Abstract
Summary
Community-based screening and treatment of women age 70–85 years at high fracture risk reduced fractures; moreover, the screening programme was cost-saving. The results support a case for a screening programme of fracture risk in older women in the UK.
Purpose
The SCOOP (screening for prevention of fractures in older women) randomised controlled trial investigated whether community-based screening could reduce fractures in women age 70–85 years. The objective of this study was to estimate the long-term cost-effectiveness of screening for fracture risk in a UK primary care setting compared with usual management, based on the SCOOP study.
Methods
A health economic Markov model was used to predict the life-time consequences in terms of costs and quality of life of the screening programme compared with the control arm. The model was populated with costs related to drugs, administration and screening intervention derived from the SCOOP study. Fracture risk reduction in the screening arm compared with the usual management arm was derived from SCOOP. Modelled fracture risk corresponded to the risk observed in SCOOP.
Results
Screening of 1,000 patients saved 9 hip fractures and 20 non-hip fractures over the remaining lifetime (mean 14 years) compared with usual management. In total, the screening arm saved costs (£286) and gained 0.015 QALYs/patient in comparison with usual management arm.
Conclusions
This analysis suggests that a screening programme of fracture risk in older women in the UK would gain quality of life and life years, and reduce fracture costs to more than offset the cost of running the programme.
Keywords: Fracture risk assessment, Cost-effectiveness, United Kingdom, FRAX, Randomized controlled trial
Introduction
It is estimated that over 500,000 fragility fractures were sustained in the UK in 2010 [1]. Fragility fractures are associated with major societal costs and individual suffering. In 2010, the monetary burden of osteoporosis was £4,397 million in the UK, the majority of which was costs related to treating fractures. In addition, approximately 160,000 QALYs were lost in the same year due to incident and previous fractures. Although many available treatments for fracture prevention have been shown to be cost-effective in patients at high risk of fracture [2], there is a large “treatment gap”, in that a minority of individuals at high fracture risk are identified and receive appropriate treatment [3].
Historically, fracture risk has been assessed mainly by the use of bone mineral density (BMD) measurement alone. BMD has been shown, however, to have low sensitivity for fracture risk in that most fragility fractures arise in individuals without densitometric osteoporosis [4]. FRAX® is a tool freely accessible on the web (www.shef.ac.uk/FRAX), which was developed to improve the prediction of fracture risk [5]. FRAX calculates the 10-year hip and major osteoporotic fracture probabilities based on a set of clinical risk factors and is recommended, for example, by the National Institute for Health and Care Excellence (NICE) and the National Osteoporosis Guidelines Group (NOGG) for the assessment of patients in the UK [6, 7]. NOGG also uses FRAX probabilities as the basis for intervention thresholds.
Despite effective assessment tools and medications for osteoporosis, screening for fracture risk is not currently advocated in many countries, including the UK, although NICE recommends assessment of fracture risk in all women aged 65 years and older. A recent randomised controlled trial, the SCOOP study, investigated whether community-based screening could reduce fractures in women age 70 to 85 years [8]. Fracture risk was evaluated for patients randomised to the screening arm using the FRAX tool. If hip fracture probability was high, the patient was invited to undergo a dual energy X-ray absorptiometry scan (DXA) to measure BMD at the femoral neck. There was a small but non-significant reduction the incidence of all osteoporosis-related fractures (HR: 0.94, 95% CI: 0.85 to 1.03) but there was a significant reduction in the risk of hip fractures (HR: 0.72, 95% CI: 0.59 to 0.89). A cost-effectiveness analysis based on the SCOOP study, using a within-trial design limited to the time frame of the trial (5 years), provided evidence that screening was likely cost-effective and an efficient use of health care resources [9].
It is important to analyse the long-term consequences of screening for fracture risk in terms of costs and health-related outcomes to inform future decisions about such clinical strategies. The objective of this study was to estimate the long-term cost-effectiveness of screening for fracture risk in a UK primary care setting compared with usual management, based on the SCOOP study.
Methods
The SCOOP study
SCOOP (‘screening for prevention of fractures in older women’) was a pragmatic, unblinded, two parallel group randomised controlled trial to assess the effectiveness and cost-effectiveness of screening to prevent fractures in older women [8]. The primary endpoint was the proportion of participants experiencing at least one osteoporosis-related fracture over the 5-year follow-up. Women age 70–85 years were recruited from primary care in seven regions in England (Norwich, Southampton, Bristol, Birmingham, Manchester, York and Sheffield). Consenting participants were randomised to either a screening arm (n=6,233) or the control arm (n=6,250).
