Abstract
Introduction
With a worldwide ageing population, the importance of the prevention and management of osteoporotic fragility fractures is increasing over time. In this review, we discuss in detail the epidemiology of fragility fractures, how this is shaped by pharmacological interventions and how novel screening programmes can reduce the clinical and economic burden of osteoporotic fractures.
Sources of data
PubMed and Google Scholar were searched using various combinations of the keywords ‘osteoporosis’, ‘epidemiology’, ‘fracture’, ‘screening’ ‘FRAX, and ‘SCOOP’.
Areas of agreement
The economic burden of osteoporosis-related fracture is significant, costing approximately $17.9 billion and £4 billion per annum in the USA and UK.
Areas of controversy
Risk calculators such as the web-based FRAX® algorithm have enabled assessment of an individual’s fracture risk using clinical risk factors, with only partial consideration of BMD.
Growing points
As with all new interventions, we await results of long-term use of osteoporosis screening algorithms and how these can be refined and incorporated into clinical practice.
Areas timely for developing research
Despite advances in osteoporosis screening, a minority of men and women at high fracture risk worldwide receive treatment. The economic and societal burden caused by osteoporosis is a clear motivation for improving the screening and management of osteoporosis worldwide.
Keywords: Osteoporosis, epidemiology, fracture
Introduction
Osteoporosis is a disease of the skeleton, characterised by micro-architectural deterioration of bone tissue and loss of bone mass. Osteoporosis (meaning ‘porous bone’) increases bone fragility and susceptibility to fracture 1. However, due to significant advances in osteoporosis management over the last 50 years – including widespread availability of various effective pharmacological therapies – it is no longer considered an inevitable consequence of ageing. Clinical diagnosis of osteoporosis is challenging: fracture-based criteria may exclude populations-at-risk who would benefit from treatment, whilst the original 1994 World Health Organisation definition by bone mineral density (BMD) alone (2.5 standard deviations below the young adult female mean) may not take account of other risk factors2. More recently, risk calculators such as the web-based FRAX® algorithm3 have enabled assessment of an individual’s fracture risk using clinical risk factors such as age and alcohol consumption, with only partial consideration of BMD. The economic burden of osteoporosis-related fracture is significant, costing approximately $17.9 billion and £4 billion per annum in the USA and UK, respectively (Table 1 summarises fracture impact across the European Union) 4,5.
Table 1. Impact of osteoporosis-related fractures across Europe. Data derived from Hernlund et al, Archives of Osteoporosis, 2013.
| Hip | Spine | Wrist | |
|---|---|---|---|
| Lifetime risk in Women (%) | 23 | 29 | 21 |
| Lifetime risk in Men (%) | 11 | 14 | 5 |
| Cases / year | 620,000 | 810,000 | 574,000 |
| Hospitalization (%) | 100 | 2-10 | 5 |
| Relative survival | 0.83 | 0.82 | 1.00 |
| Costs: All sites combined ~ €37 billion | |||
Methods
The data sources used for this review were all from published literature. PubMed and Google Scholar were searched using various combinations of the keywords ‘osteoporosis’, ‘epidemiology’, ‘fracture’, ‘screening’ ‘FRAX, and ‘SCOOP’.
Fracture Epidemiology
According to a report by the US Surgeon General 4, approximately 10 million Americans over the age of 50 have osteoporosis, with a further 34 million at risk of the disease. Osteoporotic fractures in the USA are extremely common, with an estimated 1.5 million suffering fragility fractures each year. A similar burden of disease has been observed in the UK, with epidemiological studies hypothesising that one in two women and one in five men aged over 50 years will suffer an osteoporotic fracture in their lifetime 6. Bone mass is an established determinant of bone strength, and the bone mass of an individual in later life depends upon peak skeletal growth attained during the fourth decade and the subsequent rate of bone loss thereafter 7. Logically, fracture risk should be highest when bone mass (and therefore bone strength) is lowest; indeed, fracture incidence by age has a bimodal distribution, with peaks in the young and the elderly 8. In the young, fractures occur more frequently in males, whereas from the age of 50 years onwards, fractures in females predominate and the rates become approximately twice those in men. Long bone fractures, as a result of substantial trauma, are the most common type of fracture seen in the young. However, studies suggest that (in addition to the extent of trauma) bone mass is nonetheless a relevant and important risk factor for fracture in this demographic 9. In older individuals, the forearm, hip and vertebrae are the sites most susceptible to fracture 10.
Hip Fracture
In 1990, the number of hip fractures worldwide was estimated to be 1.66 million 11, comprising around 1.19 million in women and 463,000 in men. Approximately 90% of these fractures occurred in individuals aged over 50 years, predominantly as the result of falls from standing height 12. In most populations, there is typically an exponential increase in the incidence of hip fracture with advancing age; above 50 years, hip fractures in women outnumber those in men with a ratio of two to one 8. With an ageing population the socioeconomic burden of hip fracture is likely to increase. In the UK around 79,000 individuals suffer hip fractures each year, with a cost in 2010 estimated at £3.5 billion projected to rise to £5.5 billion per year by 2025 5. In temperate climates, the number of hip fractures varies by season, with an increase in incidence during winter months. As a high proportion of these occur indoors, the cause is likely multifactorial and not simply due to slipping on icy surfaces. Factors such as fewer winter daylight hours and slowed neuromuscular reflexes may be relevant. Furthermore, the direction of fall is an important consideration, as falling sideways –resulting in a direct impact on the hip– is more likely to result in fracture than falling forwards 12.
The mortality burden of hip fracture is significant, with a rate of approximately 8% in men and 3% in women aged above 50 years and hospitalised following fracture. In the USA, approximately 31,000 annual deaths occur within 6 months of hip fracture. In the UK, observed 12-month survival rates post- hip fracture are significantly lower than expected (63.3% observed vs. 90.0% expected for men, and 74.9% observed vs. 91.1% expected for women) 6. Co-existing illnesses and poor pre-fracture functional status are key determinants of post-fracture mortality risk, which is greatest immediately post-fracture, gradually decreasing over time 13. Note, however, that an elevated risk of death has been shown to persist for up to 10 years post-fracture 14. Death following hip fracture is not solely attributable to the fracture itself; instead, prior exacerbation of other chronic comorbidities has likely contributed to reduced life expectancy and indeed, to occurrence of the hip fracture. Of all fracture types, hip fractures are associated with the highest levels of morbidity. Post-fracture complications such as bronchopneumonia, urinary tract infections and pressure sores are common. Furthermore, approximately half of those individuals who were ambulatory prior to hip fracture are unable to mobilise independently post-fracture. Notably, 55% of individuals above 90 years of age are unable to live independently following fracture and are subsequently discharged to nursing homes 15.
