Abstract
Systemic mastocytosis (SM) is frequently associated with eosinophilia. To examine its prevalence and clinical impact in all WHO classification-based subcategories, we analyzed eosinophil counts in 2350 mastocytosis patients using the dataset of the European Competence Network on Mastocytosis. Ninety percent of patients had normal eosinophil counts, 6.8% mild eosinophilia (0.5–1.5 × 109/l), and 3.1% hypereosinophilia (HE; >1.5 × 109/l). Eosinophilia/HE were mainly present in patients with advanced SM (17%/19%), and only rarely recorded in patients with indolent and smoldering SM (5%/1%), and some patients with cutaneous mastocytosis. The eosinophil count correlated with organomegaly, dysmyelopoiesis, and the WHO classification, but not with mediator-related symptoms or allergy. Eosinophilia at diagnosis had a strong prognostic impact (p < 0.0001) on overall survival (OS) and progression-free survival (PFS), with a 10-year OS of 19% for patients with HE, 70% for those with mild eosinophilia, and 88% for patients with normal eosinophil counts. In 89% of patients with follow-up data (n = 1430, censored at start of cytoreductive therapy), eosinophils remained stable. In those with changing eosinophil counts (increase/decrease or mixed pattern), OS and PFS were inferior compared with patients with stable eosinophil counts. In conclusion, eosinophilia and HE are more prevalent in advanced SM and are predictors of a worse outcome.
Introduction
Mastocytosis is a hematopoietic neoplasm characterized by expansion and accumulation of mediator-producing neoplastic mast cells in one or more organ systems, including the bone marrow (BM), skin, liver, spleen, and the gastrointestinal (GI) tract [1–4]. In the vast majority (>90%) of patients with systemic mastocytosis (SM), an activating mutation in the KIT gene (mostly D816V) is found in neoplastic mast cells [5–7]. According to the World Health Organization (WHO) 2016 classification, mastocytosis can be divided into cutaneous mastocytosis (CM), and systemic subcategories, including indolent SM (ISM), smoldering SM (SSM), and advanced SM, which comprises SM with an associated hematologic neoplasm (SM-AHN), aggressive SM (ASM), and mast cell leukemia (MCL) [1, 3, 8–10].
SM is frequently associated with eosinophilia [1]. Prior to the classification and subcategorization of SM, incidences of 19% [11] to 21% [12] were reported in smaller case series. In a more recent series of 63 patients [13], 14% of all patients with SM had eosinophilia, including all SM variants defined by the WHO. It was suggested that those patients who presented with eosinophilia had a worse overall survival (OS). In a series of 42 patients with advanced SM, the incidence of eosinophilia was higher (34%) [14]. However, the series included 12 patients with FIP1L1-PDGFRA [15] chronic eosinophilic leukemia (CEL) in which the WHO criteria for SM are not met [14].
Overall, precise data on the prevalence of eosinophilia in larger series of patients with mastocytosis are still lacking. It is also not known to which extent eosinophilia is associated with SM-related symptomatology, and it still remains unclear whether there is a firm relationship between eosinophilia and prognosis regarding progression of the disease and survival. A large database established within the registry of the European Competence Network of Mastocytosis (ECNM) [16] containing almost 3000 patients with all different categories of mastocytosis offered us the opportunity to examine the clinical impact of eosinophilia in CM and SM.
Patients and methods
ECNM registry database
The ECNM registry contains data from patients with cutaneous and SM from 25 different centers from Europe (12 different countries) and one center from the United States. For details, see [16] and the supplemental appendix. The following parameters were captured for this study: age, sex, date of diagnosis (histology based), presence of major and minor diagnostic criteria according to the WHO classification [1], WHO-based final diagnosis, laboratory values at diagnosis and during follow-up including complete blood count, serum chemistry, serum tryptase, percentage of mast cells in BM biopsy sections and BM aspirates, flow cytometry-based phenotype of mast cells, molecular and cytogenetic data, presence of hepatosplenomegaly and/or lymphadenopathy (collectively grouped as “organomegaly”), weight loss (defined as >10% loss during the last 12 months), presence and severity of mediator-related symptoms, including skin symptoms, itching, blistering, flushing, osteoporosis, bone pain, anaphylaxis, GI symptoms (diarrhea, cramping, gastric, or duodenal ulcers), allergy and specific IgE, therapy and responses, including the use of symptomatic and cytoreductive drugs.
Eosinophilia in peripheral blood was defined as eosinophil count >0.5 × 109/l, mild eosinophilia as an eosinophil count between 0.5 × 109/l and 1.5 × 109/l, and hypereosinophilia (HE) as eosinophils >1.5 × 109/l. Symptoms defined in the case record forms were dichotomized: yes or no. GI symptoms were grouped (stomach ulcer, cramping, and diarrhea).
Patients
All patients with data on eosinophils counts at diagnosis were included. When typical mast cell infiltrates were found in the skin, but BM data were not available, adult patients were included with the provisional diagnosis “mastocytosis in the skin” (MIS). These MIS patients were excluded for all analyses related to progression.
For prognostication and follow-up, at least one follow-up visit with information on eosinophils counts had to be documented. Eosinophil counts were analyzed in quarterly periods (every 3 months), starting at diagnosis. For follow-up analyses, cohorts were divided into four groups: stable course (eosinophils remained in the same group: ≤0.5; 0.5–1.5 or >1.5 × 109/l), substantial increase in eosinophil counts (marked increase resulting in a shift to a higher group: from normal to mild eosinophilia, or from mild eosinophilia to HE), marked decrease in eosinophils (shifting to a lower group), or mixed kinetics in eosinophil counts (shifts between different groups during follow-up).
Statistical analysis
In univariate analyses the influence of categorical parameters (independent variables) on the absolute number of eosinophils in blood were analyzed with the Mann–Whitney U test (in case of two groups) or the Kruskal–Wallis Test (multiple groups). The correlation of continuous parameters with absolute number of eosinophils was calculated using linear regression. For multivariate analysis, only variables that were statistically significant in univariate analysis were included. This analysis was done using a generalized linear model. To compensate for the asymmetric distribution of the data prior to statistical analysis, serum tryptase levels, white blood cell counts, platelet counts, alkaline phosphatase, and LDH were logarithmized.
