
3. Metallic trace elements
Inorganic contaminants are not biodegradable, and therefore can accumulate in the soil and enter the food chain and bioconcentrate in the environment. Historically, the most important are heavy metals, classified as chemical elements having a specific gravity higher than 4.5 g cm−3 (Kabata-Pendias, 2010). The most commonly used with this respect are Cr, Mn, Fe, Co, Ni, Cu, Zn, Hg, Cd, Pb, Sn, Mo, V. Though, likewise important from the standpoint of toxicity are metalloids like As, Se or non-metals and light metals as Al. Hence, a term metallic trace elements is more and more often used. Main source of heavy metals in sewage sludge are industrial wastewater and surface runoff. The total content varies within wide limits (from 0.5 to 2% of dry sludge). Taking into consideration the quantity of individual element it can be lined up as follows: Zn > Cu > Cr > Ni > Pb > Cd or Zn > Cr > Pb > Cu > Ni > Cd (Wilk and Gworek, 2009).
Many Member States have decided to implement stricter limit values (Table 1 ) than those stipulated by the Directive 86/278/EEC (Environment, 2014). This situation was noted particularly for mercury, but also for cadmium and nickel. In the case of zinc usually limit values close to the maximum allowed by the Directive have been adopted.
Table 1.
The content of trace elements in sewage sludge for Ireland, Italy, Russia, China and Canada.
| Trace element | unit | The range of value from UE countriesa | The limit value [Directive 86/278/EEC] | Measured values in: |
||||
|---|---|---|---|---|---|---|---|---|
| Irelandb | Italyc | Russiad | Chinae | Canadaf | ||||
| Al | % | 0.1–60 | not limited | – | – | – | – | – |
| Fe | % | 0.2–14.9 | not limited | – | – | – | – | – |
| Zn | % | 0.0–0.1 | 0.25–0.4 | 0.08 | 0.02–0.09 | 0.07–0.08 | 2.42 | 0.03 |
| Cd | mg/kg DM | 0.3–5.1 | 20–40 | 12 | 0.3–0.9 | 1.65 | 1.1 | |
| Cu | mg/kg DM | 27.3–578.1 | 1000–1750 | 520 | 90–206 | 200–300 | 3323 | 271 |
| Hg | mg/kg DM | 0.1–1.1 | 16–25 | not detected | 0.2–0.9 | 11.35 | – | 0.68 |
| Ni | mg/kg DM | 8.6–310 | 300–400 | 18 | 11–15 | 75–77 | 422 | 10.5 |
| Pb | mg/kg DM | 4.0–429.8 | 750–1200 | 252 | 80–126 | 34.7 | 69.7 | 24 |
| Ag | mg/kg DM | 0.1–14.7 | not limited | – | – | – | – | – |
| Ba | mg/kg DM | 41.5–579.9 | not limited | – | – | – | – | – |
| Mn | mg/kg DM | 75.2–959.7 | not limited | – | 103–566 | – | – | – |
| Ti | mg/kg DM | 65.2–1070.9 | not limited | – | – | – | – | – |
| V | mg/kg DM | 2.3–135.4 | not limited | – | – | – | – | – |
| As | mg/kg DM | 5.6–56.1 | not limited | not detected | – | – | – | – |
| Co | mg/kg DM | 1.5–16.7 | not limited | – | – | – | – | 2.9 |
| Cr | mg/kg DM | 10.8–1542.2 | not limited | 35 | 18–65 | 305–310 | 1983 | 20.3 |
| Mo | mg/kg DM | 1.7–12.5 | not limited | 5 | – | – | – | 3.5 |
| Se | mg/kg DM | 3.4–53.6 | not limited | 3 | – | – | – | 2.15 |
As shown by numerous studies, the total amount of metals in the sludge regulated by Directive 86/278/EEC is reduced, as indicated by the long-term analysis in Germany (Table 2 ). Since 1977, the largest decrease has been observed in the case cadmium, chrome and mercury – 95.4, 94.8 and 89.6%, respectively. It is noteworthy that the smallest divergence was noted for copper – since 1977 the content of this element has been reduced only by 22%. The content of heavy metals observed after 2000 year was lower comparing to the limit values proposed by Directive 86/278/EEC.
Table 2.
Content of selected heavy metals in sewage sludge between 1977 and 2012 in Germany in (mg/kg DM).
| Trace element | 1977a | 1982a | 1986–1990a | 2001a | 2005a | 2012b | Change between 1977 (=100%) and 2012 | Change between 2001 (=100%) and 2012 |
|---|---|---|---|---|---|---|---|---|
| Pb | 220 | 190 | 113 | 53 | 40.4 | 34 | −84.5 | −35.8 |
| Cd | 21 | 4.1 | 2.5 | 1.2 | 0.97 | 1 | −95.4 | −16.7 |
| Cr | 630 | 80 | 62 | 45 | 37.1 | 33 | −94.8 | −26.7 |
| Cu | 378 | 370 | 322 | 304 | 306.4 | 292 | −22.7 | −3.9 |
| Ni | 131 | 48 | 34 | 27 | 25.2 | 25 | −80.9 | −7.4 |
| Hg | 4.8 | 2.3 | 2.3 | 0.8 | 0.59 | 0.5 | −89.6 | −37.5 |
| Zn | 2.140 | 1.480 | 1.045 | 794 | 756.7 | 762 | −64.4 | −4.0 |
The total content of heavy metals is not a reliable indicator to assess their availability for living organisms, and thus the intrinsic toxicity. Such an assessment can be made by determining the amount of metal ions bound by the individual components (fractions) using sequence analysis. Lasheen and Ammar (2009) showed that Mn, Ni and Zn were most present in the exchangeable, carbonate and Fe/Mn-oxide forms as the most mobile fractions, while Cd, Cu, Cr and Fe were major in the organic and sulfide (exhibiting some degree of mobility), and the residual form (inert phase) which, corresponds to less mobilization. Different methods of stabilization alter both total concentration and the bioavailability of metal ions. The same authors confirmed that the use of cement kiln dust significantly reduced the availability of metals by chemical modification of their chemical speciation into less available forms. Dąbrowska and Rosińska (2012) did not observe accumulation of mobile fractions (exchangeable and carbonate) as an effect of thermophilic digestion of sewage sludge except for nickel. The highest increase of Zn, Cu, Cd and Cr concentration was observed in the form of organic-sulfide fraction, whereas in the case of Pb, the residual fraction noted the highest increase. For Ni both organic-sulfide and exchangeable–carbonate fractions were enriched. Smith (2009) in his critical review demonstrated the reduced bioavailability and crop uptake of metals from composted biosolids comparing to other types of sewage sludge. Furthermore, the use the earthworms affected metal speciation in vermicomposted sludge (Y. Zhang et al., 2008). In turn, Xiao et al. (2015) noted that after combustion process of pelletized municipal sewage sludge (MSS), the bioavailable heavy metal fractions (acid soluble/exchangeable, reducible and oxidizable fractions) were mostly transformed into the very stable heavy metal fractions (residual fractions). Healy et al. (2016) analysed metal concentrations in sewage sludge (SS) treated by thermal drying, lime stabilization, or anaerobic digestion found that Se and Sn, potentially harmful to human health, are present in SS in large concentration - much higher than their baseline amounts in soils. These metals are omitted in the regulations, just like several others, even those considered astoxic. Interesting results are shown for silver. A study financed by the US EPA (USEPA, 2009) in sludge from 74 wastewater treatment plants (WWTPs) across the USA indicates the presence of significant concentration of silver (up to 856 mg Ag/kg DM) or even titanium (to 4510 mgTi/kg DM). The Swedish Environmental Protection Agency (SEPA) has recommended a silver value of 8 mg/kg in sewage sludge destined for agricultural applications. Shamuyarira and Gumbo (2014) analysed the sewage sludge of five towns in Limpopo province of South Africa and found the silver concentration in the range of 0.22–21.93 mg/kg DM.
