Abstract
Several studies have reported associations of hypertension with cancer, but not all results were conclusive. We examined the association of systolic (SBP) and diastolic (DBP) blood pressure with the development of incident cancer at all anatomical sites in the European Prospective Investigation into Cancer and Nutrition (EPIC). Hazard ratios (HR) (95% confidence intervals) were estimated using multivariable Cox proportional hazards models, stratified by EPIC-participating centre and age at recruitment, and adjusted for sex, education, smoking, body mass index, physical activity, diabetes and dietary (in women also reproductive) factors. The study included 307,318 men and women, with an average follow-up of 13.7 (standard deviation 4.4) years and 39,298 incident cancers. We confirmed the expected positive association with renal cell carcinoma: HR=1.12 (1.08-1.17) per 10mmHg higher SBP and HR=1.23 (1.14-1.32) for DBP. We additionally found positive associations for esophageal squamous cell carcinoma (SCC): HR=1.16 (1.07-1.26) (SBP), HR=1.31 (1.13-1.51) (DBP), weaker for head and neck cancers: HR=1.08 (1.04-1.12) (SBP), HR=1.09 (1.01-1.17) (DBP) and, similarly, for skin SCC, colon cancer, post-menopausal breast cancer and uterine adenocarcinoma (AC), but not for esophageal AC, lung SCC, lung AC, or uterine endometroid cancer. We observed weak inverse associations of SBP with cervical SCC: HR=0.91 (0.82-1.00) and lymphomas: HR=0.97 (0.93-1.00). There were no consistent associations with cancers in other locations.
Our results are largely compatible with published studies and support weak associations of blood pressure with cancers in specific locations and morphologies.
Keywords: cancer, hypertension, morphology, cohort, Europe, epidemiology, association, risk factors
Introduction
Hypertension and cancer are complex multifactorial conditions. Hypertension is a worldwide public health challenge, with systolic blood pressure (SBP) above 115mmHg ranked as the leading risk factor for the global burden of disease in 2017 [1]. The global age-standardised prevalence of raised blood pressure (SBP≥140mmHg or diastolic BP DBP≥90mmHg) in adults was estimated as ≥20% in 2015 [2]. However, whilst hypertension is a major risk factor for coronary heart disease and stroke, the evidence is much weaker for an association with cancer [3].
A meta-analysis of ten longitudinal studies published in 2002 found that individuals with hypertension had higher risk of total cancer mortality: odds ratio OR=1.23 (95% confidence interval (CI) 1.11-1.36), largely explained, based on 13 case-control studies, by a positive association for renal cell carcinoma (RCC) mortality: OR=1.75 (1.61-1.90) [4]. The results of subsequent studies have confirmed that hypertension is associated with a higher incidence of RCC [5–8]. Whilst, hypertension has not attracted much attention as a risk factor for other cancers, recent meta-analyses of observational studies, although summarising only 5 to 12 prospective studies and with large between-study heterogeneity, have reported higher risks for endometrial, prostate, postmenopausal breast and colorectal cancer, comparing hypertensive with normotensive participants [9–15]. Further, the largest to date prospective study examining the association of individual components of the metabolic syndrome (including BP measurements) and cancer in over half a million participants from Norway, Sweden and Austria: the Metabolic syndrome and Cancer project (Me-Can) [16], reported positive associations of high BP with the risk of cancers in locations other than the kidney in both men (oropharynx, colon, rectum and anus, lung with larynx and trachea, bladder, malignant melanoma, non-melanoma skin cancer) and women (liver, pancreas, corpus uteri, cervix, malignant melanoma) [3].
It is not clear whether the association of hypertension with cancer is causal or it could, at least partially, be explained by reverse causality or other biases. It is, however, possible that risk factors and mechanisms of pathogenesis are shared by the two conditions. For example, it has been hypothesized that predisposition to cancer is increased by chronic inflammation [17] and vascular inflammation could be involved in the pathogenesis of hypertension [18]. Lipid peroxidation, associated with hypertension and obesity, has also been proposed as a mechanism responsible for higher risk of RCC [19]. Further, experimental studies have implicated a potential role of the renin-angiotensin-aldosterone system (which regulates BP) in the biological processes of cellular proliferation, inflammation, angiogenesis, and tissue remodeling [20]. Studies in mice have also provided preliminary evidence that blockade of the angiotensin II type 1 receptor attenuates the growth and metastatic potential of RCC [21].
In the context of the above considerations, the aim of our study was to further explore the association between hypertension and cancer. We examined whether measured SBP and DBP were associated with the risk of incident cancer at all anatomical sites in the large and well-established European Prospective Investigation into Cancer and Nutrition (EPIC) cohort, after taking into account obesity smoking and other lifestyle and dietary factors (for both men and women) and indicators of sexual maturation and reproductive life (for women), which could be potential confounders or shared risk factors for cancer and hypertension.
Materials and Methods
Study population
EPIC is an ongoing, multicentre, prospective cohort study designed to investigate the associations between diet, lifestyle, and various medical and environmental risk factors with the incidence of cancer and other diseases. The source population (the majority aged between 25 and 70 years at the time of enrolment) and data collection methods have been described in detail previously [22]. Approval for this study was obtained from the ethical review boards of the International Agency for Research on Cancer and from all the EPIC participating centres. Written informed consent was obtained from all participants before entry into the study.
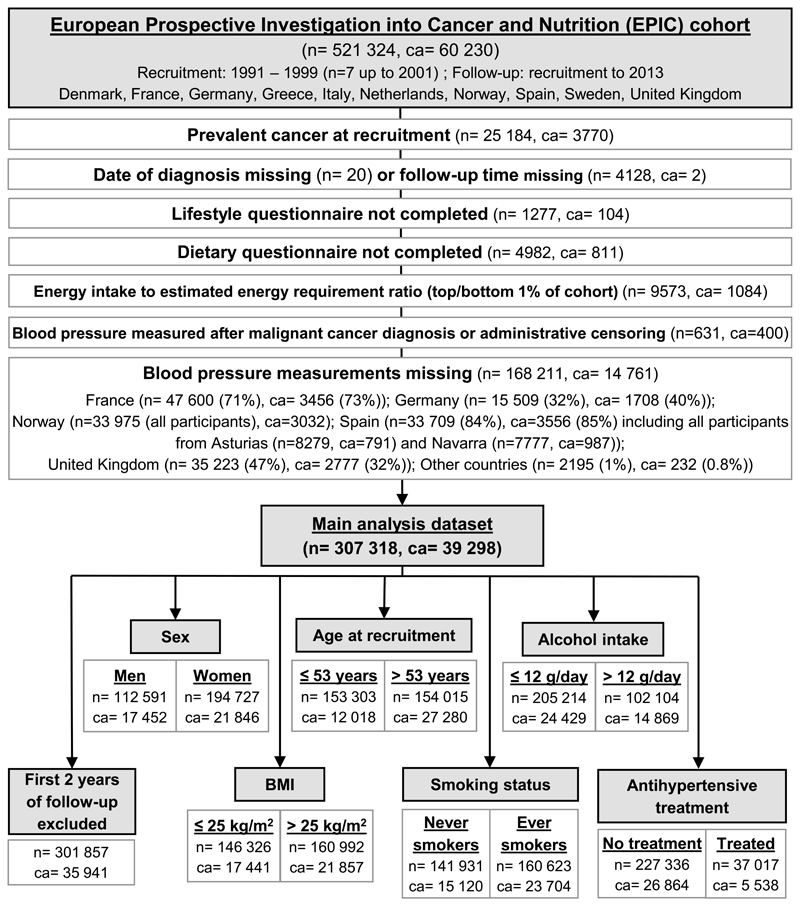
Figure 1 shows a detailed flow-chart of study participants with exclusions.
Figure 1. Flow diagram for EPIC participants included in the current study.
n – number of participants; ca – number of cancer cases.
Assessment of blood pressure and other variables
SBP and DBP were measured in millimeters of mercury (mmHg) by trained personnel. Measurements were obtained during a visit to an EPIC centre and within 6 months of recruitment in 91.5% of participants, except in France: all measurements obtained at the blood-collection visit, 3.8 years (standard deviation (SD) 1.3) after recruitment; Granada: 39.2% after 2.0 (0.9) years; San Sebastian: 78.4% after 1.6 (0.7) years; Oxford: 29.3 % after 0.8 (0.2) years. Two readings (1-5 minutes apart) were performed with a standard mercury manometer or oscillometric device on the right arm in a sitting position (after at least 5 minutes initial resting time). The average value was used as the exposure variable, assuming differences are due to random measurement error. Exceptions were the Danish and Swedish centres, where one single measurement was taken in the supine position. Mean BP (MBP) was defined as (1/3)*SBP+(2/3)*DBP [23] and not as the mean of SBP and DBP (mid-BP), used in the Me-Can study [3]. Self-reported information on treatment with antihypertensive medication (at baseline and/or in the past) was available for 264,353 participants (86.9%), of which 37,017 (14%) were receiving or had received treatment.
Information on socio-demographics, lifestyle characteristics, medical history and dietary intake was collected via questionnaires at the time of recruitment. Weight and height were measured at recruitment using a standardised protocol, except for part of the Oxford cohort and France, where height and weight were self-reported. Body mass index (BMI) was calculated as weight/height2 (kg/m2). Food and nutrient intakes were estimated from country-specific baseline dietary questionnaires [22]. A physical activity index was derived as previously described [24].