The probability of OS, event-free survival (EFS; time from diagnosis to progression or death), and progression-free survival (PFS; time from diagnosis to progression) was determined by Kaplan and Meier estimates. PFS was defined as progression from one WHO category to another (from cutaneous to systemic; from ISM to SSM or advanced SM; from SSM to advanced; within advanced SM to MCL; within SM-AHN categories from low grade MDS to high grade MDS, and all transformations into AML. For PFS analyses, patients with MIS and with MCL were excluded. Significance levels in differences concerning OS and PFS among the various patients' groups were assessed by the log rank test. In a separate multivariate analysis, the independent prognostic impact of various parameters, including the WHO category and HE on OS and EFS was analyzed.
Results
Characterization of the sample cohort
Eosinophil counts at diagnosis were available in 2350 of 2985 patients (79%). The median age of these 2350 patients was 47 years (0.1–90.0), and 44% of these patients were males. The median age varied among WHO subcategories: patients with advanced SM were older (Table 1). Ninety percent (2117/2350) of patients had normal eosinophil counts, 6.8% (159) had mild eosinophilia, and 3.1% (74) had HE (Table 1 and Fig. 1). Mild eosinophilia or HE were mainly present in patients with advanced SM (SM-AHN 17%/22%; ASM 18%/14%; MCL 10%/17%), but also in some patients with SSM (8%/6%), ISM (5%/1%), and CM (8%/0%) (Table 1 and Fig. 1). Within SM-AHN, the highest percentages of mild eosinophilia and HE were found in patients with CEL, not otherwise specified (CEL-NOS; 15 cases, 12 FIP1L1-PDGFRA negative, 3 not tested), hypereosinophilic syndrome (HES), chronic myelomonocytic leukemia, and myelodysplastic syndrome/myeloproliferative neoplasm unclassified (Table 2).
Table 1. Demographics and description of all patients at diagnosis and during follow-up according to the WHO categories.
| Mastocytoma | DCM | MPCM | MIS | ISM | SSM | ASM | MCL | SM-AHN | All patients | |
|---|---|---|---|---|---|---|---|---|---|---|
| Number (%) of patients at diagnosis | 15 (0.6) | 27 (1.1) | 280 (11.9) | 314 (13.8) | 1345 (57.0) | 53 (2.2) | 78 (3.3) | 30 (1.3) | 207 (8.8) | 2350a (100) |
| Median age at diagnosis in years (range) | 1 (0.5–15) | 31 (1–77) | 31 (0.1–80) | 41 (18–87) | 47 (17–83) | 56 (25–82) | 59 (15–83) | 56 (34–90) | 65 (22–87) | 47 (0.1–90) |
| % male | 60 | 48 | 34 | 33 | 43 | 55 | 50 | 63 | 72 | 44 |
| Median tryptase in ng/ml (range) | 3.9 (1.4–11.4) | 17.0 (2.3–94) | 8.1 (1–126) | 13.7 (1–200) | 30 (1–885) | 199.5 (21–2100) | 168 (8.9–1432) | 396.5 (74.9–4530) | 147 (1.8–1060) | 27 (1–4530) |
| Number of patients (%) | ||||||||||
| KIT D816V positive | 0 | 2 (7) | 44 (16) | 23 (7) | 951 (71) | 40 (75) | 55 (71) | 16 (53) | 156 (75) | 1287 (55) |
| KIT D816V negative | 0 | 11 (41) | 118 (42) | 20 (6) | 164 (12) | 2 (4) | 8 (10) | 11 (37) | 23 (11) | 358a (15) |
| KIT D816V not tested | 15 (100) | 14 (52) | 118 (42) | 271 (87) | 230 (17) | 11 (21) | 15 (19) | 3 (10) | 28 (14) | 705 (30) |
| Organomegaly, number (%) | 1 (7) | 2 (7) | 21 (8) | 21 (7) | 108 (8) | 35 (66) | 45 (58) | 16 (53) | 116 (56) | 365 (16) |
| Ascites, number (%) | 0 | 0 | 1b (0.3) | 0 | 4b (0.2) | 0 | 21 (27) | 7 (23) | 56 (27) | 89 (4) |
| Weight loss > 10% during last 6 monthsc, number (%) | 0 | 1c (4) | 3c (1) | 0 | 21c (2) | 11c (21) | 40 (51) | 14 (47) | 92 (44) | 182 (8) |
| Number of patients with absolute eosinophils >0.5–1.5 × 109/l (% of that category) | 1 (7) | 1 (4) | 21 (8) | 12 (4) | 67 (5) | 4 (8) | 14 (18) | 3 (10) | 37 (18) | 159 (7) |
| Number absolute eosinophils > 1.5 × 109/l (% of that category) | 0 | 0 | 0 | 0 | 10 (1) | 3 (6) | 11 (14) | 5 (17) | 45 (22) | 74 (3) |
| Number (%) of patients with follow-up eosinophil data | 3 (20) | 18 (67) | 135 (48) | 159 (51) | 897 (67) | 43 (81) | 41 (53) | 18 (60) | 115 (56) | 1430 (61) |
DCM diffuse cutaneous mastocytoma, MPCM maculopapular cutaneous mastocytoma, MIS mastocytosis in the skin, ISM indolent SM, SSM smoldering SM, ASM aggressive SM, MCL mast cell leukemia, SM-AHN SM plus associated hematological neoplasm
One patient with mast cell sarcoma was not included in this table
Ascites: 1× GI symptoms and probable Crohn’s disease; 1× probably not connected to mastocytosis, resolved during follow-up; 1× with concurrent hepatocellular carcinoma; 2× not explained
Causes of weight loss: DCM due to diet; MPCM 3× associated with severe GI symptoms (2× Crohn’s disease); ISM 1× associated with esophagitis, 2× diet, 16× with severe GI symptoms, 2× not explained; SSM 1× diet, 8× severe GI symptoms, 2× not explained
Fig. 1.