4. Organic contaminants
Over 1.5 thousand of organic compounds have already been registered. A large part of them can be found in the wastewater, and later in sewage sludge. Harrison et al. (2006) have identified the presence of 516 compounds belonging to 15 groups. Nevertheless, the authors found that more than 80% have not been tested on a large scale, but they are often of substantial toxicity as nitrosamines. During the wastewater processes and treatment of sludge, those substances can form intermediate products, often more toxic than the starting compounds. In turn, Bueno et al. (2012) during two-years of investigations of five WWTPs identified the occurrence and persistence of a group of 100 organic compounds belonging to several chemical groups (pharmaceuticals, personal care products, pesticides and metabolites). Almost simultaneously, Clarke and Smith (2011) in their critical review identified chemicals of concern ranked in decreasing order of priority: perfluorinated chemicals (PFOS, PFOA); polychlorinated alkanes (PCAs), polychlorinated naphthalenes (PCNs); organotins (OTs), polybrominated diphenyl ethers (PBDEs), triclosan (TCS), triclocarban (TCC); benzothiazoles; antibiotics and pharmaceuticals; synthetic musks; bisphenol A, quaternary ammonium compounds (QACs), steroids; phthalate acid esters (PAEs) and polydimethylsiloxanes (PDMSs). The concentrations of organic contaminants in sewage sludge are usually closely correlated with their amounts in influent wastewater. The factors affecting their processing are physicochemical properties of the compounds (molecular weight, hydrophobicity, water solubility, pKa, resistance to biodegradation) as well as the sludge characteristics (pH, organic matter, cations concentration) and the operational parameters of wastewater treatment plants (presence or absence of primary sedimentation, hydraulic residence time in different tanks, sludge residence time in bioreactors, sludge stabilization methods), (Stasinakis, 2012). Currently, the following organic pollutants have been regulated in the UE Member Countries (Table 3 ).
Table 3.
Organic pollutants regulated in the selected UE Members countries, adapted from (Mininni et al., 2015).
| Compound | Austria | Belgium | Germany | Denmark | Luxembourg | Slovenia | France |
|---|---|---|---|---|---|---|---|
| PAH Polycyclic aromatic hydrocarbons |
X | X | – | X | X | X | X |
| PCB polychlorinated biphenyl |
X | X | X | X | X | X | X |
| PCDD/F Polychlorinated dibenzo-p-dioxins/furans |
X | X | X | – | X | – | – |
| PFC perfluorinated compound |
X | X | X | – | – | – | – |
| AOX Adsorbable Organic Halides |
X | X | – | – | – | – | – |
| LAS Linear alkylbenzene sulfonate |
– | X | – | X | – | – | – |
| NPE nonylphenolethoxylates |
– | X | – | X | – | – | – |
| DEPH Di-2ethylhexyl)phthalate |
– | X | – | X | – | – | – |
“x”- means that the regulation exists, “-”- means that is not regulated.
4.1. Polycyclic aromatic hydrocarbons (PAHs)
Environmental Protection Agency (US EPA) identified sixteen PAHs as priority pollutants and seven of them are considered as probable carcinogens (those with 4 or more benzene rings like benz[a]anthracene and benzo[a]pyrene) (see Table 4). PAHs are historically most often investigated pollutants. It is found that even 95% of PAHs can be eliminated from wastewater and then associated with sewage sludge. The range of total value of PAHs detected in wastewater and sewage sludge is very wide, from 0.002 to 20 mg/kg DM.
Table 4.
The content of selected PAHs (ng/g) in different countries of EU, adapted from (Gawlik, 2012).
| compound | The range of value (ng/g) | The percentage of trialsa (%) |
|---|---|---|
| Fluoranthene | 34.5–3216.8 | 100 |
| Pyrene | 47.2–2637.0 | 100 |
| Benzo (b) fluoranthene | 25.1–1919.4 | 91 |
| Benzo (a) pyrene | 17.9–1475.5 | 100 |
| Phenanthrene | 29.9–5552.2 | 100 |
| Anthracene | 15.3–724.0 | 84 |
| Benzo (a) anthracene | 9.1–1832.6 | 97 |
| Chrysene | 21–2020.5 | 94 |
| Benzo (k) fluoranthene | 9.9–1048 | 100 |
| Benzo (a) pyrene | 18.9–1477 | 100 |
In how many samples (in percentage), taken by the researchers for analysis, investigated compounds have been detected.
4.2. Other organic contaminants
European Union's Working Document on Sludge (ENV, 2000), proposed 'limit values for concentrations of organic compounds in sludge for use on land, for certain classes of compounds: sum of halogenated organic compounds (AOX); linear alkylbenzene sulphonates (LAS); di(2-ethylhexyl)phthalate (DEHP); nonylphenol and nonylphenol ethoxylates (NPE) with 1 or 2 ethoxy groups; polychlorinated biphenyls (PCBs); and polychlorinated dibenzo-p-dioxins and -furans (PCDD/Fs). In the last years, findings from studies in Germany showed that there had been a decline of PCDD/F PCB and AOX concentration (Table 5 ), while only 3% decrease was noted for AOX. The main sources of these substances are solvents, solvent mixtures, oil and grease, resins, rubber, hydraulic oils, lubricants, plasticizers, disinfectants, wood protectives and some pesticides. According to investigations of Olofsson et al. (2012), the concentration of sewage sludge organotin compounds (OTCs) monobutyltin (MBT) and dibutyltin (DBT); perfluorooctane sulfonamide (PFOSA); polybrominated diphenylethers (PBDEs) 154 and 183; highly chlorinated PCDD/Fs (OCDD) and 1,2,4-trichlorobenzene (124CBz) decreased in time.
Table 5.
