Abstract
The dentate gyrus (DG) has a key role in hippocampal memory formation. Intriguingly, DG lesions impair many, but not all hippocampus-dependent mnemonic functions, indicating that the rest of the hippocampus (CA1-CA3) can operate autonomously under certain conditions. An extensive body of theoretical work has proposed how the architectural elements and various cell types of the DG may underlie its function in cognition. Recent studies recorded and manipulated the activity of different neuron types in the DG during memory tasks and opened exciting new insights in the mechanisms of DG computational processes, particularly for encoding, retrieval and discrimination of similar memories. Here, we review these DG-dependent mnemonic functions in light of the new findings and explore mechanistic links between cellular and network properties and the computations performed by the DG.
Introduction
Memories about ourselves and our interactions with the environment are fundamental to our life. Conscious or ‘declarative’ memories come in two flavors1: semantic memories comprise factual knowledge about the world (for example, that Brooklyn is a borough of New York), whereas episodic memories depict unique experiences (for example, your first train ride to Brooklyn). Episodic memories associate items and events with the spatial and temporal context in which they were experienced2. Memories must first be encoded2 as a permanent trace or ‘engram’, maintained and ‘consolidated’ over time 3,4 and finally recalled to become accessible to other cognitive processes.
The hippocampus, located in the brain’s temporal lobes (FIG. 1a,b), is crucial for declarative memory functions. Patients with hippocampal lesions generally retain normal intelligence2,5 but show severe memory deficits, particularly in the episodic6 and spatial domain7. They become virtually unable to encode such memories5 and their recollections of past events or spatial sceneries are schematic and lack contextual details6–8. Hippocampal neuron activity in humans and animals represents distinct memory elements, such as individuals9,10, places11,12 and associations between them12,13. The hippocampus is critical for associating individual items or events with their ‘context’, which comprises the background settings of an experience, such as a spatial environment or task-related rules14–16. Most studies discussed in this review focus on memories whose elements are organized in space. However, we assume that the neuronal mechanisms underlying such spatial memories generally apply to conscious memories organized in other, non-spatial, e.g. social- or conceptual domains16–18.
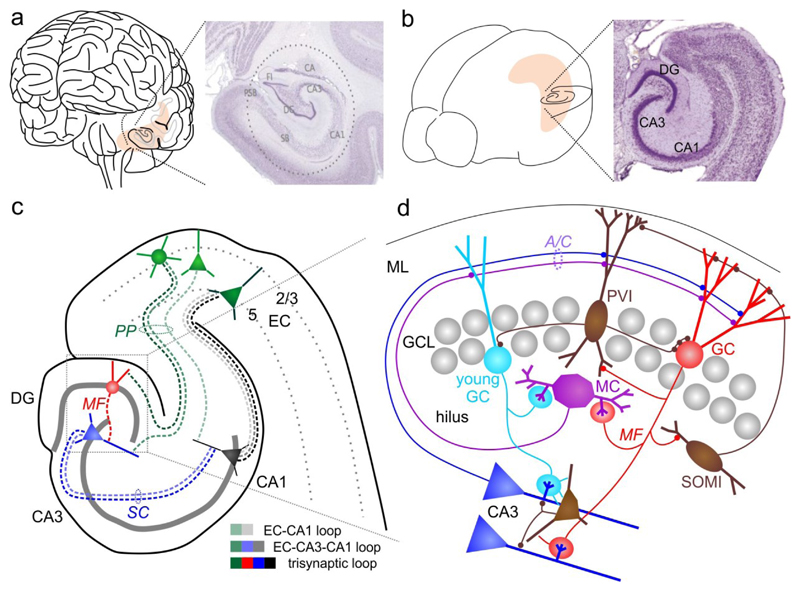
Fig. 1. Anatomical organization of the hippocampus.
a | Human brain and Nissl-stained section through the hippocampus. b | Same as in a for a mouse brain. c | Schematic of the mouse hippocampal formation and its main synaptic connections. d| Illustration of the DG microcircuitry, its intrinsic connections and outputs to CA3. A/C, associational-/commissural pathway; DG, dentate gyrus; EC, entorhinal cortex; GC, granule cell; GCL, granule cell layer; MC, mossy cell; MF, mossy fiber; ML, molecular layer; PP, perforant-path; PVI, parvalbumin-expressing interneuron; SC, schaffer-collateral; SOMI, somatostatin-positive interneuron. Photographs in a and b are derived from www.brainmaps.org and Franklin, K. B. J. & Paxinos, G., ‘The mouse brain in stereotaxic coordinates’, 3rd edition (Elsevier, New York, 2007), respectively.
The hippocampus is divided into the CA1, CA3, DG and CA2 subfields. The former three are interlinked by strong forward-connections forming the canonical ‘trisynaptic loop’ (FIG. 1c). The DG is among the few brain areas showing adult neurogenesis in most19, but not all mammalian species20, though its extent in adult humans is still debated19,21. Several mnemonic functions have been proposed for the DG22–26, including pattern separation24,27–29, pattern completion30, novelty detection31, binding of information to spatial contexts32 and working memory26. Optogenetic activation of memory-specific neuronal ensembles or ‘engrams’ in the DG can produce artificial memory recall in mice33 and fabricate mnemonic associations between separately encountered events34. The DG contains a diverse population of unique cell types (FIG. 1d; Table 1), which has been reviewed elsewhere35,36.
Table 1.
Summary of cell types of the dentate gyrus including the main synaptic input and output pathways (some subcortical input-projections were omitted for clarity) and the in vivo activity patterns.
| Cell | Synaptic inputs from | Synaptic output to | Activity in vivo | Refs. |
|---|---|---|---|---|
| Mature granule cell (GC) | - Medial- and lateral entorhinal cortex (via perforant path) - MCs (via A/C-pathway) - CA3 pyramidal cells (in the ventral DG) - medial septum |
- CA3 pyramidal cells - MCs - hilar interneurons - CA3 interneurons |
- Mean firing rate ~0.2 Hz - Strong firing-increase during non-REM sleep - Firing increase during dentate spikes and SWRs - Modulated by theta- and gamma oscillations - Long-term stable, singular place-fields - Separate populations active in completely different environments |
36,37,40–43,50,56 |
| Adult-born GC | - MCs and hilar interneurons within first week - LEC after ~3 weeks, rapid maturation - MEC beginning ~3 weeks – slow maturation until ~6 months |
- MCs and CA3 pyramidal cells after ~2 weeks - CA3 interneurons after ~2 weeks, peak at 4 weeks - Hilar interneurons delayed after >7 weeks |
- More active than mature GCs, but less spatial tuning | 38,54,81,83,87–89,91 |
| Mossy-cell (MC) | - GCs - other MCs - CA3 pyramidal cells - hilar interneurons - Potentially entorhinal cortex |
- GCs - MCs - hilar interneurons |
- Mean firing rate ~1 Hz - Modulated by theta- and gamma oscillations - Multiple place-fields per arena - Overlapping populations active in completely different environments |
39,41,42,51,52,55,93,95 |
| Parvalbumin-expressing interneuron | - GCs - MCs - hilar interneurons - entorhinal cortex - medial septum |
- GCs - MCs - hilar interneurons |
- Mean firing rate 10-20 Hz - No apparent spatial tuning - Modulated by running speed - Theta- and gamma-modulated - Strongly activated during dentate spikes and SWRs |
35,43,58,98,101,102 |
| Somatostatin- expressing interneuron | - GCs - MCs - hilar interneurons - medial septum |
- GCs - MCs - hilar interneurons - medial septum |
- Mean firing rate ~ 5 Hz - Moderate rate-increase during dentate spikes and SWR |
35,69,98,101–104 |
Recent studies used advanced electro- and optophysiological techniques to record for the first time activities of identified DG neuron classes37–43 and their synaptic connections44,45 in rodents during memory-based behaviors. Contrary to simple ideas about DG-mediated pattern separation, these studies mostly found no complete ‘global’ remapping of DG granule cells (GCs) but rather gradual activity changes between similar environments42,43. Furthermore, recent data on DG activity during memory-based discrimination and planning26,46 and GC-mediated recruitment of feed-forward inhibition in CA345,47 suggest that the DG enhances precision during memory-recall. In this review, we propose that the DG may support multiple mnemonic functions, including contextual discrimination, binding of objects and events to spatial layouts and integrating individual episodes into a framework of prior experiences. We consolidate new knowledge about the functional characteristics of different DG neuron types and their synaptic inputs and outputs to form hypotheses on the neuronal mechanisms that may support such functions during memory encoding, consolidation and recall.
1. Major cell types and their functions
1.1. Mature granule cells
Granule cells (GCs) are the DG’s principal neurons, with unique anatomical-, biophysical48 and cellular features, such as the dependence of their survival on circulating corticosteroid hormones49. GCs receive excitatory synaptic inputs from the medial and the lateral entorhinal cortex (MEC, LEC, respectively)50 via the perforant-path (PP; FIG. 1d). Mossy-cells (MCs) in the hilus, the region between the granule cell layer and CA3, form the associational-/commissural (A/C) pathway to contact GCs and hilar interneurons51,52. Furthermore, projections from CA3 pyramidal cells to GCs have been observed infrequently, mostly in the ventral DG53–55. GCs send a single mossy-fiber (MF) axon to CA3 targeting 10-15 pyramidal cells with 'giant' MF-synapses. They further contact a similar number of MCs via hilar axon collaterals and ~100-150 GABAergic interneurons in the hilus and CA356 (FIG. 1d).
MF-synapses onto CA3 pyramidal cells show low initial transmission probability, but tremendous facilitation upon high-frequency activity57. In contrast, markedly smaller MF-synapses targeting GABAergic interneurons show high initial release probability58,59 with little or no frequency-dependent facilitation. Therefore, presynaptic burst discharges >10 Hz60–62 reliably recruit CA3 pyramidal cells60, whereas low-frequency presynaptic firing at <1 Hz mostly recruits interneurons, which in turn inhibit the CA3 network59–61. Intriguingly, a recent study63 found opposite activation patterns after optogenetic stimulation of 3-5% of DG-GCs. However, disynaptic effects may have played a role in this observation. Low-frequency MF-stimulation in vitro reduces CA3 pyramidal cell recruitment by simultaneous PP-stimulation64, whereas GC ablation with the neurotoxin colchicine increases CA3 pyramidal cell discharges during spatial exploration26,65. Thus, particularly low-frequency GC activity may exert a net-inhibitory effect on CA3 by recruiting feed-forward interneurons.
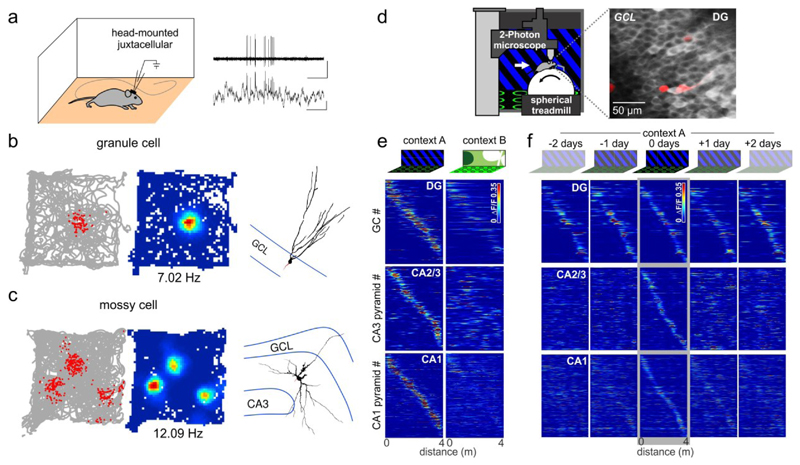
Several earlier studies recorded DG neuron activity with extracellular electrodes28,66,67. However, only recently were reliable criteria defined to distinguish GC from MC discharges (FIG. 2a,d)37–43,68,69. GC activity is exceptionally sparse37,40–43 and less than 5% of a rodent’s GCs are active when it explores a given environment43,70. These cells usually discharge when the animal is in a singular location, called their place field (FIG. 2a,b). Many GC place fields show moderate activity changes, or ‘remapping’, between similar virtual environments or chambers in the same room (FIG. 2e)38,42,43. However, another subset of GCs becomes active when animals are transferred to a different chamber located in a different room41. Thus, the degree of remapping in GCs varies from moderate, gradual changes in firing rates to pronounced, ‘global’ remapping (see Box 2) depending on the experimental conditions. In partial contrast, GC ensembles characterized by expression of the immediate-early genes (IEGs) Fos or Arc show little overlap when animals are sequentially introduced to two similar enclosures in the same room71 or two different rooms70,71. These findings could be reconciled by the observation that Fos expression in GCs is not quantitatively correlated with their firing, but rather with NMDA receptor (NMDAR) activation72 and may therefore indicate recent induction of synaptic plasticity at PP-GC inputs73. The same may apply for Arc expression74. These data suggest that IEG-expression may be more distinctive between two spatial contexts than action potentials. Activity properties observed using calcium imaging38,39,43,68 and single unit recordings of identified GCs41,42 show overall very similar results. However, some types of rapid synchronous co-activation of GCs46 and very sparse single action potentials26 may be missed by imaging techniques.
