Abstract
Introduction
Weight loss, and especially muscle loss, adversely affects skeletal health. The FRAX® tool considers baseline body mass index, but not body composition nor changes in its components over time. Our aim was to compare the independent associations between prior loss in DXA-estimated TBLM and TBFM and subsequent fracture risk.
Methods
We identified women and men age 40 years or older with two DXA assessments at least 1 year apart (median interval 3.3 years). TBLM and TBFM were estimated from weight, sex, and DXA of the spine and hip. Incident fractures and deaths were ascertained from linked population-based health services data after the date of the second DXA. Hazard ratios (HR) from Cox regression models were used to study time to fracture from prior loss in TBLM and TBFM adjusted for FRAX-related covariates.
Results
The study population consisted of 9,622 individuals (mean age 67 [SD 10] years, 95% female). We identified 692 subjects with incident major osteoporotic fracture [MOF] and 194 with hip fracture. Mean TBLM loss was significantly greater in those with incident MOF and hip fracture (P<0.001) while TBFM loss was only significantly greater in those with incident hip fracture (P<0.001). Each SD greater TBLM loss was associated with 10-13% increased MOF risk and 29-38% increased hip fracture risk, adjusted for TBFM loss and other covariates. Prior TBFM loss was not associated with fractures when adjusted for TBLM loss.
Conclusions
Prior loss in total body lean mass, but not in fat mass, is associated with increased fracture risk, particularly hip fracture, independent of other risk factors. This is consistent with the hypothesis that muscle loss (sarcopenia) adversely impacts skeletal health and fracture risk.
Keywords: Osteoporosis, Fractures, Body composition, Sarcopenia, Dual-energy x-ray absorptiometry, FRAX
Introduction
Clinical guidelines often recommend serial measurements of height and /or weight as part of routine fracture risk assessment (1, 2). The fracture risk assessment tool (FRAX®) is widely used to assess fracture risk and need for anti-osteoporosis therapy (3). Weight loss, and especially muscle loss, adversely affects skeletal health (4–10). FRAX accepts baseline height and weight as input variables but does not consider body composition nor changes in its components over time. Previously, we found that a single baseline measure of estimated total body lean mass (TBLM) and total body fat mass (TBFM) was associated with incident MOF and hip fracture risk when considered as univariate (unadjusted) predictors, but did not affect fracture risk after adjustment for baseline FRAX probability (11). We also showed that height loss was associated with increased risk for MOF and hip fracture independently from baseline fracture probability, whereas weight loss was only associated with increased risk for hip fracture (12).
The objective of the current study was to compare the association between subsequent fracture risk and prior loss in TBLM versus prior loss in TBFM estimated from DXA, adjusted for prior height loss, FRAX scores, FRAX risk factors and competing mortality. To address this question, we used a large clinical dual-energy x-ray absorptiometry (DXA) registry for the Province of Manitoba, Canada, where TBLM and TBFM have been estimated from a combination of DXA and clinical measurements. We evaluated the association between TBLM and TBFM loss on subsequent fracture risk adjusted for FRAX scores, FRAX risk factors and competing mortality.
Methods
Study Population
In the Canadian Province of Manitoba (population 1.3 million, 2017), DXA-based BMD testing is managed as an integrated clinical program (13). The program maintains a database of all DXA results, which can be linked with other provincial population-based computerized health databases through an anonymous personal identifier (database completeness and accuracy in excess of 99%) (14).
The study population consisted of all women and men age 40 years or older with two DXA scans of the lumbar spine and hip performed by the Program at least 1 year apart. The baseline DXA assessment (Visit 1) occurred between May 2004 and February 2015; the second assessment (Visit 2) occurred between April 2006 and March 2016. We excluded those without DXA of both the lumbar spine and hip, and those with other missing covariates. The study was approved by the Health Research Ethics Board for the University of Manitoba.
DXA and Body Composition Assessment
Spine and hip DXA scans were performed and analyzed in accordance with manufacturer recommendations. Femur neck T-scores were calculated from Third National Health and Nutrition Examination Surveys (NHANES III) white female reference values (15). The cross-calibrated instruments used for this study (Prodigy, iDXA, GE/Lunar Healthcare, Madison WI) exhibited stable long-term performance (coefficient of variation <0.5%).
