Abstract
Purpose
Some have questioned the usefulness of distinguishing high-trauma fractures from low-trauma fractures.
Aim
To compare BMD measurements and risk of subsequent low-trauma fracture in patients with prior high- or low-trauma fractures.
Methods
Using a clinical BMD registry for the Province of Manitoba, Canada we identified women and men age 40 years or older with fracture records from linked population-based healthcare data. Age- and sex-adjusted BMD Z-scores and covariate-adjusted hazard ratios (HR) with 95% confidence intervals (CI) for incident fracture were studied in relation to prior fracture status, categorized as high-trauma if associated with external injury codes and low-trauma otherwise.
Results
The study population consisted of 64,428 women and men with no prior fracture (mean age 63.7 years), 858 with prior high-trauma fractures (mean age 65.1 years), and 14,758 with prior low-trauma fractures (mean age 67.2 years). Mean Z-scores for those with any prior high-trauma fracture were significantly lower than in those without prior fracture (P < 0.001) and similar to those with prior low-trauma fracture. Median observation time for incident fractures was 8.8 years (total 729,069 person-years). Any prior high-trauma fracture was significantly associated with increased risk for incident major osteoporotic fracture (MOF) (adjusted HR 1.31, 95% CI 1.08–1.59) as was prior low-trauma fracture (adjusted HR 1.55, 95% CI 1.47–1.63), and there were no significant difference between the two groups (prior trauma versus low-trauma fracture P=0.093). A similar pattern was seen when incident MOF was studied in relation to prior hip fracture or prior MOF, or when the outcome was incident hip fracture or any incident fracture.
Conclusions
High-trauma and low-trauma fractures showed similar relationships with low BMD and future fracture risk. This supports the inclusion of high-trauma fractures in clinical assessment for underlying osteoporosis and in the evaluation for intervention to reduce future fracture risk.
Keywords: Osteoporosis, Fractures, Trauma, Dual-energy x-ray absorptiometry
Introduction
Osteoporosis is characterized by low bone mass and deterioration of bone tissue, leading to an increase in bone fragility and susceptibility to fracture, with substantial health consequences for the individual and society (1). Historically, the fractures that have been typically associated with osteoporosis are designated as fragility fractures (also known as low-energy or low-trauma) (2–5), though the US National Osteoporosis Foundation (NOF) guidelines have noted that most fractures in older adults are due at least in part to low bone mass, even when they result from considerable trauma (2).
Features that distinguish a low-trauma from a high-trauma fracture event are inherently arbitrary, often described as a fracture that occurs spontaneously or with minimal force (e.g., fall from standing height or less). Although simple in concept, empirical data have questioned whether distinguishing low-trauma from high-trauma fractures is clinically useful for purposes of risk assessment and treatment (6–8).
To address this question, we used a large clinical registry of patients with BMD data and linked fracture information for the province of Manitoba, Canada. Previous fractures were identified from administrative data and high-trauma events ascertained from the presence of external injury codes in association with the fracture codes. We tested the hypotheses that BMD would be normal for sex and age in those with high-trauma fractures (defined as fractures with external injury codes) but reduced in those with low-trauma fractures (defined as fractures without external injury codes), and that high-trauma fractures would not be associated with increased risk for future fractures whereas low-trauma fractures would predict increased risk for future fractures.
Methods
Study Population
In the Canadian Province of Manitoba (population 1.3 million in 2017), health services are provided to virtually all residents through a public healthcare system. DXA-based BMD testing has been managed as an integrated clinical program since 1997; criteria for testing have been published and include screening at age 65 years for women and in men and younger women with additional risk factors (9). The program maintains a database of all DXA results which can be linked with other provincial population-based computerized health databases through an anonymous personal identifier. The DXA database has completeness and accuracy in excess of 99% (10).
The study population consisted of all women and men age 40 years or older with baseline DXA scans from January 1, 1996 to March 31, 2016. We excluded those not registered for health care in Manitoba and without coverage after the baseline BMD. For those with more than one qualifying examination, only the first was included. The study was approved by the Health Research Ethics Board for the University of Manitoba.
Fracture ascertainment
Manitoba Health records for the study population were assessed for the presence of fracture diagnostic codes prior to BMD assessment. Fractures were assessed through a combination of hospital discharge abstracts (diagnoses and procedures coded using the International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] prior to 2004 and International Classification of Diseases, Tenth Revision, Canadian Enhancements [ICD-10-CA] thereafter) and physician billing claims (coded using ICD-9-CM) examining medical records back to 1987 using previously validated algorithms (11, 12). Analyses were based upon hip, clinical vertebral, forearm, and humerus fracture diagnostic codes (collectively designated “major osteoporotic fractures”, MOF) and any fracture (excluding head/neck, hands, feet and ankle). Similar definitions were used to identify incident fractures occurring after BMD assessment up to March 31, 2017, except that incident fractures with high-trauma codes were conservatively excluded due to the concern that behaviours predisposing to traumatic events might confound the assessment for osteoporosis-related events. Secondary analyses were performed to examine all incident fractures (low-trauma and high-trauma combined) and only high-trauma incident fractures. To minimize potential misclassification of prior incident fractures, we conservatively required that there be no hospitalization or physician visit(s) with the same fracture type in the six months preceding an incident fracture diagnosis.
