Abstract
Mastocytosis is a unique hematologic neoplasm with complex biology and pathology and a variable clinical course. The disease can essentially be divided into cutaneous mastocytosis (CM) and systemic mastocytosis (SM). In adults, SM is diagnosed in most cases and manifests as either indolent or advanced disease. Patients with advanced SM have an unfavorable prognosis with reduced survival. However, so far, little is known about the prevalence of various categories of SM and about prognostic factors. In an attempt to learn more about the behavior and evolution of various forms of CM and SM, the European Competence Network on Mastocytosis (ECNM) initiated a mastocytosis registry in 2012. In this article, the set up and start phase of this registry are described. Until 2018, more than 3000 patients from 12 countries and 25 centers have been enrolled. In a majority of all patients, robust follow-up data and relevant clinical end points are available. Using this data set, a series of registry projects have been launched, with the aim to validate previously identified diagnostic and prognostic variables and to identify new disease-related and patient-related parameters in various forms of mastocytosis. Moreover, the core data set of the registry will be useful to establish multiparametric scoring systems through which prognostication and individualized management of patients with mastocytosis should improve in the foreseeable future.
Keywords: Mastocytosis, Prognosis, WHO classification, Diagnostic criteria, Therapy
Introduction
Mastocytosis is a rare and complex disease characterized by abnormal expansion and accumulation of clonal mast cells (MCs) in various organs and tissue-sites.1–10 Pure cutaneous mastocytosis (CM) is usually detected in (early) childhood. The cutaneous lesions in these patients often disappear spontaneously before adolescence.7–11 In contrast, most adult patients with cutaneous lesions suffer from systemic mastocytosis (SM), a persistent neoplastic disease that usually involves multiple organs, including the skin, bone marrow (BM), liver, spleen, and/or the gastrointestinal tract.1–10 Although the BM is almost always affected in patients with SM, skin lesions may be absent. In these cases, it may take some time until the diagnosis of SM is established.
According to the criteria of the World Health Organization (WHO), indolent and advanced forms of SM have been defined.9,10,12–16 In most patients with indolent SM (ISM), the burden of neoplastic MCs is relatively low. Most patients with ISM present with skin lesions and have slightly or moderately elevated serum tryptase levels. When cutaneous lesions are absent, BM mastocytosis may be diagnosed, provided that no signs for an advanced systemic disease producing organ damage (= C-Findings) are recorded. Smoldering SM is defined by a huge burden of MCs, multilineage involvement, signs of myelodysplasia or myeloproliferation (B-Findings), and absence of C-Findings. Advanced SM includes aggressive SM (ASM), MC leukemia (MCL), and SM with an associated hematologic (non-MC) neoplasm (SM-AHN).1–8 MC sarcoma is an extremely rare local malignant MC disease.2–5,9 Many of these cases progress to MCL.9
The WHO classification is of prognostic significance concerning survival. In particular, patients with advanced SM (ASM, SM-AHN, MCL) have a poor overall survival compared with patients with CM or ISM.13–19
The diagnosis of mastocytosis is established in a step-wise approach using WHO criteria.1–3,13–16 In a first step, the patient is examined for the presence of major and minor criteria of SM. When at least 1 major and 1 minor or at least 3 minor criteria are fulfilled, the diagnosis of SM is established.1–3,13–16 The major criterion is the multifocal MC infiltrate in the BM or in another extracutaneous organ system and is assessed by histology and immunohistochemistry.10–16 Minor SM criteria relate to the atypical morphology of MC (spindle-shaped cells by histology and/or cytology), their immunological phenotype (aberrant expression of CD2 and, more frequently, of CD25), the presence of an activating KIT mutation in codon 816, and a clearly elevated serum tryptase level (>20 ng/mL).10–16
To diagnose an advanced form of mastocytosis, the presence of C-Findings (reflecting organ damage induced by MC infiltrates), evidence of MCL (leukemic expansion), or the presence of an AHN have to be documented (Table I).10–16 One or more C-Findings are sufficient to diagnose ASM.10–16 In patients in whom MCs represent more than 20% of all nucleated cells in peripheral blood or BM smears, the diagnosis MCL is appropriate. It is of particular importance to know that organ damage counts as a C-Finding only when produced by a local MC infiltrate (biopsy-based diagnosis).10–16 In case of suspected AHN, further markers and WHO criteria defining the nature and variant of the AHN have to be applied. In these patients, both the SM component and the AHN component of the neoplasm need to be defined and reported.13–16
Table I. Subvariants of advanced mastocytosis with key diagnostic features (criteria).
