Conspectus
Nanomedicine holds significant potential to improve the efficacy of cancer immunotherapy. Thus far, nanomedicines – i.e. 1-100(0) nm-sized drug delivery systems – have been primarily used to improve the balance between the efficacy and the toxicity of conjugated or entrapped chemotherapeutic drugs. The clinical performance of cancer nanomedicines has been somewhat disappointing, which is arguably mostly due to the lack of tools and technologies for patient stratification. Conversely, the clinical progress made with immunotherapy has been spectacular, achieving complete cures and inducing long-term survival in advanced-stage patients. Unfortunately, however, immunotherapy only works well in relatively small subsets of patients. Increasing amounts of pre-clinical and clinical data demonstrate that combining nanomedicine with immunotherapy can boost therapeutic outcomes, by turning “cold” non-immunoresponsive tumors and metastases into “hot” immunoresponsive lesions.
Nano-immunotherapy can be realized via three different approaches, in which nanomedicines are used (1) to target cancer cells, (2) to target the tumor immune microenvironment, and (3) to target the peripheral immune system. When targeting cancer cells, nanomedicines typically aim to induce immunogenic cell death, thereby triggering the release of tumor antigens and danger-associated molecular patterns, such as calreticulin, high mobility group box 1 protein and adenosine triphosphate. The latter serve as adjuvants to alert antigen-presenting cells to take up, process and present the former, thereby promoting the generation of CD8+ cytotoxic T cells. Nanomedicines targeting the tumor immune microenvironment potentiate cancer immunotherapy by inhibiting immunosuppressive cells, such as M1-like tumor-associated macrophages, as well as by reducing the expression of immunosuppressive molecules, such as transforming growth factor beta. In addition, nanomedicines can be employed to promote the activity of antigen-presenting cell and cytotoxic T cells in the tumor immune microenvironment. Nanomedicines targeting the peripheral immune system aim to enhance antigen presentation and cytotoxic T cell production in secondary lymphoid organs, such as lymph nodes and spleen, as well as to engineer and strengthen peripheral effector immune cell populations, thereby promoting anticancer immunity.
While the majority of immunomodulatory nanomedicines are in pre-clinical development, exciting results have already been reported in initial clinical trials. To ensure efficient translation of nano-immunotherapy constructs and concepts, we have to consider biomarkers in clinical development, to make sure that the right nanomedicine formulation is combined with the right immunotherapy in the right patient. In this context, we have to learn from currently ongoing efforts in nano-biomarker identification, as well as from partially already established immuno-biomarker-initiatives, such as the Immunoscore and the cancer immunogram. Together, these protocols will help to capture the nano-immuno-status in individual patients, enabling the identification and use of individualized and improved nanomedicine-based treatments to boost the performance of cancer immunotherapy.
1. Introduction
1.1. Cancer nanomedicine
Nanomedicine refers to as the application of nanotechnology in medicine.1 While this also entails the development of nano-sized materials and methods for the ex vivo diagnosis and staging of diseases (e.g. nanotechnologies to amplify signals in blood and/or to enable multiplexed DNA or protein detection on chips2), the term nanomedicine is traditionally used to describe to 1-100(0) nm-sized drug delivery systems that upon intravenous injection travel throughout the body to selectively accumulate in pathological regions, and to specifically elicit pharmacological effects there, while avoiding drug accumulation and drug actions elsewhere in the body.1
Nanomedicine-based tumor targeting is typically achieved via two main mechanisms, i.e. passive and active targeting. Passive targeting relies on the Enhanced Permeability and Retention (EPR) effect, which was uncovered three decades ago by Matsumura and Maeda,3 as well as by Jain and colleagues4. Active targeting relies on the decoration of nanoparticles with targeting ligands, such as antibodies or peptides, which specifically recognize receptors overexpressed at the pathological site. Both strategies have pros and cons, related e.g. to overall targeting efficiency, specific cell delivery, formulation complexity, and translational potential. These issues have recently been extensively reviewed, discussed and debated.5–7
Apart from directly killing cancer cells, nanomedicines can help to combat malignancies by modulating antitumor immune responses. This can be achieved by developing nanomedicines which (1) target cancer cells to elicit immunogenic cell death; (2) target immune cells, such as macrophages, dendritic cells and T cells, or immunosuppressive pathways in the tumor immune microenvironment; and (3) target the peripheral immune system, located e.g. in lymph nodes and in the spleen.8–11 As discussed in more detail below, ever more evidence suggests that nanomedicines can potentiate antitumor immunity and synergize with established immunotherapeutics to improve response rates and survival times.10
1.2. Cancer immunotherapy
Immunotherapy is radically reshaping the landscape of clinical cancer treatment. While the first immunomodulatory therapeutics (e.g. Coley's toxins) were described more than a century ago, immunotherapy has only very recently become broadly accepted as a “stand-alone” anticancer treatment modality.12 In several cancer types, particularly malignant melanoma and lung cancer, it outperformed standard-of-care therapy, achieving unprecedented outcomes in a number of cases, including complete regression of advanced-stage (metastasized) tumors and long-term disease-free survival.13
This immunotherapeutic revolution has profited a lot from recent advances in cancer biology and anticancer immunity, most prominently from the discovery of several dominant immunosuppressive pathways.14,15 These seminal advances have been recognized by the 2018 Nobel Prize in Physiology or Medicine, which was awarded to James Allison and Tasuku Honjo for “the discovery of cancer therapy by inhibition of negative immune regulation”. Specifically, the Nobel prize was awarded for the identification of immune checkpoints (i.e. cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and programmed death/ligand 1 (PD-1/PD-L1)), which have led to the development of antibodies targeting these checkpoints for anticancer therapy.16 Besides immune checkpoint inhibitors, which are mainly used for solid tumors, effective cancer immunotherapy has also been achieved via the use of chimeric antigen receptor (CAR) T-cell therapies, which have thus far been mainly employed for the treatment of hematological malignancies.17 Between 2014 and 2018, these two types of immunotherapy have witnessed the approval of eight new anticancer drugs by the Food and Drug Administration (FDA), i.e. six for PD-1/L1 blockade and two for CAR T-cell therapy.10 Moreover, recent advances in the development and testing of cancer vaccines, especially those based on neoantigen delivery, have demonstrated the potential of this immunotherapeutic strategy in the clinic.18
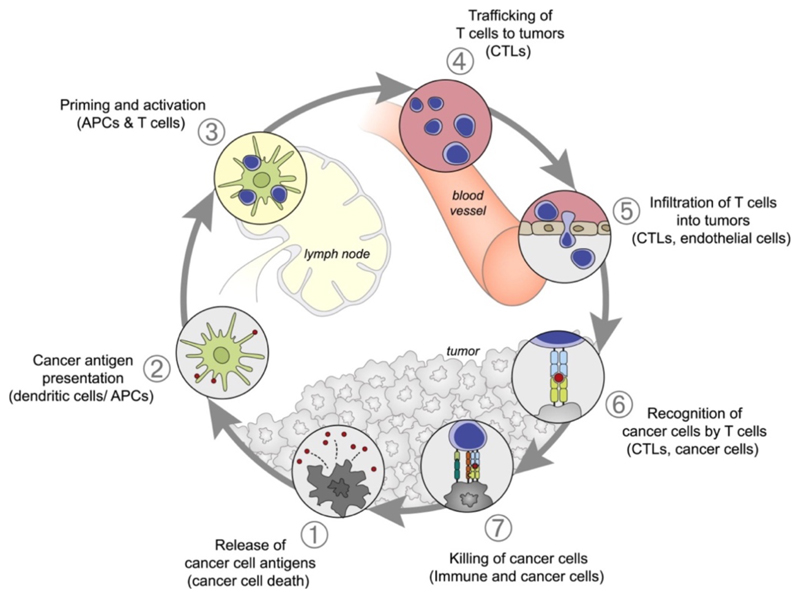
The principle(s) of cancer immunotherapy can be schematically captured in the so-called cancer-immunity cycle (Figure 1).19 This cycle starts with the release of tumor antigens, which are taken up, processed and presented by antigen-presenting cells (APCs) to naive T cells. This thereby generates cytotoxic T cells which are able to specifically recognize and kill cancer cells. The lysed cancer cells in turn release antigens and co-stimulation signals to promote another round of the immune reaction cascade. Tumors can disrupt essential elements of the cancer-immunity cycle, via a wide range of negative feedback immune regulatory pathways, which are increasingly becoming targets for cancer immunotherapy.
Figure 1. Schematic illustration of the cancer-immunity cycle.
The anticancer immune reaction starts with the release of cancer cell antigens (1), which are taken up, processed and presented by antigen-presenting cells (APCs) to naive T cells in secondary lymphoid organs, such as lymph nodes and spleen (2+3). Subsequently, cytotoxic T lymphocytes (CTLs) are generated, which migrate to and infiltrate tumors and metastases (4+5). In tumors and metastases, CTLs can then recognize (6) and kill (7) cancer cells. Reproduced with permission from ref. 19. Copyright 2013 Elsevier.