Fig. 2. Spatial, contextual and temporal firing characteristics of identified dentate gyrus neurons.
a | Schematic of juxtacellular recordings in freely moving rats and recorded signals (right). b | Left, Spikes of a granule cells (GC; red dots) plotted over the rat’s trajectory (grey line). Middle, heat-map plotting the firing frequency of this GC. Number indicates the maximum firing rate. Right, The GC was filled with neurobiotin after the recording to reconstruct the depicted morphology. c | As in b, but for a mossy cell. d | Schematic of a two-photon imaging setup for calcium imaging of DG neurons in head-fixed mice. The mice run on a treadmill to navigate along a virtual linear track displayed on monitors around them. Right, Fluorescence of a genetically encoded calcium indicator (GCaMP6f) expressed in GCs acquired in vivo. e | Mean activity (color-coded) of individual hippocampal place-cells (rows) plotted over distance on two visually different virtual linear tracks (context A and context B, respectively). The sorting of neurons is the same in both columns. A shift of place fields away from the central diagonal in the right plot signifies global remapping of the respective neuron. f | The same place cells were imaged over multiple days while the mouse ran on the same linear track on each day. Neurons were sorted by their place-fields on day 0 and this sorting is maintained for the other days shown. Note, GC place fields remain in the same location, whereas those of CA3 and CA1 cells change between days. GCL, Granule cell layer. Parts a-c are adapted from REF.41. Parts d-f are adapted from REF. 43.
Box 2. Pattern separation, remapping and their relation to discriminatory behaviors.
‘Pattern separation’ describes a network’s response to similar input activity patterns with more distinct output patterns. The opposite process, ‘pattern completion’ means retrieving a common output pattern from two similar, but distinct or degraded input patterns. These computational phenomena must be disambiguated from behaviors that require discrimination or generalization between similar stimuli201. Behavioral discrimination of similar contents very likely requires neuronal pattern separation that leads to distinctive neuronal representations in some part of the brain, but whether and where two apparently similar experiences generate a similar neuronal code that is separated by a neuronal process must always be tested experimentally. Specifically, to assess whether the DG is involved in presumed pattern-separation processes, the similarity of the PP-mediated input patterns needs to be known. Pattern separation in the hippocampus that could support contextual discrimination may be supported by multiple mechanisms: Two memories acquired in an environment could be encoded by statistically independent populations of neurons, with independent firing locations in each of the memorized experiences. Such a behaviour, termed ‘global remapping’206, may be implemented by independent attractor-states in CA3, which are classically thought to be supported by orthogonalized DG inputs22,23,172. Alternatively, a fixed set of neurons with constant firing locations can encode both memories, but change the rate within their firing fields independently between the two representations, a phenomenon termed ‘rate remapping’28,206.
A few studies have attempted to probe DG pattern separation by directly comparing the entorhinal input with DG output activity. Functional magnetic-resonance-imaging in humans discriminating similar pictures from memory shows more similar activation patterns in the entorhinal cortex than in DG and CA3, indicating that the DG-CA3 network may perform pattern separation in this task207. A patient with isolated, bilateral DG lesions, had deficits in a similar paradigm191. A series of studies in rats attempted to probe pattern separation in a graded set of growingly dissimilar spatial contexts, where local and distal cues were gradually rotated relative to each other173. Neuronal activity in the entorhinal cortex and hippocampus was recorded to determine the relative similarity of hippocampal input and output activity patterns as the contexts became more distinct. In this paradigm, MEC representations changed consistently with distal cues, whereas LEC activity was more aligned to local cues in the arena. The fact that distal CA3a activity was strongly anchored to local cues has been interpreted as pattern completion based on weakly local-cue anchored LEC inputs29,208. In contrast, activity of proximal CA3c and DG neurons displayed signs of pattern separation and did not change coherently with MEC or LEC-inputs29,173,209. A similar study reported related behaviors for DG and CA3 principal cells in a set of spatial enclosures with gradually dissimilar geometrical shapes28. These studies support the hypothesis that DG neurons perform pattern separation by transforming similar entorhinal inputs into more distinct output patterns. Future studies that disambiguate between the recorded DG neuron types could help to clarify potentially distinct contributions of GC and MC activity to this pattern separation mechanism.
A recent study observed that the field location of most place-field bearing DG GCs is stable over multiple days in a virtual-environment discrimination task, while many pyramidal cells in CA1 and CA3 changed their place field locations considerably (FIG. 2f)43. In novel environments, a small majority of GCs is less active and their overall spatial tuning is reduced43,75. Furthermore, GCs are more prone to develop new place fields in novel environments76, presumably by NMDAR-dependent strengthening of their PP-input synapses73,77,78. Thus, novelty-enhanced plasticity at PP-synapses may support the emergence of long-term stable GC place fields43.
1.2. Adult-born granule cells
Although most GCs are born prenatally, they are continuously generated throughout adulthood in many mammalian species20. Adult-born GCs show enhanced intrinsic excitability, stronger responsiveness to external inputs79 and enhanced synaptic plasticity80,81 compared with mature GCs. Some mnemonic functions, such as the discrimination of similar memories, are impaired by ablation of 4-6 week-old but not mature (>8 weeks) GCs in mice82–84.
Between 4-6 weeks, newborn mouse GCs show a distinctive connectivity pattern. Early in development, newborn GCs are contacted by MCs and GABAergic interneurons, which promote their survival and functional maturation85,86. After ~3 weeks, they receive inputs from the LEC and transiently from mature GCs and CA3 pyramidal cells54. MEC inputs are formed around the same time50,87, but remain substantially weaker than LEC inputs over several months50,54,88. After ~2 weeks, newborn GCs form synapses onto CA3 pyramidal cells and hilar MCs81,83, which have mature characteristics at ~4 weeks. At this age, MF-boutons show elevated densities of filopodial extensions, which recruit feed-forward inhibition onto CA3 pyramidal cells89. In contrast, embedding of newborn GCs into feedback-inhibitory circuits within the DG is delayed79,90,91. This creates a unique time window of enhanced excitability, plasticity and primarily feed-forward connectivity for 3-6 weeks old GCs. These cells show elevated activity levels, but less spatial tuning than their mature counterparts38. In vivo population imaging revealed that spatial activity patterns of young GCs discriminate only moderately between similar environments38. Thus, a combination of distinct structural and functional properties, such as enhanced excitability and elevated recruitment of feed-forward inhibition, may support a specialized role of young adult-born GCs. However, mature GCs can develop similar properties after behaviorally induced, plastic changes of their excitability92 and could thus carry out some adult-born GC functions, for instance in mammals lacking hippocampal neurogenesis20.
1.3. Mossy cells
MCs are glutamatergic interneurons in the hilus52. Their dendrites spread in the hilus, but occasionally extend into the molecular layer93,94. They receive monosynaptic inputs from GCs95, other MCs, CA3 pyramidal cells51,55, hilar interneurons55, the medial septum and potentially from the entorhinal cortex55,93. MCs form excitatory synapses onto interneurons and GCs in the ipsi- and contralateral DG52. Although MC axon collaterals have been observed in CA394, synaptic connections onto CA3 pyramidal cells or interneurons have so far not been described. Individual MCs show multiple place fields in experimental arenas (FIG. 2c), which display marked changes in firing rate and location upon modest environmental changes39,42, such as the introduction of novel objects96.
1.4. GABAergic inhibitory Interneurons
The DG houses several GABAergic inhibitory interneuron types35,97–99. The best investigated ones are parvalbumin-expressing interneurons (PVIs) comprising fast-spiking axo-axonic and basket-cells35. They inhibit the axon-initial segment of GCs and the perisomatic domain of GCs and interneurons, respectively100, and synchronize network activities during dentate spikes, theta- (4-10 Hz) and gamma-frequency (30-150 Hz) oscillations and sharp-wave ripples (SWRs)37,97,101.
The second major type are dendrite-targeting inhibitory cells comprising a variety of subtypes. The most prominent ones are somatostatin-expressing interneurons (SOMIs), which inhibit distal dendrites of GCs and other interneuron types near their PP-input synapses35,102. Other types of DG-SOMIs send long-range axonal projections, e.g. to the medial septum, subiculum, CA1, CA3 and the contralateral hippocampus103,104, which provide an additional output route to the MF pathway and may support information processing in neighboring hippocampal areas. Optogenetic inhibition of PVIs but not SOMIs in vitro profoundly increases GC firing-responses to PP-stimulation105. In contrast, optogenetic inhibition of SOMIs, but not PVIs strongly inhibits Fos expression in GCs in vivo106, indicating a potential role for SOMIs in controlling synaptic plasticity.
2. Synaptic inputs to the DG
2.1. Entorhinal inputs
The entorhinal cortex provides the major cortical input to the hippocampus. The MEC contains several spatially modulated cell types, such as border-, head-direction-, object-vector- and grid-cells107–109. The latter show regular, hexagonal spatial activity patterns and may provide a metric organization to the neuronal representation of space (‘where’)107,108, but also non-spatial variables such as sound110 or time-intervals111. Hippocampal neurons can nevertheless retain context-specific spatial firing in the absence of MEC-input112–114, indicating that spatial maps can be constructed from diverse input streams. However, MEC inhibition reduces long-term stability of CA1 place-fields114,115, consistent with the high temporal stability of spatial representations in the MEC116. MEC inputs might promote the high place-field stability recently reported for GCs43. Visual cue-dependent MEC-input to the DG increases over the course of spatial memory tasks44 and could thus promote increasing activation and spatial tuning of GCs during learning43.
LEC neurons preferentially represent non-spatial information117, such as objects (‘what’)118 and object-related spatial features such as egocentric bearing towards an object or previous object locations119,120. Furthermore, LEC neurons have an intrinsic code for time on scales of minutes to hours121, which is distinct from time representations in the MEC on the second-scale111. Such time-varying LEC input may give rise to the apparent instability of place fields observed in several hippocampal areas43,122,123.
2.2. Intrahippocampal DG inputs
Selective feedback-connections from MCs51,55,124 and few CA3 pyramidal cells51,53–55,125 project in the inner molecular layer and contact GCs. They can directly excite GCs126–128 but predominantly recruit di-synaptic inhibition51,124,129. While this inhibitory action has been proposed to suppress recently active GCs for enhanced network sparsity130, excitatory recruitment of GCs by the A/C-pathway may support their synchronization with bursting of CA3 pyramidal cells during SWRs131.
2.3. Subcortical inputs
While cortical and intra-hippocampal inputs convey information about discrete memory contents, subcortical inputs modulate how the DG processes this information depending on ‘global’ behavioral states, such as locomotion, arousal or sleep. Cholinergic input from the medial septum is high during spatial learning, but low during awake inactivity and slow-wave sleep132,133. Blocking acetylcholine receptors during encoding or raising acetylcholine levels during consolidation impairs spatial memory134, particularly when interfering memories require separation133. A high cholinergic tone during exploration132,133 may increase sparsity of GC firing by suppressing their recruitment through PP-inputs through cholinergic activation of hilar astrocytes which in turn release glutamate and thereby activate GABAergic interneurons133,135–137. However, acetylcholine promotes long-lasting potentiation of PP-synapses136 to prime the future recruitment of targeted GCs. Conversely, a physiological drop of cholinergic tone during non-REM sleep133 may increase GC activity42, presumably via disinhibition137.
Hippocampal dopaminergic inputs from the ventral tegmental area and the locus coeruleus138 facilitate memory acquisition and consolidation138,139, but are not required for rapid acquisition of episode-like memories138. However, locus coeruleus input enhances GC responses to PP-stimulation and promotes LTP at PP-GC synapses140. Thus, dopamine could support the slow, incremental formation of GC place fields that was observed in a longitudinal calcium-imaging study43.
3. Mnemonic operations in the DG
3.1. General considerations
For a long time it was thought that information flows unidirectionally through the hippocampus along the trisynaptic loop. However, theoretical27 and experimental data65,141–144 have later changed this view. First, individual CA3 pyramidal cells receive inputs from thousands of entorhinal- and CA3 pyramidal cells, but from only ~50 GCs145 and many of these GCs are silent during exploratory behavior43,70. Although unitary MF-synapses are stronger than PP- or recurrent-collateral inputs, only GC burst activity efficiently recruits CA3 pyramidal cells, while low-frequency firing primarily activates feed-forward inhibition59–61. Thus, GC-input as the source of CA3 place cell firing has been questioned on theoretical grounds146. Second, CA3 pyramidal cells retain moderately distorted place fields after experimental ablation of GCs26,65. Conversely, a recent study found that presynaptic GC activity poorly predicts place fields of postsynaptic MCs42. Thus, the recruitment of CA3 place-cells with established place fields may dominantly derive from PP- and recurrent CA3, rather than MF-inputs. However, MF-input could play a role in establishing novel place fields (see chapter 3.2). Finally, CA3 pyramidal cells respond to PP-stimulation in vivo prior to GCs141,142, indicating that MF-inputs may support, but not initiate CA3 pyramidal cell discharges. Thus, rather than acting as a trisynaptic chain, the hippocampus may be arranged as a set of parallel loops, with each loop being formed by a hippocampal subfield receiving direct PP-synapses and inputs from upstream hippocampal areas (FIG. 1c)65,141–143. Hence, there are several instances, where correct memory storage and retrieval can be achieved based on the PP-CA1 or PP-CA3-CA1 loop alone 143,147.
