Abstract
The five-membered nitrogen plus heteroatom rings known as azolines or in their oxidized form as azoles are very common in natural products and drugs. The oxidation of thiazoline to thiazole in the cyanobactin class of natural products is one of the several important transformations that are known to alter the biological properties of the compound. The ordering of the various chemical reactions that occur during cyanobactin biosynthesis is not fully understood. The structure of the flavin-dependent enzyme responsible for the oxidation of multiple thiazolines reveals it contains an additional domain that in other enzymes recognizes linear peptides. We characterize the activity of the enzyme on two substrates: one with a peptide leader and one without. Kinetics and biophysics reveal that the leader on the substrate is not recognized by the enzyme. The enzyme is faster on either substrate than the macrocyclase or protease in vitro. The enzyme has a preferred order of oxidation of multiple thiazolines in the same linear peptide.
Keywords: Oxidation, enzyme, macrocycles, azoles, azoline
Introduction
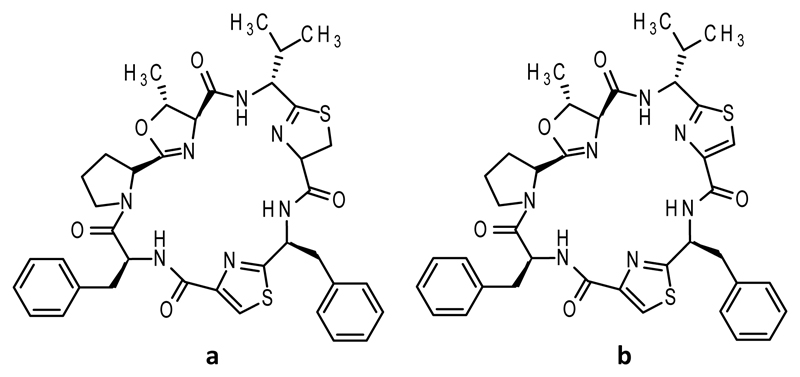
Cyclic peptides have come under intensive investigation in recent years because of the bioactivities they possess, including anticancer,(1) antibacterial,(2) and immunosuppressive.(3) Reduced structural flexibility(4) gives cyclic peptides receptor binding affinities higher than those of their linear counterparts through a lower entropic penalty upon binding.(5) Cyanobactins(6) make up a class of macrocyclic compounds, produced by cyanobacteria, and often contain posttranslational modifications, including five-membered rings (azolines and azoles(7)) that are produced from cysteine, threonine, or serines.(8) Thiazolines, methyloxazolines, and oxazolines are made by a heterocyclase and can undergo enzymatic oxidation to become thiazoles and (methyl)oxazoles.(9) Azoles are aromatic, chemically more stable, and conformationally different from azolines due to the flattening of the ring.(10) The oxidation status of thiazoline/oxazoline is known to alter the function of the parent macrocycles.(11) The most striking demonstration is an increase in cytotoxicity of two orders of magnitude against bladder carcinoma cells between lissoclinamides 4 and 5 (Figure 1), which differ only in thiazoline versus thiazole.(12)
Figure 1. Structure of lissoclinamides.
a. lissoclinamide 4 b. lissoclinamide 5. The difference is highlighted in blue.
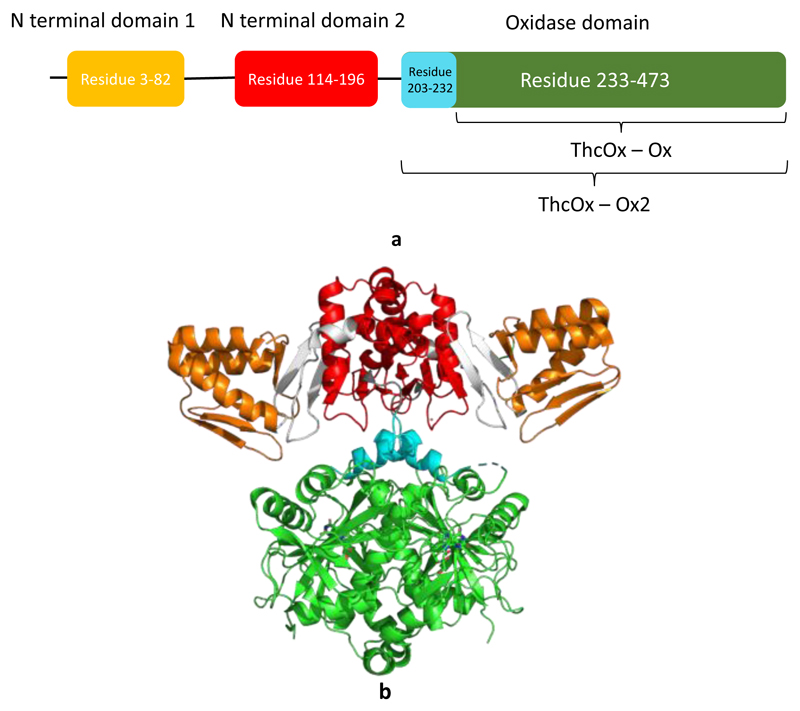
Azol(ine)s are commonly found in ribosomally synthesized and posttranslationally modified peptides (RiPPs) of the thiazole/oxazole-modified microcin (TOMM) class.(13) Microcin B17 is a classic TOMM peptide, and azoles are produced by the McbBCD complex that is comprised of a recognition domain [peptide clamp, also termed the RiPP recognition element (RRE)],(14) a heterocyclase (cyclodehydratase), and an oxidase (which would be more correctly identified as a dehydrogenase if O2 is not the acceptor).(15) BalhBCD, another TOMM biosynthetic enzyme, also operates as a tripartite complex.(16) In contrast, the cyanobactin oxidase does not appear to form a complex with the heterocyclase, which in cyanobactin biosynthetic pathways is found as a fusion of an RRE-containing domain and the YcaO (catalytic) domain. The oxidase is either fused with the macrocyclase enzyme and a domain of unknown function or found as a stand-alone protein. In addition, the cyanobactin oxidases have two RREs at the N-terminus, shown by the crystal structure of ThcOx (Figure 2b) from the cyanothecamide biosynthesis pathway.(17) The C-terminal domain shows sequence homology to other TOMM oxidases and binds flavin mononucleotide (FMN), identifying it as the catalytic domain. The functions of the RREs in ThcOx, which are highly structurally similar with the leader binding domain in LynD(18) (Figure S1), are however poorly understood. The second of the two domains at the dimeric interface has the clamping region hidden, whereas the clamp region of the N-terminal domain is exposed and may participate in substrate binding (Figure 2b)(14)
Figure 2. Crystal structure and schematic representation of ThcOx.