Vertebral Fracture
Age-standardised prevalence of vertebral fracture across Europe has been estimated to be 12.2% for men and 12.0% for women aged 50-79 years, according to data from the European Vertebral Osteoporosis Study (EVOS) 16. More recently, a UK study using GP records demonstrated an incidence rate for vertebral fracture of 7.1 per 10,000 person years in adults aged over 50 (4.6 for men, 9.4 for women) 8. For both sexes, vertebral fracture prevalence increases with age, ranging from 3% in female participants below 60 years (7.5% in men) to 19% in female participants over 70 years (20% in men) according to data from the Norwegian Tromso Study 17. The majority of vertebral deformities in men occur at younger ages, likely as a result of trauma. In elderly women, vertebral fractures usually occur due to normal activities such as lifting and bending over, as opposed to direct trauma from falling. Note that the prevalence of vertebral fracture may be underestimated as many such fractures are asymptomatic and therefore individuals do not seek medical attention. Vertebral fractures are associated with significant morbidity including back pain, kyphosis and height loss. This results in a marked reduction in quality of life as assessed by quality of life scores, which decrease as the number of vertebral fractures increases 18. In contrast to hip fractures, the risk of death following a vertebral fracture increases with time post-fracture. Data from the UK GPRD study showed that observed survival 12-months post- vertebral fracture in women was 86.5% vs 93.6% expected. At 5 years, survival was 56.5% observed and 69.9% expected 6. Like hip fractures, co-morbid conditions contribute significantly towards the risk of mortality post- vertebral fracture 14.
Distal forearm fracture
There is a gradual increase in rate of distal forearm fracture with advancing age, with occurrence higher in women than men at older ages. The incidence of distal forearm fracture has been shown to be 39.7 per 10,000 person-years in women and 8.9 per 10,000 person-years in men in the UK for individuals aged 50 years or greater 8. In contrast to both hip and vertebral fractures, distal forearm fractures do not appear to be associated with an increase in mortality 6. Distal forearm fractures also appear to have a lesser impact on activities of daily living, with few patients reporting loss of independence post-fracture. That said, approximately half of individuals report only fair- to poor- function six months post-fracture 8.
Clustering of Fractures in Individuals
There are data to suggest that if an individual suffers a fragility fracture, their risk of subsequent fracture at a different site increases. A meta-analysis conducted by Kanis and colleagues, using a population of 11 cohorts, showed that prior fracture history was associated with an 86% increase in the risk of further fracture at any new site 19. Furthermore, data from EVOS has shown that vertebral deformity has a high predictive value for future hip fracture 20 with the risk being highest immediately post- index fracture 21.
Effect of co-morbidities on osteoporosis risk
There is a well-established association between co-morbid disease and osteoporosis risk. Indeed, the FRAX algorithm asks the investigator to provide information on the presence of rheumatoid arthritis (RA), and to consider whether a number of conditions associated with “secondary osteoporosis” are present. Examples given include inflammatory bowel disease, insulin-dependent diabetes, and diseases associated with reduced mobility, such as stroke and Parkinson's disease 22. A study using participants from the Global Longitudinal Study of Osteoporosis in Women (GLOW) has demonstrated that hypertension, heart disease, asthma, chronic obstructive pulmonary disease (COPD), arthritis (reported osteoarthritis or RA), stroke, inflammatory bowel disease, Parkinson's disease, multiple sclerosis, and type I diabetes were all associated with an increased fracture risk 23. Additionally, a recent study comprising just under 20,000 adults in Germany demonstrated that 95% of the adults with osteoporosis had at least one coexisting disease, and that the odds for arthrosis, arthritis, chronic low back pain, depression and chronic heart failure, were greater for adults with osteoporosis 24. The reason for the increased propensity for individuals with co-morbid diseases to develop osteoporosis is likely multifactorial. Co-morbidities such as RA and Crohn’s disease are inflammatory conditions and studies have shown that pro-inflammatory cytokines such as TNF-α, IL-1 and IL-6 are associated with bone resorption 25, 26. Furthermore, several epidemiological studies have shown negative correlations between BMD and C-reactive protein (CRP) which is a marker of active inflammation 27–32. Additionally, bone loss in conditions such as RA, osteoarthritis, stroke and multiple sclerosis is contributed to by the decline in functional capacity and lack of exercise associated with these conditions 33.
Fracture trends over time
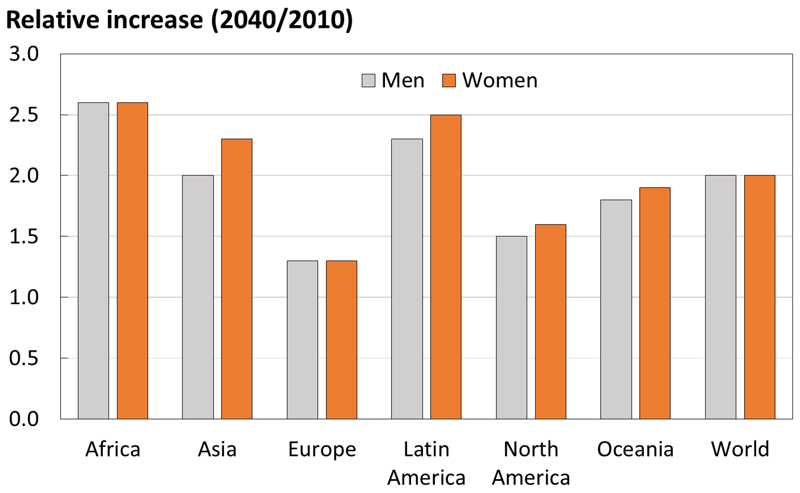
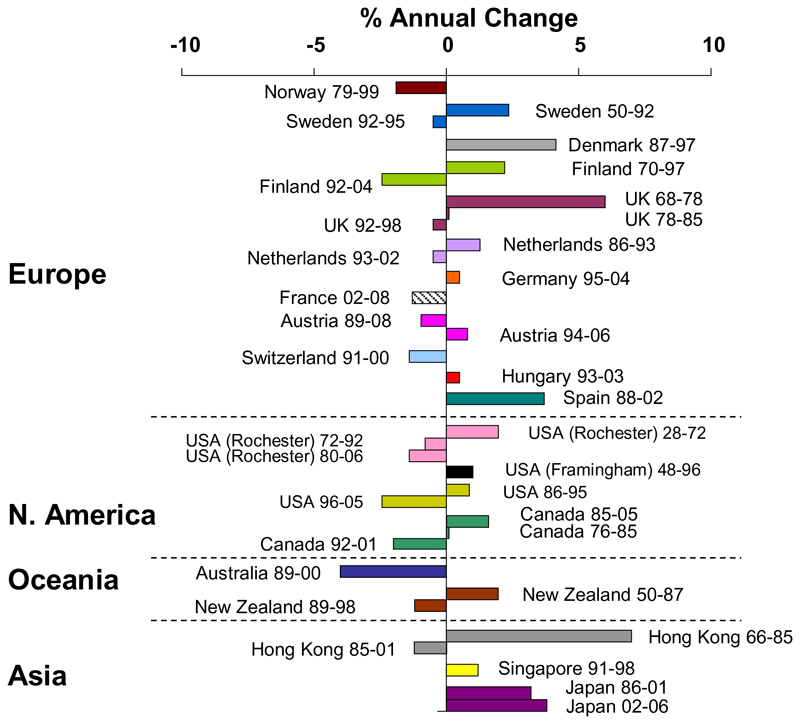
Worldwide, the proportion of individuals living to older age is increasing rapidly, with the United Nations predicting that by 2050 all major areas of the world, with the exception of Africa, will have approximately a quarter of their populations aged above 60 years 35. This ageing population demographic will likely have a significant impact on the number of hip fractures, with a conservative estimate being an increase from 1.66 million in 1990 to 6.26 million in 2050 11, 36. The number of individuals at high fracture risk worldwide is also projected to increase, the largest relative increases predicted for Africa (Figure 1). Worldwide, the incidence of age- and sex-specific vertebral, forearm and hip fractures is continuing to increase 5, 37. Conversely, the incidence of hip fracture in developed countries has stabilised over the last one to two decades (Figure 2), but is still rising in transitioning populations, likely secondary to the adoption of Westernised lifestyles 37. The reason for the stabilisation and often reduction in hip fracture incidence in developed countries is likely multifactorial. For example, the introduction of bisphosphonates in North America and Europe, the increasing prevalence of obesity in the general population and incidence and alterations in tobacco consumption might also have contributed 37.
