Abstract
In the studies presented here, dogs were vaccinated against Leishmania (Leishmania) chagasi challenge infection using a preparation of Leishmania braziliensis promastigote proteins and saponin as adjuvant (LBSap). Vaccination with LBSap induced a prominent type 1 immune response that was characterized by increased levels of interleukin (IL-) 12 and interferon gamma (IFN-γ) production by peripheral blood mononuclear cells (PBMC) upon stimulation with soluble vaccine antigen. Importantly, results showed that this type of responsiveness was sustained after challenge infection; at day 90 and 885 after L. chagasi challenge infection, PBMCs from LBSap vaccinated dogs produced more IL-12, IFN-γ and concomitant nitric oxide (NO) when stimulated with Leishmania antigens as compared to PBMCs from respective control groups (saponin, LB- treated, or non-treated control dogs). Moreover, transforming growth factor (TGF)-β decreased in the supernatant of SLcA-stimulated PBMCs in the LBSap group at 90 days. Bone marrow parasitological analysis revealed decreased frequency of parasitism in the presence of vaccine antigen. It is concluded that vaccination of dogs with LBSap vaccine induced a long-lasting type 1 immune response against L. chagasi challenge infection.
Keywords: Vaccine, Canine visceral leishmaniasis, Cytokines, Immunoprotection, Leishmania chagasi
1. Introduction
Visceral leishmaniasis with a zoonotic feature is caused by protozoan species belonging to the complex Leishmania donovani (Leishmania infantum syn. Leishmania chagasi, in Latin America) and is widely distributed in the Mediterranean Basin, Middle East, and South America (Desjeux, 2004). Canines are the main reservoir for the parasite in different geographical regions of the globe and play a relevant role in transmission to humans (Deane, 1961, Dantas-Torres, 2006). Thus, the current strategy for control of the disease includes the detection and elimination of seropositive dogs alongside vector control and therapy for human infection (Tesh, 1995). Chemotherapy in dogs still does not provide parasitological cure (Noli and Auxilia, 2005), and for this reason a vaccine against visceral leishmaniasis (VL) would be an important tool in the control of canine visceral leishmaniasis (CVL) and would also dramatically decrease the infection pressure of L. chagasi for humans (Hommel et al., 1995, Dye, 1996).
Toward this purpose, establishing biomarkers of immunogenicity is considered critical in analyzing candidate vaccines against CVL (Reis et al., 2010, Maia and Campino, 2012), and this strategy is being used to identify the pattern of immune response in dogs and to further the search for vaccine candidates against CVL (Reis et al., 2010). Several studies have reported the potential of different CVL vaccines to trigger immunoprotective mechanisms against Leishmania infection (Borja-Cabrera et al., 2002, Rafati et al., 2005, Holzmuller et al., 2005, Giunchetti et al., 2007, Lemesre et al., 2007, Araújo et al., 2008, Araújo et al., 2009, Fernandes et al., 2008, Giunchetti et al., 2008a, Giunchetti et al., 2008b).
The polarized immune response described in a mouse model during Leishmania infection (Mosman et al., 1986, Barral et al., 1993, Kane and Mosser, 2001, Murray et al., 2002, Trinchieri, 2007) does not occur in dogs, with different studies demonstrating the simultaneous presence of interferon (IFN)-γ and interleukin (IL)-10 (Chamizo et al., 2005, Lage et al., 2007, Menezes-Souza et al., 2011). In addition, a mixed profile of cytokines has been described during CVL, with high levels of IFN-γ, IL-10, and transforming growth factor (TGF)-β, concomitant with reduced expression of IL-12 according to skin parasite load (Menezes-Souza et al., 2011).
Studies evaluating other biomarkers of immunogenicity induced by the LBSap vaccine (composed of L. braziliensis promastigote proteins plus saponin as the adjuvant) have demonstrated higher levels of circulating T lymphocytes (CD5+, CD4+, and CD8+) and B lymphocytes (CD21+) and increased levels of Leishmania-specific CD8+ and CD4+ T cells (Giunchetti et al., 2007, Giunchetti et al., 2008a). LBSap vaccine is considered safe for administration, without induction of ulcerative lesions at the site of inoculation (Giunchetti et al., 2007, Vitoriano-Souza et al., 2008). Moreover, LBSap vaccinated dogs presented high IFN-γ and low IL-10 and TGF-β1 expression in the spleen, with significant reduction of parasite load in this organ (Roatt et al., 2012). Additionally, LBSap displayed a strong and sustained induction of humoral immune response, with increased levels of anti-Leishmania total IgG as well as both IgG1 and IgG2, after experimental challenge (Roatt et al., 2012).
Considering the promising results of the LBSap vaccine, we aimed to further evaluate the immunogenicity biomarkers before and after experimental L. chagasi challenge. Thus, the profile of different cytokines (IL-4, IL-10, TGF-β, IL-12, IFN-γ, and tumor necrosis factor [TNF]-α) and nitric oxide (NO) in supernatants of peripheral blood mononuclear cell (PBMC) cultures were evaluated before the first immunization (T0), 15 days after completion of the vaccine protocol (T3), and at time points 90 (T90) and 885 (T885) days after experimental L. chagasi challenge. The frequency of parasitism in the bone marrow was also evaluated until T885.
2. Materials and methods
2.1. Animals, vaccination and experimental challenge with L. chagasi plus saliva of Lutzomyia longipalpis
Twenty male and female mongrel dogs that had been born and reared in the kennels of the Instituto de Ciências Exatas e Biológicas, Universidade Federal de Ouro Preto, Ouro Preto, Minas Gerais, Brazil, were treated at 7 months with an anthelmintic and vaccinated against rabies (Tecpar, Curitiba-PR, Brazil), canine distemper, type 2 adenovirus, coronavirus, parainfluenza, parvovirus, and leptospira (Vanguard® HTLP 5/CV-L; Pfizer Animal Health, New York, NY, USA). The absence of specific anti-Leishmania antibodies was confirmed by indirect fluorescence immunoassay. Experimental dogs were divided into four experimental groups: (i) control (C) group (n = 5) received 1 ml of sterile 0.9% saline; (ii) LB group (n = 5) received 600 μg of L. braziliensis promastigote protein in 1 ml of sterile 0.9% saline; (iii) Sap group (n = 5) received 1 mg of saponin (Sigma Chemical Co., St. Louis, MO, USA) in 1 ml of sterile 0.9% saline; and (iv) LBSap group (n = 5) received 600 μg of L. braziliensis promastigote protein and 1 mg of saponin in 1 ml of sterile 0.9% saline. All animals received subcutaneous injections in the right flank at intervals of 4 weeks for a total of three injections. The challenge of experimental animals was performed after 100 days of vaccination protocol. In this sense, all dogs received intradermally 1.0 × 107 promastigotes of L. chagasi stationary phase of cultivation, in the inner side of the left ear, in addition to 5 acini of the salivary gland of L. longipalpis. This preliminary stage of the study was performed from 2005 to 2007.
2.2. Vaccine preparation
Promastigotes of L. braziliensis (MHOM/BR/75/M2903) were maintained in in vitro culture in NNN/LIT media as previously described (Giunchetti et al., 2007). Briefly, parasites were harvested by centrifugation (2000 × g, 20 min, 4 °C) from 10-day-old cultures, washed three times in saline buffer, fully disrupted by ultrasound treatment (40 W, 1 min, 0 °C), separated into aliquots, and stored at −80 °C until required for use. Protein concentration was determined according to the method of Lowry (Lowry et al., 1951). The LBSap vaccine was previously described by Giunchetti et al., 2007 and registered at the Instituto Nacional da Propriedade Industrial (Brazil) under patent number PI 0601225-6 (17 February 2006).
2.3. Blood sample collection and in vitro assays
Peripheral blood samples were collected before the first immunization (T0), 15 days after completion of the vaccine protocol (T3) and at time points of 90 (T90) and 885 (T885) days after experimental L. chagasi challenge by puncture of the jugular vein in sterile heparinized 20 ml syringes. To obtain PBMCs for the in vitro analysis, the blood collected was added over 10 ml of Ficoll-Hypaque (Histopaque® 1077, Sigma) and subjected to centrifugation at 450 × g for 40 min at room temperature. The separated PBMCs were resuspended in Gibco RPMI1640 medium, washed twice with RPMI 1640, centrifuged at 450 × g for 10 min at room temperature, homogenized, and finally resuspended in RPMI 1640 at 107 cells/ml as previously described (Giunchetti et al., 2007).