Assessment of cancer
Incident cancer cases were identified through population cancer registries in Denmark, Italy, the Netherlands, Spain, Sweden and the United Kingdom. In France, Germany and Greece, a combination of methods was used including health insurance records, cancer pathology registries and active follow-up of study participants and their next of kin. Cancer incidence data were coded according to the International Classification of Diseases for Oncology (ICD-O) [25]. The presented analyses are focused on the first primary neoplasm. Participants subsequently diagnosed with a second (or third) cancer, were censored at the date of diagnosis of the first cancer. We considered a joint group of any cancer and location (ICD-O behavioral code 3 (malignant, primary site)) and separate groups for all major anatomic sites (excluding rare morphologies), with further subdivisions for specific locations or major morphologies (Supplementary Table 1).
Statistical analyses
Hazard ratios (HRs) (95% CIs) were estimated using delayed-entry Cox proportional hazards models, with age at recruitment (5-year categories) and EPIC centre (n=25) as stratification variables. Origin of time was the date of birth, aligning individuals by birth cohort. Entry time was the date of BP measurement. Time of censorship was the date of first incidence of cancer (recruitment to 2013), or death, or last complete follow-up, whichever occurred first.
The main analyses examined the following exposures: SBP and DBP (considered in separate models and each as a continuous (per 10mmHg) variable); hypertension (defined as a dichotomous (yes/no) variable, according to BP measurements (SBP≥140mmHg, or DBP≥90mmHg) or self-reported information, and antihypertensive treatment in hypertensive individuals (a binary variable defined according to self-reported antihypertensive treatment (yes/no) for individuals fulfilling the hypertension criteria specified above). The latter analysis aimed to examine potential associations of antihypertensive drugs, as exogenous chemicals, and cancer. Although the results for hypertension (yes/no) would be useful for meta-analyses, the risk estimates per 10 mmHg, based on the complete range of SBP and DBP, would be more informative than a dichotomous simplification. The secondary analyses examined SBP and DBP as categorical variables, with categories based on the definitions of the American (ASH) [26] and the European Society of Hypertension (ESH) [27] (Supplementary Table 2), and MBP, as a continuous variable and also categorised using cohort-wide quartiles (cut-points at 88.8, 96.7, 106.0 mmHg). To test for trend, BP categories were analysed as continuous variables, after assigning participants an ordinal score.
All statistical models were adjusted for the categorical variables listed in Supplementary Table 2. Missing values were assigned to separate categories. For pre-menopausal breast cancers, analyses were restricted to participants with pre-menopausal status at recruitment (if known), or under the age of 46 (for unknown menopausal status) and were not adjusted for menopausal status or age at menopause. If breast cancer diagnosis was before 46 years of age, participants were considered “cases”, otherwise they were censored at 46 years, if not censored by age 46 for death, loss to follow-up or other cancer. For post-menopausal breast cancer, analyses were restricted to participants with physiological or surgical menopause at recruitment, or with age≥55 years (for unknown menopausal status).
Additionally, associations of BP with cancer were examined in strata according to age (cut-point at 53 years, the cohort-wide median), sex, BMI (cut-point at 25kg/m2), smoking status (ever smokers vs never smokers), alcoholic beverages intake (cut-point at 12g ethanol/day, the largest ethanol unit used in Europe) and use of antihypertensive treatment in individuals with available information for the stratifying factor. Likelihood ratio tests, comparing nested models with and without the addition of interaction terms, were used to test for statistical interactions on multiplicative scale. BP categories were included in the interaction models as ordinal variables. Examining potential biological interactions was beyond the scope of this study.
Sensitivity analyses were also performed, excluding the first two years of follow-up (to explore possible reverse causation). Crude estimates of HR (omitting the adjustment variables, but retaining the stratification by age at recruitment and study centre) were calculated to examine the influence of adjustment.
All analyses were performed with STATA version 13 software. Plots and data summaries were generated in R version 3.4.3.
Results and Discussion
Characteristics of study participants
The study cohort consisted of 307,318 individuals (63.4% women), with a mean age 52.5 (SD=9.9) years at recruitment. During an average follow-up of 13.7 (SD=4.4) years, 39,298 incident cancers were diagnosed, with major anatomical sites: breast (n=8,154 cases), prostate (n=5,848), colorectum (n=4,625) and lung (n=3,229). Mean SBP was 131.5mmHg (SD=19.7) and mean DBP was 81.1mmHg (SD=10.9). BP measurements and numbers of cancer cases are summarised by country in Supplementary Table 3. Participants with higher SBP or DBP were older, more likely to be men, to have low education or physical activity level, to have higher BMI, to have diabetes mellitus and to consume more alcohol and red meat, but less fruit and vegetables. Women with higher SBP or DBP were less likely to have ever used oral contraceptives. Cohort characteristics are summarised by hypertension and treatment status in Table 1 and by BP categories in Supplementary Table 4.
Table 1. Baseline demographic, lifestyle and reproductive characteristics by hypertension and treatment status.
| Characteristics (men and women) | Total | No hypertension | Hypertension | Untreated hypertension | Treated hypertension |
|---|---|---|---|---|---|
| Cohort size | 307318 | 174179 | 133139 | 73714 | 37017 |
| Female | 194727 (63.4) | 117264 (67.3) | 77463 (58.2) | 41779 (56.7) | 23736 (64.1) |
| Age at recruitment, years | 52.5 (9.9) | 49.8 (9.8) | 56.1 (8.7) | 54.9 (8.6) | 57.8 (7.6) |
|
| |||||
| Body Mass Index, kg/m2 | 25.8 (4.2) | 24.8 (3.7) | 27.1 (4.5) | 26.7 (4.3) | 28.2 (4.7) |
| Alcohol intake, g/day | 12.4 (17.1) | 11.2 (15.2) | 13.9 (19.2) | 15.4 (20.2) | 12.3 (18.0) |
| Fruit consumption, g/day | 230.6 (176.7) | 235.2 (182.3) | 224.6 (169.0) | 226.3 (172.6) | 230.9 (164.4) |
| Vegetable consumption, g/day | 200.9 (145.4) | 205.0 (149.9) | 195.6 (139.2) | 190.9 (138.4) | 195.9 (143.6) |
