Summary
Background
The WHO classification separates mastocytosis into distinct variants, but prognostication remains a clinical challenge. The aim of this study was to improve prognostication for patients with mastocytosis.
Methods
We analysed data of the registry of the European Competence Network on Mastocytosis including 1639 patients (age 17–90 years) diagnosed with mastocytosis according to WHO criteria between Jan 12, 1978, and March 16, 2017. Univariate and multivariate analyses with Cox regression were applied to identify prognostic variables predicting survival outcomes and to establish a prognostic score. We validated this International Prognostic Scoring System in Mastocytosis (IPSM) with data of 462 patients (age 17–79 years) from the Spanish network Red Española de Mastocitosis diagnosed between Jan 22, 1998, and Nov 2, 2017.
Findings
The prognostic value of the WHO classification was confirmed in our study (p<0·0001). For patients with non-advanced mastocytosis (n=1380), we identified age 60 years or older (HR 10·75, 95% CI 5·68–20·32) and a concentration of alkaline phosphatase 100 U/L or higher (2·91, 1·60–5·30) as additional independent prognostic variables for overall survival. The resulting scoring system divided patients with non-advanced mastocytosis into three groups: low (no risk factors), intermediate 1 (one risk factor), and intermediate 2 (two risk factors). Overall survival and progression-free survival differed significantly among these groups (p<0·0001). In patients with advanced mastocytosis (n=259), age 60 years or older (HR 2·14, 95% CI 1·42–3·22), a concentration of tryptase 125 ng/mL or higher (1·81, 1·20–2·75), a leukocyte count of 16 × 109 per L or higher (1·88, 1·27–2·79), haemoglobin of 11 g/dL or lower (1·71, 1·13–2·57), a platelet count of 100 × 109 per L or lower (1·63, 1·13–2·34), and skin involvement (0·46, 0·30–0·69) were prognostic variables. Based on these variables, a separate score for advanced mastocytosis with four risk categories was established, with significantly different outcomes for overall survival and progression-free survival (p<0·0001). The prognostic value of both scores was confirmed in 413 patients with non-advanced disease and 49 with advanced mastocytosis from the validation cohort.
Interpretation
The IPSM scores for patients with non-advanced and advanced mastocytosis can be used to predict survival outcomes and guide treatment decisions. However, the predictive value of the IPSM needs to be confirmed in forthcoming trials.
Funding
Austrian Science Fund, Deutsche Forschungsgemeinschaft, Koeln Fortune Program, Charles and Ann Johnson Foundation, Instituto de Salud Carlos III, Fondos FEDER, Research-Foundation Flanders/Fonds Wetenschappelijk Onderzoek, Clinical Research-Fund of the University Hospitals Leuven, and Research-Foundation Flanders/Fonds Wetenschappelijk Onderzoek.
Introduction
The term mastocytosis denotes a heterogeneous group of disorders characterised by abnormal expansion and accumulation of mast cells in various organs. The estimated prevalence of systemic mastocytosis is one case in 10 000 adults, and the estimated incidence amounts to one new case per 100 000 people per year.1 A diagnosis of mastocytosis is based on criteria provided by WHO.2,3 The WHO classification includes several prognostic variables and represents a well-established diagnostic method with prognostic effect. However, prognosis and survival outcomes of patients vary substantially among cases, even within WHO entities,4,5 and prediction of the clinical course and survival outcomes in individual patients is difficult. Based on the WHO classification, mastocytosis can essentially be split into non-advanced disease and advanced systemic disease.2, 3 Non-advanced mastocytosis includes patients with cutaneous mastocytosis, indolent systemic mastocytosis, and smouldering systemic mastocytosis. These patients usually have a stable clinical course and a good prognosis.4 By contrast, advanced systemic mastocytosis includes patients with aggressive systemic mastocytosis, systemic mastocytosis with an associated haematological neoplasm, and mast cell leukaemia. These patients have a poor prognosis.4, 6–9 Awareness of differences in disease biology and prognosis of patients with non-advanced mastocytosis and advanced systemic mastocytosis is important.
During the past two decades, several clinical, serological, cytomorphological, immunological, and molecular variables have been reported to be of prognostic significance in mastocytosis.10–19 Several of these variables have been included in the WHO classification, such as organomegaly or cytopenias.2,3 Other adverse prognostic variables include absence of skin lesions, multilineage involvement with KIT Asp816Val, mutations in genes other than KIT (eg, SRSF2, ASXL1, or RUNX1), increased amounts of β2-microglobulin in serum, and raised amounts of alkaline phosphatase.10,12–19 However, these variables were studied in smaller patients’ cohorts and without comparing all potential risk factors with each other in multivariate analyses. Moreover, several of these variables (eg, molecular profiling) are not available at all centres.
Several attempts have been made to improve prognostication of mastocytosis by establishing scoring systems.13,20,21 However, currently available scores are based on a limited number of patients and have not been validated in independent cohorts. More importantly, current scoring systems do not address the point that non-advanced mastocytosis and advanced systemic mastocytosis are completely different disease groups with divergent disease biology and distinct patterns of prognostic factors.13,20,21
Although the WHO classification is a well-established diagnostic approach with prognostic effect, advanced methods for prognostication in mastocytosis are scarce. The aim of this study was to confirm the prognostic effect of individual disease features and laboratory variables in patients with mastocytosis. Using data from the registry of the European Competence Network on Mastocytosis (ECNM), we identified the most discriminative prognostic variables and established a scoring system for use in daily practice. We also aimed to confirm the strength and effect of our scoring system in an independent validation cohort.
Methods
Study design and participants
Our study was retrospective in design and comprised two datasets, a test cohort and a validation cohort. We obtained data for the test cohort from the ECNM registry. We included patients with mastocytosis as per WHO criteria diagnosed between Jan 12, 1978, and March 16, 2017, at 22 centres in Europe and one US centre (Stanford; appendix p 25).6 We excluded patients who had less than 2 days of follow-up data. The ECNM registry was approved by ethics committees of the participating centres. Details about the ECNM registry, study design, eligibility, inclusion criteria, age limits, disease categories, ethics approval, informed consent, and prognostic variables are provided in the appendix (pp 2–5).
We obtained data for the validation cohort from the Spanish network Red Española de Mastocitosis (REMA). This cohort includes patients with mastocytosis as per WHO criteria diagnosed between Jan 22, 1998, and Nov 2, 2017 (appendix pp 5, 32). The REMA registry was approved by the ethics committees of the participating centres. In the REMA cohort, the following patients were excluded: patients with well-differentiated non-advanced systemic mastocytosis, patients with cutaneous mastocytosis but no bone marrow data, individuals with non-advanced systemic mastocytosis and less than 12 months of follow-up data, and patients with not enough data for analyses.
We excluded from the analysis of prognostic factors children (aged <17 years) with cutaneous mastocytosis because no bone marrow studies were available for most patients and because of the different disease biology of this group (in most patients, no KIT Asp816Val mutation is found). We also excluded patients with mast cell sarcoma because of the rarity of the disease and its unique pathology and pathogenesis (usually KIT Asp816Val is not detectable in mast cell sarcoma).
Procedures
We obtained clinical and laboratory data from both registries taken at diagnosis and during follow-up (appendix pp 26–29) in patients with indolent systemic mastocytosis, cutaneous mastocytosis (both children [aged <17 years] and adults [aged ≤17 years]), systemic mastocytosis with an associated haematological neoplasm, aggressive systemic mastocytosis, smouldering systemic mastocytosis, mast cell leukaemia, and mast cell sarcoma. Data were obtained retrospectively by chart review in the ECNM and REMA registries and were controlled regularly, with data clearing and updated follow-up once a year. We extracted data for survival outcomes and for variables to be considered as potential prognostic factors for development of the score. Details about development of the score and its validation are described in the appendix (p 6). In brief, only variables recorded in at least 70% of patients were included. Based on results of the statistical evaluation (including univariate and multivariate Cox regression), we established the scores for patients with non-advanced mastocytosis and advanced systemic mastocytosis.
Statistical analysis
We retrospectively analysed overall survival (time from diagnosis to death from any cause), progression-free survival (time from diagnosis to disease progression, defined as a shift from a low-risk disease category to a higher risk category), and event-free survival (time from diagnosis to progression or death, whichever occurred first) according to the Kaplan-Meier method with Mantel-Cox tests for group comparisons. Progression was defined as a shift from a lower risk to a higher risk category of mastocytosis. Moreover, development of an associated haematological neoplasm as well as the transformation of such an associated haematological neoplasm into a higher grade of disease (eg, from a lower grade myeloid malignancy such as myelodysplastic syndrome to acute myeloid leukaemia) counted as progression. Since mast cell leukaemia is an end-stage disease (no further progression can occur) and mastocytosis in the skin is a provisional diagnosis for patients with skin involvement but unknown or unavailable bone marrow, these two patient groups were excluded from the analysis of progression-free survival.