| Variant of advanced mastocytosis | |
|---|---|
| ASM | Criteria to diagnose SM (SM criteria) fulfilled, plus: at least 1 C-Finding (SM-induced relevant organ damage) documented (by biopsy), plus: criteria for SM-AHN or MCL not fulfilled |
| ASM-t | As for ASM, plus: MCs in BM smears* are 5%-19% of all nucleated cells |
| MCL | MCs in BM smears* are ≥20% of all nucleated BM cells |
| Aleukemic MCL | As for MCL, plus circulating MCs on PB smears are below 10% |
| Acute MCL | As for MCL, plus: organ damage caused by MCL has been documented (C-Finding(s) found) |
| Chronic MCL | As for MCL, plus: organ damage caused by MCL (C-Finding[s]) not found/documented |
| Primary MCL | As for MCL, plus: no preceding myeloid neoplasm and no preceding SM is known |
| Secondary MCL | As for MCL, plus: a preceding myeloid neoplasm/preceding SM is known |
| MCS | A local aggressive MC tumor is found, but criteria to diagnose SM/MCL are not fulfilled |
| SM-AHN† | The diagnostic criteria for SM are fulfilled, and the diagnostic criteria for a concomitant, non-MC lineage, hematologic neoplasm are also met |
ASM-t, ASM in transformation to MCL; PB, peripheral blood; MCS, mast cell sarcoma; SM-AHN, SM with associated hematologic neoplasm.
Diagnostic evaluation of the main MCL criterion is strictly bound to the BM smear, but cannot be performed by histopathological or immunohistochemical studies.
In these patients, the variant (and subvariant if applicable) of the SM and of the AHN have to be established according to WHO criteria.
The natural clinical course of mastocytosis is variable, depending on age, organs involved, subtype of disease, and other coexisting disorders, such as an IgE-dependent allergy or a preexisting osteopathy.4–8 In a vast majority of cases with pediatric CM, the disease resolves spontaneously before adolescence. Patients with CM and ISM have a normal life expectancy, whereas patients with advanced SM have a grave prognosis with reduced survival.4–8,10–19 However, the outcome of individual patients varies, even within a defined WHO category of SM, and the individual courses of the patients are unpredictable. In addition, in advanced SM, the responses to interventional treatments vary greatly among patients, ranging from resistant disease to complete and stable long-term remission.
To date, the WHO classification serves as an accepted global gold standard for prognostication in SM. Additional disease-related factors may also be of prognostic significance. However, little is known about such additional prognostic variables that may be useful for estimating survival and progression in SM. In addition, only little is known about prognostic factors that could be used to predict responses to various therapies in SM. Finally, very little is known about the incidence and prevalence of CM and SM in various regions and countries worldwide.
In 2002, the European Competence Network on Mastocytosis (ECNM) was established and then was rolled out in several European countries.20,21 In 2012, members of the ECNM established the ECNM registry, with the aim to better study basic clinical and laboratory variables and prognostic parameters in patients with CM and SM. In the current article, we describe the aims, the setup, and the start phase of this registry.
The ECNM: Historical Overview and Basic Structure
Between 1990 and 2000, European groups working in the field of MC research and mastocytosis research initiated a number of new collaborations and multicenter studies, with the aim to identify disease-specific diagnostic and prognostic parameters.22–27 The involved experts met regularly to discuss new developments in the field and novel disease-related parameters. In several of these meetings, smaller or larger series of cases with mastocytosis (BM slides and other diagnostic material) were reviewed to define the diagnostic and/or prognostic value of the proposed parameters. In several instances, experts from the United States also joined in these meetings. The most important and influential meeting was the Year 2000 Working Conference on Mastocytosis in Vienna.13 In this conference, diagnostic criteria for CM and SM were discussed and approved, and the resulting consensus classification was presented to the WHO.13 After further discussion, the concept was adopted by the WHO and since then serves as the official WHO classification of mastocytosis.14–16
In 2002, the ECNM was founded to maintain the collaborative multicenter activities in the field of mastocytosis in Europe and to further support observational studies and clinical trials as well as the WHO in the field of mastocytosis.20,21 The ECNM is a nonprofit-based consortium of physicians and researchers in Europe and is essentially based on centers of excellence and reference centers. Centers of excellence offer a broad range of diagnostic facilities and therapeutic options. In contrast, reference centers focus on a certain aspect of the disease, such as pathology, flow cytometry, molecular studies, or MC morphologies. Since 2002, the network group has organized “Annual meetings of the ECNM” and a series of working conferences. In 2008 and 2016, members of the ECNM and their US colleagues assisted in the update and refinements of the WHO classification and of related criteria.15,16 The ECNM is interconnected with experts and centers in the United States through various academic collaborations, including preclinical studies and clinical trials. Moreover, the scientific advisory board of the ECNM consists of major US authorities.