N-Terminal RREs are colored orange and red; the linker is colored gray. The oxidase domain is colored green and cyan, to illustrate the truncations made. ThcOx-Ox2 contains residues 203–473 (cyan and green), while ThcOx-Ox contains only residues 233–473 (green)
Here we report the first biochemical characterization of a cyanobactin-type oxidase. We showed that the oxidases ThcOx and ArtGox operate on leaderless and full-length linear peptide substrates. We have determined that the reaction proceeds with an order when there are multiple thiazolines in the substrate and that the order is preserved irrespective of whether the leader peptide is present. Despite the presence of RREs, no interaction was found between the leader peptide and the oxidase. The N-terminal domains instead appear to maintain the structural integrity and consequently the activity of the enzyme as a whole.
Materials and Methods
General methods
Unless specified, all chemicals were purchased from Sigma-Aldrich or Fisher Scientific. Oligonucleotides were purchased from ThermoFisher Scientific. Restriction enzymes were purchased from Promega. dNTP and DNA polymerase were purchased as part of the EMD Millipore Novagen KOD Hot Start DNA Polymerase kit. DNA sequencing was performed by GATC. Peptides were purchased from Biosynthesis.
Cloning and overexpression
ThcOx (Cyanothece sp. PCC 7425, UniprotKB entry B8HTZ1) was expressed with an N-terminal tobacco etch virus (TEV) protease-cleavable His6 tag followed by a four-glycine tag as previously described.(19) ArtGox (Arthrospira platensis, UniprotKB entry H1W8K1)(19) was cloned into a pEHISTEVSUMO vector(20) and expressed with an N-terminal His6 tag followed by a SUMO tag and TEV protease cleavage site. ThcOx-Ox (residues 233–473 of ThcOx) and ThcOx-Ox2 (residues 203–473) were cloned from full-length ThcOx into a pEHISTEV vector with an N-terminal His6 tag and a TEV protease cleavage site.(20) All proteins were expressed in Escherichia coli BL21 (DE3) cells grown in autoinduction medium by the Studier method for 48 h at 20 °C.(21) For ArtGox, ThcOx, and variants, 50 μM riboflavin was added to the expression medium.
PatE3′, PatE3KK, and ThcE1 (Figure S2 gives sequences) were cloned into a pBMS23CHIS vector(20) and expressed with a C-terminal noncleavable His6 tag in E. coli BL21 (DE3) cells. Starter cultures were grown in LB medium at 37 °C and 200 rpm until the optical density at a wavelength of 600 nm reached 0.6 and induced with 1 mM isopropyl β-d-1-thiogalactopyranoside. Cells were further incubated at 37 °C and 200 rpm for 5 h before being harvested by centrifugation at 4000 rpm for 15 min at 20 °C. Uniformly 13C- and 15N-labeled PatE2K and uniformly 15N-labeled PatE2K were expressed as previously described.(22)
Purification of proteins and peptides.
E. coli cells overexpressing ThcOx, ArtGox, and ThcOx-Ox were resuspended in lysis buffer [150 mM NaCl, 20 mM Tris-HCl (pH 8.0), 20 mM imidazole (pH 8.0), 50 μM FMN, and 3 mM 2-mercaptoethanol] and EDTA-free protease inhibitor tablets (Roche) and DNase at 0.4 mg/g of wet cell pellet. The resuspension was lysed by being passed through a cell disruptor at 30K psi (Constant Systems). The lysate was cleared by centrifugation (6000 rpm, 4 °C, 20 min) and then loaded onto a Ni Sepharose 6 Fast Flow column (GE Healthcare) equilibrated with lysis buffer. The protein was eluted with elution buffer [150 mM NaCl, 20 mM Tris-HCl (pH 8.0), 250 mM imidazole (pH 8.0), 50 μM FMN, and 3 mM 2-mercaptoethanol] and passed over a desalting column (16/10 Desalting, GE Healthcare) exchanging into desalting buffer [100 mM NaCl, 20 mM Tris-HCl (pH 8.0), 3 mM 2-mercaptoethanol, and 50 μM FMN]. TEV protease was added at a mass ratio of 1:10, and the protein was digested for 3 h at 20 °C before being loaded onto a second nickel column pre-equilibrated with desalting buffer. The eluted protein was applied directly to an anion-exchange column (HisTrap Q Sepharose FF, GF Healthcare) where it was eluted with a 0.1 to 1 M NaCl gradient. The peak fraction was then concentrated to 7.5 mL (Vivaspin concentrators, 30 kDa molecular weight cutoff) and applied to a Superdex 200 gel filtration column (GE Healthcare) equilibrated with gel filtration buffer [150 mM NaCl, 20 mM HEPES (pH 7.4), and 1 mM TCEP]. Heterocyclases LynD (UniprotKB entry A0YXD2), AcLynD, and MicD (UniprotKB entry I4F6C3) were purified as previously described.(18,19)
Cells expressing precursor peptides were resuspended in urea lysis buffer [8 M urea, 500 mM NaCl, 20 mM Tris-HCl (pH 8.0), 20 mM imidazole (pH 8.0), and 3 mM 2-mercaptoethanol] and lysed by sonication, and then the lysate was cleared by centrifugation at 19000 rpm for 20 min at 20 °C. The supernatant was filtered through 5 and 0.8 μm filters before being loaded onto a Ni-Sepharose 6 FF column (GE Healthcare) pre-equilibrated with lysis buffer. The peptide was eluted with urea elution buffer [8 M urea, 500 mM NaCl, 20 mM Tris-HCl (pH 8.0), 250 mM imidazole (pH 8.0), and 3 mM 2-mercaptoethanol]. The eluate was supplemented with 10 mM dithiothreitol (DTT) and incubated at 20 °C for 2 h to reduce all the cysteines. PatE3′ was subjected to size-exclusion chromatography (Superdex 75, GE Healthcare) in gel filtration buffer (150 mM NaCl and 20 mM HEPES). The integrity, identity, and purity of the proteins and peptides were confirmed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and mass spectrometry (MS).
