Abstract
Background
Monitoring of molecular response (MR) using quantitative polymerase chain reaction (PCR) for BCR-ABL1 is a pivotal tool for guiding tyrosine kinase inhibitor therapy and the long-term follow-up of patients with chronic myeloid leukemia (CML). Results of MR monitoring are standardized according to the International Scale (IS), and specific time-dependent molecular milestones for definition of optimal response and treatment failure have been included in treatment recommendations. The common practice to use peripheral blood (PB) instead of bone marrow (BM) aspirate to monitor the MR monitoring in CML has been questioned. Some studies described differences between BCR-ABL1 levels in paired PB and BM specimens.
Methods
We examined 631 paired PB and BM samples from 283 CML patients in a retrospective single-center study using an IS normalized quantitative reverse transcription (qRT)-PCR assay for quantification of BCR-ABL1 IS.
Results
A good overall concordance of BCR-ABL1 IS results was found, a systematic tendency towards higher BCR-ABL1 IS levels in PB was observed in samples of CML patients in a major MR. This difference was most pronounced in patients treated with imatinib for at least 1 year. Importantly, the difference resulted in a significantly lower rate of deep MR when BCR-ABL1 IS was assessed in the PB compared to BM aspirates.
Conclusions
In summary, our data suggest that the classification of deep MR in patients with CML is more stringent in PB than in BM. Our study supports the current practice to primarily use PB for long-term molecular follow-up monitoring in CML.
Keywords: BCR-ABL1, chronic myeloid leukemia, deep molecular response, quantitative PCR
Introduction
Chronic myeloid leukemia (CML) is a myeloproliferative neoplasm characterized by the presence of the BCR-ABL1 fusion gene (BCR, BCR activator of RhoGEF and GTPase, NG_009244.2; ABL1, ABL proto-oncogene 1, nonreceptor tyrosine kinase, NG_012034.1). The reciprocal translocation t(9;22)(q34;q11) is associated with the BCR-ABL1 fusion that can be detected by fluorescence in situ hybridization (FISH) or reverse transcriptase polymerase chain reaction (RT-PCR) to establish the diagnosis [1]. Tyrosine kinase inhibitors (TKI) targeting BCR-ABL1 are highly effective and have become standard of treatment for patients with CML [2]. Monitoring of molecular response (MR) by quantitative RT-PCR (qRT-PCR) is widely used as it provides important prognostic information particularly for patients undergoing TKI treatment. In fact, time-dependent molecular milestones for definition of optimal response and treatment failure are included in international treatment recommendations. In particular, the achievement of the major molecular response (MMR or MR3; 3 log reduction) is the most important milestone for CML patients undergoing TKI treatment [3, 4].
QRT-PCR for BCR-ABL1 is a highly sensitive method for detection and quantification of minimal residual disease (MRD) in CML. The amount of BCR-ABL1 mRNA is normalized to an internal reference gene, most commonly ABL1, and expressed as a percentage [5]. Tremendous efforts have been made to standardize molecular BCR-ABL1 results obtained by a variety of different assays with a substantial inter-assay variability. In particular, the development of an International Scale (IS) with the application of laboratory-specific conversion factors or the usage of reagents that have been calibrated to the World Health Organization (WHO) International Genetic Reference Panel for quantitation of BCR-ABL1 mRNA significantly improved comparability between laboratories and led to a standardized reporting of BCR-ABL1 IS levels [5, 6]. MMR corresponds to ≤0.1% BCR-ABL1 IS, and the terms MR4, MR4.5 and MR5 are used to indicate levels of MRD that are ≤0.01%, ≤0.0032%, or ≤0.001% BCR-ABL1 IS, respectively (corresponding to 4-, 4.5- or 5-log reduction from standardized baseline) [5, 7]. A 4 to 5 log reduction is of particular relevance for definition of deep molecular response (DMR) that has been established as a surrogate parameter of complete response and as a prerequisite for discontinuation of TKI treatment in CML [8, 9].
In contrast to the tremendous effort in standardization of BCR-ABL1 IS measurements, the question whether to use peripheral blood (PB) and/or bone marrow (BM) as optimal material is not finally answered. BM is generally accepted as gold standard material for determination of cytogenetic response using karyotyping in CML [10]. PB is commonly used for MR assessment as it is easier to obtain and enables frequent monitoring without the need of a more invasive BM puncture. It has also been suggested as an appropriate specimen in the European Leukemia Net (ELN) and National Comprehensive Cancer Network (NCCN) guidelines on CML [3, 4]. Despite this common practice, there are controversial studies arguing in favor of either using specifically BM or PB for MRD measurements in CML. While in general there is a good overall agreement between the level of BCR-ABL1 in PB and BM, relevant difference at specific cut-offs or time points have been described that might influence the sensitivity of molecular MRD testing in CML [11–16]. To further standardize MR testing and clinical practice in CML, the definition of an optimal specimen for the precise quantification of low levels of BCR-ABL1 mRNA is of utmost importance. The comparison of BM and PB using an IS-standardized qRT-PCR assay has not been performed in a large patient series so far and is subject of the present study.
