Abstract
Mastocytosis are a group of hematologic neoplasms characterized by an accumulation of atypical mast cells in one or several organs/tissues, often accompanied by mast cell activation. Whereas in children the disease manifestations are mostly limited to the skin, in adults the disease is usually systemic (systemic mastocytosis; SM) and involves the bone marrow and/or other internal organs. Several variants of SM have been defined. Whereas most patients have indolent SM, some patients have advanced SM, which underlines the complexity of SM. In 2002, a European consortium of clinicians and scientists initiated a multidisciplinary, multi-national cooperative network, termed the ‘European Competence Network on Mastocytosis’ (ECNM), with the aim to improve diagnosis and therapy of patients with mastocytosis and other mast cell activation disorders. Since then, members of the ECNM have organized Annual Meetings in several European countries. The present article provides a summary of advances in the field presented during the 17th Annual ECNM meeting held in Salzburg in October 2019.
Keywords: Mast cell activation, mastocytosis, KIT, classification, scoring, tyrosine-kinase inhibitors
Introduction
Mastocytosis is a term used to denote a heterogeneous group of diseases characterized by abnormal accumulation of mast cells (MCs) in one or more organs. Depending on the organ(s) and/or tissue(s) involved, mastocytosis is divided into cutaneous mastocytosis (CM), systemic mastocytosis (SM), and, rarely, localized MC tumors. Based on the 2016 classification of the World Health Organization (WHO), several variants of CM and SM have been defined (Table 1) [1,2]. The prognosis and symptoms vary among patients depending on the disease variant, the presence of an additional hematologic neoplasm (AHN) as well as the presence of comorbidities [1,2]. Independent of the variant of CM or SM, patients may suffer from mediator-related symptoms which can range from moderate to severe, or even life-threatening [3]. In some of these cases, a MC activation syndrome (MCAS) is diagnosed [4]. Advanced SM can further be divided into WHO-approved variants, including aggressive SM (ASM), SM with an associated hematologic neoplasm (SM-AHN) and MC leukemia (MCL) [5]. In these patients, MC infiltration leads to organ damage, which can manifest as cytopenia, ascites, malabsorption, lymphadenopathy, splenomegaly (hypersplenism), hepatomegaly (impaired function), or large osteolyses with pathologic fractures [5]. Whereas the prognosis in CM and indolent SM (ISM) is excellent, the prognosis in patients with advanced SM is poor [6].
Table 1. The 2016 World Health Organization (WHO) classification for mastocytosis.
| Categories | Variants | Diagnostic criteria | Prognosis |
|---|---|---|---|
| Cutaneous mastocytosis (CM) |
|
No systemic involvement (most patients are children) | Good |
| Systemic mastocytosis (SM) |
|
|
Good ± Good Depends on the type of SM and of the AHN Poor Very poor |
| Mast cell sarcoma (MCS) |
|
Very poor |
ANC: absolute neutrophil count; BM: bone marrow; GI: gastro-intestinal; Hb: hemoglobin; MCs: mast cells; MDS: myelodysplastic syndrome; MPN: myeloproliferative neoplasm; PB: peripheral blood.
B-findings: (a) BM biopsy showing >30% infiltration by MCs (focal, dense aggregates) and/or serum total tryptase level >200 ng/mL; (b) signs of dysplasia or myeloproliferation, in non-MC lineage(s), but insufficient criteria for definitive diagnosis of a hematopoietic neoplasm (AHN), with normal or slightly abnormal blood counts; (c) hepatomegaly without impairment of liver function, and/or palpable splenomegaly without hypersplenism, and/or lymphadenopathy by palpation or imaging.
C-findings: (a) BM damage caused by infiltration of neoplastic MCs with consecutive cytopenia(s) (ANC <1.0 × 109/L, Hb <100 g/L, or platelets <100 × 109/L); (b) palpable hepatomegaly with SM-related impairment of liver function, ascites, and/or portal hypertension; (c) skeletal involvement with large (several cm) osteolytic lesions and/or pathological fractures caused by local MC infiltration (d) palpable splenomegaly with hypersplenism; (e) malabsorption with weight loss due to GI MC infiltrates.
In 2002, European experts working in the field of mastocytosis established a Competence Network in Europe [7,8]. This network, termed the ‘European Competence Network on Mastocytosis’ (ECNM), was initiated as a multidisciplinary, multi-national cooperative approach to increase awareness and to improve the diagnosis and therapy of mastocytosis [7,8]. The network is composed of local centers, physicians and scientists who dedicate specifically their work to patients with mastocytosis [7,8]. Representatives of the ECNM cooperate closely with their US colleagues, with patient-organizations in Europe and in the USA, and with other scientific networks [7,8]. In 2012, the ECNM launched a mastocytosis registry [9]. As of September 2019, more than 3800 patients were included in this registry. Using the central database of this registry, cooperative multicenter studies have been conducted and several manuscripts arising from these studies have been published or submitted for publication, or are currently being prepared [9].
Members of the ECNM also organize Annual Meetings of the ECNM in Europe. In 2019, the Annual ECNM meeting took place in Salzburg (Austria) from the 3rd to the 5th of October, 2019. At this meeting, experts from various European countries and from the US presented their center-related activities and data on various projects, including those based on data from the ECNM registry. The current article summarizes advances in the science and the clinical diagnosis, prognosis, and treatment of primary MC disorders presented at the 2019 ECNM meeting. In addition, we provide an overview of ongoing activities and new concepts arising within the ECNM.