Heterocyclisation and Proteolysis
To produce substrates with thiazoline heterocycles for oxidation (Scheme S1), 100 μM PatE3′ or ThcE1 was reacted with MicD (10 μM), in gel filtration buffer (150 mM NaCl, 20 mM HEPES, and 1 mM TCEP) supplemented with MgCl2 (10 mM) and ATP (10 mM), at 27 °C for 16 h. [15N]PatE2K (175 μM) was reacted with LynD (5 μM), and PatE3KK was reacted with MicD (10 μM) for 16 h at room temperature, in 100 mM Tris (pH 8.0) supplemented with 150 mM NaCl, 10 mM ATP, 10 mM MgCl2, and 5 mM DTT. The reaction mixtures were then loaded onto a Superdex 75 column (GE Healthcare) and equilibrated in gel filtration buffer to separate MicD and LynD from the heterocyclized material (Figure S3). Heterocyclization of these substrates was confirmed by MS (Figure S4 and S6).
In cases in which leaderless substrates were used, heterocyclized peptides were incubated with trypsin (1:100 mass ratio) at 20 °C for 16 h to cleave the leader. In the case of PatE2K and PatE3′, the trypsin-treated sample was loaded onto a Ni-Sepharose 6 FF column (GE Healthcare), pre-equilibrated with gel filtration buffer. The eluate was then passed through a desalting column (16/10 Desalting, GE Healthcare) into gel filtration buffer. Proteolysis was confirmed by MS (Figures S5 and S7). In the case of PatE3KK, trypsin was inactivated by heating the sample at 100 °C for 10 min, and no purification was performed. Finally, the chemically synthesized peptide ITACITFCAYD (Biosynthesis) was incubated with AcLynD (10 μM) in gel filtration buffer supplemented with 10 mM ATP and 10 mM MgCl2 for 16 h at 20 °C, prior to reaction with ThcOx and variants (10 μM each), without purification between the steps.
Oxidation reactions
Various concentrations of enzymes and substrates were used as stated individually in the Results. ThcOx reactions were performed in 100 mM HEPES (pH 7.5) and 150 mM NaCl. ArtGox reactions were performed in 100 mM Tris (pH 8.0) supplemented with 150 mM NaCl and 2 mM FMN.
Enzyme Kinetics
Steady-state kinetics were obtained using a commercial hydrogen peroxide assay kit (Abcam, ab102500), ThcOx [100 nM (Figure S8)], and varying concentrations of the heterocycle containing full-length and leaderless PatE3′ or full-length ThcE1. The substrates were added to a Corning 96-well half-area black flat bottom polystyrene microplate. In the presence of horseradish peroxidase (HRP), the OxiRed probe produces a product with red fluorescence (excitation and emission at 535 and 587 nm, respectively) by reaction with H2O2, which is the other product of azole formation (Scheme S2). Fluorescence was measured with a SpectraMax 2e microplate reader (Molecular Devices). All experiments were performed at 25 °C. Data were corrected for the autofluorescence of ThcOx, and the substrate was used for background correction. Initial rates were plotted against substrate concentration and fitted to the Michaelis–Menten equation (below) in GraphPad Prism.
Biophysics
ITC experiments were performed on a Malvern Panalytical MicroCal iTC200 microcalorimeter. A cell concentration of 20 μM ThcOx with a syringe concentration of 400 μM PatE3′ and ThcE1 was added to the system. Both the substrate and ThcOx were in gel filtration buffer.
ThcOx-Ox was subjected to the size-exclusion chromatography multiangle light scattering (SEC-MALS)(23) for determination of molecular mass. Purified protein was loaded onto a GE Health Superdex 200 column, equilibrated in Gel filtration buffer (150 mM NaCl, 10 mM HEPES, 1 mM TCEP), attached to the Wyatt Dawn Heleos II Multi-Angle Light Scattering detector and Wyatt Optilab T-rex Refractive Index detector. Protein elution peak was recorded by differential refractive index (dRI).
ThcOx-Ox was exchanged into circular dichroism buffer [500 mM sodium phosphate buffer (pH 8.0) and 200 mM NaF] and concentrated to a final concentration of 0.2 mg/mL. The circular dichroism signal was measured and recorded by a Bio-Logic Science Instrument MOS-500 circular dichroism spectrometer.
1H–15N HSQC spectra of full-length [U-13C,15N]PatE2K were recorded on a Bruker AVANCE 500 MHz spectrometer equipped with a TXIz probe, controlled by Bruker Topspin software. A standard Bruker pulse sequence with Watergate water suppression was used with eight transients at 2048 × 128 points with spectral resolutions of 9.8 and 19.0 Hz in the direct (1H) and indirect (15N) dimensions, respectively. HSQC spectra were also recorded at 10 °C for the starting material and 12 and 60 h after the addition of 10 μM ArtGox. For sequential assignment, HNCACB spectra were acquired with 16 transients at 1024 × 50 × 128 points and spectral resolutions of 13.7, 44.6, and 157.1 Hz for the 1H, 15N, and 13C dimensions, respectively. CBCA(CO)NH spectra were acquired with eight transients at 1024 × 50 × 114 points and spectral resolutions of 13.7, 44.6, and 176.4 Hz for the 1H, 15N, and 13C dimensions, respectively. Equivalent spectra were recorded for fully oxidized [U-13C,15N]PatE2K-2het (concentration of 1.0 mM) and a sample for which the enzymatic transformation was interrupted after 12 h by gel filtration on a Superdex S75 column (GE Healthcare) in heterocyclization buffer supplemented with 5 mM FMN, thus allowing assignment of the reaction intermediate.
