Abstract
Many cancers occur from locations of inflammation due to chronic irritation and/or infection. Tumor microenvironment contains various different inflammatory cells and mediators that orchestrate diverse neoplastic processes, including proliferation, survival, adhesion and migration. In parallel, tumor cells have adapted some of the signaling molecules used by inflammatory cells, such as selectins and chemokines as well as their receptors for invasion, extravasation and subsequently metastasis. Expression and/or activation of the majority of these molecules is mediated by the proprotein convertases (PCs); proteases expressed by both tumor cells and inflammatory cells. This review analyzes the potential role of these enzymatic system in inflammation-associated cancer impacting on the malignant and metastatic potential of cancer cells, describing the possible use of PCs as a new anti-inflammatory therapeutic approach to tumor progression and metastasis.
Keywords: Protein maturation, Substrates, α1-PDX, Metastasis
Highlights
-
•
Proteins maturation by the proprotein convertases plays important role in inflammation-related cancer and metastasis.
-
•
Protein precursors require the proprotein convertases for the induction of inflammation.
-
•
Understanding of the molecular mechanism linking the proprotein convertases to inflammation will allow novel therapies.
-
•
Inhibitors of the proprotein convertases constitute great potential for cancer treatment.
Abbreviations
- PCs
the proprotein convertases
- IBD
inflammatory bowel disease
- LDLRs
low-density lipoprotein receptors
- TNFSF
Tumor necrosis factor superfamily
- THD
TNF homology domain
- TGN
trans-Golgi network
- TWEAK
TNF-like weak inducer of apoptosis
- TGF−β
Transforming growth factor-β
- BAFF
B-cell activating factor
- SLE
systemic lupus erythematosus
- RA
rheumatoid arthritis
- SS
Sjögren's syndrome
- April
A proliferation-inducing ligand
- IP-10
Interferon gamma-induced protein 10
- SDF-1
stromal cell-derived factor-1
- LTD4
Leukotriene D4
- CysLT1
Cysteinyl leukotriene receptor 1
- OPN
Osteopontin
- PAF
Platelet activating factor
- HIF-1
α, hypoxia-inducible factor-1
- UVR
Ultraviolet radiation
- TACE
(TNF-α-converting enzyme)
- IL-1Ra
IL-1 receptor antagonist
- HJV
Hemojuvelin
- TfR2
Transferrin receptor 2
- HFE
hereditary hemochromatosis
- BMP
bone morphogenetic proteins
- TME
tumor microenvironment
- MIF
migration inhibitory factor
- RNOS
reactive nitrogen species
- ROS
reactive oxygen species
1. Introduction
1.1. Inflammation and neoplasia
Although inflammation is considered as the principal host defense response to tissue damage, ischemia, infectious agents and/or various autoimmune diseases, it is also a major contributor to many diseases. Indeed, inflammation is linked to increased activation and migration/invasion of circulating immune cells including lymphocytes and macrophages that release inflammatory mediators (cytokines and cyclooxygenase derivatives). The persistent accumulation of these mediators causes various pathologies including obesity, rheumatoid arthritis, diabetes, cardiovascular disease, inflammatory bowel syndrome and cancer [[1], [2], [3]].
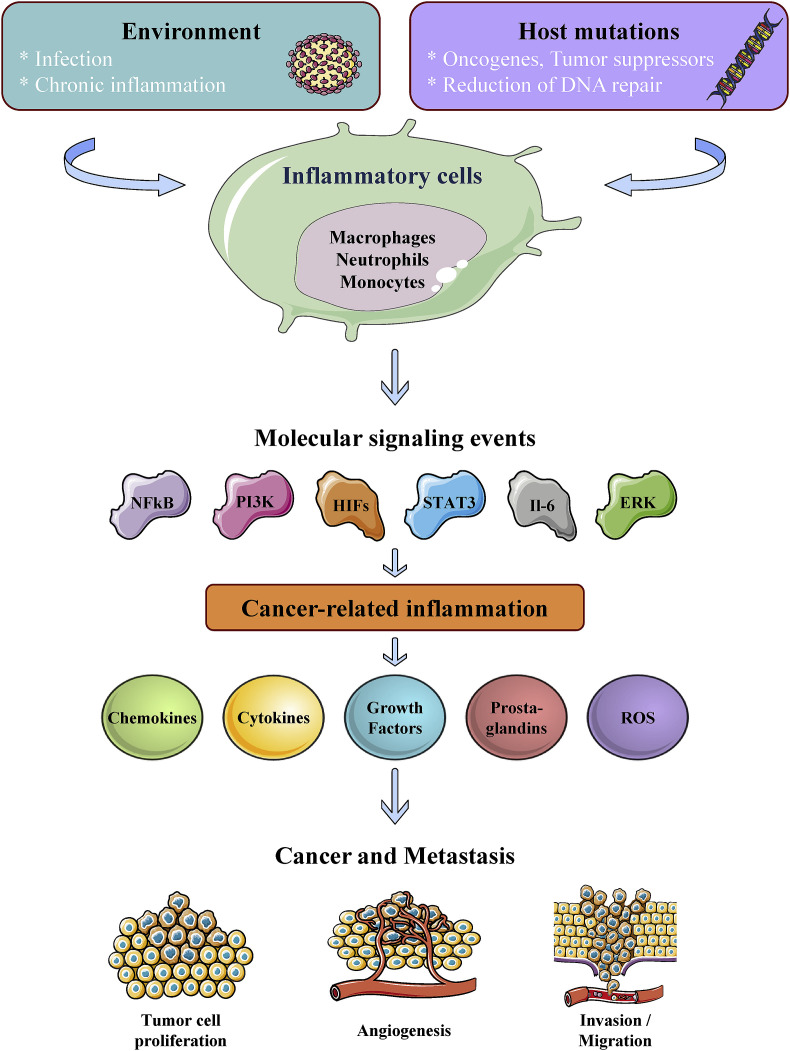
The implication of inflammation in neoplasia is based on Virchow's hypothesis from the 19th century that proposed sites of chronic inflammation to be the starting point of cancer when tissue injury and/or irritant agents increase cell proliferation [3]. It is now well established that cell proliferation alone does not provoke cancer. However, persistent proliferation of cells with damaged DNA mediated by irritants and inflammatory cells promotes a serious neoplastic risk. During combined tissue injury and inflammation, cell proliferation is enhanced while the tissue regenerates and decreases after the irritant agent is removed or the tissue is repaired. In contrast, proliferating cells that maintain DNA damage continue to proliferate during inflammation facilitating cancer initiation (Fig. 1 ). It is thus well established that inflammation is linked to increased tumor incidence and it has been long demonstrated that chronic inflammation is correlated with tumor progression and poor prognosis in cancer patients [3]. A number of chronic inflammatory diseases have been revealed to be associated with a variety of cancers (Table 1 ) and clinical studies; and experimental data were able to link tumor progression to the upregulation of various pro-inflammatory mediators, particularly during cancer progression and early events of metastases [[3], [4], [5], [6]]. Worldwide, up to 20% of cancer deaths are linked to inflammation and chronic infection [[3], [4], [5], [6], [7], [8], [9], [10]]. For example, gastric cancer was reported to occur from sites of inflammation induced by Helicobacter pylori infection [7] and hepatocarcinoma to chronic Hepatitis B or C virus infection [8]. Chronic irritation was also reported to promote cancer, such as chronic bronchitis associated with smoking, as a predisposition for lung cancer [9] and inflammatory bowel disease (IBD) that increases the risk of bowel cancer [10]. Furthermore, obesity-related inflammation is involved in various cancers [1] and rheumatoid arthritis is intimately linked to lymphoma development [11]. Therefore, chronically inflamed tissue seems to offer a favorable environment for tumor cells to originate, survive and prosper.
Fig. 1.
Schematic representation of inflammation-related pathways leading to cancer and metastasis.
Table 1.
| Inflammation/infection | Related-cancer | PCs Influence | Ref erences |
|---|---|---|---|
| Ulcerative colitis | Colorectal | + | Oncotarget. 2014 Jun 30; 5(12):4195-210. |
| Emphysema, Tobacco, tuberculosis | Lung | + | Mol Carcinog. 2017 Mar; 56(3):1182–1188. |
| Hemochromatosis, hepatitis B and C, Alcohol, obesity | Liver | – | Biomed Res Int. 2015; 2015: 148651. |
| Gastritis/helicobacter pylori | Stomach | + | Cancer Res. 2010 Jul 15; 70(14):6093-103. |
| Alcohol, pancreatitis | Pancreas | + | Mol Med Rep. 2016 Dec; 14(6):5205–5210. |
| Cervicitis/papilloma virus | Ovarian | + | Transl Oncol. 2014 May 9. |
| Prostatitis | Prostate | + | Cancer Res. 2017 Dec 15; 77(24):6863–6879. |
| Cystitis and schitosoma hematobium | Bladder | + | Oncol Lett. 2017 Jul; 14(1):1193-1199 |
| Sunburn/ultraviolet light | Melanoma | + | PLoS One. 2010 Apr 9; 5(4):e9992. |
| Esophagitis/Gastric acid, alcohol, tobacco | Esophageal | + | Acta Odontol Latinoam. 2002; 15(1–2):29–37. |
| AIDS | Non-Hodgkin's lymphoma, Kaposi's sarcoma | + | AIDS Res Hum Retroviruses. 2000 Feb 10; 16(3):227-36. |
| Rheumatoid arthritis | Non-Hodgkin's lymphoma, Hodgkin lymphoma | + | Mol Med Rep. 2015 Nov; 12(5):7681-6. d |
| Epstein-Barr virus | Nasopharyngeal carcinoma, Burkitt lymphoma | + | J Gen Virol. 2009 Mar; 90(Pt 3):591-5. d |
1.2. Inflammation during DNA damage and cell transformation
During inflammation, inflammatory cells including neutrophils, eosinophils, lymphocytes, macrophages and others were found to participate actively in the control of the malignant phenotype of tumor cells. Indeed, through the release of various mediators and/or physical interaction, inflammatory cells control tumor cell proliferation, survival and/or invasion. Usually, the initiation of neoplasia is mediated by the occurrence of various irreversible cellular changes that resulted in the induction of increased DNA damage and significant reduction of DNA repair. In the majority of cases, these irreversible modifications persist in normal tissue indefinitely, and only exposure of stressed cells to additional trauma leads to the acquisition of transformed character and ultimately tumor initiation, progression and metastasis (Fig. 1). Such trauma may occur following exposure of cells to stress mediated by chemicals and/or infectious agents or various factors released during chronic inflammation. During these processes, increased epithelial cell proliferation is crucial for the regeneration of damaged tissues. However, the increased accumulation of multiple mutations in cells due to DNA damage, in addition to their proliferative advantage constitutes a crucial step toward cell transformation. Accordingly, the amount of cell loss and the subsequent regeneration have been found to correlate in mouse model of liver cancer to tumor growth [12]. Three main mechanisms were established by which infections can mediate initiation and progression of carcinogenesis. The first mechanism is associated to the ability of infectious agents that become persistent within the host and therefore provokes chronic inflammation. This is frequently accompanied by the formation of reactive nitrogen (RNOS) and oxygen species (ROS) by leukocytes and other cells during inflammation. Repeated tissue damage and regeneration of tissue in the presence of ROS and RNOS that interact with DNA in proliferating cells resulting in continuing genomic alterations, e.g., deletions, point mutations, and/or rearrangements. A good example is the mutation in the P53 gene that was found not only in tumors but also in chronic inflammatory disease such as rheumatoid arthritis. In addition, DNA damage induced by inflammatory cells was reported to be linked to the macrophage migration inhibitory factor (MIF) released by T lymphocyte and macrophages that suppresses p53 transcriptional activity [13]. Chronic repression of p53 regulatory functions in infiltrated tissues can generate a deficient response to DNA damage, thereby amplifying accumulation of oncogenic mutations. As a second mechanism, infectious agents may directly transform cells by inserting oncogenes into the host genome and represses tumor suppressor genes. During the last mechanism, infectious agents may promote immunosuppression (e, g. human immunodeficiency virus (HIV)) and favor cancer initiation. However, virus-associated malignancies are practically rare in infected persons. This likely reflects the requirement of cofactors necessary for tumor promotion. Indeed, the inflammation induced during Rous sarcoma virus infections requires TGF-β and other cytokines produced by the inflammatory cells to mediate tumor development.