| Red meat consumption, g/day | 48.2 (36.3) | 46.5 (35.7) | 50.5 (37.0) | 53.1 (37.7) | 47.4 (34.0) |
|
| |||||
| Blood pressure, mmHg | |||||
| Systolic blood pressure | 131.5 (19.7) | 119.4 (10.9) | 147.3 (17.4) | 146.7 (15.9) | 147.9 (20.0) |
| Diastolic blood pressure | 81.1 (10.9) | 75.2 (7.5) | 88.8 (9.8) | 88.6 (9.1) | 89.0 (10.6) |
|
| |||||
| Diabetes | |||||
| Self-reported diabetes | 8588 (2.8) | 2516 (1.4) | 6072 (4.6) | 2410 (3.3) | 3067 (8.3) |
| Missing information | 30147 (9.8) | 16247 (9.3) | 13900 (10.4) | 615 (0.8) | 657 (1.8) |
|
| |||||
| Smoking status | |||||
| Never smoker | 141931 (46.2) | 80450 (46.2) | 61481 (46.2) | 33057 (44.8) | 18603 (50.3) |
| Former smoker | 86314 (28.1) | 45575 (26.2) | 40739 (30.6) | 21901 (29.7) | 11154 (30.1) |
| Current smoker (≤ 20 pack-years) | 32226 (10.5) | 21711 (12.5) | 10515 (7.9) | 6539 (8.9) | 2716 (7.3) |
| Current smoker (> 20 pack-years) | 34907 (11.4) | 19428 (11.2) | 15479 (11.6) | 9777 (13.3) | 3655 (9.9) |
| Missing information | 11940 (3.9) | 7015 (4.0) | 4925 (3.7) | 2440 (3.3) | 889 (2.4) |
|
| |||||
| Physical activity | |||||
| Inactive | 67194 (21.9) | 32680 (18.8) | 34514 (25.9) | 16013 (21.7) | 11191 (30.2) |
| Moderately inactive | 100295 (32.6) | 57476 (33.0) | 42819 (32.2) | 23828 (32.3) | 12268 (33.1) |
| Moderately active | 70977 (23.1) | 42530 (24.4) | 28447 (21.4) | 16954 (23.0) | 7300 (19.7) |
| Active | 62152 (20.2) | 36956 (21.2) | 25196 (18.9) | 15696 (21.3) | 6021 (16.3) |
| Missing information | 6700 (2.2) | 4537 (2.6) | 2163 (1.6) | 1223 (1.7) | 237 (0.6) |
|
| |||||
| Education | |||||
| None | 9377 (3.1) | 3478 (2.0) | 5899 (4.4) | 2863 (3.9) | 2973 (8.0) |
| Primary school completed | 90254 (29.4) | 43392 (24.9) | 46862 (35.2) | 25274 (34.3) | 14288 (38.6) |
| Technical/professional school | 77398 (25.2) | 43025 (24.7) | 34373 (25.8) | 18676 (25.3) | 9033 (24.4) |
| Secondary school | 53830 (17.5) | 35179 (20.2) | 18651 (14.0) | 11377 (15.4) | 4730 (12.8) |
| Longer education (inc. University) | 67606 (22.0) | 43999 (25.3) | 23607 (17.7) | 14246 (19.3) | 5875 (15.9) |
| Missing information | 8853 (2.9) | 5106 (2.9) | 3747 (2.8) | 1278 (1.7) | 118 (0.3) |
|
| |||||
| Age at first menstrual period * | |||||
| < 12 years | 27085 (13.9) | 16544 (14.1) | 10541 (13.6) | 5521 (13.2) | 3116 (13.1) |
| >=12 and < 15 years | 125828 (64.6) | 76353 (65.1) | 49475 (63.9) | 27400 (65.6) | 15450 (65.1) |
| >= 15 years | 32391 (16.6) | 17996 (15.3) | 14395 (18.6) | 7842 (18.8) | 4618 (19.5) |
| Missing information | 9423 (4.8) | 6371 (5.4) | 3052 (3.9) | 1016 (2.4) | 552 (2.3) |
|
| |||||
| Age at first full term pregnancy* | |||||
| ≤ 21 years | 36839 (18.9) | 20636 (17.6) | 16203 (20.9) | 8514 (20.4) | 5607 (23.6) |
| > 21 and ≤ 30 years | 106230 (54.6) | 62619 (53.4) | 43611 (56.3) | 23884 (57.2) | 13515 (56.9) |
| ≥ 30 years | 15709 (8.1) | 9715 (8.3) | 5994 (7.7) | 3391 (8.1) | 1689 (7.1) |
| Missing information | 35949 (18.5) | 24294 (20.7) | 11655 (15.0) | 5990 (14.3) | 2925 (12.3) |
|
| |||||
| Full-term pregnancies * | |||||
| None | 25571 (13.1) | 16963 (14.5) | 8608 (11.1) | 4966 (11.9) | 2397 (10.1) |
| One | 28796 (14.8) | 17156 (14.6) | 11640 (15.0) | 6350 (15.2) | 3656 (15.4) |
| Two | 75357 (38.7) | 45696 (39.0) | 29661 (38.3) | 16246 (38.9) | 9280 (39.1) |
| Three | 33031 (17.0) | 18740 (16.0) | 14291 (18.4) | 7483 (17.9) | 4664 (19.6) |
| Four or more | 14897 (7.7) | 7360 (6.3) | 7537 (9.7) | 3601 (8.6) | 2832 (11.9) |
| Missing information | 17075 (8.8) | 11349 (9.7) | 5726 (7.4) | 3133 (7.5) | 907 (3.8) |
|
| |||||
| Menopausal status * | |||||
| Pre-menopausal | 58190 (29.9) | 45853 (39.1) | 12337 (15.9) | 7856 (18.8) | 2450 (10.3) |
| Post-menopausal | 96806 (49.7) | 46876 (40.0) | 49930 (64.5) | 25486 (61.0) | 16640 (70.1) |
| Peri-menopausal or unknown | 33371 (17.1) | 21490 (18.3) | 11881 (15.3) | 6918 (16.6) | 3292 (13.9) |
| Surgical post-menopausal | 6360 (3.3) | 3045 (2.6) | 3315 (4.3) | 1519 (3.6) | 1354 (5.7) |
|
| |||||
| Age at menopause * | |||||
| < 40 years | 4301 (2.2) | 2106 (1.8) | 2195 (2.8) | 1067 (2.6) | 769 (3.2) |
| ≥ 40 and ≤ 46 years | 18912 (9.7) | 9058 (7.7) | 9854 (12.7) | 4754 (11.4) | 3650 (15.4) |
| > 46 and ≤ 50 years | 31996 (16.4) | 15578 (13.3) | 16418 (21.2) | 8510 (20.4) | 5579 (23.5) |
| > 50 and ≤ 56 years | 29427 (15.1) | 13238 (11.3) | 16189 (20.9) | 8327 (19.9) | 5619 (23.7) |
| > 56 years | 1997 (1.0) | 753 (0.6) | 1244 (1.6) | 595 (1.4) | 469 (2.0) |
| Missing or not applicable | 108094 (55.5) | 76531 (65.3) | 31563 (40.7) | 18526 (44.3) | 7650 (32.2) |
|
| |||||
| Oral contraceptive use * | |||||
| Never | 82568 (42.4) | 43393 (37.0) | 39175 (50.6) | 20706 (49.6) | 12755 (53.7) |
| Former | 91622 (47.1) | 58870 (50.2) | 32752 (42.3) | 18294 (43.8) | 9916 (41.8) |
| Current | 10050 (5.2) | 7434 (6.3) | 2616 (3.4) | 1829 (4.4) | 594 (2.5) |
| Missing information | 10487 (5.4) | 7567 (6.5) | 2920 (3.8) | 950 (2.3) | 471 (2.0) |
|
| |||||
| Hormone replacement therapy * | |||||
| Never | 129049 (66.3) | 79952 (68.2) | 49097 (63.4) | 26920 (64.4) | 14842 (62.5) |
| Former | 16259 (8.3) | 8638 (7.4) | 7621 (9.8) | 3937 (9.4) | 2598 (10.9) |
| Current | 30133 (15.5) | 17724 (15.1) | 12409 (16.0) | 6596 (15.8) | 3891 (16.4) |
| Missing information | 19286 (9.9) | 10950 (9.3) | 8336 (10.8) | 4326 (10.4) | 2405 (10.1) |
Reproductive characteristics in women; Categorical variables: number of individuals (percentage from total number in category (for reproductive factors in women only)); Continuous variables: mean (standard deviation).
Associations with the risk of malignant cancers
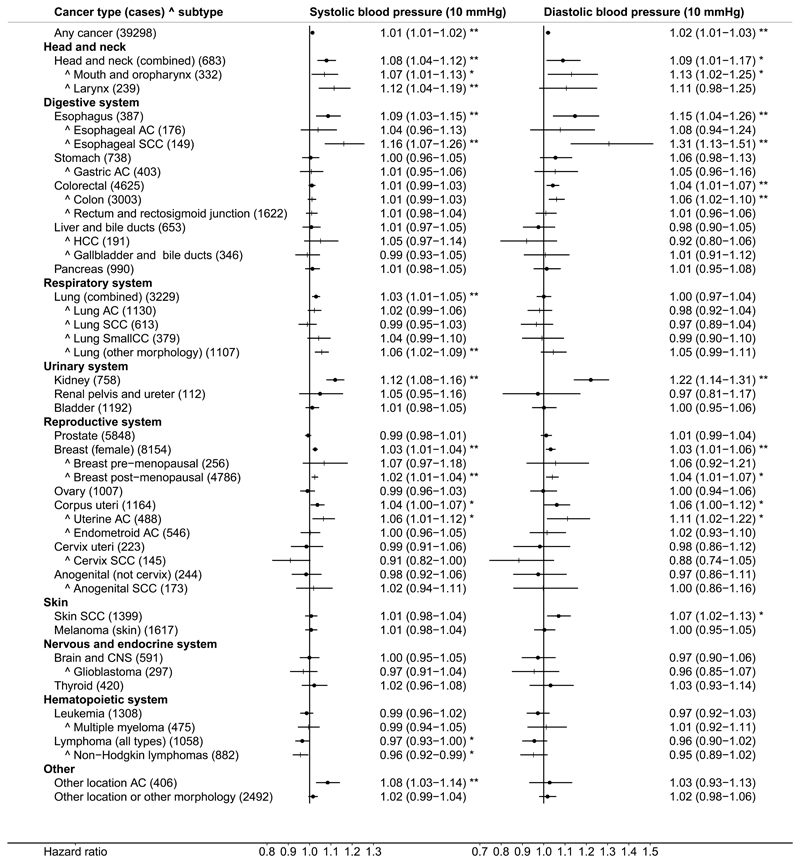
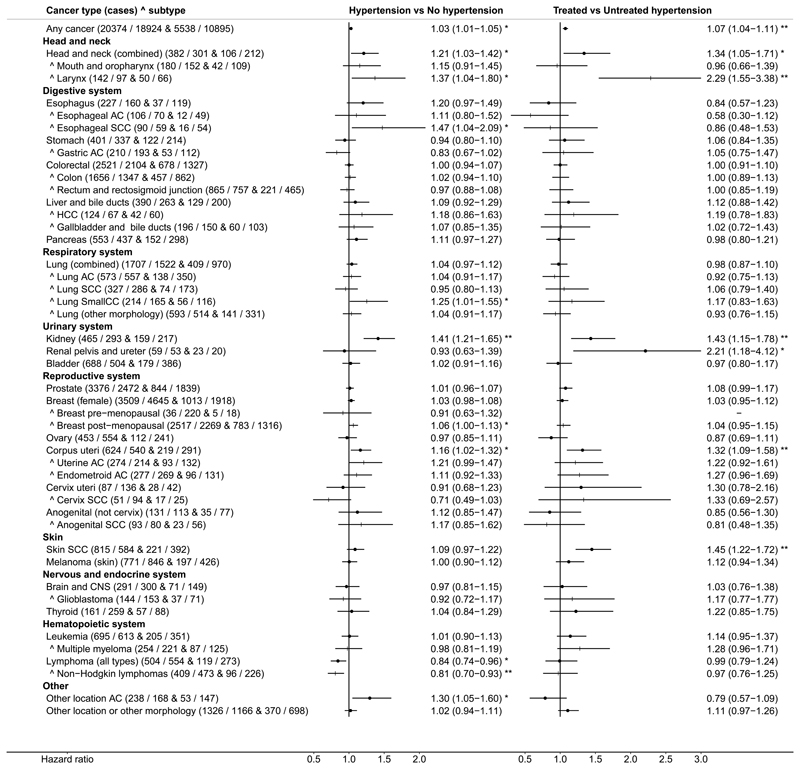
Results from all analyses are included in Supplementary Table 5. The presentation below focuses on SBP and DBP examined as continuous variables in adjusted models. Forest plots with estimates of HR (95% CI) for SBP and DBP in the total dataset are shown in Figure 2; for hypertensive status and antihypertensive treatment in Figure 3 and for sub-groups determined by sex, BMI, age at recruitment, smoking status, alcohol and antihypertensive treatment are shown in Supplementary Figure 1. There were positive associations of BP with malignant cancers in several locations and with some specific morphologies. Analyses of BP categories were largely in agreement with the findings for BP examined on a continuous scale. The main findings are presented below, within the context of large published studies and meta-analyses. Anatomical cites and cancers, for which there was no overall association, are presented in Supplementary Results and Discussion.