Nuclear magnetic resonance (NMR) experiments with the leaderless substrate were performed at 20 °C on a Bruker Ascend 700 MHz spectrometer equipped with a Prodigy TCI probe, controlled by Bruker Topspin software. For oxidation monitoring, 0.2 M cleaved peptide (see preparation of leaderless substrate) was reacted with 10 μM ArtGox in 10 mM HEPES (pH 7.4), 150 mM NaCl, 5 mM FMN, and 5% D2O at 20 °C. 1H–15N HSQC spectra were recorded before the addition of enzyme, 5 and 20 min afterward, and then every 30 min. Six transients were recorded at 1536 × 80 points with spectral resolutions of 12.8 and 26.6 Hz in the direct (1H) and indirect (15N) dimensions, respectively. 1H–15N HSQC spectra were recorded at 10 °C for the unreacted substrate and the final product. Sequential assignment of the full-length peptide was transferred to the 1H–15N HSQC spectrum at 10 °C and then to the corresponding spectrum at 20 °C. Raw spectral data were processed with Topspin and analyzed with CCPN Analysis 2.(21)
Results
The Cyanobactin Oxidase operates on both full length and ‘leaderless’ substrates but is faster on the latter
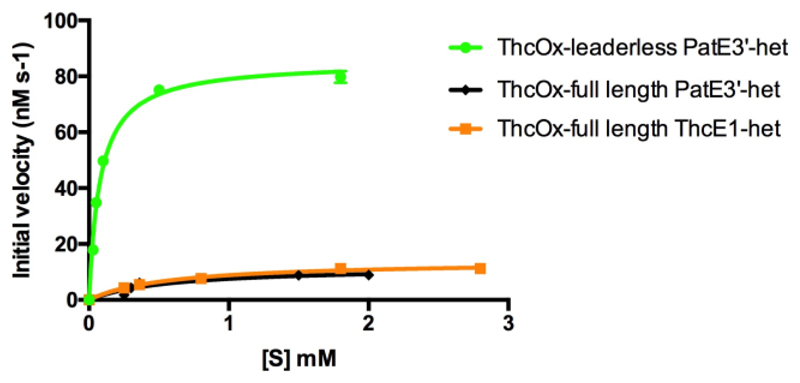
The presence of RRE in the cyanobactin oxidase, along with the fact that crystals were obtained only when the NILPQQGQPVIR peptide was added,(17) led to the hypothesis that parts of the leader peptide are involved in enzyme–substrate interaction. We predicted inactivity or reduced activity against leaderless peptides would be observed if the leader was important. ArtGox, previously shown to oxidize thiazolines in full-length (leader-containing) peptides,(19) was reacted with the four-heterocycle-containing IThetAChetIThetFChetAYDGEK peptide (Scheme S1). The loss of 4 Da in ESI-MS (Figure S9) demonstrated that two oxidation events haven taken place. Increasing the enzyme concentration to 50 μM (100 μM substrate) induced a further 2 Da mass loss (Figure S9), corresponding to the formation of one methyloxazole that is not found in arthrospiramides,(19) suggesting oxazoline oxidation is kinetically hindered. Steady-state kinetics of ThcOx were performed using full-length and trypsin-cleaved (leaderless) PatE3′ that have been heterocyclized by MicD (Figure 3 and Table 1). We observed a 7-fold increase in kcat and a 50-fold increase in kcat/Km when the leaderless peptide as opposed to the full-length substrate was used. As the leader sequence of PatE3′ is similar but not identical (Figure S2) to that of ThcE, we tested the activity of ThcOx against one of its native substrates, ThcE1. The catalytic activity toward this substrate was similar to that to PatE3′, confirming the leader does not accelerate catalytic activity.
Figure 3. Initial rates of ThcOx reaction with leaderless and full-length substrates.
Error bars represent the standard deviation from the mean (n = 2). Kinetic values derived from these plots are listed in Table 1.
Table 1. Catalytic parameters obtained from steady-state kinetic studies of ThcOx.
| Substrate | k cat (s-1) | K m (mM) | k cat/K m (s-1M-1) |
| Full ThcE1 | 0.14±0.01 | 0.58±0.06 | 240±60 |
| Full PatE3’ | 0.12±0.01 | 0.57±0.18 | 210±180 |
| Leaderless PatE3’ | 0.85±0.02 | 0.08±0.01 | 10630±20 |
Oxidation Proceeds in a Defined N-to-C Order
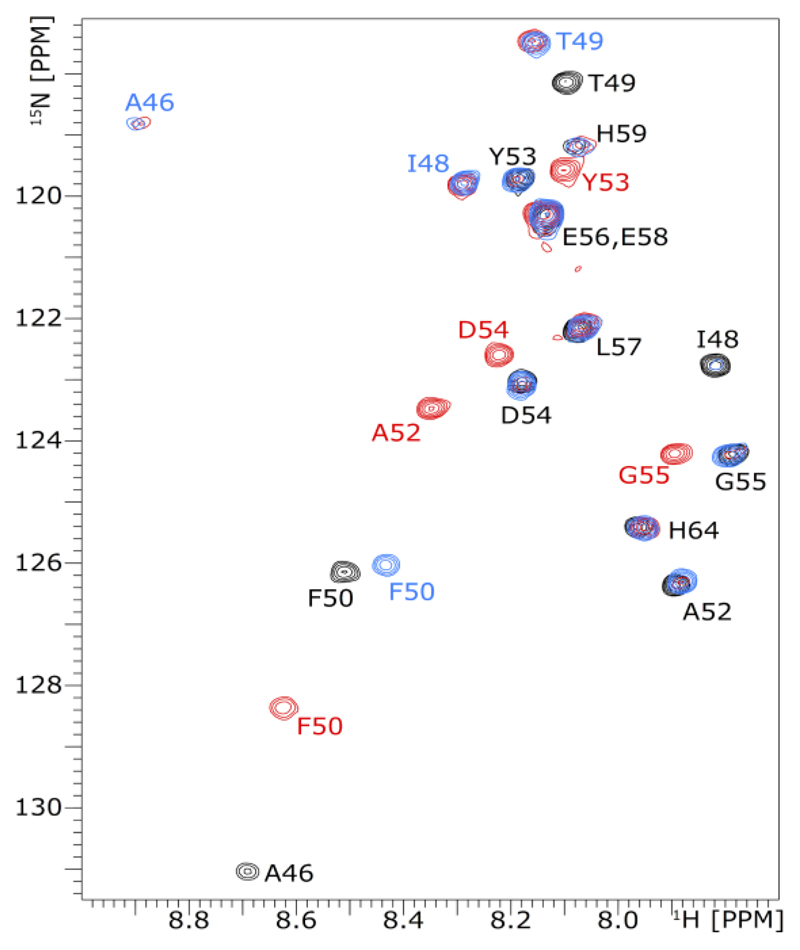
1H–15N HSQC NMR spectroscopy was employed to investigate the oxidation order of doubly heterocyclized leaderless PatE2K (Figure 4). Heterocyclized cysteines C47 and C51 were not observed in the HSQC spectra, because of the loss of their amide protons; oxidation, however, alters the chemical environment of neighboring residues A46, I48, T49, F50, A52, Y53, D54, and G55. Spectra were recorded before the addition of ArtGox and at regular intervals afterward. At 20 °C, the first oxidation at Chet47 or Thn47 happened rapidly after the addition of the enzyme and reached completion within 20 min, as demonstrated by shifting of the cross-peaks assigned to A46, I48, T49, and F50. The second oxidation step was slower, and chemical shift changes corresponding to it (changes in F50, A52, Y53, D54, and G55) became visible only after reaction for 1 h. The second reaction was complete within 16 h, yielding the final product (Figure 4). The same N-to-C order of oxidation was shown when ArtGox was reacted with full-length PatE2K (Figures S10 and S11). The consistency indicates that the reaction order is an intrinsic property of ArtGox and the core substrate sequence. Consistent with the kinetic assays (Figure 3 and Table 1), the reaction was clearly slower with the full-length substrate, with the first step taking nearly 12 h and complete oxidation taking 60 h.