1.3. Inflammation in infection-mediated cell transformation
There is a growing body of evidence that many malignancies are initiated by infections. Infection can induce cell transformation by inducing chronic inflammation, or directly by inserting active oncogenes into the host genome. It was estimated that close to 15% of malignancies resulted from infections and constitute 2.2 million cases per year [14]. However, although many kinds of infectious agents such as viruses are known to infect humans, only a small portion of infected people will develop cancer [14] (Table 1). These observations suggest the ability of these viruses to induce immune suppression [15]. Indeed, it was reported that chronic viral replication in hepatocytes may alter the levels and the profile of cytokine produced locally. Previously, such a mechanism that affects the cytokine IL-6 and STAT3 was detected downstream of H. pylori in the generation of stomach cancer [16]. In a similar manner, a hepatitis C infection was found to predispose patients to hepatocarcinoma, although the complete molecular mechanism behind this associated risk is unknown [17]. Infection of B lymphocytes by the Epstein-Barr virus induces their continued proliferation and ultimately leads to cell transformation and various cancers [18].
2. Proprotein convertases (PCs)
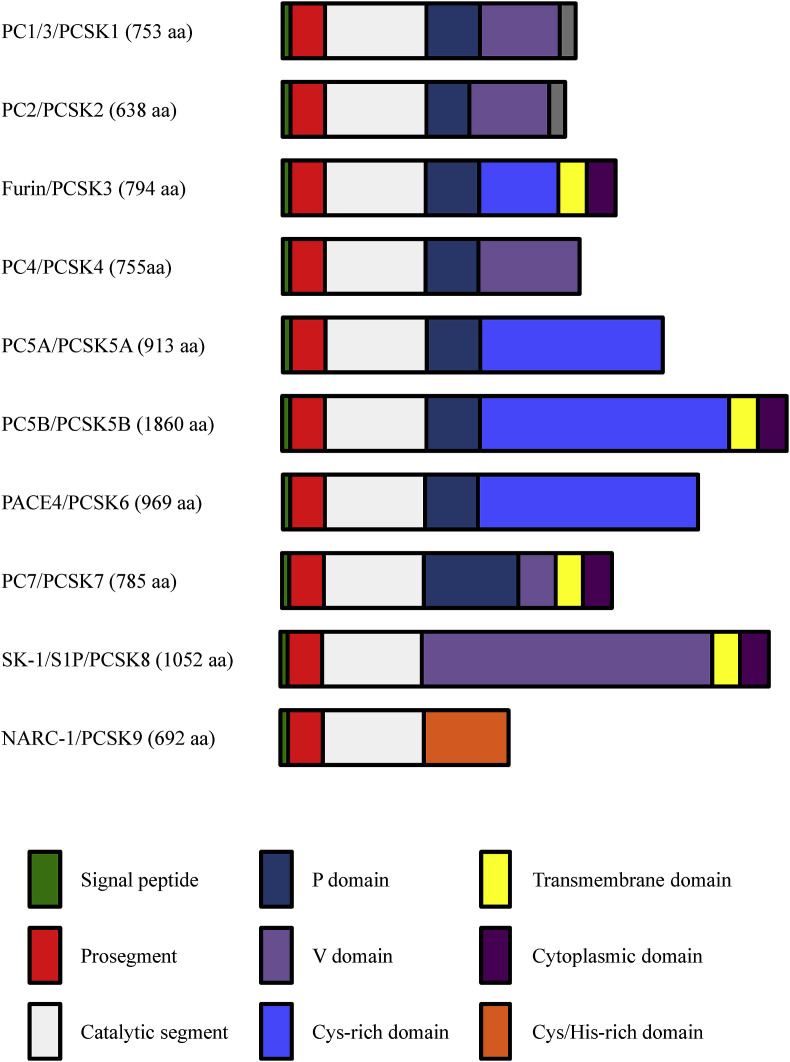
PCs are proteases involved in the proteolytic conversion of various precursor proteins into biologically active or inactive forms by limited proteolysis at one or multiple sites. To date, these enzymes constitute a family of seven known basic amino acid (aa)-specific proteinases (Furin, PC1/3, PC2, PC4, PACE4, PC5/6 and PC7 [[19], [20], [21]], as well as the two nonbasic aa-specific convertases (PCSK8, also known as subtilisin/kexin-like isozyme-1(SKI-1) and site-1 protease (S1P) [22]), and PCSK9 (known as neural apoptosis-regulated convertase-1 (NARC-1) [21]) (Fig. 2 ). PCs are implicated in the processing of multiple protein precursors, including proteases, cytokines, growth factors, and receptors at recognition sites exhibiting the general motif (K/R)-(X)n-(K/R)↓, where X is any aa except Cys and n equals 0, 2, 4, or 6 aa. PCSK8 recognizes substrates with the cleavage site(R/K)-X-(L, I, V)-Z ↓, where Z is any aa except Pro, Cys, Glu, and Val [22]. To date, no substrate has been identified for PCSK9. However, this convertase was found to cleave autocatalytically its prosegment at the motif VFAQ↓SIP, with Val at P4 being the most critical residue [23] (Fig. 2).
Fig. 2.
Schematic representation of the primary structure of the proprotein convertases (PCs). The primary structures and domains of the nine PCs including the two alternative spliced forms of PC5/6 PC5/6A and PC5/6B are shown. The signal peptide, the prosegment, and the catalytic domain with typical catalytic triad residues Asp, His, and Ser are common to all the PCs. The other domains and the number of the amino acid (aa) for each proprotein convertase (PC) are also indicated.
2.1. Proprotein convertases in health and diseases
Previously, to determine the physiological importance of PCs, these proteases were disrupted separately using complete or conditional reverse genetic approaches [24]. The varieties of the phenotypes obtained after PC knockout underline the complexity and wide array of the protein precursors that are processed by these enzymes [24]. The information gathered from the available PC null mice revealed that only the absence or dysfunction of Furin, PC5/6 and SKI-1/S1P are lethal at early embryonic stages. Knockout mice of PC1/3 and PC2 genes are viable, despite the manifestation of hormonal and/or neuroendocrine malfunctions. PC4 null mice are 75% viable and some exhibit craniofacial abnormalities. These null mice are infertile or subfertile [24]. Liver-specific Furin null mice are viable with no obvious malformation and liver specific conditional SKI-1/S1P null mice exhibit disorganized lipid and fatty acid homeostasis due to the lack of SREBP-1 and SREBP-2 processing. PCSK9 knockout mice show enhanced cholesterol uptake by liver low-density lipoprotein receptors (LDLRs). Mice with a disrupted PC7 gene (PCSK7) showed learning and memory defects [25]. In addition to their role in the maintenance of cell and tissue homeostasis, the ability of the PCs to activate various protein precursors including growth factors, receptors, adhesion molecules, matrix metalloproteinases, viral glycoproteins and bacterial toxins directly implicated them in various diseases such as obesity, diabetes, atherosclerosis, cancer, and Alzheimer's disease. For example, PCs have been linked to some neurodegenerative disorders via their direct or indirect roles in the production of amyloidogenic peptides following activation of both α- and β-secretase zymogens [24,26]. Similarly, several PCs were associated and/or implicated in arthritis, obesity and type 2 diabetes, and anxiety. Regarding bacterial toxin activation, the three known classes of bacterial toxins were described to be activated by the PCs [20]. Similarly, accumulated studies on various infectious viruses revealed that the cleavage of their glycoprotein precursors envelope by one or more PC is a required step for the acquisition of the infectious capacity of viral particles. These include the HIV-1 gp160 and surface glycoproteins of Hong Kong, Ebola virus, and the severe acute respiratory syndrome coronavirus. Other studies revealed that the inhibition of processing of these viral surface glycoproteins abrogated the virus-induced cellular cytopathicity [20].