Highlights in basic and translational research on mast cell diseases
During the Annual ECNM meeting, several groups presented new and emerging concepts in basic and translational science. As pointed out by the coordinator of the ECNM, Peter Valent, and the chair of the ECNM, Michel Arock, the ECNM plans to establish a strong basic science network in Europe that is connected with the Centers of Excellence and with Reference centers of the ECNM, and is also linked to a strong biobank system and to the ECNM registry database. In addition, the basic science section of the ECNM should promote translational research in various centers and supports ECNM-wide efforts to facilitate applications for EU grants and other multi-center grants. In Austria, the Ludwig Boltzmann Institute for Hematology and Oncology (LBI HO), headed by Peter Valent, has dedicated one project line to basic and translational research on mastocytosis [10]. For example, in collaboration with the Mannheim center of excellence and the CEREMAST center of excellence in Paris, the LBI HO has recently identified NSGSCF-repopulating neoplastic stem cells in advanced SM, including MCL [11]. The same collaborative group has also recently demonstrated that CD44 is a RAS/MEK/STAT5-dependent adhesion molecule expressed on neoplastic MCs and that levels of CD44 on MCs and neoplastic stem cells increase with disease aggressiveness [12]. Another project, presented by Martin Zenke in Salzburg, described the generation of SM-related neoplastic MCs from patient-derived inducible pluripotent stem cells (iPSC). Other presentations discussed the cellular and molecular mechanisms of mast cell activation (MCA) and the mechanisms underlying clinical symptoms in patients with mast cell activation syndromes (MCAS). In addition, the mechanisms contributing to clinical symptoms in patients with hereditary alpha-tryptasemia (HAT) were discussed [13,14]. Lawrence Schwartz presented data on α/β-tryptase hetero-tetramers in the context of HAT [13,14]. In particular, hetero-tetramers composed of 2α- and 2β-tryptase protomers (α/β-tryptase) form naturally in individuals who express α-tryptase. α/β-tryptase- hetero-tetramers, but not homotetramers, activate protease-activated receptor-2 (PAR2), a multifunctional receptor expressed on smooth muscle, neurons, and endothelial cells [15].
There is also an increasing interest of industrial partners and academic networks to collaborate with centers and groups of the ECNM. For example, representative of several companies working on the development of novel KIT-targeting drugs attended the 2019 ECNM meeting. In addition, several representatives of the European Mast Cell and Basophil Research Network (EMBRN) (a basophil/mast cell network in Europe) joined in this annual meeting of the ECNM.
Studies on mast cell activation (MCA) and mast cell activation syndromes (MCAS)
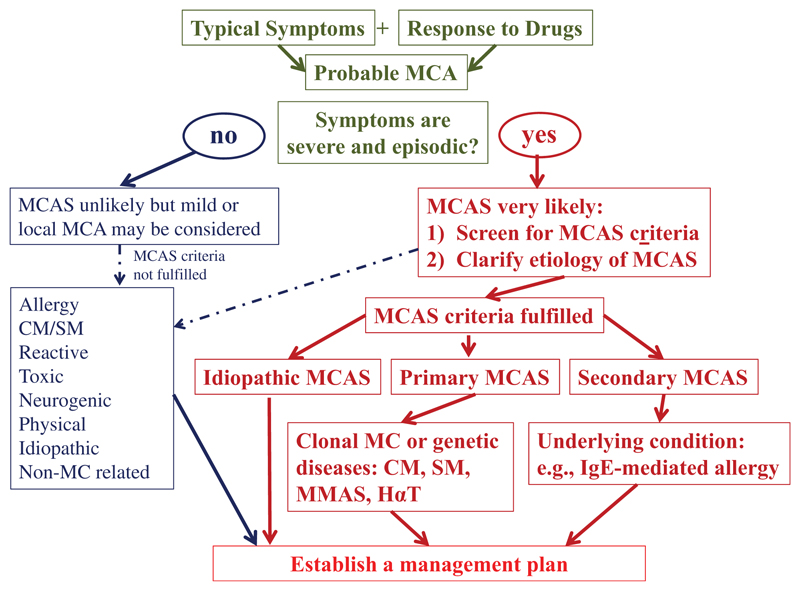
MCA can be documented in a number of physiologic and pathologic conditions [4,16–20]. Acute MCA, secondary to allergen exposure, is thus encountered in IgE-mediated allergic reactions and, when severe, may result in systemic anaphylaxis. Severe or even life-threatening MCA may be facilitated when (1) the burden of MCs is high (although anaphylactic reactions are also seen in cases with low MC counts), (2) when MCs are in a hyperactivable state, and (3) when comorbidities make the patient less tolerant to MCA events [4,16–19]. When MC involvement is documented and the reaction is severe, an MCA syndrome (MCAS) may be diagnosed [18]. Based on the recommendations of the EU/US consensus group [4,16–18], MCAS is diagnosed when the following criteria apply: (i) documented recurrent episodes of typical systemic symptoms that are produced by MC mediators and involve at least 2 organ systems, (ii) an event-related transient elevation of the serum tryptase level by at least 20% over the individual baseline plus 2 ng/mL within a 1–4 h after onset of the reaction, and (iii) a documented clinically meaningful response to drugs that either target MC-derived mediators (e.g. H1 antihistamines) and/or suppress MC activation (e.g. omalizumab) (Table 2) [18]. With regard to tryptase, it was stressed repeatedly during the meeting that an elevated basal serum tryptase level in itself is not a sufficient criterion to define MCA, as elevated basal serum tryptase levels can be found transiently or permanently during a number of conditions, including anaphylaxis, hereditary alpha-tryptasemia, mastocytosis (CM and SM), myelodysplastic syndromes, myeloproliferative neoplasms, chronic eosinophilic leukemia, chronic and acute myeloid leukemias, chronic helminth infection and chronic renal failure [14,21]. It is also important to know that based on its etiology, MCAS can be classified using criteria proposed by the consensus group (Table 3) [4,16–18]. Thus, MCAS can be divided into (i) primary (clonal (also known as monoclonal MCAS; MMAS), e.g. KIT with Gain of Function (KIT GOF) mutation, or genetic, e.g. hereditary alpha-tryptasemia)) MCAS, (ii) secondary MCA, where an allergic or other triggers of MC activation are involved, and (iii) idiopathic MCAS, presumably primary, but where neither a clonal, genetic or known agonist of MCs is found (Table 3). There are also reports suggesting the HAT can predispose to MC activation [15]. In addition, more genetic or clonal disorders associated with idiopathic MCAS may be identified in the near future. However, not all MC activation events meet criteria of MCAS. Therefore, it is important to apply and follow criteria for MCAS since more and more patients are misdiagnosed as having MCA/MCAS without having completed a thorough and appropriate medical/biological examination. To prevent misdiagnoses, a diagnostic algorithm has been proposed (Figure 1) through which a clinically relevant (systemic) MCA can be suspected and MCAS can subsequently be documented or excluded [4]. This algorithm should help guide care providers to consider the principal diagnoses that may underlie systemic MCA events, namely, severe allergy, SM and HAT [4].