Figure 4. NMR experiment tracing the oxidation of leaderless PatE2K-2het.
1H–15N HSQC spectra were recorded for doubly heterocyclized, leaderless PatE2K (black) and after it was reacted with ArtGox for 20 min (blue) and 16 h (red). Cross-peaks are annotated with their residue sequences like those of the full-length substrate; the color of a label corresponds to the spectrum where the cross-peak it designates first appears. Note the cross-peaks for A46 and G55 after 20 min and 16 h are aliased in the 15N dimension; the actual 15N chemical shifts for these signals are 133.8 and 109.3 ppm, respectively.
N-terminal Domain Maintains Structural Integrity and Enzyme Activity
As full-length peptides were worse substrates of the oxidase than their leaderless counterparts, we sought to determine if the leader peptide binds in a way that reduces enzyme activity. PatE3′ and ThcE1 that had not been heterocyclized were used to probe the binding between the leader peptide and ThcOx. However, binding with either peptide was not observed via isothermal titration calorimetry (Figure 5), suggesting that a heterocyclized core peptide alone controls the enzyme–substrate interaction. In addition, we were unable to obtain a complex structure of ThcOx with any part of the leader peptide bound by crystallography. We tested if the C-terminal domain (homologous to other TOMM oxidases) alone was sufficient for enzymatic activity. The truncated oxidase domain ThcOx-Ox2 (residues 203–473) was cloned and expressed, but it was insoluble. ThcOx-Ox (residues 233–473) was then produced (Figure S12) and purified as a colorless protein, in contrast with the yellow color of native ThcOx, which was attributed to its cofactor FMN.
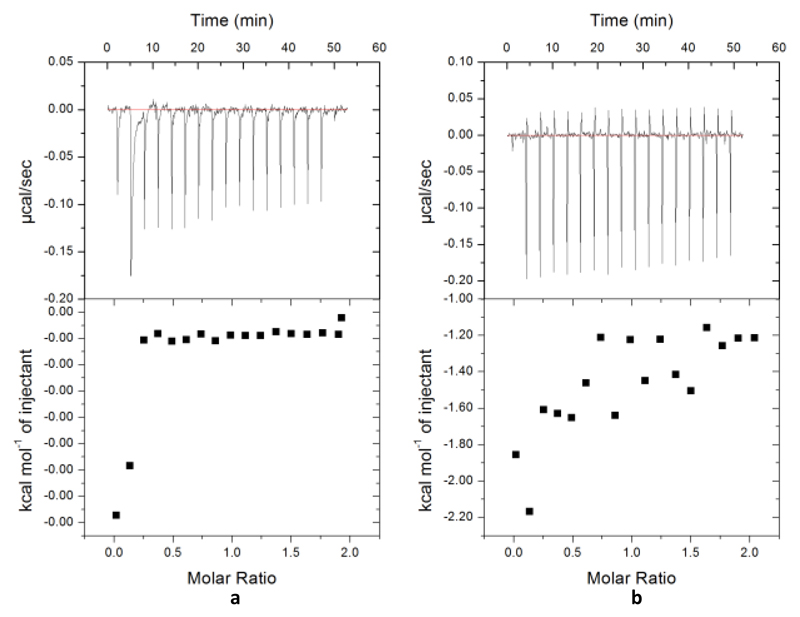
Figure 5. Calorimetric titration for the binding of peptides to ThcOx.
The top panel shows the injection of peptides (400 μM) into the cell filled with ThcOx at 20 °C, yielding an exothermic binding isotherm. The bottom panel shows the integrated data obtained from the raw data, after subtracting the heat of dilution. Experimental data were fitted by using the one set of sites model from MicroCal Origin. (a) Binding of PatE3′ with ThcOx. (b) Binding of ThcE1 and ThcOx.
The activity of ThcOx-ox was tested against ITAChetITFChetAYD, with native ThcOx as the control. While the native enzyme (10 μM) introduced a 4 Da mass loss in the peptide (Figure 6d), ThcOx-Ox at the same concentration showed no activity (Figure 6b). Even with external FMN supplementation (Figure 6c), the truncated enzyme was still unable to oxidize the thiazolines within the time scale of the experiment (16 h). The inability of exogenous FMN to rescue the reaction prompted us to think that the truncated enzyme could be unfolded or misfolded, and we therefore determined the secondary and quaternary structure of the protein with biophysical techniques. Purified ThcOx-Ox (molecular weight of 27 kDa) was subjected to SEC-MALS, which gives a solution mass of 54 kDa (Figure 7a), consistent with that of a dimer. Circular dichroism (Figure 7b) suggested there are 30% α-helices and 20% β-sheets in the protein, similar to the 29% α-helices and 22% β-sheets among residues 233–473 shown in the crystal structure of ThcOx. Taken together, we conclude that ThcOx-Ox is folded correctly overall, but FMN binding was disrupted by the truncation.
