Abstract
The most common causes of congenital myasthenic syndromes (CMS) are CHRNE mutations, and some pathogenic allelic variants in this gene are especially frequent in certain ethnic groups. In the southern region of Brazil, a study found the c.130dupG CHRNE mutation in up to 33% of families with CMS. Here, we aimed to verify the frequency of this mutation among individuals with CMS in a larger cohort of CMS patients from different areas of Brazil and to characterize clinical features of these patients. Eighty-four patients with CMS, from 72 families, were clinically evaluated and submitted to direct sequencing of the exon 2 of CHRNE. The c.130dupG mutation was found in 32 patients (23 families), with 26 patients (19 families, 26.3%) in homozygosis, confirming its high prevalence in different regions of Brazil Among the homozygous patients, the following characteristics were frequent: onset of symptoms before 2 years of age (92.3%), little functional restriction (92.3%), fluctuating symptoms (100%), ocular muscle impairment (96.1%), ptosis (100%), limb weakness (88.4%), response to pyridostigmine (100%), facial involvement (77%), and bulbar symptoms (70.8%). The pretest probability of finding at least one allele harbouring the c.130dupG mutation was 38.1%. Selecting only patients with impaired eye movement together with limb weakness and improvement with pyridostigmine, the probability increases to 72.2%. This clinical pre-selection of patients is likely a useful tool for regions where CHRNE mutations have a founder effect. In conclusion, the CHRNE mutation c.130dupG leads to fairly benign natural course of the disease with relative homogeneity.
Keywords: Congenital Myasthenic Syndrome, Neuromuscular Junction, CHRNE, Acetylcholine receptor, pyridostigmine
Introduction
Congenital myasthenic syndromes (CMS) are a group of genetic disorders in which the safety factor of the neuromuscular transmission is impaired. The phenotype is variable, making diagnosis difficult [1,2]. The increasing number of CMS causative genes adds to the difficulty of establishing the correct molecular diagnosis, and knowing the exact type of CMS has implications for appropriate care of the patients [1,3]. However, CHRNE mutations account for up to 50% of all CMS [3,4]. Other genes that more commonly lead to CMS are DOK7, COLQ, and RAPSN [5], but the frequency of the affected genes varies according to the studied geographic region and population [6,7].
Although there are several CHRNE mutations described, some pathogenic allelic variants are especially frequent in certain ethnic groups [8,9]. Nevertheless, in these cases the diagnosis can also be difficult due phenotypic variability, as some CHRNE mutations can present a high clinical heterogeneity, even when present in homozygous state [10]. In Brazil, a study with patients with CMS from the state of Paraná (southern part of the country) found the c.130dupG mutation in the CHRNE gene present in 6 out of 18 families [6]. Those patients frequently had ophthalmoplegia, palpebral ptosis, dysphagia, and proximal muscle weakness with onset before 2 years old, which are expected characteristics, considering the affected gene [1,2,3]. In the present study we aimed to verify the frequency of this mutation among individuals with CMS from different areas of Brazil and to characterize clinical features of these patients.
Patients and Methods
Eighty-four patients with CMS (49 females, 35 males) from 72 unrelated families, aged from 3 to 65 years at the time of clinical evaluation, were recruited. The parents or the patients were invited to participate in the study after informed consent was obtained, and the study was approved by the local ethics committee. The patients were evaluated at our centre in São Paulo, Hospital das Clínicas/USP (n = 61); in Salvador, at Hospital das Clínicas/UFBA (n = 2); and in Ribeirão Preto, at Hospital das Clínicas/USPRP (n = 21).
Patients were evaluated by an experienced neurologist. Data included medical history and general physical and neurological examinations. Results of ancillary exams such as muscle biopsy, nerve conduction studies and electromyography (NCS/EMG), when available, were noted. To detect the mutation c.130dupG in CHRNE (NM_000080.3), a fragment containing whole exon 2 was amplified by PCR for Sanger sequencing. Patients heterozygous for the c.130dupG mutation were screened for mutations in the whole coding CHRNE gene using primers covering all exons and splice junctions.
Clinical findings were compared only between the groups of homozygous patients for c.130dupG and the group without the mutation, with the objective to identify clinical clues for the c.130dupG mutation. Fisher's exact test was used for the comparison.