Figure 1. Number of men and women at high fracture risk in 2040 relative to 2010, by world region. (With permission from Oden et al, Osteoporosis International 2015 34).
Figure 2. Trends in hip fracture worldwide over time: annual change in age- and sex-adjusted hip fracture incidence (Reproduced with permission from Cooper et al, Osteoporosis International 2011 37).
Geography
Fracture incidence varies widely by geography, ethnicity and socioeconomic status 38. This has been demonstrated to be the case both internationally 39 and within individual countries 8. A threefold difference in the incidence of vertebral fracture between countries was demonstrated in the EVOS study, with Scandinavian countries having the highest rates, although some of these differences may be accounted for by differences in body mass index (BMI) and levels of physical activity 16. Geographical differences in hip fracture incidence are even more profound: an approximately 11-fold variation was demonstrated within Europe, which could not be accounted for by differences in activity levels, smoking, obesity, alcohol consumption, or migration status 40.
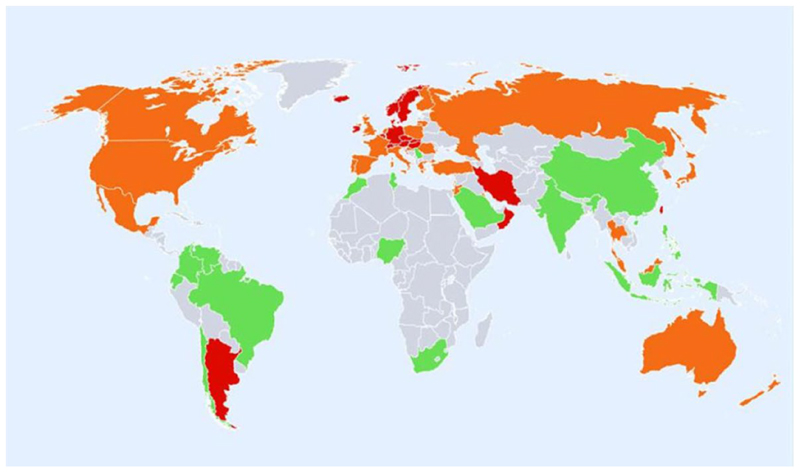
The explanation for global variation in fracture incidence is likely multifaceted, with ethnic differences in BMD, bone geometry and bone micro-architecture thought to contribute to these differences 8. Furthermore, as fracture incidence is typically higher in countries with a more northerly latitude (Figure 3), vitamin D status may be implicated 41.
Figure 3. Hip fracture rates for men and women combined in different countries of the world, categorised by risk. Countries are coded red (annual incidence >250/100,000), orange (150-250/100,000) or green (<150/100,000) where estimates are available. (Reproduced with permission from Kanis et al, Osteoporosis International 2012 39).
Early life Influences on Adult Bone Health
Osteoporosis is one of a number of diseases (including hypertension, coronary heart disease, osteoarthritis and type 2 diabetes) where low birth weight is a precursor to disease development in adulthood 42. Although variation in adult bone mass is largely attributable to genotype, evidence is accruing that interactions between genome and environment (during the intra-uterine period and early childhood) are critical for setting growth trajectory, and therefore bone mass and fracture risk in later life 42. This phenomenon has been termed ‘programming’. The link between development of osteoporosis and weight in infancy was first demonstrated in a study of 153 women born in Bath (UK) between 1968 and 1969 who were then traced and studied at 21 years of age 43. In this study, data detailing childhood growth was obtained from linked birth and school records, and associations were found between bone mineral content (BMC) at the lumbar spine and femoral neck, and weight at one year. Furthermore, these relationships were independent of adult weight and BMI. The association between an individual’s weight in infancy and their bone mass in adulthood was again observed in the UK Hertfordshire Cohort Study 44. Following this, associations between birth weight, or weight at one year, and BMC in later-life have been confirmed internationally across a range of studies, and summarised in a systematic review and meta-analysis 44. Recent findings suggest that an important early determinant of skeletal development is maternal 25(OH)-vitamin D status. The Maternal Vitamin D Osteoporosis Study (MAVIDOS) was a multicentre, double-blinded, randomised, placebo-controlled trial that recruited pregnant women from three study sites in the UK (Southampton, Oxford, and Sheffield). The findings from MAVIDOS suggest that maternal vitamin D supplementation was associated with greater bone mass at birth in babies delivered in the winter months 45. Epigenetic studies have demonstrated that sites within the retinoid X-receptor-A (RXRA) gene (important for the action of 1,25(OH)2-vitamin D and other nuclear hormones) are associated with both maternal free 25(OH)-vitamin D status and offspring bone mass 46. Maternal vitamin D supplementation in the MAVIDOS trial was associated with reduced methylation at the RXRA locus in offspring umbilical cord tissue in comparison with placebo 47.