Table 2. Consensus criteria of the EU/US group for the diagnosis of mast cell activation syndrome (MCAS).
| Criterion A | Typical clinical signs of severe, recurrent (episodic) systemic MCA are present (often as anaphylaxis) (definition of systemic: involving at least 2 organ systems among cardiovascular, cutaneous, pulmonary and gastrointestinal systems) |
| Criterion B | Involvement of MC is documented by biochemical studies: preferred marker: increase in acute serum tryptase level (ng/mL)(collected 1–4 hours after onset of symptoms) to >(2+1.2*serum baseline tryptase)a |
| Criterion C | Response of symptoms to therapy with MC-stabilizing agents, drugs directed against MC mediator production, or drugs blocking binding of these mediators to their receptorsb |
|
| |
MC: mast cells; MCA: mast cell activation.
All 3 MCAS criteria (A+B+C) must be fulfilled to call a condition MCAS.
Other MC-derived markers of MCA (histamine and histamine metabolites, PGD2 metabolites, and heparin) have also been proposed, but are less specific as compared to tryptase.
Example: histamine receptor blockers.
Table 3. Variants of mast cell activation syndrome.
| Mast cell activation syndrome (MCAS) variant | Key diagnostic variables |
|---|---|
| Primary MCAS | KIT D816V-mutated clonal MCs are found in most cases and also display CD25; also reported in cases with KIT-mutated mast cells and hereditary alpha-tryptasemiaa |
| Synonyms for KITGOF mutation-associated MCAS: Clonal MCAS | |
| Monoclonal MCAS (MMAS) | |
| (a) With cutaneous mastocytosis (CM) | CM criteria fulfilled; SM criteria not fulfilled |
| (b) With systemic mastocytosis (SM) | SM criteria fulfilled |
| (c) With only 2 minor SM criteria | Criteria to diagnose CM or SM not fulfilled |
| Secondary MCAS | Triggered by an allergen to which a subject is sensitive or to an non-IgE-dependent trigger of MC activation |
| Idiopathic MCAS | MCAS criteria are fulfilled, but no underlying reactive disease, no IgE-mediated allergy, and no monoclonal mast cells are detectablea |
GOF: gain of function.
In these patients, no activating KIT mutation at codon 816 is detected, and when tested, flow cytometry usually confirms the presence of CD25-negative (normal) mast cells.
Figure 1.
Updated diagnostic algorithm for patients with a suspected mast cell activation syndrome (MCAS). After the patient has been clinically stabilized, the physician examines potential etiologies and asks for MCAS criteria. If the symptoms are severe and episodic, the likelihood of MCAS is quite high. MCAS consensus criteria are then applied to confirm MC involvement. MCAS criteria can also be applied when the symptoms are less severe and/or atypical. However, in most of these patients, MCAS criteria are not fulfilled. In a next step, the underlying etiology is examined. At this step of the workup, it is important to screen for multiple underlying disorders, since in patients with MCAS, more than one such underlying disease may be present (e.g. mastocytosis and allergy). Regarding mastocytosis, clinical indicators are typical skin lesions, a persistently elevated serum tryptase level and detection of the KIT D816V mutant in peripheral blood cells. According to the underlying condition, MCAS is classified into primary (clonal or genetic) MCAS, secondary MCAS (IgE-dependent allergen or non-IgE-dependent trigger), and idiopathic MCAS. In patients with clonal MCAS, the final diagnosis may be CM, SM, or monoclonal MCAS defined by 2 (but not more) minor SM criteria. In a final step, the management plan is established. CM: cutaneous mastocytosis; HαT: hereditary alpha-tryptasemia; MC: mast cell; MCA: mast cell activation; MMAS: Monoclonal/primary MCAS; SM: systemic mastocytosis. Adapted from Valent et al. [4].
