Abstract
The aim of this study was to evaluate the efficacy and feasibility of intensified consolidation therapy employing fludarabine and ARA-C in cycle 1 and intermediate-dose ARA-C (IDAC) in cycles 2 through 4, in elderly acute myeloid leukemia (AML) patients and to analyze the effects of pegfilgrastim on the duration of neutropenia, overall toxicity, and hospitalization-time during consolidation in these patients. Thirty nine elderly patients with de novo AML (median age 69.9 years) who achieved complete remission (CR) after induction-chemotherapy were analyzed. To examine the effect of pegfilgrastim on neutropenia and hospitalization, we compared cycles 2 and 4 where pegfilgrastim was given routinely from day 6 (IDAC-P) with cycle 3 where pegfilgrastim was only administered in case of severe infections and/or prolonged neutropenia. All four planned cycles were administered in 23/39 patients (59.0%); 5/39 patients (12.8%) received 3 cycles, 3/39 (7.7%) 2 cycles, and 8/39 (20.5%) one consolidation-cycle. The median duration of severe neutropenia was 7 days in cycle 2 (IDAC-P), 11.5 days in cycle 3 (IDAC), and 7.5 days in cycle 4 (IDAC-P) (P <.05). Median overall survival was 1.1 years and differed significantly between patients aged <75 and ≥75 years (P <.05). The probability to be alive after 5 years was 32%. Together, intensified consolidation can be administered in AML patients ≥60, and those who are <75 may benefit from this therapy. Routine administration of pegfilgrastim during consolidation shortens the time of neutropenia and hospitalization in these patients.
1. Introduction
More than 50% of all AML patients are over 60 years at diagnosis.1–9 In a considerable number of these patients, the poor performance status or/and co-morbidities prohibit intensive myelosuppressive therapy.4–9 Most induction regimens that have been employed in elderly AML patients are the same as those applied in younger adults.1,2,8–12 However, the efficacy is poor in the elderly compared to younger patients. This appears to be due to a relatively high rate of treatment-related deaths as well as poor prognostic features.1–12
It is generally appreciated that intensive post-remission therapy is of importance to maintain remission in patients with AML.13–15 In younger patients, high-dose chemotherapy and/or allogeneic stem cell transplantation (HSCT) are established approaches.16–21 In patients aged ≥60 years, however, the consolidation strategies are discussed controversially. In many studies, post-remission treatment consists only of 1 to 2 cycles of intermediate dose chemotherapy.9–12 Only a few studies used repetitive cycles of high dose consolidation chemotherapy in elderly AML patients.22,23 Therefore, one reason for the unfavorable survival in elderly AML patients compared to younger adults may be the relatively low dose of chemotherapy applied during consolidation.
In this regard, one unresolved question is what age might be considered as an absolute upper limit to consider an intensive cytotoxic consolidation therapy. For example, high dose ARA-C may cause neurotoxicity in elderly patients with AML.24 In a previous study we have shown that repetitive cycles of intermediate dose ARA-C employing 1 g/m2 on days 1, 3, and 5 (up to four cycles) is an effective and well-tolerated consolidation in patients aged ≥60.22,23 However, although the phase of neutropenia was relatively short in these patients, neutropenic fever and severe infections were still recorded.22,23
Granulocyte colony-stimulating growth factor (G-CSF), a major regulator of neutrophil development and function, has been described to reduce the duration of chemotherapy-induced neutropenia and to prevent associated complications in AML patients.25–34 The use of G-CSF (filgrastim) is safe and is not affecting the CR rate or duration of CR.25–34 These studies have also shown that the time to neutrophil recovery is significantly shorter in AML patients receiving G-CSF compared to control patients without G-CSF support.25–34 With regard to the duration of hospitalization the data are conflicting. Some studies showed a significant shortening of the duration of hospitalization whereas other studies were unable to confirm these results.26,27,29–33 In most clinical trials, G-CSF was administered shortly after induction chemotherapy. However, only a few studies have examined the effects of G-CSF during consolidation therapy.
Pegfilgrastim (filgrastim [recombinant G-CSF] to which a 20 kilodalton poly[ethylen]glycol [PEG] molecule is bound) has been shown to be equally effective in stimulating granulopoiesis compared to non-pegylated filgrastim.35 In contrast to filgrastim, pegfilgrastim has to be administered only once per chemotherapy cycle since neutrophils are the exclusive site of pegfilgrastim clearance.
The aim of the present study were to evaluate the efficacy and feasibility of intensified consolidation elderly patients with de novo AML and to define the effect of pegfilgrastim on neutropenia and hospitalization in these patients.
2. Subjects and Methods
2.1. Patients
Patients with de novo AML aged ≥60 years (n = 64) were included in this multi-center single arm phase IV study. Patients with AML M3 according to the French-American-British (FAB) classification or acute promyelocytic leukemia (APL) with PML-RARA according to World Health Organization (WHO) criteria were excluded. Information on inclusion- and exclusion criteria is shown in Supporting Information Table S1. The study was approved by the ethics committees of the participating institutions, conducted in accordance with the Declaration of Helsinki and registered at the EMA under the EudraCT Number: 2007–005806-29. All patients gave their written informed consent to participate in this study. Due to low recruitment this trial had to be closed early (see supplemental materials).
2.2. Examinations at diagnosis and during follow up
Before and during each induction and consolidation cycle, starting at the day before chemotherapy until neutrophil recovery (absolute neutrophil count = ANC >0.5 G/L or white-blood-cell count = WBC >1.0 G/L) the following tests were performed: complete blood count and differential count, serum chemistry (twice a week), and daily temperature logs. Bone marrow (bm) aspiration was performed at diagnosis and after each induction cycle at the time of recovery or day 40, whichever was earlier. At diagnosis the karyotype (HOVON, SWOG, and ELN criteria) and molecular markers were recorded.36–38 Karyotyping was performed by conventional chromosome banding-studies and fluorescence in situ hybridization (FISH) techniques. Karyotyping results were described according to published guidelines.39 DNA and RNA were prepared from mononuclear bm cells according to established techniques.40,41 In a subgroup of patients, serum levels of G-CSF were analyzed in the follow up between days 6 and 30 after administration of G-CSF. Details regarding G-CSF-measurement are described in the supplement.
