Abstract
Background
The analytically sensitive detection of KIT D816V in blood and bone marrow is important for diagnosing systemic mastocytosis (SM). Additionally, precise quantification of the KIT D816V variant allele fraction (VAF) is relevant clinically because it helps to predict multilineage involvement and prognosis in cases of advanced SM. Digital PCR (dPCR) is a promising new method for sensitive detection and accurate quantification of somatic mutations.
Methods
We performed a validation study of dPCR for KIT D816V on 302 peripheral blood and bone marrow samples from 156 patients with mastocytosis for comparison with melting curve analysis after peptide nucleic acid-mediated PCR clamping (clamp-PCR) and allelespecific quantitative real-time PCR (qPCR).
Results
dPCR showed a limit of detection of 0.01% VAF with a mean CV of 8.5% and identified the mutation in 90% of patients compared with 70% for clamp-PCR (P < 0.001). Moreover, dPCR for KIT D816V was highly concordant with qPCR without systematic deviation of results, and confirmed the clinical value of KIT D816V VAF measurements. Thus, patients with advanced SM showed a significantly higher KIT D816V VAF (median, 2.43%) compared with patients with indolent SM (median, 0.14%; P < 0.001). Moreover, dPCR confirmed the prognostic significance of a high KIT D816V VAF regarding survival (P < 0.001).
Conclusions
dPCR for KIT D816V provides a high degree of precision and sensitivity combined with the potential for interlaboratory standardization, which is crucial for the implementation of KIT D816V allele burburden measurement. Thus, dPCR is suitable as a new method for KIT D816V testing in patients with mastocytosis.
Systemic mastocytosis (SM)6 is a hematologic neoplasm characterized by an accumulation of clonal mast cells in the bone marrow (BM) and other extracutaneous organs (1) The clinical course in SM is variable, ranging from a stable indolent form to highly aggressive disease (2). According to the WHO classification, mastocytosis can be divided into cutaneous mastocytosis, indolent SM (ISM), smoldering SM, aggressive SM, mast cell leukemia (MCL), and SM with an associated hematologic neoplasm (3–5). Based on their aggressive clinical course, aggressive SM, SM with an associated hematologic neoplasm, and MCL are collectively referred to as advanced mastocytosis (5).
Most SM patients harbor a somatic KIT 7 D816V mutation, which leads to ligand-independent activation of the oncogenic receptor tyrosine kinase (6). Depending on the technique used to detect the mutation, the reported frequency of KIT D816V is variable, ranging from 30% to 95% of all patients with SM (6–9). However, although activating codon 816 mutation of KIT is a diagnostic criterion of SM (3), no single assay for the detection of KIT mutations has been accepted as a global standard. Rather, a number of different techniques varying in terms of analytical sensitivity, specificity, and precision have been recommended (10, 11). The melting curve analysis after peptide nucleic acid (PNA)-mediated PCR clamping has been described as an analytically sensitive method for detecting KIT mutations in biopsies (12). A number of studies have reported a highly analytically sensitive quantitative real-time PCR (qPCR) method based on allele-specific primers for detection of KIT D816V (13, 14). In these studies, a high KIT D816V allele burden was associated with multilineage involvement, advanced mastocytosis, and poor outcome (14–17). In a retrospective analysis, allogeneic hematopoietic stem cell transplantation was associated with long-term survival in patients with advanced SM; however, a definitive role of hematopoietic stem cell transplantation in SM needs to be determined by a prospective trial (18). The KIT D816V-targeting tyrosine kinase inhibitor midostaurin (19) showed profound clinical efficacy in advanced SM with an overall response rate of 60% and marked reduction of BM mast cell burden and serum tryptase values in a recently reported phase II study (20). However, robust biomarkers predictive for response to midostaurin are lacking. A KIT D816V allele burden reduction of ≥25% was recently described as an independent on-treatment marker for improved overall survival in midostaurin-treated patients with advanced SM (21). Because of the availability of effective treatments, accurate quantification of KIT D816V will become more important for molecular monitoring and even minimal residual disease assessment in cases of advanced SM.
However, despite the apparent clinical need of accurate testing for KIT D816V, several issues concerning the standardization and comparability of currently available techniques remain to be solved. First, results from round-robin testing for KIT D816V quantification as an external quality assessment are lacking. Second, qPCR-based quantification typically relies on a calibrator material, and no commonly accepted calibrator for KIT D816V is available. In contrast, dilutions of KIT D816V-positive cells and cloned plasmids have been used to normalize qPCR results (13, 15). These differences in calibration material and data normalization hamper the standardization of KIT D816V quantification and complicate the comparison of results obtained in different study groups. Likewise, different calibrators have been used for quantification of BCR-ABL1 in cases of chronic myeloid leukemia, which was a major factor for poor comparability of BCR-ABL1 results between laboratories. Tremendous efforts have been made to establish an international scale for BCR-ABL1 transcripts by applying laboratory-specific conversion factors (22). International scale normalization improved the interlaboratory comparability of results, although relevant variability was still observed (23). Only recently has a certified reference material been developed as a calibrator for BCR-ABL1 quantification (24). In contrast to these efforts to test BCR-ABL1 in chronic myeloid leukemia, no high-quality calibration material is available for KIT D816V.
Digital PCR (dPCR) uses a dilution of target nucleic acid across a large number of reactions (partitions) for accurate absolute quantification of DNA molecules without the need for calibration material (25, 26). Thus, dPCR has become a new standard for quantification of mutant alleles at a low variant allele fraction (VAF) in molecular genetics (25). In the study reported here, we evaluated the performance characteristics of the PrimePCR digital droplet PCR (ddPCR) mutation assay to detect and quantify KIT D816V in patients with mastocytosis.