We applied univariate Cox regression for all potentially prognostic variables. All variables that showed prognostic significance in univariate analyses were included in multivariate analyses. These analyses were done separately for patients with non-advanced mastocytosis (excluding children with cutaneous mastocytosis) and those with advanced systemic mastocytosis. We checked the proportional hazard assumption by testing the interaction to define whether the hazards of prognostic factors change during follow-up (depending on survival time reached).
Based on results obtained in multivariate analyses, we developed two prognostic scoring systems, one for patients with non-advanced mastocytosis and one for those with advanced systemic mastocytosis.
All statistical analyses were done using GraphPad Prism version 6 (GraphPad Software, San Diego, CA, USA) and Stata version 13.1 (StataCorp, College Station, TX, USA).
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to the ECNM dataset and IA-T and AO had full access to the REMA dataset. The corresponding author had final responsibility for the decision to submit for publication.
Results
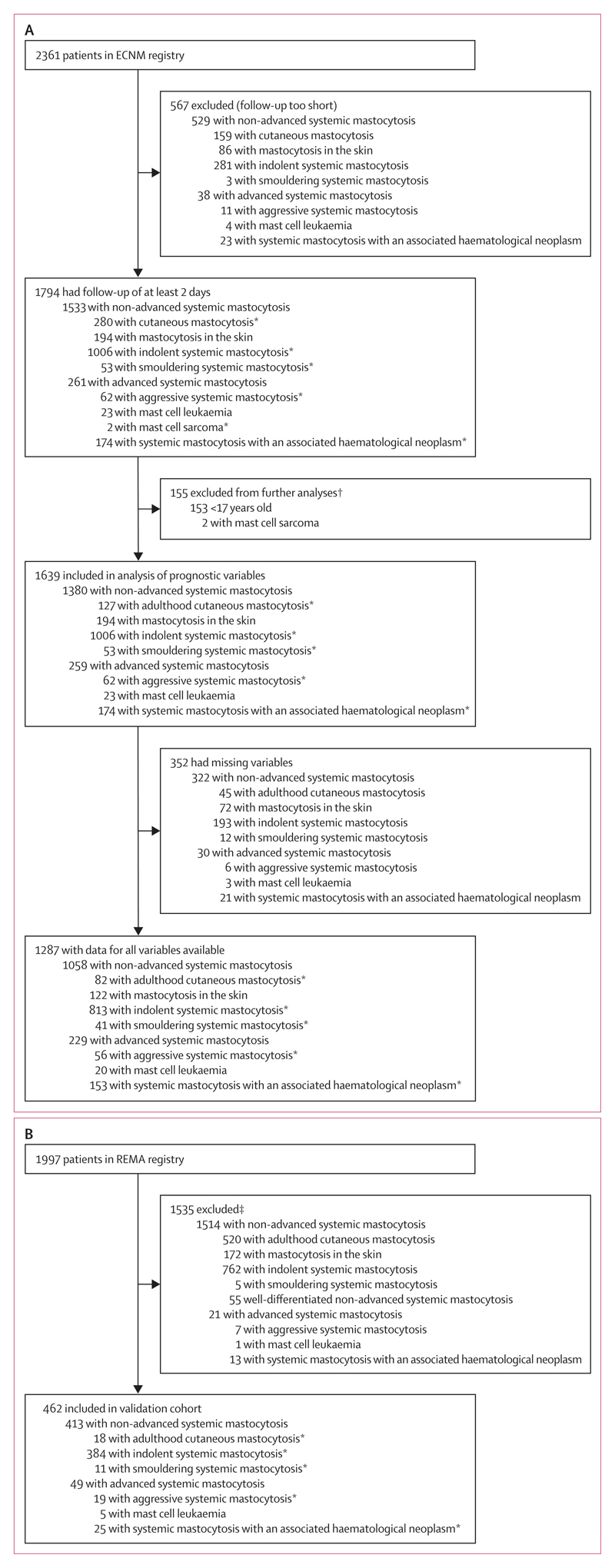
Between Jan 12, 1978, and Mar 16, 2017, 2361 patients were enrolled in the ECNM registry, of whom 567 were excluded because they were not followed up for long enough (figure 1). 1794 patients had at least 2 days of follow-up data available in the ECNM registry and comprised the test cohort. Median age at diagnosis was 46 years (range 0·15–90). Among the test cohort, 1006 had indolent systemic mastocytosis, 280 had cutaneous mastocytosis (153 children and 127 adults), 174 had systemic mastocytosis with an associated haematological neoplasm, 62 had aggressive systemic mastocytosis, 53 had smouldering systemic mastocytosis, 23 had mast cell leukaemia, and two had mast cell sarcoma. A further 194 adults had typical mast cell infiltrates in the skin, but no bone marrow examination was done (table 1; appendix pp 30, 31).
Figure 1. Study profile.
Patients were selected from the ECNM registry (A) and REMA (B). In the ECNM cohort, only patients with at least 2 days of follow-up were included. All patients were included in analyses of overall survival and event-free survival. ECNM=European Competence Network on Mastocytosis. REMA=Red Española de Mastocitosis. *Included in analyses of progression-free survival. †Children (aged <17 years) were excluded from the assessment of prognostic factors and development of the score because, for most children, no bone marrow studies were available and because the disease is different. Patients with mast cell sarcoma were excluded because of the rarity of the disease and its unique pathology and pathogenesis. ‡Those excluded were children (aged <17 years), had cutaneous mastocytosis without a bone marrow study, had less than 12 months of follow-up for non-advanced systemic mastocytosis, or did not have enough data.
Table 1. Patients’ characteristics and progressions recorded in the test and validation cohorts (pooled data).
| Childhood cutaneous mastocytosis | Adulthood cutaneous mastocytosis | Mastocytosis in the skin | Indolent systemic mastocytosis | Smouldering systemic mastocytosis | Aggressive systemic mastocytosis | Mast cell leukaemia | Mast cell sarcoma | Systemic mastocytosis with an associated haematological neoplasm | All patients | |
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Test cohort | ||||||||||
|
| ||||||||||
| Patients (n) | 153 | 127 | 194 | 1006 | 53 | 62 | 23 | 2 | 174 | 1794 |
| Age at diagnosis (years) | 1 (0·15–16) | 37 (18–76) | 43 (18–87) | 47 (17–83) | 52 (25–79) | 58 (17–83) | 54 (27–90) | 19 (8–30) | 64 (22–83) | 46 (0·15–90) |
| Female:male ratio | 1:1·2 | 1:0·5 | 1:0·5 | 1:0·7 | 1:0·7 | 1:1 | 1:1·3 | 1:0 | 1:2·5 | 1:0·8 |
| Tryptase (ng/mL) | 6 (1–98) | 9 (1–126) | 12 (1–141) | 31 (3–885) | 200 (60–819) | 175 (9–1432) | 397 (114–4530) | 3 (1–6) | 150 (2–1022) | 29 (1–4530) |
| Alkaline phosphatase (U/L) | 212 (79–1729) | 68 (33–212) | 66 (36–290) | 74 (22–317) | 114 (36–345) | 161 (63–1696) | 194 (55–1121) | 100* 201 (39–1407) | 80 (22–1729) | |
| KIT Asp816Val positive | 0/2 (0%) | 23/98 (23%) | 7/15 (47%) | 680/799 (85%) | 34/38 (89%) | 38/45 (84%) | 12/19 (63%) | 0/2 (0%) | 125/144 (87%) | 919/1162 (79%) |
| Progression† | 1/153 (1%) | 5/127 (4%) | ·· | 39/1006 (4%) | 5/53 (9%) | 11/62 (18%) | ·· | 0/2 (0%) | 27/174 (16%) | 88/1577 (6%) |
| Progression to AdvSM† | 0/153 (0%) | 0/127 (0%) | ·· | 27/1006 (3%) | 5/53 (9%) | ·· | ·· | ·· | ·· | ·· |
|
| ||||||||||
| Validation cohort | ||||||||||
|
| ||||||||||
| Patients (n) | 0 | 18 | 0 | 384 | 11 | 19 | 5 | 0 | 25 | 462 |
| Age at diagnosis (years) | ·· | 33 (17–62) | ·· | 46 (17–79) | 52 (21–72) | 61 (25–77) | 65 (27–73) | ·· | 61 (24–73) | 47 (17–79) |
| Female:male ratio | ·· | 1:0·2 | ·· | 1:0·9 | 1:1·8 | 1:1·4 | 1:1·5 | ·· | 1:3·2 | 1:1 |
| Tryptase (ng/mL) | ·· | 5 (2–19) | ·· | 29 (6–664) | 267 (161–380) | 279 (72–2036) | 196 (91–660) | ·· | 82 (6–390) | 31 (2–2036) |
| Alkaline phosphatase (U/L) | ·· | 62 (44–125) | ·· | 68 (17–332) | 133 (49–553) | 211 (68–1972) | 82 (46–365) | ·· | 124 (51–1310) | 71 (17–1972) |
| KIT Asp816Val positive | ·· | 0/11 (0%) | ·· | 333/356 (94%) | 10/11 (91%) | 17/18 (94%) | 1/5 (20%) | ·· | 18/24 (75%) | 379/425 (89%) |
| Progression† | ·· | 0/18 (0%) | ·· | 6/384 (2%) | 1/11 (9%) | 3/62 (5%) | ·· | ·· | 6/25 (24%) | 15/462 (3%) |
| Progression to AdvSM† | ·· | 0/18 (0%) | ·· | 4/384 (1%) | 1/11 (9%) | ·· | ·· | ·· | ·· | ·· |
Data are median (range) or n/N (%), unless otherwise stated. AdvSM=advanced systemic mastocytosis.