2.3. Treatment
Induction chemotherapy consisted of daunorubicin, 45 mg/m2 iv, days 1–3; ARA-C, 2 × 100 mg/m2 iv, days 1–7; etoposide, 100 mg/m2 iv days 1–5 (DAV 3 + 5 + 7). In the case of blast cell persistence a 2nd cycle employing ARA-C, 2 × 1000 mg/m2 iv days 1, 3, 5; mitoxantrone, 12 mg/m2 iv, days 3, 5 (MiDAC light) was given. In patients who did not achieve a complete remission (CR) after the 2nd cycle, a 3rd induction cycle with, fludarabine, 30 mg/m2 iv, days 1–5; ARA-C, 2000 mg/m2 iv days 1–5 with G-CSF starting on day 6 (FLAG) was administered. All patients in CR were planned to receive 4 cycles of intensive consolidation therapy. The 1st consolidation consisted of FLAG with pegfilgrastim (6 mg sc) on day 6. In the 2nd and 4th consolidation cycle, patients received ARA-C, 2 × 1000 mg/m2 iv days 1, 3,5 and pegfilgrastim 6 mg sc on day 6 (IDAC-P). In the 3rd consolidation cycle, ARA-C, 2 × 1000 mg/m2 iv days 1, 3, 5 (IDAC) was administered. To analyze the efficacy of G-CSF during consolidation, pegfilgrastim was not administered routinely in the 3rd cycle. However, G-CSF was administered in cycle 3 in patients with prolonged aplasia or severe infections or in cause of a history of severe infections during a previous cycle according to ASCO guidelines.42
2.4. Statistical analyses
The significance in differences between cycles 2 and 3, and between cycles 3 and 4 regarding the duration of severe neutropenia (ANC <0.5 or WBC <1 G/L) and the duration of hospitalization were calculated using the Friedman test and Wilcoxon test. Clinical endpoints, including overall survival (OS), continuous complete remission (CCR), and disease- free survival (DFS) were analyzed by the method of Kaplan and Meier. The differences in the survival were analyzed by cox regression (for details see supplemental materials)
3. Results
3.1. Patients′ characteristics at diagnosis
Between July 2008 and November 2012, 64 de novo non-M3 AML patients with a median age of 69.9 years (range 60.1–85.2 years; f:m-ratio: 1:1.56) were included. A majority of patients (n = 53) was <75 years, 11 patients were ≥75 years. The observation period lasted until September 2014. Diagnoses were established according to FAB and WHO (Supporting Information Table S2). In 52 patients, karyotypes were available. Patients were categorized following HOVON criteria (core binding factor, n = 1 [1.9%]; normal karyotype, n = 25 [48.1%]; non-monosomal karyotype, n = 11 [21.2%]; monosomal karyotype, n = 15 [28.8%]), modified SWOG criteria (1 favorable karyotype, n = 1 [1.9%]; intermediate/unknown karyotype, n = 41 [78.8%]; unfavorable karyotype, n = 10 [19.2%]) and ELN-criteria (favorable karyotype, n = 8 [14.8%]; intermediate karyotype, n = 29 [53.7%]; adverse karyotype, n = 19 [31.5%]).36–38 Results from molecular studies examining FLT3 ITD mutations, NPM1 mutations (NPM1-mut), and the KIT mutation D816V as well as multiplex PCR studies examining AML/ETO, CBFa/MYH11, and MLL1-AF10 were available in a subset of patients (Supporting Information Table S2). The median age of the 39 patients eligible for consolidation was 69.9 years, ranging from 60 to 78 years (<75 years, n = 34; ≥75 years, n = 5; f:m-ratio, 1:1.6).
3.2. Outcome of induction therapy
Of the 64 patients included in this trial, two patients were withdrawn during the first induction cycle because of prolonged aplasia (>40 days) and refractory thrombocytopenia resulting in a subdural hematoma in one patient. Following the induction phase, 43 patients (68%) entered complete remission (CR), 33 after 1 cycle (DAV), 9 after 2 cycles (DAV and MiDAC light), and 1 patient after 3 cycles (DAV, MiDAC-light, and FLAG) of induction therapy. In 16 patients no remission (NR) was achieved. Of these patients, 7 received one induction cycle, 3 two cycles, and 6 three cycles. Three patients died because of infections.