Immunotherapy has resulted in remarkable clinical successes, but it thus far unfortunately only works well in relatively small subsets of patients. This shortcoming is currently being addressed (and gradually tackled) via several different strategies, including the implementation of biomarkers to stratify patients with “cold” non-immunoresponsive lesions versus patients with “hot” immunoresponsive tumors and metastases.20 In addition, as will be alluded to below, many different combination immunotherapy studies are currently being explored21, including an ever-growing number of nano-immunotherapies.
1.3. Combining nanomedicine and immunotherapy
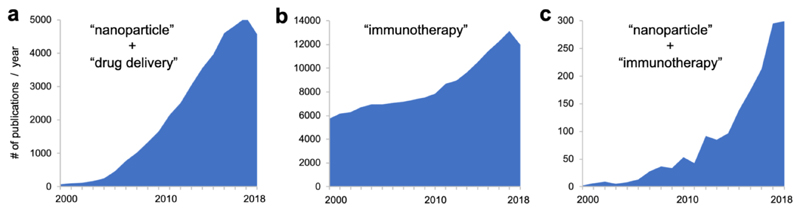
In the last couple of years, the combination of nanomedicine and immunotherapy has been gaining ever more attention. Nanomedicine has been a highly active field, as exemplified by the steadily increasing number of papers reporting on nanoparticles for drug delivery since the beginning of the new millennium (Figure 2a). Research on immunotherapy was already very active in the early 2000's, and witnessed a strong growth after 2011, the year in which the first immune checkpoint inhibitor obtained FDA approval (Figure 2b). Interestingly, in 2011, also the number of publications on nano-immunotherapy experienced a strong growth, and it started to expand exponentially from 2013 onwards (Figure 2c). This ever-expanding number reflects the high hopes that are currently associated with the use of nanomedicine formulations for individualizing and improving the outcome of cancer immunotherapy.
Figure 2. Publications on nanomedicine, immunotherapy and nano-immunotherapy.
Searches were performed in Pubmed using [(nanoparticle) AND (drug delivery) in all fields, a], [(immunotherapy) in all fields, b], and [(immunotherapy) AND (nanoparticle) in all fields, c].
In this account, we describe three main forms of cancer nano-immunotherapy, which are sub-categorized according to the targets of the nanomedicine formulations: targeting cancer cells, targeting the tumor immune microenvironment, and targeting the peripheral immune system. Among these three immunomodulatory approaches, the majority of studies focus on improving the treatment of solid tumor malignancies, and only few papers deal with hematological malignancies (mainly via targeting the peripheral immune system). These three strategies are being explored with an ever-increasing number of nanomedicines. To harness the immunomodulatory potential of nanomedicine, and to really benefit clinical immuno-oncology, nano-immunotherapy needs to be further refined. In the outlook, we therefore also propose protocols that may help to individualize and improve nano-immunotherapy, via integrating the lessons learned (on the use of biomarkers for patient stratification) in both nanomedicine and immuno-oncology. In this context, nano-immuno- biomarkers should be systemically and systematically considered, to eventually ensure efficient cancer nano-immunotherapy. Immune biomarkers should be assessed to thoroughly characterize the immune status of individual patients (and particularly of patients’ tumors and metastases). This information will be instrumental in decision-making with regard to the design of optimal nano-immunotherapy combination regimens for individual patients.
2. Nanomedicines For Cancer Immunotherapy
Cancer nanomedicine typically aims to improve the direct killing of cancer cells, by improving the delivery of chemotherapeutic drugs to tumors and metastases. In recent years, however, ever more nanomedicine formulations are used to potentiate anticancer immunity and to synergize with clinically established immunotherapeutics. In this context, three main directions are being explored, in which nanomedicines are designed to target cancer cells, to target the tumor immune microenvironment and to target the peripheral immune system.
2.1. Targeting cancer cells
Nanomedicines can be employed to promote the induction of immunogenic cell death (ICD). ICD is a specific mode of cell death which is associated with the release of tumor antigens and danger-associated molecular patterns, and it is an important trigger and enhancer of anticancer immunity. ICD is induced by certain types of chemotherapeutics (e.g. doxorubicin, oxaliplatin and cyclophosphamide), as well as by radiotherapy, photodynamic/photothermal therapy and other physical stimuli.22,23 Classical features of ICD are the translocation of calreticulin (CRT) to the cell surface, and the release of adenosine triphosphate (ATP) and the high mobility group box 1 protein (HMGB1) into the extracellular environment. These features alert the immune system, resulting in the uptake and processing of tumor antigens by APCs, and in the generation of cytotoxic T cells, which then migrate to and eradicate tumors and metastases (Figure 3a). As such, ICD is very valuable for improving the efficacy of immune checkpoint blocking therapies.24
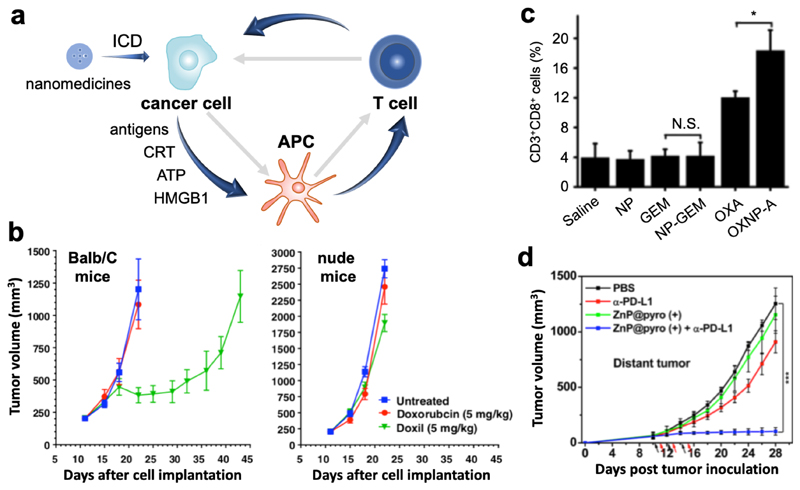
Figure 3. Nano-immunotherapy based on targeting cancer cells.
a: The cancer-immunity cycle is often impaired in tumors (depicted by grey arrows). Upon nanomedicine-based targeting of immunogenic cell death (ICD) inducing drugs to cancer cells, tumor antigens and damage-associated molecular patterns (i.e. calreticulin, adenosine triphosphate and high mobility group box 1 protein) are released. Together, these components promote antigen uptake, processing and presentation by antigen-presenting cells, which contributes to the generation of cytotoxic T cells. b: Immunopotentiation exerted by ICD-inducing nanomedicines, such as PEGylated liposomal doxorubicin (Doxil), can be exemplified by the notion that Doxil is significantly more effective in inhibiting CT26 tumor growth in immunocompetent Balb/C mice than in immunodeficient nude mice. The benefit of nanomedicine-based drug targeting to cancer cells was demonstrated by the higher efficacy and lower toxicity of Doxil as compared to free doxorubicin. Adapted with permission from ref. 25. Copyright 2015 Elsevier. c: Nanoparticle-based delivery of ICD-inducing oxaliplatin resulted in stronger immunopotentiation that oxaliplatin in free form, as exemplified by higher levels of CD8+ T cells infiltration into Pan02 tumors. In case of non-ICD-inducing gemcitabine treatment, no enhanced T cell infiltration into tumors was observed. Adapted with permission from ref. 26. Copyright 2016 Elsevier. d: Pyrolipid-loaded nanomedicines induced ICD and potentiated cancer immunity in distant tumors via the abscopal effect, which synergized with anti-PD-L1 antibodies to efficiently eradicate the tumors. Adapted with permission from ref. 27. Copyright 2013 American Chemical Society.
ICD-inducing nanomedicines, such as doxorubicin-loaded liposomes (Caelyx/Doxil®), are increasingly employed in combination with immunotherapy. An exemplary study in this regard was published by Rios-Doria and colleagues,25 who combined Doxil with several clinically relevant immunotherapeutics, including anti-PD-1, -PD-L1 and -CTLA4 antibodies, and tumor necrosis factor receptor alpha agonists. As initial evidence for Doxil-based immunopotentiation, they showed that Doxil was significantly more effective in immunocompetent than in immunodeficient mice (Figure 3b). Doxil furthermore substantially improved the efficacy of immunotherapy, in part by promoting (via ICD) the proliferation of DCs and CD8+ T cells.25 It is important to note in this regard that Doxil induced much stronger immunopotentiation than free doxorubicin administered at the same dose. Similarly beneficial effects were reported by Nie and colleagues, who showed that oxaliplatin-loaded PLGA nanoparticles more effectively induced ICD and more potently activated the immune system than free oxaliplatin (Figure 3c).26 These improvements result from more efficient targeting of chemotherapeutic drugs to cancer cells via nanomedicine formulations, and also from avoiding lymphotoxicity, which is potently induced by free chemotherapeutic drugs.