Materials and methods
Patients
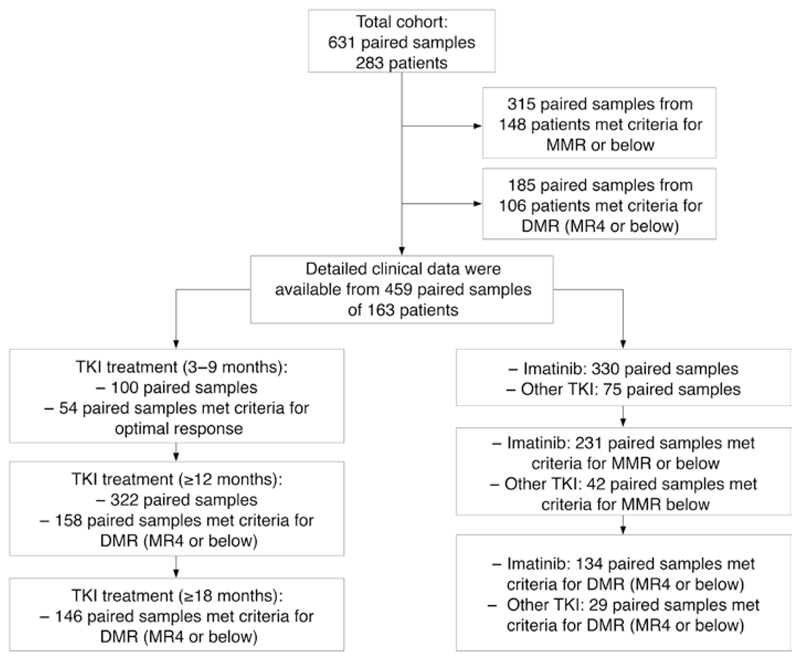
We examined 631 paired samples (BM aspirates vs. PB) from 283 CML patients (118 females, 165 males), diagnosed between October 1982 and January 2016 and stored in a local registry. PB and BM samples at diagnosis and during follow-up were obtained after informed consent was given and the study was approved by the institutional review board (EK: EK-1063/2018). Inclusion criteria were the presence of a BCR-ABL1 transcript of the major breakpoint type (e13a2 or e14a2) and a maximal diff ence of 7 days between PB and BM sampling was accepted for definition of a paired sample although 93.2% of samples were obtained with a maximal diffence of 1 day. Detailed clinical data were available in a sub-cohort of 163 patients. According to WHO criteria [1], 119 patients were diagnosed as having chronic phase (CP), eight patients had accelerated phase (AP) and three had blast phase. In 33 patients no information on the phase was available. The patients’ characteristics are shown in Table 1.
Table 1. Patients’ characteristics.
| Patients’ characteristics | Total cohort (n = 283) | Subcohort (n = 163) |
|---|---|---|
| Age, years (median and range) | 58 (19–86) | 57 (19–86) |
| Sex (female | male) | 118 | 165 | 66 | 97 |
| Disease stage at diagnosis | ||
| Chronic phase | a | 119 (73.0%) |
| Accelerated phase | a | 8 (4.9%) |
| Blast phase | a | 3 (1.8%) |
| Follow-up (months; median and range) | a | 63.2 (2.3–377.2) |
| TKI treatment duration (months; median and range) | a | 62.4 (2.1–177.9) |
| Best available response (number) | ||
| No MMR | a | 50 (30.7%) |
| MMR | a | 26 (16.0%) |
| MR4 | a | 31 (19.0%) |
| MR4.5 | a | 27 (16.6%) |
| MR5 | a | 29 (17.8%) |
Not available. MR, molecular response, MMR, mean molecular response.
Molecular analysis of BCR-ABL1
A total of 1 × 107 leukocytes from PB or aspirated BM were lysed using RLT-Puffer (Qiagen, Hildesheim, Germany). RNA extraction was performed using the QIAcube instrument with the RNe-asy Mini Kit (both Qiagen) and cDNA was generated out of 3 μg of RNA using the High-Capacity cDNA Reverse Transcription Kit with RNase Inhibitor with a random primer (Applied Biosystems, Foster City, CA, USA) according to the manufacturer’s recommendations. RNA was quantified using the NanoDrop 2000 instrument (Thermo Fisher Scientific, Waltham, MA, USA). Quantification of BCR-ABL1 mRNA was performed in duplicates with the Ipsogen BCR-ABL1 Mbcr IS-MMR KIT (Qiagen) according to the manufacturer’s recommendations and analyzed on the LightCycler® 2.0 Instrument (Roche, Basel, Switzerland) and LightCycler® Software. ABL1 was used as internal control gene and BCR-ABL1 mRNA values were normalized to the IS (BCR-ABL1 IS) using a conversion factor determined by sample exchange with the European Treatment and Outcome Study (EUTOS) for CML reference center Mannheim (Germany) [6]. A minimum of 10,000 control gene copies was required for MR assessement, and MR was diagnosed according to the standardized ELN recommendations [7].
Statistical analysis
Statistical analysis was performed using R (R: The R Project for Statistical Computing, Vienna, Austria) [17] and GraphPad Prism (GraphPad Software, La Jolla, CA, USA). Categorical data was assessed using McNemar’s chi-square (χ2)-test or Fisher’s exact test. Metric data was given as median and group diff ences were evaluated by the Wilcoxon rank test. The correlation was assessed by applying Spearman’s rank correlation coefficient (ρ). Diffences between the quantitative PCR results obtained from diffent specimen were graphically displayed using a Bland-Altman plot and statistically compared by applying ordinary least squares regression and Passing-Bablok regression on log transformed data. Differences were considered to be significant when the p-value was <0.05.
