Abstract
Lectins are proteins with a high degree of stereospecificity to recognize various sugar structures and form reversible linkages upon interaction with glyco-conjugate complexes. These are abundantly found in plants, animals and many other species and are known to agglutinate various blood groups of erythrocytes. Further, due to the unique carbohydrate recognition property, lectins have been extensively used in many biological functions that make use of protein-carbohydrate recognition like detection, isolation and characterization of glycoconjugates, histochemistry of cells and tissues, tumor cell recognition and many more. In this review, we have summarized the immunomodulatory effects of plant lectins and their effects against diseases, including antimicrobial action. We found that many plant lectins mediate its microbicidal activity by triggering host immune responses that result in the release of several cytokines followed by activation of effector mechanism. Moreover, certain lectins also enhance the phagocytic activity of macrophages during microbial infections. Lectins along with heat killed microbes can act as vaccine to provide long term protection from deadly microbes. Hence, lectin based therapy can be used as a better substitute to fight microbial diseases efficiently in future.
Keywords: Lectins, Microbes, Antimicrobial action, Immune responses
Abbreviations
- Concanavalin A
Con A
- Wheat germ aggulutinin
WGA
- Pisum sativum lectin
PSL
- Soybean lectin
SBL
- Urtica dioica agglutinin
UDA
- Galanthus nivalis agglutinin
GNA
- Ulex eueropaeus agglutinin
UEA
- Bauhinia monandra leaf lectin
BmoLL
- Lens culinais lectin
LCL
- Phytoagglutinin
PHA
- Datura stramonium agglutinin
DSA
- Vicia violsa agglutinin
VVA
- G. simplicifolia lectin-1
GSL-1
- Jack fruit lectin
JFL
- Peanut agglutinin
PNA
- Banana lectin
Ban Lec
- Carbohydrate recognition domain
CRD
- N-acetyl galactosamine
Gal NAc
- N-acetyl glucosamine
Glc NAc
- Ribosome inactivating proteins
RIP
- Pathogen associated molecular pattern
PAMP
- Pattern recognition receptors
PRR
- Enzyme linked lectin assay
ELLA
- Electrochemical impedence spectroscopy
EIS
- Maccakia amurensis leukoagglutinin
MAL
- Severe combined immune deficiency disease
SCID
- Gastrointestinal tract
GIT
- Cholecystokinin
CCK
- Lymphocyte function associated antigen 1
LFA-1
- Dioclea violacea lectin
Dvl
1. Introduction
Lectins are natural bioactive proteins and glycoproteins having the capability to bind sugars specifically (Kennedy et al., 1995; Goldstein and Hayes, 1978; Lis and Sharon, 1977; Gallagher, 1984; Brown and Hunt, 1978). These sugar-binding proteins are of non-immune origin that can agglutinate cells or precipitate glyco-conjugates (Goldstein, 1980; Nilsson, 2007). They are ubiquitous in nature and known to play pivotal role in many life processes (Kennedy et al., 1995). Hitherto, numerous lectins have been isolated from various sources such as plants, algae, fungi, body fluid of invertebrates and lower vertebrates. Lectins can be used as models to study protein-carbohydrate interactions and as subtle tool for analysing free form or lipid-bound or protein-bound carbohydrate. Owing to its carbohydrate binding specificity, lectins are further used to deliver drugs at the site of action. Many reports have shown the peculiar characteristics of plant and animal lectins as recognition molecule in cell-molecule and cell-cell interactions in various biological systems (Sharon and Lis, 2004). In addition, they also play significant role in elucidating biological processes, clinical diagnostic system and carbohydrate structure (Sharon and Lis, 2004; Moreira et al., 1991).
2. History
Since ancient times, certain plants and animals products are known to be toxic to human (Warden and Waddell, 1884). During late 19th century, while science of bacteriology had a major impact on scientific thinking and its approach, it was believed that the toxicity of the seed is due to bacterial toxin. However, this theory was refuted by Wander and Waddell in 1884 as they reported that toxicity of jequirity beans (Abrus precatorius, Leguminosae) lie in the ‘fraction’ precipitated by alcohol from aqueous extract of the bean (Warden and Waddell, 1884). Similarly, Dixson reported toxicity of castor beans (Ricinus communis, Euphorbiaceae) seed extract in 1886. Rudolf Kobert (1854–1918), a prominent pharmacologist of 19th century, engaged his medical student Peter Hermann Stillmark (1860–1923) to study castor beans in search of ‘toxic principle’ compound. In 1888, he isolated ricin from the seeds of castor bean and tested the reactivity of partially purified protein extracts towards the red blood cells from different animals i.e. horses, dogs, rabbits and cats and reported that toxic extracts from castor beans and four other Euphorbiaceae plants are proteins and have the capability to agglutinate blood cells. He assumed that ricin is an enzyme (in those days “ferment”) that shows toxicity and agglutinating property (Stillmark, 1888). Subsequently, Heinrich Hellin revealed the presence of abrin, a toxic hemagglutinin, in jequirity bean extract at University of Dorpat (Hellin, 1891). After Stillmark's discovery, Paul Ehrlich used ricin and related toxins from jequirity beans as model antigens for his immunological studies. Later, using the crude extract of ricin and abrin (as commercially available), he proposed the most fundamental principles of immunology in 1891 (Ehrlich, 1891). After few years, in 1902, Karl Landsteiner from the University of Vienna, observed the relative hemagglutinating activity of several seed extracts on erythrocytes from different animals and also compared this specificity with antibodies of animal blood serum. He observed the species specificity of plant agglutinin towards erythrocyte and discovered the human A, B and O blood groups (Landsteiner and Raubitschek, 1907). In 1954, fascinated by Landsteiner's discovery, William C. Boyd from Boston University, tested the seeds for blood group specificity. He observed agglutination in blood type A but not for B- or O- type specifically with lima bean (Phaseolus lunatus, Leguminosae) (Boyd, 1963; Boyd and Shapleigh, 1954). In 1954, Boyd and Shapleigh coined the term ‘lectin’ to describe blood group specific plant derived agglutinins after investigating the specificity for specific erythrocytes. The word ‘lectin’ is derived from latin verb legere, meaning to select, read or gather (Boyd and Shapleigh, 1954). Generally, the term was used to specify all sugar-specific agglutinins of non-immune origin, irrespective of blood type specificity and source (Brown and Hunt, 1978; Sharon and Lis, 1972). Proteins showing hemagglutinating activity are mentioned as hemagglutinins, phytohemagglutinins or phytoagglutinins (Goldstein, 1980; Allen and Brilliantine, 1969; Lis and Sharon, 1973). In 1980, Goldstein et al. defined lectins as proteins or glycoproteins of non-immune origin, associating with carbohydrates via at least two binding sites, agglutinating plant and/or animal cells, and precipitate polysaccharides, glycoproteins or glycolipids (Goldstein, 1980).
3. Nomenclature
As lectins occur in plants as well as animals, they have been distinctly designated. Some lectins name were derived from scientific denomination of the species of origin such as ricin, abrin, Concanavalin A (Con A) and favin for the lectins from Ricinus communis, Abrus precatorius, Canavalia ensiformis and Vicia faba respectively or from common name such as pea, lima-bean and soybean lectins from the seeds of Pisum sativum, Phaseolus lunatus and Glycine max respectively (Goldstein and Hayes, 1978; Gallagher, 1984; Lis and Sharon, 1973; Carrington et al., 1985). Prokop and his coworkers proposed a nomenclature for plants and animal agglutinins by employing the nomenclature of blood-group serology (Wiener, 1961, 1966). In their proposed nomenclature, lectins were classified according to their ability to agglutinate erythrocytes of different blood groups (Prokop et al., 1968). All the lectins which agglutinate type A erythrocytes such as lectins from Dolichos biflorus, Helix hortensis and Helix pomatia were termed as ADb, AHH and AHP respectively where A stand for antigen and Db, HH, HP were the initial letters of Latin names (Goldstein and Hayes, 1978). In this approach, the lectins whose carbohydrate specificities have no relationship to blood antigens could not be classified. Hence, Goldstein and Hayes proposed a system of nomenclature according to which, first the origin of the lectin was stated and this was followed by citing the sugar-binding specificity in parenthesis and then the anomeric specificity or preference was denoted if relevant. For example, Con A is described as ‘Canavalia ensiformis (α-D-Manp > α-D-Glcp > α-D-GlcNAcp)’. Major fault of this scheme was that, it did not consider the influence of lectin reactivity on either the position of the complementary sugar in carbohydrate sequence nor synergistic effects of certain neighbouring sugars (Goldstein and Hayes, 1978). Presently, most of the new lectins are cited by their genus and species names (Kennedy et al., 1995).
4. Types of lectins
Nowadays, the criteria for defining a lectin is “A lectin or glycoprotein must have a carbohydrate recognition domain, can be induced by an external stimulus distinct from an antigenic challenge and should not modify the binding carbohydrates (Goldstein, 1980; Kocourek and Horejsi, 1983; Gabius, 1997).
As lectins are produced by a wide range of living organisms from microbes to mammals, they can be grouped according to their species of origin, such as algal lectins, fungal lectins, bacterial lectins, animal lectins and plant lectins.
4.1. Algal lectins
Also known as phycolectins, show specificity for glycoproteins rather than monosaccharides. They exhibit high content of acidic amino acids and do not require metal ions for their biological activities. Algal lectins are grouped into three major categories based on carbohydrate binding properties: complex type N-glycan specific lectins, high mannose type N-glycan specific lectins, and both above type N-glycan specific lectins (Hori et al., 1990; Rogers and Hori, 1993). These lectins are used in biomedical research for their anti-inflammatory, antiviral, anti-tumor properties and the cost-effective protein system (Singh et al., 2015).
4.2. Fungal lectins
Show high specificity towards mucins and N-acetyl galactosamine (GalNAc) residues. Various fungal lectins were identified, out of which 82% are from mushrooms, 15% from microfungi (molds) and 3% from yeasts (Singh et al., 2010, 2011; Kobayashi and Kawagishi, 2014). They are usually present in fruiting bodies with a few exceptions in mycelia (Khan and Khan, 2011). They play an essential role in growth, development, morphogenesis and molecular recognition for mycorrhization (Singh et al., 2010; Khan and Khan, 2011; Varrot et al., 2013). They also participate in early stage of infection via interaction with host glycoconjugate (Singh et al., 2011; Kobayashi and Kawagishi, 2014; Khan and Khan, 2011; Varrot et al., 2013).
4.3. Bacterial lectins
Are also called adhesins as they facilitate attachment of bacteria to host cells during infection. These bind to glycan receptor through carbohydrate-recognition domain (CRD) (Hooper and Gordon, 2001). Most bacteria possess multiple adhesins with various carbohydrate specificity. Some adhesin binds to terminal sugar residues through CRD while others bind to internal sequences of linear or branched oligosaccharide chains (Nizet et al., 2017). It plays an important role in determining the tropism of symbiont or corresponding pathogen during interaction with host glycans (Hooper and Gordon, 2001). These lectins help in adhesion and symbiosis (Nizet et al., 2017; Lis and Sharon, 1986a).
4.4. Animal lectins
Are carbohydrate binding proteins with highly variable amino acid sequences that are capable to bind complex carbohydrate structures via CRD. Each animal lectin possesses its own CRD with identical sequence motif of 115–130 amino acid residues (Kilpatrick, 2002). These are divided into several families based on the CRD sequence motifs and cation requirements such as galectins (or S-type lectins), rhamnose-binding lectins (RBLs), C-type lectins (CTLs), X-type lectins (XTLs), P-type lectins, F-type lectins, and pentraxins. They are involved in development, immune response, opsonisation, phagocytosis and activation of complement pathway (Gabius, 1997; Lis and Sharon, 1986a).
4.5. Plant lectins
Possess at least one non-catalytic domain which reversibly bind to specific mono- or oligosaccharide (Damme et al., 1998; Van Damme, 2007). They were the first proteins to be studied due to the vast distribution and ease of isolation. Till date, 500 different plant lectins were isolated and characterized (Van Damme et al., 1998). These lectins are mostly found in seeds (Hořejší and Kocourek, 1978; Pueppke, 1981; Young et al., 1982; Tollefsen and Kornfeld, 1983), roots (Gade et al., 1981; Kalsi et al., 1992) storage organs (Allen and Neuberger, 1973; Cammue et al., 1986) or leaves (Cammue et al., 1985; Suzuki et al., 1979; Yanagi et al., 1990). They can help to recognize glyco-conjugate on cell surface, for separation and structural analysis of glycoprotein and oligosaccharide. Further these lectins are found to be of great importance in host-pathogen interaction, development, cell signalling and cell-cell communication (Sharon and Lis, 2004). They further provide protection to plant against harmful phytopathogenic microorganism, insect and predatory animals (Bohlool and Schmidt, 1974). Plant lectins also play a crucial role in establishing symbiotic association between host plants and nitrogen fixing microbes (Diaz et al., 1989). Before discovery of Nod factors, lectins isolated from seeds of legume plants were considered as intermediary between two symbiotic participants (Bohlool and Schmidt, 1974; Hamblin and Kent, 1973; Dazzo and Hubbell, 1975). Legume lectins specifically target and bind to carbohydrate moieties present on the bacterial surface. This interaction leads to either agglutination of bacteria far away from the root or its attachment to root epithelial cells. Bacterial interaction to root hairs promotes genesis of infection thread which is essential for expansion of effective root nodule (De Hoff et al., 2009). For instance, PSL (Pisum sativum lectin) gene when introduced to Trifolium repens (white clovers) through A. rhizogenes transformation, formed active nodules (van Eijsden et al., 1995). Other studies have also highlighted the role of cross species lectins in fostering nodulation through their rhizobia in the host plant. For instance, insertion of SBL (Soybean lectin) transgene to Lotus corniculatus (nodulated normally by Mesorhizobium loti) increased its binding affinity with Bradyrhizobium japonicum, the suitable rhizobia of soybean plant (van Rhijn et al., 1998). Similarly, insertion of PSL transgene or Glycine max lectin apyrase/GSC2 to root of transgenic rice (which generally establishes symbiotic association with mycorhiza) led to colonization of root by different rhizobia (R. leguminosarum, B. japonicum and Rhizobium species NGR234) as compared to the control roots (Sreevidya et al., 2005). Introduction of GSC2 gene into L. japonicum is also reported to increase nodule formation and infection thread progression upon inoculation with Mesorhizobium loti (McAlvin and Stacey, 2005). In addition to above, LecRK DB46, known as lectin nucleotide phosphohydrolase (LNP) has been shown to influence nodule formation whose expression level generally goes up during nitrogen limiting conditions (Etzler et al., 1999).
5. Classification and structure of plant lectins
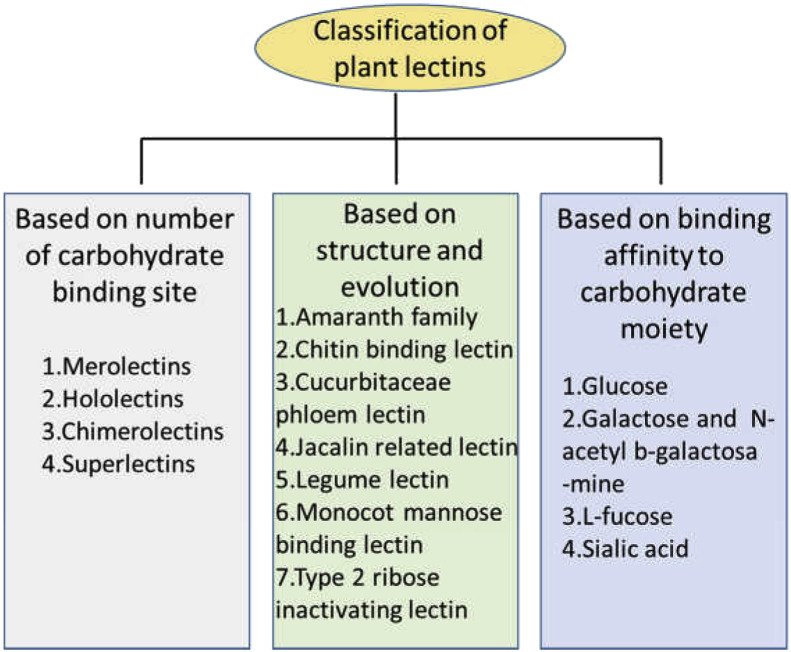
5.1. Classification of lectins on the basis of overall structure (Fig. 1)
Fig. 1.
Classification of plant lectins.
According to this way of classification Plant lectins are broadly divided into four categories.
5.1.1. Merolectins
Lectins having single carbohydrate-binding domain are characterized as merolectins. For example small chitin-binding protein from the latex of rubber plant (Hevea brasiliensis) is a merolectins. Hevein is an only carbohydrate-binding domain in this protein (Van Parijs et al., 1991). Merolectins are incapable of precipitating glyco-conjugates or agglutinating cells because they are monovalent.
5.1.2. Hololectins
Hololectins are having at least two identical and similar carbohydrate-binding domains. As hololectins are divalent and multivalent in nature they cause agglutination of cells and precipitation of glyco-conjugates.
5.1.3. Superlectins
Superlectins are a distinct class of hololectins and also considered as special group of chimerolectins. They possess two non-identical carbohydrate-binding domains that recognize structurally different sugars. For example tulip bulb lectin TxLCI, which contains two dissimilar carbohydrate-binding domains, that specifically binds mannose and GalNAc sugar residues (Van Damme et al., 1997).
5.1.4. Chimerolectins
Chimerolectins do not contain carbohydrate-domains as such but are ‘chimeric’ proteins holding a carbohydrate binding domain which is tagged with other domain having enzymatic activity. The enzymatic domain operates independently of carbohydrate binding domain. Based on number of sugar binding site present, chimerolectin can act either as merolectins or hololectins. For instance, as type 2 ribosome-inactivating proteins (RIP) are multivalent they easily agglutinate cells whereas class I plant chitinases as are monovalent do not have that activity (Barbieri et al., 1993; Collinge et al., 1993).
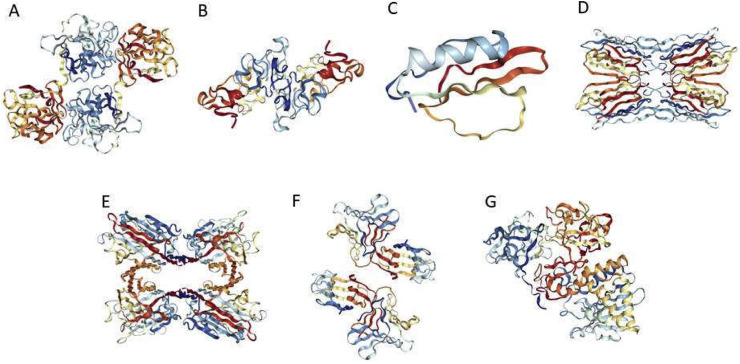
5.2. Classification of lectins based on structurally and evolutionary related proteins (Fig. 2)
Fig. 2.
Ribbon drawing of different lectins based on structural classifications. Crystal structures of amaranthin lectin (A, first member of the amaranthin family, PDB code 1JLX), WGA (B, first isolate of chitin binding lectin family, PDB code 1WGC), pumpkin lectin (C, member of cucurbitaceae phloem lectin family, PDB code 1MIT), jacalin lectin (D, members of jacalin related lectins family, PDB code 1JAC), SBL (E, member of legume lectin family, PDB code 1SBF), Galanthus nivalis agglutinin (GNA) (F, member of mannose binding lectin family, PDB code 1JPC) and ricin (G, member of Type-2 ribose inactivating lectin family, PDB code 2AAI).