3.2. Memory encoding and consolidation
Encoding
A leading hypothesis about GCs is that they recruit unique CA3 pyramidal cell ensembles during memory encoding, which then become associated with the entorhinal input pattern representing the new information27. Once synaptic plasticity has permanently strengthened the intrinsic and extrinsic connections of these ensembles64,142,148, PP-inputs may reactivate them to promote recall of the associated memory without the need for MF-inputs149. Indeed, lesion studies suggest a functional dissociation between the PP-CA3 and the PP-DG-CA3 path. MF-afferents appear to be required for memory acquisition, but not retrieval, while PP-afferents appear to initiate memory retrieval from CA3 without the necessity of MF-input150. Acute experimental DG-inactivation impairs memory encoding, but not recall in most studies151–153, although two papers154,155 that specifically inhibited IEG-expressing GCs during recall found a moderate reduction in contextual fear memory retrieval (see also chapter 3.3). Notably, GC ensembles are re-activated during retrieval of recent, but not remote memories156. Thus, GC activity may be required during initial encoding and early, intra-hippocampal consolidation of a memory but dispensable for its recall.
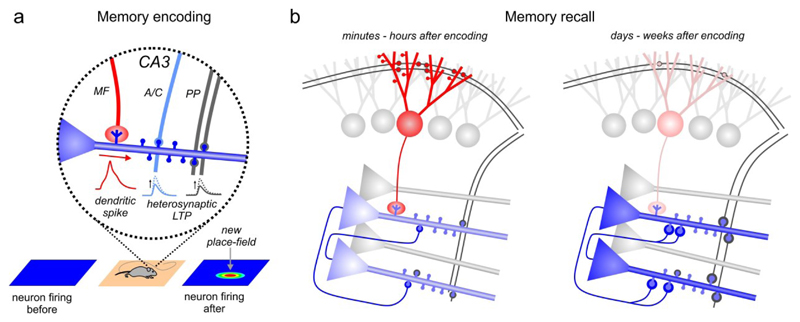
MFs can induce heterosynaptic plasticity at PP- and recurrent-inputs onto CA3 pyramidal cells64,157–159 and may thereby promote later reactivation of CA3 cells by these inputs in the absence of MF-signaling. Several forms of hippocampus-dependent learning, for example by single-trial experience, require NMDARs in CA3 pyramidal cells160,161. Plasticity of recurrent CA3- and PP-64,162, but not MF-synapses159 requires NMDARs. This supports the hypothesis that memories in CA3 are ultimately stored at PP- and recurrent connections. Strong MF-synapses at proximal pyramidal-cell dendrites can elicit large dendritic depolarizations. Such active dendritic events can support the instantaneous reorganization of pyramidal-cell place fields in CA177,78 and CA3163. Thus, MF-mediated signaling may promote heterosynaptic plasticity at PP- and recurrent inputs via active dendritic events to enable associative encoding of information between CA3 and its PP-inputs (FIG. 3a)64,157–159,164. However, MF input may not be irreplaceable in this process, as plasticity of PP- and recurrent inputs to CA3 cells can also emerge in the absence of MF input159. This could explain why under certain conditions, e.g. during contextual fear conditioning (CFC), memories can be acquired in the absence of functional GC-output15,30,144.
Fig. 3. DG functions in memory encoding and recall.
a | Schematic of memory encoding. Mossy-fiber (MF) input to a CA3 pyramidal cell dendrite can elicit dendritic spikes which may promote heterosynaptic potentiation of perforant-path (PP) and recurrent-collateral inputs. This process may potentially underlie the formation of new place-fields (lower row). b | Left, Rapid strengthening of PP-GC and MF-CA3 synapses may support the re-activation of CA3 pyramidal cells during memory recall minutes to hours after the original experience. Right, Once CA3 ensembles have been permanently established by durable plasticity of PP-CA3 and recurrent-synapses, the memory can be reliably recalled without MF input, which is reduced at this stage due to depotentiation of PP-GC synapses167.
Consolidation
Synapses between GCs and CA3 pyramidal cells encoding the same spatial context are durably strengthened starting minutes to hours after CFC47,148,165,166 and PP-inputs to GCs encoding a spatial context are transiently strengthened for ~1 week167. However, established CFC memories can be retrieved in the absence of GC-output30,144,168. MF-inputs to CA3 may hence be required for early memory consolidation. Furthermore, disruption of GC-output impairs the maturation of memory engrams in the prefrontal cortex during late- or ‘systems’ consolidation167. SWRs occur during consolidation and are required for hippocampal working-and reference memory169,170. GCs promote SWRs in CA3 during spatial working memory in an 8-arm radial maze and boost activation of CA3 ensembles, which prospectively encode the animal’s planned trajectories26 (see also Box 1). Moreover, optogenetic silencing of a subset of GCs that have been artificially incorporated into a spatial-context representation 5 minutes after CFC memory acquisition disrupts memory recall 24 hours later155. Taken together, transient increase of PP-input to, and MF-output from GCs, plays a key role in memory maintenance during memory consolidation. During initial learning, rapid plasticity at MF-CA3 pyramidal-cell synapses may act as a ‘seed’ to promote the establishment of stable CA3 assemblies, potentially even between learning trials, through the re-activation of these ensembles during SWRs26,164. Once CA3 ensembles are stabilized, recall may become independent from MF-inputs.
Box 1. The functions of the dentate gyrus in working memory.
Aside of its function in long-term memory storage4, the hippocampus is also required for short-term retention of spatial information, better known as ‘spatial working memory’ (SWM). SWM is most commonly probed in the delayed match-to-place task, in which an animal must re-visit a previously encountered location after a delay of seconds to minutes, and the non-match-to-place tasks, where the animal has to choose the alternative of two spatial choices after the delay. Furthermore, the radial 8-arm maze, in which animals must subsequently visit all arms of the maze, has a SWM component, as the animals have to remember which arms they have not yet visited in the same run. Blockade of MF-output by zinc-chelator infusion202 as well as GC-ablation25 cause impairments in these tasks. Furthermore, exercise-induced improvement of SWM is mediated by increased neurogenesis in the DG203.
Decision-specific, non-overlapping CA3 pyramidal cell ensemble sequences are activated at the choice point of a T-maze based SWM paradigm204. Whether the instantiation of such choice-specific ensemble sequences depends on DG-input remains an open question. Recordings of CA3 cells in DG lesioned animals performing an 8-arm maze working-memory task indicate that MF-input promotes the activation of working-memory-related neuronal ensembles in CA3 during synchronous, high-frequency (SWR) network oscillations26. In line with this observation, disrupting the output from DG-PVIs, which prominently support fast network oscillations97,99, impairs SWM205. Working memory maintenance and choices based on it are challenging, as the underlying neuronal representations need to be sustained internally in the absence of supporting sensory input. If stored ensemble sequences in the CA3 network are critical for SWM, it is conceivable that DG-output is necessary to promote their establishment or re-instantiation.
3.3. Pattern separation and pattern completion
DG function is characterized by the expansion of relatively few entorhinal cortex neurons onto many more GCs23,145 and by its competitive network dynamics99,171, in which active GC ensembles may suppress the activation of competing ensembles by recruiting disynaptic inhibition. It is assumed that these factors help to separate overlapping PP-input patterns and promote representations of similar memories by distinct GC assemblies to provide unique input patterns to CA3 during memory encoding23,27,172. Conversely, CA3 is assumed to retrieve previously stored activation patterns from inputs representing parts (cues) of the initially stored experience via its numerous internal recurrent connections and their associated attractor dynamics22,23,27,173. This process may underlie memory retrieval. These computational functions, termed ‘pattern separation’ and ‘pattern completion’, respectively, together with experiments that explicitly probe pattern separation in the DG by comparing its input- and output patterns, are discussed in Box 2.
However, the attractor dynamics that promote CA3 pattern completion by retrieving identical activity patterns in response to similar inputs could also cause CA3 to rapidly fall into a different activity state or attractor, if synaptic input activity patterns are sufficiently different from each other173. This network behavior has been observed in response to rapid changes of cues defining spatial contexts174. As CA3 is the only known target area of the DG175, any pattern separation process in the DG that produces differential behavior ultimately has to promote distinctive neuronal representations in CA3. Below, we outline potential mechanisms for pattern separation in the DG and how they might support the discrimination of similar memories in two DG-dependent learning paradigms.
Contextual discrimination learning
The most common behavior to probe hippocampal pattern separation is discriminative CFC, where an animal has an aversive experience (e.g. a foot-shock) in one context, but never in a slightly different, ‘safe’ context (FIG. 4a). ‘Contexts’ are usually defined by the arrangement of visual cues and environmental boundaries, which is slightly different between the fear- and safe context. Upon learning this task, animals show fear (‘freezing’) in the conditioned, but not in the safe context if they can discriminate them. The task is hippocampus-dependent176 and potentially requires separation of entorhinal input patterns encoding the similar contexts. Activity-patterns of MEC neurons and PP-axons for similar spatial contexts appear to be highly overlapping28,144, while more distinct contexts are differentially encoded already by the MEC144,177,178. Discriminatory CFC-learning has two challenges. First, animals must acquire a fear memory for the conditioned context. Second, the animals should not generalize fear to the safe context but instead form a sufficiently unrelated representation of the latter.
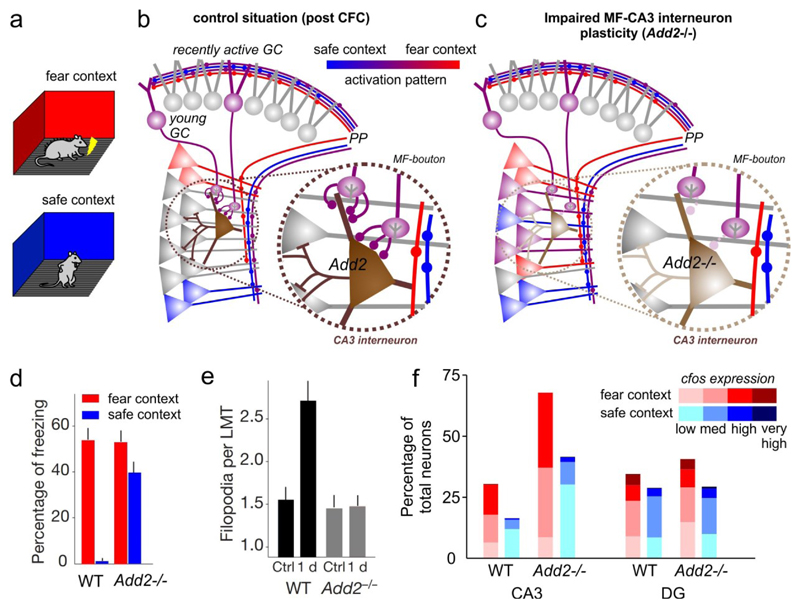
Fig. 4. DG function in discriminatory contextual fear conditioning (CFC).
a | Schematic of discriminative CFC. b | Illustration of the physiological DG and CA3 activation patterns evoked by overlapping PP-inputs encoding similar contexts. Young and mature GC show similar activity patterns in both contexts. Young- and recently-active GCs potently recruit feed-forward inhibition45,89 and may thus suppress firing of CA3 pyramidal cells with overlapping synaptic input-patterns between the two contexts. This could spare less overlapping CA3 ensembles forming context-unique representations. c | Activation pattern as in b in a mouse model where MF-plasticity onto CA3 feed-forward interneurons was disrupted genetically by knocking out the gene encoding adducin 2 (Add2)47. Releasing CA3 from MF-recruited feed-forward inhibition unmasks the activation of larger CA3 ensembles with more overlapping activity between the two contexts.
Several studies found that contextual fear per se can be acquired without DG input to CA315,30,144. Layer II cells in the MEC represent spatial contexts and their output can evoke spatial-context representations in CA3144. Contextual fear can be acquired and retrieved by mice with genetically ablated PP-GC-15 or MF-plasticity47,179 and after complete blockade of MF-transmission with tetanus-toxin30. However, increasing the size of the context-activated GC ensemble by experimental suppression of feedback inhibition enhances, whereas decreasing GC-ensemble size reduces the degree of freezing during memory recall, indicating that GC activity supports CFC encoding106. Studies in which different subsets of the GC population were acutely inhibited during contextual fear acquisition38,144,152,168 or retrieval83,152,154,155 had conflicting results. Some revealed memory impairments38,152,168, while others did not144. For example, optogenetic inhibition of GCs expressing IEGs during context-encoding154,155 or inhibition of 4-week old young GCs83, while leaving the remaining DG-output intact, impairs context-dependent memory expression. Moreover, contextual fear acquisition can be impaired by pharmacogenetically activating a random (false) GC ensemble, which may di-synaptically suppress the genuinely context-activated GCs via lateral feedback inhibition106 and could thereby impose an unrelated representation onto CA3. Unfortunately, none of the aforementioned studies simultaneously recorded neuronal firing activity during memory-affecting opto-/pharmacogenetic manipulations, which could reveal such indirect, network-mediated effects. The results above suggest that fear memories can in principle be acquired and retrieved based on the PP-CA3 loop alone30,46,144, but an erroneous output from the DG could be worse than none at all and may disrupt the residual function of the PP-CA3 loop168. Interestingly, in all mentioned studies15,30,38,47,144,168,179, mice excessively generalized fear towards a different, non-conditioned context, suggesting that DG-output is necessary for context discrimination and promotes separation of context-encoding CA3 ensembles rather than establishing the context-fear association memory per se.
What are the cellular substrates of contextual discrimination? Impaired discrimination is accompanied by stronger overlap of CA3 pyramidal cell ensemble activity15,47,180 but not of Fos-expressing GC-ensembles47. In contrast, one study investigating differences between young and aged rats found that improved discrimination was accompanied by higher GC ensemble-overlap181. Furthermore, NMDAR-ablation in CA3 pyramidal cells, which disrupts plasticity at PP- and recurrent-, but not at MF-synapses, impairs rapid discrimination of similar contexts182. Thus, CA3 ensemble composition may be the relevant arbiter of subjective context memory discrimination173. Similarly, artificially enhancing the overlap of active CA1 ensembles increases contextual fear generalization183. Thus, context discrimination may critically depend on independent, attractor-like contextual representations in CA3.
