Abstract
Agonist stimulation of G-protein-coupled receptors (GPCRs) typically results in phosphorylation and activation of ERK (Extracellular-signal Regulated Kinase) which is a member of MAP kinase (Mitogen-Activated Protein kinase) family. Detection of phosphorylated ERK1/2 MAP kinase has been widely used as readout of GPCR signaling in heterologous cells, primary cells, tissues and even in animal studies. ERK1/2 phosphorylation downstream of GPCRs is now well established to arise from the activation of both, the heterotrimeric G-proteins and β-arrestins (βarrs) with distinct spatio-temporal components. Here, we present a step-by-step protocol for measuring agonist-induced ERK1/2 MAP kinase activation downstream of GPCRs using standard Western blotting assay.
Note: ERK1/2 is also referred to as p44/42 MAP kinase. ERK1 and ERK2 are same as Mitogen-Activated Protein Kinase 3 (MAP3) and Mitogen-Activated Protein Kinase 1 (MAP1), respectively.
1. Introduction
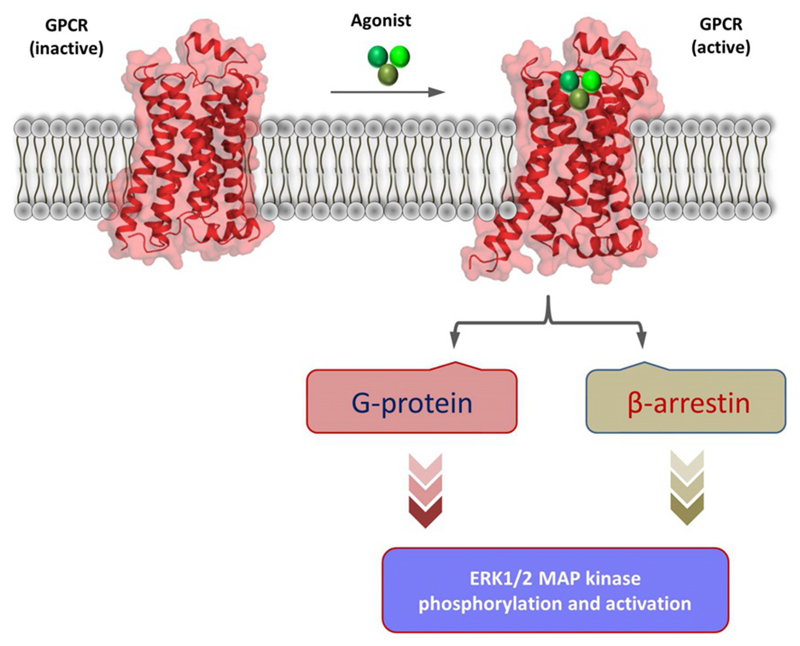
G-protein-coupled receptors (GPCRs) are present in the plasma membrane and they recognize a wide array of stimuli including hormones, lipids, peptides and chemicals through their extracellular surface (Bockaert & Pin, 1999; Ghosh, Nidhi, & Shukla, 2014; Vass et al., 2018; Weis & Kobilka, 2018). Subsequently, they convey the message across the cell membrane via activation dependent conformational changes and coupling to intracellular effectors (Bockaert & Pin, 1999; Gurevich & Gurevich, 2018; Weis & Kobilka, 2018). Upon agonist-stimulation, GPCRs typically couple to and activate heterotrimeric G-proteins (for example, Gαs, Gαi, Gαq/11) and βarrs (βarr1 and 2) (Bockaert & Pin, 1999; Gurevich & Gurevich, 2018; Shukla, Xiao, & Lefkowitz, 2011; Weis & Kobilka, 2018). Both of these signal-transducers, i.e., G-proteins and βarrs mediate a cascade of events that lead to phosphorylation of ERK1/2 MAP kinase as a key step in downstream signaling pathways (Lefkowitz & Shenoy, 2005; Luttrell & Lefkowitz, 2002; Ranjan, Dwivedi, Baidya, Kumar, & Shukla, 2017) (Fig. 1). While G-protein-dependent ERK MAP kinase phosphorylation primarily results from the activation of PKA (Protein Kinase A) and PKC (Protein Kinase C), the ability of βarrs to mediate ERK phosphorylation results from their scaffolding properties (Chen, Iverson, & Gurevich, 2018; DeWire, Ahn, Lefkowitz, & Shenoy, 2007; Gurevich, Gurevich, & Uversky, 2018).