Dot plot representing individual absolute eosinophil counts at diagnosis of mastocytosis. Data from 2350 patients of all WHO cutaneous and systemic mastocytosis subcategories. DCM diffuse cutaneous mastocytosis, MCPM maculopapular cutaneous mastocytosis, MIS mastocytosis in the skin, ISM indolent systemic mastocytosis, SSM smoldering systemic mastocytosis, ASM aggressive systemic mastocytosis, MCL mast cell leukemia, MCS mast cell sarcoma, SM-AHN systemic mastocytosis with an associated hematological neoplasm
Table 2. Eosinophilia in relation to the categories advanced mastocytosis with associated hematologic neoplasm (SM-AHN).
| Eosinophils (×109/l); median (range) | All patients with available eosinophils at diagnosis | 0.5–1.5 × 109/leosinophils | >1.5 × 109/leosinophils | |
|---|---|---|---|---|
| AML | 0.07 (0–16.65) | 14 | 1 | 2 |
| MDS | 0.05 (0–1.44) | 35 | 3 | 0 |
| MDS/MPN-U | 0.83 (0–8.70) | 23 | 5 | 8 |
| CMML | 0.29 (0–3.66) | 50 | 11 | 9 |
| MPN-U or not specifieda | 0.62 (0–19) | 24 | 6 | 9 |
| CEL-NOS/HESb | 8.55 (0.506–35) | 15 | 3 | 12 |
| CML | 0.16 | 1 | 0 | 0 |
| ET/PV | 0.17 (0–2.16) | 8 | 0 | 1 |
| PMF | 0.12 (0–1.38) | 18 | 4 | 0 |
| B-cell malignanciesc | 0.13 (0–2.38) | 14 | 3 | 2 |
| Other | 0.07 (0–16.65) | 14 | 1 | 2 |
AML acute myeloid leukemia, CEL-NOS/HES chronic eosinophil leukemia not otherwise specified/hypereosinophilia syndrome, CML chronic myeloid leukemia, CMML chronic myelomonocytic leukemia, ET essential thrombocythaemia, MDS myelodysplastic syndrome, MDS/MPN-U myelodysplastic/myeloproliferative syndrome unclassifiable, other other associated hematologic disorder not specified, PMF primary myelofibrosis, PV polycythemia vera
Including eight patients with MPNeo (myeloproliferative neoplasm with eosinophilia [29])
FIP1L1-PDGFRa results in CEL/HES patients: negative in 12; not tested in three patients
Hodgkin lymphoma (1), MGUS/multiple myeloma (4), non-Hodgkin lymphoma/CLL (9)
Eosinophilia, clinical, and laboratory parameters, and symptoms
In univariate analysis, the WHO classification, presence of dysmyelopoiesis, sex (women more frequent than men), age, KIT mutation positivity, and organomegaly (splenomegaly and/or hepatomegaly and/or lymphadenopathy) were all significantly positively correlated with eosinophilia (Table 3). In addition, most C findings (indicative of mastocytosis-induced organ damage in advanced SM) such as portal hypertension, malabsorption, ascites, and weight loss correlated with eosinophilia. Significant correlations were found between eosinophilia and white blood counts (WBC), and between alkaline phosphatase levels. In contrast, mediator-related symptoms or allergies did not correlate with eosinophilia, neither in the total group of patients nor in the subgroups analyzed, namely patients with non-advanced SM (ISM + SSM). In multivariate analysis, WHO subcategory, dysmyelopoiesis, WBC, and organomegaly remained independent variables correlating with eosinophilia (Table 3).
Table 3. Correlation between the absolute number of eosinophils and other clinical relevant parameters at diagnosis.
| Parameter | Univariate analysis p value |
Multivariate analysis p value |
|---|---|---|
| Skin lesions y/n | 0.214 | – |
| Darier's sign y/n | 0.386 | – |
| Sex female/male | 0.022 | 0.655 |
| Kit pos/neg | 0.006 | 0.385 |
| CD2 y/n | 0.691 | – |
| CD25 y/n | 0.146 | – |
| Organomegaly y/n | <0.0001 | 0.021 |
| Dysmyelopoiesis y/n | 0.019 | 0.016 |
| Ascites y/n | <0.0001 | 0.771 |
| Portal hypertension y/n | 0.052 | – |
| Malabsorption y/n | 0.001 | 0.198 |
| Weight loss y/n | <0.0001 | 0.289 |
| Mediator symptoms y/n | 0.369 | – |
| Allergies y/n | 0.871 | – |
| WHO classification | 0.004 | 0.001 |
| R/p value | ||
| Age | 0.024/0.240 | – |
| Tryptase (ln)a | 0.02/0.416 | – |
| WBC (ln) a | 0.263/<0.0001 | <0.0001 |
| Hemoglobin | 0.006/0.775 | – |
| Platelets (ln)a | 0.028/0.174 | – |
| Alkaline phosphatase (ln)a | 0.061/0.008 | 0.092 |
| LDH (ln) | 0.031/0.184 | – |
Bold face: factors that remained significant in multivariate analysis y/n yes/no
ln: to compensate for asymmetric distribution, data were logarithmized
Prognostic value of eosinophilia at diagnosis in the various categories of SM
Although at first glance, the median and mean eosinophil counts were not so different among the WHO subcategories, it turned out that the eosinophil count at diagnosis was highly prognostic for OS throughout the various WHO subcategories. In particular, patients with HE had a poor outcome (10-year OS: 19%) compared with patients with eosinophil counts ranging between 0.5–1.5 × 109/l (70%) and patients with normal eosinophil counts (88%). The same result was obtained when deleting CEL/HES patients from the AHN group (not shown).
Almost identical data were obtained for EFS (Fig. 2 and Supplementary Table S1). Finally, significant differences (p < 0.0001) were seen in 10-year PFS. In fact, PFS was 75% in patients with HE, 82% in patients with mild eosinophilia, and 91% in patients with normal eosinophil counts at diagnosis (Fig. 2 and Supplementary Table S1). In multivariate Cox regression analysis, including the WHO subcategory, it appeared that eosinophil counts remained an independent prognostic parameter concerning OS and EFS (p = 0.009).
Fig. 2.