5.2.1. Amaranthin
Lectins belonging to this family are exclusively derived from seeds of Amaranthus genus (Koeppe and Rupnow, 1988; Zenteno and Ochoa, 1988). The foremost member of this family was screened from Amaranthus caudatus seeds (Bird, 1954), hence named amaranthin. Lectins of this family are comprised of two subunits of about 33 kDa which are completely alike. The protomer of amaranthin consists of 300 amino acid residues and contains two homologous domains (N and C domain) which are linked by a short helix. Each domain is composed of six strands of antiparallel β-sheet with β-hairpin capping which forms a β-barrel assembly (Transue et al., 1997). The two protomers are linked to each other by substantial non-covalent linkage giving rise to a native homodimer. The carbohydrate binding site is formed at the interface between N- and C-domains of facing monomers. Binding of carbohydrate residues to amaranthine depends on a complex hydrogen bonding pattern with amino acid residues located on surface exposed hairpins and turns. Amaranthine is considered as GalNAc specific lectin showing higher affinity for T-antigen disaccharide Gal β(1,3) GalNAc (Rinderle et al., 1989).
5.2.2. Chitin binding lectins
All the proteins containing at least one hevein domain are classified as chitin-binding lectins. Nagata and Levine group were the first ones to isolate and characterize chitin-binding lectin, Wheat germ agglutinin (WGA) (Nagata and Burger, 1972; LeVine et al., 1972; Wright, 1990). The family of chitin binding lectins is dissimilar with reference to the molecular structure of native lectins because this family of lectins comprises merolectins and different types of chimerolectins besides hololectins. The simplest chitin lectin is composed of a single hevein domain.
A complete hevein domain contains 43 amino acid residues with 4 intrachain disulphide bridges, whereas truncated hevein domain, found in certain lectins, contains 30 amino acid residues with 3 intrachain disulphide bridges. Chitin binding lectins with two domain protomers are seen in monomeric Urtica dioica agglutinin (UDA) whereas dimeric chitin binding lectins are found in Viscum album (Beintema and Peumans, 1992; Peumans et al., 1996) as well as in Phytolacca americana lectin Pa-5/PL-D (Yamaguchi et al., 1996). Three domain protomers have been found exclusively in Phytolacca americana root lectins (Yamaguchi et al., 1995). In addition, gramineae species lectins contain four hevein domain protomers. Besides mero- and holo-lectins, family of chitin binding lectins comprises at least two different types of chimera lectins. First group is solanaceae lectins that are dimer, made up of protomers consists of an N-terminal chitin binding domain with three hevein repeats linked to an O-glycosylated serine hydroxy proline rich domain (Kieliszewski et al., 1994; Allen et al., 1996). Second group is class 1 chitinase that contains a single N-terminal hevein domain linked through a Glycine/Proline rich hinge domain to a catalytically active chimerase domain (Collinge et al., 1993; Beintema, 1994). The chitin binding lectin's carbohydrate binding sites are complex and they preferentially bind to GlcNAc (N-acetyl glucosamine) trimers or tetramers. The 3D structure of carbohydrate binding domains of the chitin binding lectins is determined primarily by the structure of hevein units. H-NMR spectroscopy demonstrated that hevein domain consists of stretch of amino acid residues located at N-terminal end of polypeptide chain that forms two strands of antiparallel β-sheet followed by a α-helix (Andersen et al., 1993). The folding of N-terminal region is stabilized by the four intra chain disulphide bonds. Both strands of antiparallel β-sheet contain several residues (such as Ser19, Trp21 and Trp23) that are involved in binding of GlcNAc-containing oligosaccharides (Asensio et al., 1995).
5.2.3. Cucurbitaceae phloem lectins
The cucurbitaceae phloem lectins are the sub-family of chitin-binding lectins. These lectins are found in phloem of the cucurbitaceae family. First lectin of this family was isolated from pumpkin (Cucurbita maxima) hence named pumpkin lectin (Hossaini, 1968; Liu et al., 1996). After this, many other lectins were isolated from the phloem of different genera of cucurbitaceae e.g. Cucurbita, Citrullus, Cucumis, Sechium, Luffa, and Coccinia species, which showed agglutinin activity (Allen et al., 1996; Read and Northcote, 1983). Generally, cucurbitaceae phloem lectins form dimer where two subunit (25 kDa each) are covalently linked through two interchain disulphide bonds (Read and Northcote, 1983).
5.2.4. Jacalin related lectins
Jacalin is a trivial name for lectin from the seeds of jack fruit (Artocarpus integrifolia). The structurally and evolutionary related lectins to the jack fruit lectins are known as jacalin-related lectins. Jacalin related lectins either belong to galactose or mannose specific subfamilies. Lectins with galactose specificity consist of four identical protomers composed of large α-chains and small β-chains containing a single sugar binding site. X-ray diffraction analysis showed that 3D structure of jacalin (Sankaranarayanan et al., 1996) is composed of three fold symmetric β-prism. Each β-prism further contains three four stranded β-sheets. Out of the twelve strands, eleven strands are constructed by α-chains whereas β-chain forms the twelfth strand. Four protomers when link to each other by non-covalent interaction, form tetrameric structure. Each protomer contains single carbohydrate binding site made up of Gly, Tyr, Trp, Asp of the α-chains which form a network of hydrogen bonds with O3, O4, O5 and O6 of methyl α-D-galactose. Galactose specific subgroup shows high affinity for Gal β (1,3) GalNAc (Sarkar et al., 1981; Sastry et al., 1986) whereas the mannose specific jacalin related lectins bind specifically to mannose and maltose and shows a high affinity for oligosaccharides (Van Damme et al., 1999; Peumans et al., 1997).
5.2.5. Legume lectins
The legume lectins are family of lectins isolated from fabaceae family. Legume lectins family is the largest family of plant lectins. Till now, 100 legume lectins have been isolated from 70 different species of this family. Robin, was the first legume lectin to be isolated from the bark of the legume tree black locust (Robinia pseudoacacia) by Power and Cambier in 1890 (Liener, 2012). Studies suggest that legume lectin family displays broader range of specificity than any other lectin family. All legume lectins are made up of protomers of 30 kDa (Olsen et al., 1997). Most legume lectins are mature protomer that composed of a single polypeptide chain of nearly 250 amino acid residues, hence are called one chain legume lectins. Upon occurrence of two different types of polypeptide forms in certain legume lectins, they are called two chain legume lectins. Further, many legume lectins are N-glycosylated and in these lectins, the protomers contain one or two glycan chains. Besides, legume lectins are the only metalloproteins among all plant lectins where the protomers contain tightly bound Mn2+ and Ca2+ ion, which are responsible for their carbohydrate binding property (Peumans et al., 2001). Both divalent cations are also essential to stabilize the functional conformation of the monosaccharide binding site (Agrawal, 1967). Besides a carbohydrate binding site, protomers contain a hydrophobic cavity mainly formed by conserved hydrophobic residues. Most legume lectins are comprised of two protomers that interact with each other in a two-fold symmetric plane. As a result, a twelve stranded β-sandwich is formed in which the two facing monomers associate by their flat bottoms and loops forming the monosaccharide binding sites that are located at the ends of dimer. A few amino acid residues found at the top of the dome shaped protomers form monosaccharide binding site. Through these sites, legume lectins bind specifically to mannose, glucose and fucose residues.
5.2.6. Monocot mannose binding lectins
Monocot mannose binding lectin is a superfamily of mannose specific lectins. They are exclusively found in the monocotyledonous plants. These are structurally and evolutionary different from the legume lectins and jacalin related lectins. First monocot mannose binding lectin was isolated form snowdrop bulb (Galanthus nivalis) hence called GNA (Van Damme et al., 1987; Wright et al., 1990). After isolation of other similar lectins from different monocot families, it is named as monocot mannose binding lectin family. The detailed information about the 3D structure of lectins of this family has been obtained by studying the structure of GNA and related lectins (Wright et al., 1990; Hester et al., 1995). Native GNA is composed of four identical non-covalently bound protomers of 109 residues (12 kDa). Each protomer is made up of three tandemly arrayed subdomains (called subdomain 1, 2 and 3). Further, each subdomain contains four stranded β-sheet and is completed by a sheet located in the extended C-terminal end of polypeptide chain. All subdomains are connected to each other by loops and form a twelve stranded β-barrel containing mannose binding sites which are located in the clefts formed by three bundles of β-sheet. A single disulphide bond is present between Cys29, Cys52 in the protomer. When four protomers (A, B, C and D) are combined together by non-covalent interactions, GNA homo-tetramer is formed that looks like a flattened crown with a central wide solvent channel of 16 A0. Four protomers, associate pairwise (that is, A with D and B with C) by hydrogen bonds into tight dimers that are stabilized by C-terminal strand exchange. Further, association of A-D and B–C dimers into tetramers involves mainly hydrophobic interactions. The carbohydrate binding site per protomer is 3, hence in total, 12 functional mannose binding sites are found in the native GNA tetramers.
5.2.7. Type-2 ribosome inactivating proteins (RIP)
RIP are known as catalytically inhibitor of eukaryotic ribosome (Barbieri et al., 1993). RIP are subdivided into two different groups, type 1 RIP and type 2 RIP. Type 1 RIPs are the small proteins having polynucleotide:adenosine glycosidase (PAG) activity and Type 2 RIPs are typical chimeric proteins containing two functionally and structurally different A and B chains harbouring an enzymatic and a carbohydrate binding activity. The N-terminal A-chain (25–30 kDa) possesses N-glycosidase activity whereas B chain (30–35 kDa) at C-terminal end contains two distinct carbohydrate binding site. Further, both the chains remain together through a disulphide bond between cysteine residues located at the C-terminal of A chain and N-terminal of B chain. The mature protomers are denoted as [A-s-s-B] pairs and it exhibits enzymatic as well as carbohydrate binding property. Native type 2 RIP can be monomeric (having one protomer pair), dimeric (two (A-s-s-B) pair) or tetrameric (having four (A-s-s-B) pairs). 3D structure information of this family has been obtained from analysis of crystallographic structure of Ricin (Rutenber and Robertus, 1991). A chain present in each protomer is made up of eight α-helices and six strands of β-sheet. N-terminal enzymatic site contains Tyr80, Tyr123, Glu177, Arg180 and Trp211 residues which is surrounded by residues namely Asn78, Arg134, Gln173, Ala178, Glu208 and Asn209 that helps in maintaining the catalytic conformation (Rutenber and Robertus, 1991; Kim and Robertus, 1992; Chaddock and Roberts, 1993). B chain of each protomer comprises of two similar domains called as domain 1 and domain 2. Each domain contains four subdomains namely λ, α, β and γ in which α, β and γ shows a high residual similarity. The B chain of ricin along with few short β-sheet strands is made up of coil structures linked by turns and loops. The folding of B chain is further stabilized by four disulphide bridges. The overall folding of Domain 1 and 2 is characterized by a typical β-trefoil structure (Murzin et al., 1992). Many of the type 2 RIP have been found to show specificity towards galactose, GalNAc or Gal/GalNAc residues (Peumans et al., 2001).
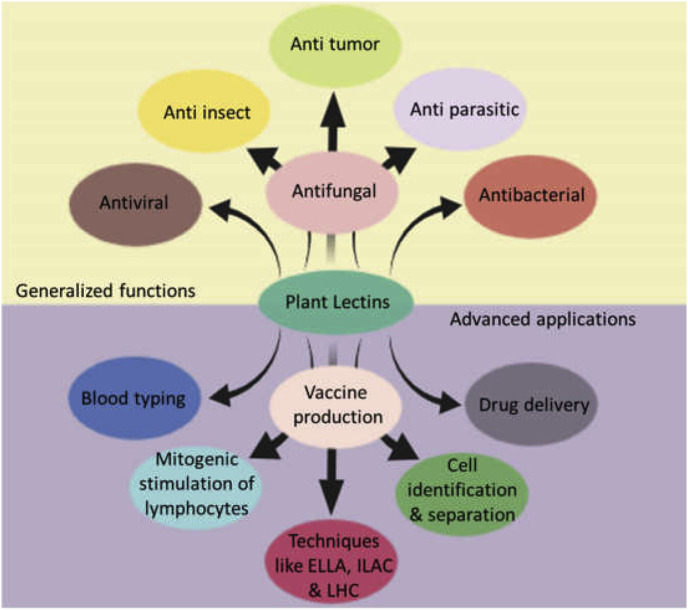
6. Generalized functions of plant lectins (Fig. 3)
Fig. 3.
Generalized and advanced applications of plant lectins.
As lectins possess the ability to bind specific carbohydrates in glyco-complexes, it has been used in many biological fields that make use of protein carbohydrate interaction. Some of the major contributions of lectins have been described below: .
6.1. Anti-insect activity of lectins
Plant lectins act as a potential insecticidal agent against a broad spectrum of insects in different orders like coleoptera, diptera, lepidoptera, hymenoptera, isoptera, neuropteran and homoptera etc. They have been successfully used previously as an alternative to conventional insect control measures (Santos et al., 2014; Hamid et al., 2013). Several plant lectins have been reported to show detrimental effect against different life stages of insect i.e. nymph, adult and oviposition by interfering with their normal physiological processes (Sadeghi et al., 2006; Lagarda-Diaz et al., 2017). Besides, in last decades, introduction of lectin gene has been found to be of great importance in providing protection from insects like aphids, moths and plant hoppers in wheat, rice, tobacco and potatoes etc. (Lam and Ng, 2011; Rüdiger and Gabius, 2001). Though the exact mechanisms of insecticidal action of plant lectins are not fully known but many studies have suggested the involvement of carbohydrate recognition property of lectin in mediating this effect (Lagarda-Diaz et al., 2017). Lectins exert its toxicity through interaction with insect gut structures in the peritrophic membrane and/or chitinous structure in the mid gut region thereby inhibiting digestion, absorption and causing nutrient deprivation that results in the death of insects (Walski et al., 2014). Other reports have also demonstrated that subcellular targets of lectins decrease food intake through its binding to either mid gut epithelium or to peritrophic matrix (Fitches et al., 1997). The surfaces of epithelial cells are rich in glycoproteins and hence provide ample targets for lectin binding. Further, these molecules cross the mid gut epithelium barrier and enter insect circulatory system that results in a toxic action due to their interference with endogenous lectins present in hemolymph that participate in self-defence (Fitches et al., 2001). In other ways, internalization of lectins by endocytic vesicles into epithelial cells lead to blockage of nuclear localization and sequence dependent nuclear protein importation that finally inhibit cell proliferation (Yu et al., 1999). Irrespective of the mechanism involved in the interaction between lectin and insect cells, for exhibiting their toxic effect, lectins must circumvent the proteolytic degradation by digestive enzymes and show resistance towards assimilatory proteins in the gut of insects. The level of resistance to digestive enzymes depends on the ability of lectins to bind to glyco-conjugates of the insect gut (Lagarda-Diaz et al., 2017). Usually the critical receptors for lectins are present on the surface of the gut epithelial cells but in some cases if lectins undergo internalization, they may interact with new set of host targets and influence particular metabolic pathways. However, such internalizations are more prone in non-legume lectins. For instance, garlic leaf lectins undergo internalization via its binding to glycosylated alkaline phosphatase harboured in insect midgut membrane. Lectins when incorporated into artificial diet or experimental environment target insects (Lagarda-Diaz et al., 2017). For example, Arisaema jacquemontii lectins (Kaur et al., 2006) adversely affected the development of Bactrocera cucurbitae larvae whereas Arisaema helleborifolium lectin (Singh et al., 2006) exerted insecticidal effect towards second instar larvae of B. curcurbitae upon its incorporation in artificial diet. Similarly, Dioscera batatas lectins blocked the arrival of Helicoverpa armigera larvae into adult by tightly binding to larval brush border and peritrophic membrane (Ohizumi et al., 2009). Annona coriacea lectin has been demonstrated to exhibit toxicity to Anagasta kuchniella by changing the gut membrane environment and interfering with digestive enzyme recycling mechanism (Coelho et al., 2007). Further, the specificity of lectins to particular sugar residues also plays an important role in mediating its anti-insect activity. One study carried out by Sadegehi et al. has demonstrated that 14 lectins with several specificities when used, all of them showed disincentive effect against oviposition of C. maculatus adults. Moreover, Talsia esculanta lectin with the ability to bind chitin and inhibited by mannose and glucose caused increased mortality in Callosobruchus maculatus and Zabrotes subfasiatus larvae (Macedo et al., 2002). Apart from these, in last decade, various plant lectins genes have been incorporated in variety of crops that has protected them from great damage caused by pests. GNA lectin when fused to an insect specific spider neurotoxin, SFTI (Segestria Florentine toxin I) carried the neurotoxin to hemolymph of lepidoptera larvae and fusion of SFTI/GNA caused toxicity in larvae (Fitches et al., 2004). Bauhina monandra leaf lectin added artificial diet showed mortality in Zabrotes subfaciatus and Callosobruchus maculatus (Braga et al., 2007). The detached leaf from transgenic tobacco plants expressing Allium sativum lectins caused reduction in weight gain, development and metamorphosis of Spodoptera littoralis larvae (Sadeghi et al., 2008). In addition to this, GNA agglutinin expressing plant showed significant reduction in the production of Rhopalosiphum maidis nymphs (Wang et al., 2005).
6.2. Anti-cancer activity of lectins
The intrinsic property of lectin to bind specific sugar residues in glycoproteins and glycolipid complex has made it a suitable candidate for use in many biomedical studies including cancer research (Hamid et al., 2013). Previously, variety of plant lectins have been successfully used as anti-tumor agent/anti neoplastic drug against various cancer types including leukaemia, sarcoma, hepatoma and breast cancer etc. (Li et al., 2008). The anticancer property of lectins is based on its ability to suppress tumor growth by inducing apoptosis and autophagy that causes down regulation of telomerase activity, inhibition of angiogenesis leading to its cytotoxicity towards cancer cells (Hamid et al., 2013; Yau et al., 2015). Further, lectins are also used to distinguish between normal and malignant cells through recognition of modified glycan structure predominantly expressed in tumor cells (Haseenabeevi et al., 1991). Apart from these, lectins after conjugation with chemotherapeutic agents act as a carrier to specifically target the tumor cells. For instance, one study carried out by Neutsch et al. have demonstrated that binding affinity of nano carrier to urothelial cells increased when they were functionalized with WGA. Further, this micro particle also showed enhanced anti neoplastic activity (Neutsch et al., 2013). Similarly, WGA tagged nanoparticles carrying thymopentin showed heightened absorption and drug delivery to intestinal mucosa in wistar rats (Yin et al., 2006). Lectins isolated from Pisum sativum seeds when encapsulated in alginate microbeads showed enhanced drug delivery to hepatocellular carcinoma (El-Aassar et al., 2014). Con A associated microsphere also displayed increased attachment and release of amoxicillin trihydrate drug in stimulated gastrointestinal fluids (Bakowsky et al., 2008; Jain et al., 2014). Bauhinia purpurea agglutinin bound liposomes were used to target human prostate cancer and this liposomal formulation was showed to repress cell proliferation after specifically binding to DU145 cells in mice (Ikemoto et al., 2016).