TBLM and TBFM (kg) were estimated for Visit 1 and Visit 2 using weight, sex, and percent fat from lumbar spine and hip DXA (R2 = 0.84 and 0.94 vs total body DXA, respectively) (11). Loss in TBLM and TBFM between visits was expressed as both absolute (kg) and annualized (kg/year) measures.
FRAX and Relevant Covariates
We adjusted for multiple covariates (incorporated as FRAX® probability) assessed at Visit 2 that could affect fracture risk independent of BMD. Ten-year probability of a MOF and hip fracture was calculated using the Canadian FRAX tool (FRAX® Desktop Multi-Patient Entry, version 3.8) as recently described (12). The Canadian FRAX tool was calibrated using nationwide hip fracture and mortality data (16) and independently validated in the general population (18;19). Weight and height were measured and recorded at the time of DXA as weight (in kilograms, floor scale) and height (in meters, wall-mounted stadiometer).
Incident fracture ascertainment
Manitoba Health records were assessed for the presence of fracture diagnostic codes occurring after the Visit 2 BMD assessment (index date) up to March 31, 2017. Fractures were assessed through a combination of hospital discharge abstracts (diagnoses and procedures coded using the International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] prior to 2004 and International Classification of Diseases, Tenth Revision, Canadian Enhancements [ICD-10-CA] thereafter) and physician billing claims (coded using ICD-9-CM) using previously validated algorithms (17, 18). We analyzed incident major osteoporotic fractures (MOF; hip, clinical vertebral, forearm, and humerus) and hip fracture alone. We required that there be no hospitalization or physician visit(s) with the same fracture type in the six months preceding an incident fracture diagnosis. Fractures with high-trauma codes were excluded from the analysis.
Statistical Analysis
Statistical analyses were performed with Statistica (Version 13.0, StatSoft Inc, Tulsa, OK). Descriptive statistics are presented as mean ± SD for continuous variables or frequency (%) for categorical variables. Time to incident fracture following the Visit 2 DXA scan (index date) was estimated using Cox proportional hazards regression. Observations were censored for death, migration out of province, or end of follow up (March 31, 2017). Absolute TBLM and TBFM as continuous measures were the primary predictor variables (both included in the analytic models), with annualized change assessed as secondary predictor variables. Graphical analyses confirmed that these approximated a normal distribution. Models were sequentially adjusted for FRAX score without BMD, FRAX with BMD, and FRAX risk factors individually including BMD. Models that included competing mortality were also assessed (19). All models were also adjusted for prior height loss since this is easily assessed and has previously been associated with incident fracture risk (12). FRAX scores were log-transformed due to a skewed distribution. We also tested for a two-way interaction between age (stratified as < 65 years [referent], 65-79 years, > 80 years) and change in TBLM and TBFM in the model that adjusted for individual FRAX risk factors including BMD. Proportionality of hazards was confirmed by testing scaled Schoenfeld residuals versus time.
Results
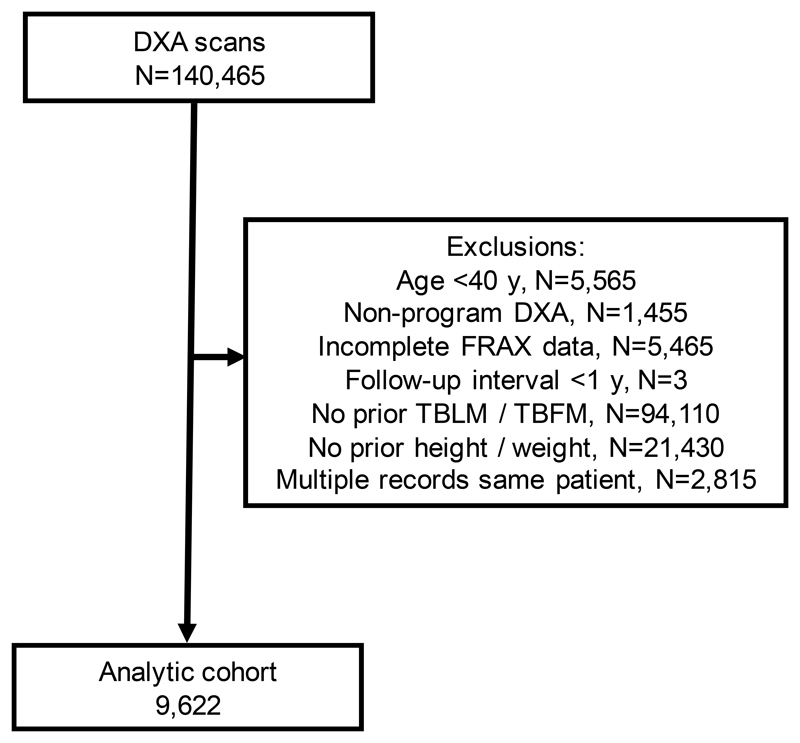
The population selection flowchart is shown in Figure 1. After exclusions, the final study population consisted of 9,622 individuals (mean age 67 [SD 10] years, 95% female) with paired measurements of estimated TBLM and TBFM (median interval 3.3 years, interquartile range 2.8-4.4 years) (Table 1). Fracture probability measured at the index date (Visit 2) was in the moderate range for MOF and was considered high for hip fracture risk, mean TBLM was 38.9 ± 5.8 kg and TBFM was 25.5 ± 9.9 kg. Mean decrease in TBLM between Visit 1 and Visit 2 was 0.61 ± 1.73 kg while there was a slight increase in TBFM of 0.05 ± 3.83 kg. Measured weight loss during the same time was 0.63 ± 5.04 kg. There was no significant correlations between baseline TBLM or TBFM with subsequent loss (r2 = 1.1% and 0.0%, respectively).