Figure 2. Forest plot of hazard ratios for continuous systolic and diastolic blood pressure.
AC – adenocarcinoma; CNS – central nervous system; HCC – hepatocellular carcinoma; SCC – squamous cell carcinoma; SmallCC – small cell carcinoma; Vertical order – determined by the hazard ratio estimates for systolic blood pressure (SBP) of the main anatomical locations (dot symbols), each followed by the relevant specific locations or morphologies marked with ^ (+ symbols) (other locations, not included in those specified, are shown last); Hazard ratios – estimates (95% confidence intervals) (per 10mmHg higher blood pressure) derived from Cox proportional hazards models, stratified by study centre and age at recruitment (5-year categories) and adjusted for potential confounders and risk factors listed in Supplementary Table 2). For cervical AC (n=37): HR=0.96 (0.79-1.17) for SBP and HR=0.84 (0.59-1.19) for DBP and for other morphology in the cervix (non-SCC and non-AC) (n=41): HR=1.28 (1.10-1.48) for SBP and HR=1.53 (1.17-2.01) for DBP (considered only in the main analyses and excluded to avoid the larger confidence intervals dominating the plot); * p<0.05, ** p<0.005.
Figure 3. Forest plot of hazard ratios for dichotomous hypertension and antihypertensive treatment.
AC – adenocarcinoma; CNS – central nervous system; HCC – hepatocellular carcinoma; SCC – squamous cell carcinoma; SmallCC – small cell carcinoma; Hypertension – defined as systolic blood pressure (SBP) ≥140 mmHg, or diastolic BP (DBP) ≥90 mmHg at the BP measurement visit, or self-reported history of hypertension; Antihypertensive treatment status – either self-reported or no treatment assumed if there was self-reported absence of diagnosis of hypertension; Cases – numbers per group (hypertension / no hypertension & treated / untreated hypertension); Vertical order – determined by the hazard ratio estimates for SBP of the main anatomical locations (dot symbols), each followed by the relevant specific locations or morphologies marked with ^ (+ symbols) (as per Figure 2) (other locations, not included in those specified, are shown last); Hazard ratios – estimates (95% confidence intervals) (per 10 mmHg higher BP) were derived from Cox proportional hazards models, stratified by study centre and age at recruitment (5-year categories) and adjusted for potential confounders and risk factors listed in Supplementary Table 2. For cervical AC (n=37): HR=1.23 (0.58-2.06) and for other morphology in the cervix (non-SCC and non-AC) (n=41): HR=1.82 (0.92-3.63) (considered only in the main analyses and omitted from the plot to avoid the larger confidence intervals dominating the plot); * p<0.05, ** p<0.005.
Kidney cancer and cancers of the renal pelvis and ureter
The results of the current analyses confirmed our previous findings [6] of a positive association of BP with the risk of RCC: HR=1.12 (1.08-1.17) for SBP and HR=1.23 (1.14-1.32) for DBP (Figure 2). We found no evidence for a difference between men (with n=431 cases) and women (n=327) (Supplementary Figure 1A,B). As cancers of the renal pelvis and ureter are mainly of transitional cell morphology, i.e. different than the parenchymal cell morphology of RCC, they were considered as a negative control and, indeed, there was no association with BP in the total dataset (n=112). There was, however, an indication for a positive association with SBP in the subgroup with BMI>25kg/m2 (n=63): HR=1.16 (1.03-1.32). The risk was also higher in treated compared to untreated hypertensive individuals: HR=2.21 (1.18-4.12) (Figure 3), although for a small number of cases.
The association of hypertension with a higher risk of RCC is well established. In 1999, Grossman et al. reported a higher risk for users of diuretics relative to nonusers: OR=1.55 (1.42-1.71), based on a meta-analysis of 9 case-control studies, and more than a two-fold increased risk, based on three cohort studies [28]. In 2007, a meta-analysis of 18 studies reported a higher risk of RCC among hypertensive patients (estimated pooled OR=1.62 (1.24-2.12)), also for treatment with diuretics and, in women, with non-diuretic drugs [7]. At the same time, Weikert et al. reported, based on 250 cases in the EPIC study, that high SBP and DBP are associated with a higher risk of RCC, both in men and women and found that individuals receiving antihypertensive treatment had higher risk only if hypertension was poorly controlled [6]. Earlier, Heath et al. had reported in a large cohort high age-adjusted risk-ratio in women: RR=3.1 (1.5-4.3), but not in men: RR=0.8 (0.4-1.3) receiving antihypertensive medication, including diuretics [29] and Grossman et al. had reported from a meta-analysis of seven case-control studies a high risk in women: averaged OR=2.01 (1.56-2.67), but slightly lower risk in men OR=1.69 (1.34-2.13), but with heterogeneity between the studies [28]. However, Haggström et al. reported for the Me-Can project a higher risk only in men (n=592): HR=1.39 (1.24-1.56) per 10mmHg higher mid-BP, but not in women (n=263): HR=1.05 (0.89-1.24) [3], with similar findings for SBP and DBP examined separately [30]. Nevertheless, a more recent meta-analysis of 18 prospective studies, with a total of 8097 cases, has confirmed an association of history of hypertension with kidney cancer: risk ratio estimate RR=1.67 (1.46-1.90) and an association also of SBP and DBP in both men and women [8], in agreement with our findings. A potential relationship between hypertension and the risk of renal pelvis and ureter cancer is less well studied, but an early case-control study reported a positive association for hypertension history longer than 5 years: OR=1.3 (1.0-1.8) and, in agreement with our findings, among users of antihypertensive drugs: OR=2.4 (1.1-4.9) [31]. However, as antihypertensive treatment may be related to the type and severity of hypertension, it is difficult to separate their effects in an observational study.
Cancers of the upper aero-digestive tract and lung
We found a positive association of BP with the risk of esophageal carcinoma, but more specifically with squamous cell carcinoma (SCC) (n=149): HR=1.16 (1.07-1.26) for SBP and HR=1.31 (1.13-1.51) for DBP and not with esophageal adenocarcinoma (AC) (n=176) (Figure 2). We also found a weak positive association for head and neck cancers (89% of which were SCC morphology): HR=1.08 (1.04-1.12) for SBP and HR=1.09 (1.01-1.17) for DBP (similarly for mouth and oropharynx and for larynx). For mouth and oropharynx, the positive associations were statistically significant only in women, in individuals older than 53 years at recruitment and for alcohol intake>12g/day and for head and neck cancers and esophageal SCC only for alcohol intake>12g/day and not below. There were, however, fewer cases among never smokers and the 95% CIs were too wide to make meaningful conclusions for smoking (Supplementary Figure 1G,H).
For lung cancer, there was no overall evidence for association of BP with SCC, AC or small cell carcinoma morphologies (Figure 2). In subgroup analyses, we observed an inverse association of DBP with lung AC among individuals with BMI>25kg/m2 (n=528): HR=0.90 (0.83-0.98) and a positive association of SBP with lung SCC in individuals receiving antihypertensive treatment (n=74): HR=1.14 (1.01-1.28). A weak positive association of SBP with the risk of total lung cancer was mainly accountable for by other morphologies (predominantly unclassified or large cell): HR=1.06 (1.02-1.09).