Sludge concentrations and changes of selected organic compounds between 1990 and 2012 in Germany.
Anaerobic process is one of the most frequently used strategy for sewage sludge stabilization, mainly at large WWTPs. The investigations of Dąbrowska and Rosińska (2012) shown that thermophilic digestion has a positive effect on degradation of PCBs: total concentration of seven PCBs was reduced by 47%. In turn Siebielska and Sidełko (2015) observed that anaerobic digestion is much more effective than composting in terms of degradadtion of PCBs. The chlorination level of PCB was limiting factor in the composting process, but did not affect the anaerobic digestion of sewage sludge.
The polybrominated diphenylethers (PBDE) are most known flame-retardants, which comprise 209 different brominated diphenylether congeners. They are used in most of the electrical and devices, textiles, furniture, cars, airplanes, colours, polymers (polyurethane foam), resins, coatings. The investigations made in Italy confirm the presence of these contaminants in sewage sludge (Cincinelli et al., 2012). Total PBDE concentrations ranged from 158.3 to 9427 ng g−1 DM, while deca-BDE (BDE-209) (concentrations ranging from 130.6 to 9411 ng g −1 DM) dominated the congener profile in all the samples, contributing between 77% and 99.8% of total PBDE (Cincinelli et al., 2012). Olofsson et al. (2012) shown that during seven years concentration of BDE 209 in sewage sludge increased by 16% year−1. In turn different brominated flame retardants (BFRs) in sewage sludge produced in 17 WWTPs located in the Northeast of Spain were determined (Gorga et al., 2013). The total of eight polybrominated diphenyl ether (PBDE) were analysed, from tri- to deca-BDEs, the emerging BFR compounds, hexabromobenzene (HBB), pentabromoethylbenzene (PBEB) and decabromodiphenylethane (DBDPE). The maximum concentration of one of the PBDE congener - BDE-209 was 2303 ng/g DW, DBDPE 257 ng/g dw, the emerging compounds HBB and PBEB–5.71 and nd–2.33 ng/g, respectively. In turn TBBPA was detected in concentration range of nd–472 ng/g dw, whereas HBCDs between nd and 97.5 ng/g dw.
From other group of organic substances detected in sewage sludge the most important are:
-
-
linear alkylbenzene sulphonates (LAS), anionic surfactants;
-
-
nonylphenol and nonylphenol-ethoxylates (NP, NPEO);
-
-
di-(2-ethylhexyl) phthalate (DEHP) and Dibutyl phthalate (DBP).
Linear alkylbenzene sulphonate (LAS), the main synthetic anionic surfactant was analysed in Spanish WWTPs. It was found that the concentration of LAS in anaerobic sewage sludge samples was 8.06 g/kg, higher than the average values noted in sewage sludge form other European countries (Cantarero et al., 2012).
5. Pharmaceuticals (PhCs) and personal care products (PCPs)
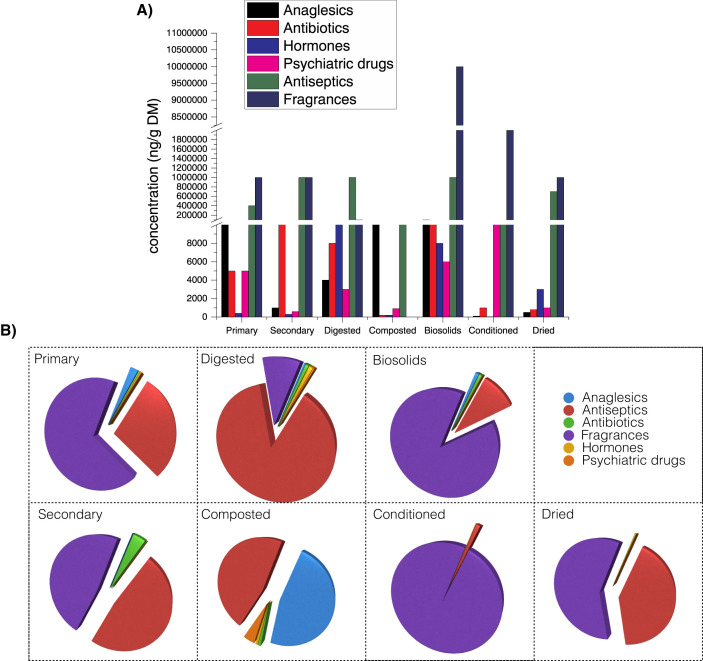
Among socalled emerging organic contaminants (ECs) the greatest interest of the researchers is paid to pharmaceuticals (PhCs) and personal care products (PCPs) (X. Wang et al., 2016). Verlicchi and Zambello (2015) based on 59 papers published between 2002 and 2015, referring to about 450 treatment trains, investigated the content of PCPs in sewage sludge found the following group: analgesics/anti-inflam, Anti-histamines, hormones, antiseptics, antianginals, anti-hypertensives, hypnotics, insect repellents, antiarrhythmics, anti neoplastics, lipid regulators, UV filters, antibiotics, antiplatelets, psychiatric drugs, synthetic musks, anticoagulants, antiprotozoals, contrast media, non-ionic surfactants, antidiabetics, beta-agonists, receptor antagonists, antiemetics, beta-blockers, stimulants, antifungals, diuretics. The concentration of PhCs and PCPs varied depending on sludge processing (Fig. 2 ). Generally the highest values were detected in biosolids, however it is very difficult to find simple correlations, showing that sludge processing has significant effect on decrease/increase the concentrations of selected group PhCs and PCPs.
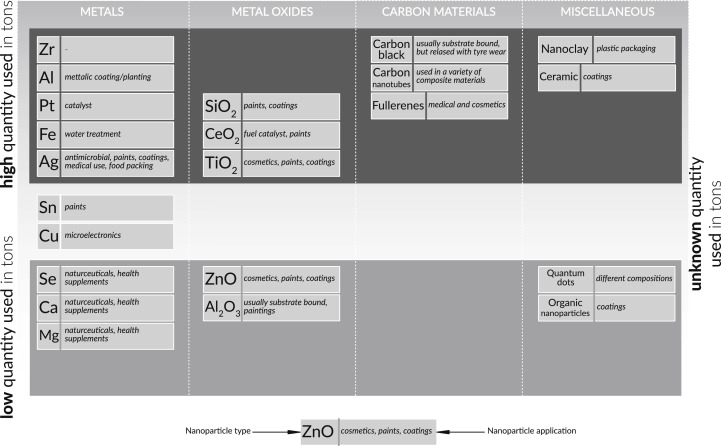
Fig. 2.
Occurrence of nanoparticles in consumer products, adapted from (Brar et al., 2010).
Pharmaceuticals are one of the most investigated compounds present in sewage sludge according to sludge adsorption during wastewater process. Bo et al. (2015) described that effective sludge adsorption can be made in the case of gemfibrozil and cholesterol due to their high log Kow values. Earlier research conducted in different EU countries has shown significant concentrations of active substances such as aspirin, diclofenac or nitrophenol; and thus essentially analgesics and antipyretics in sewage sludge (Table 6 ).