Many studies have conclusively suggested the use of lectin as antitumor agent in various aspects of cancer treatments previously. Some of them can be summarized as follows:
6.2.1. Anti-cancer activity of lectins through apoptosis and autophagy induction
Frutalin, a lectin, isolated from Artocarpus incise showed pro-apoptotic activity in HeLa cells by induction of cellular stress that lead to blockage of tumor cell proliferation (Oliveira et al., 2011). Con A, a legume lectin, that specifically binds with a mannose/glucose, has been reported to induce apoptosis in murine macrophage, PU5-1.8 cells, through mitochondrial assembly and cytochrome c release (Li et al., 2010). Astragalus membranaceus derived lectin further showed caspase dependent pro-apoptotic activity by downregulating Bcl-2 expression in chronic myeloid leukaemia cell line, K562 (Lavrik et al., 2005). Another study has also demonstrated the role of Con A treatment in triggering mitochondrial mediated apoptosis in human melanoma A375 cells through mitochondria dependent membrane potential collapse leading to cytochrome c release and caspase activation. Another lectin from Viscum album exhibited cytotoxic effect on Molt 4 cell line (a T cell derived leukaemia) (Peumans et al., 1996). Soybean lectin treatment in HeLa cells also reported to increase reactive oxygen species generation in a dose-dependent manner there by inducing apoptosis, autophagy and DNA damage in the cells (Hajela et al., 2002). Haliclona crater lectin exerted cytotoxic effect on HeLa and Fem X cells whereas dark red kidney bean agglutinin showed antineoplastic activity towards leukemic, L1210 cells (Pajic et al., 2002). Del monte banana lectin is reported to slow down the growth of L1210 and hepatoma (HepG2) cells (Cheung et al., 2009). Lectins from extra-long purple bean retarded hepatoma hepG2 cells through production of apoptotic bodies (Fang et al., 2010). Further, treatment of Korean mislotoe lectin in B16 –BL6 melanoma (Park et al., 2001) and human A253 cancer cells (Choi et al., 2004), abrus agglutinin in Dalton's lymphoma (Bhutia et al., 2008a) and HeLa cells (Bhutia et al., 2008b), Polygonatum odoratum lectin in murine fibrosarcoma L929 cells (Liu et al., 2009a), Polygonatum cyrtonema lectin in human melanoma A375 cells (Liu et al., 2009b) and French bean agglutinin in breast cancer MCF-7 cells (Lam and Ng, 2010) showed induction of apoptosis that led to the clearance of tumor cells. In addition to this, lectins also play an important role in initiating death receptor (such as FAS receptor) mediated apoptosis (Liu et al., 2009a). Furthermore, lectins could alter the signalling pathways involved in the expression of various members of different families like Bcl-2, autophagy (ATG) related, caspase, p53, ERK, Ras-Raf and BNIP3 resulting in apoptosis and autophagy induction. Anticancer effect of lectins in in vivo systems has also been well studied (Jiang et al., 2015). Lectins from Plurotus citrinopileatus (Li et al., 2008) and R. lepida (Zhang et al., 2010a) upon intraperitoneal injection in white kumming mice bearing sarcoma 180 were found to inhibit tumor growth. However, in order to induce cell death, lectin endocytosis is required. For example, Con A toxicity depends on its binding to the mannose moiety of cell membrane glycoproteins, followed by subsequent internalization and accumulation in the mitochondria that activates autophagy and degrades affected mitochondria and cell death through lysosomes (Kim et al., 1993; Chang and Lei, 2008).
6.2.2. Lectin based delivery system in cancer treatment
To broaden the usefulness of lectins in clinical settings, lectin based delivery system has been developed. Plattner et al. has demonstrated that WGA and Ulex eueropaeus agglutinin (UEA) have a strong interaction with human urinary carcinoma 5673 cells and was used to target bladder cancer cells (Plattner et al., 2008). Further, Cratylia mollis lectin when encapsulated in liposome displayed lower toxicity in liver and kidney and its anti-tumor activity ameliorated in Swiss mice bearing Sarcoma 180 (Andrade et al., 2004). Besides, biodegradable polymer wall coated Alginate/chitosan microcapsule provide stability to Mistletoe lectin and protect it from degradation in acidic pH of stomach (Lyu et al., 2004). Lectins derived from Bauhinia monandra leaf (BmoLL) and Lens culinaris (LCL) after incorporation on the surface of nanoparticles acts as a potential tool for oral drugs with controlled release (Rodrigues et al., 2003).
6.2.3. Lectins for identification of malignant tumors
Glycoproteins and glycolipids in neoplastic cells undergo changes in their glycosylation pattern that results in the generation of membrane signalling molecule capable of inducing several processes directly related to tumor development and cancer progression such as cell adhesion, angiogenesis, cellular mitosis and metastasis (Taniguchi and Korekane, 2011). These aberrant glycan patterns are considered tumor specific and it provides an indication between disease progression and prognosis of the same. The altered glycan structure expressed in tumor can be detected by using lectin (Teixeira et al., 2012). A study conducted by Litynska et al. has demonstrated the binding pattern of phytohemagglutinin (PHA) lectin and other non-legume lectins like the GNA, S. nigra lectin, MAL, Datura stramonium agglutinin (DSA) and WGA in different human melanoma cell lines and found that the extent of binding of lectins to surface exposed oligosaccharides is associated with tumor cell's ability to induce metastasis (Litynska et al., 2001). Further, expression analysis of carbohydrate antigens in human breast ductal carcinoma in situ (DCIS) using G. simplicifolia lectin-1 (GSL-1) and Vicia violsa agglutinin (VVA), revealed a positive correlation between the expression of VVA and GSL-1 and tumor grade in DCIS patients, indicating the role of antigenic determinants in cancer invasiveness (Korourian et al., 2008). Labelling of abundant N-acetylglucosamine (α1,3), N-acetylglucosamine/galactose and galactose (β1,4), N-acetylglucosamine (α,2) mannose (α1,6) residues expressed in rat prostate carcinoma by N-acetyl glucosamine specific and complex oligosaccharide specific lectin indicated that these sugar residues are responsible for dysplastic and neoplastic behaviour of the prostate cells. Con A and UEA 1 has also been used as markers of parotid gland and mucoepidermoid carcinoma with either high or low/intermediate grade of dysplasia respectively (Sobral et al., 2010). Jack fruit lectins (JFL) and peanut agglutinin (PNA) have been used for identification of various pathological stages of tumor progression in different oral mucosa lesions (Kumar et al., 2012). Above studies clearly suggest that lectins can be used to develop potential tools for cancer diagnosis and metastasis detection.
6.2.4. Use of lectins in clinical settings
Many plant lectins have been successfully used in cancer treatment in past years and some of them have shown excellent antitumor activity and are now under pre-clinical and clinical trials. Among different lectins, legume lectins, Con A, PHA and mistletoe lectins have contributed the most towards cancer therapy (Liu et al., 2010). Recent reports have elucidated the anticancer role of Con A against hepatoma in vivo because of its autophagy induction and immune stimulatory action as a mitogen of T cell (Chang and Lei, 2008; Lei and Chang, 2009). Besides, chemically modified form of Con A when administered to mice with melanoma, it augmented the anticancer ability of peripheral lymphocytes against tumor cells (Ueno et al., 2000). Similarly, PHA derived from raw kidney beans when incorporated in diet, inhibited the proliferation of murine non Hodgkin lymphoma tumor (Liu et al., 2010; Remmelink et al., 1999). On parallel lines, WGA treatment has shown to increase life expectancy of mice through modulation of host immune response (Liu et al., 2010). Administering Mistletoe lectins resulted in the reduction of tumor growth through stimulation of apoptosis and necrosis as well as arresting mitosis of tumor cells (Seifert et al., 2008). The extract from V. album coloratum (Korean misletoe) has also been shown to block tumor metastasis in colon carcinoma, lymphoma and melanoma cells in the mouse model (Yoon et al., 1999). Apart from this, many clinical trials have been carried out to check the clinical efficacy and safety of mistletoe lectins extracts on different cancer patient groups (Stauder and Kreuser, 2002). A standardized, fermented European mistletoe extact, Iscador was found to be safe and showed reduction in further tumor enhancement as compared to untreated groups when used during post-operative aftercare of patients with different stages of melanoma (Augustin et al., 2005). Aviscumine, a recombinant complement of natural mistletoe lectin I, have also been tested clinically to monitor its safety profile and optimum tolerated dose in cancer patients of different clinical stages. (Rüdiger and Gabius, 2001; Schöffski et al., 2004).
6.3. Anti-diabetic activity of lectins
Diabetes, also commonly known as Diabetes mellitus, is a metabolic disease that causes increase in blood sugar level because of no or less production and improper utilization of insulin by the body. It is divided into Type 1 and type II diabetes that almost show more or less similar medical complications such as diabetes retinopathy, diabetes neuropathy, diabetes nephropathy, risk of heart diseases and stroke. Many studies have demonstrated the beneficial effect of plant lectins in diabetic patients like Kolb H et al. has shown that Con A when treated in streptozotocin induced diabetic mice suppressed the hyperglycemia condition (Kolb et al., 1986). Aqueous extract of mistletoe (Viscum album) has also been reported to show anti-diabetic effect through stimulation of insulin production from clonal B cells (Gray and Flatt, 1999). Lectins isolated from seeds of Urtica pilulifera when used in streptozotocin induced diabetic rats showed magnified hypoglycemic effect compared to untreated rats (Kavalalı et al., 2003). Similarly, lectin from Cratavea tapia bark (Crata B) has been found to possess anti diabetic activity and it reduced the plasma glucose level and improved hepatic and nephritic complications in alloxan stimulated diabetic mice (Rocha et al., 2013). Further, lectins isolated from seeds of Abrus precaterius when used in alloxan monohydrate induced diabetic rats, decreased the serum glucose level as well as increased the body weight and food consumption (Sawant et al., 2017). Besides above reports, Leong et al. has reported that insulin packed microparticle when functionalized with lectins exhibited prolong hypoglycemic effect up to 12–14 h in diabetic rats (Leong et al., 2011; Coelho et al., 2017). WGA connected alginate microparticles also displayed enhanced oral delivery of insulin in streptozotocin induced diabetic rats (Kim et al., 2005).
6.4. Antimicrobial activity of lectins
Besides antitumor and anti-insecticidal property, many plant lectins also possess antimicrobial action against a number of bacteria (both gram positive and gram negative), fungal species and viruses. Plant lectins are generally present at the potential sites of microbial invasion and their interaction with glyco-components of microbial cell membrane surface leads to the inhibition of microbial growth and its adhesion and migration (Breitenbach Barroso Coelho et al., 2018). Though lectins are not able to alter structure and permeability of membrane or interrupt normal intracellular processes of invading microbes but they exert their microbicidal activity through some alternate mechanisms like microorganism agglutination and immobilization (Lagarda-Diaz et al., 2017).
6.5. Anti -fungal action of lectins
In spite of characterization of a large no. of lectins, only few of them show antifungal activity. Lectins exhibit fungicidal action through its attachment with chitins and other glycans on the fungal surface that affects fungal survival and other related activities. Upon attachment of lectins to hyphae, it affects the nutrient absorption and spore germination process (Hamid et al., 2013; Lis and Sharon, 1981). Further, lectin binding also causes impairment of synthesis and/or deposition of chitin in the cell wall thereby inhibiting the fungal growth (Selitrennikoff, 2001). Apart from the mechanisms discussed above, lectins also induce several morphological changes that make the fungi susceptible to different stress conditions (Ciopraga et al., 1999). Certain lectins have been reported to cause swollen hyphae, vacuolization of cell content and lysis of hyphal cell wall upon interaction and hence enhance sensitivity to osmotic shock (Lis and Sharon, 1981). Moreover, some small lectins also directly penetrate the fungal cell wall and reach cell membrane where they block the enzyme activity by binding to active sites and therefore influence cell wall morphogenesis. WGA upon addition inhibited spore germination and hyphal growth of Trichoderma viride and interrupted synthesis of chitin (Kumar et al., 2012). Lectin isolated from rhizomes of Ophiopogon japonicus exhibited antifungal activity towards phytopathogenic fungi namely Gibberella saubinetii and Rhizoctonia solani (Tian et al., 2008). Curcuma longa lectin is reported to inhibit fungal growth of Exserohilum turicicum, Fusarium oxysporum and Colectrotrichum cassiicola (Petnual et al., 2010). Lectins from potato (Gómez et al., 1995) and red kidney bean (Ye et al., 2001) also showed antifungal activity. Similarly, lectins isolated from Talisia esculenta seeds showed antifungal effect on Fusarium oxysporum, Colectrotrichum lindemuthianum, and Saccharomyces cerevisiae (Maria das Graças et al., 2002). Two novel chitin binding seeds of Artocarpus integrifolia demonstrated growth inhibition of Fusarium moniliforme and Saccharomyces cerevisae (Karnchanatat, 2012). Apart from the direct effect of lectins on fungal growth, lectin genes also impart protection in transgenic plants from fungal infection. For instance, insertion of precursor gene of stinging nettle isolectin I into tobacco plant resulted in the reduction of spore germination of Botrytis cinerea, Colletotrichum lindemuthianum and T. viride (Does et al., 1999). Legume lectins from plants such as Astragalus mongholicus, P. coccineus, Archidendron jiringa, B. ungulata, Glycine max, Indigofera heterantha and A. hypogaea displayed fungicidal action against phytopathogenic fungi including Botrytis cinerea, Fusarium oxysporum, F. moniliforme, F. solani, Colletotrichum species, Drechslera turcia, Exserohilum turcicum and pathogenic fungi like Candida albicans, Penicillium italicum and Aspergillus species (Sharon and Lis, 2001; Qadir et al., 2013; Yan et al., 2005; Chen et al., 2009; Boleti et al., 2007; Rao et al., 1998).
6.6. Antiviral activity of lectins
Plant lectins possess the ability to bind glycans present on the envelope glycoproteins from viruses thereby preventing its transmission and penetration into host cells (Barton et al., 2014; Akkouh et al., 2015). Moreover, lectins also crosslink surface viral glycans and hence prevent interactions with other co-receptors. Antiviral activity of plant lectins depends upon their carbohydrate specificity and varies between different lectins. Lectin isolated from Gerardia savaglia with D-mannose specificity was first reported to block HIV infection in H9 cells. This lectin has also been reported to prevent syncytium formation in HTLV-IIIB/H9-Jurkat cell system and HIV-1/human lymphocyte system by reacting with oligosaccharide chains of the HIV-1 gp120 envelop molecule (Müller et al., 1988). Later, different lectins like Con A, WGA, Lens culinaris agglutinin, Vicia faba agglutinin, PSL and PHA were found to be associated with inhibition of fusion between HIV infected cells and CD4+ cells by specifically binding with HIV infected cells (Hansen et al., 1989). Corona virus has also been reported to be sensitive to mannose specific lectins in severe acute respiratory syndrome. These lectins act by interfering with viral attachment in early phases of replication cycle and suppress viral development by binding at the end of viral infection cycle (Keyaerts et al., 2007).
Among non-legume lectins, griffithsin (GRFT), cyanovirin (CV–N) and banana lectin (BanLec) (Swanson et al., 2010) are known to have antiviral effect. Very often, these lectins are incorporated into vaginal and rectal gels, creams and suppositories to prevent HIV transmission (Lusvarghi and Bewley, 2016). Here, lectins bind viruses and block their entry and fusion to target cells by averting infection. Further, some lectins such as extralong autumn purple bean lectin and mushroom Russula delica lectin induced antiviral effect through inhibition of HIV-1 reverse transcriptase that results in blocking RNA to DNA conversion leading to blockage of its entry into host cell nucleus (Zhao et al., 2009).
6.7. Anti-parasitic activity of lectins
Some of the parasites that cause infections like Trypanosoma cruzi, Leishmania spp., Tetrahymena pyriformis and Giardia lamblia etc. have been reported to be affected by plant lectins due to their ability to act as adjuvants. The anti-parasitic effect of plant lectins is based on their property to bind to specific carbohydrates present in the parasite and thereby cause interference in downstream biological processes (Iordache et al., 2015). However, only a small number of plant lectins demonstrate anti-parasitic activity. One such lectin, isolated from the seeds of jackfruit, named jacalin has been reported to have adjuvant property and modulate cellular and humoral immunity. When used to treat cells infected with Trypanosoma cruzi or its antigens, it increases antibody production against the antigens of the parasite (Jandú et al., 2017a).
6.8. Antibacterial activity of plant lectins
The antibacterial activity of plant lectins is attributed to its ability to interact with a variety of complex carbohydrates present on the surface of bacteria. Mainly these carbohydrates include, lipopolysaccharides (LPS), peptidoglycans and techoic acids that interact with glycan binding site present in the lectin through hydrogen bonding. This interaction results in morphological alteration in bacteria leading to pore formation and cell wall bubbling in gram positive and negative bacteria respectively (Hamid et al., 2013; Lagarda-Diaz et al., 2017). To exert their microbicidal effect, lectins also interact with N-acetylmuramic acid (NAM), N-acetylglucosamine (NAG) and tetrapeptides linked to N-acetylmuramic acid or LPS present on the cell surface of gram positive or gram negative bacteria (Ayouba et al., 1994). Lectins were further found to inhibit adherence and invasion of bacteria like lectins from sarcotesta of Punica granatum decreased the adherence and intrusion of Salmonella enterica, S. aureus, S. marcescens and Aeromonas species in human cells (Silva et al., 2016a). Lectins isolated from Bangladeshi cultivar of potato, specifically binds to chitin and exhibited microbicidal effect against Shigella boydii, Salmonella enteritidis, E. coli and Listeria monocytogenes (Hasan et al., 2014). Another lectin from Apuleia leiocarpa seed showed inhibition in bacterial growth of gram positive bacteria like B. cereus, B. subtilis, Micrococcus luteus, Enterococcus faecalis, Streptococcus pyogenes, S. aureus and gram negative bacteria namely S. enteritidis, P. aeruginosa, E. coli, Klebsiella pnumoniae and Xanthomonas campestris (de Souza Carvalho et al., 2015). Some lectins also interfere with bacterial cell wall permeability. For example, one study conducted by Moura et al. demonstrated that use of water soluble lectin from seeds of M. oleifera against corrosive bacteria caused leakage of intracellular proteins due to the loss of wall/membrane integrity in a dose dependent manner (Moura et al., 2017). Apart from the bactericidal and bacteriostatic effect, plant lectins also inhibit bacterial biofilm formation. Since bacterial biofilm shows resistance to detergents and antibiotics, so it is imperative to deduce that use of lectins in this context would be useful to effectively reduce/control the problem of biofilm formation. Some of the prominent legume lectins derived from Canavalia ensiformis, T. foenumgraecum, Arachis hypogaea, Cajanus cajan, P. vulgaris and Pisum sativum has been reported to inhibit biofilm formation by Streptococcus mutans (Islam and Khan, 2012).
7. Immune response mediated by plant lectins
Despite the discovery of antibiotics long time back, the effective control of bacterial infections is still a tough challenge. This is because of the emergence of antibiotic resistant strains of different bacteria against the prevailing drugs that has spread all over the world (Davies and Davies, 2010). Moreover, pathogens have also evolved many strategies to circumvent host immune defences and in turn induce excessive inflammation resulting in the injury of host tissue (Chaves et al., 2016; Gomes et al., 2016). Hence, to tackle this situation efficiently, discovery of new antimicrobial drugs that can modulate the host immune response upon microbial infection without directly targeting the microbes is urgently needed. Among the natural compounds, plant lectins are known to have immuno-modulatory activity. They are capable of modulating cytokine secretion and production of other immune mediators like reactive oxygen species (ROS) and reactive nitrogen intermediates (RNI) to improve the host defences against microbial infections (Coelho et al., 2017; Souza et al., 2013; Da Silva and Correia, 2014). Plant lectins possess the property to bind to specific carbohydrate residues present in the membrane of both bacterial cells as well as immune cells of the host. Hence, antimicrobial activity of the lectins is manifested in two ways i.e. lectins can directly inhibit the attachment of bacteria to host cells by binding to bacterial cell surface or it can bind to glycan moieties expressed on the surface of immune cells to induce signal transduction and activation of effector mechanism against the invaded microbes (Souza et al., 2013).
