Abstract
Background
In indolent systemic mastocytosis (ISM), several risk factors of disease progression have been identified. Previous studies, performed with limited patient numbers, have also shown that the clinical course in ISM is stable and comparable to that of cutaneous mastocytosis (CM). The aim of this project was to compare the prognosis of patients with ISM with that of patients with CM.
Methods
We employed a dataset of 1993 patients from the registry of the European Competence Network on Mastocytosis (ECNM) to compare outcomes of ISM and CM.
Results
We found that overall survival (OS) is worse in ISM compared to CM. Moreover, in patients with typical ISM, bone marrow mastocytosis (BMM), and smoldering SM (SSM), 4.1% of disease progressions have been observed (4.9% of progressions in typical ISM group, 1.7% in BMM, and 9.4% in SSM). Progressions to advanced SM were observed in 2.9% of these patients. In contrast, six patients with CM (1.7%) converted to ISM and no definitive progression to advanced SM was found. No significant differences in OS and event-free survival (EFS) were found when comparing ISM, BMM, and SSM. Higher risk of both progression and death was significantly associated with male gender, worse performance status, and organomegaly.
Conclusion
Our data confirm the clinical impact of the WHO classification that separates ISM from CM and from other SM variants.
Keywords: cutaneous mastocytosis, indolent systemic mastocytosis, prognostication, survival, WHO classification
1. Introduction
Mastocytosis is a rare hematologic disease characterized by abnormal accumulation and expansion of tissue mast cells (MCs) in various organs.1–5 According to World Health Organization (WHO) criteria, indolent and advanced forms of mastocytosis can be distinguished.1–4 Cutaneous mastocytosis (CM) is typically found in childhood but can also be detected in adults. When detected in children, CM often resolves spontaneously before adolescence.1–5 Most adult patients have systemic mastocytosis (SM), often presenting with skin lesions and almost always with bone marrow (BM) involvement. 1–7 Indolent SM (ISM) has the highest prevalence.1–5 Bone marrow mastocytosis (BMM) represents a subvariant of ISM.1–4 In these patients, no skin lesions are detectable and the MC burden as well as serum tryptase levels are usually low. Recently, the smoldering subtype of SM (SSM), a former provisional ISM subvariant, has been designated as a distinct variant of SM by the WHO.1,2,4 Advanced mastocytosis includes aggressive SM (ASM), MC leukemia (MCL), and SM with an associated hematologic (non-MC) neoplasm (SM-AHN).1–4 The diagnosis of mastocytosis is established in a stepwise approach using the WHO classification and related diagnostic criteria.1–12
In a majority of patients with mastocytosis, including CM, ISM, and SSM, the clinical course remains stable over years or even decades.1–4,7,13–16 This contrasts with the smaller group of advanced SM, including ASM and MCL, where most patients show a more or less rapid progression of disease, resulting in organ damage.1–4,6,7,13–16 During the last decade, several clinical, serological, cytomorphological, immunological, and molecular factors have been reported to be of prognostic importance in CM and SM.1–5,16–31 A number of these prognostic variables have been included in the WHO classification.1–5
The WHO classification of mastocytosis was initially coined in 20013 and has been refined in 2008 and 2016.1,2,4 The correct classification of mastocytosis is important because it serves as a major tool of prognostication. In the 2016 update of the WHO classification, nonadvanced group was split into patients with typical ISM, patients with SSM, and patients with BMM, a subset that is still considered to represent a provisional subentity of typical ISM.1,2,4 However, so far, the clinical implications of the updated WHO classification have not been analyzed in detail.
The aim of this current project was to compare the prognosis of patients with typical ISM with that of patients with SSM, BMM, and CM regarding OS and EFS, and to perform univariate and multivariate analysis of factors associated with disease progression in these subgroups of patients. To meet this aim, we examined data in 1993 patients with mastocytosis collected in the dataset of the registry of the European Competence Network on Mastocytosis (ECNM).19,32 Details concerning the ECNM registry have been described recently by us19 and are summarized in the Supplemental Appendix.
2. Patients and Methods
2.1. Patients´ characteristics
A total of 1993 patients entered into the ECNM registry were analyzed (all the nonadvanced SM patients registered at the ECNM registry at the time of the study; Figure S1). Patients had typical ISM (n = 813), SSM (n = 56), BMM (n = 474), CM (n = 359; mastocytoma excluded), and mastocytosis in the skin (MIS; n = 291). MIS is a provisional diagnosis when no BM results are available in adults.33 In all cases, no C-findings were found, so ASM was always excluded. To analyze overall survival (OS), all patients with a follow-up of at least 1 day were included. As a result, the dataset for analyzing OS consisted of 1538 patients. The MIS cohort was excluded when calculating event-free survival (EFS). Thus, the dataset for this analysis consisted of 1325 patients. Detailed patients´ characteristics are shown in Tables 1 and 2. All patients provided written informed consent to participate.
Table 1. Patients’ characteristics. Data of patients with indolent systemic mastocytosis (ISM), bone marrow mastocytosis (BMM), and smoldering systemic mastocytosis (SSM).