Patients and Methods
Patients
We examined 302 peripheral blood (PB) and BM samples from 156 patients (85 female, 71 male) with mastocytosis diagnosed between April 1988 and April 2015 and included in a local registry. One hundred five patients were included in a previous study on the clinical significance of KIT D816V allele burden measurement (15, 27). PB and BM samples at diagnosis and during follow-up were obtained after informed consent was given, and the study was approved by the institutional review board. Details on sample collection, processing, and storage are described in the Methods file of the Data Supplement that accompanies the online version of this article at http://www.clinchem.org/content/vol64/issue3. According to WHO criteria (3, 4), 16 patients were diagnosed with cutaneous mastocytosis, 5 with mastocytosis in the skin (BM involvement not confirmed) (10), 105 with ISM, 7 with smoldering SM, 1 with bone marrow mastocytosis, 8 with aggressive SM, 2 with MCL, and 12 with SM with an associated hematologic neoplasm. The patients’ characteristics are shown in Table 1) here and in Table 1 of the online Data Supplement.
Table 1. Patients’ characteristics.
| Disease subtype | ||||
|---|---|---|---|---|
|
|
||||
| CM/MIS (n = 21) | ISM (n = 113) | Advanced SM (n = 22) | Total cohort (n = 156) | |
|
| ||||
| Age, years (median, range) | 46 (28–94) | 55 (26–91) | 68 (39–88) | 56 (26–94) |
|
| ||||
| Sex, female/male | 14/7 | 64/49 | 7/15 | 85/71 |
|
| ||||
| KIT D816V positivea | 15/21 (71%) | 110/113 (97%) | 16/22 (73%)b | 141/156 (90%) |
|
| ||||
| KIT D816V VAF, % (median, range) | 0.06 (0.004–0.51) | 0.14 (0.002–46.9) | 2.43 (0.005–46.7) | 0.14 (0.002–46.9) |
|
|
||||
CM, cutaneous mastocytosis; MIS, mastocytosis in the skin.
KIT D816V as assessed by ddPCR.
Detailed characteristics of KIT D816V-negative advanced SM patients are presented in Table 1 of the online Data Supplement.
Detection of KIT D816V
Genomic DNA was extracted from HMC-1.2 cells (28, from PB and/or BM cells, as well as from formalin-fixed paraffin-embedded (FFPE) BM sections as described in the Methods file of the online Data Supplement. Three different PCR methods were applied for analysis of KIT D816V from genomic DNA, as described in the Methods file of the online Data Supplement. Qualitative detection of KIT codon 816 mutations was performed using melting curve analysis after PNA-mediated PCR clamping essentially as described (12). KIT D816V was quantified using allele-specific qPCR basically as described (13). dPCR was performed with the PrimePCR ddPCR mutation assay for KIT wild-type and the KIT D816V point mutation (Bio-Rad Laboratories) and analyzed on a QX-200 droplet reader (Bio-Rad) according to the manufacturer’s recommendations. The KIT D816V mutation burden (VAF) was calculated by dividing the number of mutated KIT D816V copies by the total number of KIT copies, and VAF results were expressed as percent mutant alleles.
Statistical Analysis
The limit of detection (LOD) was defined as the lowest VAF at which a KIT D816V mutant amplification product was detected with a probability of at least 0.95 (LOD95) determined by replicate measurements (n = 50) of low-level positive samples according to guidelines for qualitative PCR methods (29, 30). The limit of quantification was defined as the lowest VAF at which replicates showed a CV :≤35% according to suggestions for quantitative PCR results (29). The interassay evaluation, precision, and CV of the dPCR assay were estimated using 3 reference material samples measured in 5 independent experiments. Statistical analysis was performed using R (version 3.3.0) (31) and is described in detail in the Methods file of the online Data Supplement. Differences were considered significant when the P value was <0.05.
Results
Ddpcr Analytical Sensitivity and Reproducibility for KIT D816V
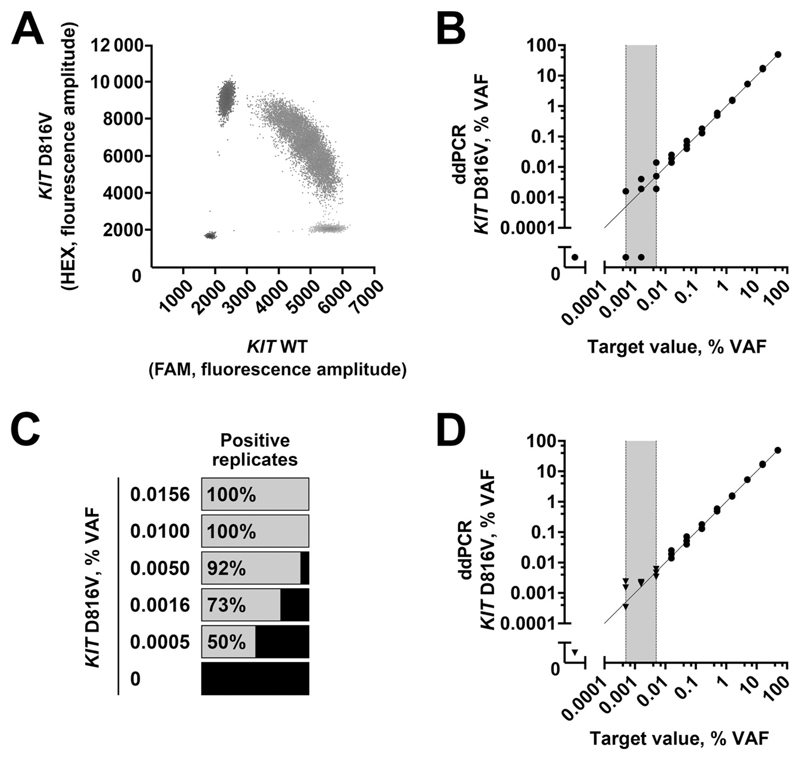
KIT D816V-positive HMC-1.2 cells showed a VAF of 50.1% ± 0.5% (mean ± SD), consistent with the heterozygous mutation status (Fig. 1A). Serial dilution experiments with these cells were performed to assess the LOD of the assay (Fig. 1B). The total number of KIT molecules per ddPCR reaction ranged from 50000 to 100000, which corresponded to a theoretically achievable VAF of 0.001% when detecting a single KIT D816V-positive molecule per reaction. No KIT D816V-positive events were detected in the negative control and a number of control individuals (n = 47; see Table 2 in the online Data Supplement). Thus, limit of blank was not applicable for LOD calculation (32), and LOD95 was determined by replicate measurements of low-level positive samples (0.0005%–0.016% VAF) (30). At 0.016% and 0.01% VAF, all replicates tested positive, whereas 4 of 50 replicates (8%) were negative at a VAF of 0.005% (Fig. 1C). Thus, the LOD95 of the assay was determined as 0.01% VAF and the limit of quantification as 0.016% (see Table 3 of the online Data Supplement). Results showed a good linear correlation (r P = 0.99) with the target value down to 0.01% (Fig. 1B; see also Table 3 of the online Data Supplement). From 0.0005% to 0.005% VAF, not all replicates were positive, likely because of stochastic effects. Merged analysis of multiple wells of ddPCR reactions was used to increase the control gene results and, thus, the assay sensitivity (33). Combined analysis of 10 wells per sample corresponded to a merged number of all KIT molecules of approximately 1000000 and improved recovery and precision at 0.005% and 0.0016% VAF (Fig. 1D). Therefore, our results showed that an LOD95 of 0.01% VAF could be achieved for KIT D816V and that combined analysis of 10 ddPCR reactions per sample could further improve the sensitivity of the assay.