Table 6.
The concentration of selected pharmaceuticals in sewage sludge (ng/g) from different UE countries, adapted from (Gawlik, 2012).
| compound | the range of value | the percentage of trials [%]a |
|---|---|---|
| acesulfame potassium | 0.1–156.7 | 91.4 |
| acetylsalicylic acid | 0.6–563 | 98.3 |
| chloramphenicol | 0–7.6 | 8.6 |
| diclofenac | 1.3–429.1 | 81 |
| ibuprofen | 0.2–108.2 | 72.4 |
| ketoprofen | 0.3–8.6 | 13.8 |
| naproxen | 0.2–9.0 | 58.6 |
| nitrophenol | 0.2–22.2 | 86.2 |
In how many samples (in percentage), taken by the researchers for analysis, investigated compounds have been detected.
Also in the USA 110 biosolids samples have been analysed for the presence of the 72 different pharmaceuticals (McClellan and Halden, 2010). The mean concentration of triclocarban and triclosan was 36 ± 8 and 12.6 ± 3.8 mg kg−1 (n = 5), respectively. On the other hand (Olofsson et al. (2012) has shown that the annual decrease of triclosan in the sludge was 65%, which is probably connected with decrease in its national usage during the seven years from 3.1 to 2.2 tonnes per year. Anaerobic digestion was the most effective technology in reducing a wide spectrum of pharmaceutical residuals (in average ca 30% reduction) (Malmborg and Magnér, 2015). Eyser et al. (2015) investigated the removal rate of 12 pharmaceuticals in sewage sludge (ibuprofen, phenazone, carbamazepine, bezafibrate, fenofibric acid, clarithromycin, roxithromycin, erythromycin, metoprolol, propranolol, diclofenac, sulfamethoxazole) as an effect of by hydrothermal carbonization (HTC). It was noted that removal rates ranged of 39% to ≥97% in spite of phenazone, which increased during the HTC process.
6. Nanoparticles
Increasing interest of using nanotechnologies contributes to entrance of nanoparticles to environment. Nanoparticles of silver, titanium dioxide and zinc oxide are increasingly used in common industrial and consumer products like textiles, cosmetics and sunscreens, thus they can easily enter to WWTPs and reside within sewage sludge as nanomaterials (Fig. 3 ). It was noted that nanoparticles such as Cu, TiO2, Ag° or CeO2 mainly eliminated from wastewater through primary and secondary treatment and then associated with the solid phases of sludge by over 80% by mass (Ganesh et al., 2010, Gómez-Rivera et al., 2012, Kaegi et al., 2011; Y. Wang et al., 2012). The research of Kim et al. (2012) found the presence of nanoparticles of silver sulphide. These nanoparticles of silver sulphide result from oxidation of silver metal to form Ag+ and precipitation of Ag + to form Ag2S which is thermodynamically stable.
Fig. 3.
Comparing the concentration (ng/g DM) (A) and percentages (B) of PhCs and PCPs in different sewage sludge types (primary, secondary, digested, composted, biosolids, conditioned, dried), adapted from (Verlicchi and Zambello, 2015).
Currently it is estimated that over 90% of silver nanoparticles and almost 100% of the silver ions could be absorbed into the sewage sludge. The microbial toxicity of silver is known for centuries. The researchers from Germany found that the predicted no-effect concentration (PNEC) (the highest concentration below which no harmful effects are expected to occur) of silver nanoparticles in soil was 0.05 mg/kg DM soil (Schlich et al., 2013). This concentrations would be equivalent for actual applications of sewage sludge in Germany, where maximum amount equal of 30 mg/kg DM of sludge for each application, based on the average application of 5 tons per hectare every three years (Schlich et al., 2013). Simultaneously, the predicted environmental concentration (PEC) of silver nanoparticles in soil treated with sewage sludge was calculated at the level 0.0015 mg/kg DM soil (Europe). With an estimated annual increase of 0.001 mg/kg DM soil, the researchers suggest the PNEC of silver nanoparticles could be exceeded in 50 years. The investigations of Kim et al. (2012) shown that TiO2 nanoparticles from biosolids can interact with toxic trace metals (as Ag) and enter of soil environment. Johnson et al. (2011) calculated Ti presence of 305 mg/kg DW in wasted sludge thus, 69 t of predicted total 347 t/year discharge of Ti in sludge could be involved.
7. Pathogens
Sewage sludge is a kind of “biological cocktail” containing a mixture of different organisms, both saprophytes and pathogens. The largest and most diverse group present in sewage sludge are heterotrophic and chemautotrophic bacteria, saprophytes and pathogens. The analysis of DNA of the biofilm and S-sludge cells (collected from an integrated fixed-film and suspended growth SBR) by Illumina MiSeq sequencing shown that the dominant genera were Gemmatimonas, Nitrosomonas, Thermomonas and Truepera in the S-sludge, and Nitrosomonas, Opitutus, Nitrospira and Truepera in the biofilm, respectively (P. Zhang et al., 2015). Main groups of pathogenic organisms present in sewage sludge are: the enteric bacteria, parasites, viruses and fungi (Table 7 ).
Table 7.
The occurrence of different group of microorganisms in sewage sludge in relation to soil biota.