Fractures were coded according to the presence of accompanying external injury codes (designated high-trauma fracture) versus absence of accompanying external injury codes (designated low-trauma fracture). The external injury codes (“E” codes from ICD-9-CM and “X-V-W” codes from ICD-10-CA) are summarized in Supplemental Table 1. If a patient’s records showed fractures of both types, then they were categorized as having sustained a low-trauma fracture. The external injury codes are only available from the hospital data, and traumatic events insufficient to produce hospitalization would not be identified. Hospital diagnosis coding (captured in the Discharge Abstract Database) is performed by professional medical abstract coders based upon a thorough review of the entire hospitalization record. The Canadian Institute for Health Information administers a Data and Information Quality Program the ensure accuracy of the DAD (https://www.cihi.ca/en/discharge-abstract-database-metadata, https://www.cihi.ca/en/submit-data-and-view-standards/data-and-information-quality).
Bone Mineral Density Measurements
Hip and spine DXA scans were performed and analyzed in accordance with manufacturer recommendations. Hip T-scores (number of SDs above or below young adult mean BMD) were calculated from NHANES III white female reference values (13). Spine T-scores were calculated from the manufacturer's white female reference values. BMD Z-scores were age- and sex-adjusted. The program’s quality assurance is under strict supervision by a medical physicist (9). The six cross-calibrated instruments used for this study (1 DPX, 3 Prodigy and 3 iDXA, GE/Lunar Healthcare, Madison WI) exhibited equivalent phantom calibration and stable long-term performance (coefficient of variation <0.5%). BMD T-scores and Z-scores from the instruments were all based upon the same reference databases. All reporting physicians and supervising technologists are required to maintain DXA certification with the International Society for Clinical Densitometry (ISCD).
Other Covariates
We considered multiple covariates that affect fracture risk independent of BMD: age, sex, body mass index (BMI), parental history of hip fracture, current smoking, long-term oral glucocorticoid use, rheumatoid arthritis diagnosis and high alcohol consumption (14). Weight and height were measured at the time of DXA, and BMI was calculated as weight (in kilograms) divided by height (in meters) squared. Other covariates were assessed using a combination of self-report at the time of DXA and hospital discharge abstracts, physician billing claims, and prescription drug records as previously described (15). Prolonged oral corticosteroid use (>90 days dispensed in the 1 year prior to DXA) was obtained from the provincial pharmacy system (16). Smoking and parental hip fracture was by self-report. High alcohol use was directly assessed from 2012 onwards and represented by a proxy variable in earlier years (alcohol substance abuse diagnosis codes). Finally, we also ascertained use of aromatase inhibitor and osteoporosis medications (>180 days dispensed in the year following DXA). Osteoporosis medications included oral or parenteral bisphosphonates (~90% of all osteoporosis medication use), raloxifene, denosumab, calcitonin, teriparatide or any systemic estrogen product. Area of residence (urban versus rural) and mean household income quintiles were defined as previously described (17–19). Mean household income for dissemination areas, the smallest geographic unit for which Census data are made available, was obtained from Canada Census public use files and subsequently used to define quintiles (five groupings of ~20 % of the population in each which are assigned an income quintile grouping from 1 to 5, stratified separately for urban and rural residency). For analytical purposes, these were combined as quintiles 1–2 (lower income quintiles) and 3–5 (higher income quintiles; reference category). The Johns Hopkins ACG® Case-Mix System (system 9) was used to develop an index of comorbidity (20). This system has been previously validated to predict premature mortality and use of medical services in Manitoba (21). Aggregated Diagnosis Groups™ (ADGs®) represent 32 comorbidity clusters of ICD diagnostic codes.
Statistical Analysis
Statistical analyses were performed with Statistica (Version 13.0, StatSoft Inc, Tulsa, OK). Descriptive statistics for demographic and baseline characteristics are presented as mean ± SD for continuous variables or frequency (%) for categorical variables. BMD Z-scores, which adjust for the effects of age and sex, were analyzed according to prior fracture status (no fracture, high-trauma and low-trauma). Mean Z-scores were compared using analysis of variance with Tukey’s test for post hoc pairwise comparisons. Cumulative incidence of fracture according to prior fracture status was constructed from Kaplan-Meier curves to time to first fracture. Curves were compared using the log-rank test. Time to incident fracture following the baseline DXA scan (index date) was studied using multivariable Cox proportional hazards regression. The exposure (predictors) were fractures occurring before the index date; outcomes were incident fractures occurring after the index date. Observations were censored at death, migration out of province (Manitoba Health registry file), or end of follow up (March 31, 2017). Models were adjusted for age, sex, BMI, parental hip fracture, smoking, glucocorticoid use, rheumatoid arthritis, other secondary causes of osteoporosis, high alcohol intake, femur neck BMD T-score, residency (rural versus urban), income level (lower versus higher), osteoporosis treatment, diagnosed breast cancer, aromatase inhibitor use, and comorbidity score. Proportionality of hazards was confirmed by testing scaled Schoenfeld residuals versus time. As a supplementary analyses, we also stratified analyses by sex and age (<65 years vs ≥65 years) and included two-way interaction terms to test for age-sex effect modification. Finally, we tested for differences in covariate-adjusted mortality according to prior fracture status (no fracture, high-trauma and low-trauma) and sensitivity of our findings to competing risk for death (22, 23).