Results
Average age at assessment of all CMS patients was 24.6 years and 24.15 years among the homozygous CHRNE c.130dupG patients. The CHRNE mutation was found in 32 patients (38.1% of the patients) from 23 different families (31.9% of the families) including 26 patients (19 families) in homozygous state (table 1). From 6 heterozygous patients, another pathogenic mutation was found in 5 (table 1). Families had segregation analysis performed, confirming compound heterozygous status. Clinical data of patients carrying the CHRNE mutation are shown in table 1.
Table 1.
Main clinical data from patients with the CHRNE c.130dupG mutation + = present; - = absent; nd = no data or not tested; EO = early onset; IP = improvement with pyridostigmine; FS = fluctuating symptoms; IEM = imparied eye movement; PIE = partially impaired eye movement; SRI= impaired eye movement sparing rectus inferior muscles; PP = ptosis; LW = limb weakness; BS = bulbar symptoms; FI = facial involvement; NPC = no progressive course; Variants found associated with c.130dupG: c.803-2A>G (previously reported as pathogenic [11]); c.858_859dup (p.leu287ser fs*14, novel); c.422T>A (p.pro141.leu, previously reported as pathogenic [12]).
| Patient / Family | Age (years)/Sex | EO | IP | FS | IEM/ PIE/ SRI | PP | LW | BS | FI | NPC | Mutation state |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1/1 | 30/M | + | + | + | +/+/+ | + | + | - | + | + | c.130dupG/c.130dupG |
| 2/1 | 29/F | + | + | + | +/+/+ | + | + | + | + | + | c.130dupG/c.130dupG |
| 3/1 | 12/M | + | + | + | +/+/+ | + | + | + | - | + | c.130dupG/c.130dupG |
| 6/3 | 28/F | + | + | + | +/+/+ | + | - | - | - | + | c.130dupG/c.130dupG |
| 7/3 | 19/F | + | + | + | +/-/- | + | + | - | - | + | c.130dupG/c.130dupG |
| 8/4 | 13/F | + | + | + | +/+/- | + | + | + | - | + | c.130dupG/c.130dupG |
| 9/5 | 32/M | + | + | + | +/+/- | + | + | + | + | + | c.130dupG/- |
| 18/14 | 12/M | + | + | + | +/+/- | + | + | + | + | + | c.130dupG/c.130dupG |
| 20/16 | 37/F | + | + | + | +/+/+ | + | + | + | + | + | c.130dupG/c.130dupG |
| 21/17 | 10/M | + | + | + | +/+/+ | + | + | + | + | - | c.130dupG/c.130dupG |
| 25/21 | 25/F | + | + | + | +/+/+ | + | + | + | + | + | c.130dupG/c.130dupG |
| 27/23 | 4/F | + | + | + | +/-/- | + | - | + | + | + | c.130dupG/c.130dupG |
| 29/25 | 34/F | + | + | + | +/+/+ | + | + | + | + | + | c.130dupG/c.130dupG |
| 34/29 | 14/F | + | + | + | +/+/+ | + | + | + | + | + | c.130dupG/c.130dupG |
| 40/34 | 64/M | + | + | + | +/+/+ | + | + | + | + | + | c.130dupG/c.803-2A>G |
| 41/34 | 54/F | + | + | + | +/+/+ | + | + | + | + | + | c.130dupG/c.803-2A>G |
| 42/35 | 8/F | + | + | + | +/+/+ | + | + | + | + | + | c.130dupG/c.130dupG |
| 43/36 | 16/F | + | + | + | +/+/+ | + | + | + | + | + | c.130dupG/c.858_859dup |
| 44/36 | 7/M | + | + | + | +/+/+ | + | - | - | + | + | c.130dupG/c.858_859dup |
| 49/41 | 38/F | + | + | + | +/+/+ | + | + | - | + | + | c.130dupG/c.130dupG |
| 50/42 | 21/F | + | + | + | +/+/+ | + | + | + | + | + | c.130dupG/c.130dupG |
| 51/42 | 28/F | + | + | + | +/+/+ | + | + | + | + | + | c.130dupG/c.130dupG |