Using a baseline risk factor questionnaire, 10-year hip fracture probability with FRAX was calculated for participants randomised to the screening arm. The risk factors included in the questionnaire and used to calculate fracture probability were age, sex, height, weight, prior fragility fracture, parental history of hip fracture, current smoking, use of oral glucocorticoids, rheumatoid arthritis, other causes of secondary osteoporosis and high daily alcohol consumption (≥3 units/day). If the hip fracture probability was high (5.2–8.5%, depending on age), the participant was invited to undergo a DXA measurement at the femoral neck. Fracture probability was then re-calculated with the inclusion of the BMD result, and individuals with a fracture risk above the intervention threshold (hip fracture probability between 5.24% and 8.99% depending on age) were advised to make an appointment with their General Practitioner (GP) to discuss potential treatment. The GP was also informed directly of the screening result.
For the participants randomised to the control arm, a letter was sent to the GP informing them of their patient’s participation in the study. No additional information was provided to these women or their GP and they received standard management. The 10-year fracture probability using FRAX was calculated for the control arm participants at the end of the study.
Characteristics were similar across groups: the mean age was 75.4 years (SD 4.2) in the screening arm and 75.5 years (SD 4.1) in the control arm; FRAX 10-year hip fracture probability in the screening arm was 8.5% (SD 7.4) and 8.5% (SD 7.3) in the control arm ; the 10-year probability of a major osteoporotic fracture (distal forearm, clinical spine, proximal humerus or hip fracture) was 19.3% in the screening arm (SD 8.9) and 19.3% (SD 8.8) in the control arm.
Screening led to a relative reduction in hip fractures of 28% compared with the control arm (HR=0.72, 95% CI 0.59 to 0.89, p=0.002). The proportion of women who experienced an osteoporosis-related fracture was similar in the screening arm to the control arm (12.9% vs. 13.6%, HR 0.94, 95% CI 0.85 to 1.03, p=0.178).
Health economic model
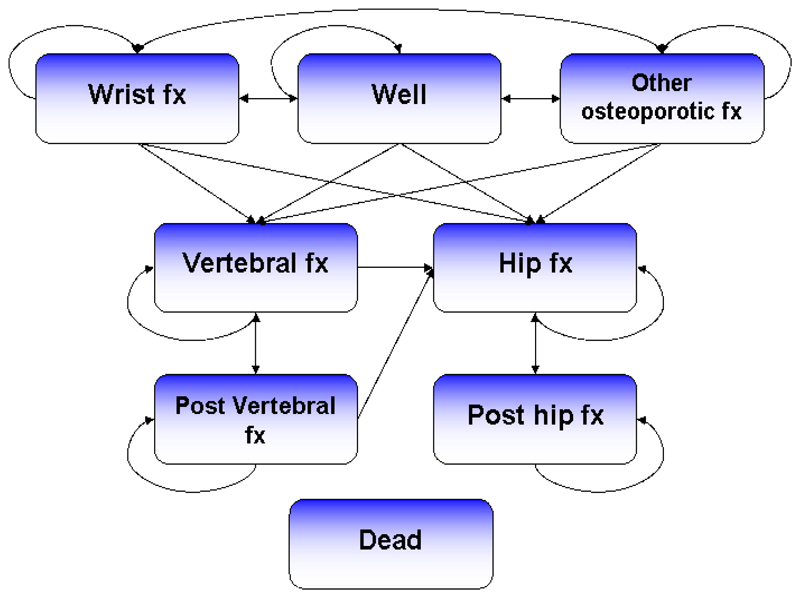
A health economic Markov model was used to predict the life-time consequences in terms of costs and quality of life of the screening programme compared with the control arm (hereafter termed usual management). The model was adapted based on previously published models of osteoporosis interventions [10–12]. The model used a six-month cycle length and the cohort was followed from study participation until death or an age of 100 years. The model consisted of eight health states, including: wrist fracture, vertebral fracture, hip fracture, other osteoporotic fracture, post-vertebral fracture, post-hip fracture, dead and well (i.e. without fracture). “Other osteoporotic fracture” was a composite health state comprising pelvis, rib, humerus, tibia, clavicle, scapula, sternum and other femoral fractures. Patients who sustained a hip/vertebral fracture transitioned to the post hip/vertebral fracture state in the cycle following the fracture and remained there until sustaining a new vertebral or hip/vertebral fracture or death occurred. All patients began in the “well” health state (including women with a past history of fracture at baseline). In each cycle, an individual was at risk of sustaining a fracture or death (Figure 1).
Fig. 1.
Model structure. Fx = fracture
Data
The model was populated with costs related to drugs, administration and screening intervention derived from the SCOOP data. Resource use and unit costs were derived from the study by Turner et al [9]. Treatment length was assumed to be as observed in the SCOOP trial, on average for 2 years and a maximum of 5 years. Fracture risk reduction in the screening arm compared with the usual management arm was derived from SCOOP data. Modelled fracture risk corresponded to the risk observed in SCOOP.