Besides for standard chemotherapeutic drugs, nanomedicines have also been employed to improve the tumor-targeted delivery and the immunotherapeutic potential of agents used for photodynamic therapy (PDT) and radiotherapy. Lin and colleagues, for instance, employed pyrolipid-loaded inorganic nanoparticles for immunoactivation via PDT. Nano-PDT treatment enhanced the serum levels of proinflammatory cytokines associated with better immunotherapy outcomes, such as tumor necrosis factor receptor alpha, interleukin 6 and interferon gamma. In addition, it significantly improved the tumor infiltration of CD4+ and CD8+ T cells. The efficient induction of anticancer immunity in the nano-PDT-treated primary tumor furthermore promoted the abscopal effect, enhancing the efficacy of anti-PD-1 therapy in distant lesions and metastases (Figure 3d).27 Not unexpectedly, nanoparticle-based PDT has also already been combined with nanoparticle-based ICD induction, as exemplified by nano-coformulations of oxaliplatin and pyrolipid28, and of doxorubicin and chlorine e629. A nice example of nano-radio-immunotherapy has been provided by Hindré and colleagues.30 The authors used lipid nanoparticles loaded with rhenium-188 for internal radiotherapy, the added value of the nanoparticles being to retain the radioisotopes in tumors after local administration in orthotopic gliomas in rats. It was found that the radiotherapeutic nanoparticles significantly increased the levels of circulating cytokines, as well as of tumor-infiltrating immune cells.
In summary, immunomodulatory nanomedicines which target cancer cells typically aim to induce ICD to potentiate the cancer-immunity cycle. In addition, nanomedicines help to improve antitumor immunity by decreasing the systemic (lymphocyte) toxicity, which also helps to potentiate immunotherapy outcomes.31 Nanomedicines do not have to be administered systemically to have a systemic effect: via the abscopal effect, locally injected or locally activated nanoparticles are able to induce systemic immunity.32 Besides inducing ICD, also other (nano-)chemotherapy effects may be useful to potentiate immunotherapy. Paclitaxel, for instance, has been shown to enhance the maturation and function of DCs33, and doxorubicin has been reported to decrease the number of myeloid-derived suppressor cells in tumors.34 It is anticipated that in the next couple of years, a significant number of (especially ICD-inducing) nanomedicine formulations will be tested in the clinic in combination with established immunotherapeutics.
2.2. Targeting the tumor immune microenvironment
A second important strategy for promoting the efficacy of anticancer immunotherapy relies on the use of nanomedicines which modulate the tumor immune microenvironment (TIME).24 In the TIME, immunosuppressive pathways and mediators are frequently upregulated, as exemplified e.g. by the increased infiltration of immunosuppressive cells, such as certain types of tumor-associated macrophages (TAM) and myeloid-derived suppressor cells (MDSC), into tumors, as well as by increased levels of soluble inhibitors, such as indoleamine 2,3-dioxygenase (IDO) and transforming growth factor beta (TGF-β). Nanomedicines modulating the TIME are designed to first accumulate in tumors via passive/active targeting mechanisms, and to then help overcome local tumor immunosuppression mediated by TAM, MDSC and/or soluble inhibitors. As a result of inhibiting immunosuppression in the TIME, the infiltration, proliferation, maturation, survival and/or activity of effector immune cells, such as cytotoxic T cells, is increased, resulting in improved immunotherapy outcomes (Figure 4a).
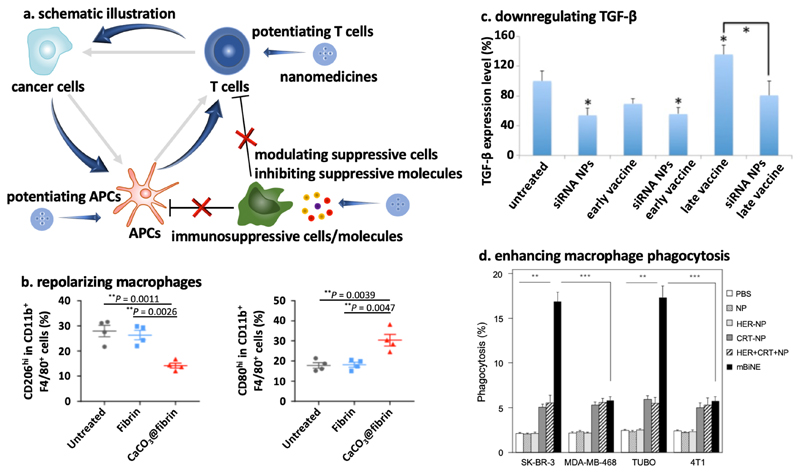
Figure 4. Nano-immunotherapy based on targeting the tumor immune microenvironment (TIME).
a: Nanomedicines can inhibit immunosuppressive cells and molecules, and they can potentiate effector immune cells (such as APCs and cytotoxic T cells). Both approaches enhance the cancer-immunity cycle. b: Immunosuppressive M2-like macrophages (left) can be decreased by CaCO3 nanoparticles, via modulating tumor acidity. At the same time, the level of immunopotentiating M1-like macrophages (right) was increased. Adapted with permission from ref. 38. Copyright 2019 Springer Nature. c: Downregulation of TGF-β levels in tumors was induced by siRNA-loaded nanoparticles, when used alone and when combined with vaccination at early or late stages of tumor development. Adapted with permission from ref. 49. Copyright 2014 American Chemical Society. d: Multivalent bi-specific nanobioconjugate engager (mBiNE) modified with calreticulin (CRT) and with antibodies targeting the human epidermal growth factor receptor (HER) trigger macrophage-mediated phagocytosis of HER2-expressing tumor cells (i.e. SK-BR-3 and TUBO). Adapted with permission from ref. 50. Copyright 2017 Springer Nature.
TAM are a major population of immune cells in tumors. The polarization of TAM in malignant lesions is typically dominated by cells with an M2-like phenotype, which is “pro-tumor”, which impedes the infiltration of effector T cells, and which prohibits efficient anti-PD-1 therapy.35 Daldrup-Link and colleagues showed that ferumoxytol, i.e. a superparamagnetic iron oxide nanoparticle formulation which is FDA-approved for the treatment of iron deficiency anemia, can convert M2-like TAM into M1-like TAM. Upon systemic injection, ferumoxytol efficiently targeted the TIME, and via altering macrophage polarization, it inhibited the growth of primary and metastatic tumors in liver and lungs.36 Analogously, Weissleder and co-workers showed that cyclodextrin nanoparticles were able to target a small molecule toll-like receptor 7/8 agonist to macrophages in the TIME, which via induction of M2 to M1 polarization improved the efficacy of checkpoint-inhibiting immunotherapy.37 Gu and colleagues developed CaCO3 nanoparticles functionalized with anti-CD47 antibodies, and demonstrated that upon local application as an in-situ forming hydrogel during tumor surgery, the CaCO3 reacted with protons in the TIME, causing the pH to increase and macrophages to polarize towards an M1 phenotype (Figure 4b).38 The anti-CD47 antibodies were incorporated to block the “don’t eat me” signal on tumor cells. Together, these two effects significantly enhanced tumor cell endocytosis by macrophages and they improved the outcome of checkpoint blockade therapy. Besides macrophages, neutrophils make up a large portion of the leukocyte population in tumors. Steinmetz, Fiering et al. engineered plant virus nanoparticles to promote the tumor infiltration of CD11b+Ly6G+ activated neutrophils, while decreasing the fraction of CD11b-Ly6G+ quiescent neutrophils, resulting in efficient immunotherapy of metastatic cancer in mice.39
Nanomedicines can also act on immunosuppressive molecules in the TIME, such IDO and TGF-β. IDO promotes the conversion of tryptophan to kynurenine, which is a potent T cell suppressing metabolite.40 Small molecule IDO inhibitors have been extensively tested in (pre-)clinical trials. They are also increasingly incorporated in nanomedicine formulations, aiming to modulate the TIME to improve outcomes of immunotherapeutic interventions. In this context, Lin et al demonstrated synergy between IDO inhibitor-loaded nanomedicines and photodynamic therapy41, as well as radiotherapy42. Nel, Meng and colleagues combined an IDO inhibitor with the ICD inducer oxaliplatin in lipid-coated mesoporous silica nanoparticles, achieving tumor eradication in a mouse model of pancreatic ductal adenocarcinoma.43 A small molecule IDO inhibitor was also combined with a peptide blocking PD-L1 in peptide-based nanoparticles that allowed for payload release in mild acidic environments in the presence of matrix metalloproteinase-2. This combination nanomedicine formulation effectively inhibited melanoma growth in mice by triggering anticancer immunity.44 TGF-β is an important immunosuppressive factor in tumors45 and attenuates the efficacy of checkpoint-inhibiting immunotherapy46. Based on this notion, Irvine and colleagues encapsulated a small molecule TGF-β inhibitor in liposomes targeting T cells.47 Nanomedicine-mediated TGF-β-inhibition resulted in significant T cell activation, both ex vivo (prior to adoptive T cell transfer) and in vivo, and controlled tumors and metastases in mice bearing B16F10 melanoma. Fahmy and colleagues co-loaded a TGF-β inhibitor and interleukin-2 in nanoparticles, and were able to alleviate immunosuppression and augment T cell proliferation.48 Huang et al. developed a TGF-β siRNA-containing nanoformulation, which achieved ~50% knockdown of TGF-β expression in tumors and which synergized with cancer vaccination, as exemplified by significantly more cytotoxic T cell infiltration and significantly enhanced tumor growth inhibition (Figure 4c).49
Immunomodulatory nanomedicines can furthermore be used to directly potentiate the function of immune cells, such as macrophages and cytotoxic T cells, in the TIME. To improve antigen presentation by macrophages, Kim and colleagues constructed a multivalent bi-specific nanobioconjugate engager (mBiNE), based on polystyrene nanoparticles modified with anti-HER2 antibodies and calreticulin.50 The mBiNE formulation efficiently targeted HER2-overexpressing tumor cells and calreticulin served as an “eat me” signal, to trigger macrophage endocytosis of tumor cells (Figure 4d). Upon intratumoral injection in mice bearing HER2high EO771/E2 tumors, mBiNE significantly increased the tumor infiltration of macrophages and T cells, the production of proinflammatory cytokines, such as IFN-γ and IL-2, and therapeutic efficacy. Effector T cells in the TIME were directly targeted by nanomedicines by Irvine and co-workers, who used liposomes loaded with IL-2 and a 4-1BB ligand to co-stimulate T-cell activation.51 The double-drug-loaded liposomes enhanced the tumor infiltration of effector T cells, which promoted cytokine production and granzyme expression, and which significantly improved antitumor immunotherapy. Recently, endosome-disrupting polymersomes were utilized by Wilson and colleagues for intracellular delivery of a stimulator of interferon genes (STING) agonist which in the native form does not cross the cell membrane. Treatment with these polymersomes substantially improved anticancer immunity and the efficacy of checkpoint blockade therapy.52
In summary, nanomedicines can potentiate anticancer immunity by modulating the TIME in two different ways, i.e. by alleviating immunosuppression or by promoting immunoactivation. These two strategies synergize with clinically established immunotherapeutics, e.g. with immune checkpoint inhibiting antibodies. Since nanomaterials typically strongly interact with immune cells in the tumor microenvironment, especially with TAM,53 targeting immune cells with nanomedicines (instead of directly targeting cancer cells) appears to be a promising future avenue for boosting local and systemic antitumor immunity.