| Characteristics | Statistics/categories | ISM | BMM | SSM | P * | P ** |
|---|---|---|---|---|---|---|
| Age (y)(n = 1085) | Median (5. perc; 95. perc) | 45.0 (24.0; 67.0) | 50.0 (27.0; 70.0) | 52.0 (30.0; 73.0) | <.001 | <.001 |
|
| ||||||
| Serum tryptase (ng/mL) (n = 1028) | Median (5. perc; 95. perc) | 35.0 (8.9; 183.0) | 27.0 (11.0; 128.0) | 200.0 (29.5; 575.0) | <.001 | <.001 |
|
| ||||||
| WBC (/μL) (n = 1027) | Median (5. perc; 95. perc) | 6600.0 (4 160.0; 10 900.0) | 6 400.0 (3 900.0; 10 600.0) | 6900.0 (2 900.0; 12 600.0) | .397 | .940 |
|
| ||||||
| Hemoglobin (g/dL) (n = 1032) | Median (5. perc; 95. perc) | 13.9 (12.0; 16.0) | 14.4 (12.3; 16.4) | 13.2 (10.4; 16.6) | <.001 | <.004 |
|
| ||||||
| Platelets (×103/μL) (n = 1025) | Median (5. perc; 95. perc) | 269.0 (179.0; 396.0) | 247.0 (162.0; 380.0) | 230.0 (112.0; 409.0) | <.001 | <.001 |
|
| ||||||
| Alkaline phosphatase (U/L) (n = 873) | Median (5. perc; 95. perc) | 75.0 (45.0; 146.0) | 71.8 (44.5; 129.0) | 114.0 (56.0; 340.0) | .133 | <.001 |
|
| ||||||
| Gender (n = 1085) | Women | 427 (65.2%) | 160 (42.4%) | 31 (58.5%) | <.001 | .370 |
| Men | 228 (34.8%) | 217 (57.6%) | 22 (41.5%) | |||
|
| ||||||
| Performance status (WHO) (n = 1085) | 0–Normal activity | 380 (58.0%) | 270 (71.6%) | 15 (28.3%) | <.001 | <.001 |
| 1–Symptoms, but fully ambulatory | 255 (38.9%) | 96 (25.5%) | 32 (60.4%) | |||
| 2–Symptoms, but in bed <50% of the day | 9 (1.4%) | 6 (1.6%) | 5 (9.4%) | |||
| 3–Needs to be in bed >50% of the day, but not bedridden | 5 (0.8%) | 3 (0.8%) | 0 (0.0%) | |||
| 5–Unknown | 6 (0.9%) | 2 (0.5%) | 1 (1.9%) | |||
|
| ||||||
| Typical skin involvement (n = 1085) | Yes | 655 (100%) | 0 (0%) | 43 (81.1%) | <.001 | <.001 |
| No | 0 (0%) | 377 (100%) | 10 (18.9%) | |||
|
| ||||||
| MC infiltrates in BM biopsy (n = 1085) | Yes | 561 (85.6%) | 284 (75.3%) | 50 (94.3%) | <.001 | 0.006 |
| No a | 68 (10.4%) | 77 (20.4%) | 0 (0.0%) | |||
| Not done a | 26 (4.0%) | 16 (4.2%) | 3 (5.7%) | |||
|
| ||||||
| KIT mutation D816V in BM (n = 1081) | Yes | 428 (65.6%) | 274 (72.9%) | 34 (64.2%) | .614 | .636 |
| No | 77 (11.8%) | 44 (11.7%) | 4 (7.5%) | |||
| Not done | 147 (22.5%) | 58 (15.4%) | 15 (28.3%) | |||
|
| ||||||
| Spleen (palpable) (n = 1056) | Yes | 23 (3.6%) | 15 (4.0%) | 27 (52.9%) | .735 | <.001 |
| No | 603 (95.1%) | 352 (94.9%) | 24 (47.1%) | |||
| Unknown | 8 (1.3%) | 4 (1.1%) | 0 (0.0%) | |||
|
| ||||||
| Hepatomegaly (palpable) (n = 1054) | Yes | 28 (4.4%) | 19 (5.1%) | 21 (41.2%) | .644 | <.001 |
| No | 597 (94.3%) | 347 (93.8%) | 29 (56.9%) | |||
| Unknown | 8 (1.3%) | 4 (1.1%) | 1 (2.0%) | |||
|
| ||||||
| Darier sign positive (n = 787) | Yes | 419 (67.0%) | 65 (53.7%) | 23 (56.1%) | 1.000 | .279 |
| No | 38 (6.1%) | 6 (5.0%) | 4 (9.8%) | |||
| Unknown | 168 (26.9%) | 50 (41.3%) | 14 (34.1%) | |||
|
| ||||||
| Lymphadenopathy (n = 1047) | Yes | 7 (1.1%) | 8 (2.2%) | 8 (15.7%) | .189 | <.001 |
| No | 590 (93.4%) | 341 (93.7%) | 37 (72.5%) | |||
| Unknown | 35 (5.5%) | 15 (4.1%) | 6 (11.8%) | |||
Note: Dataset for overall survival.
Abbreviations: BM, bone marrow; MC, mast cell; WBC, white blood count.
Patients without MC infiltrates in the bone marrow biopsy or with no bone marrow biopsy had MCs found in the bone marrow blood smear; therefore, the diagnosis of ISM or BMM has been established.
ISM vs BMM.
ISM vs SSM.
Bold P-values are statisticly significant.
Table 2. Patients’ characteristics. Data of patients with indolent systemic mastocytosis (ISM), cutaneous mastocytosis (CM), adulthood CM (aCM) and mastocytosis in the skin (MIS).
| Characteristics | Statistics/categories | ISM | CM | aCM | MIS | P * | P ** | P *** |
|---|---|---|---|---|---|---|---|---|
| Age (yrs) (n = 1108) | Median (5. perc; 95. perc) | 45.0 (24.0; 67.0) | 23.0 (0.6; 60.0) | 37.0 (22.0; 60.0) | 42.0 (17.0; 69.0) | <.001 | <.001 | .013 |
|
| ||||||||
| Serum tryptase (ng/mL) (n = 1007) | Median (5. perc; 95. perc) | 35.0 (8.9; 183.0) | 7.6 (2.7; 27.8) | 9.2 (2.7; 26.9) | 12.0 (2.7; 64.0) | <.001 | <.001 | <.001 |
|
| ||||||||
| WBC (/μL) (n = 966) | Median (5. perc; 95. perc) | 6 600.0 (4 160.0; 10 900.0) | 7 000.0 (4 510.0; 12 800.0) | 6300.0 (4 510.0; 12 800.0) | 6 690.0 (4 410.0; 11 000.0) | .025 | .484 | .343 |
|
| ||||||||
| Hemoglobin (g/dL) (n = 975) | Median (5. perc; 95. perc) | 13.9 (12.0; 16.0) | 13.5 (10.8; 15.9) | 13.9 (12.1; 15.9) | 13.8 (11.7; 15.8) | <.001 | .807 | .194 |
|
| ||||||||
| Platelets (×103/μL) (n = 967) | Median (5. perc; 95. perc) | 269.0 (179.0; 396.0) | 276.5 (173.0; 434.0) | 258.0 (173.0; 386.0) | 254.0 (183.0; 390.0) | .022 | .081 | .231 |
|
| ||||||||
| Alkaline phosphatase (U/L) (n = 754) | Median (5. perc; 95. perc) | 75.0 (45.0; 146.0) | 76.0 (40.0; 357.0) | 68.0 (40.0; 148.0) | 66.0 (42.0; 135.0) | .126 | .063 | .014 |
| Gender (n = 1108) | Women | 427 (65.2%) | 143 (59.1%) | 92 (69.2%) | 137 (64.9%) | .101 | .423 | 1.000 |
| Men | 228 (34.8%) | 99 (40.9%) | 41 (30.8%) | 74 (35.1%) | ||||
|
| ||||||||
| Performance status (WHO) (n = 1106) | 0–Normal activity | 380 (58.0%) | 166 (68.9%) | 79 (59.8%) | 153 (72.9%) | .007 | .409 | .001 |