Pharmacological Interventions for Osteoporosis
Over the past half-century, there have been rapid and marked advancements in pharmacological interventions for osteoporosis. These include calcium and vitamin D supplementation, hormonal replacement therapy, and bisphosphonates 5. Studies have shown that these interventions are effective at reducing the incidence of osteoporotic fragility fracture 48–50. The drugs most commonly used in the treatment of osteoporosis are in the bisphosphonate (formerly diphosphonates) class, which have been shown to reduce all fractures by 35%, vertebral fractures by 50% and non-vertebral fractures by 25% 49, 51. The human monoclonal antibody denosumab, which targets RANKL (receptor activator of NFκB ligand), was shown to reduce the risk of new radiographic vertebral fractures by 68% and hip fractures by 40% in the original 36-month FREEDOM trial 52. Extension of the FREEDOM trial has subsequently found that this reduction in fracture risk is sustained for at least 10 years of denosumab treatment 53. A more recent medication called teriparatide, a parathyroid hormone analogue which promotes bone formation, has been shown in clinical trials to be extremely efficacious in reducing fracture risk. For example, Kendler and colleagues showed in a multicentre, double-blinded, double-dummy, randomised controlled trial that teriparatide was more effective than risedronate, with a reduction in the risk of vertebral fractures by 64% and pooled clinical fractures by 52% over a two-year treatment period 54. The anti-sclerostin antibody romosozumab was approved for medical use in the United States and Canada in 2019. Romosozumab is a humanized monoclonal antibody which blocks sclerostin from inhibiting osteoblast maturation and function. Phase III clinical trials have demonstrated romosozumab’s ability to increase BMD at the lumbar spine and hip and reduce the risk of vertebral and clinical fractures 55. However, as blocking sclerostin leads to Wnt (wingless/integrated) activation and therefore participation in the cardiovascular remodelling process, use of romosozumab may potentially lead to adverse cardiovascular events 56. Indeed, clinical trials have demonstrated an increased risk of serious cardiovascular events among patients that received romosozumab, which warrant further investigation 55. Another new approach for the treatment of osteoporosis is the parathyroid hormone–related peptide analog abaloparatide, which was approved to treat postmenopausal osteoporosis in the United States in 2017. The ACTIVE (Abaloparatide Comparator Trial in Vertebral Endpoints) trial showed that treatment with abaloparatide (80 μg daily) for 18 months reduced new morphometric vertebral fractures (RR 0.14; p < 0.001), nonvertebral fractures (HR 0.57; p = 0.049), major osteoporotic fractures (HR 0.45; p = 0.03), and clinical fractures (RR 0.30; p < 0.001) compared to placebo 57.
The Osteoporosis Treatment Gap
Although treatment strategies for osteoporosis have been shown to be highly effective, there is evidence to suggest that only a minority of osteoporosis patients receive treatment, and therefore the personal and societal burden of fragility fractures remains high 5. A recent report issued by the US National Osteoporosis Foundation estimated that 2 million Americans had 2.3 million osteoporotic fractures in 2015 with only 9% undergoing bone mineral density testing within 6 months of the fracture. In the first 2–3 years post fracture, a second fracture occurred in 307 000 of these individuals incurring a cost of in excess of $6·3 billion 58. This untreated population of individuals with osteoporosis is referred to as ‘The Osteoporosis Treatment Gap’ and recent studies have sought to introduce interventions to reduce this. For example, fracture risk assessment tools (such as FRAX), which utilise clinical variables to provide a measure of fracture risk, have been developed to assist clinicians in identifying ‘at risk’ individuals 22. There is, however, a wide variation in the use of fracture assessment tools worldwide (1000-fold) which may be a reflection of the lack of cohesion in local guidelines or difficulty in accessing the assessment tools online or in paper format59. Despite the introduction of fracture risk assessment tools, there has been a reduction in the number of ‘at risk’ individuals receiving treatment for osteoporosis in some developed countries including the UK and USA 60, 61. This trend may reflect disproportionate highlighting in the lay-media of rare adverse events associated with bisphosphonate use, such as osteonecrosis of the jaw and atypical femoral fractures 62. There is, however, little evidence to suggest that the risk of these adverse events is significantly higher in individuals taking bisphosphonates for 10 years, compared to age-matched controls 63.
Osteoporosis screening programmes
To increase identification of individuals at risk of fracture, and therefore reduce the aforementioned osteoporosis treatment gap, robust screening programmes are required. The WHO recommends that individuals be identified as either at high, medium or low risk of fracture. Following this, they recommend that high-risk individuals be considered for treatment, low-risk individuals not be recommended for treatment and medium-risk individuals be further assessed with a measurement of BMD 64. One of the first studies to examine the effectiveness of an osteoporosis screening programme recruited a total of 4,800 women aged 45-54 years in Aberdeen, Scotland, who were subsequently randomised in equal numbers to screening or no-screening (i.e. control) groups. Post-screening, those in the lowest quartile of BMD were advised to consider hormone replacement therapy. Nine years later, the effect of screening (on the uptake of treatment and fracture incidence) was assessed by postal questionnaire. They found a 25.9% reduction in risk of fracture (any site) in the screened group 65. To identify older women with prevalent osteoporotic vertebral fractures, the Cohort for Skeletal Health in Bristol and Avon (COSHIBA) study –a randomized controlled trial of a primary-care–based screening program– was conducted. The trial comprised a total of 3,200 women aged 65 to 80 years from 15 general practices within Bristol in the UK. The major findings were that allocation to screening increased the prescription of osteoporosis medications by 124% and also reduced fracture incidence at 12-month follow-up, although this did not reach statistical significance (OR for new fracture 0.60; 95% CI, 0.35–1.03; p = 0.063) 66. The Danish Risk Stratified Osteoporosis Study Evaluation (ROSE) study found no overall effect on fracture incidence of a screening programme, but in those individuals with a FRAX ≥15%, major osteoporotic fractures, hip fractures and all fractures were reduced67. More recently, the SCreening Of Older women for the Prevention of fractures (SCOOP) trial was established to test whether a community-based screening intervention could reduce fractures in older women. The SCOOP trial was an unblinded randomised controlled trial of women aged 70-85 years in the UK. It was based in seven centres in the UK (including Birmingham, Bristol, Manchester, Norwich, Sheffield, Southampton and York) from which a total of 12,483 participants were recruited 65. Study participants were stratified into blocks according to age (70-74, 75-79, 80-85) and location of general practice. Participants were then either randomised into the control or screening arm of the study, with control arm participants receiving ‘usual care’, and the participants in the screening arm having their 10-year probability of fracture calculated using FRAX. If participants in the screening arm were assessed as having a moderate- or high-risk of fracture, a Dual-energy X-ray absorptiometry (DXA) scan was performed to calculate BMD. BMD was subsequently incorporated into the FRAX algorithm to inform primary-care treatment decisions. There was no significant difference between the two groups with respect to the proportion of individuals sustaining fragility fractures (p=0.178, HR 0.94 (0.85-1.03)), nor regarding the rate of all clinical fractures (p=0.83, HR 0.94 (0.86-1.03) (as shown in Table 2). There was, however, a reduction in the rate of hip fracture in the screening arm (p=0.002, HR 0.72 (0.59-0.89) 68. The absolute reduction in hip fracture risk was 0.9%, i.e. 111 women between the ages of 70-85 should be screened to avert a single hip fracture. Furthermore, osteoporosis medication use was significantly higher in participants in the treatment arm compared to the control arm (15% and 4% respectively) with 78% of participants in the treatment arm on anti-osteoporotic medication 6-months post-screening 68.