Clinical costs in the first and subsequent years after fracture were derived from two retrospective cohort studies that estimated fracture costs in postmenopausal women in the UK [13, 14]. In the “Well” health state, quality of life based on EQ-5D-3L was assumed to be equal to the age and gender matched general UK population taken from a study by Szende et al. [15]. Wrist, hip, vertebral and other fractures were assumed to have an impact on quality of life during the first year after fracture (weights 0.82, 0.55, 0.68 and 0.86, respectively). Hip and vertebral fractures were assumed to also have an impact on quality of life in subsequent years (weights 0.82 and 0.84, respectively). Quality of life weights, in the first year after fracture and subsequent years respectively, were derived from the International Costs and Utilities Related to Osteoporotic fractures Study (ICUROS) [16]. Annual mortality in the general female population was obtained from the Office for National Statistics dataset [17]. The relative risks of death in patients who had sustained a fracture compared with the general population were derived from a study by Jönsson et al. [12]. In agreement with previous health economic studies of osteoporotic treatments it was assumed that 30% of the excess mortality after a hip, vertebral, wrist and other osteoporotic fracture was related to the fracture event [10]. The remaining excess mortality was assumed to be related to other concomitant diseases [18, 19].
Analysis
The outcome measures were life years, quality-adjusted life years (QALYs), costs and incremental cost per QALY (ICER). Cost-effectiveness analysis evaluated the effectiveness of screening on QALYs relative to the cost compared with usual management. The intervention was assumed to be cost-effective if the ICER lay at or below NICE’s willingness-to-pay (WTP) threshold for recommending new treatment of £20,000–30,000 per QALY gained [20]. Intervention is assumed to be cost saving if it produces more health benefit at net cost savings. Costs and effects were discounted by 3.5% annually in accordance with NICE guidelines [20].
A probabilistic sensitivity analysis (PSA) was conducted by simultaneously sampling from estimated probability distributions of treatment effect of screening vs. usual management to obtain 1,000 sets of model input estimates. For each simulation, expected costs and QALYs were calculated for the screening arm and the usual management arm, respectively, along with the difference between comparators. Acceptability curves were constructed for the pairwise comparison.
Since the SCOOP study only provided evidence of an effect of screening compared with usual management for hip fracture risk reduction, a deterministic sensitivity analysis was conducted assuming that screening had an effect only on this risk.
Other deterministic sensitivity analyses were conducted to estimate the impact on the results of changing the discount rate, modelling time horizon, age, and assuming that 100% of the excess mortality of fracture was related to the fracture event.
Results
Base case scenario
Over the remaining lifetime (mean 14 years) of 1,000 women, screening saved 9 hip fractures and 20 non-hip fractures compared with usual management. Fracture-related costs were £551 lower per patient in the screening arm compared with the usual management arm (Table 1). Drug and intervention costs were £265 higher in the screening arm. In total, the screening arm was cost-saving (£286) and gained 0.015 QALYs/patient in comparison with usual management arm.
Table 1. Base case deterministic cost-effectiveness results.
| Usual management | Screening | Screening vs. usual management | |
|---|---|---|---|
|
|
|||
| Mean costs, per patient (£) | |||
| Hospitalisations | 3,059 | 2,934 | -125 |
| Nursing home | 6,056 | 5,645 | -410 |
| Outpatient | 378 | 363 | -15 |
| Total morbidity cost | 9,493 | 8,942 | -551 |
| Drugs | 12 | 43 | 31 |
| Treatment management | 92 | 326 | 234 |
| Total intervention cost | 104 | 369 | 265 |
| Total cost | 9,596 | 9,310 | -286 |
| Effects, per patient | |||
| Life years | 10.485 | 10.487 | 0.002 |
| QALYs | 7.359 | 7.374 | 0.015 |
| Cost-effectiveness ratios | |||
| Cost/Life year | Cost-saving | ||
| Cost/QALY | Cost-saving | ||
Probabilistic sensitivity analysis (PSA)
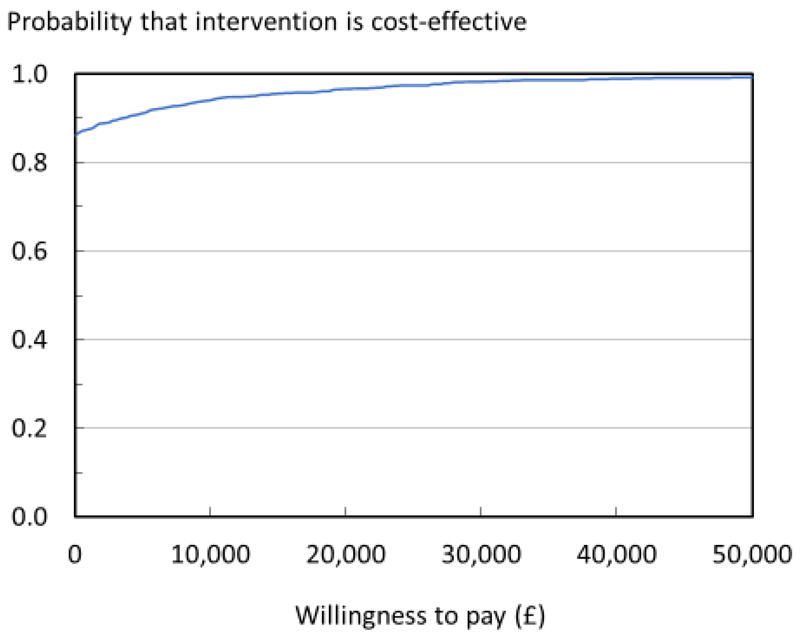
The PSA showed that the probability of screening being cost-effective at a willingness-to-pay (WTP) of £20,000 was 97% and 98% at a WTP of £30,000. In 87% of the PSA simulations, screening was cost-saving, i.e. saved costs and gained QALYs, compared with usual management. Mean difference in QALYs (screening vs. usual management) was 0.015 (95% CI 0.007, 0.023). Mean difference in costs was £-281 (95% CI -579, -77).
Deterministic sensitivity analyses