The in vitro assays were performed in 48-well flat-bottomed tissue culture plates (Costar, Cambridge, MA, USA), with each well containing 650 μl of culture medium (10% fetal bovine serum/1% streptavidin/penicillin, 2 mM l-glutamine, and 0.1% β-mercaptoethanol in RPMI 1640) and 50 μl of PBMCs (5.0 × 105 cells/well) with 100 μl of vaccine soluble antigen (VSA; L. braziliensis, 25 μg/ml) or 100 μl of soluble L. chagasi antigen (SLcA, 25 μg/ml) obtained according to Reis et al. (Reis et al., 2006a, Reis et al., 2006b). One-hundred μl of RPMI was added in place of the antigenic stimulus in the non-stimulated control cultures. Incubation was carried out in a humidified incubator with 5% CO2, at 37 °C for 5 days, after which the supernatants were collected and stored in a freezer at −80 °C for detection of cytokine and NO.
The in vitro evaluation was performed with the supernatant of PBMCs collected at T0, T3, T90 and T885, which were stored as described above.
2.4. Quantification of cytokines
Cytokine levels were determined by enzyme-linked immunosorbent assay (ELISA), purchased from R&D Systems (Minneapolis, MN, USA), according to the manufacturer's instructions. DuoSet ELISA was used for analysis of TNF-α (anti-canine TNF-α/TNFSF1A immunoassay; catalog number: DY1507); IL-10 (anti-canine IL-10, catalog number: DY735); IL-12 (anti-canine IL-12/IL-23 p40, catalog number: DY1969); and IFN-γ (anti-canine IFN-γ, catalog number: DY781B) cytokines. The level of TGF-β was quantified by ELISA using the Quantikine® kit (mouse/rat/porcine/canine TGF-β1 immunoassay, catalog number MB100B). IL-4 cytokine was evaluated using monoclonal anti-canine IL-4 antibody (catalog number: MAB7541) as capture antibody; recombinant canine IL-4 (catalog number: 754CL) for obtaining the standard curve; and biotinylated anti-canine IL-4 antibody (catalog number: BAF754), streptavidin (R&D Systems, DY998), and substrate solution (1:1 mixture of H2O2 and tetramethylbenzidine, product code 50-76-4, lot. no. RB49). Minimum sensitivity was 63 pg/ml for TNF-α, 78 pg/ml for IL-10, 62 pg/ml for IL-12, 63 pg/ml for IFN-γ, 31 pg/ml for TGF-β, and 78 pg/ml for IL-4.
All experiments were performed using 96-well plates (COSTAR®, Washington, DC), according to R&D Systems instructions. The reading was performed using the microplate automatic reader (EL800, Biotek, Winosski, VT) at a wavelength of 450 nm.
2.5. NO production
Quantification of levels of NO was performed indirectly by measuring nitrite in supernatants of PBMC cultures by Griess reaction (Green et al., 1982, Gutman and Hollywood, 1992). Duplicate samples were grown in 96-flat bottom wells (Nunc, Naperville, IL). Briefly, a 100-μl aliquot of cell-free culture supernatant was mixed with 100 μl of Griess reagent (1% sulfanylamide, 0.1% naphthylethylene-diamide-dihydrochloride, and 2.5% phosphoric acid, all from Sigma). Following 10 min of incubation at room temperature in the dark, the absorbance was measured at 540 nm by using a microplate reader (Biotek, EL800). The concentration of nitrite was determined by interpolation from a standard curve constructed by using sodium nitrite solutions of known concentration in the range 0–100 μM. To discount the interference of nitrites already present in the culture medium, data were calculated taking into account the blank for each experiment, assayed by using the medium employed for the in vitro PBMC cultures. The results were first expressed as nitrite concentration (μM).
2.6. Parasitological analyze on bone marrow samples
Bone marrow was obtained to evaluate the frequency of tissue parasitism in the different groups. Dogs were anesthetized with an intravenous dose (8 mg/kg body weight) of sodium thiopental (Thionembutal®; Abbott Laboratories, São Paulo, Brazil), and bone marrow fluid was removed from the iliac crest under aseptic conditions. The bone marrow aspirates were used to study the presence of L. chagasi parasites by PCR.
DNA of bone marrow samples was extracted by Wizard™ Genomic DNA Purification Kit (Promega, Madison, WI, USA) according to the manufacturer's instructions. PCR was performed as previously described (Degrave et al., 1994) using the primers 150 forward: [5′-GGG(G/T)AGGGGCGTTCT(G/C)CGAA-3′] and 152 reverse: [5′-(G/C)(G/C)(G/C)(A/T)CTAT(A/T)TTACACCAACCCC-3′] that amplified a DNA fragment of 120 base pairs (bp) from the conserved region of Leishmania minicircle kDNA. Briefly, the PCR assay reaction mixture contained 1.0 μl of DNA preparation, 0.2 mM dNTPs, 10 mM Tris-HCl (pH 8.0), 50 mM KCl, 1.5 mM MgCl2, 10 pmol of each primer, and 1 U Taq polymerase (Invitrogen) to a final volume of 10 μl. PCR amplification was performed in a Veriti Thermal Cycler 96-well thermocycler (Applied Biosystems®, Irvine, CA, USA), over 40 cycles consisting of 1 min at 94 °C (denaturation), 1 min at 64 °C (annealing), 1 min at 72 °C (extension), and 7 min at 72 °C (final extension). Positive [genomic DNA of L. chagasi (MHOM/BR/1972/BH46)] and negative (without DNA) controls were included in each test. Amplified fragments were analyzed by electrophoresis on 8% polyacrylamide gel and ethidium bromide-stained for the PCR product identification.
The parasitological investigation was performed until 885 days after L. chagasi challenge.
2.7. Statistical analysis
Statistical analyses were performed using Prism 5.0 software package (Prism Software, Irvine, CA, USA). Normality of the data was demonstrated using a Kolmogorov-Smirnoff test. Paired t-tests were used to evaluate differences in mean values of cytokines levels, considering the comparative analysis of T0 and T3 (Fig. 1 ) or T90 (Fig. 2 ) or T885 (Fig. 3 ), in each group evaluated. Unpaired t-tests were used to evaluate differences in mean of values of TGF-β (Table 1 ). Analysis of variance (ANOVA) test followed by Tukey's multiple comparisons were used in the evaluation between the different treatment groups for cytokines (Fig. 1, Fig. 2, Fig. 3) and nitric oxide (Fig. 4 ) analysis. Differences were considered significant when P values were <0.05.
Fig. 1.
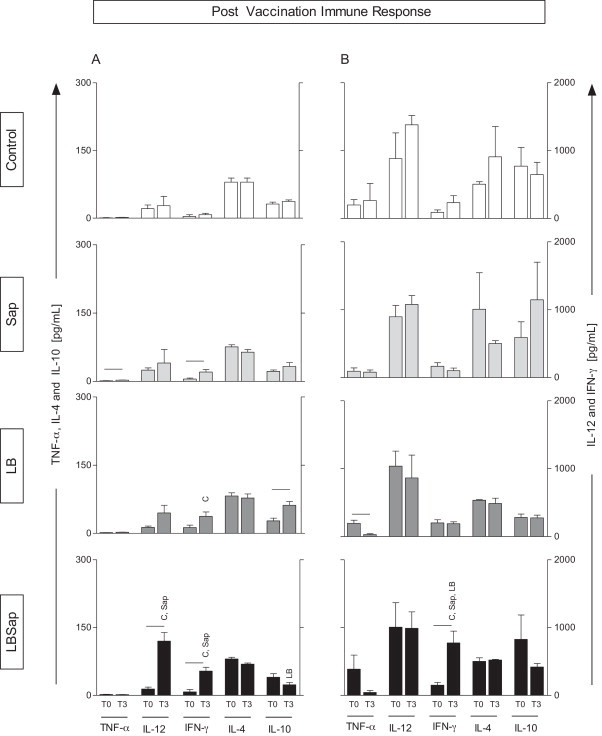
Impact of vaccination in the immune response based on the cytokine levels in the supernatant of PBMCs submitted to vaccine soluble antigen (VSA, A panels) or soluble L. chagasi antigen (SLcA, B panels). Data are displayed as a comparative analysis between the time point prior to vaccination (T0) and 15 days after completion of the vaccine protocol (T3). The groups are represented as C (control; □); Sap (saponin;  ); LB (killed L. braziliensis vaccine;
); LB (killed L. braziliensis vaccine;  ); and LBSap (killed L. braziliensis vaccine plus saponin; ■). The x-axis displays the cytokines evaluated (TNF-α, IL-12, IFN-γ, IL-4, and IL-10). The y-axis represents the mean values (pg/ml) ± SD from groups of five animals/evaluation time; the left y-axes depict the TNF-α, IL-4, and IL-10 levels, while in the right y-axes represent the IL-12 and IFN-γ cytokine levels. Significant differences (P < 0.05) between values measured at T0 (before the first dose) and T3 (15 days after the third dose) are indicated by connecting lines, whereas the symbols C, Sap, and LB indicate significant differences in relation to C, Sap, and LB, groups, respectively, at the same stimulus and time of evaluation.