Fig. 1. LOD of ddPCR for KIT D816V.
ddPCR of HMC-1.2 cells (A). Analysis of dilution series (B) complemented by merged measurement from multiple wells (D, black triangles). The gray zone of stochastic results below LOD95 is marked in gray. Qualitative results of low abundant KIT D816V samples in multiple replicates to define LOD95 (C). WT, wild type.
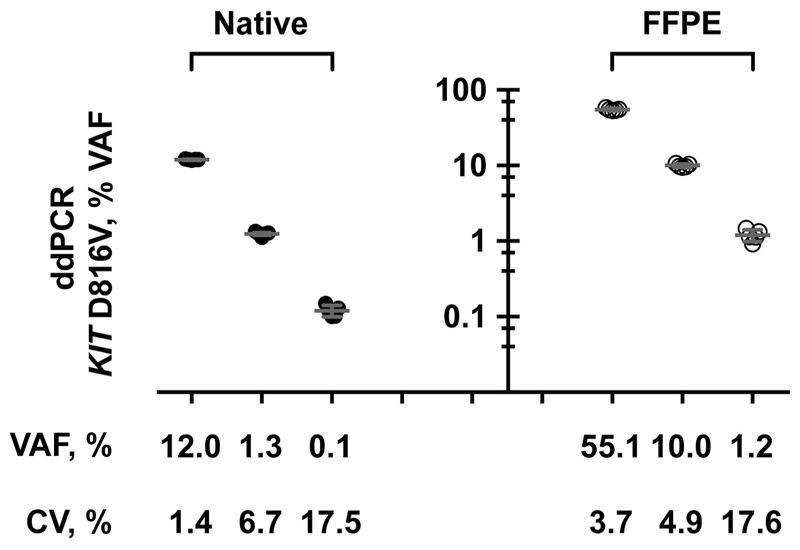
In additional validation experiments, the assay showed a mean CV of 8.5% in the interassay evaluation. As expected, the CV increased at low VAF with <20% CV at 0.1% VAF (Fig. 2). In addition, we also tested 3 DNA samples isolated from FFPE BM sections of patients with KIT D816V-positive SM and observed a mean CV of 8.7% (Fig. 2) for ddPCR results.
Fig. 2. Reproducibility of ddPCR for KIT D816V.
DNA isolated from native PB or BM aspirates (closed circles) or FFPE BM sections (open circles) of 3 patients with mastocytosis was analyzed by ddPCR in 5 independent runs. Mean VAF and corresponding CV of the samples are reported.
Concordance oF ddpcr and Qpcr-based KIT D816V Allele Burden Measurement in Mastocytosis
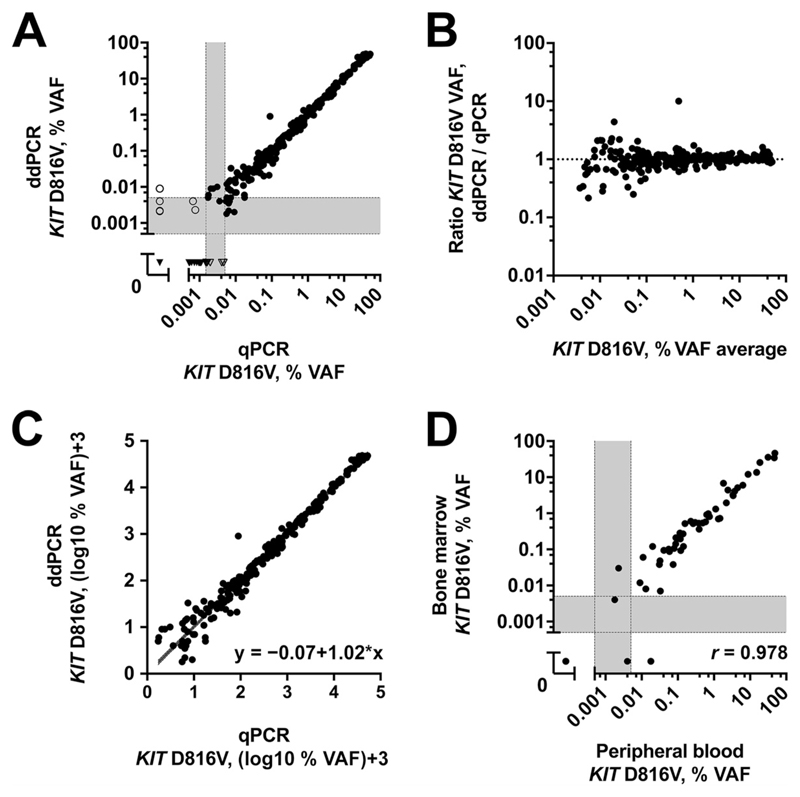
In total, 302 samples from 156 patients were measured by ddPCR and qPCR. Overall, a concordance rate of 96% was observed (Fig. 3A). Of these samples, 265 were found to be positive and 25 negative by both methods. In the gray zone of stochastic PCR results below LOD95, some discrepancies occurred; 6 samples were found to be low-level KIT D816V-positive by ddPCR but not by qPCR, whereas in 6 samples, KIT D816V low-level positivity was detected by only qPCR. Of note, all 6 samples were found to be positive for KIT D816V by ddPCR when merged analysis of multiple wells was performed (median, 0.005% VAF; range, 0.0014%–0.0067% VAF).