Fig. 1.
A schematic representation of ERK MAP kinase activation downstream of GPCRs. Upon agonist-stimulation, GPCRs typically couple to and activate heterotrimeric G-proteins and β-arrestins. Activation of each of these transducers culminate into phosphorylation and activation of ERK1/2 MAP kinase with distinct spatio-temporal pattern. The inactive and active GPCR cartoons are adapted from previously determined antagonist and agonist-bound crystal structures of the β2-adrenergic receptor, respectively (PDB ID: 2RH1 and 3P0G).
βarrs can bind to, and nucleate, various members of ERK MAP kinase cascade including MAP3K (e.g., Raf-1), MAP2K (e.g., MEK-1) and MAP1K (e.g., ERK2) (Chen et al., 2018; DeWire et al., 2007; Shukla, Singh, & Ghosh, 2014). Typically, G-protein-dependent ERK1/2 phosphorylation exhibits a relatively faster onset while that mediated by βarrs are delayed (Ahn, Shenoy, Wei, & Lefkowitz, 2004; DeWire et al., 2007). In addition, G-protein-dependent component of phosphorylated ERK1/2 is directed to the nucleus while βarr-dependent component is localized primarily in the cytoplasm (Ahn et al., 2004; DeWire et al., 2007; Reiter, Ahn, Shukla, & Lefkowitz, 2012). Therefore, distinct spatio-temporal components of ERK MAP kinase phosphorylation have been used as signatures of G-protein-dependent and βarr-dependent signaling (DeWire et al., 2007; Reiter et al., 2012; Shenoy et al., 2006). However, there are examples of GPCRs which do not strictly follow this spatio-temporal pattern of ERK1/2 phosphorylation and localization (Dwivedi, Baidya, & Shukla, 2018; Grundmann & Kostenis, 2017; Perez-Aso et al., 2013; Ranjan, Gupta, & Shukla, 2016; Segura et al., 2013; Srivastava, Gupta, Gupta, & Shukla, 2015).
Detection of ERK1/2 MAP kinase phosphorylation has emerged as a primary downstream readout (other than direct transducer coupling or second messengers) for activation of GPCRs in cellular context. For example, it is considered a quintessential signature of βarr signaling and therefore, used extensively in the characterization of βarr-biased GPCR ligands (DeWire et al., 2007; Reiter et al., 2012). Although FRET based biosensors and ELISA-based approaches for detecting ERK1/2 phosphorylation and activation have been described in the literature (Harvey et al., 2008), Western blot assay has been the most widely used strategy due to its ease and availability of high-quality commercial antibodies. Here, we present a step-by-step protocol for the detection of ERK1/2 phosphorylation using a conventional Western blotting assay. The protocol presented below is based on the experiments carried out in our laboratory for a series of GPCRs (Ghosh et al., 2017; Kumari et al., 2016, 2017). Throughout the protocol, we have highlighted specific measures that should be taken to obtain reliable data, and at the end, we provide a list of potential problems and corresponding troubleshooting steps.