How could the DG promote establishment and stabilization of such discriminative CA3 representations? Traditionally, context-specific, sparse GC populations have been proposed to recruit independent CA3 ensembles27. Intriguingly, however, the activity patterns of MCs and CA3 cells are often more distinct between similar environment than those of GCs38,42,43. Moreover, a recent study found no correlation between spatial remapping of DG cells and performance in a DG-dependent memory-discrimination task46. Thus, a substantial fraction of active mature GCs might encode common features of spatial contexts, and their activity would be more suitable to promote context generalization (FIG. 2e). It is therefore unsurprising that completely blocking mature GC-output improves contextual discrimination30.
Despite the observations summarized before, brief re-exposure to an originally fear-conditioned context transiently increases excitability in a limited GC-ensemble that expressed IEGs during initial learning and this increase in excitability improves behavioral context-discrimination92. Thus, a small subset of IEG-expressing GCs may support contextual discrimination, potentially enabled through IEG-mediated structural- and functional plasticity45,92. Adult-born GCs (4-6 week old) appear to support context discrimination, too30,83,154. Genetic or chemical ablation of neurogenesis180,184 or inhibition of young GC-output30,38 has a disruptive effect on context discrimination similar to complete DG lesions. Conversely, discrimination is improved by genetic inhibition of apoptosis in newborn GCs to increase their survival185. Thus, highly excitable young GCs38,79,80 and recently active mature GCs92 may be important for the DG context-discrimination mechanism, whereas a separate population of context invariant mature GCs seem to support context generalization30,42,43.
MF-synapses onto CA3 interneurons are rapidly strengthened after GC burst firing186 and show structural plasticity within hours after CFC, which is correlated with discrimination performance47. Moreover, genetically enhancing the stability of MF-to-CA3 interneuron synapses improves contextual discrimination and prolongs CFC memory retention45. Notably, 4-6 week-old GCs89 and GCs expressing IEGs during memory encoding45 make disproportionally more filopodial synapses onto CA3 interneurons45. Their recruitment of feed-forward inhibition47,59,60 may subsequently help to exclude pyramidal cells representing the initial context from encoding representations of similar contexts144 or suppress competing contextual representations during retrieval (FIG. 4b)168. Supporting this idea, disruption of MF-plasticity selectively onto GABAergic interneurons by knocking out adducin 2 increases CA3 ensemble-overlap during memory retrieval (FIG. 4c) and impairs contextual discrimination memory47.
Interestingly, spatial activity of MCs remaps strongly between similar contexts39,42,96 and is reminiscent of extracellular recordings which originally suggested context-discrimination via DG neurons28. ‘Backprojections’ from CA3 pyramidal cells51,130 to MCs could potentially support such context-selective activity. MC ablation causes a transient context-discrimination deficit, which recovers after 5-6 weeks187. Thus, MCs support, but are not mandatory for context discrimination memory. How could context selective MC activity be relayed to CA3? MC activity could be propagated di-synaptically via GCs, but the latter show on average less context-selective activity than MCs39. It is possible, however, that a specific subset of GCs with strong context-dependent remapping conveys contextual information from MCs to CA3. MC axons also directly extend to CA394, but monosynaptic MC-CA3 connections have so far not been described52,188. Alternatively, hippocampal interneurons might relay MC activity. They encode contextual variables, such as running, immobility189, or sensory cues190. Hilar interneurons receive MC input52,128 and project to hippocampal subfields outside the DG, including CA3103. Similar to the effect originally proposed for MF-mediated excitation27, putative context-specific inhibition from DG interneurons may suppress different parts of the CA3 pyramidal cell population and ensure that independent ensembles represent distinct contexts.
It has been proposed that DG-mediated pattern separation supports differential memory encoding27,191. However, discriminative CFC recall also requires DG activation168 and long-term plasticity at MF-synapses onto CA3 interneurons45,47. Furthermore, transient enhancement of GC excitability by re-exposure to a spatial context improves subsequent behavioral context discrimination92. These facts strongly suggest that DG output is necessary for context-discrimination during both, memory encoding and recall.
Location discrimination learning
In addition to discriminating between different spatial contexts, the DG is also required to discriminate adjacent locations within the same spatial context24, for example to memorize one rewarded arm in several adjacent ones of an 8-arm maze192 or locations of closely spaced objects24. These operations may require neuronal pattern separation processes, too, as animals must memorize and distinguish exact locations within an environment. However, unlike contextual discrimination, which involves discrete attractor-like remapping174, this type of pattern separation likely needs to be performed on a continuum of neuronal activity-patterns encoding adjacent locations. Intriguingly, both types of memory tasks are DG-dependent24,31,193 and their mechanisms share many common elements. Similar to contextual discrimination, structural MF-plasticity onto CA3 interneurons supports precise platform-location memory in the Morris water maze47. Furthermore, adult-born GCs are required for object-location memory192. Notably, their activity produces ipsi- and contralateral effects, indicating that their output may be relayed through an enhanced recruitment of CA3 pyramidal cells or contralaterally projecting MCs84. Notably, encoding, but not retrieval, of precise object locations is impaired by optogenetic MC inhibition188.
Formation and discrimination of precise location-bound memories requires binding of non-spatial information, such as objects, visual cues or a hidden platform to a spatial map-like representation, which appears to be another cognitive functions supported by the DG32. To form associations between objects or events and their location, mature GCs may provide unique representations for individual locations of the spatial map by their singular sparse place-fields41,42 to recruit distinct CA3 ensembles at each location and facilitate the binding of objects or events to these ensembles32. A subgroup of GCs that has similar place fields in different contexts39,42,43 may form a ‘reference map’ of an environment and recruit CA3 cells to become parts of common assemblies representing identical locations over multiple experiences to support e.g. precise object-location memory43,149.
3.4. Memory retrieval
In contrast to memory encoding, the DG's role in memory retrieval is more controversial. Re-activation of GCs by re-exposure to a previously explored environment declines heavily over days after memory acquisition156,167 due to downscaling of synaptic inputs and cellular excitability4,92,167. However, artificial re-activation of context-encoding GC ensembles can trigger memory retrieval33 even after several weeks when the DG is not required for natural memory recall167,194. Furthermore, restoring GC recruitment by inducing artificial PP-LTP can rescue memory deficits in models of early stage Alzheimer disease195 and optogenetic reactivation of GC engrams can trigger recall of memories, which have become inaccessible to natural recall due to pharmacologically induced amnesia166, dementia195 or infantile amnesia194. The DG may furthermore support memory-recall for behavior-planning: A specific synchronous activation pattern of DG units that had place fields during encoding predicts successful avoidance during retrieval in a flexible spatial location-learning paradigm46. A recent study further indicates, that sparse, non place field bound activity of unidentified DG units promotes the recruitment of future-trajectory encoding CA3 cells26, indicating that distinct DG activity patterns may support retrieval specifically during memory-based behavior planning.
GC activity is often sufficient, but may not always be necessary to induce memory recall. By default, memory recall may be initiated by PP-inputs to CA3 without the necessity of MF-input150,151,168. However, GC-output could provide a further partially redundant memory representation, particularly early after learning43,156, that promotes specificity of the CA3 representation47. When limited cues are available to retrieve a spatial memory, inhibition of mature GC-output impairs recall30. Similarly, mature GC-output is required to recognize a familiar context rapidly within 10 seconds of exposure30. Thus, GC input to CA3 during recall may be necessary under conditions where a faithful memory representation for an environment cannot be established in the entorhinal cortex alone due to lack of cues144 or insufficient exploration time107.
3.5. Modification of pre-existing memories
As outlined above, GCs store long-term stable mnemonic representations of spatial environments43,167 which get rapidly disconnected from experience-driven re-activation due to a degradation of PP-inputs92,156,167, thus falling into a ‘stand-by’ mode. However, brief re-exposure to the original environment can restore their intrinsic and synaptic excitability during subsequent exposures to the same environment92. What role could such ‘stand-by’ representations play? Notably, optogenetic DG inactivation impairs the ability to learn changed contingencies in a previously encountered environment46,152,168. For example, when mice must avoid an invisible ‘shock-zone’ in a circular arena, optogenetic inhibition of GCs does not impair initial learning of the shock-zone location, but when the zone is relocated, mice are impaired in learning the changed location152. Ablation of neurogenesis produces the same learning deficit, and increases the size of Arc-expressing GC ensembles after the relocation but not after the initial learning condition196. Thus, neurogenesis may help to restrict the recall-activated GC ensemble to those neurons active during the initial experience197. Furthermore, optogenetic inhibition of DG-output168 or genetic ablation of MF-plasticity179 impairs contextual fear extinction induced by re-exposure of mice to the previously fear-associated context, presumably because the novel experience of safety is not associated with the original context representation.
Taken together, GCs may support the integration of distinct, potentially interfering experiences into the same general framework or environmental representation32. This integrative function of the DG may further support the binding of episode-like representations in downstream hippocampal areas19,43,122,198,199 to a common reference. GCs which encode the same location over multiple spatial contexts or temporal instances38,39,42,43 in a ‘semantic-like’ manner could promote synaptic plasticity in CA3 cells that were part of the ensemble encoding the previous episode, to efficiently incorporate them into a new ensemble encoding the most recent encounter of the same environment. Such a mechanism could induce interference between conflicting memory representations in CA3 and allow a more recent experience to efficiently suppress the re-instantiation of an ‘outdated’, related memory (for example, the parking spot today versus the parking spot yesterday).
A new, distinct CA3 ensemble for the most recent experience could also be established by suppressing the previously active CA3 ensemble via MF-recruited feed-forward inhibition47. In this alternative scenario, the DG pattern-separation mechanism that likely supports spatial-context discrimination might also support the discrimination of separate temporal instances. Pattern separation would be required to generate independent CA3 ensembles based on completely overlapping MEC inputs representing the same environment116 (the parking lot) and temporally varying LEC inputs121 (the most recent parking position).
4. Conclusions and Perspectives
Above, we summarize recent advances in understanding mnemonic functions supported by the DG and their neuronal implementation26,43,46,92. Theoretical27 and experimental150,151 studies proposed a role for the DG in memory encoding. However, recent data indicate that GC-output is also required for short-term memory retention and consolidation26,155 and for specificity during memory retrieval26,46,47. The precise effects of DG output during memory consolidation, however, are still largely unexplored. Memory recall can often be initiated without GC-output30,168, but GCs can enhance its precision30,47,92,168. The DG may thus modify computational processes in the PP-CA3-CA1 loop (FIG. 1c) instead of merely driving CA3 activity. Future experiments will have to determine how GCs exert such modifying effects and whether they can permanently alter information processing in CA3, for example through heterosynaptic plasticity64,158. DG output appears to be important for a variety of cognitive functions, including discrimination of similar contexts15,24,30,193, binding of objects or events to locations32 and integration of new experiences (chapter 3.5).
A tentative circuit mechanism for the discrimination of memory contents, such as similar spatial contexts or individual episodes in the same environment, involves highly excitable 4-6 week-old-30,38,82,83,180,184,185 and recently Fos expressing GCs92. MFs from these types of GCs preferentially contact feed-forward inhibitory CA3 interneurons45,89, and plasticity at these synapses is critical for discriminative contextual learning45,47. MF-recruited feed-forward inhibition may suppress activation of CA3 cells that would otherwise be recruited by overlapping PP-inputs in similar contexts144 or during repeated encounters of the same environment116. This could spare unique sets of context- and episode-specific CA3 pyramidal cells to represent the distinctive elements of each memory. An open question is, whether IEG-expressing GCs show different context-dependent firing patterns than non-IEG-expressing ones, as recently observed in CA1 pyramidal cells200. Furthermore, given their role in constraining the overlap of CA3 ensembles47, future experiments are necessary to investigate the precise recruitment of feed-forward interneurons in CA3 and their effect on CA3 pyramidal cell activity during encoding and recall of hippocampal memories.
In addition to similar-content discrimination, the DG appears to support integrative memory operations, such as binding of objects and events to spatial contexts32 and integrating new experiences into existing frameworks (chapter 3.5). Several recent publications investigated the activity and function of identified DG neuron types37,38,40,41,43,46,69 and the results question particularly models of pattern separation that propose a complete orthogonalization of entorhinal inputs by GCs.27,70,201. Indeed, the assumption that similar experiences are represented by distinct, non-overlapping GC ensembles70,71, seems inconsistent with the electro- and optophysiologically measured activity of many GCs38,39,42,43. While some of them have distinctive activity profiles in different environments41, other GCs have similar place fields over distinct contexts or time points42,43. Such GCs might therefore rather support integrative- or binding-functions by recruiting overlapping CA3 ensembles to create links between shared elements of distinct memories149.
It will be important to determine the exact neuronal processes underlying these potentially distinct functions of the DG network. These functions may be executed in parallel by independent GC subpopulations, as outlined above, but could also be serially performed by the same microcircuits if behavior-dependent neuromodulation132 or differential functional dominance of synaptic input streams197 modify the relative contributions of individual circuit elements, such as young- and mature GCs, MCs and interneurons.
Glossary.
Declarative memory: A memory of facts and events that can be consciously recollected and reported to others. In contrast, implicit- or procedural knowledge is hard to put into words.
Semantic memory: A form of declarative memory that comprises general knowledge (e.g. facts, concepts) about the world, which is not bound to a distinct personal experience.