Fig. 3. Method comparison of ddPCR and qPCR for KIT D816V in mastocytosis.
Comparison of KIT D816V quantification in 302 mastocytosis samples (A). Concordant PCR results are shown in closed symbols, and discordant results in open symbols. Bland–Altman plot (B) and Passing–Bablok regression of log-transformed VAF data (C) show no deviation from linearity. Comparison of results in PB and BM aspirates (D).
Using the Bland–Altman plot, no deviation tendency between both methods was detected for high- or low-level KIT D816V allele burden samples (Fig. 3B). A high degree of correlation between the 2 methods was found in 265 double-positive samples (r P = 0.99). No systematic deviation was found in ordinary least-squares regression analysis of log-transformed data, with a slope of 1.00 (95% CI, 0.98 –1.02) for the conversion from qPCR to ddPCR (Fig. 3C). Regression coefficients of the Passing–Bablok regression presented a similar result [intercept, –0.07 (CI, –0.12 to 0.001); slope, 1.02 (CI, 1.00 –1.04); CUSUM test, P = 0.170] (34), without evidence of a systematic or proportional difference. We observed a correlation of r = 0.978 when comparing the KIT D816V VAF from patients with simultaneously obtained BM aspirate and PB samples (Fig. 3D).
Higher Detection bY DDPCR and QPCR for KIT D816V in Mastocytosis than Melting Curve Analysis after PNA-Mediated PCR Clamping
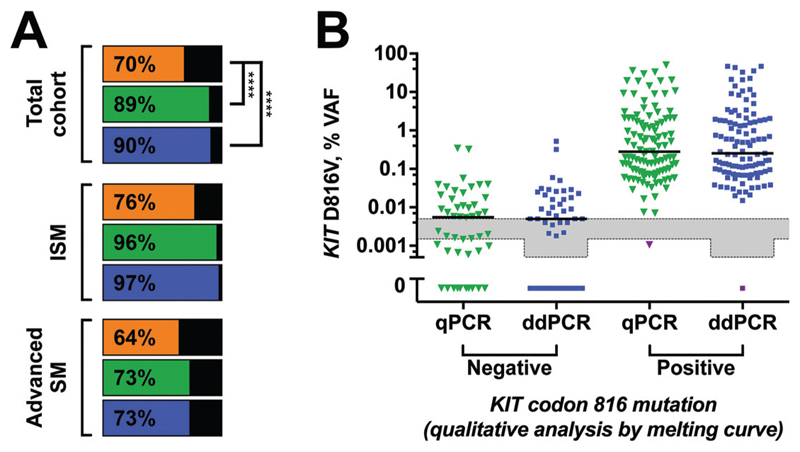
At the time of diagnosis, KIT codon 816 mutations were detectable in 110 of 156 mastocytosis patients (70%) by qualitative melting curve analysis after PNA-mediated PCR clamping, in 139 of 156 patients (89%) using qPCR, and in 141 of 156 patients (90%) using ddPCR (Fig. 4A). The difference in the positivity rate of both the qPCR and the ddPCR assay to clamp PCR was highly statistically significant in favor of the qPCR and ddPCR assay (both P < 0.001; McNemar test). In contrast, ddPCR and qPCR assay gave comparable results (P = 0.752). This held true for all subtypes of mastocytosis tested and also for separate analysis of PB and BM aspirate samples (Fig. 4A) here and also Fig. 2 in the online Data Supplement). qPCR and ddPCR failed to detect a KIT mutation in only 1 patient with MCL, for whom melting curve analysis indicated a KIT mutation at codon 816 identified as D816H (Fig. 4B). Thus, ddPCR and qPCR better detected low abundant KIT mutants in mastocytosis but did not detect other KIT mutations than D816V.
Fig. 4. Superiority of ddPCR to melting curve analysis after PNA-mediated PCR clamping for detecting KIT D816V in mastocytosis.
Percentages of KIT D816V-positive mastocytosis patients assessed by clamp-PCR (orange), qPCR (green), or ddPCR (blue) (A). KIT D816V VAF of patients stratified according to clamp-PCR results (B). One MCL patient with a KIT D816H mutation is shown in purple.