Table 2. Efficacy outcomes for the screening of older women for prevention of fracture (SCOOP) study (Shepstone et al., 2018).
| Control (n=6250) | Screening (n=6233) | Hazard ratio (95% CI) | p value | |
|---|---|---|---|---|
| Osteoporosis-related | ||||
| No Fracture | 5398 (86.4%) | 5428 (87.1%) | - | - |
| Fracture | 852 (13.6%) | 805 (12.9%) | 0.94 (0.85-1.03) | 0.178 |
| Hips | ||||
| No Fracture | 6032 (96.5%) | 6069 (97.4%) | - | - |
| Fracture | 218 (3.5%) | 164 (2.6%) | 0.72 (0.59-0.89) | 0.002 |
| All clinical | ||||
| No Fracture | 5248 (84.0%) | 5282 (84.7%) | - | - |
| Fracture | 1002 (16.0%) | 951 (15.3%) | 0.94 (0.86-1.03) | 0.183 |
| Mortality | ||||
| Survived | 5725 (91.6%) | 5683 (91.2%) | - | - |
| Died | 525 (8.4%) | 550 (8.8%) | 1.05 (0.93-1.19) | 0.436 |
Conclusion
Osteoporosis and the resultant fragility fractures have a profound impact in terms of mortality and morbidity on individuals, healthcare systems and communities as a whole. Whilst there is some evidence that in Western countries fracture incidence rates are falling, the combination of an ageing population and the adoption of a Western lifestyle in developing countries is resulting in an increase in the burden of osteoporosis worldwide. In the past quarter-of-a-century, many risk factors for loss of bone mass (and therefore fracture) have been identified, and several effective pharmacologic therapies for osteoporosis have been introduced. Nevertheless, only a minority of individuals with osteoporosis are treated and therefore resources should be focused on the identification and treatment of those at highest fracture risk.
Biography
Biographical details:
Dr Michael A. Clynes (BSc (hons), MBchB, PhD, MRCP) is a Clinical Lecturer in Rheumatology. Research interests include the developmental origin of musculoskeletal diseases.
Prof. Nicholas C. Harvey (MA, MB, BChir, PhD, FRCP) is a Professor of Rheumatology and Clinical Epidemiology. His work incorporates a lifecourse approach towards the epidemiology and determinants of osteoporotic fracture.
Dr Elizabeth M. Curtis (MA, MB, BChir, PhD, MRCP) is a Clinical Lecturer in Rheumatology. Her research interests include the early life influences on musculoskeletal health, including maternal vitamin D and epigenetic mechanisms.
Dr Nicholas R. Fuggle (BSc (hons), MBBS, MRCP) is a Clinical Research Fellow in Rheumatology. Research interests include epigenetics and musculoskeletal ageing.
Prof. Elaine M. Dennison (MA, MB BChir, MSc, PhD) is a Professor of Musculoskeletal Epidemiology. Her research interests include the epidemiology of musculoskeletal disease, particularly those conditions common in later life (osteoporosis and osteoarthritis).
Cyrus Cooper (OBE, DL, FMedSci) is Professor of Rheumatology and Director of the MRC Lifecourse Epidemiology Unit; Vice-Dean of Medicine at the University of Southampton; and Professor of Epidemiology at the Nuffield Department of Orthopaedics, Rheumatology and Musculoskeletal Sciences, University of Oxford. Professor Cooper leads an internationally competitive programme of research into the epidemiology of musculoskeletal disorders, most notably osteoporosis.
Footnotes
Conflict of interest statement:
The authors have no potential conflicts of interest.
References
- 1.Consensus development conference: diagnosis, prophylaxis, and treatment of osteoporosis. Am J Med. 1993;94:646–50. doi: 10.1016/0002-9343(93)90218-e. [DOI] [PubMed] [Google Scholar]
- 2.Kanis JA. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: synopsis of a WHO report. WHO Study Group. Osteoporos Int. 1994;4:368–81. doi: 10.1007/BF01622200. [DOI] [PubMed] [Google Scholar]
- 3.Kanis JA, Oden A, Johnell O, Johansson H, De Laet C, Brown J, Burckhardt P, Cooper C, Christiansen C, Cummings S, Eisman JA, et al. The use of clinical risk factors enhances the performance of BMD in the prediction of hip and osteoporotic fractures in men and women. Osteoporos Int. 2007;18:1033–46. doi: 10.1007/s00198-007-0343-y. [DOI] [PubMed] [Google Scholar]
- 4.Office of the Surgeon G. Reports of the Surgeon General. Bone Health and Osteoporosis: A Report of the Surgeon General. Rockville (MD): Office of the Surgeon General (US) 2004 [PubMed] [Google Scholar]
- 5.Hernlund E, Svedbom A, Ivergard M, Compston J, Cooper C, Stenmark J, McCloskey EV, Jonsson B, Kanis JA. Osteoporosis in the European Union: medical management, epidemiology and economic burden. A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA) Arch Osteoporos. 2013;8:136. doi: 10.1007/s11657-013-0136-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Staa TP, Dennison EM, Leufkens HG, Cooper C. Epidemiology of fractures in England and Wales. Bone. 2001;29:517–22. doi: 10.1016/s8756-3282(01)00614-7. [DOI] [PubMed] [Google Scholar]
- 7.Burrows M. Exercise and bone mineral accrual in children and adolescents. J Sports Sci Med. 2007;6:305–12. [PMC free article] [PubMed] [Google Scholar]
- 8.Curtis EM, van der Velde R, Moon RJ, van den Bergh JP, Geusens P, de Vries F, van Staa TP, Cooper C, Harvey NC. Epidemiology of fractures in the United Kingdom 1988-2012: Variation with age, sex, geography, ethnicity and socioeconomic status. Bone. 2016;87:19–26. doi: 10.1016/j.bone.2016.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goulding A, Jones IE, Taylor RW, Manning PJ, Williams SM. More broken bones: a 4-year double cohort study of young girls with and without distal forearm fractures. J Bone Miner Res JID - 8610640. 2000;15:2011–2018. doi: 10.1359/jbmr.2000.15.10.2011. [DOI] [PubMed] [Google Scholar]