Survival (overall, progression-free (PFS), and event-free (EFS)) according to the initial absolute numbers of eosinophils at diagnosis analyzed in all patients. For the definition of PFS, patients in the MIS group and patients with mast cell leukemia were not included. Note that for PFS the Y-axis is cut at 70%. The p value refers to the comparison of all three survival curves as assessed by log rank test
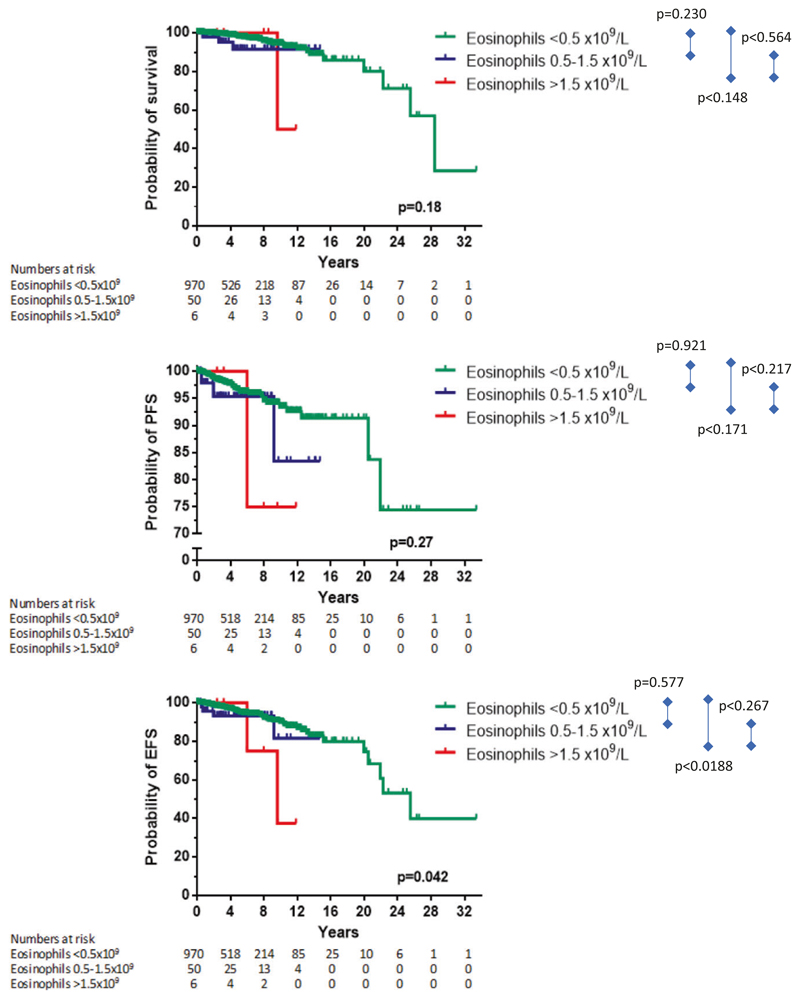
When only patients with ISM were analyzed, a prognostic role of mild eosinophilia or HE was seen for EFS (p = 0.048), but not for OS (p = 0.18) or PFS (p = 0.27; Fig. 3 and Supplementary Table S1). We also examined subcategories of patients with advanced SM, namely ASM/MCL and SM-AHN. The prognostic impact of eosinophils concerning OS and EFS was still present in patients with ASM/MCL but not in patients with SM-AHN (Supplementary Fig. S1 and Supplementary Table 1). Interestingly, in patients with ASM/MCL, it appeared that patients with mild eosinophilia had the best outcome regarding OS and EFS.
Fig. 3.
Survival (overall, progression-free (PFS), and event-free (EFS)) according to the initial absolute numbers of eosinophils at diagnosis analyzed only in the patients within the ISM category. Note that in the figure showing PFS data, the Y-axis is cut at 70%. The p value refers to the comparison of all three survival curves as assessed by log rank test
Eosinophilia during follow-up
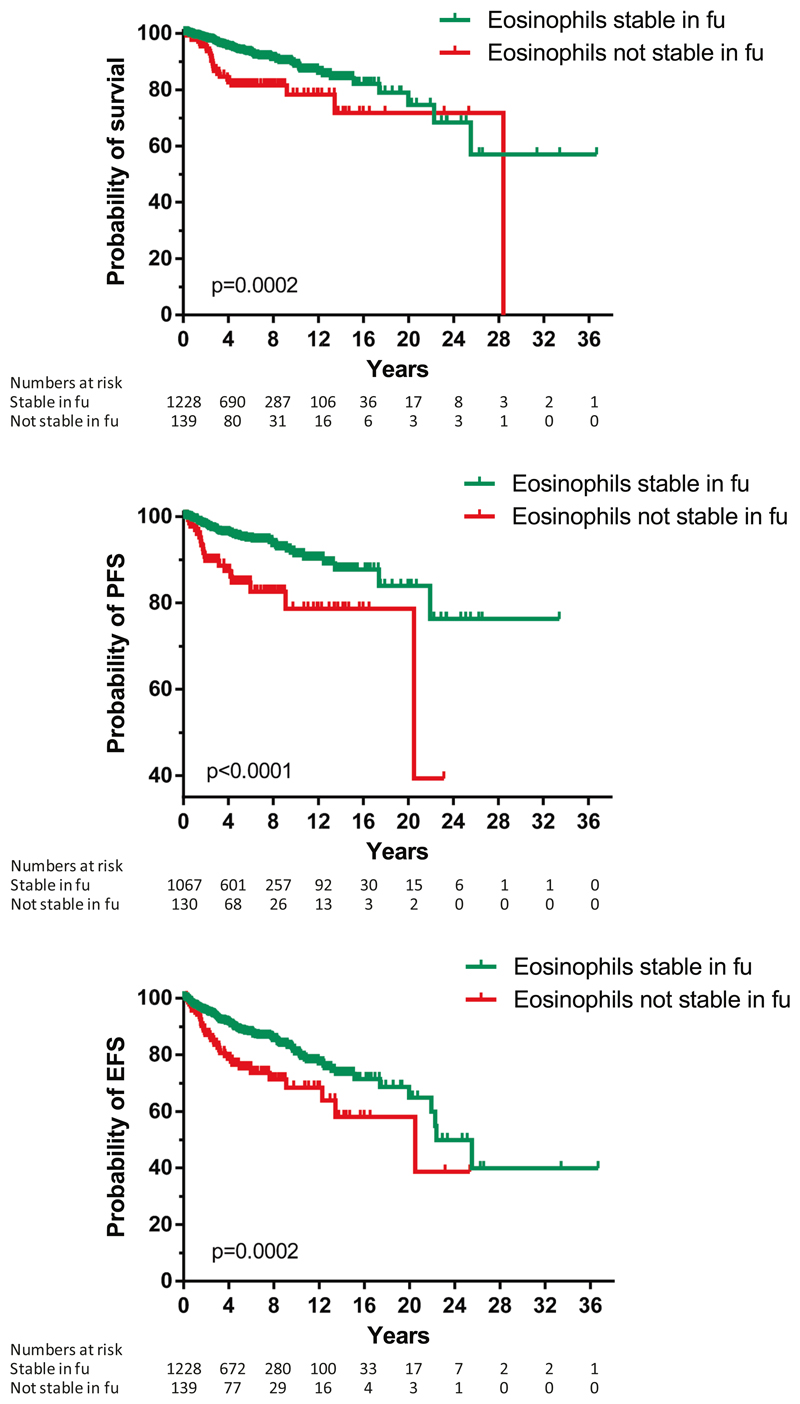
In 1430 patients, follow-up data (at least 6 months) on eosinophil counts and cytoreductive therapy were available. The median follow-up time was 4.6 years (range 0.1–36.7). We analyzed whether eosinophil counts remained stable during the follow up and categorized the dynamics of eosinophil counts into four groups as described in Materials and methods section. To avoid any influence of corticosteroids or cytoreductive therapy (e.g., interferon-alpha, cytostatic drugs, tyrosine kinase inhibitors), data were censored at the time of initiation of such treatment. It appeared that the large majority of untreated patients (90%) had stable eosinophil counts over time, whereas 3.5% (n = 48) showed an increase, 3.4% (n = 47) a decrease, and 3.2% a mixed pattern (n = 44). An increase in eosinophils as defined above was observed in patients with maculopapular CM (3%), MIS (2%), ISM (4%), SSM (11%), SM-AHN (4%), and ASM (3%). Nontreatment-related decreases in eosinophil counts were most pronounced in SM-AHN (15%) and ASM patients (18%).
Next, we examined whether a stable pattern or any change in eosinophil counts over time (increase, decrease, or mixed) were prognostic. Of note, we censored for start of cytoreductive therapy or corticosteroid use. Unexpectedly, we found that any change in eosinophil counts over time was significantly associated with an unfavorable outcome regarding OS, EFS, and PFS (Fig. 4 and Supplementary Table S1). In a multivariate analysis, the change in eosinophil counts during follow-up lost its independency for OS when the WHO classification was included, but this was not the case for PFS (p = 0.019). In a subset analysis of all these possible patterns over time, patients who showed a decrease in eosinophil counts and patients with a mixed response had the worst outcome (Fig. 5 and Supplementary Table S1). We also correlated these data with blood cell counts and observed a significant association between the changes in eosinophil counts and changes in hemoglobin, platelets, and progenitor cells, with contingency coefficients being 0.28, 0.23, and 0.30, respectively, (p < 0.001). Since we did not have sufficient data during follow-up in most patients, we could not correlate eosinophil counts with changes in organ function.
