Abstract
Basophils form a distinct cell lineage within the hematopoietic cell family. In various myeloid neoplasms, including chronic myeloid leukemia, basophilia is frequently seen. Acute and chronic basophilic leukemias, albeit rare, have also been described. However, no generally accepted criteria and classification of basophilic leukemias have been presented to date. To address this unmet need, a series of Working Conferences and other meetings were organized between March 2015 and March 2016. The current article provides a summary of consensus statements from these meetings, together with proposed criteria to delineate acute basophilic leukemia (ABL) from chronic basophilic leukemia (CBL) and primary forms of the disease where no preceding myeloid malignancy is detected, from the more common ‘secondary’ variants. Moreover, the term hyperbasophilia (HB) is proposed for cases with a persistent peripheral basophil count ⩾ 1000 per μl of blood. This condition, HB, is highly indicative of the presence of an underlying myeloid neoplasm. Therefore, HB is an important checkpoint in the diagnostic algorithm and requires a detailed hematologic investigation. In these patients, an underlying myeloid malignancy is often found and is then labeled with the appendix -baso, whereas primary cases of ABL or CBL are very rare. The criteria and classification proposed in this article should facilitate the diagnosis and management of patients with unexplained basophilia and basophil neoplasms in routine practice, and in clinical studies.
Introduction
Since their description by Paul Ehrlich, tissue mast cells and blood basophils have been the subject of intensive research. Both types of cells are of hematopoietic origin. However, although they share morphologic features, biochemical markers and IgE receptors, basophils and mast cells are derived from different precursor cell-subsets and represent two distinct lineages within the hematopoietic cell family.1–4 In contrast to mast cells, basophils usually develop and complete their differentiation in the bone marrow (BM) and are released into the peripheral blood (PB) after maturation. Basophils are derived from multipotent and bi-potent colony-forming precursor cell-units (CFU).5,6
Basophils are involved in a number of pathologic conditions, including reactive states, autoimmune diseases and neoplastic states. Especially in allergic patients, basophil activation is often documented. In patients with chronic myeloproliferative neoplasms (MPN) the numbers of basophils and their progenitors regularly increase.7–11 In BCR-ABL1+ chronic myeloid leukemia (CML), basophilia is a typical feature and is of prognostic significance.11–15 In addition, marked basophilia (⩾20%) is a criterion of disease acceleration in CML. In some of these patients, basophilia may be excessive and may even produce a clinical picture resembling (secondary) basophilic leukemia.16–20 A massive expansion of basophils is sometimes also observed in patients with advanced myelodysplastic syndromes (MDS), JAK2-mutated MPN, MDS/MPN overlap diseases and less frequently in patients with acute myeloid leukemia (AML).21–25 Basophilic leukemias have also been described, but are rare and are not well defined. In many cases, a pre-existing underlying CML is detected.16–20 In other cases, however, no Ph-chromosome or other specific cytogenetic or molecular marker is found, and the basophilic leukemia must be regarded as a primary disease.26–28 In these cases it is often difficult to distinguish between mast cell- and basophilic leukemia, especially when the cells are extremely immature.
The World Health Organization (WHO) has included acute basophilic leukemia (ABL) as a distinct entity in the classification of hematologic malignancies. However, to date, no generally accepted criteria for the diagnosis and classification of basophilic leukemias have been generated. In addition, little is known about specific biochemical, immunohistochemical and molecular markers of ABL and chronic basophilic leukemia (CBL). To address these issues, a series of Working Conferences and other meetings were organized between March 2015 and March 2016 (Supplementary Table S1). Final consensus statements and open discussion points are presented in this article. In addition, we propose criteria and a classification of basophilic leukemias, as well as a diagnostic algorithm. The application of this classification and the algorithm proposed should assist in routine practice and in the harmonization of scientific studies and clinical trials.
Origin of Basophils and Regulation of Differentiation and Function
A widely accepted hypothesis is that basophils are derived from multipotent hematopoietic stem- and progenitor cells. Various types of colony-forming precursors (CFU), including multipotent, bi-potent and lineage-restricted CFU, give rise to basophils and are detectable in the BM and PB of healthy subjects and patients with reactive or clonal states.5–11 In patients with JAK2-mutated MPN and CML, the numbers of these CFU usually increase. The most frequently detected bi-lineage precursor cell giving rise to basophils is the CFU-eo/baso.5,6 Basophil development is regulated by several cytokines. In humans, the most effective growth factor for basophils is interleukin-3 (IL-3).29–31 This cytokine promotes basophil differentiation and maturation in multi-lineage and in lineage-restricted progenitor cells, but also augments viability and activation of mature blood basophils.32,33 Other basophil growth regulators include granulocyte/macrophage colony-stimulating factor (GM-CSF), IL-5, transforming growth factor-beta (TGF-β) and thymic stromal lymphopoietin (TSLP).29–31,34–36 In mature basophils, additional factors and molecules are involved in the regulation of survival, migration, adhesion and activation.1,37–41 A compilation of clinically relevant markers and mediators expressed by basophils is provided in Table 1. Basophil-derived mediators and cytokines, like IL-4, vascular endothelial growth factor (VEGF) or hepatocyte growth factor (HGF) are considered to have an underestimated role in the pathogenesis of various reactive and neoplastic states involving basophils, including CML.
Table 1. Clinically relevant antigens expressed in basophils.
| Antigen | CD | Function | Biologic/clinical relevance |
|---|---|---|---|
| Surface | |||
| LAMP-3 | CD63 | TIMP1R | Activation antigen (basotesta) |
| C5aR | CD88 | C5aR | Complement-dependent activation |
| IL-3 RA | CD123 | IL-3 R | Basophil differentiation and viability as well as basophil activation/function |
| ENPP3 | CD203c | n.k. | Basophil detection and enumeration; and activation antigen (basotesta) |
| FcERI | n.c. | IgE-R | IgE-dependent activation |
| Cytoplasm | |||
| 2D7 | n.c. | n.k. | Basophil detection in tissue sectionsb |
| Basogranulin (=BB1) | n.c. | n.k. | Basophil detection in tissue sectionsb |
| Histamine | n.c. | Bioactive amine | Clinical symptoms of anaphylaxis |
| Tryptase (alpha pro-tryptase) | n.c. | n.k. | Marker of immature basophilsc (also expressed in mast cells) |
| HGF | n.c. | Cytokine | Mediator of angiogenesis (highly upregulated in CML) |
| VEGF | n.c. | Cytokine | Mediator of angiogenesis and vascular permeability |
| IL-4 | n.c. | Cytokine | Multifunctional regulator of the immune system |
Abbreviations: C5a, complement factor 5a; ENPP3, ectonucleotide pyrophosphatase/phosphodiesterase 3; HGF, hepatocyte growth factor; IL-3R, interleukin-3 receptor; LAMP-3, lysosome-associated membrane protein-3; n.c., not yet clustered; n.k., not know; TIMP1, tissue inhibitor of metalloproteinase 1; VEGF, vascular endothelial growth factor.
Both CD63 and CD203c are widely used as markers of IgE-dependent activation of basophils. In response to IgE-dependent activation, the levels of CD63 and CD203c on basophils increase.
Using conventional stains, basophils are not detectable in routinely processed (formalin-fixed) tissue sections, therefore, immunohistochemistry is required for basophil detection.
In CML tryptase is a valuable marker of immature basophils.