Figure 1. Population selection flowchart.
Table 1. Characteristics of the study cohort measured at the index date (Visit 2).
| Characteristic | All individuals |
|---|---|
| N=9,622 | |
| Age (years) | 67.0 ± 9.7 |
| Sex (female) | 9,112 (94.7) |
| Prior fracture | 1,748 (18.2) |
| Femoral neck T-score | -1.6 ± 0.8 |
| Height (cm) | 160.6 ± 7.1 |
| Weight (kg) | 67.2 ± 14.3 |
| FRAX MOF percent (without BMD) | 13.8 ± 9.3 |
| FRAX hip percent (without BMD) | 4.6 ± 6.5 |
| FRAX MOF percent (with BMD) | 12.0 ± 7.4 |
| FRAX hip percent (with BMD) | 3.1 ± 4.6 |
| Total Body Lean Mass (kg) | 38.9 ± 5.8 |
| Total Body Fat Mass (kg) | 25.5 ± 9.9 |
| Total Body Lean Mass, change (kg) | -0.61 ± 1.73 |
| Total Body Fat Mass, change (kg) | +0.05 ± 3.82 |
| Total Body Lean Mass, change per year (kg/y) | -0.17 ± 0.60 |
| Total Body Fat Mass, change per year (kg/y) | +0.02 ± 1.28 |
Data expressed as mean (SD) or N (percent). MOF, major osteoporotic fracture. BMD, bone mineral density.
During median follow up of 6 years (interquartile range 5-7 years) we identified 692 individuals with one or more MOF of which 194 experienced incident hip fracture. There were 726 (7.5%) who died and 239 (2.5%) who moved and were lost to follow up without sustaining MOF before the censoring date. Table 2 demonstrates the univariate association between change in TBLM and TBFM according to fracture status. There was a significantly greater reduction in TBLM (absolute and annualized) for individuals sustaining incident MOF or incident hip fracture. Change in TBFM was not associated with incident MOF, but loss in TBFM was associated with incident hip fracture.
Table 2. Unadjusted mean (± standard deviation) change in Total Body Lean Mass (TBLM) and Total Body Fat Mass (TBFM) according to incident fracture status.
| No Fracture | Fracture | p-value * | |
|---|---|---|---|
| Incident MOF | 8,940 | 682 | |
| Lean mass loss (kg) | -0.60 ± 1.73 | -0.77 ± 1.73 | 0.012 |
| Fat mass loss (kg) | +0.06 ± 3.82 | -0.19 ± 3.92 | 0.102 |
| Lean mass loss rate (kg/y) | -0.16 ± 0.6 | -0.23 ± 0.59 | 0.003 |
| Fat mass loss rate (kg/y) | +0.03 ± 1.27 | -0.06 ± 1.34 | 0.096 |
|
| |||
| Incident hip | 9,432 | 190 | |
| Lean mass loss (kg) | -0.60 ± 1.72 | -1.20 ± 2.05 | <0.001 |
| Fat mass loss (kg) | +0.07 ± 3.81 | -1.24 ± 4.34 | <0.001 |
| Lean mass loss rate (kg/y) | -0.16 ± 0.60 | -0.32 ± 0.59 | <0.001 |
| Fat mass loss rate (kg/y) | +0.03 ± 1.28 | -0.34 ± 1.22 | <0.001 |
Student t-test, No fracture vs Fracture. MOF, major osteoporotic fracture. Statistically significant (α = .05) effects in boldface.