3.3. Number of consolidation cycles
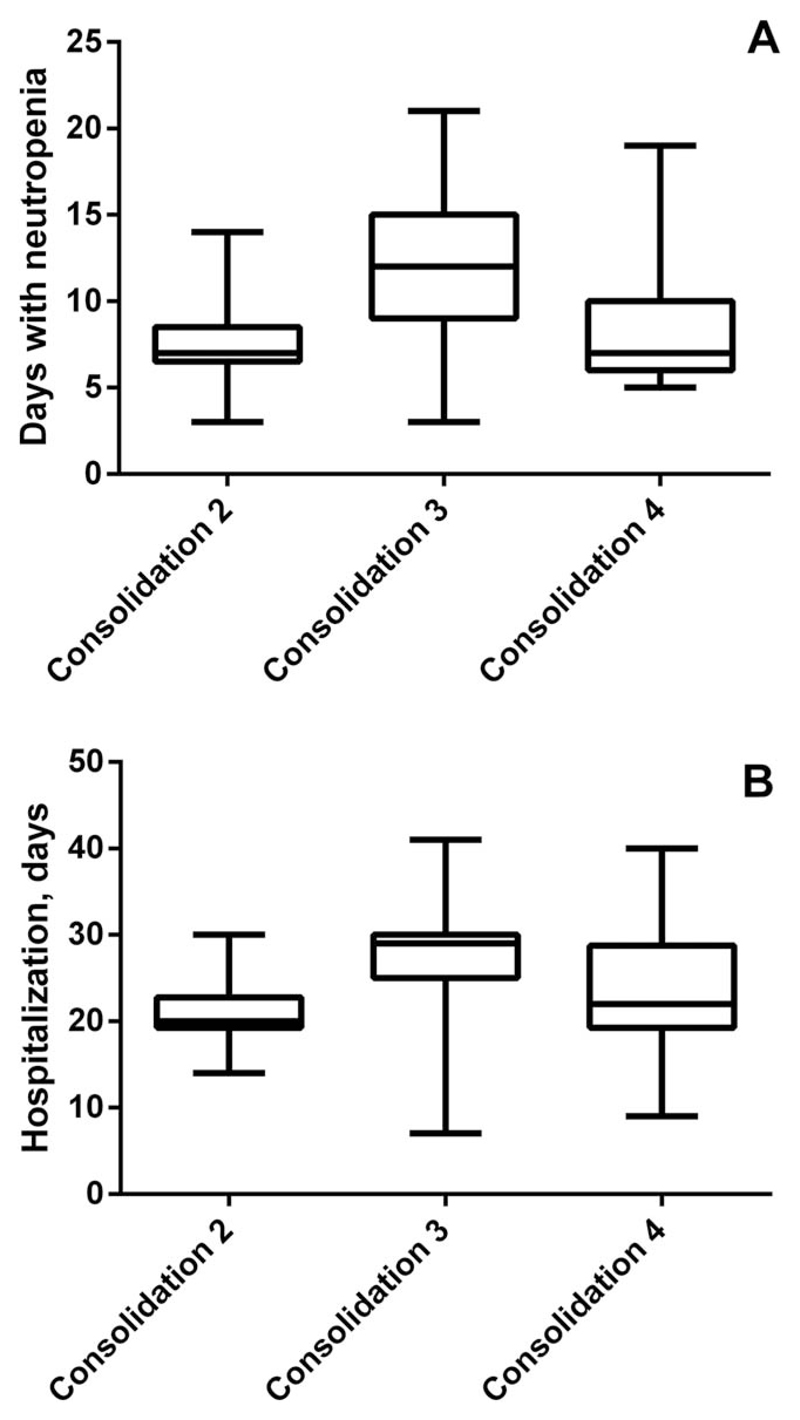
In 39/43 CR patients (91%), consolidation therapy could be initiated. In 4 patients no consolidation therapy could be given according to the study protocol. In these patients, an early relapse (n = 1), death due to a spontaneous pulmonary bleeding (n = 1), a severe protocol violation (n = 1), or aggravation of dementia (n = 1) was found. All 4 planned courses of consolidation were administered in 23/39 patients (59.0%). Five patients (12.8%) received three, and 3 patients (7.7%) two cycles. In 8 patients (20.5%), the treatment had to be stopped after the 1st consolidation. Causes to withhold further therapy are shown in Table 1. Four patients (10.2%) died in the consolidation phase (treatment related, n = 2; after complete recovery, not treatment-related, n = 2). The median duration of neutropenia was 9 days (range: 4–28 days) in consolidation 1 (FLAG), 7 days (range: 3–14 days) in consolidation 2 (IDAC-P), 11.5 days (range: 3–21 days) in consolidation 3 (IDAC), and 7.5 days (range: 5–19 days) in consolidation 4 (IDAC-P). Neutropenic fever (>38°C) occurred in 68/121 consolidation cycles (56.2%), i.e., in 19/39 patients (48.7%) during the 1st cycle, 18/31 patients (58.1%) during the 2nd cycle, 17/28 patients (60.7%) during the 3rd cycle, and 14/23 patients (60.9%) during the 4th cycle of consolidation. The median duration of fever was 1 day (range: 0–27 days). The median number of packed red cell and platelet concentrates given per consolidation cycle were 4 (range: 0–15) and 3 (range 0–13), respectively (Supporting Information Table S3).
Table 1. Causes of withdrawal from further consolidation cycles.
| Death related to therapy | Death unrelated to therapy | Relapse | HSCT | In-fection | Persisting cytopenia | All with-drawals per cycle | |
|---|---|---|---|---|---|---|---|
| 1st consolidation | 1 | 1 | 3 | 1 | 0 | 2 | 8 |
| 2nd consolidation | 0 | 1 | 2 | 0 | 0 | 0 | 3 |
| 3rd consolidation | 0 | 0 | 2 | 0 | 2 | 1 | 5 |
| 4th consolidation | 1 | 0 | 1 | 0 | 0 | 1 | 3 |
| All consolidations | 2 | 2 | 8 | 1 | 2 | 4 | 19 |
HSCT, hematopoietic stem cell transplantation.
3.4. Tolerability and safety of intensified consolidation
Severe, life-threatening adverse events occurred in seven patients. There were two cases (aged 66 and 65) with a single severe adverse event, i.e., severe vomiting and nausea in consolidation 2, severe neutropenic fever in consolidation 2, and one case (aged 68) with 2 severe events, i.e., ARA-C-induced fever and neutropenic fever in consolidation 3. One patient (aged 73) developed a severe infection with hypotension and neurologic deterioration after the first consolidation and died later despite hematopoietic recovery from a cardiac arrest. In another patient (aged 76) severe neutropenic fever occurred during consolidations 1 and 4. In the 4th consolidation cycle this patient also developed pneumonia and died from septic shock. There was one case aged 76 years with cholecystitis and ileus during the 1st consolidation and severe emesis in the 2nd cycle. In the follow up, after complete recovery he developed a fatal spontaneous intracerebral bleeding. In another patient (aged 68) severe diarrhea developed during the 1st consolidation. In the follow up this patient developed pulmonary inflammation resulting in a fatal alveolar damage. With regard to the laboratory results, liver toxicity grades II, III, and IV were found in 10 patients, 2 patients, and 2 patients respectively. Three patients had a grade II nephrotoxicity, but there were no cases of grade III or IV nephrotoxicity observed during consolidation therapy (Supporting Information Figure S1).