Our findings for esophageal cancer are in agreement with the similarly-sized Me-Can project, for which Stocks et al. reported a positive association of mid-BP with total esophageal cancer (n=285): HR=1.33 (1.13–1.57) per 10mmHg higher BP [3]. Lindkvist et al. further showed that this was accountable for by a higher risk of SCC (n=184): HR=1.30 (1.17-1.44) and not AC (n=114): HR=1.03 (0.89-1.19), with no major differences between subgroups of never, former and current smokers [32]. For head and neck cancers, Stocks et al. did report for the Me-Can project an association of mid-BP with the risk of cancers of the lip, oral cavity and pharynx, but only in men (n=561): HR=1.31 (1.15-1.48) per 10mmHg increase, and not in women (n=177): HR=1.05 (0.85-1.28). They also found a positive association for the combined group of cancer of the larynx, trachea and lung in men (n=2810): HR=1.09 (1.03-1.16) but not in women (n=905): HR=1.00 (0.92-1.10) [3]. Our study includes a similar number of lung cancer cases (n=3229), but we have examined separately cancer of the larynx and individual lung morphologies. Whilst smoking is a major risk factor for both esophageal and lung SCC cancers and can also lead to hypertension, as shown in an animal model [33] and some epidemiological studies [34, 35], we could find a positive association with hypertension only for esophageal and not for lung SCC, after adjustment for smoking. If there was any residual confounding by smoking, it is likely that we would have observed a positive association for lung, as well as for esophageal SCC. In fact, we could find no positive association of hypertension with lung SCC even without adjustment for confounders (crude HRs in Supplementary Figure 2). Further, a positive association for esophageal AC was observed only in unadjusted analyses but was lost after adjustment for confounders, indicating that the adjustment, has removed, to a great extent, the confounding by smoking. This leads us to conclude that smoking is not likely to explain the association of high BP with the risk of SCC in the upper aero-digestive tract. Alcohol, however, may have an influence (Supplementary Figure 1I,J), but further investigations are needed to clarify our observations.
Gastric and colorectal cancers
We could not find association of BP with the risk of gastric cancer (n=738) (including gastric AC (n=403)) in the total cohort (Figure 2). We only found a positive association for gastric AC among individuals receiving antihypertensive treatment (n=53): HR=1.22 (1.06-1.40) for SBP and HR=1.65 (1.27-2.15) for DBP. We also found a weak positive association of DBP with the risk of cancer of the colon (n=3003) (75% of which had AC (code 8140/3) morphology): HR=1.06 (1.02-1.10) for DBP, similarly for men and women (Supplementary Figure 1B), but not the rectum and rectosigmoid junction (n=1622) (81% AC) (Figure 2). There was also a weak positive association of SBP with the risk of colorectal cancer (similarly for colon and rectum (including rectosigmoid junction)) in men, but not in women (in men colon (n=1304): HR=1.03 (1.00-1.06) and rectum (n=876): HR=1.03 (0.99-1.07)). We also found a positive association in the subgroup analyses for participants with BMI>25kg/m2 (colon: n=1813, rectum: n=966) and for the participants who reported alcohol intake>12g/d (colon: n=1091, rectum: n=685) (Supplementary Figure 1C,D,I,J).
Our data are broadly compatible with results from previous large studies, which suggest that high BP is associated with the risk of colorectal cancer in men but not in women. In 2001 Tenenbaum et al. reported, in a cohort of patients with stable angina or previous myocardial infarction, higher risk of colon cancer (n=96) in individuals receiving diuretics compared to nonusers: HR=2.0 (1.2-3.2) [36]. More recently, in a large case-control study in Italy, Pelucchi et al. found that history of treated hypertension was associated with colorectal cancer risk in men (n=1310) OR=1.24 (1.03-1.48) but not in women (n=946) OR=0.87 (0.71-1.06) [37]. Stocks et al., considering SBP, DBP and mid-BP in a prospective study (Me-Can), also reported a positive association for cancer of the colon in men (n=1747): HR=1.10 (1.03-1.19) per 10mmHg higher mid-BP but not in women (n=1265): HR=0.95 (0.88-1.02) [3, 38], with similar findings for cancer of the rectum and anus [3]. Esposito et al., have subsequently reported in a meta-analysis based on 9 studies, a RR=1.09 (1.01-1.18) for high BP, although considering jointly men and women [13]. For gastric AC, Lindkvist et al., similar to our total cohort results, could not find in the Me-Can project evidence supporting an association with mid-BP (n=1210), but they did not consider antihypertensive treatment [39].
Breast cancer
In our study, representing a cohort with the largest to date number of breast cancer cases, we found a weak but statistically significant positive association with both, SBP and DBP (n=8154): HR=1.03 (1.01-1.04) for SBP and HR=1.03 (1.01-1.06) for DBP. The association was similar for post-menopausal cancers (n=4786), but the number of pre-menopausal cancers was considerably smaller and the 95% CIs were too wide to permit conclusions. In sub-group analyses, the positive association of SBP with the risk of post-menopausal breast cancer was retained only in ever smokers (n=2180) and there was some suggestion for a positive association of SBP with pre-menopausal breast cancer in women with alcohol intake≤12g/day (n=201) (Supplementary Figure 1G,I).
Literature reports on breast cancer are conflicting. In an early case-control study in Italy, Soler at al. described a higher risk of breast cancer in women with treated hypertension (n=3,406): OR=1.2 (1.1-1.4) and more specifically in post-menopausal (n=2184), at age 55 years or older (n=1580), in drinking women (n=2,400) and at BMI>25kg/m2 (n=1266) and not in pre- and peri-menopausal women [40]. However, Bjorge et al. in the Me-Can project did not find associations of SBP or DBP with the risk of incident breast cancer (n=4,862), however they reported a higher risk of breast cancer mortality for age≥60 years [41]. Similarly, Largent et al., defining high BP as treated hypertension in the California Teachers Study cohort (n=4,151), found a higher risk associated with antihypertensive treatment longer than 5 years: HR=1.18 (1.02-1.36) [42], but no association with hypertension overall. However, in the largest to date meta-analysis (n=11,643), Han et al. (in agreement with an earlier meta-analysis [12] and with our findings) have reported a higher risk of breast cancer in hypertensive women, based on 18 retrospective case-control studies: RR 1.29 (1.14-1.47) and on 12 prospective studies: RR=1.07 (1.01-1.14), but only for post-menopausal women (13 studies): RR=1.20 (1.09-1.31) and not for pre-menopausal (9 studies): RR=0.97 (0.84-1.12) [15].
Endometrial cancer
For cancers located in corpus uteri, we found a weak positive association, which could be traced only to AC morphology (code 8140/3): HR=1.06 (1.01-1.12) for SBP and HR=1.11 (1.02-1.22) for DBP, but not to the endometroid morphology (code 8380/3): HR=1.00 (0.96-1.05) for SBP and HR=1.02 (0.93-1.10) for DBP (Figure 2). In sub-group analyses, the association for AC morphology was retained only at BMI>25kg/m2 and in never smokers (Supplementary Figure 1C,D,G,H). The differences between morphologies, however, would need further clarification, as the relative proportions of the two morphologies differed considerably between the individual countries in the EPIC cohort (Supplementary Table 3).
Large European case-control and cohort studies (n>700 in each), although not accounting for specific morphologies, have consistently reported, in agreement with our findings, a higher risk of endometrial cancer with high BP [40, 43, 44], especially in obese women [43, 44], while a relatively smaller case-control study in the United States (n=469) found a higher risk only in women receiving thiazide diuretics [45]. Nevertheless, recent meta-analyses, have corroborated a positive association: RR=1.32 (1.12-1.56) (6 prospective studies, 1,1469 cases) [9], RR=1.81 (1.08–3.03) (5 studies, 3,112 cases) [10].
Cervical cancer
For cervical SCC (n=145), but not for total cervical cancers (n=223), we found an inverse association (Figure 2), which was especially pronounced in women with BMI>25kg/m2 (n=68): HR=0.81 (0.71-0.93) for SBP and HR=0.74 (0.57-0.95) for DBP (Supplementary Figure 1C,D), whilst we found no evidence for association of BP with cervical AC (n=37) and a positive association for the remaining morphologies (n=41): HR=1.28 (1.10-1.48) for SBP and HR=1.53 (1.17-2.01) for DBP, which would have contributed to absence of an overall association for total cervical cancers, but with a small number of cases, this could be a chance finding.
In contrast to our findings, Stocks et al. reported a higher risk of total cervical cancer in the Me-Can study (n=424): HR=1.17 (1.01-1.34) per 10mmHg higher mid-BP [3]. Further, Ulmer et al. examined individual morphological subtypes and reported, similar to esophageal cancer, a positive association for cervical SCC (n=337): HR=1.28 (1.05-1.57) per SD higher mid-BP, but not for cervical AC (n=59): HR=1.09 (0.65-1.83) [46].
Prostate cancer
Our study, based on a reasonably large number of incident cases (n=5,848), provided no evidence for association of SBP or DBP with the risk of prostate cancer: HR=0.99 (0.98-1.01) for SBP and HR=1.01 (0.99-1.04) for DBP, except for some weak inverse association, mainly with SBP, which was found only in never smokers (n=1937): HR=0.97 (0.95-1.00).
Contrary to our findings, the CONOR study (n=1974) [47] has reported a weak positive association between SBP and DBP and the risk of prostate cancer and recent meta-have confirmed this [11, 14]. Based on 10 studies (n=4343), Esposito et al. reported RR=1.15 (1.01-1.30) [11] and Gacci et al. (7 studies) reported RR=1.10 (1.01-1.19) [14]. However, the Me-Can study (n=6673) [48] found only a positive association of SBP and DBP with prostate cancer death, while Stocks et al. reported in the Swedish Construction Workers cohort an inverse association with the risk of total prostate cancer (n=10,002) and non-aggressive tumours (n=2817), but a positive association of DBP with the risk of aggressive tumours (n=2402) [49]. In the light of these discrepancies, it would be important to examine further the impact of cancer aggressiveness or grading in EPIC, but this was beyond the scope of the current study.