2.3. Targeting the peripheral immune system
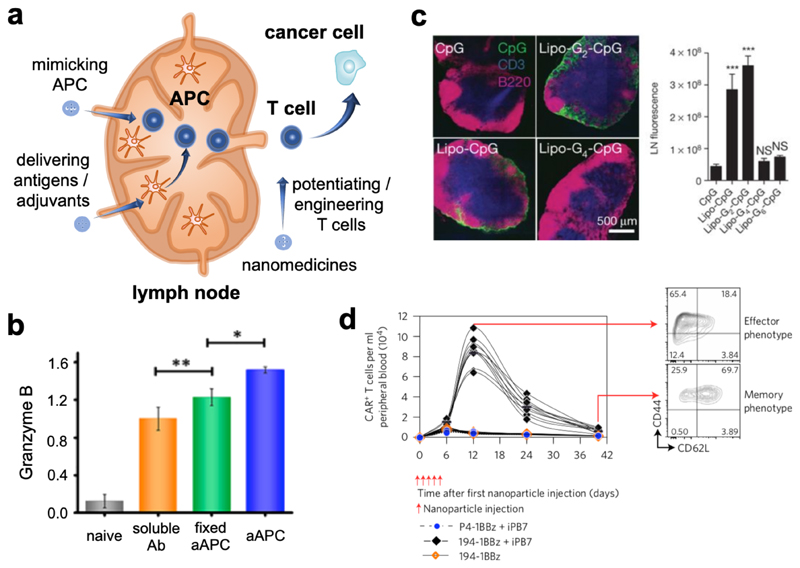
Nanomedicines targeting immune compartments located outside of tumors (here referred to as the peripheral immune system) are receiving increasing attention. The peripheral immune system, i.e. secondary lymphoid organs such as lymph nodes (LNs) and the spleen, is essential in cancer immunity, as antigen presentation and cytotoxic T cell generation take place in these compartments. In cancer occurrence and progression, the peripheral immune system is often impaired. Immunomodulatory nanomedicines can restore the functions of the peripheral immune system, e.g. by potentiating antigen presentation and by engineering T cells (Figure 5a).
Figure 5. Nano-immunotherapy based on targeting the peripheral immune system.
a: Nanomedicines can improve cytotoxic T cell generation in peripheral immune organs, such as lymph nodes, by modulating antigen presentation or by mimicking APCs. Furthermore, nanomedicines can potentiate or engineer peripheral T cells for more efficient killing of cancer cells. b: CpG motifs modified with lipid tails (Lipo-G2-CpG) efficiently targeted lymph nodes to activate APCs following intravenous injection. Adapted with permission from ref. 58. Copyright 2014. Springer Nature. c: In vivo T cell maturation and activation were more effectively achieved by artificial APCs (aAPC) with optimized surface chemistry compared to fixed aAPC and soluble activating antibodies. Adapted with permission from ref. 63. Copyright 2017 American Chemical Society. d: Peripheral T cells were engineered using intravenously injected gene delivery nanoparticles to express chimeric antigen receptors (CAR) in mice bearing B-cell acute lymphoblastic leukemia. High numbers of effector and memory T cells could be detected in peripheral blood for up to one month. Adapted with permission from ref. 66. Copyright 2017 Springer Nature.
Nanomedicines can be applied for cancer vaccination based on the notions that intradermally or subcutaneously injected nanoparticles drain to LNs, and that nanoparticulate antigens are more efficiently taken and processed by APCs than are small molecules and soluble vaccines.54 In this context, Moon and Schwendeman designed nanodiscs physically loaded with CpG and peptide antigens,55 and Hubbell and Swartz developed nanoparticles chemically conjugated with CpG56. Both nano-vaccines efficiently drained to LNs and promoted anticancer immunity upon local injection. De Geest and colleagues utilized pH-sensitive nanogels chemically entrapping the small molecule toll-like receptor 7/8 agonist imidazoquinoline for LN targeting upon local injection.57 Irvine and colleagues showed that LNs can also be targeted upon systemic injection, via modifying CpG and antigens with lipid moieties which binding to endogenous albumin (Figure 5b).58 Apart from enhancing vaccination and immunotherapy efficiency, such nano-vaccines typically significantly improve the tolerability of the adjuvants used in vaccination approaches.
Nanomedicines can also induce antitumor vaccination without using conventional adjuvants. Wang and colleagues, for instance, reported an approach to improve antigen presentation by DCs using PLGA nanoparticles, which adsorbed tumor antigens and then drained to LNs to deliver the antigens to DCs. In a bilateral tumor model, one tumor was irradiated to release antigens and injected with the nanoparticles, and strong immunity was elicited in both tumors, which synergized with anti-PD-1 antibodies.59 Gao and colleagues used nanoparticles based on pH-sensitive PEG-b-polymethacrylate polymers to deliver antigens to APCs in LNs. The antigen-loaded nanoparticles elicited stronger vaccination than free antigens combined with conventional adjuvants (e.g. polyinosinic:polycytidylic acid (i.e. poly(I:C))), likely by activating the STING pathway.60 Sahin and colleagues formulated antigen-encoding mRNAs within a lipoplex, which targeted APCs in the spleen upon intravenous injection. This nano-vaccine achieved strong immunization and de novo cytotoxic T cell generation,61 and it is currently under investigation in multiple clinical trials.
Instead of triggering APCs to present antigens to naive T cells, nanomedicines have also been designed to replace APCs, by directly generating cytotoxic T cells. Such synthetic APCs require nano-materials with sufficient flexibility and multivalency, which are crucial for forming an “immune synapse” on naive T cells.10 Rowan, Figdor and colleagues designed synthetic APCs based on poly(isocyano peptide) modified with 3-5 anti-CD3 antibodies per 150-200 nm of the polymer chain, which induced the expression of the early T cell activation marker CD69 and which promoted the production of IFN-γ.62 Xie and colleagues prepared synthetic APCs based on clusters of iron nanoparticles coated with leukocyte membranes bearing peptide-loaded major histocompatibility complex I and anti-CD28 antibodies as co-stimulatory ligands. These synthetic APCs achieved effective generation and activation of cytotoxic T cells (Figure 5c), and they induced effective tumor inhibition when administered together with T cells in tumor-bearing mice.63
Circulating T cells can be targeted by immunomodulatory nanomedicines. Irvine and colleagues prepared liposomes loaded with the cytokines IL-15 superagonist and IL-21 to target T cells, improving T cell persistence and homing to LNs and spleen.64 Tang et al. developed cytokine-based nanogels crosslinked via disulfide bonds, which were able to respond to T cell receptor signaling, triggering the release of IL-15 superagonist.65 Apart from strengthening T cells, the chimeric antigen receptor (CAR) gene transfection nanoformulation developed by Stephan et al. systemically generated CAR T cells upon intravenous injection in leukemia-bearing mice (Figure 5d), via which some of the laborious procedures for CAR T cell manufacturing can be overcome.66
Nanomedicines targeting the peripheral immune system are designed to potentiate antigen presentation and cytotoxic T cell generation, as well as to enhance the viability and activity of T cells. They can also engineer T cells to recognize and kill cancer cells. One of these formulations, i.e. antigen-encoding mRNAs within a lipoplex, has already entered clinical trials,61 in monotherapy settings and well as upon combination with established immunotherapeutics. Collectively, for nanomedicines targeting the peripheral immune system, as well as for nanomedicines targeting cancer cells and the tumor immune microenvironment, the progress made so far clearly indicates that combining nanomedicine with immunotherapy holds significant potential to improve the outcome of cancer immunotherapy in patients.