- 10.Felsenberg D, Silman AJ, Lunt M, Armbrecht G, Ismail AA, Finn JD, Cockerill WC, Banzer D, Benevolenskaya LI, Bhalla A, Bruges Armas J, et al. Incidence of vertebral fracture in europe: results from the European Prospective Osteoporosis Study (EPOS) J Bone Miner Res. 2002;17:716–24. doi: 10.1359/jbmr.2002.17.4.716. [DOI] [PubMed] [Google Scholar]
- 11.Cooper C, Campion G, Melton LJ. Hip fractures in the elderly: a world-wide projection. Osteoporos Int. 1992;2:285–289. doi: 10.1007/BF01623184. [DOI] [PubMed] [Google Scholar]
- 12.Blain H, Masud T, Dargent-Molina P, Martin FC, Rosendahl E, van der Velde N, Bousquet J, Benetos A, Cooper C, Kanis JA, Reginster JY, et al. A Comprehensive Fracture Prevention Strategy in Older Adults: The European Union Geriatric Medicine Society (EUGMS) Statement. J Nutr Health Aging. 2016;20:647–52. doi: 10.1007/s12603-016-0741-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klop C, Welsing PM, Cooper C, Harvey NC, Elders PJ, Bijlsma JW, Leufkens HG, de Vries F. Mortality in British hip fracture patients, 2000-2010: a population-based retrospective cohort study. Bone. 2014;66:171–7. doi: 10.1016/j.bone.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 14.Bliuc D, Nguyen ND, Milch VE, Nguyen TV, Eisman JA, Center JR. Mortality risk associated with low-trauma osteoporotic fracture and subsequent fracture in men and women. JAMA. 2009;301:513–521. doi: 10.1001/jama.2009.50. [DOI] [PubMed] [Google Scholar]
- 15.Chrischilles EA, Butler CD, Davis CS, Wallace RB. A model of lifetime osteoporosis impact. Arch Intern Med. 1991;151:2026–32. [PubMed] [Google Scholar]
- 16.O'Neill TW, Felsenberg D, Varlow J, Cooper C, Kanis JA, Silman AJ. The prevalence of vertebral deformity in european men and women: the European Vertebral Osteoporosis Study. J Bone Miner Res. 1996;11:1010–1018. doi: 10.1002/jbmr.5650110719. [DOI] [PubMed] [Google Scholar]
- 17.Waterloo S, Ahmed LA, Center JR, Eisman JA, Morseth B, Nguyen ND, Nguyen T, Sogaard AJ, Emaus N. Prevalence of vertebral fractures in women and men in the population-based Tromso Study. BMC Musculoskelet Disord. 2012;13:3. doi: 10.1186/1471-2474-13-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oleksik A, Lips P, Dawson A, Minshall ME, Shen W, Cooper C, Kanis J. Health-related quality of life in postmenopausal women with low BMD with or without prevalent vertebral fractures [In Process Citation] J Bone Miner Res. 2000;15:1384–1392. doi: 10.1359/jbmr.2000.15.7.1384. [DOI] [PubMed] [Google Scholar]
- 19.Kanis JA, Johnell O, De Laet C, Johansson H, Oden A, Delmas P, Eisman J, Fujiwara S, Garnero P, Kroger H, McCloskey EV, et al. A meta-analysis of previous fracture and subsequent fracture risk. Bone. 2004;35:375–82. doi: 10.1016/j.bone.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 20.Ismail AA, Cockerill W, Cooper C, Finn JD, Abendroth K, Parisi G, Banzer D, Benevolenskaya LI, Bhalla AK, Armas JB, Cannata JB, et al. Prevalent vertebral deformity predicts incident hip though not distal forearm fracture: results from the European Prospective Osteoporosis Study. Osteoporos.Int. 2001;12:85–90. doi: 10.1007/s001980170138. [DOI] [PubMed] [Google Scholar]
- 21.Johnell O, Kanis JA, Oden A, Sernbo I, Redlund-Johnell I, Petterson C, De Laet C, Jonsson B. Fracture risk following an osteoporotic fracture. Osteoporos Int. 2004;15:175–9. doi: 10.1007/s00198-003-1514-0. [DOI] [PubMed] [Google Scholar]
- 22.Kanis JA, McCloskey EV, Johansson H, Strom O, Borgstrom F, Oden A. Case finding for the management of osteoporosis with FRAX--assessment and intervention thresholds for the UK. Osteoporos Int. 2008;19:1395–408. doi: 10.1007/s00198-008-0712-1. [DOI] [PubMed] [Google Scholar]
- 23.Dennison EM, Compston JE, Flahive J, Siris ES, Gehlbach SH, Adachi JD, Boonen S, Chapurlat R, Diez-Perez A, Anderson FA, Hooven FH, Jr, et al. Effect of co-morbidities on fracture risk: findings from the Global Longitudinal Study of Osteoporosis in Women (GLOW) Bone. 2012;50:1288–93. doi: 10.1016/j.bone.2012.02.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Puth MT, Klaschik M, Schmid M, Weckbecker K, Munster E. Prevalence and comorbidity of osteoporosis- a cross-sectional analysis on 10,660 adults aged 50 years and older in Germany. BMC Musculoskelet Disord. 2018;19:144. doi: 10.1186/s12891-018-2060-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ding C, Parameswaran V, Udayan R, Burgess J, Jones G. Circulating levels of inflammatory markers predict change in bone mineral density and resorption in older adults: a longitudinal study. J Clin Endocrinol Metab. 2008;93:1952–8. doi: 10.1210/jc.2007-2325. [DOI] [PubMed] [Google Scholar]
- 26.McLean RR. Proinflammatory cytokines and osteoporosis. Curr Osteoporos Rep. 2009;7:134–9. doi: 10.1007/s11914-009-0023-2. [DOI] [PubMed] [Google Scholar]
- 27.Nakamura K, Saito T, Kobayashi R, Oshiki R, Oyama M, Nishiwaki T, Nashimoto M, Tsuchiya Y. C-reactive protein predicts incident fracture in community-dwelling elderly Japanese women: the Muramatsu study. Osteoporos Int. 2011;22:2145–50. doi: 10.1007/s00198-010-1425-9. [DOI] [PubMed] [Google Scholar]
- 28.Cauley JA, Barbour KE, Harrison SL, Cloonan YK, Danielson ME, Ensrud KE, Fink HA, Orwoll ES, Boudreau R. Inflammatory Markers and the Risk of Hip and Vertebral Fractures in Men: the Osteoporotic Fractures in Men (MrOS) J Bone Miner Res. 2016;31:2129–2138. doi: 10.1002/jbmr.2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schett G, Kiechl S, Weger S, Pederiva A, Mayr A, Petrangeli M, Oberhollenzer F, Lorenzini R, Redlich K, Axmann R, Zwerina J, et al. High-sensitivity C-reactive protein and risk of nontraumatic fractures in the Bruneck study. Arch Intern Med. 2006;166:2495–501. doi: 10.1001/archinte.166.22.2495. [DOI] [PubMed] [Google Scholar]