2. Materials
2.1. Cell Culture and DNA Transfection Related Reagents
HEK-293 cells American Type Culture Collection (ATCC)
Dulbecco’s minimum essential medium (DMEM)
Penicillin-Streptomycin (Pen-Strep)
Foetal Bovine Serum
10 × Phosphate-buffered saline
Trypsin-EDTA
Disposable Vacuum Filter Unit (0.2μm)
Tissue culture treated sterile plates (100 mm, 6 well), serological pipettes (5 mL, 10 mL, 25 mL), pipetting aid (also referred to as Steripet or pipette controller), Aerosol Barrier tips (1000 μL, 200 μL, 20 μL, 2 μL), Aspirator, Centrifuge tubes (15 mL and 50 mL), Microcentrifuge tubes (1.5 mL) and KimWipes
CO2 Incubator
Inverted Microscope (Leica)
Transfection reagent (Polyethyleneimine; PEI—linear MW 25000)
Plasmid DNA encoding the GPCR of interest
2.2. Western Blot Based ERK Assay
Cell scraper
2 × SDS-PAGE sample loading buffer (Tris, pH 6.8; Glycerol; Sodium Dodecyl Sulfate, Bromophenol blue, β-mercaptoethanol)
Dry heating bath
High speed centrifuge
Regular micro-tips (1000 μL, 200 μL, 20 μL)
Precast or manually prepared acrylamide gels for SDS-PAGE
Suitable power supply and SDS-PAGE apparatus
Protein molecular weight marker
Chemicals for different buffers (Tris-HCl, Glycine, Methanol, Tween-20, Hydrochloric acid)
Suitable power supply and Western blot apparatus for semidry transfer
PVDF membrane for protein transfer
Bovine Serum Albumin (BSA) for blocking step
Antiphopsho-ERK1/2 primary antibody (CST, catalogue number 9101)
Antitotal-ERK1/2 antibody (CST, catalogue number 9102)
Antirabbit IgG secondary antibody (Genscript, catalogue number A00098)
Enhanced chemi-luminescence (ECL) reagents
Chemi-Doc imaging system
3. Methods
3.1. Cell Culture
3.1.1. General guidelines for cell culture
It is important to maintain aseptic conditions during cell culture and hence, all procedures related to cell growth and transfection should be carried out inside a biological laminar flow cabinet. Use of personal protective equipments (PPE) in conjunction with biological cabinets can further minimize the chances of contamination. It is important to ensure that all supplies and reagents that come into contact with the cell cultures are sterile, and it is advisable to strictly follow the guidelines provided by ATCC for proper growth and maintenance of cells.
3.1.2. Preparation of growth media for HEK-293 cells
-
(1)
For the preparation of growth media, put Fetal Bovine Serum (FBS) and Pen-Strep overnight at 4°C or alternatively, incubate at 37°C in a water bath till it is completely thawed. ! CAUTION: Water in the water bath may be a potential source of contamination, and therefore, it should be changed on regular basis to avoid contamination. All the bottles (or containers) containing DMEM, FBS, Pen-Strep should be properly sprayed with 70% ethanol before they are taken inside the cell culture laminar flow cabinet.
-
(2)HEK-293 cells are typically cultured in Dulbecco’s modified Eagle’s media (DMEM) supplemented with 10% FBS and 1% Pen-Strep. For the preparation of 500 mL of complete DMEM media, FBS and Pen-Strep can be added as given in the below table.
Reagent Volume (ml) DMEM media 445 mL FBS 50 mL Pen-Strep 5 mL Once the complete DMEM media is prepared, it should be filtered using a 0.22μm filter unit and it can be subsequently stored at 4°C until further use. ! CAUTION: Before adding the FBS and Pen-Strep to DMEM, the bottles should be inverted and mixed properly. Improper mixing of FBS bottle in particular may result in the settling of serum constituents at the bottom of the bottle and it might affect the growth of the cells. It is advisable to use up the entire bottle of FBS once it is thawed because multiple freeze-thaw cycles may affect the stability of some of the constituents.
3.1.3. Growth and maintenance of HEK-293 cells
-
(1)
HEK-293 cells should be sub-cultured when they reach approximately 75–80% confluence. Aspirate the media gently from the cell culture plate and wash the cells with 2–3mL of 1 × PBS (prewarmed at 37°C). Washing the cells with PBS helps remove the residual serum and facilitates their detachment upon addition of Trypsin-EDTA. ! CAUTION: Add PBS gently from the sides of the plate in order to prevent detachment of the cells from the plate.
-
(2)
Add 2–3mL of 0.05% Trypsin-EDTA (1 ×) (prewarmed at 37°C) and incubate the plates at 37°C in the CO2 incubator for about 2–3 min. Afterwards, add 2–5 mL of prewarmed complete DMEM media to the cells, collect them in a Falcon tube, and resuspend the cells by gentle pipetting to obtain a homogeneous suspension. ! CAUTION: Do not incubate the cells in Trypsin-EDTA for more than 3–5 min as it may lyse the cells. Count the cells using either a hemocytometer or automated cell counter, whichever is available in the laboratory. For transfection next day, seed 2–2.5 × 106 cells in a 100 mm cell culture plate which typically yields 50–60% confluent cells optimal for PEI based DNA transfection. ! CAUTION: Seeding a fixed number of cells prior to transfection typically minimizes the experiment to experiment variation and ensures reproducibility of results.