Cox multivariable regression analyses were performed to assess the independent effects of prior TBLM and TBFM loss with both measures included in the analytic models (Table 3). There was greater risk of mortality during follow-up in those with prior loss in TBLM (HR 1.08, 95% CI 1.02 – 1.15 per SD) or with prior loss in TBFM (HR 1.15, 95% CI 1.08 – 1.21 per SD), therefore we also tested models that considered the effect of competing mortality. Each SD decrease in TBLM was associated with a small increased risk for incident MOF (10 – 12%) when adjusted for TBFM loss, height loss, and additionally for fracture probability or FRAX risk factors. These results were not significantly altered by considering competing mortality. TBLM loss was a stronger risk factor for incident hip fracture (29 – 38% increase per SD) and again showed a similar effect size in all of the models. In contrast, TBFM loss was not an independent risk factor for incident MOF or incident hip fracture adjusted for TBLM loss and other covariates. No significant interaction was detected between age and change in TBLM and TBFM on incident MOF or hip fracture adjusted for individual FRAX risk factors including BMD (all p-interaction > 0.1). Results were similar when TBLM loss and TBFM loss were expressed as annualized measures (Table 4) and when prior BMD loss was included as an additional covariate (data not shown).
Table 3. Multivariable adjusted hazard ratios (HR) per standard deviation (SD) with 95% confidence interval (CI) for incident fracture according to prior loss in Total Body Lean Mass (TBLM) and Total Body Fat Mass (TBFM).
| Incident MOF | Incident Hip | |||
|---|---|---|---|---|
| TBLM loss | TBFM loss | TBLM loss | TBFM loss | |
| Adjusted for: | HR per SD (95% CI) | HR per SD (95% CI) | HR per SD (95% CI) | HR per SD (95% CI) |
| FRAX without BMD, no competing mortality |
1.11
(1.00-1.22) |
0.92 (0.83-1.01) |
1.32
(1.11-1.57) |
1.03 (0.86-1.22) |
| FRAX with BMD, no competing mortality |
1.12
(1.02-1.24) |
0.93 (0.85-1.02) |
1.36
(1.15-1.62) |
1.05 (0.89-1.25) |
| FRAX without BMD, includes competing mortality | 1.10 (1.00-1.21) |
0.91
(0.83-1.00) |
1.29
(1.09-1.54) |
1.01 (0.85-1.21) |
| FRAX with BMD, includes competing mortality |
1.11
(1.01-1.22) |
0.92 (0.84-1.01) |
1.34
(1.13-1.59) |
1.03 (0.87-1.23) |
| FRAX risk factors individually with BMD |
1.13
(1.03-1.25) |
0.94 (0.86-1.03) |
1.38
(1.16-1.64) |
1.06 (0.90-1.25) |
Results from Cox regression models; height loss included as a covariate in all models. Statistically significant (α = .05) effects in boldface. MOF, major osteoporotic fracture. BMD, bone mineral density (femur neck). FRAX hip fracture probability was used for covariate adjustment in the incident hip fracture analysis; FRAX MOF probability was used for all other incident fracture analyses.
Table 4. Multivariable adjusted hazard ratios (HR) with 95% confidence intervals (CI) for incident fracture according to prior annualized loss in Total Body Lean Mass (TBLM) and Total Body Fat Mass (TBFM).