3. Outlook: Toward Individualized and Improved Nano-Immunotherapy
The combination of nanomedicine and immunotherapy has been achieving remarkable responses, particularly in pre-clinical settings. That said, it is important that only rationally designed nano-immunotherapy combinations are being explored; formulating anti-PD-(L)1 antibodies in nanomedicines, for instance, will arguably not add much value. To ensure progress in nano-immunotherapy, it will be crucial to learn from currently ongoing developments in the clinical translation of both anticancer nanomedicines and immunotherapeutics. As in almost all other areas of oncology drug development, it is becoming increasingly clear that molecularly targeted small molecule anticancer drugs, as well as nanotherapeutics and immunotherapeutics, only work well in certain sub-populations of patients. As a result of this, strategies have to be established for patient stratification in clinical trials and in clinical practice. Such strategies will be instrumental to ensure rapid and efficient clinical implementation of nano-immunotherapy.
In the case of cancer nanomedicine, we and others have been arguing that the lack of efficient clinical translation is due to the lack of tools and technologies for patient stratification, and not to poor in vivo targeting or therapeutic performance of the nanoformulation in question.6,67–69 Imaging-based biomarkers, which assist in assessing the extent of the EPR effect in patients, can help to identify the right patient population for standard nano-chemotherapy. In this context, scientists at Merrimack Pharmaceuticals have shown that ferumoxytol, i.e. Feraheme® (an iron oxide nanoparticle formulation approved for anti-anemia treatment) can be used off-label to visualize and quantify EPR-based tumor targeting in patients by means of magnetic resonance imaging, and that individuals having high levels of ferumoxytol accumulation in tumors generally responded better to nano-liposomal irinotecan chemotherapy treatment than individuals presenting with low levels of ferumoxytol accumulation in tumors.70 The same group of scientists also demonstrated that theranostic nanomedicines, i.e. a liposomal formulation containing both a drug (doxorubicin) and an imaging agent (copper-64), allows for positron emission tomography-based quantitative assessment of target site accumulation, and for correlation between target site accumulation and therapeutic response.71 Such companion diagnostic and theranostic strategies for patient stratification are crucial for ensuring proper performance in clinical trials and in routine clinical practice.
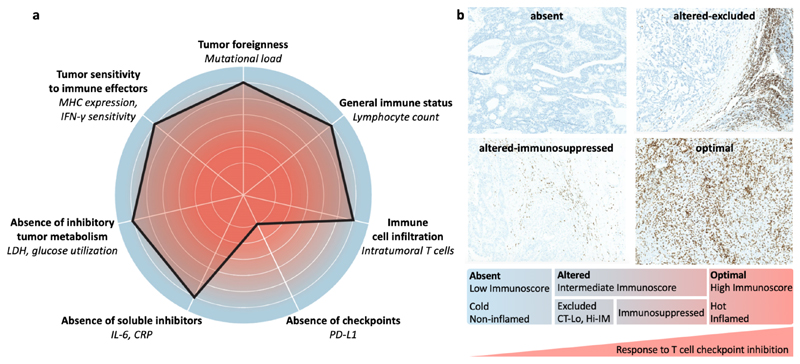
In immunotherapy, it has turned out to be at least equally important to (pre-) select the right patients for ensuring good therapeutic performance. This has already been done with individual biomarkers, such as PD-L1 expression in tumors, tumor mutational burden and microsatellite instability.72 However, on their own, these biomarkers only enable moderately efficient prediction of patient responses to immunotherapy. To improve the predictability of immune biomarkers, advanced assessment methodologies such as the cancer immunogram and the Immunoscore have been established (Figure 6). The cancer immunogram describes different parameters affecting the interaction between cancer and the immune system in individual patients, with the overall goal of identifying biomarkers to guide and improve cancer immunotherapy interventions (Figure 6a).20 The Immunoscore is based on initial observations that the location, type, density and function of immune cells predict clinical outcome in human colorectal cancer.73 This systematic histopathological analysis of the immune contexture in human tumors has gradually led to the establishment of the Immunoscore for the classification of malignant tumors, predicting not only standard survival outcomes, but also aiding to achieve more optimal responses to cancer immunotherapy.74,75
Figure 6. The cancer immunogram and Immunoscore.
a: The cancer immunogram is a scoring tool based on seven parameters related to cancer-immune system interactions. MHC: major histocompatibility complex; LDH: lactate dehydrogenase; CRP: C-reactive protein. Adapted with permission from ref. 20. Copyright 2016 The American Association for the Advancement of Science. b: The Immunoscore aims to analyze the location, type, density and function of T cells in tumors. In the most recent version, tumors are categorized in four types: absent (“cold” tumors), altered-immunosuppressed, altered-excluded and optimal (“hot” tumors). The latter have an increased likelihood to respond to immunotherapeutic interventions, such as checkpoint inhibition. CT-Lo: low T cells in the core of tumors. Hi-IM: high T cells in the invasive margin. CD3+ T cells are stained in brown and background tissue is stained in blue. Adapted with permission from ref. 76. Copyright 2019 Springer Nature.
Recently, the Immunoscore has been updated to guide decision-making in the case of combination immunotherapy.76 As exemplified by Figure 6b, four different phenotypes of tumors are defined, related to the presence and location of T cells in tumors: absent, altered-excluded, altered-immunosuppressed and optimal. In the first three phenotypes, different types of combination immunotherapy have to be considered. Analogously, in each of these situations, different strategies for nanomedicine-based enhancement of cancer immunotherapy should be conceived.
To illustrate biomarker-guided nano-immunotherapy, several clinical situations can be envisaged. For instance, if a patient presents with an altered-immunosuppressed tumor phenotype, he or she can be treated with ICD-inducing nanomedicines, to kill tumor cells and trigger the cancer-immunity cycle. This type of nano-therapy is anticipated to combine well with anti-PD-1/L1 immunotherapy. For tumors with a reasonably high level of cytotoxic T cell infiltration, but also with high levels of M2-like macrophages (which suppress anti-tumor immunity and anti-PD-1/L1 therapy77), nano-immunotherapy could involve macrophage-modulating nanomedicines, such as ferumoxytol36, as well as toll-like receptor agonist-loaded nanoparticles37. As a third example for biomarker-guided nano-immunotherapy of altered-immunosuppressed tumors, we can consider a patient with tumors characterized by a very low tumor mutational burden, and therefore by a very low level of pre-existing antitumor immunity. In this situation, ICD-inducing nanomedicines may not be very useful, since ICD cannot overcome issues related to very low numbers of clonal mutations. Alternatively, nano-vaccines containing neoepitopes seem to be a good and rational choice, as they can trigger the production and activity of cytotoxic T cells.78
For patients with an altered-excluded tumor phenotype, the extent of T cell exclusion needs to be reduced. This can for instance be achieved by nanomedicines downregulating TGF-β49, which results in less fibrotic tumors and which may promote T cells to more efficiently penetrate into tumors46. Moreover, nanomedicines which are able to actively promote degradation of the extracellular matrix, e.g. via tumor-targeted delivery of collagenase79 or via inhibiting collagen I synthesis by means of angiotensin receptor blockers80–83, can be considered. For tumors with poor T cell infiltration due to overly active pathological angiogenesis84, nanomedicines loaded with siRNA targeting VEGF (vascular endothelium growth factor)85 may be useful. Via processes such as vascular normalization and enhanced perfusion, these nano-co-treatments may improve T cell infiltration, which will help to enhance the efficacy immunotherapeutics, such as checkpoint inhibitors.
Thus far, biomarker-guided nano-immunotherapy has only been explored in a relatively small number of pre-clinical reports. For example, in two separate studies, 4T1 mouse breast cancer and B16F10 mouse melanoma models were analyzed with regard to relevant immuno-biomarkers prior to treatment.49,86 These two models were found to be characterized by high expression levels of the immunosuppressive molecules IDO and TGF-β, respectively. When tumor-bearing mice were treated with nanomedicine formulations loaded with an IDO inhibitor86 and with siRNA downregulating TGF-β49, respectively, the outcome of anti-PD-1 and nano-vaccine-based cancer immunotherapy could be strongly enhanced. These examples illustrate that initially identifying (via biomarkers) and subsequently inhibiting/targeting (using nanomedicines) specific immunosuppressive pathways which are overly active in specific tumor models - and eventually in individual patients - may be very valuable for individualizing and improving nano-immunotherapy combination regimens.