Updated prognostication tools for mastocytosis
The 2016 WHO classification of mastocytosis (Table 1) plays a pivotal role in the stratification of patients and is thus a most important initial prognostication tool [2]. However, this classification alone is not sufficient to estimate the risk of progression in individual patients with SM. For instance, although patients with ISM have in general an excellent prognosis, some of these patients may finally progress to SM-AHN, ASM or MCL [22,23]. During the past few years a number of prognostic variables predicting progression of ISM/SSM to a high-grade disease have been identified, including multi-lineage involvement of hematopoietic cells with KIT D816V, the variant allele frequency (VAF) of mutated KIT, lymphadenopathy, splenomegaly, and an elevated β2-microglobulin or alkaline phosphatase level [24–27]. However, there is still a need to establish robust and reliable prognostic scoring systems that predict: (1) the risk of progression of ISM patients into more aggressive variants of the disease, and (2) the overall survival and progression-free survival of those with advanced SM. Recently, two multi-parametric risk models have been established, leading to two similar scoring systems, both based on age, hemoglobin level, platelets count and alkaline phosphatase level: the clinical risk model (CRM) established at the Mayo Clinic on 277 patients, but not further validated [28], and the clinical risk score (CRS), presented at the ECNM 2019 meeting, based upon a German training cohort of 197 patients that was validated in a group of 149 additional patients [29].
A novel improved prognostic scoring system was also presented at the 2019 ECNM meeting: the International Prognostic Scoring System for Mastocytosis (IPSM) [30]. The IPSM was established by analyzing the prognostic relevance of clinical and laboratory parameters in 1794 mastocytosis patients collected in the ECNM registry [30]. For validation, 462 patients from the Spanish mastocytosis-network were examined. In this study, the prognostic value of the WHO classification was confirmed. However, the data generated in this study also demonstrates that in 1533 patients with non-advanced mastocytosis, two parameters, namely age ≥60 years and alkaline phosphatase ≥100 U/L, are additional independent prognostic variables for survival [30]. Thus, this new scoring system (IPSM) divides patients with non-advanced mastocytosis into 3 groups: low risk (no risk factors), intermediate risk (one risk factor), and high risk (both risk factors) [30]. Interestingly, overall survival (OS), progression-free survival (PFS), and event-free survival (EFS) differed significantly among these subgroups and between these subgroups and advanced SM [30]. Besides, in 261 patients with advanced SM, age ≥60 years (1 point), tryptase ≥125 ng/ml (1 point), leukocytes ≥16 x 109/L (1 point), hemoglobin ≤11 g/dL (1 point), platelets ≤100 x 109/L (1 point) and skin involvement (-1 point) were found to be of prognostic value, leading to the establishment of a separate score for advanced SM, which delineates 4 risk groups (AdvSM-1: –1 to 0 point; AdvSM-2: 1 point; AdvSM-3: 2–3 points and AdvSM-4: 4–5 points) of prognostic significance for OS, PFS and EFS. The value of both scores was confirmed in the Spanish validation-cohort [30]. The IPSM utilizes thus routinely applicable prognostic parameters for non-advanced mastocytosis (age and alkaline phosphatase) and for advanced SM (age, tryptase, blood counts and skin involvement), allowing to reach a robust prognostication score useful in daily clinical practice.
However, the IPSM does not take into account molecular defects frequently encountered in patients with advanced SM. Particularly in patients with SMAHN, additional molecular mutations, apart from KIT D816V, have been described and found to be of major prognostic significance [26,31–34]. Thus the mutationadjusted risk score (MARS) for advanced SM was developed with the intention to integrate clinical and mutation characteristics in the scoring system which was discussed in Salzburg [29]. The MARS study included 383 patients with advanced SM from the German Registry on Disorders of Eosinophils and Mast Cells (training set; n = 231) and several centers for mastocytosis in the United States and Europe (validation set; n = 152) [29]. The following risk factors were identified concerning overall survival (OS) in multivariate analyses: age >60 years (1 point), anemia (hemoglobin <10 g/dL) (1 point), thrombocytopenia (platelets <100 x 109/L) (1 point), presence of one high risk gene mutation (1 point) and presence of two or more high risk gene mutations in the SRSF2/ASXL1/RUNX1 (S/A/R) panel (2 points) (Table 4) [29]. By assigning hazard ratio–weighted points to these variables, the following three risk categories were defined: low risk (0 to 1 point), intermediate risk (2 points), and high risk (3 to 5 points). The MARS was independent of the WHO classification and was confirmed in an independent validation set [29]. In conclusion, the MARS is a validated, five-parameter, WHO-independent prognostic score that defines three risk groups among patients with advanced SM. However, the therapeutic/treatment implication of the various risk scores remains an open question and the implementation of the scores in prospective studies is needed.
Table 4. The Mutation-Adjusted Risk Score (MARS) for advanced SM.
| Criterion | Points |
|---|---|
| Age >60 years | 1 |
| Platelets <100 × 109/L | 1 |
| Hemoglobin <10 g/dL | 1 |
| S/A/R panel positive (1 gene)a | 1 |
| S/A/R panel positive (≥2 genes)b | 2 |
The MARS delineates 3 categories of risk for advanced SM: low risk (median overall survival (OS) not reached): 0 to 1 point; intermediate risk (median OS, 3.9 years): 2 points; and high risk (median OS, 1.9 years): 3 to 5 points.
Presence of one molecular high risk gene mutation (i.e. in SRSF2, ASXL1, and/or RUNX1; S/A/R gene panel), or
presence of two or more molecular high risk gene mutations in the S/A/R gene panel.