| 60/51 | 43/F | + | + | + | -/-/- | + | + | + | - | + | c.130dupG/c.130dupG |
| 64/55 | 34/M | - | + | + | +/nd/nd | + | + | - | + | + | c.130dupG/c.130dupG |
| 65/55 | 38/F | + | + | + | +/nd/nd | + | + | + | + | - | c.130dupG/c.130dupG |
| 66/56 | 18/F | + | + | + | +/nd/nd | + | + | - | + | + | c.130dupG/c.130dupG |
| 67/57 | 27/F | + | + | + | +/nd/nd | + | + | + | + | + | c.130dupG/c.130dupG |
| 68/58 | 34/M | + | + | + | +/nd/nd | + | + | + | + | + | c.130dupG/c.130dupG |
| 69/58 | 19/M | + | + | + | +/nd/nd | + | + | - | - | + | c.130dupG/c.130dupG |
| 70/59 | 19/F | + | nd | + | +/nd/nd | + | - | nd | + | + | c.130dupG/c.130dupG |
| 71/59 | 34/M | - | nd | + | +/nd/nd | + | + | nd | + | + | c.130dupG/c.130dupG |
| 72/60 | 5/F | + | + | + | +/nd/nd | + | + | + | + | + | c.130dupG/c.422T>A |
Among the homozygous c.130dupG mutation patients (n = 26), the following characteristics were very frequent: onset of symptoms before 2 years of age (92.3%); fluctuating symptoms (100%); ocular muscle impairment (96.1%); ptosis (100%); improvement with pyridostigmine (100%); limb weakness (88.4%); facial involvement (77%); and bulbar symptoms (70.8%). Delayed motor milestones were observed in 56.5% of the homozygous c.130dupG patients, neonatal hypotonia in 54.1%, breastfeeding difficulties in 55%, high arched palate in 66.6%, and scoliosis in 38.4%. No CHRNE c.130dupG patients presented with any congenital contractures. Twenty-two out of the 26 homozygous patients had available data of Electromiography/Nerve conduction study: 15 (68.1%) showed decremental response to 3 Hz repetitive stimulus (DRRS). Of the seven patients without DRRS, three were submitted to single fibre study, which demonstrated increased jitter in all.
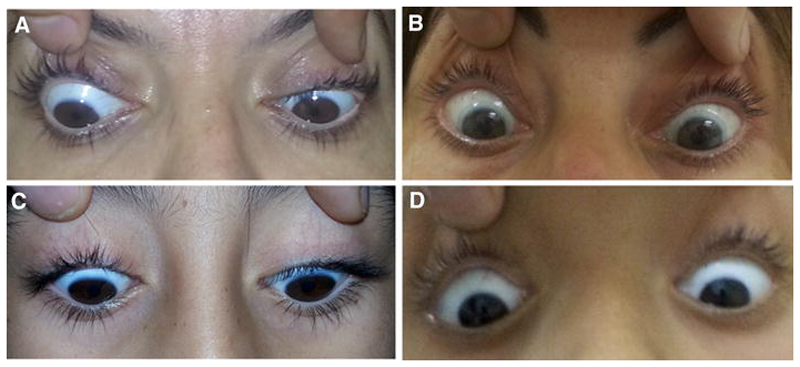
Interestingly, of the CMS patients with ocular movement impairment, most of them presented with partial, instead of complete, impairment. That was the case in 88% of the patients homozygous for the CHRNE mutation and 87% of the patients without the mutation, considering patients with ocular movement impairment. In particular, the rectus inferior muscles were the most frequently spared, being not affected or relatively less affected in 73.9% and 76.4% of the patients with and without the c.130dupG mutation, respectively (figure 1).
Figure. Relative sparing of the muscle rectus inferior among patients with partial ophtalmoparesis.
Patients presented with different levels of asymmetrical impairment of ocular movements, which are not related to age. A. Patient 6 (28 years old). B. Patient 20 (37 years old). C. Patient 42 (8 years old. D. Patient 21 (10 years old)
Comparing the patients with homozygous c.130dupG mutations to those without, the following clinical findings were statistically more frequent in the homozygous c.130dupG mutation group: impaired eye movement (p = 0.0002), facial involvement (p = 0.01), limb weakness (p = 0.03), and good response to pyridostigmine (p = 0.0001), type II selective atrophy on muscle biopsy (p = 0.0089). Frequency of clinical characteristics of all patients are shown on table 2.
Table 2.