Episodic memory: Declarative memories, which depict unique, personal experiences made in a distinct spatial and temporal context.
Engram: An experience-driven change to the brain that is stored and reactivated during memory recall. It may be a distinct neuronal ensemble, a sequential co-activation pattern of several neurons or a set of synaptic weights in a neuronal network.
Trisynaptic loop: Canonical set of forward synaptic connections from the entorhinal cortex to the dentate gyrus via the perforant-path, further to CA3 via the mossy-fibers and from there to CA1 via the Schaffer-collaterals.
Pattern separation: A network computation that generates more dissimilar output patterns in response to similar input patterns. It is hypothesized to support the cognitive discrimination of similar representations in the brain.
Pattern completion: A computation in which slightly different input patterns activate a common, stored output pattern. This could promote retrieval of a complete stored memory from inputs representing partial cues or support generalization between similar memory contents.
Place-field: The spatial location in which a hippocampal neuron called a ‘place cell’ fires action potentials.
Remapping: The process of changing place-field locations or the firing rate of neurons in a place field in response to contextual factors, such as environmental changes or altered behavioral demands.
Immediate early gene (IEGs): Genes that usually encode transcription factors (such as Fos or Arc), which are induced immediately within minutes after strong neuronal activation. High expression of immediate-early genes in a neuron is commonly used as a readout for recently elevated activity or synaptic plasticity.
NMDA Receptor: Ionotropic glutamate receptors highly prevalent at excitatory synapses in the central nervous system. They are activated by coincident presynaptic glutamate release and postsynaptic neuronal depolarization and are critical for many forms of synaptic plasticity.
Dentate spike: A brief (20-80 ms) large amplitude (1-3 mV) local-field potential activity pattern characteristic of the DG, elicited by synchronous activation of a larger group of GCs and other dentate neurons.
Sharp-wave-ripples (SWRs): Brief (40-150 ms) large-amplitude deflection of the local field potential associated with synchronous high-frequency (110-200 Hz) network oscillations that originate in CA3 and propagate into many other brain areas. SWRs occur during immobility and sleep and play an important role in memory consolidation and other cognitive processes.
Contextual fear conditioning (CFC): A behavioral paradigm in which an aversive stimulus (for example, a foot shock) is given in a defined experimental chamber. Fear memories are assessed by the amount of fear-related behavior (for example, freezing) that an animal shows after re-exposure to the same chamber.
Attractor dynamics: Tendency of a process or neuronal network to fall into a continuous or discrete set of stable states (attractors) which are relatively resistant to external perturbations. Activity states outside of these attractors are unstable and the network rapidly falls back into the nearest attractor state from them.
Morris water maze: Behavioral paradigm where an animal swims through a water basin to find a hidden platform under the water surface. Visual cues around the maze allow animals to navigate directly to the platform once they have learned the platform-location over repeated trials.
T-maze: Binary spatial decision task where an animal can go into the left or right arm of a T-shaped arena. Often used as working memory paradigm, in which the animal must choose either the same, or opposite arm to the one that was chosen on the previous trial.
Acknowledgements
The authors’ work was supported by the Deutsche Forschungsgemeinschaft (FOR2143-2, M.B.; HA 8939/1-1 T.H.), the European Research Council Advanced Grant (ERC-AdG 787450, M.B.), EMBO (ALTF 6-2019, T.H.) and the Excellence Initiative of the German Research Foundation (GSC-4, Spemann Graduate School, T.H., and Brain-Links Brain-Tools, M.B.). The authors apologize for the fact that, owing to space limits, not all relevant papers could be cited.
Footnotes
Competing interests
The authors declare no competing interests.
Bibliography
- 1.Schacter DL, Tulving E. Memory systems 1994. MIT Press; 1994. [Google Scholar]
- 2.Eichenbaum H. The cognitive neuroscience of memory: an introduction. Oxford University Press; 2012. [Google Scholar]
- 3.Squire LR. Mechanisms of memory. Science. 1986;232:1612–1619. doi: 10.1126/science.3086978. [DOI] [PubMed] [Google Scholar]
- 4.Tonegawa S, Morrissey MD, Kitamura T. The role of engram cells in the systems consolidation of memory. Nature Reviews Neuroscience. 2018;19:485–498. doi: 10.1038/s41583-018-0031-2. [DOI] [PubMed] [Google Scholar]
- 5.Corkin S. What’s new with the amnesic patient H.M.? Nat Rev Neurosci. 2002;3:153–160. doi: 10.1038/nrn726. [DOI] [PubMed] [Google Scholar]
- 6.Nadel L, Samsonovich A, Ryan L, Moscovitch M. Multiple trace theory of human memory: computational, neuroimaging, and neuropsychological results. Hippocampus. 2000;10:352–368. doi: 10.1002/1098-1063(2000)10:4<352::AID-HIPO2>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 7.Rosenbaum RS, et al. Remote spatial memory in an amnesic person with extensive bilateral hippocampal lesions. Nat Neurosci. 2000;3:1044–1048. doi: 10.1038/79867. [DOI] [PubMed] [Google Scholar]
- 8.Yonelinas AP, Ranganath C, Ekstrom AD, Wiltgen BJ. A contextual binding theory of episodic memory: systems consolidation reconsidered. Nat Rev Neurosci. 2019 doi: 10.1038/s41583-019-0150-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gelbard-Sagiv H, Mukamel R, Harel M, Malach R, Fried I. Internally generated reactivation of single neurons in human hippocampus during free recall. Science. 2008;322:96–101. doi: 10.1126/science.1164685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Okuyama T, Kitamura T, Roy DS, Itohara S, Tonegawa S. Ventral CA1 neurons store social memory. Science. 2016;353:1536–1541. doi: 10.1126/science.aaf7003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Keefe J, Dostrovsky J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 1971;34:171–175. doi: 10.1016/0006-8993(71)90358-1. [DOI] [PubMed] [Google Scholar]
- 12.Miller JF, et al. Neural activity in human hippocampal formation reveals the spatial context of retrieved memories. Science. 2013;342:1111–1114. doi: 10.1126/science.1244056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ison MJ, Quiroga R Quian, Fried I. Rapid Encoding of New Memories by Individual Neurons in the Human Brain. Neuron. 2015;87:220–230. doi: 10.1016/j.neuron.2015.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Markus EJ, et al. Interactions between location and task affect the spatial and directional firing of hippocampal neurons. J Neurosci. 1995;15:7079–7094. doi: 10.1523/JNEUROSCI.15-11-07079.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McHugh TJ, et al. Dentate Gyrus NMDA Receptors Mediate Rapid Pattern Separation in the Hippocampal Network. Science. 2007;317:94–99. doi: 10.1126/science.1140263. [ Mice with genetically ablated NR1 subunits of NMDARs at PP-GC synapses show impaired spatial context discrimination in a CFC memory task and impaired contextual remapping of CA3 pyramidal cells. ] [DOI] [PubMed] [Google Scholar]
- 16.Lisman J, et al. Viewpoints: how the hippocampus contributes to memory, navigation and cognition. Nat Neurosci. 2017;20:1434–1447. doi: 10.1038/nn.4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jensen O, Lisman JE. Hippocampal sequence-encoding driven by a cortical multi-item working memory buffer. Trends Neurosci. 2005;28:67–72. doi: 10.1016/j.tins.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 18.Buzsáki G, Moser EI. Memory, navigation and theta rhythm in the hippocampal-entorhinal system. Nat Neurosci. 2013;16:130–138. doi: 10.1038/nn.3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boldrini M, et al. Human Hippocampal Neurogenesis Persists throughout Aging. Cell Stem Cell. 2018;22:589–599.e5. doi: 10.1016/j.stem.2018.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patzke N, et al. In contrast to many other mammals, cetaceans have relatively small hippocampi that appear to lack adult neurogenesis. Brain Struct Funct. 2015;220:361–383. doi: 10.1007/s00429-013-0660-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sorrells SF, et al. Human hippocampal neurogenesis drops sharply in children to undetectable levels in adults. Nature. 2018;555:377–381. doi: 10.1038/nature25975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McNaughton BL, Morris RGM. Hippocampal synaptic enhancement and information storage within a distributed memory system. Trends in Neurosciences. 1987;10:408–415. [Google Scholar]
- 23.McNaughton B, Nadel L. Hebb-Marr Networks and the Neurobiological Representation of Action in Space. In: Gluck MA, Rumelhart DE, editors. Neuroscience and connectionist theory. L. Erlbaum Associates; 1990. [Google Scholar]
- 24.Gilbert PE, Kesner RP, Lee I. Dissociating hippocampal subregions: double dissociation between dentate gyrus and CA1. Hippocampus. 2001;11:626–636. doi: 10.1002/hipo.1077. [DOI] [PubMed] [Google Scholar]
- 25.Xavier GF, Costa VCI. Dentate gyrus and spatial behaviour. Progress in NeuroPsychopharmacology and Biological Psychiatry. 2009;33:762–773. doi: 10.1016/j.pnpbp.2009.03.036. [DOI] [PubMed] [Google Scholar]
- 26.Sasaki T, et al. Dentate network activity is necessary for spatial working memory by supporting CA3 sharp-wave ripple generation and prospective firing of CA3 neurons. Nat Neurosci. 2018;21:258–269. doi: 10.1038/s41593-017-0061-5. [ Selective ablation of GCs with colchicine reduces the occurrence of awake SWRs and the occurrence of CA3 ensembles encoding the future-trajectory in a SWM paradigm. This deficit is highly correlated with the loss of MF innervation and SWM performance. ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Treves A, Rolls ET. Computational analysis of the role of the hippocampus in memory. Hippocampus. 1994;4:374–391. doi: 10.1002/hipo.450040319. [ Seminal theoretical work outlining a model of the hippocampus’ computational function and proposing a mechanism by which DG-mediated pattern separation can enhance hippocampal storage capacity. ] [DOI] [PubMed] [Google Scholar]
- 28.Leutgeb JK, Leutgeb S, Moser M-B, Moser EI. Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science. 2007;315:961–966. doi: 10.1126/science.1135801. [ Experimental study showing that the firing patterns of DG neurons discriminate markedly between similar spatial enclosures. ] [DOI] [PubMed] [Google Scholar]
- 29.Neunuebel JP, Knierim JJ. CA3 retrieves coherent representations from degraded input: direct evidence for CA3 pattern completion and dentate gyrus pattern separation. Neuron. 2014;81:416–427. doi: 10.1016/j.neuron.2013.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakashiba T, et al. Young dentate granule cells mediate pattern separation, whereas old granule cells facilitate pattern completion. Cell. 2012;149:188–201. doi: 10.1016/j.cell.2012.01.046. [ Disruption of the output from mature- and young GCs shows that mature GCs promote rapid memory recall based on depleted cues, while young GCs facilitate the discrimination of similar spatial contexts. ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hunsaker MR, Rosenberg JS, Kesner RP. The role of the dentate gyrus, CA3a,b, and CA3c for detecting spatial and environmental novelty. Hippocampus. 2008;18:1064–1073. doi: 10.1002/hipo.20464. [DOI] [PubMed] [Google Scholar]
- 32.Lee JW, Jung MW. Separation or binding? Role of the dentate gyrus in hippocampal mnemonic processing. Neurosci Biobehav Rev. 2017;75:183–194. doi: 10.1016/j.neubiorev.2017.01.049. [DOI] [PubMed] [Google Scholar]
- 33.Liu X, et al. Optogenetic stimulation of a hippocampal engram activates fear memory recall. Nature. 2012;484:381–385. doi: 10.1038/nature11028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramirez S, et al. Creating a false memory in the hippocampus. Science. 2013;341:387–391. doi: 10.1126/science.1239073. [DOI] [PubMed] [Google Scholar]