When it was assumed that screening had an effect only on the risk of hip fracture, the QALY gained and cost-savings were lower compared with the base case scenario. However, the results showed that screening would still be cost-saving if the programme had an effect on hip fractures alone (Table 2).
Table 2. Results of deterministic sensitivity analysis assuming screening had an effect only on the risk of hip fracture.
| Usual management | Screening | Screening vs. usual management | |
|---|---|---|---|
| Total cost (£), per patient | 9,596 | 9,355 | -241 |
| QALYs, per patient | 7.359 | 7.369 | 0.011 |
| Cost/QALY | Cost-saving |
Other sensitivity analyses
The impact of changing modelling assumptions on the QALY gained of screening compared with usual management is shown in Table 3. In all scenarios, screening was cost-saving (not shown in table). The results were most sensitive to assuming a 0% discount rate on effects and costs. Results were also rather sensitive to assuming that 100% of excess mortality was attributable to fractures. Applying a 10-year time horizon had a small negative impact in this population of older women. An additional sensitivity analysis (not shown in Table 3), showed that the cost per QALY gained of screening vs. usual management was age dependent. Screening became cost-neutral at the start age of 71 years.
Table 3. Other sensitivity analyses.
| Scenario | QALY difference per patient, screening vs. usual management |
|---|---|
| Base case | 0.015 |
| 5% discount rates | 0.014 |
| 0% discount rates | 0.020 |
| 10-year modelling time horizon | 0.012 |
| 100% of excess mortality attributable to fractures | 0.019 |
Discussion
Osteoporosis is a silent disease that tends to go underdiagnosed and undertreated despite the availability of effective assessment tools and medications. This study estimated the cost-effectiveness of screening for hip fracture risk with the FRAX tool compared with usual management in UK primary care setting, using a health economic Markov model to estimate the life-time consequences of screening. The population modelled and fracture risks were based on to the SCOOP study. We found that screening is expected to gain 0.015 QALYs and save £286 per patient because of fracture risk reduction compared with usual management; the conclusions were robust across a number of sensitivity analyses. This study indicates that screening for fracture risk in elderly women is an efficient means to identify high risk patients, improve treatment uptake in these patients, and ultimately improve health outcomes.
Our study has a number of strengths and limitations. A major strength is the use of fracture risk data and costs as observed in the SCOOP pragmatic trial, permitting an accurate depiction of the relative effect of screening based in a real-world setting. The structure of the Markov model used in this study is validated and adapted based on a model used in previously published studies [12]. A limitation of the model is that it has a hierarchical structure that causes a slight underestimation of the number of less severe fractures, as patients suffering a hip or vertebral fracture cannot subsequently sustain wrist or other fractures in following cycles (i.e. remain in post hip/vertebral state). Some other assumptions were necessary to estimate the life-time consequences of screening and usual management. The most conservative assumption was that the effect of screening was only modelled for a maximum of 5 years, - the follow-up time in the SCOOP study, and thereafter women in the screening arm were assumed to be at the same fracture risk as the usual management arm. This assumption sets a ceiling on the potential gains as it ignores the fact that many high-risk women would continue on treatment for longer than 5 years and that the offset of treatment effects is not immediate. Population-based studies show long term reductions in hip fractures risk during longer exposures to anti-resorptive medications similar to those used in SCOOP [21]. The assumption that the protective effect on fracture risk ceases on the day of medication discontinuation is very conservative and further contributes to the ceiling effect. For example, oral bisphosphonates, the predominant treatment prescribed for high risk patients in SCOOP, are known to at least partially reduce bone turnover and bone mineral density for several months or years following discontinuation [22, 23], with likely persisting effects on fracture risk. The continuing therapeutic effect when treatment is stopped is consistent with recent suggestions that patients could undertake a break from bisphosphonate therapy without immediately losing fracture protection [24–27]. An assumption of a linear offset of the fracture protective effect over a few years has been built into previous models of oral bisphosphonate and has a significant impact on improving cost-effectiveness [28].