| Incident MOF | Incident Hip | |||
|---|---|---|---|---|
| TBLM loss | TBFM loss | TBLM loss | TBFM loss | |
| Adjusted for: | HR per SD (95% CI) | HR per SD (95% CI) | HR per SD (95% CI) | HR per SD (95% CI) |
| FRAX without BMD, no competing mortality |
1.05
(1.01-1.09) |
0.98 (0.92-1.04) |
1.30
(1.15-1.46) |
0.89 (0.75-1.07) |
| FRAX with BMD, no competing mortality |
1.06
(1.02-1.10) |
0.99 (0.94-1.05) |
1.31
(1.16-1.48) |
0.91 (0.76-1.08) |
| FRAX without BMD, includes competing mortality |
1.04
(1.00-1.08) |
0.96 (0.91-1.02) |
1.28
(1.14-1.42) |
0.88 (0.75-1.04) |
| FRAX with BMD, includes competing mortality | 0.98 (0.96-1.01) |
0.97 (0.92-1.03) |
1.29
(1.16-1.44) |
0.90 (0.76-1.05) |
| FRAX risk factors individually with BMD |
1.07
(1.02-1.11) |
1.00 (0.94-1.06) |
1.31
(1.16-1.47) |
0.91 (0.77-1.09) |
Results from Cox regression models; height loss included as a covariate in all models. Statistically significant (α = .05) effects in boldface. MOF, major osteoporotic fracture. BMD, bone mineral density (femur neck). FRAX hip fracture probability was used for covariate adjustment in the incident hip fracture analysis; FRAX MOF probability was used for all other incident fracture analyses.
Discussion
This longitudinal registry-based cohort analysis found that decreasing TBLM was a modest but significant risk factor for incident MOF independent of fracture probability or FRAX risk factors including BMD, with a larger magnitude of association forincident hip fracture. In contrast, TBFM loss was not an independent risk factor for any fracture type, including hip fracture. This implies that FRAX may underestimate fracture risk, particularly hip fracture risk, in individuals losing TBLM. The current report complements previous work that did not find a FRAX-independent association between a single baseline assessment of estimated TBLM or TBFM on subsequent fracture risk (11). In contrast baseline appendicular lean mass was weakly predictive of incident fracture risk independently of FRAX probability in the MrOS cohorts, but this association was removed by adjustment for femoral neck BMD (20).
Indeed, most studies to date examining the association between body composition and fracture risk have been based upon a single assessment (21). Some groups have also reported that greater TBLM is protective against fracture risk, but does not improve prediction when combined with BMD (22), whereas others have found BMD-independent reduction in fracture risk associated with greater TBLM or appendicular lean mass (23). The prospective population-based Rotterdam cohort study found a greater prevalence of fractures in individuals with lower appendicular lean mass, but this was no longer significant after correcting for age and sex (24). “Dysmobility Syndrome”, which includes low appendicular lean mass index as a component of the definition, has been associated with increased fracture risk (25). Various sarcopenia definitions were compared for prediction of incident hip fracture, but did not significantly improve fracture discrimination compared with a reference model (age and bone density) (26). Reduced measures of physical performance (but not appendicular lean mass index) was associated with increased fracture risk independent of baseline femoral neck BMD (20). TBLM assessed at a single visit was not significantly associated with hip fracture risk in 1,978 women age 50 years and older (27). Mortality risk is greater in those with declining weight, lean mass and fat mass as we noted in our study (28).
The mechanism underlying the association of TBLM loss with fracture cannot be directly ascertained from this study. Increased risk for falls is a likely candidate (29), but prospective falls data are not available for our cohort. TBLM is also strongly associated with BMD (11), and a reduction in BMD could be a contributing factor though results were adjusted for BMD at Visit 2 (index date) at the end of the period during which TBLM loss was assessed. Interestingly, TBFM loss was not associated with fracture risk, including hip fracture risk, which would argue against a simple effect from weight loss alone. Decreasing TBLM could reflect a decline in muscle mass (a component of sarcopenia), though DXA-measured lean mass in an imperfect measure of muscle mass (30). Furthermore, we did not have any objective measures of muscle strength (e.g., grip strength) or muscle function (e.g., walking time).
Strengths of our study include the large population size, long-term follow up and large number of clinical fracture events observed. Limitations include reliance on linked administrative data for ascertainment of fractures, although the procedures used have been directly validated against x-ray confirmed fractures and adopted for a national osteoporosis surveillance program (17, 18, 31). As a clinical registry, referral bias in baseline and subsequent DXA testing is to be expected. However, our cohort selection likely reflects routine clinical practice and therefore complements previous population-based cohort studies. We were unable to determine how much of the decrease in lean mass relates to muscle versus non-muscle compartments. Lifestyle factors, including diet and exercise, are unavailable through administrative data. The study cohort was over ~95% women and ~98% of European ancestry, and it was therefore not possible to study subgroup differences related to sex or race/ethnicity. Finally, we do not have information on additional changes in body composition following the index date (Visit 2) which might further contribute to fracture risk (especially progressive loss in TBLM), though from a clinical perspective future information cannot be used for clinical decision making.