); and LBSap (killed L. braziliensis vaccine plus saponin; ■). The x-axis displays the cytokines evaluated (TNF-α, IL-12, IFN-γ, IL-4, and IL-10). The y-axis represents the mean values (pg/ml) ± SD from groups of five animals/evaluation time; the left y-axes depict the TNF-α, IL-4, and IL-10 levels, while in the right y-axes represent the IL-12 and IFN-γ cytokine levels. Significant differences (P < 0.05) between values measured at T0 (before the first dose) and T3 (15 days after the third dose) are indicated by connecting lines, whereas the symbols C, Sap, and LB indicate significant differences in relation to C, Sap, and LB, groups, respectively, at the same stimulus and time of evaluation.
Fig. 2.
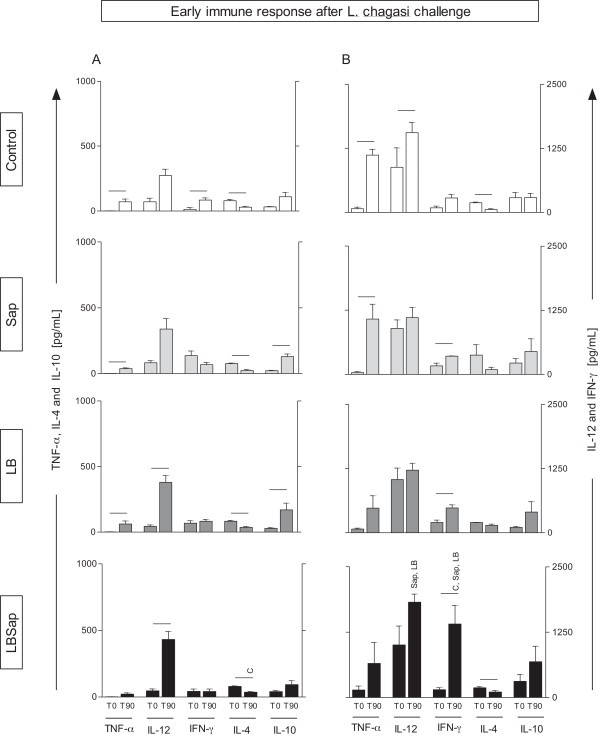
Early immune response after L. chagasi challenge in groups exposed to different vaccines with regard to cytokine levels in the supernatant of PBMCs treated with vaccine soluble antigen (VSA, A panel) or soluble L. chagasi antigen (SLcA, B panel). Data are displayed as a comparative analysis between the time point prior to vaccination (T0) and after the early time point following the L. chagasi challenge (T90). The groups are represented as C (control; □); Sap (saponin;  ); LB (killed L. braziliensis vaccine;
); LB (killed L. braziliensis vaccine;  ), LBSap (killed L. braziliensis vaccine plus saponin; ■). The x-axis depicts the cytokines evaluated (TNF-α, IL-12, IFN-γ, IL-4, and IL-10). The y-axis represents the mean values (pg/ml) ± SD from groups of five animals/evaluation time; the left y-axes illustrate the TNF-α, IL-4, and IL-10 levels, while the right y-axes represent the IL-12 and IFN-γ cytokine levels. Significant differences (P < 0.05) between values measured at T0 (before the first dose) and T90 (90 days after the L. chagasi challenge) are indicated by connecting lines, whereas the symbols C, Sap, and LB indicate significant differences in relation to C, Sap, and LB, groups, respectively, at the same stimulus and time of evaluation.
), LBSap (killed L. braziliensis vaccine plus saponin; ■). The x-axis depicts the cytokines evaluated (TNF-α, IL-12, IFN-γ, IL-4, and IL-10). The y-axis represents the mean values (pg/ml) ± SD from groups of five animals/evaluation time; the left y-axes illustrate the TNF-α, IL-4, and IL-10 levels, while the right y-axes represent the IL-12 and IFN-γ cytokine levels. Significant differences (P < 0.05) between values measured at T0 (before the first dose) and T90 (90 days after the L. chagasi challenge) are indicated by connecting lines, whereas the symbols C, Sap, and LB indicate significant differences in relation to C, Sap, and LB, groups, respectively, at the same stimulus and time of evaluation.
Fig. 3.
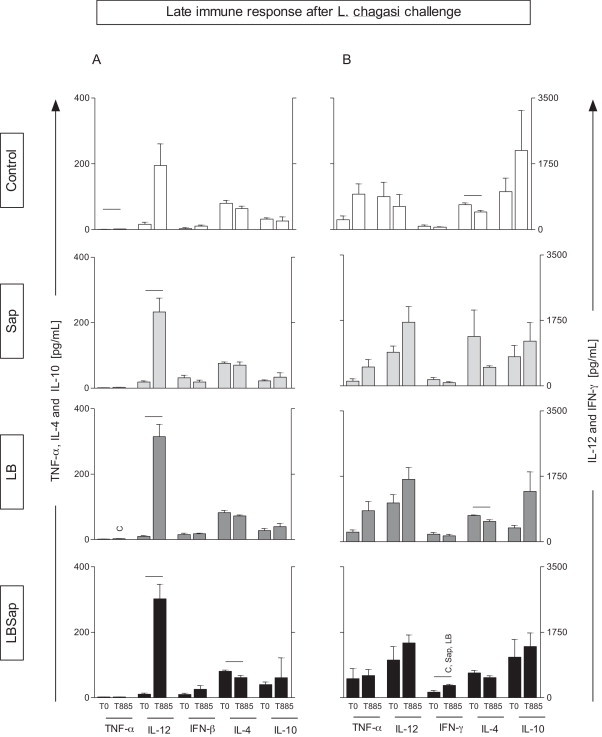
Late immune response after L. chagasi challenge in groups exposed to different vaccines with regard to cytokine levels in the supernatant of PBMCs treated with vaccine soluble antigen (VSA, A panel) or soluble L. chagasi antigen (SLcA, B panel). Data are displayed as a comparative analysis between the time point prior to vaccination (T0) and at the late time point following L. chagasi challenge (T885). The groups are represented as C (control; □); Sap (saponin;  ); LB (killed L. braziliensis vaccine;
); LB (killed L. braziliensis vaccine;  ); and LBSap (killed L. braziliensis vaccine plus saponin; ■). The x-axis displays the cytokines evaluated (TNF-α, IL-12, IFN-γ, IL-4, and IL-10). The y-axis represents the mean values (pg/ml) ± SD from groups of five animals/evaluation time; the left y-axes illustrate the TNF-α, IL-4, and IL-10 levels, while in the right y-axes represent the IL-12 and IFN-γ cytokine levels. Significant differences (P < 0.05) between values measured at T0 (before the first dose) and T885 (885 days after the L. chagasi challenge) are indicated by connecting lines, whereas the symbols C, Sap, and LB indicate significant differences in relation to C, Sap, and LB, groups, respectively, at the same stimulus and time of evaluation.
); and LBSap (killed L. braziliensis vaccine plus saponin; ■). The x-axis displays the cytokines evaluated (TNF-α, IL-12, IFN-γ, IL-4, and IL-10). The y-axis represents the mean values (pg/ml) ± SD from groups of five animals/evaluation time; the left y-axes illustrate the TNF-α, IL-4, and IL-10 levels, while in the right y-axes represent the IL-12 and IFN-γ cytokine levels. Significant differences (P < 0.05) between values measured at T0 (before the first dose) and T885 (885 days after the L. chagasi challenge) are indicated by connecting lines, whereas the symbols C, Sap, and LB indicate significant differences in relation to C, Sap, and LB, groups, respectively, at the same stimulus and time of evaluation.
Table 1.