3.5. OS and prognostic variables predicting OS
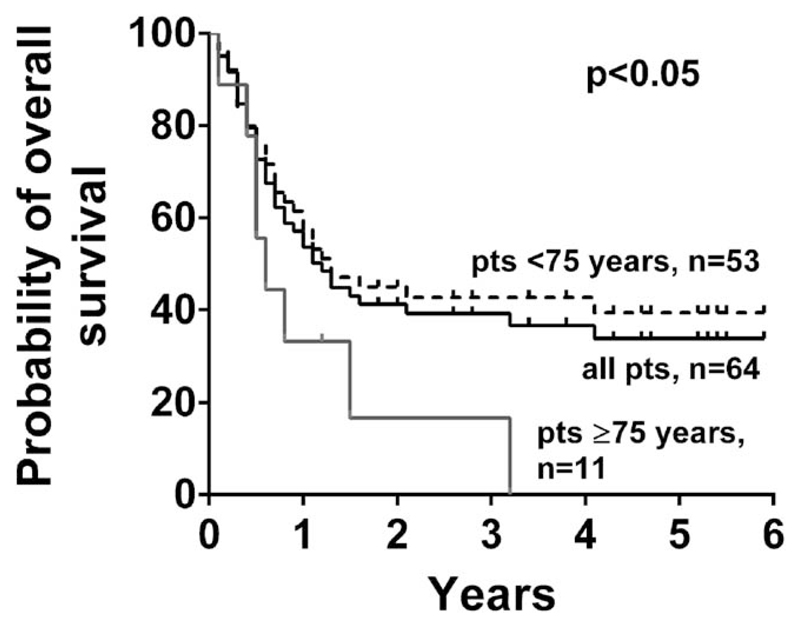
The median observation time was 1 year; 13 patients were followed for more than 4 years and 6 patients for more than 5 years. The median OS was 1.1 years and the probability to be alive 2 and 5 years after the start of therapy was 39% and 32%, respectively (Figure 1A). Three patients, who received an allogeneic HSCT were included. One of these patients was in the consolidation arm and was transplanted after consolidation 1 (OS: 1 year), in two patients the HSCT was performed as salvage therapy because of blast cell persistence following the second induction cycle (OS: 1.2 and 0.5 years, respectively). Major differences in OS were observed when comparing patients aged <75 years with patients ≥75 years. The probability of survival after 2 and 5 years was 44% and 39% in the group aged <75 years, respectively. By contrast, in the cohort aged ≥75 years (n = 11), only one patient was alive after 2 years, and no patient was alive after 5 years (Figure 1A, P <.05). Marked differences were observed between NPM1-wild type (NPM1-wt) and NPM1-mut patient, between patients carrying a FLT3 ITD mutation and FLT3 ITD negative patient but not between female and male patients (Supporting Information Table S4). A risk stratification according ELN criteria was available in 54 patients. Significant differences in OS were found between patients with favorable, intermediate or adverse karyotypes according to ELN criteria (P <.05; Supporting Information Figure S2A). All long-term survivors received at least three cycles of consolidation therapy (Supporting Information Figure S2B).
Figure 1.
Survival of patients. Overall survival as calculated according to the method of Kaplan and Meier. The survival of all patients is indicated by the black solid line. The dashed black line represents the survival of patients aged <75 years and the grey solid line the survival of patients aged ≥75 years with a median survival of 1.5 years and 0.5 years, respectively. The difference in OS, between patients aged <75 years and ≥75 years were found to be statistically significant (P <.05)
3.6. Continuous complete remission (CCR) and disease-free survival (DFS)
CCR and DFS, calculated in patients receiving at least 1 cycle of consolidation chemotherapy, were 1.25 years and 1.23 years, respectively (including one patient that underwent HSCT). The probability to be in CCR and DFS after 2 years was 37% and 35% respectively, and the probability to be alive 5 years after the start of therapy was 29% and 23%, respectively. Only 6 patients aged ≥75 years could be analyzed. Their median CCR and DFS (0.47 years; each) were markedly reduced compared to patients aged <75 years (1.25 years, each; CCR, p = n.s.; DFS, p = 0.08; Supporting Information Figure S3). Karyotyping results were available in 32/39 patients. Significant differences were observed in CCR and DFS between patients in different ELN risk groups (Supporting Information Table S5; Figure S4A, B). In addition, CCR and DFS differed between patients receiving at least 3 consolidation cycles and those receiving less than 3 consolidation cycles (Supporting Information Figure S4C,D). Finally, CCR and DFS differed among patients with NPM1-wt and NPM1-mut, and between FLT3 ITD-positive patients and FLT3 ITD-negative patients (Supporting Information Table S5).
3.7. G-CSF shortens the duration of aplasia and hospitalization
To evaluate the effect of pegfilgrastim on the duration of neutropenia and hospitalization during consolidation, we compared the consolidation cycles where G-CSF was given routinely on day 6 (IDAC-P: cycles 2 and 4) with cycle 3 where G-CSF was only given based on ASCO criteria (IDAC).42 Only patients who received all 4 planned cycles of chemotherapy per protocol were included in this analysis (n = 17). The duration of neutropenia differed significantly between consolidation 3 (11.5 days; range: 3–14 days), consolidation 2 (7 days; range 4–28 days), and consolidation 4 (7.5 days; range: 5–19 days) (P <.05 by Friedman test; Figure 2A). The different outcome concerning the phase of severe neutropenia and days of hospitalization was seen despite the low numbers of patients and although 3/17 patients (18%) received G-CSF during the 3rd consolidation according to ASCO criteria.42 The duration of hospitalization in the 17 patients treated per protocol differed significantly between consolidation 3 (29 days, range 7–41 days) and consolidation 2 (20 days, range 13–42 days), and between consolidation 3 and consolidation 4 (22 days, range: 9–40 days) (Figure 2B).
Figure 2.