- 35.Freund TF, Buzsáki G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 36.Amaral DG, Scharfman HE, Lavenex P. The dentate gyrus: fundamental neuroanatomical organization (dentate gyrus for dummies) Prog Brain Res. 2007;163:3–22. doi: 10.1016/S0079-6123(07)63001-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pernía-Andrade AJ, Jonas P. Theta-gamma-modulated synaptic currents in hippocampal granule cells in vivo define a mechanism for network oscillations. Neuron. 2014;81:140–152. doi: 10.1016/j.neuron.2013.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Danielson NB, et al. Distinct Contribution of Adult-Born Hippocampal Granule Cells to Context Encoding. Neuron. 2016;90:101–112. doi: 10.1016/j.neuron.2016.02.019. [ Two-photon calcium imaging reveals that young adult-born GCs show higher activity levels, but lower spatial tuning and similarly moderate context-selectivity compared to mature GCs. ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Danielson NB, et al. In Vivo Imaging of Dentate Gyrus Mossy Cells in Behaving Mice. Neuron. 2017;93:552–559.e4. doi: 10.1016/j.neuron.2016.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Diamantaki M, Frey M, Berens P, Preston-Ferrer P, Burgalossi A. Sparse activity of identified dentate granule cells during spatial exploration. Elife. 2016;5 doi: 10.7554/eLife.20252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.GoodSmith D, et al. Spatial Representations of Granule Cells and Mossy Cells of the Dentate Gyrus. Neuron. 2017;93:677–690.e5. doi: 10.1016/j.neuron.2016.12.026. [ Single-unit and juxtacellular recordings from DG and CA3 cells reveal that independent sets of GCs, but overlapping sets of MCs are active in widely distinct enclosures located in different rooms and demonstrate two modes of pattern separation in distinct excitatory cell populations of the DG. ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Senzai Y, Buzsáki G. Physiological Properties and Behavioral Correlates of Hippocampal Granule Cells and Mossy Cells. Neuron. 2017;93:691–704.e5. doi: 10.1016/j.neuron.2016.12.011. [ This study established and validated classification criteria for GCs and MCs in single-unit recording data and finds stronger context-dependent remapping in MCs than in GCs. ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hainmueller T, Bartos M. Parallel emergence of stable and dynamic memory engrams in the hippocampus. Nature. 2018;558:292–296. doi: 10.1038/s41586-018-0191-2. [ Chronic two-photon Calcium imaging in head-fixed mice performing a spatial learning paradigm over multiple days reveals highly stable, little context-selective place-fields in GCs, but strong remapping over days and contexts in CA3 and CA1. ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qin H, et al. A Visual-Cue-Dependent Memory Circuit for Place Navigation. Neuron. 2018;99:47–55.e4. doi: 10.1016/j.neuron.2018.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guo N, et al. Dentate granule cell recruitment of feedforward inhibition governs engram maintenance and remote memory generalization. Nat Med. 2018;24:438–449. doi: 10.1038/nm.4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Dijk MT, Fenton AA. On How the Dentate Gyrus Contributes to Memory Discrimination. Neuron. 2018;98:832–845.e5. doi: 10.1016/j.neuron.2018.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ruediger S, et al. Learning-related feedforward inhibitory connectivity growth required for memory precision. Nature. 2011;473:514–518. doi: 10.1038/nature09946. [ MF-LTP and long-lasting reversible increase in filopodial synapses onto feed-forward interneurons in CA3 is required for contextual and spatial memory precision. ] [DOI] [PubMed] [Google Scholar]
- 48.Krueppel R, Remy S, Beck H. Dendritic integration in hippocampal dentate granule cells. Neuron. 2011;71:512–528. doi: 10.1016/j.neuron.2011.05.043. [DOI] [PubMed] [Google Scholar]
- 49.Sloviter RS, et al. Selective loss of hippocampal granule cells in the mature rat brain after adrenalectomy. Science. 1989;243:535–538. doi: 10.1126/science.2911756. [DOI] [PubMed] [Google Scholar]
- 50.Woods NI, et al. Preferential Targeting of Lateral Entorhinal Inputs onto Newly Integrated Granule Cells. J Neurosci. 2018;38:5843–5853. doi: 10.1523/JNEUROSCI.1737-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Scharfman HE. The CA3 ‘backprojection’ to the dentate gyrus. Prog Brain Res. 2007;163:627–637. doi: 10.1016/S0079-6123(07)63034-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Scharfman HE. The enigmatic mossy cell of the dentate gyrus. Nat Rev Neurosci. 2016;17:562–575. doi: 10.1038/nrn.2016.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li XG, Somogyi P, Ylinen A, Buzsáki G. The hippocampal CA3 network: an in vivo intracellular labeling study. J Comp Neurol. 1994;339:181–208. doi: 10.1002/cne.903390204. [DOI] [PubMed] [Google Scholar]
- 54.Vivar C, et al. Monosynaptic inputs to new neurons in the dentate gyrus. Nat Commun. 2012;3 doi: 10.1038/ncomms2101. 1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sun Y, Grieco SF, Holmes TC, Xu X. Local and Long-Range Circuit Connections to Hilar Mossy Cells in the Dentate Gyrus. eNeuro. 2017;4 doi: 10.1523/ENEURO.0097-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Acsády L, Kamondi A, Sík A, Freund T, Buzsáki G. GABAergic cells are the major postsynaptic targets of mossy fibers in the rat hippocampus. J Neurosci. 1998;18:3386–3403. doi: 10.1523/JNEUROSCI.18-09-03386.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nicoll RA, Schmitz D. Synaptic plasticity at hippocampal mossy fibre synapses. Nat Rev Neurosci. 2005;6:863–876. doi: 10.1038/nrn1786. [DOI] [PubMed] [Google Scholar]
- 58.Hainmüller T, Krieglstein K, Kulik A, Bartos M. Joint CP-AMPA and group I mGlu receptor activation is required for synaptic plasticity in dentate gyrus fast-spiking interneurons. Proc Natl Acad Sci USA. 2014;111:13211–13216. doi: 10.1073/pnas.1409394111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zucca S, et al. Control of Spike Transfer at Hippocampal Mossy Fiber Synapses In Vivo by GABA A and GABA B Receptor-Mediated Inhibition. The Journal of Neuroscience. 2017;37:587–598. doi: 10.1523/JNEUROSCI.2057-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Henze DA, Wittner L, Buzsáki G. Single granule cells reliably discharge targets in the hippocampal CA3 network in vivo. Nat Neurosci. 2002;5:790–795. doi: 10.1038/nn887. [ Stimulation of single GCs and recordings of their CA3 targets in anaesthetized rats revealed that high-frequency GC activity recruits pyramidal cell firing while low-frequency activity primarily activates interneurons. ] [DOI] [PubMed] [Google Scholar]
- 61.Mori M, Abegg MH, Gähwiler BH, Gerber U. A frequency-dependent switch from inhibition to excitation in a hippocampal unitary circuit. Nature. 2004;431:453–456. doi: 10.1038/nature02854. [DOI] [PubMed] [Google Scholar]
- 62.Vyleta NP, Borges-Merjane C, Jonas P. Plasticity-dependent, full detonation at hippocampal mossy fiber-CA3 pyramidal neuron synapses. Elife. 2016;5 doi: 10.7554/eLife.17977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee J, et al. Transient effect of mossy fiber stimulation on spatial firing of CA3 neurons. Hippocampus. 2019 doi: 10.1002/hipo.23066. [DOI] [PubMed] [Google Scholar]
- 64.Tsukamoto M, et al. Mossy fibre synaptic NMDA receptors trigger non-hebbian long-term potentiation at entorhino-CA3 synapses in the rat. The Journal of Physiology. 2003;546:665–675. doi: 10.1113/jphysiol.2002.033803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McNaughton BL, Barnes CA, Meltzer J, Sutherland RJ. Hippocampal granule cells are necessary for normal spatial learning but not for spatially-selective pyramidal cell discharge. Exp Brain Res. 1989;76:485–496. doi: 10.1007/BF00248904. [DOI] [PubMed] [Google Scholar]
- 66.Buzsáki G, Leung LW, Vanderwolf CH. Cellular bases of hippocampal EEG in the behaving rat. Brain Res. 1983;287:139–171. doi: 10.1016/0165-0173(83)90037-1. [DOI] [PubMed] [Google Scholar]
- 67.Jung MW, McNaughton BL. Spatial selectivity of unit activity in the hippocampal granular layer. Hippocampus. 1993;3:165–182. doi: 10.1002/hipo.450030209. [DOI] [PubMed] [Google Scholar]
- 68.Pilz G-A, et al. Functional Imaging of Dentate Granule Cells in the Adult Mouse Hippocampus. J Neurosci. 2016;36:7407–7414. doi: 10.1523/JNEUROSCI.3065-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Senzai Y. Function of local circuits in the hippocampal dentate gyrus-CA3 system. Neurosci Res. 2018 doi: 10.1016/j.neures.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 70.Chawla MK, et al. Sparse, environmentally selective expression ofArc RNA in the upper blade of the rodent fascia dentata by brief spatial experience. Hippocampus. 2005;15:579–586. doi: 10.1002/hipo.20091. [DOI] [PubMed] [Google Scholar]
- 71.Deng W, Mayford M, Gage FH. Selection of distinct populations of dentate granule cells in response to inputs as a mechanism for pattern separation in mice. Elife. 2013;2:e00312. doi: 10.7554/eLife.00312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Labiner DM, et al. Induction of c-fos mRNA by kindled seizures: complex relationship with neuronal burst firing. J Neurosci. 1993;13:744–751. doi: 10.1523/JNEUROSCI.13-02-00744.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kim S, Kim Y, Lee S-H, Ho W-K. Dendritic spikes in hippocampal granule cells are necessary for long-term potentiation at the perforant path synapse. Elife. 2018;7 doi: 10.7554/eLife.35269. [ Combined dual somato-dendritic patch clamp recordings in hippocampal slices and pharmacology demonstrate that PP-LTP onto GCs requires active, NMDAR-driven dendritic events. ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ying S-W, et al. Brain-derived neurotrophic factor induces long-term potentiation in intact adult hippocampus: requirement for ERK activation coupled to CREB and upregulation of Arc synthesis. J Neurosci. 2002;22:1532–1540. doi: 10.1523/JNEUROSCI.22-05-01532.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nitz D, McNaughton B. Differential modulation of CA1 and dentate gyrus interneurons during exploration of novel environments. J Neurophysiol. 2004;91:863–872. doi: 10.1152/jn.00614.2003. [DOI] [PubMed] [Google Scholar]
- 76.Diamantaki M, Frey M, Preston-Ferrer P, Burgalossi A. Priming Spatial Activity by Single-Cell Stimulation in the Dentate Gyrus of Freely Moving Rats. Curr Biol. 2016;26:536–541. doi: 10.1016/j.cub.2015.12.053. [ Juxtacellular recordings in freely-moving mice show that permanent place-fields in GCs can be induced by strong depolarization that would be suitable to trigger dendritic depolarization. This process is facilitated in a novel environment ] [DOI] [PubMed] [Google Scholar]
- 77.Bittner KC, Milstein AD, Grienberger C, Romani S, Magee JC. Behavioral time scale synaptic plasticity underlies CA1 place fields. Science. 2017;357:1033–1036. doi: 10.1126/science.aan3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sheffield MEJ, Adoff MD, Dombeck DA. Increased Prevalence of Calcium Transients across the Dendritic Arbor during Place Field Formation. Neuron. 2017;96:490–504. doi: 10.1016/j.neuron.2017.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Marín-Burgin A, Mongiat LA, Pardi MB, Schinder AF. Unique processing during a period of high excitation/inhibition balance in adult-born neurons. Science. 2012;335:1238–1242. doi: 10.1126/science.1214956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schmidt-Hieber C, Jonas P, Bischofberger J. Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature. 2004;429:184–187. doi: 10.1038/nature02553. [DOI] [PubMed] [Google Scholar]
- 81.Toni N, Schinder AF. Maturation and Functional Integration of New Granule Cells into the Adult Hippocampus. Cold Spring Harb Perspect Biol. 2015;8 doi: 10.1101/cshperspect.a018903. a018903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Denny CA, Burghardt NS, Schachter DM, Hen R, Drew MR. 4- to 6-week-old adult-born hippocampal neurons influence novelty-evoked exploration and contextual fear conditioning. Hippocampus. 2012;22:1188–1201. doi: 10.1002/hipo.20964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gu Y, et al. Optical controlling reveals time-dependent roles for adult-born dentate granule cells. Nat Neurosci. 2012;15:1700–1706. doi: 10.1038/nn.3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhuo J-M, et al. Young adult born neurons enhance hippocampal dependent performance via influences on bilateral networks. Elife. 2016;5 doi: 10.7554/eLife.22429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Alvarez DD, et al. A disynaptic feedback network activated by experience promotes the integration of new granule cells. Science. 2016;354:459–465. doi: 10.1126/science.aaf2156. [DOI] [PubMed] [Google Scholar]
- 86.Yeh C-Y, et al. Mossy Cells Control Adult Neural Stem Cell Quiescence and Maintenance through a Dynamic Balance between Direct and Indirect Pathways. Neuron. 2018;99:493–510.e4. doi: 10.1016/j.neuron.2018.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kumamoto N, et al. A role for primary cilia in glutamatergic synaptic integration of adult-born neurons. Nat Neurosci. 2012;15:399–405. doi: 10.1038/nn.3042. S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vivar C, van Praag H. Functional circuits of new neurons in the dentate gyrus. Front Neural Circuits. 2013;7:15. doi: 10.3389/fncir.2013.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Restivo L, Niibori Y, Mercaldo V, Josselyn SA, Frankland PW. Development of Adult-Generated Cell Connectivity with Excitatory and Inhibitory Cell Populations in the Hippocampus. J Neurosci. 2015;35:10600–10612. doi: 10.1523/JNEUROSCI.3238-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dieni CV, Nietz AK, Panichi R, Wadiche JI, Overstreet-Wadiche L. Distinct determinants of sparse activation during granule cell maturation. J Neurosci. 2013;33:19131–19142. doi: 10.1523/JNEUROSCI.2289-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Temprana SG, et al. Delayed Coupling to Feedback Inhibition during a Critical Period for the Integration of Adult-Born Granule Cells. Neuron. 2015;85:116–130. doi: 10.1016/j.neuron.2014.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pignatelli M, et al. Engram Cell Excitability State Determines the Efficacy of Memory Retrieval. Neuron. 2019;101:274–284.e5. doi: 10.1016/j.neuron.2018.11.029. [ This study combines cellular physiology and behavioral experiments to demonstrate that the excitability of ‘engram’ GCs which were active at memory encoding rapidly increases after re-exposure to the memorized situation and can promote subsequent retrieval of the memorized contents ] [DOI] [PubMed] [Google Scholar]