3.2. Transfection of HEK-293 Cells Using Polyethyleneimine (PEI)
-
(1)
Weigh 100 mg of PEI linear and dissolve it in 90 mL of autoclaved Milli-Q water. A stirrer can be used to dissolve PEI properly. It may take 10–15 min for PEI powder to dissolve completely but there still might be some dissolved small particles left. HCl can be added drop-wise to dissolve the remaining PEI particles (by lowering the pH) followed by subsequent neutralization using NaOH until the pH is back to 7.0. Adjust the final volume to 100 mL by adding autoclaved Milli-Q water and filter the solution using a 0.22 μm filter. PEI solution can be stored at −20°C for up to about 6 months; however, if the transfections are to be carried out frequently, it can also be stored at 4°C for a couple of months to avoid freeze-thaw cycles. ! CAUTION: For adjusting the pH, add only a few drops of NaOH at a time and allow the solution to stir properly before checking the pH.
-
(2)
Aspirate the media from the cell culture plate, add 4–5 mL of DMEM (without FBS and Pen-Strep) and put it back into the CO2 incubator at 37°C. For a typical transfection in 100mm plate (at 50–60% confluency of cells), add 7 μg of DNA and 21 μL of PEI into 200–300 μL of DMEM, and incubate the mixture at room-temperature for 5–10 min. Subsequently, add this mixture to the cells in a drop wise manner. Approximately 6 h posttransfection, replace the media in cell culture plates with 6–10 mL of complete, and incubate the plates in the CO2 incubator for additional 18–20 h. After 24 h posttransfection, visualize the cells under the microscope for proper transfection (transfected cells typically exhibit different morphology compared to nontransfected cells), and seed approximately 1 × 106 cells per well in a six well cell culture plate following the same protocol as mentioned above in Section 3.1.3. Incubate the six well plates at 37°C in the CO2 incubator for an additional 24 h.
Notes
-
(A)
HEK-293 cells which are maintained under appropriate conditions and sub-cultured carefully exhibit better transfection efficiency and ERK response. Therefore, it is necessary to monitor the cells for their confluency, morphology and contaminations on regular basis. The confluency of cells should be in the range of 50–70% for transfection and it advisable to set-up transfection within 24 h of sub-culturing the cells.
-
(B)
Typically, a ratio of DNA:PEI is fixed at 1:3 (i.e., 7 μg of DNA and 21 μL of PEI); however, this can be further adjusted depending on the receptor if the expression level at this ratio is not optimal. Purity and integrity of the plasmid DNA is of critical importance and it should be carefully monitored by measuring the absorbance ratio at 260/280nm, and agarose gel electrophoresis.
3.3. Ligand Stimulation and Sample Preparation
-
(1)
In order to minimize the basal signal (i.e., basal level of ERK1/2 phosphorylation), a serum starvation step is typically used. For this, 48 h posttransfection aspirate the media from cells and add 1 mL of prewarmed DMEM to the wells and incubate the plates at 37°C in CO2 incubator for 4–12 h. Afterwards, stimulate the cells with the receptor ligands at desired concentrations for required time-points.
-
(2)
After ligand stimulation, place the cells in six well plate on ice, aspirate the media carefully and add 100 μL of 2 × SDS gel loading buffer to each well. Scrape the cells using a scraper, collect them in a microfuge tubes, and heat the samples at 95°C in a dry bath for 15 min followed by centrifugation at 10,000 rpm at room-temperature. The supernatant can be directly loaded on acrylamide gel for SDS-PAGE.
Notes
-
(A)
During media exchange, add the media gradually to prevent the detachment of cells. During sample preparation, wipe the scraper for different conditions with Milli-Q water or 70% ethanol to avoid any cross-contamination.
-
(B)
Duration of serum starvation can be optimized depending on the receptor. For some receptors (such as the human vasopressin receptor; V2R), even a serum starvation of 2 h is sufficient to obtain a high signal to noise ratio while for the others (such as β2 adrenergic receptor; β2AR), 8-12h of serum starvation yields a better profile.
-
(C)
For ligands that are prepared in organic solvent (e.g., DMSO), caution should be taken not to exceed the final concentration of DMSO above 0.01% as high concentrations may be toxic to the cells.