The cost-effectiveness analysis based on the SCOOP study by Turner et al [9] used, in contrast to the analysis in this paper, a within-trial design limited to the time frame of the trial (5 years). Additionally, it used questionnaires (EQ-5D-3L) to document self-reported quality of life (QoL) at 6-month intervals during the first year and then at annual intervals to derive QALYs. The results showed that the QALYs per person gained with screening was 0.0237 (95% CI: -0.0034 to 0.0508) with an incremental total health care cost of £66 (95% CI -21.7, 153) compared with usual management, providing a cost per QALY gained of £2,772. This compares with the longer-term gains of 0.015 QALYs (95% CI 0.007, 0.023) per person and a £241 (95% CI -579, -77) lower cost yielding a cost-saving result in the present analysis. Confidence intervals for the within trial analysis of the effect on QALYs are broader than the model-based values and overlap these values. The expectation, given the different study designs, would be that modelling over a longer time period (remaining lifetime) would provide larger cost-savings [29]. While we might also expect a larger average QALY gain over a longer timeframe, the within-trial analysis showed a larger apparent gain (0.0237 vs. 0.015), despite the QoL questionnaires not being administered at the time of the incident fracture events. The reason for the difference is not entirely clear. One potential explanation, besides the inherent differences in study design between modelling and within-trial analysis, could be that the within-trial study design captured an additional element of quality of life improvement resulting from screening, an improvement that hasn’t been noted in screening studies in other diseases [29, 30]. Alternatively, it could be an effect related to pharmaceutical treatment or fracture prevention, but this seems unlikely given the small proportion of patients on treatment in the screening arm (approximately 14%). It remains possible that the measured larger gain in QALYs occurred by chance, given that SCOOP was not primarily powered to detect a statistical difference in QALYs between arms, and indeed there was no evidence of such a difference as displayed in the confidence interval around the mean [9]. Disregarding the unexpected difference in QALYs gained between the two studies, it is clear that both studies used well accepted health economic methods, and both consistently indicate that a screening programme of fracture risk is a cost-effective intervention.
A small number of randomised studies have now addressed the potential benefit of population screening for fracture prevention. In the Aberdeen Prospective Osteoporosis Screening Study (APOSS), 4,800 women aged 45–54 years were screened by BMD. Osteoporosis treatment, predominantly hormone replacement therapy (HRT) was recommended to those women with BMD in the lowest quartile of the screening arm. The study showed an increase in HRT uptake in those so identified [31], and a parallel increase in uptake of other osteoporosis therapies [32]. After a follow-up of 9 years, the study reported weak evidence of a decrease in fracture rates in the screened arm, but this did not alter the UK National Screening Committee’s position of not recommending screening in osteoporosis [33]. The latter decision was underpinned by the conclusion that there was no randomised controlled trial assessing the clinical and cost-effectiveness of any current approach to screening for osteoporosis. The SCOOP study has now addressed this, and recently another study of screening using the FRAX tool has been published from Denmark [34–36]. While no formal cost-effectiveness analysis has yet been published from the latter study, the FRAX approach in both studies reduces the use of bone mineral density measurements, thus reducing this contribution to screening costs. A systematic review found that screen-and-treat strategies based on FRAX and similar tools were more cost-effective compared with selecting patients based solely on age, gender and BMD [37]. This may be explained by lower screening costs due to avoided BMD measurements, but also that FRAX and similar tools enables finding patients where it is cost-effective to treat even at lower ages (e.g. 50–60 years).
Conclusion
The burden of fragility fractures in ageing populations is high and increasing. There is a substantial treatment gap so that most women at high risk of fracture are neither identified nor treated. This analysis suggests that a screening programme for fracture risk in older women in the UK could gain quality of life and life years, and reduce fracture costs so as to more than offset the cost of running the programme.
Fig. 2.
Cost-effectiveness acceptability curve for pairwise comparison of screening vs. no screening.