Frequency of clinical features of patients with Congenital Myasthenic Syndrome
| Clinical Features | Frequency (%) in the group with homozygous c.130dupG mutation | Frequency (%) in the group without c.130dupG mutation |
|---|---|---|
| Early onset of symptoms | 92.3 | 75 |
| Fuctuation of symptoms | 100 | 86.5 |
| Ocular muscle impairment/ partial /sparing muscle rectus inferior | 96.1 / 83.3 / 72.2 | 55.7 / 43.4 / 36.9 |
| Eyelid ptosis | 100 | 86.5 |
| Limb weakness | 88.4 | 63.4 |
| Bulbar symptoms | 70.8 | 50 |
| Facial weakness | 77 | 52 |
| Little functional restriction | 92.6 | 80.7 |
| Exacerbations crisis: At least one episode/more than 2 per year | 30.7 / 7.7 | 21.1 / 7.6 |
| Delayed motor milestones | 56.5 | 53.8 |
| Neonatal hypotonia | 54.1 | 50 |
| High arched palate | 66.6 | 59.6 |
| Breastfeeding difficulties | 55 | 27.5 |
| Scoliosis | 38.4 | 28.8 |
| Congenital Contractures | 0 | 15 |
| Response to pyridostigmine | 100 | 54.1 |
| Response to adding β2-agonists: Ephedrine/salbuthamol | 100 / 100 | 100 / 100 |
| with type II selective atrophy on muscle biopsy | 66.6 | 20 |
| Electromyography and nerve conduction study: decremental response to repetitive stimulus / single fibre study whith increased jitter | 68.1 / 100 | 48.9 / 54.5 |
The pretest probability among our cohort of finding at least one allele harbouring the CHRNE c.130dupG mutation was 38.1%. Selecting only patients with impaired eye movement who improved with pyridostigmine (n = 47), the probability of finding at least one allele mutated increased to 61.7% (29/47, p = 0.0001). In patients with impaired eye movement and limb weakness who improved with pyridostigmine (n = 36), the probability of finding at least one allele with the mutation was 72.2% (26/36, p = 0.0001).
Regarding the distribution of the mutation in the country, 27 patients (22 homozygous) of 19 families were from the state of São Paulo (33.9% of São Paulo families), 4 patients (3 homozygous) of 4 families were from the state of Minas Gerais (75% of Minas Gerais families), and 1 patient (homozygous) was from the state of Bahia (incidence of 20% of Bahia families).
Discussion
CHRNE c.130dupG (p.Glu44Glyfs*3) is a known pathogenic variant [13], with an allele frequency on GenomAD of 0.00010, that leads to a lack of adult form acetylcholine receptor (AChR) on the post-synaptic membrane [14,15] and consequently impairs neuromuscular transmission. This mutation has been repeatedly identified in patients with Spain and Portugal origin [16], and was present in 6.4% of CMS patients on a Spanish cohort [17]. Mihaylova et al. showed a higher prevalence of this mutation in CMS Brazilian patients from the state of Paraná [6]. In our cohort, the mutation was found among patients from other regions of the country, confirming the high prevalence of the mutation across different regions of Brazil.
We found a pretest probability of the c.130dupG mutation of 31.9% in at least one allele of CMS patients, and when considering only homozygous patients the percentage is still high (26.4%). The percentages notably increase if we consider only patients with impaired eye movement and improvement of symptoms with pyridostigmine. This may be driven by the likely exclusion of RAPSN and DOK7 cases by this selection, as these genetic entities are more likely to not have those clinical features. In addition, for patients with impaired eye movement as well as with limb weakness and improvement with pyridostigmine, the percentage of patients with the CHRNE c.130dupG mutation is even higher (72.2% and 58.3% for one and two alleles). This extra effect can be explained by the exclusion of patients with pure ocular symptoms, which seem to have a low probability to be genetically solved [4].
The clinical characteristics of the c.130dupG patients represent a good prognosis, and are quite similar to those bearing other CHRNE mutations that lead to AChR deficiency [8,15] although some clinical features seem to be strongly linked to this specific mutation. In general, the clinical presentation of the homozygous CHRNE c.130dupG patients was fairly homogenous, contrary to the great clinical variability shown in other CMS syndromes with other homozygous mutations [10, 18,19]. One possible explanation for this difference could be the fact that the c.130dupG mutation occurs in an early portion of the expressing gene, likely resulting in a null mutation. This might lead to stronger stimulus for compensating factors compared to, for instance, more C-terminal frameshift mutations, which may result in expression of dysfunctional proteins. Therefore, in this case, patients with dysfunctional epsilon subunit would depend on other mechanisms to trigger compensating factors (which in turn might lead to more variable compensation) in comparison with patients with no epsilon subunit at all.