Prediction of Clinical Outcome in SM using KIT D816V VAF Assessed by DDPCR
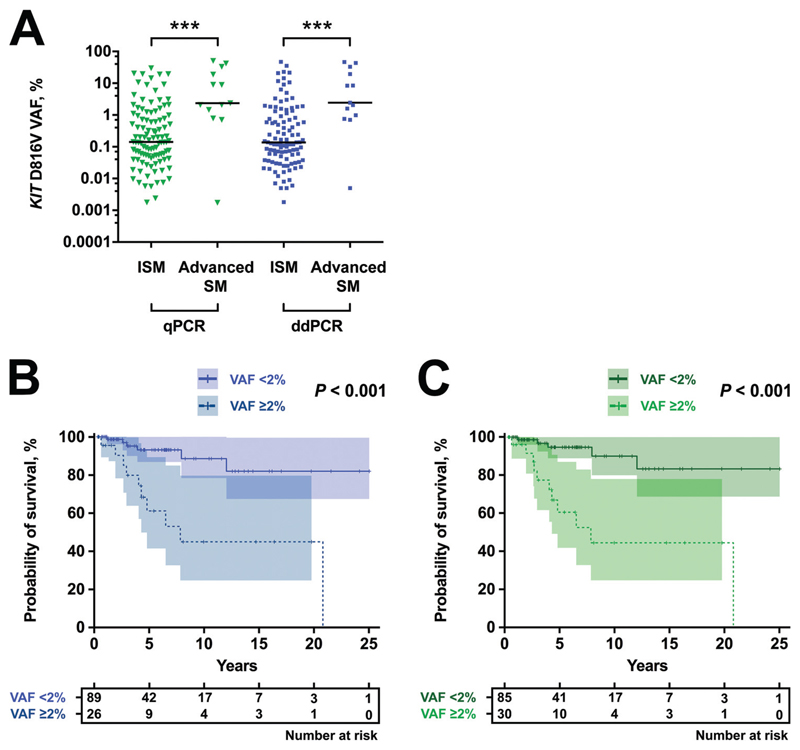
Recent data suggest that the KIT D816V allele burden correlates with WHO subgroups of mastocytosis and predicts survival in SM (15–17). To define the prognostic value of ddPCR-based VAF measurements, we correlated ddPCR results with clinical end points and compared the prognostic ability of ddPCR with qPCR-based VAF quantification. The median KIT D816V allele burden in all positive mastocytosis patients was 0.137% (range, 0.002%–46.9%) as assessed by ddPCR compared with 0.156% (range, 0.002%–50.2%) for qPCR. In line with previous data, KIT D816V VAF was higher in advanced SM compared with ISM (Fig. 5A). This difference in allele burden was highly significant when assessed by ddPCR (2.43% in advanced SM compared with 0.138% median VAF in ISM; P < 0.001) or qPCR (2.37% in advanced SM compared with 0.143% median VAF in ISM; P < 0.001).
Fig. 5. Clinical significance of ddPCR-based measurement of KIT D816V allele burden.
KIT D816V allele burden for ISM and patients with advanced SM measured by qPCR (green) and ddPCR (blue) (A). Kaplan–Meier plot for overall survival stratified for high (≥2% VAF, dotted) and low (<2% VAF, straight line) KIT D816V allele burden assessed by ddPCR (B) or qPCR (C). ***P<0.001.
We previously used a cutoff level of 2% KIT D816V mutant allele burden to separate the cohort into 2 prognostically distinct subsets of patients with mastocytosis (15). When we compared ddPCR- and qPCR-based results for KIT D816V-positive SM patients with available survival data (n = 115) at a 2% VAF cutoff, only 4 patients were categorized differentially. Survival curves for patients with <2% or ≥2% KIT D816V VAF were assessed separately for ddPCR (Fig. 5B) and qPCR (Fig. 5C). Significant differences in survival were found in results obtained by ddPCR and by qPCR (both P < 0.001; log-rank test), but no statistical difference was observed between the methods (P = 0.913; rank test according to Fleming and Harrington). In addition, we performed separate analysis of PB and BM aspirate samples, indicating a prognostic value of KIT D816V VAF measurement in both specimens (see Fig. 3 in the online Data Supplement). Thus, ddPCR confirmed the main outcome data for KIT D816V allele burden measurement in SM.
Discussion
Several different dPCR platforms have been developed that allow absolute quantification and rare event detection with high analytical sensitivity (26). Some platforms use droplets of an emulsion (ddPCR) for partition of PCR reactions, whereas others apply microchips with distinct chambers (26). We performed a validation study of PrimePCR ddPCR (Bio-Rad Laboratories) for KIT D816V in PB and BM samples from patients with mastocytosis. ddPCR could quantify KIT D816V sensitively and precisely. The LOD95 of 0.01% VAF defined in this study is in a range comparable with previously published qPCR-based testing (15). The initial report on the allelespecific qPCR assay reported an LOD of 0.003% mutated cells, which corresponds to a VAF of approximately 0.0015% (13). This is close to the theoretically achievable VAF of 1 mutated molecule in 100000 wild-type molecules and, thus, subject to stochastic distribution effects in the sample. To further increase the analytical sensitivity of the ddPCR test, a higher input of DNA is warranted. We showed that this could be achieved by simultaneous analysis of multiple wells (Fig. 1D). A similar approach has been described for patients with chronic myeloid leukemia to further increase the analytical sensitivity of dPCR for detection of BCR-ABL1 beyond MR5.0 (33). Although this approach is feasible for KIT D816V, it might not be easily applicable in routine clinical practice. Still, single-well ddPCR reaction-based analysis also showed performance characteristics that were not inferior to qPCR. In particular, the CV for ddPCR-based quantification of KIT D816V was <20% for high and low VAF, indicating higher reproducibility compared with qPCR (13).
In line with previous reports, we found a substantial number of patients with SM with KIT D816V VAF <0.1% in PB and BM aspirate (16, 17). This indicates that the high analytical sensitivity of the assays is clinically relevant for proper assessment of KIT D816V as a diagnostic criterion of SM. We show that both qPCR and ddPCR have superior analytical sensitivity over melting curve analysis after PNA-mediated PCR clamping to detect KIT D816V. Our results are comparable with the recently published qPCR data of the Spanish cohort (16). The consideration that less-sensitive molecular tests will fail to detect KIT D816V in SM is especially relevant for the next-generation sequencing-based analysis of hematologic malignancies. Larger gene panels or exome sequencing is typically performed at LODs of 1% to 5% and, thus, is not sensitive enough to detect KIT D816V in SM. Even when focusing specifically on KIT mutations, next-generation sequencing did not perform well below an LOD of 0.2% VAF because of background sequencing errors (35). Error-corrected sequencing and other bioinformatics approaches should be able to improve the analytical sensitivity of next-generation sequencing (36). However, to date, highly analytically sensitive PCR-based molecular analysis is still the gold standard for KIT D816V testing (11). Thus, dPCR is a valuable new diagnostic test for detection of KIT D816V in individuals with mastocytosis.