3.4. Western Blotting and Detection
-
(1)
For the detection of ERK1/2 phosphorylation, load 10–20 μL of the sample on SDS-PAGE gel and resolve the proteins by running the gel at 100–120 V. As the molecular weights of ERK1 and ERK2 are very close to each other (42kDa and 44kDa), it is important to run the gel long enough to visualize a clear separation. If prestained protein marker is being used, separation of different bands can be used as a proxy to roughly estimate the duration for running the gel.
-
(2)
Once proteins in the lysate are resolved by SDS-PAGE, rinse the gel in Milli-Q water to get rid of residual SDS as it can interfere with the protein transfer onto PVDF membrane followed by an additional wash in the transfer buffer. In the meantime, activate the PVDF membrane by incubating it in methanol for 2–5 min and then set-up the protein transfer on the membrane using semidry transfer assembly.
-
(3)
Once the protein transfer is done, incubate the PVDF membrane in the blocking buffer (5% BSA in 1 × TBST) for 1h at room-temperature on a rocking platform to block any nonspecific antibody binding sites on the membrane. The composition of 1 × TBST is—19 mM Tris-Cl, 137 mM NaCl, 0.1% Tween 20, pH 7.5. Subsequently, take the membrane out of the blocking buffer and incubate it with antiphospho-ERK1/2 antibody solution (1:5000–10,000 dilution) prepared in 5% BSA in TBST for 8–12h at 4°C.
-
(4)
After primary antibody incubation, wash the membrane three times (5–10 min each) with 1 × TBST under moderate shaking. Incubate the membrane with HRP-conjugated antirabbit secondary antibody (1,5000–10,000 dilution) for 1–2 h at room-temperature with moderate shaking. Subsequently, wash the membrane three times (5–10 min each) with 1 × TBST under moderate shaking and then add 1–2 mL of ECL substrate to cover the entire membrane. Capture the image with CCD camera attached to the ChemiDoc imaging system or using conventional X-ray film.
-
(5)
A key step in the Western blot based ERK assay is to probe the samples for total ERK1/2 using the same membrane as used for detecting phospho-ERK1/2 signal. For this, incubate the membrane (after phosph-ERK1/2 signal is captured) with 15–30 mL of stripping buffer (Glycine 200 mM, 0.1% SDS (w/v), 1% Tween 20 (v/v), β-Mercaptoethanol 0.08% (v/v) and pH adjusted to 2.2 with HCL) for 15–30 min followed by washing in 1 × TBST (three times, 5–10 min each) and blocking of nonspecific binding sites using 5% BSA in 1 × TBST for 1 h at room-temperature on a rocking platform. Subsequently, incubate the membrane with antitotal-ERK1/2 antibody (1:5000–10,000) for 2 h at room-temperature or 8–12 h at 4°C. Capture the signal following the protocol as described above for the phospho-ERK1/2.
-
(6)
Quantify the intensity for phospho-ERK1/2 and total-ERK1/2 bands using densitometry, plot the normalized data as phospho-ERK/total-ERK, and analyze using appropriate statistical measures.
Note
-
(A)
Transfer buffer should be prepared fresh with a final composition of 48 mM Tris-Cl, 39 mM Glycine, 20% Methanol, pH 9.2. Alternatively, a stock of 10 × transfer buffer can be prepared and stored without the methanol, and methanol can be added freshly just before setting up the transfer. Methanol helps in getting rid of any residual SDS bound to the gel, and enhances the ability of the proteins to bind the PVDF membrane, and therefore, it is a critical to activate the membrane with methanol prior to setting-up the protein transfer.
-
(B)
Nonfat dry milk powder should not be used for blocking the nonspecific binding sites on PVDF membrane as casein present in milk powder may result in high background signal. Membrane should be washed carefully and thoroughly to minimize the background signal, and the incubation with HRP-coupled secondary antibody should preferably be carried in dark. pH of the buffers should be carefully measured just before the experiment.
-
(C)
Phospho-ERK1/2 antibody may be used more than once, and therefore, it can be stored at 4°C with 0.02% NaN3 for a month or so.