Results
Differences between BCR-ABL1 IS in paired samples: PB vs. BM
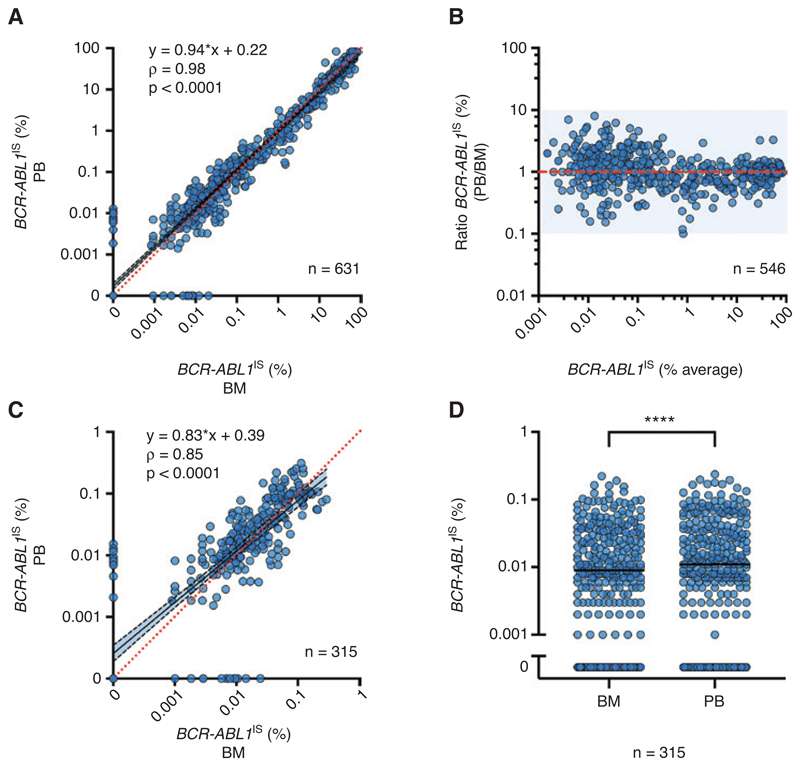
BCR-ABL1 IS was measured in 631 paired samples from 283 patients (Table 1, Figure 1). BCR-ABL1 was detected in 559 BM samples with a median BCR-ABL1 IS of 0.34% and in 563 PB samples with a median BCR-ABL1 IS of 0.30%. Overall, a high degree of correlation between the two specimens was observed (rP = 0.98), and no relevant systematic deviation was found in ordinary least squares regression analysis of log-transformed data, with a slope of 0.94 (95% confidence interval [CI] 0.92–0.96) for the conversion from BM to PB (Figure 2A). Regression coefficients of the Passing-Bablok regression disclosed a similar result (intercept: 0.0 [CI: 0.0–0.001], slope: 0.98 [CI: 0.96–1.00]), without evidence of a systematic or proportional difference. However, a Bland-Altman plot showed a deviation tendency toward higher levels in PB for samples with a low BCR-ABL1 IS level (Figure 2B). We therefore restricted the analysis to 315 samples pairs with ≤0.1% BCR-ABL1 IS in at least one material. In this subgroup of samples that met the criteria for MMR, a weaker correlation between the two types of specimens (rP = 0.85) and a systematic deviation towards slightly higher BCR-ABL1 IS results in PB for samples with low average BCR-ABL1 IS was observed with a slope of 0.83 ([95% CI 0.76−0.89] and an intercept of 0.39 [CI: 0.27−0.52] in ordinary least squares regression analysis of log-transformed data) (Figure 2C). Regression coefficients of the Passing-Bablok regression also indicated systematic deviation (intercept: 0.0 [CI: 0.0−0.0], slope: 1.33 [CI: 1.14−1.50]). In line with this observation, paired samples meeting the MMR criteria in at least one material showed significantly higher BCR-ABL1 IS levels in PB (median 0.011%) than in BM (median 0.009%; p ≤ 0.0001) (Figure 2D).
Figure 1. Grouping of timepoints, MR and treatment modalities.
MMR, major molecular response; DMR, deep molecular response; MR, molecular response; TKI, tyrosine kinase inhibitor.
Figure 2. Comparison of BCR-ABL1 IS in BM aspirate and PB in samples of patients with CML.
(A) Quantification of BCR-ABL1 IS in 631 paired samples from 283 CML patients showed a strong overall correlation (Spearman’s rank correlation ρ = 0.98). (B) Bland-Altmann plot of qPCR positive samples (n = 546) indicates a slight systematic deviation at low BCR-ABLIS values ≤0.1%. (C, D) Detailed analysis of 315 paired samples with BCR-ABL1 IS ≥0.1% in at least one specimen showed a weaker correlation (ρ = 0.85) with a systematic deviation (C) and significantly higher values in PB than in BM (D). ****p < 0.0001 in paired Wilcoxon signed-rank test. ρ, Spearman’s rho; BM, bone marrow; PB, peripheral blood; CML, chronic myeloid leukemia.