Multicenter Studies and the Dilemma of a Rare Disease with Variable Clinical Course and Complex Pathology
Between 1990 and 2005, EU and US researchers conducted a number of multicenter studies, with the aim to identify new disease-related parameters in patients with mastocytosis. These studies supported the development of diagnostic criteria that were discussed in the Year 2000 Working Conference and served as the basis for the WHO classification of the disease.13–17,27 Later, the prognostic significance of the WHO classification was confirmed by several monocenter and multicenter studies, including a series of investigations performed at the Mayo Clinic.18,19 Additional prognostic factors were also identified.28–42 However, in most instances, the number of cases was too small to reach definitive conclusions. Especially in the light of the rarity of advanced mastocytosis, the clinical, immunological, functional, and genetic heterogeneity of its variants (various forms of MCL, slowly and rapidly progressing ASM, ASM in transformation to MCL, various forms of acute and chronic myeloid AHN), and the poor prognosis of these patients (survival times of only a few months to years),10,11,16,43 it became clear that only a large-scale approach capturing information from many more patients would be sufficient to address the dilemma of studying details of such a rare and complex disease. Therefore, the ECNM consortium concluded that a registry study has to be initiated with the aim to create a robust data set and to conduct larger multicenter studies. In 2008, the ECNM registry concept and the related study contract were presented to the consortium (Annual ECNM meeting in Budapest, 2008). In 2010, a test phase was completed, and first patients were enrolled in the ECNM registry in 2011.
The Registry Consortium, Data Controlling, and Data Distribution
Until 2018, a total of 25 centers from 12 countries have joined the ECNM registry. Most of these sites are centers of excellence of the ECNM.21 In addition, 1 US center of excellence (Stanford, Calif) has joined and several other US centers of excellence have expressed their interest and will join the ECNM registry in the future. This means that most ECNM registry projects will be conducted on data collected in a joint EU/US database. The ECNM registry is based on a consortium contract that defines all rules and regulations through which patient data are collected and ECNM registry projects are selected, distributed, and conducted. In addition, the contract regulates the rights of each center and the publication policy of the consortium. The database of the ECNM registry, data storage, and data distribution comply with the rules and regulations of the data protection laws, with local ethics committee regulations, and with the Declaration of Helsinki. All patients gave their written informed consent to participate before their data were enrolled in the data set. The registry study was approved by the local ethics committee of each participating center. The data of the ECNM registry are secured on the servers of the Austrian Control Bank (Österreichische Kontrollbank OeKB). The coordinators and their team supervise data entry, data consistency, and data plausibility, to ensure data quality. Data monitoring and data clearing are conducted once a year. Data and the data set files are checked for accuracy, completeness, consistency, and plausibility as well as homogeneity (cross-country, interregional as well as geographical) in the data set. In case of unexpected results, outliers, unusual outcomes, or clearly incorrect entries, official queries are generated and sent out to individual sites. After all queries are solved, the database is checked once again before the yearly data set file is generated and sent out to the principle investigators of the ECNM registry projects.
The First 6 Years: Initiation and Distribution of Registry Projects
During the first 12 months, a pilot series of patients were enrolled and initial quality control examinations were performed. After a successful test phase, the registry rapidly recruited patients into the registry (Figure 1). Since then, recruitment is ongoing and has resulted in more than 3000 cases (and more than 5500 follow-up examinations) until 2018. A large series of parameters were captured in these examinations and follow-ups, including blood and serum parameters, MC morphology and phenotype, molecular studies, comorbidities, and signs of progression. In addition, symptomatic and interventional treatment (more than 3000 in the total cohort) and treatment responses are captured in the data registry.
Figure 1.
Recruitment of patients into the ECNM registry over time. The figure shows the number of patients with mastocytosis recruited into the ECNM registry per year. Until June 2018, more than 3000 patients have been enrolled.
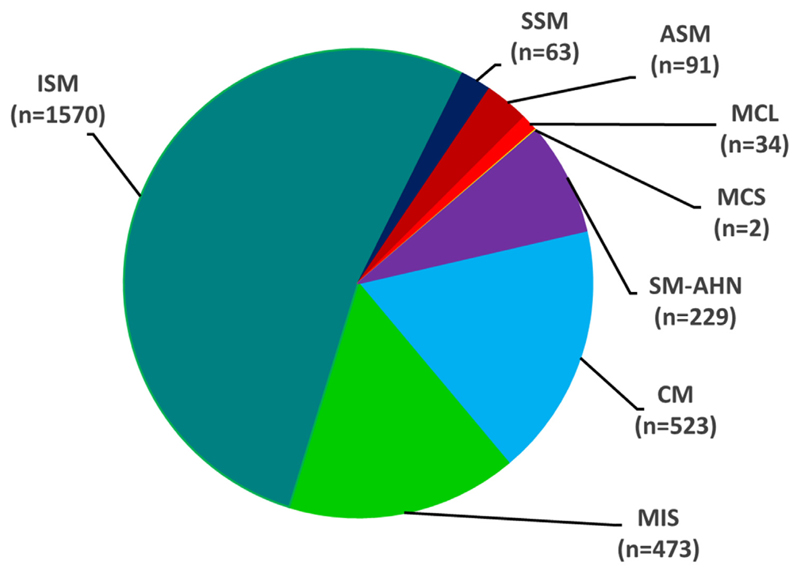
After 3 years, a first series of registry projects were defined and were then distributed among actively enrolling centers. During the following years, additional projects were formulated and until 2017, 3 series (waves) of projects with a total of 25 projects have been initiated. An overview of projects and project topics is provided in Table II. Most of these projects are expected to be finalized and published within the next couple of years. In initial calculations, the prevalence of various forms of mastocytosis and the prognostic impact of the WHO classification were confirmed. Figure 2 shows the relative distribution of patients in various categories of the disease, and Figure 3 shows the overall survival of treatment-naive patients with SM (no interventional therapies) who presented at diagnosis (1) without B- or C-Findings, (2) with 1 or more B-Finding (but no C-Findings), and (3) patients with 1 or more C-Finding (advanced SM). These data nicely confirm the prognostic impact of the WHO classification. The prognostic significance of additional, WHO-independent, parameters, such as the alkaline phosphatase level, organomegaly, or age, is currently being defined in ongoing ECNM registry projects.