| Group of microorganism | Typical subspecies in soil | Occurrence | Typical subspecies in sewage sludge | Occurrence |
|---|---|---|---|---|
| Viruses | Soil is an ecosystem that many new viral species occurred which may represent a large reservoir of theirs's diversity but most of them are not pathogenic for humans and their major role is to influence od bacterial populationh | Polio virusi | ||
| TOTAL | 87–417 × 107 cfu/ge,g,h | Coxsackiei | ||
| Influenza virusi | ||||
| Adenovirusi | 5.8–32.5 × 103 cfu/ld | |||
| Astrovirusi | ||||
| Calicivirusi | ||||
| Coronavirusi | ||||
| Enterovirusi | >8.3 cfu/10g DMd | |||
| Parwovirusi | ||||
| Reovirusi | ||||
| Rotavirusi | 26–30 × 104 cfu/ld | |||
| Norwalk virusi | ||||
| Hepatitis A virusi | ||||
| Hepatitis E virusi | ||||
| TOTAL | ||||
| Bacteria | Pseudomonas spp.b | Arizona hinshawiii | ||
| Arthrobacter spp.b | Aeromonas spp.i | |||
| Corynebacterium spp.b | Bacilluscereusi | |||
| Cellvibrio spp.b | Bacillusanthracisi | |||
| Bacillus spp.b | Brucella spp.i | |||
| Clostridium spp.b | Campylobacter jejunii | 106 gene/mld | ||
| Azotobacter spp. b | Citrobacter spp.i | |||
| Rhizobium spp.b | Clostridium botulinumi | |||
| Nitrosomonas spp.b | Clostridium perfringensi | |||
| Nitrobacter spp.b | Clostridium spp.i | 1–1000 × 103 gene/mld | ||
| Flavobacterium spp.b | Enterobacteriaceaei | 1–50 × 107 gene/mld | ||
| Thiobacillus spp.b | Escherichia colii | |||
| Desulfovibrio spp.b | Klebsiella spp.i | |||
| Escherichia spp.b | Legionella sp.i | |||
| Micrococcus spp.b | Leptospira | |||
| Sarcina spp.b | icterohaemorrhagiaei | |||
| TOTAL | 2.69–3376 × 105 cfu/ge,g | Listeria monocytogenesi | ||
| Mycobacterium tuberculosisi | ||||
| Pasteurella | ||||
| pseudotuberculosisi | ||||
| Proteus spp.i | ||||
| Providencia spp.i | ||||
| Pseudomonas aeruginosai | 5 × 104 gene/mld | |||
| Salmonella spp.i | ||||
| Serratia spp.i | ||||
| Shigella spp.i | ||||
| Staphylococcus aureusi | ||||
| Enterococcus spp.i | ||||
| Vibrio parahaemoliticusi | ||||
| Vibrio choleraei | ||||
| Yersinia enterocoliticai | ||||
| TOTAL | ||||
| Fungi | Neurospora spp.a | Absidia spp.i | ||
| Saccharomyces spp.a | Aspergillus spp.i | 102-103 cfu/g DWd | ||
| Morchella spp.a | Candida albicansi | |||
| Amanita spp.a | Candida guillermondiii | |||
| Mucor spp.a | Candida kruseii | |||
| Rhizopus spp.a | Candida tropicalisi | |||
| Penicillum spp.a | Cryptococcus neoformansi | |||
| Aspergillus spp.a | Epidermophyton spp.i | |||
| Candida spp.a | Fusarium spp.i | |||
| Penicillium brasilianumf | Geotrichum candidumi | |||
| Aspergillus fumigatusf | Microsporum spp.i | |||
| Trichoiderma spp.f | Mucor spp.i | |||
| Giberella spp.f | Penicillium spp.i | |||
| Paecilomyces spp.f | Phialophora richardsiii | |||
| Trechispora spp.f | Trichoderma spp.i | |||
| Mortirella spp.f | Trichosporon cutaneumi | |||
| Cryptococcus spp.f | Trichophyton spp.i | 114.3–752.3 × 106 cfu/mld | ||
| TOTAL | Verticillium spp.i | |||
| TOTAL | ||||
| Protozoa | Trypanosomab | Acanthomoebai | ||
| Leishamaniab | Dientamoeba fragilisi | |||
| Trichomonasb | Entamoebahystoliticai | |||
| Euglenab | Giardia lambliai | 22-32 oocysts/gd | ||
| Amoebab | Giardia intestinalisi | |||
| Entoamoebab | Isospora bellii | |||
| Balantidiumb | Naeglaria fomlerii | |||
| Parameciumb | Palantidium colii | |||
| Tetrahymenab | Sarcocystis spp.i | |||
| Spumella sp.c | Toxoplasma gondiii | |||
| Heteromita sp.c | Cryptosporidiumd | 12–17d | ||
| Goniomonas sp.c | TOTAL | 12–32d | ||
| Spongomonas sp.c | ||||
| TOTAL | ||||
| Helminths | Toxocara spp.a | 3.25 eggs/ga | Ankylostoma duodenalei | |
| Toxocara vulpisa | 2.9a | Ascaris lumbricoidesi | ||
| Ascaris spp.a | 1.75a | Echinococcus granulosusi | ||
| Trichuris spp.a | 0.2a | Echinococcus multilocularisi | ||
| Ascaris lumbricoidesa | 454.5a | Enterobium vermicularisi | ||
| TOTALa | 0.063–453.5a | Hymenolepsis spp.i | 1.5–6 eggs/ga | |
| Necator americanusi | ||||
| Strongyloides stercoralisi | ||||
| Taenia saginatai | ||||
| Taenia soliumi | ||||
| Toxocara spp.a | ||||
| Trichuris spp.a | 13–94a | |||
| Ascaris spp.a | 312–776a | |||
| TOTALa | 0-2910a,d | |||
Investigation of viral pathogen diversity in sewage sludge by metagenome analysis shown the high abundance of newly emerging viruses (e.g., Coronavirus HKU1, Klassevirus, and Cosavirus) the strong representation of respiratory viruses, and the relatively minor abundance and occurrence of Enteroviruses (Bibby and Peccia, 2013). Schlindwein et al. (2010) on the base of samples from local WWTP in Florianopolis city, Brazil noted that from four viruses the most prevalent was Adenovirus (AdV) next Rotavirus (RV), Poliovirus (PV) and hepatitis A virus (HAV) Viral viability by cell culture (ICC-PCR) was: AdV: 100%, HAV: 16.7%, PV: 91.7%, RV: 25%, respectively.
On the other hand sewage sludge can be a source for more virulent strains of commonly occurring microorganisms. According to presence of antibiotics in environment, in sewage sludge some antibiotic resistances such as multi-resistant E. coli strains can occur (Reinthaler et al., 2013). Very little is also known about ability to survive in sewage sludge and then in soil strong pathogenic strains, such as EHEC pathogen O104:H4. The bacteria was found in different period of year at four urban wastewater treatment plants in southern Poland (Fijalkowski et al., 2014).
Sewage sludge processing, mainly hygenization with the use of high temperature, anaerobic digestion, trickling filters (TF) or autothermal thermophilic aerobic digestion (ATAD) may control the presence of pathogens as E. coli or Salmonella (De los Cobos-Vasconcelos et al., 2015, Fu et al., 2014, Marin et al., 2015) and modify bacterial community structure. Stiborova et al. (2015) described that sludge after TAD treatment had considerably higher number of thermotolerant/thermophilic taxa, such as the phyla Deinococcus-Thermus and Thermotogae or the genus Coprothermobacter. However, the occurrence of fecal contamination indicators is frequently not correlated with the presence of other pathogenic microorganisms that may inhibit sewage sludge and survive the treatment processes.