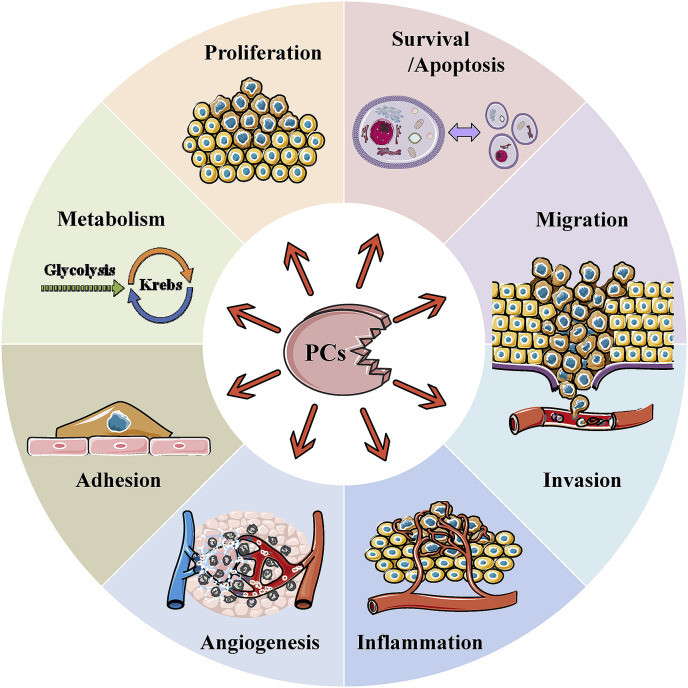
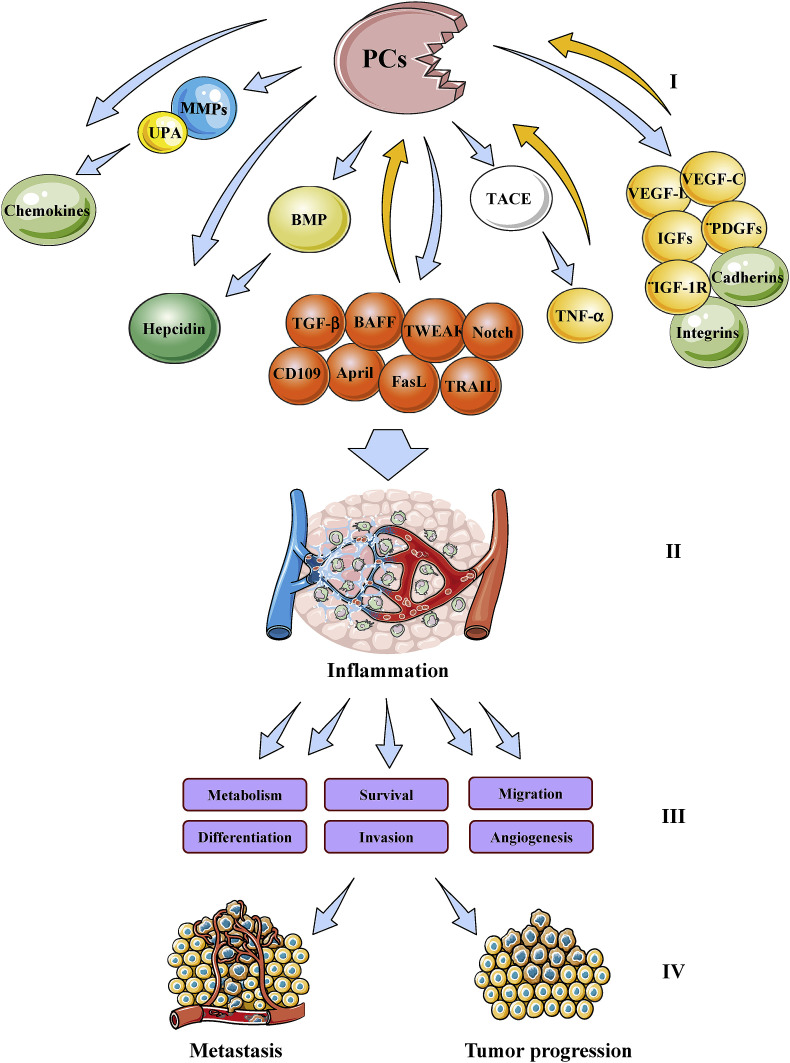
The involvement of several PCs in tumorigenesis has been extensively reviewed [[27], [28], [29], [30]]. Indeed, there is growing evidence for the implication of some of these proteases in various processes involved in tumor progression and metastasis. They include angiogenesis, immune responses and cell proliferation, migration/invasion and adhesion (Fig. 3 ). Previously, various studies revealed that the enhanced or reduced processing of several substrates of the PCs affect the malignant phenotypes and the metastatic potential of tumor cells. Overexpression of Furin was found to enhance tumor growth and aggressiveness of tumor cells [31]. On the other hand, while inhibition of PCs activity in several tumor cells using the general PC inhibitor (α1-PDX) was found to abrogate their malignant phenotype and reduce their ability to induce tumor progression, angiogenesis and metastases formation in mice [19,[32], [33], [34], [35], [36], [37]], the inhibition of the PCs in other cancer cells exacerbated their malignant phenotype [30,38]. These effects were associated with the inhibition of the processing of several molecules involved in the malignant phenotypes of tumor cells and tumorigenesis such as metalloproteases (MT1-MMP, MMP11/Str3), adhesion molecules (integrins, cadherins), cytokines (TGF family), growth factors (PDGFs, VEGF-C, VEGF-D, IGF-1), and growth factor receptors (IGF-1 receptor) [30,[37], [38], [39]] (Fig. 4 ). The expression of several PCs was examined in a variety of cancers [19,37]. In some cases, expression of a number of PCs correlated with metastatic potential of cancer cells and aggressiveness. Amongst all PCs, Furin has been the most studied in the context of tumor growth and progression and found expressed in all the analyzed human cancers. Other studies revealed that PACE4 was also increased in prostate cancer [40].
Fig. 3.
Biological functions involving the proprotein convertases (PCs) leading to cancer.
Fig. 4.
Schematic representation of inflammatory mediators and proprotein convertases (PCs) cross-talk. Substrates and downstream effectors of the PCs (I) leading to inflammation (II). The latter mediates various biological processes (III) responsible for tumor progression and metastasis (IV). The ability of some PC substrates to induce several PCs expression is also indicated.
2.2. The clinical relevance of proprotein convertases in inflammation-related malignancies
Elevated expression of different PCs was reported for a range of human cancers [19]. However, the relative importance of each PC in these cancers has not yet been clarified. The majority of these cancers are directly or indirectly linked to inflammation. Table 1 summarizes the results of various studies on the implication of the PCs in these human cancers. Initial studies revealed a high Furin expression in lung tumors [19]. Subsequently altered furin and PACE4 expression was confirmed in other inflammation-related malignancies such as colorectal, liver, ovarian and prostate cancers (Table 1). Based on these studies the expression of these PCs in tumors may constitute a significant prognostic factor independent of other conventional clinicopathological ones. Indeed, the ability of furin and PACE4 in promoting the activation of molecules involved in inflammation-related cancers suggested that the repression of these convertases could be employed at the clinical sitting. Silencing of Furin was recently employed to inhibit the activity of the immunosuppressive cytokine TGFβ1 using an autologous tumor-based strategy consisting of a plasmid that encodes granulocyte-macrophage colony-stimulating factor (GMCSF) and Furin shRNA. This vaccine was found to be efficient and safe during phase I and II clinical trials in patients with Ewing sarcoma, hepatocellular carcinoma and ovarian cancer; all inflammation-related cancers [41].
3. Cross-talk between proprotein convertases and inflammatory mediators
The regulation of PCs expression in a context of inflammation is not fully investigated. Previously, Furin gene activation was reported to be regulated by three distinct promoters, namely P1, P1A, and P1B. Only P1 promoter was revealed to bear inducible gene features, whereas P1A and P1B promoters were found to contain several Sp1-binding sites [42]. The induction of PCs expression is mediated by various cytokines such as TGF-β [43] and growth factors (e.g. PDGF) [44]. Interestingly, the majority of these mediators are well-characterized PCs substrates and have been shown to play a role in several aspects of inflammation, immune response and/or malignant transformation (Fig. 4). The best-established link between chronic inflammation and cancer is seen in colorectal cancer that develops in patients with IBD (e.g., ulcerative colitis). These patients have up to a seven-fold increased risk of developing colorectal cancer [45]. The implication of PCs in the generation of an enzyme/substrate amplification loop may lead to increase active inflammatory mediators and subsequently sustained inflammation contributing to malignancy (Fig. 4). Interestingly, the expression of several PCs, particularly Furin, was found to be altered in these patients [37]. Therapeutic strategies for the treatment or prevention of IBD aim to reduce the endogenous levels of TNF-α, which is a key pathophysiologic element of the disease [46] and involves the PCs in its full activity like other inflammatory mediators.
4. Proprotein convertase substrates and inflammation
4.1. Tumor necrosis factor superfamily (TNFSF)
The members of TNFSF are involved in the coordination of various immune and inflammatory responses [47]. Numerous studies reported their direct implication in various diseases, such as impaired immune response, septic shock, lymphoproliferation, atherosclerosis, cancer and bone and immunoinflammatory diseases [47]. Most members of the TNFSF are synthesized as type II transmembrane proteins and share a common structural motif, the TNF homology domain (THD), that mediates receptor binding [47]. The extracellular domain can be cleaved in order to generate soluble cytokines. This cleavage was found to be directly or indirectly coordinated by PCs. Many TNFSF cytokines, including TNF, FasL, TRAIL and TWEAK activate the NF-κB family of transcription factors. Interestingly, Furin expression was reported to be also induced by NF-κB activation [48], suggesting the existence of a potential Furin/TNFSF loop that use NF-κB activation for its amplification.
4.2. TNF-α
Produced by activated macrophages and pro-inflammatory T cells, TNF-α is also a pleiotropic cytokine that regulates multiple cellular functions including promotion of cell proliferation and migration, and/or differentiation [47]. TNF-α was found to stimulate both pro- and anti-apoptotic signals in tumor cells, endothelial cells, macrophages and most other cells within the tumor microenvironment. Like IL-1, TNF-α is an essential effector cytokine required for the initiation and maintenance of chronic inflammation and was considered as a good target for the treatment of various inflammatory diseases, including atherosclerosis, cardiovascular dysfunction and ischemia-reperfusion injuries. Indeed, the clinical importance and relevance of TNF-α is well demonstrated in the accomplishment of the anti-TNF-α therapies in inflammatory diseases [47]. In addition, TNF-α also induces apoptosis of activated T cells that infiltrate tumors, and therefore may affect the immune surveillance within the tumor. However, although the pro-apoptotic effects of TNF-α offer promise for point the interest in its therapeutic utility, it requires higher doses than therapeutically feasible [49]. In addition, most in vivo and clinical studies dealing with TNF-α function have revealed its pro-neoplastic functions rather than its pro-apoptotic effect on tumor cells. Indeed, following TNF-α release by inflammatory and tumor cells, the latter seemed to promote tumor survival via the induction of various antiapoptotic molecules expression regulated by NF-κB activation. The direct implication of TNFα in tumorigenesis was previously confirmed by the use of TNF-α-deficient mice or mice treated with anti-TNF-α antibodies. The latter were found to be highly protected against chemical induction of skin papillomas [50]. In addition, TNF-α was described to potentially participate in malignant transformation through its ability to induce the production of various reactive oxygen species [51]. Accordingly, abnormal presence of higher concentrations of TNF-α in cancer patients correlated with worse prognosis [52]. TNF-α signals through activation of two receptors, TNFR1 and TNFR2 [47]. TNFR1 is expressed in a wide range of cells and tissues and can be activated by soluble TNF-α, whereas TNFR2 exhibits highly restricted tissue distribution and has high affinity for membrane-associated TNF. Following its production as a protein precursor, TNF-α remains attached to the membrane and is released as a soluble active form only after its processing by the TNF-α-converting enzyme (TACE, ADAM17) [53]. The activation of TACE is mediated by Furin between the prodomain and the metalloproteinase domain [54]. In addition, TNF-α was reported to induce Furin activation that was abrogated by breeding A (Fig. 4), an inhibitor of ER-Golgi transport and monensin, that blocks Golgi-cell surface vesicular transport. These studies suggest that the Furin activation by TNF occurs during its transport from the trans-Golgi network (TGN) to the cell surface. Although the link between the TNF-α receptor and Furin activation is not well known, previous studies suggested the possible involvement of oxidative stress in these processes [55] (Fig. 4).
4.3. TNF-like weak inducer of apoptosis (TWEAK)
As a member of the TNF family, TWEAK is a multifunctional cytokine that acts via binding to a cell surface receptor named fibroblast growth factor-inducible 14 or Fn14. TWEAK was initially described as a proapoptotic factor for various tumor cell lines [56], but subsequent studies revealed that it can induce many other cellular responses, including cell proliferation, survival and differentiation. This cytokine has been studied in critical biological processes, including embryonic development, organogenesis, tissue repair and immune responses. It was also linked to the pathogenesis of ischemic stroke and several chronic inflammatory diseases, including rheumatoid arthritis, and inflammatory bowel disease. Produced as a precursor, the full-length TWEAK is processed intracellularly by Furin (Fig. 4). Membrane-anchored TWEAK can bind the Fn14 receptor on neighboring cells and activate the NF-κB signaling pathway, suggesting that membrane-anchored TWEAK can act in a juxtacrine manner to initiate cellular responses. In addition, TWEAK was found to form a cell surface fusion protein with APRIL called TWE-PRIL. The formation of this complex occurred just after the processing of APRIL by Furin [57].