Levels of TGF-β in the PBMCs from dogs before the first vaccine dose (T0), following completion of the vaccine protocol (T3), and after early (T90) and late (T885) time points following L. chagasi challenge. The results are presented with regards to stimulation with soluble L. chagasi antigen (SLcA) in the following groups: C (control) and LBSap (killed L. braziliensis vaccine plus saponin).
| Time | Groups | TGF-β (CC) | TGF-β (SLcA) |
|---|---|---|---|
| T0 | C | 1815 ± 728.5 | 2229 ± 374.4 |
| LBSap | 1735 ± 447.0 | 2027 ± 680.1 | |
| T3 | C | 3355 ± 817.2 | 2997 ± 588.8 |
| LBSap | 2642 ± 100.1 | 2553 ± 922.2 | |
| T90 | C | 2928 ± 972.7 | 3445 ± 170.7 |
| LBSap | 1688 ± 803.2 | *2278 ± 437.7 | |
| T885 | C | 1704 ± 457.7 | 1857 ± 333.8 |
| LBSap | 1437 ± 259.5 | 1427 ± 296.2 | |
Significant difference (P < 0.05) between the C and LBSap groups by different times of evaluation.
Fig. 4.
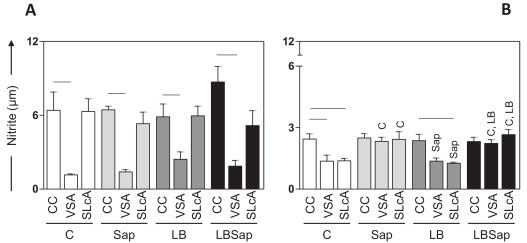
NO levels (μM) determined after early (T90; A panel) and late (T885; B panel) time points following L. chagasi challenge in culture supernatants from the different treatment groups: C (control; □); Sap (saponin;  ); LB (killed L. braziliensis vaccine;
); LB (killed L. braziliensis vaccine;  ); and LBSap (killed L. braziliensis vaccine plus saponin; ■). Significant differences (P < 0.05) between stimuli are represented by connecting lines, whereas the symbols C, Sap, and LB indicate significant differences in relation to C, Sap, and LB, groups, respectively, at the same stimulus and time of evaluation. Data are presented as mean ± SD of NO levels from groups of five animals/evaluation time. CC, control cultures.
); and LBSap (killed L. braziliensis vaccine plus saponin; ■). Significant differences (P < 0.05) between stimuli are represented by connecting lines, whereas the symbols C, Sap, and LB indicate significant differences in relation to C, Sap, and LB, groups, respectively, at the same stimulus and time of evaluation. Data are presented as mean ± SD of NO levels from groups of five animals/evaluation time. CC, control cultures.
3. Results
3.1. LBSap induced a prominent type 1 immune response elicited by higher levels of IL-12 and IFN-γ post vaccine protocol
To determine the impact of LBSap vaccination on the immune response, we evaluated the cytokine profile (TNF-α, IL-12, IFN-γ, IL-4, and IL-10) in the supernatant of PBMC stimulated with VSA (Fig. 1A) or SLcA (Fig. 1B). In this context, we performed a comparative analysis between T0 and T3, in addition to the comparisons between experimental groups at each time point. In the comparison between T0 and T3, the Sap group showed increased levels (P < 0.05) of TNF-α and IFN-γ production at T3 with VSA stimulation. Additionally, the LB group presented higher levels (P < 0.05) of IL-10 in VSA-stimulated PBMCs at T3, as compared to T0. In contrast, in SLcA-stimulated cultures, the LB group displayed lower levels of TNF-α at T3 as compared to T0 in SLcA-stimulated cultures (P < 0.05).
Interestingly, the LBSap vaccine induced higher levels of both IL-12 and IFN-γ at T3 in VSA-stimulated PBMCs. Similarly, in the presence of SLcA, increased levels (P < 0.05) of IFN-γ were observed in the LBSap group at T3.
The comparison between the experimental groups, in different time points, revealed increased levels (P < 0.05) of IFN-γ in VSA-stimulated cultures from the LB group, as compared to C group in T3. Interestingly, higher (P < 0.05) levels of this cytokine were observed in the VSA-stimulated culture of LBSap group when compared to C and Sap groups, at T3. Similarly, in SLcA-stimulated cultures, LBSap group displayed increased (P < 0.05) levels of IFN-γ in relation to C, Sap and LB groups at T3. In addition, at T3, LBSap group showed increased (P < 0.05) levels of IL-12 in relation to C and Sap groups, in addition to reduced (P < 0.05) levels of IL-10 when compared to LB group, in VSA-stimulated cultures.
3.2. Both IL-12 and IFN-γ markedly increased in response to LBSap vaccine at T90
The early immune response after L. chagasi challenge was analyzed in different groups. We determined the cytokine patterns in the supernatant of PBMCs comparing the different treatments (VSA – Fig. 2A and SLcA – Fig. 2B), different time points (T0 and T90), and different experimental groups, at each time point.
Comparison between T0 and T90 showed that the C group had increased levels of TNF-α production (P < 0.05) and lower levels of IL-4 production (P < 0.05) at T90 upon VSA and SLcA stimulation. Additionally, C group had higher levels of IL-12 in SLcA-stimulated PBMCs (P < 0.05) and higher levels of IFN-γ production in VSA-stimulated PBMCs (P < 0.05) at T90. The Sap group showed increased levels (P < 0.05) of TNF-α and IL-10 production and reduction of IL-4 levels at T90. In SLcA-stimulated cultures, the Sap group presented higher levels (P < 0.05) of TNF-α and IFN-γ. The LB group showed increased levels (P < 0.05) of TNF-α, IL-12, and IL-10 production and reduction of IL-4 in VSA-stimulated PBMCs at T90. In cultures stimulated with SLcA, the LB group shown increased levels (P < 0.05) of IFN-γ. Interestingly, the LBSap vaccine induced higher levels of IL-12 at T90 in PBMCs stimulated with VSA. Furthermore, in the presence of SLcA, LBSap vaccine induced higher levels of IFN-γ (P < 0.05). The reduced levels of IL-4, which occurred in the other groups, were retained (P < 0.05) in the LBSap group at T90 for both stimuli (VSA and SLcA).
The comparative analysis between the experimental groups showed, at T90, increased levels (P < 0.05) of IL-4, in SLcA-stimulated cultures in the LB group and VSA-stimulated cultures in the LBSap group, in relation to C group. Interestingly, the SLcA-stimulated PBMCs from LBSap group showed increased levels (P < 0.05) of IL-12 compared to LB and Sap groups at T90. Furthermore, increased levels (P < 0.05) of IFN-γ in the LBSap when compared to C, Sap and LB groups were observed.
3.3. LBSap vaccine elicited a long-lasting type 1 immune response at T885, displaying higher levels of IFN-γ
The late immune response after L. chagasi challenge was studied in different groups with regard to the cytokine levels in the supernatant of PBMCs treated with VSA (Fig. 3A) or SLcA (Fig. 3B), and the T0 and T885 data were compared, besides the comparisons between experimental groups, at each time.
In the comparison between T0 and T885, the results showed that the C group had increased levels of TNF-α in VSA-stimulated PBMCs (P < 0.05) and decreased levels of IL-4 production (P < 0.05) in the presence of SLcA at T885. The Sap and LB groups presented increased levels of IL-12 (P < 0.05) at T885 in the presence of the VSA stimulus, as compared to T0. In the presence of the SLcA stimulus, the LB group had decreased levels of IL-4 (P < 0.05) at T885, as compared to T0. Similarly, the group LBSap had decreased levels of IL-4 (P < 0.05) at T885, as compared to T0, but this difference was only observed in the presence of the VSA stimulus. Interestingly, in this group, levels of IL-12 (in the presence of VSA) and IFN-γ (in the presence of SLcA) were higher compared to T0 (P < 0.05). Whereas this was a time-delayed response post-challenge with L. chagasi (T885), this result indicates an immune response predominantly of the type 1, induced by vaccination with LBSap.
Comparative analysis between the experimental groups showed increased (P < 0.05) levels of TNF-α in VSA-stimulated cultures of LB group in relation to C group, at T885. Interestingly, at T885, increased (P < 0.05) levels of IFN-γ in LBSap group was observed in relation to C, Sap and LB groups, in SLcA-stimulated PBMCs.