8. Conclusion
Sewage sludge is one of the most important renewable source of nutrients. It is known that this material could substitute up to 60% lack of mineral phosphorus obtained from sewage sludge ash afters incineration (Guedes et al., 2014) or dried processes (Kahiluoto et al., 2015). However, development of technology and analytic techniques contribute to “discovery” of new sludge contaminants, which negatively affect environmental balance. Threats arising from excessive amounts of heavy metals are slowly replaced by specific form of selected trace elements – nanoparticles. The industry as a well known main source of contaminants is increasingly replaced by households, as in the case of pharmaceuticals (PhCs) and personal care products (PCPs). The concentrations of so called emerging contaminants (ECs) in sewage sludge are noted between few μg kg−1 (estrogens, some pharmaceuticals, PFCs) to g kg−1 (LAS). The excessive content of antibiotics are closely connected with appearance of antibiotic resistance such as multi-resistant E. coli strains or strong pathogenic strain, such as EHEC pathogen O104:H4. All of these threats should lead to stronger limits considering the direct use of sewage sludge as a fertilizer on land. Sewage sludge processes (anaerobic digestion, composting, even thermal carbonization) are not guarantee to obtain the product of high quality without contaminants. Hence, these insights should to be investigated in future studies.
Acknowledgments
The research leading to these results has received funding from the Polish-Norwegian Research Programme operated by the National Centre for Research and Development under the Norwegian Financial Mechanism 2009–2014 in the frame of Project Contract No (POL NOR/201734/76) and internal Czestochowa University of Technology grant BS PB 401/304/11.
References
- Amoah Isaac Dennis, Singh Gulshan, Stenström Thor Axel, Reddy Poovendhree. Detection and quantification of soil-transmitted helminths in environmental samples: a review of current state-of-the-art and future perspectives. Acta Trop. 2017;169:187–201. doi: 10.1016/j.actatropica.2017.02.014. [DOI] [PubMed] [Google Scholar]
- Bergs C.G. Presented at the Polish-Germany Work Group Meeting, Bonn. 2015. Sewage sludge management and the recycling of phosphorus in Germany. [Google Scholar]
- Bibby Kyle, Peccia Jordan. Identification of viral pathogen diversity in sewage sludge by metagenome analysis. Environ. Sci. Technol. 2013;47(4):1945–1951. doi: 10.1021/es305181x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bo Longli, Feng Li, Fu Jinting, Li Xuegang, Li Ping, Zhang Yahong. The fate of typical pharmaceuticals in wastewater treatment plants of Xi’an city in China. J. Environ. Chem. Eng. 2015;3(3):2203–2211. [Google Scholar]
- Brar Satinder K., Verma Mausam, Tyagi R.D., Surampalli R.Y. Engineered nanoparticles in wastewater and wastewater sludge – evidence and impacts. Waste Manag. 2010;30(3):504–520. doi: 10.1016/j.wasman.2009.10.012. [DOI] [PubMed] [Google Scholar]
- Bueno M.J. Martínez, Gomez M.J., Herrera S., Hernando M.D., Agüera A., Fernández-Alba A.R. Occurrence and persistence of organic emerging contaminants and priority pollutants in five sewage treatment plants of Spain: two years pilot survey monitoring. Environ. Pollut. 2012;164:267–273. doi: 10.1016/j.envpol.2012.01.038. [DOI] [PubMed] [Google Scholar]
- Cantarero S., Prieto C.A., López I. Occurrence of high-tonnage anionic surfactants in Spanish sewage sludge. J. Environ. Manag. 2012;95:S149–S153. doi: 10.1016/j.jenvman.2011.05.027. [DOI] [PubMed] [Google Scholar]
- Cincinelli Alessandra, Martellini Tania, Misuri Lorenza, Lanciotti Eudes, Sweetman Andy, Laschi Serena, Palchetti Ilaria. PBDEs in Italian sewage sludge and environmental risk of using sewage sludge for land application. Environ. Pollut. 2012;161:229–234. doi: 10.1016/j.envpol.2011.11.001. [DOI] [PubMed] [Google Scholar]
- Clarke Bradley O., Smith Stephen R. Review of ‘emerging’ organic contaminants in biosolids and assessment of international research priorities for the agricultural use of biosolids. Environ. Int. 2011;37(1):226–247. doi: 10.1016/j.envint.2010.06.004. [DOI] [PubMed] [Google Scholar]
- Dąbrowska L., Rosińska A. Change of PCBs and forms of heavy metals in sewage sludge during thermophilic anaerobic digestion. Chemosphere. 2012;88:168–173. doi: 10.1016/j.chemosphere.2012.02.073. [DOI] [PubMed] [Google Scholar]
- De los Cobos-Vasconcelos D., Villalba-Pastrana M.E., Noyola A. Effective pathogen removal by low temperature thermal pre-treatment and anaerobic digestion for Class A biosolids production from sewage sludge. J. Water, Sanit. Hyg. Dev. 2015;5(1):56–63. [Google Scholar]
- ENV E. 2000. Working Document on Sludge, 3rd Draft. DG ENV. E. 3/LM. [Google Scholar]
- Environment E.C.D. BIO Intelligence Service; 2014. Ex-post Evaluation of Certain Waste Stream Directives. [Google Scholar]
- Enviseng Environmental Consulting Services . Communities of Tommorow, Municipal Innovation Network; 2012. Beneficial Practice and Appropriate Technology Guide. Wastewater Treatment Plant Sludge and Biosolids. [Google Scholar]
- Eyser, Vom C., Palmu K., Schmidt T.C., Tuerk J. Pharmaceutical load in sewage sludge and biochar produced by hydrothermal carbonization. Sci. Total Environ. 2015;537:180–186. doi: 10.1016/j.scitotenv.2015.08.021. [DOI] [PubMed] [Google Scholar]
- Fijalkowski K.L., Kacprzak M.J., Rorat A. Occurrence changes of Escherichia coli(including O157:H7 serotype) in wastewater and sewage sludge by quantitation method of (EMA) real time—PCR. Desalin. Water Treat. 2014;52(19–21):3965–3972. [Google Scholar]
- Finlay B.J., Black H.I.J., Brown S., Clarke K.J., Esteban G.F., Hindle R.M., Olmo J.L., Rollett A., Vickerman K. Estimating the Growth Potential of the Soil Protozoan Community. Protist. 2000;151:367. doi: 10.1078/1434-4610-00008. [DOI] [PubMed] [Google Scholar]