Blood and lymphoid cancers
Our data revealed an inverse association of BP with the risk of all-type lymphomas (n=1058), and specifically with non-Hodgkin lymphomas (n=882): HR 0.96 (0.92-0.99) for SBP and borderline for DBP: HR=0.95 (0.89-1.02), whilst we found no evidence for association with leukaemia (n=1308), or specifically with multiple myeloma (n=475) (Figure 2).
In the Me-Can study, Nagel et al. considered a total of 2,751 cases of myeloid and lymphoid neoplasms and their results did not support associations with mid-BP, except for a suggestion, based on a small number of cases (n=46), for an inverse association with the risk of T-cell non-Hodgkin lymphomas in men: HR=0.54 (0.29-1.01) [50]. Lymphoid cells are closely involved in inflammatory processes and recent studies have specifically linked T-cell subtypes with vascular remodelling and the development of hypertension [51], so there may be some mechanistic explanation, but this would need a more detailed investigation.
Skin cancer
We found evidence for a positive association with the risk of skin SCC for DBP (n=1399): HR=1.07 (1.02-1.13) and for antihypertensive treatment among hypertensive individuals: HR=1.45 (1.22-1.72).
The Me-Can project also reported higher risk of skin SCC, but only in men (n=566): HR=1.11 (0.95–1.31) for one SD higher mid-BP and not in women (n=286): HR=0.95 (0.76-1.19) [52]. Several studies have also reported higher risk in association with antihypertensive drugs, especially diuretics [53], but there were no sufficient treatment details in EPIC to explore further.
Sensitivity analyses
Excluding the first two years of follow-up did not have material influence on the findings (Supplementary Figure 2), except for abolishing the inverse association for cervical SCC (without changing it to positive), which may be the result of selection bias introduced by the exclusion. However, crude HRs (unadjusted) (Supplementary Figure 3) had indicated some associations, which were lost or mitigated after adjustment for confounders. Thus, crude HRs indicated positive associations of SBP and DBP not only with esophageal SCC or the endometroid cancer morphology in corpus uteri, but also of the AC morphology in both locations, and further positive associations of DBP with gastric cancer (total and AC morphology) and of SBP, not only DBP, with cancers of the rectum and rectosigmoid junction, as well as with colon cancer. Additionally, crude HRs indicated positive associations of SBP and DBP with bladder cancer, of SBP with liver cancer (accountable for only by HCC) and of DBP with multiple myeloma. In addition, in the absence of adjustment for confounders a weak inverse association was observed for SBP with prostate cancer (SBP only) and DBP with lung AC. Associations observed only in crude and not in adjusted HR estimates suggest that the differences in the selection of adjustment variables in our and other studies may be responsible for some of the discrepancies in the findings. Of note, we have included adjustment for dietary factors, information on which was either not available or not included in the analyses in other published studies. This may be of particular relevance to cancers of the gastrointestinal tract. Similarly, we have used detailed information on reproductive factors in women, although adjustment did not affect our findings for breast and cervical cancers (Supplementary Figure 3).
Strengths and limitations
Our study has several strengths and limitations. Major advantages are the prospective design and the large sample size, including several European countries. Furthermore, BP was measured by trained personnel and was not self-reported. Detailed information on lifestyle, diet and, in women, reproductive history and hormonal treatments was also available, enabling adjustment for potential confounders and shared risk factors.
The main limitation of our study is that BP was measured only at one timepoint. Moreover, specific information on the type of antihypertensive medications in treated individuals was unavailable. Theoretically, antihypertensive treatment could lead to a lower “observed” BP, i.e. measured during the investigation, compared to the “underlying” BP, i.e. the BP that could be reached without treatment. If high BP is causally associated with cancer, controlling BP would mitigate the association, but if high BP and cancer share common mechanisms, the association would remain when BP is controlled, unless treatment targets the mechanism of BP development. In practice, however, a single timepoint measurement may not be representative of the commonly “observed” BP and this applies to untreated and treated individuals alike. Individuals receiving treatment are also likely to have a more sustained high BP, confirmed by a doctor. Treated individuals in our study showed, indeed, considerably higher SBP (mean difference 19.8mmHg (95% CI 19.6-20.1)) and DBP (9.5mmHg (9.4-9.6)) compared to cohort participants without antihypertensive treatment and even showed marginally higher SBP (1.2mmHg (1.0-1.4)) and DBP (0.4mmHg (0.3-0.5)) compared to untreated hypertensive individuals (self-reported or with “observed” high BP (Table 1)). Therefore, when there was a positive association for both hypertension and antihypertensive treatment, as for kidney cancer, we could not discriminate associations related to the severity and duration of high BP from association related to the administration of antihypertensive medication (Figure 3). Nevertheless, a positive association only for treated compared to untreated hypertension, as for cancers of the renal pelvis and ureter, might be more suggestive of the involvement of treatment (Figure 3). There is a growing body of literature evaluating associations between antihypertensive medication and cancer development, but with overall inconclusive findings. An involvement of drugs in cancer pathogenesis is possible, because they are exogenous chemical compounds administered often for very long time [54], but we have not reviewed this literature, because our study could not contribute reliably to the debate.
Finally, information about potential confounders and shared risk factors was self-reported, which may have contributed to misclassification bias and there are always potentially unmeasured risk factors, which may result in residual confounding.
Conclusions
The results of our study, involving over 300,000 participants, are largely compatible with published studies. We confirmed a positive association between BP and RCC and additionally found a positive association of BP with malignant cancers in several anatomical cites, including postmenopausal breast and colon cancers, and with specific morphologies, i.e. SCC in the upper aero-digestive tract and the skin or AC in corpus uteri and other unspecified locations. We also found an inverse association of BP with the risk of non-Hodgkin lymphomas and cervical SCC. These associations, however, are mainly weak and future research is required to clarify potential shared mechanisms. Admittedly, observations based on smaller number of cases could be chance findings, but they could also give some directions for further studies.
Supplementary Material
What’s New.
Our large prospective study strengthens the evidence for weak positive associations of blood pressure with the risk of cancers in several locations: kidney, colon and post-menopausal breast cancers, and with some specific morphologies: squamous cell carcinoma (SCC) in the upper aero-digestive tract and skin and adenocarcinoma in corpus uteri. We also report weak inverse associations with lymphomas and cervical SCC. Our findings render plausible a hypothesis of shared mechanisms for hypertension and cancer development.
Novelty and Impact.
Is there a link between high blood pressure and cancer? In this large, prospective study, the authors found that hypertension is indeed associated with a moderate increase in risk for several cancers, including renal, esophageal, head and neck, skin, colon, post-menopausal breast cancer, and uterine cancer. These results may potentially enhance screening and risk assessment. Further research may also identify shared mechanisms for both hypertension and cancer, such as inflammation, lipid peroxidation, etc.
Acknowledgements
The coordination of EPIC is financially supported by the European Commission (DG-SANCO) and the International Agency for Research on Cancer. The national cohorts are supported by Danish Cancer Society (Denmark); Ligue Contre le Cancer, Institut Gustave Roussy, Mutuelle Générale de l’Education Nationale, Institut National de la Santé et de la Recherche Médicale (INSERM) (France); German Cancer Aid, German Cancer Research Center (DKFZ), Federal Ministry of Education and Research (BMBF), Deutsche Krebshilfe, Deutsches Krebsforschungszentrum and Federal Ministry of Education and Research (Germany); the Hellenic Health Foundation (Greece); Associazione Italiana per la Ricerca sul Cancro-AIRC-Italy and National Research Council (Italy); Dutch Ministry of Public Health, Welfare and Sports (VWS), Netherlands Cancer Registry (NKR), LK Research Funds, Dutch Prevention Funds, Dutch ZON (Zorg Onderzoek Nederland), World Cancer Research Fund (WCRF), Statistics Netherlands (The Netherlands); ERC-2009-AdG 232997 and Nordforsk, Nordic Centre of Excellence programme on Food, Nutrition and Health (Norway); Health Research Fund (FIS), PI13/00061 to Granada, PI13/01162 to EPIC-Murcia, Regional Governments of Andalucía, Asturias, Basque Country, Murcia, Navarra, and the Catalan Institute of Oncology (Barcelona) (Spain); Swedish Cancer Society, Swedish Research Council and County Councils of Skåne and Västerbotten (Sweden); Cancer Research UK (14136 to EPIC-Norfolk; C570/A16491 and C8221/A19170 to EPIC-Oxford), Medical Research Council (1000143 to EPIC-Norfolk, MR/M012190/1 to EPIC-Oxford) (United Kingdom).
For information on how to submit an application for gaining access to EPIC data and/or biospecimens, please follow the instructions at http://epic.iarc.fr/access/index.php KKT was supported by the Hellenic Republic, General Secretary of Research and Technology, Aristeia II funding programme for his work in this paper.