- 30.Pasco JA, Kotowicz MA, Henry MJ, Nicholson GC, Spilsbury HJ, Box JD, Schneider HG. High-sensitivity C-reactive protein and fracture risk in elderly women. Jama. 2006;296:1353–5. doi: 10.1001/jama.296.11.1353. [DOI] [PubMed] [Google Scholar]
- 31.Ishii S, Cauley JA, Greendale GA, Crandall CJ, Danielson ME, Ouchi Y, Karlamangla AS. C-reactive protein, bone strength, and nine-year fracture risk: data from the Study of Women's Health Across the Nation (SWAN) J Bone Miner Res. 2013;28:1688–98. doi: 10.1002/jbmr.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dahl K, Ahmed LA, Joakimsen RM, Jorgensen L, Eggen AE, Eriksen EF, Bjornerem A. High-sensitivity C-reactive protein is an independent risk factor for non-vertebral fractures in women and men: The Tromso Study. Bone. 2015;72:65–70. doi: 10.1016/j.bone.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 33.Moayyeri A. The association between physical activity and osteoporotic fractures: a review of the evidence and implications for future research. Ann Epidemiol. 2008;18:827–35. doi: 10.1016/j.annepidem.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 34.Oden A, McCloskey EV, Kanis JA, Harvey NC, Johansson H. Burden of high fracture probability worldwide: secular increases 2010-2040. Osteoporos Int. 2015;26:2243–8. doi: 10.1007/s00198-015-3154-6. [DOI] [PubMed] [Google Scholar]
- 35.Nations U. World Population Prospects: The 2015 Revision. New York: United Nations; 2015. [Google Scholar]
- 36.Gullberg B, Johnell O, Kanis JA. World-wide projections for hip fracture. Osteoporos Int. 1997;7:407–13. doi: 10.1007/pl00004148. [DOI] [PubMed] [Google Scholar]
- 37.Cooper C, Cole ZA, Holroyd CR, Earl SC, Harvey NC, Dennison EM, Melton LJ, Cummings SR, Kanis JA. Secular trends in the incidence of hip and other osteoporotic fractures. Osteoporos Int. 2011;22:1277–1288. doi: 10.1007/s00198-011-1601-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fuggle NR, Curtis EM, Ward KA, Harvey NC, Dennison EM, Cooper C. Fracture prediction, imaging and screening in osteoporosis. Nat Rev Endocrinol. 2019;15:535–547. doi: 10.1038/s41574-019-0220-8. [DOI] [PubMed] [Google Scholar]
- 39.Kanis JA, Oden A, McCloskey EV, Johansson H, Wahl DA, Cooper C. A systematic review of hip fracture incidence and probability of fracture worldwide. Osteoporos Int. 2012;23:2239–56. doi: 10.1007/s00198-012-1964-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.lffors I, Allander E, Kanis JA, Gullberg B, Johnell O, Dequeker J, Dilsen G, Gennari C, Lopes Vaz AA, Lyritis G, et al. The variable incidence of hip fracture in southern Europe: the MEDOS Study. Osteoporos Int. 1994;4:253–63. doi: 10.1007/BF01623349. [DOI] [PubMed] [Google Scholar]
- 41.Wahl DA, Cooper C, Ebeling PR, Eggersdorfer M, Hilger J, Hoffmann K, Josse R, Kanis JA, Mithal A, Pierroz DD, Stenmark J, et al. A global representation of vitamin D status in healthy populations. Arch Osteoporos. 2012;7:155–72. doi: 10.1007/s11657-012-0093-0. [DOI] [PubMed] [Google Scholar]
- 42.Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med. 2008;359:61–73. doi: 10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cooper C, Cawley M, Bhalla A, Egger P, Ring F, Morton L, Barker D. Childhood growth, physical activity, and peak bone mass in women. J Bone Miner Res. 1995;10:940–7. doi: 10.1002/jbmr.5650100615. [DOI] [PubMed] [Google Scholar]
- 44.Baird J, Kurshid MA, Kim M, Harvey N, Dennison E, Cooper C. Does birthweight predict bone mass in adulthood? A systematic review and meta-analysis. Osteoporos Int. 2011;22:1323–34. doi: 10.1007/s00198-010-1344-9. [DOI] [PubMed] [Google Scholar]
- 45.Cooper C, Harvey NC, Bishop NJ, Kennedy S, Papageorghiou AT, Schoenmakers I, Fraser R, Gandhi SV, Carr A, D'Angelo S, Crozier SR, et al. Maternal gestational vitamin D supplementation and offspring bone health (MAVIDOS): a multicentre, double-blind, randomised placebo-controlled trial. Lancet Diabetes Endocrinol. 2016;4:393–402. doi: 10.1016/S2213-8587(16)00044-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Harvey NC, Sheppard A, Godfrey KM, McLean C, Garratt E, Ntani G, Davies L, Murray R, Inskip HM, Gluckman PD, Hanson MA, et al. Childhood bone mineral content is associated with methylation status of the RXRA promoter at birth. J Bone Miner Res. 2014;29:600–7. doi: 10.1002/jbmr.2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Curtis EM, Krstic N, Cook E, D'Angelo S, Crozier SR, Moon RJ, Murray R, Garratt E, Costello P, Cleal J, Ashley B, et al. Gestational Vitamin D Supplementation Leads to Reduced Perinatal RXRA DNA Methylation: Results From the MAVIDOS Trial. J Bone Miner Res. 2019;34:231–240. doi: 10.1002/jbmr.3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chapuy MC, Arlot ME, Duboeuf F, Brun J, Crouzet B, Arnaud S, Delmas PD, Meunier PJ. Vitamin D3 and calcium to prevent hip fractures in elderly women. N Engl J Med. 1992;327:1637–42. doi: 10.1056/NEJM199212033272305. [DOI] [PubMed] [Google Scholar]
- 49.McClung MR, Geusens P, Miller PD, Zippel H, Bensen WG, Roux C, Adami S, Fogelman I, Diamond T, Eastell R, Meunier PJ, et al. Effect of risedronate on the risk of hip fracture in elderly women. Hip Intervention Program Study Group. N Engl J Med. 2001;344:333–40. doi: 10.1056/NEJM200102013440503. [DOI] [PubMed] [Google Scholar]