In summary, change in body composition, and more specifically a loss of TBLM, is associated with increased MOF and hip fracture risk. Moreover, this increased risk is independent of fracture probability measurements generated by FRAX and individual FRAX risk factors including BMD. In contrast, loss in TBFM did not increase risk for fracture. This highlights the importance of maintaining healthy body weight, composition and muscle mass in order to reduce fracture risk.
Supplementary Material
Summary.
During median follow-up 6.0 years in 9,622 individuals, prior loss in estimated total body lean mass (TBLM), but not total body fat mass loss (TBFM), was associated with increased fracture risk, particularly for hip fracture.
Acknowledgments
The authors acknowledge the Manitoba Centre for Health Policy for use of data contained in the Population Health Research Data Repository (HIPC 2016/2017-29). The results and conclusions are those of the authors and no official endorsement by the Manitoba Centre for Health Policy, Manitoba Health, Seniors and Active Living, or other data providers is intended or should be inferred. This article has been reviewed and approved by the members of the Manitoba Bone Density Program Committee.
Funding
No funding support was received for this research. SNM is chercheur-boursier des Fonds de Recherche du Québec en Santé. LML is supported by a Tier I Canada Research Chair.
Footnotes
Disclosures:
John Schousboe: Nothing to declare in context of this paper; ad hoc consultancy to Hologic, Inc.
Suzanne Morin: Nothing to declare for the context of this paper, but has received research grants: Amgen.
Eugene McCloskey: Nothing to declare for the context of this paper, but numerous ad hoc consultancies/ speaking honoraria and/or research funding from Amgen, Bayer, General Electric, GSK, Hologic, Lilly, Merck Research Labs, Novartis, Novo Nordisk, Nycomed, Ono, Pfizer, ProStrakan, Roche, Sanofi-Aventis, Servier, Tethys, UBS and Warner-Chilcott
Nicholas Harvey: Nothing to declare for the context of this paper, but has received consultancy/ lecture fees/ honoraria/ grant funding from Alliance for Better Bone Health, Amgen, MSD, Eli Lilly, Servier, Shire, Consilient Healthcare, Radius Health, UCB, Kyowa Kirin and Internis Pharma.
John A. Kanis: Grants from Amgen, Lilly, Radius Health and non-financial support from Medimaps outside the submitted work
William Leslie, Patrick Martineau, Lisa Lix, Helena Johansson: No conflicts of interest.
Contributor Information
John T. Schousboe, Email: scho0600@umn.edu.
Suzanne N. Morin, Email: suzanne.morin@mcgill.ca.
Patrick Martineau, Email: pmartineau@manitoba-physicians.ca.
Lisa M. Lix, Email: Lisa.Lix@umanitoba.ca.
Helena Johansson, Email: helena@statiq.se.
Eugene V. McCloskey, Email: e.v.mccloskey@sheffield.ac.uk.
Nicholas C. Harvey, Email: nch@mrc.soton.ac.uk.
John A. Kanis, Email: jakanis@outlook.com.
References
- 1.Papaioannou A, Morin S, Cheung AM, Atkinson S, Brown JP, Feldman S, et al. 2010 clinical practice guidelines for the diagnosis and management of osteoporosis in Canada: summary. CMAJ. 2010;182(17):1864–73. doi: 10.1503/cmaj.100771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cosman F, de Beur SJ, LeBoff MS, Lewiecki EM, Tanner B, Randall S, et al. Clinician's Guide to Prevention and Treatment of Osteoporosis. Osteoporos Int. 2014;25(10):2359–81. doi: 10.1007/s00198-014-2794-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kanis JA, Oden A, Johansson H, Borgstrom F, Strom O, McCloskey E. FRAX and its applications to clinical practice. Bone. 2009;44(5):734–43. doi: 10.1016/j.bone.2009.01.373. [DOI] [PubMed] [Google Scholar]
- 4.Ensrud KE, Ewing SK, Stone KL, Cauley JA, Bowman PJ, Cummings SR, et al. Intentional and unintentional weight loss increase bone loss and hip fracture risk in older women. J Am Geriatr Soc. 2003;51(12):1740–7. doi: 10.1046/j.1532-5415.2003.51558.x. [DOI] [PubMed] [Google Scholar]