Several groups have checked the effect of various plant lectins in directly inhibiting the bacterial growth but only few have studied about the role of these lectins in modulating host immune system for effective clearance of the pathogen in vitro.
The innate immune system acts as the first line of defence against any infection caused by different pathogens. This system consists of few proteins and certain phagocytic cells that are not specific to any particular pathogen. These cells recognize specific conserved proteins, carbohydrate residues or lipid moieties in the bug called pathogen associated molecular patterns (PAMPs) and act to curtail the infection. The activation of innate immunity in host is a pre-requisite for the development of adaptive immunity against any pathogen. Plant lectins not only play an important role in plant defence as pattern recognition receptors (PRRs) that identify PAMPs but also as an excellent immunomodulators for activating innate immunity in animals as well.
The immunomodulatory activity of plant lectins in various immune cells have been well documented in literature that clearly suggests that many of these lectins have the ability to enhance phagocytic activity of immune cells vis-à-vis their cytokine production in response to bacterial infection (da Silva et al., 2015). Con A is one of the most widely studied plant lectin with major immunomodulatory properties (Agrawal and Goldstein, 1968). It's treatment in murine macrophages has shown to increase the expression of various toll like receptors (TLRs) (Da Silva and Correia, 2014; Sodhi et al., 2007) through JNK, p38 and NF-κB-dependent signalling pathway. Further, these treated macrophages also secreted different pro-inflammatory cytokines and nitric oxide (NO) in TLR mediated pathway that abrogated the survival of infectious pathogen (Sodhi et al., 2007; Kesherwani and Sodhi, 2007). The TLRs are a major receptor of innate immune system and upon recognizing PAMPs of different bacteria, these activate immune response against them (Sodhi et al., 2007). Thus, TLRs induction by lectin is very helpful in clearing the infection. Another group have reported the effect of Con A on Klebsiella pneumonia in mice. Pre-treatment with a single dose of Con A enhanced the mice survival by 55% whereas treatment with consecutive doses of Con A enhanced the survival by 83%. Further, it was observed that the adversity of liver necrosis was very less in Con A pre-treated mice as compared to untreated mice. The administration of Con A also lead to inhibition of liver necrosis, decrease in alanine aminotransferase and aspartate aminotransferase in blood and liver of the infected mice respectively (Kuo et al., 2007). This indicates that Con A must be enhancing the host immunity that increases the ability of host to fight against the pathogen.
ConBr, a D-glucose lectin isolated from Canavalia brasiliensis shares 99% homology and physical properties with Con A. It is reported to induce the production of cytokines like IL-2, IL-6 and IFN-γ but on the contrary inhibit IL-10 production with concomitant production of NO in murine splenocytes (de Oliveira Silva et al., 2011). It has also been shown to activate lymphocytes in vivo, cause apoptosis, produce TNF-α in PBMCs and release histamine from mast cells (Cavada et al., 2001; Lopes et al., 2005). The antibacterial effect of ConBr and CFL isolated from Cratylia argentea has also been checked in Salmonella enterica serovar Typhimurium infection (Silva et al., 2016b). It is observed that ConBr and CFL are not cytotoxic to S. enterica as such but administration of lectin a day before infection in mice showed almost 80% dose dependent survival as compared to untreated mice. Further, when 10 mg/kg lectin was administered daily for 3 days before infection, 90% and 100% survival was recorded for CFL and ConBr respectively due to decrease in the bacterial growth in peritoneal cavity, spleen, bloodstream and liver of lectin treated mice. Con Br and CFL treatment also led to reduction in expression and amounts of IL-10 and TNF-α in the peritoneal fluid (Silva et al., 2016b). Further, it is reported to abort salmonella colocalization in mice peritoneal macrophages by regulating the expression of TLR and NO (Batista et al., 2017). Besides above, lectins are also used to enhance systemic immunity when used along with orally administered antigens. Ulex europaeus agglutinin (UEA-1) oral immunization with killed Helicobacter pylori or Campylobacter jejuni induced protective responses against bacterial challenges (Chionh et al., 2009). Con A have also been reported to activate antifungal responses in mice upon C. albicans infection. This leads to increased survival of the host due to death of the pathogen via increased phagocytosis by macrophages and neutrophils (Loyola et al., 2002; Moresco et al., 2002). The ability of Con A in enhancing the ability of mouse peritoneal cells to kill pathogen by increasing the expression of mannose receptors on the cell surface is also reported (Loyola et al., 2002). Artin M and jacalin lectins, isolated from Artocarpus integrifolia (Raval et al., 2004) have been reported to contain C. albicans infection and enhance the survival of infected swiss mice (Custodio et al., 2011) by affecting Th1 and Th17 response, mannose receptor expression, TNF-α production and bacterial killing (Custodio et al., 2011; Loyola et al., 2012). Coltri et al. have reported that Artin M can also act against Paracoccidioides brasilensis and conclusively prove that Artin M treated mice had less fungal load in lungs and pulmonary lesions in comparison to untreated mice with increased production of IL-12 in TLR-2 dependent manner (Coltri et al., 2008). Another functional attribute of helping in the migration of neutrophils is also suggested for Artin M by interacting with mannose residues present in the extracellular matrix, a process known as haptotaxis (Souza et al., 2013; Ganiko et al., 2005). Another lectin shown to have antifungal activity is Cramoll, isolated from Cratylia mollis. It inhibits in vitro growth of Cryptococcus gattii when treated one day before the infection in mice. Cramoll is also reported to have immunomodulatory effect by regulating the levels of IFN-γ, IL-6 IL-10, IL-17 and ROS in mice. Cramoll is shown to potentiate the antimycotic effect of fluconazole (another antifungal drug) by many fold thus clearly suggesting the effect of this lectin in checking the spread of fungal infection (Jandú et al., 2017b).
8. Other applications
Plants are excellent source of therapeutic substances whose potential can be harnessed for developing novel drug candidates (Peumans and Damme, 1998) so that infections caused by resistant pathogens can be cured (Chandra et al., 2017). Plant derived lectins have been widely used in different technological interventions like understanding the molecular basis of interactions between protein and carbohydrate and others that are discussed in the following section.
8.1. Plant-lectins in technological interventions
In recent years, lectins have been extensively used in the field of structural as well as functional glycomics. Specificity and sensitivity of lectins further make it a valuable tool in disease diagnosis compared to instrumental techniques (Pihíková et al., 2015). In the following sections of this review, lectin based technologies are carefully addressed.
8.2. Enzyme linked lectin assay (ELLA)
ELLA has been used to detect specific carbohydrate unit on the surface of the unfixed cells. This assay adopts the principle of Enzyme linked immunosorbent assay (ELISA). But here, plant-derived lectins (e.g. PNA) are used as a capturing as well as detecting reagent (in sandwich ELLA) instead of antibody (Hashim et al., 2017). The intensity of enzyme-conjugated lectin, determines the levels of the coated glycol-conjugates. Plant Lectins are very specific for different structure of glycans hence used to detect glycan expression profile in various tissue samples in ELLA technology. This technique has high through-put potential and other advantages like it is easy to perform, very cost effective and requires minute amount of sample. This technique helps in in situ characterization and quantification of biofilm exo-polysaccharides as lectins specifically bind to saccharide residues that are most frequently encountered in biofilms matrices (Hashim et al., 2017).
8.3. Lectin blotting
Lectin blotting technique is an extension of western blotting where lectins with carbohydrate binding modules are used instead of antibodies (Hashim et al., 2017; Shan et al., 2001). Varieties of glycan structures have been detected using glycan specific lectin probes because of its potential for visualization of small amount of protein with high specificity and sensitivity. Also, it is a very convenient method for screening complex protein samples. These labelled plant lectins such as ConA, Chempedak (Artocarpus integer) galactose binding lectin (CGB) (Phang et al., 2016) in combination with specific glycosidase has been applied for the characterization of carbohydrate chain of recombinant tissue-plasminogen activator as well as erythropoietin (Kim et al., 2008).
8.4. Immobilized-lectin affinity chromatography
Immobilized-lectin affinity chromatography is a molecular-based technique for the enrichment of glycoprotein separation in the field of glycomics research (Hashim et al., 2017). This is the most widely employed separation technique that has been developed, based on the interaction between the immobilized plant lectins such as immobilized CGB or Con A (Hashim et al., 2017) onto the chosen matrix and its carbohydrate ligand (Hage et al., 2012). Chromatography column is packed with a lectin-conjugated gel matrix and the process is carried out with the washing of non-binding proteins as well as elution of the bound glycoproteins using specific carbohydrate solution. This technique may provide a new approach for analysing and identifying site specific glycosylation of many proteins using mass spectroscopy. Furthermore, this technique is broadly applicable for the purification as well as the characterization of proteins bearing O-linked 3 oligosaccharides (Hashim et al., 2017; Pang et al., 2010).
8.5. Lectin arrays based glycan profiling
With the choice and availability of bio-recognition elements (Angeloni et al., 2004), this lectin-based technique has been developed to study a challenging field of glycomics. Lectin array technology provides a rapid and sensitive characterization of carbohydrates on glycoconjugates. Different carbohydrate contents of glycoprotein or glycolipid in a single sample is being detected by using plant lectin immobilized onto a solid support at a very high density. Multiple lectins and lectin beads are used for this type of array analysis (Hashim et al., 2017).
8.6. Flow cytometry
Flow cytometry is a powerful technology that enables the quantification of structural features of different types of cells in a mixture (Zhelev et al., 2005). Unique cell surface glycan structure of certain cell types have been characterized by flow cytometry using chemically modified lectins (Lam and Ng, 2011) and this technique can also be used for cell sorting. The cells bound with plant lectins like PNA (Gillan et al., 2005) have been detected by examining fluorescence in the flow cytometer. Apart from cells, even the number of binding sites can also be correlated with the degree of fluorescence (Zhelev et al., 2005).
8.7. Lectin histochemistry
Like immunohistochemistry, labelled lectins are used as antibodies to detect specific glycan conjugated components in tissue staining procedures based on the property that glycan moieties are recognized by the individual lectins. Plant lectins such as Con A (Hashim et al., 2017) are covalently linked to different entities like fluorophores, enzymes, colloidal gold or ferritin in the direct method to detect glycoproteins in tissue specimens. On the other hand, in the more sensitive indirect labelled method, the lectin that is conjugated with a hapten, such as biotin, are recognized by enzyme-linked streptavidin. Lectin histochemistry has been tremendously used in the study of glycosylation changes related to different diseases (Sobral et al., 2010).
8.8. Electrochemical impedance spectroscopy (EIS)
EIS technique provides sensitive, specific, robust and portable bio-sensing system with combination of carbohydrate and can also be used as an effective tool to characterize and detect the surface modification and bio-recognition process (Grossi and Riccò, 2016). EIS has been employed as level-free biosensor to detect bacteria using plant lectin such as Con A. After capturing with synthetic glycans and achieving a lower detection limit of 10∧2 CFU/ml, bacteria can be detected by EIS (Guo et al., 2011). This technique is also used for the sensitive determination and discrimination of the alpha-fetoprotein (AFP) (Chen et al., 2008; Johnson et al., 1999) that was developed by employing the lectin as molecular recognition element.
8.9. Plant lectins in blood-typing
Plant lectins are capable of interacting with animal erythrocytes and other cells (Kriipe, 1956). They bind specifically to terminal sugar residues present on the surface of human blood cells. Interaction between lectins and surface sugar moieties present on the erythrocytes results in agglutination (hemagglutination) that helps to distinguish different blood groups (A, B and H blood groups). The seed derived lectins of Dolichos biflorus are the most widely used plant lectin in serology and agglutinate type A red blood cells (more avidity towards type A1) (Judd and Issitt, 1980; Boyd et al., 1961). Lectins from Vicia cracca and lima bean (Phaseolus lunatus) are also used as anti A1 reagent (Judd and Issitt, 1980; Boyd and Reguera, 1949). Plant lectins are not predominantly used to check B type blood group because of their instability, incomplete agglutinations and cross-reactive nature with other blood types. Plant lectins used as anti-H reagents such as Ulex europaeus lectin are used for resolving exceptional blood typing results and detecting water-soluble secreted substances in blood serology tests (Judd and Issitt, 1980).
8.10. Plant lectins in cell identification
Plant lectins can identify mammalian cells containing various glycoconjugates with minor structural differences (Yim et al., 2001). Plant lectin libraries are used to discriminate among a variety of cell types in a highly specific manner. For identification of a wide variety of cells, random mutations have been incorporated to the sequences of already available plant lectins thereby expanding the lectin's use in cell identification. Plant lectins such as Maccakia amurensis (MAH) and Maccakia amurensis leukoagglutinin (MAL) recognize carbohydrate chains having sialic acid and have applications in identification of various types of erythrocytes (Yim et al., 2001). Post-translational modification including glycosylation is important for the recognition of a particular cell type at a given stage of differentiation. So, the profiling of cell-surface glycans provides an important aspect in cell identification (Yim et al., 2001).
8.11. Plant lectin-mediated separation of lymphocytes
To overcome the problem of poor yield arising in the fractionation of lymphocyte subpopulations, lectin-based fractionation can be adopted. The principle of this separation is based on the ability of lymphocyte subpopulations to agglutinate in the presence of several plant lectins (Reisner et al., 1976). The agglutinated cells can be isolated from the unagglutinated population by gravity sedimentation, followed by detachment into a single viable cell by a specific inhibitor of that particular plant lectin. Among plant lectins, SBL and WGA agglutinate B lymphocytes more specifically than T lymphocytes (Reisner et al., 1976; Bourguignon et al., 1979) whereas PHA binds more preferentially to T lymphocytes (Janossy and Greaves, 1971). The separation of lymphocytes based on lectins can really be helpful in controlling graft versus host disease (GVHD) during haploidentical transplantations, where cells liable for GVHD are removed by agglutinating using SBL (Reisner et al., 1983; Friedrich et al., 1984; O'Reilly et al., 1986). Plant lectins (especially SBL) are used against immunodeficiency diseases. Patients with severe combined immune deficiency disease (SCID) can receive haploidentical bone marrow transplants in the same manner as mentioned above (O'Reilly et al., 1986; Cowan et al., 1985).
8.12. Plant lectin-mediated mitogenic stimulation of lymphocytes
Plant lectins are mitogens having the ability for stimulating lymphocyte to undergo mitosis in a calcium-dependent manner (Sharon and Lis, 2004; Alford, 1970; Whitney and Sutherland, 1972). For the first time, mitogenic activity was studied in PHA among plant lectins. Later, other plant lectins including SBL, WGA, and Con A were also found to cause mitogenic stimulation. This stimulation is a result of the interaction between plant lectins and surface sugar moieties present on the surface of lymphocytes. A novel plant lectin obtained from rhizomes of Calystegia sepium (hedge bindweed) can be used as a powerful T cell mitogen (Peumans et al., 1997). β-galactoside-specific lectin is also reported to have mitogenic activity in vascular cells (Sanford and Harris-Hooker, 1990). Studies have shown that plant lectins (PHA and Con A) stimulate mitogenic activity at the cell membrane that may be useful in studies related to membrane structure and dynamics (Barnett et al., 1974; Andersson et al., 1972; Greaves and Bauminger, 1972).
8.13. Plant-lectins in the development of nanotechnology
Lectin-carbohydrate interaction helps in the vesicular transport into or across epithelial cells besides targeting the specific cells (Bies et al., 2004). The initial mechanism involving lectin mediated bio-adhesion, shows its targeting potential at epithelial barriers (Haltner et al., 1997). Processes such as, binding, internalization and intracellular transport of lectin and lectin conjugates depend on the lectin-glycan interaction (Mathiowitz et al., 1999). These glycans are present on many glycosylated lipids of the cell membrane and on most of the cell surface glycosylated proteins (Bies et al., 2004). The attachment of drug-carrier to a specific biological location is the basic property of bio-adhesion (Jiménez-castellanos et al., 1993). To exploit this property, plant lectins have been shown to slow down the intestinal transit time and enhance the bioavailability of oral drugs (Bies et al., 2004). Tomato lectin (TP/LEA) has been used in drug-delivery to the gastrointestinal tract. Lectins are also used in conjugation with micro and nanoparticles to reduce the transit time of pharmaceutical formulations. Plant lectin like WGA is used as a good candidate for drug targeting to the blood brain barrier as it shows low cytotoxicity and high affinity for the cerebral capillary endothelium (Fischer and Kissel, 2001; Banks and Kastin, 1998). Conjugated lectins that adhere to the corneal and conjunctival epithelia, may prolong the ocular drug delivery and also may enhance the drug absorption across these epithelia (Nicholls et al., 1996; Panjwani et al., 1995). Lectins can also be used in drug delivery to the buccal cavity and also to the nasal mucosa as this type of bioadhesive system enhances the contact time between the drug or immunogen and the site of absorption (Smart, 2004). Besides that, many histological data and recent studies revealed the binding and uptake of lectin to the apical surface of the lung cells (Brück et al., 2001). This property is applied in the field of lectin mediated drug delivery as well as gene transfer studies because it enables to target and enhance the uptake of DNA (Batra et al., 1994). In drug targeting field, the use of lectin is a nestling subject that will surely grow in the years to come.
8.13.1. Techniques that signifies the use of nanoparticle-conjugated plant lectins
8.13.1.1. Electrochemical lectin based immunosensor assay
It is a technique that combines lectin based biosensor with the (Lectin-Au-Th) bioconjugates (featuring lectin and thionine levels) linked to gold nanoparticles (AuNPs). Keeping in view of different advantages associated with this technique like selectivity, sensitivity and signal amplification, it is used in most of the cancer studies (Zhang et al., 2010b).
8.13.1.2. Quartz crystal microbalance (QCM)
It is a technique used for the level-free impedimetric detection with a lectin and antibody in a sandwich configuration. It is applied for the detection as well as for the glycoprofiling (Zhang et al., 2010b; Pihikova et al., 2016).
8.14. Plant lectins as gut stimulants
Plant lectins can be used for obtaining a healthy gastrointestinal tract (GIT) (Lis and Sharon, 1986b). Plant lectins are unaffected by digestive enzymes during their passage along GIT (Pusztai et al., 1990). These lectins are responsible for various biological aspects such as stimulation of cellular protein synthesis, growth of the organs of GIT, optimization of gut bacterial flora and modulation of hormone secretion etc. Plant lectins like PHA and SBL are shown to induce hyperplastic growth in the small intestine (Pusztai et al., 1990; Bardocz et al., 1990). Above that, plant lectin also induces cholecystokinin (CCK)-mediated pancreatic growth and is a stimulant of pancreatic enzyme secretion. That is why plant lectins are used as a potent inhibitor of gastric secretion in a dose-dependent manner without affecting pepsin secretion (Kordás et al., 2000, 2001). Mucosal damage in microvilli resulting from bacterial overgrowth is also controlled by consuming purified PHA followed by better host defence mechanism by improving the gut bacterial flora (Banwell et al., 1985; Sharon and Lis, 1989).