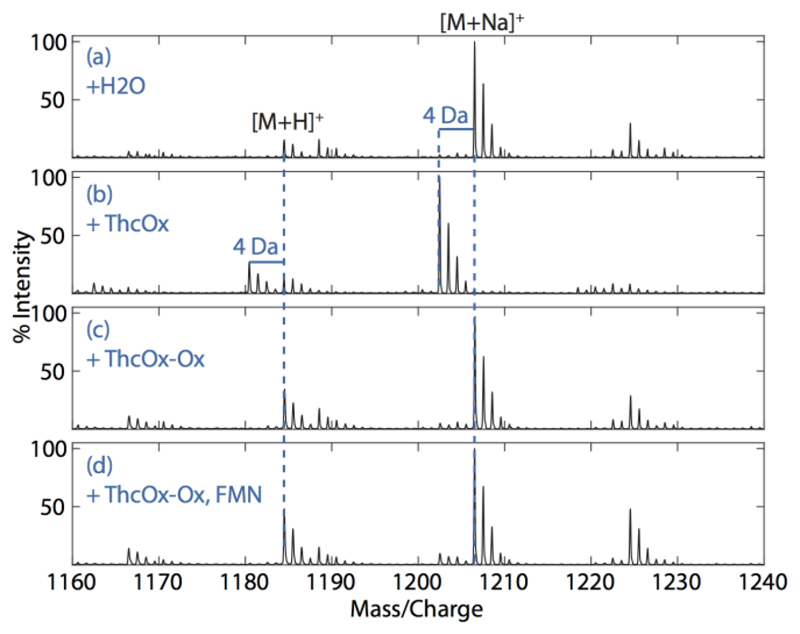
Figure 6. Mass spectra of doubly ITAChetITFChetAYD (100µM) reacted with ThcOx and ThcOx-Ox.
(a) Unoxidized peptide showing an [M + H]+ of 1184.5 Da and an [M + Na]+ of 1206.5 Da. (b) Reaction with native ThcOx resulted in a mass loss of 4 Da. (c) No mass shift was seen when the peptide was incubated with ThcOx-Ox. (d) Exogenous FMN (200 μM) was unable to rescue the reaction
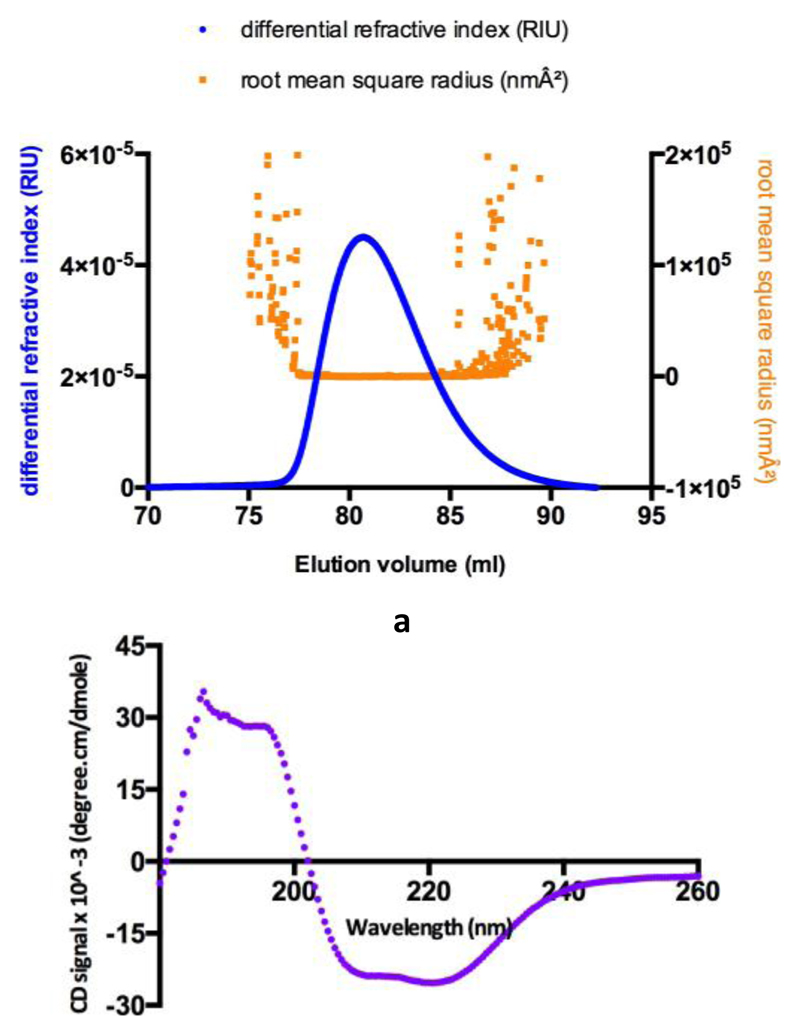
Figure 7. Biophysical analyses of ThcOx-Ox.
(a) SEC-MALS chromatograph showing elution of the protein as a homogeneous species. Changes in the refractive index (blue) show protein-mediated scattering of light. The root-mean-square radius (orange) indicates the molecular size. (b) Circular dichroism spectrum of ThcOx-Ox at a concentration of 0.2 mg/mL.
