The α-melanocyte-stimulating hormone (α-MSH) is a neuropeptide that is secreted by the cells in the intermediate lobe of the pituitary gland in the brain. In humans, α-MSH plays a pivotal role in the regulation of feeding behavior, energy homeostasis, and sexual activity, in addition to its primary function of regulating melanogenesis, the process of hair and skin pigmentation (1). α-MSH activates four of the five melanocortin receptors [melanocortin-1 receptor (MC1R), MC3R, MC4R, and MC5R but not MC2R] (1). Of these, MC4R is of peculiar interest as a drug target because mutations in this receptor are associated with different forms of obesity in humans (2). On page 428 of this issue, Yu et al. (3) present a crystal structure of human MC4R in complex with a peptide antagonist, SHU9119. This reveals a calcium-binding site on MC4R, and Ca2+ is found to be an important modulator of MC4R activation, which could potentially facilitate therapeutic development.
High-resolution structural analysis of heterotrimeric GTP-binding protein (G protein)-coupled receptors (GPCRs)—such as MC4R, the largest class of cell-surface receptors in the human body—has seen unprecedented progress in the past decade owing to numerous methodological and technological advances (4). As a result, understanding of these receptors has reached a new level with structural insights into their activation, signaling, and regulation, providing previously lacking frameworks for drug discovery (5, 6). MC4R is expressed on neurons in the hypothalamus and brainstem. It is a prototypical, rhodopsin-like class A GPCR and primarily couples to the Gαs subfamily of heterotrimeric G proteins upon agonist activation (7). Gain-of-function mutations in MC4R appear to impart a protective effect from obesity (8), whereas mutations that result in reduced Gαs coupling are associated with early onset syndromic obesity (9). The agouti-related protein (AgRP), a potent appetite-stimulating neuropeptide produced by AgRP neurons in the hypothalamus, acts as an antagonist of MC4R (10). Although α-MSH binding to MC4R leads to a reduction in appetite, the binding of AgRP exerts the opposite effect, and therefore, a balanced action of these two hormones is crucial to regulate food intake and energy homeostasis (11) (see the figure). Setmelanotide, a cyclic peptide agonist of MC4R, has been reported to exert therapeutic effects in monogenic and syndromic obesity patients, although other small-molecule MC4R agonists have not been so promising in clinical trials (2). Therefore, a better understanding of ligand-MC4R interaction is important to facilitate future drug discovery efforts.
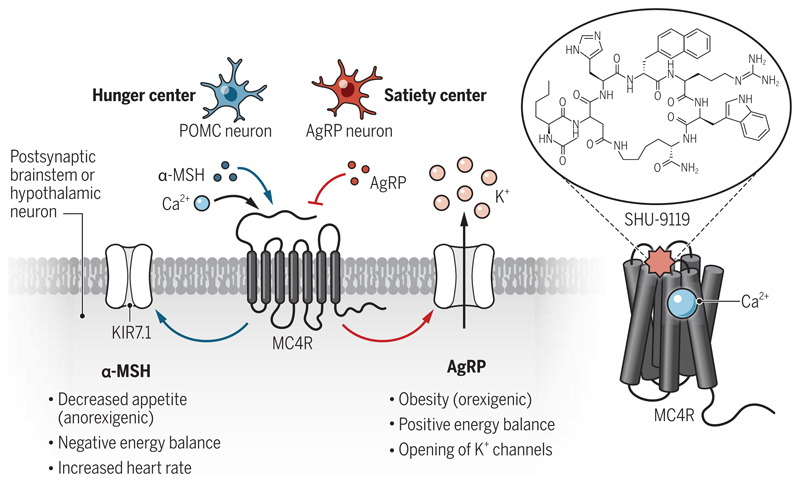
Figure. Calcium binding influences hunger signaling.
Melanocortin-4 receptor (MC4R) is expressed primarily in the hypothalamus and brainstem. It binds to two different neuropeptides, α-melanocyte–stimulating hormone [α-MSH, from proopiomelanocortin (POMC) neurons] and agouti-related protein (AgRP), which exert opposing effects on downstream responses. A crystal structure of MC4R in complex with the cyclic peptide antagonist, SHU9119, revealed a Ca2+-binding site that influences downstream signaling.
The crystal structure determined by Yu et al. uncovers the molecular details of SHU9119 binding, which makes extensive interactions with multiple residues from the amino terminus, the extracellular loop 2, and transmembrane helices of the receptor. The structure also reveals the binding of a Ca2+ ion to MC4R, coordinated by the amino acid residues from both the receptor and SHU9119. Comparison of the MC4R crystal structure with that of other class A GPCRs suggests a similarity with the lipid-activated receptors compared with the peptide-binding receptors, an observation that needs additional studies to establish physiological importance. Ca2+ also increases the binding affinity of α-MSH by more than 30-fold but has no effect on SUH9119 and AgRP binding. This effect of Ca2+ on α-MSH binding translates into potentiation of cyclic adenosine monophosphate (cAMP) response, which is a typical readout of Gαs coupling, and the closure of inward rectifier potassium channel (KIR7.1) upon α-MSH stimulation in cellular assays using a human cell line (3). This suggests that the effect of Ca2+ on α-MSH binding to MC4R enhances transducer coupling and downstream signaling.