Quantification of the KIT D816V allele burden in PB and BM has been shown to be of clinical significance (15–17). Importantly, we show that ddPCR results fo quantification of KIT D816V highly correlate with qPCR results based on the method described by Kristensen et al. (13). In particular, both tests showed interchangeable results for allele burden measurement, and all clinically relevant end points could be confirmed by ddPCR for KIT D816V. Thus, ddPCR-based studies should generally be well comparable with published results for genomic DNA-based KIT D816V allele burden measurement (15–17). However, different clinical cut-offs have been proposed to discriminate between high- and low-risk patients with SM based on KIT D816V mutant allele burden. Jara-Acevedo et al. used a 6% VAF cutoff to discriminate between mast cell-restricted vs multilineage SM (16). We used a 2% VAF cutoff to stratify overall survival in SM patients (15). Although we observed a good correlation between BM and PB samples, the ideal specimen for KIT D816V quantification is still a matter of discussion because of differences in the composition of cellular compartments. In our cohort, BM aspirate seemed slightly better in terms of detection rate and prognostic separation (see Figs. 2 and 3 in the online Data Supplement). However, differences were not significant, and PB can be obtained without an invasive procedure.
All these clinical cutoffs require further validation in prospective multicenter studies. A prerequisite for these trials and for the widespread clinical use of KIT D816V allele burden measurement is a thorough standardization between different laboratories. Likewise, tremendous efforts have been made to achieve comparability between different laboratories in BCR-ABL1 quantification, which ultimately resulted in normalization of BCR-ABL1 transcripts according to international scale (37). dPCR is a promising technology that could overcome some of the limitations concerning the comparability of PCR results. The majority of general considerations apply not only to the PrimePCR ddPCR assay (Bio-Rad Laboratories) evaluated in our study but also to other dPCR technologies. dPCR does not rely on external calibration material for absolute quantification of KIT wild-type and mutant molecules, and both alleles are detected with the same PCR assay and, thus, with the same PCR efficiency (25). In contrast, allele-specific qPCR relies on separate assays for wild-type and mutant KIT that could theoretically differ in terms of PCR efficiency (13), and correction by external calibration material potentially remains error prone (23). This is of particular relevance when analyzing DNA samples of impaired quality. In particular, DNA isolated from FFPE tissue is often highly fragmented (38). Likewise, cell-free circulating DNA in the plasma shows a typical pattern of very short fragments corresponding to single nucleosomes (39). In contrast to allele-specific qPCR-based testing, no systematic bias because of DNA fragmentation is expected for dPCR (40). Our preliminary data indicate that dPCR also sensitively and reproducibly detects and quantifies KIT D816V in FFPE material. Nevertheless, thorough validation studies of the performance characteristics of dPCR for these additional matrices are warranted before assessment of the clinical relevance of KIT D816V allele burden measurement in this specimen.
In summary, we performed a comprehensive validation of dPCR for KIT D816V in PB and BM, and showed results well comparable with established qPCR techniques. The opportunity to reliably and comparably detect and quantify KIT D816V in different specimens despite poor DNA quality is a major advantage of dPCR. Furthermore, quantification of KIT D816V is not limited by the necessity of generally accepted calibration material for assay standardization between different laboratories. In this regard, dPCR might become a new standard method for detection and quantification of KIT D816V in SM. Thus, we propose dPCR for KIT D816V testing in future external quality assessments and multicentric studies on mastocytosis to increase comparability of allele burden data from different study groups. This high standardization of KIT D816V measurement is the next crucial step toward the wide implementation of KIT D816V allele burden in clinical practice, and will further improve treatment of patients with mastocytosis.
Supplementary Material
Acknowledgments
The authors thank Jana Strasakova (Department of Laboratory Medicine, Medical University of Vienna) for technical support and John Heath for proofreading the manuscript.
Research Funding
The Austria Science Fund (FWF) project P26079-B13 and the SFB projects F4701-B20 and F4704-B20. P. Valent, Novartis, Blueprint, Deciphera.
Footnotes
Author Contributions: All authors confirmed they have contributed to the intellectual content of this paper and have met the following 3 requirements: (a) significant contributions to the conception and design, acquisition of data, or analysis and interpretation of data; (b) drafting or revising the article for intellectual content; and (c) final approval of the published article.
Authors’ Disclosures or Potential Conflicts of Interest: Upon manuscript submission, all authors completed the author disclosure form. Disclosures and/or potential conflicts of interest:
Employment or Leadership: None declared.
Consultant or Advisory Role: P. Valent, consultant in a global Novartis trial investigating the effects of midostaurin in patients with advanced systemic mastocytosis.
Stock Ownership: None declared.
Honoraria:P. Valent, Novartis, Blueprint, Deciphera; G. Hoermann, Novartis; W.R. Sperr, Novartis.
Expert Testimony: None declared.
Patents: None declared.
Role of Sponsor: The funding organizations played no role in the design of study, choice of enrolled patients, review and interpretation of data, or final approval of manuscript.