Comparison of the duration of neutropenia (A) and hospitalization (B) between the 2nd, 3rd, and 4th consolidation cycle. The box represents the 25–75% percentile in each group, the horizontal line within boxes defines the median, and the whiskers represent the range. The differences in the duration of neutropenia among Consolidation 2, 3, and 4 were found to be significant as assessed by Friedman test (P <.05)
3.8. Serum levels of G-CSF during therapy
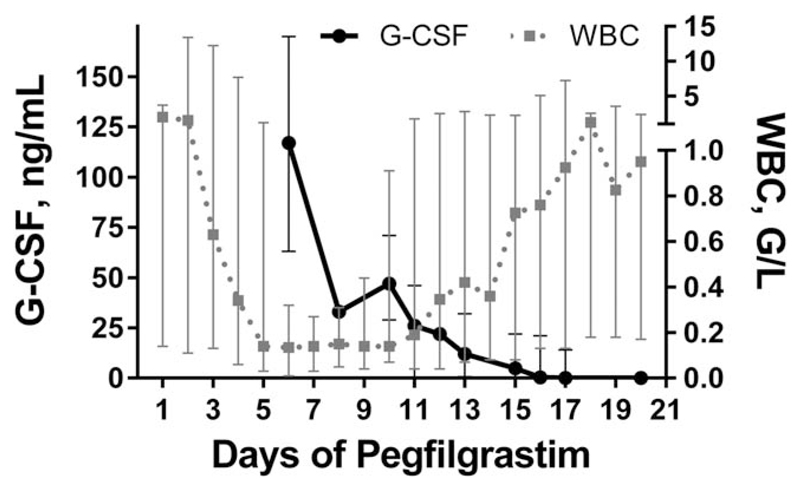
To evaluate how long plasma levels of pegfilgrastim were detectable after injection, the concentration of G-CSF was measured serially in 18 consolidation cycles (FLAG, n = 16; IDAC, n = 2). These measurements were done after administration of pegfilgrastim, i.e., between days 11 and 35 after start of chemotherapy. G-CSF was detectable in all serum samples taken before day 21 of chemotherapy, i.e., day 16 after the administration of pegfilgrastim. Thereafter, the number of samples with detectable G-CSF decreased. The ′latest′ serum samples with a detectable cytokine level were taken on day 27 of chemotherapy, i.e., day 22 after pegfilgrastim administration. There was a clear (inverse) correlation between G-CSF levels and the peripheral WBC. In patients with an early increase of WBC and granulocyte counts, there was an early decrease of G-CSF and vice versa (Figure 3).
Figure 3.
G-CSF levels and WBC counts. G-CSF levels (solid line) and WBC (dashed line) are presented as median and range in 18 consolidation cycles (FLAG, n = 16; IDAC, n = 2). Each point represents the median of the measurements available for this time point. Day 1 is the first day of pegfilgrastim administration
4. Discussion
More than 50% of all AML patients are 60 years or beyond at diagnosis.1–12,43–46 In contrast to younger patients, elderly patients with AML may not always benefit from intensive chemotherapy. In our study 68% of all patients aged ≥60 achieved a CR. Of these, 91% were eligible for intensive consolidation. Although hematologic and non-hematologic toxicity were moderate, four patients died during consolidation. No severe grade III or IV nephrotoxicity or neurotoxicity was recorded and there was only one patient with grade III and grade IV liver toxicity, each. The probability to be alive after 5 years was 32%. Patients aged <75 years showed a clearly better survival compared to those aged ≥75 years. Finally, our study also shows that the addition of pegfilgrastim during consolidation significantly shortens the duration of neutropenia and the duration of hospitalization.
The appropriate consolidation in AML patients aged ≥60 years who achieve a CR is still a matter of discussion. Many centers offer HSCT to a subset of (fit) elderly high risk AML patients.19,20,47 Contrasting this strategy, other centers offer only a few cycles of low dose chemotherapy as consolidation.13,48 Overall, the optimal type and optimal number of consolidation cycles are currently unknown. The high risk of treatment-related toxicity is the primary cause to withhold intensive consolidation in many such cases. We have previously shown, that intermediate dose intermittent ARA-C (IDAC) employing 2 × 1 g/m2 on days 1, 3, 5 is feasible in patients aged ≥60 years, even when up to 4 cycles are administered (Supporting Information Table S6).22,23 In the current study, FLAG was used as consolidation 1 instead of IDAC. This represents an intensification of consolidation chemotherapy and our data are the first to prove that such intensified consolidation therapy can be administered safely in patients aged ≥60 years. However, there were 7 cases of severe or life-threatening events resulting in 4 deaths during consolidation. This early death rate is within the range of published results11,22,23 but is still lower compared to that seen in patients undergoing HSCT.18,19 However, considering the high rate of relapses in these patients, there is a need to introduce high-risk post CR therapies, such as high-dose consolidation or allogeneic HSCT. Another strategy is to apply post-consolidation maintenance therapy.49,50
Considering the toxicity of our treatment approach, it is important to define predictive factors indicating which patients will benefit from such intensive treatment. One important risk factor is age. In fact, most (3/4) patients with fatal events were over 73 years. Together, our data show that patients aged ≥60 years can achieve long-term survival with conventional chemotherapy if treated appropriately. However, there was a significant difference in the survival between patients aged <75 and ≥75 years. Moreover, no patient aged ≥75 years had a survival of more than 4 years. Although observed in only a small cohort, these results argue against intensive consolidation in these patients. Thus, in line with published results, age and performance status were the most important predictive factors to decide which patients might be eligible for and can benefit from intensive chemotherapy.23,45,46 The karyotype is another well-established prognostic variable. The prognostic importance of karyotyping has been demonstrated in various studies.37–39,51 Similarly, in our study cohort, patients with a monosomal karyotype had the worst outcome.
In patients with intensive chemotherapy the long duration of neutropenia results in prolonged hospitalization and a high risk of neutropenic infections. It is generally accepted that the time of neutropenia can be shortened by application of G-CSF.25–34 However, whether G-CSF therapy also results in a shorter time of hospitalization in AML patients is discussed controversially.26,27,29–33 Moreover, with regard to the application of G-CSF during consolidation only limited data are available.26,52,53 We compared the duration of hospitalization and absolute neutropenia in the 2nd, 3rd, and 4th consolidation cycles. There was a significant difference in the duration of neutropenia and duration of hospitalization between the 2nd and 3rd consolidation cycles as well as between the 3rd and 4th consolidation cycles although 18% of the patients had received G-CSF during the 3rd cycle according to ASCO criteria.42 These data strongly suggest that the administration of pegylated G-CSF shortens not only the duration of neutropenia but also the duration of hospitalization during consolidation in elderly AML patients.