Basophil Morphology in Normal and Neoplastic States
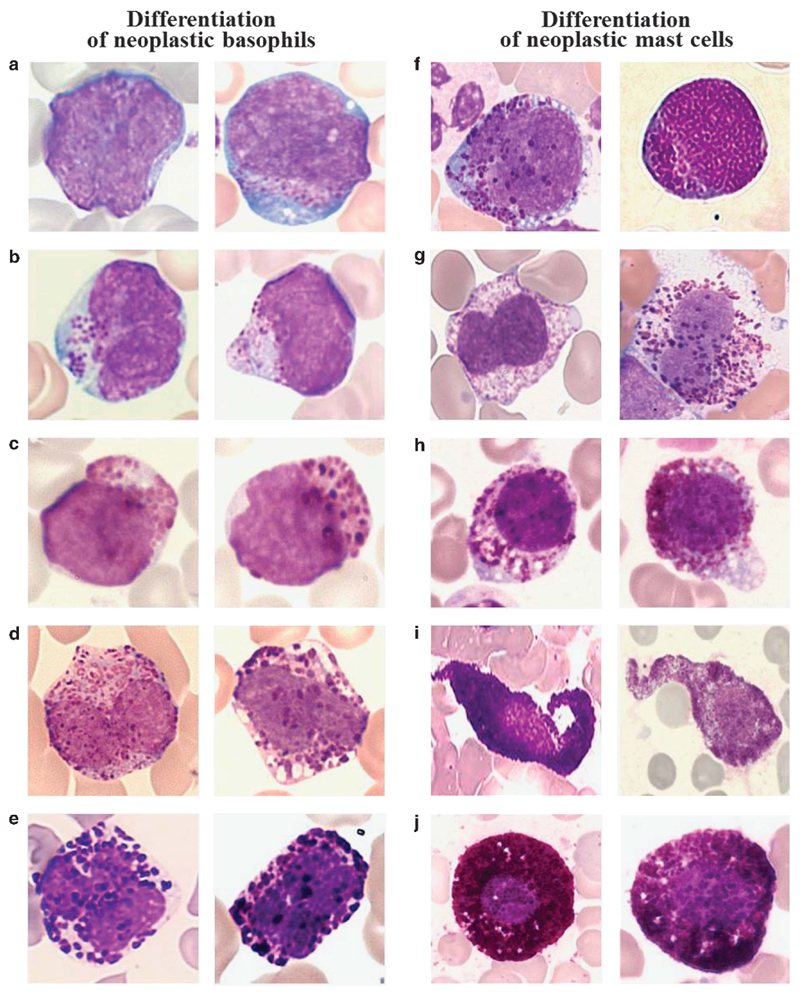
Basophil development includes a number of distinct, morphologically detectable, stages of differentiation and maturation. The most immature (morphologically visible) stage of basophil development is the metachromatically granulated blast cell (metachromatic blast). At this stage of differentiation it is not possible to differentiate between basophil- and mast cell precursors by morphological characteristics using light microscopy. Therefore, it is mandatory to analyze these cells by immunophenotyping and/or by electron microscopy.1,42–45 At the promyelocyte stage, immature (especially leukemic) basophils may also contain a few specific (metachromatic) granules that are difficult to detect amongst the abundant azurophilic (pro) granules. A next defined stage of basophil development is the basophilic myelocyte. Immature basophils, including basophilic myelocytes, exhibit a round nucleus and larger granules, whereas mast cell precursors usually have small-sized granules and, at a certain maturation stage, these cells exhibit bi- or poly-lobed nuclei (Figure 1).46 In consecutive phases of maturation, the nuclear shape of mast cell- and basophil precursor cells changes in a cell-specific manner. Whereas in mature mast cells, the nuclei are round (or oval in neoplastic mast cells), the nuclei in maturing basophils become segmented. It is therefore important to avoid confusion and to separate immature mast cells from basophils and immature basophils from mast cells in myeloid neoplasms.43 The final stage of basophil development is the basophilic granulocyte. An overview of morphologically defined stages of basophil differentiation and a comparison to mast cell stages is provided in Table 2, and typical examples are shown in Figure 1. In some myeloid neoplasms, including MDS and MDS/MPN overlap disorders, basophil maturation may be altered significantly, and signs of dysplasia are present. However, basophil dysplasia is not well defined. Dysplastic basophils may exhibit a hypogranulated cytoplasm, nuclear condensation and signs of apoptosis. An important cell type is the so-called ‘mixed granulated (eo/ba) cell’ which exhibits eosinophilic and ‘basophilic’ (dark-blue) granules. These cells are immature eosinophil-lineage cells and their ‘basophilic’ granules have no relationship with basophils.
Figure 1. Morphology of basophils and mast cells and their precursor cells.
Morphological changes typically occurring during the differentiation of neoplastic basophils (left panels) and mast cells (right panels). (a–e) Basophil-lineage cells were examined on Wright–Giemsa-stained PB smears in patients with CML at the time of acceleration and basophil expansion (a–c) or chronic phase CML with moderate basophilia (d, e). (a) Two metachromatically granulated blast cells. (b) Two basophilic promyelocytes. (c) Two basophilic myelocytes. (d) Two immature basophils with bi-lobed nuclei, and (e) two fully mature basophils with segmented nuclei. (f–j) Mast cell lineage cells on Wright–Giemsa-stained BM smears of a patient with mast cell leukemia (f–h) and a patient with indolent systemic mastocytosis (i, j) are shown. (f) Metachromatic blast cells. (g) Atypical mast cells type II (promastocytes) with bi- or poly-lobed nuclei. (h, i) Immature and more mature atypical mast cells type I with cytoplasmic extensions and hypogranulated cytoplasm, and (j) mature typical round mast cells with a round central nucleus. Note that it is impossible to distinguish between mast cells and basophils by morphology at the stage of metachromatic blasts. Images were prepared using a Basler Vision Technology A1021C camera (Ahrensburg, Germany) connected to an Olympus BX51 microscope equipped with Olympus UIS 2 U Plan FL N × 100/1.30 objective lens (Olympus, Hamburg, Germany). Images were prepared using TRIBVN ICS capture 1.6 and processed with PowerPoint software (Microsoft, Redmond, WA, USA). Original magnification: × 100.
Table 2. Stages of differentiation and maturation of basophils.
| Stage of cellular differentiation/maturation | Morphologic features | Preferred method(s) of detection |
|---|---|---|
| Myeloblast | No signs of maturation | MGG staining plus immunophenotyping |
| Metachromatic blast | Blast cell with a few metachromatic granules | MGG staining plus EMa or immunophenotyping |
| Promyelocyte | Large immature cell with primary granules; may contain a few basophilic granules | MGG staining |
| Basophil myelocyte | Mononuclear maturing cell with basophilic granules | MGG staining |
| Basophil metamyelocyte | Maturing basophil with lobed/folded nucleus | MGG staining |
| Mature blood basophil (basophil granulocyte) | Basophilic cell with segmented nucleus | MGG staining |
Abbreviations: EM, electron microscopy; MGG, May–Grünwald–Giemsa.
EM or immunophenotyping should clarify whether the cells belong to the basophil or to the mast cell lineage.