8.15. Role of plant lectins in inflammatory diseases
The role of lectins in inflammatory diseases remains controversial because some lectins are shown to inhibit inflammation whereas others are reported to induce inflammation (Gong et al., 2017). Many reports point to the fact that plant lectins are involved in the pathogenesis of various inflammatory diseases like rheumatoid arthritis, colitis, celiac disease and Alzheimer's etc. (Gong et al., 2017; Pusztai et al., 1993; Vasconcelos and Oliveira, 2004; Watzl et al., 2001; Kennedy et al., 2003). Gong et al. have shown that Con A binds to LFA-1, that leads to the internalization of Con A and activates NLRP3 inflammasome through endoplasmic reticulum stress and subsequently leads to the release of proinflammatory cytokines like IL-1β and IL-18 (Gong et al., 2017). Con A and WGA are also reported to induce intracellular Ca2+ mobilization in different cell types that is hypothesized to be a mechanism of NLRP3 inflammasome activation (Grinstein et al., 1987; Yatomi et al., 1993). Dioclea violacea (Dvl), a lectin isolated from the Fabaceae family is reported to have anti-inflammatory properties whereby it inhibits neutrophil migration upon inflammation when injected intravenously (Nascimento et al., 2018; Freitas et al., 2015). Freitas et al. have reported beneficial effects of Dvl in curing acute kidney injury due to its ability to reduce renal vascular resistance, apoptosis and ROS generation (Freitas et al., 2015). Another study has linked the anti-inflammatory effect of Dvl due to its ability of inhibiting plasma protein extravasation and leukocyte infiltration in rat model by decreasing ICAM-1 expression (Clemente-Napimoga et al., 2019). Another lectin isolated from Canavalia grandiflora (ConGF), a D-glucose/D-mannose-specific lectin is shown to inhibit the production of various pro-inflammatory cytokines like IL-1β and TNF-α thus affecting the leukocyte-endothelium interaction and neutrophil transmigration during inflammation in a dose dependent manner (Nunes et al., 2009). Hence, due to above said properties, plant lectins can be used to develop new therapies to control inflammatory disease and can act as a target for novel anti-inflammatory therapeutics.
8.16. Plant-derived lectins in vaccine production
Various traditional approaches (attenuated or killed microorganism) for vaccine production have failed against many pathogens due to number of reasons like inability to stimulate immune responses and poor potency. So, there is a necessity to administer the vaccine with potent immunomodulatory adjuvant that is capable of boosting and directing immune responses against specific diseases (Moyle, 2017). Based on their potential in pharmaceutical applications, plant lectins are being investigated as a candidate for adjuvant/carrier formulation for use in chemotherapeutic and prophylactic vaccine production (Sander et al., 2019). The potency of oral vaccine has also been increased with the lectin mediated vaccine production by targeting intestinal M-cells (Azizi et al., 2010). Some plant-derived lectins can act as TLR agonist due to its interaction with the glycosylated TLR receptor on macrophage and/or dendritic cells as has been shown in case of SBA, PNA, Con A, and PHA (Sander et al., 2019; Unitt and Hornigold, 2011).
9. Conclusion
Plant lectins are produced by a myriad of plants and can be isolated from different parts of plants like seed, leaf, flower, stem and bark etc. They differ greatly in their amino acid sequence, sugar specificity, stability, molecular weight and number of subunits. In spite of all the differences in the properties of these lectins, most of them exhibit a common biological function i.e. they have antitumor, antiviral, anti-insect, antifungal, anti-parasitic and immunomodulatory activities. They were also able to inhibit microbial infections in different animal models due to their potential to regulate immunomodulation. Some of the lectins are also used as adjuvants for the development of effective vaccine as they induce Th1 response. These immunotherapeutic studies have also improved our knowledge about the host-pathogen relationship and could be helpful to provide insights for the development of new therapeutic strategies. Plant lectins have some side effects as most are toxic immunogens but their effects have not been evaluated clinically apart from those that are used in anticancer therapy. Another demerit is the unavailability of suitable purification techniques for large scale production of many of the lectins. It is hoped that with the advancement in protein engineering and drug delivery techniques, we might overcome these difficulties in near future to produce quantities of smaller fragments of lectins that will retain the high target specificity that these fascinating molecules possess apart from their easy manipulation. Thus, the use of plant lectins as immunomodulatory agents to combat different infections and diseases will grow in years to come.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Department of Science and Technology (DST), Govt. of India (EMR/2016/000048, EEQ/2016/000205 and DST/INSPIRE/Faculty award/2014/DST/INSPIRE/04/2014/01662) is acknowledged by RD for the generous financial support to his laboratory. RD is also thankful to the Ministry of Human Resource and Development (MHRD), Govt. of India for intramural support. Financial support to SKP by Department of Bio-Technology (DBT), Govt. of India (BT/PR21318/MED/12/742/2016) and DST, Govt. of India (EMR/2016/007034) is also acknowledged. The research fellowship by DST to AB and MHRD to AM, SM, AK and LN is deeply acknowledged. DM is thankful to DST for Kishore Vaigyanik Protsahan Yojana (KVPY) fellowship.
Contributor Information
Samir K. Patra, Email: skpatra_99@yahoo.com, samirp@nitrkl.ac.in.
Rohan Dhiman, Email: dhimanr@nitrkl.ac.in, rohandhiman79@yahoo.com.
References
- Agrawal B. Isolation of concanavalin A by specific adsorption on cross-linked dextran gels. Biochim. Biophys. Acta. 1967;147:262–271. [PubMed] [Google Scholar]
- Agrawal B., Goldstein I. Protein–carbohydrate interaction. XV. The role of bivalent cations in concanavalin A–polysaccharide interaction. Can. J. Biochem. 1968;46(9):1147–1150. doi: 10.1139/o68-170. [DOI] [PubMed] [Google Scholar]
- Akkouh O. Lectins with anti-HIV activity: a review. Molecules. 2015;20(1):648–668. doi: 10.3390/molecules20010648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alford R.H. Metal cation requirements for phytohemagglutinin-induced transformation of human peripheral blood lymphocytes. J. Immunol. 1970;104(3):698–703. [PubMed] [Google Scholar]
- Allen N.K., Brilliantine L. A survey of hemagglutinins in various seeds. J. Immunol. 1969;102(5):1295–1299. [PubMed] [Google Scholar]
- Allen A.K., Neuberger A. The purification and properties of the lectin from potato tubers, a hydroxyproline-containing glycoprotein. Biochem. J. 1973;135(2):307–314. doi: 10.1042/bj1350307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen A.K. Potato lectin: a three-domain glycoprotein with novel hydroxyproline-containing sequences and sequence similarities to wheat-germ agglutinin. Int. J. Biochem. Cell Biol. 1996;28(11):1285–1291. doi: 10.1016/s1357-2725(96)00043-x. [DOI] [PubMed] [Google Scholar]
- Andersen N.H. Hevein: NMR assignment and assessment of solution-state folding for the agglutinin-toxin motif. Biochemistry. 1993;32(6):1407–1422. doi: 10.1021/bi00057a004. [DOI] [PubMed] [Google Scholar]
- Andersson J. Activation of B lymphocytes by locally concentrated concanavalin A. Eur. J. Immunol. 1972;2(3):233–235. doi: 10.1002/eji.1830020307. [DOI] [PubMed] [Google Scholar]
- Andrade C.A. Antitumor activity of Cratylia mollis lectin encapsulated into liposomes. Int. J. Pharm. 2004;278(2):435–445. doi: 10.1016/j.ijpharm.2004.03.028. [DOI] [PubMed] [Google Scholar]
- Angeloni S. Glycoprofiling with micro-arrays of glycoconjugates and lectins. Glycobiology. 2004;15(1):31–41. doi: 10.1093/glycob/cwh143. [DOI] [PubMed] [Google Scholar]
- Asensio J.L. The interaction of hevein with N‐acetylglucosamine‐containing oligosaccharides: solution structure of hevein complexed to chitobiose. Eur. J. Biochem. 1995;230(2):621–633. doi: 10.1111/j.1432-1033.1995.tb20604.x. [DOI] [PubMed] [Google Scholar]
- Augustin M. Safety and efficacy of the long-term adjuvant treatment of primary intermediate-to high-risk malignant melanoma (UICC/AJCC stage II and III) with a standardized fermented European mistletoe (Viscum album L.) extract. Arzneimittelforschung. 2005;55(01):38–49. doi: 10.1055/s-0031-1296823. [DOI] [PubMed] [Google Scholar]
- Ayouba A. Interactions of plant lectins with the components of the bacterial cell wall peptidoglycan. Biochem. Syst. Ecol. 1994;22(2):153–159. [Google Scholar]
- Azizi A. Enhancing oral vaccine potency by targeting intestinal M cells. PLoS Pathog. 2010;6(11) doi: 10.1371/journal.ppat.1001147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakowsky H. Adhesion characteristics and stability assessment of lectin-modified liposomes for site-specific drug delivery. Biochim. Biophys. Acta Biomembr. 2008;1778(1):242–249. doi: 10.1016/j.bbamem.2007.09.033. [DOI] [PubMed] [Google Scholar]
- Banks W.A., Kastin A.J. Characterization of lectin‐mediated brain uptake of HIV‐1 GP120. J. Neurosci. Res. 1998;54(4):522–529. doi: 10.1002/(SICI)1097-4547(19981115)54:4<522::AID-JNR9>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Banwell J. Intestinal microbial flora after feeding phytohemagglutinin lectins (Phaseolus vulgaris) to rats. Appl. Environ. Microbiol. 1985;50(1):68–80. doi: 10.1128/aem.50.1.68-80.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbieri L., Battelli M.G., Stirpe F. Ribosome-inactivating proteins from plants. Biochim. Biophys. Acta Rev. Biomembr. 1993;1154(3–4):237–282. doi: 10.1016/0304-4157(93)90002-6. [DOI] [PubMed] [Google Scholar]
- Bardocz S. Polyamine metabolism and uptake during Phaseolus vulgaris lectin, PHA-induced growth of rat small intestine. Digestion. 1990;46(Suppl. 2):360–366. doi: 10.1159/000200409. [DOI] [PubMed] [Google Scholar]
- Barnett R.E. Evidence that mitogenic lectins induce changes in lymphocyte membrane fluidity. Nature. 1974;249(5456):465. doi: 10.1038/249465a0. [DOI] [PubMed] [Google Scholar]
- Barton C. Activity of and effect of subcutaneous treatment with the broad-spectrum antiviral lectin griffithsin in two laboratory rodent models. Antimicrob. Agents Chemother. 2014;58(1):120–127. doi: 10.1128/AAC.01407-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batista J. Plant lectins ConBr and CFL modulate expression toll-like receptors, pro-inflammatory cytokines and reduce the bacterial burden in macrophages infected with Salmonella enterica serovar Typhimurium. Phytomedicine. 2017;25:52–60. doi: 10.1016/j.phymed.2016.12.005. [DOI] [PubMed] [Google Scholar]
- Batra R.K. Receptor-mediated gene delivery employing lectin-binding specificity. Gene Ther. 1994:255–260. [PubMed] [Google Scholar]
- Beintema J.J. Structural features of plant chitinases and chitin-binding proteins. FEBS Lett. 1994;350(2–3):159–163. doi: 10.1016/0014-5793(94)00753-5. [DOI] [PubMed] [Google Scholar]
- Beintema J.J., Peumans W.J. The primary structure of stinging nettle (Urtica dioica) agglutinin A two‐domain member of the hevein family. FEBS Lett. 1992;299(2):131–134. doi: 10.1016/0014-5793(92)80231-5. [DOI] [PubMed] [Google Scholar]
- Bhutia S.K. Antitumor and proapoptotic effect of Abrus agglutinin derived peptide in Dalton's lymphoma tumor model. Chem. Biol. Interact. 2008;174(1):11–18. doi: 10.1016/j.cbi.2008.04.043. [DOI] [PubMed] [Google Scholar]
- Bhutia S.K. Induction of mitochondria-dependent apoptosis by Abrus agglutinin derived peptides in human cervical cancer cell. Toxicol. In Vitro. 2008;22(2):344–351. doi: 10.1016/j.tiv.2007.09.016. [DOI] [PubMed] [Google Scholar]
- Bies C., Lehr C.-M., Woodley J.F. Lectin-mediated drug targeting: history and applications. Adv. Drug Deliv. Rev. 2004;56(4):425–435. doi: 10.1016/j.addr.2003.10.030. [DOI] [PubMed] [Google Scholar]
- Bird G. Observations on the interactions of the erythrocytes of various species with certain seed agglutinins. Br. J. Exp. Pathol. 1954;35(3):252. [PMC free article] [PubMed] [Google Scholar]
- Bohlool B., Schmidt E. Lectins: a possible basis for specificity in the Rhizobium—legume root nodule symbiosis. Science. 1974;185(4147):269–271. doi: 10.1126/science.185.4147.269. [DOI] [PubMed] [Google Scholar]
- Boleti A.P.d.A. Insecticidal and antifungal activity of a protein from Pouteria torta seeds with lectin-like properties. J. Agric. Food Chem. 2007;55(7):2653–2658. doi: 10.1021/jf0636317. [DOI] [PubMed] [Google Scholar]
- Bourguignon L.Y., Rader R.L., McMahon J.T. Rapid separation of mouse T and B lymphocytes using wheat germ agglutinin. J. Cell. Physiol. 1979;99(1):95–99. doi: 10.1002/jcp.1040990111. [DOI] [PubMed] [Google Scholar]
- Boyd W.C. The lectins: their present status. Vox Sanguinis. 1963;8(1):1–32. doi: 10.1111/j.1423-0410.1963.tb04146.x. [DOI] [PubMed] [Google Scholar]
- Boyd W.C., Reguera R.M. Hemagglutinating substances for human cells in various plants. J. Immunol. 1949;62(3):333–339. [PubMed] [Google Scholar]
- Boyd W.C., Shapleigh E. Specific precipitating activity of plant agglutinins (lectins) Science. 1954;119(3091) doi: 10.1126/science.119.3091.419. 419-419. [DOI] [PubMed] [Google Scholar]
- Boyd W.C., Waszczenko‐Zacharczenko E., Goldwasser S.M. List of plants tested for hemagglutinating activity. Transfusion. 1961;1(6):374–382. doi: 10.1111/j.1537-2995.1961.tb00078.x. [DOI] [PubMed] [Google Scholar]
- Braga Y.F. Insecticidal activity of 2-tridecanone against the cowpea weevil Callosobruchus maculatus (Coleoptera: Bruchidae) An Acad. Bras Ciências. 2007;79(1):35–39. doi: 10.1590/s0001-37652007000100005. [DOI] [PubMed] [Google Scholar]
- Breitenbach Barroso Coelho L. Lectins as antimicrobial agents. J. Appl. Microbiol. 2018;125(5):1238–1252. doi: 10.1111/jam.14055. [DOI] [PubMed] [Google Scholar]
- Brown J.C., Hunt R.C. International Review of Cytology. Elsevier; 1978. Lectins; pp. 277–349. [Google Scholar]
- Brück A. Lectin-functionalized liposomes for pulmonary drug delivery: interaction with human alveolar epithelial cells. J. Drug Target. 2001;9(4):241–251. doi: 10.3109/10611860108997933. [DOI] [PubMed] [Google Scholar]
- Cammue B., Peeters B., Peumans W. Isolation and partial characterization of an N-acetylgalactosamine-specific lectin from winter-aconite (Eranthis hyemalis) root tubers. Biochem. J. 1985;227(3):949–955. doi: 10.1042/bj2270949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cammue B., Peeters B., Peumans W. A new lectin from tulip (Tulipa) bulbs. Planta. 1986;169(4):583–588. doi: 10.1007/BF00392110. [DOI] [PubMed] [Google Scholar]
- Carrington D., Auffret A., Hanke D. Polypeptide ligation occurs during post-translational modification of concanavalin A. Nature. 1985;313(5997):64. doi: 10.1038/313064a0. [DOI] [PubMed] [Google Scholar]
- Cavada B.S. Revisiting proteus: do minor changes in lectin structure matter in biological activity? Lessons from and potential biotechnological uses of the Diocleinae subtribe lectins. Curr. Protein Pept. Sci. 2001;2(2):123–135. doi: 10.2174/1389203013381152. [DOI] [PubMed] [Google Scholar]
- Chaddock J.A., Roberts L.M. Mutagenesis and kinetic analysis of the active site Glu 177 of ricin A-chain. Protein Eng. Des. Sel. 1993;6(4):425–431. doi: 10.1093/protein/6.4.425. [DOI] [PubMed] [Google Scholar]
- Chandra H. Antimicrobial resistance and the alternative resources with special emphasis on plant-based antimicrobials—a review. Plants. 2017;6(2):16. doi: 10.3390/plants6020016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C.-P., Lei H.-Y. Autophagy induction in T cell-independent acute hepatitis induced by concanavalin A in SCID/NOD mice. Int. J. Immunopathol. Pharmacol. 2008;21(4):817–826. doi: 10.1177/039463200802100406. [DOI] [PubMed] [Google Scholar]
- Chaves M.M., Canetti C., Coutinho-Silva R. Crosstalk between purinergic receptors and lipid mediators in leishmaniasis. Parasites Vectors. 2016;9(1):489. doi: 10.1186/s13071-016-1781-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P. Identification of N-glycan of alpha-fetoprotein by lectin affinity microarray. J. Cancer Res. Clin. Oncol. 2008;134(8):851–860. doi: 10.1007/s00432-008-0357-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. A novel sialic acid-specific lectin from Phaseolus coccineus seeds with potent antineoplastic and antifungal activities. Phytomedicine. 2009;16(4):352–360. doi: 10.1016/j.phymed.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Cheung A.H., Wong J.H., Ng T. Musa acuminata (Del Monte banana) lectin is a fructose-binding lectin with cytokine-inducing activity. Phytomedicine. 2009;16(6–7):594–600. doi: 10.1016/j.phymed.2008.12.016. [DOI] [PubMed] [Google Scholar]
- Chionh Y.-T. M-cell targeting of whole killed bacteria induces protective immunity against gastrointestinal pathogens. Infect. Immun. 2009;77(7):2962–2970. doi: 10.1128/IAI.01522-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S.H., Lyu S.Y., Park W.B. Mistletoe lectin induces apoptosis and telomerase inhibition in human A253 cancer cells through dephosphorylation of Akt. Arch Pharm. Res. (Seoul) 2004;27(1):68. doi: 10.1007/BF02980049. [DOI] [PubMed] [Google Scholar]