Whereas G-CSF has to be administered every day due to its short half-life, pegylated G-SCF (pegfilgrastim) has the same efficacy as filgrastim, but a reduced renal clearance.35 Neutrophil-mediated clearance is the major mechanism of drug-elimination in these patients.35 We were therefore interested to see how long plasma levels of pegfilgrastim were detectable after therapy. This was of particular interest since after the start of consolidation almost normal granulocyte counts were detectable up to day 10 or day 11. Pegfilgrastim was detectable throughout the observation period until full neutrophil recovery. There was decrease of pegfilgrastim between day 6 and 12 after administration (i.e., day 12 and 18 of chemotherapy). This could be an effect of remaining granulocytes. However, it is also tempting to speculate, that this could be a sign of early regrowth of neutrophil precursors. Whether a delayed administration of pegfilgrastim would improve the efficacy remains to be elucidated. Overall, our data are in line with those that demonstrate the long-term effect of pegfilgrastim on neutrophil recovery during repetitive cycles of consolidation therapy in AML in younger patients.54
Together, our data show, that intensified consolidation chemotherapy can be administered safely in AML patients aged ≥60 years and results in an improved survival. A majority of patients received all 4 consolidation cycles although substantial toxicity was observed. Unfavorable factors concerning survival were age ≥75 years and monosomal karyotype. Thus, our data suggest that a subset of elderly patients with AML, aged 60–74, benefit from intensified consolidation therapy. The administration of pegfilgrastim during consolidation shortened not only the duration of neutropenia but also the time of hospitalization. Therefore, it seems reasonable to argue that both filgrastim and pegfilgrastim can be regarded as standard adjunct to consolidation therapy of elderly patients with AML.
Supplementary Material
Additional Supporting Information may be found online in the supporting information tab for this article
Aknowledgment
We would like to thank all co-investigators who supported this trial in the participating centers, including Ch.Sillaber, M.T.Krauth, A. Hauswirth, H.Agis, H.Gisslinger (Medical University of Vienna), D. Lutz, O.Krieger (Hospital of the Elisabethinen Linz), P.Großschmidt (Krankenhaus Hietzing, Vienna), and H.Kienzer (Kaiser-Franz-Josef-Spital, Vienna). This study was supported by Austrian Science Fund (FWF) grant F4704-B20 and by Amgen.
Funding information
Austrian Science Fund (FWF), Grant Number: F4704-B20.
References
- 1.Löwenberg B, Downing JR, Burnett A. Acute myeloid leukemia. N Engl J Med. 1999;341:1051–1062. doi: 10.1056/NEJM199909303411407. [DOI] [PubMed] [Google Scholar]
- 2.Vellenga E, Griffin JD. The biology of acute myeloid leukemia. Semin Oncol. 1987;14:365–371. [PubMed] [Google Scholar]
- 3.Sperr WR, Hauswirth AW, Wimazal F, Knöbl P, Geissler K, Valent P. Treatment concepts for elderly patients with acute myeloid leukaemia. Wien Klin Wochenschr. 2003;115:505–514. doi: 10.1007/BF03041034. [DOI] [PubMed] [Google Scholar]
- 4.Estey EH. Acute myeloid leukemia: 2013 update on risk-stratification and management. Am J Hematol. 2013;88:318–327. doi: 10.1002/ajh.23404. [DOI] [PubMed] [Google Scholar]
- 5.Löwenberg Treatment of the elderly patient with acute myeloid leukaemia. Baillieres Clin Haematol. 1996;9:147–159. doi: 10.1016/s0950-3536(96)80041-2. [DOI] [PubMed] [Google Scholar]
- 6.Hiddemann W, Kern W, Schoch C, et al. Management of acute myeloid leukemia in elderly patients. J Clin Oncol. 1999;17:3569–3576. doi: 10.1200/JCO.1999.17.11.3569. [DOI] [PubMed] [Google Scholar]
- 7.Büchner T, Hiddemann W, Schoch C, Haferlach T, Sauerland MC, Heinecke A. Acute myeloid leukaemia (AML): treatment of the older patient. Best Pract Res Clin Haematol. 2001;14:139–151. doi: 10.1053/beha.2000.0120. [DOI] [PubMed] [Google Scholar]
- 8.Estey EH. Therapeutic options for acute myelogenous leukemia. Cancer. 2001;92:1059–1073. doi: 10.1002/1097-0142(20010901)92:5<1059::aid-cncr1421>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 9.Appelbaum FR. Molecular diagnosis and clinical decisions in adult acute leukemia. Semin Hematol. 1999;36:401–410. [PubMed] [Google Scholar]
- 10.Löwenberg B, Zittoun R, Kerkhofs H, et al. On the value of intensive remission-induction chemotherapy in elderly patients of 65+ years with acute myeloid leukemia: a randomized phase III study of the European Organization for Research and Treatment of Cancer Leukemia Group. J Clin Oncol. 1989;7:1268–1274. doi: 10.1200/JCO.1989.7.9.1268. [DOI] [PubMed] [Google Scholar]
- 11.Ossenkoppele G, Loewenberg B. How I treat the older patient with acute myeloid leukemia. Blood. 2015;125:767–774. doi: 10.1182/blood-2014-08-551499. [DOI] [PubMed] [Google Scholar]
- 12.Stasi R, Venditti A, Del Poeta G, et al. Intensive treatment of patients age 60 years and older with de novo acute myeloid leukemia: analysis of prognostic factors. Cancer. 1996;77:2476–2488. doi: 10.1002/(SICI)1097-0142(19960615)77:12<2476::AID-CNCR10>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 13.Tilly H, Castaigne S, Bordessoule D, et al. Low-dose ARA-C versus intensive chemotherapy in the treatment of acute nonlymphocytic leukemia in the elderly. J Clin Oncol. 1990;8:272–279. doi: 10.1200/JCO.1990.8.2.272. [DOI] [PubMed] [Google Scholar]
- 14.Bassan R, Buelli M, Viero P, Minotti C, Barbui T. The management of acute myelogenous leukemia in the elderly: ten-year experience in 118 patients. Hematol Oncol. 1992;10:251–260. doi: 10.1002/hon.2900100503. [DOI] [PubMed] [Google Scholar]