Detection and Enumeration of Basophils: Recommended Markers and Stains
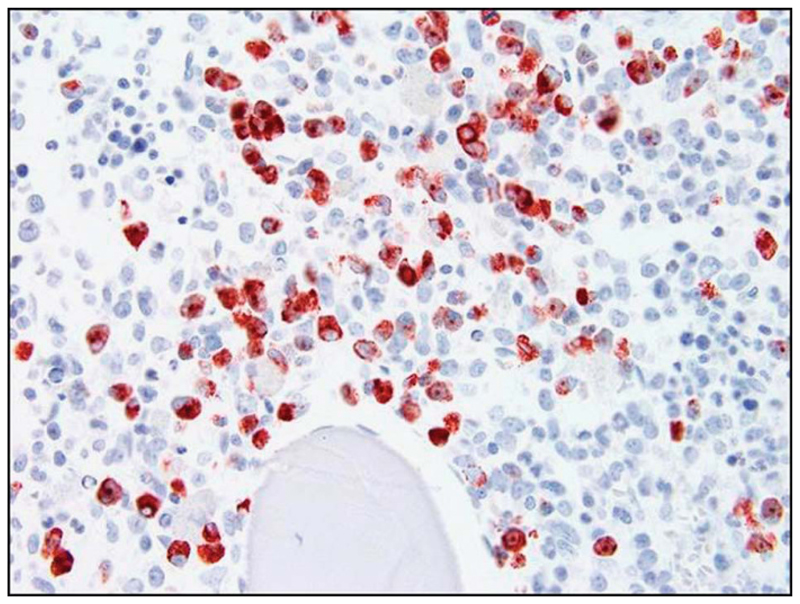
Most stains routinely applied to the morphologic identification of leukocyte subsets in BM or PB smears, such as May–Grünwald–Giemsa (MGG) or Wright–Giemsa, are sufficient for basophil detection and enumeration. However, using conventional (meta-chromatic) stains, it is impossible to detect the earliest stages of basophil development, especially when specific granulation is absent or only a few specific granules are present. Therefore, additional marker-studies are recommended for neoplastic conditions where immature metachromatic cells are found, including MDS, AML with basophilia, and accelerated phase CML. It is of utmost importance to understand that basophils are not detectable in conventional histopathological stains (H&E) in BM sections. Even in Giemsa-stained BM sections, it is impossible to detect and enumerate basophils with certainty as their granules are water-soluble and thus escape morphologic detection after tissue-fixation. By contrast, the mast cell granules are water-resistant and therefore are detectable after fixation of BM sections. As a result, the pathologist has to employ immunohistochemistry (IHC) to detect and enumerate basophils in BM sections. However, only a few IHC-stains are (more or less) specific for basophils. These IHC-stains include basogranulin, also known as BB1 antigen, the 2D7 antigen, as well as CD123 and CD203c (Table 1; Figure 2).47–49 Note that eosinophils, especially immature forms, may also be labeled by antibodies against BB1, 2D7 and CD123. Moreover, mast cells may sometimes react with these antibodies, especially in patients with mastocytosis.50 Therefore, these markers are recommended as confirmatory stains and are useful to estimate the burden of basophils in patients in whom the presence of a prominent basophil compartment (in BM or PB smears) has been documented. In addition, these antibodies may help document basophilia in BM sections, provided that eosinophils and mast cells were excluded. Mast cells are excluded by staining for CD117 (KIT) and eosinophils by their specific granulation and expression of eosinophil-specific proteins.49,51
Figure 2. Immunohistochemical detection of basophils in BM sections.
A BM section of a patient with chronic myeloid leukemia in accelerated phase (with HB) was stained with an antibody against basogranulin (BB1). As visible, the BM appeared to be heavily infiltrated by BB1+ basophils. Photographs were taken using an Olympus DP11 camera connected to an Olympus BX50F4 microscope equipped with × 100/1.35 UPlan-Apo objective lense (Olympus). Images were prepared using Adobe Photoshop CS2 software version 9.0 (Adobe Systems, San Jose, CA, USA) and processed with PowerPoint software (Microsoft). Original magnification: × 100.
Another important approach for the detection and enumeration of basophils in reactive and neoplastic states is flow cytometry. Cell surface antigens (more or less) specifically expressed on basophils include the IL-3 R alpha chain CD123, the Fcε receptor type I (IgE-R), Bsp-1 and the ecto-enzyme ENPP3, also known as CD203c (Supplementary Figure S1).1,3,24,52–55 CD203c is widely used to detect and enumerate human basophils in BM or PB. Notably, CD203c is largely specific for basophils in the PB and increases in response to IgE-dependent cell activation.54 By contrast, in BM samples, normal and neoplastic mast cells also react with antibodies against CD203c.55 Therefore, additional markers, including CD117/KIT (usually not expressed on basophils) and CD123 (usually not expressed on mast cells) should be applied when BM cells are examined (Table 3). When the aim is to enumerate basophils, unfractionated, fresh BM or PB aspirate samples (heparinized or EDTA-anticoagulated) should be used. Note that for example basophils are enriched in mononuclear cell fractions resulting in a seemingly higher percentage count.
Table 3. Recommended markers to delineate between basophils, eosinophils and mast cells.
| Technique | Cell type | Markers and expected staining reactions with cells |
|||||
|---|---|---|---|---|---|---|---|
| CD34 | BB1 | 2D7 | Tryptase | CD117/KIT | EMBP | ||
| Flow cytometry | |||||||
| Myeloblast | + | n.k. | n.k. | − (+/ − a) | + | n.k. | |
| Basophil | − | n.k. | n.k. | n.k. | − | n.k. | |
| Eosinophil | − | n.k. | n.k. | n.k. | − | n.k. | |
| Mast cell | − | n.k. | n.k. | +a | + | n.k. | |
| Immunohistochemistry | |||||||
| Myeloblast | + | − | − | − (+/ − a) | + | − | |
| Basophil | − | + | + | +/ − b | − | +/ − | |
| Eosinophil | − | + | + | − | − | + | |
| Mast cell | − | +/ − | +/ − | + | + | − | |
Abbreviations: EMBP, eosinophil major basic protein; n.k., not known.
In a subset of patients with acute myeloid leukemia, myeloblasts stain positive for tryptase by immunohistochemistry and flow cytometry; and in patients with mastocytosis, tryptase can be detected in mast cells by flow cytometry using a cytoplasmic staining protocol.
In chronic myeloid leukemia, immature basophils display substantial amounts of tryptase.
Finally, freeze-thawing should be avoided because it usually results in decreased viability and thus, lower basophil numbers. A summary of markers and marker-panels to detect basophils in the PB and BM by IHC and flow cytometry is shown in Table 3.
An important serologic parameter is the basal serum (total) tryptase level. Tryptase is produced by mast cells and immature BM basophils, and especially by neoplastic basophils.56,57 In healthy subjects, the normal basal tryptase level (basal: no anaphylaxis) ranges between 0 and 15 ng/ml (average median: 5 ng/ml). In patients with systemic mastocytosis, basal tryptase levels are almost always elevated.58,59 However, tryptase levels also increase in other myeloid neoplasms, especially when mast cells or immature basophil-committed precursor cells are involved. In BCR-ABL1+ CML, where immature basophils are typically elevated, the tryptase level is often elevated and is of prognostic significance.60 In these patients, the presence of immature (often agranular or hypogranulated) basophil-lineage cells can also be confirmed by application of CD203c (by immunohistochemistry or flow cytometry) or by electron microscopy. These patients are usually suffering from high-risk disease as defined by conventional scoring systems.60
Criteria for Basophilia and Hyperbasophilia
Absolute PB basophilia is usually defined as an absolute basophil count exceeding 100 cells per microliter blood. In some laboratories, the threshold to define normal from elevated may be lower (50 basophils/microliter). Relative blood basophilia is defined by a percentage of basophils exceeding 1% (in some laboratories 2%) of total leukocytes determined by microscopic evaluation. One major problem with these threshold-counts is that the physiologic numbers of basophils are rather low, and repeated counting may well result in different outcomes. Therefore, a generally accepted recommendation is that at least 500 nucleated leukocytes be counted on a good-quality BM or PB smear to determine the percentage of basophils. Moreover, the faculty recommends that a mild form of absolute basophilia should be separated from massive absolute basophilia, and that the latter should be referred to as hyperbasophilia (HB). Although the exact threshold to define HB may be subject of further discussion, the faculty was of the opinion that an absolute basophil count exceeding 1000 per microliter PB should be regarded as HB, provided that the blood count abnormality is persistent (documented over at least 8 weeks). The faculty also concluded that HB is almost always associated with an underlying myeloid neoplasm, and therefore is an important diagnostic checkpoint in the algorithm where additional diagnostic markers must be applied to establish the correct final diagnosis. Specifically, we recommend that all patients with unexplained (isolated) HB should undergo a thorough investigation in a step-wise process (Table 4). In a first step, non-invasive tests are applied and PB leukocytes are examined for the presence of BCR-ABL1 and JAK2 V617F. In addition, radiologic examinations and allergy diagnostics are performed and the serum tryptase level is measured. Moreover, certain infectious diseases (like tuberculosis and influenza), should be excluded. If no underlying disease is detected in these evaluations, ‘step 2’ is initiated and a BM investigation is performed, including histology and immunohistochemistry, cytogenetic analyses and molecular studies, with the aim to exclude or reveal an underlying BM neoplasm. In addition, detailed investigations for signs and symptoms of an underlying allergic disease, collagen vascular disorder, endocrinopathy or myxedema have to be initiated. In case of a suspected lymphoma, radiologic studies, computed tomography and T cell receptor and Ig receptor rearrangement analyses are mandatory. Table 4 shows a summary of investigations recommended for patients with unexplained HB.