Results
Table 1 summarizes baseline characteristics of the population according to prior fracture status. Individuals without prior fracture (N=64,626) tended to be younger, with higher femoral neck T-score and lower comorbidity score compared to those with any prior fracture. The 858 individuals with high-trauma fracture were slightly younger than the 14,758 with low-trauma fracture (65.1 ± 11.4 versus 67.2 ± 11.4 years, P<0.001) and less often female (77% versus 87.2%, P<0.001). Time since prior fracture did not differ for high-trauma fracture (median [interquartile range] 7 years [3-14]) versus low-trauma fracture (7 years [2-13]). Median age at fracture was also similar (high-trauma fracture 57 years [48-67] versus low-trauma fracture 58 years [50-68]).
Table 1. Baseline characteristics according to prior fracture status.
| Characteristic | No fracture | Any high-trauma fracture | Any low-trauma fracture |
|---|---|---|---|
| N=64,626 | N=858 | N=14,758 | |
| Age (years) | 63.7 ± 11.0 | 65.1 ± 11.4 *** | 67.2 ± 11.4 *** |
| Sex (female) | 58,540 (90.6) | 661 (77.0) *** | 12874 (87.2) *** |
| BMI (kg/m2) | 27.3 ± 8.4 | 27.4 ± 5.4 | 27.2 ± 6.8 |
| Parental hip fracture | 4850 (7.5) | 75 (8.7) | 1171 (7.9) |
| Smoking | 5954 (9.2) | 99 (11.5) * | 1873 (12.7) * |
| Glucocorticoid use | 3776 (5.8) | 48 (5.6) | 593 (4.0) |
| Rheumatoid arthritis | 1900 (2.9) | 29 (3.4) | 370 (2.5) |
| Secondary osteoporosis | 9410 (14.6) | 127 (14.8) | 2008 (13.6) |
| High alcohol use | 251 (0.4) | 15 (1.7) *** | 212 (1.4) |
| Femoral neck T-score | -1.3 ± 1.0 | -1.6 ± 1.0 *** | -1.7 ± 1.0 ** |
| Residency (rural vs urban) | 20,528 (31.8) | 358 (41.7) *** | 5040 (34.2) *** |
| Income (lower vs higher) | 21,794 (33.7) | 313 (36.5) | 5883 (39.9) * |
| Anti-osteoporosis medication use | 18,665 (28.9) | 274 (31.9) | 5292 (35.9) * |
| Breast cancer diagnosis | 4706 (7.3) | 38 (4.4) ** | 703 (4.8) |
| Aromatase inhibitor use | 2950 (4.6) | 25 (2.9) * | 364 (2.5) |
| Comorbidity score | 4.7 ± 2.7 | 5.7 ± 3.0 *** | 5.6 ± 2.9 |
Data expressed as mean (SD) or N (percent). BMI, body mass index. * P < 0.05, ** P < 0.01, *** P < 0.001; No fracture vs Trauma, Trauma vs No-trauma
Mean BMD Z-in scores individuals without previous fracture were all slightly positive (Table 2) whereas mean Z-scores for those with previous high-trauma fracture and low-trauma fracture were all negative. Mean Z-scores for those with any prior high-trauma fracture or any prior MOF were significantly less than in those with no prior fracture (P < 0.001) and were similar to those with prior low-trauma fracture (all P > 0.05). Similar results were seen when stratified by prior fracture site.
Table 2. Baseline bone mineral density Z-scores according to prior fracture status.
| No fracture | High-trauma fracture | Low-trauma fracture | |
|---|---|---|---|
| Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | |
| Any fracture | N=64,626 | N=858 | N=14758 |
| Femur neck | 0.06 (0.06:0.07) | -0.33 (-0.39:-0.26) *** | -0.25 (-0.27:-0.24) *** |
| Total hip | 0.26 (0.25:0.27) | -0.24 (-0.31:-0.16) *** | -0.16 (-0.18:-0.14) *** |
| Lumbar spine | 0.27 (0.25:0.28) | -0.18 (-0.29:-0.08) *** | -0.19 (-0.22:-0.17) *** |
|
| |||
| Any major osteoporotic fracture | N=68,095 | N=485 | N=11,662 |
| Femur neck | 0.05 (0.04:0.06) | -0.41 (-0.49:-0.32) *** | -0.28 (-0.29:-0.26) *** |
| Total hip | 0.25 (0.24:0.25) | -0.33 (-0.43:-0.23) *** | -0.19 (-0.21:-0.17) *** |
| Lumbar spine | 0.25 (0.24:0.26) | -0.25 (-0.38:-0.12) *** | -0.24 (-0.27:-0.21) *** |
|
| |||
| Hip fracture | N=78,704 | N=206 | N=1332 |
| Femur neck | 0.01 (0.01:0.02) | -0.74 (-0.88:-0.60) *** | -0.77 (-0.82:-0.73) *** |
| Total hip | 0.20 (0.19:0.21) | -0.79 (-0.94:-0.63) *** | -0.87 (-0.93:-0.82) *** |
| Lumbar spine | 0.19 (0.18:0.20) | -0.42 (-0.63:-0.21) *** | -0.41 (-0.51:-0.32) *** |
|
| |||
| Humerus fracture | N=77,872 | 74 | N=2296 |
| Femur neck | 0.01 (0.00:0.02) | -0.49 (-0.67:-0.31) *** | -0.27 (-0.31:-0.23) *** |
| Total hip | 0.19 (0.18:0.20) | -0.37 (-0.58:-0.15) ** | -0.16 (-0.21:-0.11) *** |
| Lumbar spine | 0.19 (0.18:0.20) | -0.06 (-0.42:0.30) | -0.22 (-0.28:-0.15) *** |
|
| |||
| Forearm fracture | N=73,318 | N=293 | N=6631 |
| Femur neck | 0.02 (0.02:0.03) | -0.27 (-0.37:-0.17) *** | -0.23 (-0.25:-0.21) *** |
| Total hip | 0.21 (0.20:0.22) | -0.16 (-0.29:-0.04) *** | -0.12 (-0.14:-0.09) *** |
| Lumbar spine | 0.22 (0.21:0.23) | -0.35 (-0.51:-0.18) *** | -0.26 (-0.30:-0.23) *** |
Post hoc Tukey test vs No fracture, * P < 0.05, ** P < 0.01, *** P < 0.001. High-trauma vs Low-trauma all non-significant. Number with prior high-trauma clinical vertebral fractures insufficient for analysis.