4. Troubleshooting Steps
| Potential problems | Possible cause(s) | Suggested solutions |
|---|---|---|
| Cells do not look transfected or they appear over-confluent even after transfection. | Low transfection efficiency. | Optimize DNA: PEI ratio, check the integrity and purity of DNA, and consider preparing fresh PEI if the stock is very old. |
| High signal even at '0 min' stimulation. | High level of basal activity of the receptor. | Increase the duration of serum starvation (e.g., from 6 h to 12 h). Phosphorylation of ERK1/2 may even be triggered by mechanical stress. Handle the six well plates very gently during stimulation and sample harvesting. Consider optimizing the expression level of the receptor. |
| No difference is observed between stimulated and unstimulated conditions. | Ligand used for stimulation may not be working properly. | Consider preparing a fresh stock of the ligand and optimize the concentration of the ligand used for stimulation. |
| No signal is observed on the blot or only very weak signals are observed. | Phospho-ERK1/2 antibody may have been exhausted due to multiple usages or there may be a problem during protein transfer on PVDF membrane. | Prepare fresh stock of the antibody at appropriate dilution and use the transfer of prestained protein marker bands as a proxy for protein transfer. Alternatively, the membrane can be incubated with Ponceau stain to visualize the protein transfer. It is important to remove any residual SDS from the gel with thorough washing before setting-up the transfer. |
| There is a high background throughout the membrane. | The blocking and washing steps may have been suboptimal. | Prepare fresh blocking solution and increase the washing time (e.g., from 5 min to 10 min). |
| The pattern for total-ERK1/2 is identical to phospho-ERK1/2. | The membrane may have not been stripped properly or the total-ERK1/2 antibody is not working. | Ensure that the pH of the stripping buffer is 2.2 and consider repeating the stripping step twice. Stripping buffer may also be warmed-up slightly before the use. Prepare fresh stock of total-ERK1/2 antibody at appropriate dilution. |
5. Expected Result
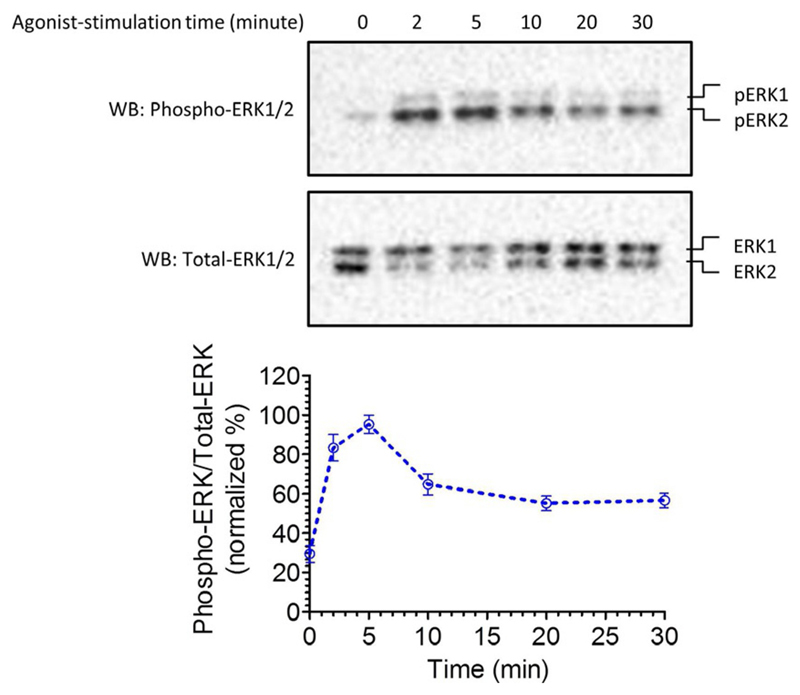
A typical ERK1/2 phosphorylation profile upon stimulation of HEK-293 cells expressing V2R with AVP, and its densitometry-based quantification is presented in Fig. 2.
Fig. 2.
A typical example of ERK MAP kinase activation upon stimulation of β2V2R. Agonist-stimulation results in robust ERK1/2 phosphorylation in the time-frame of 2–5 min which subsequently declines at later time-points. The lower panel represents densitometry-based quantification and normalization of the data.