Discussion
The cyanobactin oxidase is a fusion between two vestigial RREs(14) and an FMN-dependent oxidase domain that shows moderate sequence homology to other characterized TOMM oxidases (e.g., McbC and BalhB).(15,16) The vestigial RREs of ThcOx each possess the characteristic structure(19) of three α-helices and three β-strands. McbC and BalhB both form part of a heterocyclase–oxidase complex in conjunction with an RRE-containing protein and a YcaO (heterocyclization) protein. The RRE has been found with a variety of activities, such as in MccB [an adenylating enzyme in the Trojan horse antibiotic microcin C7 biosynthesis pathway, Protein Data Bank (PDB) entry 3H9J], LynD (a heterocyclase in the aestuaramide pathway, PDB entry 4V1T), and NisB (a dehydratase in lantibiotic nisin, PDB entry 4WD9).(14) In many enzymes, the RiPP domain was found to be able to bind the leader peptide. Because the structure of ThcOx showed it had two RREs fused to an oxidase domain (B protein), we wished to investigate their role (Figure 8).
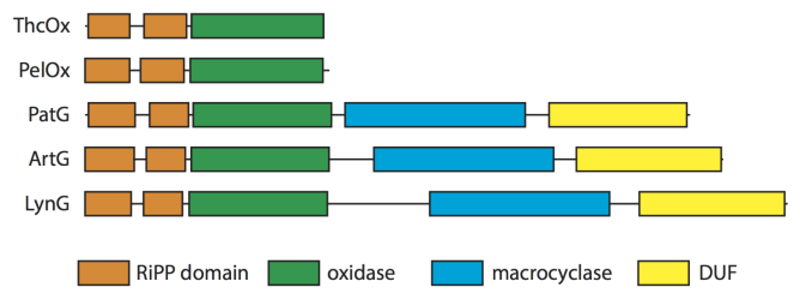
Figure 8. Domain arrangement of Cyanobactin oxidases.
ThcOx and PelOx (Nostoc sp. Peltigera membranacea cyanobiont 232) are stand-alone (putative) oxidases. PatG (Prochlorin didemni), ArtG, and LynG (Lyngbya sp. PCC 8106) are fused oxidases. DUF is a domain of unknown function. Domains were assigned by the HHpred server.(24)
Steady-state kinetics and NMR both show that the oxidase is faster and more efficient in the absence of the leader peptide on the substrate. Consistent with this, we were unable to detect any binding of the leader to ThcOx using ITC. Our NMR results showed that the oxidase reaction proceeds with an N-to-C order, the opposite of that demonstrated by the cyanobactin heterocyclase TruD.(22) Moreover, because the order is not dependent upon the leader, it is a feature of the core sequence and enzyme. The vestigial RREs in the oxidase do not act as a clamp for the oxidase, but their loss seems to destabilize the enzyme.
In the processing of cyanobactin precursor peptide in vivo, the PatA protease cleaves off the leader. The lack of leader binding by the oxidase (as well as the inefficient processing of substrates with leader) would suggest that heterocyclization is followed by protease cleavage (a slow reaction in vitro); then oxidation, and lastly macrocyclization, which is slower than any of the above-stated reactions in vitro.(25,26) Quite why the organism uses rates to regulate production is unclear, but we note the frequent presence of epimerized amino acids adjacent to the thiazoles in the many cyanobactins. Because epimerization has been assumed to be spontaneous for thiazolines, the slow enzyme rate of the macrocyclase may be related to this. The inactivity of the RREs was an unexpected result given that they are found as part of the oxidase in many cyanobactin biosynthetic pathways and cautions against automatic assignment of function. The recently identified radical SAM enzyme SuiB has also been shown to contain a vestigial N-terminal RRE but uses a different portion of the protein for substrate binding.(27) From a biotechnological perspective, the oxidase is a versatile enzyme capable of using a range of different substrates, including linear and macrocyclic peptides.(20) Its functional independence of the leader means it could be incorporated into the semisynthetic approach toward patellamide-like peptides described in previous studies.(18,19)
Supplementary Material
Acknowledgment
We thank Dr. Clarissa Czekster for assistance with kinetics, Dr Wael Houssen (University of Aberdeen) for providing the genes artG and thcOX, Dr Huangting Liu for the vector pEHISTEVSUMO vector.20
Funding Sources
The work is supported by ERC grant NCB-TNT (339367).
Abbreviations
- ThcOx
oxidase from Cyanothece PCC7425 involved in cyanothecamide biosynthesis
- ArtGox
oxidase domain from Arthrospira spirulina involved in arthrospiramide biosynthesis
- HSQC spectra
heteronuclear single-quantum correlation spectroscopy
- ESI-MS
electrospray ionization mass spectrometry
- ITC
isothermal titration calorimetry
- NMR
nuclear magnetic resonance
Footnotes
No competing financial interests have been declared.