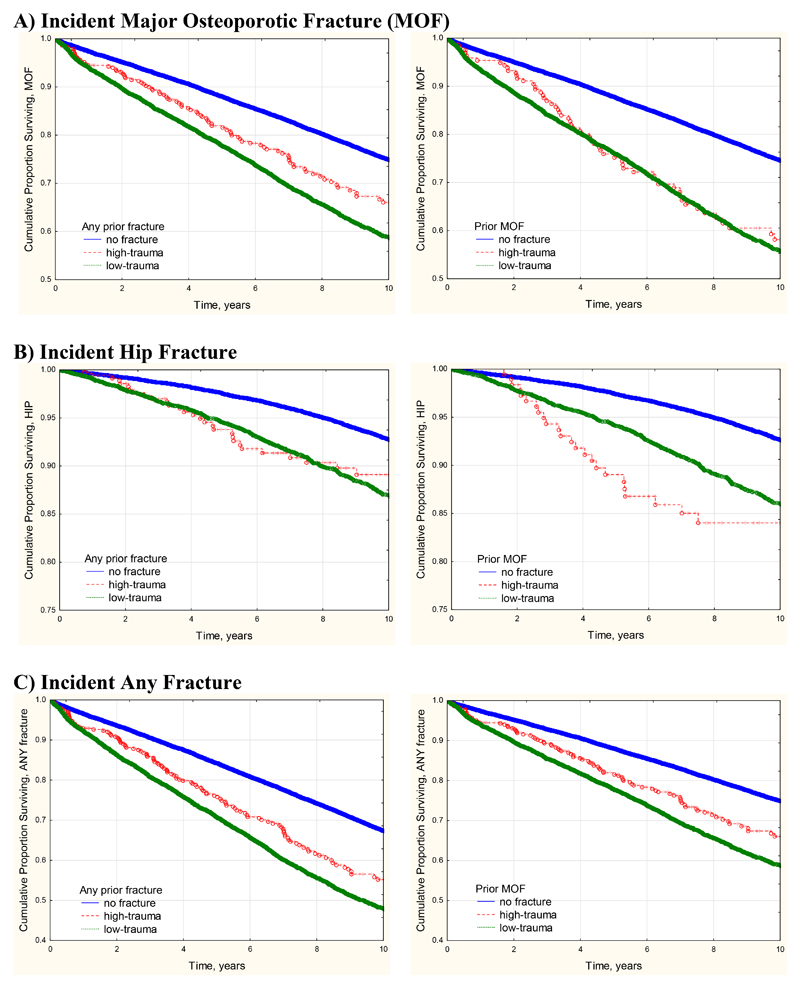
Median observation time for incident fractures was 8.8 years (interquartile range 4.8 – 13.3 years) with total observation time 729,069 person years. Incident fractures were identified in 10,595 (13.2%) based upon any fracture site, 8.063 (10.0%) based upon any MOF, and 2,498 (3.1%) based upon hip fracture. Frequency of incident fractures was significantly greater among those with prior high-trauma fracture versus no prior fracture (Table 3). Frequency of incident fractures was slightly greater among those with prior low-trauma fracture versus high-trauma fracture (statistically significant in two of six analyses). Kaplan-Meier plots of fracture-free survival showed statistically significant differences according to prior fracture status (all log-rank P < 0.001) (Figure 1). In general, curves for those with prior high-trauma fracture and low-trauma fracture tended to track each other more closely and were both significantly different from those with no prior fracture (P < 0.001). Cumulative incidence for MOF was slightly greater in those with any prior low-trauma versus high-trauma fracture (P = 0.035) but did not differ according to prior MOF (P = 0.295). There were similar findings for any incident fracture, but not for incident hip fracture.