Table II. Major topics and issues addressed in ECNM registry projects.
| Impact of age and age-related subgroups of patients with mastocytosis |
| Impact of allergic disorders and anaphylaxis in mastocytosis |
| Criteria and prognosis of BM mastocytosis |
| Clinical significance of serum tryptase levels at diagnosis and in the follow-up |
| Sex-related features and prognosis in patients with SM |
| Prognostic impact of LDH, alkaline phosphatase, and β 2MG in mastocytosis |
| Spectrum of mediator-related symptoms in mastocytosis and clinical significance |
| Treatment responses of patients with mastocytosis receiving interventional therapy |
| Clinical impact of skin lesion and correlation with disease variants |
| International Prognostic Scoring System for patients with mastocytosis |
| Frequency of SM in patients with mastocytosis in the skin |
| Clinical features and prognosis of patients with smoldering SM |
| Clinical impact of organomegaly in patients with SM |
| Clinical impact of the body mass index in patients with mastocytosis |
| Clinical features and courses of patients with various forms of ASM |
| Clinical course of patients with diverse variants of MCL |
| Features, clinical course, and prognosis in patients with typical indolent SM |
| Pure CM in adults: clinical features and course |
| Frequency, distribution, subtypes, and prognostic impact of AHN variants |
| Impact of bone lesions and related symptoms in patients with SM |
| Prognostic impact of the percent of MCs in BM smears vs BM histology |
| Diagnostic and prognostic impact of cytogenetic abnormalities in SM |
| Prognostic impact of the platelet counts in SM variants |
| Incidence of solid tumors and melanoma in patients with SM |
| Clinical impact of (hyper)eosinophilia and (hyper)basophilia in SM |
| Clinical efficacy of midostaurin and cladribine (2CdA) in advanced SM |
| Overview of reported comorbidities in the ECNM registry |
| Clinical features, course, and prognosis of pediatric mastocytosis |
β2MG, β2 Microglobulin; LDH, lactate dehydrogenase.
Figure 2.
Distribution of mastocytosis variants in the total patient cohort. The diagram shows the relative number of patients (N = 2985) in each diagnostic subgroup (variants of mastocytosis) collected in the ECNM registry. MCS, Mast cell sarcoma; MIS, mastocytosis in the skin; SM-AHN, SM with associated hematologic neoplasm; SSM, smoldering SM. MIS is diagnosed in adult patients with skin involvement in whom no BM investigation was performed and who may finally turn out to have SM or CM, based on a later BM biopsy. No children are included in the MIS group.
Figure 3.
Survival of patients with SM. A total of 1358 patients with SM with robust follow-up data were examined. Patients were split into SM cases without any B- or C-Findings (blue graph; n = 932), 1 or more B-Finding but no C-Findings (green graph; n = 206), and 1 or more C-Finding (red graph; n = 220). Patients with B- or C-Findings were often found to suffer from an associated hematologic neoplasm (SM-AHN). The survival estimates were established using the method of Kaplan and Meier. Survival was calculated from the date of diagnosis. Differences in survival were found to be statistically significant (P < .05).