DMR of CML in PB and BM
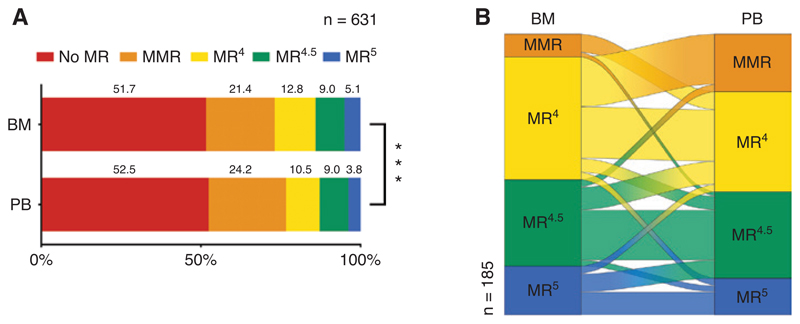
We next analyzed whether the difference between BCR-ABL1 IS in PB and BM had an impact on the MR classification of the samples. Three hundred of the 631 PB samples (47.5%) and 305/631 BM samples (48.3%) were classified as MMR or below. When taking DMR (MR4, MR4.5 or MR5) into account, a significant difference between response classification in PB and BM was observed (p <0.001, Figure 3A). In particular, a DMR of MR4 or below was found in at least one specimen of 185 paired samples (29.3%). One hundred and thirty-two paired samples (20.9%) were concordantly classified as at least MR4 in both specimens, while 53 (8.4%) were discordantly classified with significantly more samples meeting the MR4 criteria in the BM (n = 38) than in the PB (n = 15; p = 0.0025; Table 2). Individual analysis of these sample pairs in Sankey diagrams showed a substantial amount of samples that were classified as MR4 in BM but only as MMR in the PB. In contrast, samples that were classified as MR4 in PB were only rarely classified as a deeper response in BM (Figure 3B). In summary, we observed a statistically significant tendency towards deeper MR levels in the BM compared to PB.
Figure 3. Differences in MR assessed in paired samples in patients with CML.
(A) Distribution of MR data from 631 paired samples from 283 CML patients. (B) A Sankey diagram of paired samples with DMR (≥MR4) in at least one specimen. ***p < 0.001 in Fisher’s exact test. DMR, deep molecular response; MR, molecular response; MMR, major molecular response; BM, bone marrow; PB, peripheral blood; CML, chronic myeloid leukemia.
Table 2. Differences in achievement of MR4 in paired BM and PB samples of 283 CML patients.
| All samples | ≥MR4 | PB |
|
|
|---|---|---|---|---|
| No, n | Yes, n | Total, n | ||
| BM | No, n | 446 (70.7%) | 15 (2.4%) | 461 (73.1%) |
| Yes, n | 38 (6.0%) | 132 (20.9%) | 170 (26.9%) | |
| p = 0.0025 | Total, n | 484 (76.7%) | 147 (23.3%) | 631 (100%) |
Contingency table with McNemar test of all samples. BM, bone marrow; CML, chronic myeloid leukemia; MR, molecular response; PB, peripheral blood.
The depth of MR can be limited either by the level of BCR-ABL1 IS or by an insufficient number of control gene copies. When we compared BCR-ABL1 IS and ABL1 copy numbers at the MR4 cutoff, no difference in the control gene copy numbers were observed (Supplementary Figure 1A). In particular, all samples met the MR4 requirement of 10,000 ABL1 copies, while the BCR-ABL1 IS levels were higher in the PB than in the BM (Supplementary Figure 1B) – indicating that the observed differences were not observed due to technical issues.
Impact of treatment modalities on the MR in PB and BM
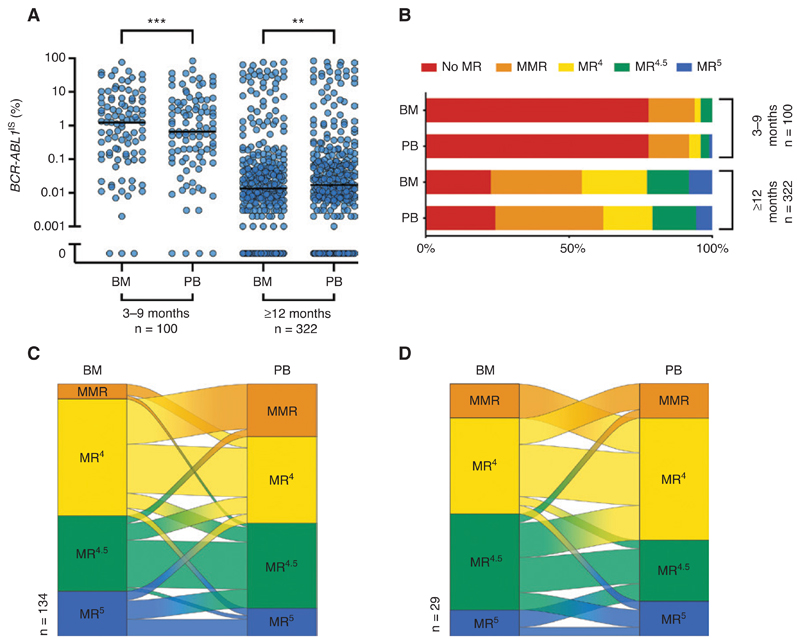
To investigate the relationship between treatment modalities and the MR in PB and BM, we next restricted the analysis to 459 samples of 163 patients with detailed clinical follow-up data available (Table 1). In the early phase of TKI treatment (3–9 months) we observed significantly higher levels of BCR-ABL1 IS in the BM (median 1.23%) than in the PB (median 0.66%, p < 0.001; Figure 4A), while contradictory results with significantly higher BCR-ABL1 IS in the PB were found after at least 12 months of treatment (median 0.014% vs. 0.017%, p = 0.001; Figure 4A). Like in the total cohort, paired samples obtained from patients after at least 12 months of TKI treatment showed a trend towards deeper MR in the BM (Figure 4B). The deviation towards higher BCR-ABL1 IS levels in PB was also confirmed when restricting the correlation analysis to samples with sustained DMR (Supplementary Figure 3). A DMR of MR4 or below was found in at least one specimen of 158 paired samples (49.1%). One hundred and twelve paired samples (34.8%) were concordantly classified as at least MR4 in both specimens, while 46 (14.3%) were discordantly classified with significantly more