- 93.Scharfman HE. Dentate hilar cells with dendrites in the molecular layer have lower thresholds for synaptic activation by perforant path than granule cells. J Neurosci. 1991;11:1660–1673. doi: 10.1523/JNEUROSCI.11-06-01660.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Soltesz I, Bourassa J, Deschênes M. The behavior of mossy cells of the rat dentate gyrus during theta oscillations in vivo. Neuroscience. 1993;57:555–564. doi: 10.1016/0306-4522(93)90005-z. [DOI] [PubMed] [Google Scholar]
- 95.Sík A, Coté A, Boldogkõi Z. Selective spread of neurotropic herpesviruses in the rat hippocampus. J Comp Neurol. 2006;496:229–243. doi: 10.1002/cne.20921. [DOI] [PubMed] [Google Scholar]
- 96.Jung D, et al. Dentate granule and mossy cells exhibit distinct spatiotemporal responses to local change in a one-dimensional landscape of visual-tactile cues. Sci Rep. 2019;9 doi: 10.1038/s41598-019-45983-6. 9545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci. 2007;8:45–56. doi: 10.1038/nrn2044. [DOI] [PubMed] [Google Scholar]
- 98.Hosp JA, et al. Morpho-physiological criteria divide dentate gyrus interneurons into classes. Hippocampus. 2014;24:189–203. doi: 10.1002/hipo.22214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Strüber M, Sauer J-F, Jonas P, Bartos M. Distance-dependent inhibition facilitates focality of gamma oscillations in the dentate gyrus. Nature Communications. 2017;8 doi: 10.1038/s41467-017-00936-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bartos M, et al. Fast synaptic inhibition promotes synchronized gamma oscillations in hippocampal interneuron networks. Proc Natl Acad Sci USA. 2002;99:13222–13227. doi: 10.1073/pnas.192233099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Szabo GG, et al. Extended Interneuronal Network of the Dentate Gyrus. Cell Rep. 2017;20:1262–1268. doi: 10.1016/j.celrep.2017.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Savanthrapadian S, et al. Synaptic properties of SOM- and CCK-expressing cells in dentate gyrus interneuron networks. J Neurosci. 2014;34:8197–8209. doi: 10.1523/JNEUROSCI.5433-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Buckmaster PS, Yamawaki R, Zhang GF. Axon arbors and synaptic connections of a vulnerable population of interneurons in the dentate gyrus in vivo. J Comp Neurol. 2002;445:360–373. doi: 10.1002/cne.10183. [DOI] [PubMed] [Google Scholar]
- 104.Yuan M, et al. Somatostatin-positive interneurons in the dentate gyrus of mice provide local- and long-range septal synaptic inhibition. Elife. 2017;6 doi: 10.7554/eLife.21105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lee C-T, et al. Causal Evidence for the Role of Specific GABAergic Interneuron Types in Entorhinal Recruitment of Dentate Granule Cells. Sci Rep. 2016;6 doi: 10.1038/srep36885. 36885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Stefanelli T, Bertollini C, Lüscher C, Muller D, Mendez P. Hippocampal Somatostatin Interneurons Control the Size of Neuronal Memory Ensembles. Neuron. 2016;89:1074–1085. doi: 10.1016/j.neuron.2016.01.024. [DOI] [PubMed] [Google Scholar]
- 107.Hafting T, Fyhn M, Molden S, Moser M-B, Moser EI. Microstructure of a spatial map in the entorhinal cortex. Nature. 2005;436:801–806. doi: 10.1038/nature03721. [DOI] [PubMed] [Google Scholar]
- 108.Hargreaves EL, Rao G, Lee I, Knierim JJ. Major dissociation between medial and lateral entorhinal input to dorsal hippocampus. Science. 2005;308:1792–1794. doi: 10.1126/science.1110449. [DOI] [PubMed] [Google Scholar]
- 109.Høydal ØA, Skytøen ER, Andersson SO, Moser M-B, Moser EI. Object-vector coding in the medial entorhinal cortex. Nature. 2019;568:400–404. doi: 10.1038/s41586-019-1077-7. [DOI] [PubMed] [Google Scholar]
- 110.Aronov D, Nevers R, Tank DW. Mapping of a non-spatial dimension by the hippocampal-entorhinal circuit. Nature. 2017;543:719–722. doi: 10.1038/nature21692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Heys JG, Dombeck DA. Evidence for a subcircuit in medial entorhinal cortex representing elapsed time during immobility. Nat Neurosci. 2018;21:1574–1582. doi: 10.1038/s41593-018-0252-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Miao C, et al. Hippocampal Remapping after Partial Inactivation of the Medial Entorhinal Cortex. Neuron. 2015;88:590–603. doi: 10.1016/j.neuron.2015.09.051. [DOI] [PubMed] [Google Scholar]
- 113.Kanter BR, et al. A Novel Mechanism for the Grid-to-Place Cell Transformation Revealed by Transgenic Depolarization of Medial Entorhinal Cortex Layer II. Neuron. 2017;93:1480–1492.e6. doi: 10.1016/j.neuron.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 114.Schlesiger MI, Boublil BL, Hales JB, Leutgeb JK, Leutgeb S. Hippocampal Global Remapping Can Occur without Input from the Medial Entorhinal Cortex. Cell Rep. 2018;22:3152–3159. doi: 10.1016/j.celrep.2018.02.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mallory CS, Hardcastle K, Bant JS, Giocomo LM. Grid scale drives the scale and long-term stability of place maps. Nat Neurosci. 2018;21:270–282. doi: 10.1038/s41593-017-0055-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Diehl GW, Hon OJ, Leutgeb S, Leutgeb JK. Stability of medial entorhinal cortex representations over time. Hippocampus. 2019;29:284–302. doi: 10.1002/hipo.23017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Keene CS, et al. Complementary Functional Organization of Neuronal Activity Patterns in the Perirhinal, Lateral Entorhinal, and Medial Entorhinal Cortices. The Journal of Neuroscience. 2016;36:3660–3675. doi: 10.1523/JNEUROSCI.4368-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Deshmukh SS, Knierim JJ. Representation of non-spatial and spatial information in the lateral entorhinal cortex. Front Behav Neurosci. 2011;5:69. doi: 10.3389/fnbeh.2011.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tsao A, Moser M-B, Moser EI. Traces of experience in the lateral entorhinal cortex. Curr Biol. 2013;23:399–405. doi: 10.1016/j.cub.2013.01.036. [DOI] [PubMed] [Google Scholar]
- 120.Wang C, et al. Egocentric coding of external items in the lateral entorhinal cortex. Science. 2018;362:945–949. doi: 10.1126/science.aau4940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tsao A, et al. Integrating time from experience in the lateral entorhinal cortex. Nature. 2018;561:57–62. doi: 10.1038/s41586-018-0459-6. [DOI] [PubMed] [Google Scholar]
- 122.Mankin EA, et al. Neuronal code for extended time in the hippocampus. Proc Natl Acad Sci USA. 2012;109:19462–19467. doi: 10.1073/pnas.1214107109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ziv Y, et al. Long-term dynamics of CA1 hippocampal place codes. Nat Neurosci. 2013;16:264–266. doi: 10.1038/nn.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Larimer P, Strowbridge BW. Nonrandom local circuits in the dentate gyrus. J Neurosci. 2008;28:12212–12223. doi: 10.1523/JNEUROSCI.3612-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ishizuka N, Weber J, Amaral DG. Organization of intrahippocampal projections originating from CA3 pyramidal cells in the rat. J Comp Neurol. 1990;295:580–623. doi: 10.1002/cne.902950407. [DOI] [PubMed] [Google Scholar]
- 126.Wu K, Canning KJ, Leung LS. Functional interconnections between CA3 and the dentate gyrus revealed by current source density analysis. Hippocampus. 1998;8:217–230. doi: 10.1002/(SICI)1098-1063(1998)8:3<217::AID-HIPO5>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 127.Hsu T-T, Lee C-T, Tai M-H, Lien C-C. Differential Recruitment of Dentate Gyrus Interneuron Types by Commissural Versus Perforant Pathways. Cereb Cortex. 2016;26:2715–2727. doi: 10.1093/cercor/bhv127. [DOI] [PubMed] [Google Scholar]
- 128.Hashimotodani Y, et al. LTP at Hilar Mossy Cell-Dentate Granule Cell Synapses Modulates Dentate Gyrus Output by Increasing Excitation/Inhibition Balance. Neuron. 2017;95:928–943.e3. doi: 10.1016/j.neuron.2017.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Buzsàki G, Eidelberg E. Commissural projection to the dentate gyrus of the rat: evidence for feed-forward inhibition. Brain Res. 1981;230:346–350. doi: 10.1016/0006-8993(81)90413-3. [DOI] [PubMed] [Google Scholar]
- 130.Myers CE, Scharfman HE. Pattern separation in the dentate gyrus: a role for the CA3 backprojection. Hippocampus. 2011;21:1190–1215. doi: 10.1002/hipo.20828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ylinen A, et al. Intracellular correlates of hippocampal theta rhythm in identified pyramidal cells, granule cells, and basket cells. Hippocampus. 1995;5:78–90. doi: 10.1002/hipo.450050110. [DOI] [PubMed] [Google Scholar]
- 132.Pych JC, Chang Q, Colon-Rivera C, Haag R, Gold PE. Acetylcholine release in the hippocampus and striatum during place and response training. Learn Mem. 2005;12:564–572. doi: 10.1101/lm.33105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Giocomo LM, Hasselmo ME. Neuromodulation by glutamate and acetylcholine can change circuit dynamics by regulating the relative influence of afferent input and excitatory feedback. Mol Neurobiol. 2007;36:184–200. doi: 10.1007/s12035-007-0032-z. [DOI] [PubMed] [Google Scholar]
- 134.Rogers JL, Kesner RP. Cholinergic modulation of the hippocampus during encoding and retrieval. Neurobiol Learn Mem. 2003;80:332–342. doi: 10.1016/s1074-7427(03)00063-7. [DOI] [PubMed] [Google Scholar]
- 135.Kahle JS, Cotman CW. Carbachol depresses synaptic responses in the medial but not the lateral perforant path. Brain Res. 1989;482:159–163. doi: 10.1016/0006-8993(89)90554-4. [DOI] [PubMed] [Google Scholar]
- 136.Burgard EC, Sarvey JM. Muscarinic receptor activation facilitates the induction of long-term potentiation (LTP) in the rat dentate gyrus. Neurosci Lett. 1990;116:34–39. doi: 10.1016/0304-3940(90)90382-j. [DOI] [PubMed] [Google Scholar]
- 137.Pabst M, et al. Astrocyte Intermediaries of Septal Cholinergic Modulation in the Hippocampus. Neuron. 2016;90:853–865. doi: 10.1016/j.neuron.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 138.Wagatsuma A, et al. Locus coeruleus input to hippocampal CA3 drives single-trial learning of a novel context. Proceedings of the National Academy of Sciences. 2018;115:E310–E316. doi: 10.1073/pnas.1714082115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Takeuchi T, et al. Locus coeruleus and dopaminergic consolidation of everyday memory. Nature. 2016;537:357–362. doi: 10.1038/nature19325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Harley C, Milway JS, Lacaille JC. Locus coeruleus potentiation of dentate gyrus responses: evidence for two systems. Brain Res Bull. 1989;22:643–650. doi: 10.1016/0361-9230(89)90084-1. [DOI] [PubMed] [Google Scholar]
- 141.Yeckel MF, Berger TW. Feedforward excitation of the hippocampus by afferents from the entorhinal cortex: redefinition of the role of the trisynaptic pathway. Proc Natl Acad Sci USA. 1990;87:5832–5836. doi: 10.1073/pnas.87.15.5832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Do VH, Martinez CO, Martinez JL, Derrick BE. Long-Term Potentiation in Direct Perforant Path Projections to the Hippocampal CA3 Region In Vivo. Journal of Neurophysiology. 2002;87:669–678. doi: 10.1152/jn.00938.2000. [ In vivo calcium imaging and optogenetic manipulations show that a subpopulation of MEC layer II neurons forms salient and distinct representations of different spatial contexts which are necessary for CFC learning. ] [DOI] [PubMed] [Google Scholar]
- 143.Kitamura T, et al. Island cells control temporal association memory. Science. 2014;343:896–901. doi: 10.1126/science.1244634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kitamura T, et al. Entorhinal Cortical Ocean Cells Encode Specific Contexts and Drive Context-Specific Fear Memory. Neuron. 2015;87:1317–1331. doi: 10.1016/j.neuron.2015.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Amaral DG, Ishizuka N, Claiborne B. Neurons, numbers and the hippocampal network. Prog Brain Res. 1990;83:1–11. doi: 10.1016/s0079-6123(08)61237-6. [DOI] [PubMed] [Google Scholar]
- 146.Urban NN, Henze DA, Barrionuevo G. Revisiting the role of the hippocampal mossy fiber synapse. Hippocampus. 2001;11:408–417. doi: 10.1002/hipo.1055. [DOI] [PubMed] [Google Scholar]
- 147.Nakashiba T, Young JZ, McHugh TJ, Buhl DL, Tonegawa S. Transgenic inhibition of synaptic transmission reveals role of CA3 output in hippocampal learning. Science. 2008;319:1260–1264. doi: 10.1126/science.1151120. [DOI] [PubMed] [Google Scholar]
- 148.Gruart A, Sánchez-Campusano R, Fernández-Guizán A, Delgado-García JM. A Differential and Timed Contribution of Identified Hippocampal Synapses to Associative Learning in Mice. Cereb Cortex. 2015;25:2542–2555. doi: 10.1093/cercor/bhu054. [DOI] [PubMed] [Google Scholar]