References
- 1.Metcalfe DD. Mast cells and mastocytosis. Blood. 2008;112:946–56. doi: 10.1182/blood-2007-11-078097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arock M, Valent P. Pathogenesis, classification and treatment of mastocytosis: state of the art in 2010 and future perspectives. Expert Rev Hematol. 2010;3:497–516. doi: 10.1586/ehm.10.42. [DOI] [PubMed] [Google Scholar]
- 3.Horny HP, Metcalfe DD, Bennett JM, Bain BJ, Akin C, Escribano L, Valent P. Mastocytosis. In: Swerdlow S, Campo E, Harris N, Jaffe E, Stefano A, Stein H, et al., editors. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th Ed. Lyon (France); IARC; 2008. pp. 54–63. [Google Scholar]
- 4.Valent P, Horny HP, Escribano L, Longley BJ, Li CY, Schwartz LB, et al. Diagnostic criteria and classification of mastocytosis: a consensus proposal. Leuk Res. 2001;25:603–25. doi: 10.1016/s0145-2126(01)00038-8. [DOI] [PubMed] [Google Scholar]
- 5.Valent P, Akin C, Metcalfe DD. Mastocytosis: 2016 updated WHO classification and novel emerging treatment concepts. Blood. 2017;129:1420–7. doi: 10.1182/blood-2016-09-731893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nagata H, Worobec AS, Oh CK, Chowdhury BA, Tannenbaum S, Suzuki Y, Metcalfe DD. Identification of a point mutation in the catalytic domain of the protooncogene c-kit in peripheral blood mononuclear cells of patients who have mastocytosis with an associated hematologic disorder. Proc Natl Acad SciUSA. 1995;92:10560–4. doi: 10.1073/pnas.92.23.10560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Longley BJ, Jr, Metcalfe DD, Tharp M, Wang X, Tyrrell L, Lu SZ, et al. Activating and dominant inactivating c-kit catalytic domain mutations in distinct clinical forms of human mastocytosis. Proc Natl Acad Sci U S A. 1999;96:1609–14. doi: 10.1073/pnas.96.4.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kahler C, Didlaukat S, Feller AC, Merz H. Sensitive and reliable detection of kit point mutation asp 816 to val in pathological material. Diagn Pathol. 2007;2:37. doi: 10.1186/1746-1596-2-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schumacher JA, Elenitoba-Johnson KS, Lim MS. Detection of the c-KIT D816V mutation in systemic mastocytosis by allele-specific PCR. J Clin Pathol. 2008;61:109–14. doi: 10.1136/jcp.2007.047928. [DOI] [PubMed] [Google Scholar]
- 10.Valent P, Akin C, Escribano L, Fodinger M, Hartmann K, Brockow K, et al. Standards and standardization in mastocytosis: consensus statements on diagnostics, treatment recommendations and response criteria. Eur J Clin Invest. 2007;37:435–53. doi: 10.1111/j.1365-2362.2007.01807.x. [DOI] [PubMed] [Google Scholar]
- 11.Arock M, Sotlar K, Akin C, Broesby-Olsen S, Hoermann G, Escribano L, et al. Kit mutation analysis in mast cell neoplasms: recommendations of the European competence network on mastocytosis. Leukemia. 2015;29:1223–32. doi: 10.1038/leu.2015.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sotlar K, Escribano L, Landt O, Mohrle S, Herrero S, Torrelo A, et al. One-step detection of c-kit point mutations using peptide nucleic acid-mediated polymerase chain reaction clamping and hybridization probes. Am J Pathol. 2003;162:737–46. doi: 10.1016/S0002-9440(10)63870-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kristensen T, Vestergaard H, Moller MB. Improved detection of the KIT D816V mutation in patients with systemic mastocytosis using a quantitative and highly sensitive real-time qPCR assay. J Mol Diagn>. 2011;13:180–8. doi: 10.1016/j.jmoldx.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Erben P, Schwaab J, Metzgeroth G, Horny HP, Jawhar M, Sotlar K, et al. The KIT D816V expressed allele burden for diagnosis and disease monitoring of systemic mastocytosis. Ann Hematol. 2014;93:81–8. doi: 10.1007/s00277-013-1964-1. [DOI] [PubMed] [Google Scholar]
- 15.Hoermann G, Gleixner KV, Dinu GE, Kundi M, Greiner G, Wimazal F, et al. The KIT D816V allele burden predicts survival in patients with mastocytosis and correlates with the WHO type of the disease. Allergy. 2014;69:810–3. doi: 10.1111/all.12409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jara-Acevedo M, Teodosio C, Sanchez-Munoz L, Alvarez-Twose I, Mayado A, Caldas C, et al. Detection of the KIT D816V mutation in peripheral blood of systemic mastocytosis: diagnostic implications. Mod Pathol. 2015;28:1138–49. doi: 10.1038/modpathol.2015.72. [DOI] [PubMed] [Google Scholar]
- 17.Broesby-Olsen S, Kristensen T, Vestergaard H, Brixen K, Moller MB, Bindslev-Jensen C. Mastocytosis Centre Odense University Hospital. KIT D816V mutation burden does not correlate to clinical manifestations of indolent systemic mastocytosis. J Allergy Clin Immunol. 2013;132:723–8. doi: 10.1016/j.jaci.2013.02.019. [DOI] [PubMed] [Google Scholar]
- 18.Ustun C, Reiter A, Scott BL, Nakamura R, Damaj G, Kreil S, et al. Hematopoietic stem-cell transplantation for advanced systemic mastocytosis. J Clin Oncol. 2014;32:3264–74. doi: 10.1200/JCO.2014.55.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gleixner KV, Mayerhofer M, Aichberger KJ, Derdak S, Sonneck K, Bohm A, et al. Pkc412 inhibits in vitro growth of neoplastic human mast cells expressing the D816V-mutated variant of KIT comparison with amn107, imatinib, and cladribine (2cda) and evaluation of cooperative drug effects. Blood. 2006;107:752–9. doi: 10.1182/blood-2005-07-3022. [DOI] [PubMed] [Google Scholar]