| 1–Symptoms, but fully ambulatory | 255 (38.9%) | 74 (30.7%) | 52 (39.4%) | 53 (25.2%) | ||||
| 2–Symptoms, but in bed <50% of the day | 9 (1.4%) | 0 (0.0%) | 0 (0.0%) | 2 (1.0%) | ||||
| 3–Needs to be in bed >50% of the day, but not bedridden | 5 (0.8%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | ||||
| 4–Unable to get out of bed | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (0.5%) | ||||
| 5–Unknown | 6 (0.9%) | 1 (0.4%) | 1 (0.4%) | 1 (0.5%) | ||||
|
| ||||||||
| MC infiltrates in BM biopsy (n = 1108)a | Yes | 561 (85.6%) | 2 (0.8%) | 2 (1.5%) | 0 (0.0%) | <.001 | <.001 | <.001 |
| No | 68 (10.4%) | 133 (55.0%) | 128 (96.2%) | 10 (4.7%) | ||||
| Not done | 26 (4.0%) | 107 (44.2%) | 3 (2.3%) | 201 (95.3%) | ||||
|
| ||||||||
| C-kit mutation D816V in BM (n = 1103) | Yes | 428 (65.6%) | 24 (10.0%) | 24 (18.3%) | 7 (3.3%) | <.001 | <.001 | <.001 |
| No | 77 (11.8%) | 81 (33.8%) | 79 (60.3%) | 9 (4.3%) | ||||
| Not done | 147 (22.5%) | 135 (56.3%) | 28 (21.4%) | 195 (92.4%) | ||||
|
| ||||||||
| Spleen (palpable) (n = 1053) | Yes | 23 (3.6%) | 3 (1.3%) | 3 (2.3%) | 0 (0.0%) | .113 | .600 | .007 |
| No | 603 (95.1%) | 208 (93.3%) | 125 (94.7%) | 165 (84.2%) | ||||
| Unknown | 8 (1.3%) | 12 (5.4%) | 4 (3.0%) | 31 (15.8%) | ||||
|
| ||||||||
| Hepatomegaly (palpable) (n = 1051) | Yes | 28 (4.4%) | 8 (3.6%) | 7 (5.3%) | 6 (3.1%) | .845 | .644 | .829 |
| No | 597 (94.3%) | 202 (91.0%) | 121 (91.7%) | 159 (81.1%) | ||||
| Unknown | 8 (1.3%) | 12 (5.4%) | 4 (3.0%) | 31 (15.8%) | ||||
|
| ||||||||
| Darier sign positive (n = 1069) | Yes | 419 (67.0%) | 178 (74.8%) | 85 (64.9%) | 141 (68.4%) | .761 | .240 | .337 |
| No | 38 (6.1%) | 18 (7.6%) | 12 (9.2%) | 17 (8.3%) | ||||
| Unknown | 168 (26.9%) | 42 (17.6%) | 34 (26.0%) | 48 (23.3%) | ||||
|
| ||||||||
| Lymphadenopathy (n = 1043) | Yes | 7 (1.1%) | 7 (3.2%) | 2 (1.6%) | 11 (5.8%) | .052 | .657 | <.001 |
| No | 590 (93.4%) | 188 (85.5%) | 122 (94.6%) | 138 (72.3%) | ||||
| Unknown | 35 (5.5%) | 25 (11.4%) | 5 (3.9%) | 42 (22.0%) | ||||
Note: Dataset for overall survival.
Abbreviations: BM, bone marrow; MC, mast cell; NA, not applicable; WBC, white blood count.
The diagnosis ISM can be established based on the presence of three minor criterion even in the absence of the major criterion (dense mast cell infiltrate); not every mast cell infiltrate is diagnostic even if it is clearly visible; a loose infiltrate without densely packed atypical mast cells does not qualify as a major SM criterion.
ISM vs CM.
ISM vs aCM.
ISM vs MIS.
Bold P-values are statisticly significant.
2.2. Clinical and laboratory parameters assessed at diagnosis
Clinical and laboratory parameters captured at diagnosis included age, gender, performance status (Eastern Cooperative Oncology Group; ECOG), presence of skin lesions, organomegaly, serum tryptase level, white blood cell count (WBC), platelet count (PLT), hemoglobin (Hb), alkaline phosphatase (ALP), presence of MC infiltrates in BM sections, and presence of KIT mutation in codon 816 in BM cells.
2.3. Estimation of survival
Comparisons of patients´ characteristics in WHO subgroups (ISM vs BMM vs SSM vs CM)1,2,4 were performed using Fisher's exact test for categorical variables and the Mann-Whitney U test for continuous variables. Primary endpoints were OS and EFS. OS was calculated from the date of diagnosis to the date of death or last visit. EFS was defined as the time from the date of diagnosis to the date of progression to more advanced or aggressive forms of mastocytosis, death, or last visit. Survival estimates were established according to the method of Kaplan and Meier and compared across different diagnostic variants using the log-rank test. Patients´ characteristics and diagnoses were evaluated for association with survival using univariate and multivariate Cox regression analyses. P < .05 were considered significant, and the outcomes were accompanied by 95% confidence intervals (CI).
3. Results
3.1. Age and gender of patients with ISM and comparison to other forms of mastocytosis
Patients with ISM differed from patients with CM or MIS regarding age (ISM: median age 45.0 years; CM: median 23.0 years; MIS: median 42.0 years; P < .001 for ISM vs CM; P = .013 for ISM vs MIS). ISM, BMM, and SSM patients were exclusively adults; patients with CM consisted of 55% adults and 45% children. As per definition, patients diagnosed with MIS included only adults as children with skin lesions are usually classified as CM without BM studies.1–3 Patients with ISM were significantly younger than patients with BMM or SSM (ISM: median 45.0 years; BMM: 50.0 years; SSM: 52.0 years; P < .001 for both comparisons: ISM vs BMM and ISM vs SSM). Performance status was significantly different in these forms of mastocytosis (the best in BMM, worse in SSM; P < .001 for all comparisons). Furthermore, patients with typical ISM differed from patients with BMM concerning gender distribution (ISM: 65.2% women; BMM: 42.4% women; P < .001) (Tables 1 and 2).