Fig. 4.
Survival (overall, progression-free (PFS), and event-free (EFS)) according to the follow-up of the absolute numbers of eosinophils. Not stable in time means that an increase (from normal range to eosinophilia, or from eosinophilia to hypereosinophilia), a decrease, or mixed pattern was observed. For the definition of PFS, patients in the MIS group and patients with mast cell leukemia were not included. Note that for PFS, the Y-axis is cut at 40%. The p value refers to the comparison of the survival curves as assessed by log rank test
Fig. 5.
Overall survival of patients who showed decrease, increase, mixed pattern, or no change (stable) of eosinophil counts during follow-up. Data were censored for start cytoreductive therapy or corticosteroids. The p value refers to the comparison of all four survival curves as assessed by log rank test
We finally analyzed whether eosinophil counts mattered in patients who had documented progression, i.e., changed from one WHO subcategory into a more advanced one. Here, we started with the progressing patients, and next analyzed the eosinophil counts. To this end, we selected 35 patients available with a follow-up of at least 2 years and data on eosinophils at diagnosis and during follow-up, up to 5 months before documented progression to avoid any potential influence of cytoreductive therapy. No relationship was seen between eosinophils counts at first visit when eosinophils were measured after diagnosis or at time of progression (p = 0.12, Supplementary Fig. S2).
Discussion
In this large dataset of 2350 patients with mastocytosis, collected in the ECNM registry, we found that mild eosinophilia and HE are frequently recorded across all WHO subcategories. The highest incidence (39%) was seen in patients with SM and an associated nonmast cell hematological neoplasm (SM-AHN), which is not surprising as AHN includes, among others, also CEL and other myeloid neoplasms associated with eosinophilia such as MPN or MPN/MDS. But incidental high eosinophil counts were even observed in patients with nonadvanced SM. We did not perform a separate analysis on patients with extremely high (far above 1.5 × 109/l) eosinophil counts, as these cases were rare and mainly observed in patients with SM-AHN.
Previous studies have shown that eosinophils are usually clonal in patients with advanced SM [17–19]. However, eosinophilia can also be caused by other pathologic conditions such as parasitosis, drug reactions, or atopia. In our dataset, the clonality of eosinophils was not assessed. Therefore, we cannot exclude with certainty that eosinophilia could have been transient and reactive in some of our patients. However, given the fact that eosinophil counts remained stable in 90% of the 1430 patients with follow-up data, and that in our registry reactive causes of eosinophilia related to allergic reactions were captured, we are confident that in the vast majority of our patients, infections or allergies did not play an important role. This notion was also supported by the observation that the WHO category of SM and/or the presence of dysmyelopoiesis, organomegaly, and WBC counts showed an independent correlation with high eosinophil counts in multivariate analysis, whereas other clinical variables, such as mediator symptoms and allergies did not correlate with eosinophil counts.