Table 3. Frequency of incident fractures (%) according to prior fracture status.
| Prior fracture | Incident fracture | No fracture | High-trauma fracture | Low-trauma fracture | ||
|---|---|---|---|---|---|---|
| N (%) | N (%) | P vs No fracture | N (%) | P vs High-trauma | ||
| Any fracture | N=64,626 | N=858 | N=14758 | |||
| Major osteoporotic fracture | 5631 (8.7) | 108 (12.6) | <0.001 | 2324 (15.7) | 0.013 | |
| Hip | 1695 (2.6) | 41 (4.8) | <0.001 | 762 (5.2) | 0.620 | |
| Any fracture | 7571 (11.7) | 148 (17.2) | <0.001 | 3084 (20.9) | 0.010 | |
|
| ||||||
| Major osteoporotic fracture | N=64,626 | N=858 | N=14758 | |||
| Major osteoporotic fracture | 6047 (8.9) | 71 (14.6) | <0.001 | 1945 (16.7) | 0.237 | |
| Hip | 1836 (2.7) | 29 (6.0) | <0.001 | 633 (5.4) | 0.600 | |
| Any fracture | 8187 (12.0) | 92 (19.0) | <0.001 | 2524 (21.6) | 0.154 | |
Estimates in bold-face font are statistically significant at α = .05.
Figure 1. Kaplan-Meier curves for fracture-free survival according to number of self-reported falls in the previous year.
Cox regression models for fracture outcomes are summarized in Table 4. Adjusted for baseline covariates, the presence of any prior high-trauma fracture was significantly associated with increased risk for incident MOF (HR 1.31, 95% CI 1.08 – 1.59) as was prior low-trauma fracture (HR 1.55, 95% CI 1.47 – 1.63), whilst no significant difference was seen for prior trauma versus low-trauma fracture (P = 0.093). A similar pattern was seen when incident MOF was studied in relation to prior hip fracture or prior MOF, or when the outcome was incident hip fracture or any incident fracture. Although HRs for high-trauma fracture tended to be slightly lower than for low-trauma fracture, in no analysis was this statistically significant. Similar results were seen in secondary analyses that considered all incident fractures (low-trauma and high-trauma combined, Supplemental Table 2). When only high-trauma incident fractures were considered (Supplemental Table 3), prior low-trauma fractures continued to predict incident high-trauma fractures with point estimates that were similar to the primary analysis.
Table 4. Adjusted hazard ratios (HR) with 95% confidence intervals (CI) for incident fracture according to prior fracture status.
| Incident fracture | Prior fracture | High-trauma fracture | Low-trauma fracture | High-trauma fracture | |||
|---|---|---|---|---|---|---|---|
| Referent: No fracture | Referent: No fracture | Referent: Low-trauma fracture | |||||
| HR (95%CI) | P | HR (95%CI) | P | HR (95%CI) | P | ||
| Major osteoporotic fracture | Any fracture |
1.31
(1.08-1.59) |
0.005 |
1.55
(1.47-1.63) |
<0.001 | 0.85 (0.70-1.03) |
0.093 |
| Major osteoporotic fracture | Hip |
1.38
(1.01-1.88) |
0.043 |
1.18
(1.04-1.35) |
0.010 | 1.16 (0.83-1.63) |
0.372 |
| Major osteoporotic fracture | Major osteoporotic fracture |
1.48
(1.17-1.88) |
<0.001 |
1.57
(1.49-1.66) |
<0.001 | 0.94 (0.74-1.19) |
0.626 |
| Hip fracture | Any fracture | 1.35 (0.99-1.84) |
0.062 |
1.34
(1.23-1.47) |
<0.001 | 1.00 (0.73-1.37) |
0.988 |
| Any fracture | Any fracture |
1.39
(1.18-1.64) |
<0.001 |
1.61
(1.54-1.68) |
<0.001 | 0.87 (0.73-1.02) |
0.090 |
Results from Cox regression model. Adjusted for age, sex, body mass index, parental hip fracture, smoking, glucocorticoid use, rheumatoid arthritis, secondary causes of osteoporosis, high alcohol intake, femur neck T-score, rural vs urban residency, lower vs higher income, osteoporosis treatment, diagnosed breast cancer, aromatase inhibitor use, Johns Hopkins ADG comorbidity score. Estimates in bold-face font are statistically significant at α = .05.
Sex- and age-stratified analyses with test for interaction are reported in Supplemental Table 4 and Table 5, respectively. There were significant sex interactions for incident MOF related to any prior fracture or prior MOF, and for incident hip fracture related to any prior fracture. HRs for both high-trauma and low-trauma fractures tender to be greater in men than women. There were significant age interactions for incident MOF related to any prior fracture or prior MOF, for incident hip fracture related to any prior fracture, and for any incident fracture related to any prior fracture. In some analyses high-trauma fractures appeared to be relatively less important than low-trauma fractures in younger versus older subjects, but this was inconsistent and potentially limited by the lower fracture rates among those age < 65 years resulting in wide CIs in the HRs for high-trauma fractures.
Adjusted for all covariates, the presence of any prior high-trauma fracture was not associated with risk for death (HR 0.98, 95% CI 0.85 – 1.14) whilst death was increased after low-trauma fracture (HR 1.16, 95% CI 1.12 – 1.21). Including competing mortality in the analysis did not appreciably change our findings. Any prior high-trauma fracture was significantly associated with increased risk for any incident fracture without and with competing mortality (HR 1.39, 95% CI 1.18 – 1.64 vs HR 1.42, 95% CI 1.21 – 1.68, respectively) as was prior low-trauma fracture (HR 1.61, 95% CI 1.54 – 1.86 vs HR 1.57, 95% CI 1.51 – 1.64, respectively). Similarly, competing mortality did not alter results with MOF as the prior fracture or incident fracture (data not shown).