3.2. Comparison of laboratory parameters in patients with ISM, BMM, and SSM
Patients with ISM had significantly higher tryptase levels compared to BMM patients and significantly lower tryptase levels compared to SSM patients (ISM: median 35.0 ng/mL; BMM: median 27.0 ng/mL; SSM: median 200.0 ng/mL; P < .001 in comparisons: ISM vs BMM, ISM vs SSM, and BMM vs SSM). Moreover, ISM patients differed from BMM and SSM patients concerning ALP activity levels (ISM: median 75.0 U/L; BMM: median 71.9 U/L; SSM: median 114.0 U/L; P < .001 for comparison ISM vs SSM). Differences in Hb levels and PLT counts were marginal, but statistically significant. There were significant differences in the presence of MC infiltrates in BM biopsy sections (P < .001 for ISM vs BMM; P = .006 for ISM vs SSM). These results are summarized in Tables 1 and 2.
3.3. Laboratory parameters also differ when comparing ISM with CM and MIS
Patients with ISM differed from patients with CM, adulthood CM (aCM; CM in patients over 18 years of age) and MIS in serum tryptase levels (medians: ISM 35.0 ng/mL; CM 7.6 ng/mL; aCM 9.2 ng/mL; MIS 12.0 ng/mL; P < .001 for comparisons: ISM vs CM, ISM vs aCM, and ISM vs MIS), and presence of the KIT mutation D816V in BM cells (P < .001 for the comparisons ISM vs CM and ISM vs aCM). Differences in Hb levels and PLT counts were marginal, but statistically significant. An interesting observation was that there was no significant difference in these parameters when comparing typical ISM with MIS patients. However, statistically significant differences were found between patients with typical ISM and patients with MIS concerning ALP (ISM: median 75.0 U/L; MIS: median 66.0 U/L; P = .014), performance status (P = .001), presence of splenomegaly (ISM: 3.6%; MIS: 0%; P = .007), and lymphadenopathy (ISM: 1.1%; MIS: 5.8%; P < .001). These results are summarized in Table 2.
3.4. Comparison of clinical parameters in patients with ISM, BMM, and SSM
As expected, given the WHO requirements for the SSM classification, patients with ISM differed from patients with SSM in the frequency of documented organomegaly. Organomegaly (B-finding) was thus more prominent in the SSM subgroup than in ISM patients (splenomegaly: ISM 3.6%, SSM 52.9%, P < .001; hepatomegaly: ISM 4.4%, SSM 41.2% yes, P < .001; and lymphadenopathy: ISM: 1.1% yes; SSM: 15.7% yes; P < .001). There were also significant differences in skin involvement (P < .001 for ISM vs SSM) (Table 1).
3.5. Overall survival is better in patients with CM compared to patients with ISM
One thousand five hundred thirty-eight patients were analyzed for OS. Median follow-up of patients with ISM (n = 655) was 4.3 years (range 0.0-31.4). Twenty-two patients (3.4%) died during this period. Causes of death were disease-related (n = 5; progression to more advanced SM); cardiovascular (n = 7); secondary cancer (n = 6); unknown (n = 4). Median OS was 28.4 years (95% CI: 24.1-32.7 years). Median follow-up of patients with BMM (n = 377) was 3.2 years (range 0.0-20.5). Thirteen patients (3.4%) died in this group. Causes of death were secondary cancer (n = 4); cardiovascular (n = 4); liver failure (n = 1); unknown (n = 4). Median OS was 19.9 years (95% CI: 15.2-24.7) in the BMM group. Median follow-up of patients with SSM (n = 53) was 4.3 years (range 0.3-22.0); five patients (9.4%) died in this cohort (disease-related cause (n = 2; progression to more advanced SM); secondary cancer (n = 1); infection (n = 1); unknown (n = 1)). At the time of analysis, median OS was not reached in the SSM group. There were no significant differences among ISM, BMM, and SSM both in length of follow-up (P = .206) and in percentage of surviving patients (P = .262). There was no significant difference in OS when comparing ISM and BMM (P = .727) and borderline when comparing ISM and SSM (P = .050) (Figures S2 and S3).
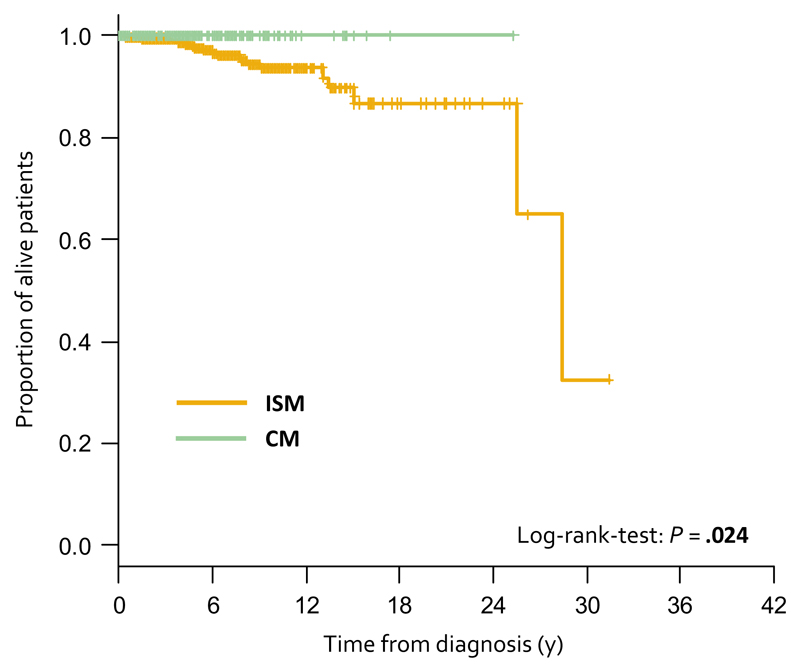
Median follow-up of patients with CM (n = 242) was 3.0 years (range 0.0-25.3 years; median in aCM was 3.1 years). No patient with childhood CM or aCM died during follow-up. Median follow-up of patients with MIS (n = 211) was 3.0 years (range 0.0-36.7 years). There were 10 (4.7%) deaths among patients with MIS. Causes of death were secondary cancer (n = 2), cardiovascular (n = 2), kidney failure (n = 1), accident (n = 1), infection (n = 1), unknown (n = 3). Median OS was not reached both in patients with CM and MIS. A significant difference in OS was found between patients with ISM and CM (P = .024), but no significant difference in OS was found when comparing ISM patients with the aCM (P = .092) and MIS subgroup (P = .17). However, there was a clear trend to better survival in aCM patients compared with ISM (Figure 1; Figures S4 and S5).
Figure 1.