4.4. Transforming growth factor-β (TGF−β)
TGF-β exerts effects on most cell types; thereby this pleiotropic cytokine has the ability to simultaneously control immunological and non-immunological processes. Indeed, TGF-β was previously reported as a key regulator of cancer progression, inflammation, and immunosuppression processes. In addition, TGF-β was found to be released not only by a variety of tumor cells but also by normal cells (macrophages and platelets and T cells). Through its ability to regulate the function of these cells and other cells such as fibroblasts and endothelial cells, this cytokine is able to create an inflammatory milieu similar to chronic inflammation typical of various diseases including cancer. TGF-β contributes to inflammation induced by tumor mediators by facilitating tissue remodeling and local suppression of antigen-specific CD8+ T-cell function. In addition, TGF-β1 was found to increase hypoxia-inducible factor-1 (HIF-1α) levels by modulating HIF-1α protein half-life through inhibition of PHD2 expression that impact on inflammation and/or tumor progression. This process enhances the expression of various genes implicated in angiogenesis, cell invasion, and chemotaxis as well as cell growth and survival [58]. Early in cancer development and premalignant lesions, TGF-β was reported to play a tumor suppressive function due to its negative effect on tumor cell growth. Previously, it was reported that genetic deletion of the TGF-β receptor in mouse models of human cancer led to increased tumor incidence and progression [59]. Similarly, numerous clinical studies have demonstrated that a higher level of TGF-β1 expression is significantly associated with an invasive phenotype of tumors in patients. In vitro, high levels of TGF-β1 are only produced by poorly differentiated tumor cells and reduced levels were detected in well-differentiated cells. TGF-β activates a heterodimeric receptor pair of TGF-β receptors I and II. The interaction of TGF-β with its receptor induces the phosphorylation of the transcription factors Smad2 and Smad3 that mediate their translocation to the nucleus and subsequently initiation of transcription [60]. Like the majority of PCs substrates, TGF-β is synthesized as an inactive precursor (proTGF-β) protein (Fig. 4). This precursor contains a prodomain sequence known as the latency associated peptide or LAP and the mature peptide. Following synthesis, two proTGF-β precursor molecules dimerized via disulfide bonds prior their cleavage by the PCs. This cleavage occurs between LAP and the mature peptide that remains as a noncovalently associated complex. The cleavage/maturation process occurs in the Golgi apparatus and/or ECM and is necessary for generation of the bioactive mature TGF-β ligand, which can then bind TGF-β receptors to trigger downstream pathways [61,62]. CD109, a glycosylphosphatidylinositol (GPI)-anchored glycoprotein upregulated in various tumor cells, was found to negatively regulate TGF-β functions directly by modulating receptor activity. CD109 was found to be processed by Furin [63]. Produced as a 205 kDa glycoprotein, CD109 is processed in the Golgi apparatus into 180 kDa and 25 kDa proteins. The two parts remain associated with GPI-anchor on the cell surface and the 180 kDa form is secreted into the culture medium. Further studies have revealed that the processing of CD109 into 180 kDa and 25 kDa proteins by Furin, followed by complex formation with the TGF-β receptor is required for TGF-β signaling in cancer cells [63].
4.5. 4.5. B-cell activating factor (BAFF, or BLyS, THANK, ALL-1, zTNF-4)
BAFF has been shown to be important for maintenance of peripheral B-cell homeostasis and enhancing antigen-specific humoral immunity, by inducing peripheral B-cell survival, proliferation and Ig secretion [64]. Furthermore, other studies have reported that elevated levels of BAFF and its receptors may be involved in the pathogenesis of B-cell-mediated autoimmune diseases, such as systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), Sjögren's syndrome (SS) and probably other diseases [65]. These studies suggest the potential use of BAFF in the activation of immune system and improvement of immunity under other circumstances. BAFF is processed by Furin at the cell surface [66], and is able to perform its function only as a soluble factor. Interestingly, Furin expression was found to be linked to soluble BAFF release [67]. Indeed, Furin expression was revealed to be regulated by JNK and p38 signaling pathways [68] that was suggested to mediate intracellular processing of membrane-bound BAFF to soluble BAFF by regulating Furin-like protease expression (Fig. 4).
4.6. A proliferation-inducing ligand (April, or TALL-2, TRDL-1, TNFSF-13a)
Like Baff, this cytokine is also involved in autoimmunity and considered as an important regulator of the immune system. In contrast to BAFF and other TNF family members, the precursor form of APRIL is cleaved in the Golgi by Furin following its synthesis and exists only as a secreted soluble ligand [69] (Fig. 4). In addition, although both APRIL and BAFF are found in various immune system cells such as monocytes, dendritic cells, macrophages and T cells, APRIL is also expressed by tumor cells.
4.7. Chemokines
Initially, the chemokines were identified as soluble factors involved in the migration of leukocytes during inflammation. To date, more than 50 chemokines are known to control the function of leukocytes and other cell types and affect the majority of tumor cells [70]. Indeed, various studies revealed that chemokine-receptor system is altered considerably in various tumor tissues. In addition to their role in regulating leukocyte recruitment, chemokines were found to directly control stromal and tumor cell behavior. The complexity of this system is not only controlled at the transcriptional level, but also involves post-translational modifications, including processing by Furin-like proteases [70,71]. Indeed, Furin was found to mediate the cleavage of the chemokine CXCL10 (also known as Interferon gamma-induced protein 10; IP-10) followed by its trimming by a basic amino acid-specific carboxypeptidase [72]. The use of the Furin inhibitor decanoyl-RVKR-chloromethylketone was found to block this process, suggesting the requirement of Furin for the initiation of this proteolytic cascade, important for the mediation of optimal activation of its receptor CXCR3 [73]. Cleavage of CXCL10 by basic amino acid-specific carboxypeptidase was suggested to mediate biological functions not usually induced by the uncleaved CXCL10, such its ability to mediate biological responses normally induced by CXCL12 (stromal cell-derived factor-1; SDF-1). CXCL12 activates CXCR4 after its cleavage by MMP-2 and mediates the highly neurotoxic effect [74]. Recently, various chemokines were found to be sensitive to cleavage by several MMPs at their N-terminal region. Indeed, CXCL8 (IL-8) cleavage by MMP-9 was found to potentiate its activity [75]. In contrast, the cleavage of CCL7 by MMP-9 and CXCL12 by MMP-2 negatively affect their activity [76]. Other proteases such as cathepsins [77], urokinase plasminogen activator, and plasmin [78] were found also to be involved in the processing of chemokines. The expression and/or the activity of these proteases were previously reported to be regulated directly or indirectly by PCs [19] (Fig. 4).
4.8. Hepcidin
Mainly expressed in the liver, the peptide hepcidin is a major regulator of systemic iron homeostasis. In septic patients, the degree of inflammation, indicated by IL-6 levels, was found to be associated with elevated concentrations of hepcidin [79]. Similarly, urine analyses of patients with severe inflammatory diseases or chronic infections have shown up to 100-fold increases in hepcidin levels; that cause iron sequestration in macrophages and decreases the absorption of iron from the small intestine, thereby causing anemia [80]. Similarly, resection of a hepcidin-producing hepatic adenoma in a patient suffering from refractory anemia leads to spontaneously reduction of anemia. Hepcidin expression was reported to be altered in various cancers [81]. This therefore suggests that hepcidin expression in a tumor cell microenvironment may initiate and/or accentuate tumorigenesis. Using in vitro and in vivo assays Furin and other PCs were found to be implicated in the proteolytic processing of the hepcidin precursor (Fig. 4). Mutation of the hepcidin precursor at the Furin cleavage site completely abolishes processing. Following ferroportin binding to hepcidin, ferroportin is internalized and degraded, leading to decreased export of cellular iron. The blockade of hepcidin precursor processing inhibits the hepcidin effect on ferroportin degradation [82]. Previously, studies on in vivo iron homeostasis revealed that the expression of hepcidin in the liver is positively regulated by HJV, TfR2, HFE and BMP. Most of the BMP tested, including BMP2, 4, 5, 6 and 9, were reported to be strong inducers of hepcidin [81,83,84]. Interestingly, Furin acts also on the maturation of these BMPs, and on other class of TGF-β molecules [19]. In the hepatic hepcidin producing HepG2 cells, Furin is also regulated by TGF-β1 through a mechanism that involves a cross-talk between Erk1/2 MAPK and SMAD2/3 pathways [85]. Similarly, the silencing of HFE and TfR2 was found to reduce both Erk phosphorylation and Furin expression. The relationship between TfR2 activity and Furin may be relevant in the regulation of hepcidin expression, since Furin has already been shown to act as a regulator of hepcidin expression and to be modulated by HIF-α in an iron-dependent manner [68]. The activity of HJV is regulated by a complex post-translational program. Its level is proteolytically controlled by matriptase-2, while its secretion and processing into soluble form is regulated by the activity of Furin [68]. Thus, the effect of Furin on hepcidin expression may be complex, resulting in activation or inhibition depending on the conditions.
5. Furin inducers and inflammation
Leukotriene D4 (LTD 4 ): Mediated by the Cysteinyl leukotriene receptor 1 (CysLT1), LTD4 induces the expression of Furin following the activation of P1 promoter [48].
Osteopontin (OPN): This extracellular matrix-associated cytokine was found to induce CD44-mediated p38 phosphorylation that induces NF-κB activation and NF-κB–dependent expression of Furin [86].
Platelet-activating factor (PAF): Known as a potent bioactive lipid mediator that accumulates rapidly after an injury, PAF was found to significantly stimulate the induction of Furin expression. This factor increases the transcriptional activator AP-1-responsive elements c-fos and c-jun.
Interleukin 12 (Il-12): Produced by monocytes, macrophages and dendritic cells, IL-12 is involved in the regulation of T-cell activation and differentiation during inflammation, in response to infection and during autoimmune diseases [87]. IL-12 is therefore generally considered to promote antitumor effects, and cancer patients have been treated with recombinant IL-12 in several clinical studies. Furin was found to be preferentially expressed in differentiated Th1 cells in a Stat4-dependant manner. Expression of Furin enhanced IFN-γ secretion, whereas inhibition of Furin blocks its production, suggesting that IL-12 induction of Furin might represent a new aspect of IFN-γ regulation and control of Th1 differentiation [88].
6. External conditions that enhance Furin activity
Hypoxia. Hypoxia is a metabolical condition, which enhances tumorigenesis, mostly due to its impact on gene expression of pro-angiogenic factors and invasion-related mediators, some of which are natural substrates for Furin. Analysis of Furin promoters revealed the presence of putative binding sites for HIF-1α, a transcription factor that plays a pivotal role in cellular adaptation to hypoxia. Indeed, Furin is remarkably increased upon hypoxic conditions [89]. Previously, induction of Furin by HIF-1 was found to correlate with an increased proteolytic activation of various Furin substrates such as MT1-MMP and TGF-β1, suggesting the existence of another mechanism used by hypoxia to induce tumorigenesis, through enhanced Furin-induced proteolytic processing of precursor proteins known to be involved in the acquisition of the malignant phenotype by tumor cells [89,90].