Abbreviations
- AC
adenocarcinoma
- ASH
American Society of Hypertension
- BP
blood pressure
- CI
confidence interval
- DBP
diastolic blood pressure
- EPIC
European Prospective Investigation into Cancer and Nutrition
- ESH
European Society of Hypertension
- HCC
hepatocellular carcinoma
- HR
hazard ratio
- MBP
mean blood pressure, derived as (1/3)*SBP+(2/3)*DBP (used in the current study)
- Me-Can
Metabolic syndrome and Cancer project
- mid-BP
mid-blood pressure, derived as the mean of SBP and DBP (used in Me-Can)
- OR
odds ratio
- RCC
renal cell carcinoma
- RR
risk ratio
- SBP
systolic blood pressure
- SCC
squamous cell carcinoma
- SD
standard deviation
Footnotes
Conflict of interest: The authors have no conflict of interest to declare.
Disclaimer
Where authors are identified as personnel of the International Agency for Research on Cancer / World Health Organization, the authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy or views of the International Agency for Research on Cancer / World Health Organization.
References
- 1.Stanaway JD, Afshin A, Gakidou E, Lim SS, Abate D, Abate KH, Abbafati C, Abbasi N, Abbastabar H, Abd-Allah F, Abdela J, et al. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. The Lancet. 2018;392:1923–1994. doi: 10.1016/S0140-6736(18)32225-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou B, Bentham J, Di Cesare M, Bixby H, Danaei G, Cowan MJ, Paciorek CJ, Singh G, Hajifathalian K, Bennett JE, Taddei C, et al. Worldwide trends in blood pressure from 1975 to 2015: a pooled analysis of 1479 population-based measurement studies with 19·1 million participants. The Lancet. 2017;389:37–55. doi: 10.1016/S0140-6736(16)31919-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stocks T, Van Hemelrijck M, Manjer J, Bjorge T, Ulmer H, Hallmans G, Lindkvist B, Selmer R, Nagel G, Tretli S, Concin H, et al. Blood pressure and risk of cancer incidence and mortality in the Metabolic Syndrome and Cancer Project. Hypertension. 2012;59:802–810. doi: 10.1161/HYPERTENSIONAHA.111.189258. [DOI] [PubMed] [Google Scholar]
- 4.Grossman E, Messerli FH, Boyko V, Goldbourt U. Is there an association between hypertension and cancer mortality? Am J Med. 2002;112:479–486. doi: 10.1016/s0002-9343(02)01049-5. [DOI] [PubMed] [Google Scholar]
- 5.Chow WH, Dong LM, Devesa SS. Epidemiology and risk factors for kidney cancer. Nat Rev Urol. 2010;7:245–257. doi: 10.1038/nrurol.2010.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weikert S, Boeing H, Pischon T, Weikert C, Olsen A, Tjonneland A, Overvad K, Becker N, Linseisen J, Trichopoulou A, Mountokalakis T, et al. Blood pressure and risk of renal cell carcinoma in the European prospective investigation into cancer and nutrition. Am J Epidemiol. 2007;167:438–446. doi: 10.1093/aje/kwm321. [DOI] [PubMed] [Google Scholar]
- 7.Corrao G, Scotti L, Bagnardi V, Sega R. Hypertension, antihypertensive therapy and renal-cell cancer: a meta-analysis. Curr Drug Saf. 2007;2:125–133. doi: 10.2174/157488607780598296. [DOI] [PubMed] [Google Scholar]
- 8.Hidayat K, Du X, Zou SY, Shi BM. Blood pressure and kidney cancer risk: meta-analysis of prospective studies. J Hypertens. 2017;35:1333–1344. doi: 10.1097/HJH.0000000000001286. [DOI] [PubMed] [Google Scholar]
- 9.Aune D, Sen A, Vatten LJ. Hypertension and the risk of endometrial cancer: a systematic review and meta-analysis of case-control and cohort studies. Sci Rep. 2017;7 doi: 10.1038/srep44808. 44808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Esposito K, Chiodini P, Capuano A, Bellastella G, Maiorino MI, Giugliano D. Metabolic syndrome and endometrial cancer: a meta-analysis. Endocrine. 2014;45:28–36. doi: 10.1007/s12020-013-9973-3. [DOI] [PubMed] [Google Scholar]
- 11.Esposito K, Chiodini P, Capuano A, Bellastella G, Maiorino MI, Parretta E, Lenzi A, Giugliano D. Effect of metabolic syndrome and its components on prostate cancer risk: meta-analysis. J Endocrinol Invest. 2013;36:132–139. doi: 10.1007/BF03346748. [DOI] [PubMed] [Google Scholar]
- 12.Esposito K, Chiodini P, Capuano A, Bellastella G, Maiorino MI, Rafaniello C, Giugliano D. Metabolic syndrome and postmenopausal breast cancer: systematic review and meta-analysis. Menopause. 2013;20:1301–1309. doi: 10.1097/GME.0b013e31828ce95d. [DOI] [PubMed] [Google Scholar]
- 13.Esposito K, Chiodini P, Capuano A, Bellastella G, Maiorino MI, Rafaniello C, Panagiotakos DB, Giugliano D. Colorectal cancer association with metabolic syndrome and its components: a systematic review with meta-analysis. Endocrine. 2013;44:634–647. doi: 10.1007/s12020-013-9939-5. [DOI] [PubMed] [Google Scholar]
- 14.Gacci M, Russo GI, De Nunzio C, Sebastianelli A, Salvi M, Vignozzi L, Tubaro A, Morgia G, Serni S. Meta-analysis of metabolic syndrome and prostate cancer. Prostate Cancer Prostatic Dis. 2017;20:146–155. doi: 10.1038/pcan.2017.1. [DOI] [PubMed] [Google Scholar]
- 15.Han H, Guo W, Shi W, Yu Y, Zhang Y, Ye X, He J. Hypertension and breast cancer risk: a systematic review and meta-analysis. Sci Rep. 2017;7 doi: 10.1038/srep44877. 44877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stocks T, Borena W, Strohmaier S, Bjorge T, Manjer J, Engeland A, Johansen D, Selmer R, Hallmans G, Rapp K, Concin H, et al. Cohort Profile: The Metabolic syndrome and Cancer project (Me-Can) Int J Epidemiol. 2010;39:660–667. doi: 10.1093/ije/dyp186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dinh QN, Drummond GR, Sobey CG, Chrissobolis S. Roles of inflammation, oxidative stress, and vascular dysfunction in hypertension. Biomed Res Int. 2014;2014 doi: 10.1155/2014/406960. 406960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gago-Dominguez M, Castelao JE, Yuan JM, Ross RK, Yu MC. Lipid peroxidation: a novel and unifying concept of the etiology of renal cell carcinoma (United States) Cancer Causes Control. 2002;13:287–293. doi: 10.1023/a:1015044518505. [DOI] [PubMed] [Google Scholar]
- 20.George AJ, Thomas WG, Hannan RD. The renin-angiotensin system and cancer: old dog, new tricks. Nat Rev Cancer. 2010;10:745–759. doi: 10.1038/nrc2945. [DOI] [PubMed] [Google Scholar]
- 21.Araujo WF, Naves MA, Ravanini JN, Schor N, Teixeira VP. Renin-angiotensin system (RAS) blockade attenuates growth and metastatic potential of renal cell carcinoma in mice. Urol Oncol. 2015;33:389 e381–387. doi: 10.1016/j.urolonc.2014.11.022. [DOI] [PubMed] [Google Scholar]
- 22.Riboli E, Hunt KJ, Slimani N, Ferrari P, Norat T, Fahey M, Charrondiere UR, Hemon B, Casagrande C, Vignat J, Overvad K, et al. European Prospective Investigation into Cancer and Nutrition (EPIC): study populations and data collection. Public Health Nutr. 2002;5:1113–1124. doi: 10.1079/PHN2002394. [DOI] [PubMed] [Google Scholar]
- 23.Safar ME, Boudier HS. Vascular development, pulse pressure, and the mechanisms of hypertension. Hypertension. 2005;46:205–209. doi: 10.1161/01.HYP.0000167992.80876.26. [DOI] [PubMed] [Google Scholar]
- 24.Wareham NJ, Jakes RW, Rennie KL, Schuit J, Mitchell J, Hennings S, Day NE. Validity and repeatability of a simple index derived from the short physical activity questionnaire used in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Public Health Nutr. 2003;6:407–413. doi: 10.1079/PHN2002439. [DOI] [PubMed] [Google Scholar]
- 25.Who. International Classification of Diseases for Oncology. [Accessed: 24/01/2019]; http://codes.iarc.fr/abouticdo.php.