- 5.Zibellini J, Seimon RV, Lee CM, Gibson AA, Hsu MS, Shapses SA, et al. Does Diet-Induced Weight Loss Lead to Bone Loss in Overweight or Obese Adults? A Systematic Review and Meta-Analysis of Clinical Trials. J Bone Miner Res. 2015;30(12):2168–78. doi: 10.1002/jbmr.2564. [DOI] [PubMed] [Google Scholar]
- 6.Compston JE, Wyman A, FitzGerald G, Adachi JD, Chapurlat RD, Cooper C, et al. Increase in Fracture Risk Following Unintentional Weight Loss in Postmenopausal Women: The Global Longitudinal Study of Osteoporosis in Women. J Bone Miner Res. 2016;31(7):1466–72. doi: 10.1002/jbmr.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson KC, Bray GA, Cheskin LJ, Clark JM, Egan CM, Foreyt JP, et al. The Effect of Intentional Weight Loss on Fracture Risk in Persons With Diabetes: Results From the Look AHEAD Randomized Clinical Trial. J Bone Miner Res. 2017;32(11):2278–87. doi: 10.1002/jbmr.3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kammire DE, Walkup MP, Ambrosius WT, Lenchik L, Shapses SA, Nicklas BJ, et al. Effect of Weight Change Following Intentional Weight Loss on Bone Health in Older Adults with Obesity. Obesity (Silver Spring) 2019 doi: 10.1002/oby.22604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Y, Hao Q, Ge M, Dong B. Association of sarcopenia and fractures in community-dwelling older adults: a systematic review and meta-analysis of cohort studies. Osteoporos Int. 2018 doi: 10.1007/s00198-018-4429-5. [DOI] [PubMed] [Google Scholar]
- 10.Wong RMY, Wong H, Zhang N, Chow SKH, Chau WW, Wang J, et al. The relationship between sarcopenia and fragility fracture-a systematic review. Osteoporos Int. 2019;30(3):541–53. doi: 10.1007/s00198-018-04828-0. [DOI] [PubMed] [Google Scholar]
- 11.Leslie WD, Orwoll ES, Nielson CM, Morin SN, Majumdar SR, Johansson H, et al. Estimated lean mass and fat mass differentially affect femoral bone density and strength index but are not FRAX independent risk factors for fracture. J Bone Miner Res. 2014;29(11):2511–9. doi: 10.1002/jbmr.2280. [DOI] [PubMed] [Google Scholar]
- 12.Leslie WD, Schousboe JT, Morin SN, Martineau P, Lix LM, Johansson H, et al. Measured height loss predicts incident clinical fractures independently from FRAX: a registry-based cohort study. Osteoporos Int. 2020 doi: 10.1007/s00198-020-05313-3. [DOI] [PubMed] [Google Scholar]
- 13.Leslie WD, Metge C. Establishing a regional bone density program: lessons from the Manitoba experience. J Clin Densitom. 2003;6(3):275–82. doi: 10.1385/jcd:6:3:275. [DOI] [PubMed] [Google Scholar]
- 14.Leslie WD, Caetano PA, Macwilliam LR, Finlayson GS. Construction and validation of a population-based bone densitometry database. J Clin Densitom. 2005;8(1):25–30. doi: 10.1385/jcd:8:1:025. [DOI] [PubMed] [Google Scholar]
- 15.Looker AC, Wahner HW, Dunn WL, Calvo MS, Harris TB, Heyse SP, et al. Updated data on proximal femur bone mineral levels of US adults. Osteoporos Int. 1998;8(5):468–89. doi: 10.1007/s001980050093. [DOI] [PubMed] [Google Scholar]
- 16.Leslie WD, Lix LM, Langsetmo L, Berger C, Goltzman D, Hanley DA, et al. Construction of a FRAX(R) model for the assessment of fracture probability in Canada and implications for treatment. Osteoporos Int. 2011;22(3):817–27. doi: 10.1007/s00198-010-1464-2. [DOI] [PubMed] [Google Scholar]
- 17.Lix LM, Azimaee M, Osman BA, Caetano P, Morin S, Metge C, et al. Osteoporosis-related fracture case definitions for population-based administrative data. BMC Public Health. 2012;12:301. doi: 10.1186/1471-2458-12-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Epp R, Alhrbi M, Ward L, Leslie WD. Radiological validation of fracture definitions from administrative data. J Bone Miner Res. 2018;33(Supp 1):S275. [Google Scholar]