Ultraviolet radiation. Ultraviolet radiation (UVR) that reaches the earth's surface consists mainly (90–95%) of UVA, along with lower (5–10%) levels of UVB [91]. It is well recognized that exposure to high doses of UVR causes cellular and molecular damage, resulting in immunosuppression and increased frequency of mutations [91]. Previously, Furin expression was reported to be induced by UVR [92]. This induction is associated with the induction of TNF-α and other cytokines involved in the activation of stress-inflammation signal transduction pathways such as p38, JNK and ERK. Indeed, UVR has been shown to be a potent inducer of TNF-α gene expression in keratinocytes [93]. When released from irradiated keratinocytes, TNF-α stimulates the migration of Langerhans cells to the lymph nodes as well as the release of other molecules that interfere with the functioning of these cells, thereby mediating an impaired immune response in the skin [94]. TNF-α is released from the surface of the keratinocytes as a mature protein by the action of the metalloproteinase TNF-α-converting enzyme (TACE), also known as ADAM-17 [95]. TACE in turn is cleaved from its preproform by the action of Furin [54,96]. Thereby, UV radiation seems to induce the expression of stress-inflammation signal molecules and their activating proteases including Furin. UVR can also increase FasL expression and release from keratinocytes, which may also result in the suppression of the host's immunity. Soluble FasL is cleaved from membrane FasL by the action of the metalloproteinase matrilysin (MMP-7) [97].
7. Proprotein convertases in intercellular communication leading to inflammation and tumor progression
The tumor microenvironment (TME) is a complex system including tumor cells, epithelial cells and stromal cells composed of immune cells, fibroblasts, endothelial cells, adipocytes, the extracellular matrix and others [34]. Intercellular communication between cancer cells and these cells plays a central role in inflammation and tumor development. Tumors surround infiltrates of immune cells and stromal cells that can either support or inhibit tumor growth and progression, depending on the secreted cytokine/chemokine within that TME and their outcome on cell activation status. Indeed, tumor cells that interact with the surrounding stroma, energetically modify the microenvironment following exchange information, and thus creating a microenvironment favorable to various events such as tumor angiogenesis, proliferation and metastasis [33, 35, 36]. TME characterized by relatively low pH [35, 46] and chronic inflammatory state [47] and always deficient in nutrients and oxygen [45]. Tumor cells use this relatively “unsympathetic” environment to mediate various processes that are related to their progression [8, 13, 48]. For example, hypoxia caused by oxygen deficiency that induces angiogenesis, and signal pathways activated by chronic inflammation, such as matrix metalloproteinase (MMP). Growth factors and tumor necrosis factor (TNF) pathways, are some of these processes involved in the regulation of tumor growth and metastasis. Cytokine-mediated communication between the tumor and the TME can also occur via angiogenesis signaling. Indeed, lymphatic vessel density, VEGF-D and VEGF-D expression, and E-cadherin expression were reported to be higher in inflammation related-tumors. TME-secreted VEGFD was shown to promote downstream TGFβ and E-cadherin signaling in cancer cells, resulting in increased cancer cell invasion into the lymphovascular system. The activity and/or the expression of the majority of these mediators involved in tumor and the TME communication are regulated by the proprotein convertases [19,[32], [33], [34], [35], [36], [37]]. These include cytokines, angiogenic factors (VEGF-C, VEGF-D), matrix metalloproteinase and adhesion molecules (Integrins, cadherins, selectins, immunoglobulins) (Fig. 4). The inhibition of their maturation by PC inhibitors was found to affect their function in vitro and within the TME. Indeed, PC inhibitors were reported to repress growth factors and receptors maturation and activity, cytokines (Il-1, TNF) production and vascular and lymphatic vessel density. Similarly, recent studies have revealed that PCs also play a crucial role in the intercellular communication between tumor cells and the immune system that plays a critically important role in proliferation, survival, invasion and metastasis of tumor cells. PCs repression was found to reduce T cell exhaustion and immune escape of cancer cells [9]. Further studies revealed that these processes were linked to PC substrates blockade such as Notch receptor [9]. Furthermore, following inflammatory stimulus, the latter interacts first with cell surface through the proteoglycan layer that includes different classes of adhesion molecules directly connected the ECM [2]. The adhesion capacity and/or the expression of these adhesion molecules are also regulated by the PCs, suggesting the direct participation of the PCs in the initial and late interaction of targeted cells with pathogenic stimulus. Thereby, these studies directly link PC activity to intercellular communication during inflammation-related cancer initiation and progression.
8. Proprotein convertases in inflammation-induced angiogenesis
The angiogenic process involves numerous cell types and mediators that interact to create the particular microenvironment appropriate for the formation of new capillaries. Angiogenesis is a complex process controlled by the involvement of different proangiogenic and antiangiogenic factors. For new blood vessel growth, the balance between these factors is altered in favor of proangiogenic factors, either by their upregulation or downregulation of molecules negatively affecting angiogenesis. Many of the proangiogenic factors are well-established PC substrates, such as VEGF-C, VEGF-D, PDGFs and IGFs (Fig. 4). The cleavage of these substrates by Furin-like enzymes was found to be crucial for the mediation of their functions in vitro and during tumor angiogenesis [19,[32], [33], [34], [35], [36], [37]]. Inhibition of Furin-like enzymatic activity in tumor cells expressing the general PCs inhibitor α1-PDX, reduces the ability of these tumor cells to form vascularized tumors in mice [[32], [33], [34]]. Macrophages, T lymphocytes and neutrophils, actively participate in the angiogenic process by secreting cytokines that affect endothelial cells functions, including their proliferation and migration. Previously, IL-1 and TNF-α, were found to have proangiogenic activity in physiological and pathological angiogenesis. IL-1 family consists of pleiotropic proinflammatory and immunoregulatory cytokines, namely, IL-1alpha and IL-1beta, and one antagonistic protein, IL-1 receptor antagonist (IL-1Rα) that represents a physiological inhibitor of preformed IL-1, which binds to IL-1 receptors without transmitting an activating signal. The activity of IL-1 and TNF-α is regulated by PCs and in vitro and in vivo analyses revealed that expression of the PC inhibitors in tumor cells reduced their ability to secrete these cytokines [32].
9. Proprotein convertases in inflammation-related metastasis
Cancer metastasis is a dynamic process involving multiple interactions between the disseminating cancer cells and their rapidly changing microenvironment. To date, the implication of tumor cells in the mediation of inflammation during colonization is well established. Indeed, the interaction of tumor cells with their newly acquired microenvironment induces the invading tumor cells to produce various cytokines and chemokines. The latter not only attract diverse leukocyte populations but also activate the endothelium of the invaded area, a crucial step for the establishment of metastases. The expression of these molecules significantly enhances the metastatic potential of tumor cells [[4], [5], [6]]. The arrest of metastatic tumor cells in hepatic circulation induces a variety of cytokines such as IL-1 and TNF-α, that in turn, induce the expression of a variety of adhesion molecules on endothelial cells including members of the selectin family [[4], [5], [6]]. These adhesion molecules facilitate tumor cell adhesion, a decisive step for liver colonization and metastasis [[4], [5], [6]]. Other studies have demonstrated that Kupffer cells are the major source of TNF-α in tumor cell inoculated livers. Comparative studies between high- and low- or non-metastatic cancer cells revealed that only the arrest of highly metastatic ones was found to cause this cascade of events and subsequently metastases formation in the liver.
In addition to the impact of these local inflammatory changes in the tumor microenvironment induced by the arrest of tumor cells in the invaded area, systemic inflammation was also reported to influence the adhesion of tumor cells to the endothelium of distant organs. Recently, several clinical observations have raised the possibility that inflammatory consequences resulting from postsurgical complications may influence cancer recurrence. Indeed, although surgical interventions remain the main potentially curative option, the latter is associated with surgical trauma resulting in significant cytokine accumulation and systemic inflammation [98].
The factors that regulate the host inflammatory response to tumor cells are only partially known. Several studies suggest that tumor cells produce soluble mediators that can directly promote cytokine release that induces the expression of adhesion molecules such as E-selectin by activated endothelial cells. Using in vitro assays, we found that medium derived from metastatic colon cancer cells is able to trigger TNF-α production by macrophages [[4], [5], [6]], suggesting that the ability of these tumors to induce a host inflammatory response in vivo is due, at least in part, to tumor-derived soluble factors. Similarly, activation of endothelial cells by media derived from tumor cells increases adhesion of tumor cells to activated endothelial cells. Various cytokines produced during inflammation induced by tumor cells such TNF-α can also inhibit tumor growth through their cytocidal and proapoptotic activities. However, the ultimate effect of TNF-α was found to depend on its concentrations, on tumor cell susceptibility, and on the stage of the disease [99]. Analysis of various colon carcinoma cell lines compared to normal colon tissue reveals their ability to secrete cytokines such as IL-1, IL-6, and TNF-α. Other mediators produced by tumor cells including growth factors such IGF-1 and VEGF were also found to be involved in the induction of E-selectin and participate in tumor cell survival and growth. Previously, the expression and/or activity of the majority of these proteins are directly or indirectly controlled by Furin-like protease activity [19]. Indeed, the suppression of PCs activity by the general PCs inhibitor α1-PDX in metastatic colon carcinoma cells attenuated significantly their ability to induce E-selectin expression and colon cancer cell adhesion on endothelial cells [32]. These studies underline the importance of PCs in the generation of active soluble molecules responsible for the induction of E-selectin, a finding that could probably be expanded to other adhesion molecules. Conditioned media derived from tumor cells expressing α1-PDX had reduced levels of TNF-α and IL-1α. Accordingly, inoculation of colon cancer cells expressing α1-PDX into hepatic circulation failed to induce significantly hepatic TNF-α and E-selectin, accompanied by reduced metastasis formation in the liver [32]. These studies indicate that PCs contribute to metastasis by enhancing the levels of active molecules involved in the first step of liver colonization by tumor cells. Following their adhesion on liver endothelial cells, the survival and growth of metastatic tumor cells requires the availability of various autocrine/paracrine growth-promoting factors and their receptors. Analysis of some of these factors revealed that their expression and/or activity are also regulated by PCs. For example, the Furin substrate IGF-1 and its receptor IGF-1R (Fig. 4) were reported to affect tumor metastasis through anti-apoptotic and pro-angiogenic activity as well as the regulation of tumor cell proliferation [32] and tumor-induced inflammatory response. The latter is achieved by suppressing the production of the secretory leukocyte protease inhibitor, an anti-inflammatory modulator [100]. This mediator was identified as an inhibitor of liver metastasis due to its ability to suppress the tumor cell-induced host proinflammatory response during the early stages of liver colonization. Inhibition of secretory leukocyte protease inhibitor production by IGF-1 allows TNF-α accumulation that initiates the molecular cascade required for metastasis formation [100]. Thereby, the inhibition of IGF-1R processing in tumor cells may also affect IGF-1 function, leading to reduced ability of tumor cells to induce cytokine and adhesion molecules cascades required for metastasis formation.