Only one of the two patients had data for alkaline phosphatase available.
Disease progression was examined by analysing progression-related events defined by a shift from a known disease category in a higher graded (more advanced) form of mastocytosis or advanced haematological malignancy during follow-up.
Between Jan 22, 1998, and Nov 2, 2017, 1997 patients were collected in the REMA registry. 1535 individuals were excluded from our analyses (figure 1); thus, the validation cohort consisted of 462 patients. Median age at diagnosis was 47 years (range 17–79). 384 patients had indolent systemic mastocytosis, 25 had systemic mastocytosis with an associated haematological neoplasm, 19 had aggressive systemic mastocytosis, 18 had cutaneous mastocytosis, 11 had smouldering systemic mastocytosis, and five had mast cell leukaemia (table 1; appendix pp 5, 32).
Median follow-up in the ECNM registry was 3·4 years (IQR 1·4–6·6; appendix pp 7, 9). The data obtained in our registry confirmed that the WHO classification defines two prognostic groups: non-advanced mastocytosis and advanced systemic mastocytosis. The non-advanced mastocytosis category comprised patients with cutaneous mastocytosis, mastocytosis in the skin, indolent systemic mastocytosis, and smouldering systemic mastocytosis. The advanced systemic mastocytosis category consisted of patients with aggressive systemic mastocytosis, mast cell leukaemia, mast cell sarcoma, and systemic mastocytosis with an associated haematological neoplasm.
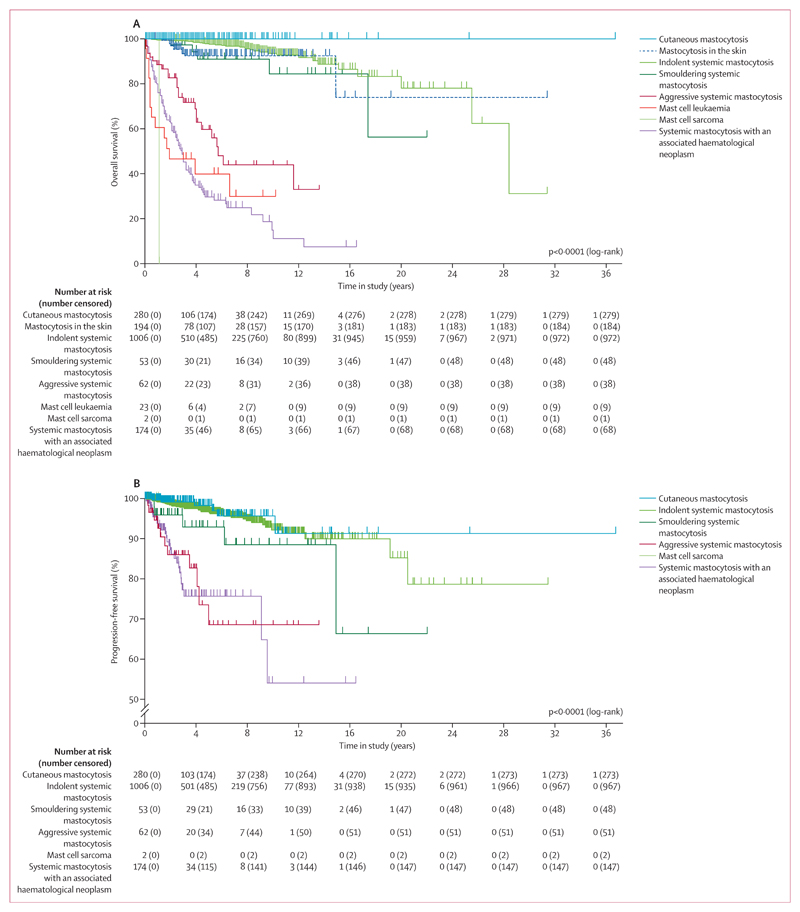
In the total cohort, median overall survival was 28·4 years (95% CI 19·5–37·0) and 10-year overall survival was 81·9% (95% CI 7·7–84·7). Differences in overall survival between WHO cohorts were significant (p<0·0001). Patients in the non-advanced mastocytosis category had improved prognosis compared with patients with advanced systemic mastocytosis (figure 2). However, subtle differences in overall survival were noted among patients with non-advanced mastocytosis. For example, individuals with cutaneous mastocytosis (both children and adults) had improved overall survival compared with all other subgroups, including patients with indolent systemic mastocytosis (figure 2A). No substantial differences were noted in overall survival when comparing cutaneous mastocytosis in children (n=153) and adults (n=127; appendix p 10). In patients with indolent systemic mastocytosis (73% with skin lesions; appendix p 30), 10-year overall survival was 93·5% (95% CI 90·1–95·8) and median overall survival was 28·4 years (95% CI 24·1–32·8). 10-year overall survival was 100% for patients with cutaneous mastocytosis, 92·5% (85·9–96·0) for those with mastocytosis in the skin, and 84·5% (61·1–84·5) for individuals with smouldering systemic mastocytosis, but median overall survival was not reached in these subgroups. Median overall survival was 5·7 years (95% CI 0·6–4·5) for patients with aggressive systemic mastocytosis, 1·9 years (0·0–5·2) for those with mast cell leukaemia, 1·1 years for one patient with mast cell sarcoma, and 2·9 years (2·5–3·3) for individuals with systemic mastocytosis with an associated haematological neoplasm (figure 2A). 10-year overall survival was 44·0% (95% CI 26·6–60·1) in patients with aggressive systemic mastocytosis, 29·9% (10·0–53·2) in those with mast cell leukaemia, 0% for mast cell sarcoma, and 11·2% (7·5–12·1) for individuals with systemic mastocytosis with an associated haematological neoplasm. In 11 (22%) of 49 patients with non-advanced mastocytosis and 109 (75%) of 145 patients with advanced systemic mastocytosis, the cause of death was related to mastocytosis (appendix pp 7, 33).
Figure 2. Survival outcomes in WHO subgroups of mastocytosis.
Kaplan–Meier curves show the probability of overall survival (A) and progression-free survival (B) in subgroups of patients with mastocytosis, defined by WHO criteria.
Progression-free survival was analysed in 1577 (88%) patients after excluding patients with mastocytosis in the skin (n=194) and mast cell leukaemia (n=23). Progression of disease was observed in 88 (6%) of 1577 patients, including 39 (4%) of 1006 patients with indolent systemic mastocytosis, five (9%) of 53 with smouldering systemic mastocytosis, 11 (18%) of 62 with aggressive systemic mastocytosis, and 27 (16%) of 174 with systemic mastocytosis with an associated haematological neoplasm (table 1, appendix p 34). Six (2%) of 280 patients with cutaneous mastocytosis developed indolent systemic mastocytosis (one [1%] of 153 children and five [4%] of 127 adults; appendix p 34). In 32 (2%) of 1339 patients with non-advanced mastocytosis, progression to advanced systemic mastocytosis was seen in 27 (3%) of 1006 patients with indolent systemic mastocytosis and five (9%) of 53 patients with smouldering systemic mastocytosis (table 1, appendix pp 34, 35). Among 194 patients with mastocytosis in the skin, 49 had a bone marrow examination during follow-up, resulting in a diagnosis of cutaneous mastocytosis in 16 patients, indolent systemic mastocytosis in 30, smouldering systemic mastocytosis in one, aggressive systemic mastocytosis in one, and systemic mastocytosis with an associated haematological neoplasm in one (appendix p 36). For all patients, progression-free survival at 10 years was 88·0% (95% CI 85·8–91·5; appendix p 9). Differences in progression-free survival between WHO cohorts were significant (p<0·0001). None of the WHO groups reached median progression-free survival during the study (figure 2B). Significant differences between WHO groups were also seen for event-free survival (p<0·0001; appendix pp 7, 11). Overall survival and progression-free survival in patients with systemic mastocytosis with an associated haematological neoplasm, according to subtype of neoplasm, are shown in the appendix (p 12).