- 26.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 27.Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, Grassi G, Heagerty AM, Kjeldsen SE, Laurent S, Narkiewicz K, et al. 2007 Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) J Hypertens. 2007;25:1105–1187. doi: 10.1097/HJH.0b013e3281fc975a. [DOI] [PubMed] [Google Scholar]
- 28.Grossman E, Messerli FH, Goldbourt U. Does diuretic therapy increase the risk of renal cell carcinoma? Am J Cardiol. 1999;83:1090–1093. doi: 10.1016/s0002-9149(99)00021-1. [DOI] [PubMed] [Google Scholar]
- 29.Heath CW, Jr, Lally CA, Calle EE, Mclaughlin JK, Thun MJ. Hypertension, diuretics, and antihypertensive medications as possible risk factors for renal cell cancer. Am J Epidemiol. 1997;145:607–613. doi: 10.1093/oxfordjournals.aje.a009157. [DOI] [PubMed] [Google Scholar]
- 30.Haggstrom C, Rapp K, Stocks T, Manjer J, Bjorge T, Ulmer H, Engeland A, Almqvist M, Concin H, Selmer R, Ljungberg B, et al. Metabolic factors associated with risk of renal cell carcinoma. PLoS One. 2013;8:e57475. doi: 10.1371/journal.pone.0057475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liaw KL, Linet MS, Mclaughlin JK, Yu MC, Schoenberg JB, Lynch CF, Niwa S, Fraumeni JF., Jr Possible relation between hypertension and cancers of the renal pelvis and ureter. Int J Cancer. 1997;70:265–268. doi: 10.1002/(sici)1097-0215(19970127)70:3<265::aid-ijc3>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 32.Lindkvist B, Johansen D, Stocks T, Concin H, Bjorge T, Almquist M, Haggstrom C, Engeland A, Hallmans G, Nagel G, Jonsson H, et al. Metabolic risk factors for esophageal squamous cell carcinoma and adenocarcinoma: a prospective study of 580,000 subjects within the Me-Can project. BMC Cancer. 2014;14:103. doi: 10.1186/1471-2407-14-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Talukder MA, Johnson WM, Varadharaj S, Lian J, Kearns PN, El-Mahdy MA, Liu X, Zweier JL. Chronic cigarette smoking causes hypertension, increased oxidative stress, impaired NO bioavailability, endothelial dysfunction, and cardiac remodeling in mice. Am J Physiol Heart Circ Physiol. 2011;300:H388–396. doi: 10.1152/ajpheart.00868.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bowman TS, Gaziano JM, Buring JE, Sesso HD. A prospective study of cigarette smoking and risk of incident hypertension in women. J Am Coll Cardiol. 2007;50:2085–2092. doi: 10.1016/j.jacc.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 35.Halperin RO, Gaziano JM, Sesso HD. Smoking and the risk of incident hypertension in middle-aged and older men. Am J Hypertens. 2008;21:148–152. doi: 10.1038/ajh.2007.36. [DOI] [PubMed] [Google Scholar]
- 36.Tenenbaum A, Motro M, Jonas M, Fisman EZ, Grossman E, Boyko V, Behar S, Reicher-Reiss H. Is diuretic therapy associated with an increased risk of colon cancer? Am J Med. 2001;110:143–145. doi: 10.1016/s0002-9343(00)00674-4. [DOI] [PubMed] [Google Scholar]
- 37.Pelucchi C, Negri E, Talamini R, Levi F, Giacosa A, Crispo A, Bidoli E, Montella M, Franceschi S, La Vecchia C. Metabolic syndrome is associated with colorectal cancer in men. Eur J Cancer. 2010;46:1866–1872. doi: 10.1016/j.ejca.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 38.Stocks T, Lukanova A, Bjorge T, Ulmer H, Manjer J, Almquist M, Concin H, Engeland A, Hallmans G, Nagel G, Tretli S, et al. Metabolic factors and the risk of colorectal cancer in 580,000 men and women in the metabolic syndrome and cancer project (Me-Can) Cancer. 2011;117:2398–2407. doi: 10.1002/cncr.25772. [DOI] [PubMed] [Google Scholar]
- 39.Lindkvist B, Almquist M, Bjorge T, Stocks T, Borena W, Johansen D, Hallmans G, Engeland A, Nagel G, Jonsson H, Selmer R, et al. Prospective cohort study of metabolic risk factors and gastric adenocarcinoma risk in the Metabolic Syndrome and Cancer Project (Me-Can) Cancer Causes Control. 2013;24:107–116. doi: 10.1007/s10552-012-0096-6. [DOI] [PubMed] [Google Scholar]
- 40.Soler M, Chatenoud L, Negri E, Parazzini F, Franceschi S, La Vecchia C. Hypertension and hormone-related neoplasms in women. Hypertension. 1999;34:320–325. doi: 10.1161/01.hyp.34.2.320. [DOI] [PubMed] [Google Scholar]
- 41.Bjorge T, Lukanova A, Jonsson H, Tretli S, Ulmer H, Manjer J, Stocks T, Selmer R, Nagel G, Almquist M, Concin H, et al. Metabolic syndrome and breast cancer in the me-can (metabolic syndrome and cancer) project. Cancer Epidemiol Biomarkers Prev. 2010;19:1737–1745. doi: 10.1158/1055-9965.EPI-10-0230. [DOI] [PubMed] [Google Scholar]
- 42.Largent JA, Bernstein L, Horn-Ross PL, Marshall SF, Neuhausen S, Reynolds P, Ursin G, Zell JA, Ziogas A, Anton-Culver H. Hypertension, antihypertensive medication use, and breast cancer risk in the California Teachers Study cohort. Cancer Causes Control. 2010;21:1615–1624. doi: 10.1007/s10552-010-9590-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bjorge T, Stocks T, Lukanova A, Tretli S, Selmer R, Manjer J, Rapp K, Ulmer H, Almquist M, Concin H, Hallmans G, et al. Metabolic syndrome and endometrial carcinoma. Am J Epidemiol. 2010;171:892–902. doi: 10.1093/aje/kwq006. [DOI] [PubMed] [Google Scholar]
- 44.Weiderpass E, Persson I, Adami HO, Magnusson C, Lindgren A, Baron JA. Body size in different periods of life, diabetes mellitus, hypertension, and risk of postmenopausal endometrial cancer (Sweden) Cancer Causes Control. 2000;11:185–192. doi: 10.1023/a:1008946825313. [DOI] [PubMed] [Google Scholar]
- 45.Fortuny J, Sima C, Bayuga S, Wilcox H, Pulick K, Faulkner S, Zauber AG, Olson SH. Risk of endometrial cancer in relation to medical conditions and medication use. Cancer Epidemiol Biomarkers Prev. 2009;18:1448–1456. doi: 10.1158/1055-9965.EPI-08-0936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ulmer H, Bjorge T, Concin H, Lukanova A, Manjer J, Hallmans G, Borena W, Haggstrom C, Engeland A, Almquist M, Jonsson H, et al. Metabolic risk factors and cervical cancer in the metabolic syndrome and cancer project (Me-Can) Gynecol Oncol. 2012;125:330–335. doi: 10.1016/j.ygyno.2012.01.052. [DOI] [PubMed] [Google Scholar]
- 47.Martin RM, Vatten L, Gunnell D, Romundstad P. Blood pressure and risk of prostate cancer: Cohort Norway (CONOR) Cancer Causes Control. 2010;21:463–472. doi: 10.1007/s10552-009-9477-x. [DOI] [PubMed] [Google Scholar]
- 48.Haggstrom C, Stocks T, Ulmert D, Bjorge T, Ulmer H, Hallmans G, Manjer J, Engeland A, Nagel G, Almqvist M, Selmer R, et al. Prospective study on metabolic factors and risk of prostate cancer. Cancer. 2012;118:6199–6206. doi: 10.1002/cncr.27677. [DOI] [PubMed] [Google Scholar]
- 49.Stocks T, Hergens MP, Englund A, Ye W, Stattin P. Blood pressure, body size and prostate cancer risk in the Swedish Construction Workers cohort. Int J Cancer. 2010;127:1660–1668. doi: 10.1002/ijc.25171. [DOI] [PubMed] [Google Scholar]
- 50.Nagel G, Stocks T, Spath D, Hjartaker A, Lindkvist B, Hallmans G, Jonsson H, Bjorge T, Manjer J, Haggstrom C, Engeland A, et al. Metabolic factors and blood cancers among 578,000 adults in the metabolic syndrome and cancer project (Me-Can) Ann Hematol. 2012;91:1519–1531. doi: 10.1007/s00277-012-1489-z. [DOI] [PubMed] [Google Scholar]
- 51.Wade B, Abais-Battad JM, Mattson DL. Role of Immune Cells in Salt-Sensitive Hypertension and Renal Injury. Current opinion in nephrology and hypertension. 2016;25:22–27. doi: 10.1097/MNH.0000000000000183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nagel G, Bjorge T, Stocks T, Manjer J, Hallmans G, Edlinger M, Haggstrom C, Engeland A, Johansen D, Kleiner A, Selmer R, et al. Metabolic risk factors and skin cancer in the Metabolic Syndrome and Cancer Project (Me-Can) Br J Dermatol. 2012;167:59–67. doi: 10.1111/j.1365-2133.2012.10974.x. [DOI] [PubMed] [Google Scholar]
- 53.Schmidt SA, Schmidt M, Mehnert F, Lemeshow S, Sorensen HT. Use of antihypertensive drugs and risk of skin cancer. J Eur Acad Dermatol Venereol. 2015;29:1545–1554. doi: 10.1111/jdv.12921. [DOI] [PubMed] [Google Scholar]
- 54.Messerli FH. Risk factors for renal cell carcinoma: hypertension or diuretics? Kidney Int. 2005;67:774–775. doi: 10.1111/j.1523-1755.2005.67190.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.