This figure is adapted from a previously published paper from our own laboratory Kumari, P., Srivastava, A., Banerjee, R., Ghosh, E., Gupta, P., Ranjan, R., et al. (2016). Functional competence of a partially engaged GPCR-beta-arrestin complex. Nature Communications, 7, 13416.
6. Additional Notes
-
(i)
The levels of phospho-ERK detected by Western blotting differ significantly for different GPCRs and perhaps reflect their coupling efficiency with G-proteins and βarrs.
-
(ii)
The protocol presented here can also be adapted to measure specific contribution of G-proteins and βarrs, for example, by using specific inhibition (using chemical inhibitors) or depletion (using siRNA) of the respective transducers.
Acknowledgment
The research program in our laboratory is supported by the DBT Wellcome Trust India Alliance (Intermediate Fellowship to AKS—IA/I/14/1/501285), Department of Biotechnology, Government of India (Innovative Young Biotechnologist Award to AKS—BT/08/IYBA/2014-3), LADY TATA Memorial Trust (Young Researcher Award to AKS), Science and Engineering Research Board (SERB) (SB/SO/BB-121/2013), Council of Scientific and Industrial Research (CSIR) (37[1637]14/EMR-II). AKS is an EMBO Young Investigator. MB and HD are supported by the National Postdoctoral Fellowships from SERB (PDF/2016/002930 and PDF/2016/002893).
References
- Ahn S, Shenoy SK, Wei H, Lefkowitz RJ. Differential kinetic and spatial patterns of beta-arrestin and G protein-mediated ERK activation by the angiotensin II receptor. The Journal of Biological Chemistry. 2004;279:35518–35525. doi: 10.1074/jbc.M405878200. [DOI] [PubMed] [Google Scholar]
- Bockaert J, Pin JP. Molecular tinkering of G protein-coupled receptors: An evolutionary success. The EMBO Journal. 1999;18:1723–1729. doi: 10.1093/emboj/18.7.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Iverson TM, Gurevich VV. Structural basis of arrestin-dependent signal transduction. Trends in Biochemical Sciences. 2018;43:412–423. doi: 10.1016/j.tibs.2018.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWire SM, Ahn S, Lefkowitz RJ, Shenoy SK. Beta-arrestins and cell signaling. Annual Review of Physiology. 2007;69:483–510. doi: 10.1146/annurev.physiol.69.022405.154749. [DOI] [PubMed] [Google Scholar]
- Dwivedi H, Baidya M, Shukla AK. GPCR signaling: The interplay of Galphai and beta-arrestin. Current Biology. 2018;28:R324–R327. doi: 10.1016/j.cub.2018.02.027. [DOI] [PubMed] [Google Scholar]
- Ghosh E, Nidhi K, Shukla AK. SnapShot: GPCR-ligand interactions. Cell. 2014;159:1712, e1711. doi: 10.1016/j.cell.2014.12.008. [DOI] [PubMed] [Google Scholar]
- Ghosh E, Srivastava A, Baidya M, Kumari P, Dwivedi H, Nidhi K, et al. A synthetic intrabody-based selective and generic inhibitor of GPCR endocytosis. Nature Nanotechnology. 2017;12:1190–1198. doi: 10.1038/nnano.2017.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundmann M, Kostenis E. Temporal Bias: Time-encoded dynamic GPCR signaling. Trends in Pharmacological Sciences. 2017;38:1110–1124. doi: 10.1016/j.tips.2017.09.004. [DOI] [PubMed] [Google Scholar]
- Gurevich VV, Gurevich EV. GPCRs and signal transducers: Interaction stoichiometry. Trends in Pharmacological Sciences. 2018;39:672–684. doi: 10.1016/j.tips.2018.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurevich VV, Gurevich EV, Uversky VN. Arrestins: Structural disorder creates rich functionality. Protein & Cell. 2018;9:1–18. doi: 10.1007/s13238-017-0501-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey CD, Ehrhardt AG, Cellurale C, Zhong H, Yasuda R, Davis RJ, et al. A genetically encoded fluorescent sensor of ERK activity. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:19264–19269. doi: 10.1073/pnas.0804598105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari P, Srivastava A, Banerjee R, Ghosh E, Gupta P, Ranjan R, et al. Functional competence of a partially engaged GPCR-beta-arrestin complex. Nature Communications. 2016;7 doi: 10.1038/ncomms13416. 13416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari P, Srivastava A, Ghosh E, Ranjan R, Dogra S, Yadav PN, et al. Core engagement with beta-arrestin is dispensable for agonist-induced vasopressin receptor endocytosis and ERK activation. Molecular Biology of the Cell. 2017;28:1003–1010. doi: 10.1091/mbc.E16-12-0818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefkowitz RJ, Shenoy SK. Transduction of receptor signals by beta-arrestins. Science. 2005;308:512–517. doi: 10.1126/science.1109237. [DOI] [PubMed] [Google Scholar]
- Luttrell LM, Lefkowitz RJ. The role of beta-arrestins in the termination and transduction of G-protein-coupled receptor signals. Journal of Cell Science. 2002;115:455–465. doi: 10.1242/jcs.115.3.455. [DOI] [PubMed] [Google Scholar]
- Perez-Aso M, Segura V, Monto F, Barettino D, Noguera MA, Milligan G, et al. The three alpha1-adrenoceptor subtypes show different spatio-temporal mechanisms of internalization and ERK1/2 phosphorylation. Biochimica et Biophysica Acta. 2013;1833:2322–2333. doi: 10.1016/j.bbamcr.2013.06.013. [DOI] [PubMed] [Google Scholar]
- Ranjan R, Dwivedi H, Baidya M, Kumar M, Shukla AK. Novel structural insights into GPCR-beta-arrestin interaction and signaling. Trends in Cell Biology. 2017;27:851–862. doi: 10.1016/j.tcb.2017.05.008. [DOI] [PubMed] [Google Scholar]
- Ranjan R, Gupta P, Shukla AK. GPCR signaling: Beta-arrestins kiss and remember. Current Biology. 2016;26:R285–R288. doi: 10.1016/j.cub.2016.02.056. [DOI] [PubMed] [Google Scholar]
- Reiter E, Ahn S, Shukla AK, Lefkowitz RJ. Molecular mechanism of beta-arrestin-biased agonism at seven-transmembrane receptors. Annual Review of Pharmacology and Toxicology. 2012;52:179–197. doi: 10.1146/annurev.pharmtox.010909.105800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segura V, Perez-Aso M, Monto F, Carceller E, Noguera MA, Pediani J, et al. Differences in the signaling pathways of alpha(1A)- and alpha(1B)-adrenoceptors are related to different endosomal targeting. PLoS One. 2013;8:e64996. doi: 10.1371/journal.pone.0064996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy SK, Drake MT, Nelson CD, Houtz DA, Xiao K, Madabushi S, et al. Beta-arrestin-dependent, G protein-independent ERK1/2 activation by the beta2 adrenergic receptor. The Journal of Biological Chemistry. 2006;281:1261–1273. doi: 10.1074/jbc.M506576200. [DOI] [PubMed] [Google Scholar]
- Shukla AK, Singh G, Ghosh E. Emerging structural insights into biased GPCR signaling. Trends in Biochemical Sciences. 2014;39:594–602. doi: 10.1016/j.tibs.2014.10.001. [DOI] [PubMed] [Google Scholar]
- Shukla AK, Xiao K, Lefkowitz RJ. Emerging paradigms of beta-arrestin-dependent seven transmembrane receptor signaling. Trends in Biochemical Sciences. 2011;36:457–469. doi: 10.1016/j.tibs.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava A, Gupta B, Gupta C, Shukla AK. Emerging functional divergence of beta-arrestin isoforms in GPCR function. Trends in Endocrinology and Metabolism. 2015;26:628–642. doi: 10.1016/j.tem.2015.09.001. [DOI] [PubMed] [Google Scholar]
- Vass M, Kooistra AJ, Yang D, Stevens RC, Wang MW, de Graaf C. Chemical diversity in the G protein-coupled receptor superfamily. Trends in Pharmacological Sciences. 2018;39:494–512. doi: 10.1016/j.tips.2018.02.004. [DOI] [PubMed] [Google Scholar]
- Weis WI, Kobilka BK. The molecular basis of G protein-coupled receptor activation. Annual Review of Biochemistry. 2018;87:897–919. doi: 10.1146/annurev-biochem-060614-033910. [DOI] [PMC free article] [PubMed] [Google Scholar]