10. Conclusions and future directions
Cancer-related inflammation is a substantial healthcare issue and an important challenge regarding the elucidation of the basic mechanisms of inflammation-induced tumorigenesis. Mainly because the pathogenesis of inflammation-related cancer initiation and progression is partly known and only reduced therapeutic approaches for the treatment of cancers that arise in the setting of prolonged inflammation. As reviewed here, we mentioned a variety of chronic inflammatory conditions and infections that are directly or indirectly responsible for various types of cancer. We highlighted the importance of protein maturation in an increasing number of cellular pathways mediating cancers and metastasis that occur in the setting of chronic inflammation. In particular, we reviewed current knowledge implicating the activation of various inflammatory mediators by the PCs, including cytokines, growth factors/their receptors, adhesion molecules and MMPs as central regulators in inflammation-associated cancer (Fig. 4). To date, although much information is available about what the PCs activate and what promotes inflammation-associated cancer, a number of crucial puzzle pieces are missing regarding PC substrates involvement in inflammation and the role of each PC in these processes remains to be elucidated. In this regard, future in vivo studies utilizing small molecules targeting PC activity or murine transgenic approaches will be necessary to advance our understanding of the field. Strategies aimed at regulating PCs activity may prove beneficial in reducing malignant transformation after exposure to irritant agents or infection, and host mutations that result in inflammation-associated cancer. Importantly, the significance of these investigations is that they may provide the molecular rationale for developing urgently needed and novel strategies for cancer prevention and treatment.
Declaration of competing interest
The authors declare no conflicts of interest.
Acknowledgements
This paper was supported by the La ligue contre le cancer, Siric Brio and Nouvelle Région Aquitaine (France). The authors thank Pippa McKelvie (Napier, New Zealand) for reading the article.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.canlet.2019.12.027.
Contributor Information
Geraldine Siegfried, Email: geraldine.siegfried@inserm.fr.
Jean Descarpentrie, Email: descarpentrie.jean@gmail.com.
Serge Evrard, Email: S.Evrard@bordeaux.unicancer.fr.
Abdel-Majid Khatib, Email: majid.khatib@u-bordeaux.fr.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Saltiel A.R., Olefsky J.M. Inflammatory mechanisms linking obesity and metabolic disease. J. Clin. Investig. 2017;127:1–4. doi: 10.1172/JCI92035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Falconer J., Murphy A.N., Young S.P., Clark A.R., Tiziani S., Guma M., Buckley C.D. Review: synovial cell metabolism and chronic inflammation in rheumatoid arthritis. Arthritis Rheum. 2018;70:984–999. doi: 10.1002/art.40504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Korniluk A., Koper O., Kemona H., Dymicka-Piekarska V. From inflammation to cancer. Ir. J. Med. Sci. 2017;186:57–62. doi: 10.1007/s11845-016-1464-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khatib A.M., Kontogiannea M., Fallavollita L., Jamison B., Meterissian S., Brodt P. Rapid induction of cytokine and E-selectin expression in the liver in response to metastatic tumor cells. Cancer Res. 1999;59:1356–1361. PubMed 10096570. [PubMed] [Google Scholar]
- 5.Khatib A.M., Fallavollita L., Wancewicz E.V., Monia B.P., Brodt P. Inhibition of hepatic endothelial E-selectin expression by C-raf antisense oligonucleotides blocks colorectal carcinoma liver metastasis. Cancer Res. 2002;62:5393–5398. PubMed 12359742. [PubMed] [Google Scholar]
- 6.Khatib A.M., Auguste P., Fallavollita L., Wang N., Samani A., Kontogiannea M., Meterissian S., Brodt P. Characterization of the host proinflammatory response to tumor cells during the initial stages of liver metastasis. Am. J. Pathol. 2005;167:749–759. doi: 10.1016/S0002-9440(10)62048-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miftahussurur M., Yamaoka Y., Graham D.Y. Helicobacter pylori as an oncogenic pathogen, revisited. Expert Rev. Mol. Med. 2017;19:e4. doi: 10.1017/erm.2017.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fung J., Lai C.L., Yuen M.F. Hepatitis B and C virus-related carcinogenesis. Clin Microbiol Infect. 2009;15:964–970. doi: 10.1111/j.1469-0691.2009.03035.x. [DOI] [PubMed] [Google Scholar]
- 9.Gomes M., Teixeira A.L., Coelho A., Araújo A., Medeiros R. The role of inflammation in lung cancer. Adv. Exp. Med. Biol. 2014;816:1–23. doi: 10.1007/978-3-0348-0837-8_1. [DOI] [PubMed] [Google Scholar]
- 10.Feagins L.A., Souza R.F., Spechler S.J. Carcinogenesis in IBD: potential targets for the prevention of colorectal cancer. Nat. Rev. Gastroenterol. Hepatol. 2009;6:297–305. doi: 10.1038/nrgastro.2009.44. [DOI] [PubMed] [Google Scholar]
- 11.De Cock D., Hyrich K. Malignancy and rheumatoid arthritis: Epidemiology, risk factors and management. Best Pract. Res. Clin. Rheumatol. 2018;32:869–886. doi: 10.1016/j.berh.2019.03.011. [DOI] [PubMed] [Google Scholar]
- 12.Murata M. Inflammation and cancer. Environ. Health Prev. Med. 2018;23:50. doi: 10.1186/s12199-018-0740-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shimizu T. The Role of Macrophage Migration Inhibitory Factor (MIF) in Ultraviolet Radiation-Induced Carcinogenesis. Cancers. 2010;2:1555–1564. doi: 10.3390/cancers2031555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Plummer M., de Martel C., Vignat J., Ferlay J., Bray F., Franceschi S. Global burden of cancers attributable to infections in 2012: a synthetic analysis. Lancet Glob Health. 2016;4:609–616. doi: 10.1016/S2214-109X(16)30143-7. [DOI] [PubMed] [Google Scholar]
- 15.Barber G.N. STING: infection, inflammation and cancer. Nat. Rev. Immunol. 2015;15:760–770. doi: 10.1038/nri3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tebbutt N.C., Giraud A.S., Inglese M., Jenkins B., Waring P., Clay F.J., Malki S., Alderman B.M., Grail D., Hollande F., Heath J.K., Ernst M. Reciprocal regulation of gastrointestinal homeostasis by SHP2 and STAT-mediated trefoil gene activation in gp130 mutant mice. Nat. Med. 2002;8:1089–1097. doi: 10.1038/nm763. [DOI] [PubMed] [Google Scholar]
- 17.Yang J.D., Hainaut P., Gores G.J., Amadou A., Plymoth A., Roberts L.R. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat. Rev. Gastroenterol. Hepatol. 2019;16:589–604. doi: 10.1038/s41575-019-0186-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murray P.G., Young L.S. An etiological role for the Epstein-Barr virus in the pathogenesis of classical Hodgkin lymphoma. Blood. 2019;134:591–596. doi: 10.1182/blood.2019000568. [DOI] [PubMed] [Google Scholar]
- 19.Khatib A.M., Siegfried G., Chrétien M., Metrakos P., Seidah N.G. Proprotein convertases in tumor progression and malignancy: novel targets in cancer therapy. Am. J. Pathol. 2002;160:1921–1935. doi: 10.1016/S0002-9440(10)61140-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bontemps Y., Lapierre M., Siegfried G., Calvo F., Khatib A.M. Inhibitory feature of the proprotein convertases prosegments. Med Chem. 2008;4:116–120. doi: 10.2174/157340608783789176. [DOI] [PubMed] [Google Scholar]
- 21.Seidah N.G., Benjannet S., Wickham L., Marcinkiewicz J., Jasmin S.B., Stifani S., Basak A., Prat A., Chretien M. The secretory proprotein convertase neural apoptosis-regulated convertase 1 (NARC-1): liver regeneration and neuronal differentiation. Proc Natl Acad Sci U S A. 2003;100:928–933. doi: 10.1073/pnas.0335507100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seidah N.G., Mowla S.J., Hamelin J., Mamarbachi A.M., Benjannet S., Touré B.B., Basak A., Munzer J.S., Marcinkiewicz J., Zhong M., Barale J.C., Lazure C., Murphy R.A., Chrétien M., Marcinkiewicz M. Mammalian subtilisin/kexin isozyme SKI-1: A widely expressed proprotein convertase with a unique cleavage specificity and cellular localization. Proc Natl Acad Sci U S A. 1999;96:1321–1326. doi: 10.1073/pnas.96.4.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Naureckiene S., Ma L., Sreekumar K., Purandare U., Lo C.F., Huang Y., Chiang L.W., Grenier J.M., Ozenberger B.A., Jacobsen J.S., Kennedy J.D., DiStefano P.S., Wood A., Bingham B. Functional characterization of Narc 1, a novel proteinase related to proteinase K. Arch. Biochem. Biophys. 2003;420:55–67. doi: 10.1016/j.abb.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 24.Scamuffa N., Calvo F., Chrétien M., Seidah N.G., Khatib A.M. Proprotein convertases: lessons from knockouts. FASEB J. 2006;20:1954–1963. doi: 10.1096/fj.05-5491rev. [DOI] [PubMed] [Google Scholar]
- 25.Wetsel W.C., Rodriguiz R.M., Guillemot J., Rousselet E., Essalmani R., Kim I.H., Bryant J.C., Marcinkiewicz J., Desjardins R., Day R., Constam D.B., Prat A., Seidah N.G. Disruption of the expression of the proprotein convertase PC7 reduces BDNF production and affects learning and memory in mice. Proc Natl Acad Sci U S A. 2013;110:17362–17367. doi: 10.1073/pnas.1314698110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lahlil R., Calvo F., Khatib A.M. The potential anti-tumorigenic and anti- metastatic side of the proprotein convertases inhibitors. Recent Pat Anticancer Drug Discov. 2009;4:83–91. doi: 10.2174/157489209787002470. [DOI] [PubMed] [Google Scholar]
- 27.Couture F., Kwiatkowska A., Dory Y.L., Day R. Therapeutic uses of furin and its inhibitors: a patent review. Expert Opin Ther Pat. 2015;25:379–396. doi: 10.1517/13543776.2014.1000303. [DOI] [PubMed] [Google Scholar]
- 28.Couture F., Sabbagh R., Kwiatkowska A., Desjardins R., Guay S.P., Bouchard L., Day R. PACE4 undergoes an oncogenic alternative splicing switch in cancer. Cancer Res. 2017;77:6863–6879. doi: 10.1158/0008-5472.CAN-17-1397. [DOI] [PubMed] [Google Scholar]