Table 4. Investigations recommended in patients with HBa .
Primary, non-invasive
|
Secondary (when no BCR-ABL1 is found and no other underlying cause of HB is identified after primary testing)
|
Abbreviations: CALR, calreticulin; FISH, fluorescence in situ hybridization; IgE, immunoglobulin E; RT-PCR, reverse transcriptase polymerase chain reaction; MDS, myelodysplastic syndromes; MPN, myeloproloferative neoplasms; NGS, next-generation sequencing studies.
HB is defined as persistent basophilia with a basophil count of > 1000 per μl of blood.
Additional FISH studies are recommended when conventional karyotyping is inconclusive or did not work (no growth of cells) and molecular studies did not reveal a specific aberration. Depending on the clinical presentation (for example, signs of MDS) and presence of (additional) laboratory abnormalities such as eosinophilia, the FISH panel should cover MDS-related molecular aberrations (standard MDS panel according to local institutional guidelines) and MPN-related lesions, including FIP1L1-PDGFRA (CHIC2 del), PDGFRB rearrangements (5q33), FGFR1 rearrangements (8p11), MYB-GATA1, monosomy 7 and 7q del (cen 7/7q31), trisomy 8 (cen 8), trisomy 9 (cen 9) and 20q deletion (20q21).
Global Classification of Basophil Disorders
In general, diseases involving basophils can be divided into reactive and neoplastic states, and into conditions presenting with basophil activation, an increase in basophil numbers, or both (Supplementary Table S2). Reactive states are usually associated with basophil activation rather than a marked increase in basophils unless affected tissues and cells produce basophilopoietic cytokines such as IL-3. Likewise, in patients with allergic disorders, basophil numbers in the BM and PB are usually normal or slightly elevated. However, in a few patients, presumably those in whom T cell-derived cytokines (like IL-3) are produced, marked or even massive basophilia, often in association with eosinophilia, may develop. However, this type of basophilia is usually transient and disappears as soon as the allergic state resolves or can be brought under control. By contrast, a marked persistent basophilia (HB) is always suspicious and potentially indicative of the presence of a myeloid neoplasm. In these patients, basophils may increase over time and may lead to substantial leukocytosis or even a clinical picture resembling basophilic leukemia. Basophilic leukemias are very rare, however, and often develop on the basis of a pre-existing underlying disease, such as BCR-ABL1+ CML. A global classification of basophil disorders is provided in Supplementary Table S2. As mentioned, it is important to differentiate between reactive and clonal states, and between true basophilic leukemia and myeloid neoplasms accompanied by HB. For the latter group of patients we propose to add the appendix ‘baso’ to the principle diagnosis ( = underlying myeloid neoplasm), an example being: MDS with HB = MDS-baso. However, as soon as the diagnostic criteria of basophilic leukemia are fulfilled in these patients (⩾ 40% basophils) the diagnosis changes to secondary (acute or chronic) basophilic leukemia.
Proposed Diagnostic Criteria and Classification of Basophilic Leukemias
Basophilic leukemias are extremely rare conditions. The WHO classification includes ABL but does not include a chronic variant or secondary variant of the disease. During the preparation of this consensus article, the faculty reviewed 40 unpublished cases with basophilic leukemia or CML in accelerated phase with marked basophilia, as well as a series of published cases of basophilic leukemias (Table 5). On the basis of these analyses, the faculty concluded that a pre-requisite for the diagnosis of ‘basophilic leukemia’ is the presence of HB, and, in addition, the percentage of basophils must be ⩾40%. Moreover, basophils must belong to the malignant clone as evidenced by (i) the (immature) morphology of basophils, (ii) the type of underlying neoplasm (myeloid) if present and (iii) the presence of a clonal (cytogenetic or molecular) marker. For example, if a patient is suffering from an acute T lymphoblastic leukemia (ALL) and basophils represent 50% of all blood leukocytes, the diagnosis is ALL-baso (but not secondary basophilic leukemia) unless basophils are demonstrated to be clonal (leukemic) cells by cytogenetic or molecular studies. By contrast, if a patient suffers from lymphoid blast phase of CML, and basophils increase to 50%, the final diagnosis should be lymphoid blast phase CML with secondary basophilic leukemia. The same holds true for any type of myeloid neoplasm where basophils are ⩾ 40% of total leukocytes.
Table 5. Basophilic leukemias (BL) described in the literaturea .
| Case # | Underlying primary diagnosisb | Type of BL | Age (yrs) | Sex (m/f) | Karyotype | WBC G/L | % BA in PB | % Blasts in PB | % BA in BM | % Blasts in BM | Primary therapy | Remission status | Survival (months) | Reference #a,c |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| #1 | CML-BP | ABL | 37 | f | 46XX, t(9;22) | 57.8 | 49 | 45 | 20.6 | 66 | Poly-CT | NR | 16 | S1 |
| #2 | n.r. | BL | 59 | f | n.r. | 70 | 82 | n.r. | 28 | n.r. | n.r. | n.r. | 3 | S2 |
| #3 | n.r. | BL | 39 | m | 48XY, complex | 27.2 | 77 | n.r. | 27.8 | n.r. | n.r. | n.r. | 2.5 | S3 |
| #4 | CML-AP | BL | 31 | m | 48XY, complex +8,+19, t(9;22) | 34 | 78 | n.r. | 57 | n.r. | n.r. | n.r. | 3 | S4 |
| #5 | n.r. | BL | 46 | m | n.r. | 13.6 | 78 | n.r. | 37 | n.r. | n.r. | n.r. | 3 | S5 |
| #6 | n.r. | BL | 27 | m | 51XY, complex | 17.7 | 45 | n.r. | n.r. | n.r. | n.r. | n.r. | 2.5 | S6 |
| #7 | n.r. | BL | 55 | m | n.r. | 24.3 | 52 | n.r. | 4.2 | n.r. | n.r. | n.r. | 2 | S7 |
| #8 | CML-AP | BL | 77 | f | 46XX, complex with t(9;22) | 93 | 40 | n.r. | 33.2 | n.r. | n.r. | n.r. | 5 | S8 |
| #9 | CML-AP | BL | 50 | f | 47XX, complex with t(9;22) | 47.9 | 47 | n.r. | 29 | n.r. | n.r. | n.r. | 1.5 | S9 |
| #10 | n.r. | BL | 43 | m | n.r. | 20.5 | 94 | n.r. | n.r. | n.r. | n.r. | n.r. | 6 | S10 |
| #11 | CML-BP | ABL | 26 | f | Complex with t(9;22) | 35 | 57 | n.r. | 26 | 33 | Poly-CT | n.r. | 4 | S11 |
| #12 | AML | ABL | 7 | m | 46XY, t(8;21) | 75 | 2 | 54 | 40 | 3 | Poly-CT | NR | 2 | S12 |
| #13 | n.r. | CBL | 68 | f | 47, XX, der(6) | 57.9 | 84 | 0 | 75 | <5 | HU | CR | >36 | S13 |
| #14 | CML-BP | ABL | 44 | f | 47, XX, +19 t(9;22), | 23 | 40 | 0 | 80 | 20 | n.r. | n.r. | n.r. | S14 |
| #15 | MDS | CBL | 84 | f | Complex | 34.2 | 44 | 0 | n.r. | n.r. | HU | n.r. | >3 | S15 |
| #16 | CML-BP | ABL | 30 | m | Complex with t(9;22) | 109.7 | 90 | >20 | n.r. | n.r. | n.r. | n.r. | n.r. | S16 |
Abbreviations: BM, bone marrow; CR, complete remission; f, female; HU, hydroxyurea; m, male; MDS, myelodysplastic syndrome; n.r., not reported; NR, no remission; PB, peripheral blood; poly-CT, polychemotherapy; WBC, white blood count; yrs, years.
Only cases that were described/classified as basophilic leukemia by the reporting authors and were presented with a percentage count of at least 40% basophils (BA) were selected (of ≈50 cases screened). BA included mature and immature basophilic cells as well as metachromatic (basophilic) blasts.