samples meeting the MR4 criteria in the BM than in the PB (p = 0.0007; Table 3). As expected, DMR was only rarely observed in the early phase of TKI treatment (3–9 months) (Figure 4B). We therefore analyzed whether the specimen had an impact on the 1% BCR-ABL1 IS cut-off that is used for definition of optimal response at 6 months of TKI treatment according to ELN guidelines [3]. An MR below 1% BCR-ABL1 IS was found in 54 paired samples (54.0%): 46 paired samples (46.0%) were classified as <1% BCR-ABL1 IS in both types of specimens. Eight samples (8.0%) were discordantly classified with a not significant trend towards more PB samples meeting the definition of optimal response (p = 0.08; Supplementary Table 1).
Figure 4. Effect of treatment modalities on the difference of BCR-ABL1 IS between specimens.
(A, B) Differences of BCR-ABL1 IS between BM and PB at early time points of TKI treatment (3–9 months, left) differ from that observed in long-term follow-up (≥12 months, right) (A); effect on the distribution of MR classification (B). (C, D) A Sankey diagram of paired samples with DMR (≥MR4) in at least one specimen for patients receiving imatinib (n = 134, C) or other TKI (n = 29, D). **p < 0.01, ***p < 0.001. BM, bone marrow; DMR, deep molecular response; PB, peripheral blood; MR, molecular response; MMR, major molecular response; TKI, tyrosine kinase inhibitor.
Table 3. Differences in achievement of MR4 in paired BM and PB samples of 134 CML patients in long-term follow-up.
| ≥12 months | ≥MR4 | PB |
Total, n | |
|---|---|---|---|---|
| No, n | Yes, n | |||
| BM | No, n | 164 (50.9%) | 11 (3.4%) | 175 (54.3%) |
| Yes, n | 35 (10.9%) | 112 (34.8%) | 147 (45.7%) | |
| p = 0.0007 | Total, n | 199 (61.8%) | 123 (38.2%) | 322 (100%) |
Contingency table with McNemar test of samples from patients undergoing TKI treatment for ≥12 months. BM, bone marrow; CML, chronic myeloid leukemia; PB, peripheral blood.
Finally, we stratified this cohort (459 samples of 163 patients with detailed clinical follow-up data available) into paired samples under treatment with imatinib (n = 330) or other TKI (n = 75) (54 samples without TKI treatment). Patients treated with imatinib represented the majority of our cases and the shift towards higher BCR-ABL1 transcripts in PB vs. BM were confirmed (Supplementary Figure 2A). In particular, detailed comparison of DMR in 134 paired samples of imatinib-treated patients with MR4 in at least one specimen showed a substantial amount of samples that were classified as MR4 in BM but only as MMR in the PB (Figure 4C) and significantly more samples met the MR4 criteria in the BM than in the PB (p = 0.0015; Supplementary Table 2). In contrast, only minor differences between BCR-ABL1 IS levels in PB and BM were observed for other TKI (Supplementary Figure 2B). In particular, we did not observe a difference for the DMR response defined as MR4 or below between the two types of specimen (PB vs. BM) (Figure 4D; Supplementary Table 3). In summary, the observed differences between MR in PB and BM were most pronounced in patients treated with imatinib for at least 12 months while a limited number of samples from patients treated with other TKI showed no obvious difference between BCR-ABL1 IS levels in both specimens.
Discussion
We systematically compared molecular quantification of BCR-ABL1 in 631 paired PB and BM samples from 283 CML patients using an IS normalized qRT-PCR assay. While a good overall correlation of BCR-ABL1 IS results was found, a systematic tendency towards higher BCR-ABL1 IS levels in the PB was observed for CML patients in MMR. This resulted in a significantly higher rate of DMR (MR4) when assessed in the BM in comparison to the PB in these patients. This effect was most pronounced for patients treated with imatinib for at least 1 year, while contrary results with slightly higher BCR-ABL1 IS levels in the BM were found in the first months of treatment.