- 29.Creemers J.W., Khatib A.M. Knock-out mouse models of proprotein convertases: unique functions or redundancy? Front. Biosci. 2008;13:4960–4971. doi: 10.2741/3055. [DOI] [PubMed] [Google Scholar]
- 30.Declercq J., Brouwers B., Pruniau V.P., Stijnen P., Tuand K., Meulemans S., Prat A., Seidah N.G., Khatib A.M., Creemers J.W. Liver-specific inactivation of the proprotein convertase FURIN leads to increased hepatocellular carcinoma growth. BioMed Res. Int. 2015;2015:148651. doi: 10.1155/2015/148651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bassi D.E., Mahloogi H., Lopez De Cicco R., Klein-Szanto A. Increased furin activity enhances the malignant phenotype of human head and neck cancer cells. Am. J. Pathol. 2003;162:439–447. doi: 10.1016/s0002-9440(10)63838-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.N. Scamuffa, G. Siegfried, Y. Bontemps, L. Ma, A. Basak, G. Cherel, F. Calvo, N.G. Seidah, and A.M. Khatib. Selective inhibition of proprotein convertases represses the metastatic potential of human colorectal tumor cells. J. Clin. Investig.. 118 (20081) 352-363. DOI: 10.1172/JCI32040. [DOI] [PMC free article] [PubMed]
- 33.Siegfried G., Basak A., Cromlish J., Benjannet S., Marcinkiewicz J., Chrétien M., Seidah N.G., Khatib A.M. The secretory proprotein convertases furin, PC5, and PC7 activate VEGF-C to induce tumorigenesis. J. Clin. Investig. 2003;111:1723–1732. doi: 10.1172/JCI17220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Siegfried G., Khatib A.M., Benjannet S., Chrétien M., Seidah N.G. The proteolytic processing of pro-platelet-derived growth factor-A at RRKR(86) by members of the proprotein convertase family is functionally correlated to platelet-derived growth factor-A-induced functions and tumorigenicity. Cancer Res. 2003;63:1458–1463. PMID:12670890. [PubMed] [Google Scholar]
- 35.Khatib A.M., Siegfried G., Prat A., Luis J., Chrétien M., Metrakos P., Seidah N.G. Inhibition of proprotein convertases is associated with loss of growth and tumorigenicity of HT-29 human colon carcinoma cells: importance of insulin-like growth factor-1 (IGF-1) receptor processing in IGF-1-mediated functions. J. Biol. Chem. 2001;276:30686–30693. doi: 10.1074/jbc.M101725200. [DOI] [PubMed] [Google Scholar]
- 36.Sfaxi F., Scamuffa N., Lalou C., Ma J., Metrakos P., Siegfried G., Ragg H., Bikfalvi A., Calvo F., Khatib A.M. Repression of liver colorectal metastasis by the serpin Spn4A a naturally occurring inhibitor of the constitutive secretory proprotein convertases. Oncotarget. 2014:4195–4210. doi: 10.18632/oncotarget.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tomé M., Pappalardo A., Soulet F., López J.J., Olaizola J., Leger Y., Dubreuil M., Mouchard A., Fessart D., Delom F., Pitard V., Bechade D., Fonck M., Rosado J., Ghiringhelli F., Déchanet-Merville J., Soubeyran I., Siegfried G., Evrard S., Khatib A.M. Inactivation of proprotein convertases in T cells inhibits PD-1 expression and creates a favorable immune microenvironment in colorectal cancer. Cancer Res. 2019;79:5008–5021. doi: 10.1158/0008-5472.CAN-19-0086. [DOI] [PubMed] [Google Scholar]
- 38.Lapierre M., Siegfried G., Scamuffa N., Bontemps Y., Calvo F., Seidah N.G., Khatib A.M. Opposing function of the proprotein convertases furin and PACE4 on breast cancer cells' malignant phenotypes: role of tissue inhibitors of metalloproteinase-1. Cancer Res. 2007;67:9030–9034. doi: 10.1158/0008-5472.CAN-07-0807. [DOI] [PubMed] [Google Scholar]
- 39.Siegfried G., Basak A., Prichett-Pejic W., Scamuffa N., Ma L., Benjannet S., Veinot J.P., Calvo F., Seidah N., Khatib A.M. Regulation of the stepwise proteolytic cleavage and secretion of PDGF-B by the proprotein convertases. Oncogene. 2005;24:6925–6935. doi: 10.1038/sj.onc.1208838. [DOI] [PubMed] [Google Scholar]
- 40.D'Anjou F., Routhier S., Perreault J.P., Latil A., Bonnel D., Fournier I., Salzet M., Day R. Molecular validation of PACE4 as a target in prostate cancer. Transl Oncol. 2011;4:157–1572. doi: 10.1593/tlo.10295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ghisoli M., Barve M., Mennel R., Lenarsky C., Horvath S., Wallraven G., Pappen B.O., Whiting S., Rao D., Senzer N., Nemunaitis J. Three-year Follow up of GMCSF/bi-shRNA(furin) DNA- transfected Autologous Tumor Immunotherapy (Vigil) in Metastatic Advanced Ewing's Sarcoma. Mol. Ther. 2016;24:1478–1483. doi: 10.1038/mt.2016.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ayoubi T.A., Creemers J.W., Roebroek A.J., Van de Ven W.J. Expression of the dibasic proprotein processing enzyme furin is directed by multiple promoters. J. Biol. Chem. 1994;269:9298–9303. PMID:8132667 HighWire - PDF. [PubMed] [Google Scholar]
- 43.Blanchette F., Rudd P., Grondin F., Attisano L., Dubois C.M. Involvement of Smads in TGFbeta1-induced furin (fur) transcription. J. Cell. Physiol. 2001;188:264–273. doi: 10.1002/jcp.1116. [DOI] [PubMed] [Google Scholar]
- 44.Bando M., Matsuoka A., Tsuji A., Matsuda Y. The proprotein convertase PACE4 is upregulated by PDGF-BB in megakaryocytes: gene expression of PACE4 and furin is regulated differently in Dami cells. J. Biochem. 2002;132:127–134. doi: 10.1093/oxfordjournals.jbchem.a003189. [DOI] [PubMed] [Google Scholar]
- 45.Ekbom A., Helmick C., Zack M., Adami H.O. Ulcerative colitis and colorectal cancer. A population-based study. N Engl J Med. 1990;323:1228–1233. doi: 10.1056/NEJM199011013231802. [DOI] [PubMed] [Google Scholar]
- 46.Nielsen O.H. New strategies for treatment of inflammatory bowel disease. Front. Med. 2014;1:3. doi: 10.3389/fmed.2014.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sedger L.M., McDermott M.F. TNF and TNF-receptors: From mediators of cell death and inflammation to therapeutic giants - past, present and future. Cytokine Growth Factor Rev. 2014;25:453–472. doi: 10.1016/j.cytogfr.2014.07.016. [DOI] [PubMed] [Google Scholar]
- 48.Kumar V., Behera R., Lohite K., Karnik S., Kundu G.C. p38 kinase is crucial for osteopontin-induced furin expression that supports cervical cancer progression. Cancer Res. 2010;70:10381–10391. doi: 10.1158/0008-5472.CAN-10-1470. [DOI] [PubMed] [Google Scholar]
- 49.Mocellin S., Rossi C.R., Pilati P., Nitti D. Tumor necrosis factor, cancer and anticancer therapy. Cytokine Growth Factor Rev. 2005;16:35–53. doi: 10.1016/j.cytogfr.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 50.Moore R.J., Owens D.M., Stamp G., Arnott C., Burke F., East N., Holdsworth H., Turner L., Rollins B., Pasparakis M., Kollias G., Balkwill F. Mice deficient in tumor necrosis factor-alpha are resistant to skin carcinogenesis. Nat. Med. 1999;5:828–831. doi: 10.1038/10552. [DOI] [PubMed] [Google Scholar]
- 51.Szlosarek P., Charles K.A., Balkwill F.R. Tumour necrosis factor-alpha as a tumour promoter. Eur. J. Cancer. 2006;42:745–750. doi: 10.1016/j.ejca.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 52.Szlosarek P.W., Balkwill F.R. Tumour necrosis factor alpha: a potential target for the therapy of solid tumours. Lancet Oncol. 2003;4:565–573. doi: 10.1016/s1470-2045(03)01196-3. [DOI] [PubMed] [Google Scholar]
- 53.Black R.A., Rauch C.T., Kozlosky C.J., Peschon J.J., Slack J.L., Wolfson M.F., Castner B.J., Stocking K.L., Reddy P., Srinivasan S., Nelson N., Boiani N., Schooley K.A., Gerhart M., Davis R., Fitzner J.N., Johnson R.S., Paxton R.J., March C.J., Cerretti D.P. A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature. 1997;385:729–733. doi: 10.1038/385729a0. [DOI] [PubMed] [Google Scholar]
- 54.Srour N., Lebel A., McMahon S., Fournier I., Fugère M., Day R., Dubois C.M. TACE/ADAM-17 maturation and activation of sheddase activity require proprotein convertase activity. FEBS Lett. 2003;554:275–283. doi: 10.1016/s0014-5793(03)01159-1. [DOI] [PubMed] [Google Scholar]
- 55.Singh I., Pahan K., Khan M., Singh A.K. Cytokine-mediated induction of ceramide production is redox- sensitive. Implications to proinflammatory cytokine-mediated apoptosis in demyelinating diseases. J. Biol. Chem. 1998;273:20354–20362. doi: 10.1074/jbc.273.32.20354. [DOI] [PubMed] [Google Scholar]
- 56.Chicheportiche Y., Bourdon P.R., Xu H., Hsu Y.M., Scott H., Hession C., Garcia I., Browning J.L. TWEAK, a new secreted ligand in the tumor necrosis factor family that weakly induces apoptosis. J. Biol. Chem. 1997;272:32401–32410. doi: 10.1074/jbc.272.51.32401. [DOI] [PubMed] [Google Scholar]
- 57.Daridon C., Youinou P., Pers J.O. BAFF, APRIL, TWE-PRIL: who's who? Autoimmun. Rev. 2008;7:267–271. doi: 10.1016/j.autrev.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 58.McMahon S., Charbonneau M., Grandmont S., Richard D.E., Dubois C.M. Transforming growth factor beta1 induces hypoxia-inducible factor-1 stabilization through selective inhibition of PHD2 expression. J. Biol. Chem. 2006;281:24171–24181. doi: 10.1074/jbc.M604507200. [DOI] [PubMed] [Google Scholar]
- 59.Bierie B., Moses H.L. Tumour microenvironment: TGFbeta: the molecular Jekyll and Hyde of cancer. Nat. Rev. Cancer. 2006;6:506–520. doi: 10.1038/nrc1926. [DOI] [PubMed] [Google Scholar]
- 60.Massague J. TGFbeta signalling in context. Nat. Rev. Mol. Cell Biol. 2012;13:616–630. doi: 10.1038/nrm3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Miyazono K., Ichijo H., Heldin C.H. Transforming growth factor-beta: latent forms, binding proteins and receptors. Growth Factors. 1993;8:11–22. doi: 10.3109/08977199309029130. [DOI] [PubMed] [Google Scholar]
- 62.Khalil N. TGF-beta: from latent to active. Microb. Infect. 1999;1:1255–1263. doi: 10.1016/s1286-4579(99)00259-2. [DOI] [PubMed] [Google Scholar]
- 63.Hagiwara S., Murakumo Y., Mii S., Shigetomi T., Yamamoto N., Furue H., Ueda M., Takahashi M. Processing of CD109 by furin and its role in the regulation of TGF-beta signaling. Oncogene. 2010;29:2181–2191. doi: 10.1038/onc.2009.506. [DOI] [PubMed] [Google Scholar]