The underlying diagnoses were either reported by the authors or were ‘established’ based on information provided in the literature and the currently available WHO criteria. Regarding basophilic leukemia, patients were classified as ABL or CBL based on the percentage of blasts (<20% =CBL; ⩾20%= ABL). Cases without a reported blast count were classified as basophilic leukemia (BL).
References are included in the Supplementary Material.
In general, basophilic leukemias should be divided into primary and secondary forms and acute and chronic variants (Table 6). In patients with primary basophilic leukemia, no underlying myeloid BM neoplasm is detected. By contrast, in patients with secondary basophilic leukemia, an underlying BM neoplasm with clonal expansion of basophils can be documented. In most cases, a BCR-ABL1+ CML will be diagnosed.16–20 However, other myeloid neoplasms, such as MDS and MPN, may also transform into a secondary basophilic leukemia.21–25 In patients with CML, the question remains whether the condition should indeed be called secondary basophilic leukemia of CML. In fact, based on the WHO definition, these patients are suffering from accelerated phase CML (when basophils are ⩾20%; and also even when basophils exceed 40%). The faculty is of the opinion that the use of the term ‘secondary basophilic leukemia’ may be justified when basophils are ⩾40% in these cases, but that in all these patients, the underlying disease should be mentioned first in the final report. Example: accelerated phase of BCR-ABL1+ CML with secondary basophilic leukemia. Here, it is also important to note that the diagnosis of primary basophilic leukemia changes to secondary basophilic leukemia as soon as BCR-ABL1 p210 is detected. An equally important aspect in the final diagnosis is to distinguish between ABL and CBL. In patients with ABL, the percentage of blast cells (myeloblasts plus metachromatic blasts) is ⩾20% (Table 6). By contrast, in patients with CBL, blast cells are < 20%. A summary of the proposed categories of basophilic leukemia is provided in Table 6.
Table 6. Proposed classification and criteria of basophilic leukemiasa .
| Disease variant | Proposed criteria |
|---|---|
| ABL | Myeloblasts+metachromatic blasts ⩾ 20% and basophilsb ⩾ 40% of nucleated BM or PB cells (and HB criteria are fulfilled) |
| Primary ABL | - No preceding or underlying BM neoplasm |
| Secondary ABLc | - Known preceding/underlying BM neoplasmd |
| CBL | Myeloblasts+metachromatic blasts < 20% and basophils ⩾40% of nucleated BM or PB cells (and HB criteria are fulfilled) |
| Primary CBL | - No preceding or underlying BM neoplasm |
| Secondary CBLc | - Known preceding/underlying BM neoplasmd |
Abbreviations: ABL, acute basophilic leukemia; BM, bone marrow; CBL, chronic basophilic leukemia; HB, hyperbasophilia; PB, peripheral blood; WHO, World Health Organization.
The diagnosis basophilic leukemia (ABL or CBL) is established on the basis of the criteria shown in this table, investigations proposed in Table 4, and exclusion of reactive HB. A diagnostic algorithm is shown in Supplementary Figure S2.
In ABL, many or even most of the basophils may be quite immature cells. When all these cells are metachromatic blasts, they can only be regarded (counted) as ‘basophils’ when the basophil lineage has been confirmed by immunophenotyping or electron microscopy.
Secondary BL variants should be further sub-classified according to the type of preceding or underlying BM neoplasm—these neoplasms should be classified according to the WHO proposal.
The presence of the Ph-chromosome or BCR-ABL1 p210 counts as a definitive sign of an underlying BM neoplasm even if no known prephase of overt CML had been diagnosed before. In these patients, the treatment plan also needs to be adjusted according to the detection of BCR-ABL1.
Molecular Markers and Cytogenetic Variables
A number of cytogenetic defects have been described in patients with ABL or CBL. In many cases a complex karyotype and one or more Ph-chromosome(s) are detected.16–20 In these CML patients as well as in those with lower basophil counts (criteria for ABL or CBL not fulfilled) basophils display BCR-ABL1.61 However, apart from the Ph-chromosome, only a very few other specific karyotype anomalies have been described in basophilic leukemias. One is the t(X;6)(p11;q23) translocation occurring in male infants with ABL.62 In these patients, immature metachromatic (basophilic) blast cells display the MYB-GATA1 fusion gene.62 Otherwise, no recurrent chromosome or molecular defects have been identified in ABL or CBL. In one patient with a myeloid/lymphoid neoplasm with PDGFRB rearrangement (according to WHO) resembling a CBL, a PRKG2-PDGFRB fusion gene was detected, and this patient was responsive to imatinib.63 An unresolved question is why some patients with CML develop massive basophilia, whereas others do not develop basophilia even if their disease progresses. Recent data suggest that IKAROS alterations may be associated with disease acceleration and basophil expansion in CML.64 Additional genes and altered molecular pathways responsible for basophil expansion in CML may be detected when next-generation sequencing (NGS) approaches are routinely applied in these patients. However, it should also be noted that the prognosis of all these basophilic leukemia variants is grave independent of the type of molecular lesion detected.
The faculty also discussed molecular markers and assays as well as cytogenetic studies that should be applied in patients with suspected basophilic leukemia. All faculty members agreed that conventional karyotyping and probing for the Ph-chromosome and for BCR-ABL1 and JAK2 V617F by PCR is standard in patients with suspected basophilic leukemia. Additional cytogenetic and molecular studies, such as fluorescence in situ hybridization (FISH) and sequencing of typically mutated genes (MDS/AML panel, MPN panel) should also be performed following local guidelines and according to additional findings in PB investigations. Likewise, in patients presenting with HB and eosinophilia, PB cells should also be examined for the presence of the FIP1L1-PDGFR1 fusion gene (Table 4). In fact there are rare patients with a FIP1L1-PDGFRA+ myeloid neoplasm with hypereosinophilia (HE) as well as HB (P.B., unpublished observation). In patients in whom no clonal aberration indicative for an underlying myeloid neoplasm is found in such studies, BM and PB cells should be examined for additional cytogenetic and molecular markers. Similarly, in those with eosinophilia and/or lymphadenopathy, cells should be examined for the presence of clonal T cells and immunoglobulin rearrangements as well as for the KIT D816V mutation. Finally, when all attempts to document an underlying clonal BM disease have been unsuccessful, the etiology of HB has to be re-considered and the condition re-examined using markers of reactive diseases, including allergic conditions, autoimmune disorders, intoxication and infectious diseases.
Differential Diagnoses
A number of differential diagnoses (DD) have to be considered in patients with suspected ABL or CBL. Major DD to be considered in suspected ABL include blast phase of CML, myelomastocytic leukemia, AML with t(8;21) and basophilia (AML-baso), monocytic/monoblastic AML with basophilia, acute promyelocytic leukemia (APL) and aggressive systemic mastocytosis (ASM) or mast cell leukemia (MCL) accompanied by AML (ASM-AML/MCL-AML). Alternative diagnoses to be considered in suspected CBL include MPN or MDS with basophilia (MPN-baso; MDS-baso), ASM in transformation (ASM-t), MCL, APL and accelerated phase CML. In rare cases, a massive reactive expansion of polyclonal basophils may mimic CBL. However, this form of HB usually disappears after the underlying process (for example, allergy or infection) has been brought under control with therapy or has resolved spontaneously.