Despite the tremendous effort in the standardization of BCR-ABL1 measurement and the common practice to use PB for MR assessment, there has been an ongoing discussion about the potential differences between BM and PB as specimens for MRD measurement in CML [11–16]. Stock et al. reported frequent differences between PB and BM BCR-ABL1 measurements in 36 paired samples with a trend towards lower values in PB samples obtained during treatment compared to the corresponding BM values (of note, 34 of the 36 paired on-treatment samples were studied between 3 and 9 months of TKI treatment). Subsequently, the authors recommended caution against interchanging BM with PB sampling for MRD monitoring during treatment of CML [11]. In contrast, Jiang et al. reported conflicting results in 634 paired on-treatment samples where the level of BCR-ABL1 was significantly lower in PB than in BM for samples with a <2 log reduction and significantly higher for samples with a ≥2 log reduction. In total, the reported depth of the MR in PB was lower than that in BM (corresponding to significantly higher levels of BCR-ABL1 in PB compared to BM) [12]. Although the results of these studies seem to be conflicting at first glance, they fit very well to our results when the duration of TKI treatment is taken into account. We also observed slightly higher BCR-ABL1 IS levels in BM samples at an early time point of TKI treatment (3–9 months) while BCR-ABL1 IS levels in the PB were significantly higher compared to BM in samples after a longer duration of TKI treatment (≥12 months). In summary, our data confirm results from a previous study indicating that the differences between of BCR-ABL1 in PB and BM were associated with the depth of MR during imatinib therapy [12]. Although differences were small in the majority of samples, we and others have observed differences of up to 1 log between BCR-ABL1 IS in PB and BM in some paired samples [11–16]. While in general both cell sources are considered suitable for molecular analysis, our results also argue against an uncritical interchanging of BM and PB results for MRD testing. Differences in the individual follow-up of a patient need to be critically evaluated when changing the type of specimen and should be confirmed before making a treatment decision. This is also in line with previous recommendations stating that the serial use of qRT-PCR values based on interchangeable use of PB and BM can lead to misinter-pretation of results [18].
Although our data show that BCR-ABL1 IS results in PB and BM are not completely equal, the relevance of the observed differences for response classification and monitoring in CML remains uncertain. Regarding the early phase of TKI treatment, we observed a slight trend towards higher rates of achieved molecular milestones (e.g. <1% BCR-ABL1 IS) in PB than in the BM that did not meet statistical significance. At the MMR cut-off of 0.1% BCR-ABL1 IS we observed a very good correlation between BM and PB that is in line with previous studies [19]. In comparison to previous data, our study was designed to detect potential differences in long-term follow-up (due to the median time from diagnosis to sampling of 48 months). Long-term molecular follow-up has become increasingly important as it was realized that assessment of DMR is a prerequisite for the discontinuation of TKI treatment in CML [9]. It is of pivotal importance to standardize the assessment of BCR-ABL1 as much as possible to minimize the effects of testing conditions on MR classification – this also includes the standardization of the specimen for DMR assessment. Although MR is commonly assessed in PB and recommended as appropriate material by international guidelines, additional testing of BCR-ABL1 IS in the BM has been discussed [11–16]. NCCN guidelines on CML state that a major advantage of qPCR is the strong correlation between results obtained from PB and BM, allowing molecular monitoring without BM aspiration. They do not discourage molecular testing in the BM [4]. ELN guidelines are more in favor of PB and state that PB is suitable for analysis of BCR-ABL1 transcripts in chronic-phase CML and that PB should routinely be used for monitoring, as PB samples correlate with clinical response and are easy to collect on a regular basis in patients within chronic phase CML [3]. Recommendations on discontinuation of TKI treatment and DMR do not always specify the specimen [4, 9, 20, 21]. In our cohort, we observed a trend towards a deeper MR classification in BM compared to PB. A substantial amount of paired samples were classified as MR4 only in BM but not in the PB. This argues for a slightly higher sensitivity to detect low level MRD in PB than in BM and supports the current practice to primarily use PB for molecular long-term follow-up in CML. The use of PB might not only be sufficient but even superior.
The reason for the differences between BCR-ABL1 IS in the BM and PB observed in this study remains unclear. We were able to exclude technical reasons as the majority of samples were obtained in-house without a delay in transportation, a defined amount of RNA was used for molecular testing and no differences in the control gene copy number were observed. Optimal performance of the molecular analysis is a prerequisite to detect low amounts of BCR-ABL1 in the PB and alterations of the protocol for RNA isolation, cDNA synthesis and PCR can substantially effect the sensitivity of the assay [22]. Thus, although sub-optimal analysis conditions might compromise the claim of superiority for BCR-ABL1 IS in the PB, this is obviously not an issue in our study. Potential biological reasons for the differences are not well understood. Differences in the amount of BCR-ABL1 transcripts as well as the dynamic of response to TKI treatment have been discussed between BM precursors and more mature myeloid cells [12, 23–25]. In our study, patients undergoing TKI treatment with imatinib represented the majority of the study cohort. We cannot exclude that a faster or deeper MR with second generation TKI influences the differences between BCR-ABL1 IS levels of the specimens. Indeed, we did not observe the same effect under TKI treatment with nilotinib or dasatinib. However, the number of samples under second generation TKI was limited. Likewise, treatment free remission was not assessed within our study.
In summary, our data support the common practice to use PB for assessment of DMR in CML. BM puncture is not necessary for additional molecular analysis of BCR-ABL1 IS in the BM and can be limited to cases with the suspicion of treatment failure for additional morphologic and cytogenetic assessments.
Supplementary Material
The online version of this article offers supplementary material (https://doi.org/10.1515/cclm-2019-1172).
Acknowledgments
The authors thank Jana Strasakova (Department of Laboratory Medicine, Medical University of Vienna) for technical support.