Discussion
In contradiction with our hypothesis, we found that BMD in individuals with high-trauma fractures was similarly reduced as in those with low-trauma fractures, and that high-trauma fractures also increased risk for future fractures similar to low-trauma fractures. Our findings complement and support similar previous results from cohort studies and randomized clinical trials (6–8).
Mackey et al (6) analyzed the data from large community-dwelling cohorts from the United States (8,022 women from the Study of Osteoporotic Fractures, 5,995 men from the Osteoporotic Fractures in Men study) and found that initial high-trauma non-vertebral fractures were associated with low bone mineral density (BMD) and subsequent increase fracture risk to the same extent as low-trauma fractures. Similar findings had been noted in the Geelong Osteoporosis Study (7). Pooling study-level data from 5 randomized fracture-prevention trials (N= 30,118 women), antiresorptive treatments were found to reduce incident non-vertebral fracture risk regardless of the degree of trauma (8). Our findings support a more inclusive approach to prior fracture information that also extends to the routine clinical practice setting.
Ensrud et al (24) has previously shown that men are much more likely to sustain MOF associated with high-trauma than women. Specifically, the odds ratio of a fracture related to high-trauma fracture among men was more than 3 times greater than among women adjusted for other risk factors. Our findings were similar with a higher proportion of men in the high-trauma fracture group (23.0%) versus the low-trauma fracture group (12.8%).
Our findings have significant implications when it comes to assessment of fracture risk in patients with prior fracture and suggests that identifying a traumatic mechanism may be less important than previous considered, and support previous suggestions that high-trauma fractures be included as outcomes in osteoporosis trials, observational studies and fracture risk assessment (6, 8, 25–27). This potentially simplifies the collection of prior fracture information when used as an input to fracture risk assessment tools. Assessment of fracture mechanism and degree of trauma in clinical practice is difficult and inherently arbitrary. Most would agree that fall off a roof is a major traumatic event. Fall stepping off a curb or on the last step of a staircase is barely greater than standing height. There is no natural point that separates these two extremes. Similar difficulties arise in the setting of fractures related to sports or collisions, where a wide spectrum of energy mechanisms can exist. A fracture deemed high-trauma typically does not prompt further investigation for underlying osteoporosis and may inadvertently contribute to the post-fracture care gap (28). Although high-trauma fractures represent only a minority of the overall fracture burden, they may have a disproportionally large impact on osteoporosis management by creating confusion in the minds of patients and healthcare providers, leading some to suggest that attempts to define the level of trauma leading to fracture are counterproductive and that all fractures in older adults warrant evaluation for possible underlying osteoporosis (29). Such a strategy, if supported by consistent evidence, might simplify clinical practice guidelines.
The size of the cohort, long term follow-up and large number of incident fracture events are strengths to this analysis. Access to linked administrative data since 1987 also avoids recall bias. Limitations are also acknowledged. Although the presence of external injury codes ascertained from hospitalization data are likely to be indicative of a high-trauma mechanism, their absence may not exclude a high-trauma mechanism despite the designation of "low-trauma fracture". Notably, we found that among women with prior fracture, 4.9% were associated with trauma codes which is slightly lower than in the Study of Osteoporotic Fractures (6.3 – 8.9%) (6, 24). Among men, we found that of those with prior fractures 9.5% were associated with high-trauma codes, compared with 14.6 – 21.3% in the MrOS cohort (6, 24). It is likely that trauma codes appearing in hospitalization records reflect a more severe degree of injury. However, even among those with prior hip fractures, which are solely defined from hospitalization records, we found that incident MOF was significantly increased after both high-trauma (adjusted HR 1.38, 95% CI 1.01-1.88) and low-trauma events (1.18, 1.04-1.35). Administrative data are unable to directly assess the fracture mechanism and level of trauma. Our study did not prospectively recruit patients after fracture, and we cannot exclude differential referral such that patients with the highest level of trauma may not be referred for BMD. However, this would not affect the observed association between prior low-trauma fracture and incident high-trauma fracture. Finally, our analysis did not consider the time since prior fracture, but this did not differ for high-trauma fracture versus low-trauma fracture.
In summary, we found that high-trauma and low-trauma fractures showed similar relationships with low BMD and future fracture risk. This supports the inclusion of high-trauma fractures in clinical assessment for underlying osteoporosis and in the evaluation for intervention to reduce future fracture risk.
Supplementary Material
Mini-Abstract.
Prior high-trauma fractures identified through health services data are associated with low bone mineral density (BMD) and future fracture risk to the same extent as fractures without high-trauma.
Acknowledgments
The authors acknowledge the Manitoba Centre for Health Policy for use of data contained in the Population Health Research Data Repository (HIPC 2016/2017-29). The results and conclusions are those of the authors and no official endorsement by the Manitoba Centre for Health Policy, Manitoba Health, Seniors and Active Living, or other data providers is intended or should be inferred. This article has been reviewed and approved by the members of the Manitoba Bone Density Program Committee.