3.4. Impaired levels of TGF-β were the hallmark of the LBSap vaccine at T90
The levels of TGF-β are shown in Table 1, which focuses on the analysis using supernatant of PBMCs simulated with SLcA. We evaluated the data using a comparative analysis between the control and LBSap groups at T0 and T3 as well as at T90 and T885 for the L. chagasi challenge.
Interestingly, there was a decrease in TGF-β in the group immunized with LBSap compared to group C at T90.
3.5. LBSap enhanced NO production at T885 in both VSA- and SLcA-stimulated cultures
Since the production of NO is considered to be a key element in mechanisms that mediate the elimination of intracellular pathogens, the levels of antimicrobial oxidant produced by in vitro antigen-stimulated PBMCs derived from dogs vaccinated with LBSap were determined (Fig. 4).
At T90 a reduction (P < 0.05) was observed in the levels of the reactive NO in VSA-stimulated cultures compared to the respective control cultures of the groups C, Sap, LB, and LBSap (Fig. 4A).
At T885, significantly increased nitrite levels (P < 0.05) in the VSA- and SLcA-stimulated cultures were observed in the Sap group compared with cultures receiving the same stimuli in the C and LB groups. SLcA-stimulated cultures in the C and LB groups showed a significant reduction of NO levels when compared to the respective control cultures (Fig. 4B). In addition, the C group presented higher levels of NO in control cultures in relation to VSA-stimulated cultures (Fig. 4B). Interestingly, in the LBSap group, higher (P < 0.05) levels of NO levels were recorded in the supernatant of SLcA- and VSA-stimulated cultures at T885 when compared with cultures receiving the same stimuli in groups C and LB.
3.6. Bone marrow parasitological analysis revealed decreased frequency of parasitism in the presence of vaccine antigen
The parasitological investigation was performed until 885 days after L. chagasi challenge. By T885 two dogs from group C, four dogs from group Sap, and one dog each from the LB and LBSap groups were diagnosed as positive. It is interesting to note also, that until the period in which they were accompanied (T885) all experimental groups remained asymptomatic.
4. Discussion
Increased VL incidence in the world and especially in Brazil have motivated studies and evaluations of anti-CVL vaccines because of the epidemiological importance of dogs in the biological cycle of the parasite (Palatnik-de-Sousa, 2012). Aiming to guide the rationale for developing anti-CVL vaccines, studies have been performed to identify biomarkers of immunogenicity before and after L. chagasi challenge (Gutman and Hollywood, 1992, Reis et al., 2010). Type 1 and type 2 immune responses and immunomodulatory cytokines are considered the main targets for identifying resistance biomarkers following vaccination against CVL (Reis et al., 2010, Fernandes et al., 2008, Carrillo et al., 2007, De Lima et al., 2010).
Results from previous studies using LBSap, the anti-CVL vaccine, showed high immunogenic potential, with induction of increased levels of circulating T lymphocytes (CD5+, CD4+, and CD8+) and B lymphocytes (CD21+), and higher levels of CD4+ and CD8+ T cells that were Leishmania specific (Giunchetti et al., 2007, Roatt et al., 2012). In these studies, LBSap vaccine elicited strong antigenicity related to the increased levels of anti-Leishmania IgG isotypes after vaccination (Giunchetti et al., 2007), and a strong and sustained induction of humoral immune response after experimental challenge, with increased levels of anti-Leishmania total IgG, IgG1 and IgG2 (Roatt et al., 2012). Furthermore, LBSap vaccinated dogs presented high IFN-γ and low IL-10 and TGF-β1 expression in spleen with significant reduction of parasite load in this organ (Roatt et al., 2012). In addition, LBSap vaccine displayed safety and security for the administration (Giunchetti et al., 2007, Vitoriano-Souza et al., 2008, Moreira et al., 2009).
However, there are few studies evaluating the cytokine profiles associated with CVL and in anti-CVL vaccines, which might serve as biomarkers to identify resistance and susceptibility. Thus, this study aimed to evaluate the cytokine profile and NO induced by immunization before and after experimental challenge with L. chagasi and sand fly saliva. In addition, the frequency of bone marrow parasitism was included in the evaluation.
We thus performed a comparative analysis of the cytokine profile before immunization (T0), after completion of the vaccine protocol (T3), and at early (T90) and late (T885) time points after experimental challenge with L. chagasi. The production of distinct cytokines was evaluated during the vaccination protocol and after L. chagasi and sand fly saliva experimental challenge.
The analysis of IL-4 levels has been considered a morbidity marker during ongoing CVL (Quinnel et al., 2001, Brachelente et al., 2005, Chamizo et al., 2005), as well as in a murine models of VL (Miralles et al., 1994). We observed that the group vaccinated with LBSap showed increased levels of IL-4 as compared to the C group. However, increased levels of IFN-γ in the LBSap group were also observed. According to Manna et al. (2008), it is possible to maintain a standard of resistance in CVL even in the presence of IL-4, as long as there are elevated levels of IFN-γ. Nevertheless, our results do not suggest a typical profile linking this cytokine with a resistance or susceptibility pattern in CVL. Similar to our study, a previous study (Manna et al., 2006) did not associate IL-4 with resistance or susceptibility to natural L. chagasi infection in CVL. In contrast, levels of IL-4 in splenocytes from dogs naturally infected with L. chagasi and presenting different clinical signs, indicated that this cytokine could be a biomarker present during the course of infection in CVL (Lage et al., 2007).
Similarly, IL-10 has also been associated with susceptibility to CVL (Pinelli et al., 1999, Lage et al., 2007, Alves et al., 2009, Boggiatto et al., 2010) and human VL (Nylen and Sacks, 2007). Our data showed increased levels of IL-10 at T3 and T90 in the LB group and at T90 in the Sap group. In contrast, we observed decreased levels of IL-10 in LBSap in relation to the LB group at T3 in VSA-stimulated PBMCs. We hypothesize that lower levels of IL-10 during the immunization protocol and the lack of significance in IL-10 levels after experimental challenge with L. chagasi in the LBSap contributes to the establishment of a more efficient immune response in these vaccinated dogs.
In addition, the cytokine TGF-β has been associated with progression of Leishmania infection in a murine model (Barral et al., 1993, Virmondes-Rodrigues et al., 1998, Gantt et al., 2003). Few studies have been performed in CVL; however, existing studies show increased levels of TGF-β in both asymptomatic and symptomatic dogs naturally infected with L. chagasi (Correa et al., 2007). Our results displayed decreased levels of TGF-β in SLcA-stimulated cultures of LBSap group at T90. These results suggest that vaccination with LBSap may trigger reduced TGF-β production after experimental challenge. In fact, a previous work (Alves et al., 2009) reported high levels of TGF-β associated with increased parasite load in lymph nodes from symptomatic dogs naturally infected with L. chagasi and an association between this cytokine and CVL morbidity. Therefore, it is possible that the reduced levels of TGF-β, associated with higher levels of IL-12 and IFN-γ, after L. chagasi and sand fly saliva challenge, would contribute to establishing immunoprotective mechanisms induced by LBSap vaccination.
Type 1 cytokines have also been considered as a prerequisite for evaluating immunogenicity before and after L. chagasi experimental challenge in anti-CVL vaccine clinical trials (Reis et al., 2010). Thus, we analyzed TNF-α, IL-12, and IFN-γ levels.
Some studies have established that TNF-α together with IFN-γ are associated with a resistance profile against CVL (Pinelli et al., 1994, Pinelli et al., 1999, Chamizo et al., 2005, Carrillo et al., 2007, Alves et al., 2009). However, it is not a consensus that TNF-α profile would be a good indicator of resistance or susceptibility after L. chagasi infection, considering the similar levels of TNF-α showed in dogs presenting distinct clinical signs (De Lima et al., 2007, Lage et al., 2007). Moreover, LBSap group did not present any differences in TNF-α levels when compared to other experimental groups. In fact, our data were similar to Leishmune® results, that did not present differences in the expression of this molecule (Araújo et al., 2009, De Lima et al., 2010). In addition, assessment of IL-12 levels in the group immunized with LBSap revealed increased levels of this cytokine at T3, T90, and T885 in the presence of VSA stimulation, compared to T0. Interestingly, higher levels of IL-12 after vaccine protocol in relation to C and LB group (T3, in VSA-stimulated cultures), and in the early period post challenge in relation to Sap and LB groups (T90, in SLcA-stimulated cultures) was the hallmark of LBSap group. Since this cytokine has been associated with protection in CVL (Strauss-Ayali et al., 2005, Menezes-Souza et al., 2011), high levels of IL-12 and impaired TGF-β production would indicate the establishment of immunoprotective mechanisms induced by LBSap vaccination.