Taking everything together, several systems and strategies are envisaged for the use of nanomedicines to improve the efficacy of anti-cancer immunotherapy. In the last couple of years, significant advances have been made at the pre-clinical level, and promising initial proof-of-concept has been achieved in the clinic. To promote progress in nano-immunotherapy, biomarkers have to be established, to identify which immunosuppressive or immunoactivating cells or pathways have to be targeted to ensure optimal therapeutic outcomes. These insights will guide the design and development of immuno-modulatory nanomedicines, they will promote the clinical translation of nanomedicines in general, and - most importantly - they will contribute to the establishment of better treatments for cancer patients.
Acknowledgements
The authors gratefully acknowledge financial support by the European Research Council (ERC: Starting Grant NeoNaNo (309495) and Proof-of-Concept Grants CONQUEST (680882) and PIcelles (813086)), by the European Union (EU-EFRE: European Fund for Regional Development: I3-STM (0800387)), by the German Research Foundation (DFG: SFB 1066-1/-2: Nanodimensional polymer therapeutics for tumor therapy; and GRK/RTG 2375: Tumor-targeted Drug Delivery (331065168)), and by the Aachen Interdisciplinary Center for Clinical Research (IZKF; Project O3-2).
Biographical Information
Yang Shi obtained a PhD in pharmaceutics at Utrecht University in 2014. After an associate professorship at South China University of Technology, he took up his current position as a group leader at RWTH Aachen University Clinic in 2016. His research focuses on nanomedicines and macroscale materials for cancer chemotherapy and immunotherapy.
Twan Lammers obtained a DSc in Radiation Oncology from Heidelberg University in 2008 and a PhD in Pharmaceutics from Utrecht University in 2009. In 2014, he was promoted to full professor at the faculty of medicine at RWTH Aachen University Clinic. His primary research interests include image-guided drug delivery and tumor-targeted combination therapies.
References
- 1.Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2007;2:751–760. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 2.Stoeva SI, Lee JS, Smith JE, Rosen ST, Mirkin CA. Multiplexed detection of protein cancer markers with biobarcoded nanoparticle probes. J Am Chem Soc. 2006;128:8378–8379. doi: 10.1021/ja0613106. [DOI] [PubMed] [Google Scholar]
- 3.Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46:6387–6392. [PubMed] [Google Scholar]
- 4.Gerlowski LE, Jain RK. Microvascular permeability of normal and neoplastic tissues. Microvasc Res. 1986;31:288–305. doi: 10.1016/0026-2862(86)90018-x. [DOI] [PubMed] [Google Scholar]
- 5.Lammers T, Kiessling F, Hennink WE, Storm G. Drug targeting to tumors: principles, pitfalls and (pre-) clinical progress. J Control Release. 2012;161:175–187. doi: 10.1016/j.jconrel.2011.09.063. [DOI] [PubMed] [Google Scholar]
- 6.Lammers T, Kiessling F, Ashford M, Hennink W, Crommelin D, Storm G. Cancer nanomedicine: is targeting our target? Nat Rev Mater. 2016;1:16069. doi: 10.1038/natrevmats.2016.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilhelm S, Tavares AJ, Dai Q, Ohta S, Audet J, Dvorak HF, Chan WC. Analysis of nanoparticle delivery to tumours. Nat Rev Mater. 2016;1:16014. [Google Scholar]
- 8.Wang C, Ye Y, Hu Q, Bellotti A, Gu Z. Tailoring biomaterials for cancer immunotherapy: Emerging trends and future outlook. Adv Mater. 2017;29 doi: 10.1002/adma.201606036. 1606036. [DOI] [PubMed] [Google Scholar]
- 9.Irvine DJ, Hanson MC, Rakhra K, Tokatlian T. Synthetic nanoparticles for vaccines and immunotherapy. Chem Rev. 2015;115:11109–11146. doi: 10.1021/acs.chemrev.5b00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun Q, Barz M, De Geest BG, Diken M, Hennink WE, Kiessling F, Lammers T, Shi Y. Nanomedicine and macroscale materials in immuno-oncology. Chem Soc Rev. 2019;48:351–381. doi: 10.1039/c8cs00473k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang W, Von Roemeling CA, Chen Y, Qie Y, Liu X, Chen J, Kim BY. Designing nanomedicine for immuno-oncology. Nat Biomed Eng. 2017;1 0029. [Google Scholar]
- 12.Hoos A. Development of immuno-oncology drugs - from CTLA4 to PD1 to the next generations. Nat Rev Drug Discov. 2016;15:235–247. doi: 10.1038/nrd.2015.35. [DOI] [PubMed] [Google Scholar]
- 13.Drake CG, Lipson EJ, Brahmer JR. Breathing new life into immunotherapy: review of melanoma, lung and kidney cancer. Nat Rev Clin Oncol. 2014;11:24–37. doi: 10.1038/nrclinonc.2013.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Couzin-Frankel J. Cancer immunotherapy. Science. 2013;342:1432–1433. doi: 10.1126/science.342.6165.1432. [DOI] [PubMed] [Google Scholar]
- 15.Ledford H. Cancer treatment: the killer within. Nature. 2014;508:24–26. doi: 10.1038/508024a. [DOI] [PubMed] [Google Scholar]
- 16.Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018;359:1350–1355. doi: 10.1126/science.aar4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosenberg SA, Restifo NP. Adoptive cell transfer as personalized immunotherapy for human cancer. Science. 2015;348:62–68. doi: 10.1126/science.aaa4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schumacher TN, Scheper W, Kvistborg P. Cancer Neoantigens. Annu Rev Immunol. 2019;37:173–200. doi: 10.1146/annurev-immunol-042617-053402. [DOI] [PubMed] [Google Scholar]
- 19.Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39:1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 20.Blank CU, Haanen JB, Ribas A, Schumacher TN. The “cancer immunogram”. Science. 2016;352:658–660. doi: 10.1126/science.aaf2834. [DOI] [PubMed] [Google Scholar]
- 21.Mahoney KM, Rennert PD, Freeman GJ. Combination cancer immunotherapy and new immunomodulatory targets. Nat Rev Drug Discov. 2015;14:561–584. doi: 10.1038/nrd4591. [DOI] [PubMed] [Google Scholar]
- 22.Duan X, Chan C, Lin W. Nanoparticle-mediated immunogenic cell death enables and potentiates cancer immunotherapy. Angew Chem Int Ed Engl. 2018;58:670–680. doi: 10.1002/anie.201804882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Galluzzi L, Buqué A, Kepp O, Zitvogel L, Kroemer G. Immunogenic cell death in cancer and infectious disease. Nat Rev Immunol. 2017;17:97–111. doi: 10.1038/nri.2016.107. [DOI] [PubMed] [Google Scholar]
- 24.Binnewies M, Roberts EW, Kersten K, Chan V, Fearon DF, Merad M, Coussens LM, Gabrilovich DI, Ostrand-Rosenberg S, Hedrick CC, Vonderheide RH, et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med. 2018;24:541–550. doi: 10.1038/s41591-018-0014-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rios-Doria J, Durham N, Wetzel L, Rothstein R, Chesebrough J, Holoweckyj N, Zhao W, Leow CC, Hollingsworth R. Doxil synergizes with cancer immunotherapies to enhance antitumor responses in syngeneic mouse models. Neoplasia. 2015;17:661–670. doi: 10.1016/j.neo.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao X, Yang K, Zhao R, Ji T, Wang X, Yang X, Zhang Y, Cheng K, Liu S, Hao J, Ren H, et al. Inducing enhanced immunogenic cell death with nanocarrier-based drug delivery systems for pancreatic cancer therapy. Biomaterials. 2016;102:187–197. doi: 10.1016/j.biomaterials.2016.06.032. [DOI] [PubMed] [Google Scholar]
- 27.Duan X, Chan C, Guo N, Han W, Weichselbaum RR, Lin W. Photodynamic therapy mediated by nontoxic core-shell nanoparticles synergizes with immune checkpoint blockade to elicit antitumor immunity and antimetastatic effect on breast cancer. J Am Chem Soc. 2016;138:16686–16695. doi: 10.1021/jacs.6b09538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.He C, Duan X, Guo N, Chan C, Poon C, Weichselbaum RR, Lin W. Core-shell nanoscale coordination polymers combine chemotherapy and photodynamic therapy to potentiate checkpoint blockade cancer immunotherapy. Nat Commun. 2016;7 doi: 10.1038/ncomms12499. 12499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang G, Xu L, Chao Y, Xu J, Sun X, Wu Y, Peng R, Liu Z. Hollow MnO2 as a tumor-microenvironment-responsive biodegradable nano-platform for combination therapy favoring antitumor immune responses. Nat Commun. 2017;8 doi: 10.1038/s41467-017-01050-0. 902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vanpouille-Box C, Lacoeuille F, Belloche C, Lepareur N, Lemaire L, LeJeune JJ, Benoit JP, Menei P, Couturier OF, Garcion E, Hindré F. Tumor eradication in rat glioma and bypass of immunosuppressive barriers using internal radiation with 188Re-lipid nanocapsules. Biomaterials. 2011;32:6781–6790. doi: 10.1016/j.biomaterials.2011.05.067. [DOI] [PubMed] [Google Scholar]