Research funding
This study was supported by the Austria Science Fund (FWF) project P26079-B13, the SFB projects Funder Id: http://dx.doi.org/10.13039/501100002428, F4701-B20 and F4704-B20, and the Medical-Scientific Fund of the Mayor of Vienna.
Footnotes
Author contributions: G.G., M.G., N.W., S.M.H.T.W., S.G.K.M, G.M.-H., C.M., and G.H. performed molecular tests and analyzed the data. G.G., H.E., W.R.S., P.V., and G.H. obtained and analyzed clinical data. G.G., F.R., and W.R.S. performed statistical analyses. G.G., P.V., and G.H. designed the study and wrote the paper. All authors revised and approved the manuscript.
Employment or leadership: None declared.
Honorarium: None declared.
Competing interests: The funding organization(s) played no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the report for publication.
Conflict of interest: P.V. served as a consultant in a global Novartis trial investigating the effects of midostaurin in patients with advanced systemic mastocytosis and received honoraria and research grants from Novartis, Blueprint, Pfizer, Ariad, Incyte, Celgene, and Deciphera. received honoraria from Novartis. G.H. received honoraria and research grants from Novartis. The authors declare no other competing financial interests.
Contributor Information
Georg Greiner, Department of Laboratory Medicine, Medical University of Vienna, Vienna, Austria; Ludwig Boltzmann Institute for Hematology and Oncology, Medical University of Vienna, Vienna, Austria.
Franz Ratzinger, Department of Laboratory Medicine, Medical University of Vienna, Vienna, Austria; Ihr Labor, Medical Diagnostic Laboratories, Vienna, Austria.
Michael Gurbisz, Department of Laboratory Medicine, Medical University of Vienna, Vienna, Austria.
Nadine Witzeneder, Department of Laboratory Medicine, Medical University of Vienna, Vienna, Austria; Department of Internal Medicine I, Division of Hematology and Hemostaseology, Medical University of Vienna, Vienna, Austria.
Hossein Taghizadeh, Department of Internal Medicine I, Division of Oncology, Medical University of Vienna, Vienna, Austria.
Harald Esterbauer, Department of Laboratory Medicine, Medical University of Vienna, Vienna, Austria; Ludwig Boltzmann Institute for Hematology and Oncology, Medical University of Vienna, Vienna, Austria.
Christine Mannhalter, Department of Laboratory Medicine, Medical University of Vienna, Vienna, Austria.
References
- 1.Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391–405. doi: 10.1182/blood-2016-03-643544. [DOI] [PubMed] [Google Scholar]
- 2.O’Brien SG, Guilhot F, Larson RA, Gathmann I, Baccarani M, Cervantes F, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;348:994–1004. doi: 10.1056/NEJMoa022457. [DOI] [PubMed] [Google Scholar]
- 3.Baccarani M, Deininger MW, Rosti G, Hochhaus A, Soverini S, Apperley JF, et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood. 2013;122:872–84. doi: 10.1182/blood-2013-05-501569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Radich JP, Deininger M, Abboud CN, Altman JK, Berman E, Bhatia R, et al. Chronic Myeloid Leukemia, Version 1.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2018;16:1108–35. doi: 10.6004/jnccn.2018.0071. [DOI] [PubMed] [Google Scholar]
- 5.Cross NC, White HE, Colomer D, Ehrencrona H, Foroni L, Gottardi E, et al. Laboratory recommendations for scoring deep molecular responses following treatment for chronic myeloid leukemia. Leukemia. 2015;29:999–1003. doi: 10.1038/leu.2015.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muller MC, Cross NC, Erben P, Schenk T, Hanfstein B, Ernst T, et al. Harmonization of molecular monitoring of CML therapy in Europe. Leukemia. 2009;23:1957–63. doi: 10.1038/leu.2009.168. [DOI] [PubMed] [Google Scholar]
- 7.Cross NC, White HE, Muller MC, Saglio G, Hochhaus A. Standardized definitions of molecular response in chronic myeloid leukemia. Leukemia. 2012;26:2172–5. doi: 10.1038/leu.2012.104. [DOI] [PubMed] [Google Scholar]
- 8.Saussele S, Richter J, Guilhot J, Gruber FX, Hjorth-Hansen H, Almeida A, et al. Discontinuation of tyrosine kinase inhibitor therapy in chronic myeloid leukaemia (EURO-SKI): a prespecified interim analysis of a prospective, multicentre, non-randomised, trial. Lancet Oncol. 2018;19:747–57. doi: 10.1016/S1470-2045(18)30192-X. [DOI] [PubMed] [Google Scholar]
- 9.Mahon FX, Rea D, Guilhot J, Guilhot F, Huguet F, Nicolini F, et al. Discontinuation of imatinib in patients with chronic myeloid leukaemia who have maintained complete molecular remission for at least 2 years: the prospective, multicentre Stop Imatinib (STIM) trial. Lancet Oncol. 2010;11:1029–35. doi: 10.1016/S1470-2045(10)70233-3. [DOI] [PubMed] [Google Scholar]