- Ciopraga J. Fusarium sp. growth inhibition by wheat germ agglutinin. Biochim. Biophys. Acta Gen. Subj. 1999;1428(2–3):424–432. doi: 10.1016/s0304-4165(99)00085-9. [DOI] [PubMed] [Google Scholar]
- Clemente-Napimoga J.T. Dioclea violacea lectin ameliorates inflammation in the temporomandibular joint of rats by suppressing intercellular adhesion molecule-1 expression. Biochimie. 2019;158:34–42. doi: 10.1016/j.biochi.2018.12.007. [DOI] [PubMed] [Google Scholar]
- Coelho M.B., Marangoni S., Macedo M.L.R. Insecticidal action of Annona coriacea lectin against the flour moth Anagasta kuehniella and the rice moth Corcyra cephalonica (Lepidoptera: Pyralidae) Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2007;146(3):406–414. doi: 10.1016/j.cbpc.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Coelho L.C.B.B. Lectins, interconnecting proteins with biotechnological/pharmacological and therapeutic applications. Evid. Based Complement Altern. Med. 2017;2017 doi: 10.1155/2017/1594074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collinge D.B. Plant chitinases. Plant J. 1993;3(1):31–40. doi: 10.1046/j.1365-313x.1993.t01-1-00999.x. [DOI] [PubMed] [Google Scholar]
- Coltri K.C. Therapeutic administration of KM+ lectin protects mice against Paracoccidioides brasiliensis infection via interleukin-12 production in a toll-like receptor 2-dependent mechanism. Am. J. Pathol. 2008;173(2):423–432. doi: 10.2353/ajpath.2008.080126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan M.J. Haploidentical bone marrow transplantation for severe combined immunodeficiency disease using soybean agglutinin-negative, T-depleted marrow cells. J. Clin. Immunol. 1985;5(6):370–376. doi: 10.1007/BF00915333. [DOI] [PubMed] [Google Scholar]
- Custodio L.A. Protective effect of Artin M from extract of Artocarpus integrifolia seeds by Th1 and Th17 immune response on the course of infection by Candida albicans. Int. Immunopharmacol. 2011;11(10):1510–1515. doi: 10.1016/j.intimp.2011.05.005. [DOI] [PubMed] [Google Scholar]
- Da Silva L.C.N., Correia M.T.D.S. Plant lectins and Toll-like receptors: implications for therapy of microbial infections. Front. Microbiol. 2014;5:20. doi: 10.3389/fmicb.2014.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva L.C.N. Immunomodulatory effects of pCramoll and rCramoll on peritoneal exudate cells (PECs) infected and non-infected with Staphylococcus aureus. Int. J. Biol. Macromol. 2015;72:848–854. doi: 10.1016/j.ijbiomac.2014.09.045. [DOI] [PubMed] [Google Scholar]
- Damme E.J.V. Plant lectins: a composite of several distinct families of structurally and evolutionary related proteins with diverse biological roles. Crit. Rev. Plant Sci. 1998;17(6):575–692. [Google Scholar]
- Davies J., Davies D. Origins and evolution of antibiotic resistance. Microbiol. Mol. Biol. Rev. 2010;74(3):417–433. doi: 10.1128/MMBR.00016-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dazzo F.B., Hubbell D.H. Cross-reactive antigens and lectin as determinants of symbiotic specificity in the Rhizobium-clover association. Appl. Environ. Microbiol. 1975;30(6):1017–1033. doi: 10.1128/am.30.6.1017-1033.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Hoff P.L., Brill L.M., Hirsch A.M. Plant lectins: the ties that bind in root symbiosis and plant defense. Mol. Genet. Genom. 2009;282(1):1–15. doi: 10.1007/s00438-009-0460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira Silva F. Immunostimulatory activity of ConBr: a focus on splenocyte proliferation and proliferative cytokine secretion. Cell Tissue Res. 2011;346(2):237–244. doi: 10.1007/s00441-011-1239-x. [DOI] [PubMed] [Google Scholar]
- de Souza Carvalho A. Purification, characterization and antibacterial potential of a lectin isolated from Apuleia leiocarpa seeds. Int. J. Biol. Macromol. 2015;75:402–408. doi: 10.1016/j.ijbiomac.2015.02.001. [DOI] [PubMed] [Google Scholar]
- Diaz C.L. Root lectin as a determinant of host–plant specificity in the Rhizobium–legume symbiosis. Nature. 1989;338(6216):579. [Google Scholar]
- Does M.P. Processing, targeting, and antifungal activity of stinging nettle agglutinin in transgenic tobacco. Plant Physiol. 1999;120(2):421–432. doi: 10.1104/pp.120.2.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich P. Experimentelle untersuchungen über immunität. I. Ueber ricin. Dtsch. Med. Wochenschr. 1891;17(32):976–979. [Google Scholar]
- El-Aassar M. Microencapsulation of lectin anti-cancer agent and controlled release by alginate beads, biosafety approach. Int. J. Biol. Macromol. 2014;69:88–94. doi: 10.1016/j.ijbiomac.2014.05.031. [DOI] [PubMed] [Google Scholar]
- Etzler M.E. A nod factor binding lectin with apyrase activity from legume roots. Proc. Natl. Acad. Sci. 1999;96(10):5856–5861. doi: 10.1073/pnas.96.10.5856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang E.F. A lectin with anti-HIV-1 reverse transcriptase, antitumor, and nitric oxide inducing activities from seeds of Phaseolus vulgaris cv. extralong autumn purple bean. J. Agric. Food Chem. 2010;58(4):2221–2229. doi: 10.1021/jf903964u. [DOI] [PubMed] [Google Scholar]
- Fischer D., Kissel T. Histochemical characterization of primary capillary endothelial cells from porcine brains using monoclonal antibodies and fluorescein isothiocyanate-labelled lectins: implications for drug delivery. Eur. J. Pharm. Biopharm. 2001;52(1):1–11. doi: 10.1016/s0939-6411(01)00159-x. [DOI] [PubMed] [Google Scholar]
- Fitches E., Gatehouse A.M., Gatehouse J.A. Effects of snowdrop lectin (GNA) delivered via artificial diet and transgenic plants on the development of tomato moth (Lacanobia oleracea) larvae in laboratory and glasshouse trials. J. Insect Physiol. 1997;43(8):727–739. doi: 10.1016/s0022-1910(97)00042-5. [DOI] [PubMed] [Google Scholar]
- Fitches E. In vitro and in vivo binding of snowdrop (Galanthus nivalis agglutinin; GNA) and jackbean (Canavalia ensiformis; Con A) lectins within tomato moth (Lacanobia oleracea) larvae; mechanisms of insecticidal action. J. Insect Physiol. 2001;47(7):777–787. doi: 10.1016/s0022-1910(01)00068-3. [DOI] [PubMed] [Google Scholar]
- Fitches E. Fusion proteins containing insect-specific toxins as pest control agents: snowdrop lectin delivers fused insecticidal spider venom toxin to insect haemolymph following oral ingestion. J. Insect Physiol. 2004;50(1):61–71. doi: 10.1016/j.jinsphys.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Freitas F.P. Dioclea violacea lectin ameliorates oxidative stress and renal dysfunction in an experimental model of acute kidney injury. Am. J. Tourism Res. 2015;7(12):2573. [PMC free article] [PubMed] [Google Scholar]
- Friedrich W. Immunoreconstitution in severe combined immunodeficiency after transplantation of HLA-haploidentical, T-cell-depleted bone marrow. The Lancet. 1984;323(8380):761–764. doi: 10.1016/s0140-6736(84)91277-7. [DOI] [PubMed] [Google Scholar]
- Gabius H.J. Animal lectins. Eur. J. Biochem. 1997;243(3):543–576. doi: 10.1111/j.1432-1033.1997.t01-1-00543.x. [DOI] [PubMed] [Google Scholar]
- Gade W. The isolation and characterization of a root lectin from soybean (Glycine max (L), cultivar Chippewa) J. Biol. Chem. 1981;256(24):12905–12910. [PubMed] [Google Scholar]
- Gallagher J.T. Carbohydrate-binding properties of lectins: a possible approach to lectin nomenclature and classification. Biosci. Rep. 1984;4(8):621–632. doi: 10.1007/BF01121015. [DOI] [PubMed] [Google Scholar]
- Ganiko L. Lectin KM+-induced neutrophil haptotaxis involves binding to laminin. Biochim. Biophys. Acta Gen. Subj. 2005;1721(1–3):152–163. doi: 10.1016/j.bbagen.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Gillan L., Evans G., Maxwell W. Flow cytometric evaluation of sperm parameters in relation to fertility potential. Theriogenology. 2005;63(2):445–457. doi: 10.1016/j.theriogenology.2004.09.024. [DOI] [PubMed] [Google Scholar]
- Goldstein I.J. What should be called a lectin? Nature. 1980;285:66. [Google Scholar]
- Goldstein I.J., Hayes C.E. Advances in Carbohydrate Chemistry and Biochemistry. Elsevier; 1978. The lectins: carbohydrate-binding proteins of plants and animals; pp. 127–340. [DOI] [PubMed] [Google Scholar]
- Gomes P.S. Immune escape strategies of malaria parasites. Front. Microbiol. 2016;7:1617. doi: 10.3389/fmicb.2016.01617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez E. Advances in Mucosal Immunology. Springer; 1995. Intestinal and systemic immune responses in rats to dietary lectins; pp. 533–536. [DOI] [PubMed] [Google Scholar]
- Gong T. Plant lectins activate the NLRP3 inflammasome to promote inflammatory disorders. J. Immunol. 2017;198(5):2082–2092. doi: 10.4049/jimmunol.1600145. [DOI] [PubMed] [Google Scholar]
- Gray A., Flatt P. Insulin-secreting activity of the traditional antidiabetic plant Viscum album (mistletoe) J. Endocrinol. 1999;160(3):409–414. doi: 10.1677/joe.0.1600409. [DOI] [PubMed] [Google Scholar]
- Greaves M.F., Bauminger S. Activation of T and B lymphocytes by insoluble phytomitogens. Nat. New Biol. 1972;235(55):67–70. doi: 10.1038/newbio235067a0. [DOI] [PubMed] [Google Scholar]
- Grinstein S. Mechanism of activation of lymphocyte Na+/H+ exchange by concanavalin A. A calcium-and protein kinase C-independent pathway. J. Biol. Chem. 1987;262(31):15277–15284. [PubMed] [Google Scholar]
- Grossi M., Riccò B. Electrical impedance spectroscopy (EIS) for biological analysis and food characterization: a review. Journal of Sensors and Sensor Systems. 2016;6:303–325. [Google Scholar]
- Guo X. Carbohydrate-based label-free detection of Escherichia coli ORN 178 using electrochemical impedance spectroscopy. Anal. Chem. 2011;84(1):241–246. doi: 10.1021/ac202419u. [DOI] [PubMed] [Google Scholar]
- Hage D.S. Pharmaceutical and biomedical applications of affinity chromatography: recent trends and developments. J. Pharm. Biomed. Anal. 2012;69:93–105. doi: 10.1016/j.jpba.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajela K. The biological functions of MBL-associated serine proteases (MASPs) Immunobiology. 2002;205(4–5):467–475. doi: 10.1078/0171-2985-00147. [DOI] [PubMed] [Google Scholar]
- Haltner E., Easson J.H., Lehr C.-M. Lectins and bacterial invasion factors for controlling endo-and transcytosis of bioadhesive drug carrier systems. Eur. J. Pharm. Biopharm. 1997;44(1):3–13. [Google Scholar]
- Hamblin J., Kent S. Possible role of phytohaemagglutinin in Phaseolus vulgaris L. Nat. New Biol. 1973;245(140):28–30. doi: 10.1038/newbio245028a0. [DOI] [PubMed] [Google Scholar]
- Hamid R. Lectins: proteins with diverse applications. J. Appl. Pharm. Sci. 2013;3(4.1):93–103. [Google Scholar]
- Hansen J.-E. Correlation between carbohydrate structures on the envelope glycoprotein gp120 of HIV-1 and HIV-2 and syncytium inhibition with lectins. AIDS (London, England) 1989;3(10):635–641. doi: 10.1097/00002030-198910000-00003. [DOI] [PubMed] [Google Scholar]
- Hasan I., Ozeki Y., Kabir S.R. 2014. Purification of a Novel Chitin-Binding Lectin with Antimicrobial and Antibiofilm Activities from a Bangladeshi Cultivar of Potato (Solanum tuberosum) [PubMed] [Google Scholar]
- Haseenabeevi V. Plant lectins--histochemical and cytochemical applications in oncology. Indian J. Dent. Res.: official publication of Indian Society for Dental Research. 1991;2(3–4):45–53. [PubMed] [Google Scholar]
- Hashim O.H., Jayapalan J.J., Lee C.-S. Lectins: an effective tool for screening of potential cancer biomarkers. PeerJ. 2017;5 doi: 10.7717/peerj.3784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellin H. Dorpat; 1891. Der giftige Eiweisskörper Abrin und seine Wirkung auf das Blut. [Google Scholar]
- Hester G. Structure of mannose-specific snowdrop (Galanthus nivalis) lectin is representative of a new plant lectin family. Nat. Struct. Biol. 1995;2(6):472. doi: 10.1038/nsb0695-472. [DOI] [PubMed] [Google Scholar]
- Hooper L.V., Gordon J.I. Glycans as legislators of host–microbial interactions: spanning the spectrum from symbiosis to pathogenicity. Glycobiology. 2001;11(2):1R–10R. doi: 10.1093/glycob/11.2.1r. [DOI] [PubMed] [Google Scholar]
- Hori K., Miyazawa K., Ito K. Some common properties of lectins from marine algae. Hydrobiologia. 1990;204(1):561–566. [Google Scholar]
- Hossaini A. Hemolytic and hemagglutinating activities of 222 plants. Vox Sanguinis. 1968;15(6):410–417. doi: 10.1111/j.1423-0410.1968.tb04083.x. [DOI] [PubMed] [Google Scholar]
- Hořejší V., Kocourek J. Studies on lectins: XXXVII. Isolation and characterization of the lectin from Jimson-weed seeds (Datura stramonium L.) Biochim. Biophys. Acta Protein Struct. 1978;532(1):92–97. [PubMed] [Google Scholar]
- Ikemoto K. Bauhinia purprea agglutinin‐modified liposomes for human prostate cancer treatment. Cancer Sci. 2016;107(1):53–59. doi: 10.1111/cas.12839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iordache F. Antimicrobial and antiparasitic activity of lectins. Curr. Pharmaceut. Biotechnol. 2015;16(2):152–161. doi: 10.2174/138920101602150112151907. [DOI] [PubMed] [Google Scholar]
- Islam B., Khan A.U. Protein Purification. 2012. Lectins: to combat infections. (IntechOpen) [Google Scholar]
- Jain S.K. Lectin conjugated gastro-retentive microspheres of amoxicillin for effective treatment of Helicobacter pylori. Curr. Sci. 2014:267–276. [Google Scholar]
- Jandú J.J. Targeting the immune system with plant lectins to combat microbial infections. Front. Pharmacol. 2017;8:671. doi: 10.3389/fphar.2017.00671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jandú J.J. Treatment with pCramoll alone and in combination with fluconazole provides therapeutic benefits in C. gattii infected mice. Frontiers in cellular and infection microbiology. 2017;7:211. doi: 10.3389/fcimb.2017.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janossy G., Greaves M. Lymphocyte activation: I. Response of T and B lymphocytes to phytomitogens. Clin. Exp. Immunol. 1971;9(4):483. [PMC free article] [PubMed] [Google Scholar]
- Jiang Q.L. Plant lectins, from ancient sugar‐binding proteins to emerging anti‐cancer drugs in apoptosis and autophagy. Cell Prolif. 2015;48(1):17–28. doi: 10.1111/cpr.12155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez-castellanos M.R., Zia H., Rhodes C. Mucoadhesive drug delivery systems. Drug Dev. Ind. Pharm. 1993;19(1–2):143–194. [Google Scholar]
- Johnson P. Glycan composition of serum alpha-fetoprotein in patients with hepatocellular carcinoma and non-seminomatous germ cell tumour. Br. J. Canc. 1999;81(7):1188. doi: 10.1038/sj.bjc.6690828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judd W.J., Issitt P.D. The role of lectins in blood group serology. CRC Crit. Rev. Clin. Lab. Sci. 1980;12(3):171–214. doi: 10.3109/10408368009108729. [DOI] [PubMed] [Google Scholar]
- Kalsi G. Isolation and characterization of a lectin from peanut roots. Biochim. Biophys. Acta Gen. Subj. 1992;1117(2):114–119. doi: 10.1016/0304-4165(92)90067-5. [DOI] [PubMed] [Google Scholar]
- Karnchanatat A. InTech; 2012. Antimicrobial Activity of Lectins from Plants. Antimicrobial Agents; pp. 145–178. [Google Scholar]
- Kaur M. A tuber lectin from Arisaema jacquemontii Blume with anti-insect and anti-proliferative properties. BMB Reports. 2006;39(4):432–440. doi: 10.5483/bmbrep.2006.39.4.432. [DOI] [PubMed] [Google Scholar]
- Kavalalı G. Hypoglycemic activity of Urtica pilulifera in streptozotocin-diabetic rats. J. Ethnopharmacol. 2003;84(2–3):241–245. doi: 10.1016/s0378-8741(02)00315-x. [DOI] [PubMed] [Google Scholar]
- Kennedy J. Lectins, versatile proteins of recognition: a review. Carbohydr. Polym. 1995;26(3):219–230. [Google Scholar]
- Kennedy J.F., Bandaiphet C. In: Reviews in Food and Nutrition Toxicity: Volume 1-VR Preedy. Watson R.R., editor. Taylor & Francis; London: 2003. p. 221. xii+ 458 pp., ISBN 0-415-28025-7,@ $115.00. Carbohydrate Polymers, 2004. 2(58) [Google Scholar]
- Kesherwani V., Sodhi A. Differential activation of macrophages in vitro by lectin Concanavalin A, Phytohemagglutinin and Wheat germ agglutinin: production and regulation of nitric oxide. Nitric Oxide. 2007;16(2):294–305. doi: 10.1016/j.niox.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Keyaerts E. Plant lectins are potent inhibitors of coronaviruses by interfering with two targets in the viral replication cycle. Antivir. Res. 2007;75(3):179–187. doi: 10.1016/j.antiviral.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan F., Khan M.I. Fungal lectins: current molecular and biochemical perspectives. Int. J. Biol. Chem. 2011;5(1):20. [Google Scholar]
- Kieliszewski M.J., Showalter A.M., Leykam J.F. Potato lectin: a modular protein sharing sequence similarities with the extensin family, the hevein lectin family, and snake venom disintegrins (platelet aggregation inhibitors) Plant J. 1994;5(6):849–861. doi: 10.1046/j.1365-313x.1994.5060849.x. [DOI] [PubMed] [Google Scholar]
- Kilpatrick D.C. Animal lectins: a historical introduction and overview. Biochim. Biophys. Acta Gen. Subj. 2002;1572(2–3):187–197. doi: 10.1016/s0304-4165(02)00308-2. [DOI] [PubMed] [Google Scholar]
- Kim Y., Robertus J.D. Analysis of several key active site residues of ricin A chain by mutagenesis and X-ray crystallography. Protein Eng. Des. Sel. 1992;5(8):775–779. doi: 10.1093/protein/5.8.775. [DOI] [PubMed] [Google Scholar]
- Kim M. Lectin-induced apoptosis of tumour cells. Glycobiology. 1993;3(5):447–453. doi: 10.1093/glycob/3.5.447. [DOI] [PubMed] [Google Scholar]