- 149.Rolls ET. The storage and recall of memories in the hippocampo-cortical system. Cell and Tissue Research. 2017 doi: 10.1007/s00441-017-2744-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lee I, Kesner RP. Encoding versus retrieval of spatial memory: double dissociation between the dentate gyrus and the perforant path inputs into CA3 in the dorsal hippocampus. Hippocampus. 2004;14:66–76. doi: 10.1002/hipo.10167. [DOI] [PubMed] [Google Scholar]
- 151.Lassalle JM, Bataille T, Halley H. Reversible inactivation of the hippocampal mossy fiber synapses in mice impairs spatial learning, but neither consolidation nor memory retrieval, in the Morris navigation task. Neurobiol Learn Mem. 2000;73:243–257. doi: 10.1006/nlme.1999.3931. [DOI] [PubMed] [Google Scholar]
- 152.Kheirbek MA, et al. Differential Control of Learning and Anxiety along the Dorsoventral Axis of the Dentate Gyrus. Neuron. 2013;77:955–968. doi: 10.1016/j.neuron.2012.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Madroñal N, et al. Rapid erasure of hippocampal memory following inhibition of dentate gyrus granule cells. Nature Communications. 2016;7 doi: 10.1038/ncomms10923. 10923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Denny CA, et al. Hippocampal memory traces are differentially modulated by experience, time, and adult neurogenesis. Neuron. 2014;83:189–201. doi: 10.1016/j.neuron.2014.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Park S, et al. Neuronal Allocation to a Hippocampal Engram. Neuropsychopharmacology. 2016;41:2987–2993. doi: 10.1038/npp.2016.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Tayler KK, Tanaka KZ, Reijmers LG, Wiltgen BJ. Reactivation of neural ensembles during the retrieval of recent and remote memory. Curr Biol. 2013;23:99–106. doi: 10.1016/j.cub.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 157.McMahon DBT, Barrionuevo G. Short- and Long-Term Plasticity of the Perforant Path Synapse in Hippocampal Area CA3. Journal of Neurophysiology. 2002;88:528–533. doi: 10.1152/jn.2002.88.1.528. [DOI] [PubMed] [Google Scholar]
- 158.Kobayashi K, Poo M. Spike train timing-dependent associative modification of hippocampal CA3 recurrent synapses by mossy fibers. Neuron. 2004;41:445–454. doi: 10.1016/s0896-6273(03)00873-0. [DOI] [PubMed] [Google Scholar]
- 159.Rebola N, Carta M, Mulle C. Operation and plasticity of hippocampal CA3 circuits: implications for memory encoding. Nature Reviews Neuroscience. 2017;18:208–220. doi: 10.1038/nrn.2017.10. [DOI] [PubMed] [Google Scholar]
- 160.Nakazawa K, et al. Requirement for Hippocampal CA3 NMDA Receptors in Associative Memory Recall. Science. 2002;297:211–218. doi: 10.1126/science.1071795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Nakazawa K, et al. Hippocampal CA3 NMDA receptors are crucial for memory acquisition of one-time experience. Neuron. 2003;38:305–315. doi: 10.1016/s0896-6273(03)00165-x. [DOI] [PubMed] [Google Scholar]
- 162.Zalutsky RA, Nicoll RA. Comparison of two forms of long-term potentiation in single hippocampal neurons. Science. 1990;248:1619–1624. doi: 10.1126/science.2114039. [DOI] [PubMed] [Google Scholar]
- 163.Diamantaki M, et al. Manipulating Hippocampal Place Cell Activity by Single-Cell Stimulation in Freely Moving Mice. Cell Rep. 2018;23:32–38. doi: 10.1016/j.celrep.2018.03.031. [DOI] [PubMed] [Google Scholar]
- 164.Buzsáki G. Two-stage model of memory trace formation: a role for ‘noisy’ brain states. Neuroscience. 1989;31:551–570. doi: 10.1016/0306-4522(89)90423-5. [ Fundamental theoretical study postulating a two-stage memory model of memory formation involving heterosynaptic plasticity at recurrent- and PP-synapses by MF-input as a mechanism of initial memory storage. ] [DOI] [PubMed] [Google Scholar]
- 165.Hagena H, Manahan-Vaughan D. Learning-facilitated synaptic plasticity at CA3 mossy fiber and commissural-associational synapses reveals different roles in information processing. Cereb Cortex. 2011;21:2442–2449. doi: 10.1093/cercor/bhq271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ryan TJ, Roy DS, Pignatelli M, Arons A, Tonegawa S. Engram cells retain memory under retrograde amnesia. Science. 2015;348:1007–1013. doi: 10.1126/science.aaa5542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kitamura T, et al. Engrams and circuits crucial for systems consolidation of a memory. Science. 2017;356:73–78. doi: 10.1126/science.aam6808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Bernier BE, et al. Dentate Gyrus Contributes to Retrieval as well as Encoding: Evidence from Context Fear Conditioning, Recall, and Extinction. The Journal of Neuroscience. 2017;37:6359–6371. doi: 10.1523/JNEUROSCI.3029-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Girardeau G, Benchenane K, Wiener SI, Buzsáki G, Zugaro MB. Selective suppression of hippocampal ripples impairs spatial memory. Nat Neurosci. 2009;12:1222–1223. doi: 10.1038/nn.2384. [DOI] [PubMed] [Google Scholar]
- 170.Jadhav SP, Kemere C, German PW, Frank LM. Awake hippocampal sharp-wave ripples support spatial memory. Science. 2012;336:1454–1458. doi: 10.1126/science.1217230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.de Almeida L, Idiart M, Lisman JE. The input-output transformation of the hippocampal granule cells: from grid cells to place fields. J Neurosci. 2009;29:7504–7512. doi: 10.1523/JNEUROSCI.6048-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Marr D. Simple memory: a theory for archicortex. Philos Trans R Soc Lond, B, Biol Sci. 1971;262:23–81. doi: 10.1098/rstb.1971.0078. [DOI] [PubMed] [Google Scholar]
- 173.Knierim JJ, Neunuebel JP. Tracking the flow of hippocampal computation: Pattern separation, pattern completion, and attractor dynamics. Neurobiology of Learning and Memory. 2016;129:38–49. doi: 10.1016/j.nlm.2015.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Jezek K, Henriksen EJ, Treves A, Moser EI, Moser M-B. Theta-paced flickering between place-cell maps in the hippocampus. Nature. 2011;478:246–249. doi: 10.1038/nature10439. [DOI] [PubMed] [Google Scholar]
- 175.Amaral DG, Lavenex P. Hippocampal Neuroanatomy. In: Andersen P, editor. The hippocampus book. Oxford University Press; 2007. pp. 37–114. [Google Scholar]
- 176.Fanselow MS. Contextual fear, gestalt memories, and the hippocampus. Behav Brain Res. 2000;110:73–81. doi: 10.1016/s0166-4328(99)00186-2. [DOI] [PubMed] [Google Scholar]
- 177.Marozzi E, Ginzberg LL, Alenda A, Jeffery KJ. Purely Translational Realignment in Grid Cell Firing Patterns Following Nonmetric Context Change. Cereb Cortex. 2015;25:4619–4627. doi: 10.1093/cercor/bhv120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Diehl GW, Leutgeb S. Spatial representations in medial entorhinal cortex remain stable over the course of hours in contrast to hippocampal CA1 and CA2 network ensembles. 2017 in. [Google Scholar]
- 179.Jones BW, et al. Targeted deletion of AKAP7 in dentate granule cells impairs spatial discrimination. Elife. 2016;5 doi: 10.7554/eLife.20695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Niibori Y, et al. Suppression of adult neurogenesis impairs population coding of similar contexts in hippocampal CA3 region. Nature Communications. 2012;3 doi: 10.1038/ncomms2261. 1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Marrone DF, Adams AA, Satvat E. Increased pattern separation in the aged fascia dentata. Neurobiol Aging. 2011;32:2317.e23–32. doi: 10.1016/j.neurobiolaging.2010.03.021. [DOI] [PubMed] [Google Scholar]
- 182.Cravens CJ, Vargas-Pinto N, Christian KM, Nakazawa K. CA3 NMDA receptors are crucial for rapid and automatic representation of context memory. Eur J Neurosci. 2006;24:1771–1780. doi: 10.1111/j.1460-9568.2006.05044.x. [DOI] [PubMed] [Google Scholar]
- 183.Cai DJ, et al. A shared neural ensemble links distinct contextual memories encoded close in time. Nature. 2016;534:115–118. doi: 10.1038/nature17955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Tronel S, et al. Adult-born neurons are necessary for extended contextual discrimination. Hippocampus. 2012;22:292–298. doi: 10.1002/hipo.20895. [DOI] [PubMed] [Google Scholar]
- 185.Sahay A, et al. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature. 2011;472:466–470. doi: 10.1038/nature09817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Neubrandt M, et al. Single Bursts of Individual Granule Cells Functionally Rearrange Feedforward Inhibition. The Journal of Neuroscience. 2018;38:1711–1724. doi: 10.1523/JNEUROSCI.1595-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Jinde S, et al. Hilar mossy cell degeneration causes transient dentate granule cell hyperexcitability and impaired pattern separation. Neuron. 2012;76:1189–1200. doi: 10.1016/j.neuron.2012.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Bui AD, et al. Dentate gyrus mossy cells control spontaneous convulsive seizures and spatial memory. Science. 2018;359:787–790. doi: 10.1126/science.aan4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Arriaga M, Han EB. Dedicated Hippocampal Inhibitory Networks for Locomotion and Immobility. The Journal of Neuroscience. 2017;37:9222–9238. doi: 10.1523/JNEUROSCI.1076-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Lovett-Barron M, et al. Dendritic inhibition in the hippocampus supports fear learning. Science. 2014;343:857–863. doi: 10.1126/science.1247485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Baker S, et al. The Human Dentate Gyrus Plays a Necessary Role in Discriminating New Memories. Curr Biol. 2016;26:2629–2634. doi: 10.1016/j.cub.2016.07.081. [DOI] [PubMed] [Google Scholar]
- 192.Clelland CD, et al. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325:210–213. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Morris AM, Churchwell JC, Kesner RP, Gilbert PE. Selective lesions of the dentate gyrus produce disruptions in place learning for adjacent spatial locations. Neurobiol Learn Mem. 2012;97:326–331. doi: 10.1016/j.nlm.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Guskjolen A, et al. Recovery of “Lost” Infant Memories in Mice. Current Biology. 2018;28:2283–2290.e3. doi: 10.1016/j.cub.2018.05.059. [DOI] [PubMed] [Google Scholar]
- 195.Roy DS, et al. Memory retrieval by activating engram cells in mouse models of early Alzheimer’s disease. Nature. 2016;531:508–512. doi: 10.1038/nature17172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Burghardt NS, Park EH, Hen R, Fenton AA. Adult-born hippocampal neurons promote cognitive flexibility in mice. Hippocampus. 2012;22:1795–1808. doi: 10.1002/hipo.22013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Luna VM, et al. Adult-born hippocampal neurons bidirectionally modulate entorhinal inputs into the dentate gyrus. Science. 2019;364:578–583. doi: 10.1126/science.aat8789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Manns JR, Howard MW, Eichenbaum H. Gradual changes in hippocampal activity support remembering the order of events. Neuron. 2007;56:530–540. doi: 10.1016/j.neuron.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Mau W, et al. The Same Hippocampal CA1 Population Simultaneously Codes Temporal Information over Multiple Timescales. Curr Biol. 2018;28:1499–1508.e4. doi: 10.1016/j.cub.2018.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Tanaka KZ, et al. The hippocampal engram maps experience but not place. Science. 2018;361:392–397. doi: 10.1126/science.aat5397. [DOI] [PubMed] [Google Scholar]
- 201.Santoro A. Reassessing pattern separation in the dentate gyrus. Frontiers in Behavioral Neuroscience. 2013;7 doi: 10.3389/fnbeh.2013.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Frederickson RE, Frederickson CJ, Danscher G. In situ binding of bouton zinc reversibly disrupts performance on a spatial memory task. Behav Brain Res. 1990;38:25–33. doi: 10.1016/0166-4328(90)90021-6. [DOI] [PubMed] [Google Scholar]
- 203.Choi J-H, et al. Interregional synaptic maps among engram cells underlie memory formation. Science. 2018;360:430–435. doi: 10.1126/science.aas9204. [DOI] [PubMed] [Google Scholar]
- 204.Johnson A, Redish AD. Neural ensembles in CA3 transiently encode paths forward of the animal at a decision point. J Neurosci. 2007;27:12176–12189. doi: 10.1523/JNEUROSCI.3761-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Murray AJ, et al. Parvalbumin-positive CA1 interneurons are required for spatial working but not for reference memory. Nat Neurosci. 2011;14:297–299. doi: 10.1038/nn.2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Leutgeb S, et al. Independent codes for spatial and episodic memory in hippocampal neuronal ensembles. Science. 2005;309:619–623. doi: 10.1126/science.1114037. [DOI] [PubMed] [Google Scholar]
- 207.Bakker A, Kirwan CB, Miller M, Stark CEL. Pattern separation in the human hippocampal CA3 and dentate gyrus. Science. 2008;319:1640–1642. doi: 10.1126/science.1152882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Neunuebel JP, Yoganarasimha D, Rao G, Knierim JJ. Conflicts between local and global spatial frameworks dissociate neural representations of the lateral and medial entorhinal cortex. J Neurosci. 2013;33:9246–9258. doi: 10.1523/JNEUROSCI.0946-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Lee H, Wang C, Deshmukh SS, Knierim JJ. Neural Population Evidence of Functional Heterogeneity along the CA3 Transverse Axis: Pattern Completion versus Pattern Separation. Neuron. 2015;87:1–13. doi: 10.1016/j.neuron.2015.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]