Author Contributions
S.G. and Y.G. contributed equally to this work. S.G., Y.G., U.S.-L., and J.H.N. performed experiments, analyzed data, and wrote the paper. A.F.B. had performed the initial experiments. All authors have given approval to the final version of the manuscript
References
- 1.Hong J, Luesch H. Largazole: from discovery to broad-spectrum therapy. Nat Prod Rep. 2012;29:449–456. doi: 10.1039/c2np00066k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yamada K, Kodaira M, Shinoda S, Komagoe K, Oku H, Katakai R, Katsu T, Matsuo I. Structure–activity relationships of gramicidin S analogs containing (β-3-pyridyl)-α,β-dehydroalanine residues on membrane permeability. Medchemcomm. 2011;2:644. [Google Scholar]
- 3.Schreiber SL, Crabtree GR. The mechanism of action of cyclosporin A and FK506. Immunol Today. 1992;13:136–142. doi: 10.1016/0167-5699(92)90111-J. [DOI] [PubMed] [Google Scholar]
- 4.Namjoshi S, Benson HAE. Cyclic peptides as potential therapeutic agents for skin disorders. Biopolymers. 2010;94:673–680. doi: 10.1002/bip.21476. [DOI] [PubMed] [Google Scholar]
- 5.Davies JS. The cyclization of peptides and depsipeptides. J Pept Sci. 2003;9:471–501. doi: 10.1002/psc.491. [DOI] [PubMed] [Google Scholar]
- 6.Ehrlich A, Heyne H-U, Winter R, Beyermann M, Haber H, Carpino LA, Bienert M. Cyclization of all-L-Pentapeptides by Means of 1-Hydroxy-7-azabenzotriazole-Derived Uronium and Phosphonium Reagents. J Org Chem. 1996;61:8831–8838. doi: 10.1021/jo951108d. [DOI] [PubMed] [Google Scholar]
- 7.Nielsen DS, Hoang HN, Lohman R-J, Hill TA, Lucke AJ, Craik DJ, Edmonds DJ, Griffith DA, Rotter CJ, Ruggeri RB, Price DA, et al. Improving on nature: making a cyclic heptapeptide orally bioavailable. Angew Chemie Int Ed. 2014;53:12059–12063. doi: 10.1002/anie.201405364. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt EW, Nelson JT, Rasko Da, Sudek S, Eisen Ja, Haygood MG, Ravel J. Patellamide A and C biosynthesis by a microcin-like pathway in Prochloron didemni, the cyanobacterial symbiont of Lissoclinum patella. Proc Natl Acad Sci U S A. 2005;102:7315–7320. doi: 10.1073/pnas.0501424102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Czekster CM, Ge Y, Naismith JH. Mechanisms of cyanobactin biosynthesis. Curr Opin Chem Biol. 2016;35:80–88. doi: 10.1016/j.cbpa.2016.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katritzky AR. Handbook of heterocyclic chemistry. Elsevier; 2010. [Google Scholar]
- 11.Houssen WE, Jaspars M. Azole-based cyclic peptides from the sea squirt Lissoclinum patella: Old scaffolds, new avenues. ChemBioChem. 2010;11:1803–1815. doi: 10.1002/cbic.201000230. [DOI] [PubMed] [Google Scholar]
- 12.Hawkins CJ, Lavin MF, Marshall KA, Van den Brenk AL, Watters DJ. Structure-activity relationships of the lissoclinamides: cytotoxic cyclic peptides from the ascidian Lissoclinum patella. J Med Chem. 1990;33:1634–1638. doi: 10.1021/jm00168a016. [DOI] [PubMed] [Google Scholar]
- 13.Melby JO, Nard NJ, Mitchell DA. Thiazole/oxazole-modified microcins: complex natural products from ribosomal templates. Curr Opin Chem Biol. 2011;15:369–378. doi: 10.1016/j.cbpa.2011.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burkhart BJ, Hudson GA, Dunbar KL, Mitchell DA. A prevalent peptide-binding domain guides ribosomal natural product biosynthesis. Nat Chem Biol. 2015;11:564–570. doi: 10.1038/nchembio.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li YM, Milne JC, Madison LL, Kolter R, Walsh CT. From peptide precursors to oxazole and thiazole-containing peptide antibiotics: microcin B17 synthase. Science. 1996;274:1188–93. doi: 10.1126/science.274.5290.1188. [DOI] [PubMed] [Google Scholar]
- 16.Melby JO, Dunbar KL, Trinh NQ, Mitchell DA. Selectivity, Directionality, and Promiscuity in Peptide Processing from a Bacillus sp. Al Hakam Cyclodehydratase. J Am Chem Soc. 2012;134:5309–5316. doi: 10.1021/ja211675n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bent AF, Mann G, Houssen WE, Mykhaylyk V, Duman R, Thomas L, Jaspars M, Wagner A, Naismith JH. Structure of the cyanobactin oxidase ThcOx from Cyanothece sp. PCC 7425, the first structure to be solved at Diamond Light Source beamline I23 by means of S-SAD. Acta Crystallogr Sect D Struct Biol. 2016;72:1174–1180. doi: 10.1107/S2059798316015850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koehnke J, Mann G, Bent AF, Ludewig H, Shirran S, Botting CH, Lebl T, Houssen WE, Jaspars M, Naismith JH. Structural analysis of leader peptide binding enables leader-free cyanobactin processing. Nat Chem Biol. 2015;11:558–563. doi: 10.1038/nchembio.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Houssen WE, Bent AF, McEwan AR, Pieiller N, Tabudravu J, Koehnke J, Mann G, Adaba RI, Thomas L, Hawas UW, Liu H, et al. An Efficient Method for the In Vitro Production of Azol(in)e-Based Cyclic Peptides. Angew Chemie Int Ed. 2014;53:14171–14174. doi: 10.1002/anie.201408082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu H, Naismith JH. A simple and efficient expression and purification system using two newly constructed vectors. Protein Expr Purif. 2009;63:102–111. doi: 10.1016/j.pep.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Studier FW. Protein production by auto-induction in high-density shaking cultures. Protein Expr Purif. 2005;41:207–234. doi: 10.1016/j.pep.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 22.Koehnke J, Bent AF, Zollman D, Smith K, Houssen WE, Zhu X, Mann G, Lebl T, Scharff R, Shirran S, Botting CH, et al. The cyanobactin heterocyclase enzyme: a processive adenylase that operates with a defined order of reaction. Angew Chem Int Ed Engl. 2013;52:13991–6. doi: 10.1002/anie.201306302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Folta-Stogniew E. New and Emerging Proteomic Techniques. Humana Press; New Jersey: 2006. Oligomeric States of Proteins Determined by Size-Exclusion Chromatography Coupled With Light Scattering, Absorbance, and Refractive Index Detectors; pp. 97–112. [DOI] [PubMed] [Google Scholar]
- 24.Soding J, Biegert A, Lupas AN. The HHpred interactive server for protein homology detection and structure prediction. Nucleic Acids Res. 2005;33:W244–W248. doi: 10.1093/nar/gki408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee J, McIntosh J, Hathaway BJ, Schmidt EW. Using marine natural products to discover a protease that catalyzes peptide macrocyclization of diverse substrates. J Am Chem Soc. 2009;131:2122–2124. doi: 10.1021/ja8092168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McIntosh JA, Robertson CR, Agarwal V, Nair SK, Bulaj GW, Schmidt EW. Circular logic: Nonribosomal peptide-like macrocyclization with a ribosomal peptide catalyst. J Am Chem Soc. 2010;132:15499–15501. doi: 10.1021/ja1067806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davis KM, Schramma KR, Hansen WA, Bacik JP, Khare SD, Seyedsayamdost MR, Ando N. Structures of the peptide-modifying radical SAM enzyme SuiB elucidate the basis of substrate recognition. Proc Natl Acad Sci. 2017;114:10420–10425. doi: 10.1073/pnas.1703663114. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.