IFN-γ is considered an important pro-inflammatory cytokine for establishing protective immunity against the Leishmania parasite, inducing NO synthesis, and activating microbicidal function in macrophages (Trinchieri et al., 1993, Reiner and Locksley, 1995). Thus, NO is considered one of the most important molecules responsible for killing intracellular parasites such as those of the Leishmania genus (Heinzel et al., 1989, Bogdan, 2001, Sisto et al., 2001, Gradoni and Ascenzi, 2004). In this context, we found that the LBSap group had increased levels of IFN-γ after the vaccine protocol (T3), presenting sustained improvement at the early (T90) and late (T885) time points after L. chagasi experimental challenge in the presence of the SLcA stimulus, compared to T0. Interestingly, after the vaccination protocol (T3), the LBSap group showed increased levels in IFN-γ in VSA or SLcA- stimulated cultures compared to other groups. Moreover, in both early (T90) and late (T885) period post challenge, the LBSap group remained producing increased levels of Leishmania-specific IFN-γ, as compared to the respective stimulated cultures (VSA or SLcA) from the other groups. Furthermore, the increased IFN-γ levels at T885 was concomitant with higher NO amounts in cultures stimulated with SLcA and VSA. Since IFN-γ is associated with a resistance profile to Leishmania infection in different experimental models (Squires et al., 1989, Andrade et al., 1999, Murray et al., 1992, Carrillo et al., 2007, Fernandes et al., 2008), our data revealed an intense Leishmania-specific induction of IFN-γ after immunization with LBSap.
Considering the lack of a sufficient amount of biological material, we performed PCR analysis to assess the parasite burden. However, only the LBSap and LB groups showed one dog each with positive parasitological results, which may indicate that the antigen of L. braziliensis can induce protection after experimental L. chagasi challenge. Further investigations will focus on the efficacy of the LBSap vaccination in protecting against an experimental challenge with L. chagasi, using quantitative PCR.
In conclusion, our data point to a prominent type 1 immune response is elicited by higher levels of IL-12 and IFN-γ following complete vaccination and after L. chagasi challenge. Additionally, the levels of TGF-β are reduced in the early immune response after L. chagasi challenge, while NO production is enhanced at a late time point following L. chagasi challenge. Furthermore, based on bone marrow parasitological analysis, the frequency of parasitism is decreased in the presence of the vaccine antigen. Thus, LBSap vaccine appears to elicit prominent, long-lasting type 1 immunogenicity.
Acknowledgments
The authors are grateful for the use of the facilities at CEBIO, Universidade Federal de Minas Gerais and Rede Mineira de Bioterismo (FAPEMIG). This work was supported by Fundação de Amparo a Pesquisa do Estado de Minas Gerais, Brazil (grant: CBB-APQ-02473-10; CBB-APQ-00356-10-PPSUS; CBB-APQ-01052-11), Conselho Nacional de Desenvolvimento Científico e Tecnológico- CNPq, Brazil (grant: 403485/2008-8-PAPES V/FIOCRUZ; 473234/2010-6; 560943/2010-5) and CAPES. RCO, OAMF, RTF, CMC, ABR and RCG are grateful to CNPq for fellowships. The authors also thank the Boldface Editors for the critical reading of the manuscript, editorial suggestions and changes.
References
- Alves C.F., De Amorim I.F., Moura E.P., Ribeiro R.R., Alves C.F., Michalick M.S., Kalapothakis E., Bruna-Romero O., Tafuri W.L., Teixeira M.M., Melo M.N. Expression of IFN-gamma, TNF-alpha IL-10 and TGF-beta in lymph nodes associates with parasite load and clinical form of disease in dogs naturally infected with Leishmania (Leishmania) chagasi. Vet. Immunol. Immunopathol. 2009;128:349–358. doi: 10.1016/j.vetimm.2008.11.020. [DOI] [PubMed] [Google Scholar]
- Andrade H.M., Toledo V.P.C.P., Mayrink W., Genaro O. Evaluation of the immune response and production of interferon in canine visceral leishmaniasis. Rev. Med. Vet. 1999;50:809–814. [Google Scholar]
- Araújo M.S., de Andrade R.A., Vianna L.R., Mayrink W., Reis A.B., Sathler-Avelar R., Teixeira-Carvalho A., Andrade M.C., Mello M.N., Martins-Filho O.A. Despite Leishvaccine and Leishmune trigger distinct immune profiles, their ability to activate phagocytes and CD8+ T-cells support their high-quality immunogenic potential against canine visceral leishmaniasis. Vaccine. 2008;26:2211–2224. doi: 10.1016/j.vaccine.2008.02.044. [DOI] [PubMed] [Google Scholar]
- Araújo M.S., de Andrade R.A., Sathler-Avelar R., Teixeira-Carvalho A., Andrade M.C., Vianna L.R., Mayrink W., Reis A.B., Malaquias L.C.C., Mello M.N., Martins-Filho A.O. T-cell-derived cytokines, nitric oxide production by peripheral blood monocytes and seric anti-Leishmania (Leishmania) chagasi IgG subclass patterns following immunization against canine visceral leishmaniasis using Leishvaccine and Leishmune. Vaccine. 2009;27:1008–1017. doi: 10.1016/j.vaccine.2008.11.104. [DOI] [PubMed] [Google Scholar]
- Barral A., Barral-Netto M., Yong E.C., Brownell C.E., Twardizik D.R., Reed S.G. Transforming growth factor beta as a virulence mechanism for Leishmania braziliensis. Proc. Natl. Acad. Sci. U.S.A. 1993;90:3442–3446. doi: 10.1073/pnas.90.8.3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdan C. Nitric oxide and the immune response. Nat. Immunol. 2001;2:907–916. doi: 10.1038/ni1001-907. [DOI] [PubMed] [Google Scholar]
- Boggiatto P.M., Ramer-Tait A., Metz K., Kramer E.E., Gibson-Corley K., Mullin K., Hostetter J.M., Gallup J.M., Jones D.E., Petersen C.A. Immunologic indicators of clinical progression during canine Leishmania infantum infection. Clin. Vacc. Immunol. 2010;17:267–273. doi: 10.1128/CVI.00456-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borja-Cabrera G.P., Correia-Pontes N.N., da Silva V.O., Paraguai de Souza E., Santos W.R., Gomes E.M., Luz K.G., Palatnik M., Palatnik de Sousa C.B. Long lasting protection against canine kala-azar using the FML-QuilA saponin vaccine in an endemic area of Brazil (São Gonçalo do AmaranteRN) Vaccine. 2002;20:3277–3284. doi: 10.1016/s0264-410x(02)00294-3. [DOI] [PubMed] [Google Scholar]
- Brachelente C., Muller N., Doherr M.G., Sattler U., Welle M. Cutaneous Leishmaniasis in naturally infected dogs is associated with a T helper-2- biased immune response. Vet. Pathol. 2005;42:166–175. doi: 10.1354/vp.42-2-166. [DOI] [PubMed] [Google Scholar]
- Carrillo E., Ahmed S., Goldsmith-Pestana K., Nieto J., Osorio Y., Travic B., Moreno J., McMahon-Pratt D. Immunogenicity of the P-8 amastigote antigen in the experimental model of canine visceral leishmaniasis. Vaccine. 2007;25:1534–1543. doi: 10.1016/j.vaccine.2006.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamizo C., Moreno J., Alvar J. Semi-quantitative analysis of cytokine expression in asymptomatic canine leishmaniasis. Vet. Immunol. Immunopathol. 2005;103:67–75. doi: 10.1016/j.vetimm.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Correa A.P., Dossi A.C., Vasconcelos R.O., Munari D.P., De Lima V.M.F. Evaluation of transformation growth factor beta1, interleukin-10, and interferon-gamma in male symptomatic and asymptomatic dogs naturally infected by Leishmania (Leishmania) chagasi. Vet. Parasitol. 2007;143:267–274. doi: 10.1016/j.vetpar.2006.08.023. [DOI] [PubMed] [Google Scholar]