Strategic Aims for the Next 5 Years
During the past few years, our understanding and knowledge regarding the pathogenesis, evolution, progression, and management of various forms of mastocytosis have increased substantially.7–16 At the same time, the number of (diagnostic and potential prognostic) markers and technologies also increased. Finally, the number of treatment options and drugs with documented beneficial effects in patients with mastocytosis increased.7–16 Based on these developments, management and treatment of patients with mastocytosis is currently changing from rather stringent algorithms toward a more personalized (individualized) way of management. The coordinators of the ECNM registry and the registry consortium have the plan to address these new developments in several ways. First, the ECNM registry will include a series of novel potentially diagnostic and prognostic markers (eg, genetic and molecular markers, including somatic mutation profiles) and disease-related features (eg, comorbidities, established markers of allergy, mediator-induced symptoms, markers of osteopathy and osteoporosis, or quality-of-life parameters), so that these variables can be included in forth-coming registry studies and multiparametric scoring systems. Second, the ECNM registry consortium has the plan to link the data registry of the ECNM with a robust multicentric biobank system. Third, the ECNM registry data set will be used to start a call for basic science projects aimed at defining novel mechanisms of disease progression or the occurrence of certain clinically relevant comorbidities. One special focus in these projects will be primary MC activation syndromes (MCAS) defined by severe symptoms induced by MC-derived mediators.44–48 The ECNM registry will include data on MCAS parameters and will try to capture prognostic variables and clinical end points in patients with MCAS as well as responses of patients with MCAS to various therapies. In addition, the registry consortium will establish novel, multiparameter-based score systems through which the prognosis and patient selection in various forms of mastocytosis will improve substantially. Finally, the registry will collect data on patients treated with symptomatic and/or interventional therapies, with the aim to define prognostic parameters predicting (good) responses to these treatments.
Concluding Remarks and Future Perspectives
The ECNM registry is a major tool and basis for the identification and retrospective validation of established and new diagnostic and prognostic parameters as well as potential targets in patients with mastocytosis. Moreover, based on the registry data set, improved prognostic scoring models will be established. As a result, diagnosis, prognostication, and management of patients with mastocytosis should improve in the future. Registry-based data and information should also lead to precision medicine-based selection and treatment of individual patients with this disease.
Acknowledgments
We thank all centers and experts in the ECNM registry group for contributing data and patients to the registry. In addition, we thank the scientific advisors of the ECNM for their contribution to the project-discussion. Finally, we thank all technicians, study coordinators, study nurses, and colleagues for data entry into the registry system. Our special thanks for data management and data controlling go to Susanne Herndlhofer, Nadja Jaekel, Bouktit Hassiba, Gabriele Stefanzl, Hana Škabrahová, Gulkan Ozkan, Tarik Tiryaki, Nicole Cabral do O, Deborah Christen, Anne Simonowski, Luigi Scaffidi, Cecilia Spina, Agnes Bretter-klieber, Orsolya Pilikta, Lilla Kurunci, Kerstin Hamberg Levedahl, Pietro Benvenuti, Gregor Verhoef, Peter Vandenberghe, Christine Breynaert, Dominique Bullens, Gülkan Özkan, Tarik Tiryaki, and Stephanie Pulfer.
This work was supported by the Austrian Science Fund (FWF), grant nos. F4701-B20 and F4704-B20 (to P.V.), the German Research Council (Deutsche For-schungsgemeinschaft; grant no. HA 2393/6-1 and Excellence Cluster Inflammation at Interfaces to K.H. and grant no. RA 2838 to A.I.), the University of Luebeck (to K.H.), the Koeln Fortune Program, Faculty of Medicine, University of Cologne (grant no. 216/2016 to A.I.), and the Charles and Ann Johnson Foundation (to J.G.). V.S. is a Senior Clinical Researcher of the Research Foundation Flanders/Fonds Wetenschappelijk Onderzoek (FWO: 1804518N).
Abbreviations used
- AHN
Associated hematologic neoplasm
- ASM
Aggressive systemic mastocytosis
- BM
Bone marrow
- CM
Cutaneous mastocytosis
- ECNM
European Competence Network on Mastocytosis
- ISM
Indolent systemic mastocytosis
- MC
Mast cell
- MCAS
Mast cell activation syndrome
- MCL
Mast cell leukemia
- SM
Systemic mastocytosis
- WHO
World Health Organization
Footnotes
Conflicts of interest: The authors declare that they have no relevant conflict of interest.