Overall survival (OS) of indolent systemic mastocytosis patients (ISM, n = 655) and cutaneous mastocytosis patients (CM, n = 242). P = .024 as determined by log-rank-test. Hazard ratio (HR) 0.0 (95% confidence interval: 0.0)
3.6. Progression of disease and event-free survival
A total of 1325 patients were analyzed for EFS. Median follow-up of patients with typical ISM (n = 653) was 4.2 years (range 0.0-31.4); patients with BMM (n = 377) 3.2 years (range 0.0-20.5); and patients with SSM (n = 53) 4.3 years (range 0.3-22.0). In these patients, progressions were as follows: typical ISM to SSM (n = 11; 1.7%), typical ISM to aggressive SM (ASM) (n = 8; 1.2%), typical ISM to SM-AHN (n = 12; 1.8%), typical ISM to MC sarcoma (MCS like progression fulfilling ASM criteria) (n = 1; 0.1%), SSM to ASM (n = 4; 7.5%), SSM to SM-AHN (n = 1; 1.9%), BMM to ASM (n = 1; 0.2%), BMM to SM-AHN (n = 5; 1.3%), and BMM to SSM (n = 1; 0.2%). Median follow-up of patients with CM (n = 242) was 2.8 years (aCM also 2.8 years). Only six patients with CM (1.7%; all aCM patients) eventually converted to ISM, and no further progression to advanced SM was found. Summary of progressions is shown in Table 3. No statistically significant difference was found when comparing EFS in ISM, BMM, SSM, CM, and aCM. EFS estimates are shown in Figures S6–S10.
Table 3. Disease progressions.
| Progressions | Progressions to advanced SM | P-value (ISM vs CM) | |||
|---|---|---|---|---|---|
| ISM (n = 1083) | Typical ISM (n = 653) | 4.9% | 4.1% | 2.9% <.001 | |
| BMM (n = 377) | 1.7% | ||||
| SMM (n = 53) | 9.4% | ||||
|
| |||||
| CM (n = 242) | 1.7% | 0 | |||
Abbreviations: BMM, bone marrow mastocytosis; CM, cutaneous mastocytosis; ISM, indolent systemic mastocytosis; SMM, smoldering systemic mastocytosis.
3.7. Identification of independent prognostic variables in multivariate analyses
In multivariate analyses including parameters shown in Tables 1 and 2, age (HR 1.06, 1.04-1.09; P < .001), gender (female gender protective; HR 0.5, 0.28-0.86; P = .013), performance status (HR 2.11, 1.19-3.72; P = .010), and lymphadenopathy (HR 4.96, 1.76-13.96; P = .002) were statistically significant predictors of EFS (Table 4). Regarding OS, performance status (HR 2.02, 1.05-3.91; P = .036); splenomegaly (HR 3.39, 1.15-10.03 P = .027), and age (HR 1.11, 1.08-1.14; P < .001) were found to be significant. Results of multivariate analyses are summarized in Table 3.
Table 4. Multivariate analysis of the influence of selected parameters on event-free survival (EFS) and overall survival (OS).
| Multivariate analysis of the influence of selected parameters on EFS |
Multivariate analysis of the influence of selected parameters on OS |
|||
|---|---|---|---|---|
| Variable | HR (95% CI) | P-value | HR (95% CI) | P-value |
| Age (y) | 1.06 (1.04; 1.09) | <.001 | 1.11 (1.08; 1.14)a | <.001 |
| Serum tryptase (ng/mL) | 1.00 (1.00; 1.00) a | .093 | 1.00 (1.00; 1.00)a | .253 |
| Platelets (×103/μL) | 1.00 (0.99; 1.00) a | .485 | 1.00 (1.00; 1.00)a | .943 |
| Gender (female vs male) | 0.50 (0.28; 0.86) | .013 | 0.54 (0.27; 1.05) | .070 |
| Performance status: symptoms/need to be in bed (vs normal activity) | 2.11 (1.19; 3.72) | .010 | 2.02 (1.05; 3.91) | .036 |
| Spleen (palpable) (yes vs no) | 1.37 (0.55; 3.41) | .492 | 3.39 (1.15; 10.03) | .027 |
| Hepatomegaly (palpable) (yes vs no) | 1.99 (0.86; 4.60) | .106 | 1.36 (0.48; 3.85) | .563 |
| Lymphadenopathy (yes vs no) | 4.96 (1.76; 13.96) | .002 | 0.54 (0.27; 1.05) | .070 |
Note: Results of Cox regression multivariate analysis of the influence of selected parameters on PFS and OS in indolent systemic mastocytosis (ISM), bone marrow mastocytosis (BMM), smoldering systemic mastocytosis (SMM) and cutaneous mastocytosis (CM). Statistically significant parameters (P < .05) from univariate Cox analyses were put into multivariate Cox analysis. Performance status is binarized: symptoms/need to be in bed vs normal activity. N = 1043.
Abbreviations: HR, hazard ratio; CI, confidence interval.
Elevated vs normal.
Bold P-values are statisticly significant.
4. Discussion
The term mastocytosis denotes a heterogeneous group of rare disorders characterized by abnormal growth and accumulation of MCs in various organs.1–5 Indolent SM (ISM) is the most prevalent subtype of mastocytosis in adults. However, so far, little is known about the clinical outcome of this group, especially when comparing to adult patients with CM, BMM, or SSM. In the present study, we examined clinical and laboratory features as well as the prognosis of patients with typical ISM and compared the outcomes in this group with OS and EFS in patients with CM, BMM, and SSM. These studies were performed in 1993 patients with mastocytosis collected in the ECNM registry (no well-differentiated SM among these patients). Our data show that ISM is a unique subset with distinct clinical features and distinct prognosis which is worse compared to CM regarding OS. However, our data also show that the differences in OS and EFS are subtle if any when comparing typical ISM with BMM and SSM.
There has been a long-lasting debate about the necessity to perform a BM biopsy in all adult patients with MIS and about the clinical and prognostic implications of separating CM from SM. In particular, it was not clear whether patients with ISM have a less favorable prognosis compared to CM—actually the OS in ISM seems to be similar to that in the healthy, age-matched population.6 We found that patients with ISM have inferior OS compared to CM patients. However, there was also a significant difference in age when comparing these patients. In particular, patients in the ISM subgroup were significantly older compared to patients with CM which is due to the fact that in the CM group, 45% of the patients were children. On the other hand, adult patients with CM were also detected in the registry, and these patients were also found to have a better prognosis compared to ISM patients. Most importantly, even in adults with CM, no cases of death were recorded, contrasting the ISM group. Based on this observation, it seems clear that definitive statements about the prognosis in MIS can only be delivered when a BM biopsy is performed to clarify the final correct diagnosis: SM vs CM. Therefore, we recommend a BM study in all adult patients with MIS.