In patients with indolent systemic mastocytosis or smouldering systemic mastocytosis, alkaline phosphatase of 100 U/L or higher was associated with a significantly increased risk of disease progression to advanced systemic mastocytosis (appendix p 37). Age 60 years or older (HR 4·90, 95% CI 2·51–9·40) and alkaline phosphatase of 100 U/L or higher (2·10, 1·07–4·05) were identified as significant (independent) predictors of evolution to higher grade mastocytosis in patients with non-advanced systemic mastocytosis; these same variables were also predictive of overall survival (HR 10·75, 95% CI 5·68–20·32; and 2·91, 1·60–5·30, respectively; table 2). Based on these variables, we established a simple score, the International Prognostic Scoring System for Mastocytosis (IPSM), and applied it in 1058 of 1380 patients with non-advanced mastocytosis in whom data for all relevant prognostic variables were available. Patients with non-advanced mastocytosis without additional risk factors comprised the low-risk group (median age 43 years), and those with one or two risk factors formed the intermediate-risk group 1 (referred to as int-1; median age 56 years) and intermediate risk-group 2 (referred to as int-2; median age 64 years), respectively.
Table 2. Effect of individual risk factors on overall survival and identification of prognostic variables.
| Patients (n) | Risk population | Risk of patients with non-advanced mastocytosis |
Risk of patients with advanced systemic mastocytosis |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Univariate analysis |
Multivariate analysis |
Univariate analysis |
Multivariate analysis |
|||||||
| HR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value | |||
| Male sex | 1641 | ·· | 2·01 (1·14–3·55) | 0·016 | 1·47 (0·67–3·14) | 0·35 | 1·74 (1·20–2·54) | 0·004 | 1·12 (0·67–1·86) | 0·48 |
|
| ||||||||||
| Age (years) | 1641 | ≥60 | 1·11 (1·08–1·14) | <0·0001 | 10·75 (5·68–20·32) | <0·0001 | 1·04 (1·03–1·06) | <0·0001 | 2·14 (1·42–3·22) | <0·0001 |
|
| ||||||||||
| Tryptase (ng/mL) | 1530 | ≥125 | 3·01 (1·59–5·70) | <0·0001 | 1·61 (0·72–3·72) | 0·24 | 1·66 (1·18–2·35) | 0·0004 | 1·81 (1·20–2·75) | 0·005 |
|
| ||||||||||
| Leukocytes (× 109 per L) | 1543 | ≥16 | 2·08 (0·26–16·65) | 0·491 | ·· | ·· | 1·94 (1·20–3·14) | 0·007 | 1·88 (1·27–2·79) | 0·002 |
|
| ||||||||||
| Haemoglobin (g/dL) | 1550 | ≤11·0 | 0·01 (0·00–0·31) | 0·019 | ·· | ·· | 0·01 (0·00–0·04) | <0·0001 | 1·71 (1·13–2·57) | 0·011 |
|
| ||||||||||
| Platelets (× 109 per L) | 1543 | ≤100 | 0·05 (0·01–0·23) | <0·0001 | 5·78 (0·56–59·52) | 0·14 | 0·17 (0·11–0·27) | <0·0001 | 1·63 (1·13–2·34) | 0·009 |
|
| ||||||||||
| Lactate dehydrogenase (U/L) | 1226 | ≥260 | 0·46 (0·04–5·29) | 0·535 | ·· | ·· | 2·51 (1·27–4·98) | 0·008 | 1·36 (0·82–2·28) | 0·19 |
|
| ||||||||||
| Alkaline phosphatase (U/L) | 1295 | ≥100 | 15·19 (3·93–58·71) | <0·0001 | 2·91 (1·60–5·30) | <0·0001 | 2·16 (1·35–3·46) | 0·001 | 0·74 (0·39–1·40) | 0·11 |
|
| ||||||||||
| Calcium (mg/dL) | 1216 | ≤8·7 | 0·01 (0·00–0·01) | 0·003 | 0·97 (0·22–4·22) | 0·93 | 0·01 (0·00–0·01) | <0·0001 | 1·48 (0·91–2·41) | 0·12 |
|
| ||||||||||
| Neutrophils (%) | 1466 | ≥50 | 11·11 (0·72–171·57) | 0·085 | 2·58 (0·90–7·44) | 0·081 | 0·66 (0·30–1·46) | 0·307 | ·· | ·· |
|
| ||||||||||
| Monocytes (%) | 1420 | ≥0·32 | 16·74 (0·19–1451·66) | 0·216 | ·· | ·· | 3·20 (1·06–9·69) | 0·040 | 1·43 (0·53–3·83) | 0·44 |
|
| ||||||||||
| Eosinophils granulocytes (%) | 1432 | ·· | 1·01 (0·94–1·10) | 0·749 | ·· | ·· | 1·00 (0·99–1·02) | 0·408 | ·· | ·· |
|
| ||||||||||
| Skin involvement | 1641 | ·· | 1·06 (0·50–2·28) | 0·877 | ·· | ·· | 0·44 (0·31–0·65) | <0·0001 | 0·46 (0·30–0·69) | <0·0001 |
|
| ||||||||||
| Organomegaly* | 1464 | ·· | 3·05 (1·56–5·94) | 0·001 | 1·28 (0·56–2·94) | 0·51 | 1·06 (0·68–1·66) | 0·782 | ·· | ·· |
|
| ||||||||||
| Mediator symptoms | 1639 | ·· | 0·66 (0·36–1·20) | 0·171 | ·· | ·· | 0·61 (0·43–0·85) | 0·004 | 0·87 (0·52–1·48) | 0·25 |
|
| ||||||||||
| Allergy | 1418 | ·· | 0·74 (0·39–1·43) | 0·376 | ·· | ·· | 0·43 (0·22–0·84) | 0·014 | 0·48 (0·20–1·19) | 0·23 |
Prognostic variables were examined for their statistical power and independence from each other and from the WHO classification by univariate and multivariate analysis. HR=hazard ratio.
Organomegaly (ie, enlarged spleen, enlarged liver, enlarged lymph nodes, or a combination).
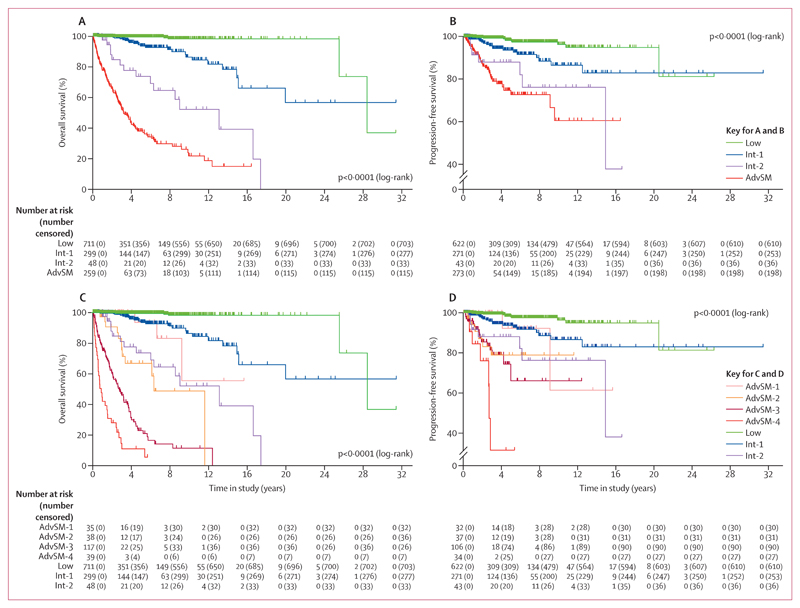
Overall survival at 10 years was 98·1% (95% CI 95·2–99·3) in the low-risk group, 87·1% (77·2–91·9) in the int-1 group, 52·1% (29·4–70·7) in the int-2-risk group, and 22·0% (13·9–21·2) in patients with advanced systemic mastocytosis (p<0·0001; figure 3A). Significant differences were also observed in progression-free survival at 10 years: 96·3% (95% CI 92·2–98·3) in lowrisk patients, 86·7% (77·9–92·2) in int-1 patients, 76·3% (52·2–89·4) in int-2 patients, and 61·1% (42·0–75·6) in patients with advanced systemic mastocytosis (figure 3B). The significance of the score was also confirmed for overall survival in mastocytosis in the skin and indolent systemic mastocytosis (p<0·0001) and for progression-free survival in cutaneous mastocytosis (p<0·0001), indolent systemic mastocytosis (p=0·0006), and smouldering systemic mastocytosis (p=0·0011; appendix pp 13, 14). In patients with smouldering systemic mastocytosis, the differences in overall survival were not significant (p=0·093), which might be attributable to the relatively low number of patients with this disorder. The score was also of prognostic significance regarding event-free survival in all subgroups (appendix pp 8, 15, 16).
Figure 3. Survival outcomes according to the IPSM score in patients with non-advanced and advanced systemic mastocytosis.
Kaplan–Meier curves show the probability of overall survival (A, C) and progression-free survival (B, D) in patients with non-advanced and advanced systemic mastocytosis, defined by the IPSM.