- 31.Mathios D, Kim JE, Mangraviti A, Phallen J, Park CK, Jackson CM, Garzon-Muvdi T, Kim E, Theodros D, Polanczyk M, Martin AM, et al. Anti-PD-1 antitumor immunity is enhanced by local and abrogated by systemic chemotherapy in GBM. Sci Transl Med. 2016;8:370ra180. doi: 10.1126/scitranslmed.aag2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mulder WJ, Gnjatic S. Cancer immunotherapy: From local to global. Nat Nanotechnol. 2017;12:840–841. doi: 10.1038/nnano.2017.196. [DOI] [PubMed] [Google Scholar]
- 33.Pfannenstiel LW, Lam SS, Emens LA, Jaffee EM, Armstrong TD. Paclitaxel enhances early dendritic cell maturation and function through TLR4 signaling in mice. Cell Immunol. 2010;263:79–87. doi: 10.1016/j.cellimm.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alizadeh D, Trad M, Hanke NT, Larmonier CB, Janikashvili N, Bonnotte B, Katsanis E, Larmonier N. Doxorubicin eliminates myeloid-derived suppressor cells and enhances the efficacy of adoptive T-cell transfer in breast cancer. Cancer Res. 2013;74:104–118. doi: 10.1158/0008-5472.CAN-13-1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peranzoni E, Lemoine J, Vimeux L, Feuillet V, Barrin S, Kantari-Mimoun C, Bercovici N, Guérin M, Biton J, Ouakrim H, Régnier F, et al. Macrophages impede CD8 T cells from reaching tumor cells and limit the efficacy of anti-PD-1 treatment. Proc Natl Acad Sci USA. 2018;115:E4041–E4050. doi: 10.1073/pnas.1720948115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zanganeh S, Hutter G, Spitler R, Lenkov O, Mahmoudi M, Shaw A, Pajarinen JS, Nejadnik H, Goodman S, Moseley M, Coussens LM, et al. Iron oxide nanoparticles inhibit tumour growth by inducing pro-inflammatory macrophage polarization in tumour tissues. Nat Nanotechnol. 2016;11:986–994. doi: 10.1038/nnano.2016.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rodell CB, Arlauckas SP, Cuccarese MF, Garris CS, Li R, Ahmed MS, Kohler RH, Pittet MJ, Weissleder R. TLR7/8-agonist-loaded nanoparticles promote the polarization of tumour-associated macrophages to enhance cancer immunotherapy. Nat Biomed Eng. 2018;2:578–588. doi: 10.1038/s41551-018-0236-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen Q, Wang C, Zhang X, Chen G, Hu Q, Li H, Wang J, Wen D, Zhang Y, Lu Y, Yang G, et al. In situ sprayed bioresponsive immunotherapeutic gel for post-surgical cancer treatment. Nat Nanotechnol. 2018;14:89–97. doi: 10.1038/s41565-018-0319-4. [DOI] [PubMed] [Google Scholar]
- 39.Lizotte P, Wen A, Sheen M, Fields J, Rojanasopondist P, Steinmetz N, Fiering S. In situ vaccination with cowpea mosaic virus nanoparticles suppresses metastatic cancer. Nat Nanotechnol. 2016;11:295–303. doi: 10.1038/nnano.2015.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prendergast GC, Malachowski WP, DuHadaway JB, Muller AJ. Discovery of IDO1 inhibitors: from bench to bedside. Cancer Res. 2017;77:6795–6811. doi: 10.1158/0008-5472.CAN-17-2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lu K, He C, Guo N, Chan C, Ni K, Weichselbaum RR, Lin W. Chlorin-based nanoscale metal-organic framework systemically rejects colorectal cancers via synergistic photodynamic therapy and checkpoint blockade immunotherapy. J Am Chem Soc. 2016;138:12502–12510. doi: 10.1021/jacs.6b06663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu K, He C, Guo N, Chan C, Ni K, Lan G, Tang H, Pelizzari C, Fu YX, Spiotto MT, Weichselbaum RR, et al. Low-dose X-ray radiotherapy-radiodynamic therapy via nanoscale metal-organic frameworks enhances checkpoint blockade immunotherapy. Nat Biomed Eng. 2018;2:600–610. doi: 10.1038/s41551-018-0203-4. [DOI] [PubMed] [Google Scholar]
- 43.Lu J, Liu X, Liao YP, Salazar F, Sun B, Jiang W, Chang CH, Jiang J, Wang X, Wu AM, Meng H, et al. Nano-enabled pancreas cancer immunotherapy using immunogenic cell death and reversing immunosuppression. Nat Commun. 2017;8 doi: 10.1038/s41467-017-01651-9. 1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cheng K, Ding Y, Zhao Y, Ye S, Zhao X, Zhang Y, Ji T, Wu H, Wang B, Anderson GJ, Ren L, et al. Sequentially responsive therapeutic peptide assembling nanoparticles for dual-targeted cancer immunotherapy. Nano Lett. 2018;18:3250–3258. doi: 10.1021/acs.nanolett.8b01071. [DOI] [PubMed] [Google Scholar]
- 45.Tauriello DV, Palomo-Ponce S, Stork D, Berenguer-Llergo A, Badia-Ramentol J, Iglesias M, Sevillano M, Ibiza S, Cañellas A, Hernando-Momblona X, Byrom D, et al. TGF-β drives immune evasion in genetically reconstituted colon cancer metastasis. Nature. 2018;554:538–543. doi: 10.1038/nature25492. [DOI] [PubMed] [Google Scholar]
- 46.Mariathasan S, Turley SJ, Nickles D, Castiglioni A, Yuen K, Wang Y, Kadel EE, III, Koeppen H, Astarita JL, Cubas R, Jhunjhunwala S, et al. TGF-β attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature. 2018;554:544–548. doi: 10.1038/nature25501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zheng Y, Tang L, Mabardi L, Kumari S, Irvine DJ. Enhancing adoptive cell therapy of cancer through targeted delivery of small-molecule immunomodulators to internalizing or noninternalizing receptors. ACS Nano. 2017;11:3089–3100. doi: 10.1021/acsnano.7b00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Park J, Wrzesinski SH, Stern E, Look M, Criscione J, Ragheb R, Jay SM, Demento SL, Agawu A, Limon PL, Ferrandino AF, et al. Combination delivery of TGF-β inhibitor and IL-2 by nanoscale liposomal polymeric gels enhances tumour immunotherapy. Nat Mater. 2012;11:895–905. doi: 10.1038/nmat3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu Z, Wang Y, Zhang L, Huang L. Nanoparticle-delivered transforming growth factor-β siRNA enhances vaccination against advanced melanoma by modifying tumor microenvironment. ACS Nano. 2014;8:3636–3645. doi: 10.1021/nn500216y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yuan H, Jiang W, von Roemeling CA, Qie Y, Liu X, Chen Y, Wang Y, Wharen RE, Yun K, Bu G, Knutson KL, et al. Multivalent bi-specific nanobioconjugate engager for targeted cancer immunotherapy. Nat Nanotechnol. 2017;12:763–769. doi: 10.1038/nnano.2017.69. [DOI] [PubMed] [Google Scholar]
- 51.Zhang Y, Li N, Suh H, Irvine DJ. Nanoparticle anchoring targets immune agonists to tumors enabling anti-cancer immunity without systemic toxicity. Nat Commun. 2018;9 doi: 10.1038/s41467-017-02251-3. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shae D, Becker KW, Christov P, Yun DS, Lytton-Jean AKR, Sevimli S, Ascano M, Kelley M, Johnson DB, Balko JM, Wilson JT. Endosomolytic polymersomes increase the activity of cyclic dinucleotide STING agonists to enhance cancer immunotherapy. Nat Nanotechnol. 2019;14:269–278. doi: 10.1038/s41565-018-0342-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim HY, Li R, Ng TS, Courties G, Rodell CB, Prytyskach M, Kohler RH, Pittet MJ, Nahrendorf M, Weissleder R. Quantitative Imaging of Tumor-Associated Macrophages and Their Response to Therapy Using 64Cu-Labeled Macrin. ACS Nano. 2018;12:12015–12029. doi: 10.1021/acsnano.8b04338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Swartz MA, Hirosue S, Hubbell JA. Engineering approaches to immunotherapy. Sci Transl Med. 2012;4:148rv9. doi: 10.1126/scitranslmed.3003763. [DOI] [PubMed] [Google Scholar]