- Fu B., Jiang Q., Liu H., Liu H., Liu H. Occurrence and reactivation of viable but non-culturable E. coli in sewage sludge after mesophilic and thermophilic anaerobic digestion. Biotechnol. Lett. 2014;36:273–279. doi: 10.1007/s10529-013-1361-9. [DOI] [PubMed] [Google Scholar]
- Ganesh Rajagopalan, Smeraldi Josh, Hosseini Turaj, Khatib Leila, Olson Betty H., Rosso Diego. Evaluation of nanocopper removal and toxicity in municipal wastewaters. Environ. Sci. Technol. 2010;44(20):7808–7813. doi: 10.1021/es101355k. [DOI] [PubMed] [Google Scholar]
- Gawlik B. Presented at the Proceedings of Workshop DG ENV and DG JRC. 2012. FATE SEES Results of a Pan-European Snapshot of randomly taken sewage sludge sample. [Google Scholar]
- Gerardi M.H., Zimmerman M.C. John Wiley & Sons, Inc.; Hoboken, NJ, USA: 2005. Wastewater Pathogens. [Google Scholar]
- Gianico A., Braguglia C.M., Mascolo G., Mininni G. Partitioning of nutrients and micropollutants along the sludge treatment line: a case study. Environ. Sci. Pollut. Res. 2013;20(9):6256–6265. doi: 10.1007/s11356-013-1686-x. [DOI] [PubMed] [Google Scholar]
- Gorga Marina, Martínez Elena, Ginebreda Antoni, Eljarrat Ethel, Barceló Damià. Determination of PBDEs, HBB, PBEB, DBDPE, HBCD, TBBPA and related compounds in sewage sludge from Catalonia (Spain) Sci. Total Environ. 2013;444:51–59. doi: 10.1016/j.scitotenv.2012.11.066. [DOI] [PubMed] [Google Scholar]
- Gómez-Rivera Francisco, Field James A., Brown Dustin, Sierra-Alvarez Reyes. Fate of cerium dioxide (CeO2) nanoparticles in municipal wastewater during activated sludge treatment. Bioresour. Technol. 2012;108:300–304. doi: 10.1016/j.biortech.2011.12.113. [DOI] [PubMed] [Google Scholar]
- Guedes P., Couto N., Ottosen L.M., Ribeiro A.B. Phosphorus recovery from sewage sludge ash through an electrodialytic process. Waste Manag. 2014;34(5):886–892. doi: 10.1016/j.wasman.2014.02.021. [DOI] [PubMed] [Google Scholar]
- Harrison E.Z., Oakes S.R., Hysell M., Hay A. Organic chemicals in sewage sludges. Sci. Total Environ. 2006;367(2–3):481–497. doi: 10.1016/j.scitotenv.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Healy M.G., Fenton O., Forrestal P.J., Danaher M., Brennan R.B., Morrison L. Metal concentrations in lime stabilised, thermally dried and anaerobically digested sewage sludges. Waste Manag. 2016;48:404–408. doi: 10.1016/j.wasman.2015.11.028. [DOI] [PubMed] [Google Scholar]
- Johnson A.C., Bowes M.J., Crossley A., Jarvie H.P., Jurkschat K., Jürgens M.D., Lawlor A.J., Park B., Rowland P., Spurgeon D., Svendsen C., Thompson I.P., Barnes R.J., Williams R.J., Xu N. An assessment of the fate, behaviour and environmental risk associated with sunscreen TiO2 nanoparticles in UK field scenarios. Sci. Total Environ. 2011;409(13):2503–2510. doi: 10.1016/j.scitotenv.2011.03.040. [DOI] [PubMed] [Google Scholar]
- Kabata-Pendias A. fourth ed. CRC Press; 2010. Trace Elements in Soils and Plants. [Google Scholar]
- Kaegi Ralf, Voegelin Andreas, Sinnet Brian, Zuleeg Steffen, Hagendorfer Harald, Burkhardt Michael, Siegrist Hansruedi. Behavior of metallic silver nanoparticles in a pilot wastewater treatment plant. Environ. Sci. Technol. 2011;45(9):3902–3908. doi: 10.1021/es1041892. [DOI] [PubMed] [Google Scholar]
- Kahiluoto H., Kuisma M., Ketoja E., Salo T., Heikkinen J. Phosphorus in manure and sewage sludge more recyclable than in soluble inorganic fertilizer. Environ. Sci. Technol. 2015;49(4):2115–2122. doi: 10.1021/es503387y. [DOI] [PubMed] [Google Scholar]
- Kim B., Murayama M., Colman B.P., Hochella M.F. Characterization and environmental implications of nano- and larger TiO2 particles in sewage sludge, and soils amended with sewage sludge. J. Environ. Monit. 2012;14:1128–1136. doi: 10.1039/c2em10809g. [DOI] [PubMed] [Google Scholar]
- Kimura M., Jia Z.-J., Nakayama N., Asakawa S. Ecology of viruses in soils: past, present and future perspectives. Soil Sci. Plant Nutr. 2010;54:1–32. [Google Scholar]
- Lasheen M.R., Ammar N.S. Assessment of metals speciation in sewage sludge and stabilized sludge from different Wastewater Treatment Plants, Greater Cairo, Egypt. J. Hazard. Mater. 2009;164(2–3):740–749. doi: 10.1016/j.jhazmat.2008.08.068. [DOI] [PubMed] [Google Scholar]
- LeBlanc R.J., Matthews P., Richard R.P. Global atlas of excreta, wastewater sludge, and biosolids management: moving forward the sustainable and welcome uses of a global resource. Choice Rev. Online. 2009 [Google Scholar]
- Li Yongchun, Li Yongfu, Chang Scott X., Liang Xue, Qin Hua, Chen Junhui, Xu Qiufang. Linking soil fungal community structure and function to soil organic carbon chemical composition in intensively managed subtropical bamboo forests. Soil Biol. Biochem. 2017;107:19–31. [Google Scholar]
- Madsen E.L. 2015. Environmental Microbiology: from Genomes to Biogeochemistry. [Google Scholar]
- Malmborg Jonas, Magnér Jörgen. Pharmaceutical residues in sewage sludge: effect of sanitization and anaerobic digestion. J. Environ. Manag. 2015;153:1–10. doi: 10.1016/j.jenvman.2015.01.041. [DOI] [PubMed] [Google Scholar]
- Marchal V., Dellink R., Van Vuuren D., Clapp C., Chateau J., Magné B., van Vliet J. Organization for Economic Co-operation and Development (OECD); 2011. OECD Environmental Outlook to 2050. [Google Scholar]
- Marín I., Goñi P., Lasheras A.M., Ormad M.P. Efficiency of a Spanish wastewater treatment plant for removal potentially pathogens: characterization of bacteria and protozoa along water and sludge treatment lines. Ecol. Eng. 2015;74:28–32. [Google Scholar]
- McClellan K., Halden R.U. Pharmaceuticals and personal care products in archived U.S. biosolids from the 2001 EPA National Sewage Sludge Survey. Water Res. 2010;44(2):658–668. doi: 10.1016/j.watres.2009.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mininni G., Blanch A.R., Lucena F., Berselli S. EU policy on sewage sludge utilization and perspectives on new approaches of sludge management. Environ. Sci. Pollut. Res. Int. 2015;22(10):7361–7374. doi: 10.1007/s11356-014-3132-0. [DOI] [PubMed] [Google Scholar]