Previous studies have identified Na+-binding sites in multiple class A GPCRs, which has emerged as an important paradigm of allosteric modulation of these receptors, harboring inherent potential to influence the efficacy and selectivity of GPCR-targeting drugs (12, 13). Moreover, a Zn2+-binding site has been visualized in the crystal structure of the human platelet-activating factor receptor (PAF1R), also a class A GPCR (14). However, the Ca2+-binding site in MC4R is distinct from the Na+- and Zn2+-binding sites and therefore hints at a much broader role and distinct modes of action of ionic cofactors in GPCR activation, signaling, and regulation than anticipated. It is also intriguing that Ca2+ selectively modulates α-MSH binding to MC4R but not the binding of AgRP. Thus, it is possible that the ionic cofactors may have an untapped potential in the context of GPCR-biased agonism, the ability of certain ligands to preferentially engage different downstream signaling pathways and a framework that holds tremendous potential for drug discovery (6,15).
Furthermore, the MC4R structure allows the mapping of the gain-of-function and loss-of-function mutations in the receptor that are linked to syndromic obesity on a structural template, which may lead to testable hypotheses in order to better understand the links between receptor activation and disease manifestation (8, 9). For example, it allows visualization of how these mutations may potentially affect the intra- and interhelical interactions in the receptor, modulating receptor activation, transducer coupling, and downstream signaling response. This study also raises several interesting questions for future studies. For example, how generic is the role of Ca2+ on the ligand binding and activation of different subtypes of the melanocortin receptors, and by extension, to other GPCRs? Taken together with previous observations of Na+- and Zn2+-binding sites on other GPCRs, it is possible that different ions provide an additional level of regulation for different receptors depending on the tissue context and physiological settings. Answering these questions will require comprehensive structural, pharmacological, and in vivo analysis on multiple receptor systems.
Acknowledgments
The authors are supported by the Wellcome Trust/Department of Biotechnology (DBT) India Alliance (IA/I/14/1/501285); Department of Science and Technology, India Ministry of Science and Technology (DST/SJF/LSA-03/2017-18); Science and Engineering Research Board (SERB) (EMR/2017/003804); DBT (BT/PR29041/BRB/10/1697/2018), Council of Scientific and Industrial Research [37(1730)/19/EMR-II]; and Indian Institute of Technology Kanpur.
References and Notes
- 1.Anderson EJ, et al. J Mol Endocrinol. 2016;56 doi: 10.1530/JME-16-0014. T157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kühnen P, Krude H, Biebermann H. Trends Mol Med. 2019;25:136. doi: 10.1016/j.molmed.2018.12.002. [DOI] [PubMed] [Google Scholar]
- 3.Yu J, et al. Science. 2020;368:428. doi: 10.1126/science.aaz8995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghosh E, Kumari P, Jaiman D, Shukla AK. Nat Rev Mol Cell Biol. 2015;16:69. doi: 10.1038/nrm3933. [DOI] [PubMed] [Google Scholar]
- 5.Chan HCS, Li Y, Dahoun T, Vogel H, Yuan S. Trends Biochem Sci. 2019;44:312. doi: 10.1016/j.tibs.2018.11.011. [DOI] [PubMed] [Google Scholar]
- 6.Wootten D, Christopoulos A, Marti-Solano M, Babu MM, Sexton PM. Nat Rev Mol Cell Biol. 2018;19:638. doi: 10.1038/s41580-018-0049-3. [DOI] [PubMed] [Google Scholar]
- 7.Tao YX. Endocr Rev. 2010;31:506. doi: 10.1210/er.2009-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lotta LA, et al. Cell. 2019;177:597. doi: 10.1016/j.cell.2019.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lubrano-Berthelier C, et al. J Clin Endocrinol Metab. 2006;91:1811. doi: 10.1210/jc.2005-1411. [DOI] [PubMed] [Google Scholar]
- 10.Ollmann MM, et al. Science. 1997;278:135. doi: 10.1126/science.278.5335.135. [DOI] [PubMed] [Google Scholar]
- 11.Pritchard LE, White A. Peptides. 2005;26:1759. doi: 10.1016/j.peptides.2004.11.036. [DOI] [PubMed] [Google Scholar]
- 12.Katritch V, et al. Trends Biochem Sci. 2014;39:233. doi: 10.1016/j.tibs.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zarzycka B, Zaidi SA, Roth BL, Katritch V. Pharmacol Rev. 2019;71:571. doi: 10.1124/pr.119.017863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cao C, et al. Nat Struct Mol Biol. 2018;25:488. doi: 10.1038/s41594-018-0068-y. [DOI] [PubMed] [Google Scholar]
- 15.Kumari P, Ghosh E, Shukla AK. Trends Mol Med. 2015;21:687. doi: 10.1016/j.molmed.2015.09.002. [DOI] [PubMed] [Google Scholar]