At first glance, the median and mean eosinophil counts were not so different among the WHO subcategories. However, upon a closer look, it turned out that in the various WHO subcategories, varying subgroups of patients exist with higher or lower eosinophil counts—which is obviously a prognostic pattern. In this regard it is worth noting that in an earlier proposal for classification of mastocytosis by Metcalfe et al., SM with eosinophilia and lymphadenopathy was presented as a separate category of SM based on unique clinical features and an unfavorable outcome [20, 21]. A similar association between eosinophilia and abdominal lymphadenopathy was seen in a German series of patients with advanced SM [22]. In line with this observation, our data show that elevated eosinophil counts correlate with the presence of organomegaly including lymphadenopathy at diagnosis and are highly prognostic for OS and PFS. However, although both correlate with advanced SM and a poor prognosis and eosinophil products are well known to cause tissue remodeling and damage, it remains unknown whether HE and eosinophil products contribute directly to organomegaly or organ damage in patients with advanced SM.
The prognostic impact of eosinophilia in patients with mastocytosis has also been examined by others [13], but the number of patients was too small to draw definitive conclusions. In our study, with data on 2350 patients available, we observed an impressive relationship between eosinophilia and outcomes, defined as OS, EFS, or PFS. In contrast, no prognostic relationship was found when the 42 patients (with 12 harboring the FIP1L1-PDGFRA fusion) from the Mayo clinic series were analyzed [14], perhaps due to the fact that those 12 classical CEL patients had an excellent prognosis.
The relationship between SM and eosinophilia is interesting, as eosinophils and mast cells can coexist in both malignant (clonal) and benign conditions [23]. This is partly explained by the immunoregulatory effects of eosinophils via numerous cytokines that act upon mast cells [24–26]. On the other hand, mast cells also produce many cytokines and mediators [4, 27], some of which may prolong viability of eosinophils [25]. However, many of our SM patients did not have eosinophilia, and there was no clear correlation between eosinophilia and mast cell activation, mediator-related symptoms or allergy. Therefore, we believe that in most cases, eosinophils are part of the disease clone. Indeed, in some patients with advanced mastocytosis, the clonal relationship has been convincingly proven [19] and the same holds true for patients with indolent disease, in whom the presence of the KIT D816V mutation could be demonstrated in the circulating myeloid cells [28] and also in eosinophils [17]. It would obviously be interesting to document that patients with clonal eosinophilia are at higher risk for progression compared with patients with reactive (transient) eosinophilia, but due to a lack of data on eosinophil clonality, we could not confirm this hypothesis.
An interesting observation was that a change, especially a decrease in eosinophil counts during follow-up, results in a poor outcome. So far, the underlying mechanism is not known. One possibility may be that other AHN cells (blasts) replaced eosinophils during progression. Alternatively, cells were recruited from progenitor cells to differentiate more into mast cells or other myeloid cells leading to a decrease in eosinophil counts. A third possibility would be drug effects, but this possibility seems rather unlikely. In fact, we carefully censored for cytoreductive therapies interfering with eosinophil counts. However, since this is a registry-based project, we cannot exclude with certainty that some of the patients in whom eosinophil counts decreased had received cytoreductive therapies or glucocorticosteroids, which were not reported. Overall, however, we believe that the decrease in eosinophils was directly a consequence of disease progression. A similar decrease in differentiated cells is seen in the blast phase of chronic myeloid leukemia where basophils first increase during acceleration but then decrease during transition into blast phase. Whatever the mechanism is, based on our findings, we believe that eosinophil counts should be carefully examined during follow-up and whenever an otherwise unexplained decrease or increase in eosinophil counts is observed, the physician should be aware of additional signs of disease progression, such as an increase in serum tryptase levels or alkaline phosphatase levels or occurrence of C findings.
We collected data from 15 patients with SM-CEL-NOS/HES. The link between CEL and SM and the terminology around eosinophil disorders can be confusing. The terminology of eosinophilic disorders was refined in a Working Conference on Eosinophil Disorders and Syndromes in 2011 [29, 30]. In fact, two distinct categories should be recognized: FIP1L1/PDGFRA-associated CEL patients with an increase of BM mast cells [31] and KIT D816V-positive SM with an associated AHN resembling CEL [25, 32]. It can be difficult to differentiate SM-CEL from the FIP1L1/PDGFRA-associated CEL, because in the latter disease, elevated tryptase can be found in addition with other minor criteria for SM, such as spindle-shaped mast cells coex-pressing CD25. However, these mast cells very rarely carry the KIT D816V mutation, are usually present in an interstitial pattern in the BM compared with the dense multifocal aggregates of SM, and FIP1L1/PDGFRA-positive disease is usually not associated with the mediator symptoms of SM [25, 32, 33]. In our series, none of the patients with SM-CEL/HES carried a FIP1L1/PDGFRA fusion gene.
In conclusion, mild eosinophilia and HE are frequently recorded in patients with mastocytosis, especially in those with advanced SM. Eosinophilia is not related to mediator-related symptoms or allergy, but is strongly related with adverse prognosis. In particular, eosinophilia at diagnosis and deviations in eosinophil counts during follow-up are strong predictors of poor PFS and OS. These observations have clinical implications and should assist in the optimal prognostication and management of patients with mastocytosis.
Supplementary Material
The online version of this article (https://doi.org/10.1038/s41375-019-0632-4) contains supplementary material, which is available to authorized users.
Acknowledgements
This work was supported by the Austrian Science Fund (FWF), SFB grants F4701-B20 and F4704-B20 (to PV), by the Deutsche Forschungsgemeinschaft (DFG, RA 2838 to AI), by the Koeln Fortune Program, Faculty of Medicine, University of Cologne (216/2016 to AI), and by the ´Charles and Ann Johnson Foundation’ (to JG). CB is supported by the Clinical Research Fund of the University Hospital Leuven. We thank all technicians, study coordinators, study nurses, and colleagues for data entry into the registry system. Our special thanks for statistical help to Michael Kundi, and to data management and data controlling go to: Susanne Herndlhofer, Nadja Jaekel, Hassiba Bouktit, Gabriele Stefanzl, Hana Škabrahová, Gulkan Ozkan, Tarik Tiryaki, Nicole Cabral do O, Deborah Christen, Anne Simonowski, Cecilia Spina, Agnes Bretterklieber, Orsolya Pilikta, Lilla Kurunci, Kerstin Hamberg-Levedahl, Pietro Benvenuti, Gregor Verhoef, Peter Vandenberghe, Dominique Bullens, Anna Van Hoolst, Nele Philips, Toon Ieven, Gülkan Özkan, and Stephanie Pulfer.