Interestingly, most of the patients presented with partial instead of complete ocular movement impairment, a characteristic not explored in other clinical studies. The ocular muscle most frequently spared was the rectus inferior, which might indicate that it tends to be the latest affected, once virtually every ocular muscle tends to be eventually paretic. This finding was similar in the groups with and without the c.130dupG mutation, indicating it is probably a general CMS feature rather than specific for CHRNE. It would be interesting to compare this feature with other diseases, and see, for instance, if it can help to clinically differentiate CMS from mitochondrial myopathy and congenital myopathy, which sometimes can be difficult to do in clinical practice [20]. Regarding therapeutics, we verified a high rate of response to anticholinesterase medication and an additional benefit to β2-agonists among the homozygous CHRNE c.130dupG patients. Most likely, this may not be specific for the c.130dupG mutation, as β2-agonists were shown to be beneficial to patients with AChR deficiency as an add-on therapy [21].
The mutation c.130dupG in the CHRNE gene seems to have a high prevalence in different regions of Brazil, and to lead to expected CHRNE-related characteristics, with relative homogeneity and a fairly benign natural course of the disease. Careful selection of patients with a few simple clinical features can lead to a percentage as high as 72.2% of patients with the mutation, which could be a useful tool for the first screening of CMS patients, including in different countries where other CHRNE mutations also have a founder effect.
Acknowledgements
HL received funding from the Medical Research Council (MRC) Centre for Neuromuscular Diseases UK (reference G1002274, grant ID 98482), by the MRC (grant ID G0900872), the Wellcome Trust, and the European Commission through the projects NeurOmics (No. 305121) and RD-Connect (No. 305444).
Footnotes
Ethical Standards
All human studies have been approved by the locals ethics committee and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki.
Conflicts of interest
The authors declare that they have no conflict of interest.
References
- 1.Engel A, Shen X-M, Secen D, Sine S. Congenital myasthenic syndromes: pathogenesis, diagnosis, and treatment. Lancet Neurology. 2015;14:421–434. doi: 10.1016/S1474-4422(15)00010-1. [DOI] [PubMed] [Google Scholar]
- 2.Finlayson S, Beeson D, Palace J. Congenital myasthenic syndromes: an update. Practical Neurology. 2013;13:80–91. doi: 10.1136/practneurol-2012-000404. [DOI] [PubMed] [Google Scholar]
- 3.McMacken G, Abicht A, Evangelista T, Spendiff S, Lochmüller H. The Increasing Genetic and Phenotypical Diversity of Congenital Myasthenic Syndromes. Neuropediatrics. 2017;48:294–308. doi: 10.1055/s-0037-1602832. [DOI] [PubMed] [Google Scholar]
- 4.Abicht A, Dusl M, Gallenmüller C, et al. Congenital myasthenic syndromes: achievements and limitations of phenotype-guided gene-after-gene sequencing in diagnostic practice: a study of 680 patients. Human Mutation. 2012;33:1474–1484. doi: 10.1002/humu.22130. eat al. [DOI] [PubMed] [Google Scholar]
- 5.Abicht A, Müller JS, Lochmüller H. Congenital Myasthenic Syndromes. In: Adam MP, Ardinger HH, Pagon RA, et al., editors. GeneReviews®. Seattle (WA): University of Washington, Seattle; 2003. May 9, [acessed 10/10/2017]. 1993-2017. [Updated 2016 Jul 14]. [Internet] [Google Scholar]
- 6.Mihaylova V, Scola RH, Gervini B, et al. Molecular characterisation of congenital myasthenic syndromes in Southern Brazil. Journal of Neurology Neurosurgery and Psychiatry. 2010;81:973–977. doi: 10.1136/jnnp.2009.177816. [DOI] [PubMed] [Google Scholar]