Acknowledgements
Ethical approval was obtained from the North Western - Haydock Research Ethics Committee of England in September 2007 (REC 07/H1010/70). The trial was registered on the International Standard Randomised Controlled Trial Register in June 2007 (ISRCTN 55814835). The Arthritis Research United Kingdom (ARUK), formerly the Arthritis Research Campaign (ARC), and the Medical Research Council (MRC) of the UK jointly funded this trial.
Footnotes
Disclosures
C Cooper has received consultancy fees and honoraria from Amgen, Danone, Eli Lilly, GlaxoSmithKline, Medtronic, Merck, Nestlé, Novartis, Pfzer, Roche, Servier, Shire, Takeda, and UCB. N Harvey has received consultancy, lecture fees, and honoraria from Alliance for Better Bone Health, Amgen, MSD, Eli Lilly, Servier, Shire, UCB, Consilient Healthcare, and Internis Pharma JA Kanis reports grants from UCB, Amgen and Radius Health outside the submitted work. E McCloskey has been, or currently is, an adviser or speaker for and has received research support from ActiveSignal, Amgen, AstraZeneca, Consilient Healthcare, GlaxoSmithKline, Hologic, Internis, Eli Lilly, Medtronic, Merck, Novartis, Pfzer, Roche, Sanof-Aventis, Servier, Synexus, Tethys, UCB, and Warner Chilcott; and has received research support from I3 Innovus, International Osteoporosis Foundation, and Unilever. E Söreskog and F Borgström have previously consulted for companies marketing products for osteoporosis. T O’Neill has received consultancy fees from UCB and research support from Amgen outside the submitted work.
S Clarke, I Harvey, R Holland, A Howe, H Johansson, T Marshall, T Peters, N Redmond, L Shepstone have nothing to disclose.
References
- 1.Svedbom A, Hernlund E, Ivergard M, Compston J, Cooper C, Stenmark J, McCloskey EV, Jonsson B, Kanis JA, IOF, EURPo Osteoporosis in the European Union: a compendium of country-specific reports. Arch Osteoporos. 2013;8:137. doi: 10.1007/s11657-013-0137-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kanis JA, Cooper C, Rizzoli R, Reginster JY, on behalf of the Scientific Advisory Board of the European Society for, C, Economic Aspects of, O, the Committees of Scientific, A, and National Societies of the International Osteoporosis, F European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporosis International. 2019;30(1):3–44. doi: 10.1007/s00198-018-4704-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kanis JA, Borgstrom F, Compston J, Dreinhofer K, Nolte E, Jonsson L, Lems WF, McCloskey EV, Rizzoli R, Stenmark J. SCOPE: a scorecard for osteoporosis in Europe. Arch Osteoporos. 2013;8:144. doi: 10.1007/s11657-013-0144-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kanis JA, Johnell O, Oden A, Johansson H, McCloskey E. FRAX and the assessment of fracture probability in men and women from the UK. Osteoporos Int. 2008;19(4):385–97. doi: 10.1007/s00198-007-0543-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kanis JA, Oden A, Johnell O, Johansson H, De Laet C, Brown J, Burckhardt P, Cooper C, Christiansen C, Cummings S, Eisman JA, et al. The use of clinical risk factors enhances the performance of BMD in the prediction of hip and osteoporotic fractures in men and women. Osteoporos Int. 2007;18(8):1033–46. doi: 10.1007/s00198-007-0343-y. [DOI] [PubMed] [Google Scholar]
- 6.National Institute for Health and Care Excellence (NICE) NICE clinical guideline 146. [cited Access 2014];Osteoporosis: assessing the risk of fragility fracture. 2014 of Medium], Last Update. [PubMed] [Google Scholar]
- 7.Compston J, Cooper A, Cooper C, Gittoes N, Gregson C, Harvey N, Hope S, Kanis JA, McCloskey EV, Poole KES, Reid DM, et al. UK clinical guideline for the prevention and treatment of osteoporosis. Arch Osteoporos. 2017;12(1):43. doi: 10.1007/s11657-017-0324-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shepstone L, Lenaghan E, Cooper C, Clarke S, Fong-Soe-Khioe R, Fordham R, Gittoes N, Harvey I, Harvey N, Heawood A, Holland R, et al. Screening in the community to reduce fractures in older women (SCOOP): a randomised controlled trial. Lancet. 2018;391(10122):741–747. doi: 10.1016/S0140-6736(17)32640-5. [DOI] [PubMed] [Google Scholar]