- 15.Embury SH, Elias L, Heller PH, Hood CE, Greenberg PL, Schrier SL. Remission maintenance therapy in acute myelogenous leukemia. West J Med. 1977;126:267–272. [PMC free article] [PubMed] [Google Scholar]
- 16.Cassileth PA, Harrington DP, Hines JD, et al. Maintenance chemotherapy prolongs remission duration in adult acute nonlymphocytic leukemia. J Clin Oncol. 1988;6:583–587. doi: 10.1200/JCO.1988.6.4.583. [DOI] [PubMed] [Google Scholar]
- 17.Cassileth PA, Lynch E, Hines JD, et al. Varying intensity of postremission therapy in acute myeloid leukemia. Blood. 1992;79:1924–1930. [PubMed] [Google Scholar]
- 18.Stone RM, Berg DT, George SL, et al. Postremission therapy in older patients with de novo acute myeloid leukemia: a randomized trial comparing mitoxantrone and intermediate-dose ARA-Ce with standard-dose ARA-C. Blood. 2001;98:548–553. doi: 10.1182/blood.v98.3.548. [DOI] [PubMed] [Google Scholar]
- 19.Greinix HT, Nachbaur D, Krieger O, et al. Factors affecting long-term outcome after allogeneic haematopoietic stem cell transplantation for acute myelogenous leukaemia: a retrospective study of 172 adult patients reported to the Austrian Stem Cell Transplantation Registry. Br J Haematol. 2002;117:914–923. doi: 10.1046/j.1365-2141.2002.03532.x. [DOI] [PubMed] [Google Scholar]
- 20.Sierra J, Storer B, Hansen JA, et al. Unrelated donor marrow transplantation for acute myeloid leukemia: an update of the Seattle experience. Bone Marrow Transplant. 2000;26:397–404. doi: 10.1038/sj.bmt.1702519. [DOI] [PubMed] [Google Scholar]
- 21.Boehm A, Piribauer M, Geissler K, et al. High dose intermittent ARA-C (HiDAC) for consolidation in patients with de novo AML: a single center experience. Leuk Res. 2005;29:609–615. doi: 10.1016/j.leukres.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 22.Sperr WR, Piribauer M, Wimazal F, Fonatsch C, Thalhammer-Scherrer R, et al. A novel effective and safe consolidation for patients with AML aged over 60: intermediate dose ARA-C (2 x 1 g/m2 on days 1, 3, and 5) Clin Cancer Res. 2004;10:3963–3971. doi: 10.1158/1078-0432.CCR-04-0185. [DOI] [PubMed] [Google Scholar]
- 23.Sperr WR, Zach O, Pöll I, et al. Karyotype plus NPM1 mutation status defines a group of elderly patients with AML (≥ 60 Years) who benefit from intensive post-induction consolidation therapy. Am J Hematol. 2016;91:1239–1245. doi: 10.1002/ajh.24560. [DOI] [PubMed] [Google Scholar]
- 24.Mayer RJ, Davis RB, Schiffer CA, et al. Intensive postremission chemotherapy in adults with acute myeloid leukemia. N Engl J Med. 1994;331:896–903. doi: 10.1056/NEJM199410063311402. [DOI] [PubMed] [Google Scholar]
- 25.Archimbaud E, Ottmann OG, Yin JA, et al. A randomized, double-blind, placebo-controlled study with pegylated recombinant human megakaryocyte growth and development factor (PEG-rHuMGDF) as an adjunct to chemotherapy for adults with de novo acute myeloid leukemia. Blood. 1999;94:3694–3701. [PubMed] [Google Scholar]
- 26.Heil G, Hoelzer D, Sanz MA, et al. A randomized, double-blind, placebo controlled, phase III study of filgrastim in remission Induction and Consolidation therapy for adults with de novo acute myeloid leukemia. Blood. 1997;90:4710–4718. [PubMed] [Google Scholar]
- 27.Dombret H, Chastang C, Fenaux P, et al. A controlled study of recombinant human granulocyte colony-stimulating factor in elderly patients after treatment for acute myelogenous leukemia. AML Cooperative Study Group. N Engl J Med. 1995;332:1678–1683. doi: 10.1056/NEJM199506223322504. [DOI] [PubMed] [Google Scholar]
- 28.Godwin JE, Kopecky KJ, Head DR, et al. A double-blind placebo-controlled trial of granulocyte colony-stimulating factor in elderly patients with previously untreated acute myeloid leukemia: a Southwest oncology group study (9031) Blood. 1998;91:3607–3615. [PubMed] [Google Scholar]
- 29.Bradstock K, Matthews J, Young G, et al. Australian Leukaemia Study Group. Effects of glycosylated recombinant human granulocyte colony-stimulating factor after high-dose cytarabine-based induction chemotherapy for adult acute myeloid leukaemia. Leukemia. 2001;15:1331–1338. doi: 10.1038/sj.leu.2402218. [DOI] [PubMed] [Google Scholar]
- 30.Goldstone AH, Burnett AK, Wheatley K, Smith AG, Hutchinson RM, Clark RE. Medical Research Council Adult Leukemia Working Party. Attempts to improve treatment outcomes in acute myeloid leukemia (AML) in older patients: the results of the United Kingdom Medical Research Council AML11 trial. Blood. 2001;98:1302–1311. doi: 10.1182/blood.v98.5.1302. [DOI] [PubMed] [Google Scholar]
- 31.Usuki K, Urabe A, Masaoka T, et al. Efficacy of granulocyte colony-stimulating factor in the treatment of acute myelogenous leukaemia: a multicentre randomized study. Br J Haematol. 2002;116:103–112. doi: 10.1046/j.1365-2141.2002.03251.x. [DOI] [PubMed] [Google Scholar]
- 32.Amadori S, Suciu S, Jehn U, et al. Use of glycosylated recombinant human G-CSF (lenograstim) during and/or after induction chemotherapy in patients 61 years of age and older with acute myeloid leukemia: final results of AML-13, a randomized phase-3 study. Blood. 2005;106:27–34. doi: 10.1182/blood-2004-09-3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wheatley K, Goldstone AH, Littlewood T, Hunter A, Burnett AK. Randomized placebo-controlled trial of granulocyte colony stimulating factor (G-CSF) as supportive care after induction chemotherapy in adult patients with acute myeloid leukaemia: a study of the United Kingdom Medical Research Council Adult Leukaemia Working Party. Br J Haematol. 2009;146:54–63. doi: 10.1111/j.1365-2141.2009.07710.x. [DOI] [PubMed] [Google Scholar]