Highlights on new therapeutic concepts in mastocytosis
ISM therapy is mainly focused on symptom relief and based on histamine 1 receptor (H1R) and H2R blockers and on MC-stabilizing agents [1,2]. More recently the leukotriene receptor antagonist montelukast, which acts by blocking action of MC-derived leukotrienes, and the humanized monoclonal antibody directed against IgE, omalizumab (Xolair®), which inhibits MC activation, have been introduced for the treatment of severe mediator-related symptoms in mastocytosis [35–37]. Some of the cytoreductive agents, like cladribine, are also known to reduce mediator-related symptoms by reducing the MC burden and by directly blocking MC activation in patients with SM [38–40]. However, these cytoreductive drugs also have (sometimes severe) side effects and therefore usually are avoided in ISM patients who respond to less intensive therapies [40–42]. On the other hand, the use of such drugs in ISM patients with symptoms refractory to maximal mediator-directed management is still debatable. It is also worth noting that some of the KIT-targeted tyrosine kinase inhibitors (TKIs), like midostaurin, can counteract mediator release in MCs and mediatorrelated symptoms in patients with SM [43,44]. Moreover, a recent phase 2 trial has demonstrated that midostaurin may be efficacious in ISM patients with severe MCA symptoms refractory to antihistamine medications [45].
Regarding advanced SM, during the 2019 ECNM meeting, data were presented to suggest that only a minority of ASM patients with signs of rapid progression respond to monotherapy with cladribine [40,41]. Correspondingly, most cladribine responders have slowly progressing SM. Patients with slowly progressing ASM without codon 816 KIT mutations may also respond to imatinib or others KIT wild-type-targeting TKIs [46,47]. This is usually not the case in SM patients in whom neoplastic cells display the KIT D816V mutant which confers resistance to these TKIs. For those patients, midostaurin is currently the only drug specifically approved for advanced SM and is often considered as first line treatment option in slowly progressing patients [44,48–50]. However, not all patients with advanced SM respond to midostaurin, especially when the disease is rapidly progressing, whereas other patients relapse during midostaurin [44,48–50], pointing to the need of developing more potent inhibitors. In this context, avapritinib, specifically designed to inhibit KIT D816V, exhibits10-fold more potency against KIT D816V than midostaurin in biochemical assays. In an ongoing phase I trial, patients treated with avapritinib exhibited marked reduction in both symptoms as well as reductions of bone marrow MCs, serum tryptase level, spleen volume, and KIT D816V mutant allele burden [51,52]. Adverse effects include expected toxicities such as myelosuppression and periorbital edema, but also low-grade cognitive impairment. Several episodes of small intraparenchymal brain hemorrhages (the majority of which have been identified on imaging and are asymptomatic) have occurred primarily in patients with moderate to severe thrombocytopenia (platelet count <50 x 109/L) [51]. Of note, KIT D816V-negative AHN may develop during treatment with avapritinib [51]. Although considerable excitement about avapritinib exists, more data are needed to assess long-term responses and adverse effects of this novel TKI. Whatsoever, treatment responses to TKIs are generally still poor in patients with highly aggressive variants of the disease (ASM in transformation and MCL), independent of the type of therapy and age [1,53]. In these patients, poly-chemotherapy and subsequent allogeneic hematopoietic stem cell transplantation (HSCT) is often recommended with the hope to achieve complete remission, despite the fact that not all patients with advanced SM can be cured by HSCT [54]. In the future avapritinib and other strong KIT D816V inhibitors may be combined with chemotherapy or even replace chemotherapy or HSCT in such patients. It is also worth noting that numerous patients are not eligible for HSCT because of advanced age or because of comorbidities [54]. The remission rate after allo-HSCT is substantially higher in patients with ASM and SM-AHN compared to patients who are suffering from (acute) MCL [54]. Moreover, the outcome after HSCT is better and more durable in those who did respond to previous cytoreductive therapy or have stable diseases [54]. In each case, the risk of relapse and progression must be balanced against side effects and the risk of transplant-related mortality when discussing HSCT in patients with advanced SM. An open question is whether avapritinib can be used instead of SCT or as preparation for HSCT in patients with advanced SM. Another open question is whether patients eligible for HSCT may benefit from a continuous treatment with a KIT-targeted TKI post-HSCT.
Concluding remarks
Mastocytosis is a rare and complex disease with a heterogeneous clinical presentation and variable prognosis, depending on the variant of the disease and response to treatment. Whereas the KIT D816V mutation alone is detected in indolent SM, additional mutations in other genes are considered to trigger malignant cell growth in advanced SM and to worsen the prognosis in these patients. In CM and ISM, patients suffer primarily from symptoms due to MC-derived mediators, which can manifest as MCAS, whereas in advanced SM, organ impairment is found and is usually an indication to start an interventional anti-neoplastic therapy. To better understand the pathophysiology of mastocytosis and to establish standards and criteria for classification and prognosis in mastocytosis, the ECNM was established in 2002. Since then, the ECNM has organized a series of Conferences, Workshops, and annual meetings, as well as scientific projects in the field of mastocytosis in Europe, the USA and other non-European countries. In addition, the ECNM has established a registry which includes more than 3800 patients and serves as the basis of several cooperative multicenter studies. In 2019, the Annual ECNM meeting was organized in Salzburg by Karl Sotlar. More than 160 attendees from all parts of Europe and from the USA participated, exchanging ideas and discussing and/or presenting data. Topics of particular interest included (1) new developments in basic and translational science, (2) a better definition and improved diagnostic criteria of mast cell activation and MCAS, (3) new prognostic tools in mastocytosis and (4) new therapeutic concepts in mastocytosis and MCAS. The inter-disciplinary, cooperative network of the ECNM and the newly generated American Initiative in Mast Cell Diseases (AIM) will guarantee that these important topics will be subject of forthcoming studies in preclinical and clinical research and will be translated into clinical practice whenever possible.