References
- 1.Escribano L, Akin C, Castells M, Orfao A, Metcalfe DD. Mastocytosis: current concepts in diagnosis and treatment. Ann Hematol. 2002;81:677–90. doi: 10.1007/s00277-002-0575-z. [DOI] [PubMed] [Google Scholar]
- 2.Valent P, Akin C, Sperr WR, Arock M, Lechner K, Bennett JM, et al. Diagnosis and treatment of systemic mastocytosis: state of the art. Br J Haematol. 2003;122:695–717. doi: 10.1046/j.1365-2141.2003.04575.x. [DOI] [PubMed] [Google Scholar]
- 3.Akin C, Metcalfe DD. Systemic mastocytosis. Annu Rev Med. 2004;55:419–32. doi: 10.1146/annurev.med.55.091902.103822. [DOI] [PubMed] [Google Scholar]
- 4.Valent P, Sperr WR, Schwartz LB, Horny HP. Diagnosis and classification of mast cell proliferative disorders: delineation from immunologic diseases and non-mast cell hematopoietic neoplasms. J Allergy Clin Immunol. 2004;114:3–11. doi: 10.1016/j.jaci.2004.02.045. [DOI] [PubMed] [Google Scholar]
- 5.Horny HP, Sotlar K, Valent P. Mastocytosis: state of the art. Pathobiology. 2007;74:121–32. doi: 10.1159/000101711. [DOI] [PubMed] [Google Scholar]
- 6.Metcalfe DD. Mast cells and mastocytosis. Blood. 2008;112:946–56. doi: 10.1182/blood-2007-11-078097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arock M, Valent P. Pathogenesis, classification and treatment of mastocytosis: state of the art in 2010 and future perspectives. Expert Rev Hematol. 2010;3:497–516. doi: 10.1586/ehm.10.42. [DOI] [PubMed] [Google Scholar]
- 8.Sperr WR, Valent P. Diagnosis, progression patterns and prognostication in mastocytosis. Expert Rev Hematol. 2012;5:261–74. doi: 10.1586/ehm.12.12. [DOI] [PubMed] [Google Scholar]
- 9.Monnier J, Georgin-Lavialle S, Canioni D, Lhermitte L, Soussan M, Arock M, et al. Mast cell sarcoma: new cases and literature review. Oncotarget. 2016;7:66299–309. doi: 10.18632/oncotarget.11812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Valent P, Akin C, Metcalfe DD. Mastocytosis: 2016 updated WHO classification and novel emerging treatment concepts. Blood. 2017;129:1420–7. doi: 10.1182/blood-2016-09-731893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Valent P, Akin C, Hartmann K, Nilsson G, Reiter A, Hermine O, et al. Advances in the classification and treatment of mastocytosis: current status and outlook toward the future. Cancer Res. 2017;77:1261–70. doi: 10.1158/0008-5472.CAN-16-2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hartmann K, Escribano L, Grattan C, Brockow K, Carter MC, Alvarez-Twose I, et al. Cutaneous manifestations in patients with mastocytosis: consensus report of the European Competence Network on Mastocytosis; the American Academy of Allergy, Asthma & Immunology; and the European Academy of Allergology and Clinical Immunology. J Allergy Clin Immunol. 2016;137:35–45. doi: 10.1016/j.jaci.2015.08.034. [DOI] [PubMed] [Google Scholar]
- 13.Valent P, Horny HP, Escribano L, Longley BJ, Li CY, Schwartz LB, et al. Diagnostic criteria and classification of mastocytosis: a consensus proposal. Leuk Res. 2001;25:603–25. doi: 10.1016/s0145-2126(01)00038-8. [DOI] [PubMed] [Google Scholar]
- 14.Valent P, Horny H-P, Li CY, Longley JB, Metcalfe DD, Parwaresch RM, et al. Mastocytosis. In: Jaffe ES, Harris NL, Stein H, Vardiman JW, editors. World Health Organization (WHO) classification of tumours of haematopoietic and lymphoid tissues. Lyon, France: IARC Press; 2001. pp. 291–302. [Google Scholar]
- 15.Horny HP, Akin C, Metcalfe DD, Bain BJ, Akin C, Escribano L, et al. Mastocytosis (mast cell disease) In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al., editors. World Health Organization (WHO) classification of tumours. Pathology & genetics. Tumours of haematopoietic and lymphoid tissues. Lyon, France: IARC Press; 2008. pp. 54–63. [Google Scholar]
- 16.Horny HP, Akin C, Arber DA, Peterson LA, Tefferi A, Metcalfe DD, et al. Mastocytosis. In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al., editors. WHO classification of tumours of haematopoietic and lymphoid tissues. Lyon, France: IARC Press; 2017. pp. 62–9. [Google Scholar]
- 17.Valent P, Akin C, Sperr WR, Escribano L, Arock M, Horny HP, et al. Aggressive systemic mastocytosis and related mast cell disorders: current treatment options and proposed response criteria. Leuk Res. 2003;27:635–41. doi: 10.1016/s0145-2126(02)00168-6. [DOI] [PubMed] [Google Scholar]