- 9.Turner DA, Khioe RFS, Shepstone L, Lenaghan E, Cooper C, Gittoes N, Harvey NC, Holland R, Howe A, McCloskey E, O’Neill TW, et al. The Cost-Effectiveness of Screening in the Community to Reduce Osteoporotic Fractures in Older Women in the UK: Economic Evaluation of the SCOOP Study. J Bone Miner Res. 2018;33(5):845–851. doi: 10.1002/jbmr.3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Strom O, Borgstrom F, Sen SS, Boonen S, Haentjens P, Johnell O, Kanis JA. Cost-effectiveness of alendronate in the treatment of postmenopausal women in 9 European countries--an economic evaluation based on the fracture intervention trial. Osteoporos Int. 2007;18(8):1047–61. doi: 10.1007/s00198-007-0349-5. [DOI] [PubMed] [Google Scholar]
- 11.Borgstrom F, Jonsson B, Strom O, Kanis JA. An economic evaluation of strontium ranelate in the treatment of osteoporosis in a Swedish setting: based on the results of the SOTI and TROPOS trials. Osteoporos Int. 2006;17(12):1781–93. doi: 10.1007/s00198-006-0193-z. [DOI] [PubMed] [Google Scholar]
- 12.Jonsson B, Strom O, Eisman JA, Papaioannou A, Siris ES, Tosteson A, Kanis JA. Cost-effectiveness of Denosumab for the treatment of postmenopausal osteoporosis. Osteoporos Int. 2011;22(3):967–82. doi: 10.1007/s00198-010-1424-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gutierrez L, Roskell N, Castellsague J, Beard S, Rycroft C, Abeysinghe S, Shannon P, Gitlin M, Robbins S. Clinical burden and incremental cost of fractures in postmenopausal women in the United Kingdom. Bone. 2012;51(3):324–31. doi: 10.1016/j.bone.2012.05.020. [DOI] [PubMed] [Google Scholar]
- 14.Gutierrez L, Roskell N, Castellsague J, Beard S, Rycroft C, Abeysinghe S, Shannon P, Robbins S, Gitlin M. Study of the incremental cost and clinical burden of hip fractures in postmenopausal women in the United Kingdom. J Med Econ. 2011;14(1):99–107. doi: 10.3111/13696998.2010.547967. [DOI] [PubMed] [Google Scholar]
- 15.Szende A, Janssen B, Cabases J. Self-Reported Population Health: An International Perspective based on EQ-5D. Springer Netherlands; 2014. [PubMed] [Google Scholar]
- 16.Kanis JA, Johansson H, Oden A, Harvey NC, Gudnason V, Sanders KM, Sigurdsson G, Siggeirsdottir K, Fitzpatrick LA, Borgstrom F, McCloskey EV. Characteristics of recurrent fractures. Osteoporos Int. 2018;29(8):1747–1757. doi: 10.1007/s00198-018-4502-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dataset. National life tables: Great Britain (2014-2016) [Accessed 2018-11-27]; Available from: https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/lifeexpectancies/datasets/nationallifetablesgreatbritainreferencetables.
- 18.Parker MJ, Anand JK. What is the true mortality of hip fractures? Public Health. 1991;105(6):443–6. doi: 10.1016/s0033-3506(05)80614-6. [DOI] [PubMed] [Google Scholar]
- 19.Kanis JA, Oden A, Johnell O, De Laet C, Jonsson B, Oglesby AK. The components of excess mortality after hip fracture. Bone. 2003;32(5):468–73. doi: 10.1016/s8756-3282(03)00061-9. [DOI] [PubMed] [Google Scholar]
- 20.National Institute for Health and Care Excellence (NICE) Guide to the methods of technology appraisal 2013. https://www.nice.org.uk/process/pmg9/chapter/foreword. [PubMed]
- 21.Abrahamsen B, Eiken P, Prieto-Alhambra D, Eastell R. Risk of hip, subtrochanteric, and femoral shaft fractures among mid and long term users of alendronate: nationwide cohort and nested case-control study. BMJ. 2016;353:i3365. doi: 10.1136/bmj.i3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Black DM, Schwartz AV, Ensrud KE, Cauley JA, Levis S, Quandt SA, Satterfield S, Wallace RB, Bauer DC, Palermo L, Wehren LE, et al. Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA. 2006;296(24):2927–38. doi: 10.1001/jama.296.24.2927. [DOI] [PubMed] [Google Scholar]
- 23.Ensrud KE, Barrett-Connor EL, Schwartz A, Santora AC, Bauer DC, Suryawanshi S, Feldstein A, Haskell WL, Hochberg MC, Torner JC, Lombardi A, et al. Randomized trial of effect of alendronate continuation versus discontinuation in women with low BMD: results from the Fracture Intervention Trial long-term extension. J Bone Miner Res. 2004;19(8):1259–69. doi: 10.1359/JBMR.040326. [DOI] [PubMed] [Google Scholar]