Funding
Karin Hartmann receives research support from Euroimmun and Thermofisher. Karl Sotlar receives research support by Novartis. Peter Valent is supported by the Austrian Science Funds (FWF) project F4704-B20. The other authors declare no funding in relation with the manuscript.
Footnotes
Disclosure statement
Michel Arock receives consultant Fees from Blueprint Medicine, Deciphera and Novartis. Karin Hartmann receives consultant Fees from Allergopharma, ALK, Blueprint, Deciphera, Menarini and Novartis, lectures Fees from Blueprint and Novartis and travel Support from ALK. Karl Sotlar receives speakers Honoraria from Nanostring, Novartis, Pfizer and Beckman-Coulter, consultant Fees from Novartis, Pfizer and Nanostring, and travel Support from Nanostring and Novartis. Peter Valent receives consultant Fees from Novartis, Blueprint and Deciphera. The other authors declare no conflict of interest in relation with the manuscript.
References
- [1].Valent P, Akin C, Hartmann K, et al. Advances in the classification and treatment of mastocytosis: current status and outlook toward the future. Cancer Res. 2017;77(6):1261–1270. doi: 10.1158/0008-5472.CAN-16-2234. [Review] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Valent P, Akin C, Metcalfe DD. Mastocytosis: 2016 updated WHO classification and novel emerging treatment concepts. Blood. 2017;129(11):1420–1427. doi: 10.1182/blood-2016-09-731893. [Review] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Valent P. Mastocytosis: a paradigmatic example of a rare disease with complex biology and pathology. Am J Cancer Res. 2013;3(2):159–172. [PMC free article] [PubMed] [Google Scholar]
- [4].Valent P, Akin C, Bonadonna P, et al. Proposed diagnostic algorithm for patients with suspected mast cell activation syndrome. J Allergy Clin Immunol Pract. 2019;7(4):1125–1133. doi: 10.1016/j.jaip.2019.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ustun C, Arock M, Kluin-Nelemans HC, et al. Advanced systemic mastocytosis: from molecular and genetic progress to clinical practice. Haematologica. 2016;101(10):1133–1143. doi: 10.3324/haematol.2016.146563. [DOI] [PubMed] [Google Scholar]
- [6].Pardanani A. Systemic mastocytosis in adults: 2019 update on diagnosis, risk stratification and management. Am J Hematol. 2019;94(3):363–377. doi: 10.1002/ajh.25371. [DOI] [PubMed] [Google Scholar]
- [7].Valent P, Arock M, Bischoff SC, et al. The European Competence Network on Mastocytosis (ECNM) Wien Klin Wochenschr. 2004;116(19–20):647–651. doi: 10.1007/s00508-004-0253-3. [DOI] [PubMed] [Google Scholar]
- [8].Valent P, Arock M, Bonadonna P, et al. European Competence Network on Mastocytosis (ECNM): 10year jubilee, update, and future perspectives. Wien Klin Wochenschr. 2012;124(23–24):807–814. doi: 10.1007/s00508-012-0293-z. [DOI] [PubMed] [Google Scholar]
- [9].Valent P, Oude Elberink JNG, Gorska A, et al. The data registry of the European Competence Network on Mastocytosis (ECNM): set up, projects, and perspectives. J Allergy Clin Immunol Pract. 2019;7(1):81–87. doi: 10.1016/j.jaip.2018.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Valent P, Hadzijusufovic E, Grunt T, et al. Ludwig Boltzmann Cluster Oncology (LBC ONC): first 10 years and future perspectives. Wien Klin Wochenschr. 2018;130(17–18):517–529. doi: 10.1007/s00508-018-1355-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Eisenwort G, Sadovnik I, Schwaab J, et al. Identification of a leukemia-initiating stem cell in human mast cell leukemia. Leukemia. 2019;33:2673–2684. doi: 10.1038/s41375-019-0460-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Mueller N, Wicklein D, Eisenwort G, et al. CD44 is a RAS/STAT5-regulated invasion receptor that triggers disease expansion in advanced mastocytosis. Blood. 2018;132(18):1936–1950. doi: 10.1182/blood-2018-02-833582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Sabato V, Van De Vijver E, Hagendorens M, et al. Familial hypertryptasemia with associated mast cell activation syndrome. J Allergy Clin Immunol. 2014;134(6):1448–1450 e3. doi: 10.1016/j.jaci.2014.06.007. [DOI] [PubMed] [Google Scholar]
- [14].Lyons JJ. Hereditary alpha tryptasemia: genotyping and associated clinical features. Immunol Allergy Clin North Am. 2018;38(3):483–495. doi: 10.1016/j.iac.2018.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Le QT, Lyons JJ, Naranjo AN, et al. Impact of naturally forming human alpha/beta-tryptase heterotetramers in the pathogenesis of hereditary alpha-tryptasemia. J Exp Med. 2019;216(10):2348–2361. doi: 10.1084/jem.20190701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Akin C, Valent P, Metcalfe DD. Mast cell activation syndrome: proposed diagnostic criteria. J Allergy Clin Immunol. 2010;126(6):1099–1104. doi: 10.1016/j.jaci.2010.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Valent P, Akin C, Arock M, et al. Definitions, criteria and global classification of mast cell disorders with special reference to mast cell activation syndromes: a consensus proposal. Int Arch Allergy Immunol. 2012;157(3):215–225. doi: 10.1159/000328760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Valent P. Mast cell activation syndromes: definition and classification. Allergy. 2013;68(4):417–424. doi: 10.1111/all.12126. [DOI] [PubMed] [Google Scholar]