- 10.Baccarani M, Cortes J, Pane F, Niederwieser D, Saglio G, Apperley J, et al. Chronic myeloid leukemia: an update of concepts and management recommendations of European LeukemiaNet. J Clin Oncol. 2009;27:6041–51. doi: 10.1200/JCO.2009.25.0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stock W, Yu D, Karrison T, Sher D, Stone RM, Larson RA, et al. Quantitative real-time RT-PCR monitoring of BCR-ABL in chronic myelogenous leukemia shows lack of agreement in blood and bone marrow samples. Int J Oncol. 2006;28:1099–103. [PubMed] [Google Scholar]
- 12.Jiang Q, Zhao XY, Qin YZ, Liu YR, Lai YY, Jiang B, et al. The differences and correlations of BCR-ABL transcripts between peripheral blood and bone marrow assays are associated with the molecular responses in the bone marrow for chronic myelogenous leukemia. Am J Hematol. 2012;87:1065–9. doi: 10.1002/ajh.23321. [DOI] [PubMed] [Google Scholar]
- Thomas JS, Jagasia M, Vnencak-Jones CL. Comparison of BCR/ ABL1 mRNA levels by quantitative real-time PCR in peripheral blood and bone marrow specimens of patients with chronic myelogenous leukemia. Leuk Lymphoma. 2017;58:1–4. doi: 10.1080/10428194.2017.1287362. [DOI] [PubMed] [Google Scholar]
- 14.Lin F, Goldman JM, Cross NC. A comparison of the sensitivity of blood and bone marrow for the detection of minimal residual disease in chronic myeloid leukaemia. Br J Haematol. 1994;86:683–5. doi: 10.1111/j.1365-2141.1994.tb04812.x. [DOI] [PubMed] [Google Scholar]
- 15.Kiss TL, Xu WM, Jamal N, Messner HA. Comparative testing of peripheral blood and bone marrow for BCR-ABL transcripts in patients post allogeneic bone marrow transplantation and during interferon treatment for chronic myeloid leukemia. Leuk Lymphoma. 1999;34:493–500. doi: 10.3109/10428199909058476. [DOI] [PubMed] [Google Scholar]
- 16.Ballestrero A, Cirmena G, Dominietto A, Garuti A, Rocco I, Cea M, et al. Peripheral blood vs. bone marrow for molecular monitoring of BCR-ABL1 levels in chronic myelogenous leukemia, a retrospective analysis in allogeneic bone marrow recipients. Int J Lab Hematol. 2010;32:387–91. doi: 10.1111/j.1751-553X.2009.01198.x. [DOI] [PubMed] [Google Scholar]
- 17.Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2005. [Google Scholar]
- 18.Hughes T, Deininger M, Hochhaus A, Branford S, Radich J, Kaeda J, et al. Monitoring CML patients responding to treatment with tyrosine kinase inhibitors: review and recommendations for harmonizing current methodology for detecting BCR-ABL transcripts and kinase domain mutations and for expressing results. Blood. 2006;108:28–37. doi: 10.1182/blood-2006-01-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akard LP, Cortes JE, Albitar M, Goldberg SL, Warsi G, Wetzler M, et al. Correlations between cytogenetic and molecular monitoring among patients with newly diagnosed chronic myeloid leukemia in chronic phase: post hoc analyses of the Rationale and Insight for Gleevec High-Dose Therapy study. Arch Pathol Lab Med. 2014;138:1186–92. doi: 10.5858/arpa.2013-0584-OA. [DOI] [PubMed] [Google Scholar]
- 20.Hehlmann R, Muller MC, Lauseker M, Hanfstein B, Fabarius A, Schreiber A, et al. Deep molecular response is reached by the majority of patients treated with imatinib, predicts survival, and is achieved more quickly by optimized high-dose imatinib: results from the randomized CML-study IV. J Clin Oncol. 2014;32:415–23. doi: 10.1200/JCO.2013.49.9020. [DOI] [PubMed] [Google Scholar]
- 21.Hughes TP, Ross DM. Moving treatment-free remission into mainstream clinical practice in CML. Blood. 2016;128:17–23. doi: 10.1182/blood-2016-01-694265. [DOI] [PubMed] [Google Scholar]
- 22.Jeromin S, Eder C, Haferlach C, Haferlach T, Kern W. Impact of assay procedures on detection of MR(4.5) status in chronic myeloid leukemia: optimization of cDNA synthesis. Int J Lab Hematol. 2019;41:e109–12. doi: 10.1111/ijlh.13004. [DOI] [PubMed] [Google Scholar]
- 23.Kramer A, Loffler H, Bergmann J, Hochhaus A, Hehlmann R. Proliferating status of peripheral blood progenitor cells from patients with BCR/ABL-positive chronic myelogenous leukemia. Leukemia. 2001;15:62–8. doi: 10.1038/sj.leu.2402005. [DOI] [PubMed] [Google Scholar]
- 24.Martinelli G, Iacobucci I, Rosti G, Pane F, Amabile M, Castagnetti F, et al. Prediction of response to imatinib by prospective quantitation of BCR-ABL transcript in late chronic phase chronic myeloid leukemia patients. Ann Oncol. 2006;17:495–502. doi: 10.1093/annonc/mdj106. [DOI] [PubMed] [Google Scholar]
- 25.Abe A, Minami Y, Hayakawa F, Kitamura K, Nomura Y, Murata M, et al. Retention but significant reduction of BCR-ABL transcript in hematopoietic stem cells in chronic myelogenous leukemia after imatinib therapy. Int J Hematol. 2008;88:471–5. doi: 10.1007/s12185-008-0221-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.