Upper panels (A, B) show that patients with non-advanced systemic mastocytosis at low risk (no risk factors [low]) and intermediate risk (one [int-1] or two [int-2] additional risk factors) differed significantly and had a favourable outcome compared with patients with advanced systemic mastocytosis (AdvSM). Lower panels (C, D) show that patients with advanced systemic mastocytosis and no additional risk factors (AdvSM-1) differed significantly from those with one (AdvSM-2), two to three (AdvSM-3), or four to five (AdvSM-4) risk factors. IPSM=international prognostic scoring system for mastocytosis.
In patients with advanced systemic mastocytosis, age 60 years or older (HR 2·14, 95% CI 1·42–3·22), tryptase 125 ng/mL or higher (1·81, 1·20–2·75), leukocytes 16 × 109 per L or higher (1·88, 1·27–2·79), haemoglobin 11 g/dL or lower (1·71, 1·13–2·57) platelets 100 × 109 per L or lower (1·63, 1·13–2·34), and skin involvement (0·46, 0·30–0·69) were independent prognostic factors for overall survival in multivariate analyses (table 2). These variables were used to optimise scoring in patients with advanced systemic mastocytosis. The score was applied in 229 (88%) of 259 patients for whom data for all variables were available. Every risk factor with an HR greater than 1·50 scored 1 point, and risk factors with an HR of 0·50 or lower scored –1 point. By adding all risk points, four different risk groups were established. Patients with scores from –1 to 0 points (no risk factors) were grouped in advanced systemic mastocytosis 1 (referred to as AdvSM-1), those with a score of 1 point (one risk factor) were in the advanced systemic mastocytosis 2 (AdvSM-2) group, individuals with scores of 2–3 points (two or three risk factors) were grouped in advanced systemic mastocytosis 3 (AdvSM-3), and patients with a score of 4 or 5 points (four or five risk factors) were included in the advanced systemic mastocytosis 4 (AdvSM-4) group.
These groupings were of prognostic significance for overall survival (p<0·0001; figure 3C). Overall survival of patients in risk groups AdvSM-1 and AdvSM-2 was similar to that of patients with non-advanced mastocytosis in the int-1 and int-2 risk groups, respectively (figure 3C). The significance of the score for advanced systemic mastocytosis was also confirmed for progression-free survival and event-free survival (p<0·0001; figure 3D; appendix p 17) and for the individual WHO entities of aggressive systemic mastocytosis, mast cell leukaemia, and systemic mastocytosis with an associated haematological neoplasm (appendix pp 18–20).
The IPSM was validated using data from the REMA registry for 413 (89%) patients with non-advanced mastocytosis and 49 (11%) with advanced systemic mastocytosis. Both scores showed significant results in the validation sample (p<0·0001 for overall survival, p<0·0001 for progression-free survival, and p<0·0001 for event-free survival), confirming the prognostic value and usefulness of the IPSM (appendix pp 21–23).
Discussion
Using data from a large cohort of patients in the ECNM registry, we identified independent prognostic variables for patients with non-advanced mastocytosis (age and alkaline phosphatase) and advanced systemic mastocytosis (tryptase, blood counts, and absence of skin involvement). Based on these variables, we established a simple prognostic score system, referred to as IPSM. The predictive value of this new score was confirmed in an independent validation cohort provided by the REMA.
So far, it remains unknown whether adults with cutaneous mastocytosis have a favourable outcome compared to patients with indolent systemic mastocytosis. In our study, patients with adulthood cutaneous mastocytosis had good overall survival, which was similar to that recorded in children with cutaneous mastocytosis and improved compared with overall survival of patients with indolent systemic mastocytosis or mastocytosis in the skin. The improved overall survival seen in children and adults with cutaneous mastocytosis could be explained by the lower numbers of cases with disease progression. Patients with mastocytosis in the skin and indolent systemic mastocytosis had similar overall survival, suggesting that most patients with mastocytosis in the skin might indeed suffer from indolent systemic mastocytosis, whereas adults with cutaneous mastocytosis have a better prognosis. So far, it remains unknown why adults with cutaneous mastocytosis have a better overall survival compared to patients with indolent systemic mastocytosis or mastocytosis in the skin. One explanation could be that indolent systemic mastocytosis is a more advanced disease with higher risk of progression to advanced systemic mastocytosis. An alternative explanation could be the higher median age of patients with mastocytosis in the skin (age 43 years) and indolent systemic mastocytosis (age 47 years) compared to adults with cutaneous mastocytosis (age 37 years).
Several clinical and laboratory parameters are prognostic variables for mastocytosis.7,10,12–19 In our study, amounts of alkaline phosphatase 100 U/L or higher and age 60 years or older were the two major independent predictors of survival in patients with non-advanced mastocytosis and were used to establish the IPSM. Alkaline phosphatase has already been shown to be of prognostic value in systemic mastocytosis in previous studies.12,13,20 A rise in the amount of alkaline phosphatase could reflect mastocytosis-mediated organ damage in the bones, liver, or both.13,20 High amounts of alkaline phosphatase found in some patients with non-advanced mastocytosis might, therefore, indicate clinically silent organ involvement. Such occult organ involvement could produce raised amounts of alkaline phosphatase even before the disease progresses to advanced systemic mastocytosis. Indeed, we found significantly more progressions in indolent or smouldering systemic mastocytosis to advanced systemic mastocytosis in patients with amounts of alkaline phosphatase of 100 U/L or higher. Surprisingly, in patients with advanced systemic mastocytosis, alkaline phosphatase was not of prognostic value, contrasting with findings of previous studies.13,20–22 However, in previous studies, the prognostic value of alkaline phosphatase was examined in the overall cohort of patients with non-advanced mastocytosis and advanced systemic mastocytosis,20,21 whereas in our study, patients with non-advanced mastocytosis and advanced systemic mastocytosis were analysed separately. Amounts of alkaline phosphatase might also fluctuate over time in individual patients, which could represent a limitation of this variable. However, a constantly increasing amount of alkaline phosphatase must raise suspicion of disease progression.
Analysing the median age of our patients, we saw that low-risk patients were younger (age 43 years) than patients in the int-1 group (age 56 years) and the int-2 group (age 64 years). A simple explanation for this observation could be that reduced life expectancy is mainly attributable to the older age of these patients. However, not only overall survival but also progression-free survival significantly differed among these patients. Thus, our results cannot only be accounted for by differences in the natural age-dependent life expectancy. With respect to progression-free survival, increased clonal instability in advanced age might contribute to higher progression rates. Indeed, the number of mutations in haemopoietic stem cells increases with age.23
Organomegaly has been shown to be of prognostic importance in systemic mastocytosis.13,20 In our study, organomegaly was not an independent prognostic factor for overall survival, which could be accounted for by the fact that organomegaly is represented in the WHO classification as either a B finding (ie, without organ damage) or as a C finding (ie, with organ damage caused by neoplastic mast cell infiltration).2,3
Patients with advanced systemic mastocytosis sometimes do not have skin involvement.2,3 In our study, the absence of skin lesions was of prognostic importance in the multivariate analysis of patients with advanced systemic mastocytosis, but not in the multivariate analysis of patients with non-advanced mastocytosis. This result has several explanations. First, it is well known that skin lesions are preferentially absent in patients with rapidly progressive aggressive systemic mastocytosis and mast cell leukaemia. Second, a subgroup of patients with indolent systemic mastocytosis have no skin lesions and their clinical course remains stable. Contrasting patients with advanced systemic mastocytosis, individuals with indolent systemic mastocytosis without skin lesions have a low mast cell burden and a favourable prognosis and are currently classified as isolated bone marrow mastocytosis as per the WHO classification.2,3
In patients with advanced systemic mastocytosis, disease biology and predictive variables are different from those of patients with non-advanced mastocytosis. Therefore, we analysed the effect of potential prognostic factors in patients with advanced systemic mastocytosis separately. Again, age was of prognostic importance. Other prognostic independent variables in patients with advanced systemic mastocytosis included elevated tryptase, abnormal blood counts, and absence of skin involvement. Using these variables, patients with advanced systemic mastocytosis were split into four risk groups, with significant differences with respect to overall survival, event-free survival, and progression-free survival. The prognosis of patients in the int-1 and int-2 risk groups overlapped with that of patients in the AdvSM-1 and AdvSM-2 groups, respectively. This observation supports the strengths and clinical relevance of the IPSM. Thus, the IPSM might identify patients at higher risk than expected by WHO classification.