We also found that there is no significant difference when OS is compared in patients with MIS and ISM, which may point to the fact that many patients with the provisional diagnosis of MIS have in fact an unrecognized (not yet diagnosed) SM, mostly ISM. This assumption is supported by the fact that indeed ISM is diagnosed in many patients once these adult patients agree to a BM biopsy study.
In the recent WHO update, ISM has been separated from SSM and from BMM, which was recognized as a provisional subset of ISM and is now considered a provisional SM variant. Unexpectedly, the OS in these subgroups (ISM, BMM, and SSM) was very similar. Regarding OS of ISM patients, we confirmed data of a previous study published by the Mayo group.6,34 In the Mayo cohort, OS of patients with ISM was 25.1 years and thus slightly shorter compared to an OS of 28.4 years in our study. The prognosis of SSM patients also appeared to be better in our analysis, with a median OS not reached in our study and a median OS of 10 years in the Mayo cohort.6,34 These differences may have several explanations. One could be that the awareness for mastocytosis and therapy has improved in recent years (when the ECNM registry was established), whereas the Mayo data are based in part on a cohort of cases that was collected in an earlier time period. Another possibility may be that our patients were diagnosed at an earlier time point in their lifetime compared to the Mayo patients.
So far, most studies evaluating risk factors in SM were based on less than 400 patients, which is an important point as SM is an extremely heterogeneous disease, and even in defined subtypes, the clinical course and organ involvements may differ substantially between patients’ subsets. In the current study, 1538 patients were examined for OS and 1325 for EFS. In multivariate analysis, gender, performance status, and lymphadenopathy were statistically significant predictors of EFS; and performance status, splenomegaly, and lymphadenopathy for OS. So far, it is unclear whether all adult patients with MIS and lower tryptase level should have a BM investigation in order to define whether they are suffering from CM or SM, or even an advanced form of SM. Our data show that the OS is worse in adult patients with ISM compared to adults with CM, although statistical significance was not reached.
In the new WHO classification, typical ISM has been separated from SSM.1,2,4 Although several observations have already supported this split, validation was still lacking. Our study confirms that the proposed split into ISM, SSM, and BMM is meaningful. In fact, slightly higher progression rates and slightly shorter OS were found in the SSM cohort compared to ISM. On the other hand, these differences were not significant statistically and no differences were found when comparing OS and EFS in ISM and BMM patients. In this regard, it should be mentioned that BMM is still regarded a special subvariant of ISM. On the other hand, BMM patients exhibit unique clinical features and are frequently overlooked, which is a clinical challenge as exactly these patients may suffer from severe systemic mediator-induced symptoms, especially when a concomitant allergy is present.
The Spanish Network on Mastocytosis published that ISM in adults has a low disease progression rate and that a majority of their patients had a normal life expectancy.16 According to their analysis, the presence of KIT mutations in multiple hematopoietic lineages and increase in serum beta2-microglobulin are the most powerful independent parameters predicting conversion into a more aggressive form of disease.16 Multilineage involvement and beta2-microglobulin concentrations were not analyzed in our study but may correlate with the smoldering state (SSM) of SM. Therefore, we believe that indeed multilineage involvement with KIT D816V is of prognostic significance in SM. More recently, this assumption has also been confirmed in several independent studies, including studies examining the mutant allele burden in SM. For example, Hoermann et al published that KIT D816V allele burden predicts survival in SM and correlates with the WHO type of the disease, and with the tryptase levels.29 In our study, the KIT D816V allele burden was not analyzed. However, we believe that multilineage involvement and the KIT D816V allele burden should be added to prognostication and should probably also count as signs or even criterion of the smoldering state in SM in the future.
An interesting aspect in our study was that the prognosis of patients with SSM was similar to OS and EFS compared to ISM which contrasts previous studies.16 There are several explanations for this discrepancy. First, it may well be that only a subset of patients with SSM (eg those with clearly elevated KIT D816V burden and/or multilineage involvement and/or elevated ALP) has a higher risk to progress. Alternatively, the different numbers of patients analyzed in the different studies may explain this discrepancy.
An interesting aspect was that the progression patterns were different when comparing patients with CM, BMM, ISM, and SSM. For example, in CM, only six patients developed typical ISM and no further progression of these “CM-to-ISM” patients was seen. In BMM, progression to ASM, SSM, and SM-AHN, but no progression to typical ISM with skin lesions was found. In this regard, it is worth noting that many patients with ASM and SM-AHN lack skin lesions. By contrast, in typical ISM, transitions to SSM, ASM, MCS (MCS like progression fulfilling ASM criteria), and SM-AHN were recorded. Together, these data suggest that clinically relevant progression may occur in ISM and even in BMM. In BMM, a close follow-up may especially be required in cases with higher or rapidly increasing tryptase levels, in order to detect or exclude disease transformation.
5. Conclusions
Our data confirm the clinical impact of the WHO classification that separates CM from ISM variants. The different prognosis of ISM and CM gives a sufficient reason for a BM investigation in all adult patients with skin lesions (MIS) and point at the need to recommend a regular follow-up in these cases. Precise differentiation of individual SM subgroups is also recommended for routine clinical practice. On the other hand, we also show that although ISM, BMM, and SSM display unique clinical features, especially the performance status which is worse in SSM, the prognosis is similar regarding OS and EFS.
Supplementary Material
Funding information
Supported by the project CEITEC 2020 [LQ1601] from the Ministry of Education, Youth and Sports of the Czech Republic under National Sustainability, TA CR Center of Competence project (TE02000058), MH CR – DRO (FNBr, 65269705), by Deutsche Forschungsgemeinschaft (DFG, RA 2838 to AI), by the Koeln Fortune Program, Faculty of Medicine, University of Cologne (216/2016 to AI) and by the Austrian Science Fund (FWF) grant SFB F4704-B20. Also supported by the Czech Leukemia Study Group for Life (CELL).