- Kim B.-Y. Bioadhesive interaction and hypoglycemic effect of insulin-loaded lectin–microparticle conjugates in oral insulin delivery system. J. Control. Release. 2005;102(3):525–538. doi: 10.1016/j.jconrel.2004.10.032. [DOI] [PubMed] [Google Scholar]
- Kim H.J., Lee S.J., Kim H.-J. Antibody-based enzyme-linked lectin assay (ABELLA) for the sialylated recombinant human erythropoietin present in culture supernatant. J. Pharm. Biomed. Anal. 2008;48(3):716–721. doi: 10.1016/j.jpba.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y., Kawagishi H. Lectins. Springer; 2014. Fungal lectins: a growing family; pp. 15–38. [DOI] [PubMed] [Google Scholar]
- Kocourek J., Horejsi V. Walter de Gruyter; Berlin, Germany: 1983. A Note on the Recent Discussion on Definition of the Term ‘lectin’; pp. 3–6. [Google Scholar]
- Koeppe S.J., Rupnow J. Purification and characterization of a lectin from the seeds of amaranth (Amaranthus cruentus) J. Food Sci. 1988;53(5):1412–1417. [Google Scholar]
- Kolb H. Suppression of low-dose streptozotocin-induced diabetes by immunomodulatory lectins. Diabetes Res. 1986;3(4):183–186. [PubMed] [Google Scholar]
- Kordás K. Diverse effects of phytohaemagglutinin on gastrointestinal secretions in rats. J. Physiol. Paris. 2000;94(1):31–36. doi: 10.1016/s0928-4257(99)00106-0. [DOI] [PubMed] [Google Scholar]
- Kordás K. Phytohaemagglutinin inhibits gastric acid but not pepsin secretion in conscious rats. J. Physiol. Paris. 2001;95(1–6):309–314. doi: 10.1016/s0928-4257(01)00043-2. [DOI] [PubMed] [Google Scholar]
- Korourian S. Expression analysis of carbohydrate antigens in ductal carcinoma in situ of the breast by lectin histochemistry. BMC Canc. 2008;8(1):136. doi: 10.1186/1471-2407-8-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriipe M. Ferdinand Enke; Stuttgart: 1956. Blutgruppenspezifische Pflanzliche Eiweissk6rper (Phytagglutine) [Google Scholar]
- Kumar K.K. Biological role of lectins: a review. Journal of orofacial sciences. 2012;4(1):20. [Google Scholar]
- Kuo C.-F. Concanavalin A protects mice from a lethal inoculation of intragastric Klebsiella pneumoniae and reduces the induced liver damage. Antimicrob. Agents Chemother. 2007;51(9):3122–3130. doi: 10.1128/AAC.01379-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagarda-Diaz I., Guzman-Partida A., Vazquez-Moreno L. Legume lectins: proteins with diverse applications. Int. J. Mol. Sci. 2017;18(6):1242. doi: 10.3390/ijms18061242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam S.K., Ng T.B. First report of a haemagglutinin-induced apoptotic pathway in breast cancer cells. Biosci. Rep. 2010;30(5):307–317. doi: 10.1042/BSR20090059. [DOI] [PubMed] [Google Scholar]
- Lam S.K., Ng T.B. Lectins: production and practical applications. Appl. Microbiol. Biotechnol. 2011;89(1):45–55. doi: 10.1007/s00253-010-2892-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landsteiner K., Raubitschek H. Observations on hemolysis and hemagglutination. C. Bakt. 1907;45:660. [Google Scholar]
- Lavrik I.N., Golks A., Krammer P.H. Caspases: pharmacological manipulation of cell death. J. Clin. Investig. 2005;115(10):2665–2672. doi: 10.1172/JCI26252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei H.-Y., Chang C.-P. Lectin of Concanavalin A as an anti-hepatoma therapeutic agent. J. Biomed. Sci. 2009;16(1):10. doi: 10.1186/1423-0127-16-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong K.H. Lectin-functionalized carboxymethylated kappa-carrageenan microparticles for oral insulin delivery. Carbohydr. Polym. 2011;86(2):555–565. [Google Scholar]
- LeVine D., Kaplan M.J., Greenaway P.J. The purification and characterization of wheat-germ agglutinin. Biochem. J. 1972;129(4):847–856. doi: 10.1042/bj1290847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y. A novel lectin with potent antitumor, mitogenic and HIV-1 reverse transcriptase inhibitory activities from the edible mushroom Pleurotus citrinopileatus. Biochim. Biophys. Acta Gen. Subj. 2008;1780(1):51–57. doi: 10.1016/j.bbagen.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Li C.-Y. Concanavalin A, from an old protein to novel candidate anti-neoplastic drug. Curr. Mol. Pharmacol. 2010;3(3):123–128. doi: 10.2174/1874467211003030123. [DOI] [PubMed] [Google Scholar]
- Liener I. Elsevier; 2012. The Lectins: Properties, Functions, and Applications in Biology and Medicine. [Google Scholar]
- Lis H., Sharon N. The biochemistry of plant lectins (phytohemagglutinins) Annu. Rev. Biochem. 1973;42(1):541–574. doi: 10.1146/annurev.bi.42.070173.002545. [DOI] [PubMed] [Google Scholar]
- Lis H., Sharon N. The Antigens. Elsevier; 1977. Lectins: their chemistry and application to immunology; pp. 429–529. [Google Scholar]
- Lis H., Sharon N. Proteins and Nucleic Acids. Elsevier; 1981. Lectins in higher plants; pp. 371–447. [Google Scholar]
- Lis H., Sharon N. 1986. Applications of Lectins. The Lectins: Properties, Functions and Applications in Biology and Medicine; pp. 293–370. [Google Scholar]
- Lis H., Sharon N. Lectins as molecules and as tools. Annu. Rev. Biochem. 1986;55(1):35–67. doi: 10.1146/annurev.bi.55.070186.000343. [DOI] [PubMed] [Google Scholar]
- Litynska A. Comparison of the lectin-binding pattern in different human melanoma cell lines. Melanoma Res. 2001;11(3):205–212. doi: 10.1097/00008390-200106000-00001. [DOI] [PubMed] [Google Scholar]
- Liu J. Solution structure and backbone dynamics of recombinant Cucurbita maxima trypsin inhibitor-V determined by NMR spectroscopy. Biochemistry. 1996;35(5):1516–1524. doi: 10.1021/bi952466d. [DOI] [PubMed] [Google Scholar]
- Liu B. Induction of apoptosis by Polygonatum odoratum lectin and its molecular mechanisms in murine fibrosarcoma L929 cells. Biochim. Biophys. Acta Gen. Subj. 2009;1790(8):840–844. doi: 10.1016/j.bbagen.2009.04.020. [DOI] [PubMed] [Google Scholar]
- Liu B. Molecular mechanisms of Polygonatum cyrtonema lectin-induced apoptosis and autophagy in cancer cells. Autophagy. 2009;5(2):253–255. doi: 10.4161/auto.5.2.7561. [DOI] [PubMed] [Google Scholar]
- Liu B., Bian H.-j., Bao J.-k. Plant lectins: potential antineoplastic drugs from bench to clinic. Cancer Lett. 2010;287(1):1–12. doi: 10.1016/j.canlet.2009.05.013. [DOI] [PubMed] [Google Scholar]
- Lopes F. Differential effect of plant lectins on mast cells of different origins. Braz. J. Med. Biol. Res. 2005;38(6):935–941. doi: 10.1590/s0100-879x2005000600016. [DOI] [PubMed] [Google Scholar]
- Loyola W. Concanavalin A enhances phagocytosis and killing of Candida albicans by mice peritoneal neutrophils and macrophages. FEMS Immunol. Med. Microbiol. 2002;33(3):201–208. doi: 10.1111/j.1574-695X.2002.tb00591.x. [DOI] [PubMed] [Google Scholar]
- Loyola W. Artin M enhances TNF-α production and phagocytosis of Candida albicans mediated by dectin-1 and mannose receptors. Int. Immunopharmacol. 2012;12(2):378–383. doi: 10.1016/j.intimp.2011.12.010. [DOI] [PubMed] [Google Scholar]
- Lusvarghi S., Bewley C. Griffithsin: an antiviral lectin with outstanding therapeutic potential. Viruses. 2016;8(10):296. doi: 10.3390/v8100296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyu S.-Y. Preparation of alginate/chitosan microcapsules and enteric coated granules of mistletoe lectin. Arch Pharm. Res. (Seoul) 2004;27(1):118. doi: 10.1007/BF02980057. [DOI] [PubMed] [Google Scholar]
- Macedo M.L.R. Talisia esculenta lectin and larval development of Callosobruchus maculatus and Zabrotes subfasciatus (Coleoptera: Bruchidae) Biochim. Biophys. Acta Gen. Subj. 2002;1571(2):83–88. doi: 10.1016/s0304-4165(02)00155-1. [DOI] [PubMed] [Google Scholar]
- Maria das Graças M.F. Isolation and partial characterization of a novel lectin from Talisia esculenta seeds that interferes with fungal growth. Plant Physiol. Biochem. 2002;40(1):61–68. [Google Scholar]
- Mathiowitz E., Chickering D.E., III, Lehr C.-M. CRC Press; 1999. Bioadhesive Drug Delivery Systems: Fundamentals, Novel Approaches, and Development. [Google Scholar]
- McAlvin C.B., Stacey G. Transgenic expression of the soybean apyrase in Lotus japonicus enhances nodulation. Plant Physiol. 2005;137(4):1456–1462. doi: 10.1104/pp.104.055939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira R.d.A. vol. 86. Memórias do Instituto Oswaldo Cruz; 1991. pp. 211–218. (Plant Lectins, Chemical and Biological Aspects). [DOI] [PubMed] [Google Scholar]
- Moresco T. Phagocytic and candidacidal activities of macrophages from suckling and adult mice pretreated with concanavalin-A. Med. Mycol. 2002;40(4):393–397. doi: 10.1080/mmy.40.4.393.397. [DOI] [PubMed] [Google Scholar]
- Moura M. Multi‐effect of the water‐soluble Moringa oleifera lectin against Serratia marcescens and Bacillus sp.: antibacterial, antibiofilm and anti‐adhesive properties. J. Appl. Microbiol. 2017;123(4):861–874. doi: 10.1111/jam.13556. [DOI] [PubMed] [Google Scholar]
- Moyle P.M. Biotechnology approaches to produce potent, self-adjuvanting antigen-adjuvant fusion protein subunit vaccines. Biotechnol. Adv. 2017;35(3):375–389. doi: 10.1016/j.biotechadv.2017.03.005. [DOI] [PubMed] [Google Scholar]
- Müller W. The D-mannose-specific lectin from Gerardia savaglia blocks binding of human immunodeficiency virus type I to H9 cells and human lymphocytes in vitro. J. Acquir. Immune Defic. Syndr. 1988;1(5):453–458. [PubMed] [Google Scholar]
- Murzin A.G., Lesk A.M., Chothia C. β-trefoil fold: patterns of structure and sequence in the kunitz inhibitors interleukins-1β and 1α and fibroblast growth factors. J. Mol. Biol. 1992;223(2):531–543. doi: 10.1016/0022-2836(92)90668-a. [DOI] [PubMed] [Google Scholar]
- Nagata Y., Burger M.M. Wheat germ agglutinin isolation and crystallization. J. Biol. Chem. 1972;247(7):2248–2250. [PubMed] [Google Scholar]
- Nascimento A.P.M. Anti-glioma properties of DVL, a lectin purified from Dioclea violacea. Int. J. Biol. Macromol. 2018;120:566–577. doi: 10.1016/j.ijbiomac.2018.08.106. [DOI] [PubMed] [Google Scholar]
- Neutsch L. Synergistic targeting/prodrug strategies for intravesical drug delivery—lectin-modified PLGA microparticles enhance cytotoxicity of stearoyl gemcitabine by contact-dependent transfer. J. Control. Release. 2013;169(1–2):62–72. doi: 10.1016/j.jconrel.2013.04.004. [DOI] [PubMed] [Google Scholar]
- Nicholls T.J. Lectins in ocular drug delivery: an investigation of lectin binding sites on the corneal and conjunctival surfaces. Int. J. Pharm. 1996;138(2):175–183. [Google Scholar]
- Nilsson C.L. Lectins. Elsevier; 2007. Lectins: analytical tools from nature; pp. 1–13. [Google Scholar]
- Nizet V., Varki A., Aebi M. Essentials of Glycobiology [Internet] third ed. Cold Spring Harbor Laboratory Press; 2017. Microbial lectins: hemagglutinins, adhesins, and toxins. [PubMed] [Google Scholar]
- Nunes B.S. Lectin extracted from Canavalia grandiflora seeds presents potential anti-inflammatory and analgesic effects. Naunyn Schmiedeberg's Arch. Pharmacol. 2009;379(6):609. doi: 10.1007/s00210-009-0397-9. [DOI] [PubMed] [Google Scholar]
- O'Reilly R. Evaluation of HLA‐haplotype disparate parental marrow grafts depleted of T lymphocytes by differential agglutination with a soybean lectin and E‐Rosette depletion for the treatment of severe combined immunodeficiency 1. Vox Sanguinis. 1986;51:81–86. doi: 10.1111/j.1423-0410.1986.tb02013.x. [DOI] [PubMed] [Google Scholar]
- Ohizumi Y. Mannose-binding lectin from yam (Dioscorea batatas) tubers with insecticidal properties against Helicoverpa armigera (Lepidoptera: noctuidae) J. Agric. Food Chem. 2009;57(7):2896–2902. doi: 10.1021/jf8040269. [DOI] [PubMed] [Google Scholar]
- Oliveira C. Cytotoxic effects of native and recombinant frutalin, a plant galactose-binding lectin, on HeLa cervical cancer cells. BioMed Res. Int. 2011;2011 doi: 10.1155/2011/568932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen L.R. X-ray crystallographic studies of unique cross-linked lattices between four isomeric biantennary oligosaccharides and soybean agglutinin. Biochemistry. 1997;36(49):15073–15080. doi: 10.1021/bi971828+. [DOI] [PubMed] [Google Scholar]
- Pajic I. A novel lectin from the sponge Haliclona cratera: isolation, characterization and biological activity. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2002;132(2):213–221. doi: 10.1016/s1532-0456(02)00068-6. [DOI] [PubMed] [Google Scholar]
- Pang W.W. Can the acute-phase reactant proteins be used as cancer biomarkers? Int. J. Biol. Mark. 2010;25(1):1–11. doi: 10.1177/172460081002500101. [DOI] [PubMed] [Google Scholar]
- Panjwani N., Ahmad S., Raizman M.B. Cell surface glycoproteins of corneal epithelium. Investig. Ophthalmol. Vis. Sci. 1995;36(2):355–363. [PubMed] [Google Scholar]
- Park W.-B. Inhibition of tumor growth and metastasis by Korean mistletoe lectin is associated with apoptosis and antiangiogenesis. Cancer Biother. Radiopharm. 2001;16(5):439–447. doi: 10.1089/108497801753354348. [DOI] [PubMed] [Google Scholar]
- Petnual P., Sangvanich P., Karnchanatat A. A lectin from the rhizomes of turmeric (Curcuma longa L.) and its antifungal, antibacterial, and α-glucosidase inhibitory activities. Food Science and Biotechnology. 2010;19(4):907–916. [Google Scholar]
- Peumans W.J., Damme E.J.V. Plant lectins: versatile proteins with important perspectives in biotechnology. Biotechnol. Genet. Eng. Rev. 1998;15(1):199–228. [Google Scholar]
- Peumans W.J. Isolation and partial characterization of a small chitin-binding lectin from mistletoe (Viscum album) FEBS Lett. 1996;396(2–3):261–265. doi: 10.1016/0014-5793(96)01108-8. [DOI] [PubMed] [Google Scholar]
- Peumans W.J. Isolation of a novel plant lectin with an unusual specificity from Calystegia sepium. Glycoconj. J. 1997;14(2):259–265. doi: 10.1023/a:1018502107707. [DOI] [PubMed] [Google Scholar]
- Peumans W.J. The Molecular Immunology of Complex Carbohydrates—2. Springer; 2001. Classification of plant lectins in families of structurally and evolutionary related proteins; pp. 27–54. [DOI] [PubMed] [Google Scholar]
- Phang W.-M. Secretion of N-and O-linked glycoproteins from 4T1 murine mammary carcinoma cells. Int. J. Med. Sci. 2016;13(5):330. doi: 10.7150/ijms.14341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pihíková D., Kasák P., Tkac J. Glycoprofiling of cancer biomarkers: label-free electrochemical lectin-based biosensors. Open chemistry. 2015;13(1) doi: 10.1515/chem-2015-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pihikova D. Sweet characterisation of prostate specific antigen using electrochemical lectin‐based immunosensor assay and MALDI TOF/TOF analysis: focus on sialic acid. Proteomics. 2016;16(24):3085–3095. doi: 10.1002/pmic.201500463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plattner V.E. Targeted drug delivery: binding and uptake of plant lectins using human 5637 bladder cancer cells. Eur. J. Pharm. Biopharm. 2008;70(2):572–576. doi: 10.1016/j.ejpb.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Prokop O., Uhlenbruck G., Köhler W. A new source of antibody‐like substances having anti‐blood group specificity: a discussion on the specificity of helix agglutinins. Vox Sanguinis. 1968;14(5):321–333. doi: 10.1111/j.1423-0410.1968.tb01722.x. [DOI] [PubMed] [Google Scholar]
- Pueppke S.G. Comparison of subunit compositions and isolectin profiles of the seed lectins purified from Glycine max and G. soja. Soybean Genet. Newsl. (U.S. Dep. Agric. Agric. Res. Serv.) 1981;8(1):17. [Google Scholar]
- Pusztai A. Relationship between survival and binding of plant lectins during small intestinal passage and their effectiveness as growth factors. Digestion. 1990;46(Suppl. 2):308–316. doi: 10.1159/000200402. [DOI] [PubMed] [Google Scholar]
- Pusztai A. Antinutritive effects of wheat-germ agglutinin and other N-acetylglucosamine-specific lectins. Br. J. Nutr. 1993;70(1):313–321. doi: 10.1079/bjn19930124. [DOI] [PubMed] [Google Scholar]
- Qadir S. Evaluation of antimicrobial activity of a lectin isolated and purified from Indigofera heterantha. Adv. Biosci. Biotechnol. 2013;4(11):999. [Google Scholar]
- Rao V., Lam K., Qasba P.K. Three dimensional structure of the soybean agglutinin-Gal/GalNAc complexes by homology modeling. J. Biomol. Struct. Dyn. 1998;15(5):853–860. doi: 10.1080/07391102.1998.10508207. [DOI] [PubMed] [Google Scholar]
- Raval S. A database analysis of jacalin-like lectins: sequence–structure–function relationships. Glycobiology. 2004;14(12):1247–1263. doi: 10.1093/glycob/cwh140. [DOI] [PubMed] [Google Scholar]
- Reisner Y., Ravid A., Sharon N. Use of soybean agglutinin for the separation of mouse B and T lymphocytes. Biochem. Biophys. Res. Commun. 1976;72(4):1585–1591. doi: 10.1016/s0006-291x(76)80195-7. [DOI] [PubMed] [Google Scholar]
- Read S.M., Northcote D.H. Subunit structure and interactions of the phloem proteins of Cucurbita maxima (pumpkin) Eur. J. Biochem. 1983;134(3):561–569. doi: 10.1111/j.1432-1033.1983.tb07603.x. [DOI] [PubMed] [Google Scholar]
- Reisner Y. Transplantation for severe combined immunodeficiency with HLA-A, B, D, DR incompatible parental marrow cells fractionated by soybean agglutinin and sheep red blood cells. Blood. 1983;61(2):341–348. [PubMed] [Google Scholar]