- Nikovski G.N., Kalinichenko K.V. Biotechnology of utilization of municipal wastewater sediments. Biotechnol. Acta. 2014;7:21–32. [Google Scholar]
- Olofsson U., Bignert A., Haglund P. Time-trends of metals and organic contaminants in sewage sludge. Water Res. 2012;46(15):4841–4851. doi: 10.1016/j.watres.2012.05.048. [DOI] [PubMed] [Google Scholar]
- Reinthaler F.F., Galler H., Feierl G., Haas D., Leitner E., Mascher F., Melkes A., Posch J., Pertschy B., Winter I., Himmel W., Marth E., Zarfel G. Resistance patterns of Escherichia coli isolated from sewage sludge in comparison with those isolated from human patients in 2000 and 2009. J. Water Health. 2013;11(1):13–20. doi: 10.2166/wh.2012.207. [DOI] [PubMed] [Google Scholar]
- Romdhana Mohamed Hédi, Lecomte Didier, Ladevie Bruno, Sablayrolles Caroline. Monitoring of pathogenic microorganisms contamination during heat drying process of sewage sludge. Process Saf. Environ. Prot. 2009;87(6):377–386. [Google Scholar]
- Schlich Karsten, Klawonn Thorsten, Terytze Konstantin, Hund-Rinke Kerstin. Hazard assessment of a silver nanoparticle in soil applied via sewage sludge. Environ. Sci. Eur. 2013;25(1):17. [Google Scholar]
- Schlindwein A.D., Rigotto C., Simões C.M.O., Barardi C.R.M. Detection of enteric viruses in sewage sludge and treated wastewater effluent. Water Sci. Technol. 2010;61:537–544. doi: 10.2166/wst.2010.845. [DOI] [PubMed] [Google Scholar]
- Shamuyarira K., Gumbo J. Assessment of heavy metals in municipal sewage sludge: a case study of Limpopo province, South Africa. Int. J. Environ. Res. Public Health 2014. 2014;11 doi: 10.3390/ijerph110302569. 2569–2579 11, 2569–2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebielska Izabela, Sidełko Robert. Polychlorinated biphenyl concentration changes in sewage sludge and organic municipal waste mixtures during composting and anaerobic digestion. Chemosphere. 2015;126:88–95. doi: 10.1016/j.chemosphere.2014.12.051. [DOI] [PubMed] [Google Scholar]
- Smith S. A critical review of the bioavailability and impacts of heavy metals in municipal solid waste composts compared to sewage sludge. Environ. Int. 2009;35:142–156. doi: 10.1016/j.envint.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Srinivasiah Sharath, Bhavsar Jaysheel, Thapar Kanika, Liles Mark, Schoenfeld Tom, Wommack K. Eric. Phages across the biosphere: contrasts of viruses in soil and aquatic environments. Res. Microbiol. 2008;159(5):349–357. doi: 10.1016/j.resmic.2008.04.010. [DOI] [PubMed] [Google Scholar]
- Stasinakis Athanasios S. Review on the fate of emerging contaminants during sludge anaerobic digestion. Bioresour. Technol. 2012;121:432–440. doi: 10.1016/j.biortech.2012.06.074. [DOI] [PubMed] [Google Scholar]
- Stiborova Hana, Wolfram Jan, Demnerova Katerina, Macek Tomas, Uhlik Ondrej. Bacterial community structure in treated sewage sludge with mesophilic and thermophilic anaerobic digestion. Folia Microbiol. 2015;60(6):531–539. doi: 10.1007/s12223-015-0396-9. [DOI] [PubMed] [Google Scholar]
- Tyagi Vinay Kumar, Lo Shang-Lien. Sludge: a waste or renewable source for energy and resources recovery? Renew. Sustain. Energy Rev. 2013;25:708–728. [Google Scholar]
- USEPA . 2009. Targeted National Sewage Sludge Survey Sampling and Analysis Technical Report. [Google Scholar]
- Verlicchi P., Zambello E. Pharmaceuticals and personal care products in untreated and treated sewage sludge: occurrence and environmental risk in the case of application on soil — a critical review. Sci. Total Environ. 2015;538:750–767. doi: 10.1016/j.scitotenv.2015.08.108. [DOI] [PubMed] [Google Scholar]
- Wang Xingdong, Li Chunxing, Zhang Bin, Lin Jingjiang, Chi Qiaoqiao, Wang Yin. Migration and risk assessment of heavy metals in sewage sludge during hydrothermal treatment combined with pyrolysis. Bioresour. Technol. 2016;221:560–567. doi: 10.1016/j.biortech.2016.09.069. [DOI] [PubMed] [Google Scholar]
- Wang Yifei, Westerhoff Paul, Hristovski Kiril D. Fate and biological effects of silver, titanium dioxide, and C60 (fullerene) nanomaterials during simulated wastewater treatment processes. J. Hazard. Mater. 2012;201–202:16–22. doi: 10.1016/j.jhazmat.2011.10.086. [DOI] [PubMed] [Google Scholar]
- Wiechmann B., Dienemann C., Kabbe C., Brandt S., Vogel I., Roskosch A. Umweltbundesamt (UBA); Germany: 2013. Sewage sludge management in Germany. [Google Scholar]
- Wilk, M., Gworek, B., 2009. Heavy metals in sewage sludge. Ochr. (Środow. Zasob. Natur).
- Williamson K.E., Radosevich M., Wommack K.E. Abundance and diversity of viruses in six Delaware soils. Appl. Environ. Microbiol. 2005;71(6):3119–3125. doi: 10.1128/AEM.71.6.3119-3125.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Zhihua, Yuan Xingzhong, Li Hui, Jiang Longbo, Leng Lijian, Chen Xiaohong, Zeng Guangming, Li Fei, Cao Liang. Chemical speciation, mobility and phyto-accessibility of heavy metals in fly ash and slag from combustion of pelletized municipal sewage sludge. Sci. Total Environ. 2015;536:774–783. doi: 10.1016/j.scitotenv.2015.07.126. [DOI] [PubMed] [Google Scholar]
- Zhang Peng, Guo Jin-Song, Shen Yu, Yan Peng, Chen You-Peng, Wang Han, Yang Ji-Xiang, Fang Fang, Li Chun. Microbial communities, extracellular proteomics and polysaccharides: a comparative investigation on biofilm and suspended sludge. Bioresour. Technol. 2015;190:21–28. doi: 10.1016/j.biortech.2015.04.058. [DOI] [PubMed] [Google Scholar]
- Zhang Yong, Zhao Lihong, Wang Yao, Yang Baoyu, Chen Shiyun. Enhancement of heavy metal accumulation by tissue specific co-expression of iaaM and ACC deaminase genes in plants. Chemosphere. 2008;72(4):564–571. doi: 10.1016/j.chemosphere.2008.03.043. [DOI] [PubMed] [Google Scholar]
- Zhen Guangyin, Lu Xueqin, Kato Hiroyuki, Zhao Youcai, Li Yu-You. Overview of pretreatment strategies for enhancing sewage sludge disintegration and subsequent anaerobic digestion: current advances, full-scale application and future perspectives. Renew. Sustain. Energy Rev. 2017;69:559–577. [Google Scholar]