Abbreviations
- ALP
alkaline phosphatase
- ASM
aggressive systemic mastocytosis
- BM
bone marrow
- BMM
bone marrow mastocytosis
- CI
confidence interval
- CM
cutaneous mastocytosis
- ECNM
the European Competence Network on Mastocytosis
- ECOG
the Eastern Cooperative Oncology Group
- EFS
event-free survival
- Hb
hemoglobin
- HR
hazard ratio
- ISM
indolent systemic mastocytosis
- MC
mast cell
- MCL
mast cell leukemia
- MCS
mast cell sarkoma
- MIS
mastocytosis in skin
- OS
overall survival
- PLT
platelet count
- SM
systemic mastocytosis
- SM-AHN
systemic mastocytosis with an associated hematologic neoplasm
- SMM
smoldering systemic mastocytosis
- WBC
white blood count
- WHO
the World Health Organization
Footnotes
Conflict of interest
WRS received honoraria from Novartis, Pfizer, AbbVie, Daiichi Sankyo, Amgen, Thermo Fisher, Diciphera, Incyte, Celgene, Jazz and travel grants from Pfizer and Roche. HOE received honoraria from ALK-Abelló, Chiesi, MEDA Pharma, Novartis, Blueprint. KVG received honoraria from Novartis, Roche, BMS, Sanofi, Incyte and travel grants from Roche and AbbVie. ML received honoraria from Novartis. KH received honoraria from Novartis, ALK, Blueprint, Deciphera and research funding from Euroimmun. MB received honoraria from Amgen, Incyte, Pfizer and research funding from Novartis. CE received honoraria from Novartis and Pfizer. MJ received honoraria from Novartis, Blueprint, Deciphera. RZ is consultant Deciphera and Novartis. AZ received honoraria or participated in trials from AbbVie, Almirall, Beiersdorf Dermo Medical, Bencard Allergie, BMS, Celgene, Eli Lilly, GSK, Janssen-Cilag, Miltenyi Biotec, Novartis, Sanofi-Aventis, Takeda Pharma. MT received honoraria from Deciphera, Blueprint, Novartis. NB received honoraria from Astra Zeneca, Amgen, Novartis and BMS and research funding from Novartis. JP received honoraria from Alexion, BMS, Boehringer Ingelheim, Grünenthal, MSD, Novartis, Pfizer, Chugai. DF received honoraria from Novartis, Pfizer, Roche travel grants from Roche. VS received honoraria from Novartis, Termofisher, Shire, Stallergens. KB received honoraria from Novartis, Phadia (Thermo Fisher), Meda, BioMarin Pharmaceutical Inc outside. BA received honoraria from Novartis. AR received honoraria from Novartis, BMS, Deciphera, Blueprint, Baxalta/Shire and research funding from Novartis. JG received honoraria from Blueprint, Deciphera, Gilead, Incyte, Novartis and research funding from Blueprint, Celgene, CTI BioPharma, Deciphera, Gilead, Incyte, Pharmacyclics, Promedior, Seattle Genetics. JM received honoraria from for Novartis, Gilead, BMS. MD received honoraria from for Roche, AbbVie, Novartis, Gilead, AOP Pharmaceuticals, Janssen-Cilag. PV has received honoraria from Novartis, Pfizer, Deciphera, Incyte, Blueprint, Celgene and research funds from Pfizer, Incyte, Celgene. JT, LN, AG, AI, CP, LM, ABF, FC, KS, RP, MN, PB, ASY, HH, MM, NJ, AK, OH, MA, AB, HCKN have nothing to disclose.
Author Contributions
JT, WRS, MD, PV designed analyses, interpreted results, and wrote the manuscript; LN performed statistical analyses; HOE, KVG, AG, ML, KH, AI, MB, CP, CE, LM, ABF, KS, MJ, RZ, PB, FC, AZ, MT, RP, NvB, ASY, HH, MM, JP, NJ, AK, OH, MA, DF, VS, KB, EA, MN, BvA, AR, JG, KCN, and JM collected data, supervised the study, and edited the manuscript.
References
- 1.Horny H-P, Akin C, Arber DA, et al. Mastocytosis. In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Arber DA, Hasserjian RP, LeBeau MM, Orazi A, et al., editors. WHO classification of tumours of haematopoietic and lymphoid tissues. Lyon: IARC Press; 2017. pp. 61–70. [Google Scholar]
- 2.Valent P, Akin C, Metcalfe DD. Mastocytosis: 2016 updated WHO classification and novel emerging treatment concepts. Blood. 2017;129(11):1420–1427. doi: 10.1182/blood-2016-09-731893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Valent P, Horny H-P, Escribano L, et al. Diagnostic criteria and classification of mastocytosis: a consensus proposal. Leuk Res. 2001;25:603–605. doi: 10.1016/s0145-2126(01)00038-8. [DOI] [PubMed] [Google Scholar]
- 4.Valent P, Akin C, Hartmann K, et al. Advances in the classification and treatment of mastocytosis: current status and outlook toward the future. Cancer Res. 2017;77(6):1261–1270. doi: 10.1158/0008-5472.CAN-16-2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sperr WR, Valent P. Diagnosis, progression patterns and prognostication in mastocytosis. Expert Rev Hematol. 2012;5:261–274. doi: 10.1586/ehm.12.12. [DOI] [PubMed] [Google Scholar]
- 6.Lim K-H, Tefferi A, Lasho TL, et al. Systemic mastocytosis in 342 consecutive adults: survival studies and prognostic factors. Blood. 2009;113(23):5727–5736. doi: 10.1182/blood-2009-02-205237. [DOI] [PubMed] [Google Scholar]
- 7.Arock M, Valent P. Pathogenesis, classification and treatment of mastocytosis: state of the art in 2010 and future perspectives. Expert Rev Hematol. 2010;3:497–516. doi: 10.1586/ehm.10.42. [DOI] [PubMed] [Google Scholar]
- 8.Sperr WR, Escribano L, Jordan J-H, et al. Morphologic properties of neoplastic mast cells: delineation of stages of maturation and implication for cytological grading of mastocytosis. Leuk Res. 2001;25:529–536. doi: 10.1016/s0145-2126(01)00041-8. [DOI] [PubMed] [Google Scholar]
- 9.Escribano L, Díaz-Agustín B, Bellas C, et al. Utility of flow cytometric analysis of mast cells in the diagnosis and classification of adult mastocytosis. Leuk Res. 2001;25:563–570. doi: 10.1016/s0145-2126(01)00050-9. [DOI] [PubMed] [Google Scholar]
- 10.Longley BJ, Reguera MJ, Ma Y. Classes of c-KIT activating mutations: proposed mechanisms of action and implications for disease classification and therapy. Leuk Res. 2001;25:571–576. doi: 10.1016/s0145-2126(01)00028-5. [DOI] [PubMed] [Google Scholar]