- [19].Theoharides TC, Valent P, Akin C. Mast cells, mastocytosis, and related disorders. N Engl J Med. 2015;373(2):163–172. doi: 10.1056/NEJMra1409760. [DOI] [PubMed] [Google Scholar]
- [20].Weiler CR, Austen KF, Akin C, et al. AAAAI Mast Cell Disorders Committee Work Group Report: mast cell activation syndrome (MCAS) diagnosis and management. J Allergy Clin Immunol. 2019;144(4):883–896. doi: 10.1016/j.jaci.2019.08.023. [DOI] [PubMed] [Google Scholar]
- [21].Valent P, Bonadonna P, Hartmann K, et al. Why the 20% + 2 tryptase formula is a diagnostic gold standard for severe systemic mast cell activation and mast cell activation syndrome. Int Arch Allergy Immunol. 2019;180(1):44–51. doi: 10.1159/000501079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Lim KH, Tefferi A, Lasho TL, et al. Systemic mastocytosis in 342 consecutive adults: survival studies and prognostic factors. Blood. 2009;113(23):5727–5736. doi: 10.1182/blood-2009-02-205237. [DOI] [PubMed] [Google Scholar]
- [23].Escribano L, Alvarez-Twose I, Sanchez-Munoz L, et al. Prognosis in adult indolent systemic mastocytosis: a long-term study of the Spanish Network on Mastocytosis in a series of 145 patients. J Allergy Clin Immunol. 2009;124(3):514–521. doi: 10.1016/j.jaci.2009.05.003. [DOI] [PubMed] [Google Scholar]
- [24].Teodosio C, Garcia-Montero AC, Jara-Acevedo M, et al. An immature immunophenotype of bone marrow mast cells predicts for multilineage D816V KIT mutation in systemic mastocytosis. Leukemia. 2012;26(5):951–958. doi: 10.1038/leu.2011.293. [DOI] [PubMed] [Google Scholar]
- [25].Erben P, Schwaab J, Metzgeroth G, et al. The KIT D816V expressed allele burden for diagnosis and disease monitoring of systemic mastocytosis. Ann Hematol. 2014;93(1):81–88. doi: 10.1007/s00277-013-1964-1. [DOI] [PubMed] [Google Scholar]
- [26].Jawhar M, Schwaab J, Hausmann D, et al. Splenomegaly, elevated alkaline phosphatase and mutations in the SRSF2/ASXL1/RUNX1 gene panel are strong adverse prognostic markers in patients with systemic mastocytosis. Leukemia. 2016;30(12):2342–2350. doi: 10.1038/leu.2016.190. [DOI] [PubMed] [Google Scholar]
- [27].Greiner G, Gurbisz M, Ratzinger F, et al. Molecular quantification of tissue disease burden is a new biomarker and independent predictor of survival in mastocytosis. Haematologica. 2019 doi: 10.3324/haematol.2019.217950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Pardanani A, Shah S, Mannelli F, et al. Mayo alliance prognostic system for mastocytosis: clinical and hybrid clinical-molecular models. Blood Adv. 2018;2(21):2964–2972. doi: 10.1182/bloodadvances.2018026245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Jawhar M, Schwaab J, Alvarez-Twose I, et al. MARS: Mutation-Adjusted Risk Score for advanced systemic mastocytosis. J Clin Oncol. 2019;37(31):2846–2856. doi: 10.1200/JCO.19.00640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Sperr WR, Kundi M, Alvarez-Twose I, et al. International prognostic scoring system for mastocytosis (IPSM): a retrospective cohort study. Lancet Haematol. 2019;6(12):e638. doi: 10.1016/S2352-3026(19)30166-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Traina F, Visconte V, Jankowska AM, et al. Single nucleotide polymorphism array lesions, TET2, DNMT3A, ASXL1 and CBL mutations are present in systemic mastocytosis. PloS One. 2012;7(8):e43090. doi: 10.1371/journal.pone.0043090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Hanssens K, Brenet F, Agopian J, et al. SRSF2-p95 hotspot mutation is highly associated with advanced forms of mastocytosis and mutations in epigenetic regulator genes. Haematologica. 2014;99(5):830–835. doi: 10.3324/haematol.2013.095133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Schwaab J, Schnittger S, Sotlar K, et al. Comprehensive mutational profiling in advanced systemic mastocytosis. Blood. 2013;122(14):2460–2466. doi: 10.1182/blood-2013-04-496448. [DOI] [PubMed] [Google Scholar]
- [34].Jawhar M, Schwaab J, Meggendorfer M, et al. The clinical and molecular diversity of mast cell leukemia with or without associated hematologic neoplasm. Haematologica. 2017;102(6):1035–1043. doi: 10.3324/haematol.2017.163964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Tolar J, Tope WD, Neglia JP. Leukotriene-receptor inhibition for the treatment of systemic mastocytosis. N Engl J Med. 2004;350(7):735–736. doi: 10.1056/NEJM200402123500723. [DOI] [PubMed] [Google Scholar]