- 24.Adams AL, Adams JL, Raebel MA, Tang BT, Kuntz JL, Vijayadeva V, McGlynn EA, Gozansky WS. Bisphosphonate Drug Holiday and Fracture Risk: A Population-Based Cohort Study. J Bone Miner Res. 2018;33(7):1252–1259. doi: 10.1002/jbmr.3420. [DOI] [PubMed] [Google Scholar]
- 25.Adler RA, El-Hajj Fuleihan G, Bauer DC, Camacho PM, Clarke BL, Clines GA, Compston JE, Drake MT, Edwards BJ, Favus MJ, Greenspan SL, et al. Managing Osteoporosis in Patients on Long-Term Bisphosphonate Treatment: Report of a Task Force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2016;31(1):16–35. doi: 10.1002/jbmr.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McClung M, Harris ST, Miller PD, Bauer DC, Davison KS, Dian L, Hanley DA, Kendler DL, Yuen CK, Lewiecki EM. Bisphosphonate therapy for osteoporosis: benefits, risks, and drug holiday. Am J Med. 2013;126(1):13–20. doi: 10.1016/j.amjmed.2012.06.023. [DOI] [PubMed] [Google Scholar]
- 27.Curtis JR, Westfall AO, Cheng H, Delzell E, Saag KG. Risk of hip fracture after bisphosphonate discontinuation: implications for a drug holiday. Osteoporos Int. 2008;19(11):1613–20. doi: 10.1007/s00198-008-0604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jonsson B, Kanis J, Dawson A, Oden A, Johnell O. Effect and offset of effect of treatments for hip fracture on health outcomes. Osteoporos Int. 1999;10(3):193–9. doi: 10.1007/s001980050215. [DOI] [PubMed] [Google Scholar]
- 29.Edelman D, Olsen MK, Dudley TK, Harris AC, Oddone EZ. Impact of diabetes screening on quality of life. Diabetes Care. 2002;25(6):1022–6. doi: 10.2337/diacare.25.6.1022. [DOI] [PubMed] [Google Scholar]
- 30.Collins RE, Lopez LM, Marteau TM. Emotional impact of screening: a systematic review and meta-analysis. BMC Public Health. 2011;11:603. doi: 10.1186/1471-2458-11-603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Torgerson DJ, Thomas RE, Campbell MK, Reid DM. Randomized trial of osteoporosis screening. Use of hormone replacement therapy and quality-of-life results. Arch Intern Med. 1997;157(18):2121–5. doi: 10.1001/archinte.157.18.2121. [DOI] [PubMed] [Google Scholar]
- 32.Barr RJ, Stewart A, Torgerson DJ, Reid DM. Population screening for osteoporosis risk: a randomised control trial of medication use and fracture risk. Osteoporos Int. 2010;21(4):561–8. doi: 10.1007/s00198-009-1007-x. [DOI] [PubMed] [Google Scholar]
- 33.Peto L, Allaby M. Screening for osteoporosis in postmenopausal women. [cited Access 2013];A report for the UK National Screening Committee. 2013 of Medium], Last Update, Available from: https://legacyscreening.phe.org.uk/policydb_download.php?doc=270. [Google Scholar]
- 34.Hoiberg MP, Rubin KH, Holmberg T, Rothmann MJ, Moller S, Gram J, Bech M, Brixen K, Hermann AP. Use of antiosteoporotic medication in the Danish ROSE population-based screening study. Osteoporos Int. 2019;30(6):1223–1233. doi: 10.1007/s00198-019-04934-7. [DOI] [PubMed] [Google Scholar]
- 35.Rubin KH, Rothmann MJ, Holmberg T, Hoiberg M, Moller S, Barkmann R, Gluer CC, Hermann AP, Bech M, Gram J, Brixen K. Effectiveness of a two-step population-based osteoporosis screening program using FRAX: the randomized Risk-stratified Osteoporosis Strategy Evaluation (ROSE) study. Osteoporos Int. 2018;29(3):567–578. doi: 10.1007/s00198-017-4326-3. [DOI] [PubMed] [Google Scholar]
- 36.Rothmann MJ, Moller S, Holmberg T, Hojberg M, Gram J, Bech M, Brixen K, Hermann AP, Gluer CC, Barkmann R, Rubin KH. Non-participation in systematic screening for osteoporosis-the ROSE trial. Osteoporos Int. 2017;28(12):3389–3399. doi: 10.1007/s00198-017-4205-y. [DOI] [PubMed] [Google Scholar]
- 37.Muller D, Pulm J, Gandjour A. Cost-effectiveness of different strategies for selecting and treating individuals at increased risk of osteoporosis or osteopenia: a systematic review. Value Health. 2012;15(2):284–98. doi: 10.1016/j.jval.2011.11.030. [DOI] [PubMed] [Google Scholar]