- Dantas-Torres F. Leishmune vaccine: the newest tool for prevention and control of canine visceral leishmaniosis and its potential as a transmission-blocking vaccine. Vet. Parasitol. 2006;141:1–8. doi: 10.1016/j.vetpar.2006.05.001. [DOI] [PubMed] [Google Scholar]
- De Lima V.M.F., Peiro J.R., Vasconcelos R.O. IL-6 and TNF-α production during active canine visceral leishmaniasis. Vet. Immunol. Immunopathol. 2007;115:189–193. doi: 10.1016/j.vetimm.2006.10.003. [DOI] [PubMed] [Google Scholar]
- De Lima V.M., Ikeda F.A., Rossi C.N., Feitosa M.M., Vasconcelos R.O., Nunes C.M., Goto H. Diminished CD4+/CD25+ T cell and increased IFN-γ levels occur in dogs vaccinated with Leishmune® in an endemic area for visceral leishmaniasis. Vet. Immunol. Immunopathol. 2010;135:296–302. doi: 10.1016/j.vetimm.2009.12.008. [DOI] [PubMed] [Google Scholar]
- Deane L.M. Reservoirs of Leishmania donovani in Brazil. Rev. Assoc. Med. Bras. 1961;7:161–169. [PubMed] [Google Scholar]
- Degrave W., Fernandes O., Thiemanm O., Wincker P., Britto C., Cardoso A., Pereira J.B., Bozza M., Lopes U., Morel C. Detection of Trypanosoma cruzi and Leishmania using the polymerase chain reaction. Mem. Inst. Oswaldo Cruz. 1994;89:367–368. doi: 10.1590/s0074-02761994000300013. [DOI] [PubMed] [Google Scholar]
- Desjeux P. Leishmaniasis: current situation and new perspectives. Comp. Immun. Microbiol. Infect. Dis. 2004;27:305–318. doi: 10.1016/j.cimid.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Dye C. The logic of visceral leishmaniasis control. Am. J. Trop. Med. Hyg. 1996;55:125–130. doi: 10.4269/ajtmh.1996.55.125. [DOI] [PubMed] [Google Scholar]
- Fernandes A.P., Costa M.M.S., Coelho E.A.F., Michalickc M.S.M., de Freitas E., Melo M.N., Tafuri W.L., Resende D.M., Hermonte V., Abrantese C.F., Gazzinelli R.T. Protective immunity against challenge with Leishmania (Leishmania) chagasi in beagle dogs vaccinated with recombinant A2 protein. Vaccine. 2008;26:5888–5895. doi: 10.1016/j.vaccine.2008.05.095. [DOI] [PubMed] [Google Scholar]
- Gantt K.R., Schultz-Cherry S., Rodriguez N., Jeronimo S.M.B., Nascimento E.T., Goldman T.L., Recker T.J., Miller M.A., Wilson M.E. Activation of TGF-beta by Leishmania chagasi: importance for parasite survival in macrophages. J. Immunol. 2003;170:2613–2620. doi: 10.4049/jimmunol.170.5.2613. [DOI] [PubMed] [Google Scholar]
- Giunchetti R.C., Correa-Oliveira R., Martins-Filho O.A., Teixeira-Carvalho A., Roatt B.M., Aguiar-Soares R.D.O., Vitoriano-Souza J., Moreira N.D., Malaquias L.C., Castro L.L.M., Lana M., Reis A.B. Immunogenicity of a killed Leishmania vaccine with saponin adjuvant in dogs. Vaccine. 2007;25:7674–7686. doi: 10.1016/j.vaccine.2007.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giunchetti R.C., Correa-Oliveira R., Martins-Filho O.A., Teixeira-Carvalho A., Roatt B.M., Aguiar-Soares R.D., Coura-Vital O., Abreu W., Malaquias R.T., Gontijo L.C., Broskyn N.F., Oliveira C., Costa C.I., Lana D.J., Reis M.A.B. A killed Leishmania vaccine with sand fly saliva extract and saponin adjuvant displays immunogenicity in dogs. Vaccine. 2008;26:623–638. doi: 10.1016/j.vaccine.2007.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giunchetti R.C., Reis A.B., Silveira-Lemos D., Martins-Filho A.O., Corrêa-Oliveira R., Vale A.M., Quetz J.S., Bueno L.L., França-Silva J.C., Nascimento E., Mayrink W., Fujiwara R.T. Antigenicity of a whole parasite vaccine as promising candidate against canine leishmaniasis. Res. Vet. Sci. 2008;85:106–112. doi: 10.1016/j.rvsc.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Gradoni L., Ascenzi P. Nitric oxide and anti-protozoan chemotherapy. Parasitologia. 2004;46:101–103. [PubMed] [Google Scholar]
- Green L.C., Wagner D.A., Glogowski J., Skipper P.L., Wishnok J.S., Tannenbaum S.R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- Gutman S.I., Hollywood C.A. Simple, rapid method for determining nitrates and nitrites in biological fluids. Clin. Chem. 1992;38:2152. [PubMed] [Google Scholar]
- Heinzel F.P., Sadick M.D., Holaday B.J., Coffmanj R.L., Locksley R.M. Reciprocal expression of interferon gamma or interleukin 4 during the resolution or progression of murine leishmaniasis. Evidence for expansion of distinct helper T cell subsets. J. Exp. Med. 1989;169:59–72. doi: 10.1084/jem.169.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzmuller P., Cavaleyra M., Moreaux J., Kovacic R., Vincendeau P., Papierok G., Lemesre J.L. Lymphocytes of dogs immunised with purified excreted-secreted antigens of Leishmania infantum coincubated with Leishmania infected macrophages produce IFN gamma resulting in nitric oxide-mediated amastigote apoptosis. Vet. Immunol. Immunopathol. 2005;106:247–257. doi: 10.1016/j.vetimm.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Hommel M., Jaffe C.L., Travi B., Milon G. Experimental models for leishmaniasis and for testing anti-leishmanial vaccines. Ann. Trop. Med. Parasitol. 1995;89:55–73. doi: 10.1080/00034983.1995.11813015. [DOI] [PubMed] [Google Scholar]
- Kane M.M., Mosser D.M. The role of IL-10 in promoting disease progression in leishmaniasis. J. Immunol. 2001;166:1141–1147. doi: 10.4049/jimmunol.166.2.1141. [DOI] [PubMed] [Google Scholar]
- Lage R.S., Oliveira G.C., Busek S.U., Guerra L.L., Giunchetti R.C., Corrêa-Oliveira R., Reis A.B. Analysis of the cytokine profile in spleen cells from dogs naturally infected by Leishmania chagasi. Vet. Immunol. Immunopathol. 2007;115:135–145. doi: 10.1016/j.vetimm.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Lemesre J.L., Holzmuller P., Gonçalves R.B., Bourdoiseau G., Hugnet G., Cavaleyra M., Papierok G. Long-lasting protection against canine visceral leishmaniasis using the LiESAp-MDP vaccine in endemic areas of France: double-blind randomised efficacy field trial. Vaccine. 2007;25:4223–4234. doi: 10.1016/j.vaccine.2007.02.083. [DOI] [PubMed] [Google Scholar]
- Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Maia C., Campino L. Cytokine and phenotypic cell profiles of Leishmania infantum infection in the dog. J. Trop. Med. 2012 doi: 10.1155/2012/541571. 7 pp. (Article ID 541571) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manna L., Reale S., Viola E., Vitale F., Foglia Manzillo V., Pavone L.M., Caracappa S., Gravino A.E. Leishmania DNA load and cytokine expression levels in asymptomatic naturally infected dogs. Vet. Parasitol. 2006;142(3–4):271-280. doi: 10.1016/j.vetpar.2006.06.028. [DOI] [PubMed] [Google Scholar]
- Manna L., Reale S., Picillo E., Vitale F., Gravino A.E. Interferon-gamma (INF-γ) IL-4 expression levels and Leishmania DNA load as prognostic markers for monitoring response to treatment of leishmaniotic dogs with miltefosine and allopurinol. Cytokine. 2008;44:288–292. doi: 10.1016/j.cyto.2008.08.017. [DOI] [PubMed] [Google Scholar]
- Menezes-Souza D., Corrêa-Oliveira R., Guerra-Sá R., Giunchetti R.C., Teixeira-Carvalho A., Martins-Filho A.O., Oliveira G.C., Reis A.B. Cytokine and transcription factor profiles in the skin of dogs naturally infected by Leishmania (Leishmania) chagasi presenting distinct cutaneous parasite density and clinical status. Vet. Parasitol. 2011;177:39–49. doi: 10.1016/j.vetpar.2010.11.025. [DOI] [PubMed] [Google Scholar]