- [36].Broesby-Olsen S, Vestergaard H, Mortz CG, et al. Omalizumab prevents anaphylaxis and improves symptoms in systemic mastocytosis: efficacy and safety observations. Allergy. 2018;73:230–238. doi: 10.1111/all.13237. [DOI] [PubMed] [Google Scholar]
- [37].Lemal R, Fouquet G, Terriou L, et al. Omalizumab therapy for mast cell-mediator symptoms in patients with ISM, CM, MMAS, and MCAS. J Allergy Clin Immunol Pract. 2019;7(7):2387–2395 e3. doi: 10.1016/j.jaip.2019.03.039. [DOI] [PubMed] [Google Scholar]
- [38].Kluin-Nelemans HC, Oldhoff JM, Van Doormaal JJ, et al. Cladribine therapy for systemic mastocytosis. Blood. 2003;102(13):4270–4276. doi: 10.1182/blood-2003-05-1699. [DOI] [PubMed] [Google Scholar]
- [39].Bohm A, Sonneck K, Gleixner KV, et al. In vitro and in vivo growth-inhibitory effects of cladribine on neoplastic mast cells exhibiting the imatinib-resistant KIT mutation D816V. Exp Hematol. 2010;38(9):744–755. doi: 10.1016/j.exphem.2010.05.006. [DOI] [PubMed] [Google Scholar]
- [40].Barete S, Lortholary O, Damaj G, et al. Long-term efficacy and safety of cladribine (2-CdA) in adult patients with mastocytosis. Blood. 2015;126(8):1009–1016. doi: 10.1182/blood-2014-12-614743. quiz 1050. [DOI] [PubMed] [Google Scholar]
- [41].Akin C. Cladribine for mastocytosis: benefits and risks. Blood. 2015;126(8):931–932. doi: 10.1182/blood-2015-06-649525. [DOI] [PubMed] [Google Scholar]
- [42].Alstadhaug KB, Halstensen R Fykse, Odeh F. Progressive multifocal leukoencephalopathy in a patient with systemic mastocytosis treated with cladribine. J Clin Virol. 2017;88:17–20. doi: 10.1016/j.jcv.2016.12.005. [DOI] [PubMed] [Google Scholar]
- [43].Krauth MT, Mirkina I, Herrmann H, et al. Midostaurin (PKC412) inhibits immunoglobulin E-dependent activation and mediator release in human blood basophils and mast cells. Clin Exp Allergy. 2009;39(11):1711–1720. doi: 10.1111/j.1365-2222.2009.03353.x. [Research Support, Non-U.S. Gov’t] [DOI] [PubMed] [Google Scholar]
- [44].Valent P, Akin C, Hartmann K, et al. Midostaurin: a magic bullet that blocks mast cell expansion and activation. Ann Oncol. 2017;28(10):2367–2376. doi: 10.1093/annonc/mdx290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].van Anrooij B, Oude Elberink JNG, Span LFR, et al. Midostaurin in patients with indolent systemic mastocytosis: an open-label phase 2 trial. J Allergy Clin Immunol. 2018;142(3):1006–1008. doi: 10.1016/j.jaci.2018.06.003. [DOI] [PubMed] [Google Scholar]
- [46].Georgin-Lavialle S, Lhermitte L, Suarez F, et al. Mast cell leukemia: identification of a new c-Kit mutation, dup(501-502), and response to masitinib, a c-Kit tyrosine kinase inhibitor. Eur J Haematol. 2012;89(1):47–52. doi: 10.1111/j.1600-0609.2012.01761.x. [DOI] [PubMed] [Google Scholar]
- [47].Alvarez-Twose I, Matito A, Morgado JM, et al. Imatinib in systemic mastocytosis: a phase IV clinical trial in patients lacking exon 17 KIT mutations and review of the literature. Oncotarget. 2017;8(40):68950–68963. doi: 10.18632/oncotarget.10711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Gotlib J, Kluin-Nelemans HC, George TI, et al. Efficacy and safety of midostaurin in advanced systemic mastocytosis. N Engl J Med. 2016;374(26):2530–2541. doi: 10.1056/NEJMoa1513098. [DOI] [PubMed] [Google Scholar]
- [49].DeAngelo DJ, George TI, Linder A, et al. Efficacy and safety of midostaurin in patients with advanced systemic mastocytosis: 10-year median follow-up of a phase II trial. Leukemia. 2018;32(2):470–478. doi: 10.1038/leu.2017.234. [DOI] [PubMed] [Google Scholar]
- [50].Kim ES. Midostaurin: first global approval. Drugs. 2017;77(11):1251–1259. doi: 10.1007/s40265-017-0779-0. [Review] [DOI] [PubMed] [Google Scholar]
- [51].DeAngelo DJ, Quiery AT, Radia D, et al. Clinical activity in a Phase 1 study of Blu-285, a potent, highly-selective inhibitor of KIT D816V in Advanced Systemic Mastocytosis (AdvSM) Blood. 2017;130(Suppl 1):2–2. [Google Scholar]
- [52].Radia D, Deininger M, Gotlib J, et al. Avapritinib, a potent and selective inhibitor of KIT D816V, induces complete and durable responses in patients with advanced systemic mastocytosis. HemaSphere. 2019;3(Suppl 1):368. [Google Scholar]
- [53].Jawhar M, Schwaab J, Naumann N, et al. Response and progression on midostaurin in advanced systemic mastocytosis: KIT D816V and other molecular markers. Blood. 2017;130(2):137–145. doi: 10.1182/blood-2017-01-764423. [DOI] [PubMed] [Google Scholar]
- [54].Ustun C, Gotlib J, Popat U, et al. Consensus opinion on allogeneic hematopoietic cell transplantation in advanced systemic mastocytosis. Biol Blood Marrow Transplant. 2016;22(8):1348–1356. doi: 10.1016/j.bbmt.2016.04.018. [DOI] [PubMed] [Google Scholar]