- 19.Leslie WD, Lix LM, Wu X, Manitoba Bone Density P Competing mortality and fracture risk assessment. Osteoporos Int. 2013;24(2):681–8. doi: 10.1007/s00198-012-2051-5. [DOI] [PubMed] [Google Scholar]
- 20.Harvey NC, Oden A, Orwoll E, Lapidus J, Kwok T, Karlsson MK, et al. Measures of physical performance and muscle strength as predictors of fracture risk independent of FRAX, falls, and aBMD: A meta-analysis of the Osteoporotic Fractures in Men (MrOS) Study. J Bone Miner Res. 2018;33(12):2150–7. doi: 10.1002/jbmr.3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Padilla Colon CJ, Molina-Vicenty IL, Frontera-Rodriguez M, Garcia-Ferre A, Rivera BP, Cintron-Velez G, et al. Muscle and Bone Mass Loss in the Elderly Population: Advances in diagnosis and treatment. J Biomed (Syd) 2018;3:40–9. doi: 10.7150/jbm.23390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cronholm F, Rosengren BE, Nilsson JA, Ohlsson C, Mellstrom D, Ribom E, et al. The fracture predictive ability of a musculoskeletal composite score in old men - data from the MrOs Sweden study. BMC Geriatr. 2019;19(1):90. doi: 10.1186/s12877-019-1106-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sornay-Rendu E, Duboeuf F, Boutroy S, Chapurlat RD. Muscle mass is associated with incident fracture in postmenopausal women: The OFELY study. Bone. 2017;94:108–13. doi: 10.1016/j.bone.2016.10.024. [DOI] [PubMed] [Google Scholar]
- 24.Trajanoska K, Schoufour JD, Darweesh SK, Benz E, Medina-Gomez C, Alferink LJ, et al. Sarcopenia and Its Clinical Correlates in the General Population: The Rotterdam Study. J Bone Miner Res. 2018;33(7):1209–18. doi: 10.1002/jbmr.3416. [DOI] [PubMed] [Google Scholar]
- 25.Buehring B, Hansen KE, Lewis BL, Cummings SR, Lane NE, Binkley N, et al. Dysmobility Syndrome Independently Increases Fracture Risk in the Osteoporotic Fractures in Men (MrOS) Prospective Cohort Study. J Bone Miner Res. 2018;33(9):1622–9. doi: 10.1002/jbmr.3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cawthon PM, Blackwell TL, Cauley J, Kado DM, Barrett-Connor E, Lee CG, et al. Evaluation of the Usefulness of Consensus Definitions of Sarcopenia in Older Men: Results from the Observational Osteoporotic Fractures in Men Cohort Study. J Am Geriatr Soc. 2015;63(11):2247–59. doi: 10.1111/jgs.13788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McLean RR, Kiel DP, Berry SD, Broe KE, Zhang X, Cupples LA, et al. Lower Lean Mass Measured by Dual-Energy X-ray Absorptiometry (DXA) is Not Associated with Increased Risk of Hip Fracture in Women: The Framingham Osteoporosis Study. Calcif Tissue Int. 2018;103(1):16–23. doi: 10.1007/s00223-017-0384-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee CG, Boyko EJ, Nielson CM, Stefanick ML, Bauer DC, Hoffman AR, et al. Mortality risk in older men associated with changes in weight, lean mass, and fat mass. J Am Geriatr Soc. 2011;59(2):233–40. doi: 10.1111/j.1532-5415.2010.03245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Balogun S, Winzenberg T, Wills K, Scott D, Jones G, Aitken D, et al. Prospective associations of low muscle mass and function with 10-year falls risk, incident fracture and mortality in community-dwelling older adults. The journal of nutrition, health & aging. 2017;21(7):843–8. doi: 10.1007/s12603-016-0843-6. [DOI] [PubMed] [Google Scholar]
- 30.Buehring B, Siglinsky E, Krueger D, Evans W, Hellerstein M, Yamada Y, et al. Comparison of muscle/lean mass measurement methods: correlation with functional and biochemical testing. Osteoporos Int. 2018;29(3):675–83. doi: 10.1007/s00198-017-4315-6. [DOI] [PubMed] [Google Scholar]
- 31.O'Donnell S, Canadian Chronic Disease Surveillance System Osteoporosis Working G Use of administrative data for national surveillance of osteoporosis and related fractures in Canada: results from a feasibility study. Arch Osteoporos. 2013;8:143. doi: 10.1007/s11657-013-0143-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.