- Remmelink M. In vitro influence of lectins and neoglycoconjugates on the growth of three human sarcoma cell lines. J. Cancer Res. Clin. Oncol. 1999;125(5):275–285. doi: 10.1007/s004320050274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinderle S.J. Isolation and characterization of amaranthin, a lectin present in the seeds of Amaranthus caudatus, that recognizes the T-(or cryptic T)-antigen. J. Biol. Chem. 1989;264(27):16123–16131. [PubMed] [Google Scholar]
- Rocha A.A. Lectin from Crataeva tapia bark improves tissue damages and plasma hyperglycemia in alloxan-induced diabetic mice. Evid. Based Complement Altern. Med. 2013;2013:869305. doi: 10.1155/2013/869305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues J. Novel core (polyester)-shell (polysaccharide) nanoparticles: protein loading and surface modification with lectins. J. Control. Release. 2003;92(1–2):103–112. doi: 10.1016/s0168-3659(03)00296-7. [DOI] [PubMed] [Google Scholar]
- Rogers D., Hori K. Marine algal lectins: new developments. Hydrobiologia. 1993;260(1):589–593. [Google Scholar]
- Rüdiger H., Gabius H.-J. Plant lectins: occurrence, biochemistry, functions and applications. Glycoconj. J. 2001;18(8):589–613. doi: 10.1023/a:1020687518999. [DOI] [PubMed] [Google Scholar]
- Rutenber E., Robertus J.D. Structure of ricin B‐chain at 2.5 Å resolution. Proteins: Structure, Function, and Bioinformatics. 1991;10(3):260–269. doi: 10.1002/prot.340100310. [DOI] [PubMed] [Google Scholar]
- Sadeghi A. Deterrent activity of plant lectins on cowpea weevil Callosobruchus maculatus (F.) oviposition. Phytochemistry. 2006;67(18):2078–2084. doi: 10.1016/j.phytochem.2006.06.032. [DOI] [PubMed] [Google Scholar]
- Sadeghi A. Ectopically expressed leaf and bulb lectins from garlic (Allium sativum L.) protect transgenic tobacco plants against cotton leafworm (Spodoptera littoralis) Transgenic Res. 2008;17(1):9. doi: 10.1007/s11248-007-9069-z. [DOI] [PubMed] [Google Scholar]
- Sander V.A., Corigliano M.G., Clemente M. Promising plant-derived adjuvants in the development of coccidial vaccines. Frontiers in veterinary science. 2019;6 doi: 10.3389/fvets.2019.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford G.L., Harris-Hooker S. Stimulation of vascular cell proliferation by beta-galactoside specific lectins. FASEB J. 1990;4(11):2912–2918. doi: 10.1096/fasebj.4.11.2379767. [DOI] [PubMed] [Google Scholar]
- Sankaranarayanan R. A novel mode of carbohydrate recognition in jacalin, a Moraceae plant lectin with a β-prism fold. Nat. Struct. Biol. 1996;3(7):596. doi: 10.1038/nsb0796-596. [DOI] [PubMed] [Google Scholar]
- Santos A.F. Lectins: function, structure, biological properties andpotential applications. Curr. Top. Pept. Protein Res. 2014;15:41–62. [Google Scholar]
- Sarkar M., Wu A.M., Kabat E.A. Immunochemical studies on the carbohydrate specificity of Maclura pomifera lectin. Arch. Biochem. Biophys. 1981;209(1):204–218. doi: 10.1016/0003-9861(81)90273-3. [DOI] [PubMed] [Google Scholar]
- Sastry M. Analysis of saccharide binding to Artocarpus integrifolia lectin reveals specific recognition of T-antigen (beta-D-Gal (1----3) D-GalNAc) J. Biol. Chem. 1986;261(25):11726–11733. [PubMed] [Google Scholar]
- Sawant S.S., Randive V.R., Kulkarni S.R. Lectins from seeds of Abrus precatorius: evaluation of antidiabetic and antihyperlipidemic potential in diabetic rats. Asian J. Pharmaceut. Res. 2017;7(2):71–80. [Google Scholar]
- Schöffski P. Phase I trial of intravenous aviscumine (rViscumin) in patients with solid tumors: a study of the European organization for research and treatment of cancer new drug development group. Ann. Oncol. 2004;15(12):1816–1824. doi: 10.1093/annonc/mdh469. [DOI] [PubMed] [Google Scholar]
- Seifert G. Molecular mechanisms of mistletoe plant extract-induced apoptosis in acute lymphoblastic leukemia in vivo and in vitro. Cancer Lett. 2008;264(2):218–228. doi: 10.1016/j.canlet.2008.01.036. [DOI] [PubMed] [Google Scholar]
- Selitrennikoff C.P. Antifungal proteins. Appl. Environ. Microbiol. 2001;67(7):2883–2894. doi: 10.1128/AEM.67.7.2883-2894.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan S., Tanaka H., Shoyama Y. Enzyme-linked immunosorbent assay for glycyrrhizin using anti-glycyrrhizin monoclonal antibody and an eastern blotting technique for glucuronides of glycyrrhetic acid. Anal. Chem. 2001;73(24):5784–5790. doi: 10.1021/ac0106997. [DOI] [PubMed] [Google Scholar]
- Sharon N., Lis H. Lectins: cell-agglutinating and sugar-specific proteins. Science. 1972;177(4053):949–959. doi: 10.1126/science.177.4053.949. [DOI] [PubMed] [Google Scholar]
- Sharon N., Lis H. Lectins as cell recognition molecules. Science. 1989;246(4927):227–234. doi: 10.1126/science.2552581. [DOI] [PubMed] [Google Scholar]
- Sharon N., Lis H. The Molecular Immunology of Complex Carbohydrates—2. Springer; 2001. The structural basis for carbohydrate recognition by lectins; pp. 1–16. [DOI] [PubMed] [Google Scholar]
- Sharon N., Lis H. History of lectins: from hemagglutinins to biological recognition molecules. Glycobiology. 2004;14(11):53R–62R. doi: 10.1093/glycob/cwh122. [DOI] [PubMed] [Google Scholar]
- Silva P.M. The juicy sarcotesta of Punica granatum contains a lectin that affects growth, survival as well as adherence and invasive capacities of human pathogenic bacteria. Journal of functional foods. 2016;27:695–702. [Google Scholar]
- Silva A.F. Comparison of immunomodulatory properties of mannose-binding lectins from Canavalia brasiliensis and Cratylia argentea in a mice model of Salmonella infection. Int. Immunopharmacol. 2016;31:233–238. doi: 10.1016/j.intimp.2015.12.036. [DOI] [PubMed] [Google Scholar]
- Singh K. A tuber lectin from Arisaema helleborifolium Schott with anti-insect activity against melon fruit fly, Bactrocera cucurbitae (Coquillett) and anti-cancer effect on human cancer cell lines. Arch. Biochem. Biophys. 2006;445(1):156–165. doi: 10.1016/j.abb.2005.10.021. [DOI] [PubMed] [Google Scholar]
- Singh R.S., Bhari R., Kaur H.P. Mushroom lectins: current status and future perspectives. Crit. Rev. Biotechnol. 2010;30(2):99–126. doi: 10.3109/07388550903365048. [DOI] [PubMed] [Google Scholar]
- Singh R.S., Bhari R., Kaur H.P. Current trends of lectins from microfungi. Crit. Rev. Biotechnol. 2011;31(3):193–210. doi: 10.3109/07388551.2010.505911. [DOI] [PubMed] [Google Scholar]
- Singh R.S., Thakur S.R., Bansal P. Algal lectins as promising biomolecules for biomedical research. Crit. Rev. Microbiol. 2015;41(1):77–88. doi: 10.3109/1040841X.2013.798780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart J.D. Lectin-mediated drug delivery in the oral cavity. Adv. Drug Deliv. Rev. 2004;56(4):481–489. doi: 10.1016/j.addr.2003.10.016. [DOI] [PubMed] [Google Scholar]
- Sobral A.P.V. ConA and UEA-I lectin histochemistry of parotid gland mucoepidermoid carcinoma. J. Oral Sci. 2010;52(1):49–54. doi: 10.2334/josnusd.52.49. [DOI] [PubMed] [Google Scholar]
- Sodhi A., Tarang S., Kesherwani V. Concanavalin A induced expression of Toll-like receptors in murine peritoneal macrophages in vitro. Int. Immunopharmacol. 2007;7(4):454–463. doi: 10.1016/j.intimp.2006.11.014. [DOI] [PubMed] [Google Scholar]
- Souza M.A. The immunomodulatory effect of plant lectins: a review with emphasis on ArtinM properties. Glycoconj. J. 2013;30(7):641–657. doi: 10.1007/s10719-012-9464-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreevidya V. Expression of the legume symbiotic lectin genes psl and gs52 promotes rhizobial colonization of roots in rice. Plant Sci. 2005;169(4):726–736. [Google Scholar]
- Stauder H., Kreuser E.-D. Mistletoe extracts standardised in terms of mistletoe lectins (ML I) in oncology: current state of clinical research. Oncology Research and Treatment. 2002;25(4):374–380. doi: 10.1159/000066058. [DOI] [PubMed] [Google Scholar]
- Stillmark H. University of Dorpat; Dorpat, Estonia: 1888. Über Ricin ein giftiges Ferment aus den Samen von Ricinus communis L. und einige anderen Euphorbiaceen. MD Thesis. [Google Scholar]
- Suzuki I. Purification and characterization of two lectins from Aloe arborescens Mill. J. Biochem. 1979;85(1):163–171. doi: 10.1093/oxfordjournals.jbchem.a132306. [DOI] [PubMed] [Google Scholar]
- Swanson M.D. A lectin isolated from bananas is a potent inhibitor of HIV replication. J. Biol. Chem. 2010;285(12):8646–8655. doi: 10.1074/jbc.M109.034926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi N., Korekane H. Branched N-glycans and their implications for cell adhesion, signaling and clinical applications for cancer biomarkers and in therapeutics. BMB reports. 2011;44(12):772–781. doi: 10.5483/bmbrep.2011.44.12.772. [DOI] [PubMed] [Google Scholar]
- Teixeira E.H. Carbohydrates-Comprehensive Studies on Glycobiology and Glycotechnology. 2012. Biological applications of plants and algae lectins: an overview. (IntechOpen) [Google Scholar]
- Tian Q. Purification, characterization and molecular cloning of a novel mannose-binding lectin from rhizomes of Ophiopogon japonicus with antiviral and antifungal activities. Plant Sci. 2008;175(6):877–884. [Google Scholar]
- Tollefsen S., Kornfeld R. Isolation and characterization of lectins from Vicia villosa. Two distinct carbohydrate binding activities are present in seed extracts. J. Biol. Chem. 1983;258(8):5165–5171. [PubMed] [Google Scholar]
- Transue T.R. Structure of benzyl T-antigen disaccharide bound to Amaranthus caudatus agglutinin. Nat. Struct. Biol. 1997;4(10):779–783. doi: 10.1038/nsb1097-779. [DOI] [PubMed] [Google Scholar]
- Ueno T. Polyethylene glycol-modified concanavalin A as an effective agent to stimulate anti-tumor cytotoxicity. Cancer Detect. Prev. 2000;24(1):100–106. [PubMed] [Google Scholar]
- Unitt J., Hornigold D. Plant lectins are novel Toll-like receptor agonists. Biochem. Pharmacol. 2011;81(11):1324–1328. doi: 10.1016/j.bcp.2011.03.010. [DOI] [PubMed] [Google Scholar]
- Van Damme E. Comprehensive glycoscience-from chemistry to systems biology; 2007. Carbohydrate-protein Interactions: Plant Lectins; pp. 563–599. [Google Scholar]
- Van Damme E.J., Allen A.K., Peumans W.J. Isolation and characterization of a lectin with exclusive specificity towards mannose from snowdrop (Galanthus nivalis) bulbs. FEBS Lett. 1987;215(1):140–144. [Google Scholar]
- Van Damme E.J. Isolation and molecular cloning of a novel type 2 ribosome-inactivating protein with an inactive B chain from elderberry (Sambucus nigra) bark. J. Biol. Chem. 1997;272(13):8353–8360. doi: 10.1074/jbc.272.13.8353. [DOI] [PubMed] [Google Scholar]
- Van Damme E.J. John Wiley & Sons; 1998. Handbook of Plant Lectins: Properties and Biomedical Applications. [Google Scholar]
- Van Damme E.J. Characterization and molecular cloning of the lectin from Helianthus tuberosus. Eur. J. Biochem. 1999;259(1‐2):135–142. doi: 10.1046/j.1432-1327.1999.00013.x. [DOI] [PubMed] [Google Scholar]
- van Eijsden R.R. Sugar-binding activity of pea (Pisum sativum) lectin is essential for heterologous infection of transgenic white clover hairy roots by Rhizobium leguminosarum biovar viciae. Plant Mol. Biol. 1995;29(3):431–439. doi: 10.1007/BF00020975. [DOI] [PubMed] [Google Scholar]
- Van Parijs J. Hevein: an antifungal protein from rubber-tree (Hevea brasiliensis) latex. Planta. 1991;183(2):258–264. doi: 10.1007/BF00197797. [DOI] [PubMed] [Google Scholar]
- van Rhijn P., Goldberg R.B., Hirsch A.M. Lotus corniculatus nodulation specificity is changed by the presence of a soybean lectin gene. The Plant Cell. 1998;10(8):1233–1249. doi: 10.1105/tpc.10.8.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varrot A., Basheer S.M., Imberty A. Fungal lectins: structure, function and potential applications. Curr. Opin. Struct. Biol. 2013;23(5):678–685. doi: 10.1016/j.sbi.2013.07.007. [DOI] [PubMed] [Google Scholar]
- Vasconcelos I.M., Oliveira J.T.A. Antinutritional properties of plant lectins. Toxicon. 2004;44(4):385–403. doi: 10.1016/j.toxicon.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Walski T., Van Damme E.J., Smagghe G. Penetration through the peritrophic matrix is a key to lectin toxicity against Tribolium castaneum. J. Insect Physiol. 2014;70:94–101. doi: 10.1016/j.jinsphys.2014.09.004. [DOI] [PubMed] [Google Scholar]
- Wang Z. Enhancement of resistance to aphids by introducing the snowdrop lectin genegna into maize plants. J. Biosci. 2005;30(5):627–638. doi: 10.1007/BF02703563. [DOI] [PubMed] [Google Scholar]
- Warden C.J.H., Waddell L.A. Bengal Secretariat Press; 1884. The Non-bacillar Nature of Abrus-Poison: with Observations on its Chemical and Physiological Properties. [Google Scholar]
- Watzl B. Dietary wheat germ agglutinin modulates ovalbumin-induced immune responses in Brown Norway rats. Br. J. Nutr. 2001;85(4):483–490. doi: 10.1017/s0007114501000721. [DOI] [PubMed] [Google Scholar]
- Whitney R.B., Sutherland R.M. Requirement for calcium ions in lymphocyte transformation stimulated by phytohemagglutinin. J. Cell. Physiol. 1972;80(3):329–337. doi: 10.1002/jcp.1040800303. [DOI] [PubMed] [Google Scholar]
- Wiener A.S. Principles of blood group serology and nomenclature: a critical review. Transfusion. 1961;1(5):308–320. doi: 10.1111/j.1537-2995.1961.tb00063.x. [DOI] [PubMed] [Google Scholar]
- Wiener A. The law of specificity, with special reference to the blood groups. Exp. Med. Surg. 1966;24(2):134. [PubMed] [Google Scholar]
- Wright C.S. 2· 2 Å resolution structure analysis of two refined N-acetylneuraminyl-lactose—wheat germ agglutinin isolectin complexes. J. Mol. Biol. 1990;215(4):635–651. doi: 10.1016/S0022-2836(05)80174-3. [DOI] [PubMed] [Google Scholar]
- Wright C.S., Kaku H., Goldstein I.J. Crystallization and preliminary X-ray diffraction results of snowdrop (Galanthus nivalis) lectin. J. Biol. Chem. 1990;265(3):1676–1677. [PubMed] [Google Scholar]
- Yamaguchi K.-I., Mori A., Funatsu G. The complete amino acid sequence of lectin-C from the roots of pokeweed (Phytolacca americana) Biosci. Biotechnol. Biochem. 1995;59(7):1384–1385. doi: 10.1271/bbb.59.1384. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K.-i., Mori A., Funatsu G. Amino acid sequence and some properties of lectin-D from the roots of pokeweed (Phytolacca americana) Biosci. Biotechnol. Biochem. 1996;60(8):1380–1382. doi: 10.1271/bbb.60.1380. [DOI] [PubMed] [Google Scholar]
- Yan Q. A novel homodimeric lectin from Astragalus mongholicus with antifungal activity. Arch. Biochem. Biophys. 2005;442(1):72–81. doi: 10.1016/j.abb.2005.07.019. [DOI] [PubMed] [Google Scholar]
- Yanagi K. Purification and characterization of anti-N lectin from Vicia unijuga leaves. Int. J. Biochem. 1990;22(1):43–52. doi: 10.1016/0020-711x(90)90076-f. [DOI] [PubMed] [Google Scholar]
- Yatomi Y. Wheat germ agglutinin-induced intracellular calcium mobilization in human platelets: suppression by staurosporine and resistance to cyclic AMP inhibition. Biochem. Biophys. Res. Commun. 1993;191(2):453–458. doi: 10.1006/bbrc.1993.1239. [DOI] [PubMed] [Google Scholar]
- Yau T. Lectins with potential for anti-cancer therapy. Molecules. 2015;20(3):3791–3810. doi: 10.3390/molecules20033791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X. Isolation of a homodimeric lectin with antifungal and antiviral activities from red kidney bean (Phaseolus vulgaris) seeds. J. Protein Chem. 2001;20(5):367–375. doi: 10.1023/a:1012276619686. [DOI] [PubMed] [Google Scholar]
- Yim M., Ono T., Irimura T. Mutated plant lectin library useful to identify different cells. Proc. Natl. Acad. Sci. 2001;98(5):2222–2225. doi: 10.1073/pnas.041621998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y. Preparation and evaluation of lectin-conjugated PLGA nanoparticles for oral delivery of thymopentin. J. Control. Release. 2006;116(3):337–345. doi: 10.1016/j.jconrel.2006.09.015. [DOI] [PubMed] [Google Scholar]
- Yoon T.J. Lectins isolated from Korean mistletoe (Viscum album coloratum) induce apoptosis in tumor cells. Cancer Lett. 1999;136(1):33–40. doi: 10.1016/s0304-3835(98)00300-0. [DOI] [PubMed] [Google Scholar]
- Young N.M. Structural comparison of the lectin from sainfoin (Onobrychis viciifolia) with concanavalin A and other D-mannose specific lectins. Can. J. Biochem. 1982;60(10):933–941. doi: 10.1139/o82-120. [DOI] [PubMed] [Google Scholar]
- Yu L.-G. Edible mushroom (Agaricus bisporus) lectin, which reversibly inhibits epithelial cell proliferation, blocks nuclear localization sequence-dependent nuclear protein import. J. Biol. Chem. 1999;274(8):4890–4899. doi: 10.1074/jbc.274.8.4890. [DOI] [PubMed] [Google Scholar]
- Zenteno E., Ochoa J.-L. Purification of a lectin from Amaranthus leucocarpus by affinity chromatography. Phytochemistry. 1988;27(2):313–317. [Google Scholar]
- Zhang G. First isolation and characterization of a novel lectin with potent antitumor activity from a Russula mushroom. Phytomedicine. 2010;17(10):775–781. doi: 10.1016/j.phymed.2010.02.001. [DOI] [PubMed] [Google Scholar]
- Zhang X. Lectin-based biosensor strategy for electrochemical assay of glycan expression on living cancer cells. Anal. Chem. 2010;82(22):9455–9460. doi: 10.1021/ac102132p. [DOI] [PubMed] [Google Scholar]
- Zhao J., Wang H., Ng T. Purification and characterization of a novel lectin from the toxic wild mushroom Inocybe umbrinella. Toxicon. 2009;53(3):360–366. doi: 10.1016/j.toxicon.2008.12.009. [DOI] [PubMed] [Google Scholar]
- Zhelev Z. Fabrication of quantum dot–lectin conjugates as novel fluorescent probes for microscopic and flow cytometric identification of leukemia cells from normal lymphocytes. Chem. Commun. 2005;(15):1980–1982. doi: 10.1039/b419305a. [DOI] [PubMed] [Google Scholar]