Footnotes
Author contributions HCK-N had the conceptual idea, helped with the analyses, and wrote the paper. WRS did all analyses, was responsible for data collection and correction in the ECNM database, designed all figures, and cowrote the article. AI and KH did the initial analyses and corrected the final version of the manuscript. BvA and LRFS were helpful in discussions and helped with entry of patient data and corrected the final version of the manuscript. AG, MN, ML, LS, RZ, PB, CP, CE, LM, KS, NvB, RP,MT, AR, JS, MJ, FC, ABF, KB, AZ, DF, AK, ASY, MD, MM, HH, JP, VS, EA, DN, CB, JV, LM, VK, OLy, TJ, OH, JR, MA, and JG contributed with patient data and corrected the final version of the manuscript. PV contributed to the study plan, cowrote the manuscript, and supervised the ECNM database.
Conflict of interest HCK-N: institutional financial support from Novartis to perform a phase II trial with midostaurin. WRS: honoraria from Novartis, Pfizer, AbbVie, Daiichi Sankyo, Amgen, Thermo Fisher, Deciphera, Incyte, Celgene, and Jazz. BvA: financial support from Novartis for research and advisory boards. KH: research grant: Euroimmun Lectures, and consulting: ALK, Blueprint, Deciphera, and Novartis. CE, DF, and JR: advisory board Novartis. KS: travel expenses from Novartis. NvB: institutional financial support from Novartis. MT: advisory board/honoraria: Deciphera and Novartis. JP: funding to support conduct of clinical trial: Blueprint Medicines and Deciphera; advisory board/honoraria: Blueprint Medicines and Novartis. OH: research funding support from AB science and Novartis. Advisory board of AB science. JG: funding to support conduct of clinical trial: Blueprint Medicines and Deciphera; advisory board/honoraria: Blueprint Medicines, Deciphera, and Allakos. VS is a senior clinical researcher of the Research Foundation Flanders/Fonds Wetenschappelijk Onderzoek (FWO: 1804518N).
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Horny H-P, Akin C, Arber DA, Peterson LC, Tefferi A, Metcalfe DD, et al. Mastocytosis. In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri S, Stein H, et al., editors. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed. Lyon: International Agency for Research on Cancer; 2017. pp. 62–9. [Google Scholar]
- 2.Theoharides TC, Valent P, Akin C. Mast cells, mastocytosis, and related disorders. N Engl J Med. 2015;373:163–72. doi: 10.1056/NEJMra1409760. [DOI] [PubMed] [Google Scholar]
- 3.Valent P, Akin C, Hartmann K, Nilsson G, Reiter A, Hermine O, et al. Advances in the classification and treatment of mastocytosis: current status and outlook toward the future. Cancer Res. 2017;77:1261–70. doi: 10.1158/0008-5472.CAN-16-2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Butterfield JH, Ravi A, Pongdee T. Mast cell mediators of significance in clinical practice in mastocytosis. Immunol Allergy Clin North Am. 2018;38:397–410. doi: 10.1016/j.iac.2018.04.011. [DOI] [PubMed] [Google Scholar]
- 5.Arock M, Sotlar K, Akin C, Broesby-Olsen S, Hoermann G, Escribano L, et al. KIT mutation analysis in mast cell neoplasms: recommendations of the European Competence Network on Mastocytosis. Leukemia. 2015;29:1223–32. doi: 10.1038/leu.2015.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Longley BJ, Tyrrell L, Lu SZ, Ma YS, Langley K, Ding TG, et al. Somatic c-KIT activating mutation in urticaria pigmentosa and aggressive mastocytosis: Establishment of clonality in a human mast cell neoplasm. Nat Genet. 1996;12:312–4. doi: 10.1038/ng0396-312. [DOI] [PubMed] [Google Scholar]
- 7.Nagata H, Worobec AS, Semere T, Metcalfe DD. Elevated expression of the proto-oncogene c-kit in patients with mastocytosis. Leukemia. 1998;12:175–81. doi: 10.1038/sj.leu.2400906. [DOI] [PubMed] [Google Scholar]
- 8.Horny H-P, Metcalfe DD, Bennett JM, Bain BJ, Akin C, Escribano L, et al. Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed. Lyon: International Agency for Research of Cancer; 2008. Mastocytosis; pp. 54–63. [Google Scholar]
- 9.Valent P, Akin C, Escribano L, Fodinger M, Hartmann K, Brockow K, et al. Standards and standardization in mastocytosis: consensus statements on diagnostics, treatment recommendations and response criteria. Eur J Clin Investig. 2007;37:435–53. doi: 10.1111/j.1365-2362.2007.01807.x. [DOI] [PubMed] [Google Scholar]
- 10.Valent P, Arock M, Akin C, Sperr WR, Reiter A, Sotlar K, et al. The classification of systemic mastocytosis should include mast cell leukemia (MCL) and systemic mastocytosis with a clonal hematologic non-mast cell lineage disease (SM-AHNMD) Blood. 2010;116:850–1. doi: 10.1182/blood-2010-05-285270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Travis WD, Li C-Y, Bergstralh MS, Yam LT, Swee RG. Systemic mast cell disease. Analysis of 58 cases and literature review. Medicine. 1988;67:345–68. [PubMed] [Google Scholar]
- 12.Lawrence JB, Friedman BS, Travis WD, Chinchilli VM, Metcalfe DD, Gralnick HR. Hematologic manifestations of systemic mast cell disease: a prospective study of laboratory and morphologic features and their relation to prognosis. Am J Med. 1991;91:612–24. doi: 10.1016/0002-9343(91)90214-i. [DOI] [PubMed] [Google Scholar]