- 64.Rolink A.G., Tschopp J., Schneider P., Melchers F. BAFF is a survival and maturation factor for mouse B cells. Eur. J. Immunol. 2002;32:2004–2010. doi: 10.1002/1521-4141(200207)32:7<2004::AID-IMMU2004>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 65.Mariette X., Roux S., Zhang J., Bengoufa D., Lavie F., Zhou T., Kimberly R. The level of BLyS (BAFF) correlates with the titre of autoantibodies in human Sjogren's syndrome. Ann. Rheum. Dis. 2003;62:168–171. doi: 10.1136/ard.62.2.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schneider P., MacKay F., Steiner V., Hofmann K., Bodmer J.L., Holler N., Ambrose C., Lawton P., Bixler S., Acha-Orbea H., Valmori D., Romero P., Werner-Favre C., Zubler R.H., Browning J.L., Tschopp J. BAFF, a novel ligand of the tumor necrosis factor family, stimulates B cell growth. J. Exp. Med. 1999;189:1747–1756. doi: 10.1084/jem.189.11.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Assi L.K., Wong S.H., Ludwig A., Raza K., Gordon C., Salmon M., Lord J.M., Scheel-Toellner D. Tumor necrosis factor alpha activates release of B lymphocyte stimulator by neutrophils infiltrating the rheumatoid joint. Arthritis Rheum. 2007;56:1776–1786. doi: 10.1002/art.22697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Thompson C., McMahon S., Bossé Y., Dubois C.M., Stankova J., Rola-Pleszczynski M. Leukotriene D4 up-regulates furin expression through CysLT1 receptor signaling. Am. J. Respir. Cell Mol. Biol. 2008;39:227–234. doi: 10.1165/rcmb.2007-0293OC. [DOI] [PubMed] [Google Scholar]
- 69.López-Fraga M., Fernández R., Albar J.P., Hahne M. Biologically active APRIL is secreted following intracellular processing in the Golgi apparatus by furin convertase. EMBO Rep. 2001;2:945–951. doi: 10.1093/embo-reports/kve198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rossi D., Zlotnik A. The biology of chemokines and their receptors. Annu Rev Immunol. 2000;18:217–242. doi: 10.1146/annurev.immunol.18.1.217. [DOI] [PubMed] [Google Scholar]
- 71.Hughes C.E., Nibbs R.J.B. A guide to chemokines and their receptors. FEBS J. 2018;285:2944–2971. doi: 10.1111/febs.14466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hensbergen P.J., Verzijl D., Balog C.I., Dijkman R., van der Schors R.C., van der Raaij-Helmer E.M., van der Plas M.J., Leurs R., Deelder A.M., Smit M.J., Tensen C.P. Furin is a chemokine-modifying enzyme: in vitro and in vivo processing of CXCL10 generates a C-terminally truncated chemokine retaining full activity. J. Biol. Chem. 2004;279:13402–13411. doi: 10.1074/jbc.M312814200. [DOI] [PubMed] [Google Scholar]
- 73.Campanella G.S., Lee E.M., Sun J., Luster A.D. CXCR3 and heparin binding sites of the chemokine IP-10 (CXCL10) J. Biol. Chem. 2003;278:17066–17074. doi: 10.1074/jbc.M212077200. [DOI] [PubMed] [Google Scholar]
- 74.Zhang K., McQuibban G.A., Silva C., Butler G.S., Johnston J.B., Holden J., Clark-Lewis I., Overall C.M., Power C. HIV-induced metalloproteinase processing of the chemokine stromal cell derived factor-1 causes neurodegeneration. Nat. Neurosci. 2003;6:1064–1071. doi: 10.1038/nn1127. [DOI] [PubMed] [Google Scholar]
- 75.Van den Steen P.E., Proost P., Wuyts A., Van Damme J., Opdenakker G. Neutrophil gelatinase B potentiates interleukin-8 tenfold by aminoterminal processing, whereas it degrades CTAP-III, PF-4, and GRO-alpha and leaves RANTES and MCP-2 intact. Blood. 2000;96:2673–2681. PMID: 11023497. [PubMed] [Google Scholar]
- 76.McQuibban G.A., Gong J.H., Tam E.M., McCulloch C.A., Clark-Lewis I., Overall C.M. Inflammation dampened by gelatinase A cleavage of monocyte chemoattractant protein-3. Science. 2000;289:1202–1206. doi: 10.1126/science.289.5482.1202. [DOI] [PubMed] [Google Scholar]
- 77.Wolf M., Clark-Lewis I., Buri C., Langen H., Lis M., Mazzucchelli L. Cathepsin D specifically cleaves the chemokines macrophage inflammatory protein-1 alpha, macrophage inflammatory protein-1 beta, and SLC that are expressed in human breast cancer. Am. J. Pathol. 2003;162:1183–1190. doi: 10.1016/s0002-9440(10)63914-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vakili J., Ständker L., Detheux M., Vassart G., Forssmann W.G., Parmentier M. Urokinase plasminogen activator and plasmin efficiently convert hemofiltrate CC chemokine 1 into its active. J. Immunol. 2001;167:3406–3413. doi: 10.4049/jimmunol.167.6.3406. [DOI] [PubMed] [Google Scholar]
- 79.Sebastiani G., Wilkinson N., Pantopoulos K. Pharmacological targeting of the hepcidin/ferroportin Axis. Front. Pharmacol. 2016;7:160. doi: 10.3389/fphar.2016.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nemeth E., Valore E.V., Territo M., Schiller G., Lichtenstein A., Ganz T. Hepcidin, a putative mediator of anemia of inflammation, is a type II acute-phase protein. Blood. 2003;101:2461–2463. doi: 10.1182/blood-2002-10-3235. [DOI] [PubMed] [Google Scholar]
- 81.Vela D., Vela-Gaxha Z. Differential regulation of hepcidin in cancer and non- cancer tissues and its clinical implications. Exp. Mol. Med. 2018;50:436. doi: 10.1038/emm.2017.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Scamuffa N., Basak A., Lalou C., Wargnier A., Marcinkiewicz J., Siegfried G., Chrétien M., Calvo F., Seidah N.G., Khatib A.M. Regulation of prohepcidin processing and activity by the subtilisin-like proprotein convertases Furin, PC5, PACE4 and PC7. Gut. 2008;57:1573–1582. doi: 10.1136/gut.2007.141812. [DOI] [PubMed] [Google Scholar]
- 83.De Domenico I., Ward D.M., Kaplan J. Hepcidin regulation: ironing out the details. J. Clin. Investig. 2007;117:1755–1758. doi: 10.1172/JCI32701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Silva B., Faustino P. An overview of molecular basis of iron metabolism regulation and the associated pathologies. Biochim. Biophys. Acta. 2015;1852:1347–1359. doi: 10.1016/j.bbadis.2015.03.011. [DOI] [PubMed] [Google Scholar]
- 85.Silvestri L., Pagani A., Camaschella C. Furin-mediated release of soluble hemojuvelin: a new link between hypoxia and iron homeostasis. Blood. 2008;111:924–931. doi: 10.1182/blood-2007-07-100677. [DOI] [PubMed] [Google Scholar]
- 86.Bazan H.E., Tao Y., Bazan N.G. Platelet-activating factor induces collagenase expression in corneal epithelial cells. Proc. Natl. Acad. Sci. U. S. A. 1993;90:8678–8682. doi: 10.1073/pnas.90.18.8678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Berraondo P., Etxeberria I., Ponz-Sarvise M., Melero I. Revisiting interleukin-12 as a cancer immunotherapy agent. Clin. Cancer Res. 2018;24:2716–2718. doi: 10.1158/1078-0432.CCR-18-0381. [DOI] [PubMed] [Google Scholar]
- 88.McMahon S., Grondin F., McDonald P.P., Richard D.E., Dubois C.M. Hypoxia-enhanced expression of the proprotein convertase furin is mediated by hypoxia-inducible factor-1: impact on the bioactivation of proproteins. J Biol Chem. 2005;280:6561–6569. doi: 10.1074/jbc.M413248200. [DOI] [PubMed] [Google Scholar]
- 89.Ma J., Evrard S., Badiola I., Siegfried G., Khatib A.M. Regulation of the proprotein convertases expression and activity during regenerative angiogenesis: Role of hypoxia-inducible factor (HIF) Eur. J. Cell Biol. 2017;96:457–468. doi: 10.1016/j.ejcb.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 90.Einspahr J.G., Stratton S.P., Bowden G.T., Alberts D.S. Chemoprevention of human skin cancer. Crit Rev Oncol Hematol. 2002;41:269–285. doi: 10.1016/s1040-8428(01)00185-8. [DOI] [PubMed] [Google Scholar]
- 91.Skiba B., Neill B., Piva T.J. Gene expression profiles of TNF-alpha, TACE, furin, IL-1beta and matrilysin in UVA- and UVB-irradiated HaCat cells. Photodermatol Photoimmunol Photomed. 2005;21:173–182. doi: 10.1111/j.1600-0781.2005.00162.x. [DOI] [PubMed] [Google Scholar]
- 92.Köck A., Schwarz T., Kirnbauer R., Urbanski A., Perry P., Ansel J.C., Luger T.A. Human keratinocytes are a source for tumor necrosis factor alpha: evidence for synthesis and release upon stimulation with endotoxin or ultraviolet light. J Exp Med. 1990;172:1609–1614. doi: 10.1084/jem.172.6.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schwarz T. Photoimmunosuppression. Photodermatol Photoimmunol Photomed. 2002;18:141–145. doi: 10.1034/j.1600-0781.2002.180307.x. [DOI] [PubMed] [Google Scholar]
- 94.Black R.A. Tumor necrosis factor-alpha converting enzyme. Int. J. Biochem. Cell Biol. 2002;34:1–5. doi: 10.1016/s1357-2725(01)00097-8. [DOI] [PubMed] [Google Scholar]
- 95.Pearton D.J., Nirunsuksiri W., Rehemtulla A., Lewis S.P., Presland R.B., Dale B.A. Proprotein convertase expression and localization in epidermis: evidence for multiple roles and substrates. Exp. Dermatol. 2001;10:193–203. doi: 10.1034/j.1600-0625.2001.010003193.x. [DOI] [PubMed] [Google Scholar]
- 96.Tanaka M., Itai T., Adachi M., Nagata S. Downregulation of Fas ligand by shedding. Nat. Med. 1998;4:31–36. doi: 10.1038/nm0198-031. [DOI] [PubMed] [Google Scholar]
- 97.Bazzoni F., Beutler B. The tumor necrosis factor ligand and receptor families. N Engl J Med. 1996;334:1717–1725. doi: 10.1056/NEJM199606273342607. [DOI] [PubMed] [Google Scholar]
- 98.Yamada A., Komaki Y., Patel N., Komaki F., Aelvoet A.S., Tran A.L., Pekow J., Dalal S., Cohen R.D., Cannon L., Umanskiy K., Smith R., Hurst R., Hyman N., Rubin D.T., Sakuraba A. Risk of postoperative complications among inflammatory bowel disease patients treated preoperatively with vedolizumab. Am. J. Gastroenterol. 2017;112:1423–1429. doi: 10.1038/ajg.2017.201. [DOI] [PubMed] [Google Scholar]
- 99.Nagahiro I., Andou A., Aoe M., Sano Y., Date H., Shimizu N. Pulmonary function, postoperative pain, and serum cytokine level after lobectomy: a comparison of VATS and conventional procedure. Ann. Thorac. Surg. 2001;72:362–365. doi: 10.1016/s0003-4975(01)02804-1. [DOI] [PubMed] [Google Scholar]
- 100.Wang N., Thuraisingam T., Fallavollita L., Ding A., Radzioch D., Brodt P. The secretory leukocyte protease inhibitor is a type 1 insulin-like growth factor receptor-regulated protein that protects against liver metastasis by attenuating the host proinflammatory response. Cancer Res. 2006;66:3062–3070. doi: 10.1158/0008-5472.CAN-05-2638. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.