A Practical Approach to Basophilia and HB: Recommended Diagnostic Algorithm
In the initial exploration and in the short term follow-up, it is important to delineate between transient and persistent (hyper) basophilia. Transient HB is indicative of a reactive underlying process, whereas persistent HB (for at least 8 weeks) must be regarded as highly suspicious (indicative) of a neoplastic (usually myeloid) disease, especially when other blood count abnormalities are also present. In all patients with unexplained persistent basophilia (even if not reaching the HB-threshold) a number of parameters should be determined in PB samples, including the serum tryptase level, BCR-ABL1 and JAK2 V617F (Table 4). In cases with concomitant eosinophilia, leukocytes should also be examined for expression of the FIPL1-PDGFRA fusion gene. In addition, the spleen size should be determined by palpation and ultrasound. Finally, a BM examination should be performed in all cases with unexplained HB, even if other blood counts are normal and the above-mentioned parameters did not reveal an underlying hematopoietic neoplasm. BM investigations should include a thorough histopathological and immunohistochemical investigation (Table 3), cytomorphologic inspection of BM smears, molecular studies and chromosome analyses. In HB patients in whom no BM neoplasm can be detected, other rare causes of persistent basophilia must be considered. These conditions include chronic infections, chronic autoimmune processes, or uncontrolled atopic disorders. In several of these conditions, basophilia may be accompanied by eosinophilia. The measurement of basophilopoietic and eosinophilopoietic cytokines (IL-3, GM-CSF, IL-5) in such conditions is of academic interest (and confirms the reactive nature of the condition) but is not regarded as standard. In rare cases, an underlying T cell neoplasm (such as adult T cell leukemia/lymphoma) may be detected.65 However, no specific (basophil-related) symptoms are found in these patients. A diagnostic algorithm for patients with HB is shown in Supplementary Figure S2.
Therapeutic Options in Patients With Basophilic Leukemias
Regardless of the underlying neoplasm, the prognosis of patients with CBL or ABL is poor.11–14,66 The survival time in these patients ranges from 2 to > 36 months in CBL and 2 to 16 months in ABL. Therefore, these patients are often regarded as candidates for stem cell transplantation. However, not all patients are eligible for high-dose therapy. When stem cell transplantation is not possible, patients with ABL should receive poly-chemotherapy, targeted drugs, or palliative therapy. In non-transplantable patients with accelerated or blast phase CML treatment with second- or third generation TKI is usually recommended regardless of the basophil count (including those with basophils ⩾ 40%). However, in other (non-transplantable) patients with ABL or CBL, no specific targets may be detected. These patients are candidates for experimental drugs or palliative cytoreductive treatment. Regardless of the nature (acute or chronic) and underlying disease (if any), all patients with basophilic leukemias (by definitions provided in this article) have an increased risk to develop histamine-related symptoms, especially when cytoreductive or targeted therapies are applied. Therefore, these patients should receive prophylactic histamine receptor (HR) antagonists (HR1 and HR2 blocker) as long as HB is present.
Concluding Remarks and Future Perspectives
Persistent HB is an important checkpoint in clinical hematology. In most cases, an underlying myeloid neoplasm is detected or was known before HB developed. Accordingly, HB can be classified into primary HB and secondary HB. We propose that myeloid neoplasms presenting with HB and less than 40% basophils in their differential counts be labeled with the appendix ‘-baso’ (example: MDS-baso) and those with basophils ⩾40% termed secondary basophilic leukemia. In a subset of patients with basophilic leukemia, no underlying myeloid neoplasm will be detected; these cases should be classified as primary basophilic leukemia. In addition, basophilic leukemia (both primary and secondary) should be divided into ABL and CBL, based on the percentage of blasts (myeloblasts and metachromatic blasts). Our proposal to classify basophil transformation in myeloid neoplasms and basophilic leukemias with defined criteria should assist in daily practice and lead to a commonly used nomenclature and should thereby support harmonizing research in these fascinating disease-entities.
Supplementary Material
Supplementary Information accompanies this paper on the Leukemia website (http://www.nature.com/leu)
Acknowledgements
This study was supported by the Austrian Science Fund (FWF) Grant #SFB-F4704-B20. DDM was supported by the Division of Intramural Research, NIAID/NIH.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
Author Contributions
All co-authors contributed by establishing the concept, by participating in essential discussions, by writing parts of the document and by correcting and approving the final version of the manuscript. Consensus statements were based on a 100% agreement (all faculty members agreed) and only those statements were included as consensus in this article.
References
- 1.Valent P, Bettelheim P. Cell surface structures on human basophils and mast cells: biochemical and functional characterization. Adv Immunol. 1992;52:333–423. doi: 10.1016/s0065-2776(08)60879-2. [DOI] [PubMed] [Google Scholar]
- 2.Agis H, Willheim M, Sperr WR, Wilfing A, Krömer E, Kabrna E, et al. Monocytes do not make mast cells when cultured in the presence of SCF. Characterization of the circulating mast cell progenitor as a c-kit+, CD34+, Ly − , CD14 − , CD17 − , colony-forming cell. J Immunol. 1993;151:4221–4227. [PubMed] [Google Scholar]
- 3.Agis H, Füreder W, Bankl HC, Kundi M, Sperr WR, Willheim M, et al. Comparative immunophenotypic analysis of human mast cells, blood basophils and monocytes. Immunology. 1996;87:535–543. doi: 10.1046/j.1365-2567.1996.493578.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kempuraj D, Saito H, Kaneko A, Fukagawa K, Nakayama M, Toru H, et al. Characterization of mast cell-committed progenitors present in human umbilical cord blood. Blood. 1999;93:3338–3346. [PubMed] [Google Scholar]
- 5.Denburg JA, Richardson M, Telizyn S, Bienenstock J. Basophil/mast cell precursors in human peripheral blood. Blood. 1983;61:775–780. [PubMed] [Google Scholar]
- 6.Leary AG, Ogawa M. Identification of pure and mixed basophil colonies in culture of human peripheral blood and marrow cells. Blood. 1984;64:78–83. [PubMed] [Google Scholar]
- 7.Denburg JA, Telizyn S, Belda A, Dolovich J, Bienenstock J. Increased numbers of circulating basophil progenitors in atopic patients. J Allergy Clin Immunol. 1985;76:466–472. doi: 10.1016/0091-6749(85)90728-6. [DOI] [PubMed] [Google Scholar]
- 8.Otsuka H, Dolovich J, Befus D, Bienenstock J, Denburg J. Peripheral blood basophils, basophil progenitors, and nasal metachromatic cells in allergic rhinitis. Am Rev Respir Dis. 1986;133:757–762. [PubMed] [Google Scholar]
- 9.Arnalich F, Lahoz C, Larrocha C, Zamorano AF, Jimenez C, Gasalla R, et al. Incidence and clinical significance of peripheral and bone marrow basophilia. J Med. 1987;18:293–303. [PubMed] [Google Scholar]