- 13.Bohm A, Fodinger M, Wimazal F, Haas OA, Mayerhofer M, Sperr WR, et al. Eosinophilia in systemic mastocytosis: clinical and molecular correlates and prognostic significance. J Allergy Clin Immunol. 2007;120:192–9. doi: 10.1016/j.jaci.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 14.Pardanani A, Lim KH, Lasho TL, Finke C, McClure RF, Li CY, et al. Prognostically relevant breakdown of 123 patients with systemic mastocytosis associated with other myeloid malignancies. Blood. 2009;114:3769–72. doi: 10.1182/blood-2009-05-220145. [DOI] [PubMed] [Google Scholar]
- 15.Walz C, Score J, Mix J, Cilloni D, Roche-Lestienne C, Yeh RF, et al. The molecular anatomy of the FIP1L1-PDGFRA fusion gene. Leukemia. 2009;23:271–8. doi: 10.1038/leu.2008.310. [DOI] [PubMed] [Google Scholar]
- 16.Valent P, Oude Elberink JNG, Gorska A, Lange M, Zanotti R, van AB, et al. The Data Registry of the European Competence Network on Mastocytosis (ECNM): set up, projects, and perspectives. J Allergy Clin Immunol Pr. 2019;7:81–9. doi: 10.1016/j.jaip.2018.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garcia-Montero AC, Jara-Acevedo M, Teodosio C, Sanchez ML, Nunez R, Prados A, et al. KIT mutation in mast cells and other bone marrow hematopoietic cell lineages in systemic mast cell disorders: a prospective study of the Spanish Network on Mastocytosis (REMA) in a series of 113 patients. Blood. 2006;108:2366–72. doi: 10.1182/blood-2006-04-015545. [DOI] [PubMed] [Google Scholar]
- 18.Mayado A, Teodosio C, Dasilva-Freire N, Jara-Acevedo M, Garcia-Montero AC, Alvarez-Twose I, et al. Characterization of CD34(+) hematopoietic cells in systemic mastocytosis: potential role in disease dissemination. Allergy. 2018;73:1294–304. doi: 10.1111/all.13413. [DOI] [PubMed] [Google Scholar]
- 19.Pardanani A, Reeder T, Li CY, Tefferi A. Eosinophils are derived from the neoplastic clone in patients with systemic mastocytosis and eosinophilia. Leuk Res. 2003;27:883–5. doi: 10.1016/s0145-2126(03)00065-1. [DOI] [PubMed] [Google Scholar]
- 20.Metcalfe DD. Classification and diagnosis of mastocytosis: Current status. J Investig Dermatol. 1991;96:2S–4S. [PubMed] [Google Scholar]
- 21.Metcalfe DD. The liver, spleen, and lymph nodes in mastocytosis. J Investig Dermatol. 1991;96:45S–6S. [PubMed] [Google Scholar]
- 22.Schwaab J, Umbach R, Metzgeroth G, Naumann N, Jawhar M, Sotlar K, et al. KIT D816V and JAK2 V617F mutations are seen recurrently in hypereosinophilia of unknown significance. Am J Hematol. 2015;90:774–7. doi: 10.1002/ajh.24075. [DOI] [PubMed] [Google Scholar]
- 23.Metcalfe DD. Mast cells and mastocytosis. Blood. 2008;112:946–56. doi: 10.1182/blood-2007-11-078097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akuthota P, Wang HB, Spencer LA, Weller PF. Immunoregulatory roles of eosinophils: a new look at a familiar cell. Clin Exp Allergy. 2008;38:1254–63. doi: 10.1111/j.1365-2222.2008.03037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kovalszki A, Weller PF. Eosinophilia in mast cell disease. Immunol Allergy Clin North Am. 2014 May;34:357–64. doi: 10.1016/j.iac.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weller PF. The immunobiology of eosinophils. N Engl J Med. 1991;324:1110–8. doi: 10.1056/NEJM199104183241607. [DOI] [PubMed] [Google Scholar]
- 27.Butterfield JH. Systemic mastocytosis: clinical manifestations and differential diagnosis. Immunol Allergy Clin North Am. 2006;26:487–513. doi: 10.1016/j.iac.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 28.Akin C, Kirshenbaum AS, Semere T, Worobec AS, Scott LM, Metcalfe DD. Analysis of the surface expression of c-kit and occurrence of the c-kit Asp816Val activating mutation in T cells, B cells, and myelomonocytic cells in patients with mastocytosis. Exp Hematol. 2000;28:140–7. doi: 10.1016/s0301-472x(99)00145-9. [DOI] [PubMed] [Google Scholar]
- 29.Valent P, Klion AD, Horny HP, Roufosse F, Gotlib J, Weller PF, et al. Contemporary consensus proposal on criteria and classification of eosinophilic disorders and related syndromes. J Allergy Clin Immunol. 2012;130:607–12. doi: 10.1016/j.jaci.2012.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Valent P, Gleich GJ, Reiter A, Roufosse F, Weller PF, Hellmann A, et al. Pathogenesis and classification of eosinophil disorders: a review of recent developments in the field. Expert Rev Hematol. 2012;5:157–76. doi: 10.1586/ehm.11.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bain BJ, Horny H-P, Arber DA, Tefferi A, Hasserjian RP. Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed. Lyon: Internation Agency for Research on Cancer (IARC); 2017. Myeloid/lymphoid neoplasms with eosinophilia and rearrangement of PDGFRA, PDGFRB or FGFR1, or with PCM1-JAK2; pp. 72–9. [Google Scholar]
- 32.Maric I, Robyn J, Metcalfe DD, Fay MP, Carter M, Wilson T, et al. KIT D816V-associated systemic mastocytosis with eosinophilia and FIP1L1/PDGFRA-associated chronic eosinophilic leukemia are distinct entities. J Allergy Clin Immunol. 2007;120:680–7. doi: 10.1016/j.jaci.2007.05.024. [DOI] [PubMed] [Google Scholar]
- 33.Sotlar K, Colak S, Bache A, Berezowska S, Krokowski M, Bultmann B, et al. Variable presence of KIT(D816V) in clonal haematological non-mast cell lineage diseases associated with systemic mastocytosis (SM-AHNMD) J Pathol. 2010;220:586–95. doi: 10.1002/path.2677. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.