Several attempts have been made to develop prognostic scoring systems in mastocytosis.13,20,21 However, these scores have limitations. First, most score studies have included relatively low numbers of patients and no validation cohort.13,20,21 Second, most of these studies did not take the different disease biology of non-advanced mastocytosis and advanced systemic mastocytosis into account. Moreover, these score models are based on datasets that include more than 50% of patients with advanced systemic mastocytosis, whereas in our cohort only 15% of all patients had advanced systemic mastocytosis, which is closer to the real-life situation (appendix p 39).13,20,21 The strength of the IPSM is that it is based on an unbiased statistical approach in more than 1000 patients, including all WHO variants of systemic mastocytosis at frequencies seen in daily practice. Moreover, prognostic factors were ascertained separately in patients with non-advanced mastocytosis and advanced systemic mastocytosis to establish optimal scoring models for both cohorts. Finally, our score was validated by an independent cohort from the REMA registry. The fact that the ECNM registry contains patients from many different centres and, thus, all categories of the disease in a rather balanced form also supports the strength of the IPSM. The IPSM incorporates the WHO classification and a few other simple variables, which provides a practicable method ready for use in patients with non-advanced mastocytosis and advanced systemic mastocytosis.
Other prognostic variables have been analysed previously in patients with systemic mastocytosis. Elevated β2-microglobulin, multilineage involvement, the KIT Asp816Val allele burden, and mutations in additional genes are of prognostic importance.13–16,24 Some of these markers, including β2-microglobulin or multilineage involvement, are not measured in daily practice in most centres, as confirmed by our study. Moreover, KIT Asp816Val is often analysed by conventional PCR but not by quantitative PCR. Other molecular markers were only available for a few patients in the ECNM registry, which shows that their use is still restricted to specialised centres. Moreover, molecular abnormalities are preferentially detected in patients who have advanced systemic mastocytosis, such as systemic mastocytosis with an associated haematological neoplasm, and are, therefore, not always WHO-independent variables. Finally, no standardised methodology for mutation analysis in mastocytosis is available to date. Nevertheless, such additional variables, and multilineage involvement, will soon be standardised and could support prognostication in the future.
In patients with non-advanced mastocytosis, progression to high-risk mastocytosis is a rare event and quality of life is most important and probably the key variable to look at when planning treatment.25,26 Indeed, most patients die from causes other than mastocytosis. However, it is important to identify those few cases who are at risk to progress after some time. Since patients with non-advanced mastocytosis in the int-1 or int-2 risk groups have a higher risk of progression to advanced systemic mastocytosis, these patients need to be monitored closely to detect progression and to define the right time for intervention.
Considering the availability of new disease-modifying approaches in advanced systemic mastocytosis, our score could be important for making treatment decisions in daily practice: first, the WHO-based diagnosis is established and, second, our score is applied. Patients with advanced systemic mastocytosis usually need cytoreductive treatment, but the exact type of treatment depends on patient-related factors, disease aggressiveness, and the presence or type of associated haematological neoplasm.27 At present, therapeutic options for slowly progressing advanced systemic mastocytosis include (offlabel) interferon alfa, cladribine, and midostaurin, which was approved for use by the US Food and Drug Administration and the European Medicines Agency in advanced systemic mastocytosis in 2017.27–29 By contrast, in patients with advanced systemic mastocytosis with rapid progression or mast cell leukaemia, polychemotherapy and, if possible, haemopoietic stem-cell transplantation are considered.30 However, the disease-modifying or curative potential of treatment has to be weighed against side-effects, and the IPSM might help with patients’ selection. For example, in older patients with slowly progressing advanced systemic mastocytosis in a low-risk group according to the IPSM, treatment with midostaurin or cladribine could be a reasonable option.29
Our study has several limitations. Because of the retrospective nature of data collection, only variables used in daily routine are regularly captured. Likewise, we cannot exclude that other markers (eg, β2-microglobulin, which was only available in 21% of all patients) would have added to prognostication when examined in all individuals and included in a scoring system. Moreover, patients are usually added to registries over a prolonged period, and diagnostic assays, standards, and sensitivity of molecular tests can change. Further, standards of clinical assessment and staging can change and might lead to earlier disease detection. For example, in patients with non-advanced mastocytosis, a diagnostic delay was sometimes suspected because first symptoms are reported by patients long before a diagnosis is established. In some patients, the initial diagnosis might have been cutaneous mastocytosis but, because of delay, the patient already had indolent systemic mastocytosis when diagnosed. However, it is almost impossible to prove that self-reported symptoms are related to mastocytosis. In the ECNM registry, data were entered in a standardised way in a central web-based registry and were checked regularly for correctness and plausibility. To guarantee data quality, a yearly data-clearing process was done. Thus, our score is based on a robust and representative database.
In summary, the IPSM can optimise prognostication of patients with non-advanced and advanced systemic mastocytosis to better guide future interventions in these patients.
Supplementary Material
Research in context.
Evidence before the study
We searched PubMed for articles published before June 7, 2019, containing information about individual prognostic variables and scoring systems established in mastocytosis. We did not restrict our search by language or type of article. Prognostication in mastocytosis is based mainly on the WHO classification.
Several prognostic variables have been identified, including absence of skin lesions, multilineage involvement with KIT Asp816Val, mutations in genes other than KIT, raised amounts of β2-microglobulin in serum, or increased alkaline phosphatase. However, these variables have only been validated in a limited number of patients and only a few prognostic scoring systems are available. The Mayo score includes the WHO classification, age, platelet count, anaemia, alkaline phosphatase, and somatic mutations as major prognostic variables. Similar variables were identified and used in the Mannheim score. The Mayo and Mannheim scoring systems are based on a limited number of cases and have not been validated in independent cohorts so far. Previous prediction models have not addressed the important point that patients with non-advanced systemic mastocytosis and advanced systemic mastocytosis are subgroups of disease with completely different biology, disease course, survival outcomes, and patterns of prognostic factors.
Added value of this study
We established a new score, termed the International Prognostic Scoring System for Mastocytosis (IPSM), that was optimised for prognostication in patients with non-advanced mastocytosis and advanced systemic mastocytosis. This new score improves prognostication when compared with the WHO classification and other scoring models. Validation of our score by an independent sample-cohort confirmed its prognostic value.
Implications of all the available evidence
Several clinical studies have analysed the importance of prognostic variables in patients with mastocytosis, and attempts have been made to develop a multiparametric scoring system in this disease. In these scores, multilineage involvement of leukocytes with KIT Asp816Val, the KIT Asp816Val allele burden, or mutations in additional genes were included as prognostic variables. However, such markers are only available in a few specialised centres. Moreover, multilineage involvement and additional molecular abnormalities are preferentially detected in patients who have advanced systemic mastocytosis; thus, the contribution of these variables is limited. We based our new scoring system on simple variables that are checked regularly in daily practice. The resulting score (IPSM) is a useful and practicable method to identify patients with a higher risk of progression or death than would have been expected from the WHO classification. As our score is simple and ready for use, it should add substantially to management and patients’ selection for various treatments in daily practice.
Acknowledgments
This work was supported by the Austrian Science Fund (SFB grants F4701-B20 and F4704-B20, to PV), Deutsche Forschungsgemeinschaft (RA 2838, to AI), Koeln Fortune Program, Faculty of Medicine, University of Cologne (216/2016, to AI), Charles and Ann Johnson Foundation (to JG), and Instituto de Salud Carlos III and fondos FEDER (PI16/00642 and CB16/12/00400, to AO). VS is a senior clinical researcher of the Research Foundation Flanders/Fonds Wetenschappelijk Onderzoek (1804518N). CB is supported by the Clinical Research Fund of the University Hospitals Leuven. We thank all centres and experts in the European Competence Network on Mastocytosis (ECNM) registry group for contributing data and patients; the scientific advisors of the ECNM for their contribution to the project discussion; all technicians, study coordinators, study nurses, and colleagues for data entry into the registry system; and Susanne Herndlhofer, Nadja Jaekel, Hassiba Bouktit, Gabriele Stefanzl, Hana Škabrahová, Gulkan Ozkan, Tarik Tiryaki, Nicole Cabral do O, Luigi Scaffidi Cecilia Spina, Kerstin Hamberg-Levedahl, Pietro Benvenuti, Gregor Verhoef, Peter Vandenberghe, Dominique Bullens, Marie-Anne Morren, Nele Philips, and Stephanie Pulfer for data management and data controlling. This paper is dedicated to Luis Escribano and his contributions to ECNM and the development of our ECNM registry projects.
Footnotes
Contributors
WRS and PV designed the study. WRS, MK, and PV contributed to data analysis and data interpretation. WRS, IA-T, BvA, JNGOE, AG, MN, KVG, EH, RZ, PB, MB, CP, AI, CE, SM, KS, NvB, RP, MJ, ABF, FC, KB, AZ, DF, AJK, ASY, MD, HH, JP, VS, AB, DN, CB, KH, MT, BN, AR, AO, OH, JG, MA, HCK-N, and PV contributed to data collection. WRS, AR, AO, JG, MA, HCK-N, and PV contributed to writing of the report. All authors critically reviewed the report and approved the final version.