- 10.Gibson PG, Manning PJ, O'Byrne PM, Girgis-Gabardo A, Dolovich J, Denburg JA, et al. Allergen-induced asthmatic responses. Relationship between increases in airway responsiveness and increases in circulating eosinophils, basophils, and their progenitors. Am Rev Respir Dis. 1991;143:331–335. doi: 10.1164/ajrccm/143.2.331. [DOI] [PubMed] [Google Scholar]
- 11.Denburg JA, Wilson WE, Bienenstock J. Basophil production in myeloproliferative disorders: increases during acute blastic transformation of chronic myeloid leukemia. Blood. 1982;60:113–120. [PubMed] [Google Scholar]
- 12.Denburg JA, Browman G. Prognostic implications of basophil differentiation in chronic myeloid leukemia. Am J Hematol. 1988;27:110–114. doi: 10.1002/ajh.2830270208. [DOI] [PubMed] [Google Scholar]
- 13.Hasford J, Pfirrmann M, Hehlmann R, Allan NC, Baccarani M, Kluin-Nelemans JC, et al. A new prognostic score for survival of patients with chronic myeloid leukemia treated with interferon alfa. Writing Committee for the Collaborative CML Prognostic Project Group. J Natl Cancer Inst. 1998;90:850–858. doi: 10.1093/jnci/90.11.850. [DOI] [PubMed] [Google Scholar]
- 14.Steegmann JL, Odriozola J, Rodriguez-Salvanés F, Giraldo P, García-Laraña J, Ferro MT, et al. Stage, percentage of basophils at diagnosis, hematologic response within six months, cytogenetic response in the first year: the main prognostic variables affecting outcome in patients with chronic myeloid leukemia in chronic phase treated with interferon-alpha. Results of the CML89 trial of the Spanish Collaborative Group on interferon-alpha2a and CML. Haematologica. 1999;84:978–987. [PubMed] [Google Scholar]
- 15.Hasford J, Baccarani M, Hoffmann V, Guilhot J, Saussele S, Rosti G, et al. Predicting complete cytogenetic response and subsequent progression-free survival in 2060 patients with CML on imatinib treatment: the EUTOS score. Blood. 2011;118:686–692. doi: 10.1182/blood-2010-12-319038. [DOI] [PubMed] [Google Scholar]
- 16.Nissenblatt MJ. Basophilic transformation of chronic myelogenous leukemia. South Med J. 1980;73:1316–1319. doi: 10.1097/00007611-198010000-00008. [DOI] [PubMed] [Google Scholar]
- 17.Ozaki M, Kanemitsu N, Yasukawa M, Fujita S. Basophilic crisis of chronic myelogenous leukemia. Jpn J Med. 1989;28:67–71. doi: 10.2169/internalmedicine1962.28.67. [DOI] [PubMed] [Google Scholar]
- 18.Yamauchi K, Arimori S. Basophilic crisis in chronic myelogenous leukemia: case report and literature review in Japan. Jpn J Med. 1990;29:334–340. doi: 10.2169/internalmedicine1962.29.334. [DOI] [PubMed] [Google Scholar]
- 19.Xue YQ, Guo Y, Lu DR, Gu J, Lu DW, Gong JX, et al. A case of basophilic leukemia bearing simultaneous translocations t(8; 21) and t(9; 22) Cancer Genet Cytogenet. 1991;51:215–221. doi: 10.1016/0165-4608(91)90134-g. [DOI] [PubMed] [Google Scholar]
- 20.Pidala J, Pinilla-Ibarz J, Cualing HD. A case of acute basophilic leukemia arising from chronic myelogenous leukemia with development of t(7; 8)(q32;q13) Cancer Genet Cytogenet. 2008;182:46–49. doi: 10.1016/j.cancergencyto.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 21.Rojas-Atencio A, Urdaneta K, Soto-Quintana M, Alvarez Nava F, Cañizales J, Solis E. Trisomy 19 and t(9;22) in a patient with acute basophilic leukemia. Case Rep Pathol. 2011;2011 doi: 10.1155/2011/269491. 269491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sugimoto N, Ishikawa T, Gotoh S, Shinzato I, Matsushita A, Nagai K, et al. Primary myelofibrosis terminated in basophilic leukemia and successful allogeneic bone marrow transplantation. Int J Hematol. 2004;80:183–185. doi: 10.1532/ijh97.03153. [DOI] [PubMed] [Google Scholar]
- 23.Gupta R, Jain P, Anand M. Acute basophilic leukemia: case report. Am J Hematol. 2004;76:134–138. doi: 10.1002/ajh.10446. [DOI] [PubMed] [Google Scholar]
- 24.Wimazal F, Baumgartner C, Sonneck K, Zauner C, Geissler P, Schur S, et al. Mixed-lineage eosinophil/basophil crisis in MDS: a rare form of progression. Eur J Clin Invest. 2008;38:447–455. doi: 10.1111/j.1365-2362.2008.01950.x. [DOI] [PubMed] [Google Scholar]
- 25.Tang G, Woods LJ, Wang SA, Brettler D, Andersen M, Miron PM, et al. Chronic basophilic leukemia: a rare form of chronic myeloproliferative neoplasm. Hum Pathol. 2009;40:1194–1199. doi: 10.1016/j.humpath.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 26.Servitzoglou M, Grenzelia M, Baka M, Harisi M, Pourtsidis A, Bouhoutsou D, et al. A novel karyotype in acute myeloid leukemia with basophilia. Pediatr Hematol Oncol. 2014;31:149–156. doi: 10.3109/08880018.2014.883655. [DOI] [PubMed] [Google Scholar]
- 27.Shin SY, Koo SH, Kwon KC, Park JW, Ko CS, Jo DY. Monosomy 7 as the sole abnormality of an acute basophilic leukemia. Cancer Genet Cytogenet. 2007;172:168–171. doi: 10.1016/j.cancergencyto.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 28.Kritharis A, Brody J, Koduru P, Teichberg S, Allen SL. Acute basophilic leukemia associated with loss of gene ETV6 and protean complications. J Clin Oncol. 2011;29:e623–e626. doi: 10.1200/JCO.2010.34.5710. [DOI] [PubMed] [Google Scholar]
- 29.Saito H, Hatake K, Dvorak AM, Leiferman KM, Donnenberg AD, Arai N, et al. Selective differentiation and proliferation of hematopoietic cells induced by recombinant human interleukins. Proc Natl Acad Sci USA. 1988;85:2288–2292. doi: 10.1073/pnas.85.7.2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Valent P, Schmidt G, Besemer J, Mayer P, Zenke G, Liehl E, et al. Interleukin-3 is a differentiation factor for human basophils. Blood. 1989;73:1763–1769. [PubMed] [Google Scholar]
- 31.Mayer P, Valent P, Schmidt G, Liehl E, Bettelheim P. The in vivo effects of recombinant human interleukin-3: demonstration of basophil differentiation factor, histamine-producing activity, and priming of GM-CSF-responsive progenitors in nonhuman primates. Blood. 1989;74:613–621. [PubMed] [Google Scholar]
- 32.Valent P, Besemer J, Muhm M, Majdic O, Lechner K, Bettelheim P. Interleukin 3 activates human blood basophils via high-affinity binding sites. Proc Natl Acad Sci USA. 1989;86:5542–5546. doi: 10.1073/pnas.86.14.5542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kurimoto Y, de Weck AL, Dahinden CA. Interleukin 3-dependent mediator release in basophils triggered by C5a. J Exp Med. 1989;170:467–479. doi: 10.1084/jem.170.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Denburg JA, Silver JE, Abrams JS. Interleukin-5 is a human basophilopoietin: induction of histamine content and basophilic differentiation of HL-60 cells and of peripheral blood basophil-eosinophil progenitors. Blood. 1991;77:1462–1468. [PubMed] [Google Scholar]
- 35.Sillaber C, Geissler K, Scherrer R, Kaltenbrunner R, Bettelheim P, Lechner K, et al. Type beta transforming growth factors promote interleukin-3 (IL-3)-dependent differentiation of human basophils but inhibit IL-3-dependent differentiation of human eosinophils. Blood. 1992;80:634–641. [PubMed] [Google Scholar]
- 36.Siracusa MC, Saenz SA, Hill DA, Kim BS, Headley MB, Doering TA, et al. TSLP promotes interleukin-3-independent basophil haematopoiesis and type 2 inflammation. Nature. 2011;477:229–233. doi: 10.1038/nature10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Valent P, Bettelheim P. The human basophil. Crit Rev Oncol Hematol. 1990;10:327–352. doi: 10.1016/1040-8428(90)90009-h. [DOI] [PubMed] [Google Scholar]
- 38.Bochner BS, Sterbinsky SA, Knol EF, Katz BJ, Lichtenstein LM, MacGlashan DW, Jr, et al. Function and expression of adhesion molecules on human basophils. J Allergy Clin Immunol. 1994;94:1157–1162. doi: 10.1016/0091-6749(94)90326-3. [DOI] [PubMed] [Google Scholar]