- 20.Gotlib J, Kluin-Nelemans HC, George TI, Akin C, Sotlar K, Hermine O, et al. Efficacy and safety of midostaurin in advanced systemic mastocytosis. N Engl J Med. 2016;374:2530–41. doi: 10.1056/NEJMoa1513098. [DOI] [PubMed] [Google Scholar]
- 21.Jawhar M, Schwaab J, Naumann N, Horny HP, Sotlar K, Haferlach T, et al. Response and progression on midostaurin in advanced systemic mastocytosis: KIT D816V and other molecular markers. Blood. 2017;130:137–45. doi: 10.1182/blood-2017-01-764423. [DOI] [PubMed] [Google Scholar]
- 22.Branford S, Cross NC, Hochhaus A, Radich J, Saglio G, Kaeda J, et al. Rationale for the recommendations for harmonizing current methodology for detecting BCR-ABL transcripts in patients with chronic myeloid leukaemia. Leukemia. 2006;20:1925–30. doi: 10.1038/sj.leu.2404388. [DOI] [PubMed] [Google Scholar]
- 23.Scott S, Travis D, Whitby L, Bainbridge J, Cross NCP, Barnett D. Measurement of BCR-ABL1 by RT-qPCR in chronic myeloid leukaemia: findings from an international EQA programme. Br J Haematol. 2017;177:414–22. doi: 10.1111/bjh.14557. [DOI] [PubMed] [Google Scholar]
- 24.Cross NC, White HE, Ernst T, Welden L, Dietz C, Saglio G, et al. Development and evaluation of a secondary reference panel for BCR-ABL1 quantification on the international scale. Leukemia. 2016;30:1844–52. doi: 10.1038/leu.2016.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hindson BJ, Ness KD, Masquelier DA, Belgrader P, Heredia NJ, Makarewicz AJ, et al. High-throughput droplet digital PCR system for absolute quantitation of DNA copy number. Anal Chem. 2011;83:8604–10. doi: 10.1021/ac202028g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huggett JF, Cowen S, Foy CA. Considerations for digital PCR as an accurate molecular diagnostic tool. Clin Chem. 2015;61:79–88. doi: 10.1373/clinchem.2014.221366. [DOI] [PubMed] [Google Scholar]
- 27.Greiner G, Witzeneder N, Berger A, Schmetterer K, Eisenwort G, Schiefer AI, et al. Ccl2 is a KIT D816V-dependent modulator of the bone marrow microenvironment in systemic mastocytosis. Blood. 2017;129:371–82. doi: 10.1182/blood-2016-09-739003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Butterfield JH, Weiler D, Dewald G, Gleich GJ. Establishment of an immature mast cell line from a patient with mast cell leukemia. Leuk Res. 1988;12:345–55. doi: 10.1016/0145-2126(88)90050-1. [DOI] [PubMed] [Google Scholar]
- 29.Forootan A, Sjöback R, Björkman J, Sjögreen B, Linz L, Kubista M. Methods to determine limit of detection and limit of quantification in quantitative real-time PCR (qPCR) Biomol Detect Quantif. 2017;12:1–6. doi: 10.1016/j.bdq.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grohmann L, Broll H, Dagand E, Hildebrandt S, Hu¨ bert P, Kiesecker H, et al. Guidelines for the single-laboratory validation of qualitative real-time PCR methods. Braunschweig (Germany): Bundesamt für Verbraucherschutz und Lebensmittelsicherheit (BVL) 2016 [Google Scholar]
- 31. R Development Core Team. R: a language and environment for statistical computing. Vienna (Austria): the R Foundation for Statistical Computing. 2005 [Google Scholar]
- 32.Linnet K, Kondratovich M. Partly nonparametric approach for determining the limit of detection. Clin Chem. 2004;50:732–40. doi: 10.1373/clinchem.2003.029983. [DOI] [PubMed] [Google Scholar]
- 33.Andersen RF, Pallisgaard N. Exceeding MR5.0 sensitivity in routine BCR-ABL1 analysis using multiplex digital PCR. Blood. 2014;124:2. [Google Scholar]
- 34.Dobben de Bruyn CSv. Cumulative sum tests: theory and practice. London (UK): Griffin; 1968. [Google Scholar]
- 35.Kristensen T, Broesby-Olsen S, Vestergaard H, Bindslev-Jensen C, Moller MB. Targeted ultradeep next-generation sequencing as a method for KIT D816V mutation analysis in mastocytosis. Eur J Haematol. 2016;96:381–8. doi: 10.1111/ejh.12601. [DOI] [PubMed] [Google Scholar]
- 36.Young AL, Challen GA, Birmann BM, Druley TE. Clonal haematopoiesis harbouring AML-associated mutations is ubiquitous in healthy adults. Nat Commun. 2016;7 doi: 10.1038/ncomms12484. 12484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cross NC, Hochhaus A, Muller MC. Molecular monitoring of chronic myeloid leukemia: principles and interlaboratory standardization. Ann Hematol. 2015;94(Suppl 2):S219–25. doi: 10.1007/s00277-015-2315-1. [DOI] [PubMed] [Google Scholar]
- 38.Ahmad-Nejad P, Duda A, Sucker A, Werner M, Bronsert P, Stickeler E, et al. Assessing quality and functionality of DNA isolated from FFPE tissues through external quality assessment in tissue banks. Clin Chem Lab Med. 2015;53:1927–34. doi: 10.1515/cclm-2014-1202. [DOI] [PubMed] [Google Scholar]
- 39.Ivanov M, Baranova A, Butler T, Spellman P, Mileyko V. Non-random fragmentation patterns in circulating cellfree DNA reflect epigenetic regulation. BMC Genomics. 2015;16(Suppl 13):S1. doi: 10.1186/1471-2164-16-S13-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dietrich D, Uhl B, Sailer V, Holmes EE, Jung M, Meller S, Kristiansen G. Improved PCR performance using template DNA from formalin-fixed and paraffin-embedded tissues by overcoming PCR inhibition. PLoS One. 2013;8:e77771. doi: 10.1371/journal.pone.0077771. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.