- 55.Kuai R, Ochyl LJ, Bahjat KS, Schwendeman A, Moon JJ. Designer vaccine nanodiscs for personalized cancer immunotherapy. Nat Mater. 2017;16:489–496. doi: 10.1038/nmat4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thomas SN, Vokali E, Lund AW, Hubbell JA, Swartz MA. Targeting the tumor-draining lymph node with adjuvanted nanoparticles reshapes the anti-tumor immune response. Biomaterials. 2014;35:814–824. doi: 10.1016/j.biomaterials.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 57.Nuhn L, Vanparijs N, De Beuckelaer A, Lybaert L, Verstraete G, Deswarte K, Lienenklaus S, Shukla NM, Salyer AC, Lambrecht BN, Grooten J, et al. pH-degradable imidazoquinoline-ligated nanogels for lymph node-focused immune activation. Proc Natl Acad Sci USA. 2016;113:8098–8103. doi: 10.1073/pnas.1600816113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu H, Moynihan KD, Zheng Y, Szeto GL, Li AV, Huang B, Van Egeren DS, Park C, Irvine DJ. Structure-based programming of lymph-node targeting in molecular vaccines. Nature. 2014;507:519–522. doi: 10.1038/nature12978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Min Y, Roche KC, Tian S, Eblan MJ, McKinnon KP, Caster JM, Chai S, Herring LE, Zhang L, Zhang T, De Simone JM, et al. Antigen-capturing nanoparticles improve the abscopal effect and cancer immunotherapy. Nat Nanotechnol. 2017;12:877–882. doi: 10.1038/nnano.2017.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Luo M, Wang H, Wang Z, Cai H, Lu Z, Li Y, Du M, Huang G, Wang C, Chen X, Porembka MR, et al. A STING-activating nanovaccine for cancer immunotherapy. Nat Nanotechnol. 2017;12:648–654. doi: 10.1038/nnano.2017.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kranz LM, Diken M, Haas H, Kreiter S, Loquai C, Reuter KC, Meng M, Fritz D, Vascotto F, Hefesha H, Grunwitz C, et al. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature. 2016;534:396–401. doi: 10.1038/nature18300. [DOI] [PubMed] [Google Scholar]
- 62.Mandal S, Eksteen-Akeroyd ZH, Jacobs MJ, Hammink R, Koepf M, Lambeck AJ, van Hest JC, Wilson CJ, Blank K, Figdor CG. Therapeutic nanoworms: towards novel synthetic dendritic cells for immunotherapy. Chem Sci. 2013;4:4168–4174. [Google Scholar]
- 63.Zhang Q, Wei W, Wang P, Zuo L, Li F, Xu J, Xi X, Gao X, Ma G, Xie HY. Biomimetic magnetosomes as versatile artificial antigen-presenting cells to potentiate T-cell-based anticancer therapy. ACS Nano. 2017;11:10724–10732. doi: 10.1021/acsnano.7b04955. [DOI] [PubMed] [Google Scholar]
- 64.Stephan MT, Moon JJ, Um SH, Bershteyn A, Irvine DJ. Therapeutic cell engineering with surface-conjugated synthetic nanoparticles. Nat Med. 2010;16:1035–1041. doi: 10.1038/nm.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tang L, Zheng Y, Melo MB, Mabardi L, Castaño AP, Xie YQ, Li N, Kudchodkar SB, Wong HC, Jeng EK, Maus MV, et al. Enhancing T cell therapy through TCR-signaling-responsive nanoparticle drug delivery. Nat Biotechnol. 2018;36:707–716. doi: 10.1038/nbt.4181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Smith TT, Stephan SB, Moffett HF, McKnight LE, Ji W, Reiman D, Bonagofski E, Wohlfahrt ME, Pillai SP, Stephan MT. In situ programming of leukaemia-specific T cells using synthetic DNA nanocarriers. Nat Nanotechnol. 2017;12:813–820. doi: 10.1038/nnano.2017.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shi J, Kantoff PW, Wooster R, Farokhzad OC. Cancer nanomedicine: progress, challenges and opportunities. Nat Rev Cancer. 2017;17:20–37. doi: 10.1038/nrc.2016.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lammers T, Yokota-Rizzo L, Storm G, Kiessling F. Personalized nanomedicine. Clin Cancer Res. 2012;15:4889–4894. doi: 10.1158/1078-0432.CCR-12-1414. [DOI] [PubMed] [Google Scholar]
- 69.Lammers T, Aime S, Hennink WE, Storm G, Kiessling F. Theranostic nanomedicine. Acc Chem Res. 2011;44:1029–1038. doi: 10.1021/ar200019c. [DOI] [PubMed] [Google Scholar]
- 70.Ramanathan RK, Korn R, Raghunand N, Sachdev JC, Newbold RG, Jameson G, Fetterly GJ, Prey J, Klinz SG, Kim J, Cain J, et al. Correlation between ferumoxytol uptake in tumor lesions by MRI and response to nanoliposomal irinotecan in patients with advanced solid tumors: a pilot study. Clin Cancer Res. 2017;15:3638–3648. doi: 10.1158/1078-0432.CCR-16-1990. [DOI] [PubMed] [Google Scholar]
- 71.Lee H, Shields AF, Siegel BA, Miller KD, Krop I, Ma CX, LoRusso PM, Munster PN, Campbell K, Gaddy DF, Leonard SC, et al. 64Cu-MM-302 positron emission tomography quantifies variability of enhanced permeability and retention of nanoparticles in relation to treatment response in patients with metastatic breast cancer. Clin Cancer Res. 2017;23:4190–4202. doi: 10.1158/1078-0432.CCR-16-3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Topalian SL, Taube JM, Anders RA, Pardoll DM. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer. 2016;16:275–287. doi: 10.1038/nrc.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoué F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 74.Fridman WH, Pages F, Sautes-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12:298–306. doi: 10.1038/nrc3245. [DOI] [PubMed] [Google Scholar]
- 75.Galon J, Mlecnik B, Bindea G, Angell HK, Berger A, Lagorce C, Lugli A, Zlobec I, Hartmann A, Bifulco C, Nagtegaal ID, et al. Towards the introduction of the 'Immunoscore'in the classification of malignant tumours. J Pathol. 2014;232:199–209. doi: 10.1002/path.4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Galon J, Bruni D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat Rev Drug Discov. 2019;18:197–218. doi: 10.1038/s41573-018-0007-y. [DOI] [PubMed] [Google Scholar]
- 77.Arlauckas SP, Garris CS, Kohler RH, Kitaoka M, Cuccarese MF, Yang KS, Miller MA, Carlson JC, Freeman GJ, Anthony RM, Weissleder R, et al. In vivo imaging reveals a tumor-associated macrophage-mediated resistance pathway in anti-PD-1 therapy. Sci Transl Med. 2017;9:eaal3604. doi: 10.1126/scitranslmed.aal3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sahin U, Türeci Ö. Personalized vaccines for cancer immunotherapy. Science. 2018;359:1355–1360. doi: 10.1126/science.aar7112. [DOI] [PubMed] [Google Scholar]
- 79.Villegas MR, Baeza A, Vallet-Regí M. Hybrid collagenase nanocapsules for enhanced nanocarrier penetration in tumoral tissues. ACS Appl Mater Interfaces. 2015;7:24075–24081. doi: 10.1021/acsami.5b07116. [DOI] [PubMed] [Google Scholar]
- 80.Zhang L, Wang Y, Yang Y, Liu Y, Ruan S, Zhang Q, Tai X, Chen J, Xia T, Qiu Y, Gao H, et al. High tumor penetration of paclitaxel loaded pH sensitive cleavable liposomes by depletion of tumor collagen I in breast cancer. ACS Appl Mater Interfaces. 2015;7:9691–9701. doi: 10.1021/acsami.5b01473. [DOI] [PubMed] [Google Scholar]
- 81.Diop-Frimpong B, Chauhan VP, Krane S, Boucher Y, Jain RK. Losartan inhibits collagen I synthesis and improves the distribution and efficacy of nanotherapeutics in tumors. Proc Natl Acad Sci USA. 2011;108:2909–2914. doi: 10.1073/pnas.1018892108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stylianopoulos T, Munn LL, Jain RK. Reengineering the physical microenvironment of tumors to improve drug delivery and efficacy: from mathematical modeling to bench to bedside. Trends Cancer. 2018;4:292–319. doi: 10.1016/j.trecan.2018.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sun Q, Ojha T, Kiessling F, Lammers T, Shi Y. Enhancing tumor penetration of nanomedicines. Biomacromolecules. 2017;18:1449–1459. doi: 10.1021/acs.biomac.7b00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Huang Y, Kim BYS, Chan CK, Hahn SM, Weissman IL, Jiang W. Improving immune-vascular crosstalk for cancer immunotherapy. Nat Rev Immunol. 2018;18:195–203. doi: 10.1038/nri.2017.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pittella F, Miyata K, Maeda Y, Suma T, Watanabe S, Chen Q, Christie RJ, Osada K, Nishiyama N, Kataoka K. Pancreatic cancer therapy by systemic administration of VEGF siRNA contained in calcium phosphate/charge-conversional polymer hybrid nanoparticles. J Control Release. 2012;161:868–874. doi: 10.1016/j.jconrel.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 86.Lu J, Liu X, Liao YP, Wang X, Ahmed A, Jiang W, Ji Y, Meng H, Nel AE. Breast cancer chemo-immunotherapy through liposomal delivery of an immunogenic cell death stimulus plus interference in the IDO-1 pathway. ACS Nano. 2018;12:11041–11061. doi: 10.1021/acsnano.8b05189. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]