- 39.Bochner BS, Schleimer RP. Mast cells, basophils, and eosinophils: distinct but overlapping pathways for recruitment. Immunol Rev. 2001;179:5–15. doi: 10.1034/j.1600-065x.2001.790101.x. [DOI] [PubMed] [Google Scholar]
- 40.Falcone FH, Haas H, Gibbs BF. The human basophil: a new appreciation of its role in immune responses. Blood. 2000;96:4028–4038. [PubMed] [Google Scholar]
- 41.Arock M, Schneider E, Boissan M, Tricottet V, Dy M. Differentiation of human basophils: an overview of recent advances and pending questions. J Leukoc Biol. 2002;71:557–564. [PubMed] [Google Scholar]
- 42.Kurosawa H, Eguchi M, Sakakibara H, Takahashi H, Furukawa T. Ultrastructural cytochemistry of congenital basophilic leukemia. Am J Pediatr Hematol Oncol. 1987;9:27–32. doi: 10.1097/00043426-198721000-00006. [DOI] [PubMed] [Google Scholar]
- 43.Agis H, Beil WJ, Bankl HC, Füreder W, Sperr WR, Ghannadan M, et al. Mast cell-lineage versus basophil lineage involvement in myeloproliferative and myelodysplastic syndromes: diagnostic role of cell-immunophenotyping. Leuk Lymphoma. 1996;22:187–204. doi: 10.3109/10428199609051750. [DOI] [PubMed] [Google Scholar]
- 44.Duchayne E, Demur C, Rubie H, Robert A, Dastugue N. Diagnosis of acute basophilic leukemia. Leuk Lymphoma. 1999;32:269–278. doi: 10.3109/10428199909167387. [DOI] [PubMed] [Google Scholar]
- 45.Shvidel L, Shaft D, Stark B, Shtalrid M, Berrebi A, Resnitzky P. Acute basophilic leukaemia: eight unsuspected new cases diagnosed by electron microscopy. Br J Haematol. 2003;120:774–781. doi: 10.1046/j.1365-2141.2003.04167.x. [DOI] [PubMed] [Google Scholar]
- 46.Sperr WR, Escribano L, Jordan JH, Schernthaner GH, Kundi M, Horny HP, et al. Morphologic properties of neoplastic mast cells: delineation of stages of maturation and implication for cytological grading of mastocytosis. Leuk Res. 2001;25:529–536. doi: 10.1016/s0145-2126(01)00041-8. [DOI] [PubMed] [Google Scholar]
- 47.Agis H, Krauth MT, Böhm A, Mosberger I, Müllauer L, Simonitsch-Klupp I, et al. Identification of basogranulin (BB1) as a novel immunohistochemical marker of basophils in normal bone marrow and patients with myeloproliferative disorders. Am J Clin Pathol. 2006;125:273–281. doi: 10.1309/M9FQ-MQGF-6616-7N2X. [DOI] [PubMed] [Google Scholar]
- 48.Agis H, Krauth MT, Mosberger I, Müllauer L, Simonitsch-Klupp I, Schwartz LB, et al. Enumeration and immunohistochemical characterisation of bone marrow basophils in myeloproliferative disorders using the basophil specific monoclonal antibody 2D7. J Clin Pathol. 2006;59:396–402. doi: 10.1136/jcp.2005.029215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Valent P, Orazi A, Büsche G, Schmitt-Gräff A, George TI, Sotlar K, et al. Standards and impact of hematopathology in myelodysplastic syndromes (MDS) Oncotarget. 2010;1:483–496. doi: 10.18632/oncotarget.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Idoate MA, Echeveste J, Gil P, Sanz ML, Ferrer M. Expression of the basophil-specific antibodies 2D7 and BB1 in patients with cutaneous Mastocytosis. J Investig Allergol Clin Immunol. 2013;23:392–397. [PubMed] [Google Scholar]
- 51.Noguchi H, Kephart GM, Colby TV, Gleich GJ. Tissue eosinophilia and eosinophil degranulation in syndromes associated with fibrosis. Am J Pathol. 1992;140:521–528. [PMC free article] [PubMed] [Google Scholar]
- 52.Bodger MP, Newton LA. The purification of human basophils: their immunophenotype and cytochemistry. Br J Haematol. 1987;67:281–284. doi: 10.1111/j.1365-2141.1987.tb02348.x. [DOI] [PubMed] [Google Scholar]
- 53.Valent P. Immunophenotypic characterization of human basophils and mast cells. Chem Immunol. 1995;61:34–48. [PubMed] [Google Scholar]
- 54.Bühring HJ, Simmons PJ, Pudney M, Müller R, Jarrossay D, van Agthoven A, et al. The monoclonal antibody 97A6 defines a novel surface antigen expressed on human basophils and their multipotent and unipotent progenitors. Blood. 1999;94:2343–2356. [PubMed] [Google Scholar]
- 55.Hauswirth AW, Escribano L, Prados A, Nuñez R, Mirkina I, Kneidinger M, et al. CD203c is overexpressed on neoplastic mast cells in systemic mastocytosis and is upregulated upon IgE receptor cross-linking. Int J Immunopathol Pharmacol. 2008;21:797–806. doi: 10.1177/039463200802100404. [DOI] [PubMed] [Google Scholar]
- 56.Foster B, Schwartz LB, Devouassoux G, Metcalfe DD, Prussin C. Characterization of mast-cell tryptase-expressing peripheral blood cells as basophils. J Allergy Clin Immunol. 2002;109:287–293. doi: 10.1067/mai.2002.121454. [DOI] [PubMed] [Google Scholar]
- 57.Samorapoompichit P, Kiener HP, Schernthaner GH, Jordan JH, Agis H, Wimazal F, et al. Detection of tryptase in cytoplasmic granules of basophils in patients with chronic myeloid leukemia and other myeloid neoplasms. Blood. 2001;98:2580–2583. doi: 10.1182/blood.v98.8.2580. [DOI] [PubMed] [Google Scholar]
- 58.Schwartz LB, Sakai K, Bradford TR, Ren S, Zweiman B, Worobec AS, et al. The alpha form of human tryptase is the predominant type present in blood at baseline in normal subjects and is elevated in those with systemic mastocytosis. J Clin Invest. 1995;96:2702–2710. doi: 10.1172/JCI118337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sperr WR, Jordan JH, Fiegl M, Escribano L, Bellas C, Dirnhofer S, et al. Serum tryptase levels in patients with mastocytosis: correlation with mast cell burden and implication for defining the category of disease. Int Arch Allergy Immunol. 2002;128:136–141. doi: 10.1159/000059404. [DOI] [PubMed] [Google Scholar]
- 60.Sperr WR, Pfeiffer T, Hoermann G, Herndlhofer S, Sillaber C, Mannhalter C, et al. Serum-tryptase at diagnosis: a novel biomarker improving prognostication in Ph(+) CML. Am J Cancer Res. 2014;5:354–362. [PMC free article] [PubMed] [Google Scholar]
- 61.Bodger MP, Morris CM, Kennedy MA, Bowen JA, Hilton JM, Fitzgerald PH. Basophils (Bsp-1+) derive from the leukemic clone in human myeloid leukemias involving the chromosome breakpoint 9q34. Blood. 1989;73:777–781. [PubMed] [Google Scholar]
- 62.Quelen C, Lippert E, Struski S, Demur C, Soler G, Prade N, et al. Identification of a transforming MYB-GATA1 fusion gene in acute basophilic leukemia: a new entity in male infants. Blood. 2011;117:5719–5722. doi: 10.1182/blood-2011-01-333013. [DOI] [PubMed] [Google Scholar]
- 63.Lahortiga I, Akin C, Cools J, Wilson TM, Mentens N, Arthur DC, et al. Activity of imatinib in systemic mastocytosis with chronic basophilic leukemia and a PRKG2-PDGFRB fusion. Haematologica. 2008;93:49–56. doi: 10.3324/haematol.11836. [DOI] [PubMed] [Google Scholar]
- 64.Beer PA, Knapp DJ, Miller PH, Kannan N, Sloma I, Heel K, et al. Disruption of IKAROS activity in primitive chronic-phase CML cells mimics myeloid disease progression. Blood. 2015;125:504–515. doi: 10.1182/blood-2014-06-581173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Takasaki Y, Iwanaga M, Tsukasaki K, Kusano M, Sugahara K, Yamada Y, et al. Impact of visceral involvements and blood cell count abnormalities on survival in adult T-cell leukemia/lymphoma (ATLL) Leuk Res. 2007;31:751–757. doi: 10.1016/j.leukres.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 66.Matsushima T, Handa H, Yokohama A, Nagasaki J, Koiso H, Kin Y, et al. Prevalence and clinical characteristics of myelodysplastic syndrome with bone marrow eosinophilia or basophilia. Blood. 2003;101:3386–3390. doi: 10.1182/blood-2002-03-0947. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.