- 34.Beksac M, Ali R, Ozcelik T, et al. Short and long term effects of granulocyte colony-stimulating factor during induction therapy in acute myeloid leukemia patients younger than 65: results of a randomized multicenter phase III trial. Leuk Res. 2011;35:340–345. doi: 10.1016/j.leukres.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 35.Sierra J, Szer J, Kassis J, et al. A single dose of pegfilgrastim compared with daily filgrastim for supporting neutrophil recovery in patients treated for low-to-intermediate risk acute myeloid leukemia: results from a randomized, double-blind, phase 2 trial. BMC Cancer. 2008;195 doi: 10.1186/1471-2407-8-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Breems DA, Van Putten WL, De Greef GE, et al. Monosomal karyotype in acute myeloid leukemia: a better indicator of poor prognosis than a complex karyotype. J Clin Oncol. 2008;26:4791–4797. doi: 10.1200/JCO.2008.16.0259. [DOI] [PubMed] [Google Scholar]
- 37.Leith CP, Kopecky KJ, Godwin J, et al. Acute myeloid leukemia in the elderly: assessment of multidrug resistance (MDR1) and cytogenetics distinguishes biologic subgroups with remarkably distinct responses to standard chemotherapy. A Southwest Oncology Group study. Blood. 1997;89:3323–3329. [PubMed] [Google Scholar]
- 38.Döhner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129:424–447. doi: 10.1182/blood-2016-08-733196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.ISCN (2013) An international system for human cytogenetic nomenclature (2013) In: Shaffer LG, McGowan-Jordan J, Schmid M, editors. Basel: S Karger; 2013. pp. 39–87. [Google Scholar]
- 40.Kainz B, Heintel D, Marculescu R, et al. Variable prognostic value of FLT3 internal tandem duplications in patients with de novo AML and normal karyotype, t(15;17), t(8;21) or inv(16) Hematol J. 2002;3:283–289. doi: 10.1038/sj.thj.6200196. [DOI] [PubMed] [Google Scholar]
- 41.Schnittger S, Schoch C, Kern W, et al. Nucleophosmin gene mutations are predictors of favorable prognosis in acute myelogenous leukemia with a normal karyotype. Blood. 2005;106:3733–3739. doi: 10.1182/blood-2005-06-2248. [DOI] [PubMed] [Google Scholar]
- 42.Smith TJ, Khatcheressian J, Lyman GH, et al. 2006 update of recommendations for the use of white blood cell growth factors: an evidence-based clinical practice guideline. J Clin Oncol. 2006;24:3187–3205. doi: 10.1200/JCO.2006.06.4451. [DOI] [PubMed] [Google Scholar]
- 43.Pinto A, Zagonel V, Ferrara F. Acute myeloid leukemia in the elderly: biology and therapeutic strategies. Crit Rev Oncol Hematol. 2001;39:275–287. doi: 10.1016/s1040-8428(00)00122-0. [DOI] [PubMed] [Google Scholar]
- 44.Büchner T, Hiddemann W, Berdel W, et al. Acute myeloid leukemia: treatment over 60. Rev Clin Exp Hematol. 2002;6:46–59. doi: 10.1046/j.1468-0734.2002.00059.x. [DOI] [PubMed] [Google Scholar]
- 45.Juliusson G, Lazarevic V, Hoerstedt AS, Hagberg O, Hoeglund M. Swedish Acute Leukemia Registry Group. Acute myeloid leukemia in the real world: why population-based registries are needed. Blood. 2012;119:890–899. doi: 10.1182/blood-2011-12-379008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Juliusson G. Swedish AML Group. Most 70- to 79-year-old patients with acute myeloid leukemia do benefit from intensive treatment. Blood. 2011;117:3473–3474. doi: 10.1182/blood-2010-11-321737. [DOI] [PubMed] [Google Scholar]
- 47.Niederwieser D, Lange T, Cross M, Basara N, Al-Ali H. Reduced intensity conditioning (RIC) haematopoietic cell transplants in elderly patients with AML. Best Pract Res Clin Haematol. 2006;19:825–838. doi: 10.1016/j.beha.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 48.Rowe JM. Treatment of acute myelogenous leukemia in older adults. Leukemia. 2000;14:480–487. doi: 10.1038/sj.leu.2401539. [DOI] [PubMed] [Google Scholar]
- 49.Stone RM, Mandrekar SJ, Sanford BL, et al. Midostaurin plus Chemotherapy for Acute Myeloid Leukemia with a FLT3 Mutation. N Engl J Med. 2017 doi: 10.1056/NEJMoa1614359. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brune M, Castaigne S, Catalano J, et al. Improved leukemia-free survival after postconsolidation immunotherapy with histamine dihydrochloride and interleukin-2 in acute myeloid leukemia: results of a randomized phase 3 trial. Blood. 2006;108:88–96. doi: 10.1182/blood-2005-10-4073. [DOI] [PubMed] [Google Scholar]
- 51.Grimwade D, Walker H, Harrison G, et al. The predictive value of hierarchical cytogenetic classification in older adults with acute myeloid leukemia (AML): analysis of 1065 patients entered into the United Kingdom Medical Research Council AML11 trial. Blood. 2001;98:1312–1320. doi: 10.1182/blood.v98.5.1312. [DOI] [PubMed] [Google Scholar]
- 52.Moore JO, Dodge RK, Amrein PC, et al. Granulocyte-colony stimulating factor (filgrastim) accelerates granulocyte recovery after intensive postremission chemotherapy for acute myeloid leukemia with aziridinyl benzoquinone and mitoxantrone: Cancer and Leukemia Group B Study 9022. Blood. 1997;89:780–788. [PubMed] [Google Scholar]
- 53.Harousseau JL, Witz B, Lioure B, et al. Granulocyte colony-stimulating factor after intensive consolidation chemotherapy in acute myeloid leukemia: results of a randomized trial of the Groupe Ouest-Est Leucémies Aigues Myeloblastiques. J Clin Oncol. 2000;18:780–787. doi: 10.1200/JCO.2000.18.4.780. [DOI] [PubMed] [Google Scholar]
- 54.Jaramillo S, Benner A, Krauter J, et al. Condensed versus standard schedule of high-dose cytarabine consolidation therapy with pegfilgrastim growth factor support in acute myeloid leukemia. Blood. 2016;128:337. doi: 10.1038/bcj.2017.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.