SNM is chercheur-boursier des Fonds de Recherche du Québec en Santé. LML is supported by a Tier I Canada Research Chair.
Funding
No funding support was received for this research.
Footnotes
Disclosures:
Suzanne Morin: Nothing to declare for the context of this paper, but has received research grants: Amgen, Merck.
Eugene McCloskey: Nothing to declare for the context of this paper, but numerous ad hoc consultancies/ speaking honoraria and/or research funding from Amgen, Bayer, General Electric, GSK, Hologic, Lilly, Merck Research Labs, Novartis, Novo Nordisk, Nycomed, Ono, Pfizer, ProStrakan, Roche, Sanofi-Aventis, Servier, Tethys, UBS and Warner-Chilcott
Nicholas Harvey: Nothing to declare for the context of this paper, but has received consultancy/ lecture fees/ honoraria/ grant funding from Alliance for Better Bone Health, Amgen, MSD, Eli Lilly, Servier, Shire, UCB, Consilient Healthcare, Radius Health, Kyowa Kirin and Internis Pharma.
John A. Kanis: Grants from Amgen, Lilly, Radius Health and non-financial support from Medimaps outside the submitted work
William Leslie, Patrick Martineau, Lisa Lix, Helena Johansson: No conflicts of interest.
Details of Contributors and Guarantor:
Authors' roles: conception, design, analysis, drafting the article (WDL), interpretation of data (All Authors); critically revising the article for important intellectual content (All Authors); final approval of the version to be published (All Authors); and agreement to be accountable for all aspects of the work (All Authors). WDL had full access to all the data in the study and takes the responsibility for the integrity of the data and the accuracy of the data analysis.
Contributor Information
John T. Schousboe, Email: scho0600@umn.edu.
Suzanne N. Morin, Email: suzanne.morin@mcgill.ca.
Patrick Martineau, Email: pmartineau@manitoba-physicians.ca.
Lisa M. Lix, Email: Lisa.Lix@umanitoba.ca.
Helena Johansson, Email: helena@statiq.se.
Eugene V. McCloskey, Email: e.v.mccloskey@sheffield.ac.uk.
Nicholas C. Harvey, Email: nch@mrc.soton.ac.uk.
John A. Kanis, Email: jakanis@outlook.com.
References
- 1.Compston JE, McClung MR, Leslie WD. Osteoporosis. The Lancet. 2019;393(10169):364–76. doi: 10.1016/S0140-6736(18)32112-3. [DOI] [PubMed] [Google Scholar]
- 2.Cosman F, de Beur SJ, LeBoff MS, Lewiecki EM, Tanner B, Randall S, et al. Clinician's Guide to Prevention and Treatment of Osteoporosis. Osteoporos Int. 2014;25(10):2359–81. doi: 10.1007/s00198-014-2794-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Papaioannou A, Morin S, Cheung AM, Atkinson S, Brown JP, Feldman S, et al. 2010 clinical practice guidelines for the diagnosis and management of osteoporosis in Canada: summary. CMAJ. 2010;182(17):1864–73. doi: 10.1503/cmaj.100771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Compston J, Cooper A, Cooper C, Gittoes N, Gregson C, Harvey N, et al. UK clinical guideline for the prevention and treatment of osteoporosis. Arch Osteoporos. 2017;12(1):43. doi: 10.1007/s11657-017-0324-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qaseem A, Snow V, Shekelle P, Hopkins R, Jr, Forciea MA, Owens DK, et al. Pharmacologic treatment of low bone density or osteoporosis to prevent fractures: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2008;149(6):404–15. [PubMed] [Google Scholar]
- 6.Mackey DC, Lui LY, Cawthon PM, Bauer DC, Nevitt MC, Cauley JA, et al. High-trauma fractures and low bone mineral density in older women and men. JAMA. 2007;298(20):2381–8. doi: 10.1001/jama.298.20.2381. [DOI] [PubMed] [Google Scholar]
- 7.Sanders KM, Pasco JA, Ugoni AM, Nicholson GC, Seeman E, Martin TJ, et al. The exclusion of high trauma fractures may underestimate the prevalence of bone fragility fractures in the community: the Geelong Osteoporosis Study. J Bone Miner Res. 1998;13(8):1337–42. doi: 10.1359/jbmr.1998.13.8.1337. [DOI] [PubMed] [Google Scholar]
- 8.Mackey DC, Black DM, Bauer DC, McCloskey EV, Eastell R, Mesenbrink P, et al. Effects of antiresorptive treatment on nonvertebral fracture outcomes. J Bone Miner Res. 2011;26(10):2411–8. doi: 10.1002/jbmr.446. [DOI] [PubMed] [Google Scholar]
- 9.Leslie WD, Metge C. Establishing a regional bone density program: lessons from the Manitoba experience. J Clin Densitom. 2003;6(3):275–82. doi: 10.1385/jcd:6:3:275. [DOI] [PubMed] [Google Scholar]
- 10.Leslie WD, Caetano PA, Macwilliam LR, Finlayson GS. Construction and validation of a population-based bone densitometry database. J Clin Densitom. 2005;8(1):25–30. doi: 10.1385/jcd:8:1:025. [DOI] [PubMed] [Google Scholar]