- 11.Schwartz LB, Metcalfe DD, Miller JS, Earl H, Sullivan T. Tryptase levels as an indicator of mast-cell activation in systemic anaphylaxis and mastocytosis. N Engl J Med. 1987;316:1622–1626. doi: 10.1056/NEJM198706253162603. [DOI] [PubMed] [Google Scholar]
- 12.Sperr WR, Jordan J-H, Fiegl M, et al. Serum tryptase levels in patients with mastocytosis: correlation with mast cell burden and implication for defining the category of disease. Int Arch Allergy Immunol. 2002;128:136–141. doi: 10.1159/000059404. [DOI] [PubMed] [Google Scholar]
- 13.Akin C, Metcalfe DD. Systemic mastocytosis. Annu Rev Med. 2004;55:419–432. doi: 10.1146/annurev.med.55.091902.103822. [DOI] [PubMed] [Google Scholar]
- 14.Metcalfe DD. Mast cells and mastocytosis. Blood. 2008;112(4):946–956. doi: 10.1182/blood-2007-11-078097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Valent P, Sperr WR, Akin C. How I treat patients with advanced systemic mastocytosis. Blood. 2010;116(26):5812–5817. doi: 10.1182/blood-2010-08-292144. [DOI] [PubMed] [Google Scholar]
- 16.Escribano L, Álvarez-Twose I, Sánchez-Muñoz L, et al. Prognosis in adult indolent systemic mastocytosis: a long-term study of the Spanish Network on Mastocytosis in a series of 145 patients. J Allergy Clin Immunol. 2009;124(3):514–521. doi: 10.1016/j.jaci.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 17.Sperr WR, Horny HP, Valent P. Spectrum of associated clonal hematologic non-mast cell lineage disorders occurring in patients with systemic mastocytosis. Int Arch Allergy Immunol. 2002;127(2):140–142. doi: 10.1159/000048186. [DOI] [PubMed] [Google Scholar]
- 18.Metcalfe DD. Classification and diagnosis of mastocytosis: current status. J Invest Dermatol. 1991;96(3):2S–4S. [PubMed] [Google Scholar]
- 19.Valent P, Oude Elberink JNG, Gorska A, et al. The data registry of the European Competence Network on Mastocytosis (ECNM): set up, projects and perspectives. J Allergy Clin Immunol Pract. 2019;7(1):81–87. doi: 10.1016/j.jaip.2018.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lennert K, Parwaresch MR. Mast cells and mast cell neoplasia: a review. Histopathology. 1979;3(5):349–365. doi: 10.1111/j.1365-2559.1979.tb03017.x. [DOI] [PubMed] [Google Scholar]
- 21.Sotlar K, Cerny-Reiterer S, Petat-Dutter K, et al. Aberrant expression of CD30 in neoplastic mast cells in high-grade mastocytosis. Mod Pathol. 2011;24(4):585–595. doi: 10.1038/modpathol.2010.224. [DOI] [PubMed] [Google Scholar]
- 22.Valent P, Cerny-Reiterer S, Herrmann H, et al. Phenotypic heterogeneity and target expression profiles of normal and neoplastic mast cells. Best Pract Res Clin Haematol. 2010;23(3):369–378. doi: 10.1016/j.beha.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 23.Escribano L, Orfao A, Díaz-Agustin B, et al. Indolent systemic mast cell disease in adults: immunophenotypic characterization of bone marrow mast cells and its diagnostic implications. Blood. 1998;91(8):2731–2736. [PubMed] [Google Scholar]
- 24.Escribano L, Orfao A, Villarrubia J, et al. Sequential immunophenotypic analysis of mast cells in a case of systemic mast cell disease evolving to a mast cell leukemia. Cytometry. 1997;30(2):98–102. doi: 10.1002/(sici)1097-0320(19970415)30:2<98::aid-cyto4>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 25.Teodosio C, Mayado A, Sa´nchez-Mun~oz L, et al. The immunophenotype of mast cells and its utility in the diagnostic work-up of systemic mastocytosis. J Leukoc Biol. 2015;97:49–59. doi: 10.1189/jlb.5RU0614-296R. [DOI] [PubMed] [Google Scholar]
- 26.Pardanani A, Reichard KK, Zblewski D, et al. CD123 immunostaining patterns in systemic mastocytosis: differential expression in disease subgroups and potential prognostic value. Leukemia. 2016;30:914–918. doi: 10.1038/leu.2015.348. [DOI] [PubMed] [Google Scholar]
- 27.Pardanani A, Finke C, Abdelrahman RA, et al. Increased circulating IL-2Ra (CD25) predicts poor outcome in both indolent and aggressive forms of mastocytosis: a comprehensive cytokine–phenotype study. Leukemia. 2013;27:1430–1433. doi: 10.1038/leu.2013.11. [DOI] [PubMed] [Google Scholar]
- 28.Hoermann G, Gleixner KV, Dinu GE, et al. The KIT D816V allele burden predicts survival in patients with mastocytosis and correlates with the WHO type of the disease. Allergy. 2014;69(6):810–813. doi: 10.1111/all.12409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schwaab J, Schnittger S, Sotlar K, et al. Comprehensive mutational profiling in advanced systemic mastocytosis. Blood. 2013;122(14):2460–2466. doi: 10.1182/blood-2013-04-496448. [DOI] [PubMed] [Google Scholar]
- 30.Jawhar M, Schwaab J, Schnittger S, et al. Additional mutations in SRSF2, ASXL1 and/or RUNX1 identify a high-risk group of patients with KIT D816V+ advanced systemic mastocytosis. Leukemia. 2016;30:136–143. doi: 10.1038/leu.2015.284. [DOI] [PubMed] [Google Scholar]
- 31.Greiner G, Witzeneder N, Berger A, et al. CCL2 is a KIT D816V-dependent modulator of the bone marrow microenvironment in systemic mastocytosis. Blood. 2017;129(3):371–382. doi: 10.1182/blood-2016-09-739003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Valent P, Arock M, Bonadonna P, et al. European Competence Network on Mastocytosis (ECNM): 10-year jubilee, update, and future perspectives. Wien Klin Wochenschr. 2012;124(23–24):807–814. doi: 10.1007/s00508-012-0293-z. [DOI] [PubMed] [Google Scholar]
- 33.Hartmann K, Escribano L, Grattan C, et al. Cutaneous manifestations in patients with mastocytosis: Consensus report of the European Competence Network on Mastocytosis; the American Academy of Allergy, Asthma & Immunology; and the European Academy of Allergology and Clinical Immunology. J Allergy Clin Immunol. 2016;137(1):35–45. doi: 10.1016/j.jaci.2015.08.034. [DOI] [PubMed] [Google Scholar]
- 34.Pardanani A. Systemic mastocytosis in adults: 2015 update on diagnosis, risk stratification, and management. Am J Hematol. 2015;90:250–262. doi: 10.1002/ajh.23931. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.