Declaration of interests
WRS declares honoraria from AbbVie, Amgen, Celgene, Daiichi Sankyo, Deciphera, Incyte, Jazz, Novartis, Pfizer, and Thermo Fisher; and travel grants from Pfizer and Roche. BvA, IA-T, and ASY declare honoraria from Novartis. KVG declares honoraria from Pfizer, Novartis, Roche, BMS, Sanofi, and Incyte; and travel grants from Roche and AbbVie. RZ declares honoraria from Deciphera, Novartis, and Takeda. MB declares honoraria from Pfizer, Amgen, and Incyte; and research funding from Novartis. CE declares honoraria from Novartis and Pfizer. KS declares honoraria from Novartis; and travel grants from AbbVie. NvB declares honoraria from AstraZeneca, Amgen, Novartis, and BMS; and research funding from Novartis. AZ declares honoraria from or has participated in trials for AbbVie, Almirall, Beiersdorf Dermo Medical, Bencard Allergie, BMS, Celgene, Eli Lilly, GSK, Janssen-Cilag, Miltenyi Biotec, Novartis, Sanofi-Aventis, and Takeda Pharma. DF declares honoraria from Novartis, Pfizer, and Roche; and travel grants from Roche. JP declares honoraria from Exion, BMS, Boehringer Ingelheim, Grünenthal, MSD, Novartis, Pfizer, Chugai, and Roche. DN declares research funding from Novartis; and non-financial support from Amgen and Cellectis. CB declares honoraria from Thermo Fisher Phadia; and travel grants from Shire/Takeda, HAL, and ALK. KH declares honoraria from ALK, Blueprint, Deciphera, and Novartis; and research funding from Euroimmun. MT declares honoraria from Blueprint, Deciphera, and Novartis. AR declares honoraria from Blueprint, Deciphera, Incyte, and Novartis. AO declares honoraria from Becton Dickinson, Janssen, and Novartis; research funding from Becton Dickinson, Cytognos, and Immunostep; and is an inventor on patents licensed to Cytognos, Immunostep, Fagotrace, and Becton-Dickinson that return royalties to the University of Salamanca and EuroFlow. OH declares honoraria from ABscience; and research funding from Alexion, Celgene, and Novartis. JG declares honoraria and research funding from Blueprint, Deciphera, and Novartis. MA declares honoraria from Deciphera, Allakos, and Blueprint; and research funding from Allakos and Blueprint. HCK-N declares research funding from Novartis. PV declares honoraria from Blueprint, Celgene, Deciphera, Incyte, Novartis, and Pfizer; and research funding from Pfizer, Incyte, and Celgene. MK, JNGOE, AG, MN, EH, PB, CP, AI, SM, RP, MJ, ABF, FC, KB, AJK, MD, HH, VS, AB, and BN declare no competing interests.
Contributor Information
Wolfgang R Sperr, Department of Internal Medicine I, Division of Hematology and Hemostaseology, and Ludwig Boltzmann Institute for Hematology and Oncology, Medical University of Vienna, Vienna, Austria.
Prof Michael Kundi, Institute of Environmental Health Medical University of Vienna, Vienna, Austria.
Ivan Alvarez-Twose, Instituto de Estudios de Mastocitosis de Castilla La Mancha (CLMast), Hospital Virgen del Valle, Toledo, Spain.
Bjorn van Anrooij, Department of Hematology, University Medical Center Groningen, University of Groningen, Groningen, Netherlands; Department of Allergology, University Medical Center Groningen, University of Groningen, Groningen, Netherland.
Joanna N G Oude Elberink, Department of Allergology, University Medical Center Groningen, University of Groningen, Groningen, Netherlands.
Aleksandra Gorska, Department of Allergology, Medical University of Gdańsk, Gdańsk, Poland.
Prof Marek Niedoszytko, Department of Allergology, Medical University of Gdańsk, Gdańsk, Poland.
Karoline V Gleixner, Department of Internal Medicine I, Division of Hematology and Hemostaseology, and Ludwig Boltzmann Institute for Hematology and Oncology, Medical University of Vienna, Vienna, Austria.
Emir Hadzijusufovic, Department of Internal Medicine I, Division of Hematology and Hemostaseology, and Ludwig Boltzmann Institute for Hematology and Oncology; Medical University of Gdansk, Gdansk, Poland; Internal Medicine Small Animals, University Clinic for Small Animals, Department/University Clinic for Companion Animals and Horses, University of Veterinary Medicine, Vienna, Austria.
Roberta Zanotti, Section of Hematology, Department of Medicine, Verona University Hospital, Verona, Italy.
Patrizia Bonadonna, Section of Hematology, Department of Medicine and Allergy Unit,Verona University Hospital, Verona, Italy.
Massimiliano Bonifacio, Section of Hematology, Department of Medicine and Allergy Unit, Verona University Hospital, Verona, Italy.
Cecelia Perkins, Verona University Hospital, Verona, Italy; Division of Hematology, Department of Medicine, Stanford University School of Medicine, Stanford, CA, USA.
Anja Illerhaus, Department of Dermatology, University of Cologne, Cologne, Germany.
Chiara Elena, Department of Molecular Medicine and Department of Hematology Oncology, University of Pavia and Fondazione IRCCS Policlinico San Matteo, Pavia, Italy.
Serena Merante, Department of Molecular Medicine and Department of Hematology Oncology, University of Pavia and Fondazione IRCCS Policlinico San Matteo, Pavia, Italy.
Khalid Shoumariyeh, Department of Hematology, Oncology and Stem Cell Transplantation, Medical Center, Faculty of Medicine, University of Freiburg, Freiburg, Germany.
Prof Nikolas von Bubnoff, Department of Hematology, Oncology and Stem Cell Transplantation, Medical Center, Faculty of Medicine, University of Freiburg, Freiburg, Germany; Department of Hematology and Oncology, Medical Center, University of Schleswig-Holstein, Campus Lübeck, Lübeck, Germany.
Roberta Parente, Division of Allergy and Clinical Immunology, University of Salerno, Salerno, Italy.
Mohamad Jawhar, III Medizinische Klinik, Universitätsmedizin Mannheim, Universität Heidelberg, Mannheim, Germany.
Anna Belloni Fortina, Pediatric Dermatology Unit, Department of Medicine, University of Padua, Padua, Italy.
Francesca Caroppo, Pediatric Dermatology Unit, Department of Medicine, University of Padua, Padua, Italy.
Knut Brockow, Department of Dermatology and Allergy, School of Medicine, Technical University of Munich, Munich, Germany.
Alexander Zink, Department of Dermatology and Allergy, School of Medicine, Technical University of Munich, Munich, Germany.
David Fuchs, University Clinic for Hematology and Internal Oncology, Kepler University Hospital, Johannes Kepler University, Linz, Austria.
Alex J Kilbertus, University Clinic for Hematology and Internal Oncology, Department of Dermatology and Venerology, Kepler University Hospital, Johannes Kepler University, Linz, Austria.
Akif Selim Yavuz, Kepler University Hospital, Johannes Kepler University, Linz, Austria; Division of Hematology, Istanbul Medical School, University of Istanbul, Istanbul, Turkey.
Michael Doubek, University Hospital and CEITEC Masaryk University, Brno, Czech Republic.
Hans Hägglund, Division of Hematology, Department of Medical Sciences Uppsala University, Uppsala, Sweden.
Jens Panse, Department of Oncology, Haemostaseology and Stem Cell Transplantation, University Hospital RWTH Aachen Haematology, Aachen, Germany.
Vito Sabato, Faculty of Medicine and Health Sciences, Department of Immunology-Allergology-Rheumatology, University of Antwerp and Antwerp University Hospital, Antwerp, Belgium.
Agnes Bretterklieber, Department of Dermatology and Venereology, University Hospital Graz, Graz, Austria.
Prof Dietger Niederwieser, University Hospital of Leipzig, Leipzig, Germany.
Christine Breynaert, KU Leuven Department of Microbiology, Immunology and Transplantation, Allergy and Clinical Immunology Research Group and MASTeL, University Hospitals Leuven, Leuven, Belgium.
Prof Karin Hartmann, Department of Dermatology, University of Cologne, Cologne, Germany; Division of Allergy, Department of Dermatology, and Department of Biomedicine, University of Basel, Basel, Switzerland.
Prof Massimo Triggiani, Division of Allergy and Clinical Immunology, University of Salerno, Salerno, Italy.
Boguslaw Nedoszytko, Department of Dermatology, Medical University of Gdańsk, Gdańsk, Poland.
Andreas Reiter, III Medizinische Klinik, Universitätsmedizin Mannheim, Universität Heidelberg, Mannheim, Germany.
Prof Alberto Orfrao, Centro de Investigación del Cáncer/IBMCC (USAL/CSIC), CIBERONC and IBSAL, Departamento de Medicina and Servicio General de Citometría, University of Salamanca, Salamanca, Spain.
Prof Olivier Hermine, Imagine Institute Université Paris Descartes, Sorbonne, Paris Cité, Centre national de référence des mastocytoses, Paris, France.
Prof Jason Gotlib, Verona University Hospital, Verona, Italy; Division of Hematology, Department of Medicine, Stanford University School of Medicine, Stanford, CA, USA.