- 18.Lim KH, Tefferi A, Lasho TL, Finke C, Patnaik M, Butterfield JH, et al. Systemic mastocytosis in 342 consecutive adults: survival studies and prognostic factors. Blood. 2009;113:5727–36. doi: 10.1182/blood-2009-02-205237. [DOI] [PubMed] [Google Scholar]
- 19.Pardanani A, Lim KH, Lasho TL, Finke CM, McClure RF, Li CY, et al. WHO subvariants of indolent mastocytosis: clinical details and prognostic evaluation in 159 consecutive adults. Blood. 2010;115:150–1. doi: 10.1182/blood-2009-10-249979. [DOI] [PubMed] [Google Scholar]
- 20.Valent P, Arock M, Bischoff SC, Bühring HJ, Brockow K, Escribano L, et al. The European Competence Network on Mastocytosis (ECNM) Wien Klin Wochenschr. 2004;116:647–51. doi: 10.1007/s00508-004-0253-3. [DOI] [PubMed] [Google Scholar]
- 21.Valent P, Arock M, Bonadonna P, Brockow K, Broesby-Olsen S, Escribano L, et al. European Competence Network on Mastocytosis (ECNM): 10-year jubilee, update, and future perspectives. Wien Klin Wochenschr. 2012;124:807–14. doi: 10.1007/s00508-012-0293-z. [DOI] [PubMed] [Google Scholar]
- 22.Horny HP, Sillaber C, Menke D, Kaiserling E, Wehrmann M, Stehberger B, et al. Diagnostic value of immunostaining for tryptase in patients with mastocytosis. Am J Surg Pathol. 1998;22:1132–40. doi: 10.1097/00000478-199809000-00013. [DOI] [PubMed] [Google Scholar]
- 23.Escribano L, Orfao A, Díaz-Agustin B, Villarrubia J, Cerveró C, López A, et al. Indolent systemic mast cell disease in adults: immunophenotypic characterization of bone marrow mast cells and its diagnostic implications. Blood. 1998;91:2731–6. [PubMed] [Google Scholar]
- 24.Sperr WR, Escribano L, Jordan JH, Schernthaner GH, Kundi M, Horny HP, et al. Morphologic properties of neoplastic mast cells: delineation of stages of maturation and implication for cytological grading of mastocytosis. Leuk Res. 2001;25:529–36. doi: 10.1016/s0145-2126(01)00041-8. [DOI] [PubMed] [Google Scholar]
- 25.Fritsche-Polanz R, Jordan JH, Feix A, Sperr WR, Sunder-Plassmann G, Valent P, et al. Mutation analysis of C-KIT in patients with myelodysplastic syndromes without mastocytosis and cases of systemic mastocytosis. Br J Haematol. 2001;113:357–64. doi: 10.1046/j.1365-2141.2001.02783.x. [DOI] [PubMed] [Google Scholar]
- 26.Escribano L, Díaz-Agustín B, Bellas C, Navalón R, Nuñez R, Sperr WR, et al. Utility of flow cytometric analysis of mast cells in the diagnosis and classification of adult mastocytosis. Leuk Res. 2001;25:563–70. doi: 10.1016/s0145-2126(01)00050-9. [DOI] [PubMed] [Google Scholar]
- 27.Valent P, Escribano L, Parwaresch RM, Schemmel V, Schwartz LB, Sotlar K, et al. Recent advances in mastocytosis research: summary of the Vienna Mastocytosis Meeting 1998. Int Arch Allergy Immunol. 1999;120:1–7. doi: 10.1159/000024214. [DOI] [PubMed] [Google Scholar]
- 28.Böhm A, Födinger M, Wimazal F, Haas OA, Mayerhofer M, Sperr WR, et al. Eosinophilia in systemic mastocytosis: clinical and molecular correlates and prognostic significance. J Allergy Clin Immunol. 2007;120:192–9. doi: 10.1016/j.jaci.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 29.Pardanani A, Lim KH, Lasho TL, Finke C, McClure RF, Li CY, et al. Prognostically relevant breakdown of 123 patients with systemic mastocytosis associated with other myeloid malignancies. Blood. 2009;114:3769–72. doi: 10.1182/blood-2009-05-220145. [DOI] [PubMed] [Google Scholar]
- 30.Escribano L, Alvarez-Twose I, Sánchez-Muñoz L, Garcia-Montero A, Núñez R, Almeida J, et al. Prognosis in adult indolent systemic mastocytosis: a long-term study of the Spanish Network on Mastocytosis in a series of 145 patients. J Allergy Clin Immunol. 2009;124:514–21. doi: 10.1016/j.jaci.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 31.Nedoszytko B, Niedoszytko M, Lange M, van Doormaal J, Gleń J, Zablotna M, et al. Interleukin-13 promoter gene polymorphism -1112C/T is associated with the systemic form of mastocytosis. Allergy. 2009;64:287–94. doi: 10.1111/j.1398-9995.2008.01827.x. [DOI] [PubMed] [Google Scholar]
- 32.Pardanani A, Tefferi A. Systemic mastocytosis in adults: a review on prognosis and treatment based on 342 Mayo Clinic patients and current literature. Curr Opin Hematol. 2010;17:125–32. doi: 10.1097/MOH.0b013e3283366c59. [DOI] [PubMed] [Google Scholar]
- 33.Schwaab J, Schnittger S, Sotlar K, Walz C, Fabarius A, Pfirrmann M, et al. Comprehensive mutational profiling in advanced systemic mastocytosis. Blood. 2013;122:2460–6. doi: 10.1182/blood-2013-04-496448. [DOI] [PubMed] [Google Scholar]