- 50.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. Jama. 2002;288:321–33. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 51.Hodsman AB, Hanley DA, Josse R. Do bisphosphonates reduce the risk of osteoporotic fractures? An evaluation of the evidence to date. Cmaj. 2002;166:1426–30. [PMC free article] [PubMed] [Google Scholar]
- 52.Cummings SR, San Martin J, McClung MR, Siris ES, Eastell R, Reid IR, Delmas P, Zoog HB, Austin M, Wang A, Kutilek S, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756–65. doi: 10.1056/NEJMoa0809493. [DOI] [PubMed] [Google Scholar]
- 53.Bone HG, Wagman RB, Brandi ML, Brown JP, Chapurlat R, Cummings SR, Czerwinski E, Fahrleitner-Pammer A, Kendler DL, Lippuner K, Reginster JY, et al. 10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol. 2017;5:513–523. doi: 10.1016/S2213-8587(17)30138-9. [DOI] [PubMed] [Google Scholar]
- 54.Kendler DL, Marin F, Zerbini CAF, Russo LA, Greenspan SL, Zikan V, Bagur A, Malouf-Sierra J, Lakatos P, Fahrleitner-Pammer A, Lespessailles E, et al. Effects of teriparatide and risedronate on new fractures in post-menopausal women with severe osteoporosis (VERO): a multicentre, double-blind, double-dummy, randomised controlled trial. Lancet. 2018;391:230–240. doi: 10.1016/S0140-6736(17)32137-2. [DOI] [PubMed] [Google Scholar]
- 55.Shakeri A, Adanty C. Romosozumab (sclerostin monoclonal antibody) for the treatment of osteoporosis in postmenopausal women: A review. J Popul Ther Clin Pharmacol. 2020;27:e25–e31. doi: 10.15586/jptcp.v27i1.655. [DOI] [PubMed] [Google Scholar]
- 56.Asadipooya K, Weinstock A. Cardiovascular Outcomes of Romosozumab and Protective Role of Alendronate. Arterioscler Thromb Vasc Biol. 2019;39:1343–1350. doi: 10.1161/ATVBAHA.119.312371. [DOI] [PubMed] [Google Scholar]
- 57.Miller PD, Hattersley G, Riis BJ, Williams GC, Lau E, Russo LA, Alexandersen P, Zerbini CA, Hu MY, Harris AG, Fitzpatrick LA, et al. Effect of Abaloparatide vs Placebo on New Vertebral Fractures in Postmenopausal Women With Osteoporosis: A Randomized Clinical Trial. Jama. 2016;316:722–33. doi: 10.1001/jama.2016.11136. [DOI] [PubMed] [Google Scholar]
- 58.Compston J. Reducing the treatment gap in osteoporosis. Lancet Diabetes Endocrinol. 2020;8:7–9. doi: 10.1016/S2213-8587(19)30378-X. [DOI] [PubMed] [Google Scholar]
- 59.Curtis EM, Moon RJ, Harvey NC, Cooper C. The impact of fragility fracture and approaches to osteoporosis risk assessment worldwide. Bone. 2017;104:29–38. doi: 10.1016/j.bone.2017.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Solomon DH, Johnston SS, Boytsov NN, McMorrow D, Lane JM, Krohn KD. Osteoporosis medication use after hip fracture in U.S. patients between 2002 and 2011. J Bone Miner Res. 2014;29:1929–37. doi: 10.1002/jbmr.2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van der Velde RY, Wyers CE, Teesselink E, Geusens P, van den Bergh JPW, de Vries F, Cooper C, Harvey NC, van Staa TP. Trends in oral anti-osteoporosis drug prescription in the United Kingdom between 1990 and 2012: Variation by age, sex, geographic location and ethnicity. Bone. 2017;94:50–55. doi: 10.1016/j.bone.2016.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Adler RA, El-Hajj Fuleihan G, Bauer DC, Camacho PM, Clarke BL, Clines GA, Compston JE, Drake MT, Edwards BJ, Favus MJ, Greenspan SL, et al. Managing Osteoporosis in Patients on Long-Term Bisphosphonate Treatment: Report of a Task Force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2016;31:16–35. doi: 10.1002/jbmr.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.LeBlanc ES, Rosales AG, Black DM, Genant HK, Dell RM, Friess DM, Boardman DL, Bauer DC, de Papp A, Santora AC, Orwoll ES. Evaluating Atypical Features of Femur Fractures: How Change in Radiological Criteria Influenced Incidence and Demography of Atypical Femur Fractures in a Community Setting. J Bone Miner Res. 2017;32:2304–2314. doi: 10.1002/jbmr.3221. [DOI] [PubMed] [Google Scholar]
- 64.Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study Group. World Health Organ Tech Rep Ser. 1994;843:1–129. [PubMed] [Google Scholar]
- 65.Barr RJ, Stewart A, Torgerson DJ, Reid DM. Population screening for osteoporosis risk: a randomised control trial of medication use and fracture risk. Osteoporos Int. 2010;21:561–8. doi: 10.1007/s00198-009-1007-x. [DOI] [PubMed] [Google Scholar]
- 66.Clark EM, Gould V, Morrison L, Ades AE, Dieppe P, Tobias JH. Randomized controlled trial of a primary care-based screening program to identify older women with prevalent osteoporotic vertebral fractures: Cohort for Skeletal Health in Bristol and Avon (COSHIBA) J Bone Miner Res. 2012;27:664–71. doi: 10.1002/jbmr.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rubin KH, Rothmann MJ, Holmberg T, Hoiberg M, Moller S, Barkmann R, Gluer CC, Hermann AP, Bech M, Gram J, Brixen K. Effectiveness of a two-step population-based osteoporosis screening program using FRAX: the randomized Risk-stratified Osteoporosis Strategy Evaluation (ROSE) study. Osteoporos Int. 2018;29:567–578. doi: 10.1007/s00198-017-4326-3. [DOI] [PubMed] [Google Scholar]
- 68.Shepstone L, Lenaghan E, Cooper C, Clarke S, Fong-Soe-Khioe R, Fordham R, Gittoes N, Harvey I, Harvey N, Heawood A, Holland R, et al. Screening in the community to reduce fractures in older women (SCOOP): a randomised controlled trial. Lancet. 2018;391:741–747. doi: 10.1016/S0140-6736(17)32640-5. [DOI] [PubMed] [Google Scholar]