Prof Michel Arock, Imagine Institute Université Paris Descartes, Sorbonne, Paris Cité, Centre national de référence des mastocytoses, Paris, France and Department of Hematological Biologie, Pitié-Salpêtrière Hospital, Paris Sorbonne University, Paris UMR8113, Ecole, France.
Prof Hanneke C Kluin-Nelemans, Department of Hematology, University Medical Center Groningen, University of Groningen, Groningen, Netherlands.
Peter Valent, Department of Internal Medicine I, Division of Hematology and Hemostaseology, and Ludwig Boltzmann Institute for Hematology and Oncology, Medical University of Vienna, Vienna, Austria.
References
- 1.Cohen SS, Skovbo S, Vestergaard H, et al. Epidemiology of systemic mastocytosis in Denmark. Br J Haematol. 2014;166:521–28. doi: 10.1111/bjh.12916. [DOI] [PubMed] [Google Scholar]
- 2.Valent P, Akin C, Metcalfe DD. Mastocytosis: 2016 updated WHO classification and novel emerging treatment concepts. Blood. 2017;129:1420–27. doi: 10.1182/blood-2016-09-731893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Valent P, Horny HP, Escribano L, et al. Diagnostic criteria and classification of mastocytosis: a consensus proposal. Leuk Res. 2001;25:603–25. doi: 10.1016/s0145-2126(01)00038-8. [DOI] [PubMed] [Google Scholar]
- 4.Lim KH, Tefferi A, Lasho TL, et al. Systemic mastocytosis in 342 consecutive adults: survival studies and prognostic factors. Blood. 2009;113:5727–36. doi: 10.1182/blood-2009-02-205237. [DOI] [PubMed] [Google Scholar]
- 5.Valent P, Akin C, Escribano L, et al. Standards and standardization in mastocytosis: consensus statements on diagnostics, treatment recommendations and response criteria. Eur J Clin Invest. 2007;37:435–53. doi: 10.1111/j.1365-2362.2007.01807.x. [DOI] [PubMed] [Google Scholar]
- 6.Valent P, Oude Elberink JNG, Gorska A, et al. The data registry of the European Competence Network on Mastocytosis (ECNM): set up, projects, and perspectives. J Allergy Clin Immunol Pract. 2019;7:81–87. doi: 10.1016/j.jaip.2018.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sperr WR, Escribano L, Jordan JH, et al. Morphologic properties of neoplastic mast cells: delineation of stages of maturation and implication for cytological grading of mastocytosis. Leuk Res. 2001;25:529–36. doi: 10.1016/s0145-2126(01)00041-8. [DOI] [PubMed] [Google Scholar]
- 8.Akin C, Metcalfe DD. Systemic mastocytosis. Annu Rev Med. 2004;55:419–32. doi: 10.1146/annurev.med.55.091902.103822. [DOI] [PubMed] [Google Scholar]
- 9.Metcalfe DD. Mast cells and mastocytosis. Blood. 2008;112:946–56. doi: 10.1182/blood-2007-11-078097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Escribano L, Díaz-Agustín B, Bellas C, et al. Utility of flow cytometric analysis of mast cells in the diagnosis and classification of adult mastocytosis. Leuk Res. 2001;25:563–70. doi: 10.1016/s0145-2126(01)00050-9. [DOI] [PubMed] [Google Scholar]
- 11.Sperr WR, Jordan JH, Fiegl M, et al. Serum tryptase levels in patients with mastocytosis: correlation with mast cell burden and implication for defining the category of disease. Int Arch Allergy Immunol. 2002;128:136–41. doi: 10.1159/000059404. [DOI] [PubMed] [Google Scholar]
- 12.Escribano L, Alvarez-Twose I, Sánchez-Muñoz L, et al. Prognosis in adult indolent systemic mastocytosis: a long-term study of the Spanish Network on Mastocytosis in a series of 145 patients. J Allergy Clin Immunol. 2009;124:514–21. doi: 10.1016/j.jaci.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 13.Jawhar M, Schwaab J, Hausmann D, et al. Splenomegaly, elevated alkaline phosphatase and mutations in the SRSF2/ASXL1/RUNX1 gene panel are strong adverse prognostic markers in patients with systemic mastocytosis. Leukemia. 2016;30:2342–50. doi: 10.1038/leu.2016.190. [DOI] [PubMed] [Google Scholar]
- 14.Teodosio C, Mayado A, Sanchez-Muñoz L, et al. The immunophenotype of mast cells and its utility in the diagnostic work-up of systemic mastocytosis. J Leukoc Biol. 2015;97:49–59. doi: 10.1189/jlb.5RU0614-296R. [DOI] [PubMed] [Google Scholar]
- 15.Pardanani A, Reichard KK, Zblewski D, et al. CD123 immunostaining patterns in systemic mastocytosis: differential expression in disease subgroups and potential prognostic value. Leukemia. 2016;30:914–18. doi: 10.1038/leu.2015.348. [DOI] [PubMed] [Google Scholar]
- 16.Hoermann G, Gleixner KV, Dinu GE, et al. The KIT D816V allele burden predicts survival in patients with mastocytosis and correlates with the WHO type of the disease. Allergy. 2014;69:810–13. doi: 10.1111/all.12409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwaab J, Schnittger S, Sotlar K, et al. Comprehensive mutational profiling in advanced systemic mastocytosis. Blood. 2013;122:2460–66. doi: 10.1182/blood-2013-04-496448. [DOI] [PubMed] [Google Scholar]
- 18.Jawhar M, Schwaab J, Schnittger S, et al. Additional mutations in SRSF2, ASXL1 and/or RUNX1 identify a high-risk group of patients with KIT D816V+ advanced systemic mastocytosis. Leukemia. 2016;30:136–43. doi: 10.1038/leu.2015.284. [DOI] [PubMed] [Google Scholar]
- 19.Greiner G, Witzeneder N, Berger A, et al. CCL2 is a KIT D816V-dependent modulator of the bone marrow microenvironment in systemic mastocytosis. Blood. 2017;129:371–82. doi: 10.1182/blood-2016-09-739003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pardanani A, Lasho TL, Reichard KK, Hanson CA, Tefferi A. World Health Organization class-independent risk categorization in mastocytosis. Blood Cancer J. 2019;9:29. doi: 10.1038/s41408-019-0189-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pardanani A, Shah S, Mannelli F, et al. Mayo alliance prognostic system for mastocytosis: clinical and hybrid clinical-molecular models. Blood Adv. 2018;2:2964–72. doi: 10.1182/bloodadvances.2018026245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jawhar M, Schwaab J, Naumann N, et al. Response and progression on midostaurin in advanced systemic mastocytosis: KIT D816V and other molecular markers. Blood. 2017;130:137–45. doi: 10.1182/blood-2017-01-764423. [DOI] [PubMed] [Google Scholar]
- 23.Steensma DP, Bejar R, Jaiswal S, et al. Clonal hematopoiesis of indeterminate potential and its distinction from myelodysplastic syndromes. Blood. 2015;126:9–16. doi: 10.1182/blood-2015-03-631747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muñoz-González JI, Jara-Acevedo M, Alvarez-Twose I, et al. Impact of somatic and germline mutations on the outcome of systemic mastocytosis. Blood Adv. 2018;2:2814–28. doi: 10.1182/bloodadvances.2018020628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siebenhaar F, von Tschirnhaus E, Hartmann K, et al. Development and validation of the mastocytosis quality of life questionnaire: MC-QoL. Allergy. 2016;71:869–77. doi: 10.1111/all.12842. [DOI] [PubMed] [Google Scholar]
- 26.Hermine O, Lortholary O, Leventhal PS, et al. Case-control cohort study of patients’ perceptions of disability in mastocytosis. PLoS One. 2008;3:e2266. doi: 10.1371/journal.pone.0002266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gotlib J, Kluin-Nelemans HC, George TI, et al. Efficacy and safety of midostaurin in advanced systemic mastocytosis. N Engl J Med. 2016;374:2530–41. doi: 10.1056/NEJMoa1513098. [DOI] [PubMed] [Google Scholar]
- 28.Kluin-Nelemans HC, Jansen JH, Breukelman H, et al. Response to interferon alfa-2b in a patient with systemic mastocytosis. N Engl J Med. 1992;326:619–23. doi: 10.1056/NEJM199202273260907. [DOI] [PubMed] [Google Scholar]
- 29.Barete S, Lortholary O, Damaj G, et al. Long-term efficacy and safety of cladribine (2-CdA) in adult patients with mastocytosis. Blood. 2015;126:1009–16. doi: 10.1182/blood-2014-12-614743. [DOI] [PubMed] [Google Scholar]
- 30.Ustun C, Reiter A, Scott BL, et al. Hematopoietic stem-cell transplantation for advanced systemic mastocytosis. J Clin Oncol. 2014;32:3264–74. doi: 10.1200/JCO.2014.55.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.