- 34.Damaj G, Joris M, Chandesris O, Hanssens K, Soucie E, Canioni D, et al. ASXL1 but not TET2 mutations adversely impact overall survival of patients suffering systemic mastocytosis with associated clonal hematologic non-mast-cell diseases. PLoS One. 2014;9:e85362. doi: 10.1371/journal.pone.0085362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sánchez-Muñoz L, Teodosio C, Morgado JM, Perbellini O, Mayado A, Alvarez-Twose I, et al. Flow cytometry in mastocytosis: utility as a diagnostic and prognostic tool. Immunol Allergy Clin North Am. 2014;34:297–313. doi: 10.1016/j.iac.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 36.Hanssens K, Brenet F, Agopian J, Georgin-Lavialle S, Damaj G, Cabaret L, et al. SRSF2-p95 hotspot mutation is highly associated with advanced forms of mastocytosis and mutations in epigenetic regulator genes. Haematologica. 2014;99:830–5. doi: 10.3324/haematol.2013.095133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoermann G, Gleixner KV, Dinu GE, Kundi M, Greiner G, Wimazal F, et al. The KIT D816V allele burden predicts survival in patients with mastocytosis and correlates with the WHO type of the disease. Allergy. 2014;69:810–3. doi: 10.1111/all.12409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jawhar M, Schwaab J, Schnittger S, Meggendorfer M, Pfirrmann M, Sotlar K, et al. Additional mutations in SRSF2, ASXL1 and/or RUNX1 identify a high-risk group of patients with KIT D816V(+) advanced systemic mastocytosis. Leukemia. 2016;30:136–43. doi: 10.1038/leu.2015.284. [DOI] [PubMed] [Google Scholar]
- 39.Jawhar M, Schwaab J, Hausmann D, Clemens J, Naumann N, Henzler T, et al. Splenomegaly, elevated alkaline phosphatase and mutations in the SRSF2/ASXL1/RUNX1 gene panel are strong adverse prognostic markers in patients with systemic mastocytosis. Leukemia. 2016;30:2342–50. doi: 10.1038/leu.2016.190. [DOI] [PubMed] [Google Scholar]
- 40.Pardanani A, Reichard KK, Zblewski D, Abdelrahman RA, Wassie EA, Morice Ii WG, et al. CD123 immunostaining patterns in systemic mastocytosis: differential expression in disease subgroups and potential prognostic value. Leukemia. 2016;30:914–8. doi: 10.1038/leu.2015.348. [DOI] [PubMed] [Google Scholar]
- 41.Pardanani A, Lasho T, Elala Y, Wassie E, Finke C, Reichard KK, et al. Next-generation sequencing in systemic mastocytosis: derivation of a mutation-augmented clinical prognostic model for survival. Am J Hematol. 2016;91:888–93. doi: 10.1002/ajh.24426. [DOI] [PubMed] [Google Scholar]
- 42.Naumann N, Jawhar M, Schwaab J, Kluger S, Lübke J, Metzgeroth G, et al. Incidence and prognostic impact of cytogenetic aberrations in patients with systemic mastocytosis. Genes Chromosomes Cancer. 2018;57:252–9. doi: 10.1002/gcc.22526. [DOI] [PubMed] [Google Scholar]
- 43.Valent P, Sotlar K, Sperr WR, Escribano L, Yavuz S, Reiter A, et al. Refined diagnostic criteria and classification of mast cell leukemia (MCL) and myelomastocytic leukemia (MML): a consensus proposal. Ann Oncol. 2014;25:1691–700. doi: 10.1093/annonc/mdu047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Valent P, Akin C, Escribano L, Födinger M, Hartmann K, Brockow K, et al. Standards and standardization in mastocytosis: consensus statements on diagnostics, treatment recommendations and response criteria. Eur J Clin Invest. 2007;37:435–53. doi: 10.1111/j.1365-2362.2007.01807.x. [DOI] [PubMed] [Google Scholar]
- 45.Akin C, Valent P, Metcalfe DD. Mast cell activation syndrome: proposed diagnostic criteria. J Allergy Clin Immunol. 2010;126:1099–1104.e4. doi: 10.1016/j.jaci.2010.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Valent P, Akin C, Arock M, Brockow K, Butterfield JH, Carter MC, et al. Definitions, criteria and global classification of mast cell disorders with special reference to mast cell activation syndromes: a consensus proposal. Int Arch Allergy Immunol. 2012;157:215–25. doi: 10.1159/000328760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Valent P. Mast cell activation syndromes: definition and classification. Allergy. 2013;68:417–24. doi: 10.1111/all.12126. [DOI] [PubMed] [Google Scholar]
- 48.Akin C. Mast cell activation syndromes presenting as anaphylaxis. Immunol Allergy Clin North Am. 2015;35:277–85. doi: 10.1016/j.iac.2015.01.010. [DOI] [PubMed] [Google Scholar]