- 11.Lix LM, Azimaee M, Osman BA, Caetano P, Morin S, Metge C, et al. Osteoporosis-related fracture case definitions for population-based administrative data. BMC Public Health. 2012;12:301. doi: 10.1186/1471-2458-12-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Epp R, Alhrbi M, Ward L, Leslie WD. Radiological validation of fracture definitions from administrative data. J Bone Miner Res. 2018;33(Supp 1):S275. [Google Scholar]
- 13.Looker AC, Wahner HW, Dunn WL, Calvo MS, Harris TB, Heyse SP, et al. Updated data on proximal femur bone mineral levels of US adults. Osteoporos Int. 1998;8(5):468–89. doi: 10.1007/s001980050093. [DOI] [PubMed] [Google Scholar]
- 14.Kanis JA. Assessment of osteoporosis at the primary health-care level. Technical Report. Published by the University of Sheffield; 2007. Accessible at http://www.shef.ac.uk/FRAX/pdfs/WHO_Technical_Report.pdf. [Google Scholar]
- 15.Leslie WD, Lix LM, Johansson H, Oden A, McCloskey E, Kanis JA, et al. Independent clinical validation of a Canadian FRAX tool: fracture prediction and model calibration. J Bone Miner Res. 2010;25(11):2350–8. doi: 10.1002/jbmr.123. [DOI] [PubMed] [Google Scholar]
- 16.Kozyrskyj AL, Mustard CA. Validation of an electronic, population-based prescription database. Ann Pharmacother. 1998;32(11):1152–7. doi: 10.1345/aph.18117. [DOI] [PubMed] [Google Scholar]
- 17.Mustard CA, Derksen S, Berthelot JM, Wolfson M. Assessing ecologic proxies for household income: a comparison of household and neighbourhood level income measures in the study of population health status. Health Place. 1999;5(2):157–71. doi: 10.1016/s1353-8292(99)00008-8. [DOI] [PubMed] [Google Scholar]
- 18.Manitoba Centre for Health Policy. Income quintiles. [Last accessed February 10, 2019];2003 Available from: http://mchp-appserv.cpe.umanitoba.ca/viewDefinition.php?definitionID=102882.
- 19.Brennan SL, Yan L, Lix LM, Morin SN, Majumdar SR, Leslie WD. Sex- and age-specific associations between income and incident major osteoporotic fractures in Canadian men and women: a population-based analysis. Osteoporos Int. 2015;26(1):59–65. doi: 10.1007/s00198-014-2914-z. [DOI] [PubMed] [Google Scholar]
- 20.Smith NS, Weiner JP. Applying population-based case mix adjustment in managed care: the Johns Hopkins Ambulatory Care Group system. Manag Care Q. 1994;2(3):21–34. [PubMed] [Google Scholar]
- 21.Reid RJ, Roos NP, MacWilliam L, Frohlich N, Black C. Assessing population health care need using a claims-based ACG morbidity measure: a validation analysis in the Province of Manitoba. Health Serv Res. 2002;37(5):1345–64. doi: 10.1111/1475-6773.01029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leslie WD, Lix LM, Wu X, Manitoba Bone Density P Competing mortality and fracture risk assessment. Osteoporos Int. 2013;24(2):681–8. doi: 10.1007/s00198-012-2051-5. [DOI] [PubMed] [Google Scholar]
- 23.Satagopan JM, Ben-Porat L, Berwick M, Robson M, Kutler D, Auerbach AD. A note on competing risks in survival data analysis. Br J Cancer. 2004;91(7):1229–35. doi: 10.1038/sj.bjc.6602102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ensrud KE, Blackwell TL, Cawthon PM, Bauer DC, Fink HA, Schousboe JT, et al. Degree of Trauma Differs for Major Osteoporotic Fracture Events in Older Men Versus Older Women. J Bone Miner Res. 2016;31(1):204–7. doi: 10.1002/jbmr.2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kanis JA, Oden A, Johnell O, Jonsson B, de Laet C, Dawson A. The burden of osteoporotic fractures: a method for setting intervention thresholds. Osteoporos Int. 2001;12(5):417–27. doi: 10.1007/s001980170112. [DOI] [PubMed] [Google Scholar]
- 26.Hernlund E, Svedbom A, Ivergard M, Compston J, Cooper C, Stenmark J, et al. Osteoporosis in the European Union: medical management, epidemiology and economic burden. A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA) Arch Osteoporos. 2013;8(1–2):136. doi: 10.1007/s11657-013-0136-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grigorie D, Sucaliuc A, Johansson H, Kanis JA, McCloskey E. Incidence of hip fracture in Romania and the development of a Romanian FRAX model. Calcif Tissue Int. 2013;92(5):429–36. doi: 10.1007/s00223-013-9697-7. [DOI] [PubMed] [Google Scholar]
- 28.Khosla S, Cauley JA, Compston J, Kiel DP, Rosen C, Saag KG, et al. Addressing the Crisis in the Treatment of Osteoporosis: A Path Forward. J Bone Miner Res. 2016 doi: 10.1002/jbmr.3074. [DOI] [PubMed] [Google Scholar]
- 29.Binkley N, Blank RD, Leslie WD, Lewiecki EM, Eisman JA, Bilezikian JP. Osteoporosis in Crisis: It's Time to Focus on Fracture. J Bone Miner Res. 2017;32(7):1391–4. doi: 10.1002/jbmr.3182. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.