- 7.Aharoni S, Sadeh M, Shapira Y, et al. Congenital myasthenic syndrome in Israel: Genetic and clinical characterization. Neuromuscular Disorders. 2017;27:136–140. doi: 10.1016/j.nmd.2016.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abicht A, Stucka R, Karcagi V, et al. A common mutation (epsilon1267delG) in congenital myasthenic patients of Gypsy ethnic origin. Neurology. 1999;53:1564–1569. doi: 10.1212/wnl.53.7.1564. [DOI] [PubMed] [Google Scholar]
- 9.Beeson D, Hantai D, Lochmuller H, Engel AG. 126th International Workshop: congenital myasthenic syndromes, 24-26 September 2004, Naarden, the Netherlands. Neuromuscular Disorders. 2005;15:498–512. doi: 10.1016/j.nmd.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 10.Natera-de Benito D, Domínguez-Carral J, Muelas N, Nascimento A, Ortez C, Jaijo T, Arteaga R, Colomer J, Vilchez JJ. Phenotypic heterogeneity in two large Roma families with a congenital myasthenic syndrome due to CHRNE 1267delG mutation. A long-term follow-up. Neuromuscul Disord. 2016;26(11):789–795. doi: 10.1016/j.nmd.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 11.Barisic N, Schmidt C, Sidorova OP, et al. Congenital myasthenic syndrome (CMS) in three European kinships due to a novel splice mutation (IVS7-2A/G) in the epsilon acetylcholine receptor (AChR) subunit gene. Neuropediatrics. 2002;33:249–254. doi: 10.1055/s-2002-36738. [DOI] [PubMed] [Google Scholar]
- 12.Ohno K, Wang HL, Milone M, et al. Congenital myasthenic syndrome caused by decreased agonist binding affinity due to a mutation in the acetylcholine receptor epsilon subunit. Neuron. 1996;17:157–70. doi: 10.1016/s0896-6273(00)80289-5. [DOI] [PubMed] [Google Scholar]
- 13.Ohno K, Anlar B, Ozdirim E, Brengman JM, DeBleecker JL, Engel AG. Myasthenic Syndromes in Turhsh Kinships Due to Mutations in the Acetylcholine Receptor. Annals of Neurology. 1998;44:234–41. doi: 10.1002/ana.410440214. [DOI] [PubMed] [Google Scholar]
- 14.Croxen R, Vincent A, Newsom-Davis J, Beeson D. Myasthenia gravis in a women with congenital AChR deficiency due to epislon-subunit mutations. Neurology. 2002;58:1563–1565. doi: 10.1212/wnl.58.10.1563. [DOI] [PubMed] [Google Scholar]
- 15.Burke G, Cossins J, Maxwell S, et al. Distinct phenotypes of congenital acetylcholine receptor deficiency. Neuromuscular Disorders. 2004;14:356–364. doi: 10.1016/j.nmd.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 16.Beeson D, Hantaï D, Lochmüller H, Engel AG. 126th International Workshop: congenital myasthenic syndromes, 24-26 September 2004, Naarden, the Netherlands. Neuromuscul Disord. 2005;15(7):498–512. doi: 10.1016/j.nmd.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 17.Natera-de Benito D, Töpf A, Vilchez JJ, González-Quereda L, Domínguez-Carral J, Díaz-Manera J, Ortez C, Bestué M, Gallano P, Dusl M, Abicht A, et al. Molecular characterization of congenital myasthenic syndromes in Spain. Neuromuscular Disorders. 2017;27:1087–1098. doi: 10.1016/j.nmd.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 18.Mihaylova V, Müller JS, Vilchez JJ, et al. Clinical and molecular genetic findings in COLQ-mutant congenital myasthenic syndromes. Brain. 2008;131:747–759. doi: 10.1093/brain/awm325. [DOI] [PubMed] [Google Scholar]
- 19.Müller JS, Mildner G, Müller-Felber W, et al. Rapsyn N88K is a frequent cause of congenital myasthenic syndromes in European patients. Neurology. 2003;60:1805–1810. doi: 10.1212/01.wnl.0000072262.14931.80. [DOI] [PubMed] [Google Scholar]
- 20.Neto O Abath, Heise CO, Moreno CA, et al. Nonlethal CHRNA1-Related Congenital Myasthenic Syndrome with a Homozygous Null Mutation. The Canadian Journal of Neurological Sciences. 2017;44:125–127. doi: 10.1017/cjn.2016.322. [DOI] [PubMed] [Google Scholar]
- 21.Rodríguez Cruz PM, Palace J, Ramjattan H, Jayawant S, Robb SA, Beeson D. Salbutamol and ephedrine in the treatment of severe AChR deficiency syndromes. Neurology. 2015;85:1043–1047. doi: 10.1212/WNL.0000000000001952. [DOI] [PMC free article] [PubMed] [Google Scholar]