- Miralles G.D., Stoeckle M.Y., McDermott D.F., Finkelman F.D., Murray H.W. Induction of Th1 and Th2 cell-associated cytokines in experimental visceral leishmaniasis. Infect. Immun. 1994;62:1058–1063. doi: 10.1128/iai.62.3.1058-1063.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira N.D., Giunchetti R.C., Carneiro C.M., Vitoriano-Souza J., Roatt B.M., Malaquias L.C., Corrêa-Oliveira R., Reis A.B. Histological study of cell migration in the dermis of hamsters after immunisation with two different vaccines against visceral leishmaniasis. Vet. Immunol. Immunopathol. 2009;128:418–424. doi: 10.1016/j.vetimm.2008.11.030. [DOI] [PubMed] [Google Scholar]
- Mosmann T.R., Cherwinski H., Bond M.W., Giedlin M.A., Coffman R.L. Two types of murine helper T cell clone I. Definition according to profiles of lymphokine activities and secreted proteins. J. Immunol. 1986;136:2348–2357. [PubMed] [Google Scholar]
- Murray H.W., Squires K.E., Miralles C.D., Stoeckle M.Y., Granger A.M., Granelli-Piperno A., Bogdan C. Acquired resistance and granuloma formation in experimental visceral leishmaniasis differential T cell and lymphokine roles in initial versus established immunity. J. Immunol. 1992;148:1858–1863. [PubMed] [Google Scholar]
- Murray H.W., Lu C.M., Mauze S., Freeman S., Moreira A.L., Kaplan G., Coffman R.T. Interleukin-10 (IL-10) in experimental visceral leishmaniasis and IL-10 receptor blockade as immunotherapy. Infect. Immun. 2002;70:6284–6293. doi: 10.1128/IAI.70.11.6284-6293.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noli C., Auxilia S.T. Treatment of canine Old World visceral leishmaniasis: a systematic review. Vet. Dermatol. 2005;16:213–232. doi: 10.1111/j.1365-3164.2005.00460.x. [DOI] [PubMed] [Google Scholar]
- Nylen S., Sacks D. Interleukin-10 and the pathogenesis of human visceral leishmaniasis. Trends Immunol. 2007;28:378–384. doi: 10.1016/j.it.2007.07.004. [DOI] [PubMed] [Google Scholar]
- Palatnik-de-Sousa C.B. Vaccines for canine leishmaniasis. Front. Immunol. 2012;3:69. doi: 10.3389/fimmu.2012.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinelli E., Killick-Kendrick R., Wagenaar J., Bernadina W., del Real G., Ruitenberg J. Cellular and humoral immune responses in dogs experimentally and naturally infected with Leishmania infantum. Infect. Immun. 1994;62:229–235. doi: 10.1128/iai.62.1.229-235.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinelli E., Van Der Kaaij S.Y., Splappendel R., Fragio C., Ruitemberg E.J., Bernadina W., Ruttem V.P.M.G. Detection of canine cytokine gene expression by reverse transcription-polymerase chain reaction. Vet. Immunol. Immunopathol. 1999;69:121–126. doi: 10.1016/s0165-2427(99)00048-3. [DOI] [PubMed] [Google Scholar]
- Quinnell R.J., Courtenay O., Shaw M.A., Day M.J., Garcez L.M., Dye C., Kaye P.M. Tissue cytokine responses in canine visceral leishmaniais. J. Infect. Dis. 2001;183:1421–1424. doi: 10.1086/319869. [DOI] [PubMed] [Google Scholar]
- Rafati S., Nakhaee A., Taheri T., Taslimi Y., Darabi H., Eravani D., Sanos S., Kaye P., Taghikhani M., Jamshidi S., Rad M.A. Protective vaccination against experimental canine visceral leishmaniasis using a combination of DNA and protein immunization with cysteine proteinases type I and II of L. infantum. Vaccine. 2005;23(28):3716–3725. doi: 10.1016/j.vaccine.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Reiner S.L., Locksley R.M. The regulation of immunity to Leishmania major. Annu. Rev. Immunol. 1995;13:151–177. doi: 10.1146/annurev.iy.13.040195.001055. [DOI] [PubMed] [Google Scholar]
- Reis A.B., Martins-Filho O.A., Teixeira-Carvalho A., Carvalho M.G., Mayrink W., Franca-Silva J.C., Giunchetti R.C., Mayirink W., Genaro O., Correa-Oliveira R., Martins-Filho O. Parasite density and impaired biochemical/hematological status are associated with severe clinical aspects of canine visceral leishmaniasis. Res. Vet. Sci. 2006;81:68–75. doi: 10.1016/j.rvsc.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Reis A.B., Teixeira-Carvalho A., Vale A.M., Marques M.J., Giunchetti R.C., Mayrink W., Guerra L.L., Andrade R.A., Correa-Oliveira R., Martins-Filho O. Isotype patterns of immunoglobulins: hallmarks for clinical status and tissue parasite density in Brazilian dogs naturally infected by Leishmania (Leishmania) chagasi. Vet. Immunol. Immunopathol. 2006;112:102–116. doi: 10.1016/j.vetimm.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Reis A.B., Giunchetti R.C., Carrilo E., Martins-Filho A.O., Moreno J. Immunity to Leishmania and the rational search for vaccines against canine leishmaniasis. Trends Parasitol. 2010;26(7):341–349. doi: 10.1016/j.pt.2010.04.005. [DOI] [PubMed] [Google Scholar]
- Roatt B.M., Aguiar-Soares R.D.O., Vitoriano-Souza J., Coura-Vital W., Braga S.L., Corrêa-Oliveira R., Martins-Filho O.A., Teixeira-Carvalho A., Lana M., Gontijo N.F., Marques M.J., Giunchetti R.C., Reis A.B. Performance of LBSap vaccine after intradermal challenge with L. infantum and saliva of Lu. longipalpis: immunogenicity and parasitological evaluation. PLoS ONE. 2012 doi: 10.1371/journal.pone.0049780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisto M., Brandonisio O., Panaro M.A., Acquafredda A., Leogrande D., Fasanella A., Trotta T., Mitolo V.F. Inducible nitric oxide synthase expression in Leishmania-infected dog macrophages. Comp. Immunol. Microbiol. Infect. Dis. 2001;24:247–254. doi: 10.1016/s0147-9571(01)00013-3. [DOI] [PubMed] [Google Scholar]
- Squires K.E., Schreiber R.D., McElrath M.J., Rubin B.Y., Anderson S.L., Murray H.W. Experimental visceral leishmaniasis: role of endogenous IFN-gamma in host defense and tissue granulomatous response. J. Immunol. 1989;143:4244–4249. [PubMed] [Google Scholar]
- Strauss-Ayali D., Baneth G., Shora S., Okanoc F., Jaffe C.L. Interleukin-12 augments a Th1-type immune response manifested as lymphocyte proliferation and interferon gamma production in Leishmania infantum-infected dogs. Int. J. Parasitol. 2005;35:63–73. doi: 10.1016/j.ijpara.2004.10.015. [DOI] [PubMed] [Google Scholar]
- Tesh R.B. Control of zoonotic visceral leishmaniasis: is it time to change strategies? Am. J. Trop. Med. Hyg. 1995;52:287–292. doi: 10.4269/ajtmh.1995.52.287. [DOI] [PubMed] [Google Scholar]
- Trinchieri G. Interleukin-10 production by effector T cells: Th1 cells show self control. J. Exp. Med. 2007;204:239–243. doi: 10.1084/jem.20070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinchieri G., Rengaraju M., D’Andrea A., Valiante N.M., Kubin M., Aste M., Chehime J. Producer cells of interleukin 12. Parasitol. Today. 1993;9(3):97. doi: 10.1016/0169-4758(93)90215-2. [DOI] [PubMed] [Google Scholar]
- Virmondes-Rodrigues J.R., Da Silva J.S., Campos-Neto A. Transforming growth factor beta and immunosuppression in experimental visceral leishmaniasis. Infect. Immun. 1998;66:1233–1236. doi: 10.1128/iai.66.3.1233-1236.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitoriano-Souza J., Reis A.B., Moreira N.D., Giunchetti R.C., Correa-Oliveira R., Carneiro C.M. Kinetics of cell migration to the dermis and hypodermis in dogs vaccinated with antigenic compounds of Leishmania braziliensis plus saponin. Vaccine. 2008;26:3922–3931. doi: 10.1016/j.vaccine.2008.04.084. [DOI] [PMC free article] [PubMed] [Google Scholar]


